Continuous flow microchannel synthesis process of flavonoid compounds
A flavonoid compound and flow synthesis technology, applied in chemical/physical processes, chemical/physical/physical chemical processes, chemical/physical/physical chemical reactors, etc., can solve the problem of unfriendly environment, hazards to operators, and long preparation time and other problems, to achieve the effect of improving the conversion rate, broad application prospects, and shortening the preparation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]Embodiment 1, the continuous flow microchannel synthesis technique of diosmin
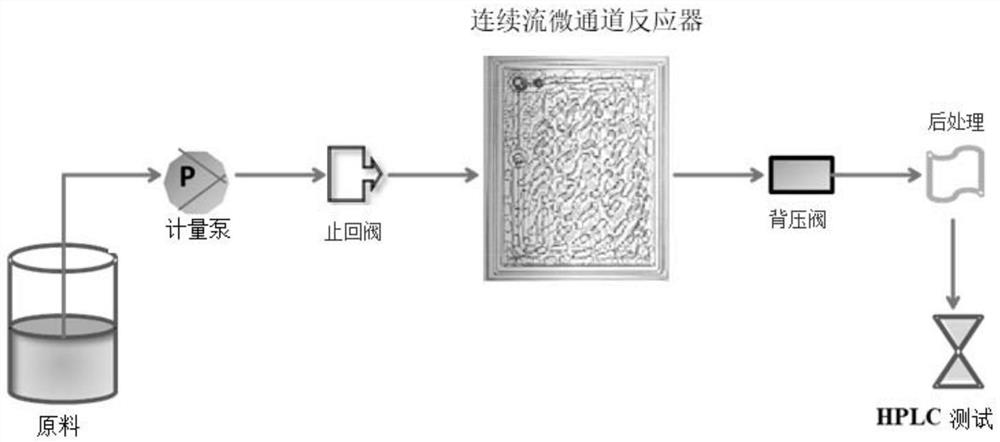
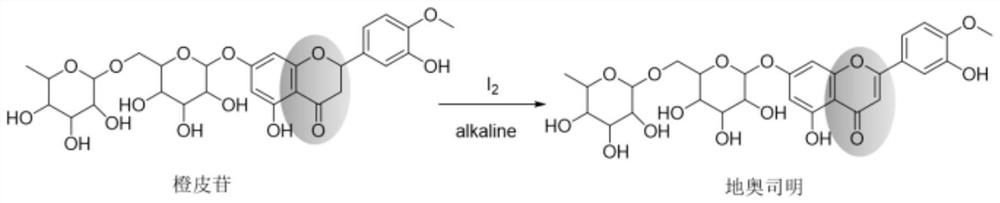
[0044] Using a continuous flow microchannel reactor, according to figure 1 The process flow shown prepares diosmin, and the reaction scheme is as follows figure 2 shown. The specific operation is as follows:
[0045] Step 1: Reaction liquid pretreatment: Mix hesperidin, iodine simple substance, and solvent pyridine evenly, and stir at 10°C under normal pressure for 30 minutes to obtain a reaction liquid; wherein, the raw material concentration (hesperidin / pyridine, mass-volume ratio) is 50 mg / ml, the raw material molar ratio (iodine / hesperidin) is 1.5:1.
[0046] Step 2: Feed the reaction solution obtained after stirring in step 1 to the continuous flow microreactor for reaction. The reaction conditions of the continuous flow microchannel are: volume flow rate of reaction solution 1.0mL / min, pressure 10Bar, reaction temperature 170°C, reaction time 2.7 min.
[0047] Step 3: post-proces...
Embodiment 2
[0051] Embodiment 2, the continuous flow microchannel synthetic technique of flavonoid compound
[0052] Using a continuous flow microchannel reactor, according to figure 1 The process flow shown prepares diosmin, and the reaction scheme is as follows:
[0053]
[0054] The structure of the compound shown in Formula A is as shown in Table 1, m, n, R in the compound shown in Formula B 1 , R 2 , R 3 With m, n, R in the compound shown in formula A 1 , R 2 , R 3 same.
[0055] The specific operation is as follows:
[0056] Step 1: Reaction solution pretreatment: Mix the compound shown in formula B, iodine element, and solvent pyridine evenly, and stir at 10°C under normal pressure for 30 minutes to obtain the reaction solution; wherein, the raw material concentration (hesperidin / pyridine, mass volume ratio) The molar ratio of raw materials (iodine / hesperidin) is 1.5:1.
[0057] Step 2: Feed the reaction solution obtained after stirring in step 1 to the continuous flow ...
experiment example 1
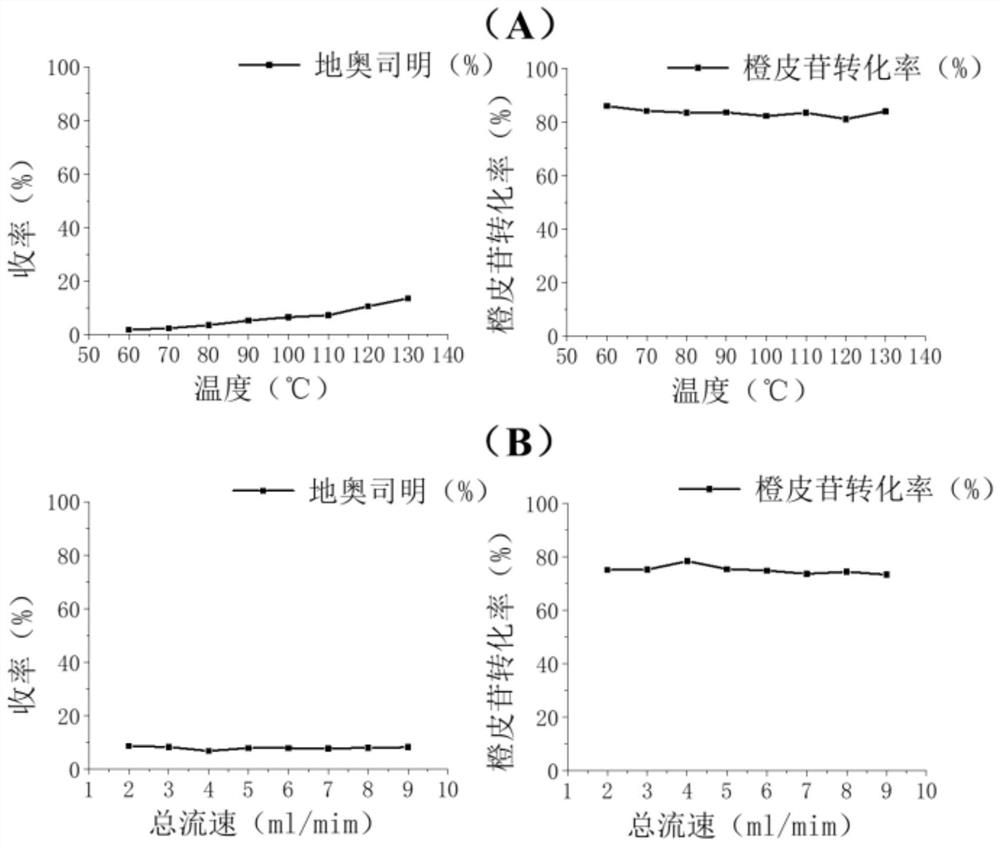
[0064] Experimental example 1. Screening experiment of continuous flow microchannel synthesis process under different conditions under normal pressure
[0065] 1. Experimental method
[0066] (1) Screening experiments at different reaction temperatures:
[0067] Mix hesperidin, elemental iodine, and solvent pyridine evenly to obtain a reaction solution; wherein the concentration of raw materials (hesperidin / pyridine, mass-to-volume ratio) is 50 mg / ml, and the molar ratio of raw materials (elemental iodine / hesperidin) is 1.1: 1;
[0068] The reaction solution was fed to the continuous flow microreactor for reaction, and the continuous flow microchannel reaction conditions were (see Table 2): reaction solution volume flow rate 1.2mL / min, normal pressure, reaction temperature 60-130°C, reaction time 135s.
[0069] Then follow the same method as Step 3 of Example 1 for post-processing to obtain the target product diosmin. The yield of diosmin under each condition is shown in Ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com