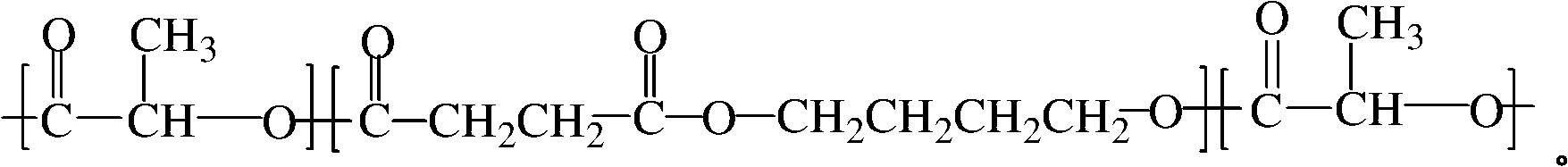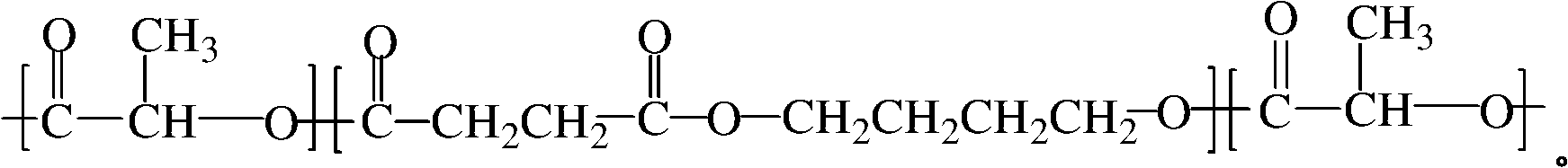Patents
Literature
2324 results about "Succinates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Derivatives of SUCCINIC ACID. Included under this heading are a broad variety of acid forms, salts, esters, and amides that contain a 1,4-carboxy terminated aliphatic structure.
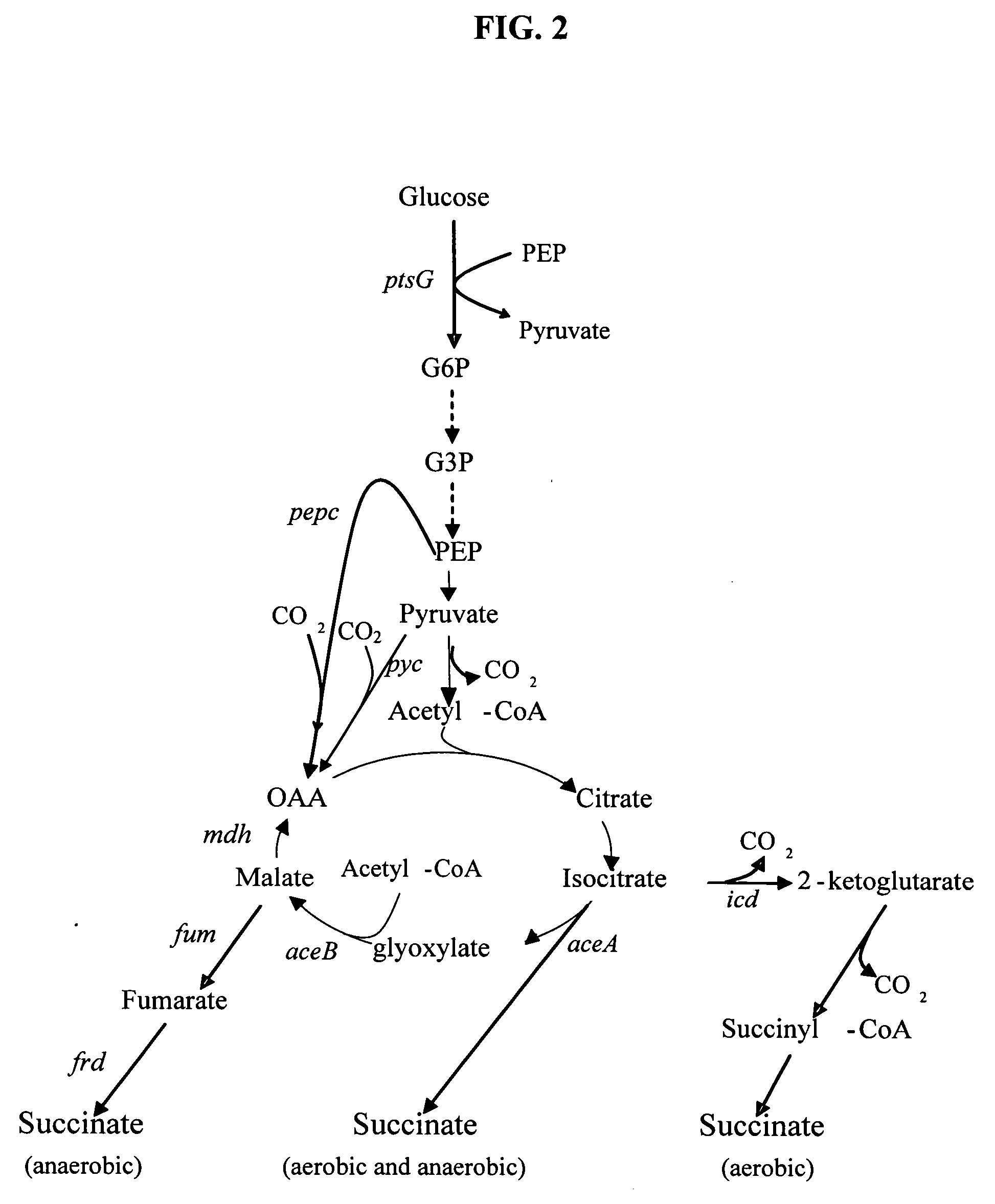
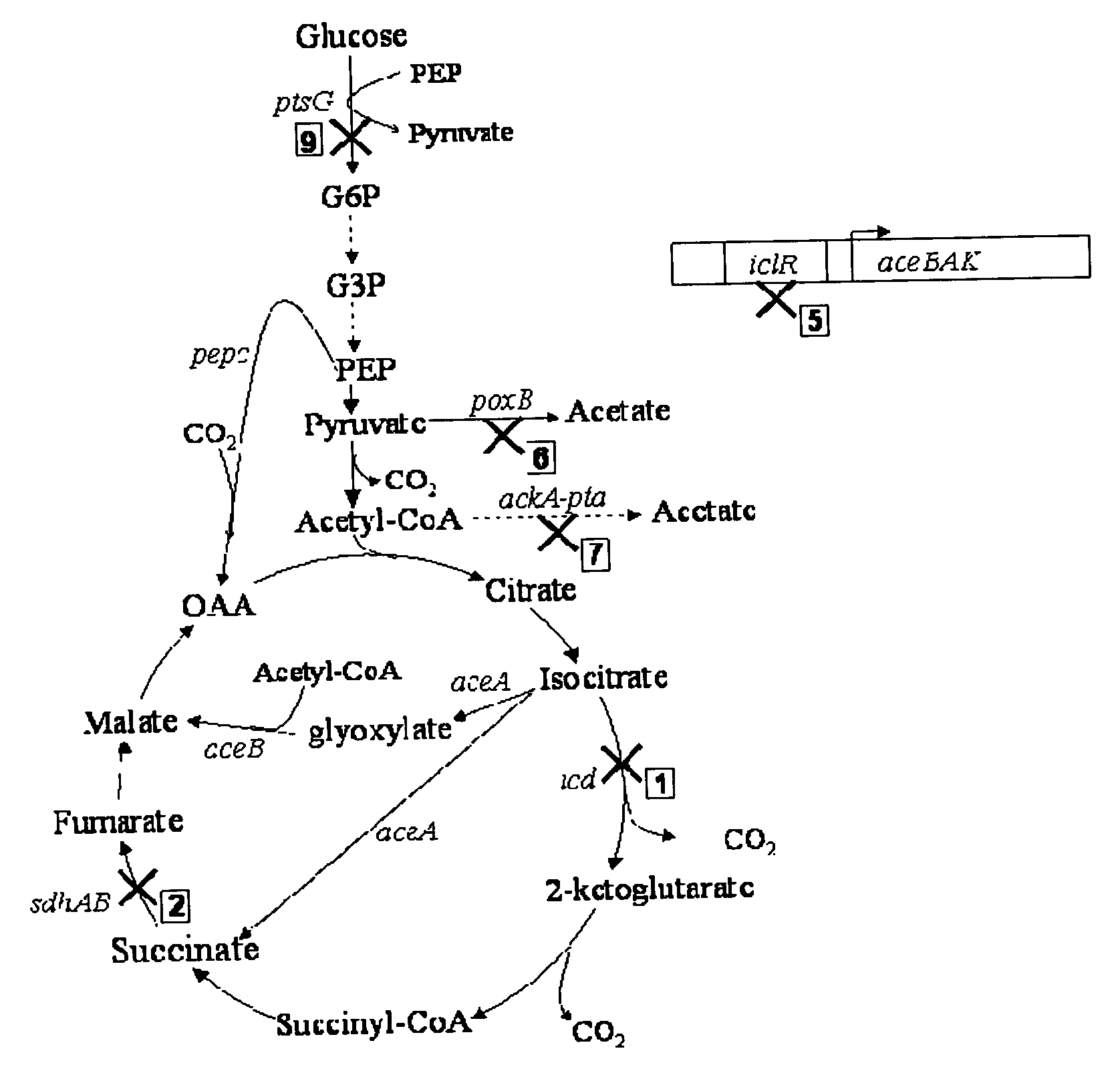
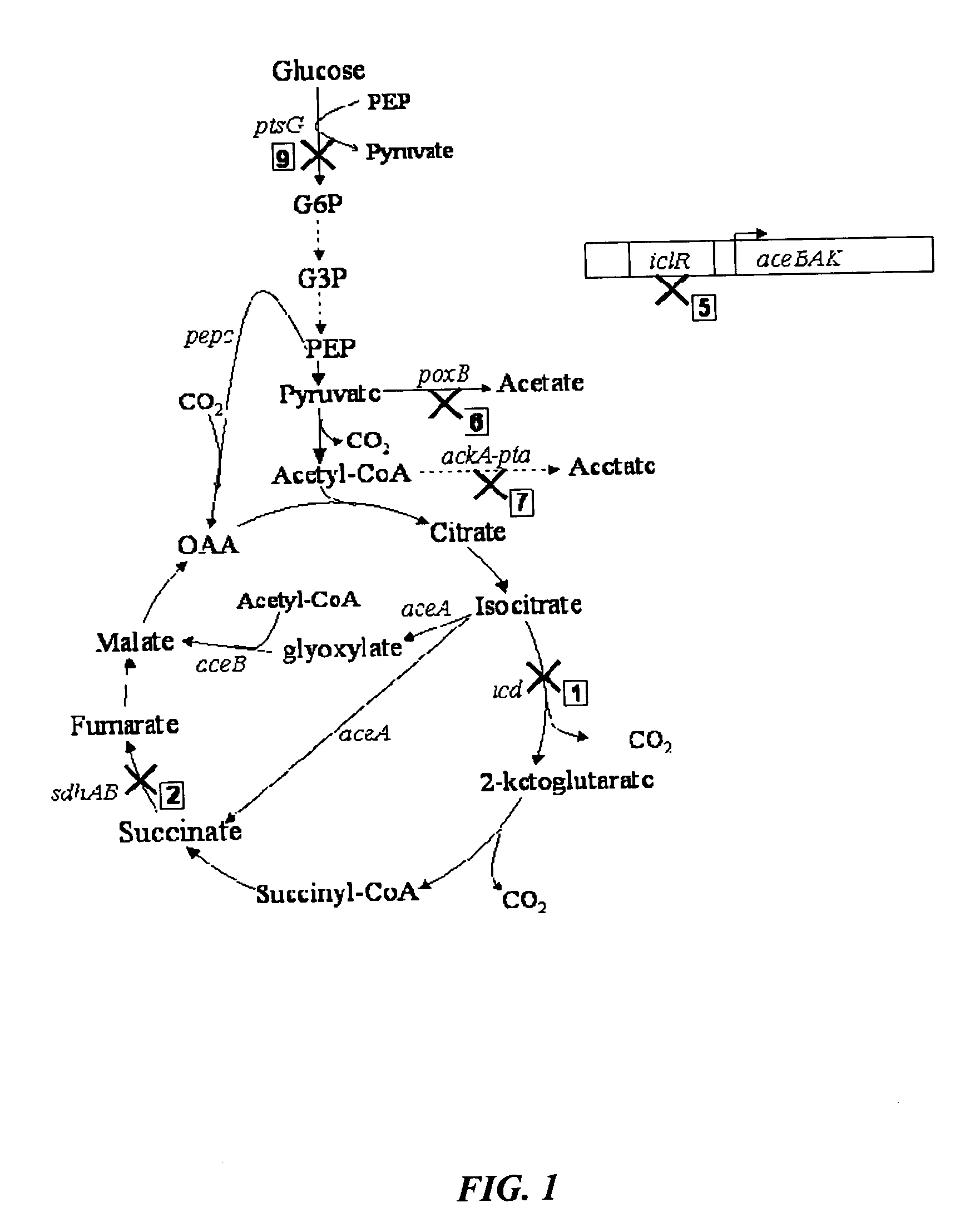
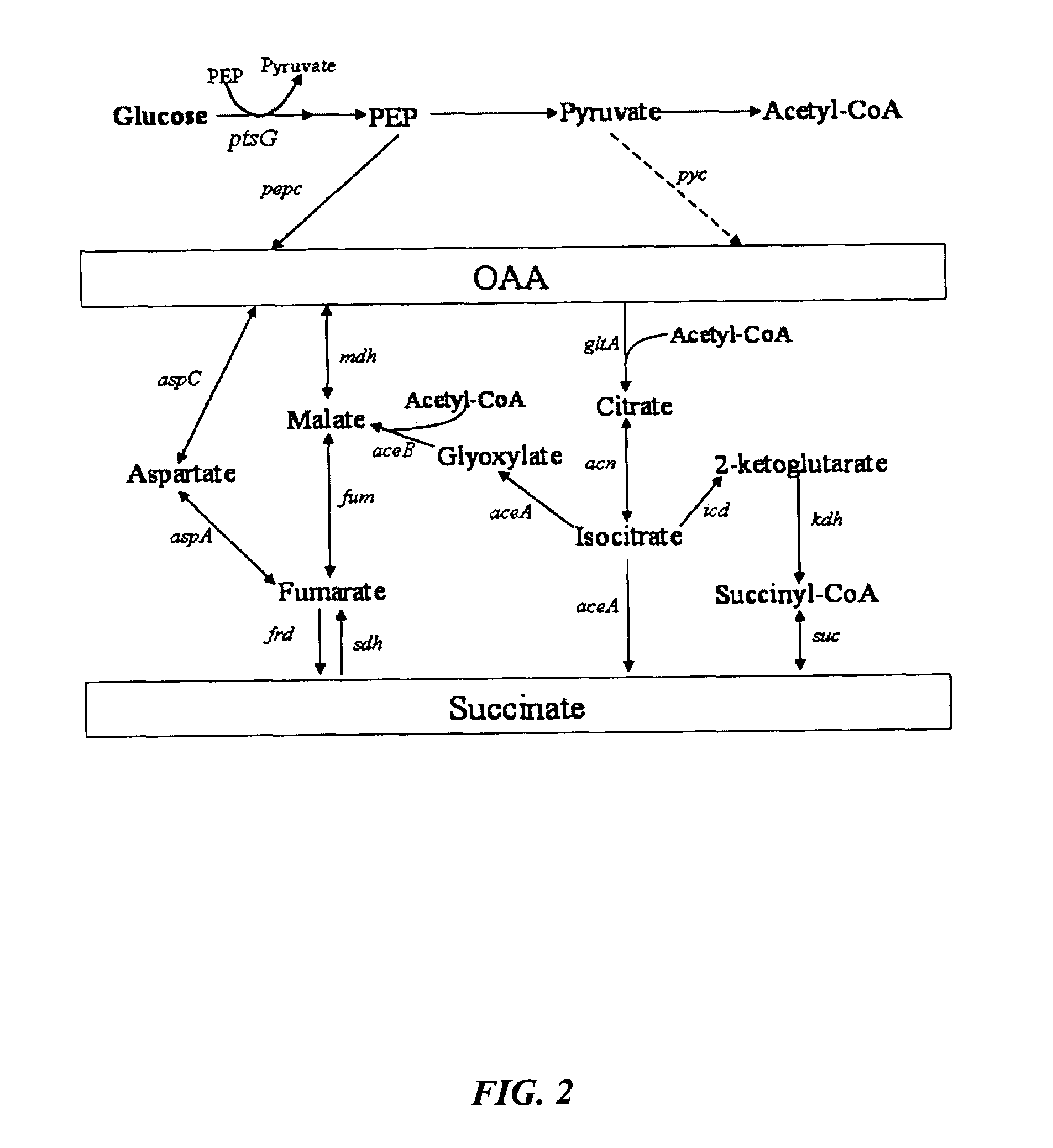
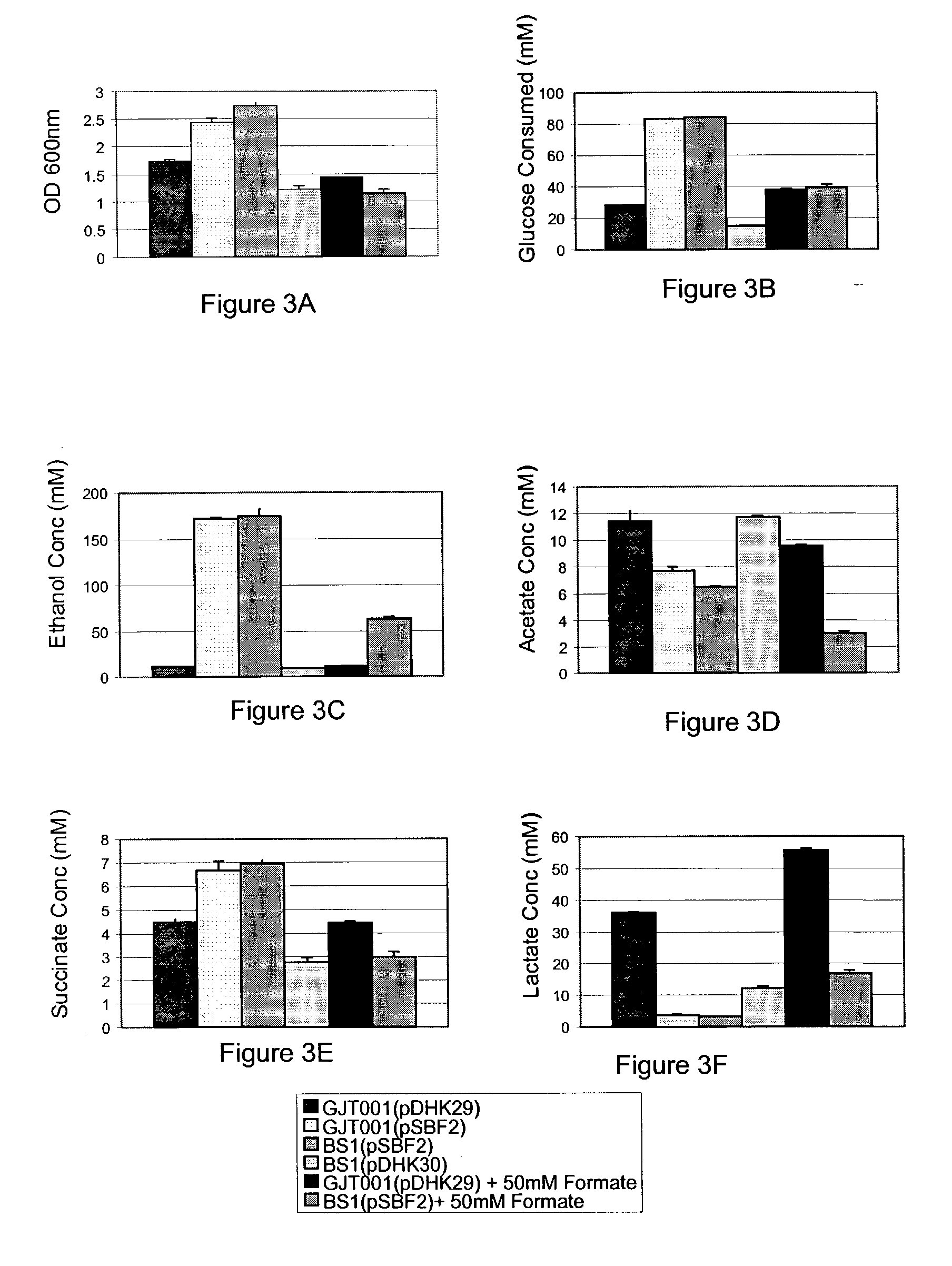
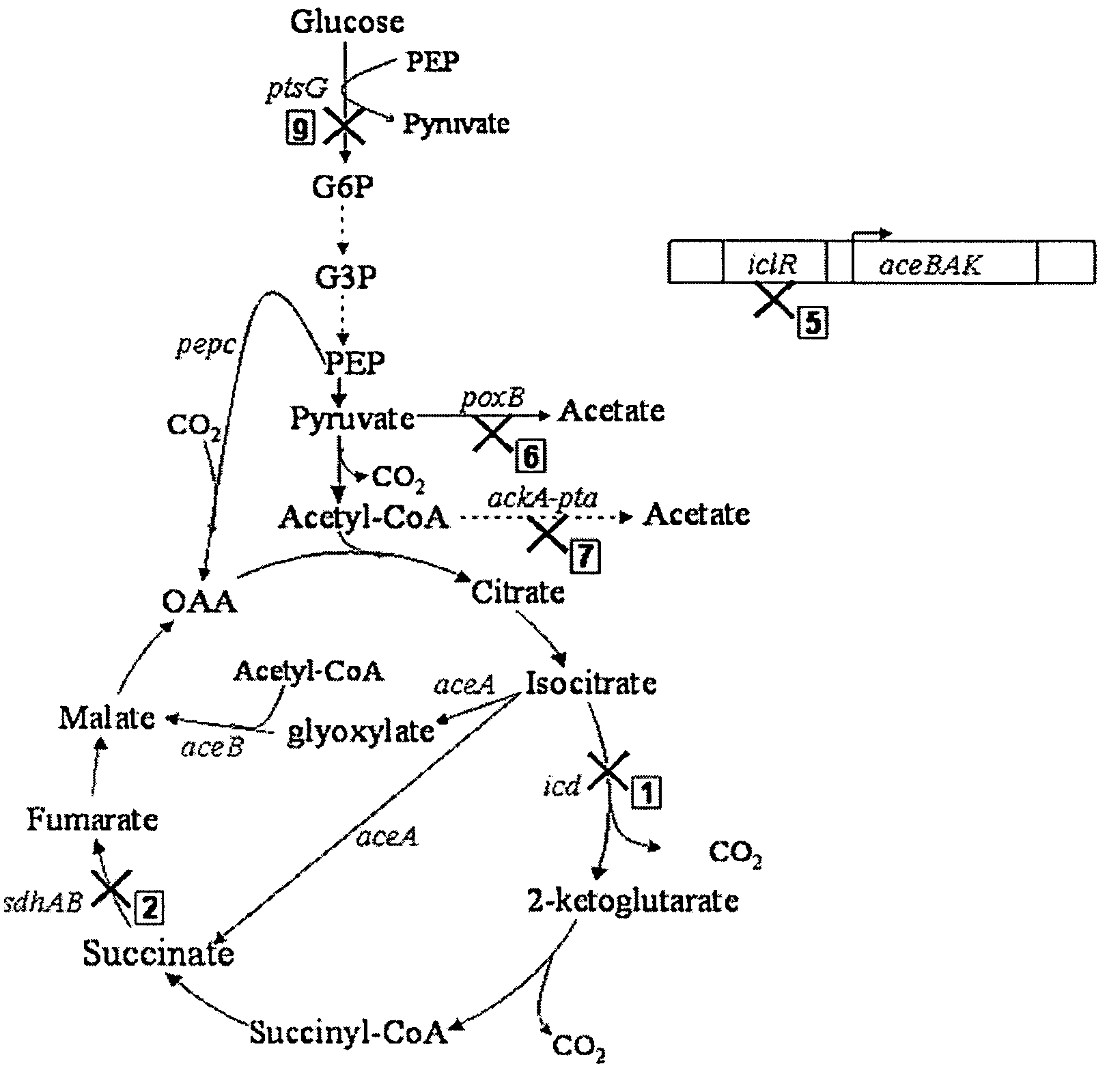
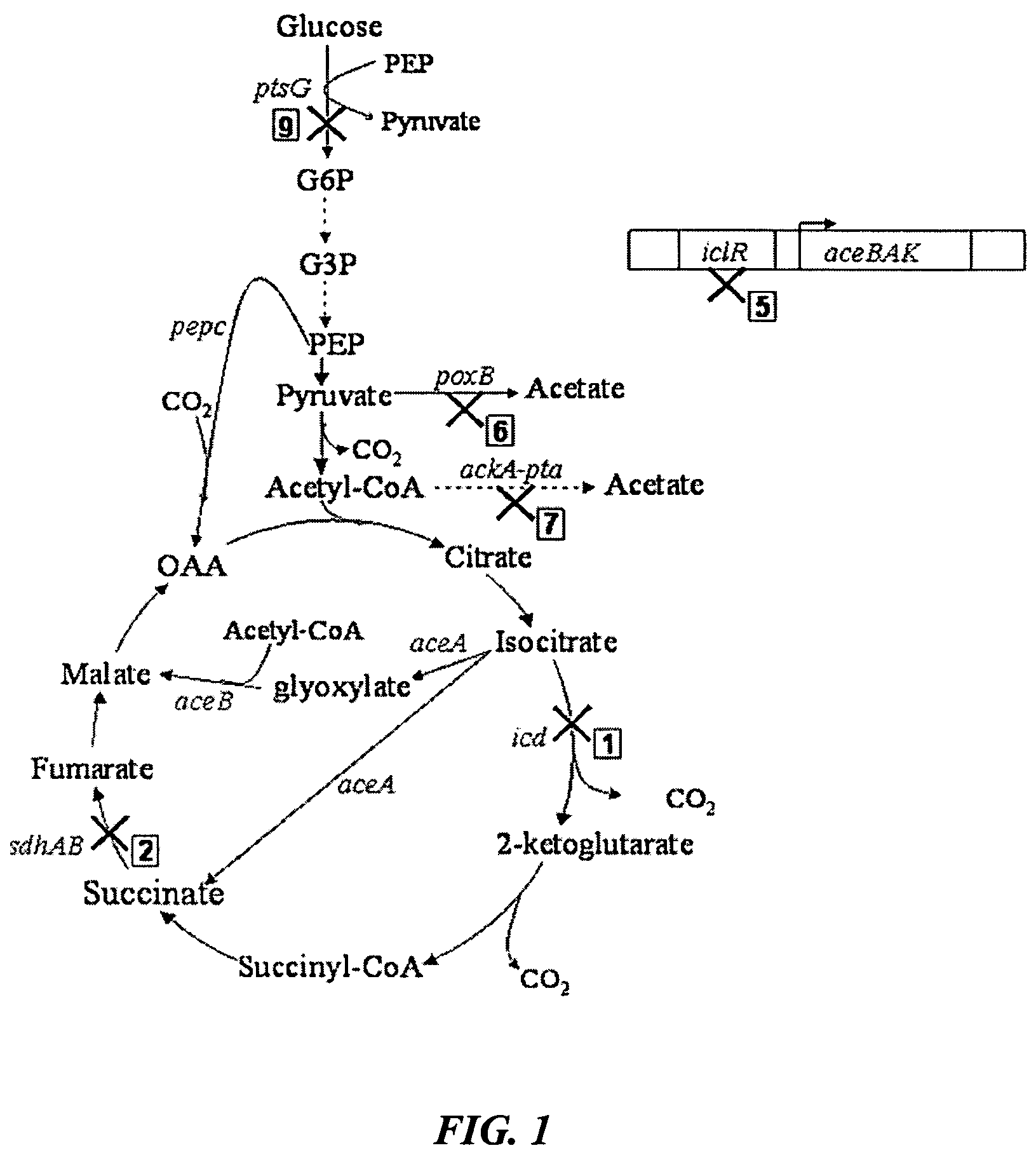
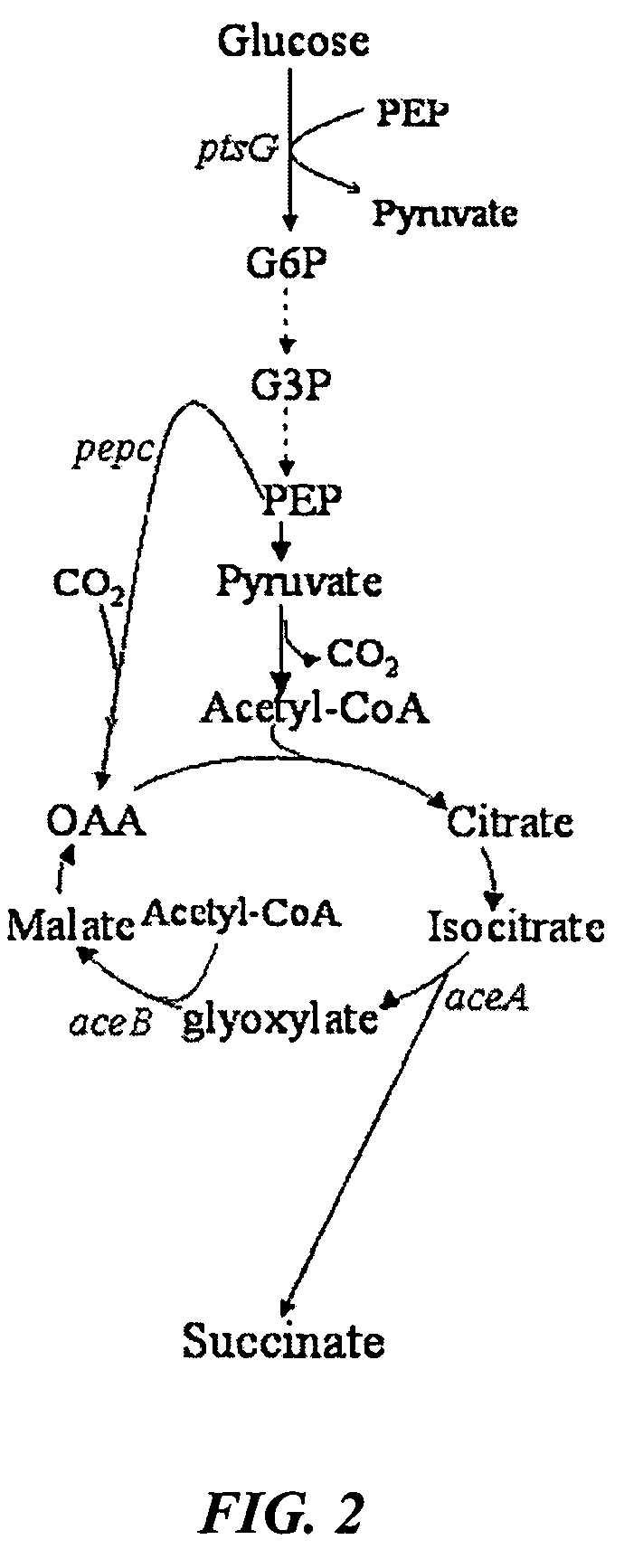
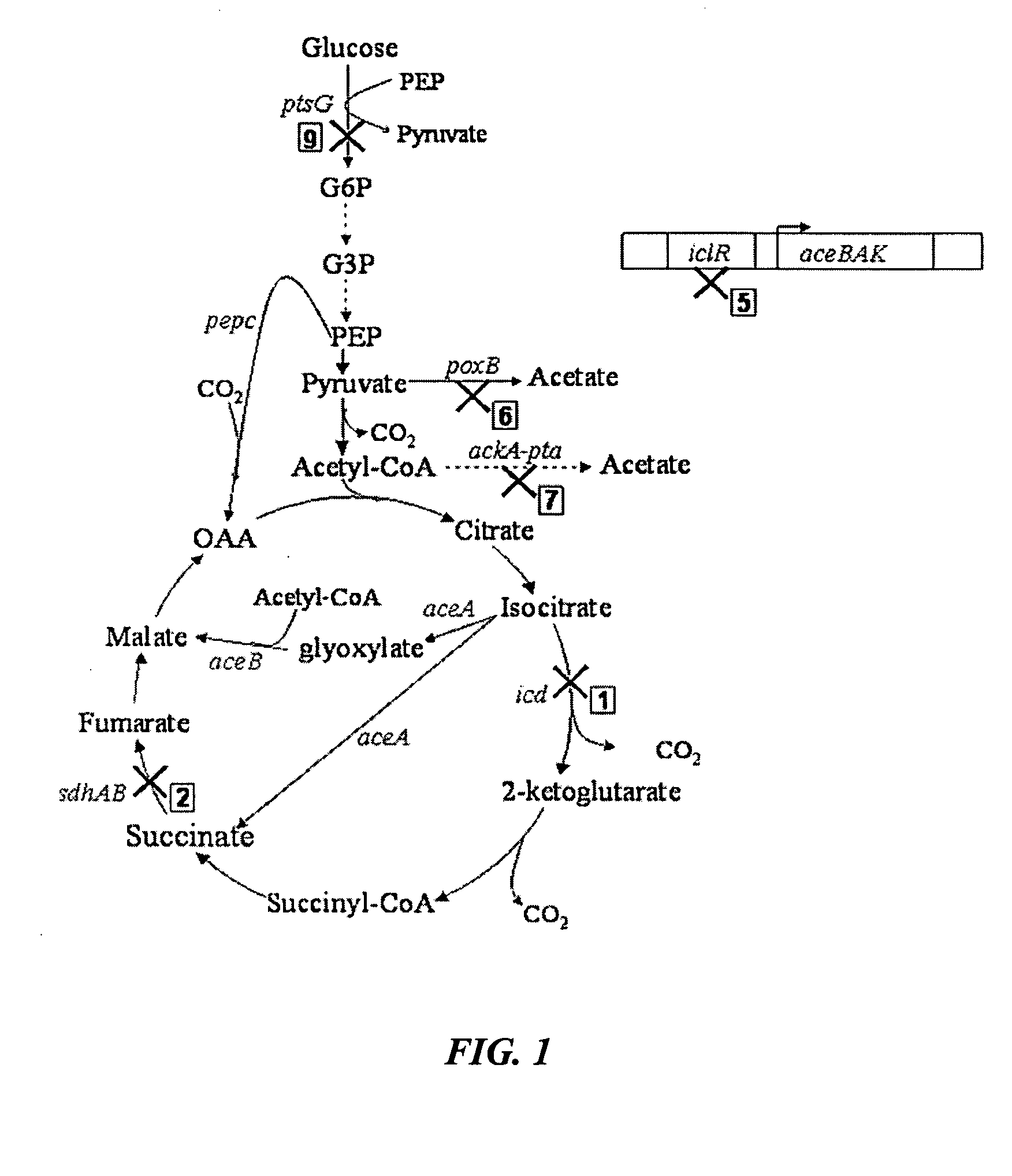
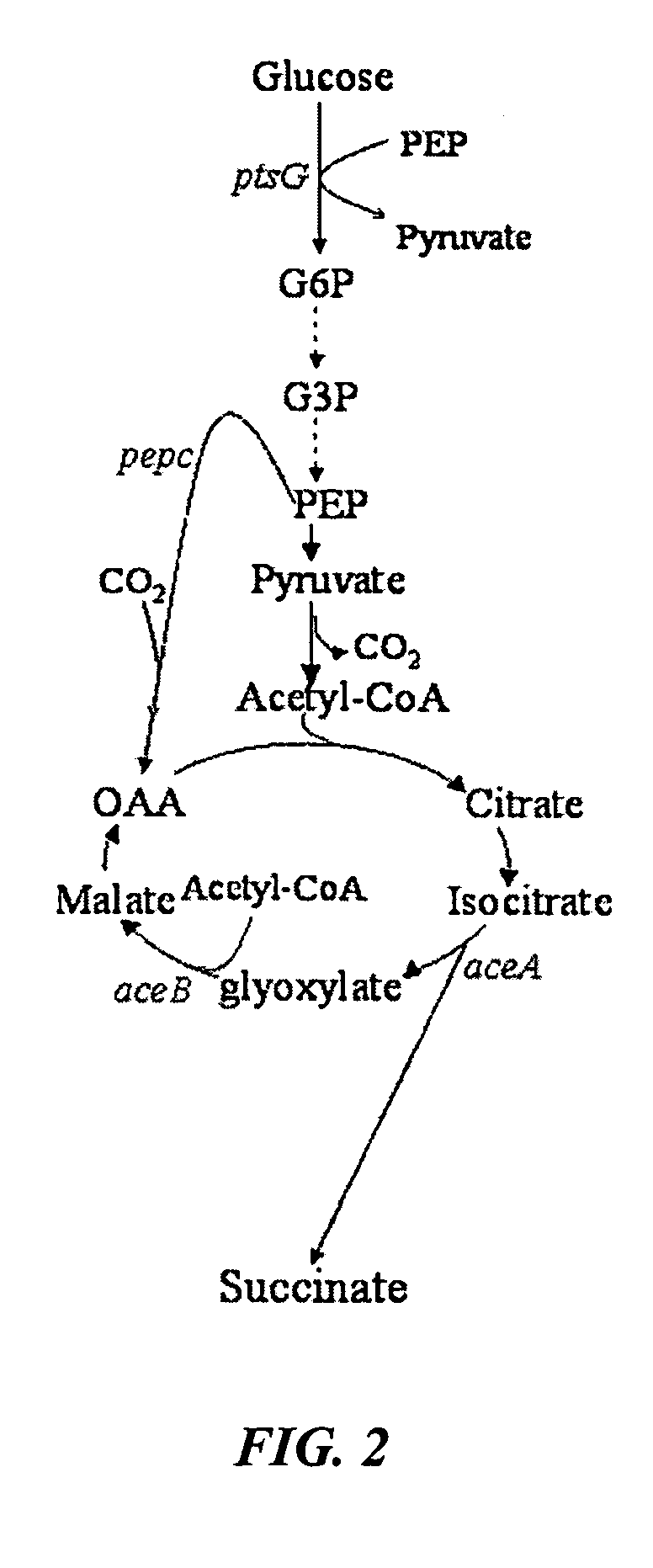
Methods and organisms for the growth-coupled production of succinate
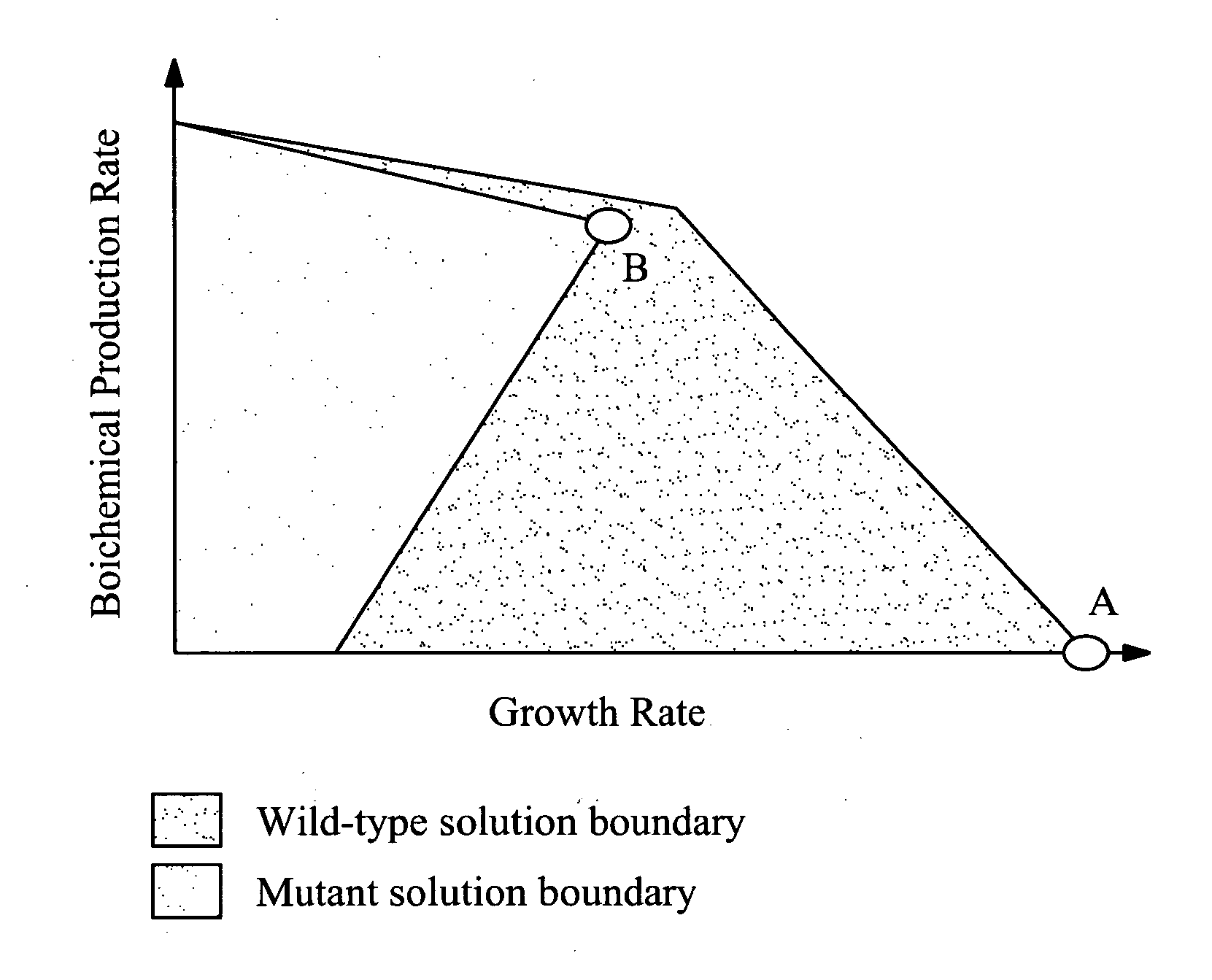
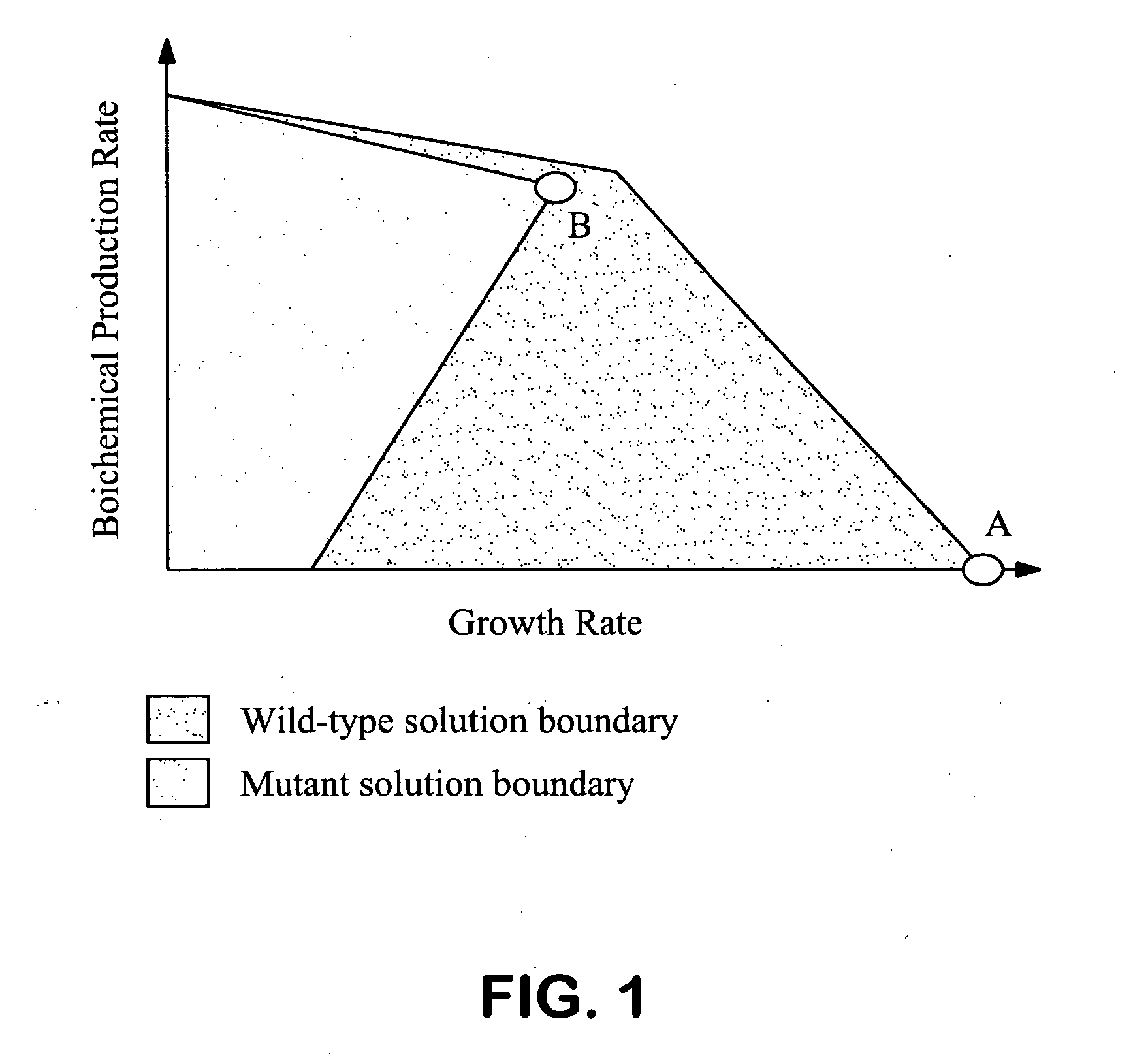
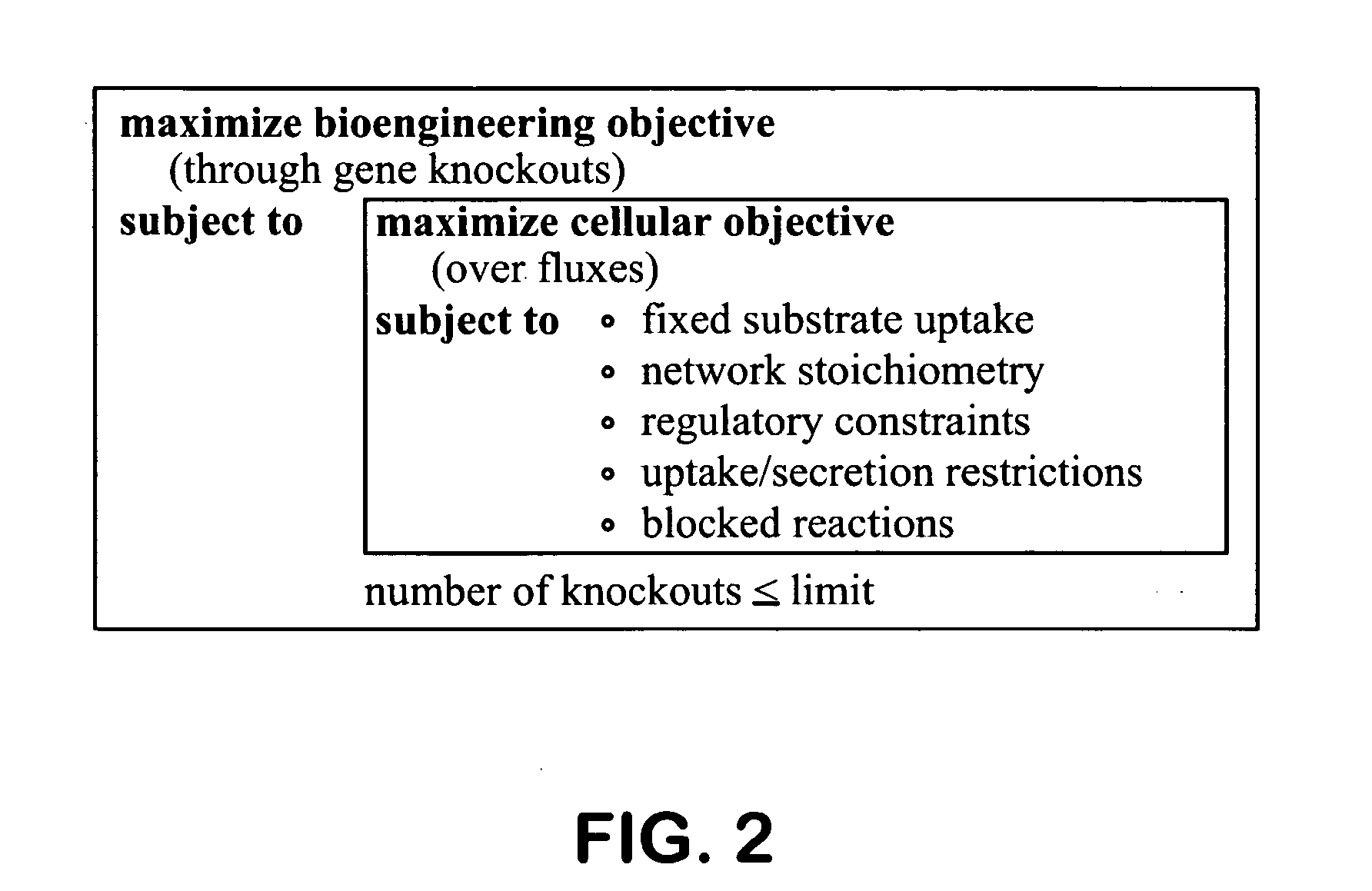
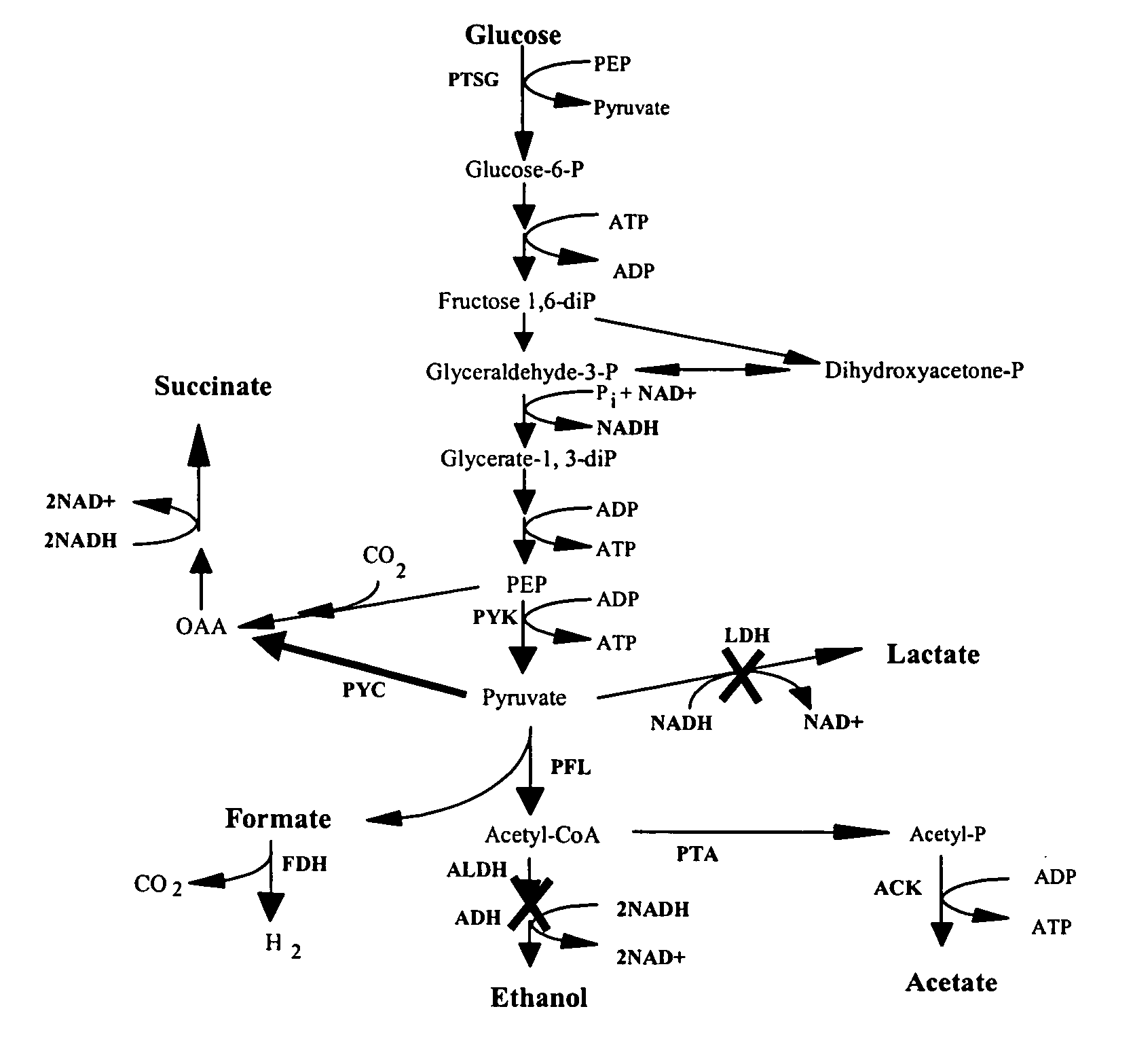
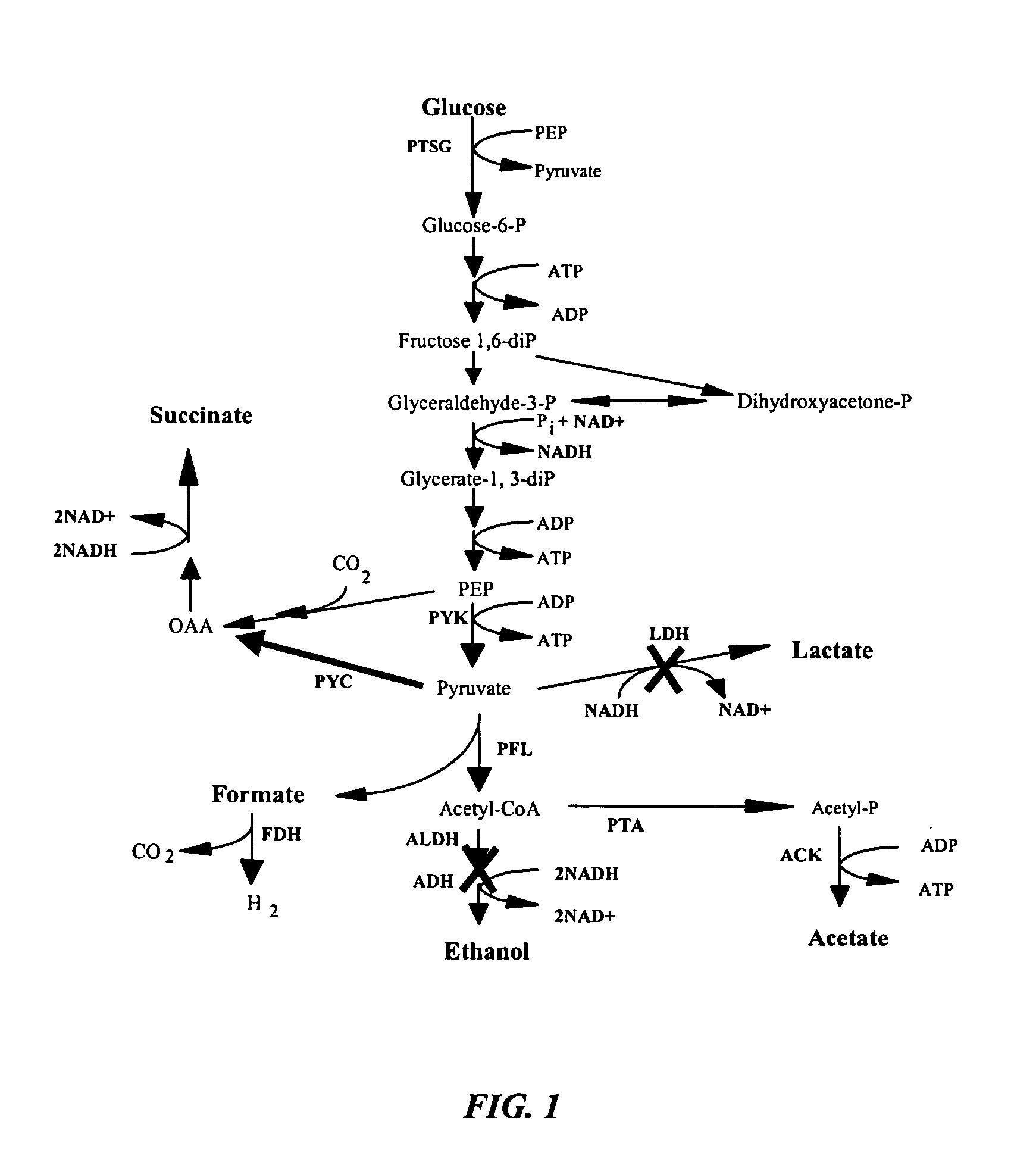
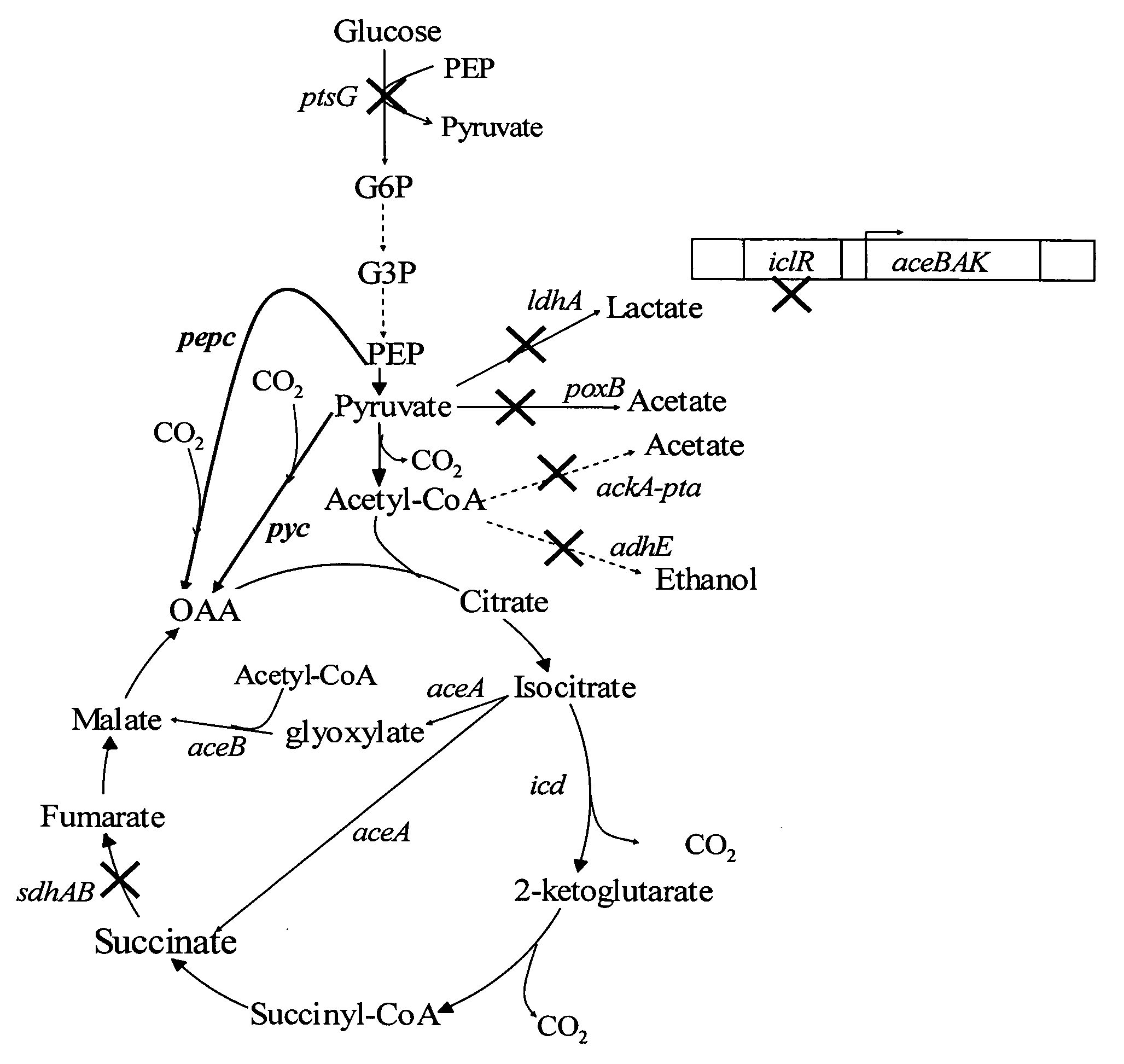
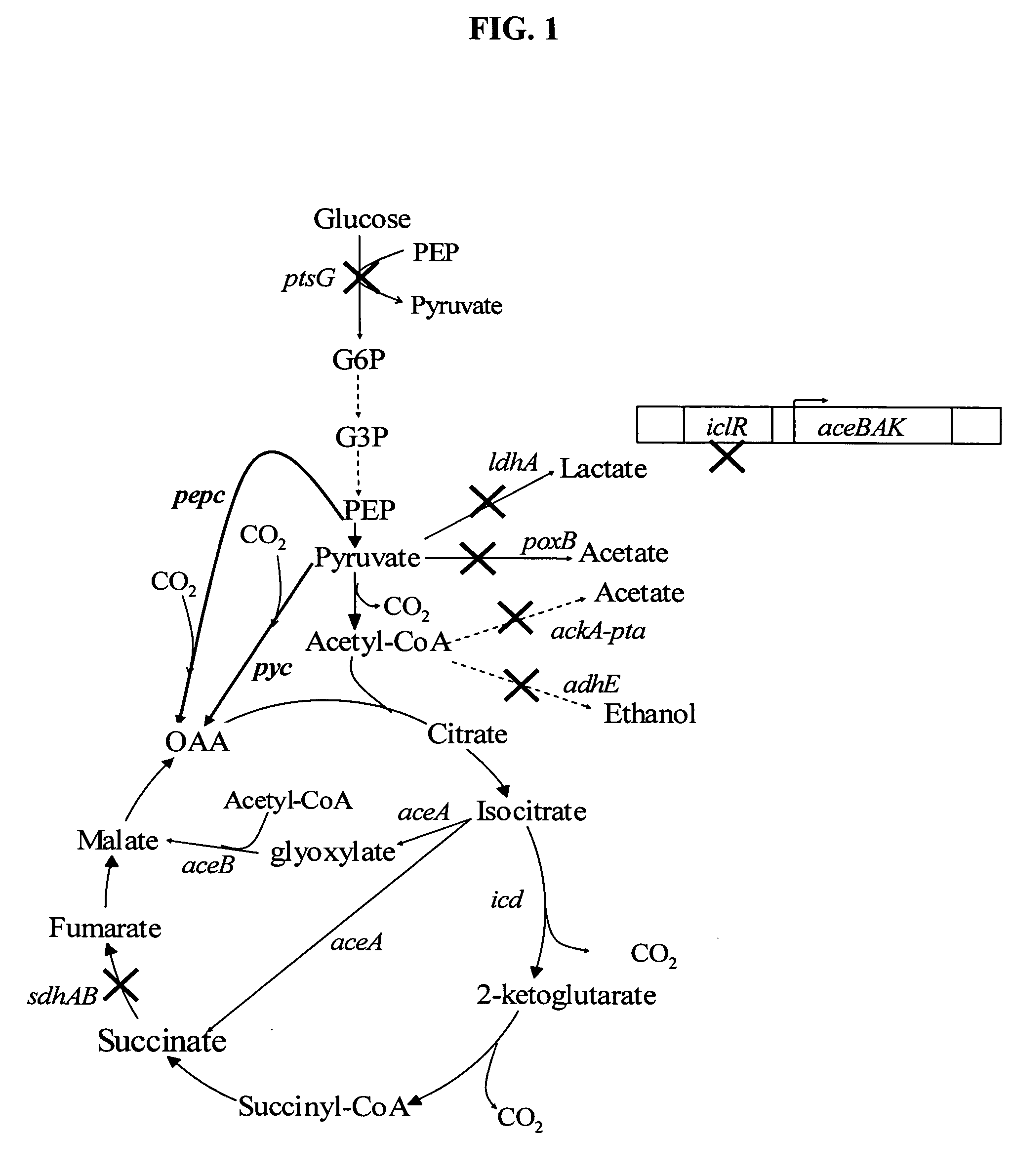
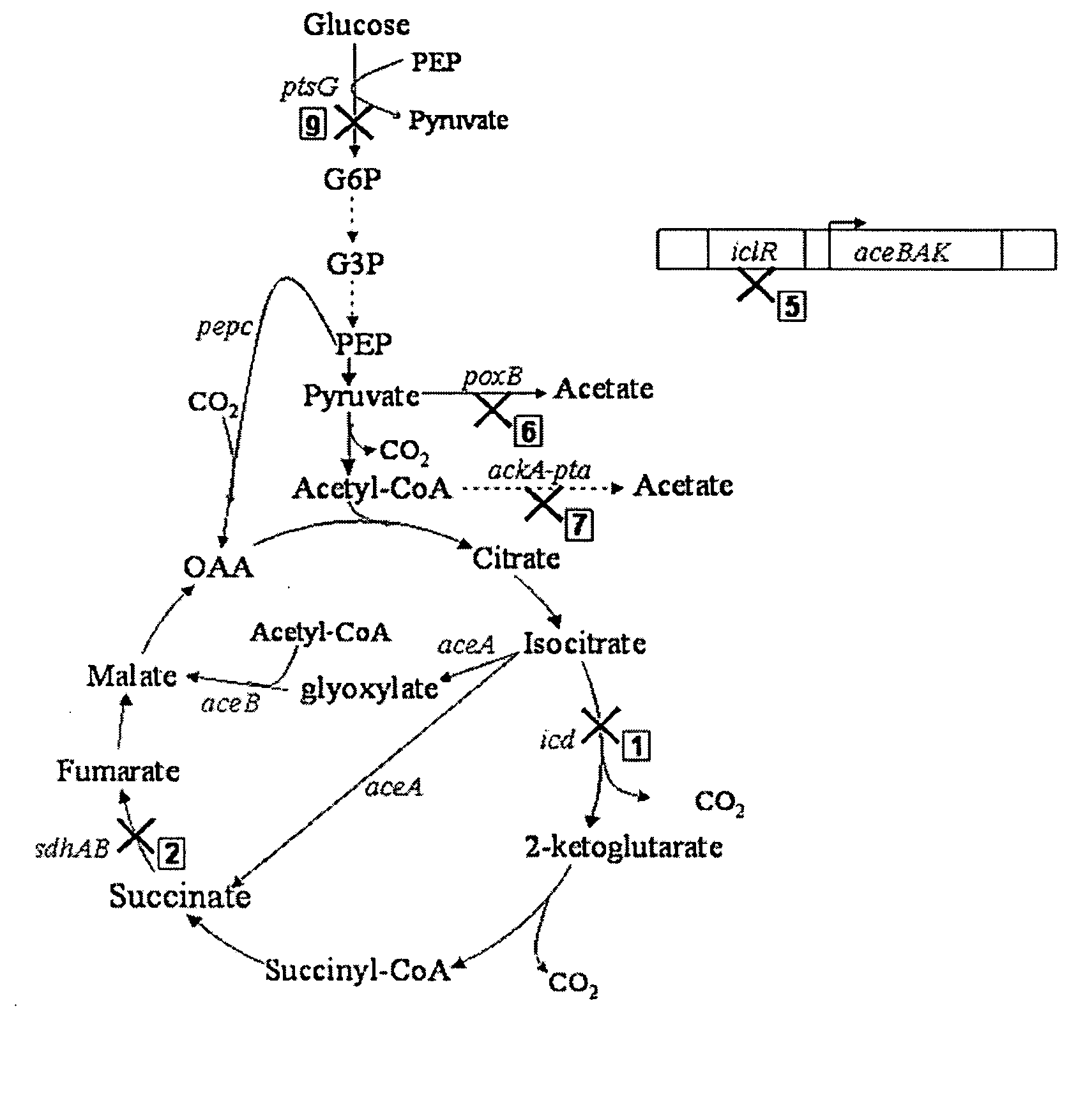
The invention provides a non-naturally occurring microorganism comprising one or more gene disruptions encoding an enzyme associated with growth-coupled production of succinate when an activity of the enzyme is reduced, whereby the one or more gene disruptions confers stable growth-coupled production of succinate onto the non- naturally occurring microorganism. Also provided is a non-naturally occurring microorganism comprising a set of metabolic modifications obligatory coupling succinate production to growth of the microorganism, the set of metabolic modifications comprising disruption of one or more genes selected from the set of genes comprising: (a) adhE, ldhA; (b) adhE, ldhA, acka-pta; (c) pfl, ldhA; (d) pfl, ldhA, adhE; (e) acka-pta, pykF, atpF, sdhA; (f) acka-pta, pykF, ptsG, or (g) acka-pta, pykF, ptsG, adhE, ldhA, or an ortholog thereof, wherein the microorganism exhibits stable growth-coupled production of succinate. Additionally provided is a non-naturally occurring microorganism having the genes encoding the metabolic modification (e) acka-pta, pykF, atpF, sdhA that further includes disruption of at least one gene selected from pyka, atpH, sdhB or dhaKLM; a non-naturally occurring microorganism having the genes encoding the metabolic modification (f) ackA-pta, pykF, ptsG that further includes disruption of at least one gene selected from pykA or dhaKLM, or a non-naturally occurring microorganism having the genes encoding the metabolic modification (g) ackA-pta, pykF, ptsG, adhE, ldhA that further includes disruption of at least one gene selected from pykA or dhaKLM. The disruptions can be complete gene disruptions and the non-naturally occurring organisms can include a variety of prokaryotic or eukaryotic microorganisms. A method of producing a non-naturally occurring microorganism having stable growth-coupled production of succinate also is provided. The method includes: (a) identifying in silico a set of metabolic modifications requiring succinate production during exponential growth, and (b) genetically modifying a microorganism to contain the set of metabolic modifications requiring succinate production.
Owner:GENOMATICA INC
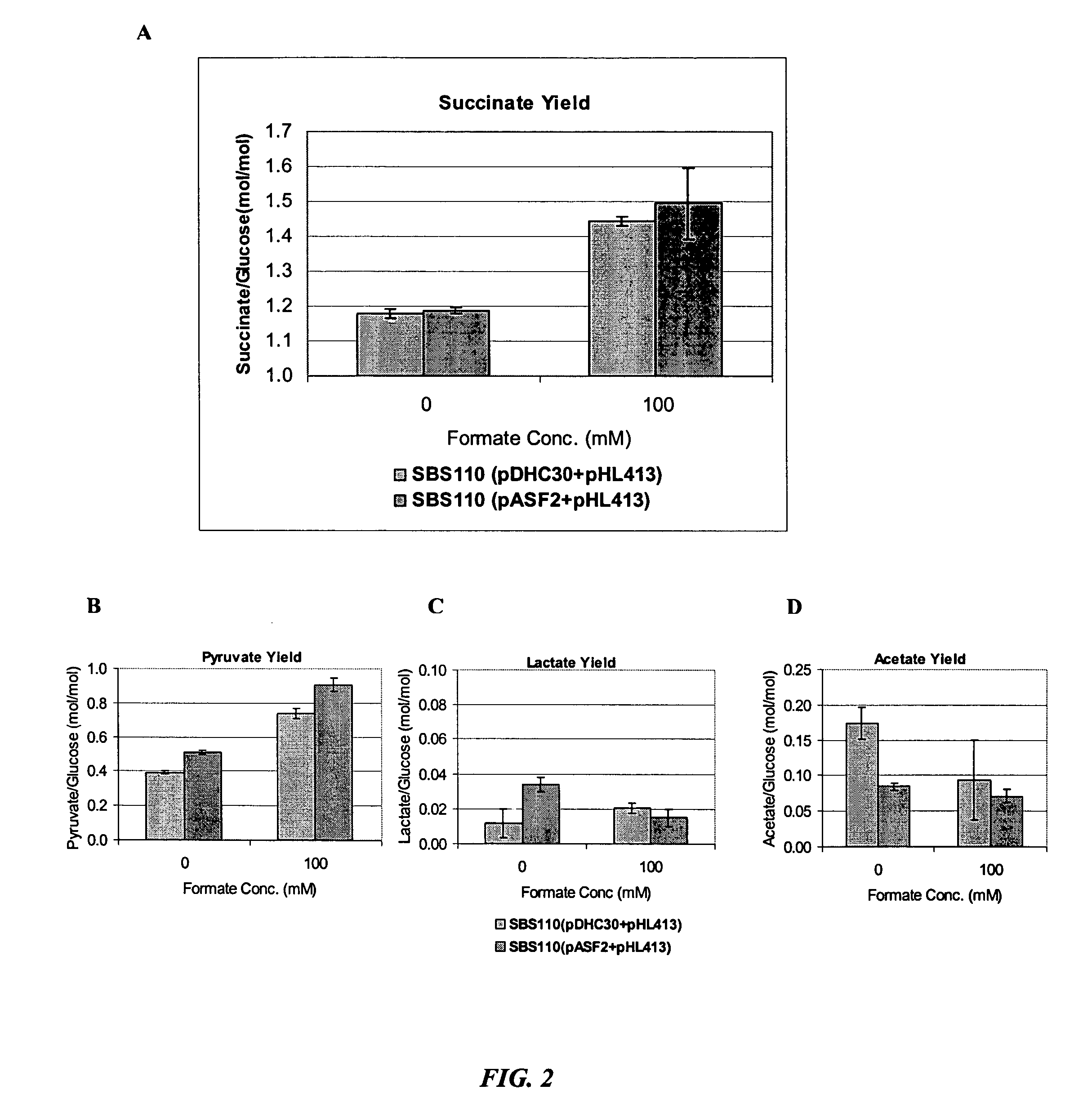
High molar succinate yield bacteria by increasing the intracellular NADH availability
InactiveUS20050042736A1Increase productionEnhanced intracellular levelSugar derivativesBacteriaMicrobiologySuccinates
The invention relates to increasing the yield of succinate in bacteria by increasing the intracellular availability of cofactors such as NADH.
Owner:RICE UNIV
High succinate producing bacteria
InactiveUS20060073577A1Increase productionQuick implementationBacteriaFermentationMicrobiologySuccinic acid
The invention relates to a hybrid succinate production system that has a high capacity to produce succinate under aerobic and anaerobic conditions. The metabolic engineering of a hybrid bacterial succinate production system that can function under both aerobic and anaerobic conditions makes the production process more efficient, and the process control and optimization less difficult.
Owner:RICE UNIV
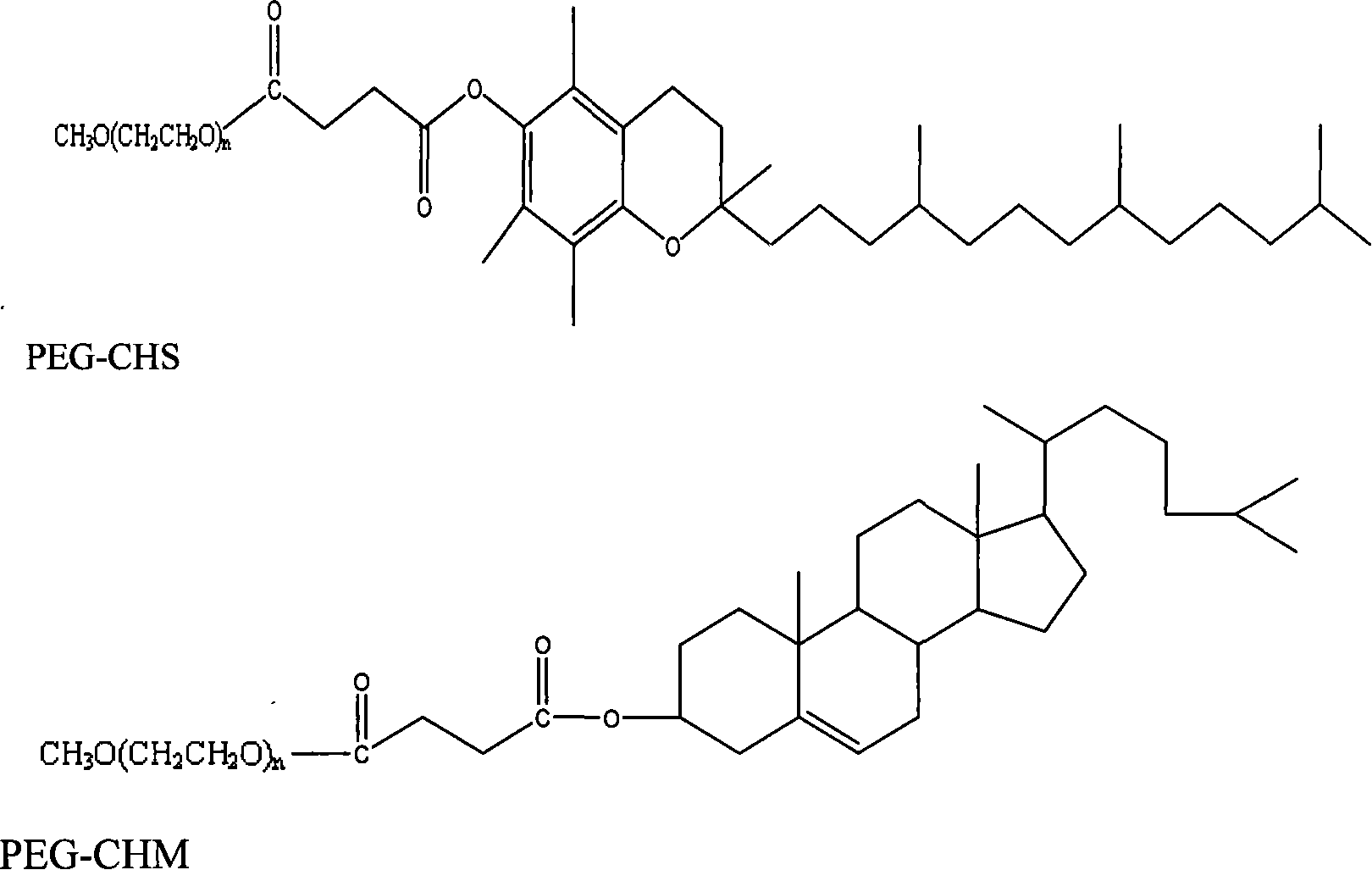
Tocopheryl polyethylene glycol succinate powder and process for preparing same
InactiveUS20070184117A1Without compromising handling characteristicPowder deliveryOrganic active ingredientsPolymer scienceTocopherol polyethylene glycol succinate
A powdered tocopheryl polyethylene glycol succinate (TPGS™) having an average particle size of less than about 1000 microns. In one embodiment, the powdered tocopheryl polyethylene glycol succinate is prepared by a process that includes atomizing a fluidic tocopheryl polyethylene glycol succinate into an environment suitable for solidifying the atomized tocopheryl polyethylene glycol succinate. In another embodiment, the powdered tocopheryl polyethylene glycol succinate is prepared by a process of applying a force to a solid tocopheryl polyethylene glycol succinate starting material that is sufficient to produce a powdered product.
Owner:EASTMAN CHEM CO
Aerobic succinate production in bacteria
Methods of increasing yields of succinate using aerobic culture methods and a multi-mutant E. coli strain are provided. Also provided is a mutant strain of E. coli that produces high amounts of succinic acid.
Owner:RICE UNIV
Use of phosphoketolase for producing useful metabolites
Owner:AJINOMOTO CO INC
Recycling system for manipulation of intracellular NADH availability
ActiveUS7256016B2Increases intracellular availabilityImprove usabilityBacteriaProtozoaVitaminBiology
The present invention describes a novel recombinant NADH recycling system that is used as a process for producing reduced compounds. In a specific embodiment, the reduced compounds include ethanol, succinate, lactate, a vitamin, a pharmaceutical and a biodegraded organic molecule. The NADH recycling system effects metabolic flux of reductive pathways in aerobic and anaerobic environments.
Owner:RICE UNIV
Aerobic succinate production in bacteria
Methods of increasing yields of succinate using aerobic culture methods and a multi-mutant E. coli strain are provided. Also provided is a mutant strain of E. coli that produces high amounts of succinic acid.
Owner:RICE UNIV
Simultaneous anaerobic production of isoamyl acetate and succinic acid
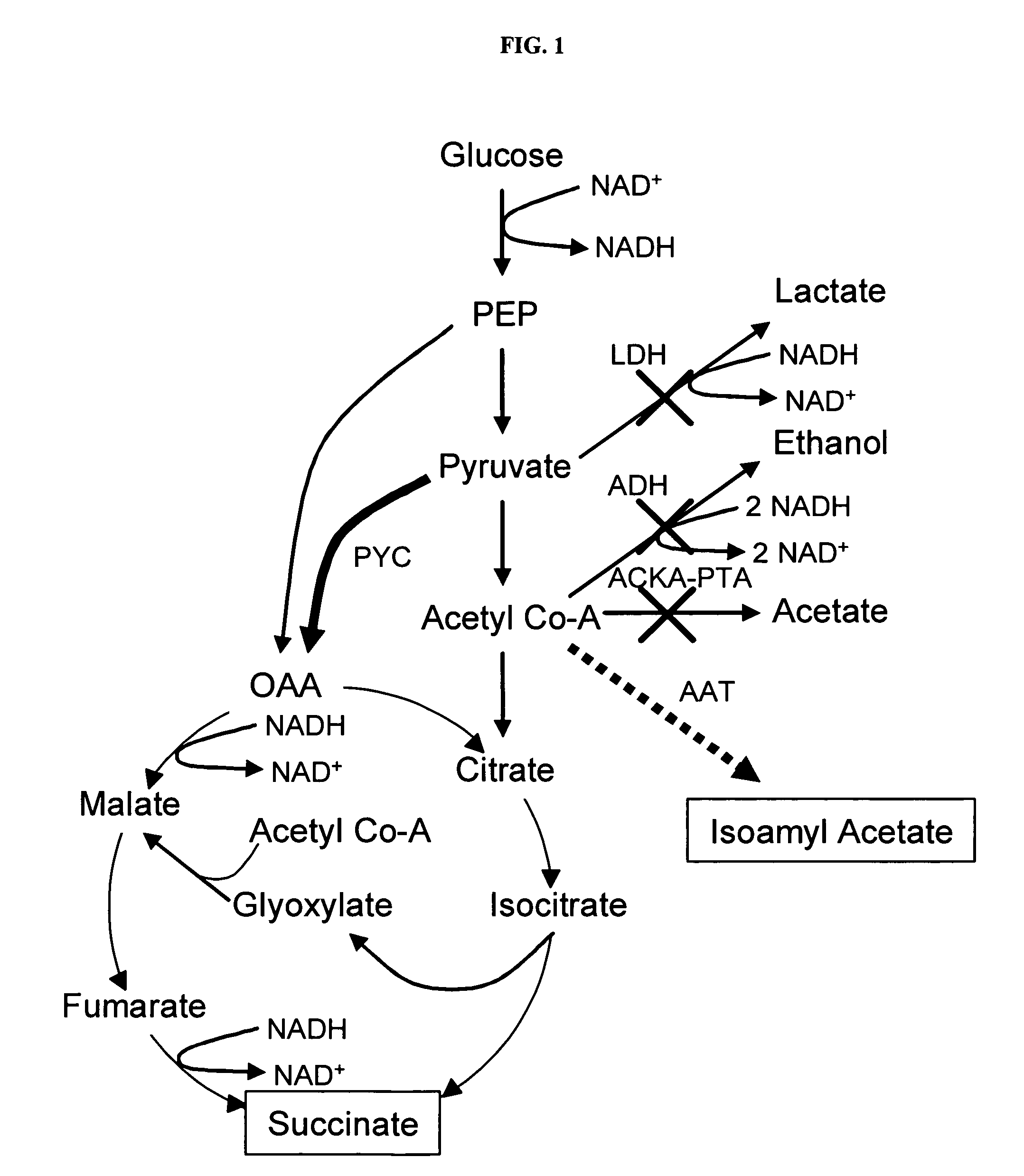
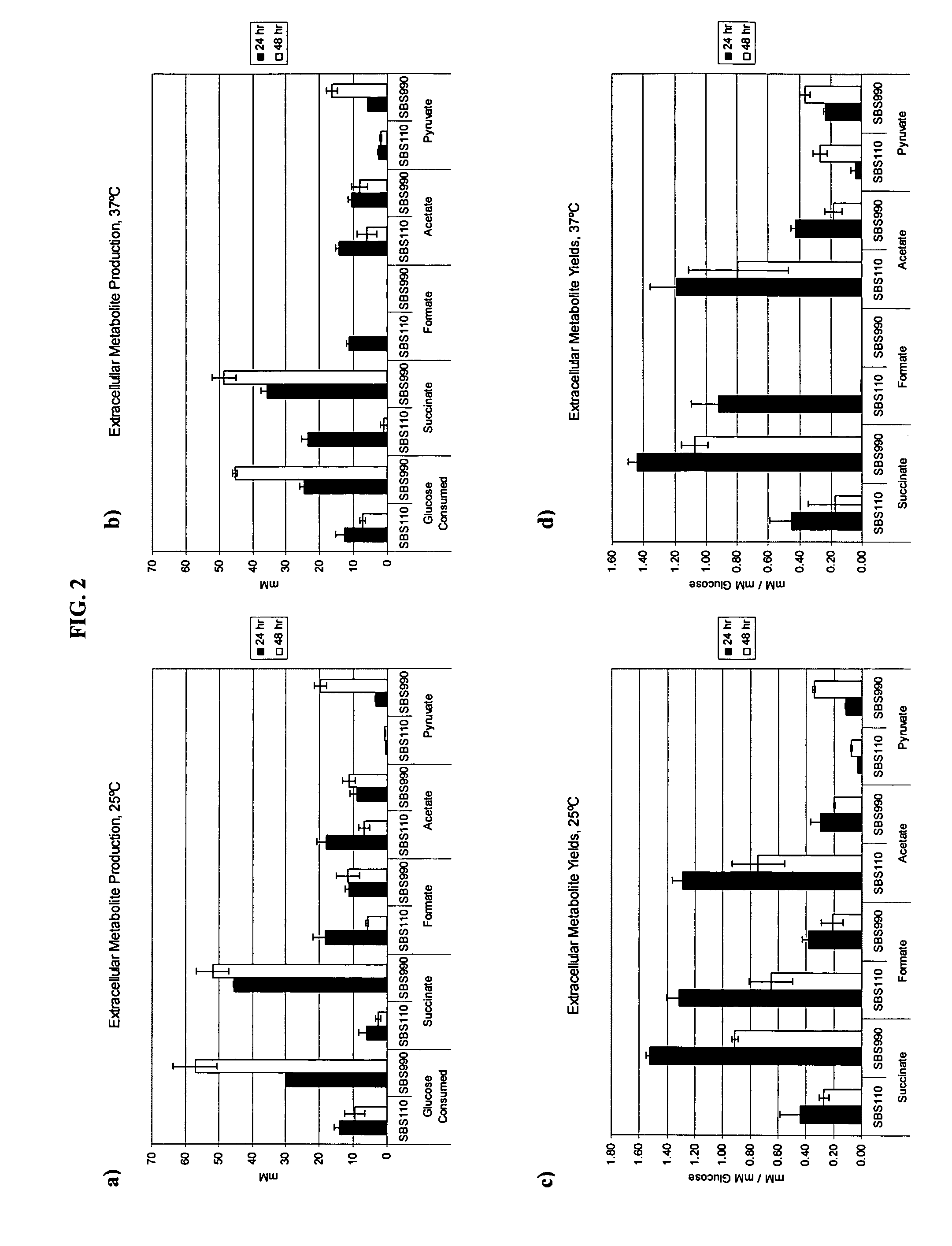
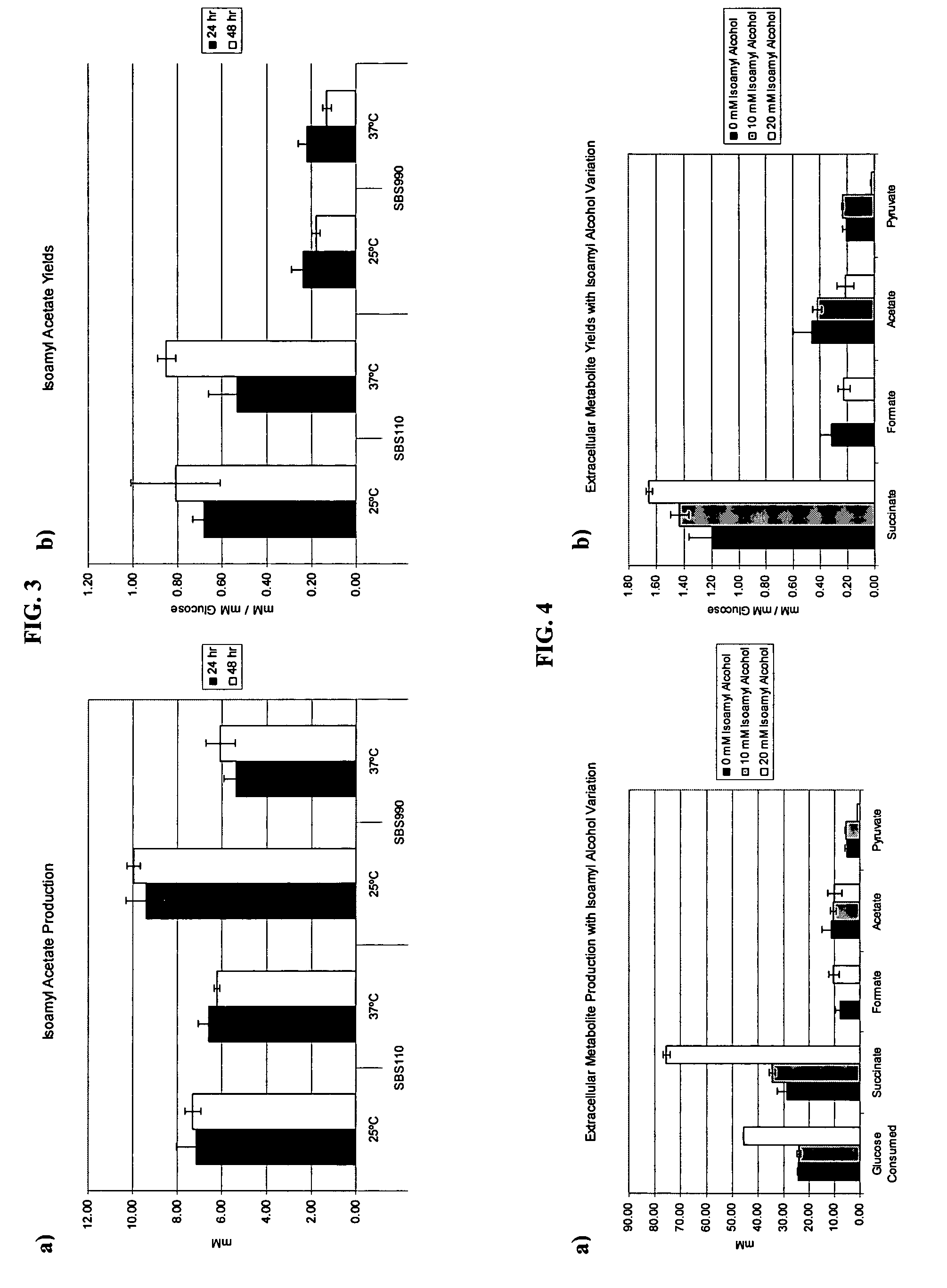
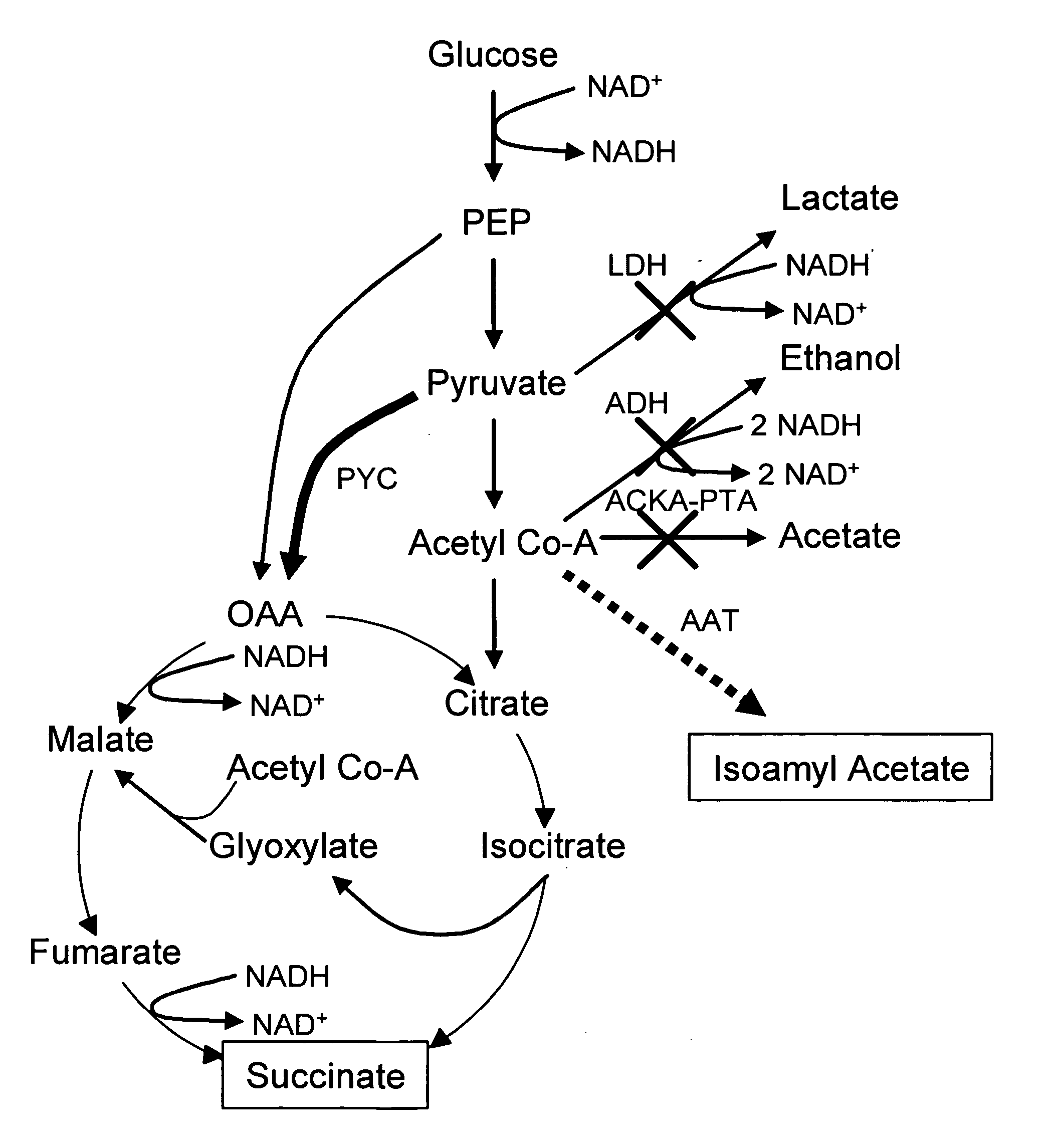
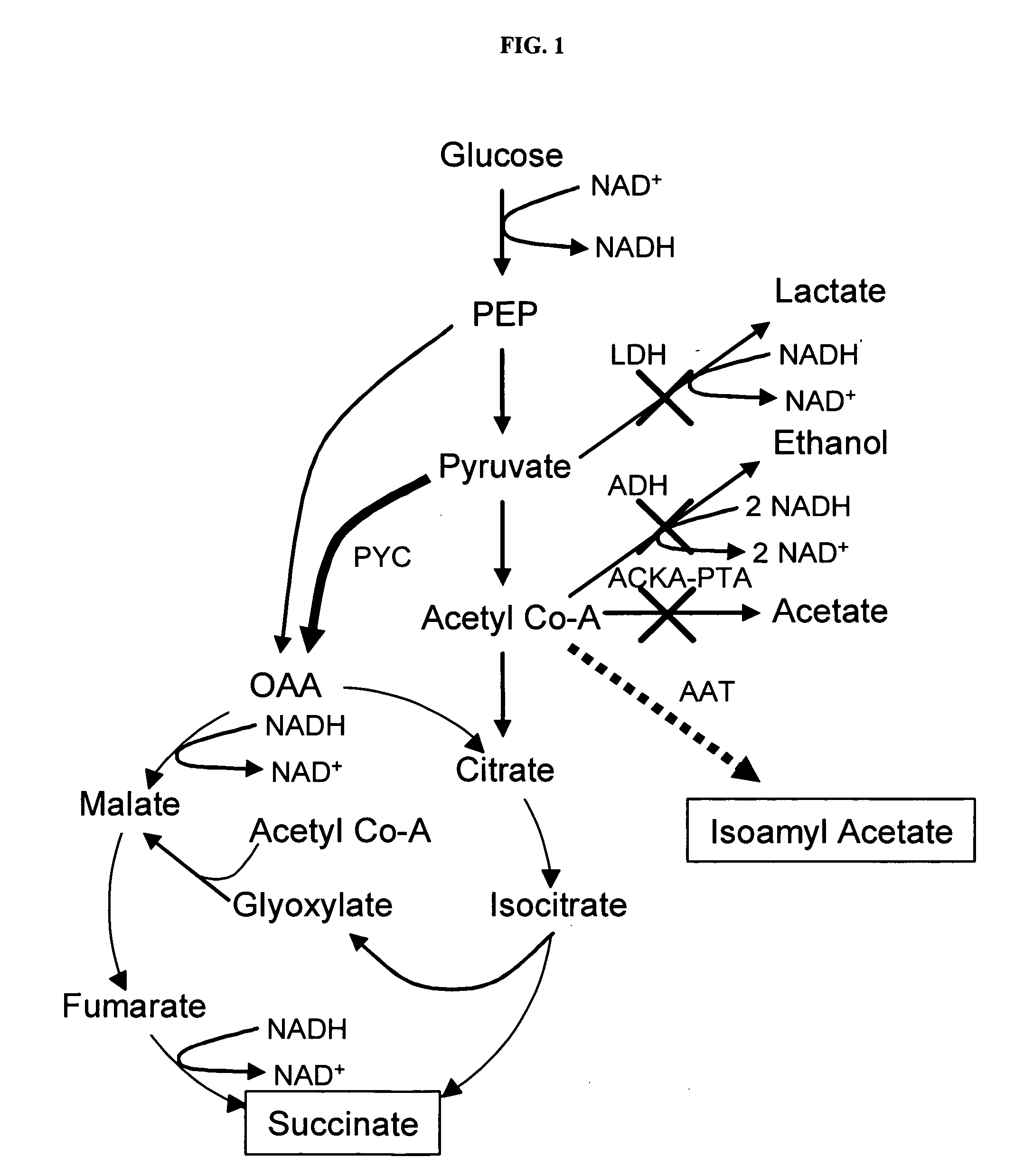
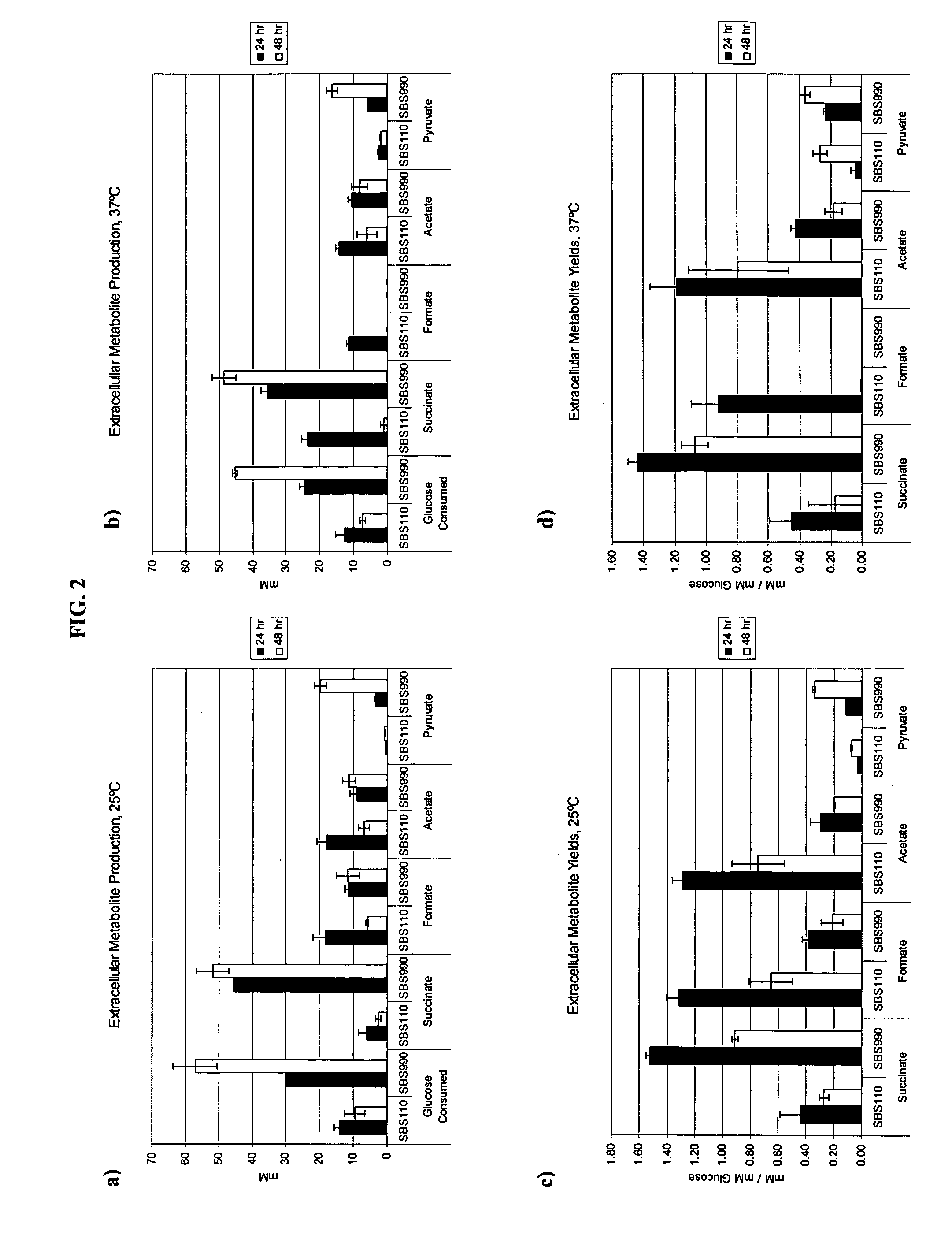
In vivo method of producing esters from acetyle coA, such as isoamyl acetate and succinate, has been developed by producing null mutants in pathways that use acetyl coA and by overexpressing products that use NADH and in order to maintain the proper redox balance between NADH and NAD+. The method is exemplified with null mutations in ldhA, adhE, ackA-pta and overexpression of pyruvate carboxylase and alcohol acetyltransferase. This strain produces higher levels of both isoamyl acetate and succinate.
Owner:RICE UNIV
Simultaneous anaerobic production of isoamyl acetate and succinic acid
InactiveUS20060141594A1Maximizing isoamyl acetate productionEasy to separateBacteriaTransferasesSuccinic acidPyruvate synthesis
In vivo method of producing esters from acetyle coA, such as isoamyl acetate and succinate, has been developed by producing null mutants in pathways that use acetyl coA and by overexpressing products that use NADH and in order to maintain the proper redox balance between NADH and NAD+. The method is exemplified with null mutations in ldhA, adhE, ackA-pta and overexpression of pyruvate carboxylase and alcohol acetyltransferase. This strain produces higher levels of both isoamyl acetate and succinate.
Owner:RICE UNIV
Beverages containing water-soluble vitamin E
The present invention relates to beverage compositions that have the added benefit of providing a water-soluble source of Vitamin E. For example, d,l- or d-α-tocopheryl polyethylene glycol 1000 succinate (such as Vitamin E TPGS™) may be used to provide tocopheryl (i.e., vitamin E) content to beverages while concurrently maintaining beverage clarity when desired. Various additives may also be used in the beverage compositions of the present invention, such as flavoring agents, colorants, preservatives, sweeteners as well as other vitamins, minerals and / or electrolytes.
Owner:COOK PHILLIP MICHAEL
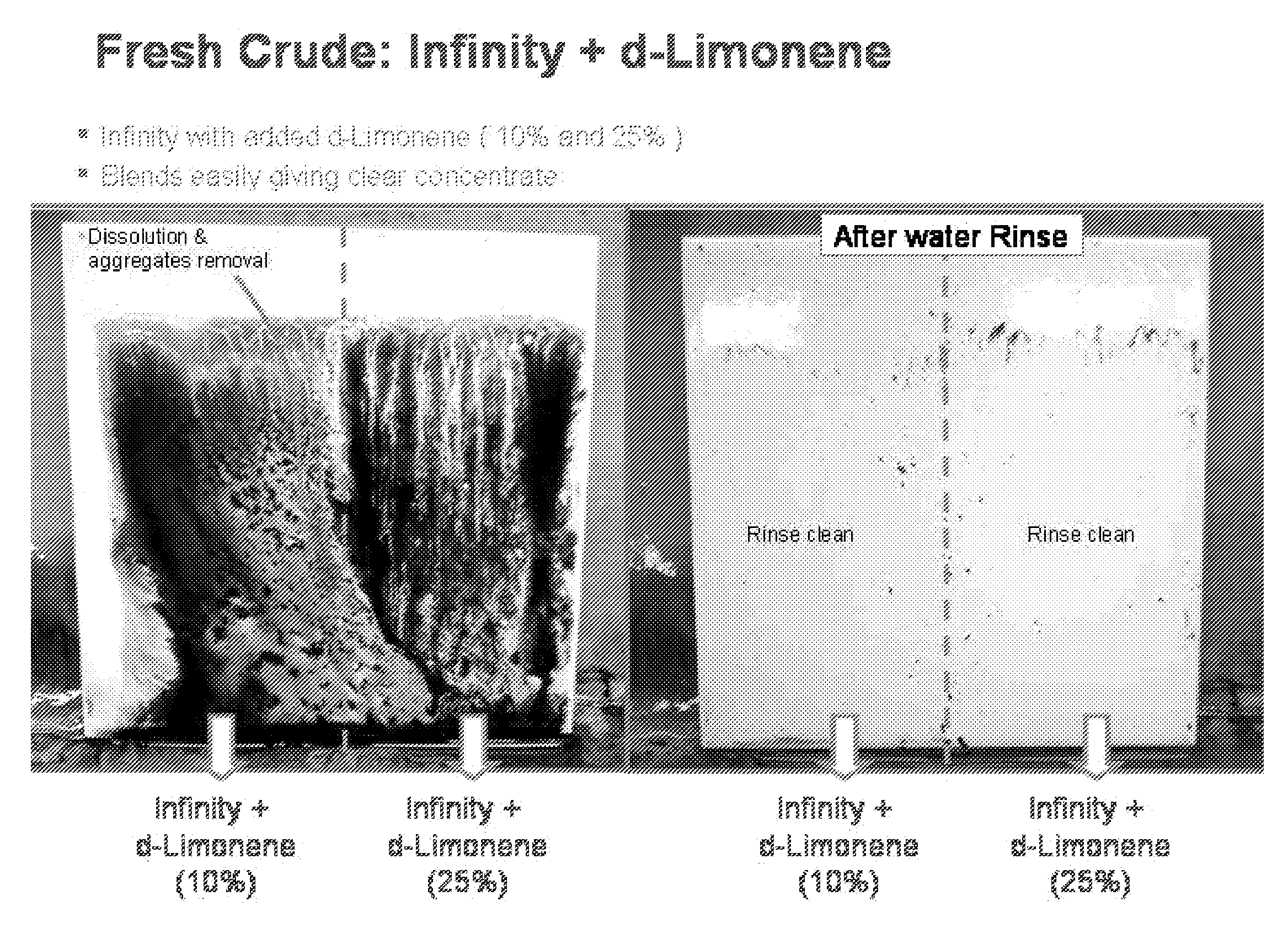
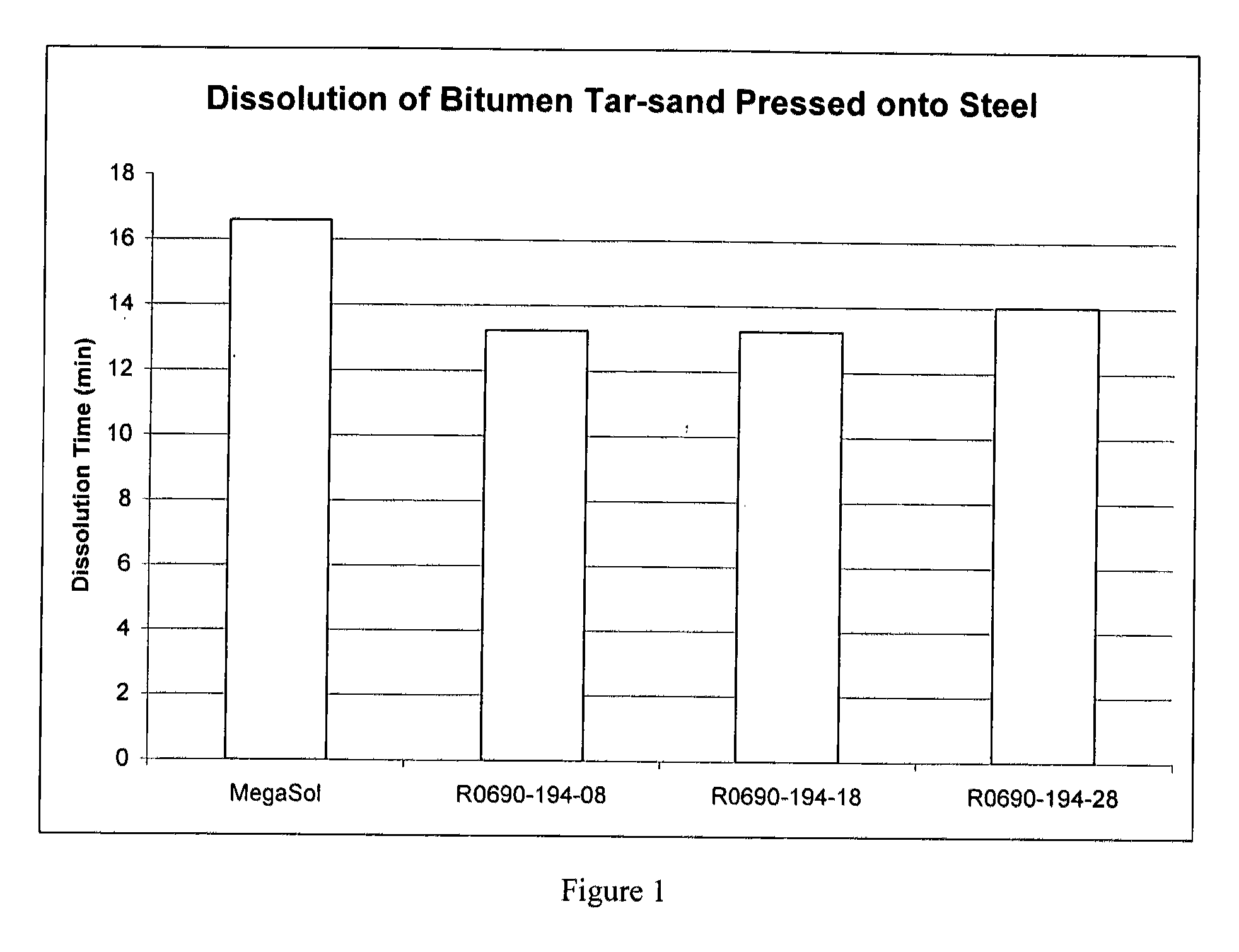
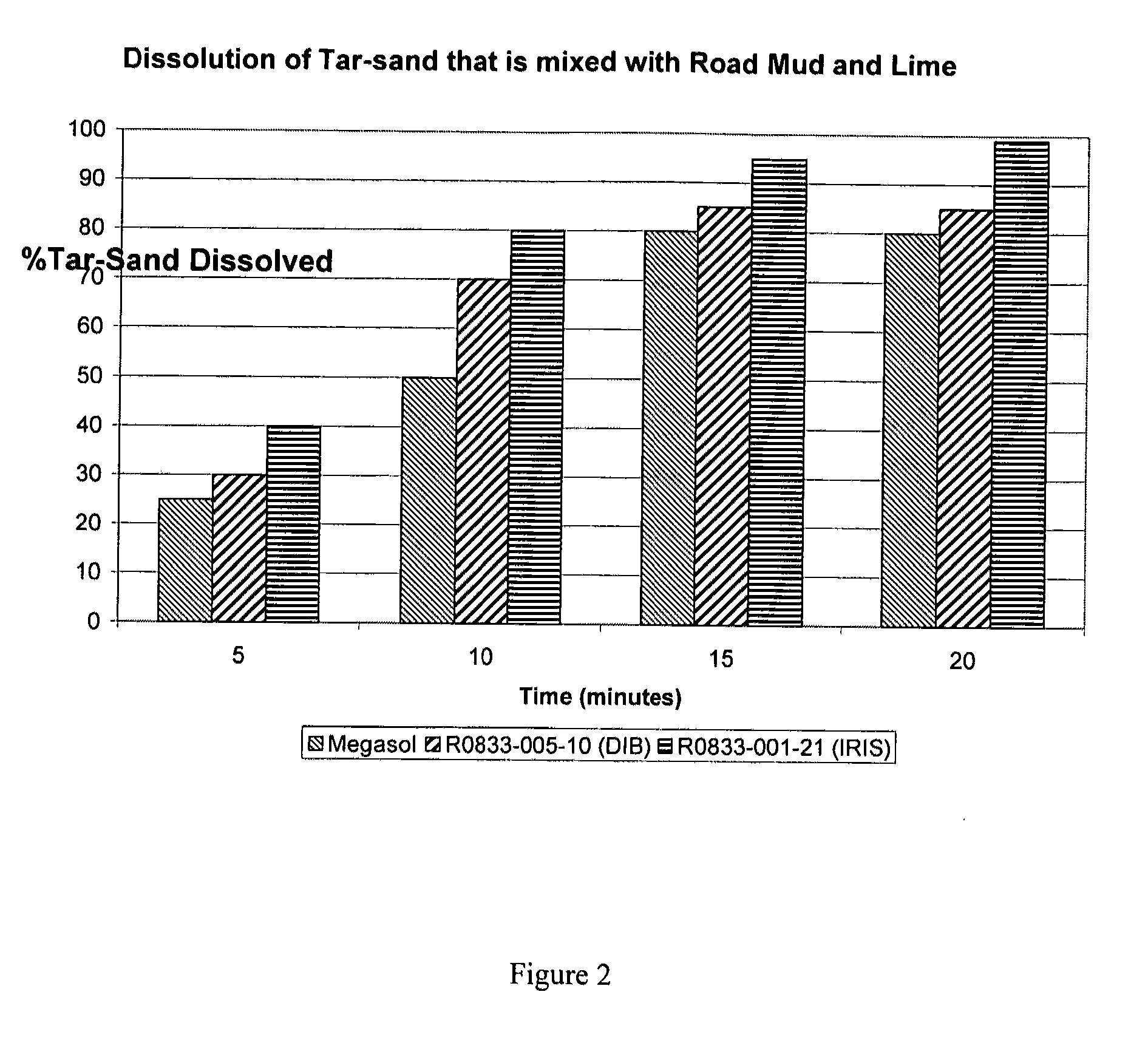
Dibasic esters utilized as terpene co-solvents, substitutes and/or carriers in tar sand/bitumen/asphaltene cleaning applications
ActiveUS20120149626A1Reduce concentrationImprove environmental conditionsOrganic detergent compounding agentsNon-ionic surface-active compoundsCarrier fluidFuel oil
A heavy oil cleaning composition comprising: a) a blend of dibasic esters comprising dialkyl methylglutarate and at least one of a dialkyl adipate or dialkyl ethylsuccinate; b) at least one terpene; and c) at least one surfactant. Also described are methods for delivering a solvent at reduced concentration comprising the steps of: a) obtaining a terpene-based solvent; and b) mixing the terpene-based solvent with a carrier fluid (the carrier fluid comprising a microemulsion of i) a blend of dibasic esters selected from the group consisting of dialkyl methylglutarate, dialkyl adipate, dialkyl ethylsuccinate, dialkyl succinate, dialkyl glutarate and any combination thereof, ii) at least one surfactant selected from the group consisting of a terpene alkoxylate, an alcohol alkoxylate and any combination thereof; and iii) water) in order to obtain a mixture to clean heavy oils.
Owner:RHODIA OPERATIONS SAS
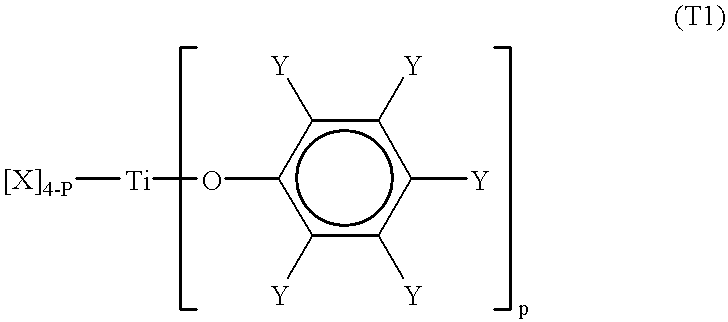
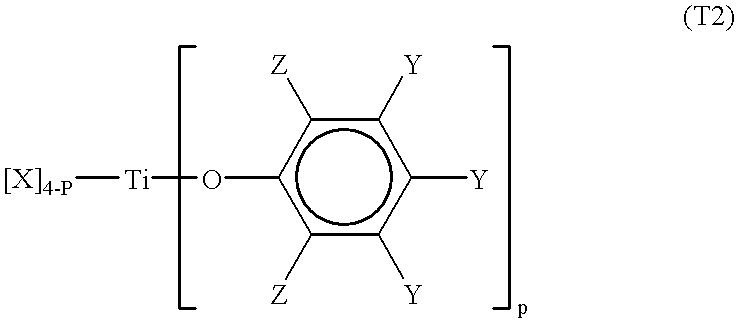
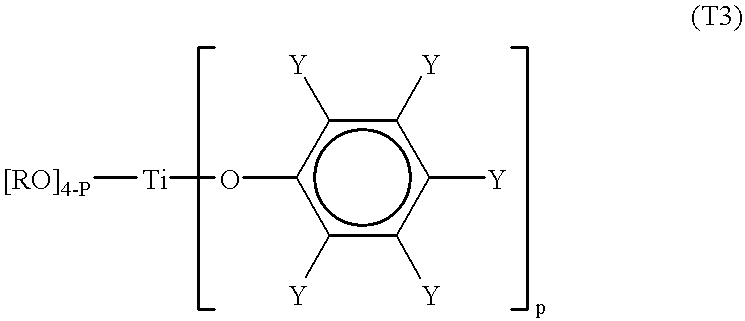
Processes for the preparation of a monodisperse polymers, processes for the continuous polymerization of cyclic monomers, and polymers prepared thereby
The present invention relates to a method for preparing lactone polymers, carbonate polymers, lactone-carbonate block copolymers and lactone-arbonate random copolymers via a ring-opening addition reaction of a lactone monomer, a cyclic carbonate monomer or a mixture thereof using an initiator and in the presence of a specific titanium-type Lewis acid catalyst. The resulting polymers have a molecular weight distribution (Mw / Mn) approximately equal to 1 or a extremely high purity of single-structure components. The polymer molecules range in an oligomer region to a molecular weight of approximately 200,000. The present invention also relates to a method for preparing lactone polymers, carbonate polymers and lactone-carbonate copolymers having a narrow molecular weight distribution and which can be obtained via a continuous polymerisation of a lactone monomer and / or a cyclic carbonate monomer using an initiator in an extruder in the presence of a specific titanium-type Lewis acid catalyst or an aluminium-type Lewis acid catalyst. The present invention also relates to these resulting polymers.
Owner:DAICEL CHEM IND LTD
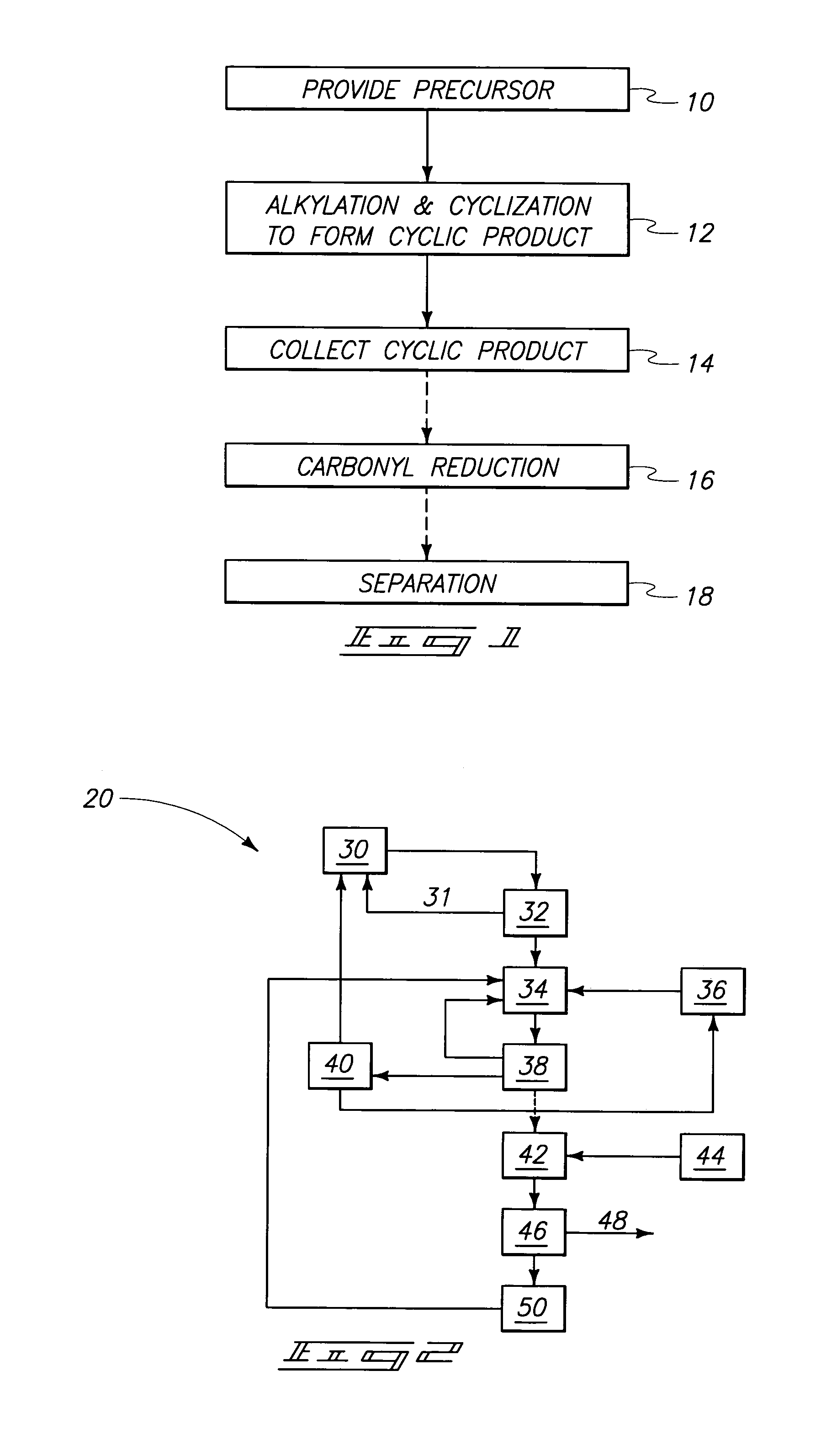
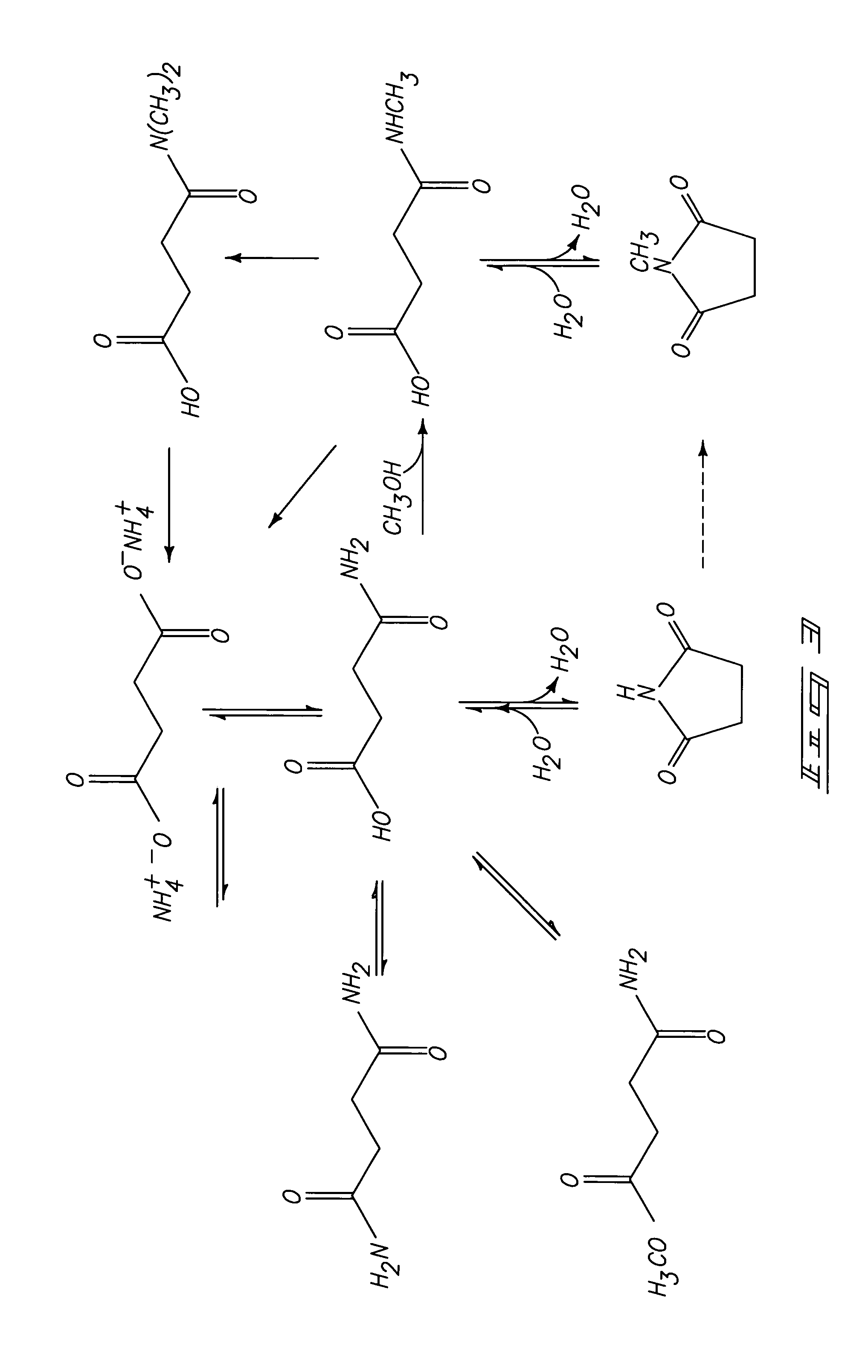
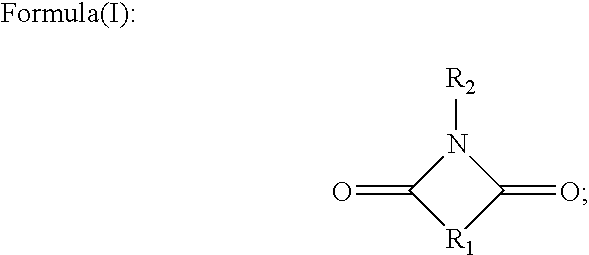
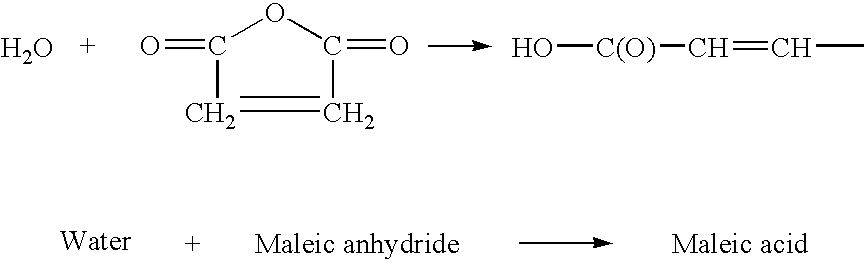
Process for producing cyclic compounds
The invention includes methods of processing an initial di-carbonyl compound by conversion to a cyclic compound. The cyclic compound is reacted with an alkylating agent to form a derivative having an alkylated ring nitrogen. The invention encompasses a method of producing an N-alkyl product. Ammonia content of a solution is adjusted to produce a ratio of ammonia to di-carboxylate compound of from about 1:1 to about 1.5:1. An alkylating agent is added and the initial compound is alkylated and cyclized. The invention includes methods of making N-methyl pyrrolidinone (NMP). Aqueous ammonia and succinate is introduced into a vessel and ammonia is adjusted to provide a ratio of ammonia to succinate of less than 2:1. A methylating agent is reacted with succinate at a temperature of from greater than 100° C. to about 400° C. to produce N-methyl succinimide which is purified and hydrogenated to form NMP.
Owner:BATTELLE MEMORIAL INST
Aerobic succinate production in bacteria
Methods of increasing yields of succinate using aerobic culture methods and a multi-mutant E. coli strain are provided. Also provided is a mutant strain of E. coli that produces high amounts of succinic acid.
Owner:RICE UNIV
Mixed glue bundle pharmaceutical preparations produced in combination use of multiple surfactant and processes for their preparation
InactiveCN101138550AStrong dilution stabilityReduce viscosityPharmaceutical non-active ingredientsLiposomal deliverySolubilityMixed micelle
The present invention relates to a mixed micelle medicine preparation and a preparation method, which is prepared by the combination of various kinds of surface acting agents. The mixed micelle consists of the polyethylene glycol-12-hydroxy stearate and the other surface acting agents of one kind or various kinds. The other surface acting agents comprise phospholipid, VE Macrogol succinate, Macrogol-VE-carbonate and Macrogol-VE-succinate. In addition, the mixed micelle also comprises drugs, solvent, a stabilizer with or without other components and a PH conditioner. The amount of the polyethylene glycol-12-hydroxy stearate in the prescription is 4 percentage to 40 percentage, W / V: the amount of the phospholipid is 0 percentage to 30 percentage, W / V: the amount of the activator is 0 percentage to 30 percentage, W / V: the amount of the drug is 0.001 percentage to 10 percentage, W / V: the amount of the solvent is 0 percentage to 90 percentage, W / V. The medicine comprises the hydrophobicity drug and the lip solubility drug, but the medicine is not restricted by the both kinds of drugs. The present invention has the following advantages. Firstly, the preparation has good dilution stability, which can improve the defect in the present preparation and can meet the demanding for clinical drug administration. Secondly, the toxicity is low and the chemical stability is excellent.
Owner:SHENYANG PHARMA UNIVERSITY
Alkyl polyglycoside derived sulfosuccinates
ActiveUS7087571B1Cosmetic preparationsOrganic detergent compounding agentsGlycoside formationAlkyl polyglycoside
The invention relates to a series of polyglycoside derivatives that contain water-soluble sulfosuccinate groups introduced into the molecule by reaction with the hydroxyl groups present in the starting polyglycoside molecule, with the chloro material. The preferred products have more than one water-soluble group per molecule and are made with mild reagents to avoid discoloration and mal odor. The most preferred products have between 2 and 3 functional groups per molecule.
Owner:SURFATECH
Medical hydrogel composition, medical hydrogel as well as preparation method and application of medical hydrogel
ActiveCN105963792ASmall degree of swellingGood biocompatibilitySurgical adhesivesAbsorbent padsSuccinimidyl carbonateBiocompatibility Testing
The invention relates to a medical hydrogel composition, a medical hydrogel as well as a preparation method and an application of the medical hydrogel. The medical hydrogel composition consists of a first component and a second component, wherein the first component includes polylysine and polyethyleneimine; the second component includes one or more of 4arm-polyethylene glycol-succinimidyl glutarate, 4arm-polyethylene glycol-succinimidyl succinate and 4arm-polyethylene glycol-succinimidyl carbonate, wherein the polymerization degree of the polylysine is above 20, preferably 25-35. The medical hydrogel disclosed by the invention is low in swelling degree, which is just 10-50%, and the medical hydrogel is applicable to narrow parts that cranial, spinal and peripheral nerves are densely distributed. In addition, the medical hydrogen disclosed by the invention is good in biocompatibility and is excellent in antibacterial property and biodegradability. Moreover, the medical hydrogel disclosed by the invention is also non-irritant to tissues.
Owner:MEDPRINSHENZHEN REGENERATIVEA MEDICAL TECH CO LTD +1
Linseed oil microcapsule powder and its prepn
InactiveCN101019838AImprove stabilityKeep aliveOrganic active ingredientsPharmaceutical non-active ingredientsFreeze-dryingHigh pressure
The present invention is linseed oil microcapsule powder and its preparation, and features that modified polysaccharide, sodium octyl alkenyl succinate starch, is used as capsule wall material for forming microcapsule in preparing linseed oil microcapsule powder. The preparation process includes adding linseed oil into the solution of sodium octyl alkenyl succinate starch, stirring to form homogeneous emulsion, high pressure homogenizing to obtain linseed oil emulsion of granularity smaller than 1000 nm, and final spray drying or freeze drying to obtain white linseed oil microcapsule powder with high stability and high flowability. The linseed oil microcapsule powder has high linseed oil activity, high stability and high bioavailability, and may be applied in food and health product.
Owner:TSINGHUA UNIV +1
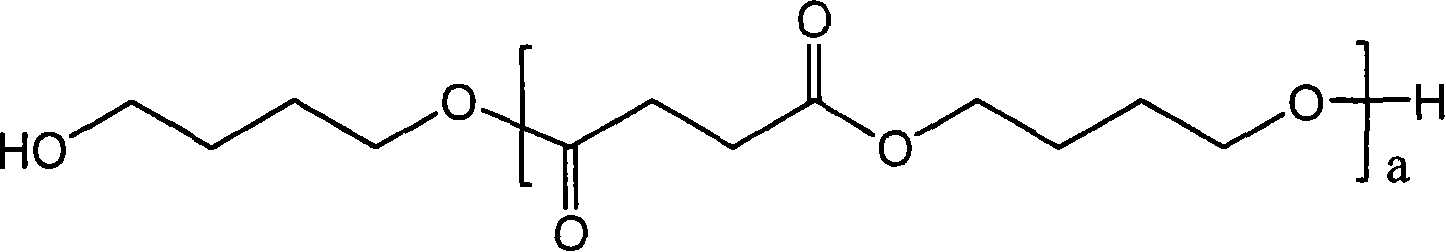
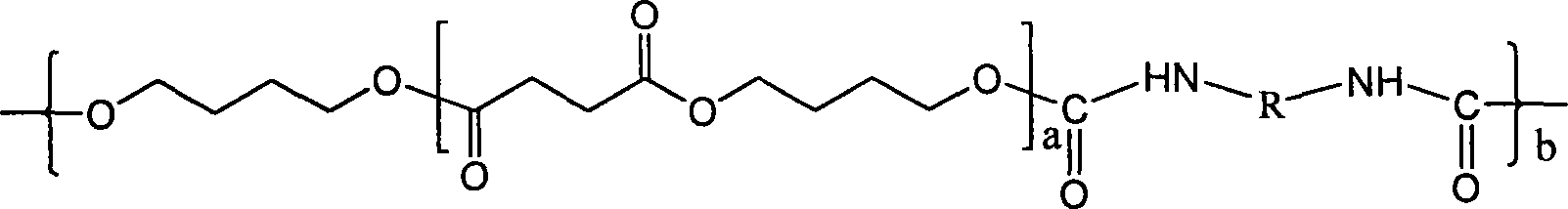
High molecular weight poly(butylene succinate) and preparation method thereof
ActiveCN101077905AHigh molecular weightNarrow molecular weight distributionPolymer scienceButanediol
The present invention relates to one kind of high molecular weight polybutanediol succinate and its preparation process, and features that bihydroxy terminated butanediol succinate, especially low molecular weight bihydroxy terminated butanediol succinate, as the pre-polymer and diisocyanate as the chain extender in 0.1-13 % are molten and reacted to prepare polybutanediol succinate with low cost, great molecular weight, narrow molecular weight distribution and excellent mechanical performance. The polybutanediol succinate has number average molecular weight greater than 5.5x10<4>, molecular weight distribution of 1.4-1.8, tensile strength of 39-58 MPa, and elongation at break of 300-700 %.
Owner:SICHUAN UNIV
Coated preparation soluble in the lower digestive tract
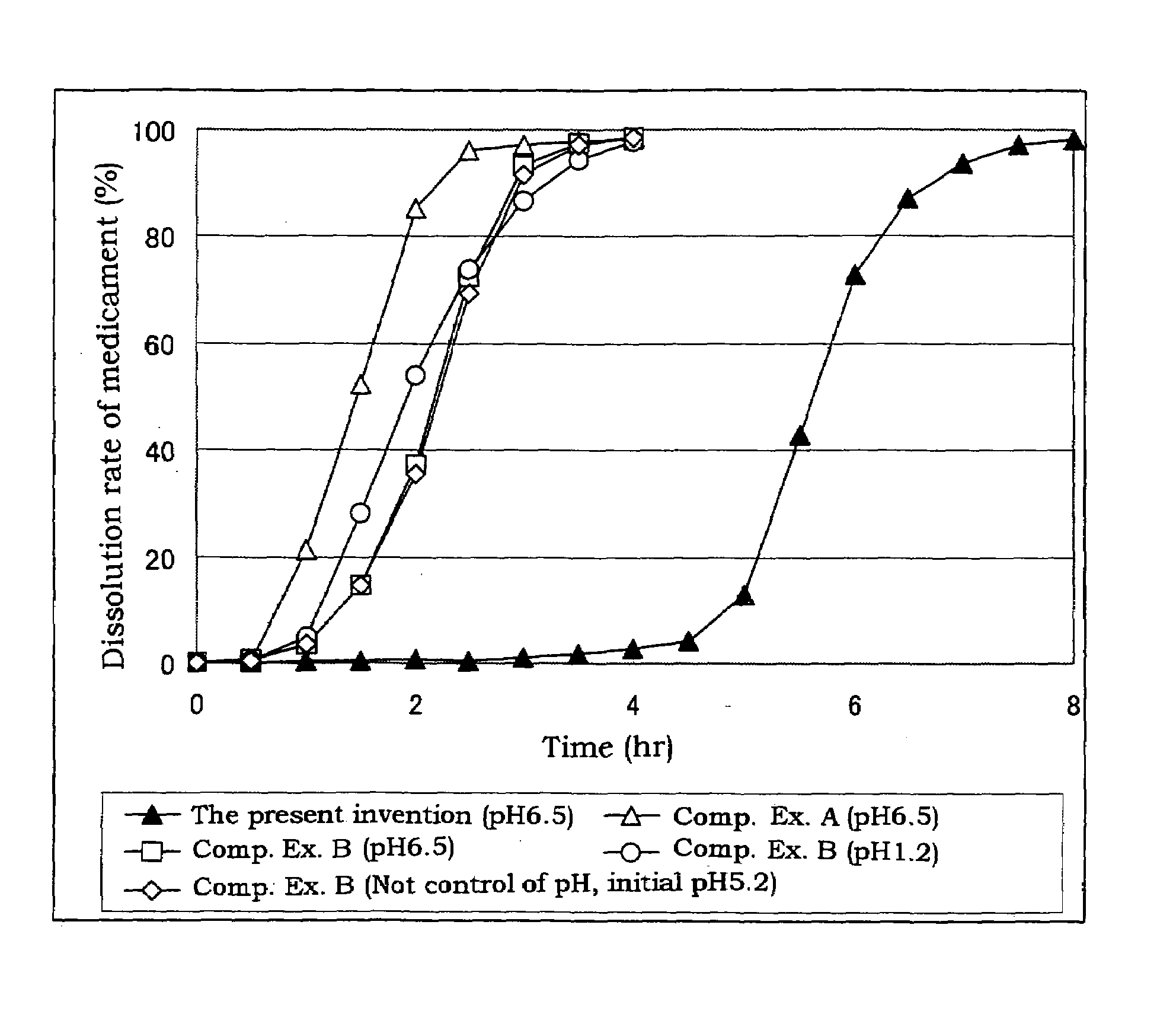
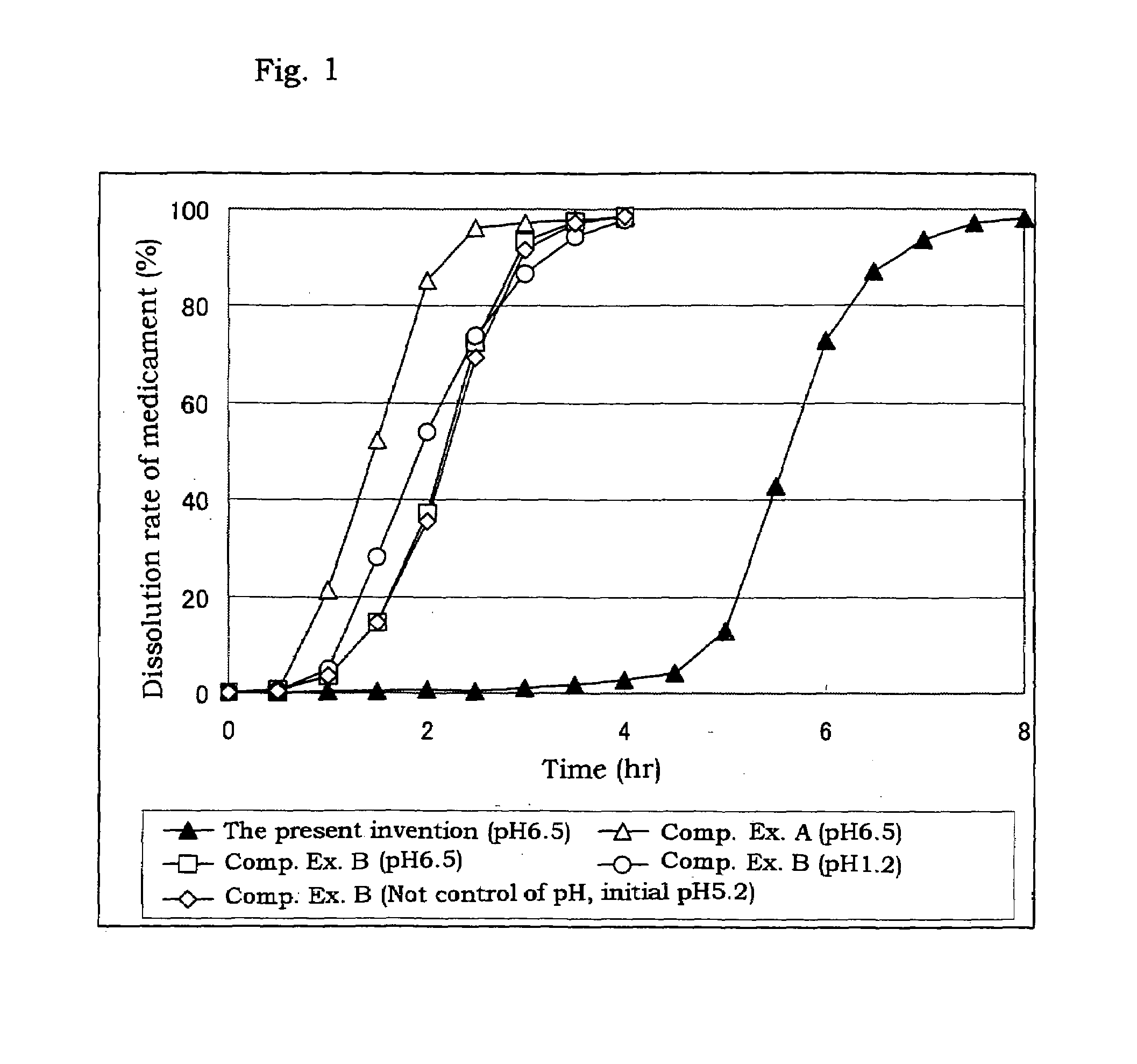
A coating dispersion soluble in the lower digestive tract which is prepared by blending a hydroxypropyl methylcellulose acetate succinate (HPMCAS) soluble at around pH 7 with a conventional plasticizer and an anion surfactant and further adding an acid, wherein the HPMCAS has an average particle size of 10 μm or less and is dispersed at a concentration of 2 to 20% by weight, and the acid is used in an amount of 1 to 10 parts by weight per 100 parts by weight of HPMCAS, and a sustained release coated preparation capable of releasing a medicament in the large intestine at the lower digestive tract, which is prepared by coating a medicament-containing solid preparation such as a granular core with the coating dispersion.
Owner:OTSUKA PHARM CO LTD
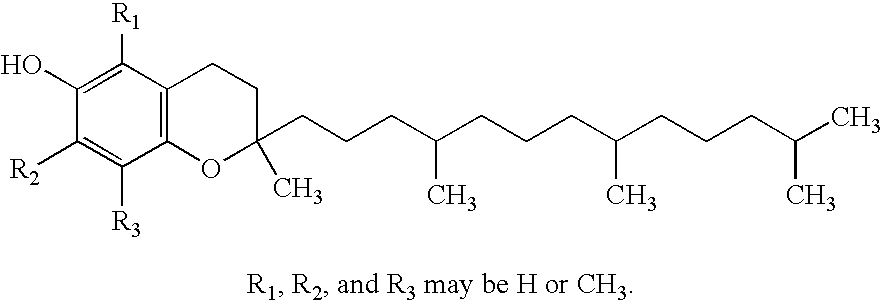
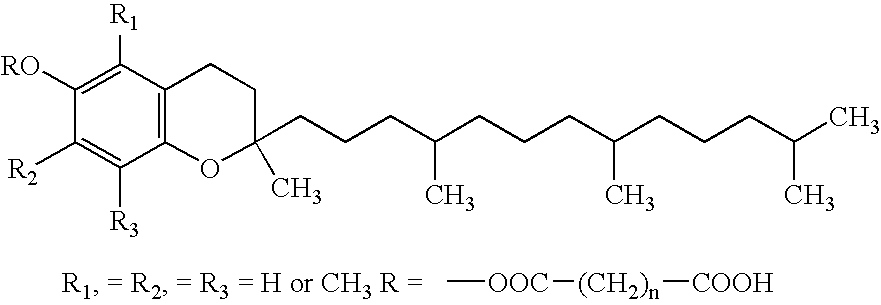
Multiple antioxidant micronutrients
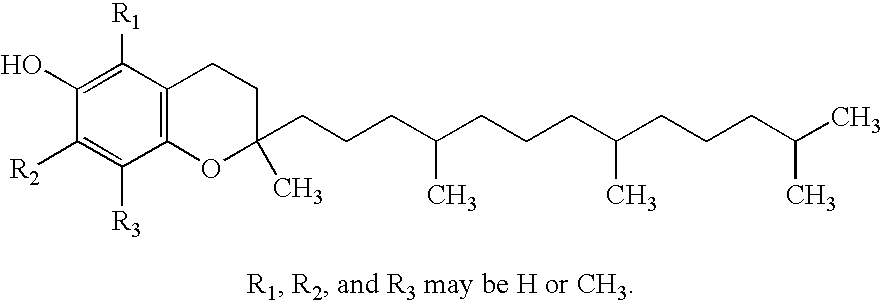
A method for administering an antioxidant composition to humans according to their age and sex is disclosed wherein the method comprises administering to said humans a daily dose of a multiple antioxidant micronutrient composition comprising vitamin A (palmitate), beta carotene (from natural d. salina), vitamin C (calcium ascorbate), vitamin D-3 (cholecalciferol), natural source vitamin E including both d-alpha tocopheryl and d-alpha tocopheryl acid succinate, thiamine mononitrate, riboflavin, niacinamide ascorbate, d-calcium pantothenate, pyridoxine hydrochloride, cyanocobalamin, folic acid (folacin), d-biotin, selenium (1-seleno methionine), chromium picolinate, zinc glycinate, calcium citrate, and magnesium citrate. For persons over the age of about 51, the composition preferably further comprises one or more of co-enzyme Q10, N-acetyl cysteine, and alpha lipoic acid. Preferably, also, vitamin D is added for women over the age of about 36.
Owner:NEW AGE HEALTH SCI INC
Continuously-produced full-degradable starch-based plastic alloy and preparation method thereof
The invention discloses a continuously-produced full-degradable starch-based plastic alloy and a preparation method thereof. The starch-based plastic alloy contains the following components in parts by weight: 40-90 parts of starch, 5-60 parts of poly(butylene succinate), 5-60 parts of polylactic acid, 10-40 parts of plasticizer, 0.1-10 parts of compatibilizer and 1-10 parts of processing acid. The preparation method comprises the steps of mixing starch, plasticizer and part of processing aid, and extruding to obtain thermoplastic starch particles; then, mixing the thermoplastic starch particles with poly(butylene succinate), polylactic acid, compatibilizer and the residual processing aid, and extruding to obtain the starch-based plastic alloy. The starch-based plastic alloy disclosed by the invention is favorable in hydrophobicity and strength, good in component compatibility, favorable in thermoplastic processability, capable of realizing continuous production and processing as well as simple and feasible in technical process.
Owner:EAST CHINA UNIV OF SCI & TECH
Catalyst that can be used in hydrotreatment, comprising metals of groups viii and VIB, and preparation with acetic acid and dialkyl succinate c1-c4
ActiveUS20130008829A1Improve catalytic performanceHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationAcetic acidCobalt
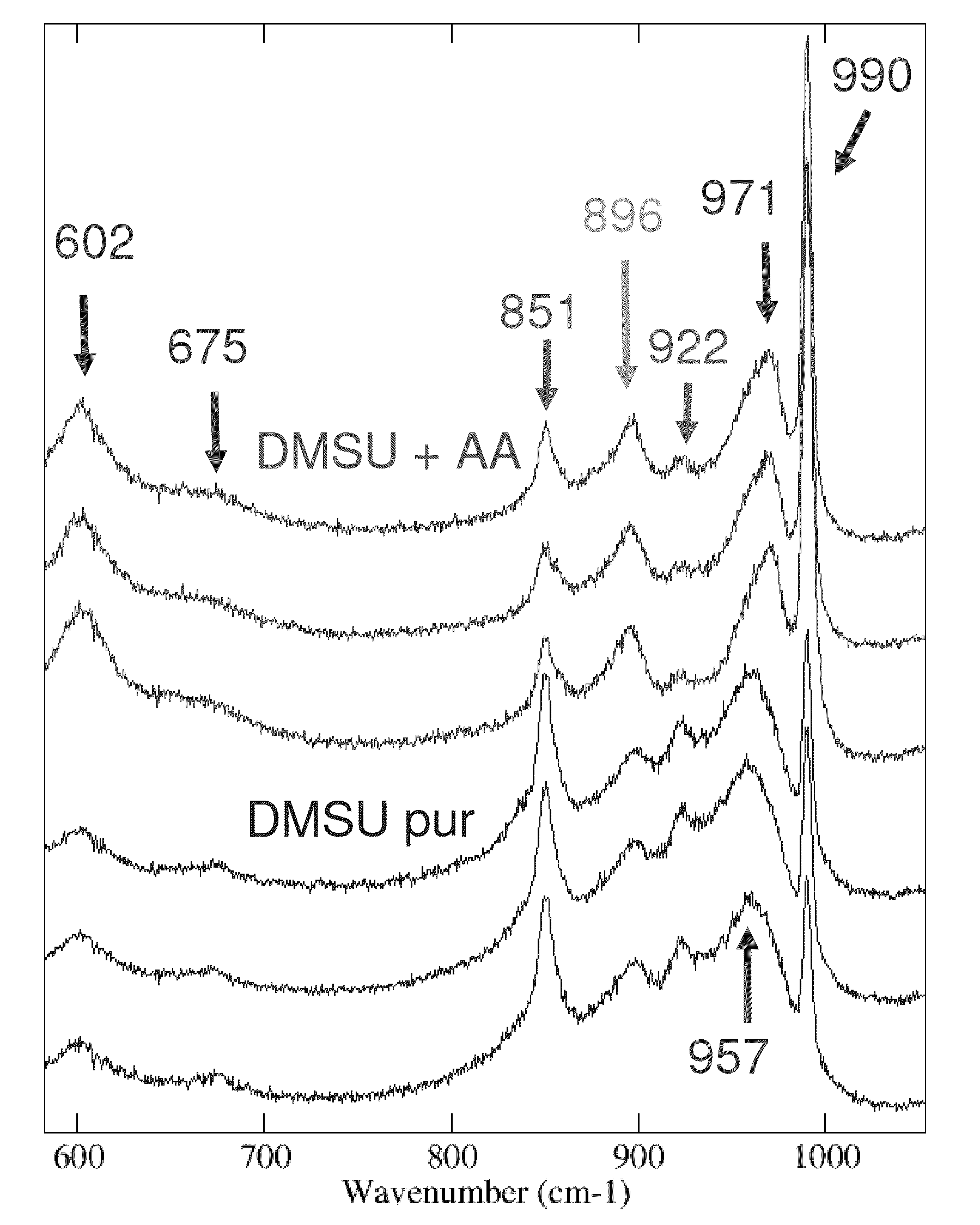
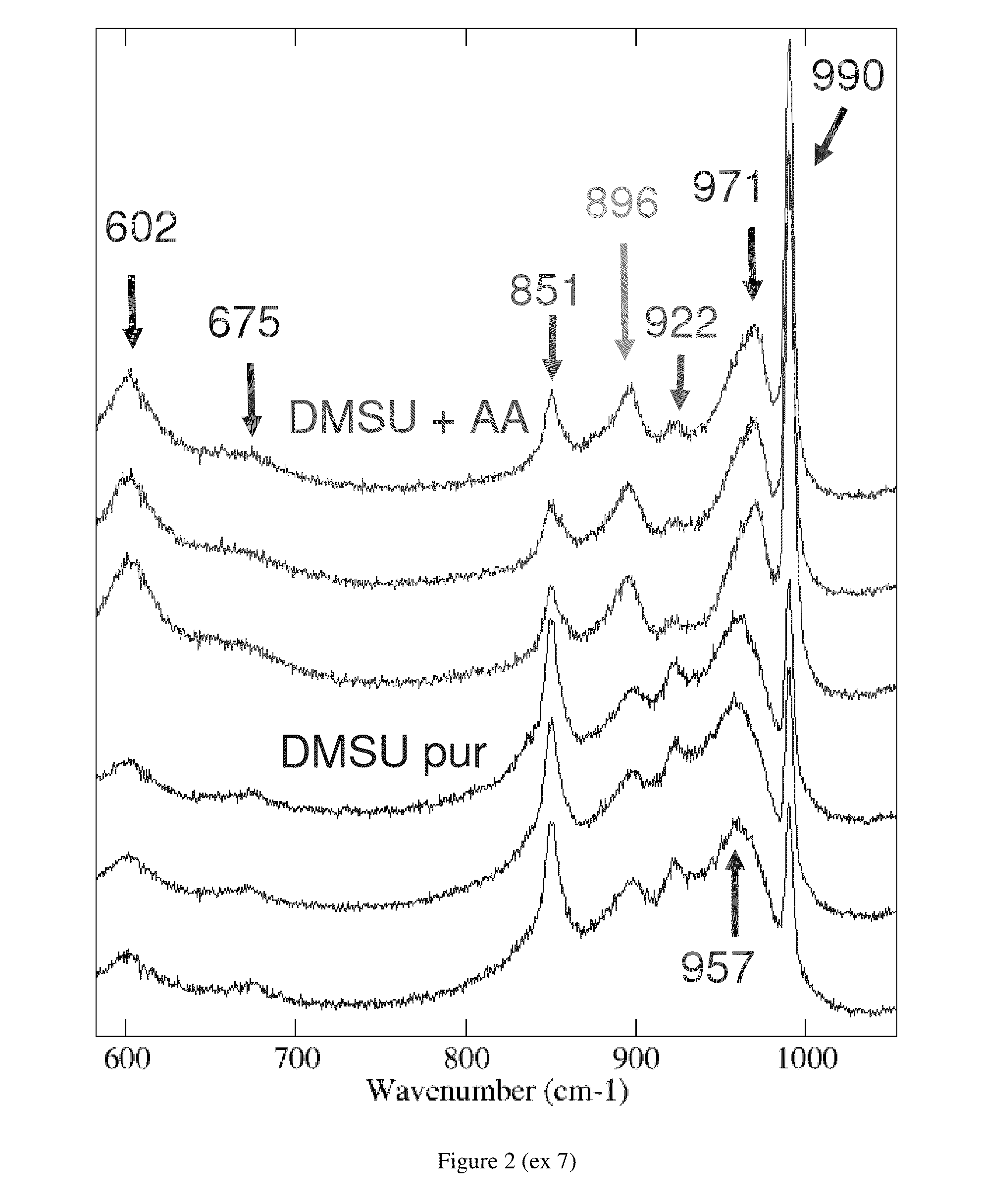
The invention relates to a catalyst usable in hydrotreatment processes, which comprises an alumina-based amorphous support, phosphorus, a C1-C4 dialkyl succinate, acetic acid and a hydro-dehydrogenizing function comprising at least one group VIII element and at least one group VIB element, preferably made up of cobalt and molybdenum, a catalyst whose Raman spectrum comprises the most intense bands characteristic of the Keggin heteropolyanions (974 and / or 990 cm−1), C1-C4 dialkyl succinate and acetic acid (896 cm−1). Preferably, the dialkyl succinate concerned is dimethyl succinate and its main band is at 853 cm−1.The invention also relates to the method of preparing said catalyst, wherein a catalytic precursor comprising the group VIB and group VIII elements, in particular the molybdenum-cobalt pair, and phosphorus, introduced by impregnation, then dried at a temperature below 180° C., is impregnated by the C1-C4 dialkyl succinate, the acetic acid and the phosphorus compound, if the latter has not been entirely introduced beforehand, then, after maturation, dried at a temperature below 180° C. prior to being optionally sulfurized.The invention also relates to the use of this catalyst in any hydrotreatment process.
Owner:TOTAL RAFFINAGE MARKETING +1
Polylactic acid toughening modifier and preparation method thereof
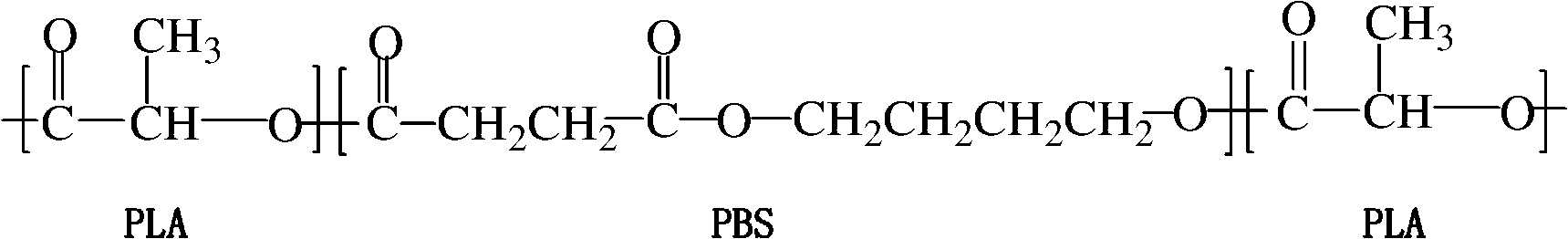
The invention relates to a polylactic acid toughening modifier in the technical field of high polymer material and a preparation method thereof. Lactide ring-opening polymerization is initiated on the terminal hydroxyl of poly (butylene succinate), and purification is carried out, thus obtaining the polylactic acid toughening modifier, the chemical structural formula is as follows. The modifier of the invention prepared by adopting degradable material can solve transport phenomenon and diosmose in the prior art.
Owner:SHANGHAI JIAO TONG UNIV
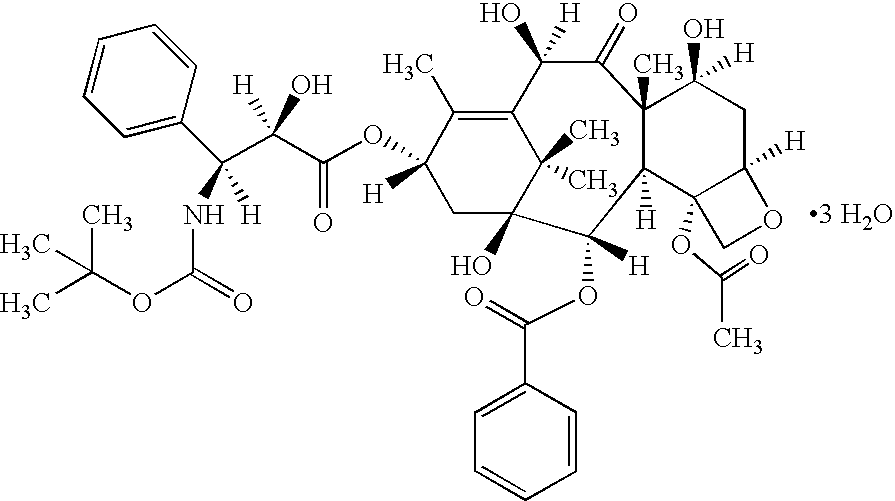
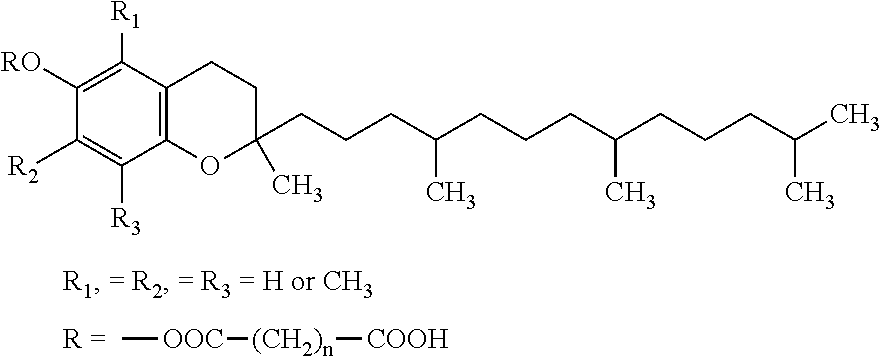
Vitamin E succinate stabilized pharmaceutical compositions, methods for the preparation and the use thereof
The present invention provides vitamin E succinate (VES)-stabilized compositions, methods for the preparation thereof and methods useful for the in vivo delivery of substantially water insoluble and optionally chemically unstable pharmacologically active agents (such as docetaxel).
Owner:CHEN ANDREW XIAN
Anticancer oral formulation
InactiveUS20100010059A1Improve oral bioavailabilityBiocideDispersion deliveryPolyethylene glycolSuccinates
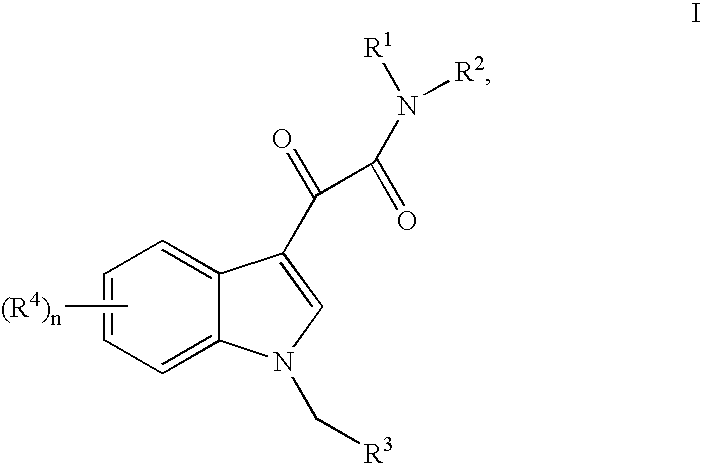
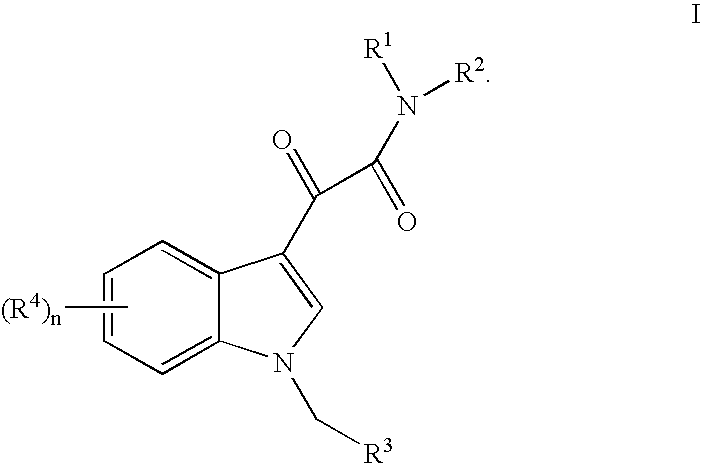
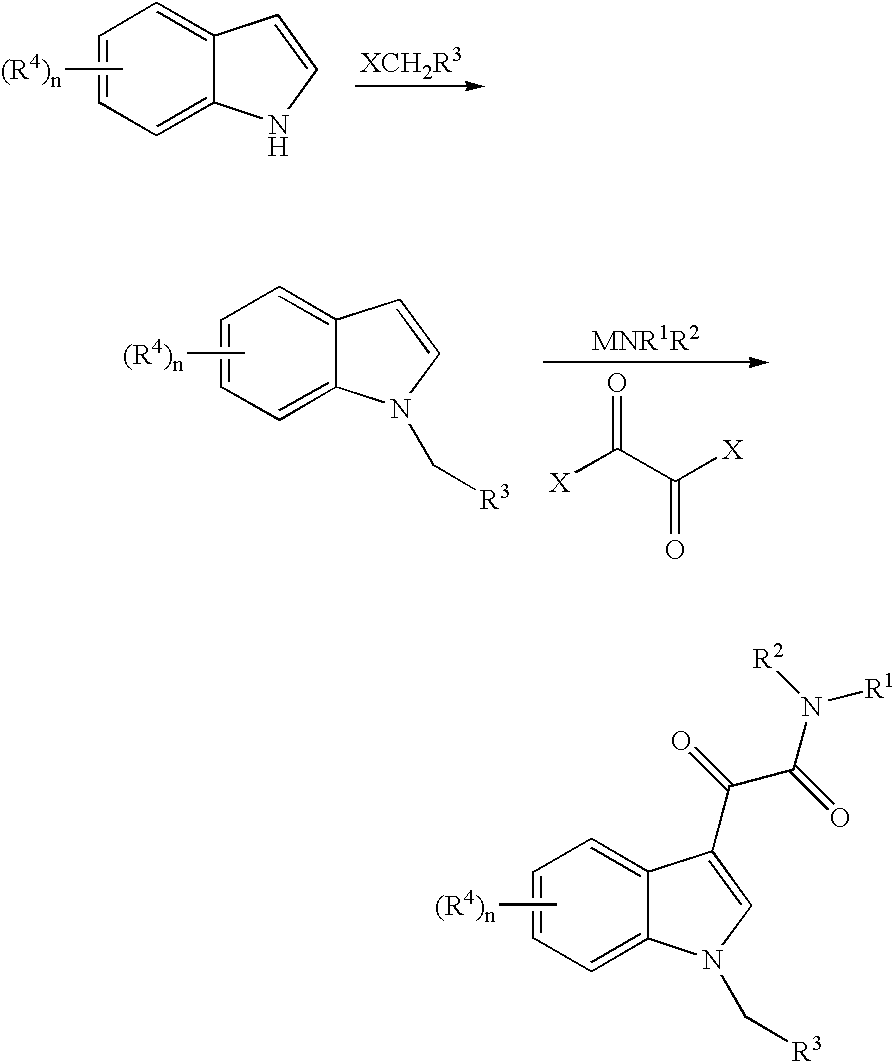
This invention relates to an oral formulation containing an effective amount of the compound of the following formula I:d-alpha-tocopheryl polyethylene glycol 1000 succinate (“TPGS”); and 2-(2-ethoxyethoxy)ethanol (“Transcutol”). R1 through R4 and n are defined herein. Also disclosed is a method of treating cancer by administering this formula to a subject orally.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Vitamin E succinate stabilized pharmaceutical compositions, methods for the preparation and the use thereof
The present invention provides vitamin E succinate (VES)-stabilized compositions, methods for the preparation thereof and methods useful for the in vivo delivery of substantially water insoluble and optionally chemically unstable pharmacologically active agents (such as docetaxel).
Owner:CHEN ANDREW XIAN
Succinate salt of O-desmethyl-venlafaxine
InactiveUS7026508B2Suitable for useImprove bioavailabilityOrganic active ingredientsNervous disorderSuccinatesDesmethyl
A novel salt of O-desmethyl venlafaxine is provided, O-desmethylvenlafaxine succinate. Pharmaceutical compositions, dosage forms and methods of use are also provided.
Owner:WYETH LLC
Multiparticulate O-desmethylvenlafaxine salts and uses thereof
InactiveUS20050175698A1Undesirable characteristicDosing is convenientBiocideNervous disorderSide effectFormate
A multiparticulate O-desmethylvenlafaxine (ODV) succinate or formate is described. Methods of treating depression and reducing the gastrointestinal side-effects of ODV are also described.
Owner:WYETH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com