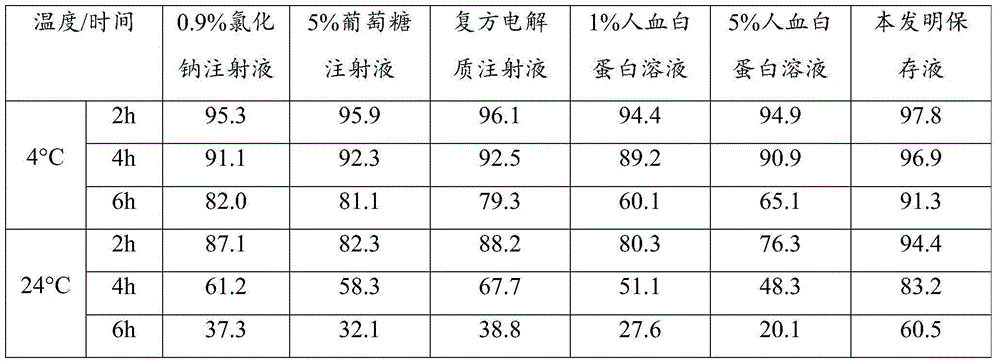
Patents
Literature
267 results about "L-Glutamin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
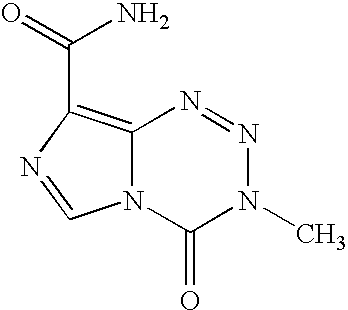
L-glutamine, or just glutamine, is an amino acid. Amino acids are nutrients that help synthesize protein in the human body for nutrition. They can be found in protein-rich foods, including those from both plants and animals.
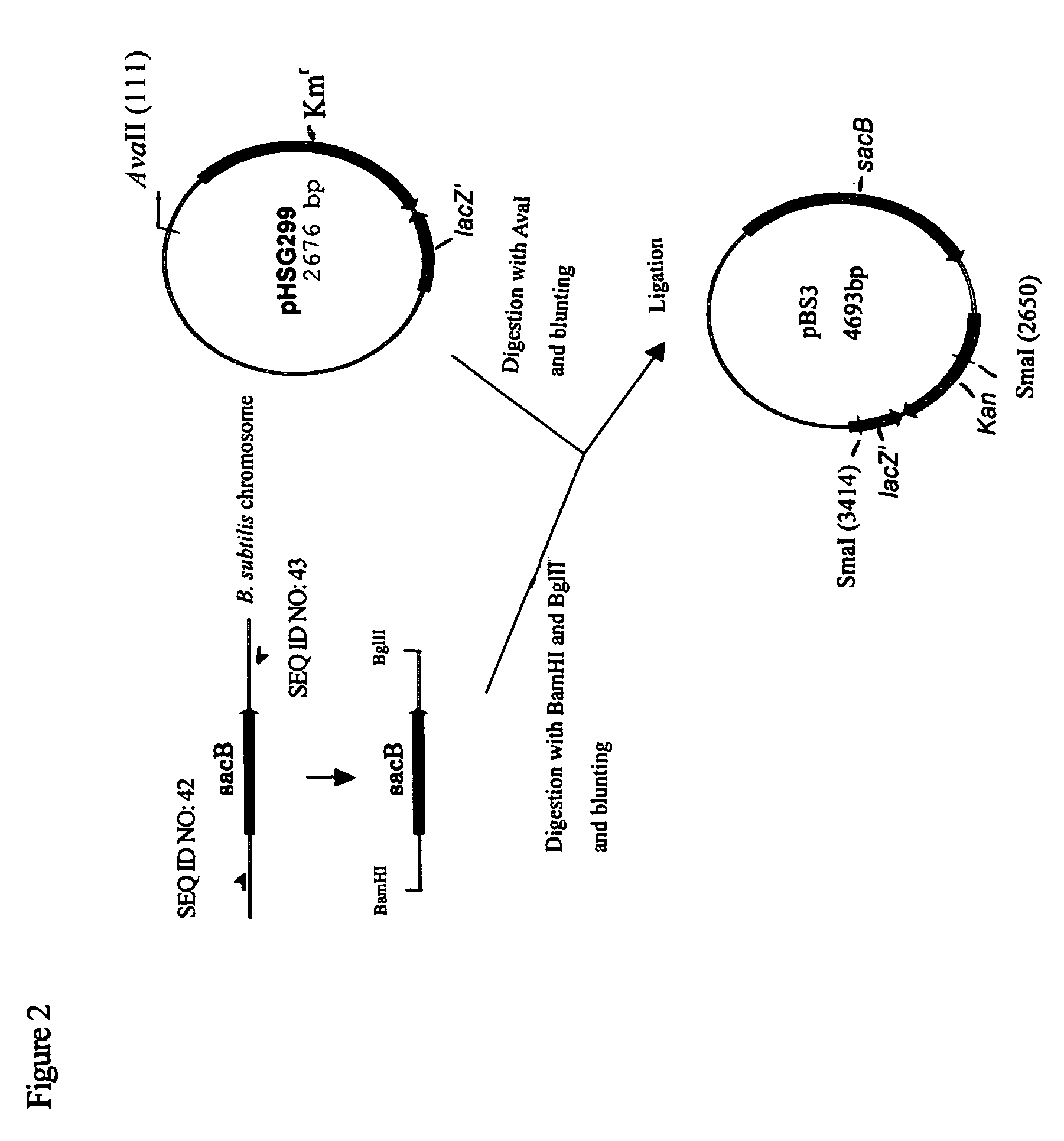
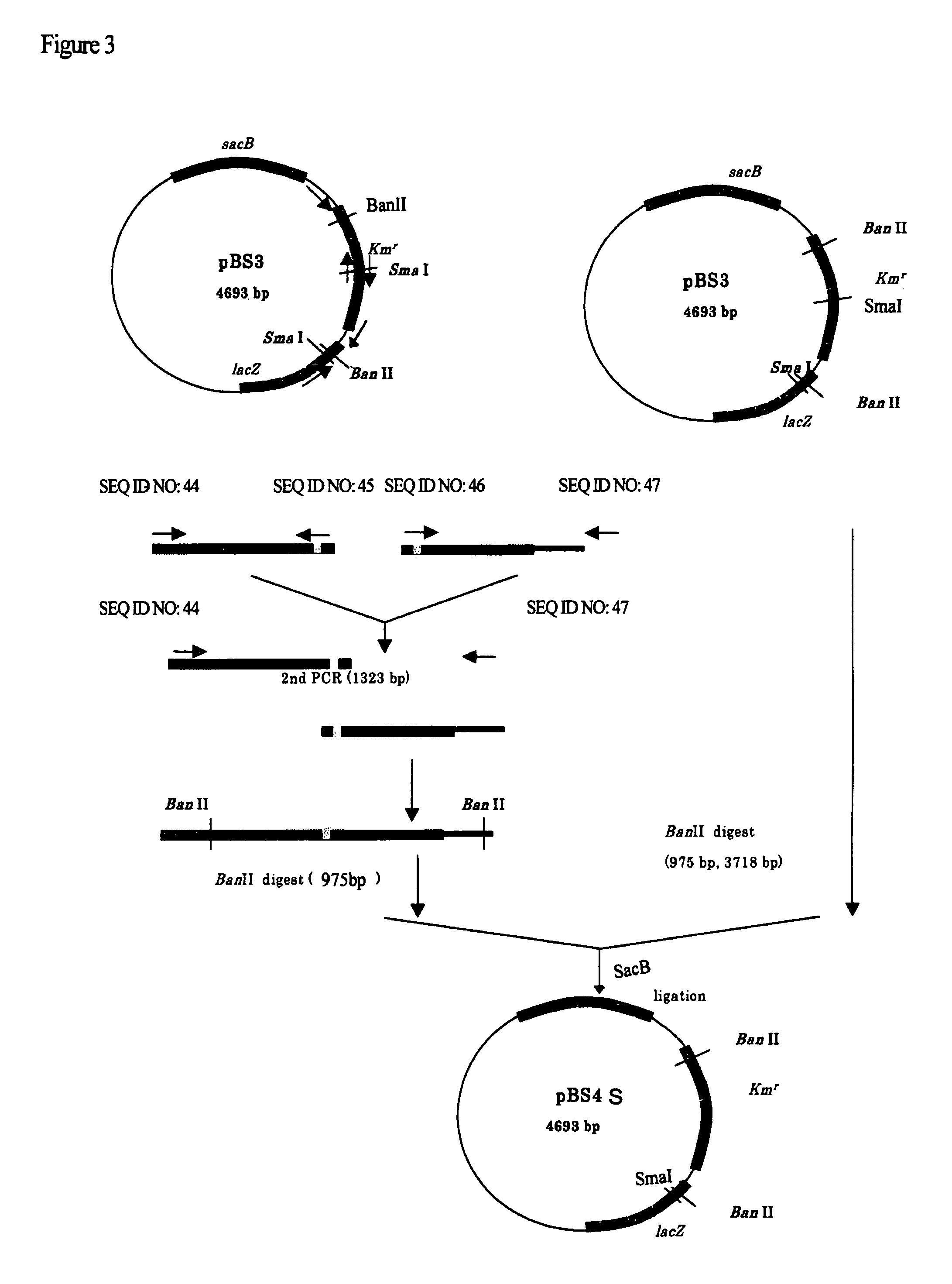
Use of phosphoketolase for producing useful metabolites
Owner:AJINOMOTO CO INC
Enzyme composition for improving food digestion
InactiveUS20080199448A1Improving food absorptionPromote digestionOrganic active ingredientsPeptide/protein ingredientsProteinase activityAdditive ingredient
An orally administered composition for improving food absorption and digestion contains therapeutically effective dosages of digestive enzymes and L-glutamine as active ingredients. The digestive enzymes include at least one each of a lipase, a protease, and an amylase, and at least a portion of each of these enzymes is enteric coated.
Owner:NUTRI-HEALTH SUPPLEMENTS LLC
Composition for an in vitro fertilization medium
InactiveUS6130086AImprove stabilityIncrease stimulationCulture processMedical devicesArginineTryptophan
PCT No. PCT / JP96 / 02503 Sec. 371 Date Mar. 2, 1998 Sec. 102(e) Date Mar. 2, 1998 PCT Filed Sep. 4, 1996 PCT Pub. No. WO97 / 08946 PCT Pub. Date Mar. 13, 1997The present invention aims to provide a medium composition for in vitro fertilization, in particular, a composition usable in the culture of ova or early embryos which are fertilized eggs, the preparation or culture of sperm, and the pre-treatment of ova or sperm. The composition comprises, as its essential components, L-phenylalanine, L-tryptophan, L-lysine, L-threonine, L-valine, L-methionine, L-isoleucine, L-leucine, L-proline, glycine, L-alanine, L-tyrosine, L-histidine, L-arginine, L-taurine, L-aspartic acid, L-serine, L-asparagine, L-glutamic acid, L-glutamine and L-cystine, provided that at least a part of the L-cystine may be replaced by L-cysteine.
Owner:FUSO PHARMA INDS
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Serum-free medium for culturing placenta mesenchymal stem cells
ActiveCN103805562AIncrease growth rateMaintain stem cell propertiesSkeletal/connective tissue cellsFibroblast growth factor receptor 2Cell culture media
The invention discloses a serum-free medium for culturing placenta mesenchymal stem cells. The serum-free medium takes a DMEM (Dulbecco Modified Eagle Medium) culture solution as a basis and also contains a fibroblast growth factor receptor 2, growth hormone, insulin, transferrin, glutathione, BMP-4, L-glutamine, sodium pyruvate, non-essential amino acids and beta-mercaptoethanol. According to various serum-free media provided by the invention, growth and proliferation of the placenta mesenchymal stem cells in a serum-free medium system can be effectively promoted, the placenta mesenchymal stem cells have higher growth and proliferation rate in the serum-free medium system compared with a serum cell culture medium, the characteristics of the stem cells are preserved, the serum-free medium has multiple differential potentials, and the stem cells can be directionally induced into fat cells and osteoblasts.
Owner:章毅 +10
Pharmaceutical formulations of antineoplastic agents and processes of making and using the same
In its several embodiments, this invention discloses a pharmaceutical formulation comprising at least one antineoplastic agent or a pharmaceutically acceptable salt thereof, and at least one dissolution enhancing agent sufficient to substantially dissolve said at least one antineoplastic agent in at least one aqueous diluent, wherein said dissolution enhancing agent is urea, L-histidine, L-threonine, L-asparagine, L-serine, L-glutamine or mixtures thereof; a lyophilized powder comprising said pharmaceutical formulation, and articles of manufacture thereof.
Owner:MERCK SHARP & DOHME LLC
Dietary Supplement Cognitive Support System
The present invention relates to a nutritional supplement composition, comprising a therapeutically effective amounts of Vitamin C, Vitamin D3, Thiamin, Riboflavin, Niacin, Vitamin B6, Folic acid, Vitamin B12, Pantothenic acid, Calcium, Magnesium, Zinc, Chromium, Sugar, Protein, Acetyl-L-Carnitine, Dimethylaminoethanol complex, Phosphatidylserine complex, L-Glutamine, N-Acetyl-L-Tyrosine, L-Phenylalanine, Taurine, Methionine, Valine, Isoleucine, 5 Hydroxytryptophan, L-Taurine, N-Acetyl-Tyrosine, N-Acetyl-L-Cysteine, Alpha Lipoic Acid, Alpha Glycerylphosphoricholine complex, Bacopa Monnieri extract, Gingko Biloba extract, Passion flower, Lemon Balm, Gotu Kola, Ashwagandha, Choline Bitartrate complex, Panax Ginseng extract, Turmeric, Organic freeze dried fruit juice blends (concord grape, red raspberry, pineapple, cranberry, acai, pomegranate, acerola cherry, bilberry, lingonberry, black currant, aronia, sour cherry, black raspberry), Organic freeze dried greens blends (barley grass, broccoli, beet, carrot, alfalfa, oat), and Protein digestive enzyme blends (Protease 4.5, peptidase, bromelain, protease 6.0, protease 3.0, L planatrum, B bifidum) in a mixture to provide optimal cognitive function.
Owner:FANTZ DAVID R
Cryopreservation solution of tissue engineering products and application method thereof
InactiveCN101720753AResuscitation is easy to useLow toxicityDead animal preservationWater bathsSucrose
A cryopreservation solution of tissue engineering products uses DMEM culture solution as a basic solvent which is added with vitamin B, vitamin C, chondroitin sulfate, beta-integrin, cromolyn sodium, cytochalasin B, L-glutamine, bovine serum albumin, fetal bovine serum, trehalose, sodium carbonate, polysucrose-70, and dimethyl sulfoxide added during freezing storage. The cryopreservation solution provided by the invention has little toxicity to cells and long storage time, and can be stored for six months under 80 DEG C below zero, and 12 to 18 months in liquid nitrogen; and the cell viability of the resuscitated cells can be over 60%. The stored tissues can be simply and conveniently used after being resuscitated for only three to five min in water bath under the temperature of 37 DEG C and being washed by sterilized saline water. The invention can be widely used, and is suitable for tissue engineering skin, tissue engineering cornea, tissue engineering blood vessel, tissue engineering nerve and the like, and also is applicable to the cryopreservation of normal tissues.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY +1
Food product and process for manufacturing same
This invention is concerned with packaged food products which contain specific combinations of functional additives aimed at addressing specific health indicators, in particular flatulence, gastro-intestinal health, stress and immune system responsiveness, in pet animals. There is provided a commercially packaged mammal pet food product that includes a manufactured, shelf-life stable food substrate and a combination of functional additives. The functional additives include at least one non-palatable plant-based remedy and / or dietary fiber source that are present to strengthen and / or maintain a specified health indicator of a mammal pet animal. The food product is portioned and packaged with the functional additives being present in predetermined concentrations and amounts sufficient to be effective in achieving said indications on regular feeding of the pet animal with said food product. The food substrate is present in a proportion sufficient to mask the flavor and / or odor of the non-palatable additive and is made-up of a unique combination of materials that are able to be processed at lower temperatures to preserve the natural botanical functional additive's activity. Functional additives intended to address dietary flatulence problems include a combination of Yucca extract, charcoal and salts of zinc, such as zinc acetate. Functional additives to promote or maintain gastro-intestinal health include a combination of L-glutamine, D-glucosamine sulphate, sugar beet pulp, slippery elm. Functional additives to strengthen or maintain a pet animal's natural body defenses include a combination of vitamin E, vitamin B complex, primrose oil vitamin C and Marigold meal. Functional additives to promote or maintain reduction of stress and / or improved behavior of a pet animal include a combination of Valerian root extract, Kava root extract, vitamin B complex and magnesium salt.
Owner:EFFEM FOODS
Complete medium and human amnion-derived mesenchymal stem cell culture method
The invention discloses a complete medium and a human amnion-derived mesenchymal stem cell (hAMSCs) culture method. The complete medium is prepared by adding 3 to 10 percent of autologous umbilical cord blood serum into low-sugar Dulbecco minimum essential medium solution according to a volume ratio. The culture method comprises: (1) separation; (2) primary culture; and (3) subculture. The methodusing the complete medium in the hAMSCs culture has the advantages that: the risk of using fetal calf serum is avoided; although the need of adding L-glutamine, non-essential amino acid, 2-mercapitoethanol, pyruvic acid and the like is obviated, the high proliferation properties and phenotypic characteristics of the hAMSCs and expression of multilineage differentiation marker genes sand proteins of some stem cells can still be retained; and in subculture, the wall adherence fastness of the hAMSCs is much lower than that in fetal bovine serum (FBS) culture, the digestion time is reduced obviously, and the damage of trypsinization to cells and loss of cells are reduced.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Composite functional amino acid feed additive and application
InactiveCN104351478AImprove conversion efficiencyImprove securityAnimal feeding stuffDiseaseArginine
The invention discloses a composite functional amino acid feed additive and an application. The composite functional amino acid feed additive is prepared from the raw materials of L-glutamic acid, L-glutamine, glycine, L-arginine and L-cysteine at a certain weight ratio; the preparation method comprises the following steps: step A, respectively weighing the L-glutamic acid, the L-glutamine, the glycine, the L-arginine and the L-cysteine; step B, sequentially feeding the weighed amino acids into a mixer and then uniformly mixing to obtain a composite amino acid feed additive. The invention also discloses the application of the composite amino acid feed additive to livestock and aquatic livestock feeds. The composite functional amino acid feed additive is reasonable in formula, convenient to use, high in safety, free of resistance to drugs and capable of improving animal intestinal health, promoting growth, improving feed conversion efficiency and reducing incidence of diseases, so the composite functional amino acid feed additive can be used for effectively replacing an antibacterial growth promoting agent.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Cell culture medium containing growth factors and L-glutamine
InactiveUS6838284B2Shorten the timeQuick distinctionMicroorganismsCulture processCulture cellCell culture media
The invention relates to a culture medium for culturing cells, in particular human cells in a process for tissue engineering bone. The medium comprises glucose, a mineral, a vitamin, a growth factor and L-glutamine, wherein the L-glutamine is present in a concentration of at least 300 mg / L.
Owner:OCTOPLUS SCI
Nutrient compositions and methods for sustenance and promotion of positive metabolic energy levels in a targeted manner
Nutrient compositions and methods that sustain and promote positive metabolic energy levels in a targeted manner are disclosed. Methods utilize endogenous energy stores (fat oxidation), increase use of those stores (increasing transport rate), increase available energy (increasing the ability to perform ADP to ATP phosphorylation,) as well as decrease catabolism and increase protein synthesis. Compositions are also disclosed, and include Mono- or Dicreatine-HMB salt; Putrescine Dihydrochloride; Alanine; L-Glutamine, which may be combined with Alanine in a 1:2 to 2:1 molecular ratio; Trimethylglycine; and Guanidinopropionic Acid.
Owner:SELLO AZUL
Oral amino acid composition
InactiveUS20050032898A1Reduce the amount requiredEfficient reductionOrganic active ingredientsBiocideGlutethimideArginine
Amino acid compositions which contain 10 to 40 parts by weight of L-arginine, 10 to 40 parts by weight of L-glutamine, 5 to 20 parts by weight of L-valine, 8 to 30 parts by weight of L-isoleucine, and 10 to 35 parts by weight of L-leucine may be taken orally and are effective for reducing body fat without dietary restrictions and exercise.
Owner:AJINOMOTO CO INC
L-Amino Acid-Producing Bacterium and a Method for Producing L-Amino Acid
ActiveUS20070254345A1Improved ability to produce L-amino acidsEfficient productionBacteriaFermentationArginineL-Glutamin
Coryneform bacteria are described that have an ability to produce L-amino acids and are modified so that acetyl-CoA hydrolase activity is decreased. The bacteria are used to produce L-amino acids generated by a biosynthetic pathway in which pyruvic acid is an intermediate, such as L-glutamic acid, L-arginine, L-glutamine, L-proline, L-alanine, L-valine, and L-lysine.
Owner:AJINOMOTO CO INC
Serum-free adipose tissue-derived mesenchymal stem cell culture medium
ActiveCN103255103AAvoid exogenous contaminationAvoid the influence of cultivationSkeletal/connective tissue cellsPenicillinCuticle
The invention relates to a serum-free adipose tissue-derived mesenchymal stem cell culture medium, which consists of a basic culture medium and added ingredients, wherein the basic culture medium is DMEM-LG, and the added ingredients and the content of each added ingredient are shown as follows: 5 to 20ng / mL of alkaline fiberblast growth factors, 5 to 20ng / mL of epidermal growth factors, 100U / mL of penicillin, 100 micrograms / mL of streptomycin, 50 to 200 micrograms / mL of heparin, 2 to 8mM of L-glutamine, 100 to 300 microM of 2-mercaptoethanol, 500 to 2000U / mL of leukaemia inhibitory factors and 0.5 to 2mM of sodium pyruvate. The serum-free adipose tissue-derived mesenchymal stem cell culture medium does not contain the serum, so that the inter-batch difference and the influence of the serum component on the cell culture can be avoided; the exogenous pollution of the serum and the toxicity of the serum on the cells can be avoided; and the ingredients are clear, so that the research of the psychological regulation mechanism of the cells can be facilitated.
Owner:冯文峰
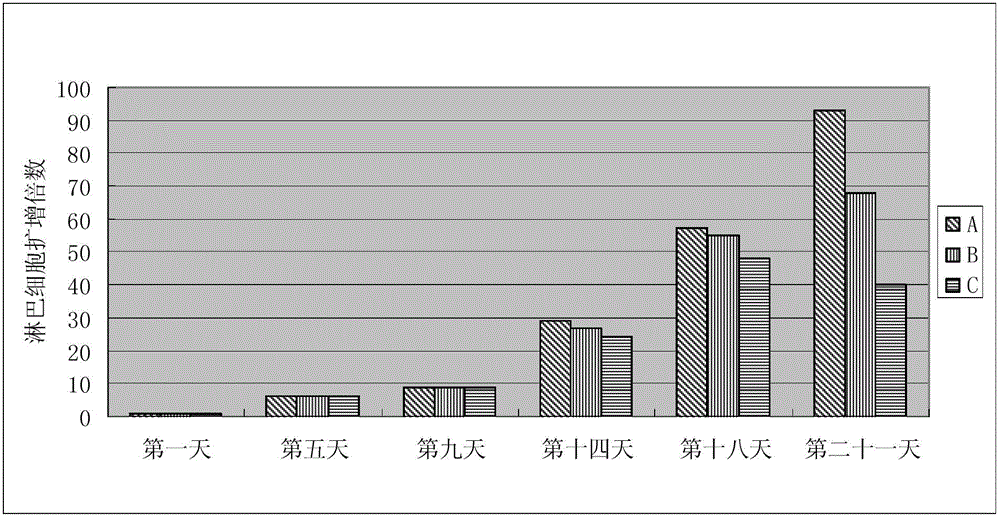
Animal source-free and serum-free culture medium of lymphocyte
InactiveCN103146648AGood amplification factorEnhance cell viabilityBlood/immune system cellsCell phenotypeSodium bicarbonate
The invention relates to the biological field and discloses an animal source-free and serum-free culture medium of lymphocyte. The culture medium disclosed by the invention essentially consists of IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, human transferrin, human serum albumin, 2-mercaptoethanol, N-acetyl-cysteine, lipid, amino acid, vitamin, microelement, ferric citrate, hydrocortisone, cholamine and non-essential amino acid. The serum-free culture medium disclosed by the invention has the advantages of clear chemical components, no animal source, no serum, safe and ideal culture cells; the instability caused by the doping of animal components and batches is avoided; the result of culturing lymphocyte shows that the total number of the cells and the cell phenotypes are normal; and the serum-free culture medium disclosed by the invention has a good industrial application prospect.
Owner:BEIJING JING MENG STEM CELL TECH
Artificial particle feed for juvenile turbot
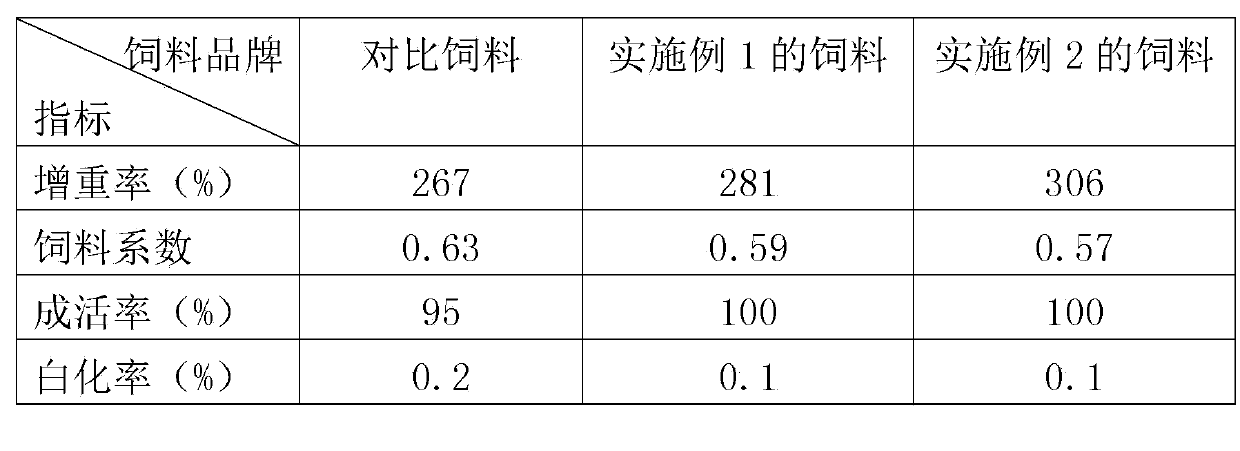
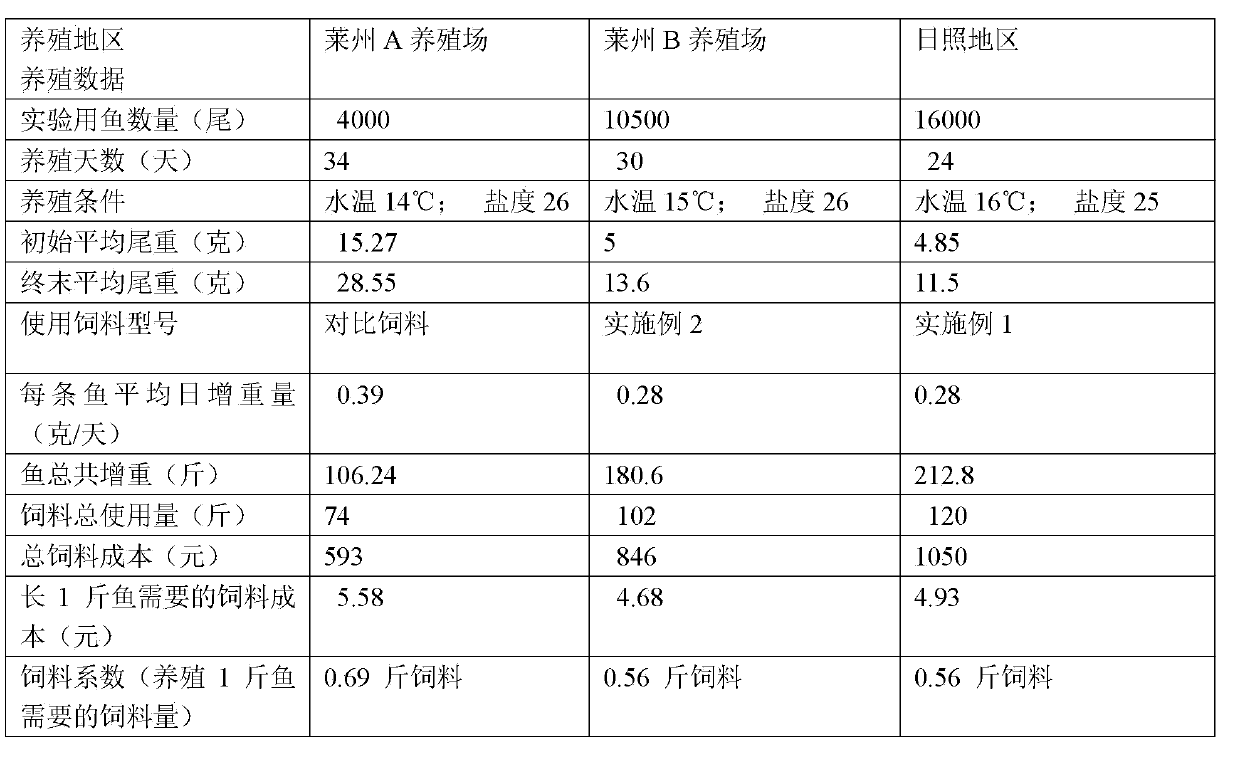
The invention relates to an artificial particle feed for juvenile turbot. The artificial particle feed is characterized by being prepared from whitefish powder, euphausiid powder, scallop powder, beer yeast, starch, cod liver oil, yolk powder, whey powder, L-alanyl-L-glutamine, choline chloride, soybean lecithin, schizochytrium limacinum powder, haematococcus pluvialis powder, compound vitamins, compound mineral substances, Chinese herbal medicines and sodium alginate. As the various raw materials are added into the feed, the comprehensiveness of nutrition is guaranteed. An immunopotentiator is added into the feed, so that the non-specificity immunity of fish bodies is enhanced. The feed is green and pollution-free. The schizochytrium limacinum powder and the haematococcus pluvialis powder are added into the feed, and the content of vitamin E is improved, so that the whitening rate of the juvenile fish is effectively reduced. The Chinese herbal medicine components are added, so that the immunity of the juvenile fish can be improved and parasite diseases in a juvenile fish growing phase are prevented from happening.
Owner:QINGDAO QIHAO NUTRITION TECH
Method for preparing lenalidomide
InactiveCN104311536ASignificant technological progressOptimize the process routeCarbamic acid derivatives preparationOrganic compound preparationDicarbonateL-Glutamin
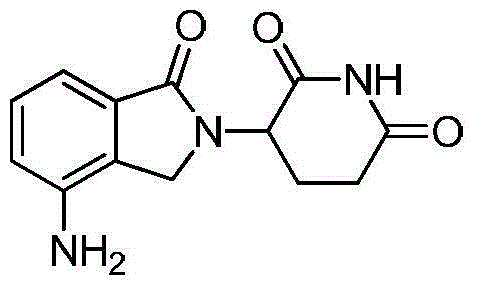
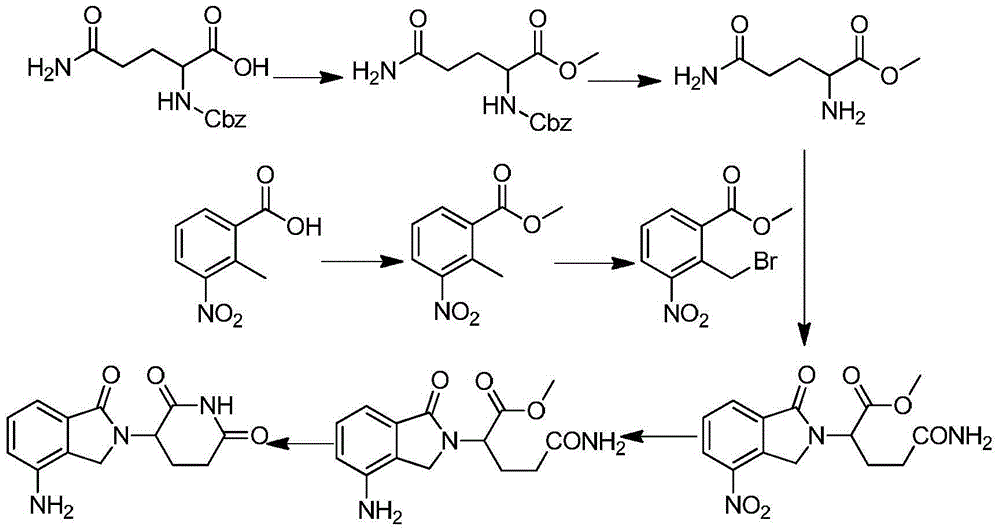
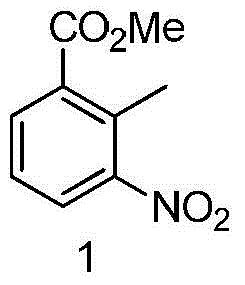
The invention discloses a method for preparing lenalidomide. The method comprises the following steps: firstly, etherifying 2-methyl-3-nitrobenzoic acid to obtain 2-methyl-3-nitrobenzoic acid methyl ester, brominating to obtain 2-brooethyl-3-nitrobenzoic acid methyl ester, reacting L-glutamine and tert-butyl dicarbonate to obtain N-Boc glutamic acid, acquiring 3-amino-2,6-piperidine diketone protected by Boc from N-Boc-glutamic acid in the presence of a condensing agent and a catalyst, further reacting with acid to prepare 3-amino-2,6-piperidine diketone hydrochloride, reacting 3-amino-2,6-piperidine diketone with 2-brooethyl-3-nitrobenzoic acid methyl ester so as to obtain 3-(4-nitryl-1,3 dihydro-1-oxo-2 hydrogen-isobenzazole-2-yl) piperidine-2,6-diketone, and finally reducing, thereby obtaining lenalidomide. The method disclosed by the invention is high in product yield.
Owner:SHANGHAI INST OF TECH
Low-fat low-osmosis low-residue type enteral nutritional powder for patients with inflammatory bowel diseases
ActiveCN105054025AImprove tolerancePromote recoveryVitamin food ingredientsInorganic compound food ingredientsWhey protein powderL-Glutamin
The invention provides a low-fat low-osmosis low-residue type enteral nutritional powder for patients with inflammatory bowel diseases. The powder is prepared from protein, fat, carbohydrate, vitamin complex, minerals and epigallocatechin gallate, and does not contain gluten, wherein the protein is supplied by whey protein powder and L-glutamine; the fat is supplied in a mode of long-chain fatty acid and medium-chain fatty acid, and a ratio of (omega-6) to medium-chain fatty acid to (omega-3) to (omega-9) is 40% to 40% to 10% to 10%; and the carbohydrate is maltodextrin. The enteral nutritional powder is suitable for people who are relatively poor in gastroenteric function and easily have diarrhea or people requiring low-fat low-residue diet, such as patients with inflammatory bowel diseases, pancreatitis patients who are in transition of initial oral diet, heart failure patients, patients who initially use enteral nutrition after fasting, patients with cholecystitis or gall-stone, patients subjected to cholecystectomy, obesity people, patients in preparation of bowel surgery, patients after gastrointestinal surgery and the like. The enteral nutritional powder can be used for supplying balanced nutrition support, is easy to brew and store, is convenient to control dosage and osmotic pressure, and can be used for improving the living quality of patients.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Therapeutic Delivery System Comprising a High Molecular Weight Peg-Like Compound
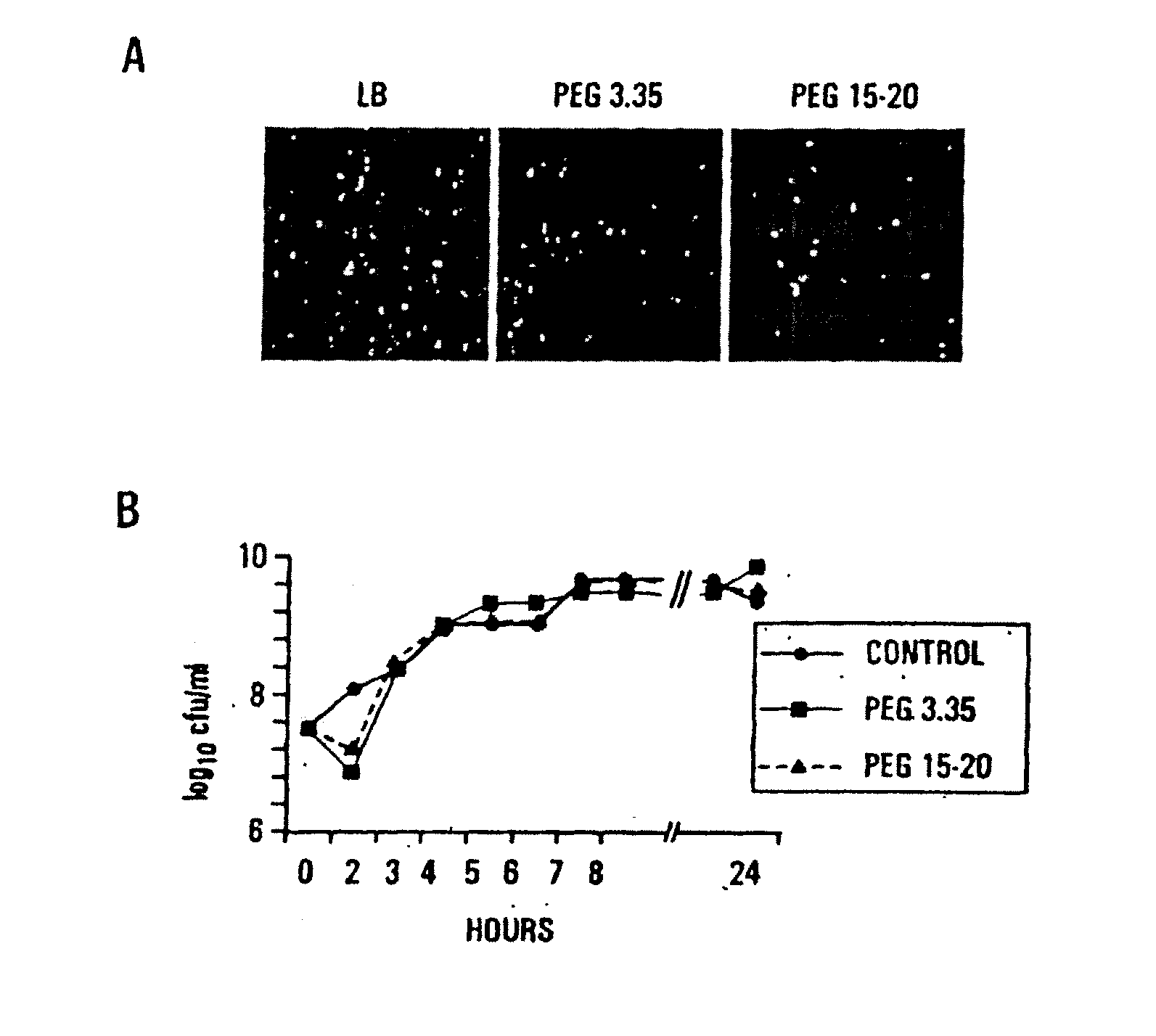
InactiveUS20080206188A1Suppresses virulence expressionEasy to operateAntibacterial agentsNervous disorderEpitheliumPolyethylene glycol
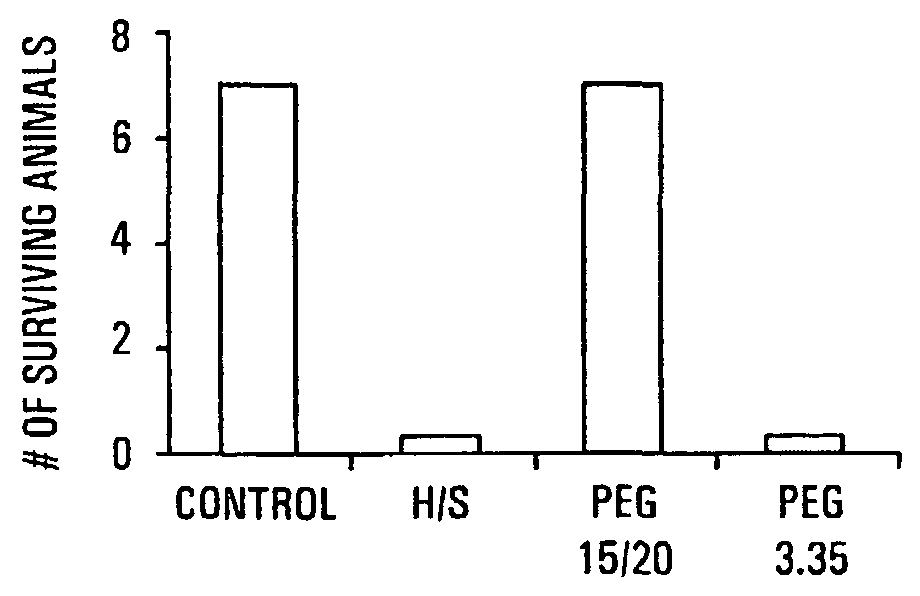
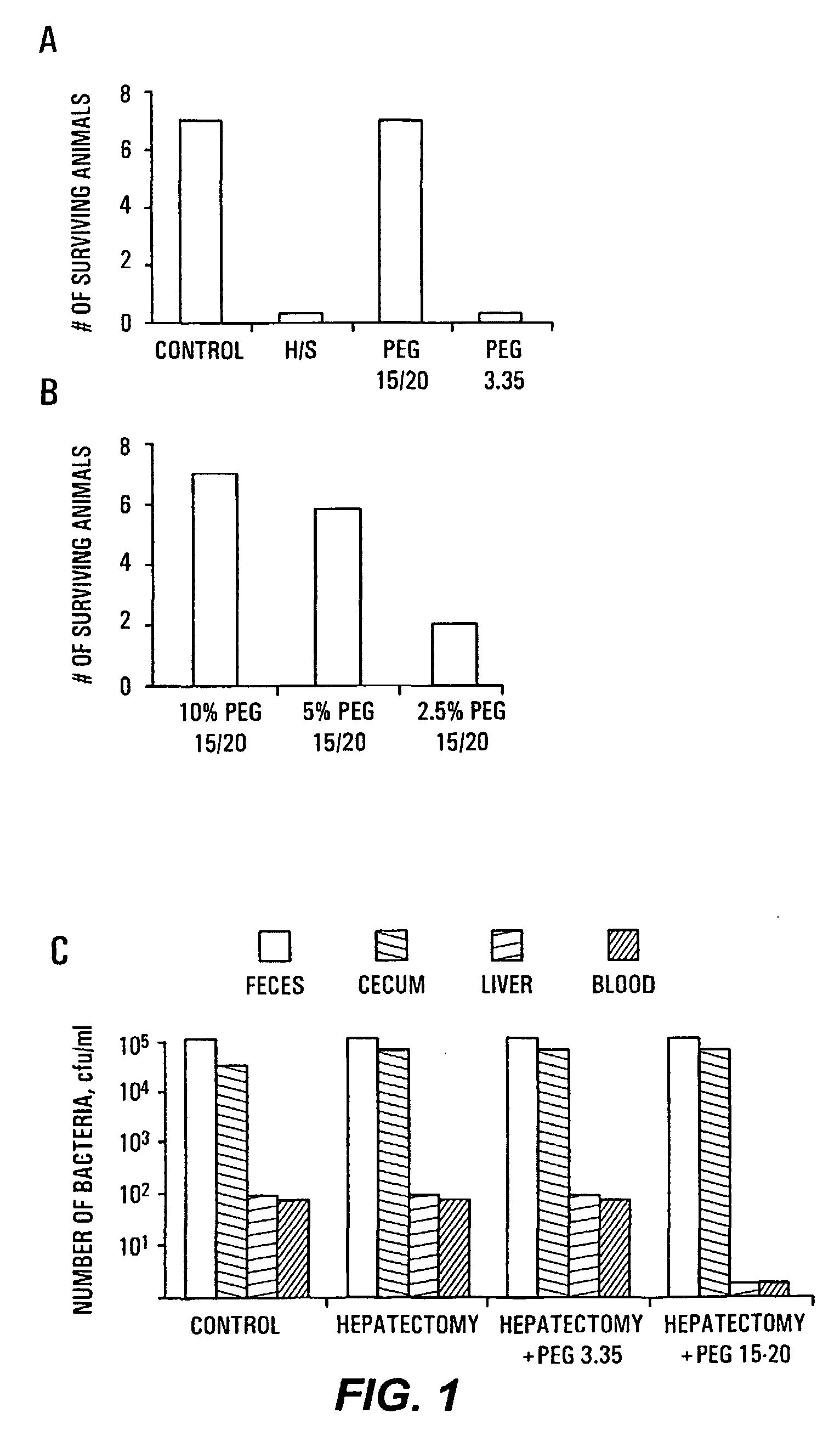
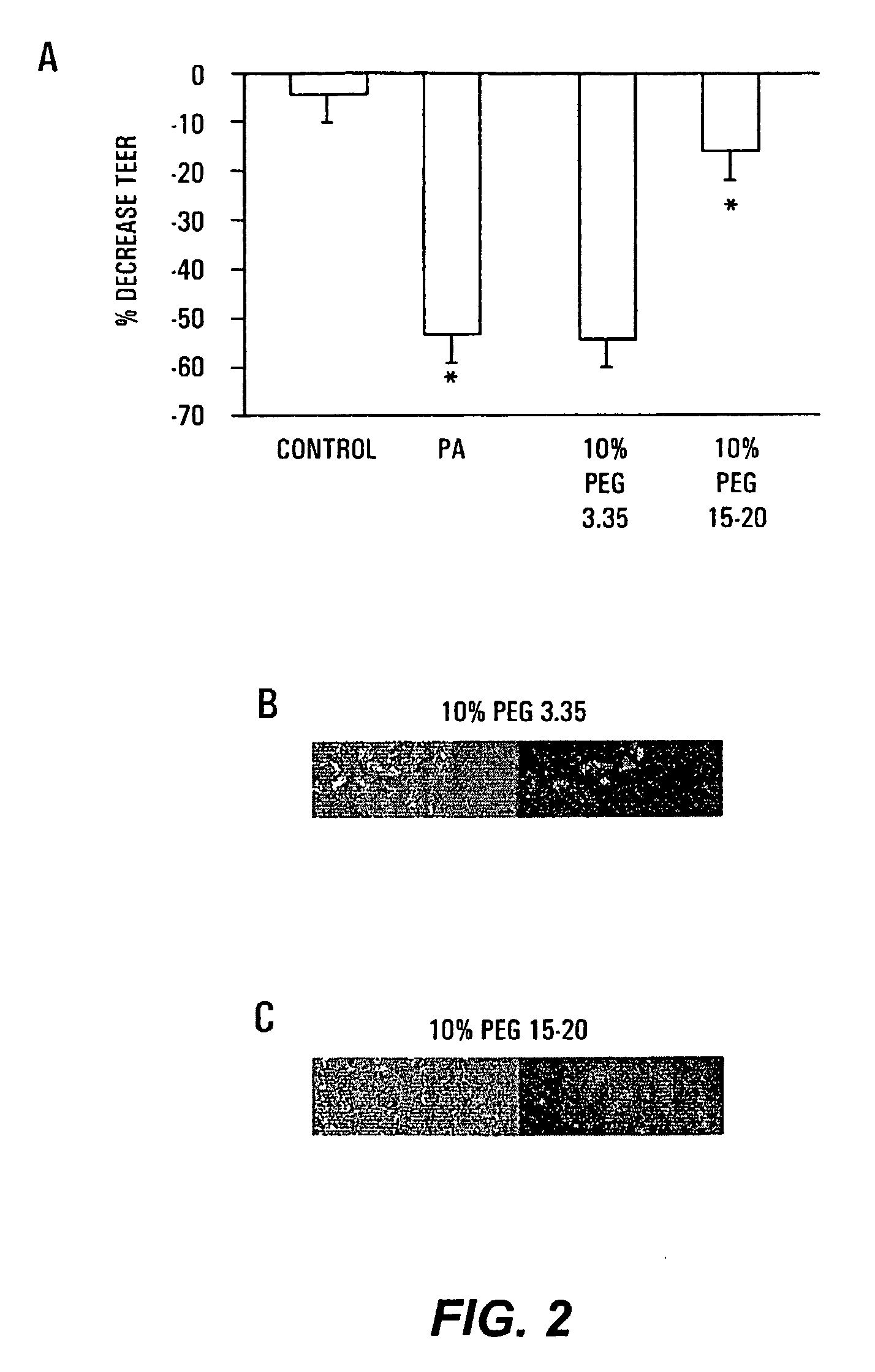
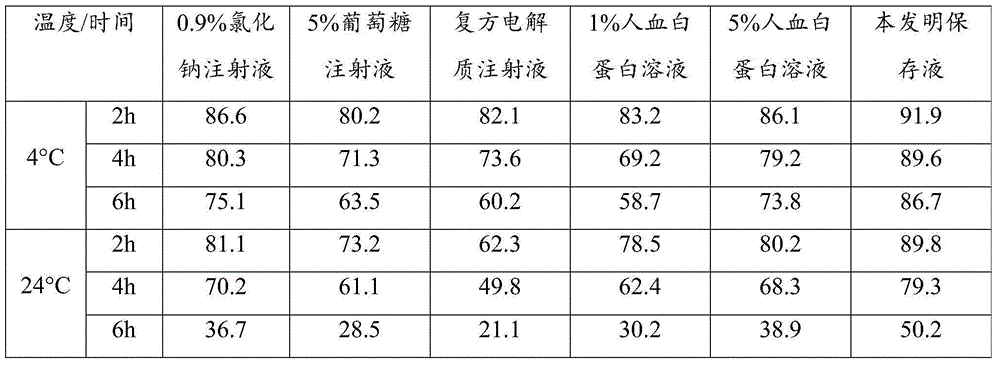
The present invention provides a system for delivering a wide range of chemical and biological therapeutics, including protein therapeutics, via transepithelial routes. The system comprises a high molecular weight polyethylene glycol-like (HMW PEG-like) compound for use with a therapeutic compound. Optionally, the system comprises a composition containing one or more HMW PEGlike compounds and one or more therapeutics, supplemented with a protective polymer such as dextran and / or essential pathogen nutrients such as L-glutamine. Administered alone, the HMW PEG-like compounds also provide therapeutic benefits. Also provided are methods for preventing or treating epithelial diseases, disorders, or conditions, such as an epithelium at risk of developing gut-derived sepsis attributable to an intestinal pathogen, as well as methods for monitoring the administration of HMW PEG-like compounds.
Owner:UNIVERSITY OF CHICAGO
Preparation method of argireline
The invention belongs to the technical field of medicinal chemistry and discloses a preparation method of argireline. The preparation method provided by the invention comprises the steps: activating resin to obtain amino resin; gradually enabling the obtained amino resin, guanidyl-protected L-arginine, the other guanidyl-protected L-arginine and side chain amino protected L-glutamine to be subjected to the first coupling reaction to obtain tripeptide resin; gradually enabling the obtained tripeptide resin, L-methionine, side chain carboxyl protected L-glutamic acid and side chain carboxyl protected N-acetyl-L-glutamic acid to be subjected to second coupling reaction to obtain hexapeptide resin; cracking the obtained hexapeptide resin to obtain a first product; and purifying the obtained first product to obtain the argireline. The preparation method provided by the invention is used for preparing the argireline through solid-phase synthesis so as to be simple in operation, capable of remarkably increasing the yield of the argireline and greatly improving the purity of the argireline, suitable for large-scale industrial production and more beneficial to the popularization and application of the argireline.
Owner:浙江华军药业有限公司
Pharmaceutical formulations of antineoplastic agents and processes of making and using the same
In its several embodiments, this invention discloses a pharmaceutical formulation comprising at least one antineoplastic agent or a pharmaceutically acceptable salt thereof, and at least one dissolution enhancing agent sufficient to substantially dissolve said at least one antineoplastic agent in at least one aqueous diluent, wherein said dissolution enhancing agent is urea, L-histidine, L-threonine, L-asparagine, L-serine, L-glutamine or mixtures thereof; a lyophilized powder comprising said pharmaceutical formulation, and articles of manufacture thereof.
Owner:MERCK SHARP & DOHME LLC
Paster for treating dental ulcer and preparation method thereof
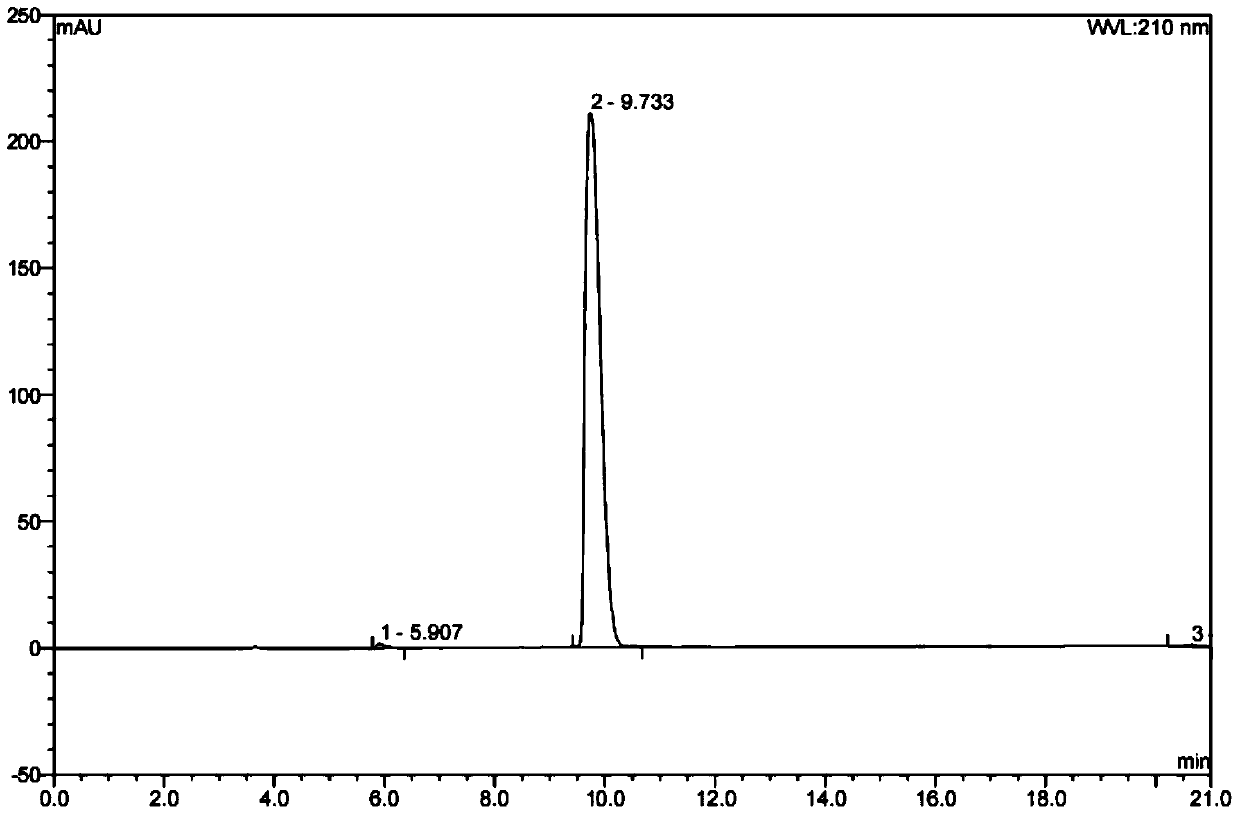
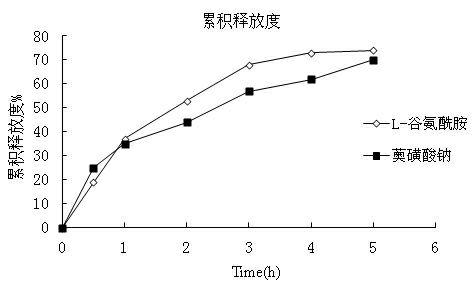
InactiveCN102600122ASimple preparation processStable drug releaseDigestive systemAnhydride/acid/halide active ingredientsCurative effectL-Glutamin
The invention aims to provide a paster for treating dental ulcer and a preparation method thereof. Aiming at the defects of the existing dental ulcer preparation, the oral paster containing L-glutamine and sodium azulenesulfonate has the advantages of simple preparation process, stable drug release, durable action to ulcer position and remarkable curative effect.
Owner:沈阳长秀医药有限公司
Compositions and methods for treating traumatic brain injury
ActiveUS20140170211A1Symptoms improvedPromote healingBiocideKetone active ingredientsL-GlutaminTaurine
The disclosure provides compositions treating traumatic brain injuries such as concussions. In one embodiment, the composition comprises phosphatidylserine, phosphatidylcholine, quercetin, astaxanthin, R-alpha lipoic acid, N-acetyl cysteine, taurine, L-glutamine, carnitine, D-ribose, creatine, epigallocatechin gallate, melatonin, ginkgo leaf extract, curcumin and L-glycine. The disclosure also provides methods for treating traumatic brain injuries such as concussions by administering an effective amount of the compositions described within.
Owner:HAVN LIFE SCI INC
Serum-free preservation liquid for umbilical cord mesenchymal stem cells and application of serum-free preservation liquid
The invention provides serum-free preservation liquid for umbilical cord mesenchymal stem cells and application of the serum-free preservation liquid. The serum-free preservation liquid comprises normal saline with a NaCl mass fraction of 0.85-0.92%, insulin, estrogen, progestogen, hydrocortisone, sodium selenite, adenosine, N-acetylcysteine and L-glutamine. The insulin, the estrogen and the progestogen are added into the normal saline. Compared with serum preservation liquid, the serum-free preservation liquid and the application have the advantages that material sources capable of generating harm due to toxin with diversified components can be reduced to the greatest extent; the serum-free preservation liquid contains functional components with few components, basal metabolism of the cells can be maintained, and the cells are smooth in performance without huge variation; certain basic active nutrient substances and energy substances can be provided by the components, so that low metabolism level can be kept, the activity of the cells is in a stable state, shape variation or differentiation or the like due to growth and cultivation can be prevented, protection effects can be realized for the cells, the activity and the quality of the transplanted umbilical cord cells can be kept in a long-acting manner, and transplanted cell therapy clinical effects can be improved.
Owner:SHEN ZHEN ISTEM REGENERATIVE MEDICINE SCI TECH CO LTD
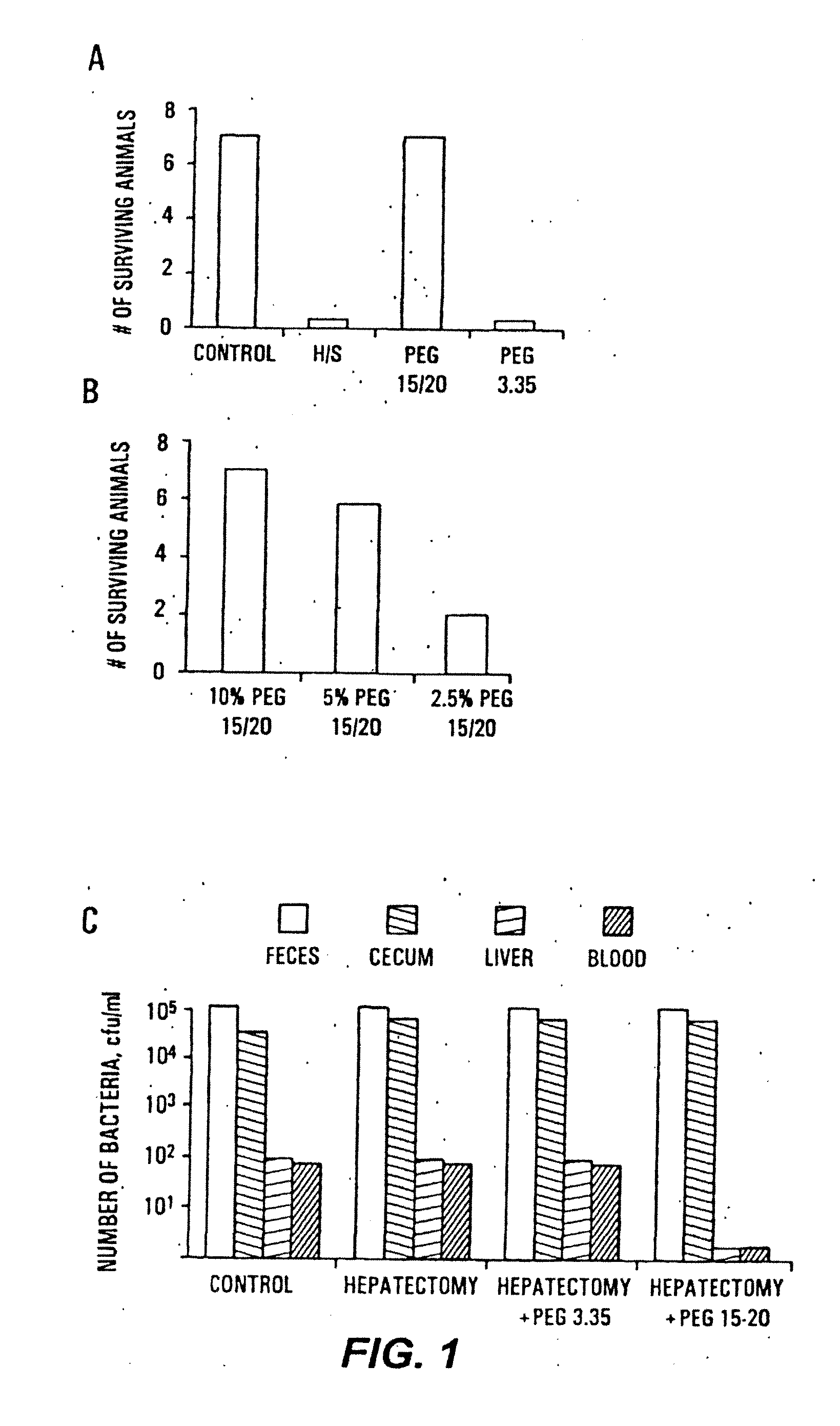
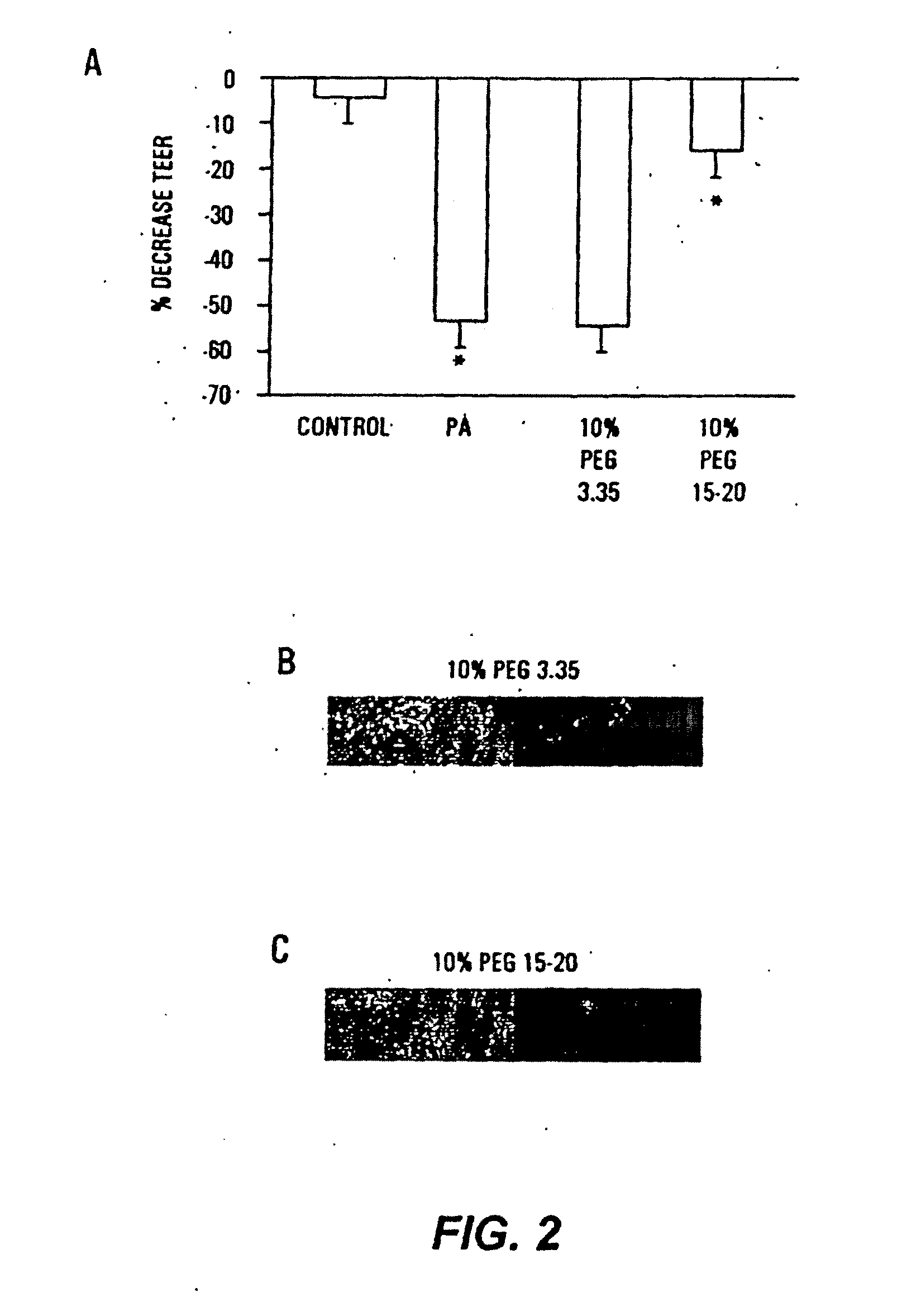
Methods for preventing and treating radiation-induced epithelial disorders
InactiveUS20120078017A1Suppresses virulence expressionAmeliorating and eliminating untoward consequenceAntibacterial agentsOrganic chemistryDiseasePolyethylene glycol
The present invention provides methods of protecting irradiated eukaryotic cells such as irradiated mammalian epithelial cells, from the deleterious effect of microbial pathogens such as Pseudomonas aeruginosa. The invention also provides methods of protecting irradiated organisms from such deleterious effects, resulting in reduced mortality and morbidity. Further provided are kits containing relatively high molecular weight biocompatible polymers such as polyethylene glycol, optionally supplemented with a protective polymer such as dextran and / or essential pathogen nutrients such as L-glutamine, along with instructions for administration to organisms to be exposed to radiation.
Owner:UNIVERSITY OF CHICAGO
Non-animal-source serum-free culture medium for umbilical cord blood stem cells
ActiveCN102827810AAvoid instabilityClear natureBlood/immune system cellsCell phenotypeLipid formation
The invention relates to the field of biology, and discloses a non-animal-source serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, phytohaemagglutinin (PHA), lipid, amino acid, vitamins, trace elements, interleukin-3(IL-3), stem cell factor, (SCF), Fit3-L, IL-6 and granulocyte colony-stimulating factor (G-CSF). The non-animal source serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation, avoids the doped animal components and unstability of batches, and the results of cultured umbilical cord blood stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the non-animal-source serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Antialcoholismic composition and preparation method thereof
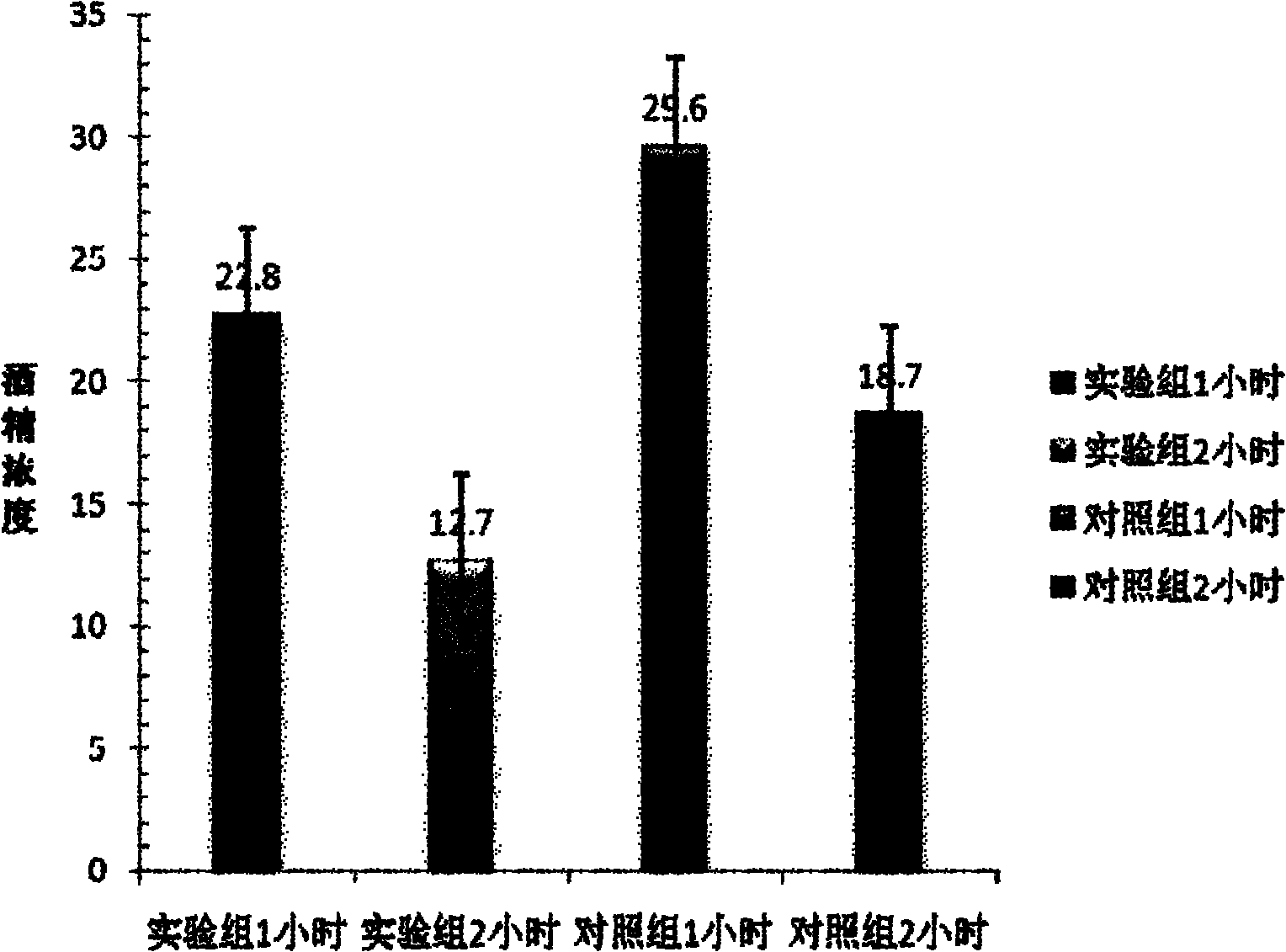
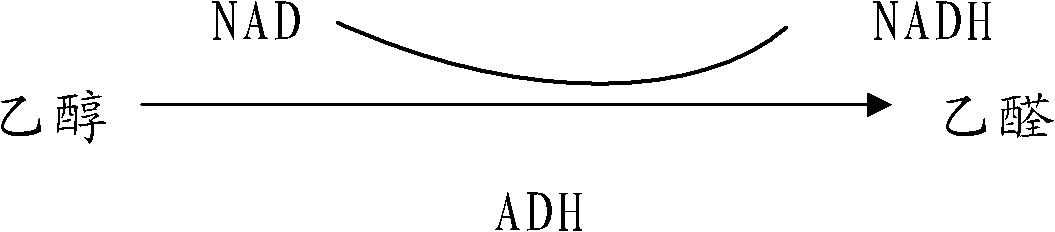
ActiveCN102038867APromote alcohol metabolismReduce absorptionNervous disorderHeterocyclic compound active ingredientsMonkshoodsAlcoholisms
The invention relates to the field of medicaments and discloses an antialcoholismic composition. The raw materials of the antialcoholismic composition comprise: one or a mixture of more than two of S-adenosyl methionine, cysteine and methionine, one of a mixture of D-glyceric acid and D-glycerate, one or a mixture of more than two of succinic acid, fumaric acid, citric acid, oxaloacetic acid and malic acid, one or a mixture of more than two of vitamin B1, vitamin B6 and vitamin B2, one or a mixture of coenzyme Q10 or coenzyme I, L-glutamine, fortune eupatorium herb, artemisia capillaries, white paeony root and root of tall monkshood. The invention also provides a preparation method of the antialcoholismic composition. The antialcoholismic composition disclosed by the invention is taken after wine drinking for reducing absorption of alcohol, accelerating alcohol metabolism, as well as tonifying stomach and spleen and preventing drunkenness and alcoholism. The antialcoholismic composition is quick in effectiveness and has a bright application prospect.
Owner:BEIJING ADINOVO TECH
Preparation method of N(2)-L-alanyl-L-glutamine
ActiveCN103626839APrice stabilityMarket sales trend ups and downsPeptide preparation methodsL-alanyl-l-glutamineChloride
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of N(2)-L-alanyl-L-glutamine. The preparation method of the N(2)-L-alanyl-L-glutamine comprises the following steps of (1) preparing L-phthaloyl-alanyl chloride; (2) preparing phthaloyl-L-alanyl-L-glutamic acid; (3) preparing phthaloyl-L-alanyl-L-glutamic acid anhydride; (4) preparing phthaloyl-L-alanyl-L-glutamine; (5) preparing an N(2)-L-alanyl-L-glutamine crude product; (6) preparing an N(2)-L-alanyl-L-glutamine refined product. The product obtained through the final deprotection process of the preparation method as a pilot plant test or a production scale process route is higher in purity; the liquid-phase purity of the product is higher than 99.9% through primary purification, and the product is low in impurity content.
Owner:JINAN CHENGHUI SHUANGDA CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com