Diosmin-containing medicinal composition
A technology of diosmin and its composition, which is applied in the field of pharmaceutical compositions containing diosmin, can solve problems such as blood in the stool and pain symptoms without obvious effect, achieve significant therapeutic effect, good stability, and improve curative effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of tablets
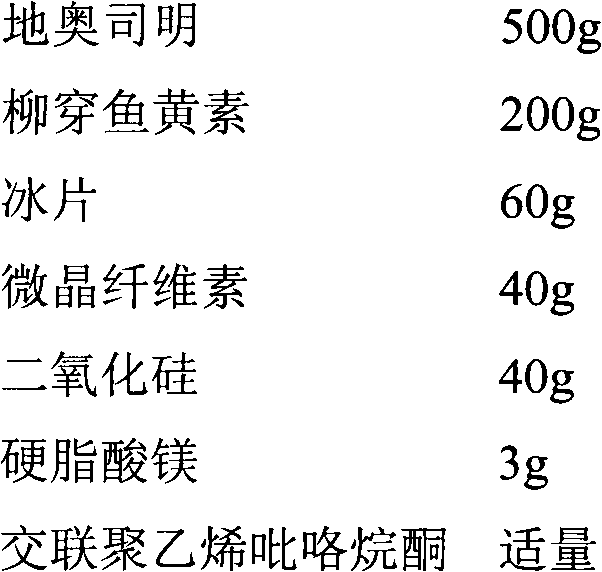
[0021] 1. Prescription (1000 tablets)
[0022]
[0023] 2. Experimental steps:
[0024] (1) Pulverization: Diosmin, ryuflavin, and borneol were pulverized to obtain particle sizes of 0.5 μm, 0.5 μm, and 1 μm, respectively.
[0025] (2) Granulation: Weigh 500g of crushed diosmin, 200g of salixin, 40g of microcrystalline cellulose, 40g of silicon dioxide, use cross-linked polyvinylpyrrolidone as a binder, and wet granulate. A 24-mesh sieve, dried at 65°C, granulated, added 60 g of borneol and 3 g of magnesium stearate, and mixed well.
[0026] (3) Tableting: get the tablet core.
[0027] (4) Coating: Film-coating the tablet core to obtain film-coated tablets.
Embodiment 2
[0028] Example 2 Preparation of tablets
[0029] 1. Prescription (1000 tablets)
[0030]
[0031] 2. Experimental steps:
[0032] (1) Pulverization: Diosmin, ryuflavin, and borneol were pulverized to obtain particle sizes of 10 μm, 10 μm, and 20 μm, respectively.
[0033] (2) Granulation: Weigh 100g of pulverized diosmin, 100g of salixin, 15g of microcrystalline cellulose, 15g of silicon dioxide, 0.5% sodium carboxymethyl starch solution as a binder, and wet process The granules are passed through a 24-mesh sieve, dried at 60°C, and granulated, 50 g of borneol and 1 g of magnesium stearate are added, and mixed evenly.
[0034] (3) Tableting: get the tablet core.
[0035] (4) Coating: Film-coating the tablet core to obtain film-coated tablets.
Embodiment 3
[0036] Example 3 Preparation of capsules
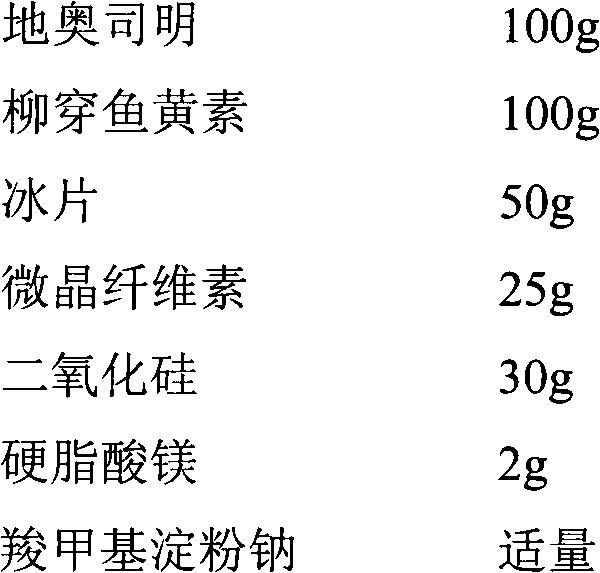
[0037] 1. Prescription (1000 capsules)
[0038]
[0039] 2. Experimental steps:
[0040] (1) Pulverization: Diosmin, ryuflavin, and borneol were pulverized to obtain particle sizes of 0.5 μm, 0.5 μm, and 1 μm, respectively.
[0041] (2) Granulation: Weigh 100g of pulverized diosmin, 100g of salixin, 25g of microcrystalline cellulose, 30g of silicon dioxide, and use 1% sodium carboxymethyl starch solution as binder, and prepare by wet method The granules are passed through a 24-mesh sieve, dried at 70°C, and granulated, 50 g of borneol and 2 g of magnesium stearate are added, and mixed evenly.
[0042] (3) Filling: get capsules.
[0043] (4) Packing, ready to go.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com