Synthesis method of diosmin raw medicine meeting EP7 version quality standards
A technology of diosmin and its synthesis method, which is applied in the field of synthesis of diosmin raw materials that meet the quality standards of EP7 edition, and can solve the problems of difficult recovery of high-boiling point organic solvents, high solvent residues, and difficult solutions of solvent residues
Inactive Publication Date: 2012-09-05
长沙富能生物技术有限公司
View PDF6 Cites 14 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
X is to use high-boiling point DMF as the reaction solvent, the reaction temperature is above 120 ℃, and the reaction time is 8-12 hours.
High reaction temperature, long reaction time, high energy consumption and high cost
Literature "Luo Meng. Synthesis and research of diosmin [J] Chemical Intermediates, 2004, 6: 29-30. 》reported that the preparation of diomin is mostly prepared by one-step iodine dehydrogenation of hesperidin. This method uses pyridine as a solvent, and if it is scaled up to industrialization, it will seriously pollute the environment
The Journal of Fourth Military Medical University (J Fourth Mil Med—Univ) 2008, 29— (21) reported "The Improvement of Diosmin Synthetic Process Exactly Feng Xuan, Zhu Xingmei, Li Xiaoye" utilizes the physical characteristics of Diosmin soluble in dilute alkali, Alkaline-added ethanol solvent is used as the reaction solvent, which solves the problem of solvent residue, but the reaction temperature is above 150°C, and it is difficult to control the conventional heating method in production, requiring high temperature and high pressure equipment
In conclusion, according to market research and analysis, China's diosmin industry currently has high energy consumption, serious environmental pollution, low yield, difficult solvent residues, and weak technological innovation capabilities.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
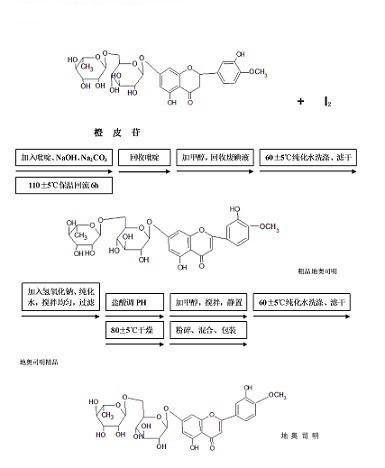
The invention discloses a synthesis method of diosmin. A microwave-assisted heating method is adopted, I2 and NaI are used as catalysts, the ethanol solution of K2CO3 and NaOH and a pyridine mixed solvent are used as reaction solvents for establishing a reaction system, hesperidin is dehydrogenized to prepare diosmin in one step, and the dehydrogenation process avoids high temperature and avoids use of a large amount of high-boiling-point toxic solvents such as pyridine, dimethyl sulfoxide and the like in the reaction solvent system. Through the invention, the total synthesis yield of the diosmin is more than 80%, and the product quality meets the EP7.0 standards. The method disclosed by the invention has the technical characteristics of easiness in acquisition of raw materials, mild reaction conditions, short reaction time, simplicity and convenience in operation, low production cost, good product quality and the like, and is suitable for industrialized production.
Description
[0001] technical field [0001] Biomedicine, chemical synthesis. Background technique Diosmin: alias 7-rutinoside, diosmin, English name Diosmin, chemical name 3',5,7-trihydroxy-4'-methoxyflavone, molecular formula C 28 h 32 o 15 , molecular weight 608.54, CAS number: 520-27-4, EINECS number: 208-289-7. Off-white or light yellow microcrystalline powder, almost insoluble in water, soluble in dimethyl sulfoxide, almost insoluble in ethanol, soluble in dilute alkaline solution. 1. It has a vitamin P-like effect, can reduce vascular fragility and abnormal permeability, and is also used as an adjuvant treatment for the prevention and treatment of hypertension and arteriosclerosis. The effect of treating capillary fragility is stronger than that of rutin and hesperidin, and has Features of low toxicity. 2. After orthopedic surgery, the change of lower extremity pain was basically the same as the change of swelling subsided. Compared with the control group, the micronized...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07H17/07C07H1/00
Inventor 阳水刘毛东雷国平朱贞钰
Owner 长沙富能生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com