Serum-free medium of immune cells
A technology of serum-free culture medium and immune cells, which is applied in the fields of biology and medicine, can solve the problems of not supporting the growth of immune cells well, the source of human AB serum is difficult, and the supply is limited, so as to achieve stable properties, reduce accidental infections, The effect of simple ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation of 1000ml serum-free medium
[0021] Mix one liter bag of IMDM dry powder (Gibco), 10g bovine serum albumin (Roche), 10mg fatty acid (sigma), 20mg cholesterol (sigma), 20mg insulin (sigma), 15mg transferrin (sigma), 5mg 2-mercapto Ethanol (Amresco), 434mg Propandipeptide (Sigma), 20mg Glycerides (Sigma), 3.024g NaHCO 3 (sigma); add ultrapure water to 1000ml, stir to dissolve, adjust the pH value to about 7.0, filter and sterilize with a 0.22μm filter membrane, and store at 4°C.
Embodiment 2
[0022] Example 2: Isolation of hematopoietic stem cells
[0023] Under sterile conditions, take 50-80ml of umbilical cord blood from full-term cesarean section or full-term normal pregnant women with negative hepatitis B virus test, put it in a blood collection bag containing CPDA1 compound anticoagulant, and store it at 4°C. All samples should be processed within 12 hours Separation; one portion of cord blood is divided into three. Each portion was diluted with normal saline 1:1, and the diluted umbilical cord blood was slowly added to the upper layer containing Ficoll Hypaque human lymphocyte separation medium along the tube wall (the ratio of the two was 1:1), and its relative density was 1.077g / L. Carry out gradient centrifugation (2000r / min×30), take the cloudy mononuclear cell layer in the middle, centrifuge and wash twice with IMDM culture medium, each time at 1000r / min, and centrifuge for 5min. After discarding the supernatant, resuspend the cells with serum-free medi...
Embodiment 3
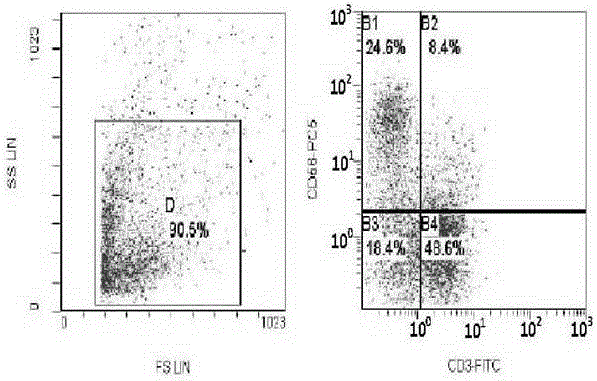
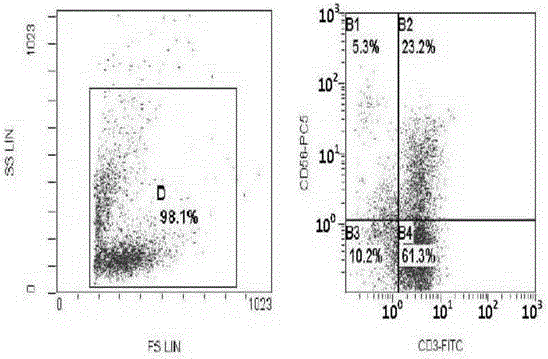
[0026] Embodiment 3: CIK cell induction and testing
[0027] Under sterile conditions, take 50-80ml of umbilical cord blood from full-term cesarean section or full-term normal pregnant women with negative hepatitis B virus test, put it in a blood collection bag containing CPDA1 compound anticoagulant, and store it at 4°C. All samples should be processed within 12 hours Separation; one portion of cord blood is divided into three. Each portion was diluted with normal saline 1:1, and the diluted umbilical cord blood was slowly added to the upper layer containing Ficoll Hypaque human lymphocyte separation medium along the tube wall (the ratio of the two was 1:1), and its relative density was 1.077g / L. Carry out gradient centrifugation (2000r / min×30), take the cloudy mononuclear cell layer in the middle, centrifuge and wash twice with IMDM culture medium, each time at 1000r / min, and centrifuge for 5min. After discarding the supernatant, adjust the cells to 1×10 with IMDM medium 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com