Preparation method of phenolic acid-modified chitosan coating liquid for fresh keeping of edible fungus
A chitosan and edible fungus technology, applied in the field of food science, can solve the problems of low antioxidant activity, unfavorable large-scale production and application, etc., achieve high scavenging activity, improve water solubility and antioxidant activity, and have good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthetic process of chitosan-protocatechuic acid graft copolymer:
[0027] (1) Weigh 0.5g chitosan and dissolve it in 25mL acetic acid solution, stir magnetically until it is completely dissolved, and simultaneously weigh 1.16g protocatechuic acid and dissolve it in 3mL absolute ethanol;
[0028] (2) Weigh 4.3g EDC and dissolve it in 20mL MES buffer, shake to dissolve EDC completely;
[0029] (3) Add the completely dissolved protocatechuic acid solution into the reactor, immediately add 0.86g NHS and adjust the pH value of the solution to 5.5, then place it in an ice-water bath, and react with magnetic stirring for 1 hour;
[0030] (4) Add the fully dissolved chitosan solution into the reactor, and react for 12 hours at 20° C.;
[0031] (5) Put the reaction solution into a dialysis bag, first dialyze with tap water for 48 hours, and then dialyze with deionized water for 24 hours;
[0032] (6) Chitosan-protocatechuic acid graft copolymer is obtained after the dial...
Embodiment 2
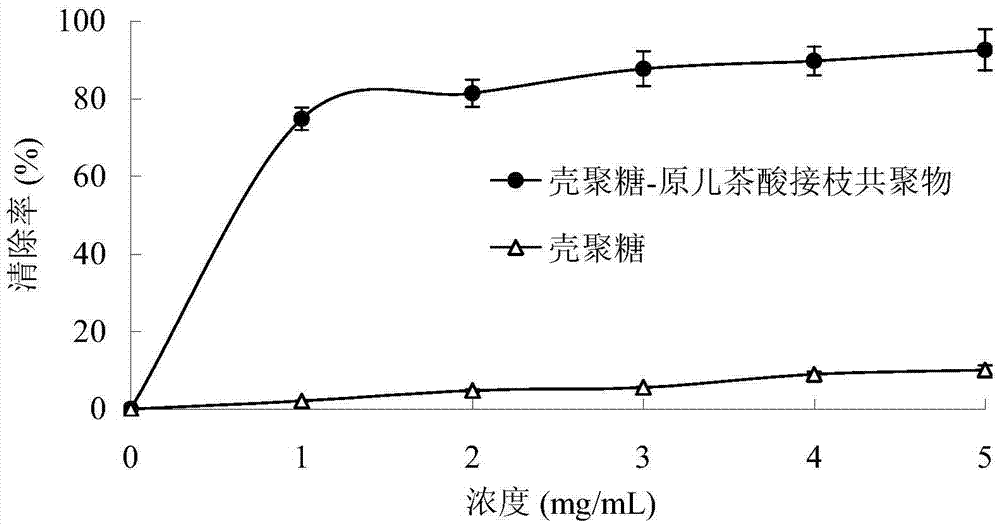
[0034] Structure and In Vitro Antioxidant Activity Determination of Chitosan-Protocatechuic Acid Graft Copolymer:
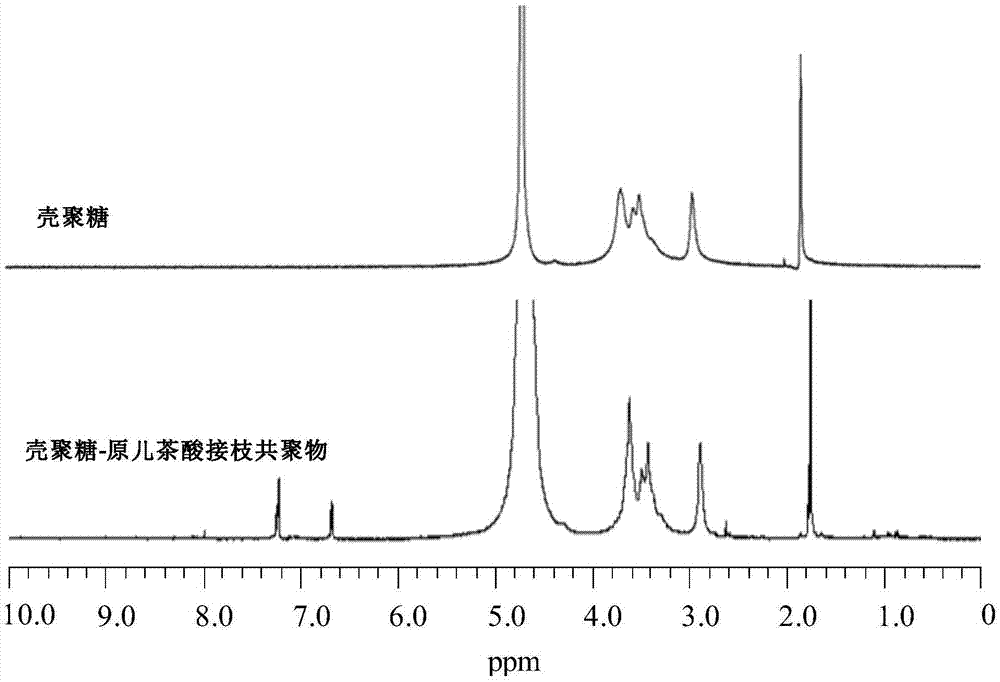
[0035] (1) Accurately weigh 5 mg of chitosan and chitosan-protocatechuic acid graft copolymer samples, dissolve them in 0.5 mL of heavy water, and measure their H NMR spectra at 25°C.
[0036] (2) chitosan and chitosan-protocatechuic acid graft copolymer 1 H NMR spectrum as figure 1 Shown, the peak of chitosan at δ=1.7ppm place corresponds to the methyl proton of acetylglucosamine residue, the peak at δ=3.3ppm place corresponds to the proton on the pyranose ring C-3; The peak at 3.4ppm corresponds to the proton on the pyranose ring C-4; the peak at δ=3.7ppm corresponds to the proton on the pyranose ring C-5; the peak at δ=4.3ppm corresponds to the pyranose ring Proton on C-6 of the furanose ring; the weak peak at δ=2.8 ppm corresponds to the proton on C-2; the peak at δ=4.7 ppm corresponds to the proton on C-1. However, in the chitosan-protocatechuic acid graf...
Embodiment 3
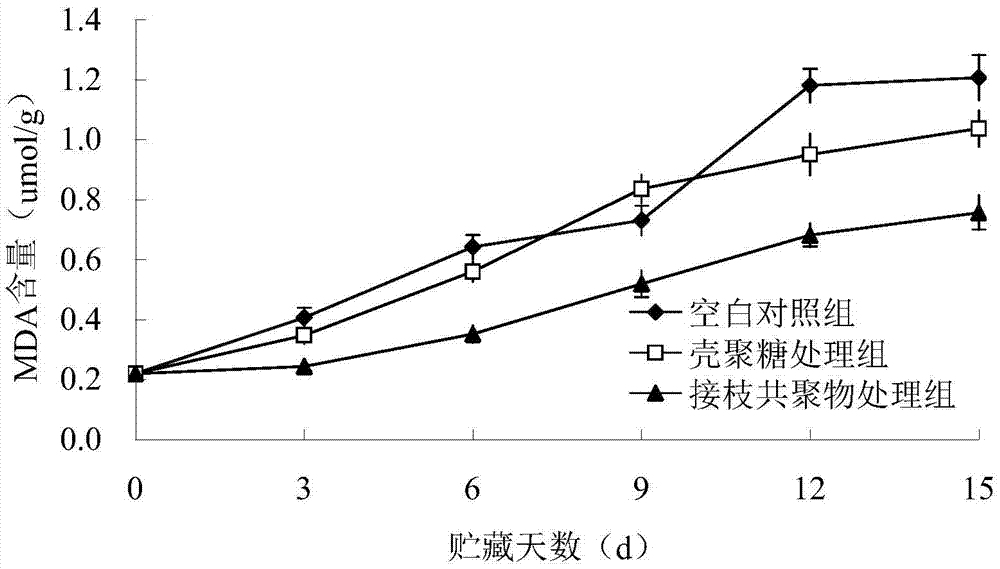
[0041] Fresh-keeping effect of chitosan-protocatechuic acid graft copolymer on edible fungi (Pleurotus eryngii).
[0042] (1) On the day of harvest, the edible fungi should be placed in a cold chain transport device at 5-8°C, with culture medium on the roots, and processed immediately after being transported to the laboratory. Discard the root culture medium, and select edible fungi with complete mushroom bodies, white color, no pests and diseases, no mechanical damage, and fruit bodies with basically the same size.
[0043] (2) Randomly divide 45 edible fungi into 3 parts, that is, 3 kinds of treatments: treat with 10g / L chitosan-protocatechuic acid graft copolymer film respectively; Membrane treatment; and the blank group without treatment. Use a polyethylene film bag seal bag with a size of 27cm×28cm to store at a humidity of 95%, and store at 4°C. Each treatment is divided into 5 groups (bags), and samples are taken every 3 days, and 3 edible fungi are randomly sampled ea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grafting rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com