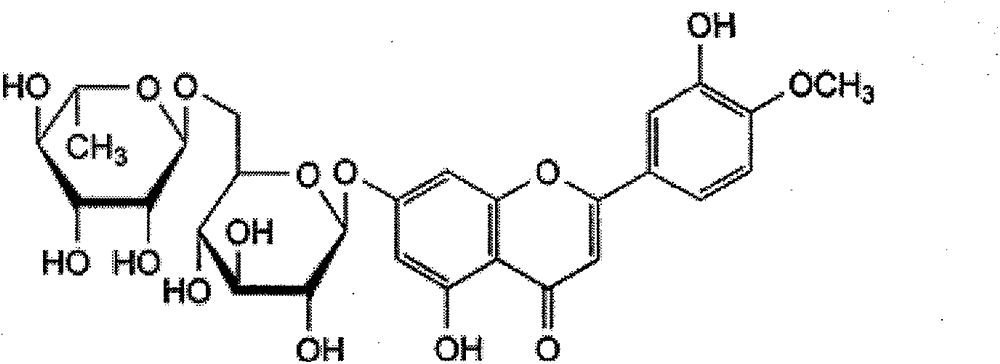
Preparation method of pure micronized diosmin
A technology of micronization of diosmin, applied in chemical instruments and methods, preparation of sugar derivatives, organic chemistry, etc., can solve problems such as unknown content, many reagents, unsuitable for industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] Method 1: Install a mechanical stirrer, a condenser, and a thermometer in a 250mL three-necked bottle, then add 50g of hesperidin and 250mL of N,N-dimethylformamide, heat up and stir until the hesperidin is completely dissolved. Add 50 mL of petroleum ether and 25 g of dibutyltin oxide, raise the temperature to 90°C and reflux for 2-5 hours, cool down to below 10°C, and add dropwise 30 mL of N,N-dimethylformamide containing 10 mL of acetic anhydride. After the dropwise addition, keep the reaction at 10°C for 1-3h, then raise the temperature to 20-30°C for 1-2h. After the reaction, add a small amount of water and mix well, extract three times with 100, 80, and 60mL petroleum ether, and keep the lower layer liquid as the reaction product. The petroleum ether liquid is distilled under reduced pressure, and the distillate is petroleum ether. The concentrated liquid is added dropwise 95mL of 20% NaOH solution, a white precipitate of dibutyltin oxide was formed, filtered, was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com