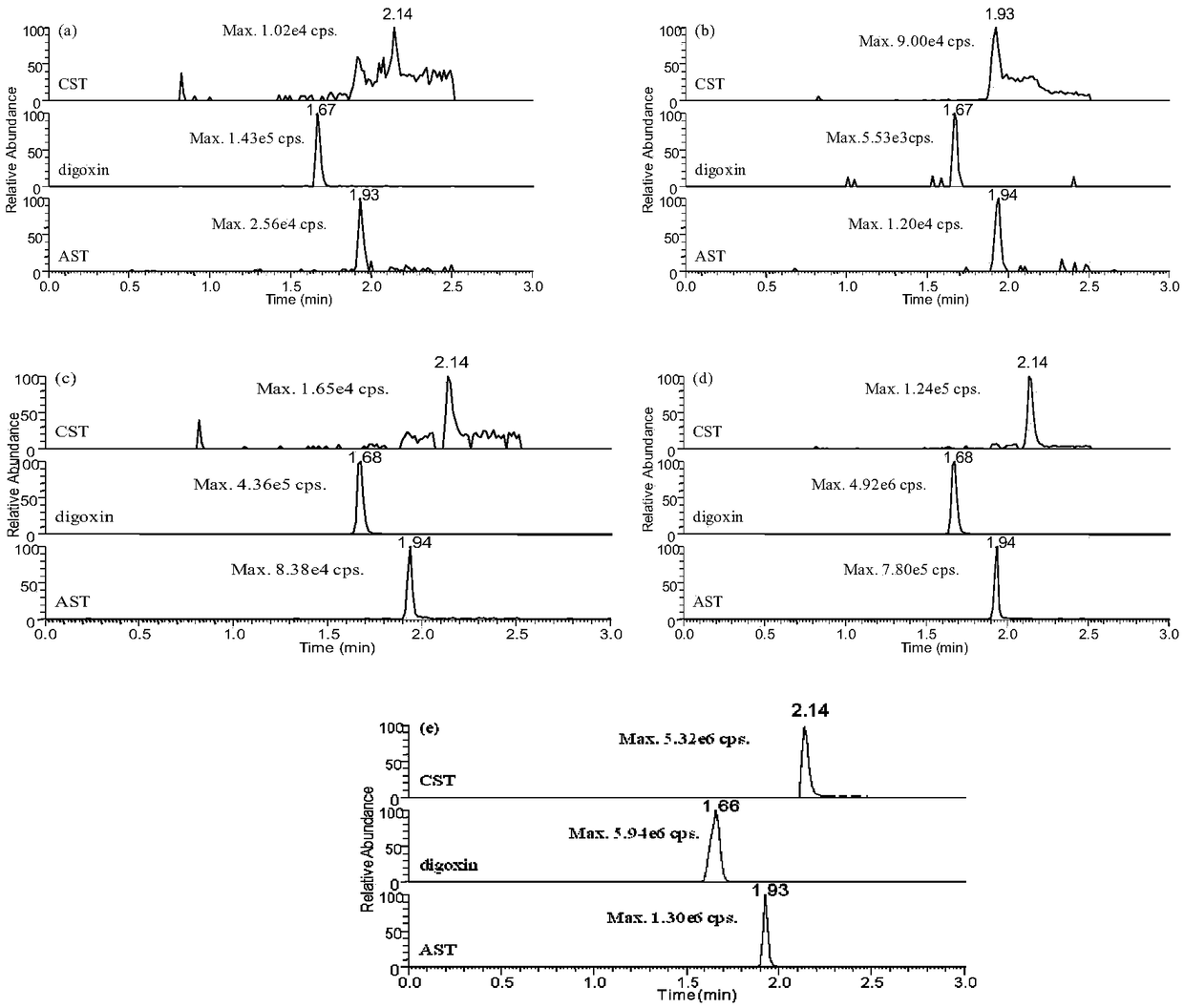
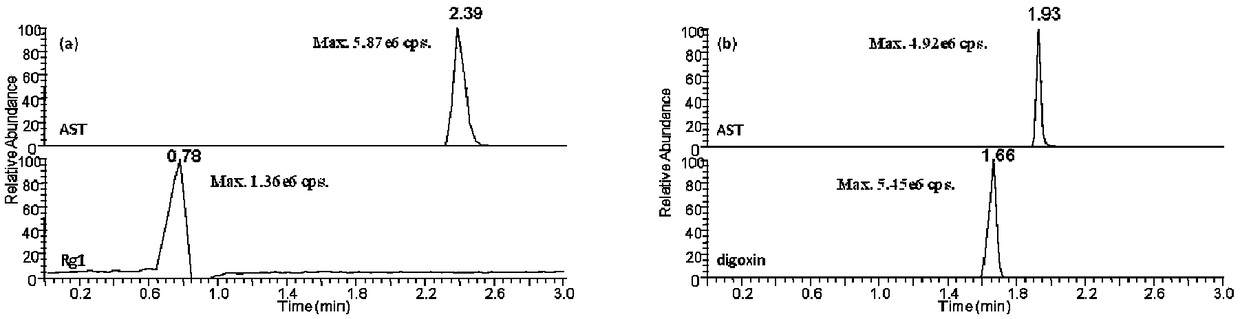
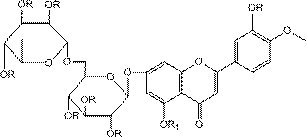
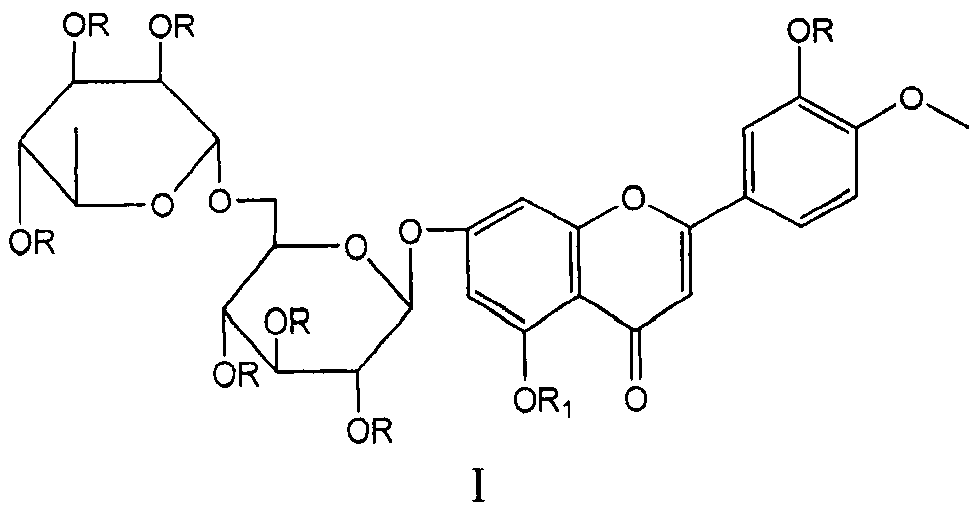
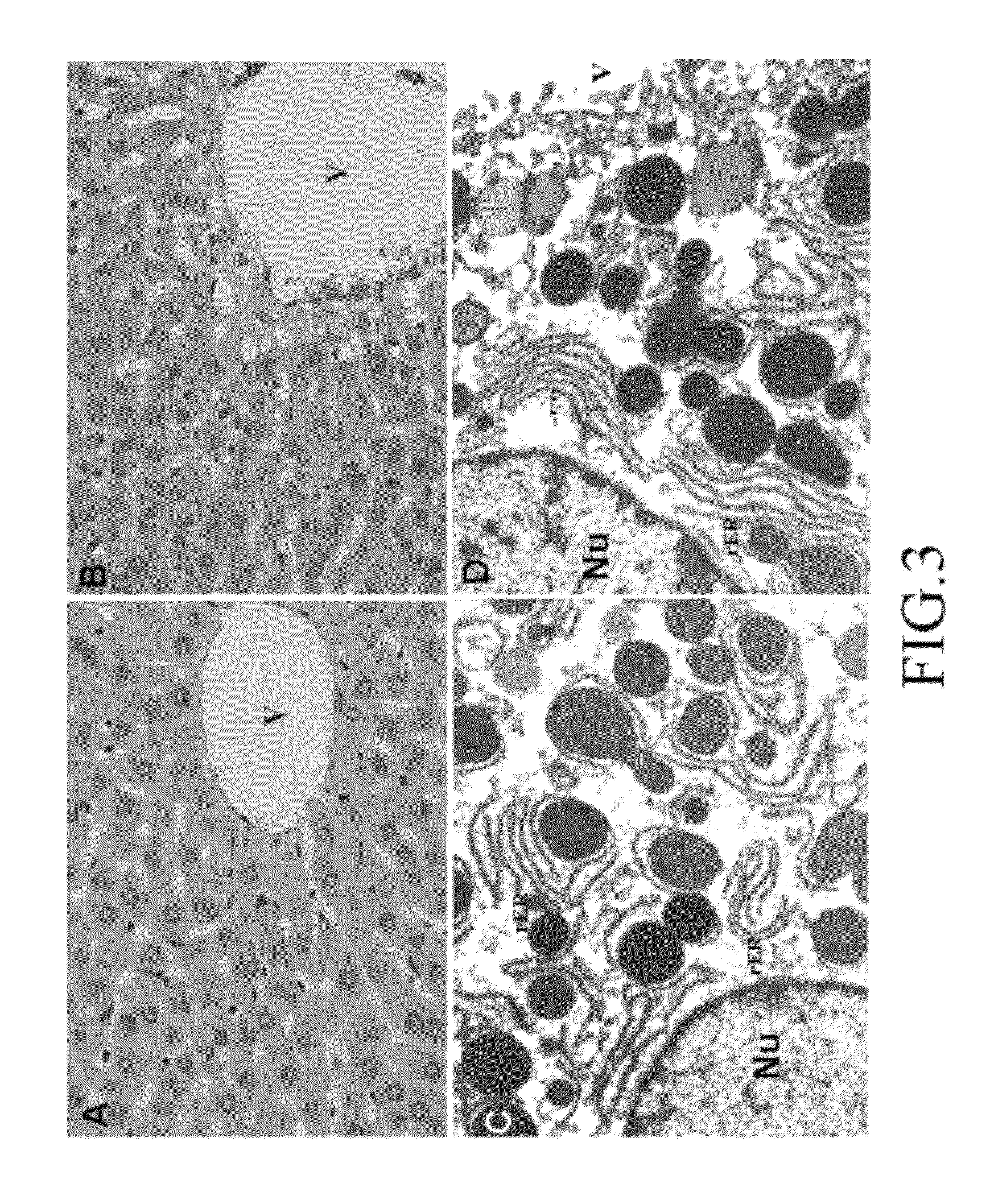
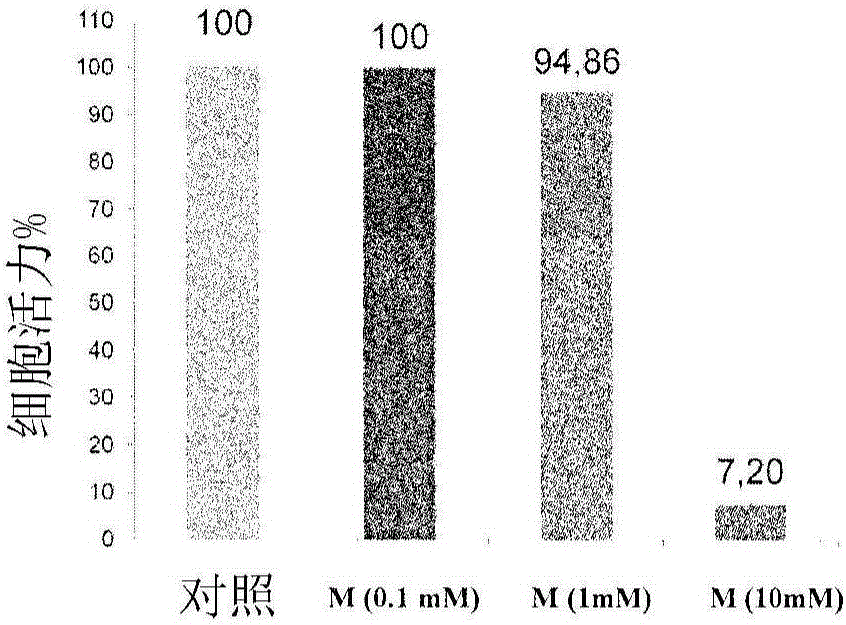
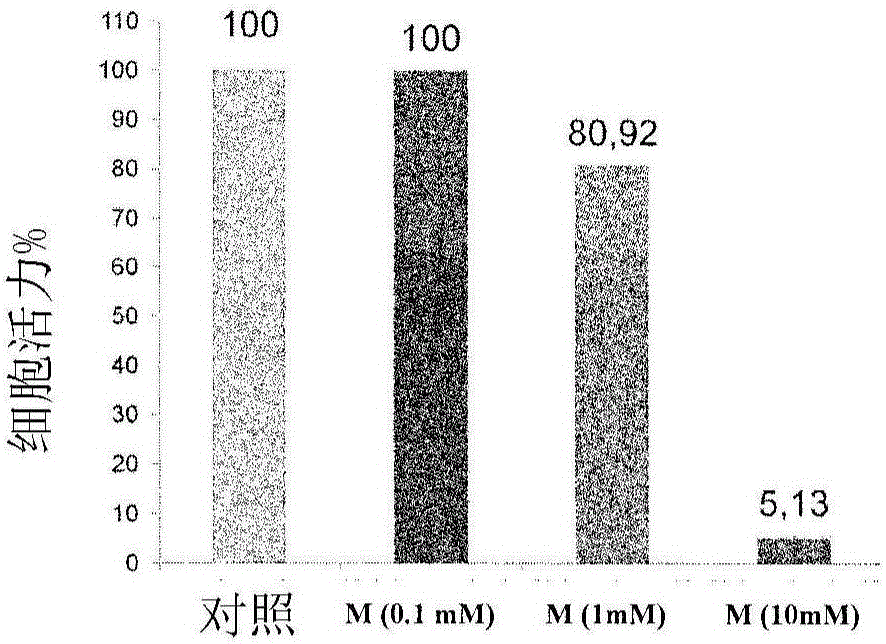
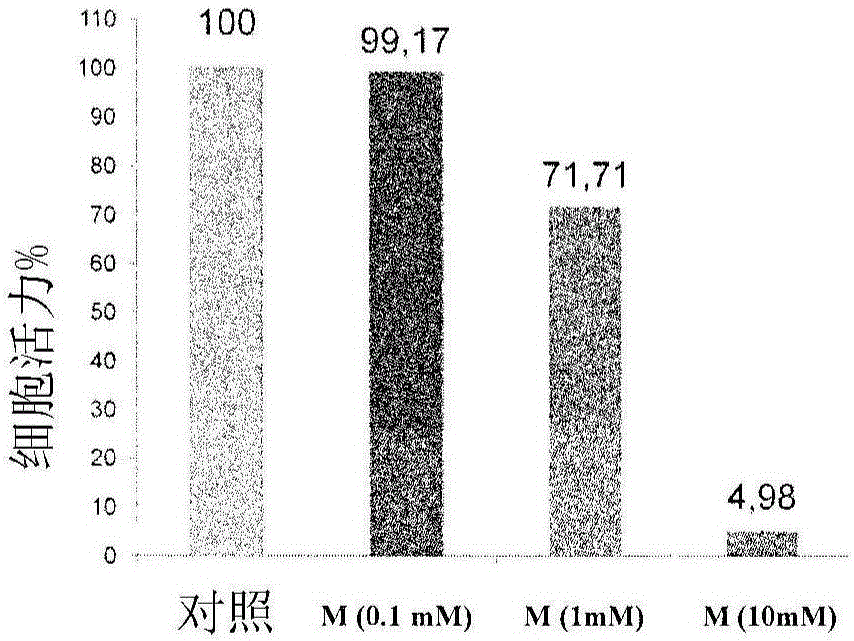
Patents
Literature
70 results about "Diosmin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
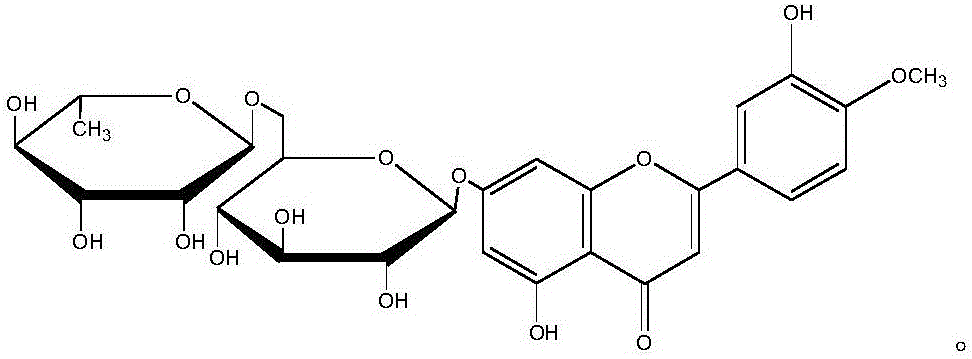
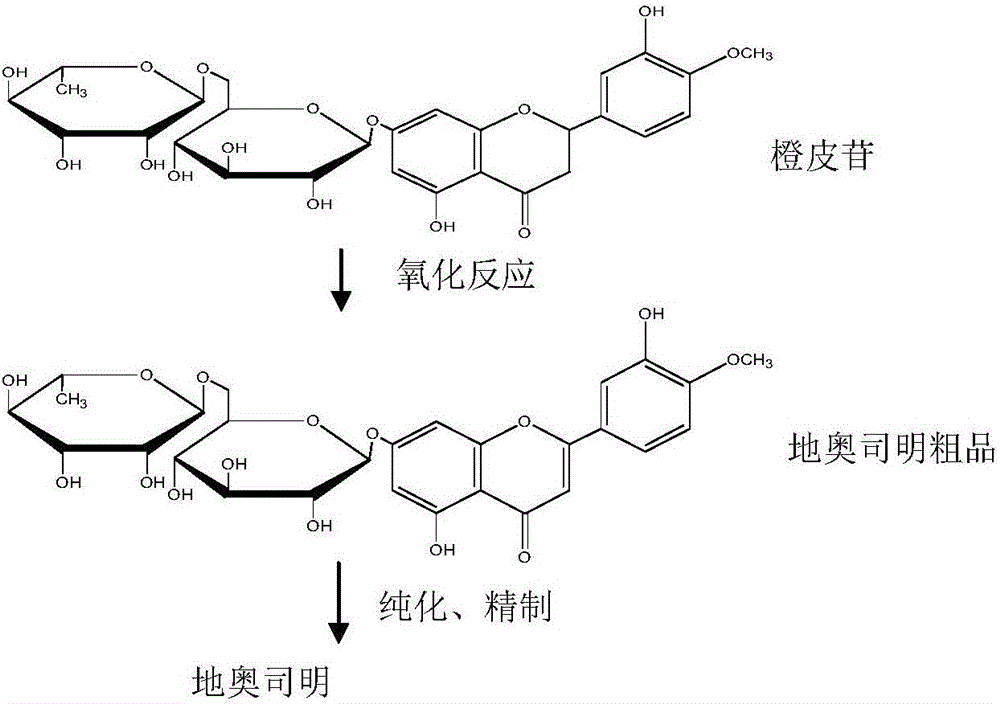
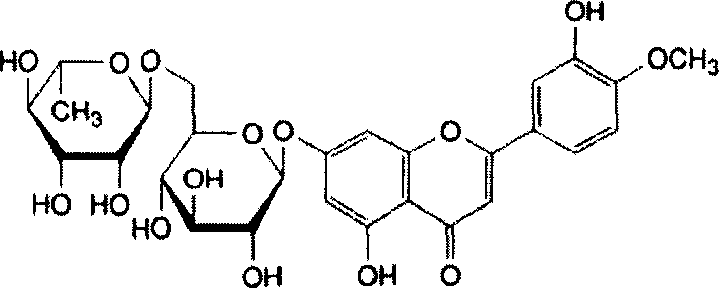
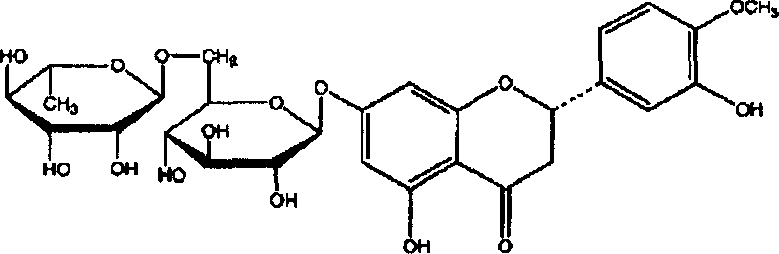
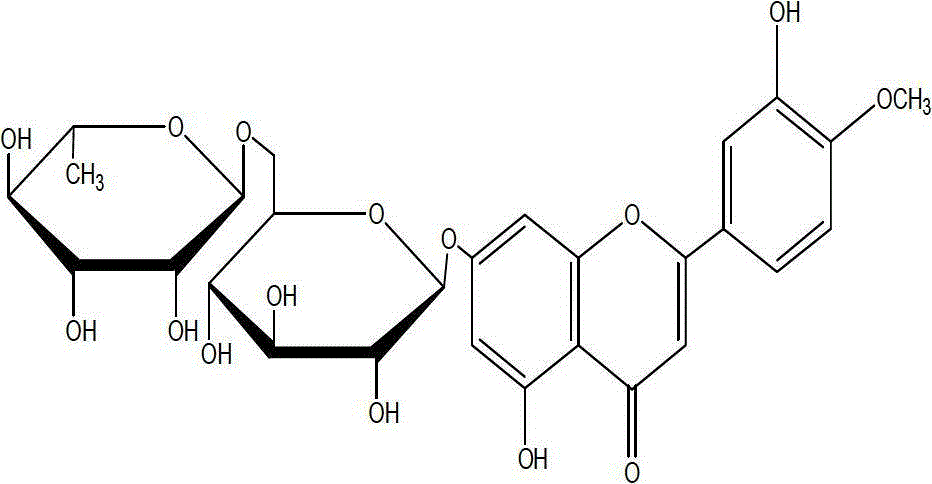
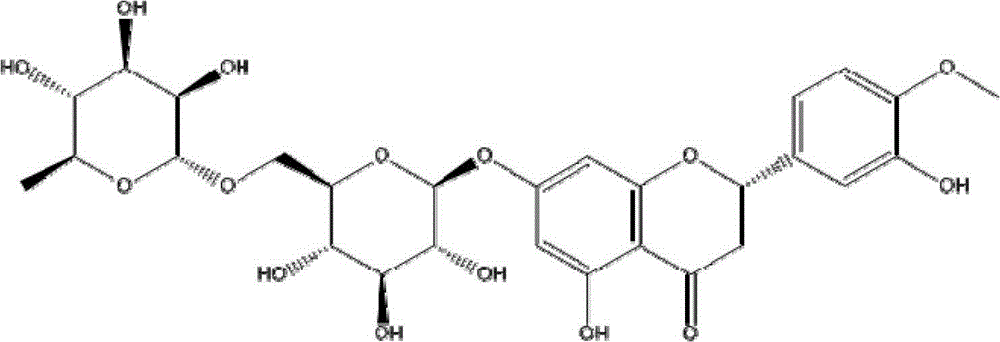
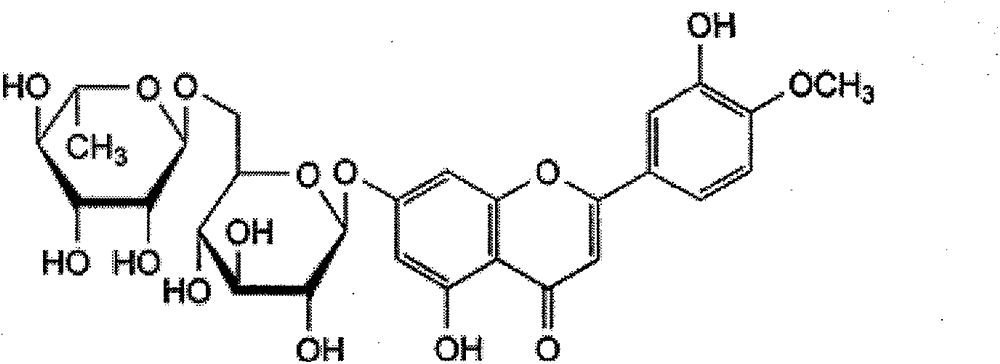
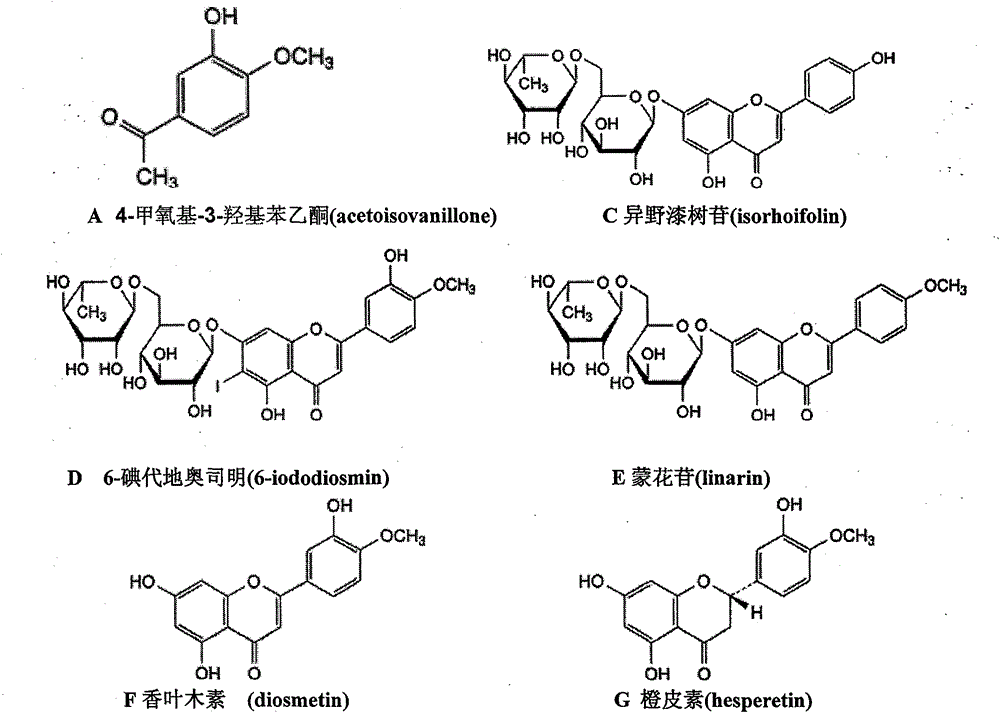
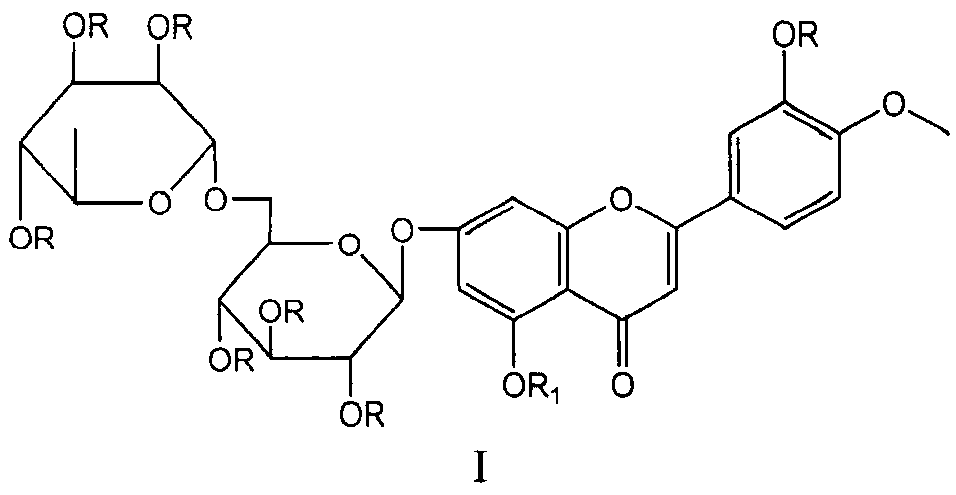
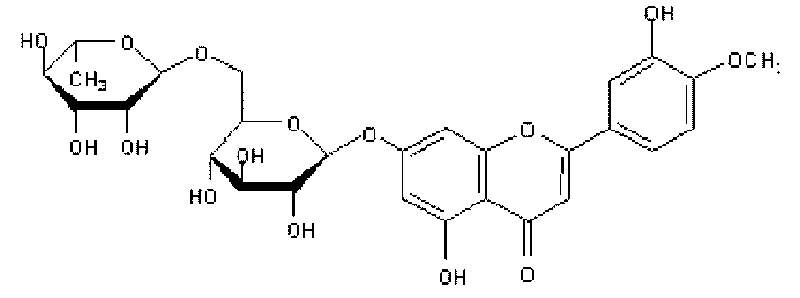
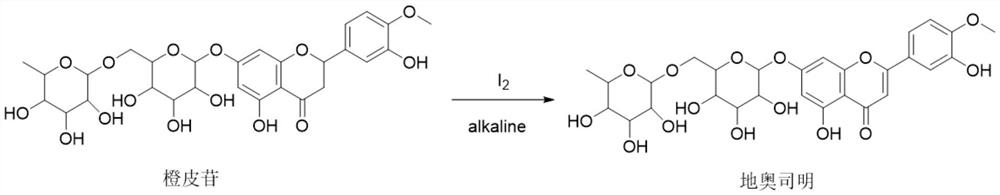
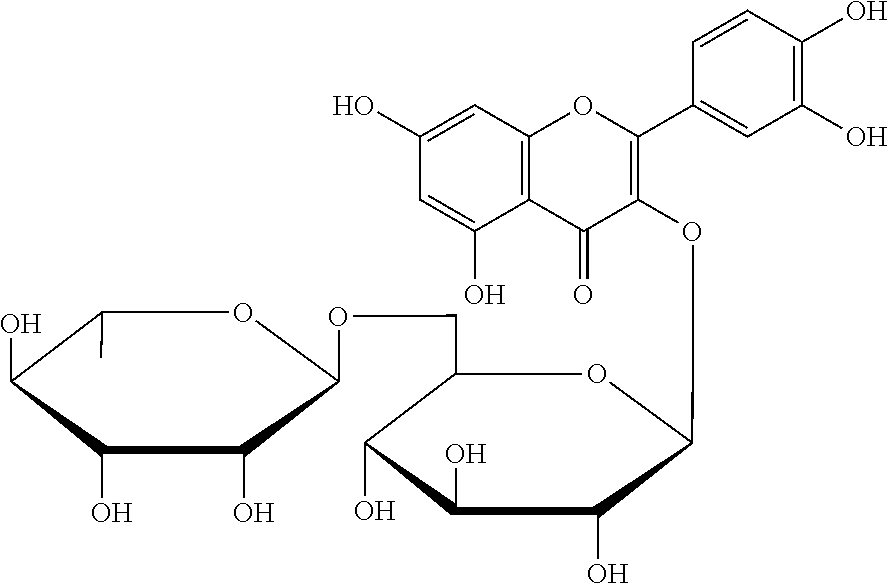
Diosmin (diosmetin 7-O-rutinoside), a flavone derivative also known as venosmine, is a glycoside of diosmetin, which in turn is the 4'-methoxy derivative of luteolin. Diosmin is naturally occurring, mainly in the citrus family rutaceae, but also in herbs such as Teucrium gnaphalodes.
Nutraceutical composition and method of use for treatment / prevention of cancer
ActiveUS20100209388A1Easy to understandReduce weightOrganic active ingredientsBiocideMyrrh1,4-Benzoquinone
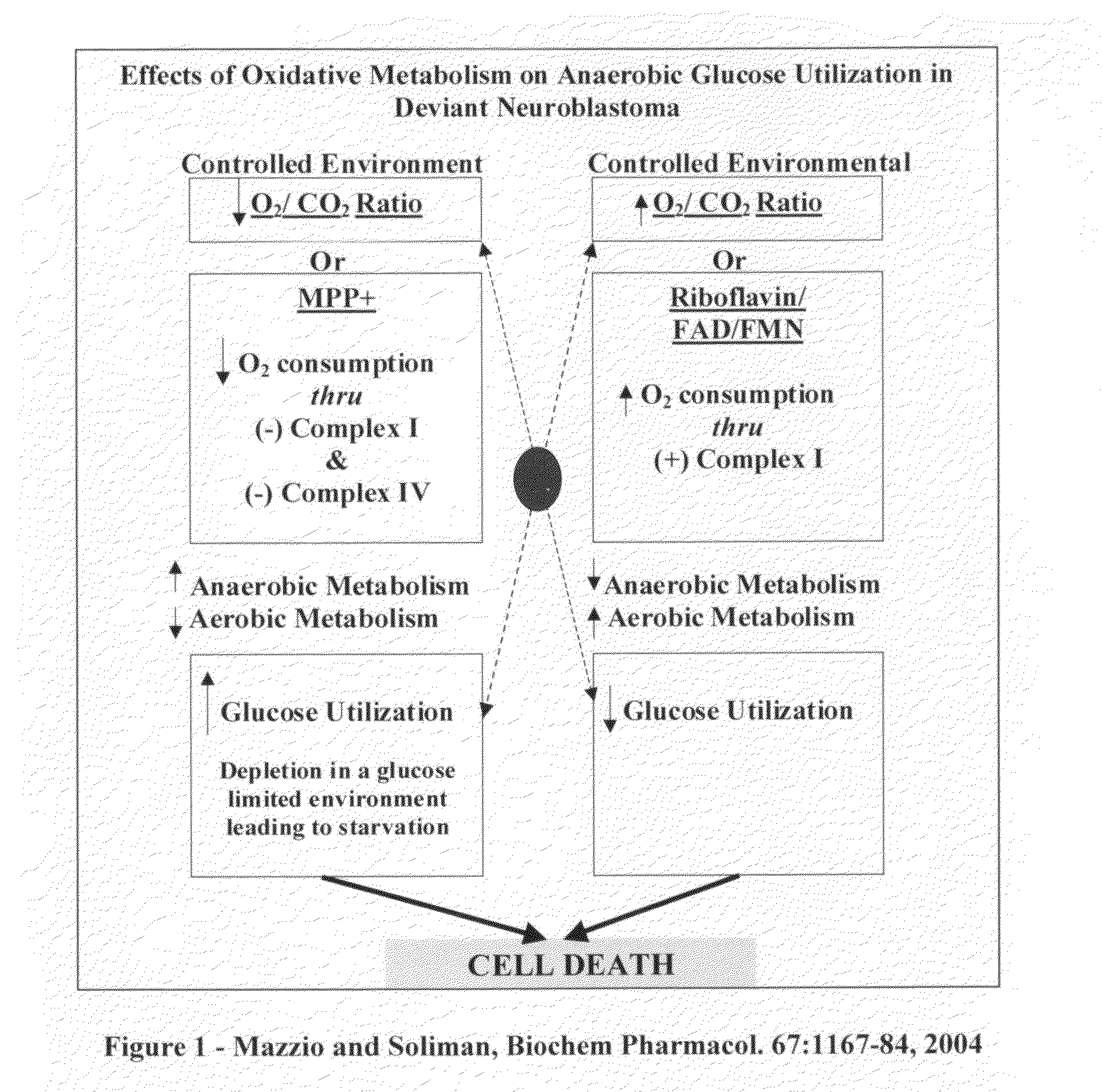
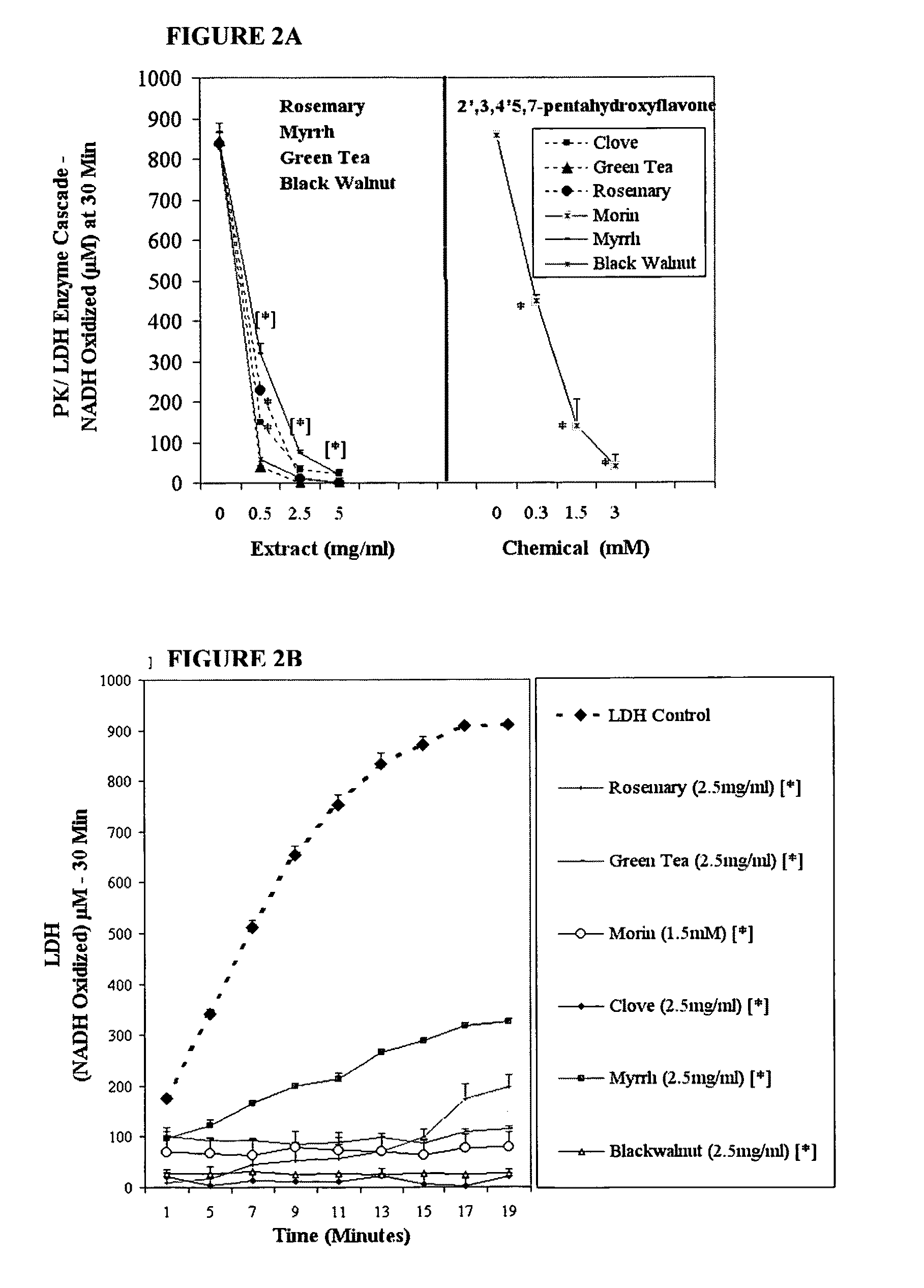
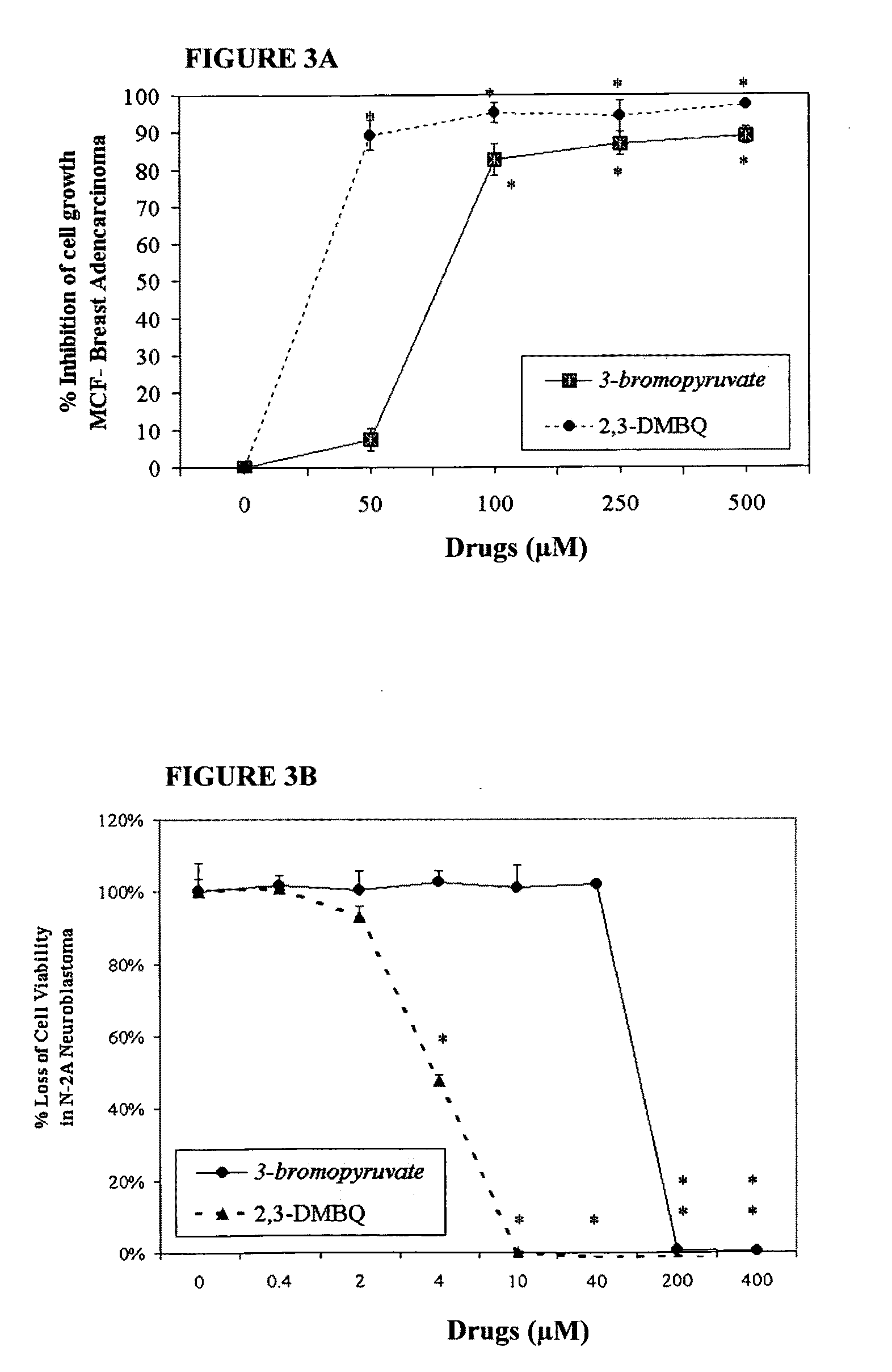
The invention describes a nutraceutical composition and method for preventing / treating cancer comprised of A) quinones (2,3-dimethoxy-5-methyl-1,4-benzoquinone, thymoquinone, tocopherolquinone) B) compounds capable of maximizing oxidative mitochondrial function preferably riboflavin, FAD, FMN, 6,7-Dimethyl-8-(1-D-ribityl)lumazine, ribitol, 5,6-dimethylbenzimidazole, tetrahydrobiopterin, vitamin B1, lipoic acid, biotin, vitamin B6, vitamin B12, folate, B3, C and pantothenate C) lactic acid dehydrogenase inhibitors; 2′,3,4′5,7-pentahydroxyflavone, epigallocatechin gallate, quercetin, citric acid, rosemary, black walnut, clove, nutmeg, licorice root, coriander, cinnamon, ginger root, myrrh gum and green tea D) alkalizing agents: aloe vera, chlorella, wheat grass, apple cider vinegar, burdock root, kudzu root, alfalfa, barley grass, spirulina, parsley leaf, calcium, magnesium, potassium or bicarbonate salts E) potent tumoricidal herbs; gromwell root, wild yam, beth root, teasel root, balm of gilead bud, frankincense, bakuchi seed, dichroa root, kochia seed, kanta kari, sweet myrrh, galbanum, garcinia fruit, mace, white sage and tumoricial plant derived constituents: gambogic acid, shikonin, diosmin or boswellic acid F) an antiproliferative herb (speranskia or goldenseal) and G) a pharmaceutically acceptable carrier.
Owner:FLORIDA A&M UNIVERSITY
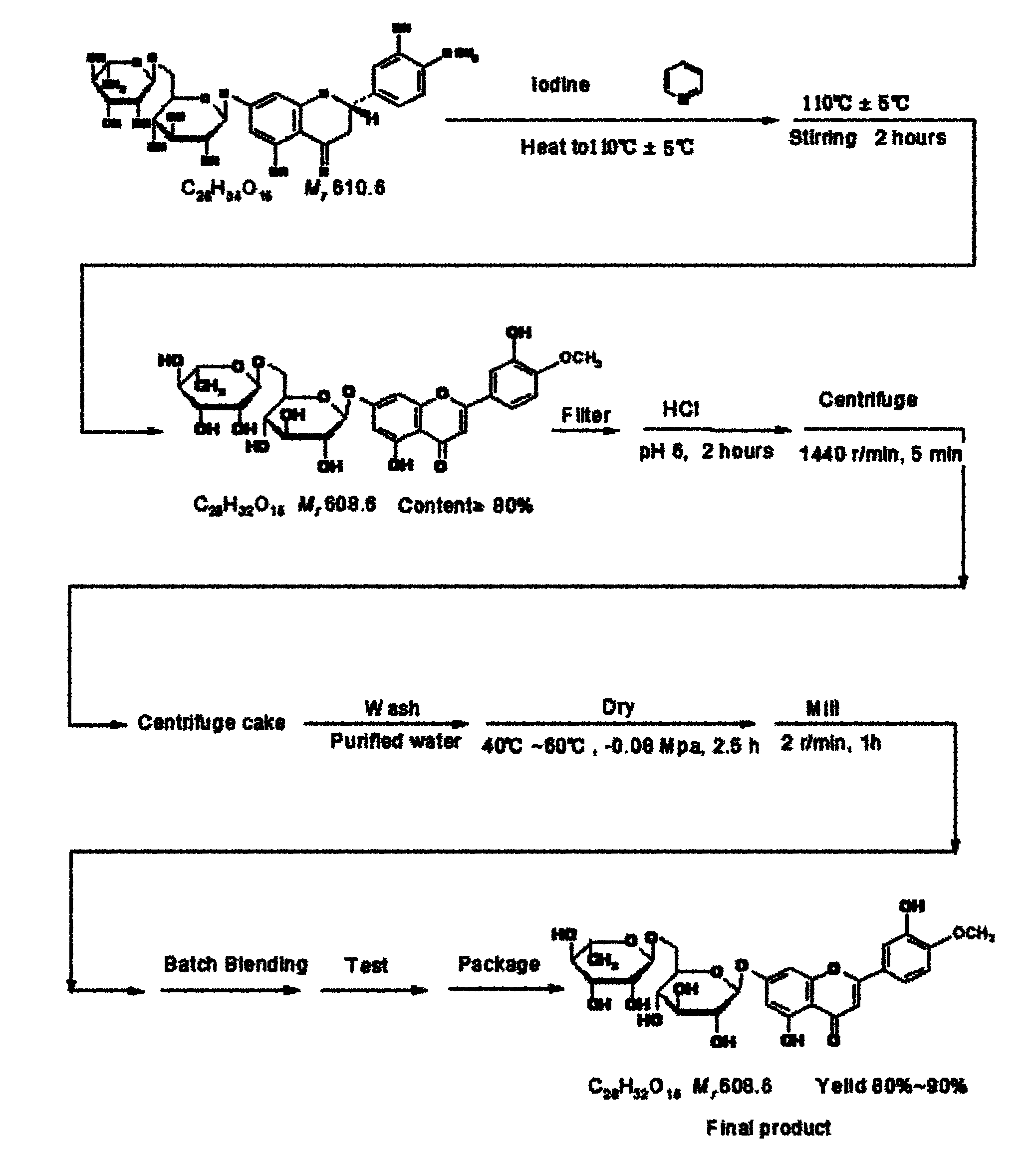
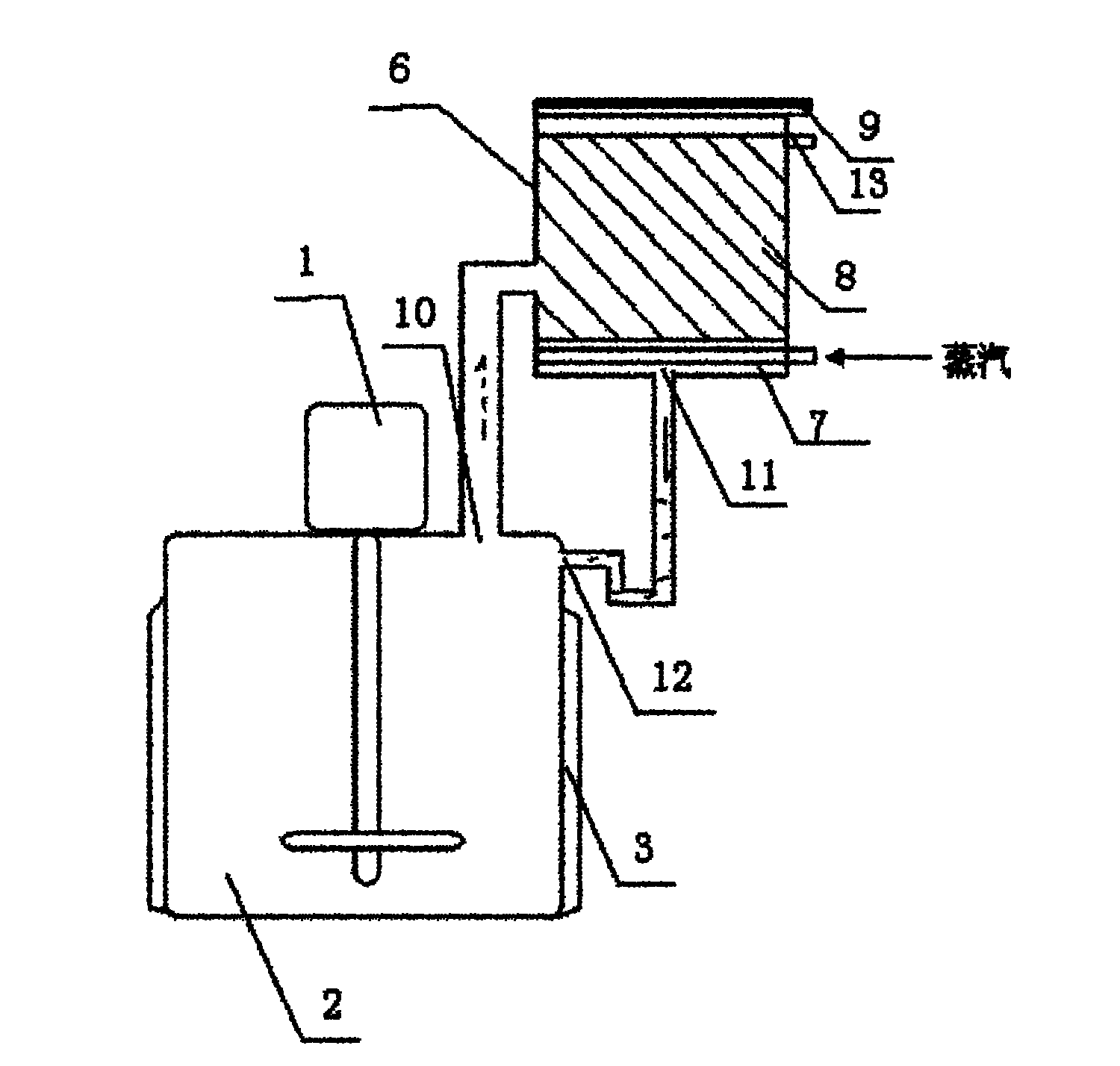
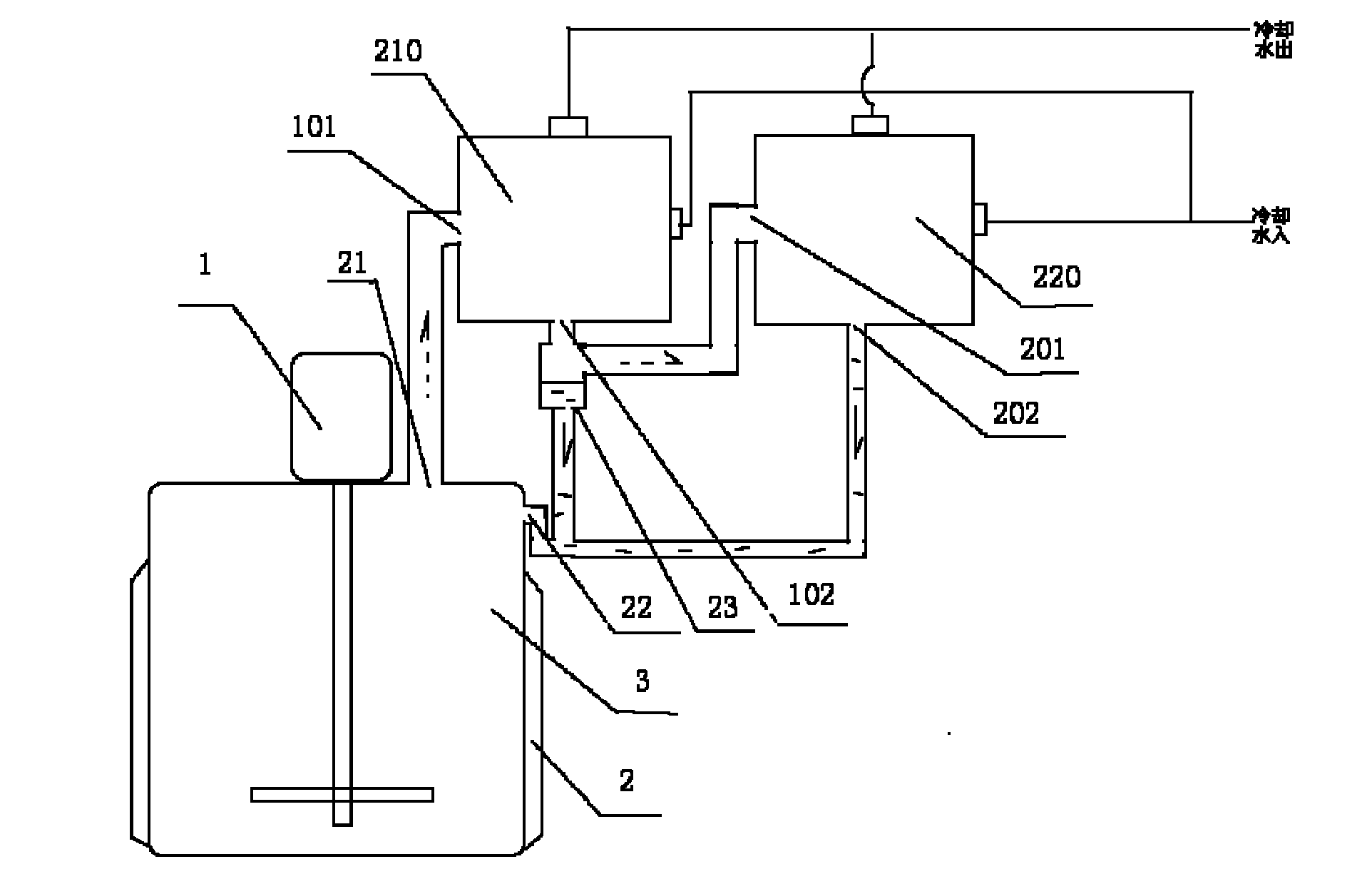
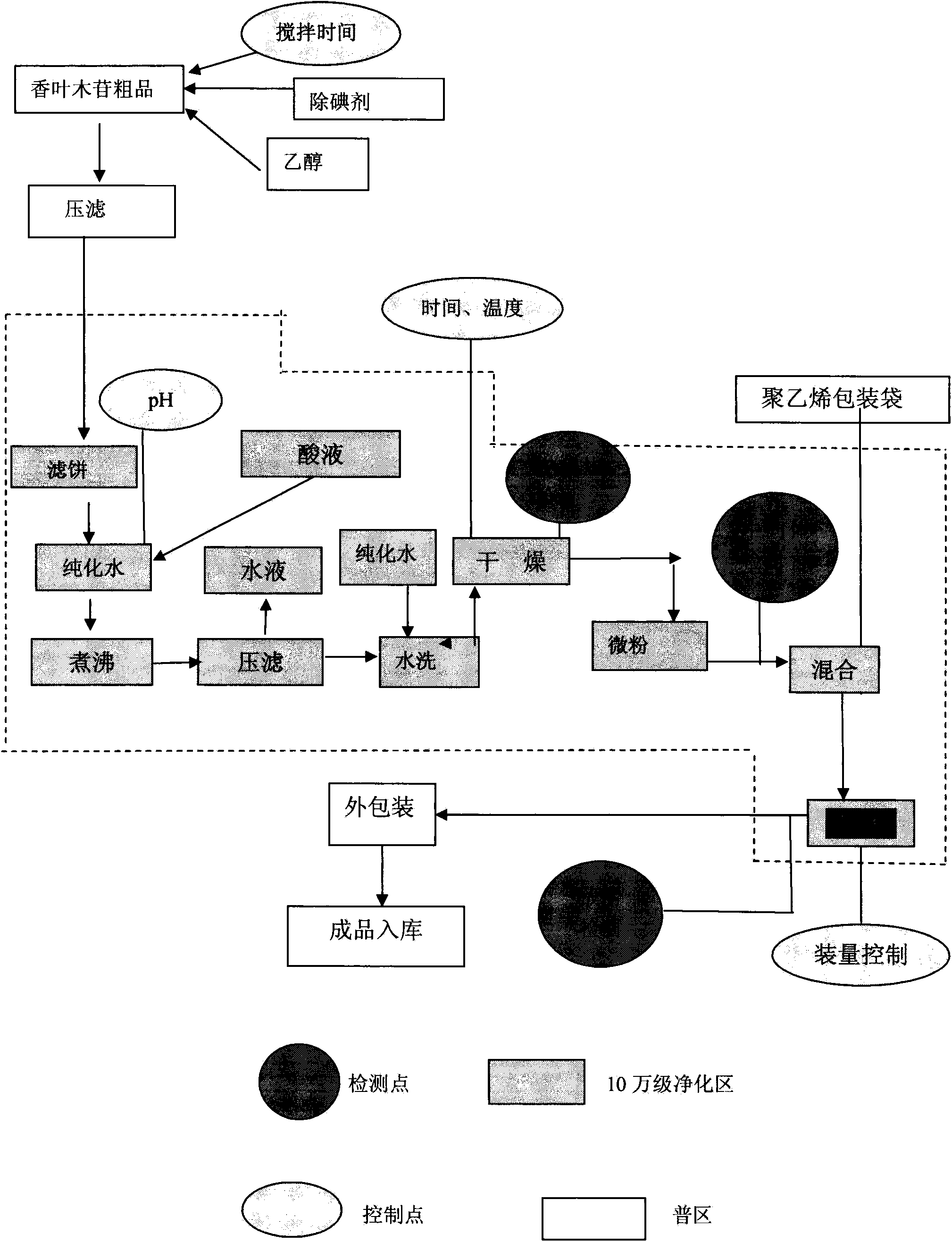
Diosmin producing process
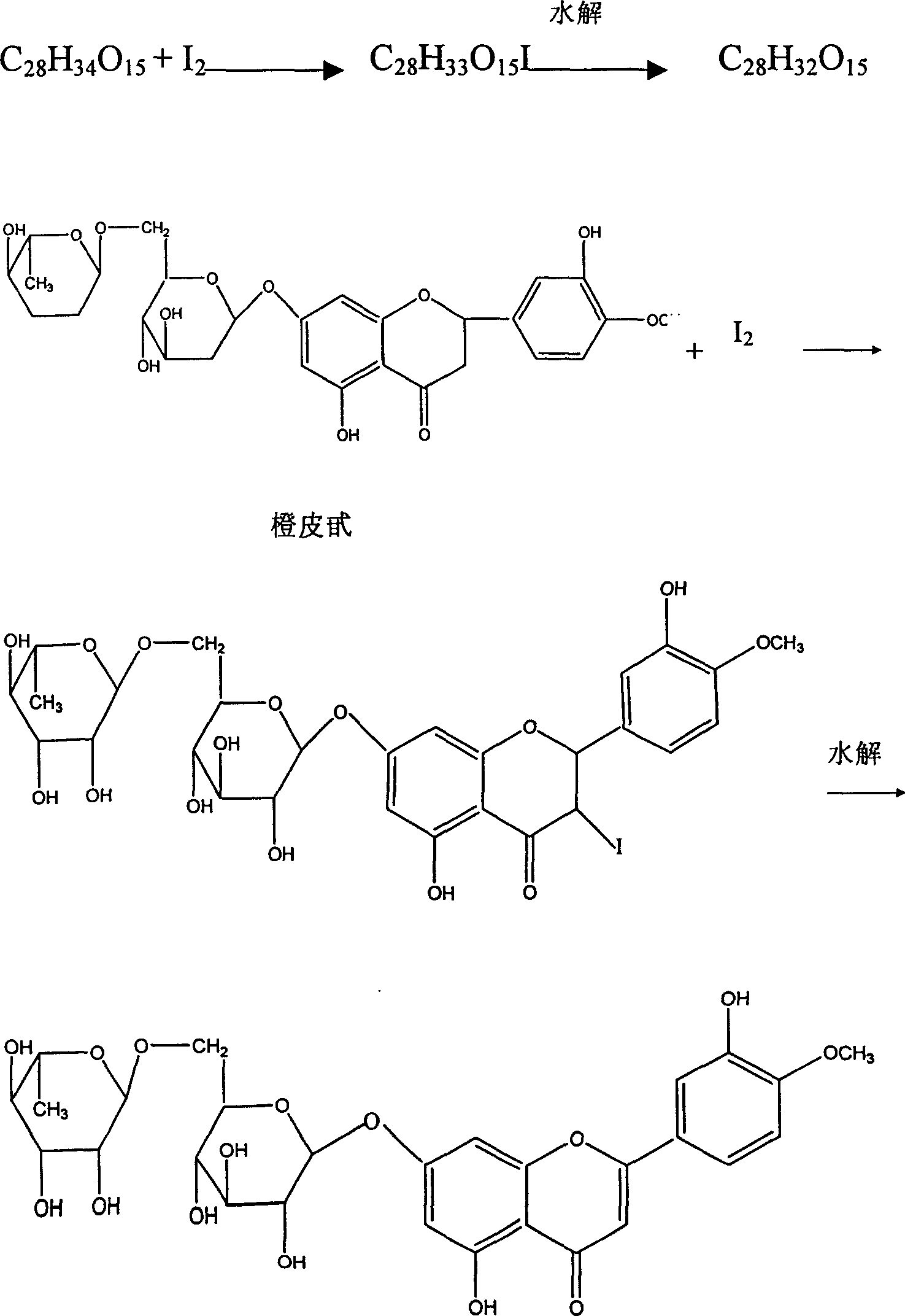
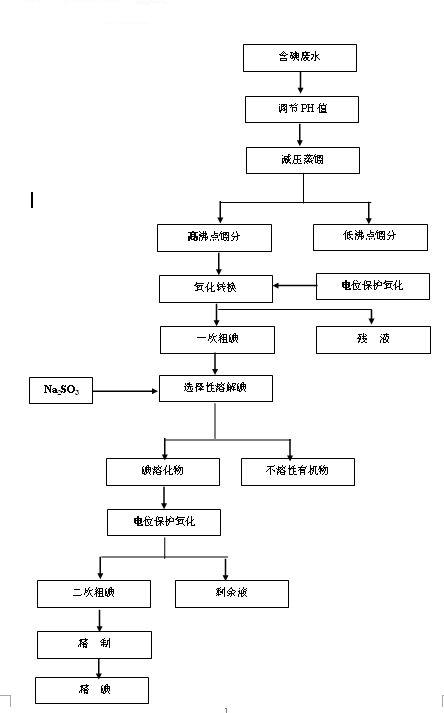
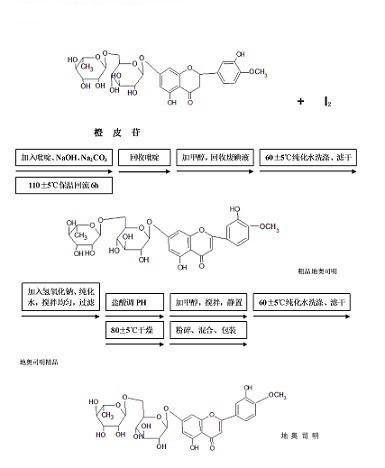
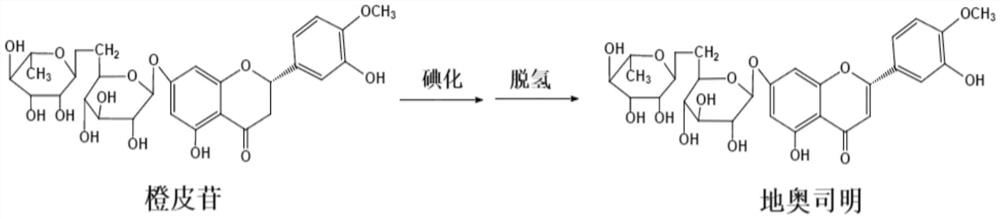
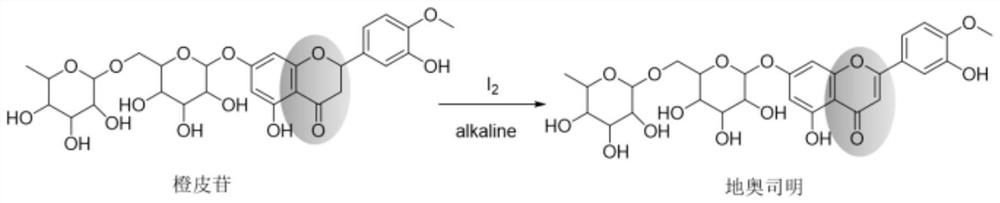
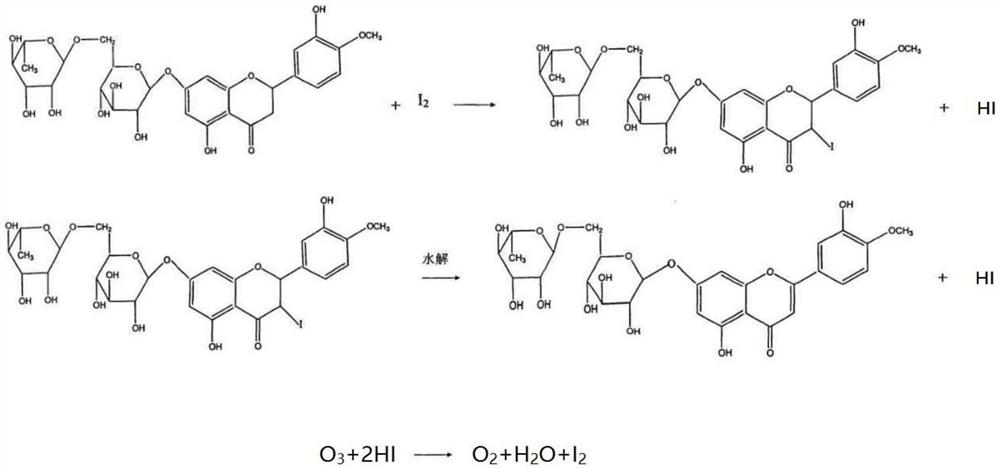
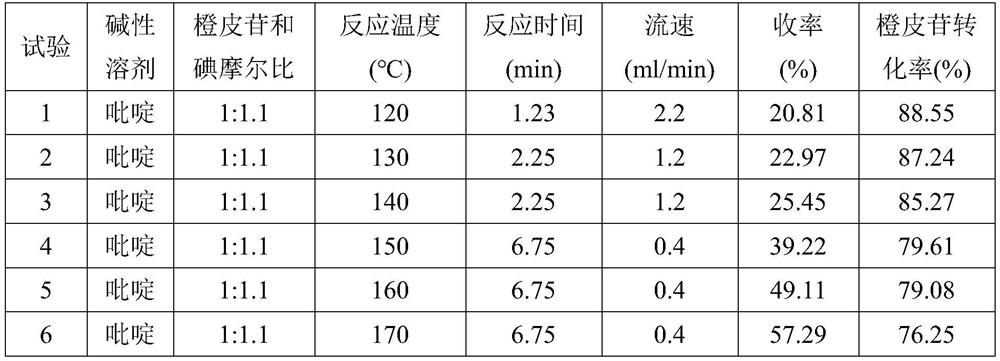
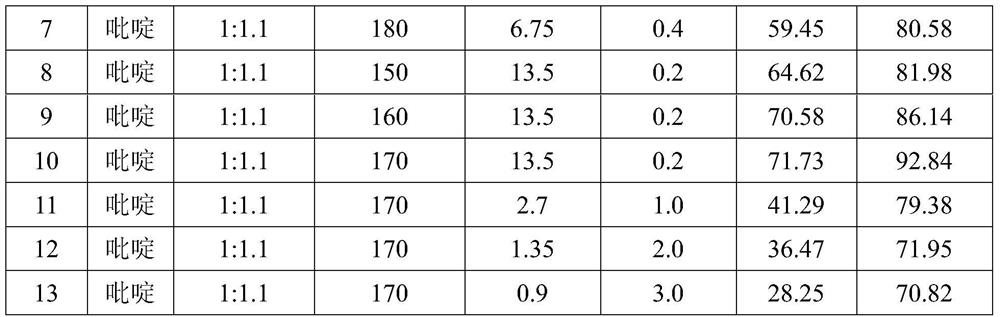
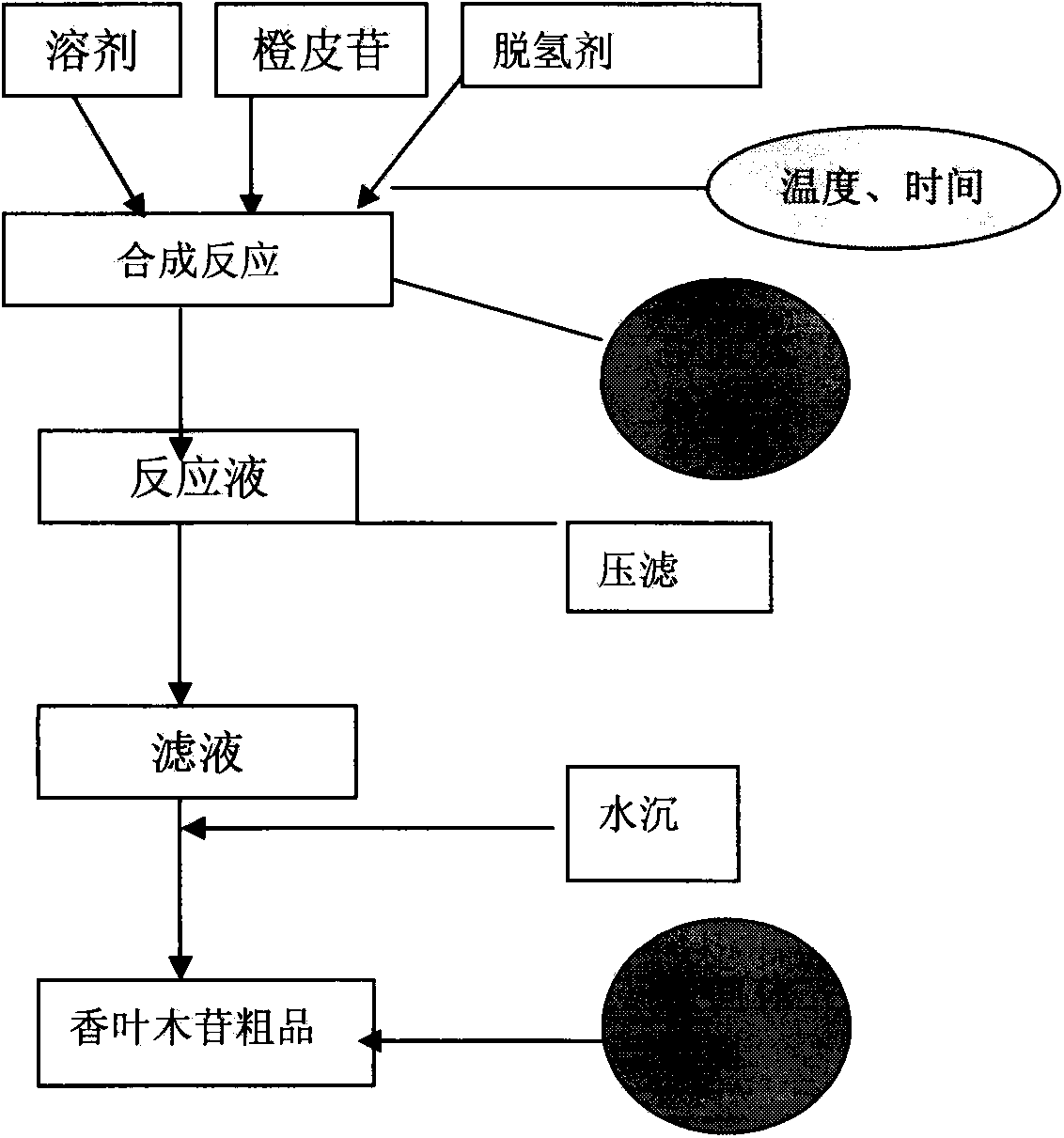
The Diosmin producing process includes the following steps: adding high boiling point solvent into hesperidin, vacuum self distillation to eliminate crystal water from hesperidin, adding reaction solvent in the amount of 3-20 times weight of hesperidin and iodine in 0.3-0.6 time to react at 80-120 deg.c for 8-20 hr, vacuum concentrating, adding water solution of alkali and hydrolyzing at 30-80 deg.c for 1-8 hr, regulating pH value with dilute acid solution to acidity, filtering to obtain solid, water washing to neutrality, and drying to obtain Diosmin product. The process has low cost, high product purity and environment friendship.
Owner:SICHUAN XIELI PHARM CO LTD
Method for producing diosmin
InactiveCN102070689AImprove conversion rateHigh yieldSugar derivativesSugar derivatives preparationDiosminMaterial consumption
The invention discloses a method for producing diosmin. The method for producing the diosmin comprises the following production procedures of: performing a main reaction; cleaning; purifying; drying; grinding and mixing; and recovering iodine. According to the technical scheme, the conversion rate of an organic raw material hesperidin is over 95 percent; yield and content are high; the iodine, pyridine, methanol and ethanol can be recovered and reused on line and the recovery rate is 90 percent; material consumption and production cost are low; and the method has the advantages of simple technological process and stable process.
Owner:HUNAN YUANTONG PHARMA
Method for preparing green and economic diosmin
ActiveCN105732744AReduce high temperature impurity contentHigh recovery rateSugar derivativesIodineDiosminIodine
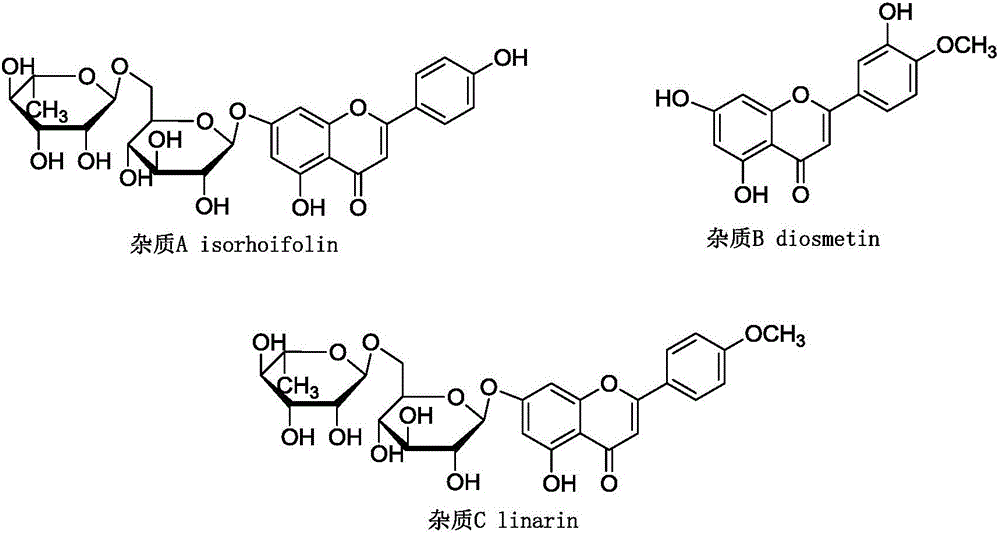
The invention discloses a method for preparing green and economic diosmin. The method comprises three main steps of preparation, recycling and refining. Compared with a conventional method, the method thoroughly avoids use of pyridine and inorganic base, the production environment is greatly improved, the aftertreatment is simple, iodine and solvents can be recycled, the reaction conditions are gentle, the production cost is low, and meanwhile in the method, a crude product is not refined in a classic alkali solution acidification mode instead of a mode that diosmin is separated out, then the purity of a finished product can be up to 99%, the product quality can be improved, and moreover the method is simple and convenient to operate and applicable to industrial production.
Owner:NANJING CHIA TAI TIANQING PHARMA
Synthesis method of diosmin raw medicine meeting EP7 version quality standards
InactiveCN102653549AResidue reductionMeet quality requirementsSugar derivativesSugar derivatives preparationPtru catalystDiosmin
The invention discloses a synthesis method of diosmin. A microwave-assisted heating method is adopted, I2 and NaI are used as catalysts, the ethanol solution of K2CO3 and NaOH and a pyridine mixed solvent are used as reaction solvents for establishing a reaction system, hesperidin is dehydrogenized to prepare diosmin in one step, and the dehydrogenation process avoids high temperature and avoids use of a large amount of high-boiling-point toxic solvents such as pyridine, dimethyl sulfoxide and the like in the reaction solvent system. Through the invention, the total synthesis yield of the diosmin is more than 80%, and the product quality meets the EP7.0 standards. The method disclosed by the invention has the technical characteristics of easiness in acquisition of raw materials, mild reaction conditions, short reaction time, simplicity and convenience in operation, low production cost, good product quality and the like, and is suitable for industrialized production.
Owner:长沙富能生物技术有限公司
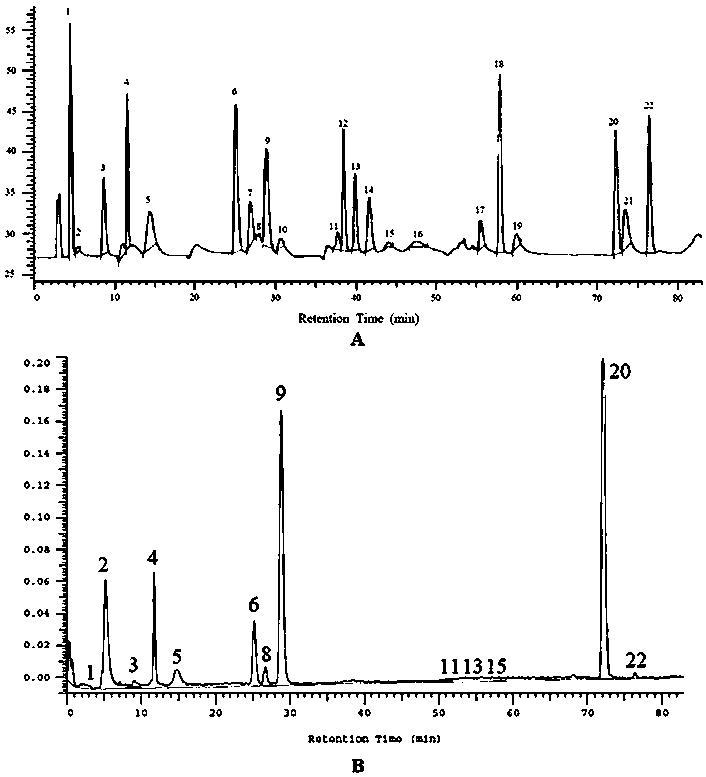
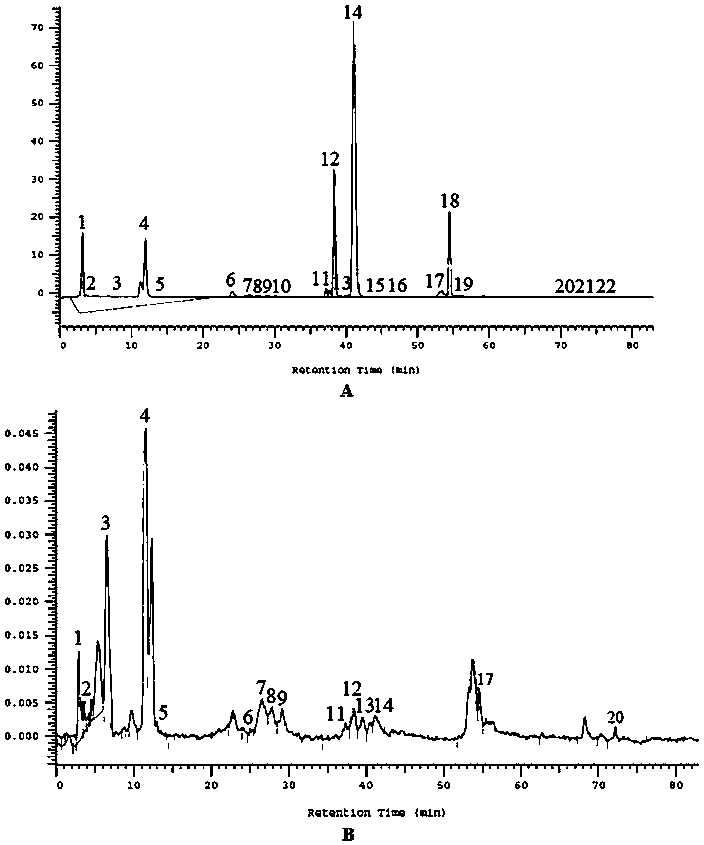
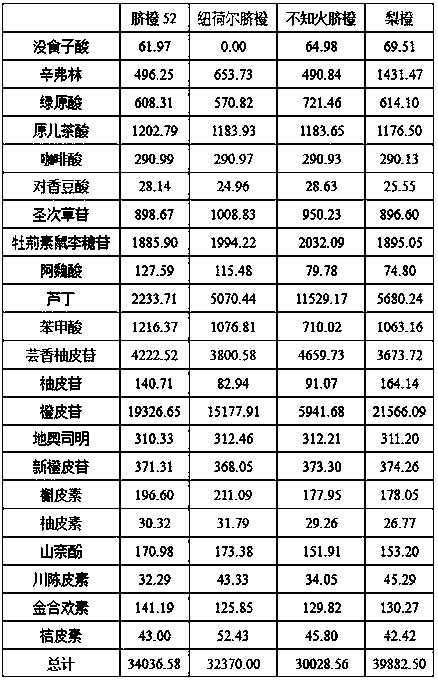
Method for simultaneously determining twenty-two flavones and phenolic acids in citrus fruits
The invention discloses a method for simultaneously determining twenty-two flavones and phenolic acids in citrus fruits by adopting high performance liquid chromatography-diode array detector-fluorescence detector (HPLC-DAD-FLD). The method is capable of simultaneously determining twenty-two phenolic compounds in citrus fruits such as gallic acid, synephrine, chlorogenic acid, protocatechuic acid,caffeic acid, p-coumaric acid, rhamnosylvitexin, eriocitrin, ferulic acid, rutin, benzoic acid, narirutin, naringin, hesperidin, diosmin, neohesperidin, quercetin, naringenin, kaempferol, nobiletin,hesperetin, acacetin and the like, derivatization is not needed, and the method is high in accuracy, high in sensitivity and excellent in repeatability.
Owner:INST OF AGRI ENG TECH FUJIAN ACAD OF AGRI SCI
Preparation method of diosmin
InactiveCN104250276AHigh purityReduce dosageSugar derivativesSugar derivatives preparationDiosminReaction intermediate
Relating to drug preparation, the invention discloses a preparation method of diosmin. The method comprises the steps of: (1) adding hesperidin into a mixed solvent of pyridine and dimethyl sulfoxide, then adding potassium iodide and sulfuric acid to carry out dehydrogenation reaction to obtain a reaction intermediate product; (2) adding a sodium hydroxide-containing methanol solution into the reaction intermediate product to react; and (3) then adding acid to adjust the pH value, and conducting standing crystallization, thus obtaining diosmin. Compared with existing preparation methods, the preparation method provided by the invention has the characteristics of low cost, simple process and high product purity, and is suitable for industrial mass production.
Owner:成都华康生物工程有限公司
Micronize diosmin and hesperidine composition suppository
InactiveCN1823807AAppropriate hardnessAppropriate toughnessOrganic active ingredientsSuppositories deliveryGynecologyDiosmin
A composite suppository for treating pile, phlebitis and phlebeurysma is prepared from micro-granular diosmin and hesperidin, matrix for suppository and additive.
Owner:刘展欣
Synthesis method of diosmin
InactiveCN102875621AReduce usageSugar derivativesSugar derivatives preparationDiosminSynthesis methods
The invention discloses a synthesis method of a medicinal compound, particularly a synthesis method of diosmin. The diosmin is prepared by heating a mixture of hesperidin, iodine, inorganic alkaline reagent and reaction solvent to 80-100 DEG C. The synthesis method of diosmin can effectively avoid using pyridine, thereby overcoming the defects of low safety and high solvent residues in the product in the existing synthesis method.
Owner:CHENGDU LANQI PHARMA
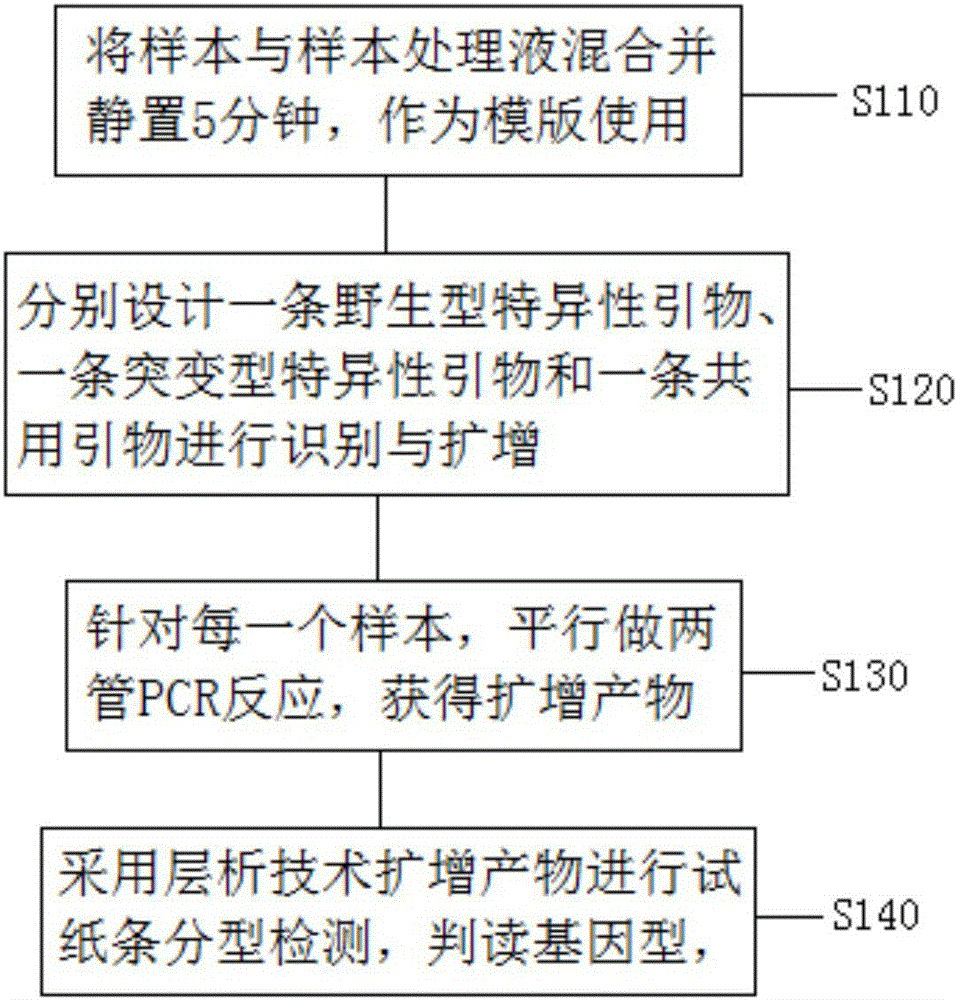
Extraction-free direct-amplification rapid detection method for SNP typing of human methylenetetrahydrofolate reductase (MTHFR) gene via test strips
InactiveCN106498074AImprove detection efficiencyReduce cross contaminationMicrobiological testing/measurementBiotin-streptavidin complexDiosmin
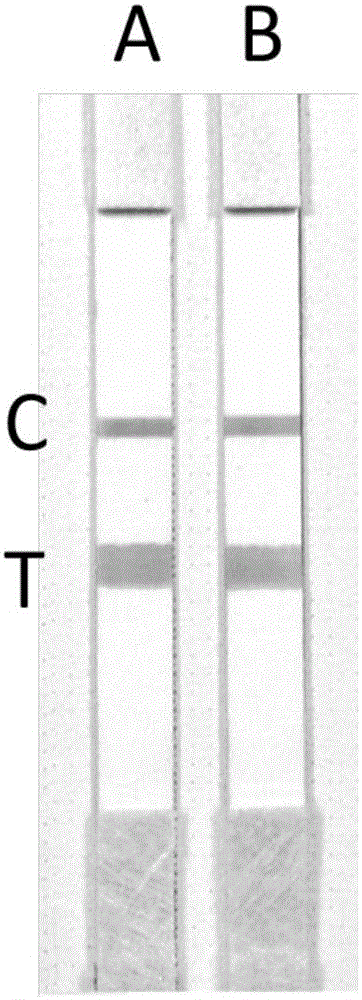
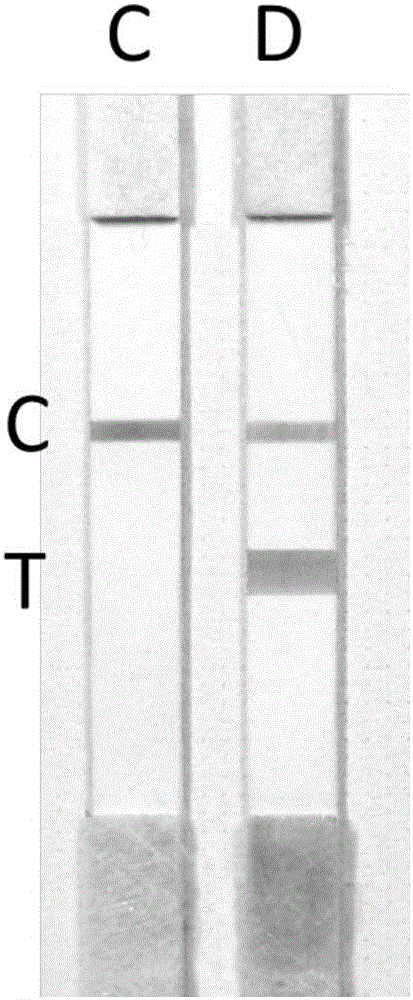
The invention provides an extraction-free direct-amplification rapid detection method for SNP typing of a human MTHFR gene via test strips. The method comprises the following steps: step 1, mixing samples with a sample treatment fluid and carrying out standing for 5 min, wherein obtained mixtures are used as templates; step 2, separately designing a wild specific primer, a mutant specific primer and a shared primer for identification and amplification of the C677T SNP site of a to-be-detected MTHFR gene, wherein the 5'-terminals of the wild specific primer and the mutant specific primer are both labeled with digoxin and the 5'-terminal of the shared primer is both labeled with biotin; step 3, carrying out parallel two-tube PCR reactions on each sample so as to obtain amplification products; and step 4, subjecting the amplification products obtained in the step 3 to typing detection via the test strips by using chromatographic techniques and action between digoxin and a digoxin monoclonal antibody and between biotin and streptavidin, and then judging and determining genotypes.
Owner:金磁(苏州)纳米科技有限公司
Pegylated insulin-like-growth-factor assay
The current invention reports an immunoassay for the determination of PEGylated insulin-like-growth-factor employing an anti-(polyethylene glycol) antibody and an anti-digoxygenin antibody for the detection of an insulin-like-growth-factor / insulin-like-growth-factor-binding-protein-complex.
Owner:F HOFFMANN LA ROCHE & CO AG
Food composition for functional foods and nutritional supplements
InactiveUS20100016246A1Good water solubilityBioavailabilityBiocideDough treatmentVascular diseaseNutrition supplementation
A food composition for functional foods and nutritional supplements is for preventing, reducing or treating cardiovascular diseases or mild manifestations thereof, obtained from the combination of extracts derived from citrus species, which contain between 25 and 80% by weight of hesperidin; between and 10% by weight of isonaringin; between 0.5 and 1% by weight of eriocitrin; and between 0.1 and 1% by weight of diosmin and the use of the composition in functional foods and nutritional supplements.
Owner:FURFURAL ESPANOL
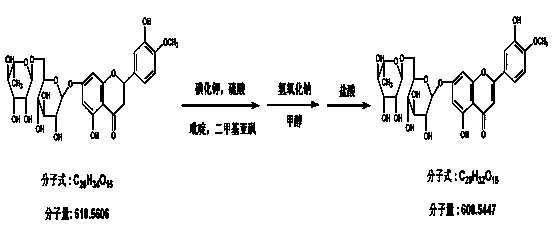
Preparation method of pure micronized diosmin
The invention relates to a preparation method of pure micronized diosmin, which comprises the following steps: 1) hesperidin phenolic hydroxyl group protection; 2) halogenating reaction; 3) protecting group removal and double bond generation; and 4) drying to obtain the micropowder. The six secondary hydroxyl groups on the hesperidin glycosyl group have low activity, the two hydroxyl groups on 6- and 7'- sites of the flavone parent nucleus are subjected to hydroxyl group protection, the flavone parent nucleus is halogenated to generate halogenated diosmin, and the protecting group removal is performed to obtain the diosmin.
Owner:李玉山
Method for extracting hesperidin from fructus aurantii immaturus
ActiveCN111732622AIncrease contentHigh yieldSugar derivativesSugar derivatives preparationBiotechnologyBiochemical engineering
The invention provides a method for extracting hesperidin from fructus aurantii immaturus. The method comprises the following steps of degreasing, enzymolysis, impurity removal, percolation, diatomiteadsorption and two-step pH regulation, thereby finally obtaining the fructus aurantii immaturus hesperidin. The method for extracting high-quality hesperidin from fructus aurantii immaturus providedby the invention is coherent and simple in process, high in operability, low in acid and alkali consumption, low in sewage discharge, low in production cost, free of toxic and harmful chemical solvents, safe, environmentally friendly, high in hesperidin content, high in yield and suitable for industrial production. In addition, the hesperidin product produced by the method is high in content and does not contain isonaringin. The hesperidin prepared by the method is used for synthesizing diosmin, and the diosmin product prepared from the hesperidin as a raw material is high in purity and good in drug effect due to no interference of isonaringin.
Owner:HUNAN HUACHENG BIOTECH
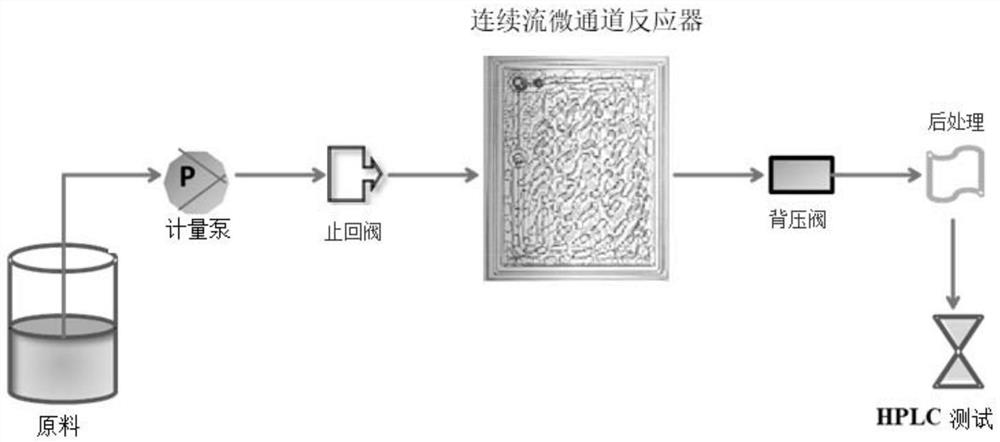
Continuous flow microchannel synthesis process of flavonoid compounds
PendingCN112979603AShort manufacturing timeImprove conversion rateSugar derivativesChemical/physical/physico-chemical microreactorsXanthonoidDiosmin
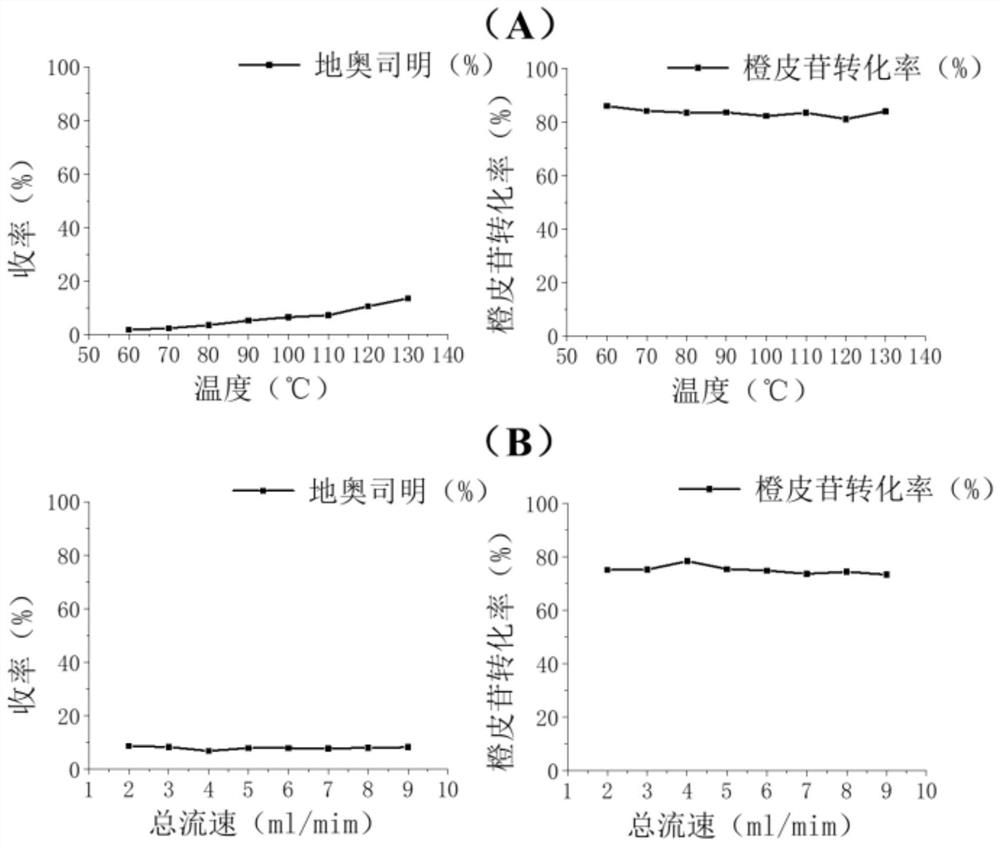
The invention provides a continuous flow microchannel synthesis process of flavonoid compounds. According to the process, hesperidin and iodine elementary substance are used as raw materials and react in a continuous flow microchannel reactor in the presence of a reaction solvent to synthesize the flavonoid compound as shown in a formula A. Compared with a traditional kettle-type preparation process, the process disclosed by the invention has the advantages that the preparation time is obviously shortened, and the conversion rate of raw materials and the yield of products are obviously improved; and especially, when the diosmin is prepared under optimal process conditions of continuous flow microchannel synthesis, the conversion rate of the raw material hesperidin is as high as 96.48%, and the yield of the product diosmin is as high as 81.96%. The continuous flow micro-channel synthesis process provided by the invention is beneficial to realizing safe, efficient and rapid industrial production of flavonoid compounds, and has a wide application prospect.
Owner:宜宾西华大学研究院 +1
Diosmin-containing medicinal composition
InactiveCN102423317AGood curative effectGood treatment effectHydroxy compound active ingredientsCardiovascular disorderTreatment effectDiosmin
The invention relates to a diosmin-containing medicinal composition, which has beneficial effect to treatment of hemorrhoids, in particular a remarkable treatment effect to hematochezia and pains caused by internal hemorrhoids, by means of combination of diosmin, pectolinarigenin and borneol. The diosmin-containing medicinal composition has the advantages of good stability, high bioavailability and simple preparation process, and is suitable for large-scale industrial production.
Owner:NANJING CHIA TAI TIANQING PHARMA
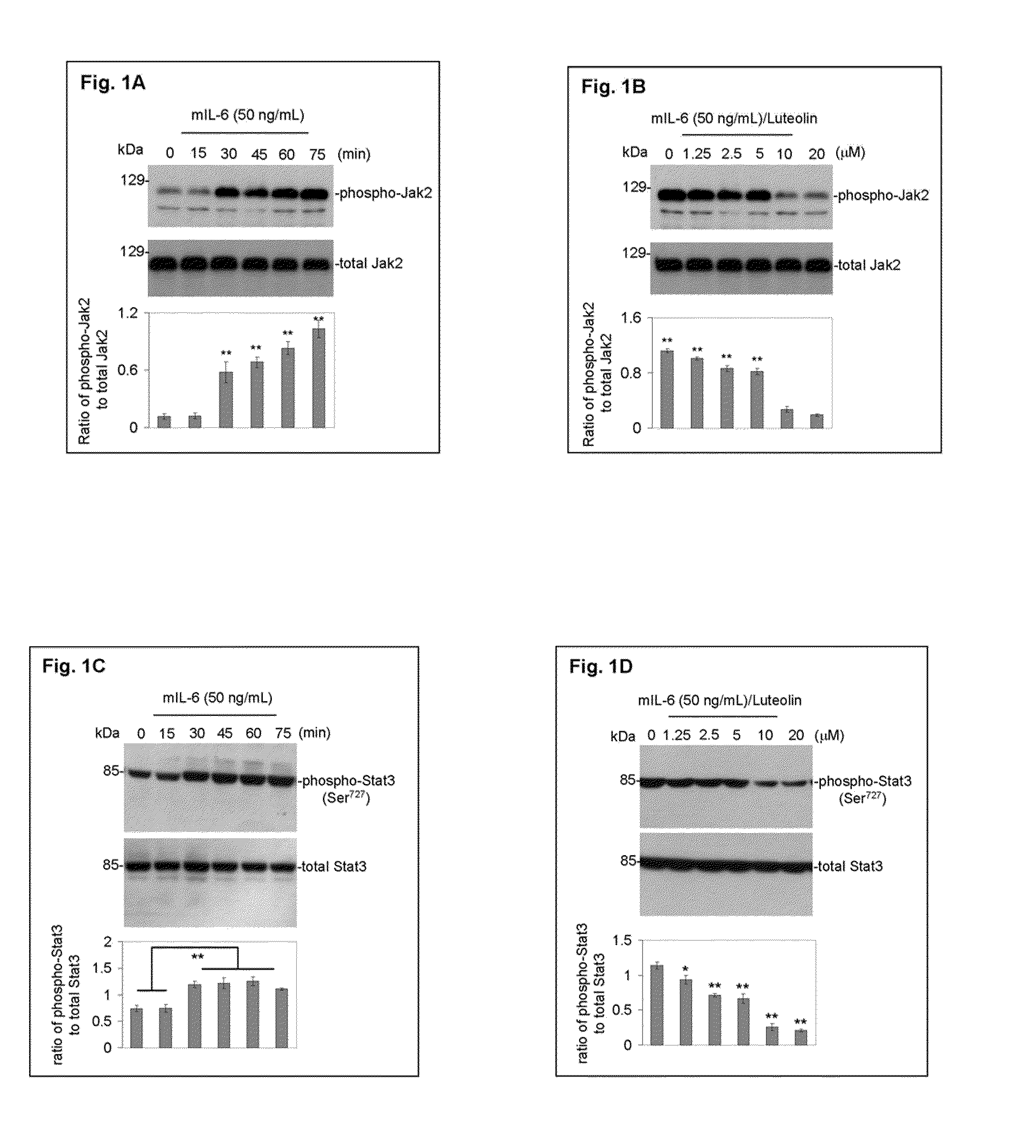
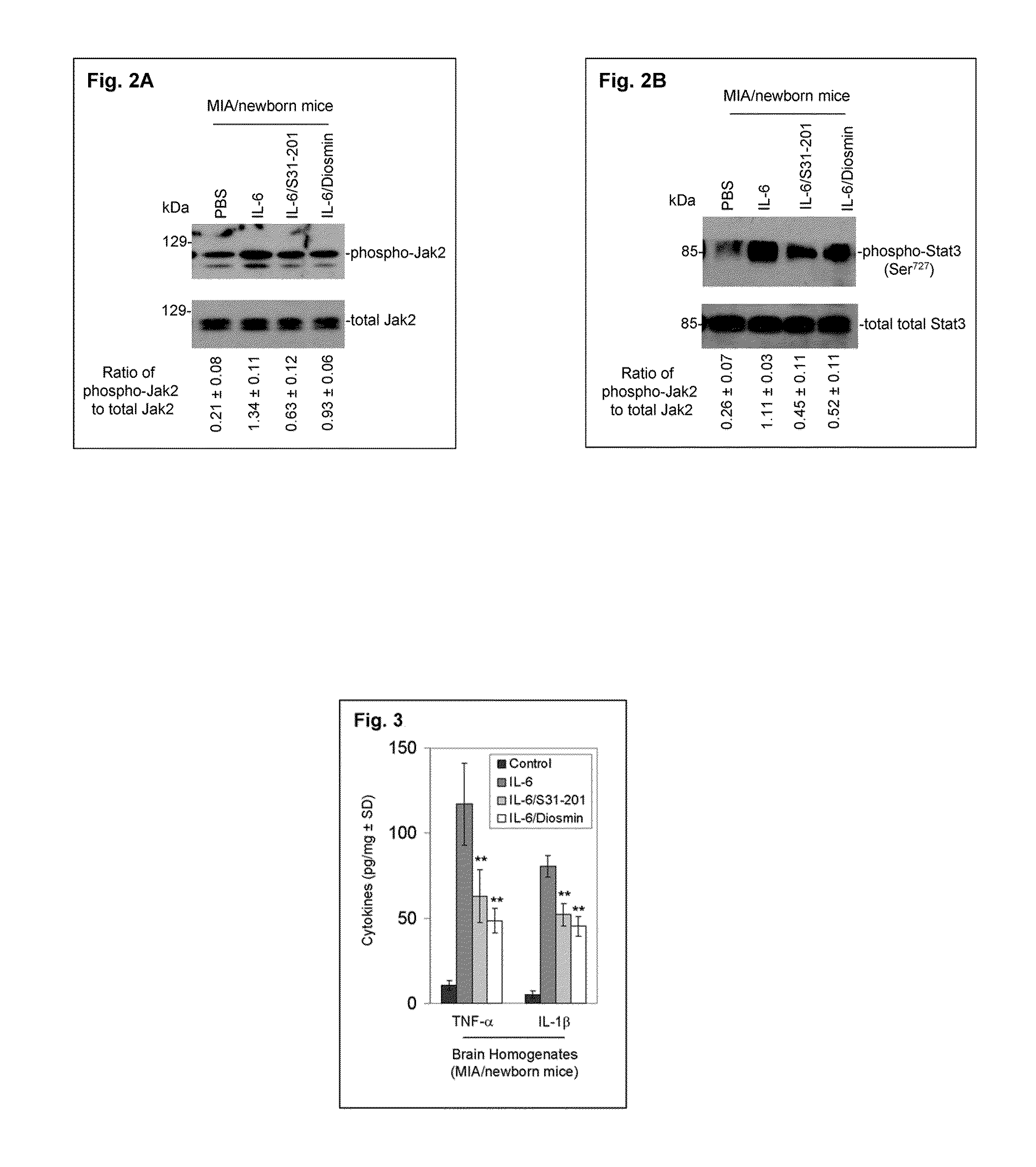
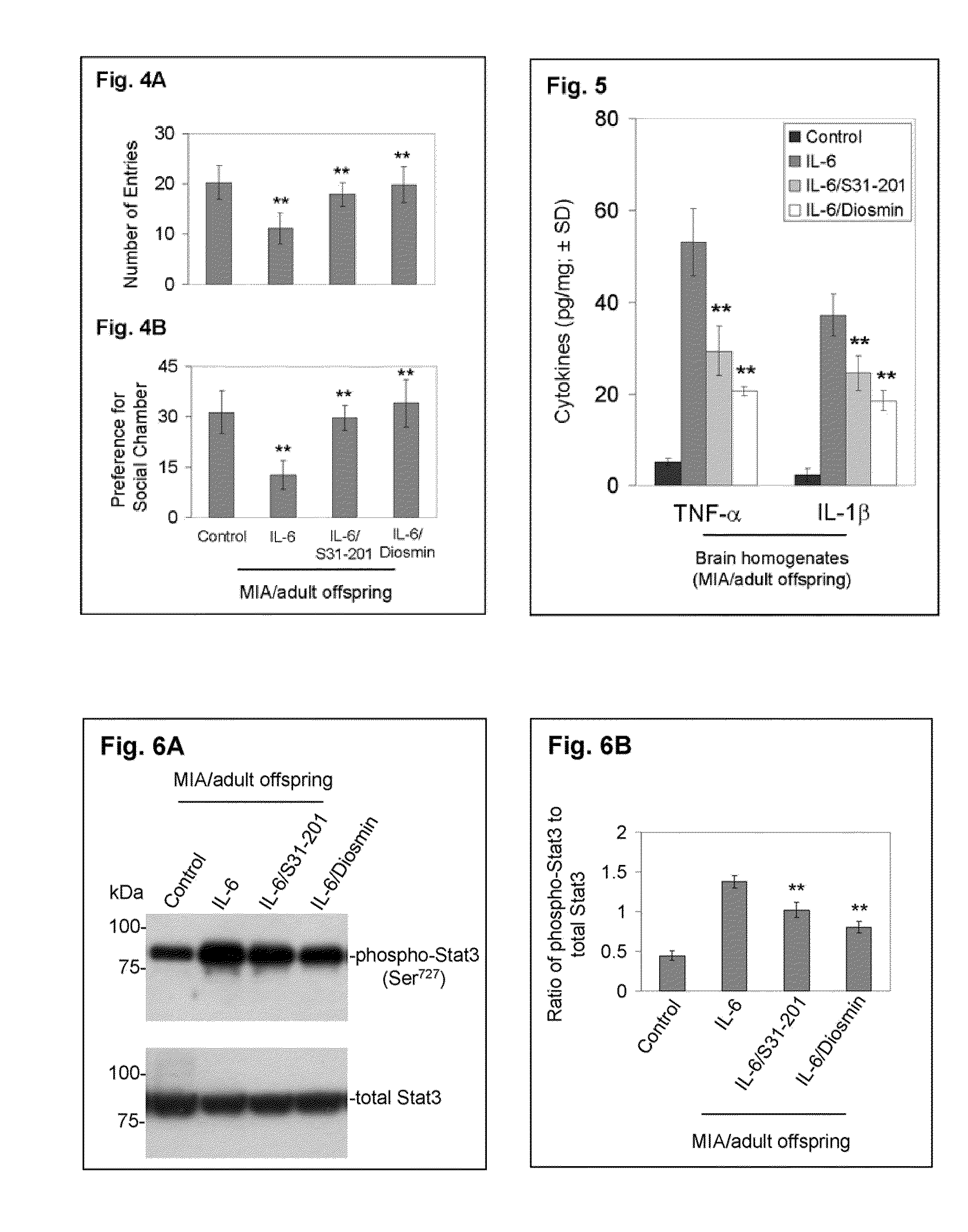
Luteolin and diosmin/diosmetin as novel stat3 inhibitors for treating autism
The present invention includes methods for the treatment of autoimmune disorders such as autism, schizophrenia, and type 1 diabetes. Flavonoids, luteolin, diosmin, and diosmin's aglycone form, diosmetin, were found to inhibit activation / phosphorylation of STAT3 induced by IL-6 in cultured neuronal cells. Furthermore, mice treated with diosmin showed a significant reduction of autistic phenotype induced by IL-6 through inhibition of STAT3 activation.
Owner:UNIV OF SOUTH FLORIDA
Method for preparing Galium verum general flavone
InactiveCN102038782AEfficient removalMitigate adsorption interferenceAntipyreticDigestive systemOrganic solventDiosmin
The invention discloses a Method for preparing Galium verum general flavone, belonging to the technical field of plant extraction. The Galium verum general flavone mainly contains rutin, Palustroside, Diosmin, Diosmetin (2->1) glucose arabinose, isoquercitrin, Diosmetin 7-O-beta-D ascorbyl glucoside and the like. The general flavone in the Galium verum is obtained by being extracted by water, extracted by organic solvent, condensed, decontaminated and adsorbed and eluted by macroporous resin. The prepared Galium verum general flavone contains 50-95% of Galium verum. In the method, petroleum ether is adopted to extract and remove fat soluble impurities; column-loading buffer effectively removes impurities, resin adsorption interference can be lightened, and thus resin has strong adsorption capability on flavone. The method has low cost and reasonable process and can adapt to large-scale production.
Owner:NANJING ZELANG MEDICAL TECH
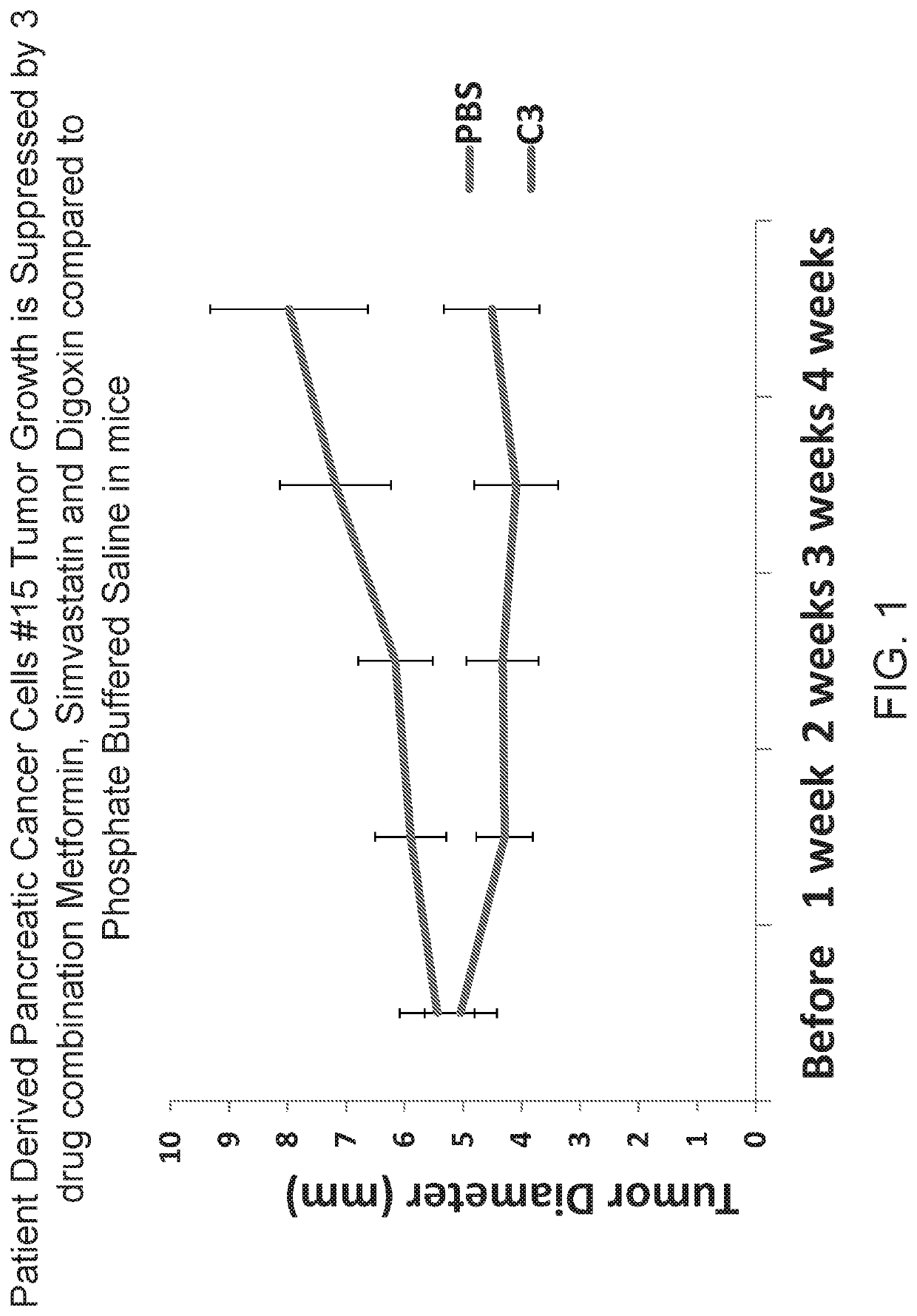
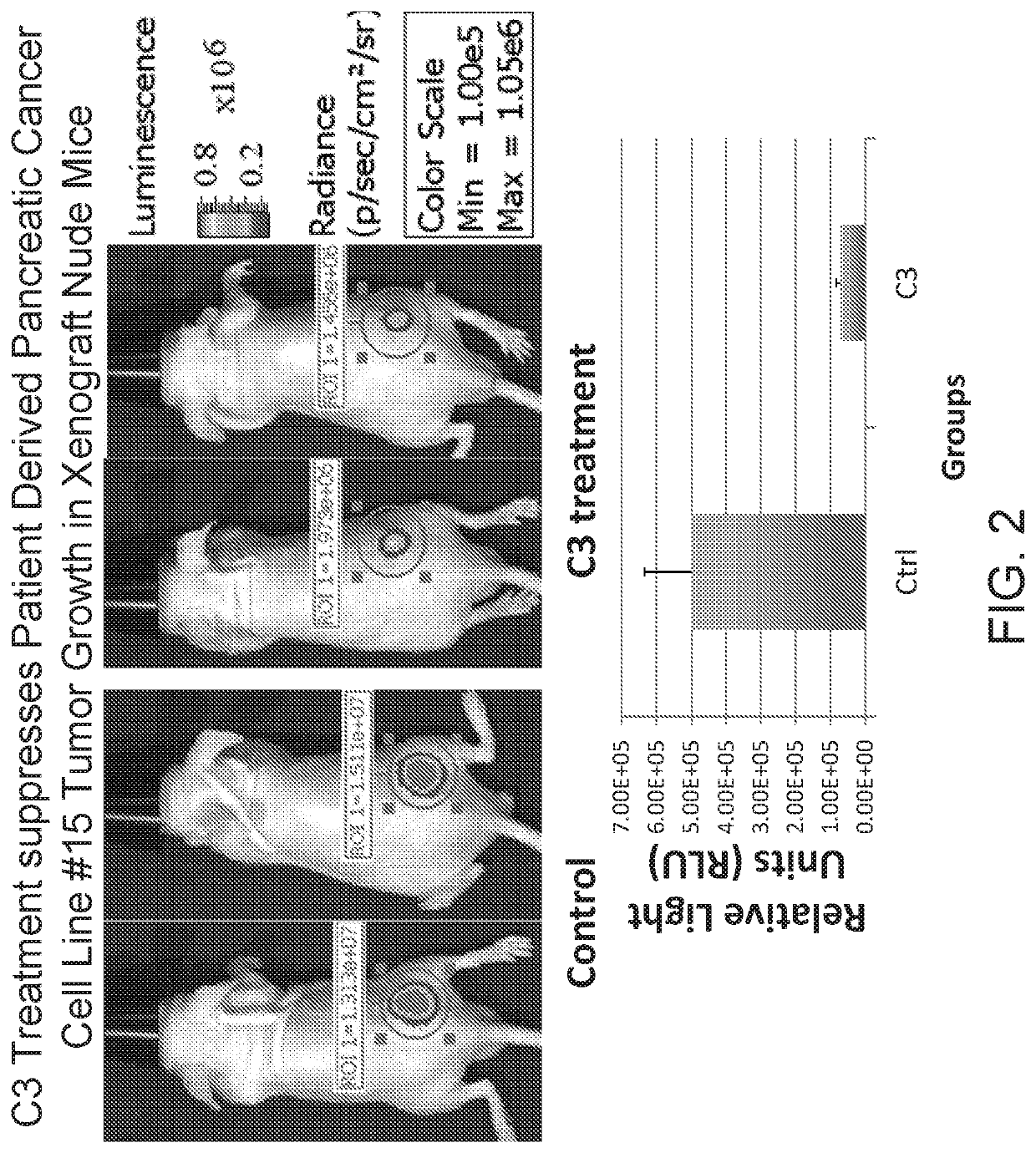
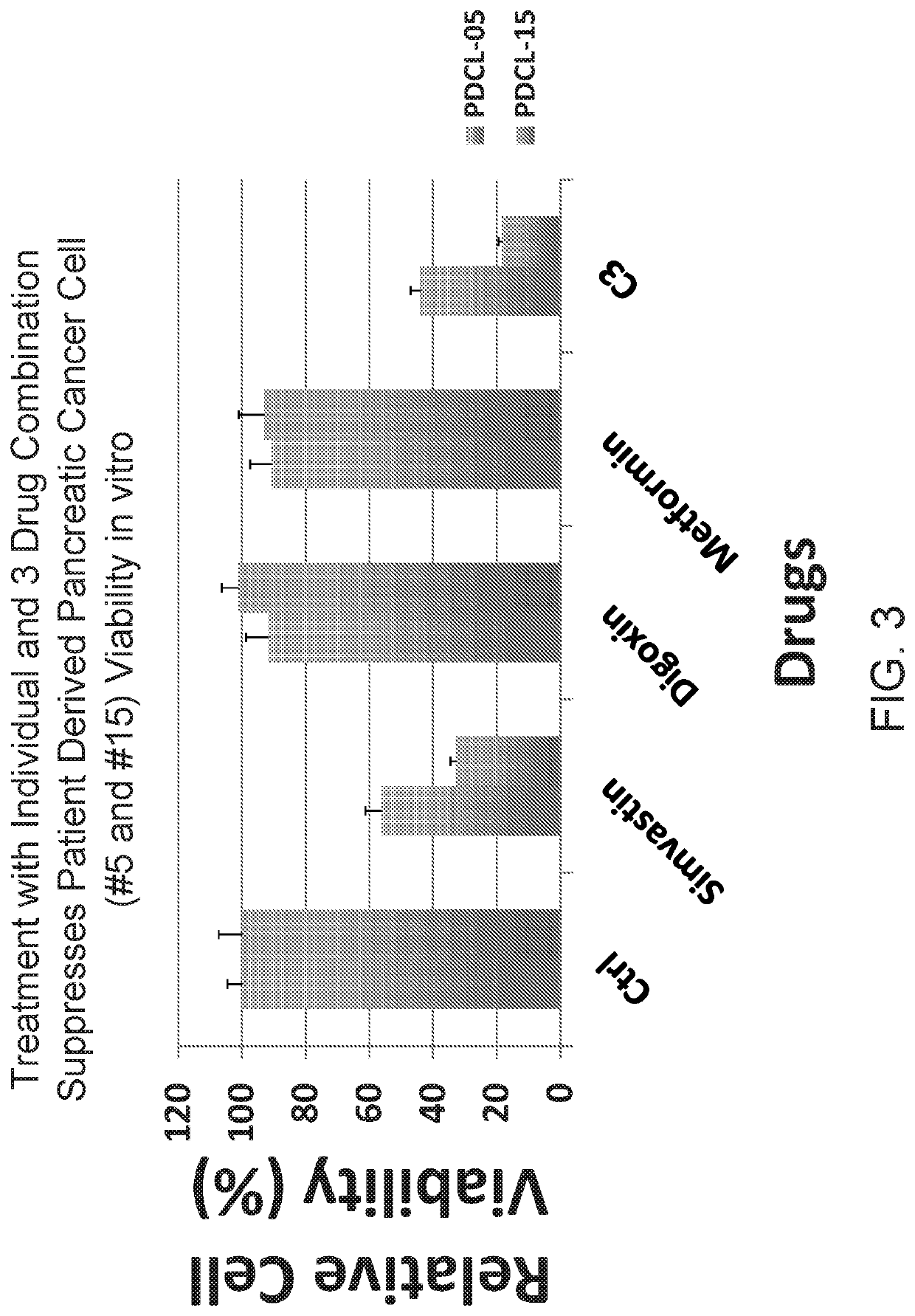
Triple drug combination (metformin, simvastatin, digoxin) for targeted treatment of pancreatic cancer
ActiveUS20190358193A1Increase heightPrevent proliferationOrganic active ingredientsAntineoplastic agentsDiosminPancreatic cancer cell
A combination of three well-known and FDA approved compounds has been discovered to significantly suppress the proliferation of pancreatic cancer cells in clinically relevant models of pancreatic cancer. Embodiments of the invention include compositions of matter comprising a combination of agents such as metformin, simvastatin, and digoxin as well as methods of treating cancers using such agents. Illustrative methods include combining a population of pancreatic cancer cells with amounts of metformin, simvastatin, and digoxin sufficient to inhibit expression of BIRC5 protein in the population of pancreatic cancer cells, thereby inhibiting the growth of the population of pancreatic cancer cells.
Owner:RGT UNIV OF CALIFORNIA
Low-cost preparation method of diosmin
PendingCN113292618AReduce dosageLow costSugar derivativesSugar derivatives preparationDiosminPyridine
The invention discloses a low-cost preparation method of diosmin. The low-cost preparation method of diosmin comprises the following steps: dissolving hesperidin and iodine in pyridine; performing heating and introducing ozone until the concentration of hesperidin is lower than 5%; adjusting the pH value to be neutral, and performing filtering to obtain a first filtered substance; washing the first filtered substance with deionized water, adding alkali for dissolving, and performing filtering to obtain filtrate; and acidizing and recrystallizing the filtrate to obtain diosmin. The method has the effects of reducing the dosage of iodine and lowering the cost.
Owner:HUNAN YUANTONG PHARMA
Method for simultaneously quantitatively detecting astragaloside-IV and cycloxanthine in mouse plasma
The present invention establishes a simultaneous quantification method for mouse plasma based on UPLC-HRMS, in which the method targets astragaloside IV and cycloxanthine that is the main metabolite of astragaloside IV. The quantitative time of the method is 3 mins, digoxin is used as an internal standard, and only 20 [mu]L of mouse plasma is needed, thus having the advantages of rapidity, high sensitivity and strong specificity. After being precipitated by the protein, the sample is filtered by dephospholipidation plate, which effectively reduces the matrix effect of endogenous metabolites ofphospholipids in plasma on the analyte. Ultra-high performance C18 column is used as the analytical column to detect two kinds of analytes and internal standards in the electrospray ion source positive ion selective ion monitoring mode. The linear range of the two analytes is 1-200ng / mL, the intra-day and inter-day precision is <=8.6%, and the precision is <=8.8%, which indicates that the methodhas good precision and accuracy. The method for simultaneously quantitatively detecting astragaloside-IV and cycloxanthine in mouse plasma was successfully applied to the pharmacokinetic study of astragaloside IV mice.
Owner:MINZU UNIVERSITY OF CHINA
Medicinal composition containing diosmin sulfate derivatives and application thereof
ActiveCN108992456AAlleviate symptoms of poisoningRestoring impaired liver functionOrganic active ingredientsDigestive systemImpaired liver functionMedicine
The invention provides a medicinal composition containing diosmin sulfate derivatives and application thereof. The derivatives or the medicinal composition thereof can be used for preventing or treating toxication. The diosmin derivatives and the medicinal composition which are provided by the invention have excellent detoxication, can obviously reduce toxication caused by various factors, effectively recover the impaired liver function, and provide a new medicine selection for clinically preventing or treating toxication or peptic ulcer combined toxication.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Measuring method for total cardiac glycoside content in Zhuang medicine streptocaulon griffithii
PendingCN107621455ASimple methodAccurate methodColor/spectral properties measurementsDiosminMedicine
The invention discloses a measuring method for total cardiac glycoside content in Zhuang medicine streptocaulon griffithii. According to the method, color-developing agents are added into samples, thecontent of total cardiac glycoside in the streptocaulon griffithii is measured by an ultraviolet spectrophotometry after the samples develop color, test conditions include that Digoxin serves as a reference substance, alkaline picric acid solution serves as the color-developing agents, detection is performed under the wave length of 415nm, the content of the reference substance and the absorbancehave a good linear relation within the range of 0-0.08mg / ml, a regression equation y=14.968x+0.008 (r=0.9969), accuracy RSD=0.1%, average sample recovery ratio is 95.51%, RSD is 2.16% (n=6), and thetotal cardiac glycoside content in the streptocaulon griffithii is 2.07mg / g. The method is simple, rapid, accurate and good in repeatability and can be used for measuring the total cardiac glycoside content in the streptocaulon griffithii, and quality basis is provided for evaluation and control of the streptocaulon griffithii.
Owner:PHARMA FACTORY OF GUANGXI TRADITIONAL CHINESE MEDICAL UNIV
Application of diosmin in manufacturing medicaments
The invention disclose application of diosmin in manufacturing medicaments, i.e. 3',5,7-trihydrocy-4-methoxyflavone7-rutinoside is taken as a medicine for treating lumbar spinal stenosis. The medicine has better and more obvious curative effect on treating the lumbar spinal stenosis than a traditional medicine.
Owner:杨义靖
Flavonoid treatment of glycogen synthase kinase-based disease
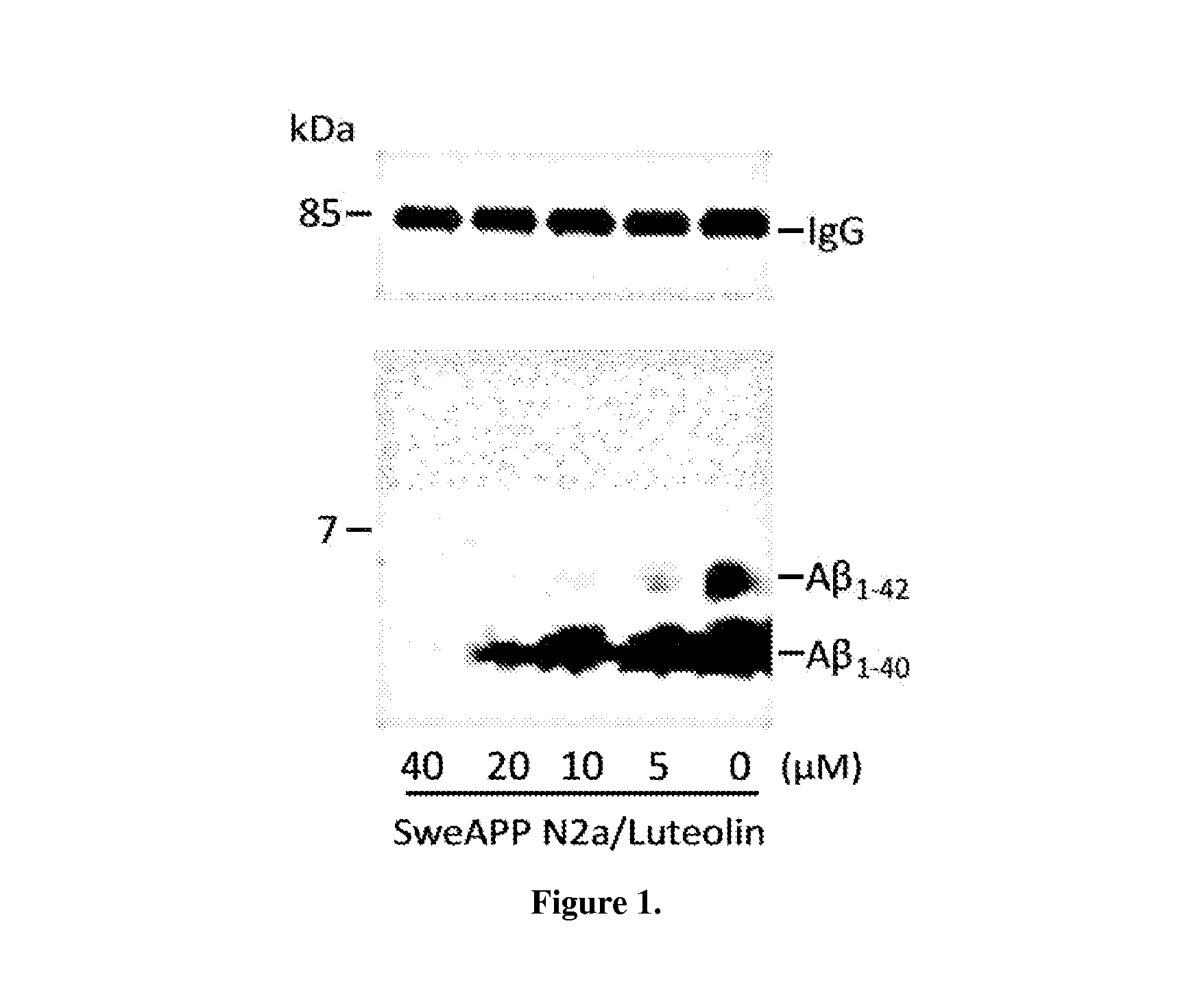
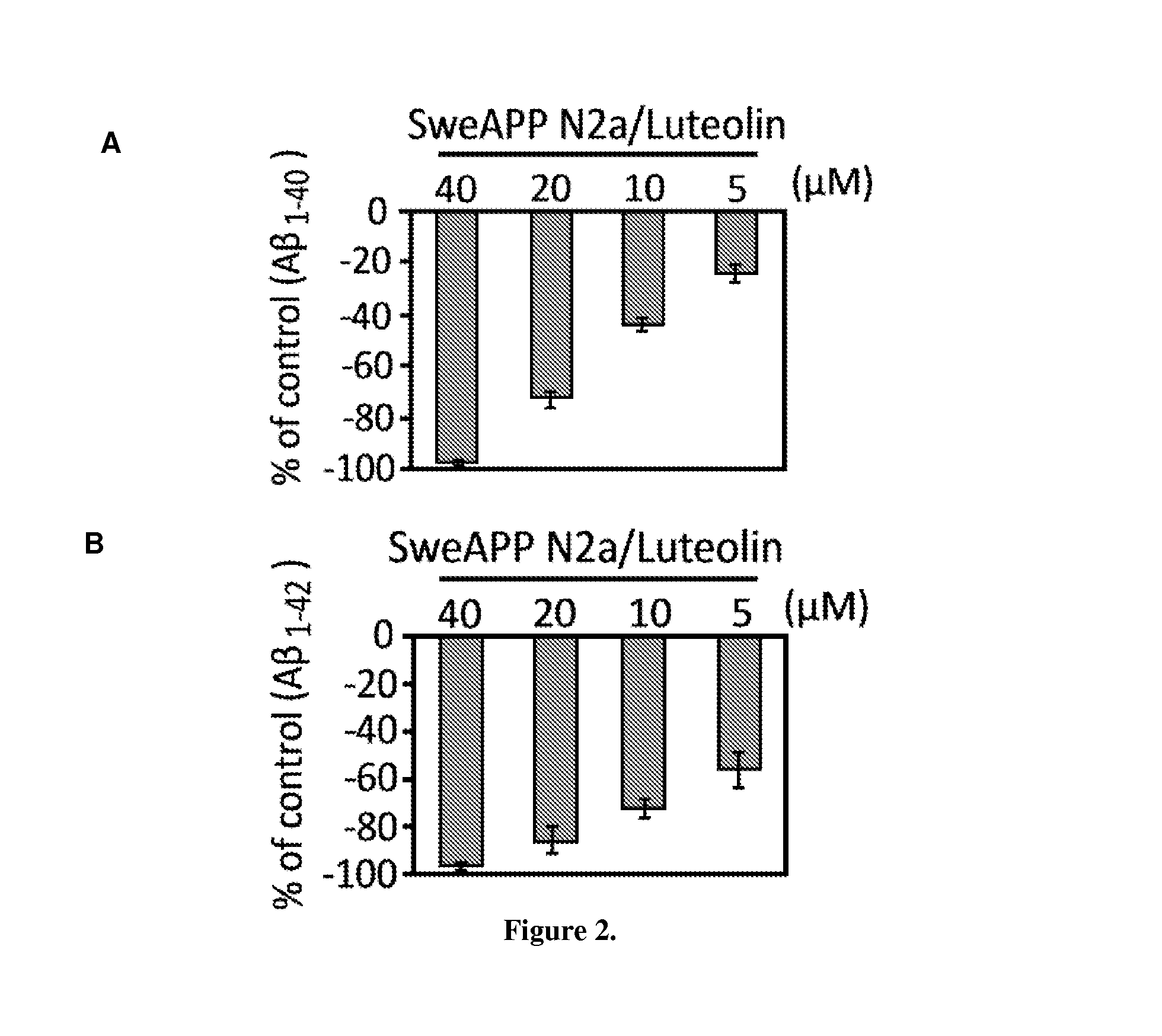
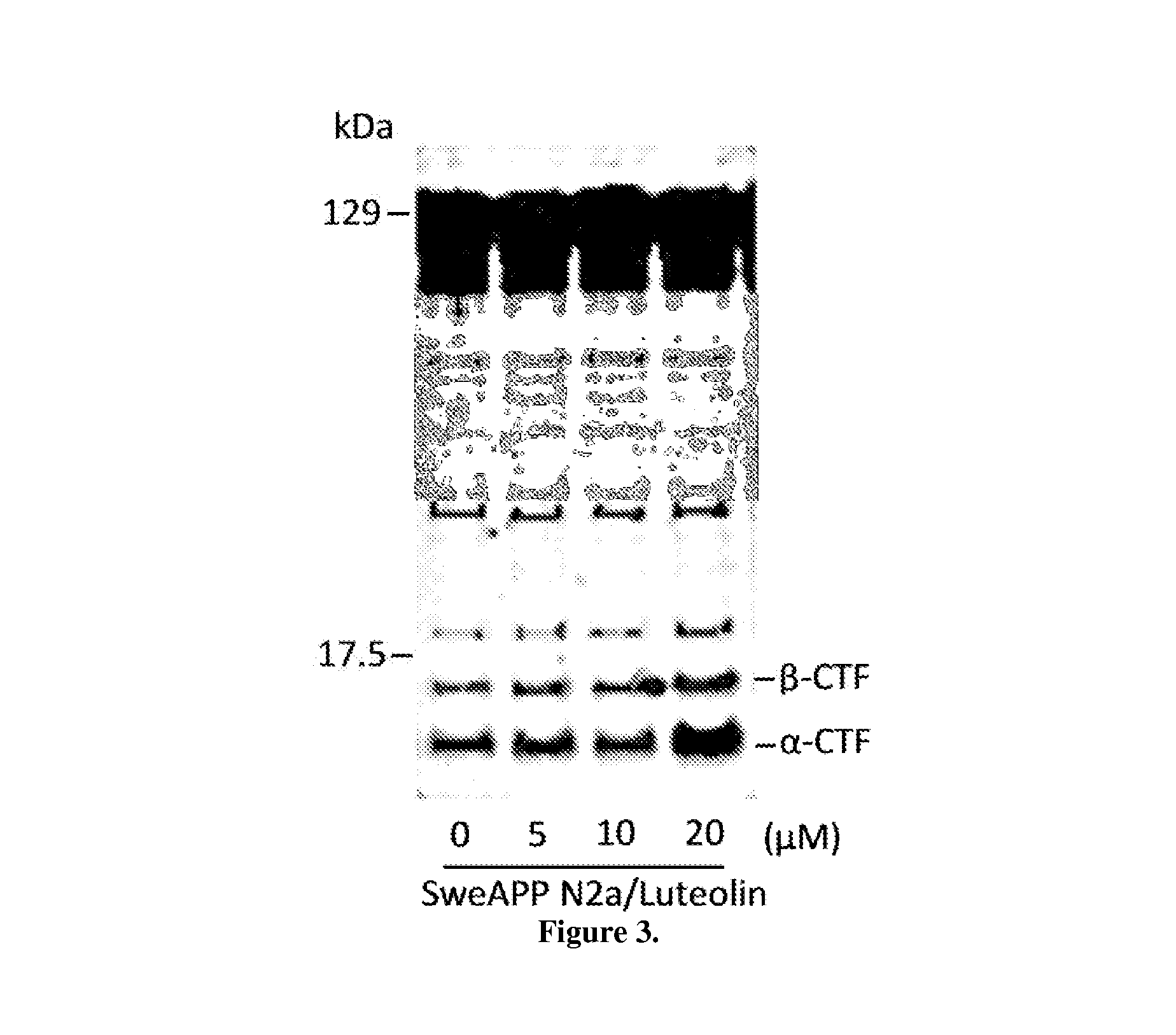
The flavonoid luteolin reduces amyloid-β peptide (Aβ) generation. Luteolin is also a selective GSK-3 inhibitor that 1) decreases amyloidogenic γ-secretase APP processing, and 2) promotes presenilin 1 (PS1) carboxyl-terminal fragment (CTF) phosphorylation. GSK-3α activity is essential for both PS1 CTF phosphorylation states and PS1-APP interaction. These findings were validated in vivo, using a Tg2576 Alzheimer's Disease model system. Luteolin treatment decreased soluble Aβ levels, reduced GSK-3 activity, and disrupted PS1-APP association. In addition, Tg2576 mice treated with diosmin, a glycoside of a flavone structurally and functionally similar to luteolin (diosmetin), displayed significantly reduced Aβ pathology as well.
Owner:UNIV OF SOUTH FLORIDA
Low side effect pharmaceutical composition containing isoniazid
ActiveUS8304394B2Reduce INH-induced side-effectsEliminate side effectsAntibacterial agentsBiocideDaidzeinDiosmin
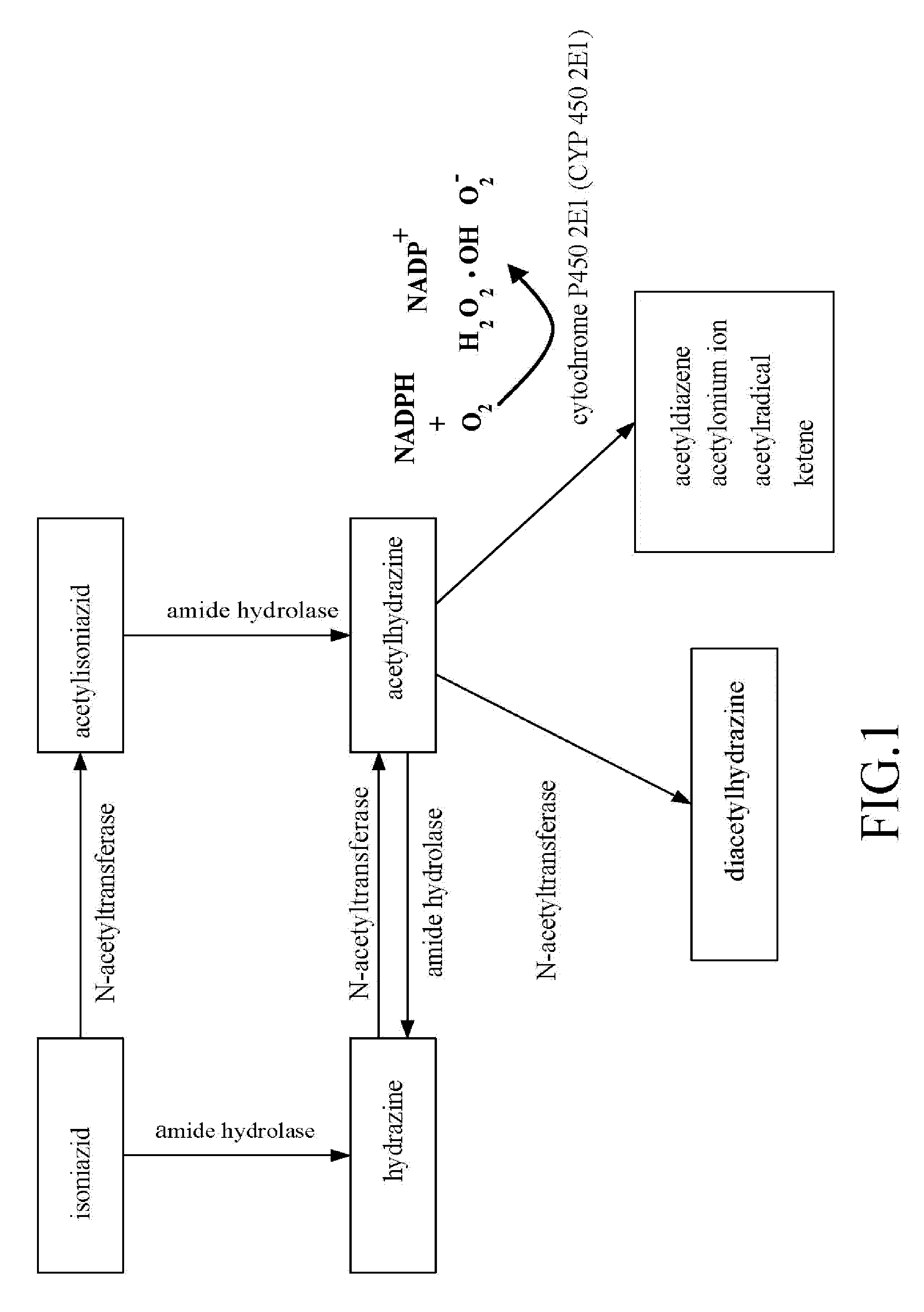
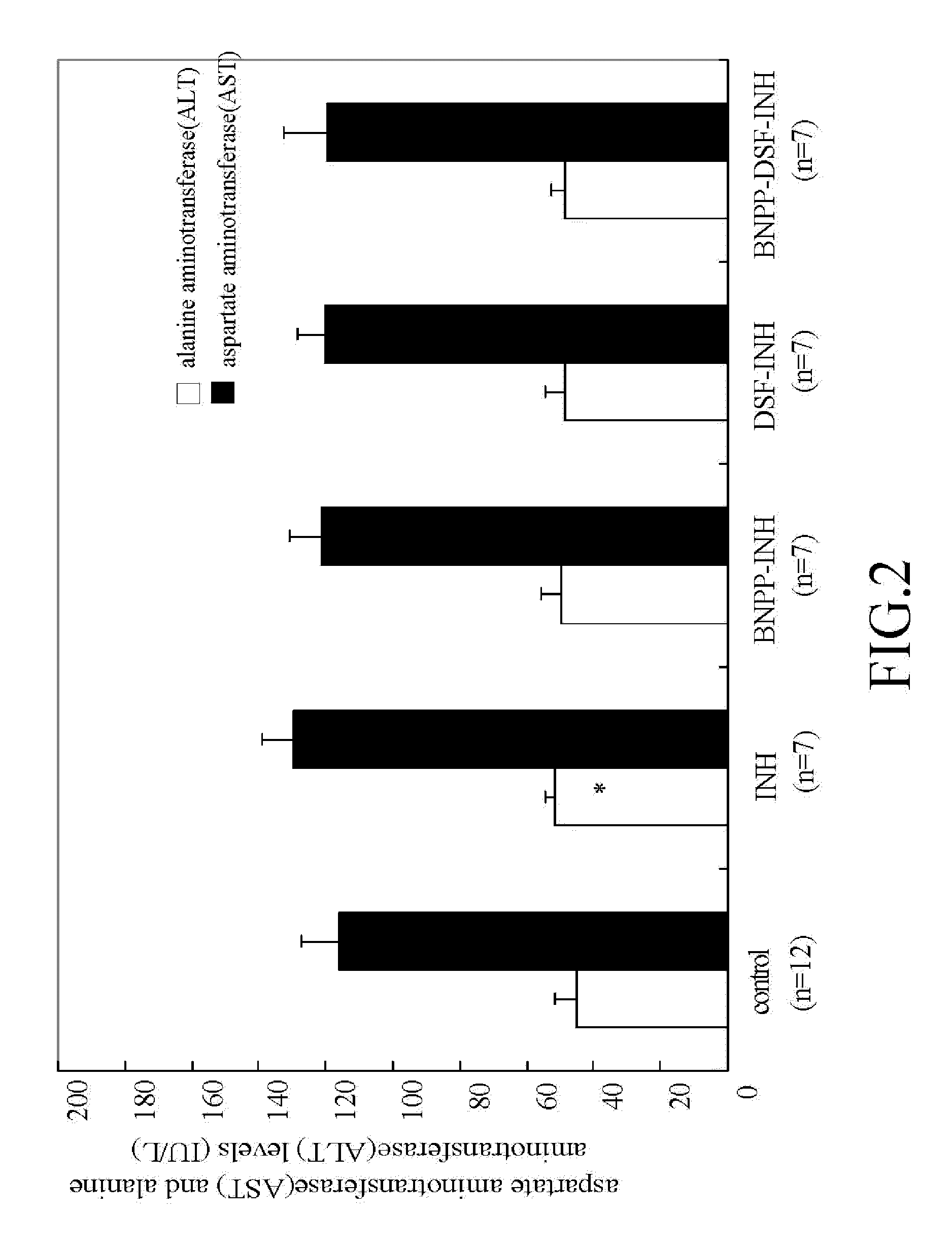
The present invention features a novel, low side-effect pharmaceutical compound complex, comprising the pharmaceutically effective dose of isoniazid (INH) and pharmaceutically effective dose of one of the following compounds. Said compound was selected from the following groups of compounds: Nordihydroguaiaretic acid, Trans-Cinnamaldehyde, Daidzein, Isovitexin, Kaempferol, disulfuram, β-Myrcene, Quercetin, (−)-Epigallocatechin-3-gallate, (+)-Limonene, Myricetin, Quercitrin, Luteolin-7-Glucoside, Morin, Neohesperidin, Hesperidin, Capillarisin, (−)-Epigallocatechin, Luteolin, Hyperoside, Ethyl Myristate, Tamarixetin, Phloretin, Baicalein, Rutin, Baicalin, Apigenin, Naringenin, Hesperetin, (+)-Epicatechin, (−)-Epicatechin-3-gallat, Isoliquritigenin, Silybin, Vitexin, Genistein, Isorhamnetin, gallic acid, Diosmin, 6-Gingerol, (+)-Taxifolin, Wongonin, Protocatechuic acid, (+)-Catechin, β-naphthoflavone, Embelin, Trans-Cinnamic acid, (−)-Epicatechin, Phloridzin, Puerarin, Umbelliferone, Brij 58, Brij 76, Brij 35, Tween 20, Tween 80, Tween 40, PEG 2000, PEG 400, Pluornic F68, and PEG 4000. The novel, low side-effect compound complex which contains pharmaceutically effective doses of isoniazid (INH), disulfuram (DSF) and / or a third compound, bis-nitrophenyl phosphate (BNPP) can reduce isoniazid (INH)-induced side effects, e.g. hepatotoxicity, etc.
Owner:INT EDUCATION FOUND
Compositions comprising melatonin and flavonoids for use in the treatment of tumours resistant to chemotherapy
InactiveCN106535939AImprove effectivenessOrganic active ingredientsAntineoplastic agentsDiosminMedicine
The present invention relates to compositions comprising at least one flavonoid of natural or synthetic origin in association with melatonin for use to increase the effectiveness of chemotherapeutic treatments used in human and veterinary medicine for the treatment of tumours, in particular for the treatment of tumours resistant to the chemotherapeutic agents currently in use. The at least one flavonoid is selected from the group comprising or, alternatively, consisting of rutin, oxerutin, diosmin and hesperidin, preferably rutin.
Owner:PROBIOTICAL
Method for preparing diosmin by adopting continuous flow microreactor
PendingCN113698440AShort reaction timeReact SafeSugar derivativesChemical/physical/physico-chemical microreactorsChemical synthesisMicroreactor
The invention provides a method for preparing diosmin by adopting a continuous flow microreactor, and belongs to the technical field of chemical synthesis. The method comprises the following steps: dissolving hesperidin and iodine in an alkaline solvent, and introducing into a continuous flow microreactor for reaction, thereby obtaining the hesperidin; wherein a molar ratio of the hesperidin to the iodine is 1: (1-1.6). According to the method, product conversion rate and yield which are equivalent to production conditions of a traditional process are realized, reaction time is shortened to 2.7 minutes from 8-20 hours of the traditional process, the reaction process is safer, more efficient and quicker, therefore, intrinsic safety of diosmin industrial production is realized, and market competitiveness is further enhanced.
Owner:XIHUA UNIV +1
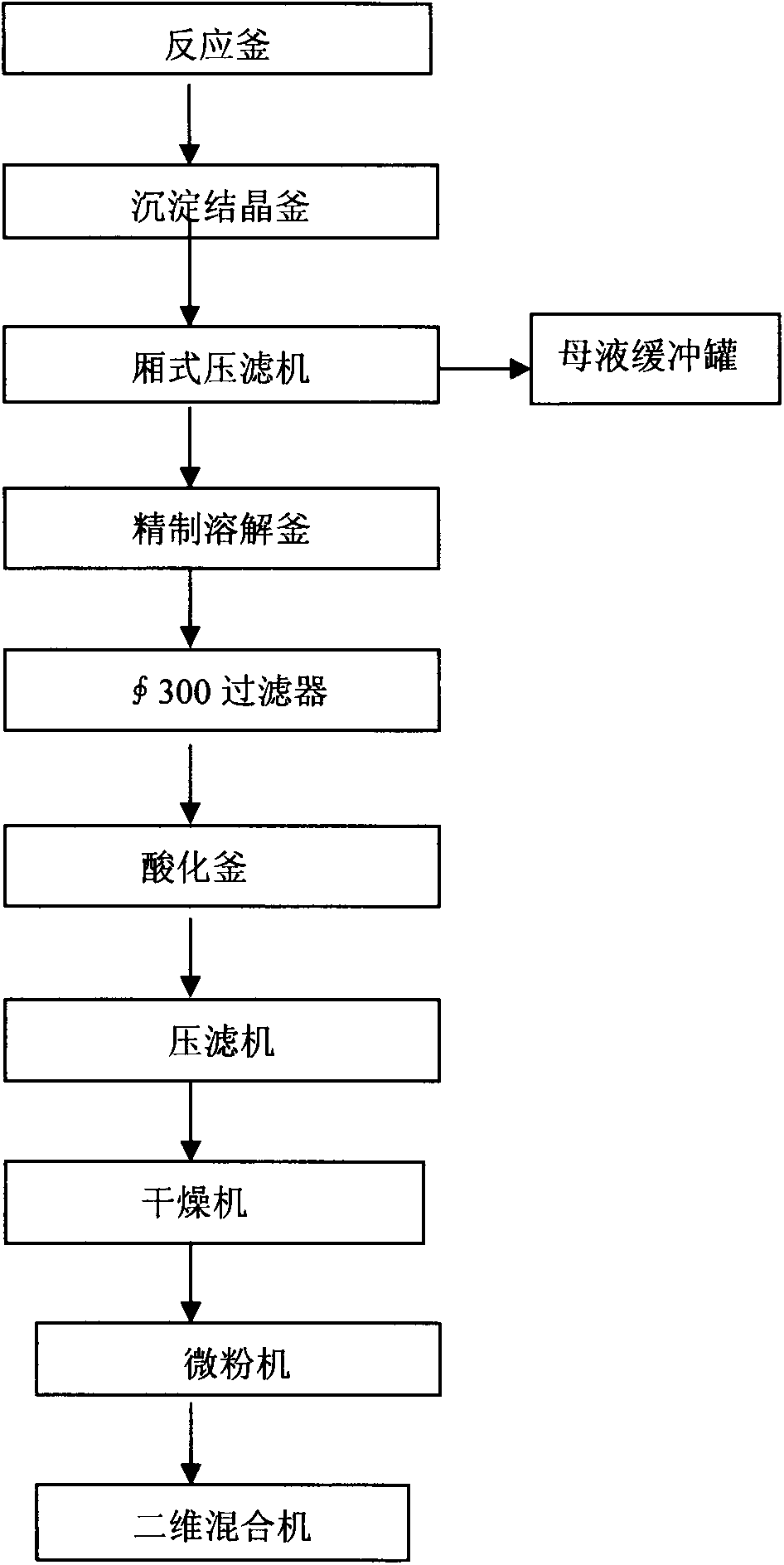
Novel production technology of diosmin
The invention discloses a novel production technology of diosmin, which comprises the following steps that a dehydrogenation reaction is performed; a crude diosmin product is prepared, dried, and subjected to iodine residual removal; a solvent residual of diosmin is removed; a fine diosmin product is dried, and subjected to a diosmin micronization technology; and micronized diosmin meeting a requirement of EP8.0 (European Pharmacopoeia 8.0) is obtained. The novel production technology has the characteristic of sociometric sustainable development; no harmful effect is exerted on an environment in a production process; more importantly, the consumption of a dehydrogenation agent is reduced to be 2-5% of that of hesperidin in the production process, so that a raw material resource is used fully to the greatest extent; the solvent and iodine residuals are removed effectively; and development requirements of a resource-saving and environment-friendly society are met.
Owner:陕西惠丰制药有限公司
Compositions for use in the treatment of tumors resistant to chemotherapy
The use of at least one flavonoid of natural or synthetic origin in association with cyclophosphamide and / or methotrexate to increase the effectiveness of chemotherapeutic treatments used in human and veterinary medicine for the treatment of tumors is described, in particular in case of resistance to the chemotherapeutic agents currently in use. At least one flavonoid herein described is selected from the group comprising or, alternatively, consisting of rutin, oxerutin, diosmin, troxerutin and hesperidin.
Owner:PROBIOTICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com