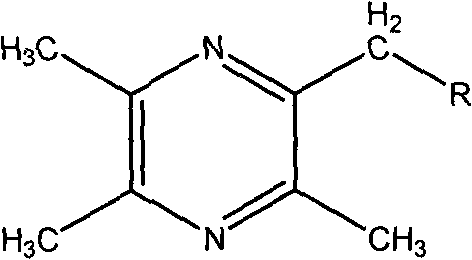
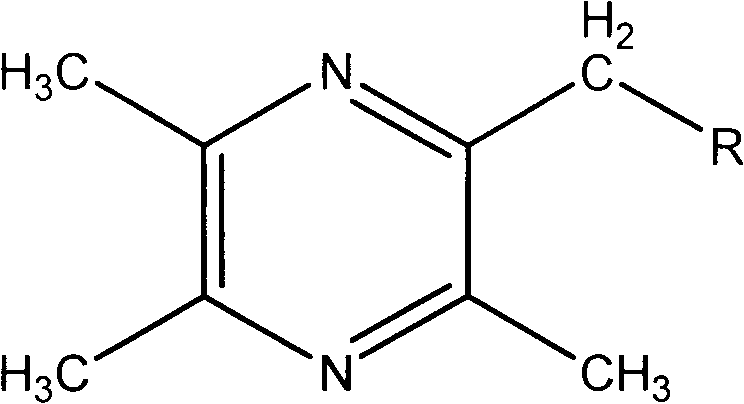
Medicine for treating ischemic brain injury stroke and sequela of ischemic brain injury stroke and preparation method for medicine
A technology of compounds and organic solvents, applied in the field of inventions involving the fields of chemistry and biological sciences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0031] Preparation Example 1 The preparation of 2-bromomethyl-3,5,6-trimethylpyrazine intermediate
[0032] Dissolve dehydrated ligustrazine 10g in 60mlCCl4, then drop into NBS 9.17g according to the molar ratio ligustrazine: NBS=1: 0.7 (can add a small amount of benzoyl peroxide as a free radical initiator), under the irradiation of an incandescent lamp, reflux React for 10-12 hours, cool, concentrate, pump off excess ligustrazine under reduced pressure in a water bath at 60-70°C, and place the residue in a refrigerator. 7.75 g of a light red semi-oil was obtained, with a yield of 70%.
preparation Embodiment 2
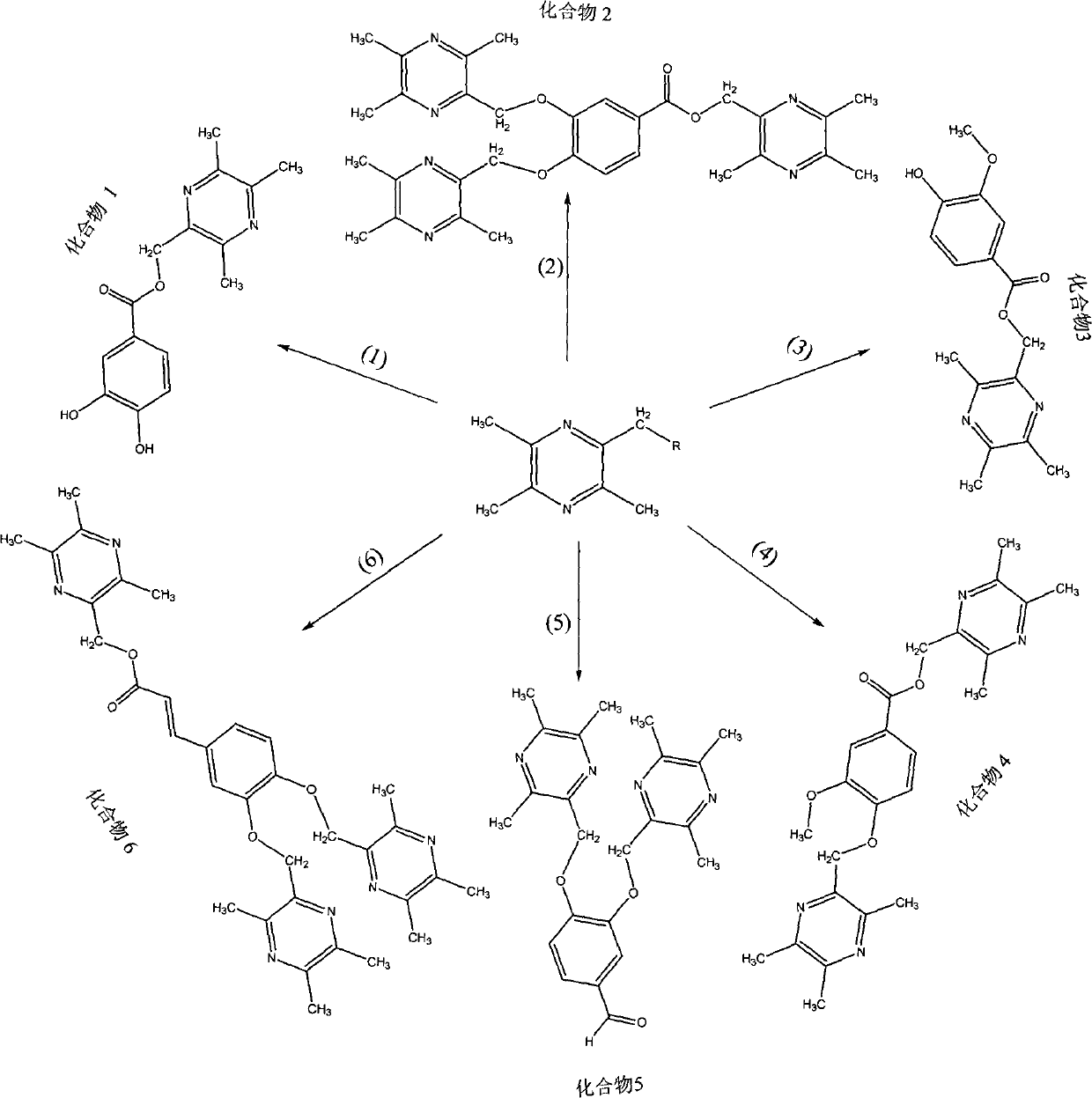
[0033] Preparation Example 2 Synthesis of LQC-T1 (Compound 1)
[0034] Place 3.26mmol of 2-bromomethyl-3,5,6-trimethylpyrazine prepared in Example 1 and 3.40mmol of protocatechuic acid prepared in Example 1 into a 50ml round bottom flask, add 30ml xylene , after the mixture is dissolved, add 3mmol of triethylamine, heat and reflux for 12h, TLC monitors that the reaction raw materials disappear substantially, stop the reaction, add 30ml of ethyl acetate to the reaction solution, concentrate under reduced pressure, add 4ml of methanol to the residue to dissolve, add 2.0g of silica gel to reduce The mixed sample was evaporated to dryness, and the eluent was petroleum ether: acetone = 5:1, and 0.45 g of white powder was obtained, with a yield of 48.0%, and a melting point of 219.2 to 220.1° C.; the hydrogen spectrum and carbon spectrum data of compound 1 were as follows:
[0035] 1 HNMR (500MH z , DMSO-d 6 ): 7.353~6.802(m, 3H, Ar-H), 5.323(s, 2H, O-CH 2 ), 2.503(s, 3H, 6-CH ...
preparation Embodiment 3
[0037] Preparation Example 3 Synthesis of LQC-T2 (Compound 2)
[0038] Place 4.6mmol of 2-bromomethyl-3,5,6-trimethylpyrazine and 1.5mmol of protocatechuic acid prepared in Example 1 into a 25ml round bottom flask, add 14ml of DMF and wait for the mixture to dissolve, then add 10mmol of Potassium carbonate, N 2 Protected at 85°C and stirred for 1.5h, TLC monitored that the reaction raw materials basically disappeared, stopped the reaction, filtered to remove potassium carbonate, diluted the reaction solution with saturated aqueous sodium bicarbonate solution, extracted three times with ethyl acetate, evaporated the combined extracts to dryness, and redissolved the residue in a small amount of acetone. Add 3.0 g of silica gel and evaporate to dryness under reduced pressure to mix the sample. The eluent is petroleum ether: acetone = 6:1 to obtain 0.36 g of white powder with a yield of 43.1% and a melting point of 202.2-203.0°C.
[0039] The hydrogen spectrum and carbon spectrum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com