Method for producing polythiol compound for optical materials and composition comprising same for optical materials
A technology of optical materials and manufacturing methods, applied in the fields of mercaptan preparation, optics, sulfide preparation, etc., can solve the problems of resin hue difference and achieve the effect of good hue and low yellowness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of 1,2-bis[(2-mercaptoethyl)thio]-3-mercaptopropane
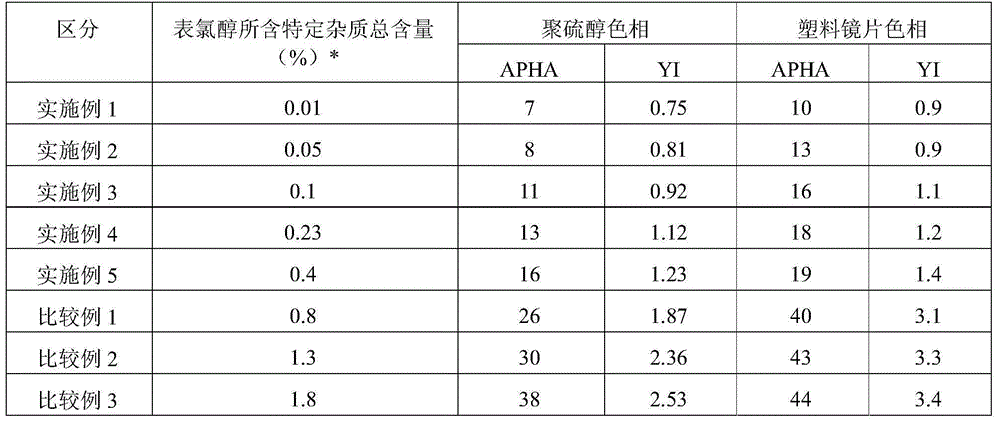
[0038] 676 g (8.65 mol) of 2-mercaptoethanol with a purity of 99.9% or higher and 340 g of water were placed in a 10-liter, 5-zone reaction flask equipped with a stirrer, an exchange cooling water separator, a nitrogen purge (purge) tube and a thermometer. At 30°C, 691.2 g (1.08 mol) of a 25% by weight aqueous sodium hydroxide solution was added dropwise over 30 minutes, and then 399.6 g (4.32 mol) of epichlorohydrin was added dropwise over 3 hours at the same temperature, and left for 1 hour. . The purity of the used epichlorohydrin is 99.9% or more, and its content is composed of acrolein (acrolein), allyl chloride (acrolein), 1,2-dichloropropane, 2,3-dichloropropene, 2-methyl group -2-pentanol, 2-chloroaryl alcohol, cis-1,3-dichloropropene, trans-1,3-dichloropropene, 1,3-dichloroisopropanol, 1,2,3 - The total content of impurities composed of trichloropropane and 2,3-dichloropropanol (hereinafter ref...
Embodiment 2
[0042] Except that the total content of specific impurities contained in the epichlorohydrin used was 0.05% by weight, it was the same as Example 1, and the same method as Example 1 was used to manufacture 1,2-bis[(2-mercaptoethyl thio]-3-mercaptopropane-based polythiol. The obtained polythiol mainly composed of 1,2-bis[(2-mercaptoethyl)thio]-3-mercaptopropane had an APHA of 8 and a YI value of 0.81. Using this polythiol, a plastic lens was produced in the same manner as in Example 1 and evaluated. The evaluation results are shown in Table 1.
Embodiment 3
[0044] Except that the total content of specific impurities contained in the epichlorohydrin used was 0.01% by weight, it was the same as Example 1, and the same method as Example 1 was used to manufacture 1,2-bis[(2-mercaptoethyl thio]-3-mercaptopropane-based polythiol. The obtained polythiol mainly composed of 1,2-bis[(2-mercaptoethyl)thio]-3-mercaptopropane had an APHA of 11 and a YI value of 0.92. Using this polythiol, a plastic lens was produced in the same manner as in Example 1 and evaluated. The evaluation results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com