Patents
Literature
97 results about "2-Mercaptoethanol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
2-Mercaptoethanol (also β-mercaptoethanol, BME, 2BME, 2-ME or β-met) is the chemical compound with the formula HOCH₂CH₂SH. ME or βME, as it is commonly abbreviated, is used to reduce disulfide bonds and can act as a biological antioxidant by scavenging hydroxyl radicals (amongst others). It is widely used because the hydroxyl group confers solubility in water and lowers the volatility. Due to its diminished vapor pressure, its odor, while unpleasant, is less objectionable than related thiols.
Sensor with improved shelf life
InactiveUS20080121533A1Weather/light/corrosion resistanceVolume/mass flow measurementMetal electrodesEthylamine
Owner:LIFESCAN INC
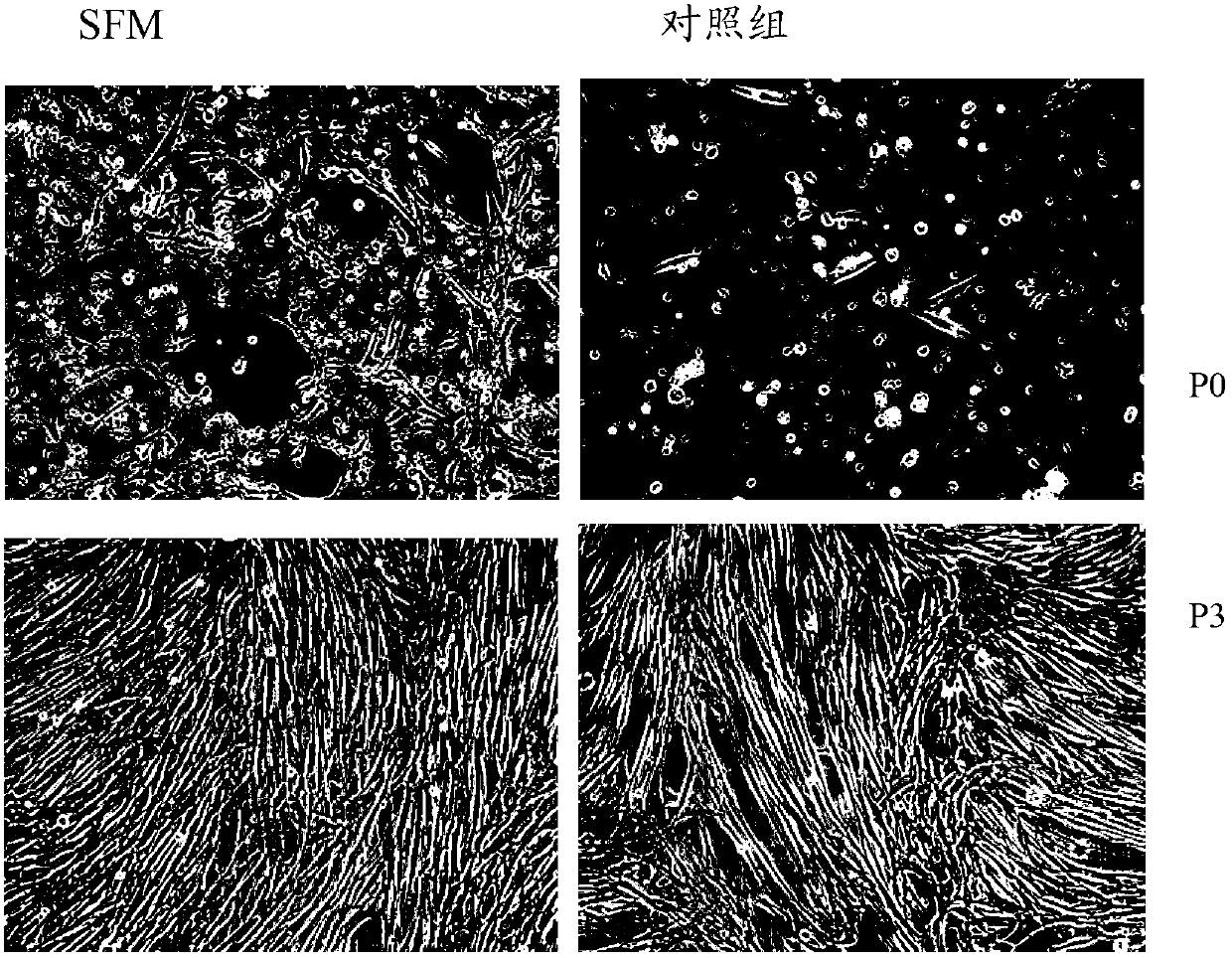
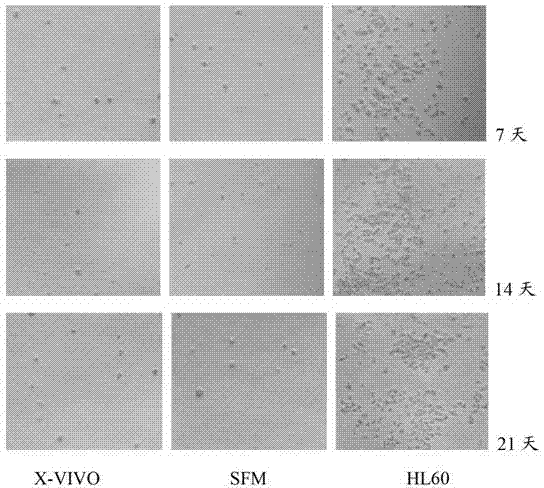
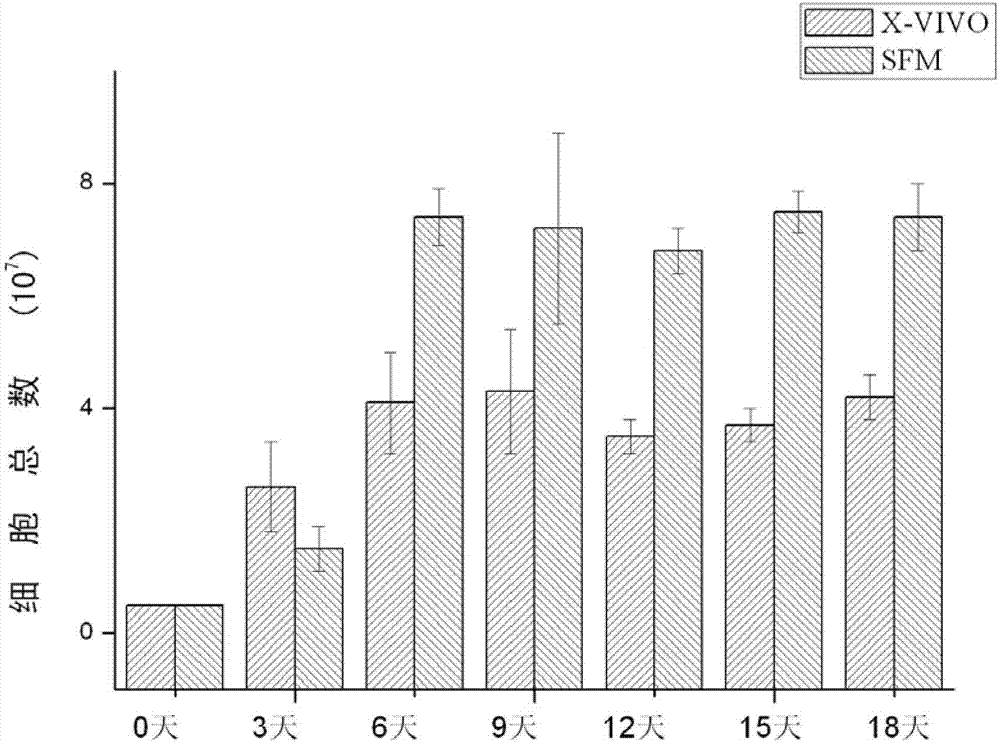
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Complete medium and human amnion-derived mesenchymal stem cell culture method
The invention discloses a complete medium and a human amnion-derived mesenchymal stem cell (hAMSCs) culture method. The complete medium is prepared by adding 3 to 10 percent of autologous umbilical cord blood serum into low-sugar Dulbecco minimum essential medium solution according to a volume ratio. The culture method comprises: (1) separation; (2) primary culture; and (3) subculture. The methodusing the complete medium in the hAMSCs culture has the advantages that: the risk of using fetal calf serum is avoided; although the need of adding L-glutamine, non-essential amino acid, 2-mercapitoethanol, pyruvic acid and the like is obviated, the high proliferation properties and phenotypic characteristics of the hAMSCs and expression of multilineage differentiation marker genes sand proteins of some stem cells can still be retained; and in subculture, the wall adherence fastness of the hAMSCs is much lower than that in fetal bovine serum (FBS) culture, the digestion time is reduced obviously, and the damage of trypsinization to cells and loss of cells are reduced.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Preparation method of waterproof soyabean protein adhesive
InactiveCN102002338AImprove water-resistant bonding performanceAntisepticProtein adhesivesGlue/gelatin preparationFiberAdhesive
The invention relates to a preparation method of a waterproof soyabean protein adhesive, comprising the following steps of: adding soyabean protein and water, which is used as a solvent, to a reaction vessel, then sequentially adding urea, sodium dodecyl benzene sulfonate and 2-mercaptoethanol, and fully and uniformly mixing all components by utilizing high-shear dispersive action in the condition of room temperature for reaction to obtain a soyabean protein emulsion; and then adding glycerol to enhance the compatibility of the soyabean protein emulsion with epoxy resins added subsequently; sequentially adding the epoxy resin and a curing agent of triethylene diamine hexahydrate to the reaction vessel, uniformly mixing for reacting at 40-80 DEG C to prepare the waterproof soyabean protein adhesive. With the method, the soyabean protein adhesive with excellent waterproof property is synthesized, can not generate poisonous and harmful matters such as formaldehyde, phenol and the like, is environmental-protection and sanitary when in use, and can be applied to the fields of woody laminated boards, shaving boards, fiber boards and the like.
Owner:NANJING FORESTRY UNIV
Serum-free adipose tissue-derived mesenchymal stem cell culture medium
ActiveCN103255103AAvoid exogenous contaminationAvoid the influence of cultivationSkeletal/connective tissue cellsPenicillinCuticle
The invention relates to a serum-free adipose tissue-derived mesenchymal stem cell culture medium, which consists of a basic culture medium and added ingredients, wherein the basic culture medium is DMEM-LG, and the added ingredients and the content of each added ingredient are shown as follows: 5 to 20ng / mL of alkaline fiberblast growth factors, 5 to 20ng / mL of epidermal growth factors, 100U / mL of penicillin, 100 micrograms / mL of streptomycin, 50 to 200 micrograms / mL of heparin, 2 to 8mM of L-glutamine, 100 to 300 microM of 2-mercaptoethanol, 500 to 2000U / mL of leukaemia inhibitory factors and 0.5 to 2mM of sodium pyruvate. The serum-free adipose tissue-derived mesenchymal stem cell culture medium does not contain the serum, so that the inter-batch difference and the influence of the serum component on the cell culture can be avoided; the exogenous pollution of the serum and the toxicity of the serum on the cells can be avoided; and the ingredients are clear, so that the research of the psychological regulation mechanism of the cells can be facilitated.
Owner:冯文峰
Method of preparing vinyl alcohol with low degree of polymerization
The invention relates to a method for producing low polymerization degree polyvinyl alcohol including: mixing the monomer vinyl acetate with solvent carbinol in a mass ratio of 60-80:20, adding ignition primer diisopropyl azodicarboxylate taking 0.1-0.5% of the monomer mass and chain transfer agent 2-mercaptoethanol taking 0.1-0.5% of the monomer mass, producing low polymerization degree polyvinyl acetate by solution polymerization method; dissolving the low polymerization degree polyvinyl acetate in the carbinol to get polyvinyl acetate carbinol solution, adding NaOH carbinol solution, alcoholysis to get low polymerization degree polyvinyl alcohol. The inventive method can produce low polymerization degree polyvinyl alcohol with a low polymerization degree of 50-500.
Owner:ZHONGBEI UNIV
Animal source-free and serum-free culture medium of lymphocyte
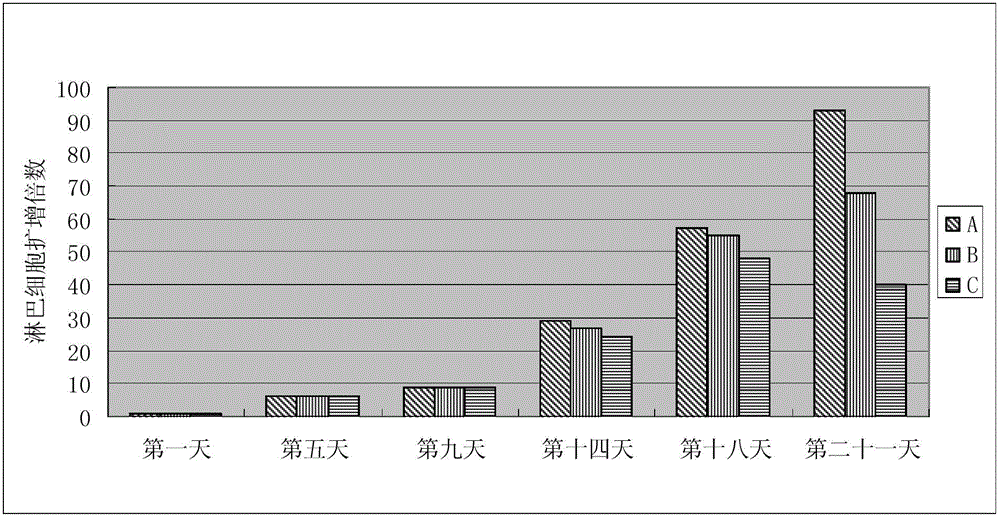
InactiveCN103146648AGood amplification factorEnhance cell viabilityBlood/immune system cellsCell phenotypeSodium bicarbonate
The invention relates to the biological field and discloses an animal source-free and serum-free culture medium of lymphocyte. The culture medium disclosed by the invention essentially consists of IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, human transferrin, human serum albumin, 2-mercaptoethanol, N-acetyl-cysteine, lipid, amino acid, vitamin, microelement, ferric citrate, hydrocortisone, cholamine and non-essential amino acid. The serum-free culture medium disclosed by the invention has the advantages of clear chemical components, no animal source, no serum, safe and ideal culture cells; the instability caused by the doping of animal components and batches is avoided; the result of culturing lymphocyte shows that the total number of the cells and the cell phenotypes are normal; and the serum-free culture medium disclosed by the invention has a good industrial application prospect.
Owner:BEIJING JING MENG STEM CELL TECH
Extraction method of plant total protein and special extract for the same
InactiveCN1908007AHigh extraction rateQuality improvementPreparing sample for investigationPlant peptidesEthylenediamineHalophyte
the invention discloses an extracting method of total plant protein and specific extracting liquid, which comprises the following parts with pH value at 7.5-8.5: 80-120mM EDTA, 80-120mM trimethyl aminomethane, 40-60mM sodium tetraborate and 40-60mM vitamin C, 0.8-1.2% polyvinyl polypyrrolidon,0.8-1.2% Triton-100, 1.5-2.5% 2-mercaptoethanol and 28-32% saccharose solution.
Owner:INST OF BOTANY CHINESE ACAD OF SCI
Human serum-free culture medium and preparation method thereof
ActiveCN102191215AExcellent proliferation rateImprove securityBlood/immune system cellsLipid formationCatalase
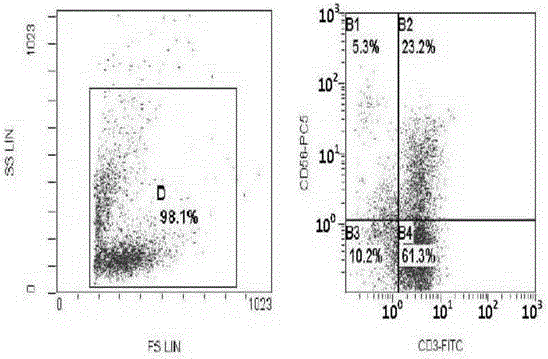
The invention discloses a human serum-free culture medium and a preparation method thereof. The human serum-free culture medium uses eleven raw materials, namely human serum albumin solution for treatment, human recombinant insulin solution, human transferrin solution, human cholesterol solution, human catalase solution, 2-mercaptoethanol solution, ascorbic acid solution, linoleic acid solution, ethanolamine solution, human vitronectin solution and L-glutamine solution; the added protein and lipid are both from the blood plasma, serum or tissue of human, the protein is pharmaceutical-grade orhighly purified human protein or human recombinant protein, the protein and lipid do not contains any animal component, the other components all meet the United States Pharmacopoeia or national standards; and the human serum-free culture medium is qualified through the cell culture test and is clinical, safe and reasonable human serum-free culture medium. The proliferation rate of the CIK cell cultured and inducted by the human serum-free culture medium is better than that of the CIK cell cultured and inducted by the culture medium with serum, the cell CD3+CD56+ percentage and the killing rate to the K562 leukemic cell are similar to that of the culture medium with serum. By adopting the human serum-free culture medium, the safety and standardization of cell therapy can be increased.
Owner:湘雅生物医药(湖州)有限公司
Detection method for gamma-aminobutyric acid content in food by using high performance liquid chromatography method
InactiveCN102230921AMeet studySatisfy productivityComponent separationGamma-Aminobutyric acidDistilled water
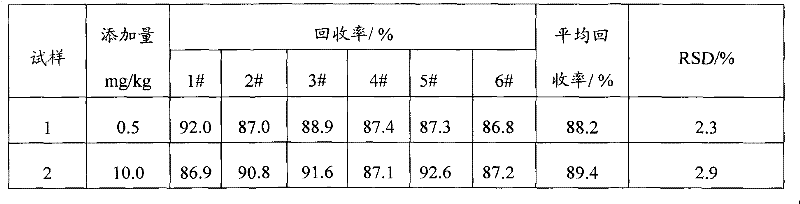
The invention relates to a detection method for gamma-aminobutyric acid (GABA) content in food by using high performance liquid chromatography (HPLC) method. The method is characterized by: carrying out sufficient whirling through double distilled water, and an ultrasonic extraction for a sample requiring detection, then carrying out a centrifugating to obtain supernatant, followed by filtering the supernatant to obtain the sample liquid; weighing the GABA standard substance, then dissolving the GABA through the double distilled water, and carrying out a metered volume for the GABA solution to adopt the solution as a standard stock solution, further dissolving the standard stock solution through the double distilled water to obtain a standard working solution having a concentration gradient; adopting o-phthalaldehyde and 2-mercaptoethanol as derivating agents, and carrying out a derivation for the sample liquid through a HPLC equiped with a online derivation apparatus, followed by carrying out a quantitative detection through a external standard method. The method for detecting the GABA content in the food is quick and effective, and has a linearity range of 0.5-1000 mg / L, a recovery rate more than 88.2%, a relatibe standard deviation (RSD) less than 5%. In addition, with the present invention, a reliable and convenient method is provided for detecting the GABA content in the food,; requirements of researches and productions are met.
Owner:宁波谱尼测试技术有限公司
Extracting method of polysaccharide and polyphenol plant genomes
The invention belongs to the technical field of molecular biology and discloses an extracting method of polysaccharide and polyphenol plant genomes. The method includes: grinding plant materials, then adding a nucleic acid separation buffer solution and 2-mercaptoethanol, well mixing, and placing into water bath; centrifuging in a centrifuge, and then discarding supernate; adding a 1x PBS solution, well mixing on a grinding instrument in an oscillation manner, and discarding supernate after centrifuging; adding preheated 3x CTAB lysate into sediment, well mixing, and performing splitting decomposition under water bath; adding equal-volume phenol / chloroform / isoamyl alcohol mixed liquor, well mixing and centrifuging; taking supernate, adding equal-volume chloroform / isoamyl alcohol mixed liquor, well mixing and centrifuging; taking supernate, adding NaCl and icy isopropanol, well mixing, and precipitating for 1-3 hours; taking out and centrifuging, then washing sediment with ethanol, and adding TE for dissolving after air drying so as to complete the extracting process. The extracting method has the advantages that polysaccharides and polyphenols can be removed effectively, the influence of the polysaccharides and polyphenols on nucleic acid extraction is reduced, and DNA quality and concentration are increased.
Owner:SHANGHAI PASSION BIOTECHNOLOGY CO LTD
Non-animal-source serum-free culture medium for umbilical cord blood stem cells
ActiveCN102827810AAvoid instabilityClear natureBlood/immune system cellsCell phenotypeLipid formation
The invention relates to the field of biology, and discloses a non-animal-source serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, phytohaemagglutinin (PHA), lipid, amino acid, vitamins, trace elements, interleukin-3(IL-3), stem cell factor, (SCF), Fit3-L, IL-6 and granulocyte colony-stimulating factor (G-CSF). The non-animal source serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation, avoids the doped animal components and unstability of batches, and the results of cultured umbilical cord blood stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the non-animal-source serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
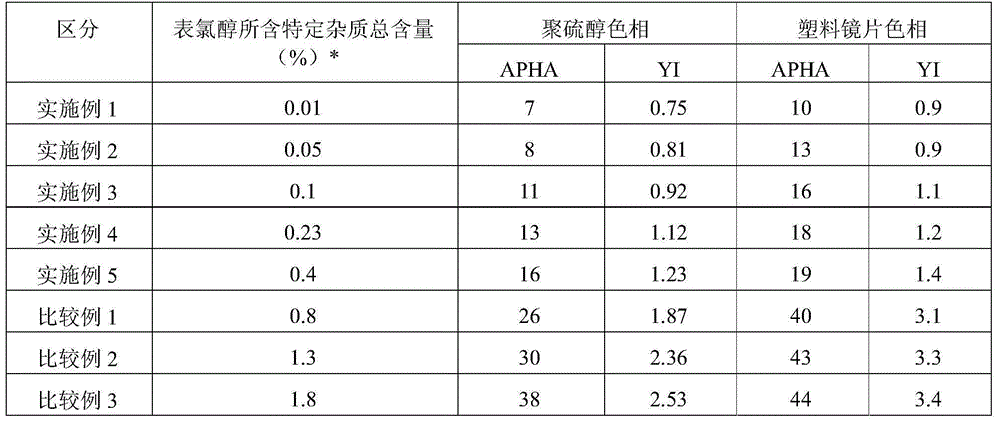
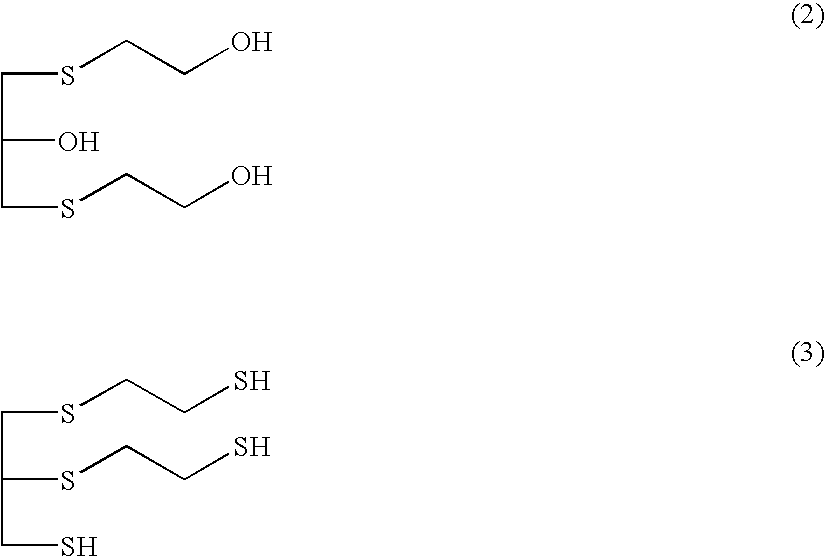
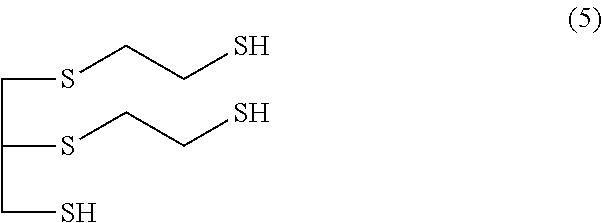
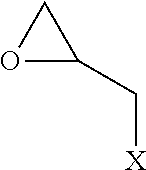
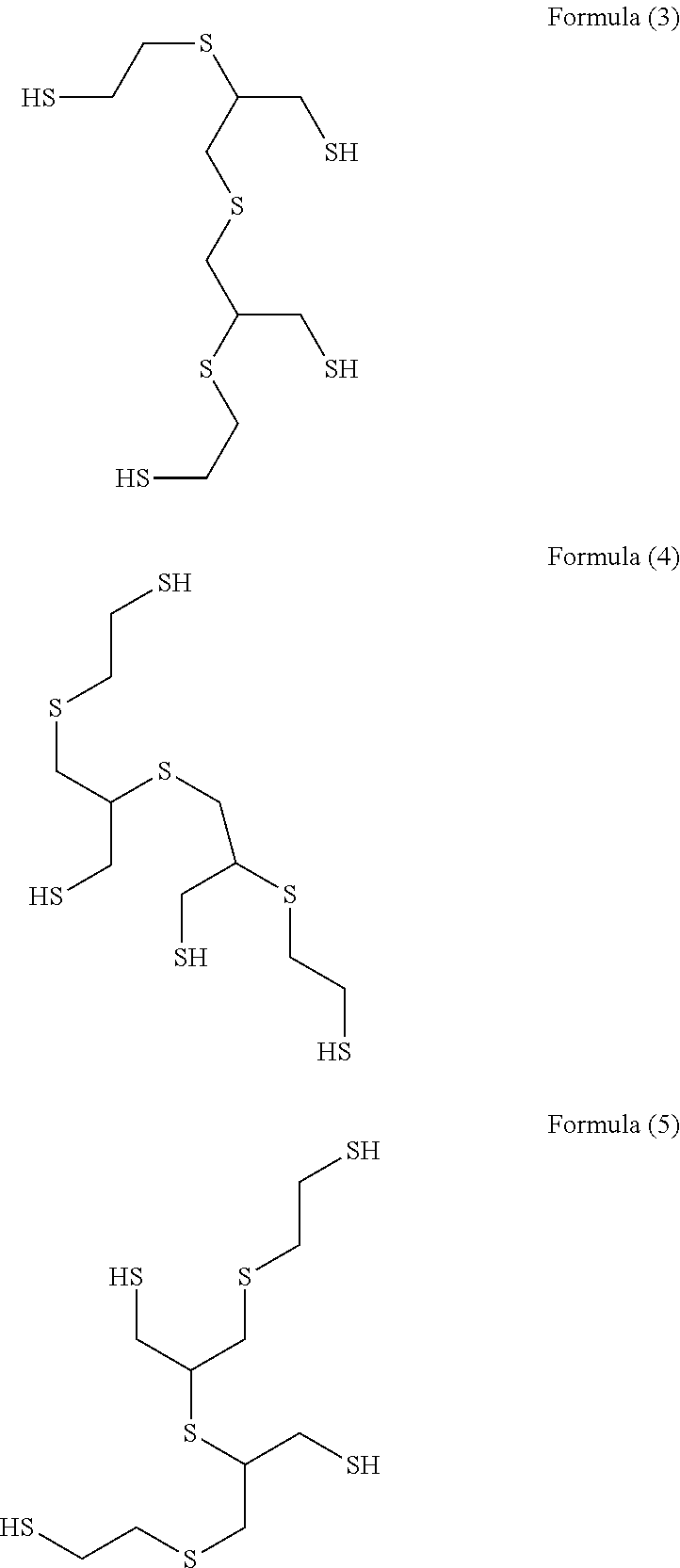
Method for producing polythiol compound for optical materials and composition comprising same for optical materials
ActiveCN104066716AInhibit coloringLow yellownessThiol preparationSulfide preparationChemical compound2-Mercaptoethanol
The present invention relates to a polythiol compound for optical materials exhibiting desirable characteristics from a coloring perspective, a composition comprising same for optical materials, and a method for producing optical materials. The present invention provides a method for producing a polythiol compound for optical materials which reacts epichlorohydrin compound having total impurities content of less than 0.5 wt% and 2-Mercaptoethanol. The present invention allows obtaining a polythiol compound exhibiting suppressed coloration, and by using same, allows obtaining a urethane group exhibiting suppressed coloration, low yellowness and good coloring. The polythiol compound according to the present invention can be used in the production of a variety of optical materials such as a urethane group, and optical lenses having good coloring, obtained by means of the present invention, can replace existing lenses and be widely used in diverse fields, particularly as lenses for glasses, polarized lights and cameras.
Owner:MITSUI CHEM INC
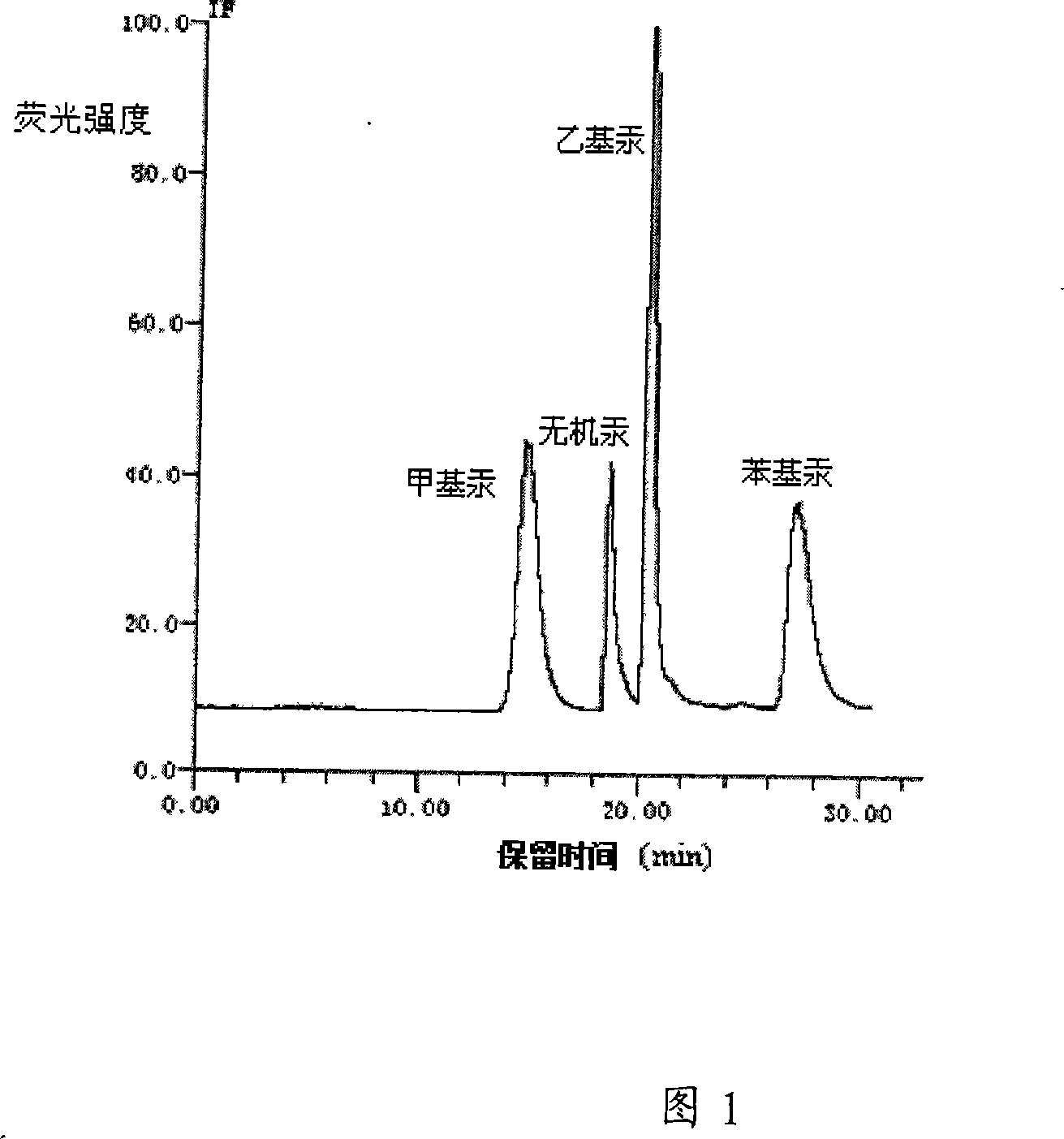
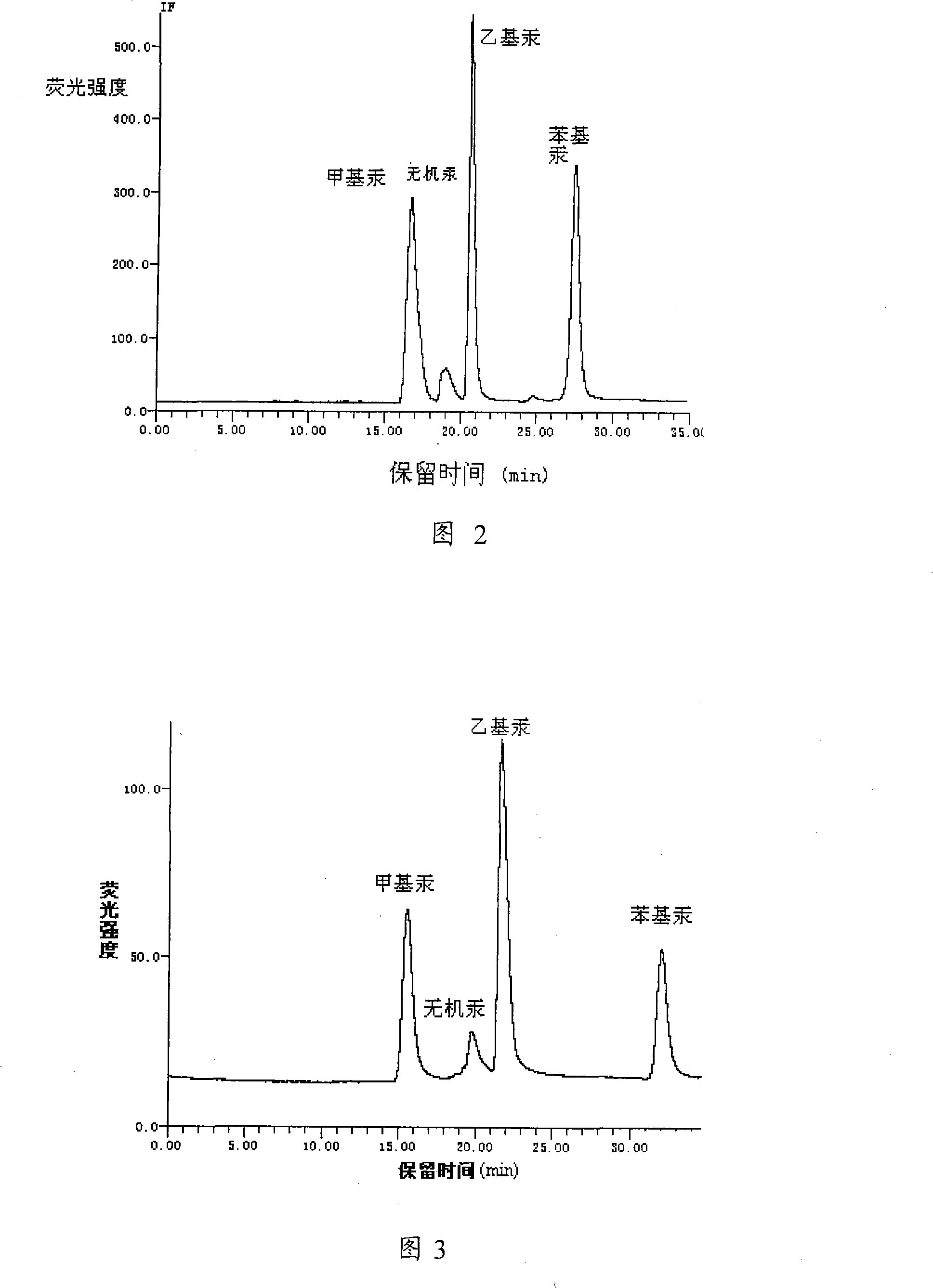
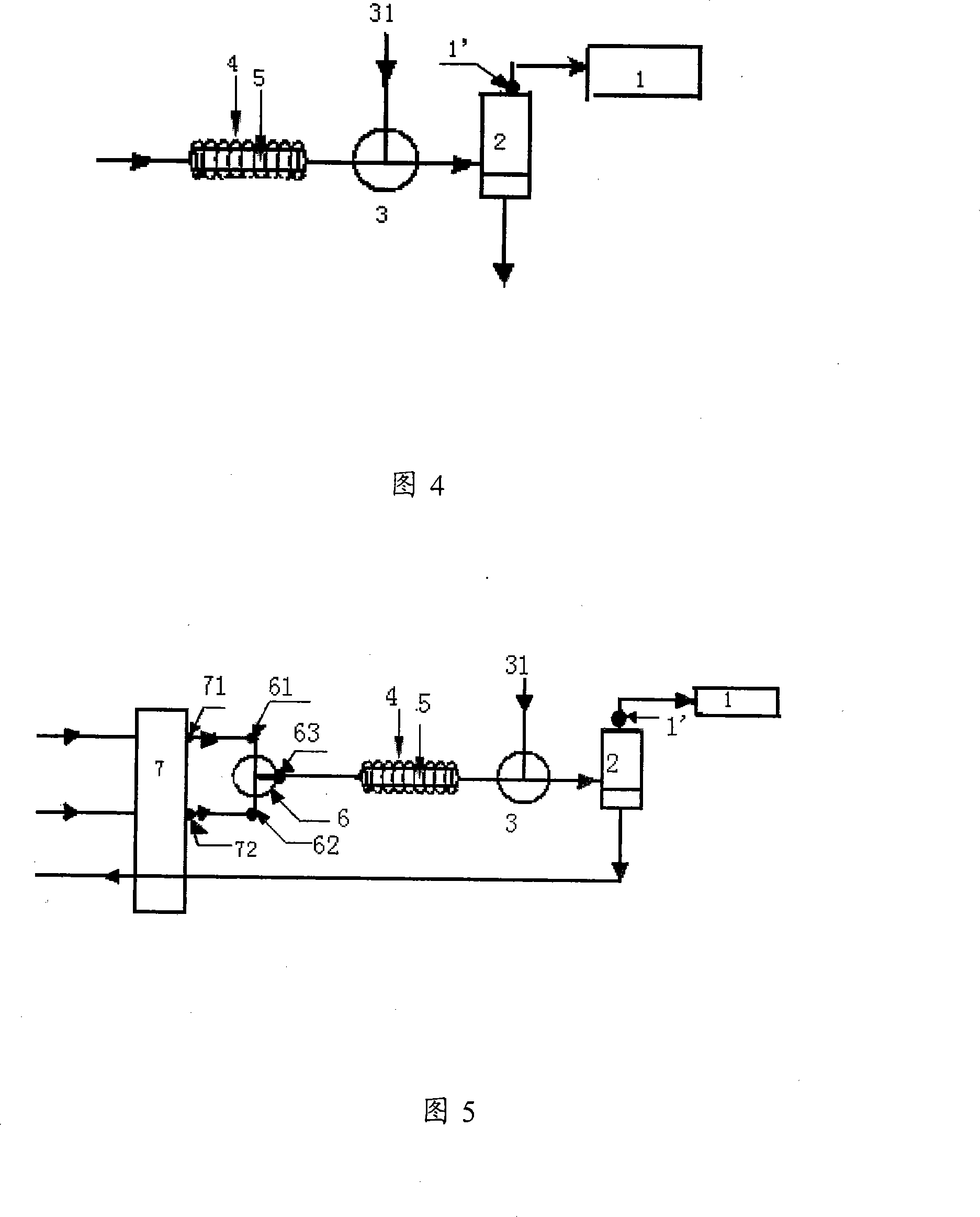
Method and equipment for separating and detecting organo-mercuric compound content
The invention provides a test method for organic mercury compounds, which comprises the following steps: first the organic mercury sample and separates the sample are extracted through high performance liquid chromatography, the mobile phase of which comprises:2-mercaptoethanol, one or more lower fatty acids and lower fatty acid salts, leads the chromatographic column draw off to cause chemical reaction under the ultraviolet light, the reaction solution is mixed with inert gases, the gas-liquid mixture is separated through a gas-liquid separator, the highly performance liquid chromatography chart is obtained through testing the separated sample gas phase through atomic fluorescence spectrometer; according to the standard curve between the organic mercury density and the peak area, the organic mercury concentration in the sample to be tested is figured out through the separated sample gas phase peak area in the highly performance liquid chromatography chart. The invention provides a separating and testing method for a plurality of organic mercury in the sample to be tested.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Placenta preserving fluid
ActiveCN103704206AReduced activityThe solution cannot be stored for a long timeDead animal preservationPenicillinPhosphate
The invention relates to placenta preserving fluid with pH value of 7.33-7.83; the placenta preserving fluid per 1,000ml contains 10-15mmol of potassium dihydrogen phosphate, 30-40mmol of sodium chloride, 40-50mmol of potassium chloride, 8-10mmol of magnesium sulfate, 70-100mmol of histidine, 1.5-2.0mmol of allopurinol, 3mmol of reduced glutathione, 1-8ml of 2-mercaptoethanol, 8-10mmol of adenosine, 1,000,00-1,000,000U of penicillin, 60-100mg of streptomycin and 50-70mg of gentamicin. The placenta preserving fluid can store the delivered placenta for a long time, prevent from reduction of cell activity and control the bacteria contamination rate of the placenta to be in a low level.
Owner:章毅 +10
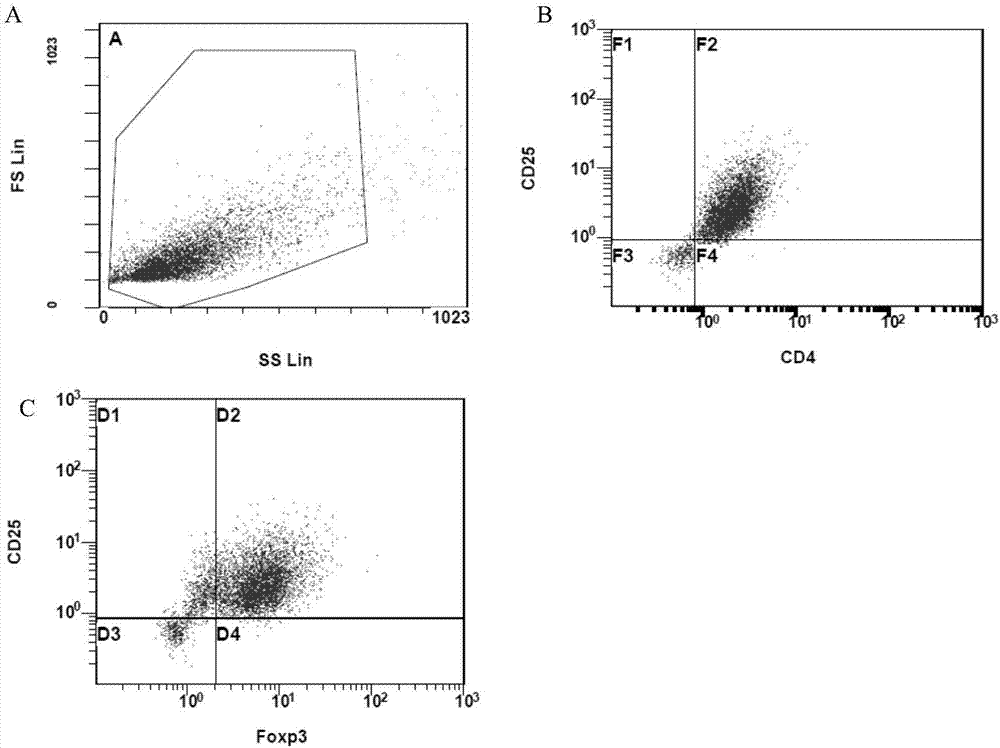
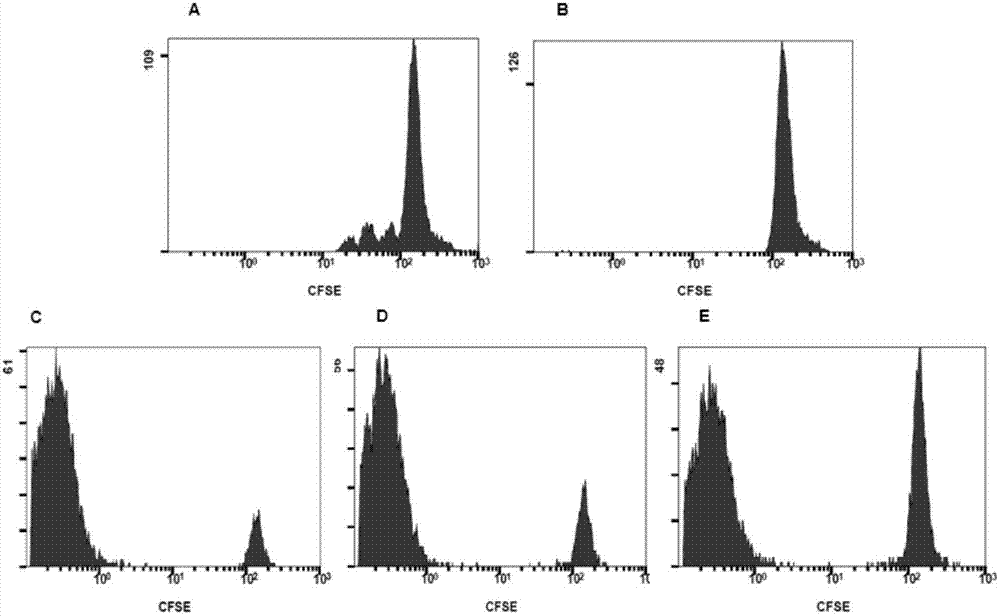
Adult regulatory T cell in-vitro amplification culture medium and application method thereof
ActiveCN104278012AHigh amplification rateReduce oxidative damageBlood/immune system cellsDiseaseImmunologic disorders
The invention provides an adult regulatory T cell in-vitro amplification culture medium and an application method thereof. The adult regulatory T cell in-vitro amplification culture medium comprises transform growth factor-beta (1-4 ng / ml), bone morphogenesis protein 4 (8-12 ng / ml), recombinant human interleukin-2 (300-500U / ml), rapamycin (80-100nM / ml), al-trans vitamin A acid (1-4 uM / mL), 4-hydroxyethylpiperazinoethylsulfonic acid (20-30mM / ml), L-glutamine (2mM / ml), 2-mercaptoethanol (40-50uM / ml), 5% human AB type serum, CD3CD28 magnetic bead, penicillin (50U / ml) and streptomycin (50ug / ml). When being used for performing amplification culture and induced differentiation on regulatory T cells, the culture medium can shorten the amplification and differentiation time of the regulatory T cells, and can obtain the high-purity Foxp3CD4+CD25+CD127-regulatory T cells. Therefore, the regulatory T cells can be used in the aspect of clinical treatment of anti-transplantation immunity rejection, autoimmune disease, allergic disease and the like to perform a novel clinical cell therapy.
Owner:HUNAN XENO LIFE SCI
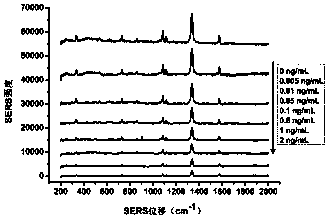
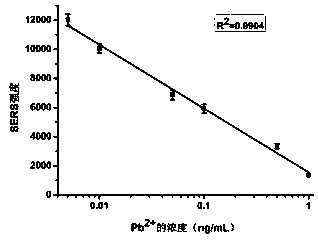
SERS method for detection of lead ions based on Ag@Au nanoparticles
InactiveCN107703115ARealize quantitative detectionMaterial nanotechnologyNanomedicine2-MercaptoethanolComposite nanoparticles
The invention discloses an SERS method for detection of lead ions based on Ag@Au nanoparticles, and belongs to the field of biological detection. By means of a principle that the presence of Pb<2+> can accelerate leaching of sodium thiosulfate and 2-mercaptoethanol on nano gold, Pb<2+> with different concentrations is added to Ag@Au composite nanoparticles containing sodium thiosulfate and 2-mercaptoethanol, and a gold shell of the Ag@Au nanoparticles is leached so as to cause change of the thickness of the gold shell; beacon molecules are added, and then different Raman signal intensities areproduced. According to the correspondence relationship between the Pb<2+> concentration and the SERS signal intensity, the Pb<2+> can be quantitatively detected. The method has the advantages of highdetection speed, low detection cost, ultra high sensitivity and good specificity, and can satisfy the detection requirements of Pb<2+> in actual samples.
Owner:杨蕾
Preparation method of emulsification compatibilization soybean protein adhesive
InactiveCN102433099AIncrease added valueImproves water-resistant bond strengthProtein adhesivesGlue/gelatin preparationEpoxyWater resistant
The invention relates to a preparation method of an emulsification compatibilization soybean protein adhesive. According to the preparation method, a renewable resource soybean protein is used as a raw material, water is used as a solvent, and carbamide, sodium dodecyl benzene sulfonate and 2-mercaptoethanol are selected to modify the soybean protein to destroy hydrogen bonds and disulfide bonds in the soybean protein, so polypeptide chains are fully stretched, and hydrophobic groups are exposed; the soybean protein is emulsified by OP-10 and is blended with an epoxy resin to improve the water-resistant adhesion strength of the adhesive; and simultaneously triethylenediamine hexahydrate is selected as a curing agent to accelerate the curing speed of the adhesive. The obtained adhesive hasexcellent water-resistant adhesion strength, the cost of the adhesive is low because a large amount of water is used as the solvent, and the deeply processed product of the soybean protein, which hasindustrialization values, is obtained, so the added value of the soybean protein is improved. The adhesive which has the advantages of environmental protection, health and no generation of toxic and harmful substances of formaldehyde, phenol and the like can be applied to fields of wood plywoods, shaving boards, fiberboards and the like.
Owner:NANJING FORESTRY UNIV
Serum-free medium of immune cells
ActiveCN104371973ASmall batch-to-batch varianceSimple ingredientsBlood/immune system cellsSerum free mediaL-alanyl-l-glutamine
The invention discloses a serum-free medium for culturing immune cells. The medium includes a basic medium, bovine serum albumin, fatty acids, cholesterols, insulin, transferrin, 2-mercaptoethanol, N-(2)-L-alanyl-L-glutamine and glyceride. The serum-free medium has the advantages of simple component, stable properties and high CIK induction efficiency, and CIK cells cultured through the medium have the advantages of small batch difference, stable quality, convenient quality control, and reduction of accidental infection of patients, and are of great significance to promoting and applying immunotherapy.
Owner:EASTERN UNION STEM CELL & GENE ENG
Strain liquid crystal dimming display glassware and manufacturing method thereof
ActiveCN103760713AUnbreakableInhibition of dissolutionNon-linear opticsLiquid-crystal displayShear stress
The invention provides strain liquid crystal dimming display glassware which is light and portable and can be in a scattering mist state with a pattern display effect under an ordinary condition. After shearing stress is exerted on the glassware, the glassware can be in mist semi-transparent state, a pattern is blanked, and the glassware can be applied to various display pattern product manufacturing effectively, is not easy to crack and has wide application prospects and markets. The invention further provides a manufacturing method of the strain liquid crystal dimming display glassware. A polymer dispersed liquid crystal layer is clamped between two pieces of organic glass and is formed by mixing a prepolymer, nematic phase liquid crystal and a gasket material. The mass ratio of the prepolymer to the nematic phase liquid crystal is 13:7-15:7. The major components of the prepolymer are, by mass, 30% of alkoxy phenylacrylic acid ester, 60% of trimethylolpropane acrylic ester, 4% of chemigum, 4% of 2-mercaptoethanol and 2% of 1173 photoinitiator.
Owner:佛山犀马精细化工有限公司
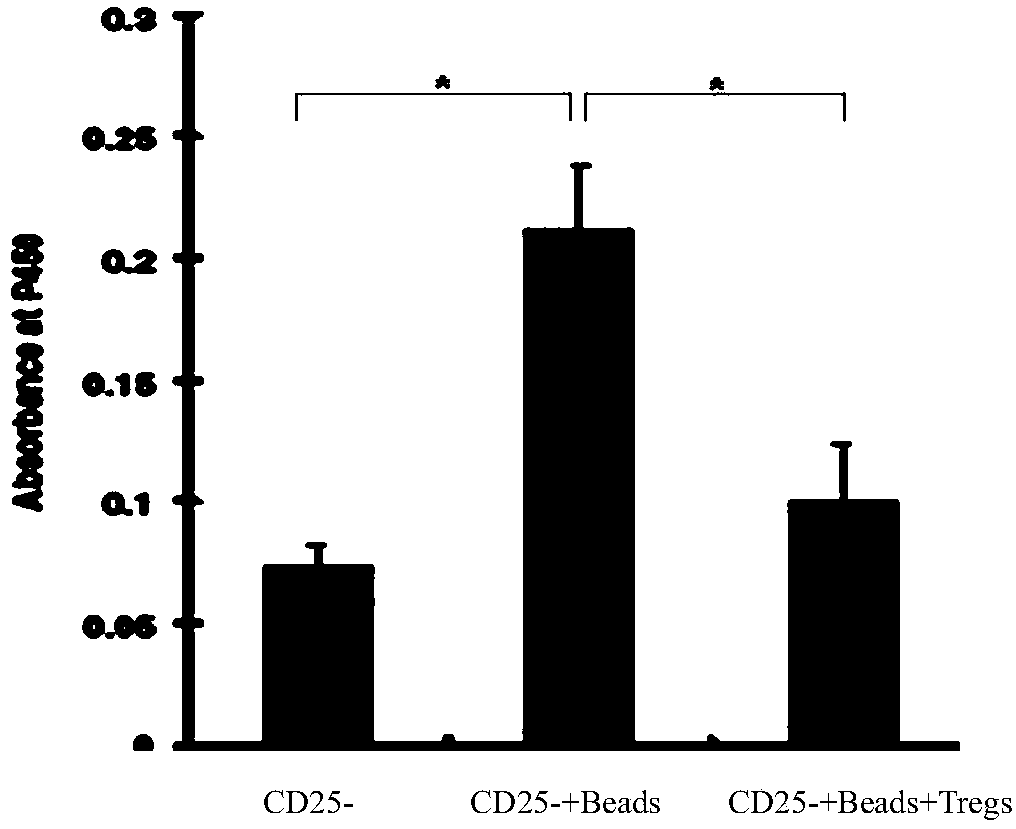
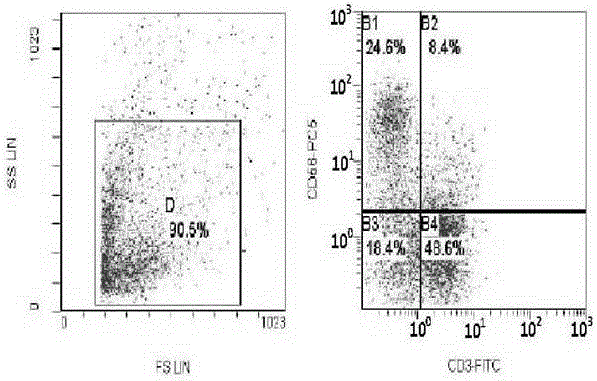
Application of regulatory T cells in preparing drug for treating autoimmune diabetes and expanding cultivation solution and method thereof
ActiveCN107349219AGood curative effectTherapy is safeMetabolism disorderCulture processRegulatory T cellPenicillin
The invention discloses application of regulatory T cells in preparing a drug for treating autoimmune diabetes and an expanding cultivation solution and method thereof. The expanding cultivation solution of the regulatory T cells is prepared from, by volume, 35.274-42.69 parts of serum-free cultivation solutions, 1.25-2.5 parts of 4-(2-hydroxyerhyl)piperazine-1-erhanesulfonic acid buffer solution, 0.5-1 part of penicillin-streptomycin solution, 0.5-1 part of L-glutamine, 0.05-0.1 part of 2-mercaptoethanol, 0.00769-0.01154 part of recombinant interleukin-2, 0.001-0.01 part of rapamycin and 5-10 parts of AB serum. According to the application of the regulatory T cells in preparing the drug for treating the autoimmune diabetes, through targeted research on the regulatory T cells and the pathogenesis of the autoimmune diabetes, applicants find that the regulatory T cells has relatively good therapeutic effects at the aspect of treating the autoimmune diabetes.
Owner:CENT SOUTH UNIV
Process for producing polythiol compound for optical materials, and polymerizable composition containing polythiol compound
InactiveUS20090082544A1Good colorColor deteriorationThiol preparationOrganic compound preparationPolyol2-Mercaptoethanol
Owner:MITSUI CHEM INC
Cornea mid-term preservation liquid
ActiveCN101524063AImprove preservation qualitySlow down metabolismDead animal preservationDexamethasone2-Mercaptoethanol
The invention relates to a cornea mid-term preservation liquid. 1000ml of preservation liquid contains 0.5-1.5g of DMEM culture medium, 1-10g of hydroxypropyl methylcellulose, 0.1-10ml of 2-mercaptoethanol, 10-20g of dextran (with the molecular weight of 40000), 1-3ml of nonessential amino acid, 10-32mmol of Hepes buffer solution and 0.01-0.05mmol of dexamethasone, 50-70mg of gentamicin. The pH value of the preservation liquid is 7.2-8.0, and the permeation pressure is 350-400mmol / LH2O. The cornea mid-term preservation liquid not only has good cornea storage quality, but also has the advantage of good cost performance and is the cornea mid-term preservation liquid which is widely applicable to the eye bank at home and abroad.
Owner:深圳端利生物集团有限公司
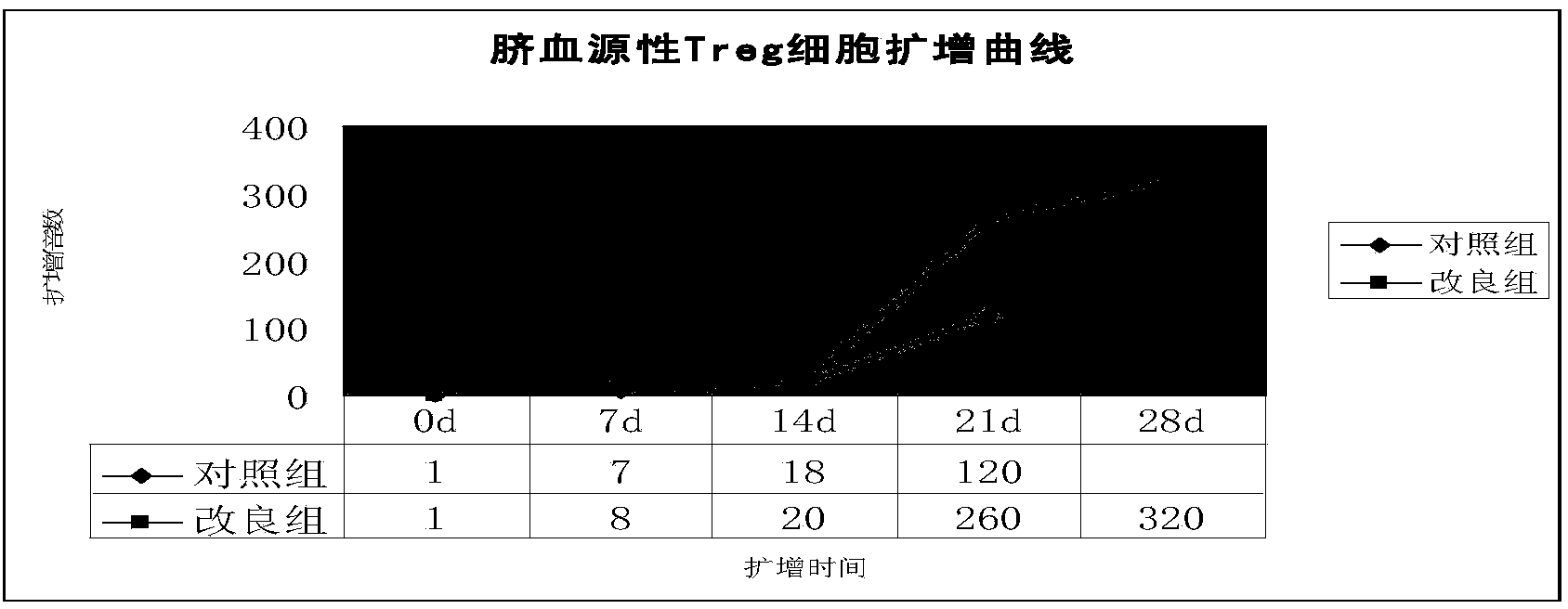
Improved expansion culture medium for regulatory T cells of human cord blood origin and application method of expansion culture medium
ActiveCN104357389AReduce riskPromote growth rateBlood/immune system cellsCulture mediumsClinical disease
The invention relates to an improved expansion culture medium for regulatory T cells of human cord blood origin and an application method of the expansion culture medium. According to the expansion culture medium, heparin anticoagulated autologous cord blood plasma accounting for 10%-12% of the volume of a culture medium, CD3-CD28 antibody co-expressed immunomagnetic beads, recombinant human interleukin 2, 2-mercaptoethanol, rapamycin, HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) and gentamicin are added into the RPMI (Roswell Park Memorial Institute)1640 culture medium; and then separated cell suspension is inoculated in a 96-well plates with a U-shaped bottom, hole-division expansion can be performed every 1-2 days, and the expansion period is 3-4 weeks. All reagents in the culture system reach the GMP (good manufacturing practice) level or are originated from autologous cord blood, so that risks caused by ingredients of animal origin are avoided, and the regulatory T cells can be used for a third-party unrelated donor and directly applied to clinical disease treatment; and compared with a traditional culture system, Treg cells (the regulatory T cells) expanded by the improved culture medium is excellent in aspects of growth speed, purity, activity, lymphocyte inhibition function and the like, and the Treg cells are expected to be used as the regulatory T cells of the third-party unrelated donor and applied to the clinical disease treatment.
Owner:HUNAN XENO LIFE SCI
Serum-free umbilical cord mesenchymal stem cell culture medium
InactiveCN110317779AIncrease growth rateMaintain propertiesCulture processSkeletal/connective tissue cellsVitamin CCuticle
The invention discloses a serum-free umbilical cord mesenchymal stem cell culture medium. The culture medium is used for culturing umbilical cord mesenchymal stem cells and comprises a serum-free basic culture medium and additive components, wherein the addition components include a recombinant human fibroblast growth factor (hFGF), a recombinant human epidermal growth factor (hEGF), insulin hI, L-glutamine, 2-mercaptoethanol, sodium selenite, transferrin, human serum albumin, fibronectin, astragalus polysaccharide APS, IL-3, IL-6, IL-r, a ginseng extract, a rose extract, a jasmine flower extract and vitamin C.
Owner:GUANGDONG AIE BIOSCIENCE CO LTD
Process for producing polythiol compound for optical materials, and polymerizable composition containing polythiol compound
InactiveUS20100280213A1Good colorThiol preparationOrganic compound preparationPolyol2-Mercaptoethanol
Owner:MITSUI CHEM INC
Method for producing polythiol compound, polymerizable composition for optical material, and uses thereof
ActiveUS20150133692A1Improve production stabilityFluctuation in product qualityOrganic compound preparationThiol preparationPolyol2-Mercaptoethanol
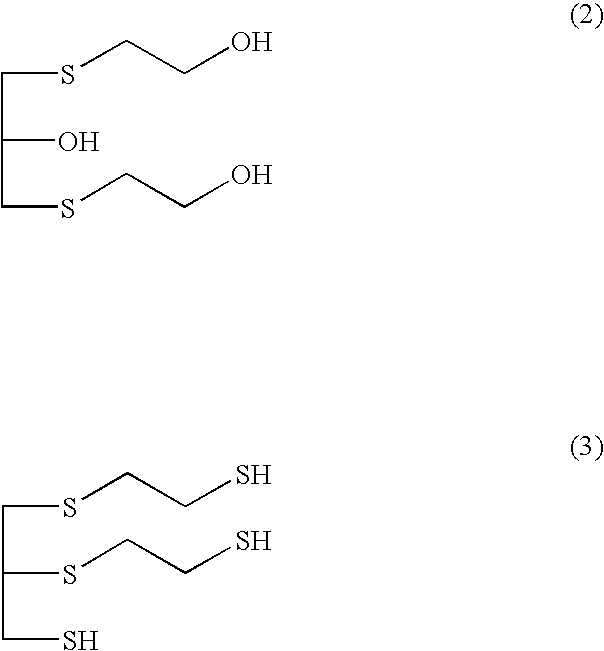
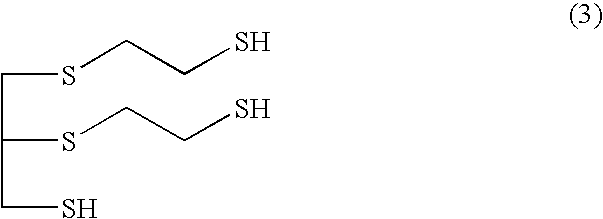
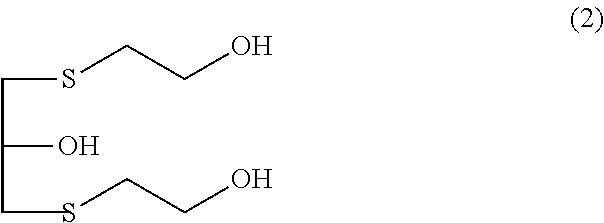
Provided is a method for producing a polythiol compound, comprising: a step for reacting 2-mercaptoethanol with a defined epihalohydrin compound at a temperature of 10° C. to 50° C. to obtain a defined polyalcohol compound; a step for reacting the polyalcohol compound thus obtained with thiourea in the presence of hydrogen chloride to obtain an isothiuronium salt; a step for adding, while maintaining a reaction solution containing the isothiuronium salt thus obtained at a temperature of 15° C. to 60° C., aqueous ammonia to the reaction solution within 80 minutes, thereby hydrolyzing the isothiuronium salt to obtain a defined polythiol compound; and a step for adding hydrochloric acid which is a concentration of 25% to 36% to the solution containing the polythiol compound thus obtained, washing the solution at a temperature of 10° C. to 50° C. to purify the polythiol compound.
Owner:MITSUI CHEM INC
Preparation method of CdS/carbon nano tube/polyacrylonitrile hybrid nano-fiber
InactiveCN102877151ASimple processIncrease productionOrganic-compounds/hydrides/coordination-complexes catalystsFilament/thread formingN dimethylformamideOrganic dye
The invention relates to a preparation method of CdS / carbon nano tube / polyacrylonitrile hybrid nano-fiber. The technical scheme is as follows: the preparation method comprises the following steps: by taking CdCl2, Na2S and 2-mercaptoethanol as raw materials, and N,N-dimethylformamide (DMF) and water as solvents, synthesizing CdS-OH / DMF suspension; uniformly dispersing the carbon nano tube (CNT) in the CdS-OH / DMF suspension through ultrasonic, slowly stirring CNT / Cds-OH / DMF suspension into the prepared polyacrylonitrile / DMF solution, stirring and ultrasonically treating to obtain mixed spinning liquid; and obtaining the hybrid nano-fiber by adopting an electrostatic spinning technology. According to the invention, a solution growth method and the electrostatic spinning method are combined, the process is simple, and the prepared hybrid nano-fiber has the characteristics of large specific surface area and good mechanical property. Meanwhile, the preparation method has efficient performance of photocatalytic degradation of organic dye, and the hybrid nano-fiber is easier to recycle than a powdery photocatalytic material.
Owner:FUJIAN NORMAL UNIV
Method for producing polythiol compound, method for producing curable composition, and method for producing cured product
ActiveUS20180362699A1Low heat resistanceHigh tensile strengthHydropoly/poly sulfide preparationSulfide preparation1-Propanol2-Mercaptoethanol
Provided is a method for producing a polythiol compound, including reacting 2-mercaptoethanol with an epihalohydrin, wherein the reaction is carried out in the presence of a halide selected from the group consisting of a 2,3-dihalogeno-1-propanol and an allyl halide, and an amount of the halide in the reaction is more than 0.50% by mass and 10.00% by mass or less with respect to the total amount of the halide and the epihalohydrin. Also provided are a method for producing a curable composition, including producing a polythiol compound by the abovementioned production method, and a method for producing a cured product, including producing a curable composition by the abovementioned production method.
Owner:HOYA LENS THAILAND LTD
Iron control agent
ActiveUS8003581B1Minimize and eliminate sludge formationInhibition formationCleaning apparatusFluid removalSulfite saltTin(II) chloride
An iron control agent capable of reducing ferric iron containing compounds to ferrous iron containing compounds in an acidic solution, such as one used for formation acidizing. The iron control agent comprises a combination of a sulfur dioxide, sulfurous acid, sulfite salts, bisulfite salts, or thiosulfate salts or mixtures thereof, with a source of copper ions and a source of iodine or iodine ions. The iron control agent may also include small amounts of an adjunct such as stannous chloride, 2-mercaptoethanol, and thioglycolic acid and its salts.
Owner:ENERGY SOLUTIONS (US) LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com