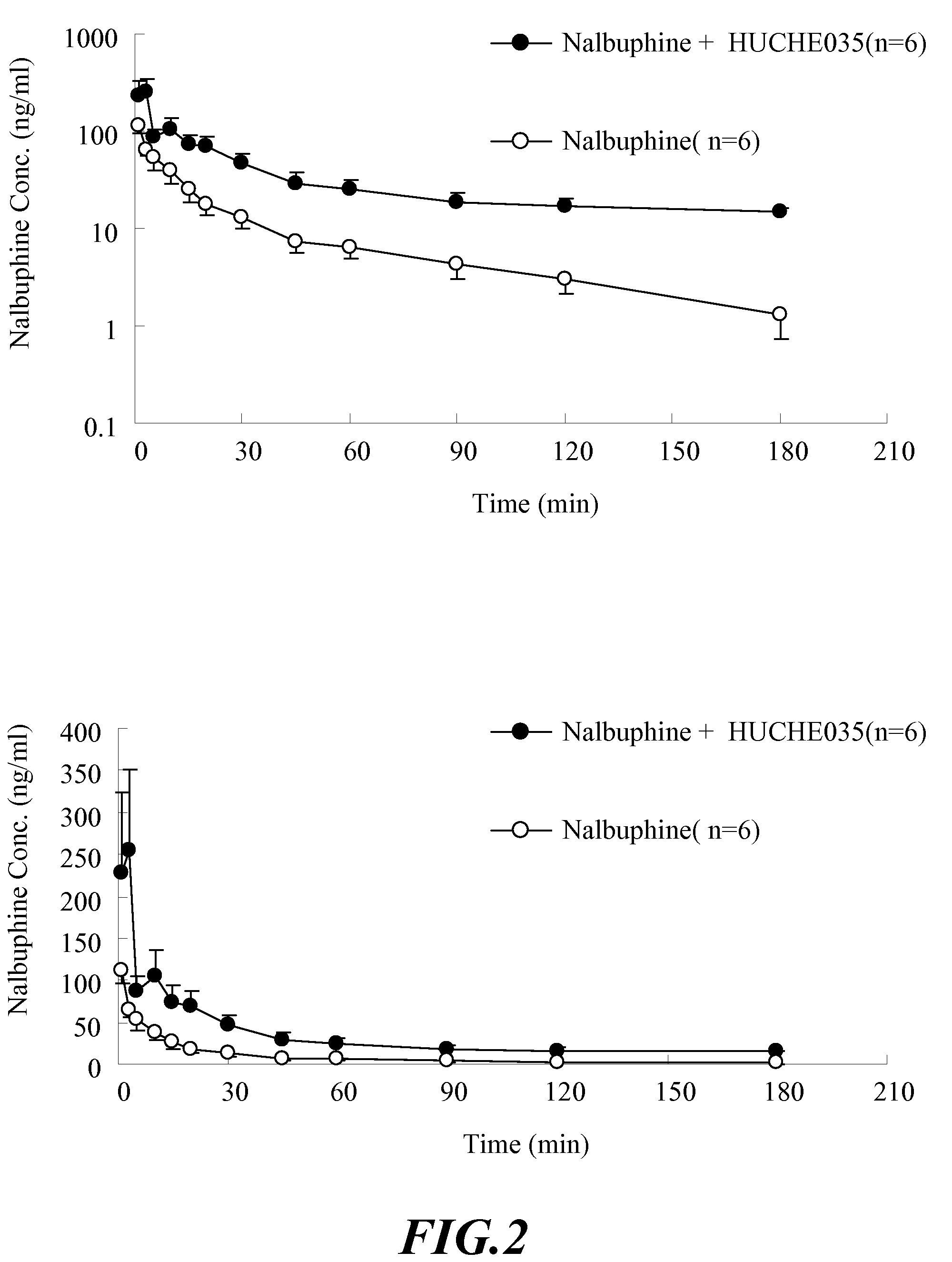
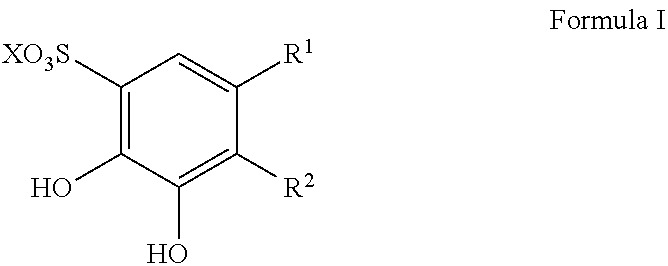
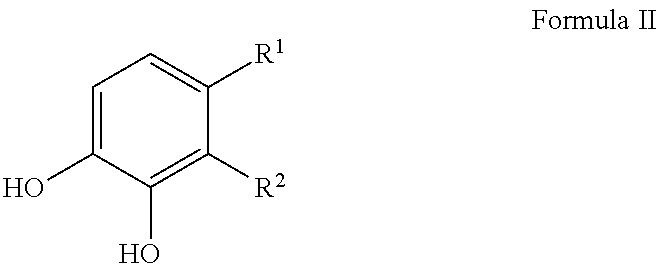
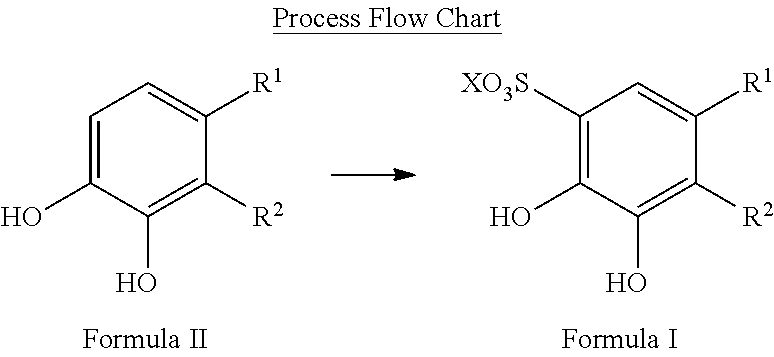
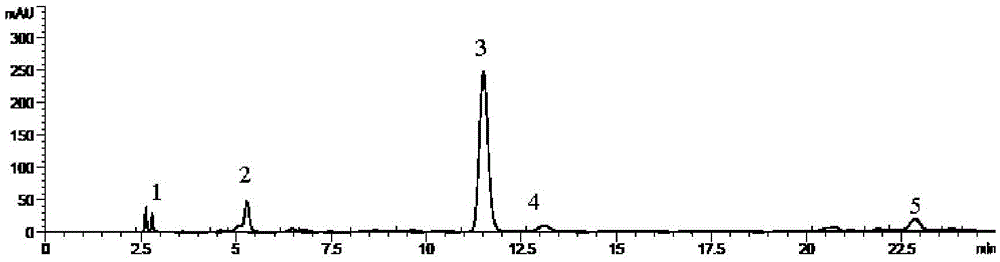
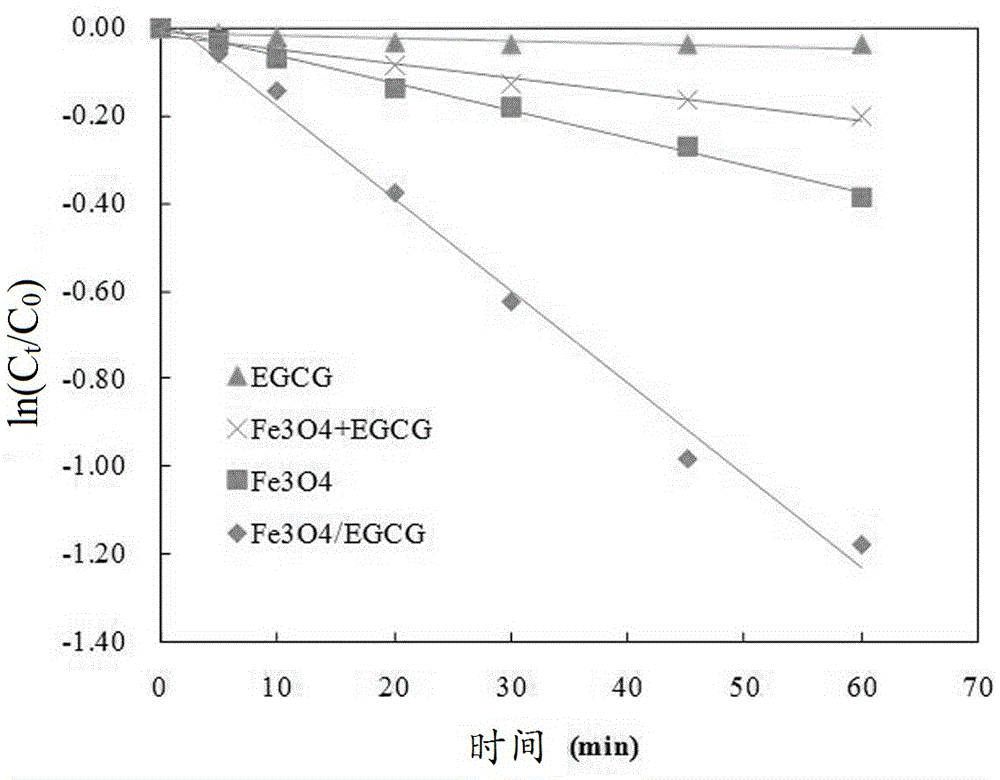
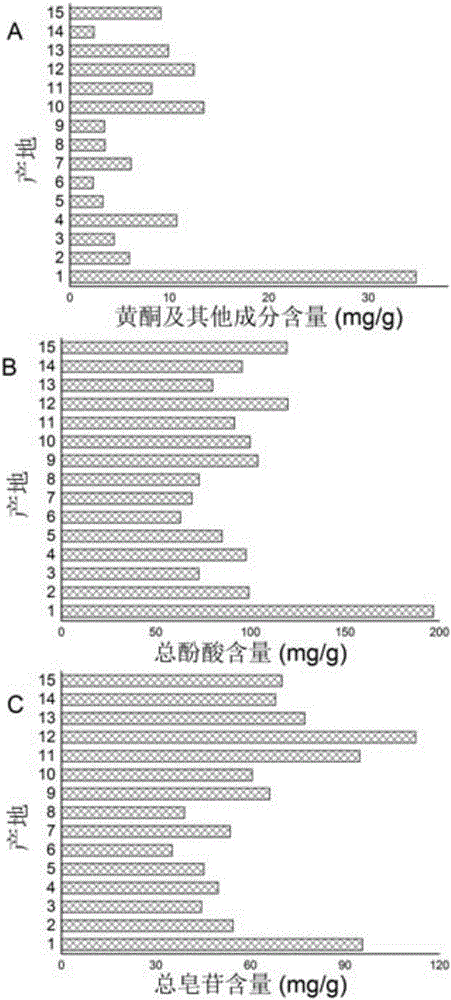
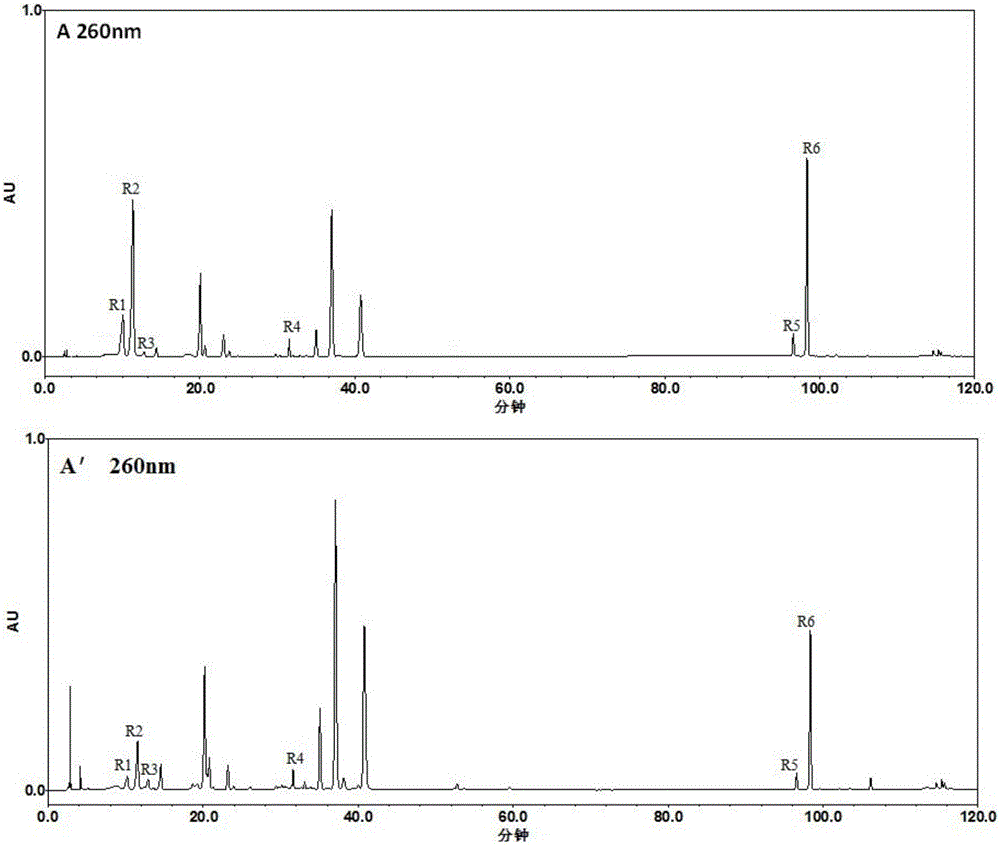
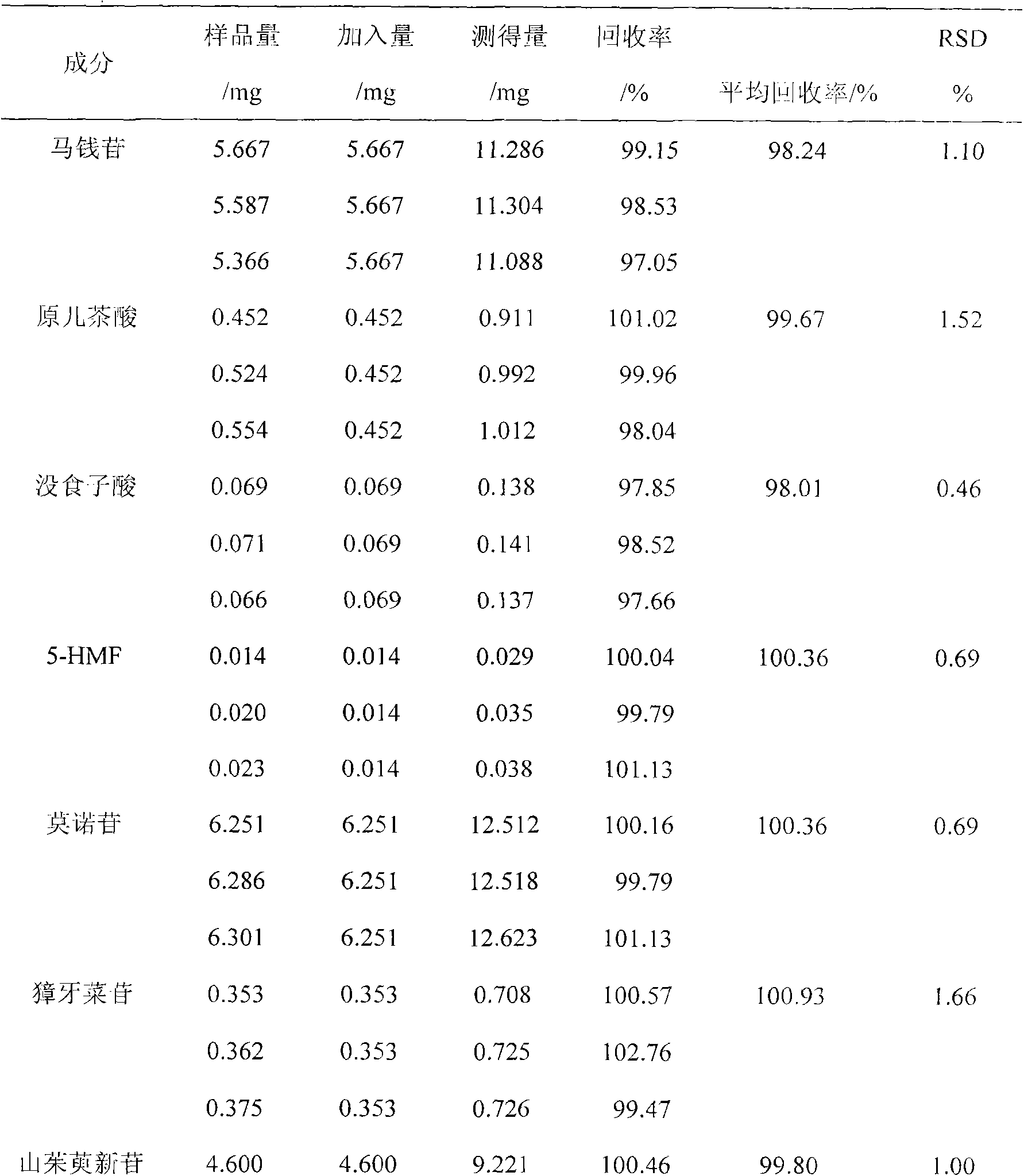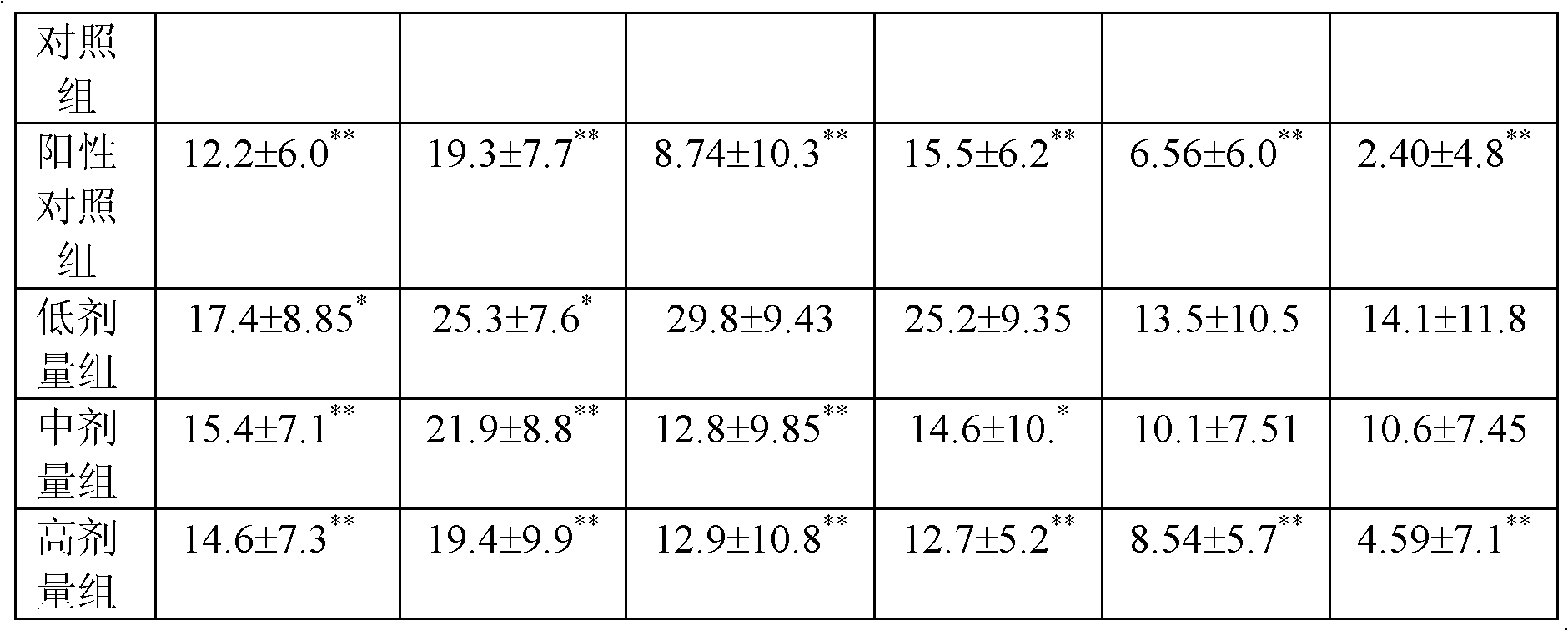Patents
Literature
306 results about "Protocatechuic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
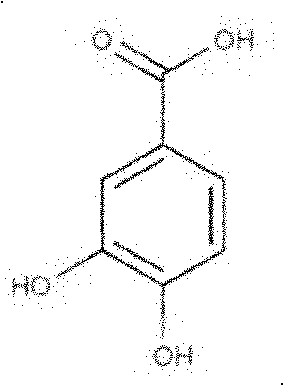
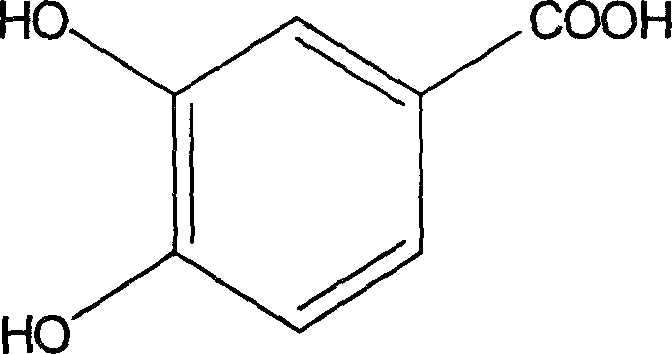
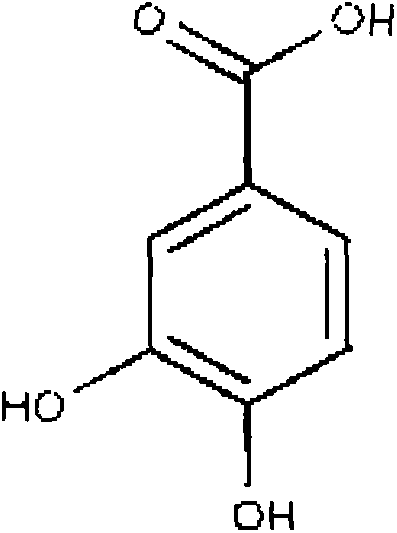
Protocatechuic acid (PCA) is a dihydroxybenzoic acid, a type of phenolic acid. It is a major metabolite of antioxidant polyphenols found in green tea. It has mixed effects on normal and cancer cells in in vitro and in vivo studies.
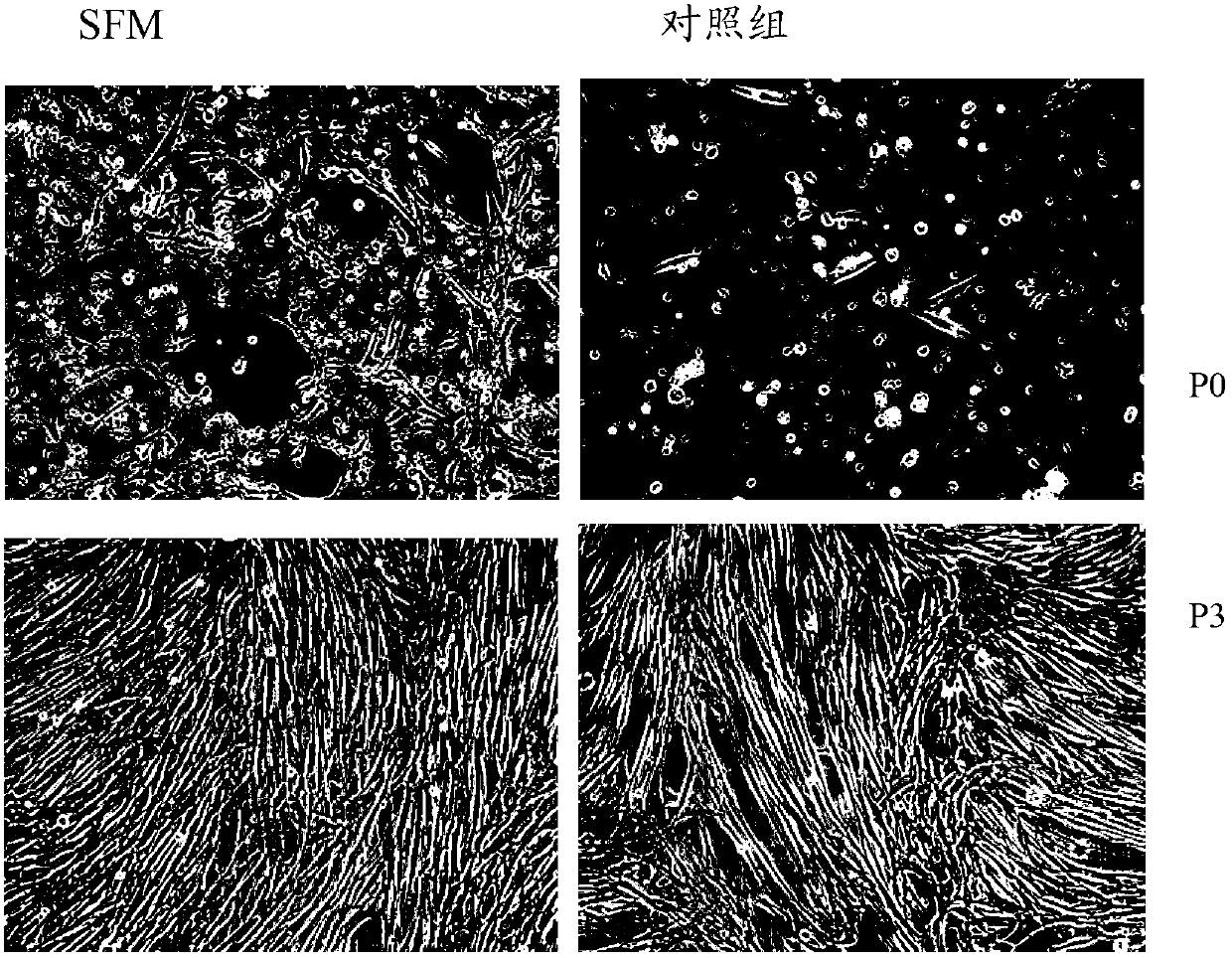
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Inhibitors and Enhancers of Uridine Diphosphate-Glucuronosyltransferase 2B (UGT2B)
ActiveUS20090074708A1Increase heightReduced activityBiocideHydroxy compound active ingredientsPolyethylene glycolEriodictyol
A UGT2B inhibitor capable of increasing the bio-availability of a drug, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: capillarisin, isorhamnetin, β-naphthoflavone, α-naphthoflavone, hesperetin, terpineol, (+)-limonene, β-myrcene, swertiamarin, eriodictyol, cineole, apigenin, baicalin, ursolic acid, isovitexin, lauryl alcohol, puerarin, trans-cinnamaldehyde, 3-phenylpropyl acetate, isoliquritigenin, paeoniflorin, gallic acid, genistein, glycyrrhizin, protocatechuic acid, ethyl myristate, umbelliferone, PEG (Polyethylene glycol) 400, PEG 2000, PEG 4000, Tween 20, Tween 60, Tween 80, BRIJ® 58, BRIJ® 76, Pluronic® F68, Pluronic® F127, and a combination thereof. A UGT2B enhancer capable of enhancing a clearance rate of morphine-like analgesic agents, is a compound in a free base or a pharmaceutically acceptable salt form that is selected from the group consisting of: nordihydroguaiaretic acid, wogonin, trans-cinnamic acid, baicalein, quercetin, daidzein, oleanolic acid, homoorientin, hesperetin, narigin, neohesperidin, (+)-epicatechin, hesperidin, liquiritin, eriodictyol, formononetin, quercitrin, genkwanin, kaempferol, isoquercitrin, (+)-catechin, naringenin, daidzin, (−)-epicatechin, luteolin-7-glucoside, ergosterol, rutin, luteolin, ethyl myristate, apigenin, 3-phenylpropyl acetate, umbelliferone, glycyrrhizin, protocatechuic acid, poncirin, isovitexin, 6-gingerol, cineole, genistein, trans-cinnamaldehyde, and a combination thereof.
Owner:NAT DEFENSE MEDICAL CENT
Sulfonation of polyhydroxyaromatics
The present invention provides improved process for the sulfonation of hydroxyaromatics amenable to direct isolation of the sulfonylated hydroxyaromatics in their free-acid forms. The process allows for the recyclization of sulfuric acid and minimizes waste. The starting materials are from a renewal resource, e.g., biomass, and contain detectable 14C up to a 14C content of 0.0000000001% (one part per trillion). The products made include sulfonated catechol, disulfonated pyrogallol and sulfonated protocatechuic acid.
Owner:AMYRIS INC
Acorus gramineus total phenylpropanoid extraction and total phenols extraction and method for preparing simultaneously
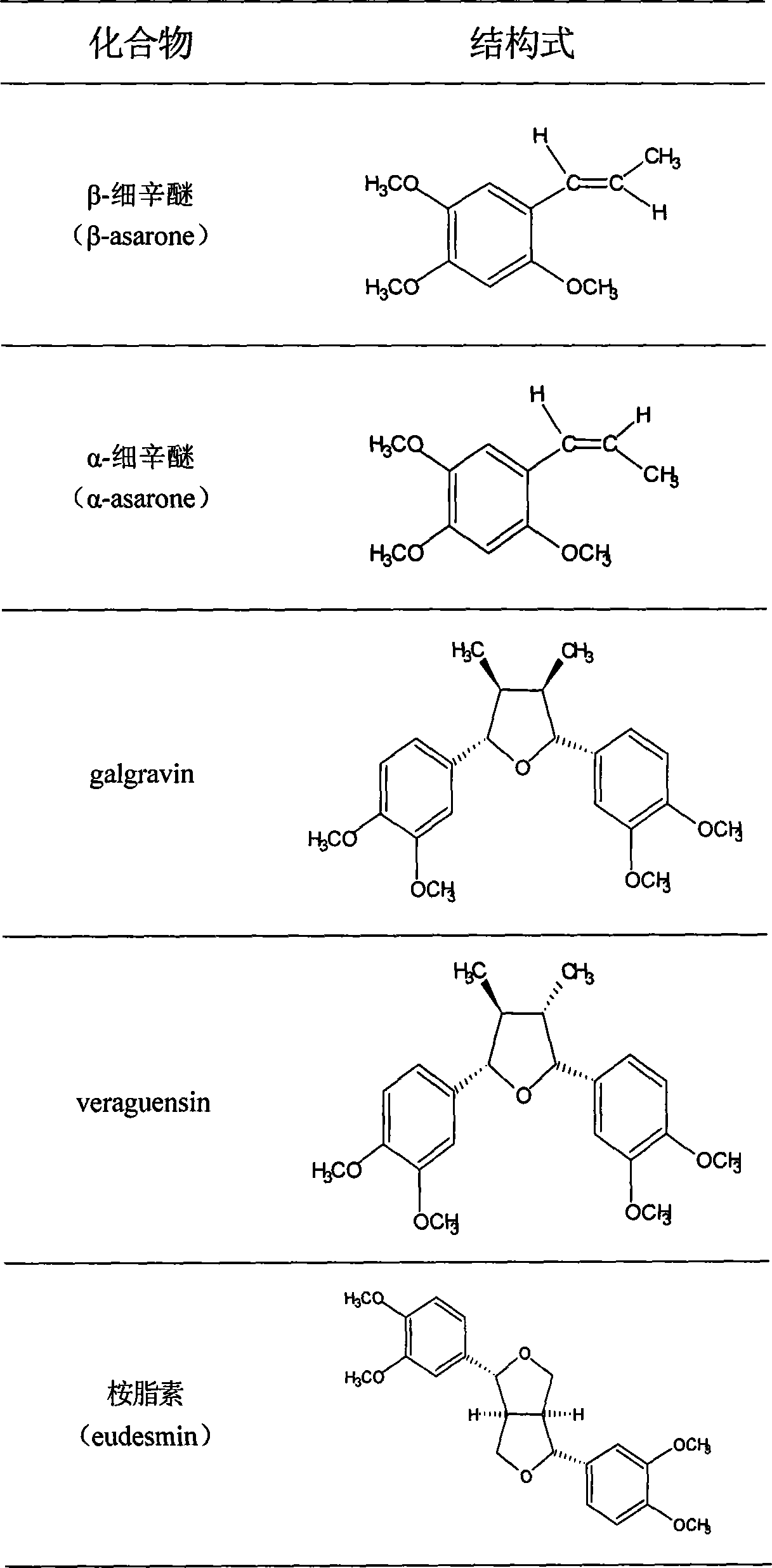
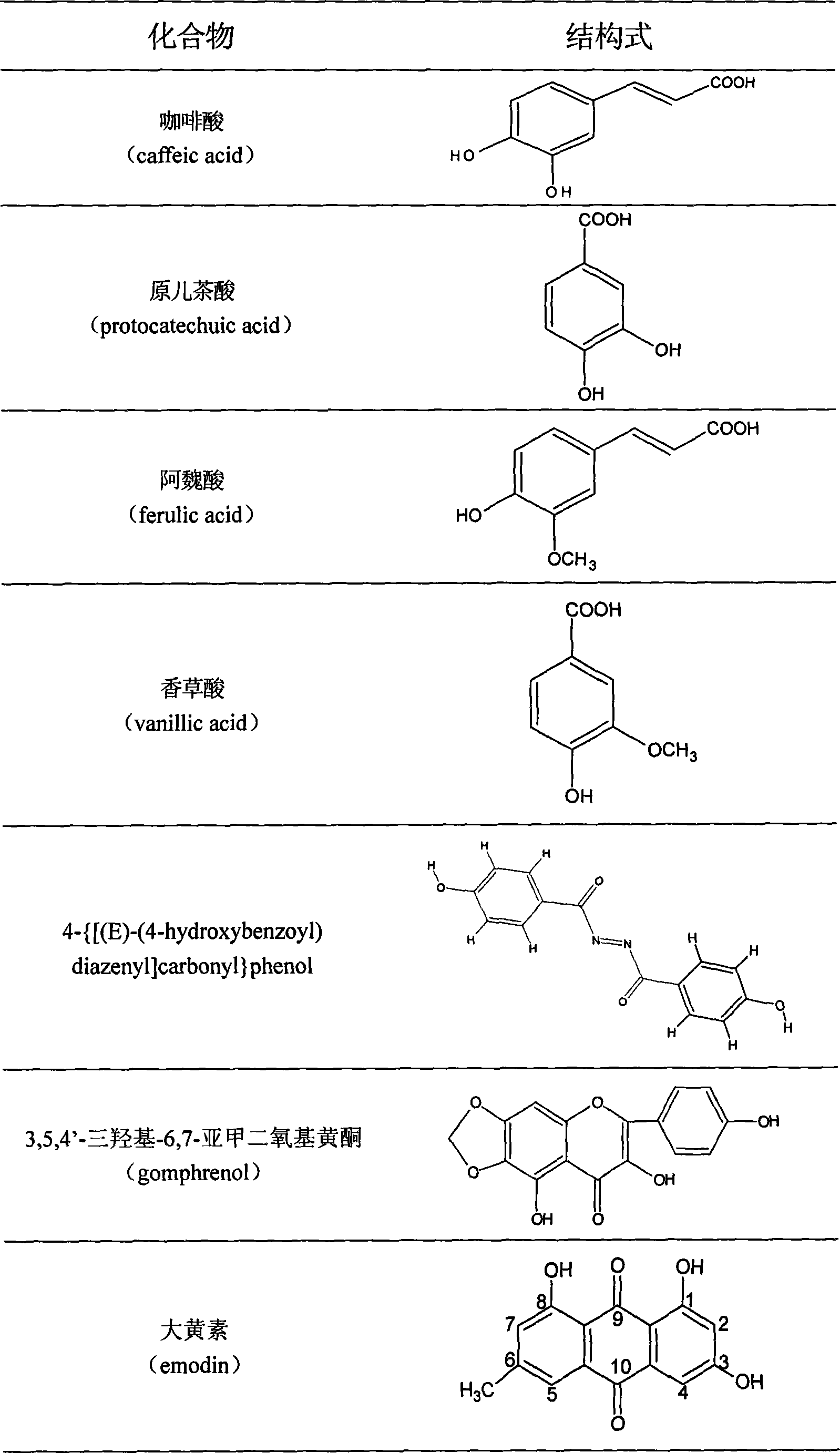
The invention discloses total phenyl propanoid extract and total phenol extract extracted from grassleaved sweetflag rhizome and its preparation method. Total phenyl propanoid extract mainly comprises beta -asaricin, alpha -asaricin, eudesmin, galgravin, veraguensin and other derivant with veraguensin as mother nuclide. Total phenol extract mainly comprises caffeic acid, protocatechuic acid, ferulic acid, vanillic acid and glucosides with vanillic acid as mother nuclide and other derivants. Total phenyl propanoid extract and total phenol extract of grassleaved sweetflag rhizome can be extracted through one or more of methods of solvent-extraction, solvent extraction, precipitation method, macroporous adsorbent resin, extraction by supercritical fluid, column chromatography, and liquid-liquid countercurrent distribution chromatography. Total percentage content of phenyl propanoids in the obtained total phenyl propanoid extract of Grassleaved sweetflag rhizome is 5-100% (w / w), wherein content of beta -asaricin and alpha -asaricin occupy 5-100% (w / w).Total percentage content of phenols in total phenol extract of grassleaved sweetflag rhizome is 5-100% (w / w), wherein content of caffeic acid and protocatechuic acid occupy 5-100% (w / w).
Owner:石任兵 +1
Method for extracting compounds using folium orthosiphoni leaf and stem as raw material
InactiveCN1775712AOrganic compounds purification/separation/stabilisationCarboxylic compound separation/purificationCaffeic acidIsoflavones
The invention provides a method for extracting compound from kidney tea stalks and leaves, crushing the stalks and leaves, successively adopting water extraction, alcohol extraction, and ethyl acetate to extract water soluble, alcohol soluble and fat soluble compounds, which are rosemary acid, caffeic acid, bear fruit acid, Yuan er tea acid, isoflavone, rosemary acid acetate and other phenolic compounds and flavone compounds. The invention is a kidney tea compound extracting method, effective and applied to industrial production.
Owner:吴振 +1
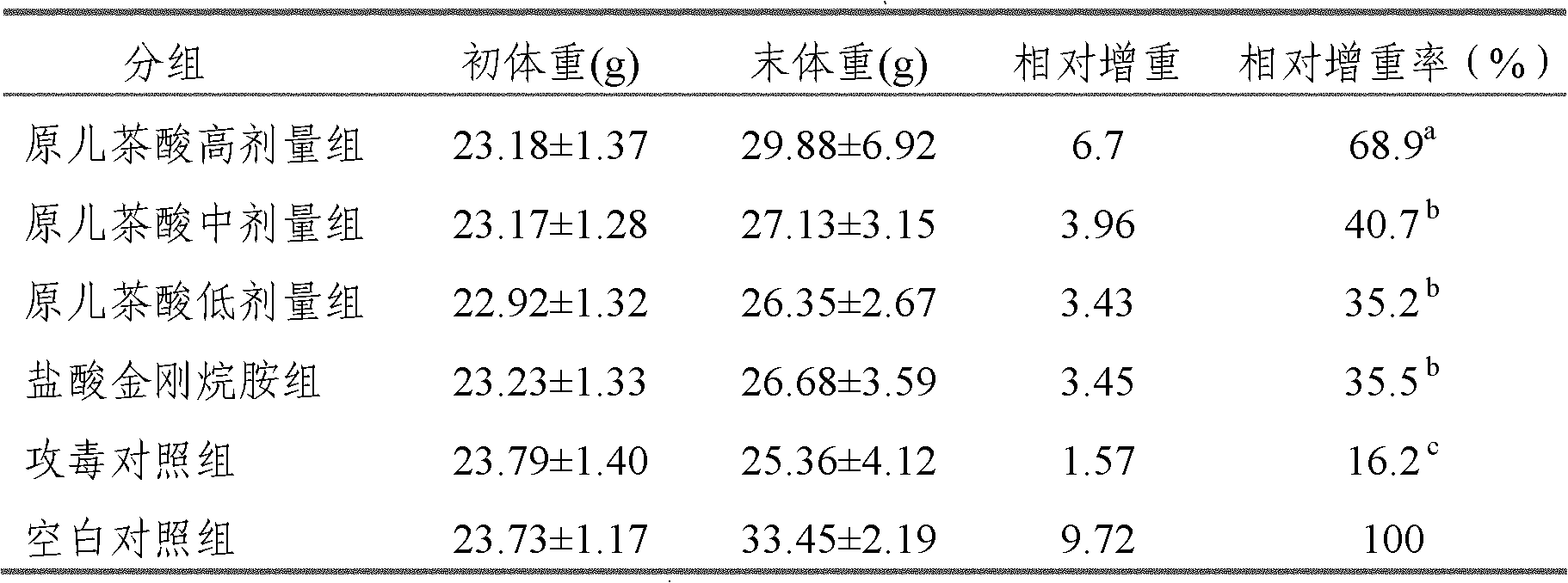
Application of protocatechuic acid in preparation of drugs for preventing and controlling livestock and poultry virus infectious diseases
ActiveCN102151256AReduce artificial infectionReduce mortalityOrganic active ingredientsPowder deliveryInfectious bronchitis virusAvian influenza virus
The invention provides an application of protocatechuic acid in the preparation of drugs for preventing and controlling livestock and poultry virus infectious diseases. The viruses include infectious bursal disease virus, avian influenza virus, infectious bronchitis virus and / or porcine transmissible gastroenteri tis virus. Meanwhile, the invention also relates to a protocatechuic acid preparation and a preparation method thereof.
Owner:CHINA AGRI UNIV
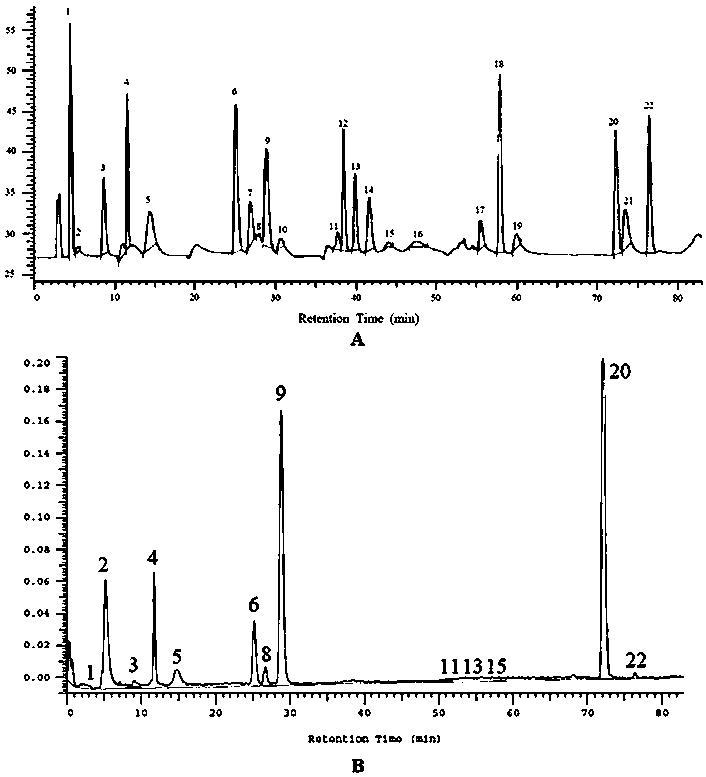
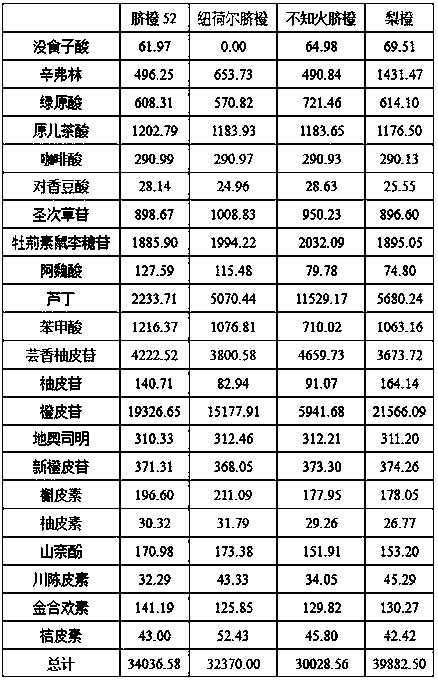
Method for simultaneously determining twenty-two flavones and phenolic acids in citrus fruits
The invention discloses a method for simultaneously determining twenty-two flavones and phenolic acids in citrus fruits by adopting high performance liquid chromatography-diode array detector-fluorescence detector (HPLC-DAD-FLD). The method is capable of simultaneously determining twenty-two phenolic compounds in citrus fruits such as gallic acid, synephrine, chlorogenic acid, protocatechuic acid,caffeic acid, p-coumaric acid, rhamnosylvitexin, eriocitrin, ferulic acid, rutin, benzoic acid, narirutin, naringin, hesperidin, diosmin, neohesperidin, quercetin, naringenin, kaempferol, nobiletin,hesperetin, acacetin and the like, derivatization is not needed, and the method is high in accuracy, high in sensitivity and excellent in repeatability.
Owner:INST OF AGRI ENG TECH FUJIAN ACAD OF AGRI SCI
Escherichia coli for producing adipic acid precursor namely cis,cis-muconic acid and application of escherichia coli
Cis,cis-muconic acid is an important chemical organic acid, has a wide range of application and can be used as a precursor of adipic acid. The cis,cis-muconic acid can be used for producing polymers including nylon 66 and the like after being converted into adipic acid through hydrogenation. The invention provides a recombinant escherichia coli TIB-LEc340 CGMCC No 6435 for producing cis,cis-muconic acid, wherein the recombinant escherichia coli is established by the steps of combining 3-dehydrogenated shikimic acid dehydrase which comes from different microbes and is optimized by codons, protocatechuic acid decarboxylase, catechol 1,2-dioxidase and artificially designed constitutive promoters and terminators into a heterologous expression module, and then introducing the heterologous expression module into shikimic acid dehydrogenase mutated escherichia coli. The escherichia coli TIB-LEc340 CGMCC No 6435 is fermented for 16-120 hours at 30-40 DEG C to obtain fermentation liquor, and the maximum content of cis,cis-muconic acid in the fermentation liquor can reach 52g / L, so that the escherichia coli has a wide application prospect.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
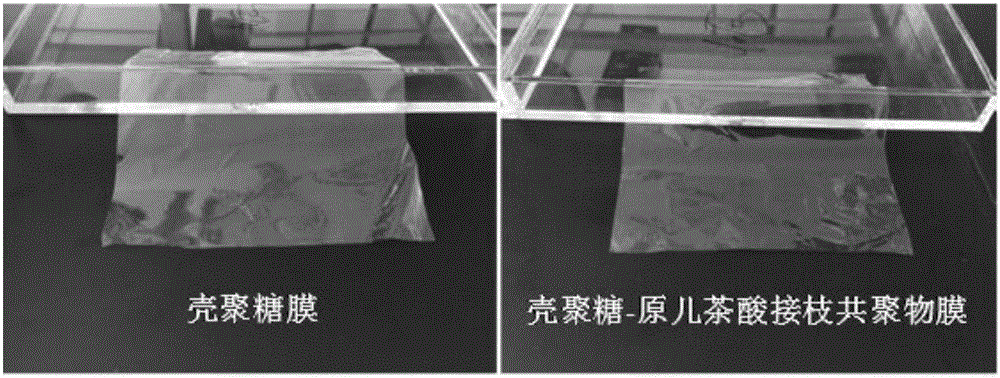
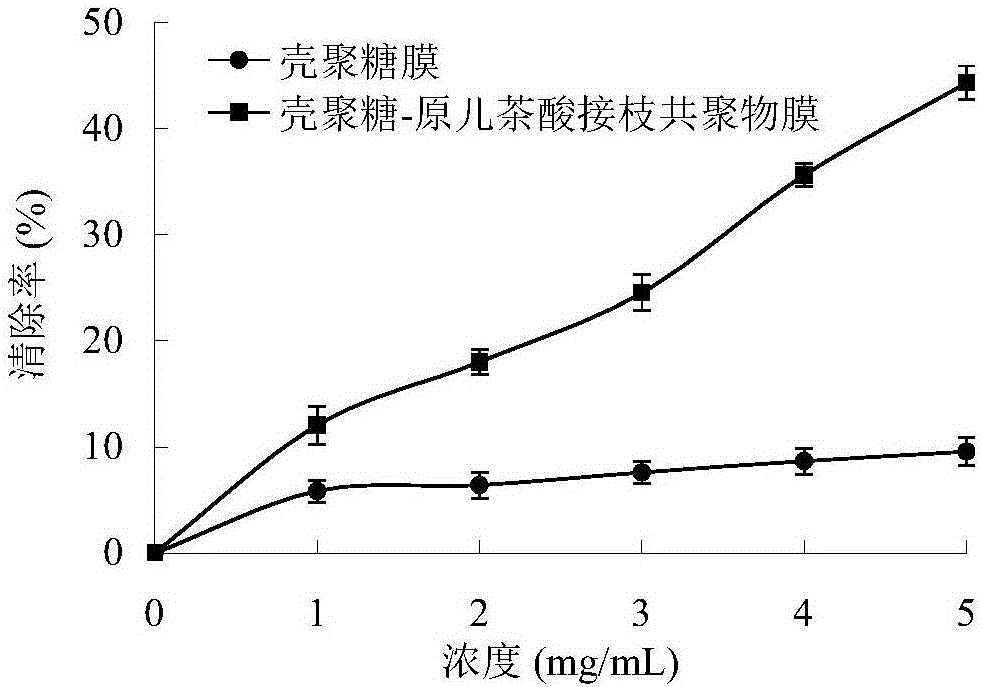
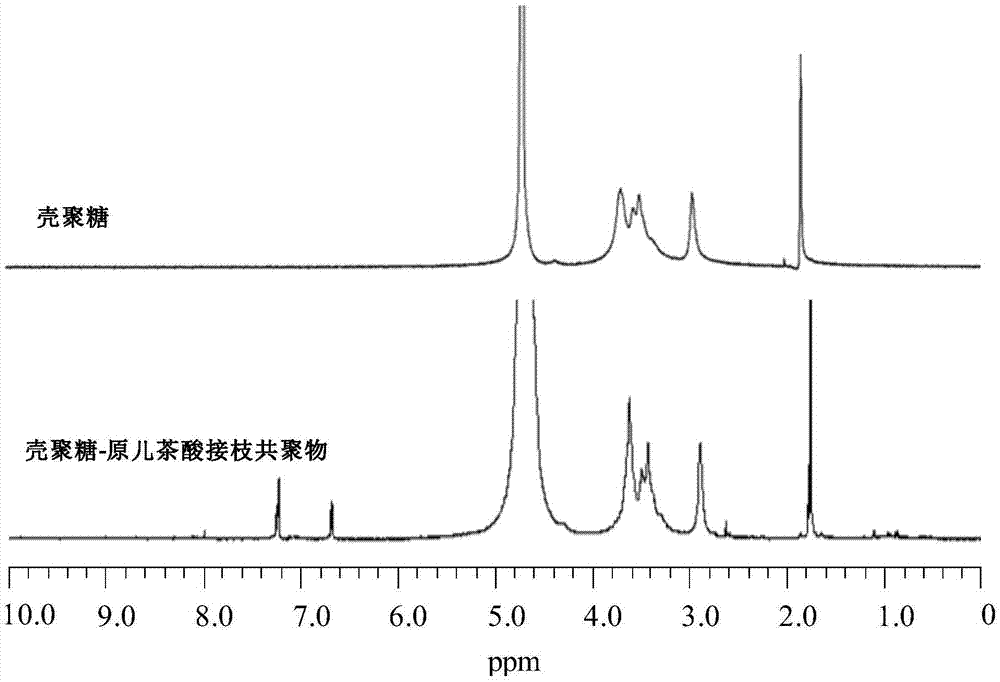
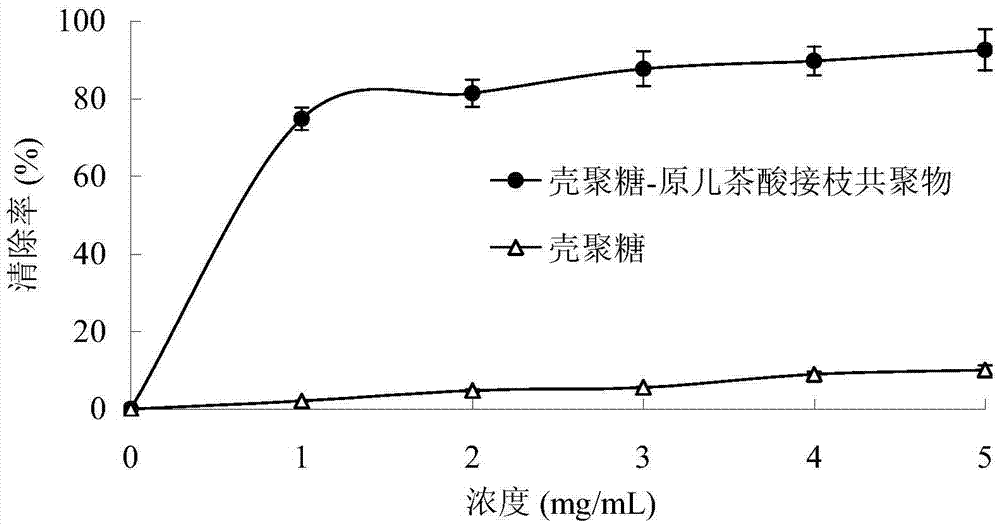
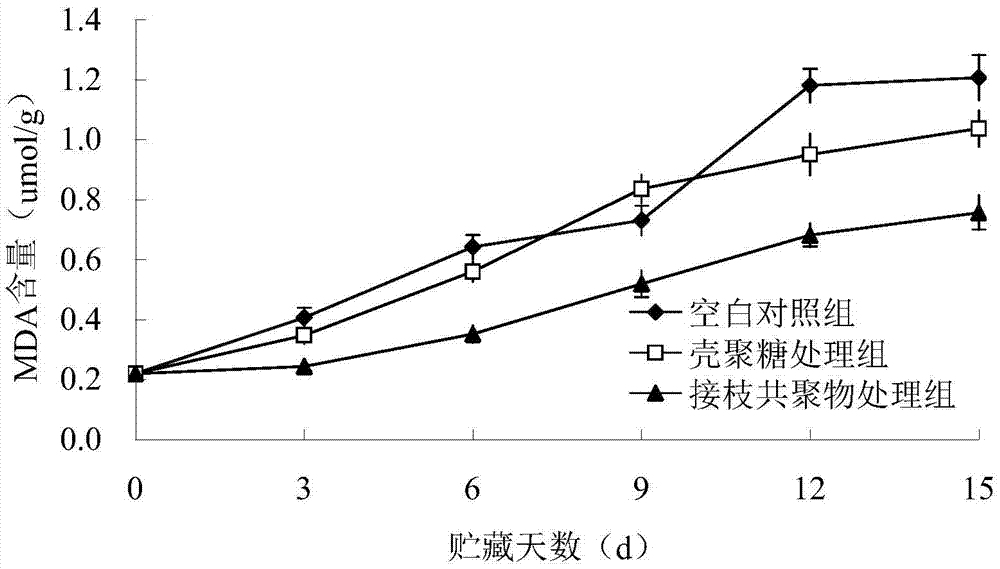
Preparation method of chitosan-protocatechuic acid graft copolymer film
InactiveCN106220876AEasy to synthesizeReduce usageFlexible coversWrappersPolymer scienceFreeze-drying
The invention provides a preparation method of a chitosan-protocatechuic acid graft copolymer film. The preparation method comprises the following steps: using chitosan and protocatechuic acid molecules as reaction substrates, using EDC / NHS as a crosslinking agent, synthesizing a chitosan-protocatechuic acid graft copolymer, and performing freeze drying to prepare a film forming solution with the proper concentration; removing bubbles, drying, soaking till the film forming solution is neutral, and performing film uncovering to finally obtain the chitosan-protocatechuic acid graft copolymer film. The preparation method is low in investment, and is suitable for use in large-scale production. The chitosan-protocatechuic acid graft copolymer film prepared by the preparation method is significantly improved in mechanical properties and physical and chemical properties, is low in water content, good in water resistance, low in transparency and high in tensile strength, has relatively high antioxidant activity, provides a very good way for artificially synthesizing a novel food fresh-keeping film, widens the application range of a chitosan film in food storage and food fresh keeping, improves the value of chitosan, and improves economic benefits of a food processing enterprise.
Owner:YANGZHOU UNIV
Method for determining content of organic acid in ginkgo leaf extract
The invention discloses a method for determining the content of organic acid in a ginkgo leaf extract. The method comprises the following steps: pretreatment of a ginkgo leaf extract sample; preparation of a test article solution, high performance liquid chromatograph analysis and the like. The method is characterized in that ultrasonic extraction is carried out by using an aqueous solution containing bovine serum albumin in a pretreatment process of the ginkgo leaf extract sample, so that the interference of procyanidine is removed; a sample is prepared by using an aqueous solution containing 0.1 percent of phosphoric acid in a preparation process of the testing article solution, so that the interference of part of impure peaks is removed, and the chromatographic behavior and the separation effect are greatly improved. After the method is adopted for analyzing, an organic acid pattern of the ginkgo leaf extract can be obtained, and the contents of five organic acid components such as shikimic acid, gallic acid, protocatechuic acid, 6-hydroxykynurenic acid and p-hydroxybenzonic acid can be simultaneously quantitated.
Owner:云南康恩贝植物研究院有限公司
Preparation method of protocatechualdehyde
InactiveCN1951892ANo residueSimple processOrganic compound preparationCarbonyl compound preparationAlcoholLow density
The invention discloses a preparing method of protocatechuic acid, which comprises the following steps: adsorbing extracted salvia miltiorrhizae alkaline through weak base resin; adjusting pH value of eluent at 1-4; eluting through pure water or acid first and low-density alcohol then; collects alcohol cleaning solution; condensing; cooling; fitting for large scale of industrial manufacturing.
Owner:SHANDONG LUYE PHARMA CO LTD
Green tea extract-ferroferric oxide composite catalyst and application thereof
ActiveCN105688991AStrong reductionHigh catalytic efficiencyOrganic-compounds/hydrides/coordination-complexes catalystsWater contaminantsSodium acetatePolyethylene glycol
The invention discloses a green tea extract-ferroferric oxide composite catalyst and application thereof. A preparation method of the green tea extract-ferroferric oxide composite catalyst comprises the steps of dissolving FeCl3.6H2O, sodium acetate and polyethylene glycol into ethylene glycol, then placing the mixture into a reaction kettle, raising the temperature to 195-205 DEG C, preserving the temperature for 8-72 hours, and then conducting natural cooling to reach the room temperature, so that black solid is obtained; cleaning the black solid with ethanol and ultra-pure water respectively, and conducting vacuum drying, so that ferroferric oxide powder is obtained; weighing ferroferric oxide and green tea extract according to the mass ratio of 1: 5-5: 1, placing the ferroferric oxide and the green tea extract in the ultra-pure water, conducting ultrasonic treatment for 0.5-2 hours, and conducting solid-liquid separation; conducting vacuum drying on obtained solid, so that the green tea extract-ferroferric oxide composite catalyst is obtained, wherein the green tea extract is one or more of protocatechuic acid, epigallocatechin, gallate and epicatechin. The green tea extract has great reductibility, and ferroferric oxide catalysis efficiency can be improved; the composite catalyst is green and environmentally friendly and convenient to separate, and secondary pollution cannot be caused.
Owner:HUNAN UNIV
Quality control method for simultaneous realization of content analysis and similarity evaluation of 18 components in Ilex kudingcha
ActiveCN106198782ARealize simultaneous content determinationComprehensive evaluationComponent separationIlex kudingchaHydroxytyrosol
The invention discloses a quality control method for simultaneous realization of content analysis and similarity evaluation of 18 components in Ilex kudingcha. Rutin, isochlorogenic acid A and kudinoside A are used as internal references; correction factors of rutin for 6-hydroxy-7,7a-dihydro-2(6H)-benzofuran and hydroxytyrosol glucoside, correction factors of isochlorogenic acid A for protocatechuic acid, kudinoside E, kudinoside D, neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, caffeic acid, isochlorogenic acid B, and isochlorogenic acid C, and correction factors of kudinoside A for latifoloside G, kudinoside G, ilex kudingcha ilexoside T and latifoloside H are calculated, and the factors are used as constants for determining content. Only three common reference substances are needed for simultaneous determination of contents of 18 kinds of components in ilex kudingcha, quality of the ilex kudingcha can be rapidly, economically and scientifically controlled, and further cluster analysis, main component analysis and similarity calculation of medicinal materials can be carried out by using contents of the 18 kinds of components, in order to comprehensively control quality of ilex kudingcha.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Method for measuring contents of multiple components in Shenxiong glucose injection
The invention belongs to the technical field of quality control of Chinese materia medica preparations, and provides a method for measuring contents of multiple components in Shenxiong glucose injection. According to the invention, the HPLC (high performance liquid chromatography) is adopted to measure contents of six compounds, namely tanshinol, ligustrazine hydrochloride, protocatechuic acid, rosmarinic acid, danshinolic acid B and danshinolic acid A, in the Shenxiong glucose injection under the same chromatographic condition. Compared with the present simple content measurement method for a single component, the method provided by the invention is better in integrity, characteristics and stability, can comprehensively reflect the interior quality of the preparations, and provides test base for further improving the quality control level of the Shenxiong glucose injection and ensuring the quality stability of the products.
Owner:GUIZHOU JINGFENG INJECTION
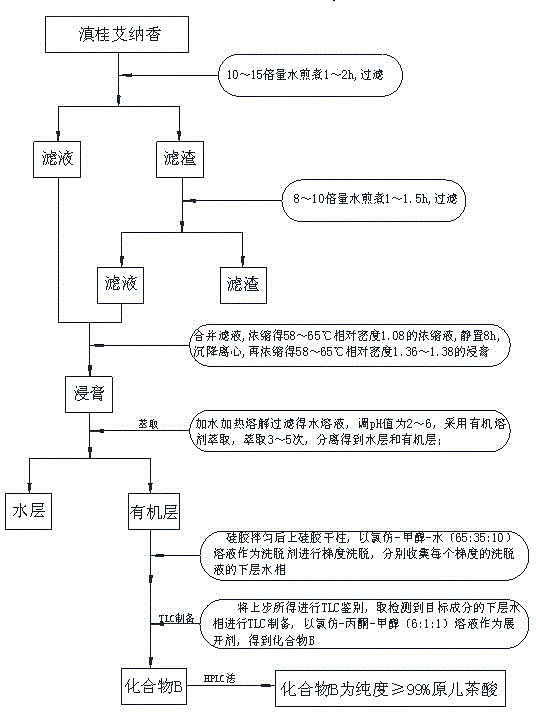
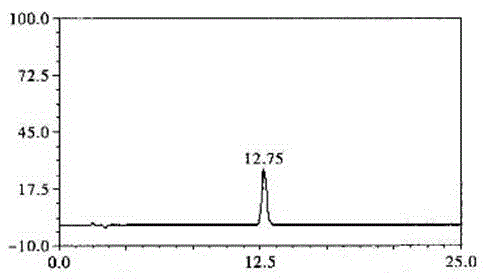
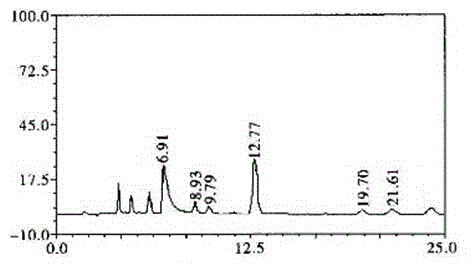
Technological method for extraction of protocatechuic acid from Blumea riparia (Bl.) DC
ActiveCN104098465AHigh purityEfficient separationCarboxylic compound separation/purificationChlorogenic acidElution
Belonging to the technical field of traditional Chinese medicine extraction, the invention discloses a technological method for extraction of protocatechuic acid from Blumea riparia (Bl.) DC. The method for preparation of a protocatechuic acid monomer consists of: water extraction, concentration, extraction, elution, and TLC preparation and purification. A compound B is determined as protocatechuic acid with a concentration of equal to or more than 99% by HPLC analysis, and is the protocatechuic acid monomer. The technological method for extraction of chlorogenic acid from Blumea riparia (Bl.) DC solves the problems of low preparation purity, small preparation quantity, complex preparation process, and difficult realization of industrialization production. By means of water extraction, concentration, extraction, elution and other processes, high extraction purity and a simple preparation process can be realized. Thus, the method is suitable for popularization, and meets the need of people for protocatechuic acid.
Owner:广西万寿堂药业有限公司
Novel method for simultaneously measuring contents of multiple active ingredients of dogwood
ActiveCN103175924ASimple methodAccurate methodComponent separationGallic acid esterAdditive ingredient
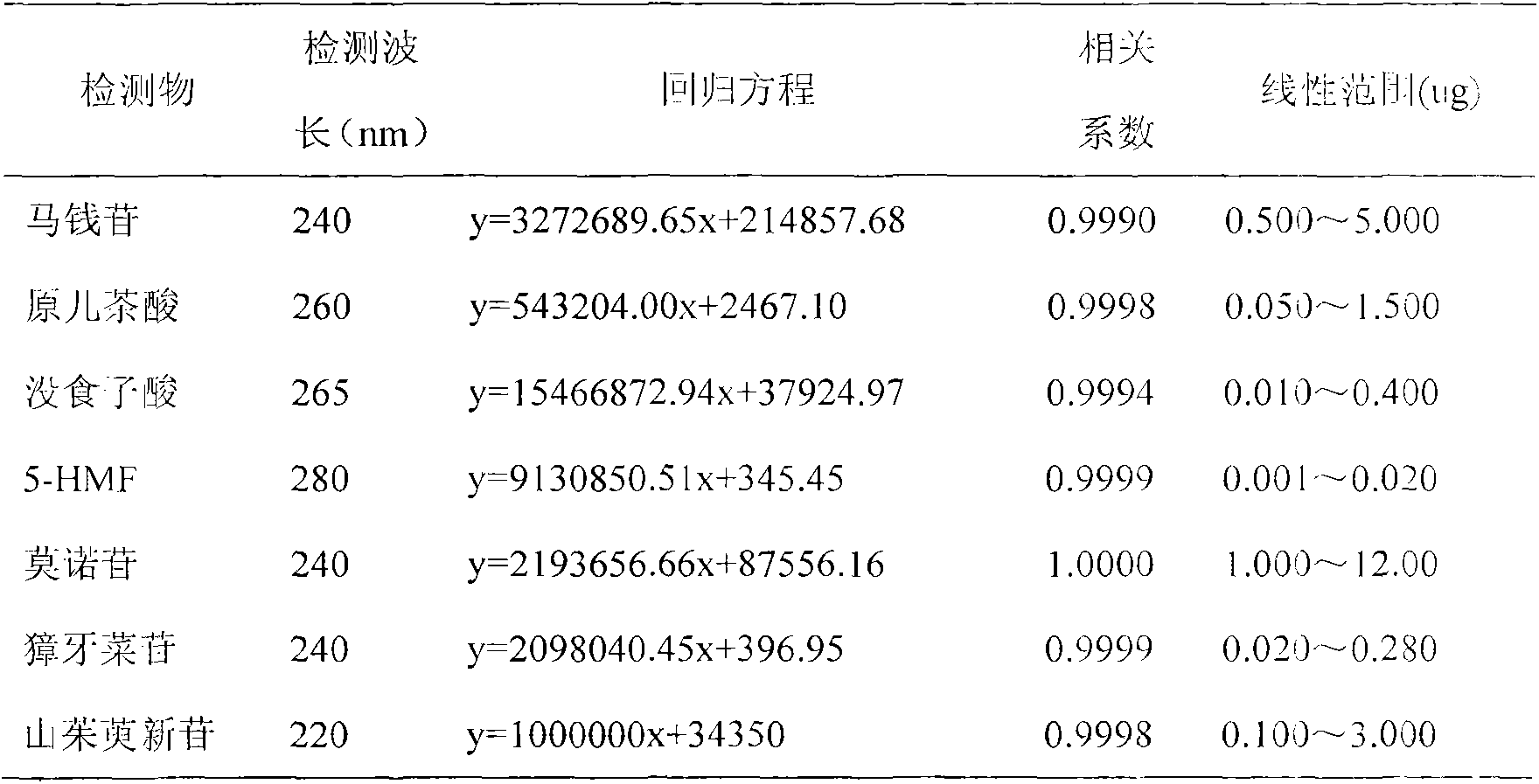
The invention discloses a novel method for simultaneously measuring contents of multiple active ingredients of dogwood. The method is used for simultaneously measuring the contents of seven ingredients, namely loganin, gallic acid, 5-HMF (hydroxymethyl furfural), protocatechuic acid, morroniside, sweroside and cor-nuside, in dogwood medicinal materials from four main producing areas by utilizing a high performance liquid diode array detection method. The method comprises the steps of: carrying out gradient elution at the flow speed of 1.0 mL.min-1 by utilizing a Phenoemenex C18 polar chromatographic column and taking acetonitrile and 0.1% methanoic acid solution as mobile phases, wherein the column temperature is 30 DEG C, the detection wavelengths are 220 nm, 240 nm, 260 nm, 265 nm and 280 nm. The contents of the loganin, the gallic acid, the 5-HMF, the protocatechuic acid, the morroniside, the sweroside and the cor-nuside in each sample are respectively 0.22-0.800 mg / g, 0.002-0.024 mg / g, 0.069-3.775 mg / g, 8.192-28.111 mg / g, 0.048-0.598 mg / g, 1.228-9.311 mg / g and 1.450-8.381 mg / g. The method is simple and accurate, is good in reproducibility and is capable of providing bases for comprehensively evaluating and controlling the quality of dogwood decoction pieces.
Owner:SHAANXI NORMAL UNIV
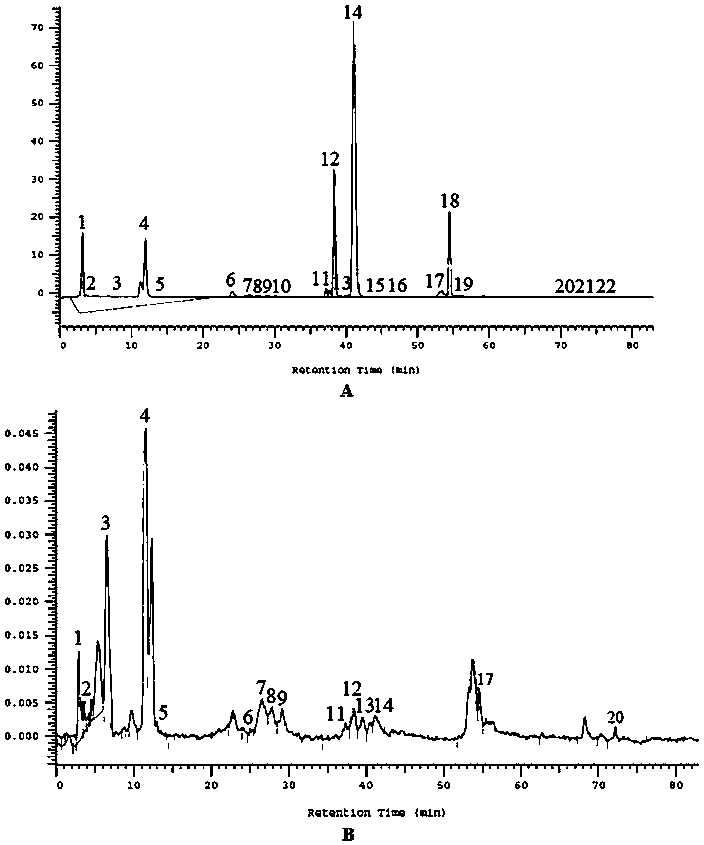
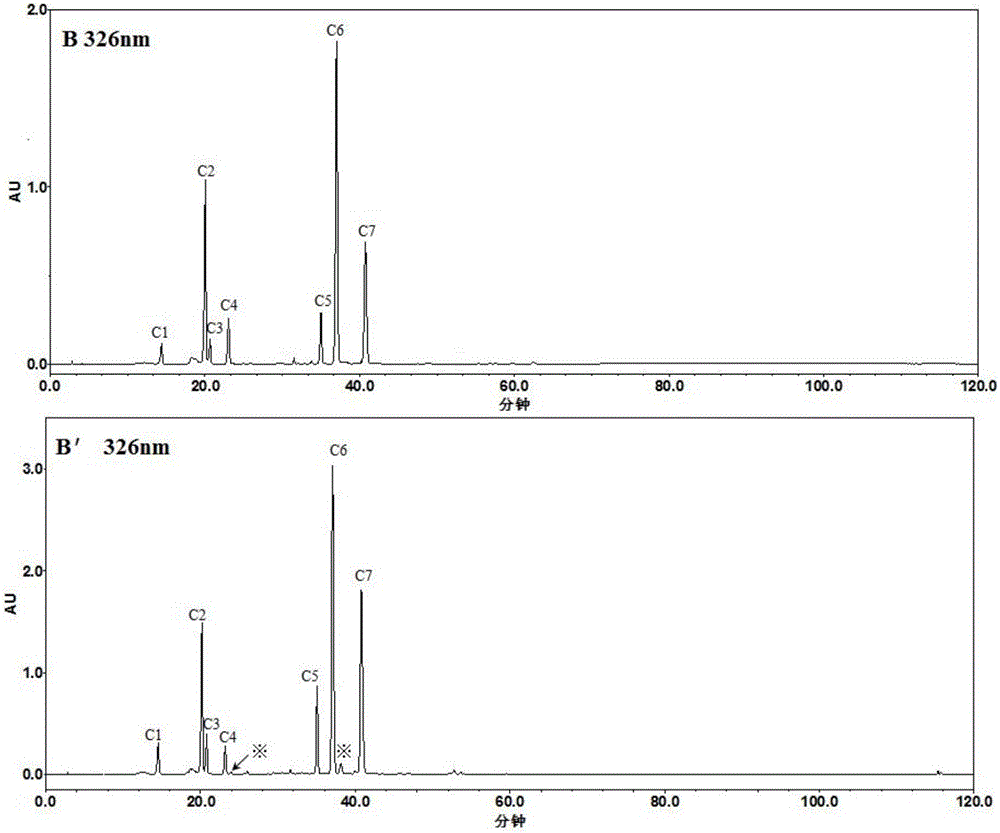
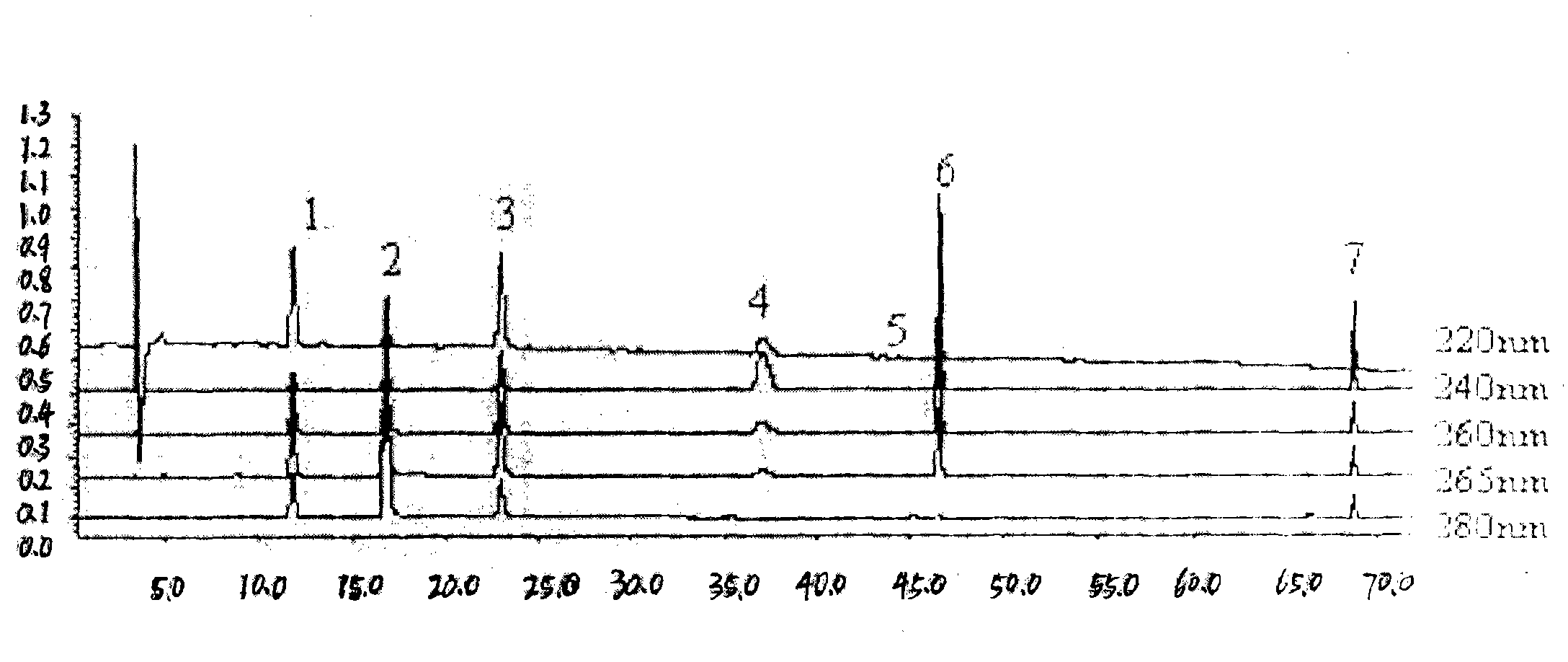
Method for establishing HPLC fingerprint spectrum of Zhuang medicinal material Blumea riparia (Bl.) DC
ActiveCN104458993AFully react chemical compositionInformativeComponent separationHplc fingerprintChemical composition
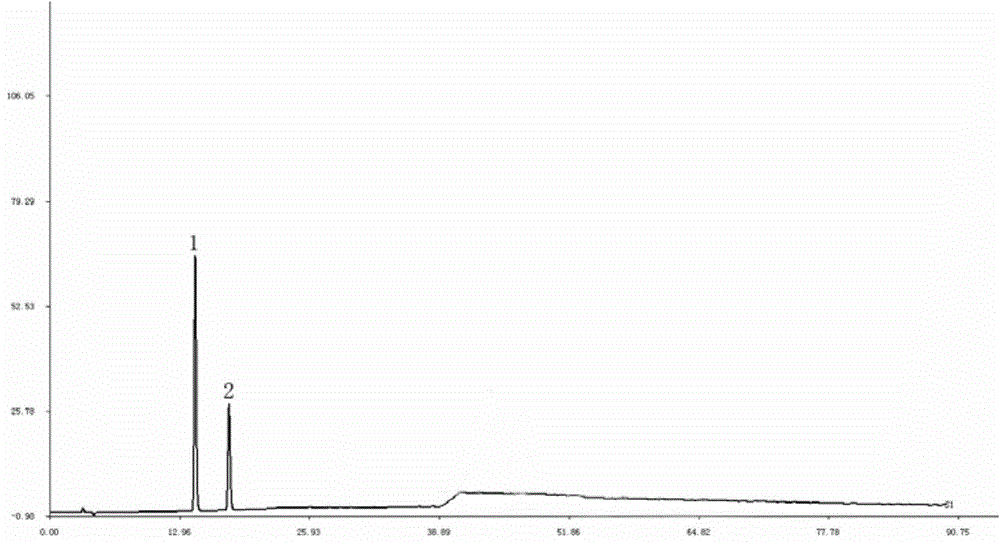
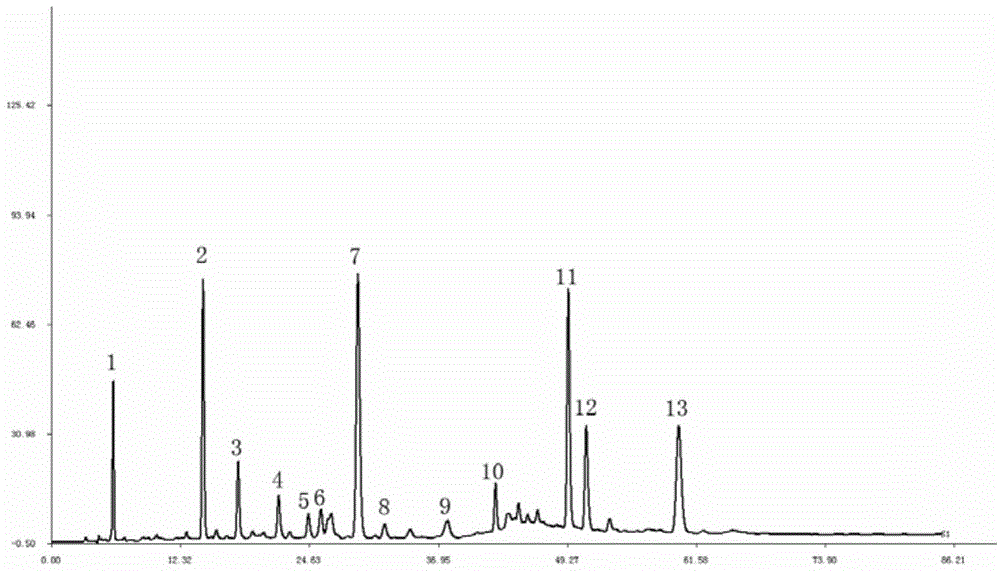
The invention discloses a method for establishing an HPLC fingerprint spectrum of a Zhuang medicinal material Blumea riparia (Bl.) DC. By adopting a high-performance liquid chromatography, and using main ingredients such as protocatechuic acid and protocatechuic aldehyde of Blumea riparia (Bl.) DC as reference substances, a fingerprint spectrum of a common pattern of the Zhuang medicine Blumea riparia (Bl.) DC medicinal material is obtained. The fingerprint spectrum has 13 chromatographic peaks, can fully reflect the chemical compositions of the Blumea riparia (Bl.) DC medicinal material, and is rich in information amount, the method has good reproducibility, a more powerful theoretical basis is provided for controlling the quality of the medicinal material and identifying the advantages and disadvantages of the medicinal material, the quality control and the level of true and false identification of the Zhuang medicine Blumea riparia (Bl.) DC medicinal material are improved, and the authenticity and the advantages and disadvantages of the Blumea riparia (Bl.) DC can be quickly and accurately identified.
Owner:广西万寿堂药业有限公司
Method for preparing cuprous oxide nanometer wire by utilizing plant phenolic acid
ActiveCN104477968ARaw materials are easy to getNo toxicityMaterial nanotechnologyCopper oxides/halidesGallic acid esterCupric nitrate
Aiming at the shortcomings of an existing technology for preparing a cuprous oxide nanometer wire, the invention discloses a method for preparing the cuprous oxide nanometer wire by utilizing plant phenolic acid and belongs to the field of a nanometer material and a novel material. The method comprises the following steps: heating a plant phenolic acid solution to the temperature of 50 to 95 DEG C under an alkaline condition by taking natural plant phenolic acids including protocatechuic acid, gallic acid and ellagic acid as a reducing agent; dropwise adding inorganic salt solutions of copper sulfate, copper chloride, cupric nitrate and copper acetate into the plant phenolic acid solution, reacting for 0.5-5 hours and directly precipitating to obtain a cuprous oxide nanometer wire material. The method is environmental friendly, simple in technological process, capable of directly finishing the technological process by one step in an alkaline solution, free of toxin or low in toxicity in raw materials, low in cost, pure in product and suitable for large-scale production.
Owner:NORTHEASTERN UNIV LIAONING
Bee glue flavone extract preparation method, pharmaceutical preparation and its new medical uses
InactiveCN1544429AAnti-myocardial ischemia effect is obviousReduced activityOrganic active ingredientsOrganic chemistryPropolisPesticide residue
The invention provides a process for preparing bee glue flavone extract, its medicinal preparation and novel medical use, wherein the preparation process comprises, freezing and crushing the bee glue into coarse powder, charging in alcohol proportionally for lixiviating, filtering and combining the filter liquor, reclaiming the solvent by depression, condensing into thick grease, decompressing and drying, disintegrating into fines, dissolving by chloroform-ethanol miscible liquid, and extracting with sodium-hydroxide solution of equal volume, adjusting pH to 4-8 by charging in hydrochloric acid into alkaline extract liquor, obtaining deposition, filtering by suction, rinshing to neutral, decompressing and drying, disintegrating into fines, thus the product according to the invention can be obtained.
Owner:CHANGCHUNG COLLEGE OF TRADITIONAL CHINESE MEDIICINE +1
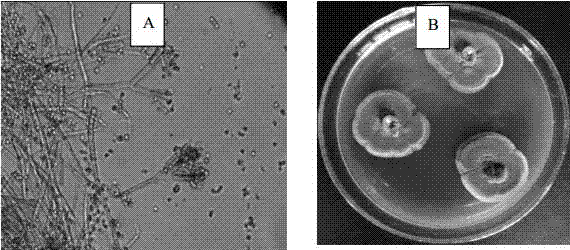
Fungus with strong weed suppression effect screened from passion fruit rhizosphere soil
InactiveCN107164234AHas an anti-herb effectEnvironmentally safeBiocideFungiSalicylic acidAspergillus sydowii
The invention belongs to the field of microbial herbicides and particularly relates to weed suppression fungus screened from passion fruit rhizosphere soil. Lettuce (compositae), barnyard grass (gramineae) and lysimachia barystachys (gramineae) are taken as receptors separately, biological test is carried out on the weed suppression ability of a fungus strain fermentation liquid, and the condition that the weed suppression fungus has a very high inhibition rate on root length and plant height of two receptors is found out. The condition that protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, salicylic acid and cinnamic acid contain phenolic acid with a weed suppression effect is detected in the fermentation liquid. The strain is named FJ-01-BXG-08. The analysis and identification results on the morphology and ITS sequence of the strain show that the strain is one of Aspergillus sydowii (aspergillus sydowii), and strong weed suppression fungus aspergillus sydowii (Aspergillus sydowii) is screened from eucalyptus soil for the first time.
Owner:FUJIAN AGRI & FORESTRY UNIV
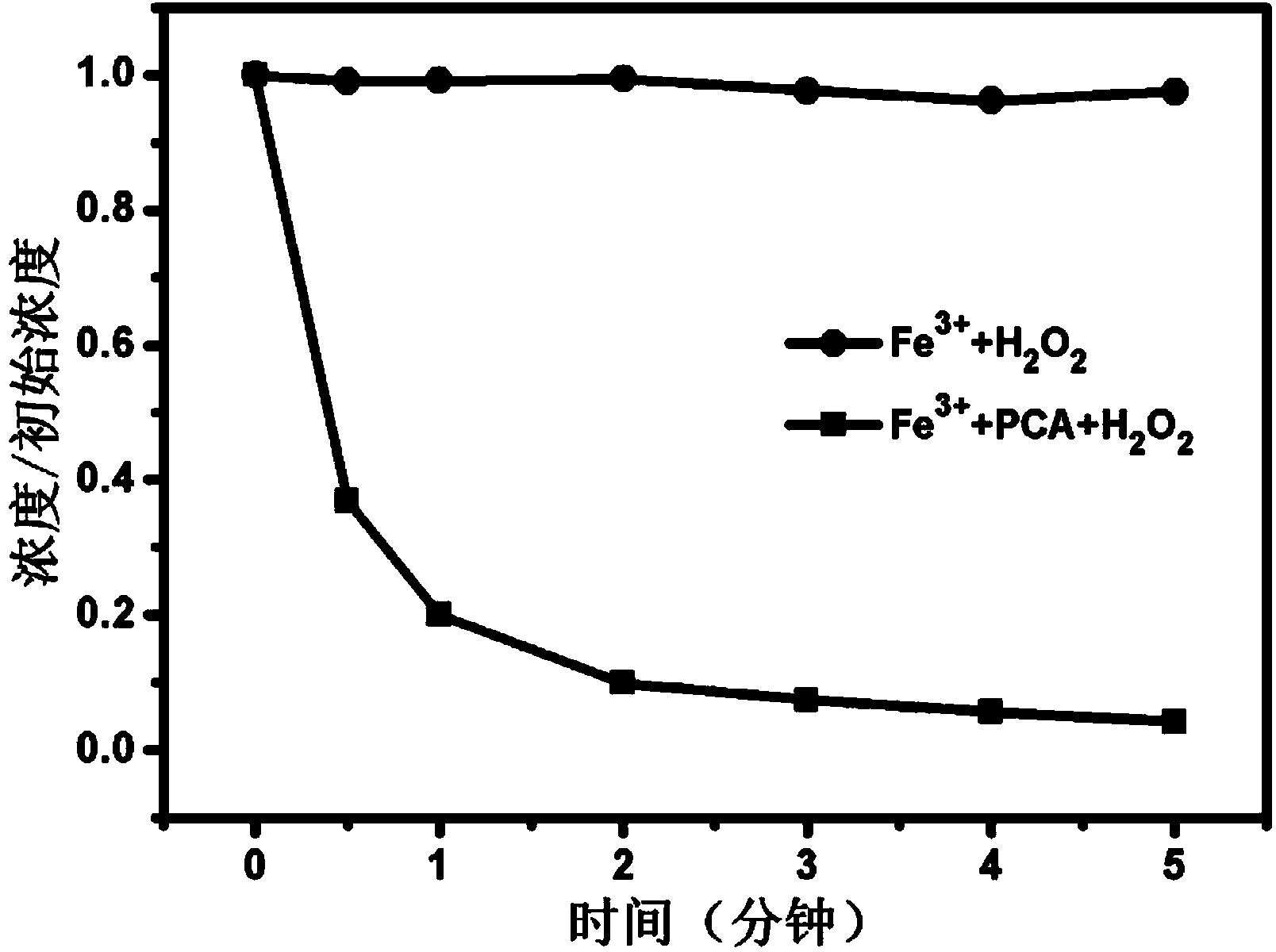
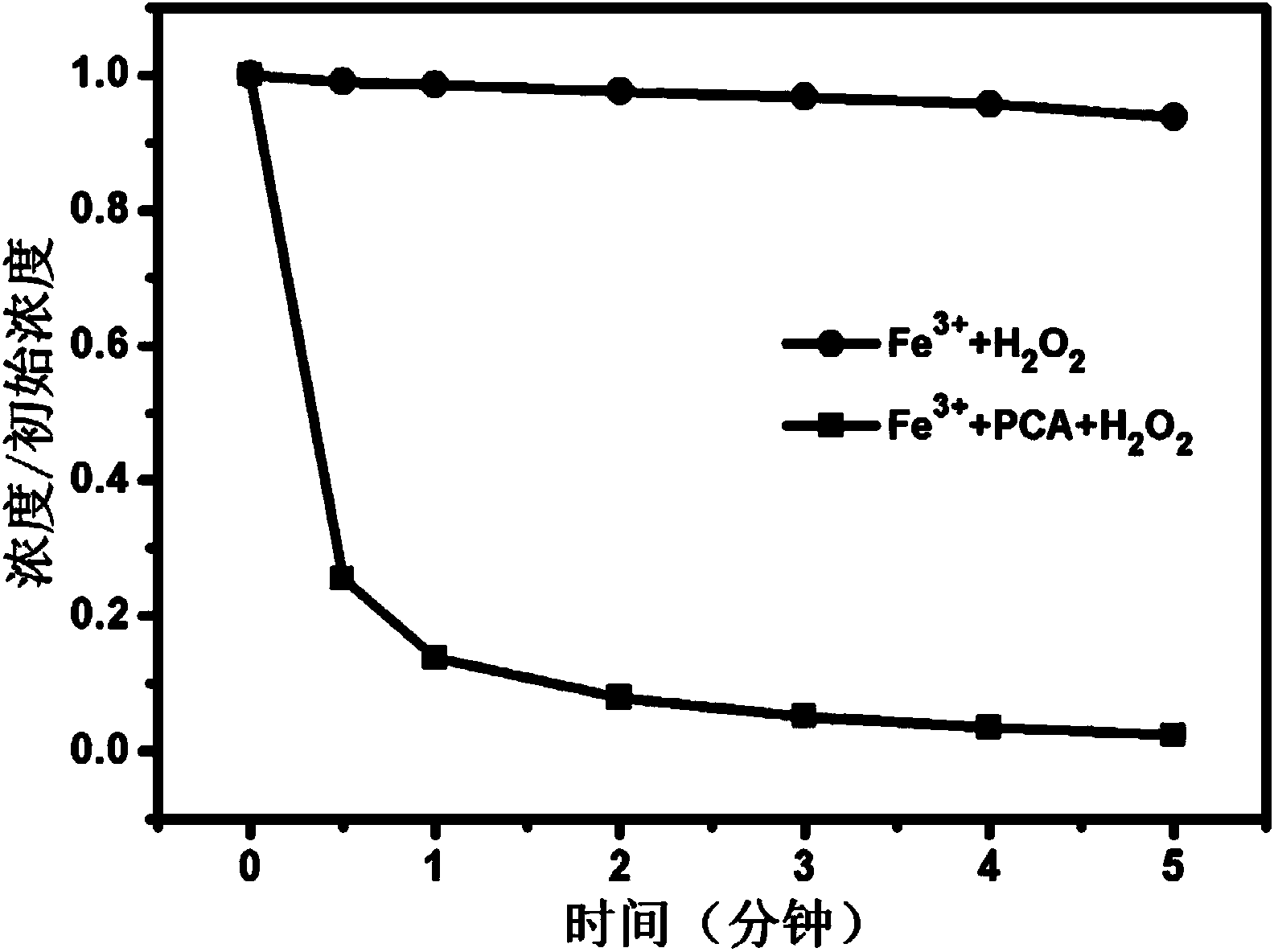
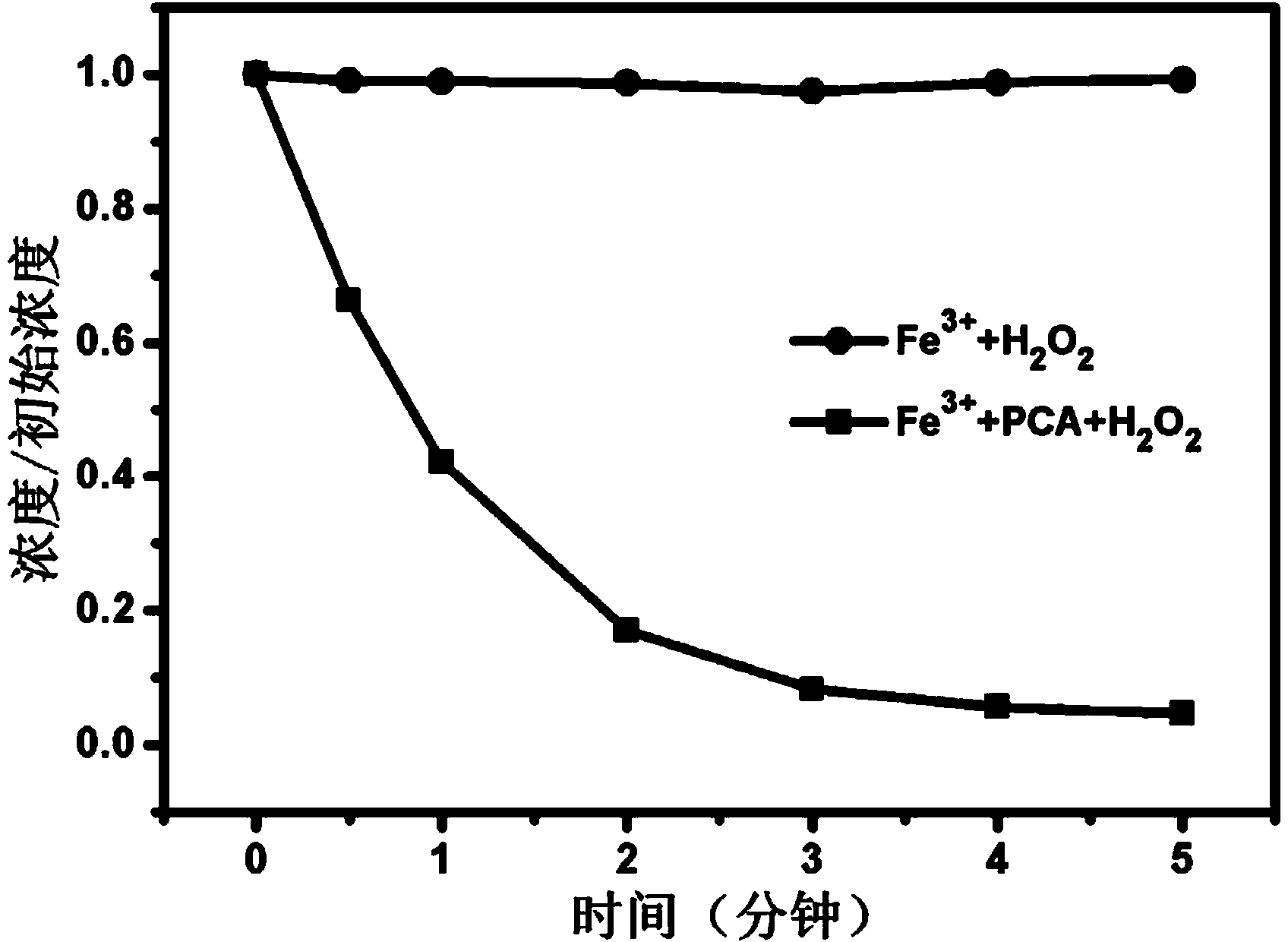
Method for promoting Fe(III)/H2O2 system to repair organism polluted water by utilizing protocatechuic acid
ActiveCN104310567AIncreased degradation rateDegradation without selectivityWater treatment compoundsWater contaminantsEnvironmental engineeringOrganism
The invention relates to a method for promoting an Fe(III) / H2O2 system to repair organism polluted water by utilizing protocatechuic acid. The method comprises the following steps: adding the protocatechuic acid and a Fe3+ substance into the organism polluted water to be repaired; and controlling the system to enable the system is under the acid condition, adding H2O2 to carry out repair of the organism polluted water. The method solves the problems of easiness for precipitation of Fe3+ and difficulty in circulation of Fe3+ / Fe2+ in conventional Fenton and Fenton-like systems and has the advantages of high efficiency, non preference, environmental-friendliness, no secondary pollution and the like when being used for repairing the organism polluted water.
Owner:HUAZHONG NORMAL UNIV
Medicine for treating ischemic brain injury stroke and sequela of ischemic brain injury stroke and preparation method for medicine
The invention discloses a structural general formula of a compound LQC-T and the synthesis and the application of the compound LQC-T. Pharmacological experiments prove that the compound has an obvious effect of promoting new vessels of chick chorioallantoic membranes to grow; a compound LQC-T4 has an obvious medicinal effect of treating ischemic brain injury stroke and the sequela of the ischemic brain injury stroke; the maximal LQC-T4 single-day dose of mice is 5,400mg / kg; and the compound is extremely high in safety and can be used for preparing a medicine for treating the ischemic brain injury stroke and the sequela of the ischemic brain injury stroke after toxic responses do not occur after continuous observation within 1 to 4 days. According to the structural formula of the compound LQC-T, R is protocatechuic acid, protocatechuic aldehyde, vanillic acid, gallic acid, caffeic acid, ferulic acid and other aromatic organic acids or phenols or structural analogues of the organic acids or phenols.
Owner:薪火炙药(北京)科技有限公司
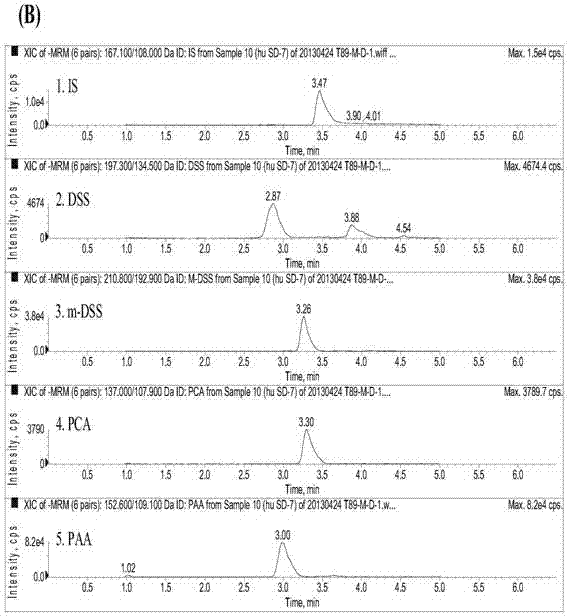
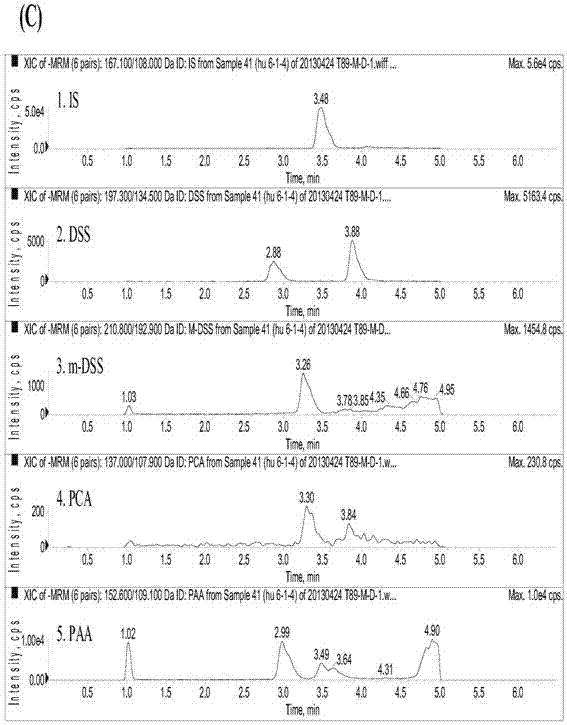
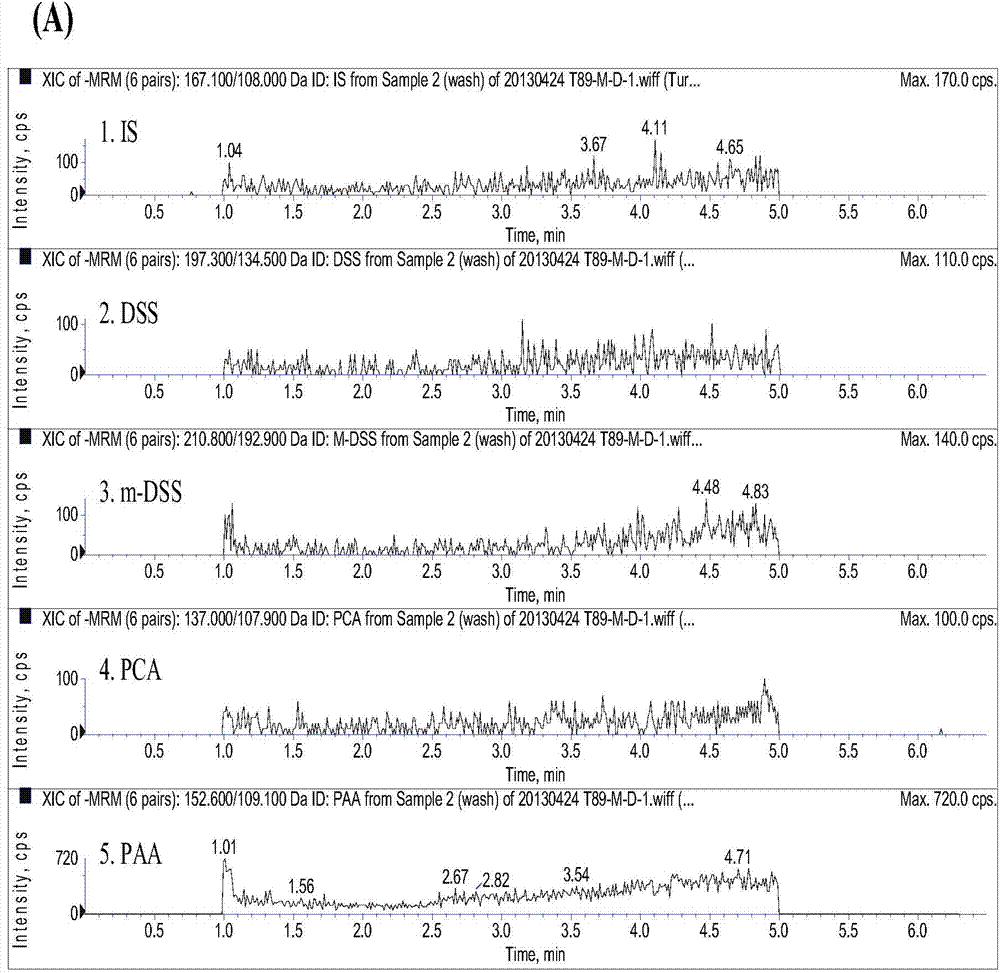
Method for measuring danshensu, m-methyl-danshensu, protocatechualdehyde, and protocatechuic acid in human blood plasma
The invention relates to a method for measuring drug contents, particularly to a method for measuring danshensu, m-methyl-danshensu, protocatechualdehyde, and protocatechuic acid in the human blood plasma. The method comprises the following steps: 1) preparation of reference substance storing solutions: weighting reference substances of danshensu, m-methyl-danshensu, protocatechualdehyde, and protocatechuic acid, and dissolving the reference substances with methanol to obtain reference substance storing solutions of danshensu, m-methyl-danshensu, protocatechualdehyde, and protocatechuic acid; 2) preparation of an internal standard solution: dissolving an internal standard substance of vanillic acid with methanol to obtain the internal standard solution; 3) a treatment method of a blood plasma sample: taking the blood plasma sample, adding the internal standard solution, methanol, hydrochloric acid, and ethyl acetate into the blood plasma sample, performing uniform mixing, performing centrifugation of the obtained mixture to obtain a supernatant ethyl acetate layer, drying the ethyl acetate layer, re-dissolving the ethyl acetate layer with methanol, and performing centrifugation of the obtained solution to obtain supernatant liquid of the blood plasma sample; and 4) content measurement: by adopting LC-MS / MS chromatography, injecting the reference substance storing solutions and the supernatant liquid of the blood plasma sample into a chromatographic instrument to obtain a chromatogram, and calculating concentrations of danshensu, m-methyl-danshensu, protocatechualdehyde, and protocatechuic acid in the human blood plasma according to the areas of chromatographic peaks in the chromatogram.
Owner:TIANJIN TASLY PHARMA CO LTD
Preparation method of phenolic acid-modified chitosan coating liquid for fresh keeping of edible fungus
InactiveCN106977622ASolve the low grafting rateSolve pollutionAcidic food ingredientsFruits/vegetable preservation by coatingSolubilityChitosan coating
The invention discloses a preparation method of a phenolic acid-modified chitosan coating liquid for fresh keeping of edible fungus. According to the preparation method, mainly chitosan and protocatechuic acid molecules are adopted as reaction substrates, and a chitosan-protocatechuic acid grafted copolymer is synthesized by using an EDC-mediated cross-linking reaction. According to the present invention, the synthesis process is simple, and the obtained chitosan-protocatechic acid grafted copolymer has characteristics of good water solubility, high grafting rate and high antioxidant activity, and can significantly improve the storage quality of edible fungus and prolong the shelf life; with the preparation method, the problems of poor water solubility, low oxidation activity and the like of the chitosan can be solved so as to improve the performance of the chitosan and expand the application in the storage and the fresh keeping of food; and the method is less investment, is suitable for large-scale production, and has excellent application prospect.
Owner:YANGZHOU UNIV
Rice wine brewing method for reducing content of ethyl carbamate through protocatechuic acid
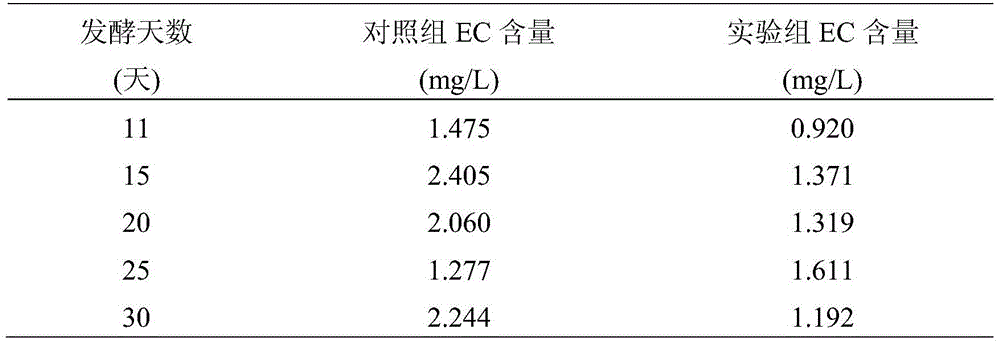
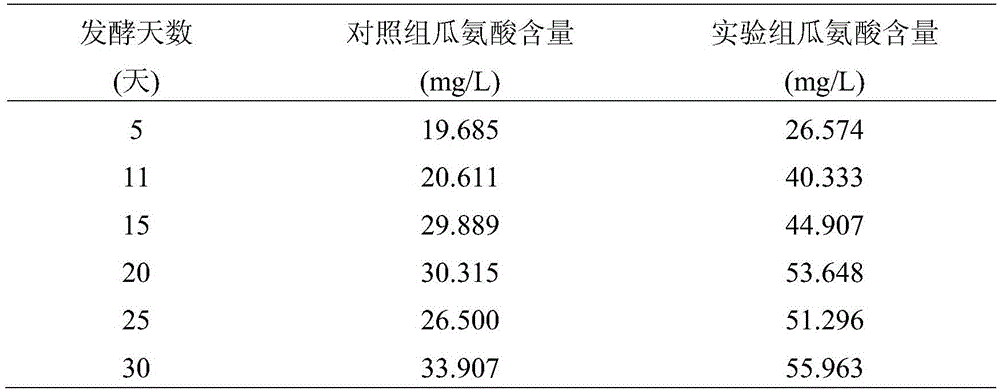
InactiveCN105543033AReduce contentInhibit transformationAlcoholic beverage preparationMicroorganism based processesSteepingCitrulline
The invention discloses a rice wine brewing method for reducing the content of ethyl carbamate through protocatechuic acid. The method includes the steps of rice steeping, rice steaming, water spraying, cylinder falling for nest building, wheat cojic and water adding, fermentation and after-treatment; protocatechuic acid is added to fermentation liquor 2-5 days after cylinder falling for nest building. Protocatechuic acid is added at the fermentation stage, so that conversion of precursor substances citrulline and urea of ethyl carbamate into ethyl carbamate in the rice wine brewing process is effectively restrained, and then the content of ethyl carbamate in rice wine is reduced. Protocatechuic acid belongs to inherent phenolic substances in the rice wine, multiple biological activities are achieved, no follow-up treatment is needed during addition of protocatechuic acid, and the defects of an existing way for degrading ethyl carbamate can be effectively overcome.
Owner:ZHEJIANG UNIV
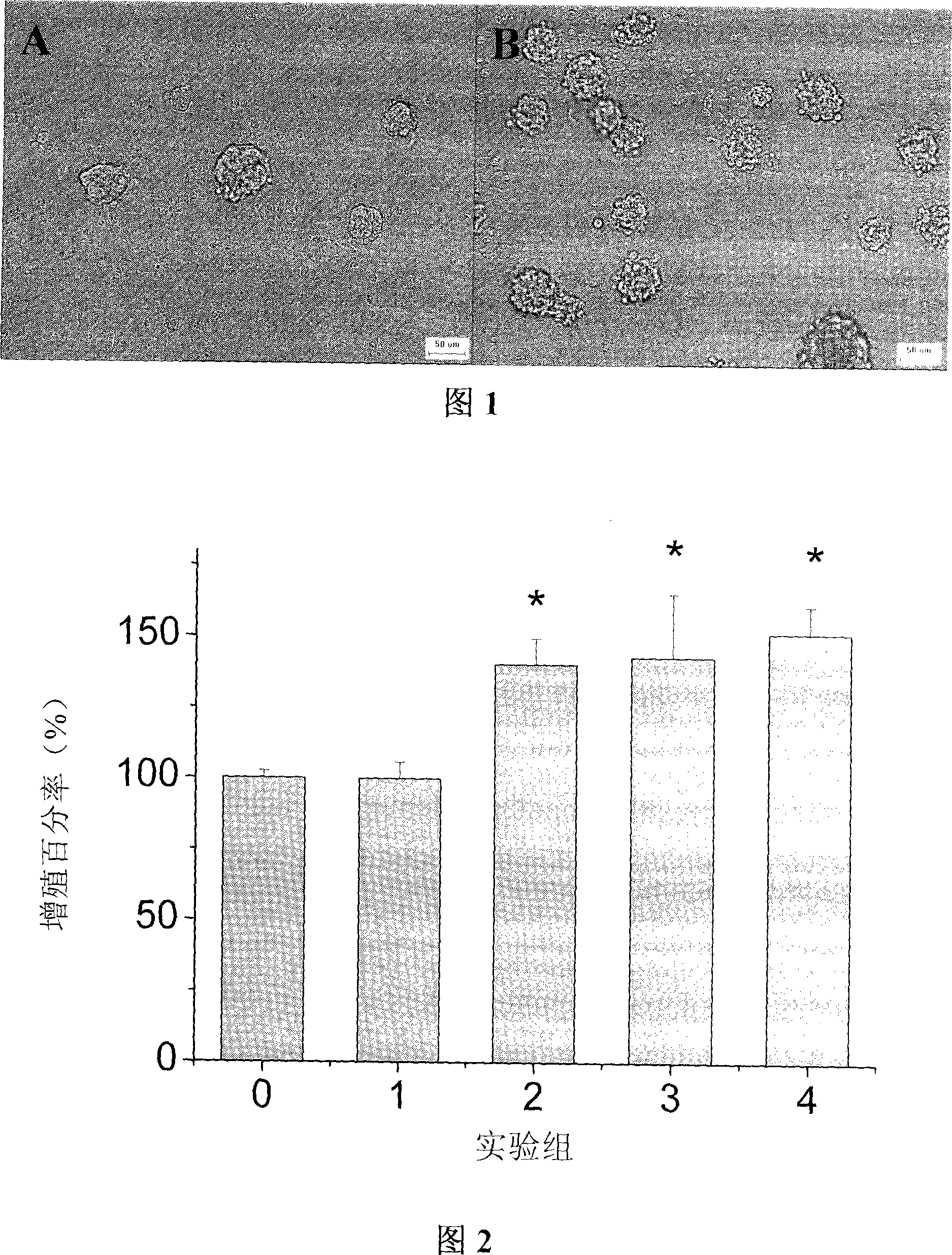
Use of protocatechuic acid in accelerating nerve stem cell multiplication in vitro and inducement differentiation
InactiveCN101104845APromotes significant proliferationKeep proliferatingNervous disorderUnknown materialsIntellectual propertyProviding material
The invention belonging to the cell biology and pharmacology technical field relates to the purpose of a Chinese traditional medicine monomeric compound, in particularly to the new purpose of a protocatechuic acid in the aspect of promoting the external proliferation and induction-differentiation of a nerve stem cell. The invention is characterized in the application of producing medicine to treat the neurodegenerative diseases by nerve stem cell transplantation, in the application of producing neurenergen and cell differential agent for restoring damaged nerve tissues and reconstructing nerve functions, and in the application of constructing high efficient medical screening model to initially screen and appraise the efficacy of medicine. The invention confirms the new phamacoloic activity of the protocatechuic acid, providing material basis with regard to prevention effect of the Chinese traditional medicine, and providing reference for the development of new medicine with independent intellectual property rights.
Owner:DALIAN UNIV OF TECH
Application of protocatechuic acid as feed additive in livestock breeding
InactiveCN103749981APromote growth and developmentImprove conversion rateAnimal feeding stuffChemical synthesisFood additive
The invention provides application of protocatechuic acid as a feed additive in livestock breeding, the protocatechuic acid is used for promoting the growth and development of animals, improving the feed conversion rate, improving ketone body quality and meat quality, comprehensively enhancing animal immunization function and capacity of preventing disease; the toxic and side effect of chemically synthesized organic acid and food potential risk are overcome, the disadvantages that the Chinese herbal medicine additives are complex in component, the use is inconvenient, and the quality standard is hard to control are avoided, the research and development prospect is positive, and the clinical application is wide.
Owner:JIANGSU ACAD OF AGRI SCI
Application of pawpaw total phenolic acid extract in preparation of arthritis prevention and treatment medicaments or food
InactiveCN102631434AGood treatment effectInhibit early inflammatory responseAntipyreticAnalgesicsChlorogenic acidMedicine
The invention relates to the technical field of medicaments and particularly relates to application of a pawpaw total phenolic acid extract in preparation of arthritis prevention and treatment medicaments or food, which is characterized in that the arthritis is gouty arthritis or rheumatoid arthritis. According to the invention, a pawpaw total phenolic acid extract is prepared from pawpaw fruit, and according to component analysis, the pawpaw extract comprises the following main components: protocatechuic acid, chlorogenic acid and other phenolic acid compounds, wherein the protocatechuic acid accounts for 30% to 35%, the chlorogenic acid accounts for 20% to 25%, and the phenolic acid compounds account for 50% to 60% of the pawpaw extract. Therefore, the product is called the pawpaw total phenolic acid extract. Animal experiments prove that the pawpaw total phenolic acid extract disclosed by the invention has obvious activity for preventing and treating the gouty arthritis or the rheumatoid arthritis, thus being used for preparing the medicament or food for preventing and treating the gouty arthritis or the rheumatoid arthritis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Technology of preparing 3,4-dihydroxybenzoic acid by using longan shells
InactiveCN107266308AReduce pollutionReduce wasteCarboxylic compound separation/purificationAcid preparationsMacroporous resin
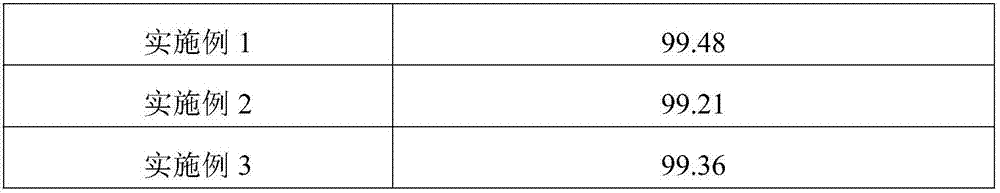
The invention discloses a technology of preparing 3,4-dihydroxybenzoic acid by using longan shells, and belongs to the technical field of 3,4-dihydroxybenzoic acid preparation. The 3,4-dihydroxybenzoic acid is prepared by the following steps: crushing longan shells, performing ultrasonic extraction, performing first decoloration, performing coarse crystallization, performing second decoloration, performing separation and purification by a macroporous resin, performing concentration and crystallization, and performing drying. The 3,4-dihydroxybenzoic acid prepared by the technology provided by the invention has purity of 99.21% or above, and can be used as a qualitative or quantitative reference substance. The technology provided by the invention can effectively extract the 3,4-dihydroxybenzoic acid in the longan shells, and is beneficial for reducing environmental pollution and resource waste caused by longan shells.
Owner:广西高企科技有限公司
Solid preparation of 'Fuxiekang' and preparation method
InactiveCN1626179AGood dispersionShort disintegration timeUnknown materialsPill deliveryBlumeaSpray drying
A Chinese medicine 'Fuxuekang' in the form of tablet or capsule is prepared from balsamiferous blumea and protocatechuic acid through extracting of balsamiferous blumea, drying, and proportionally mixing it with protocatechuic acid.
Owner:BEIJING BOERDA BIO TECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com