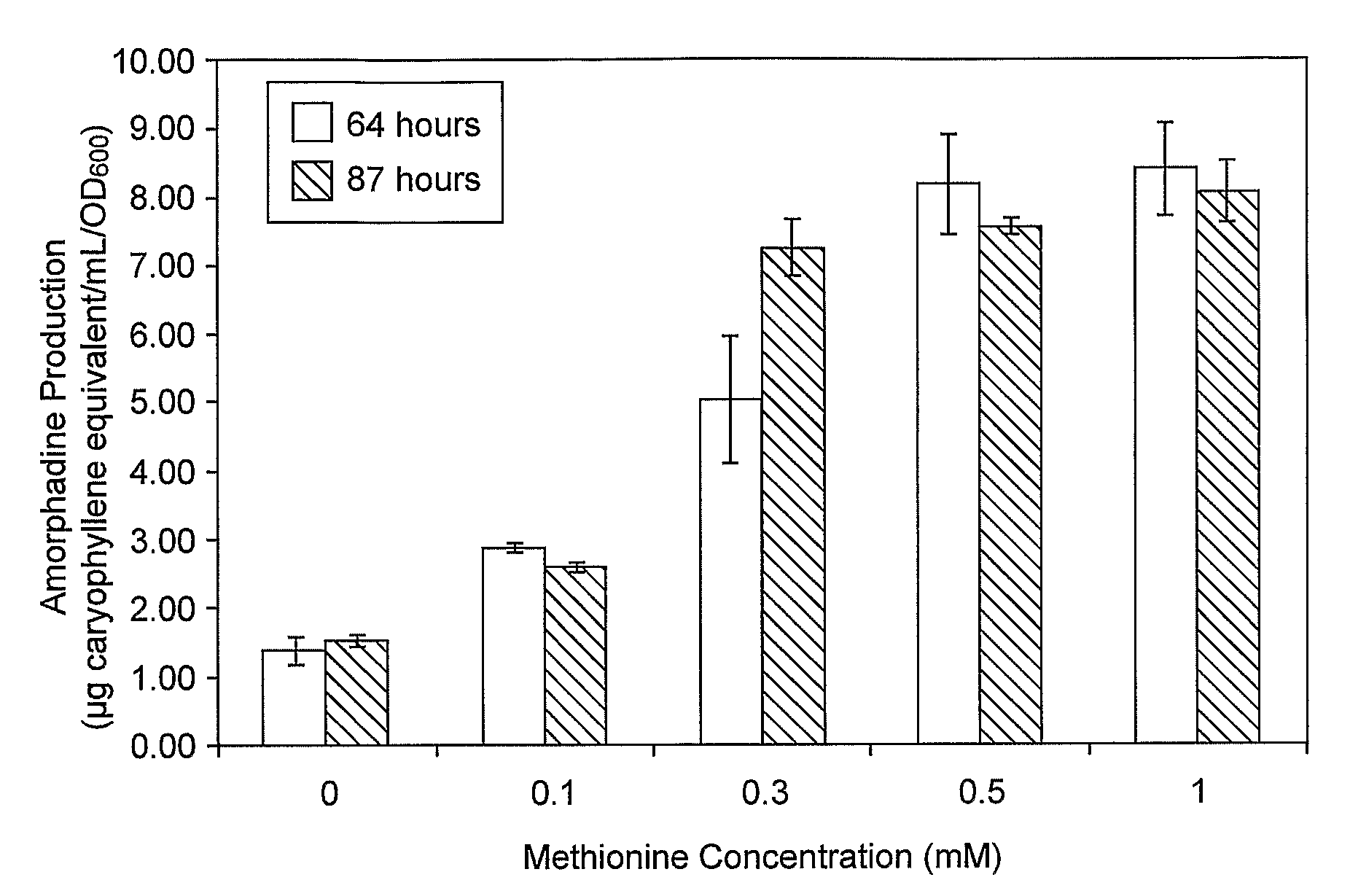
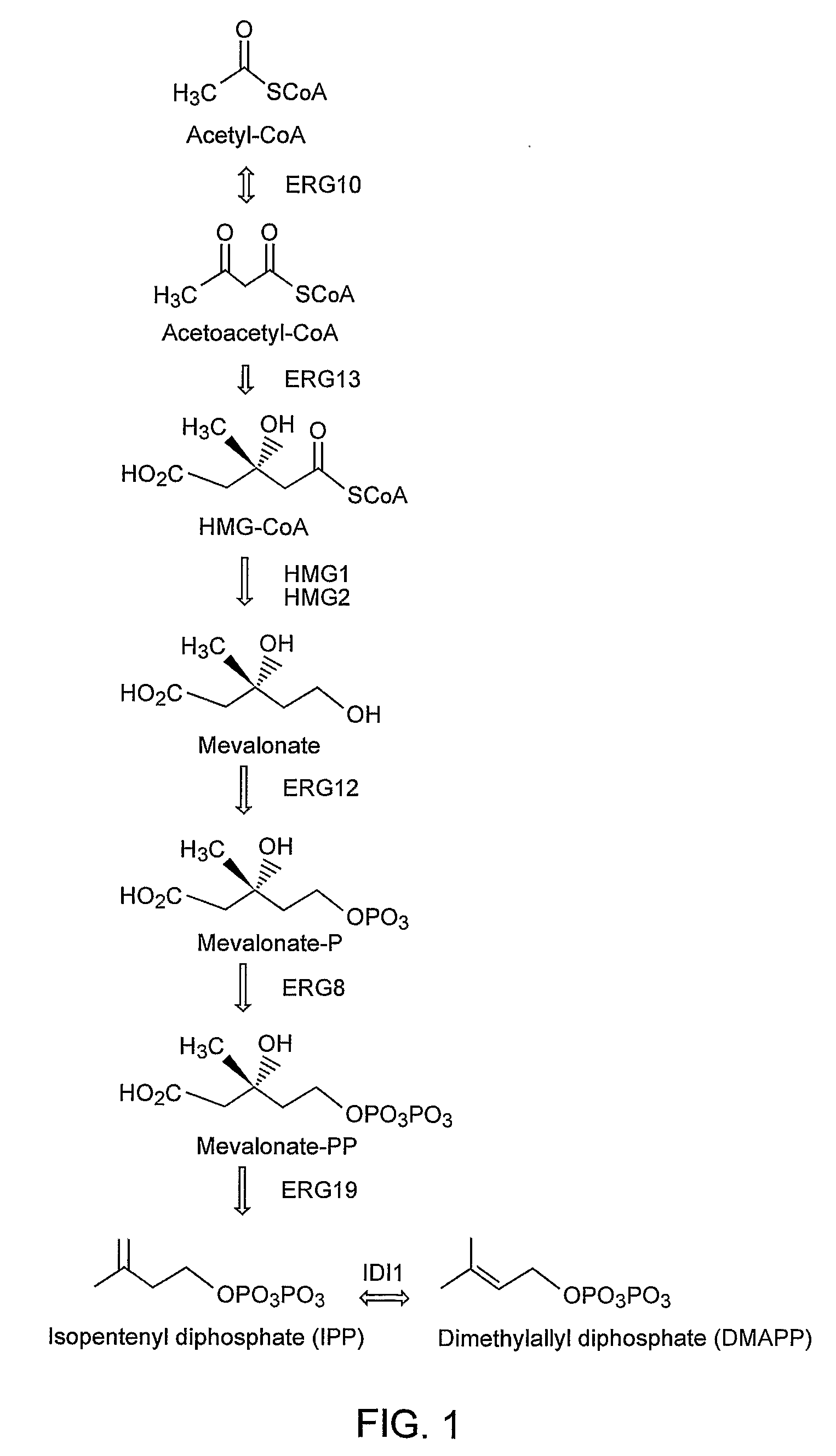
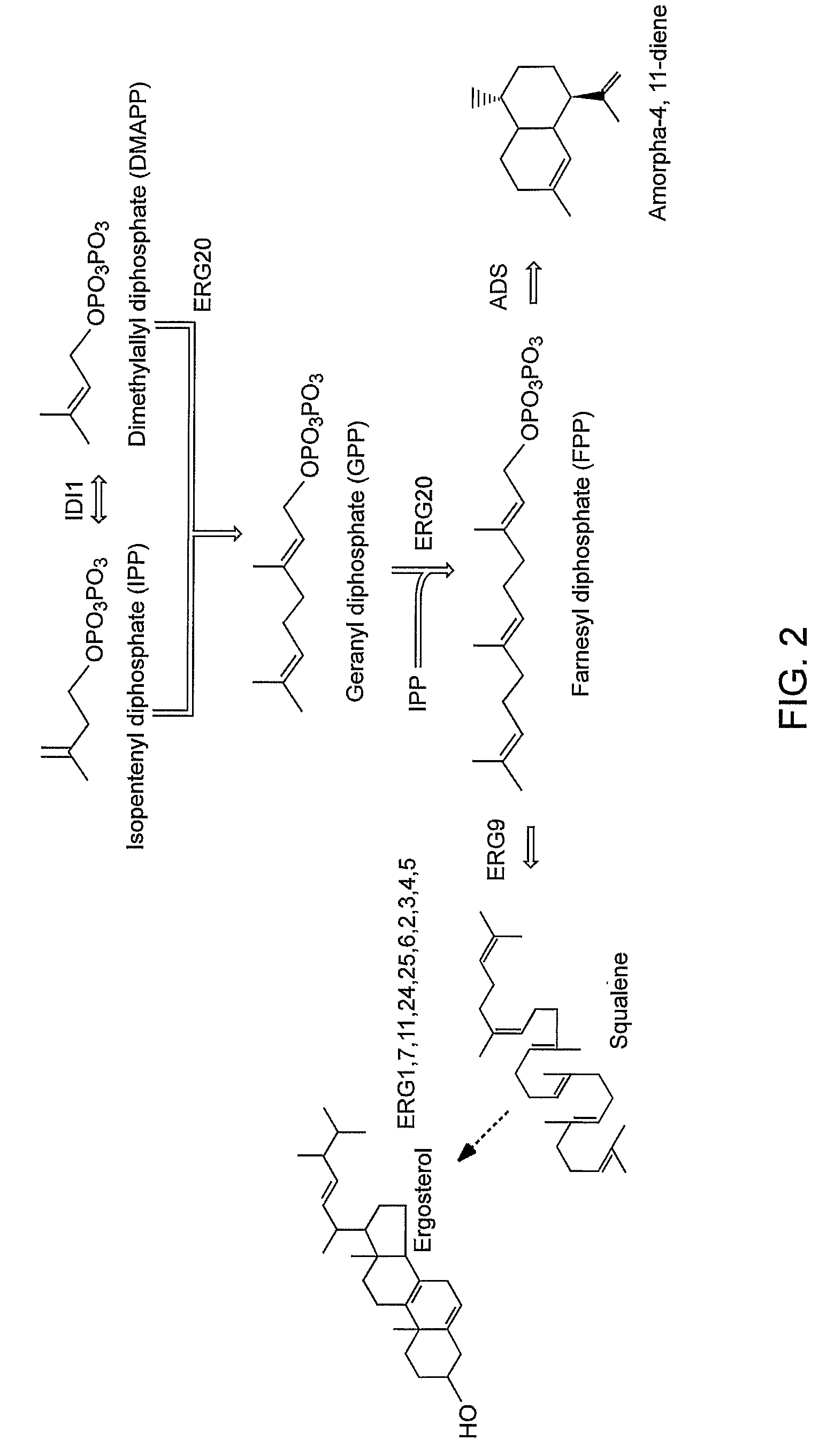
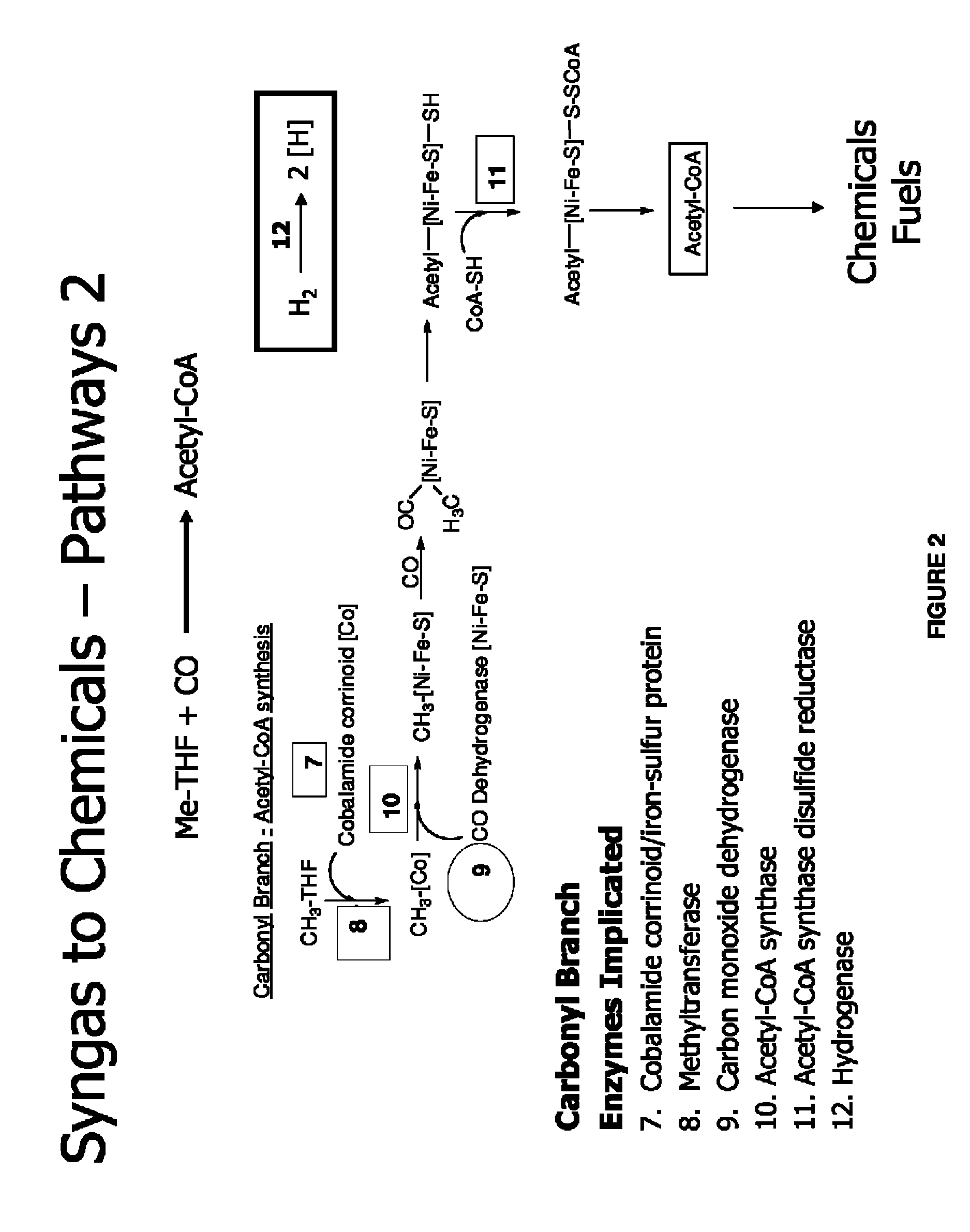
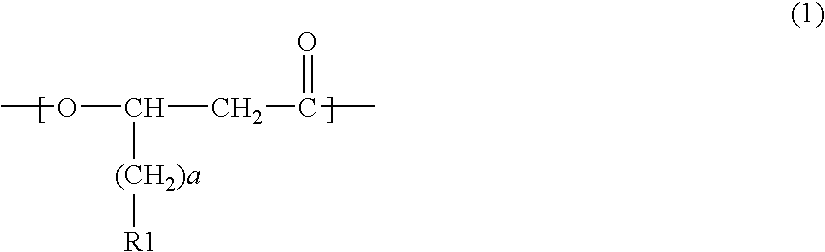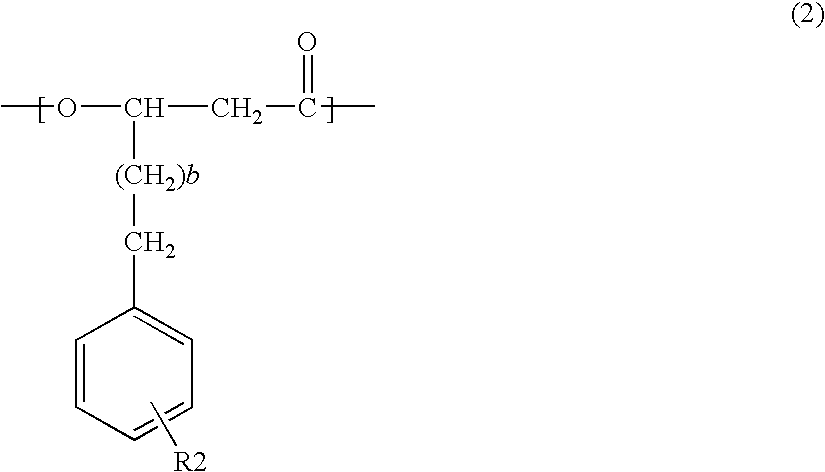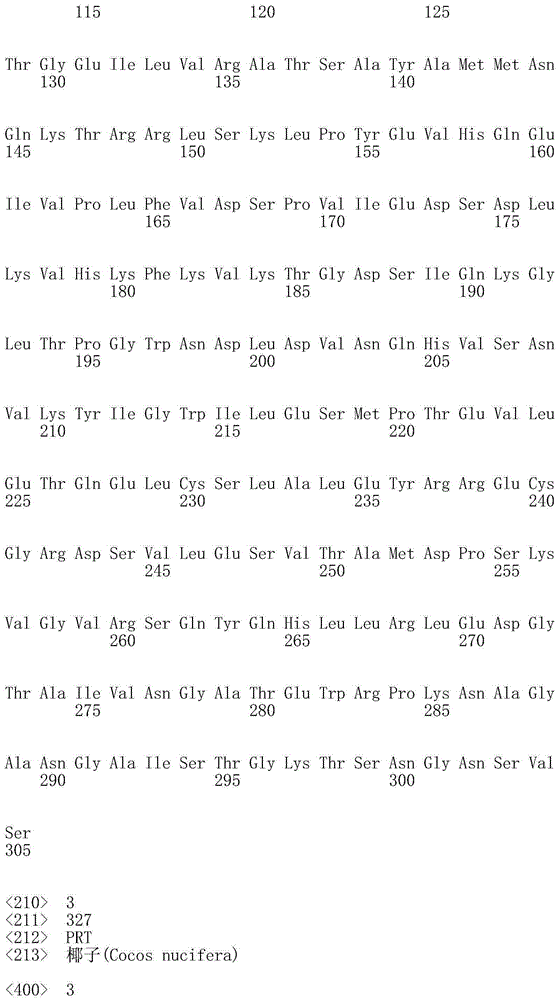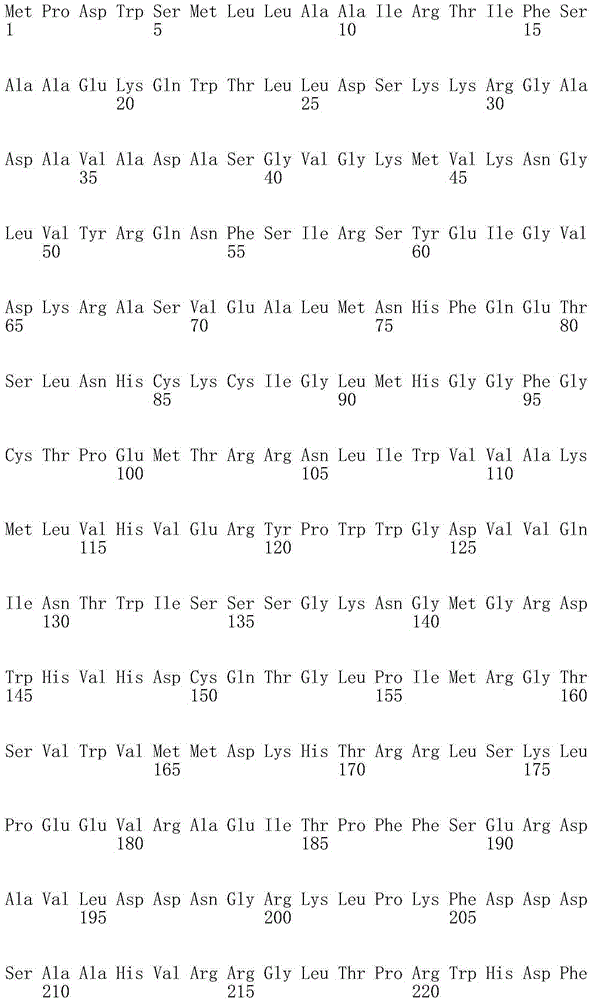Patents
Literature
126 results about "Acetyl-CoA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
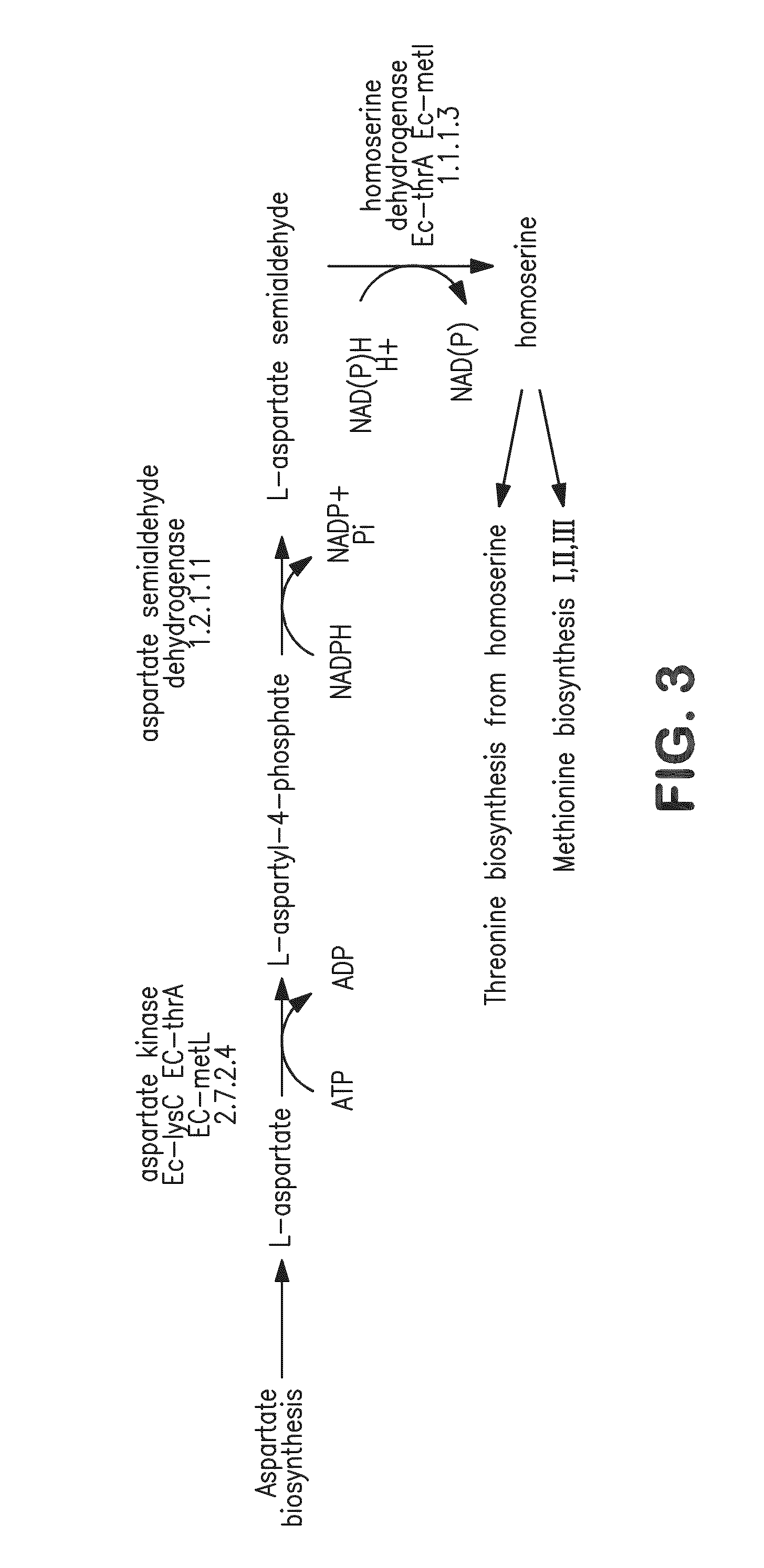
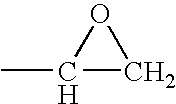
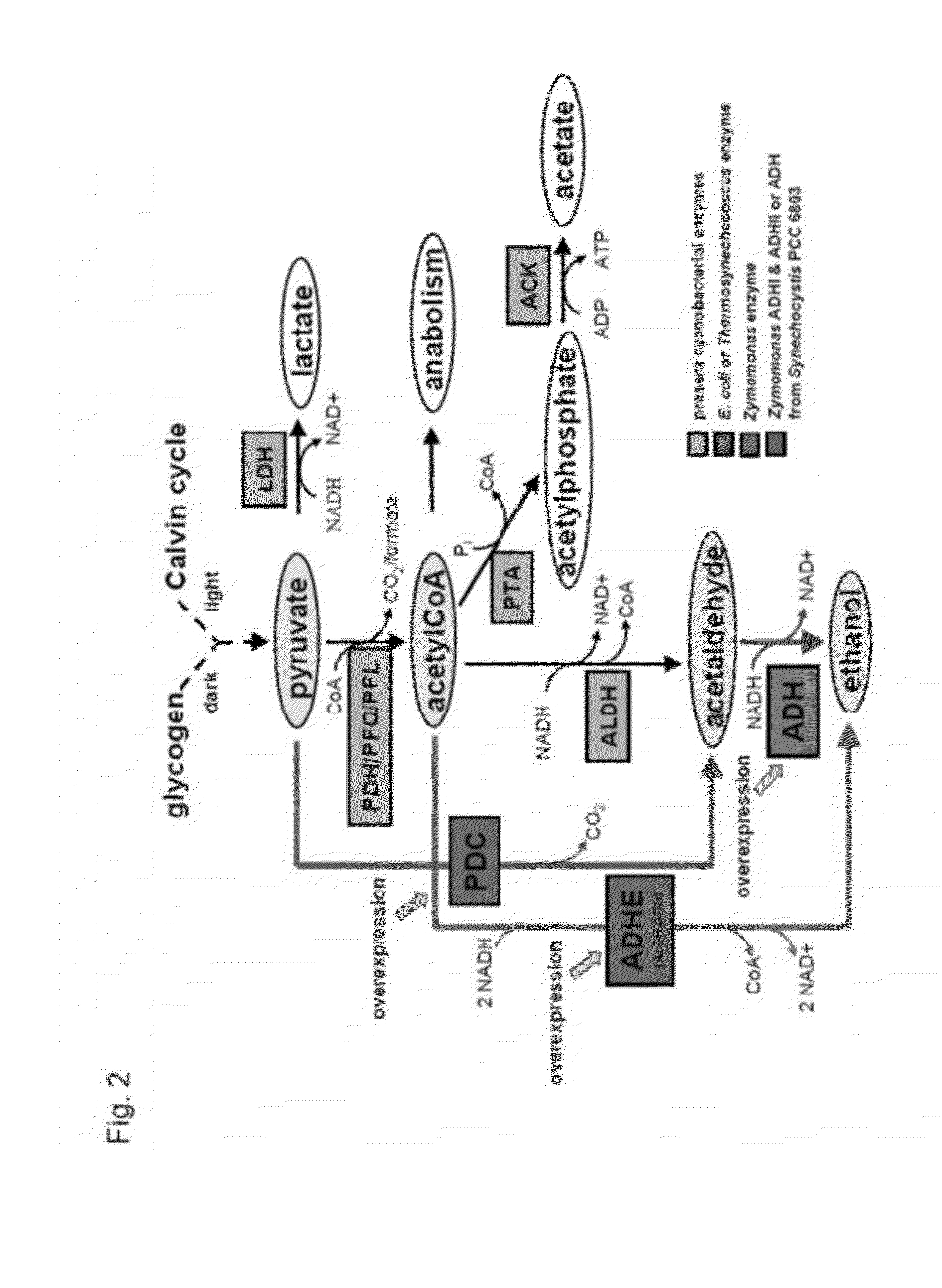
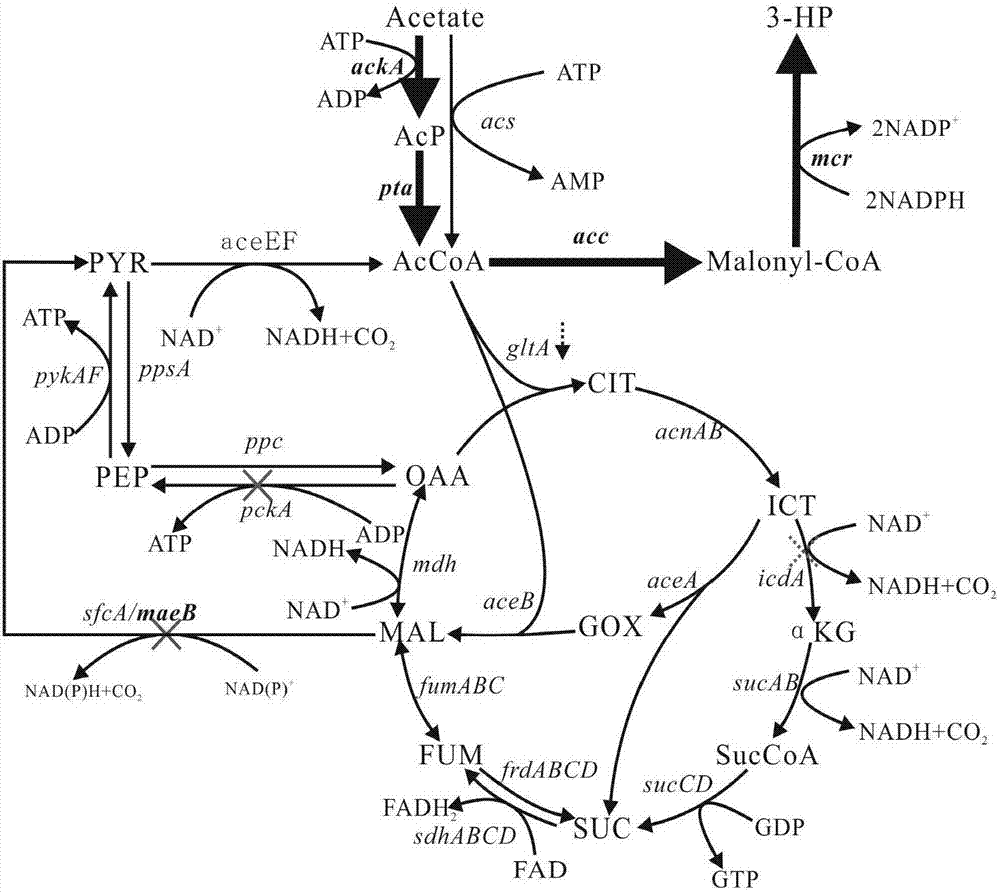
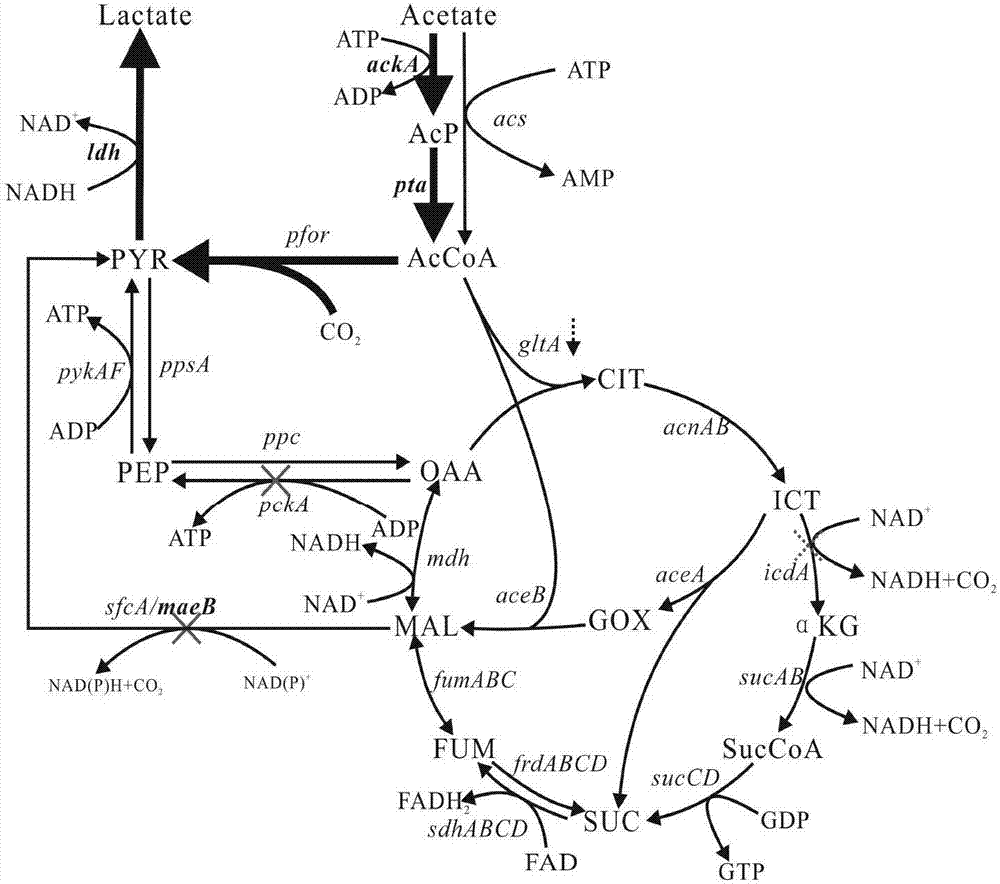
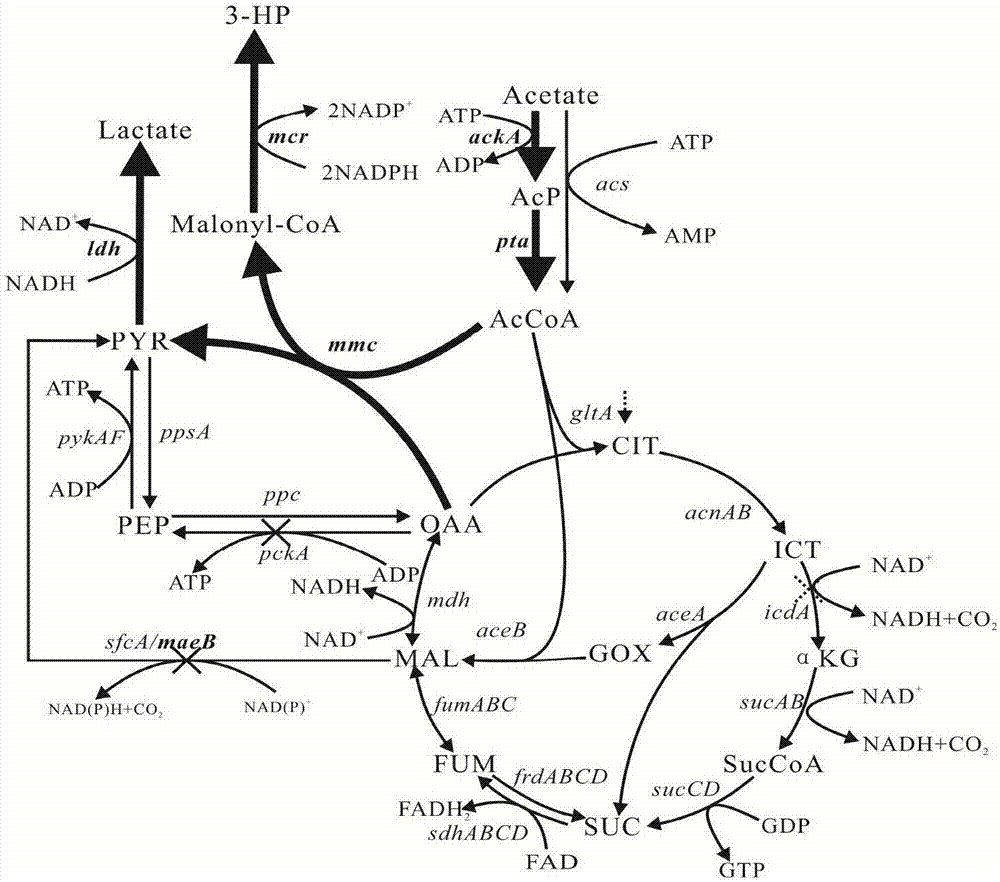
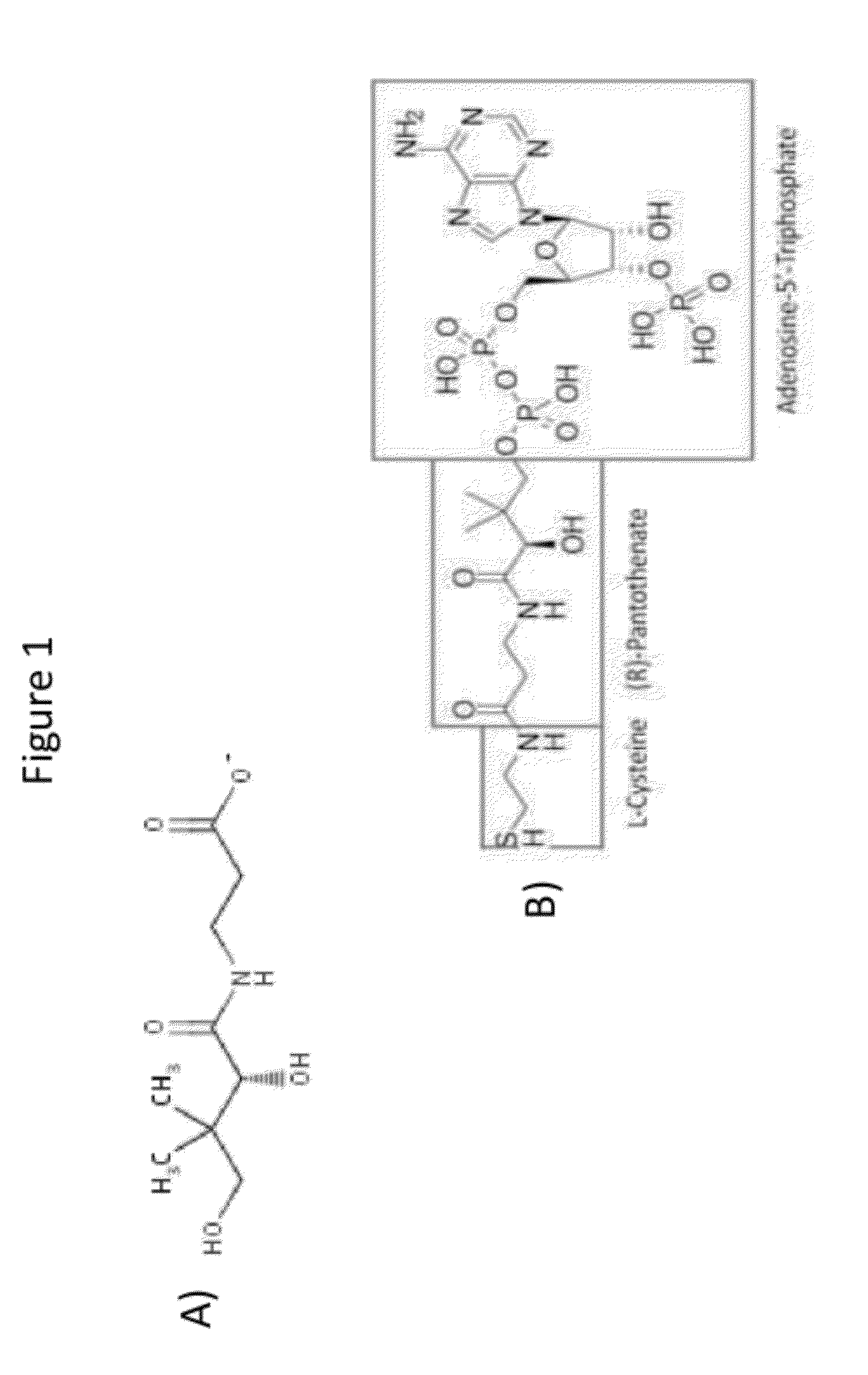
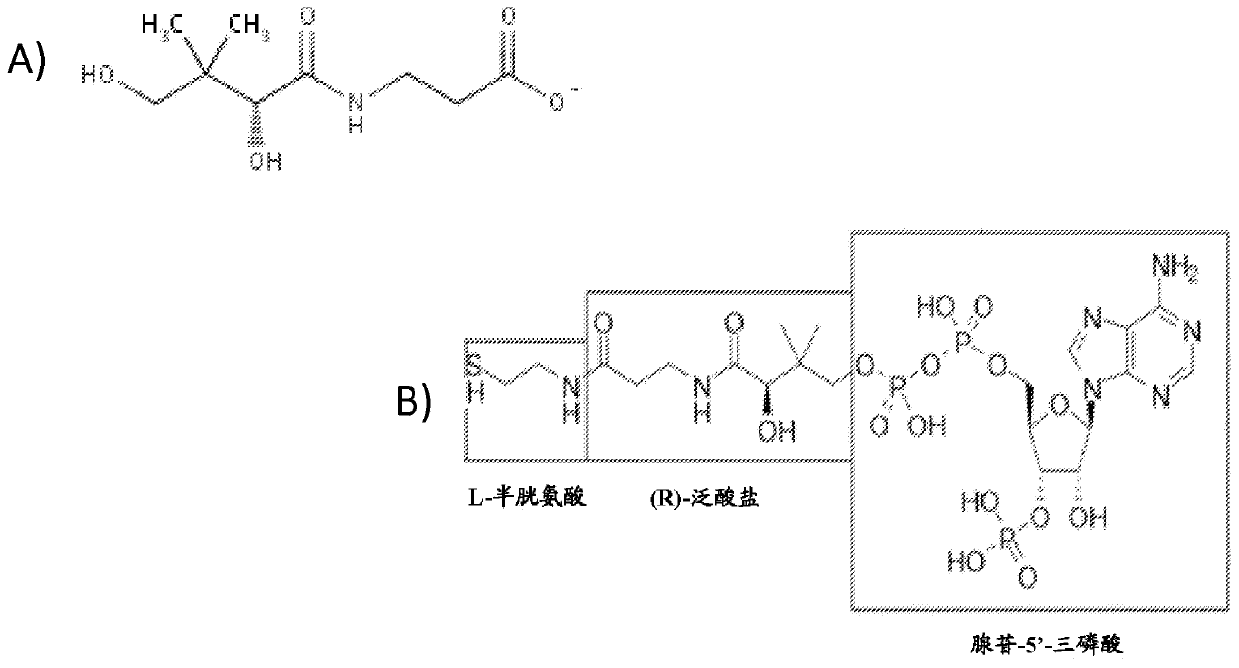
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for energy production. Coenzyme A (CoASH or CoA) consists of a β-mercaptoethylamine group linked to the vitamin pantothenic acid through an amide linkage and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol).
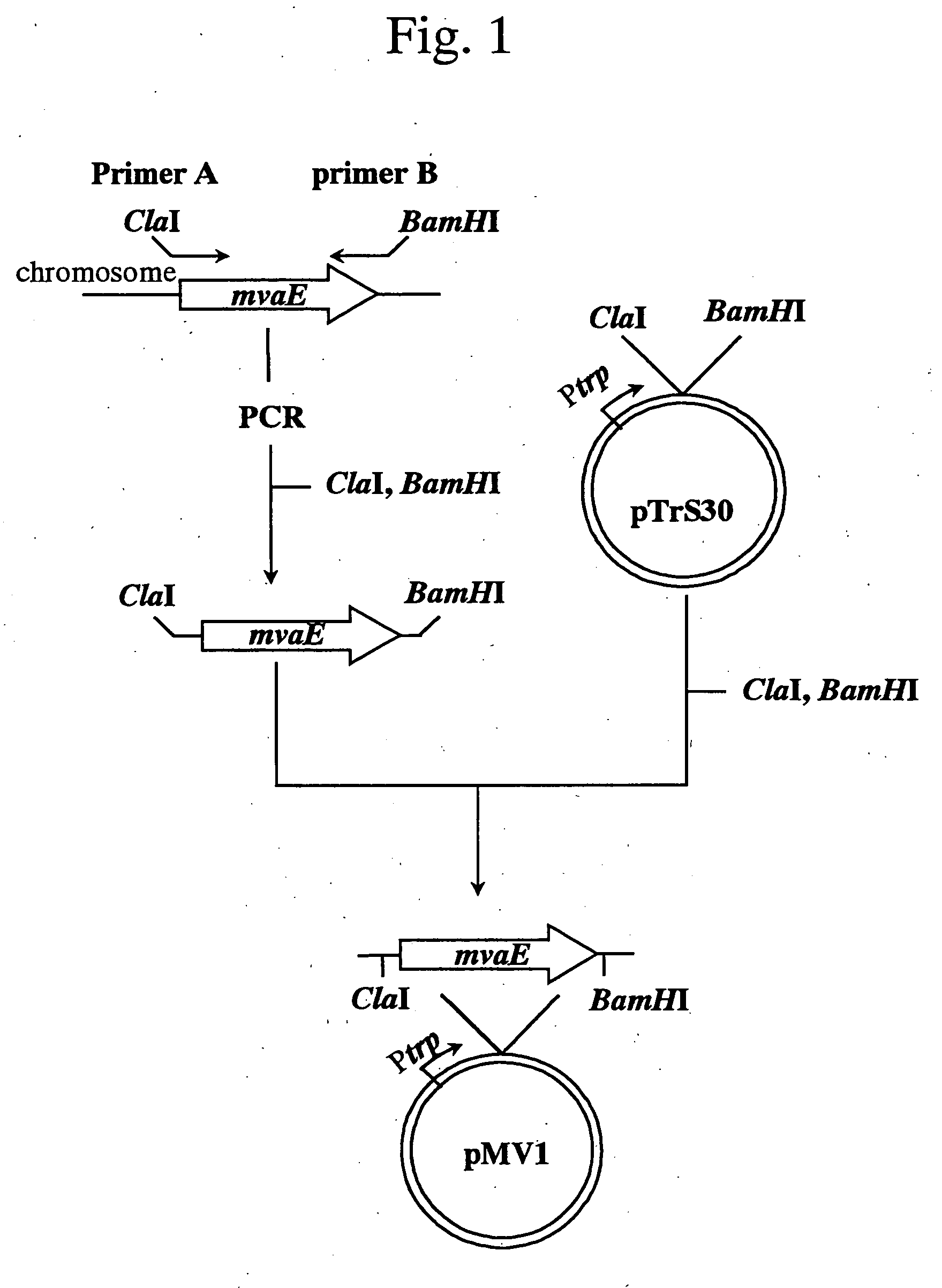
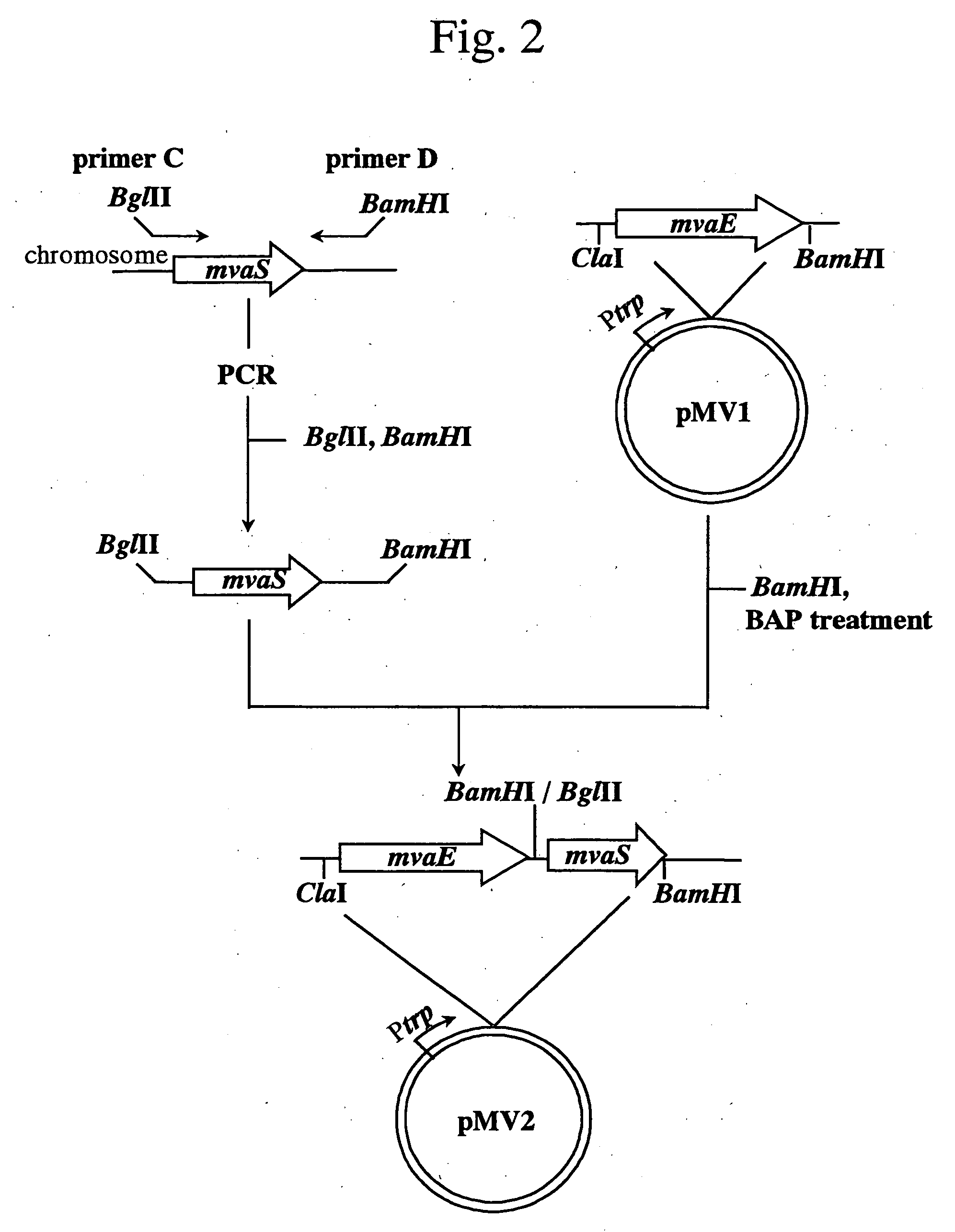
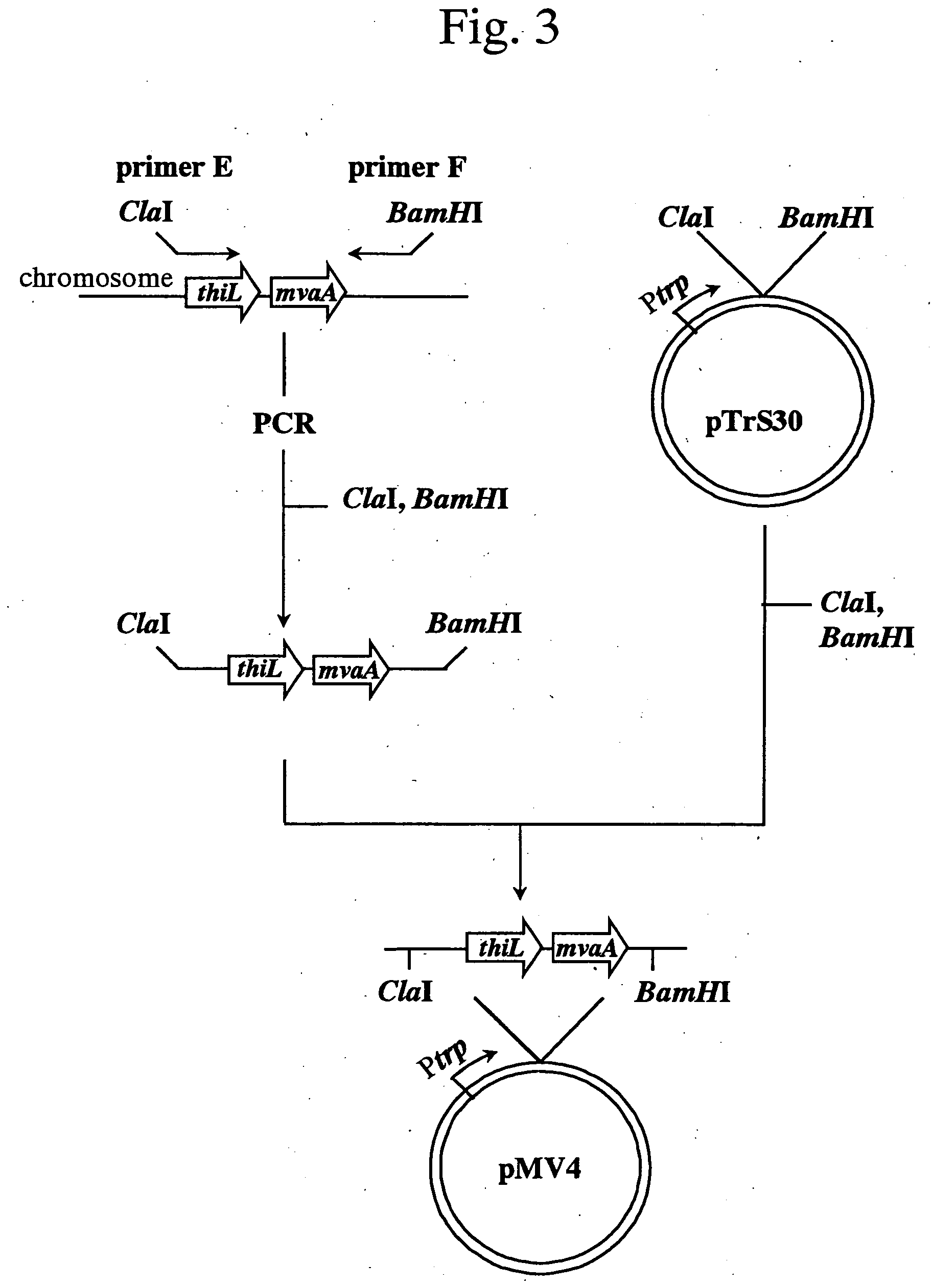
Process for producing mevalonic acid
InactiveUS20050287655A1Avoid difficult choicesHigh activityBacteriaGenetic engineeringMicroorganismMicrobiology
A microorganism having the ability to biosynthesize mevalonic acid from acetyl-CoA is obtained by transfecting a DNA participating in biosynthesis of mevalonic acid from acetyl-CoA into a host microorganism, preferably into a microorganism having only a non-mevalonic acid pathway, to express the DNA therein. Mevalonic acid can be efficiently produced by culturing the microorganism, and recovering mevalonic acid that is produced and accumulated in a large amount in the culture.
Owner:KYOWA HAKKO KOGYO CO LTD
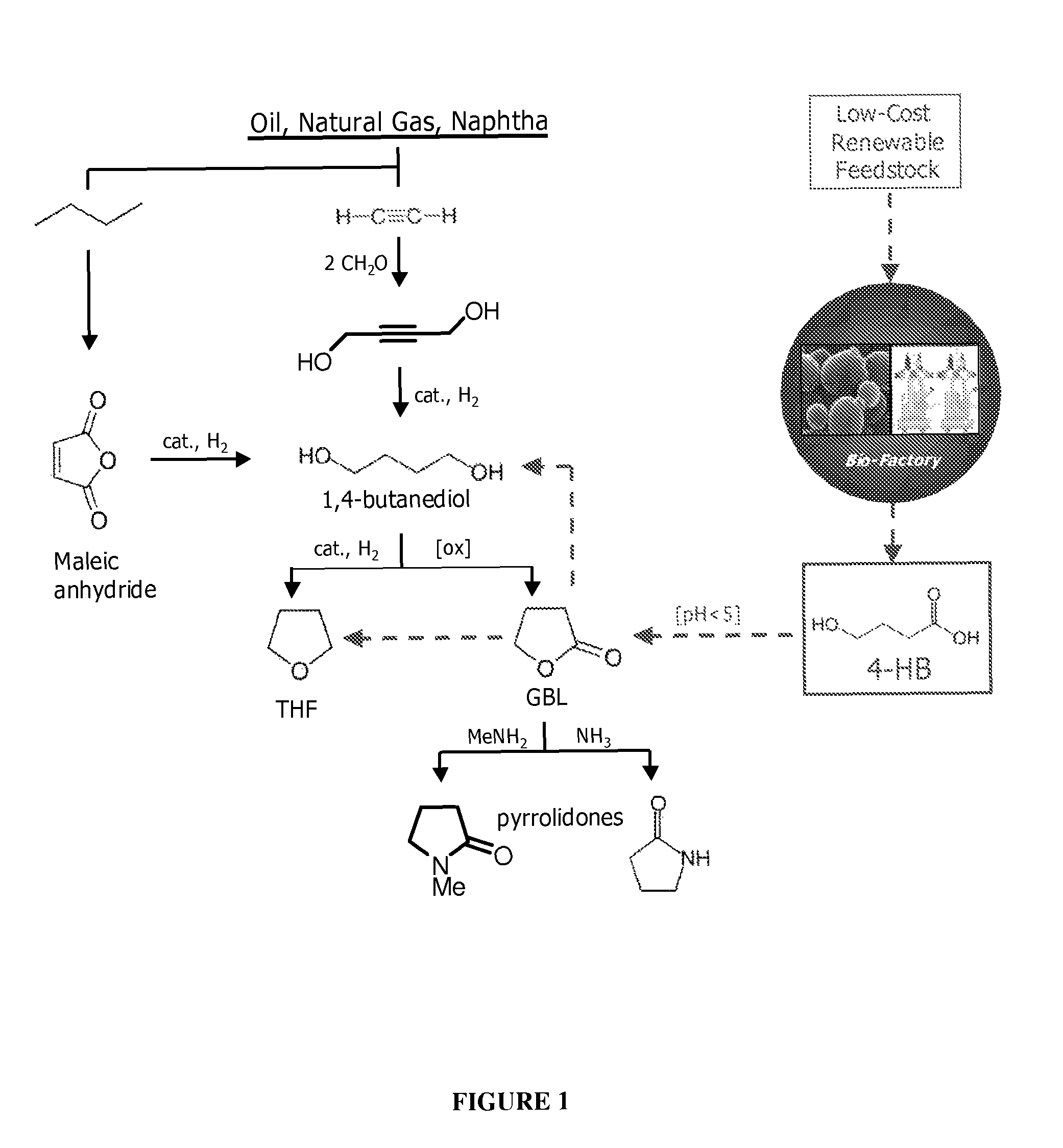
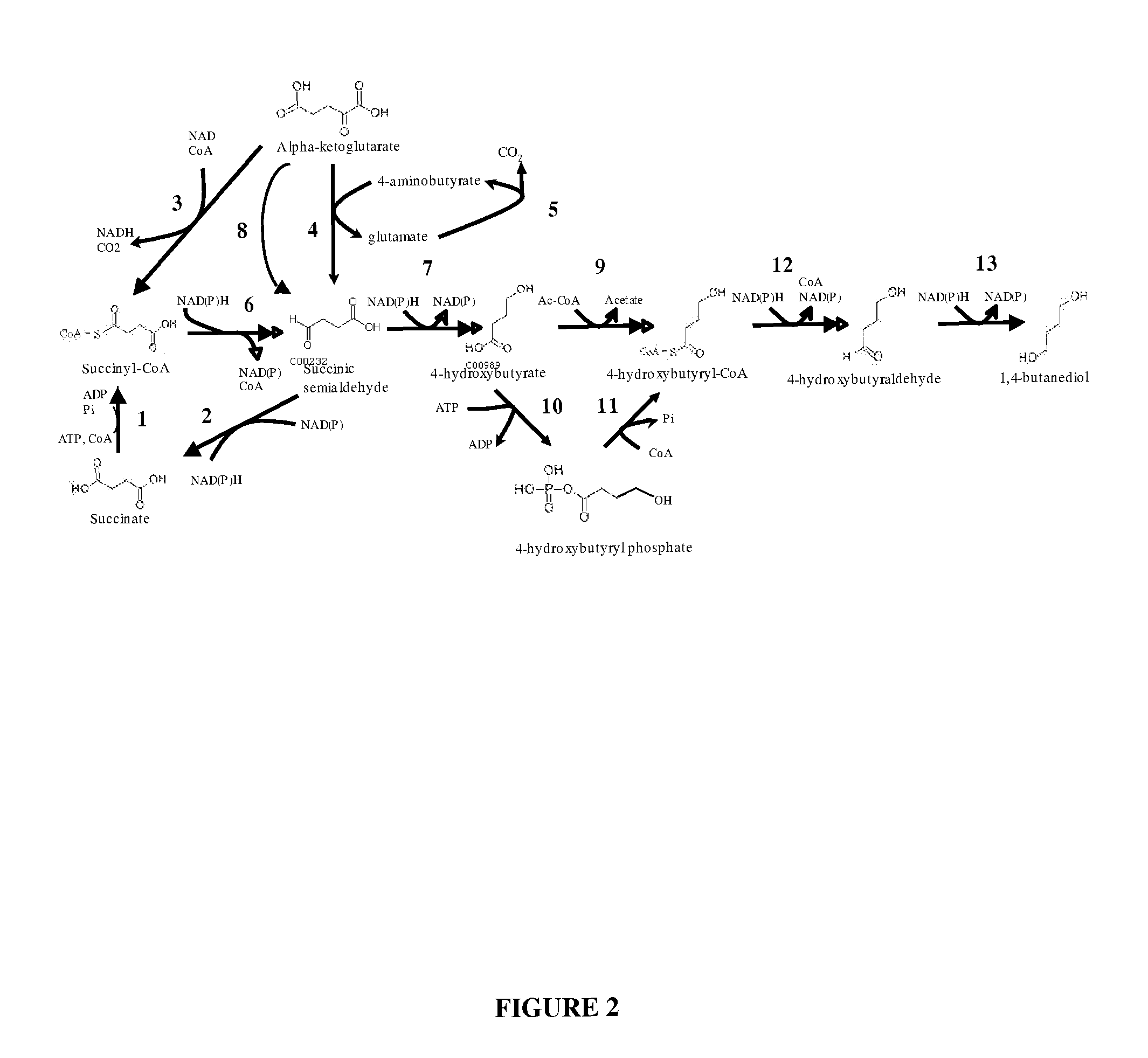
Compositions and methods for the biosynthesis of 1,4-butanediol and its precursors
The invention provides a non-naturally occurring microbial organism having 4-hydroxybutanoic acid (4-HB) and 1,4-butanediol (1,4-BDO) biosynthetic pathways. The pathways include exogenous nucleic acids encoding a) an α-ketoglutarate decarboxylase; b) a 4-hydroxybutanoate dehydrogenase; c) a 4-hydroxybutyryl-CoA:acetyl-CoA transferase or a butyrate kinase and a phosphotransbutyrylase; d) an aldehyde dehydrogenase, and e) an alcohol dehydrogenase, wherein the exogenous nucleic acids are expressed in sufficient amounts to produce 1,4-butanediol (1,4-BDO). Also provide is a method for the production of 1,4-BDO. The method includes culturing the non-naturally occurring microbial organism having 4-HB and 1,4-BDO biosynthetic pathways substantially anaerobic conditions for a sufficient period of time to produce 1,4-BDO.
Owner:GENOMATICA INC
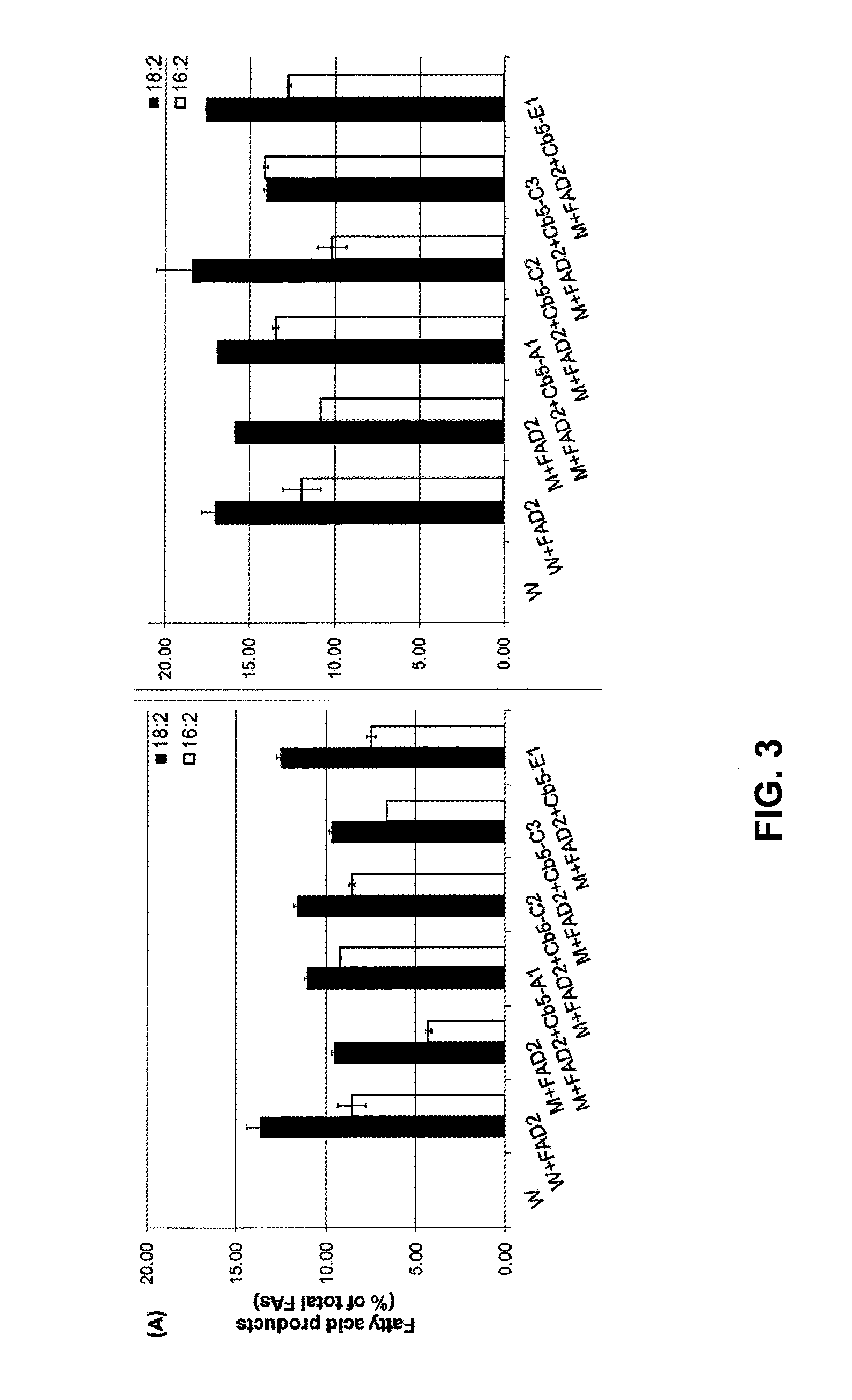
Plant Genes Associated With Seed Oil Content And Methods Of Their Use
InactiveUS20110191904A1High oil contentRaise the ratioOther foreign material introduction processesFermentationBiotechnologyReticulum cell
Cytochrome b5 (Cb5) is a haem-binding protein located in the endoplasmic reticulum (ER) and the outer mitochondrial membranes of higher eukaryotes. In higher plants, animals, and fungi, the ER resident Cb5 has been shown to play a role in desaturation of acyl CoA fatty acids. Higher plants Cb5 isoforms from plants such as soybean or Arabidopsis are capable of modulating omega-3 desaturation. Co-expression of certain Cb5 isoforms with FAD3 in a host plant results in increased production of seed oil content as well as altered ratio between different fatty acids. It is also disclosed here that overexpression of Yarrowia ACL enzymes in the plastids of a host plant helps boost the synthesis of acetyl CoA, which in turn, may lead to increased synthesis of fatty acids and enhanced oil accumulation in the seeds.
Owner:UNIVERSITY OF MISSOURI
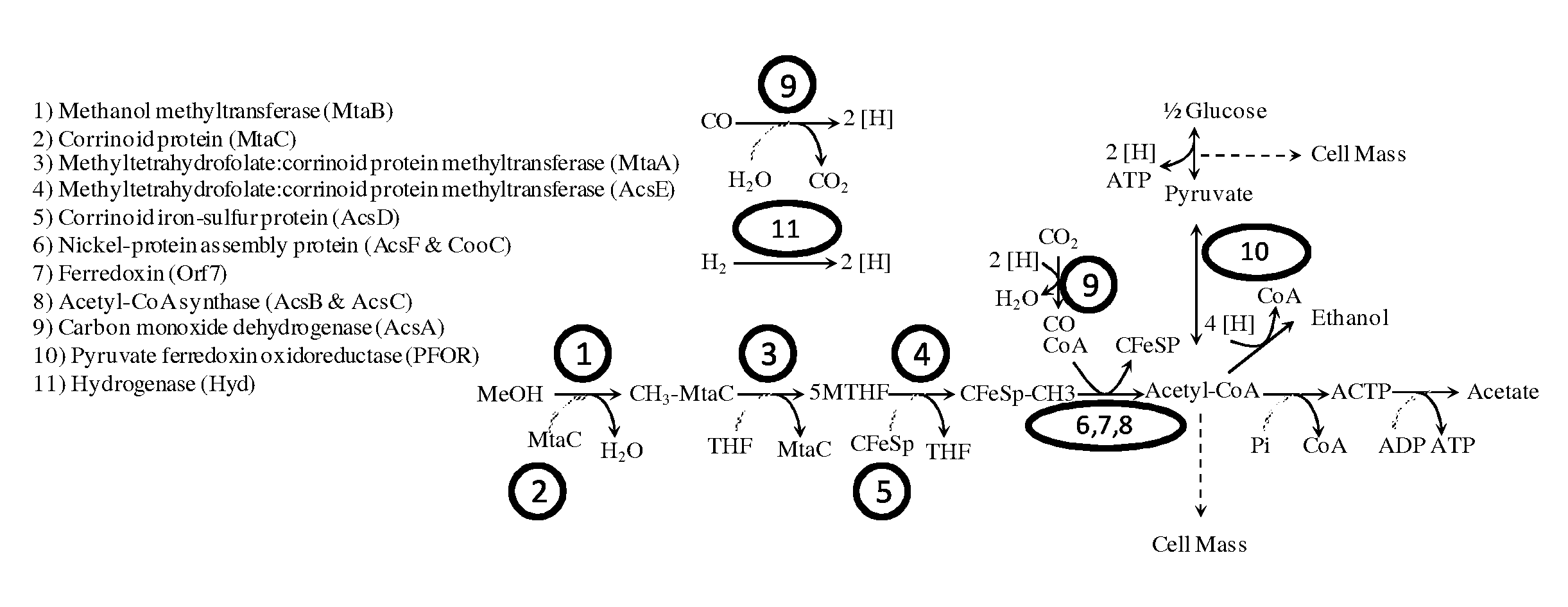
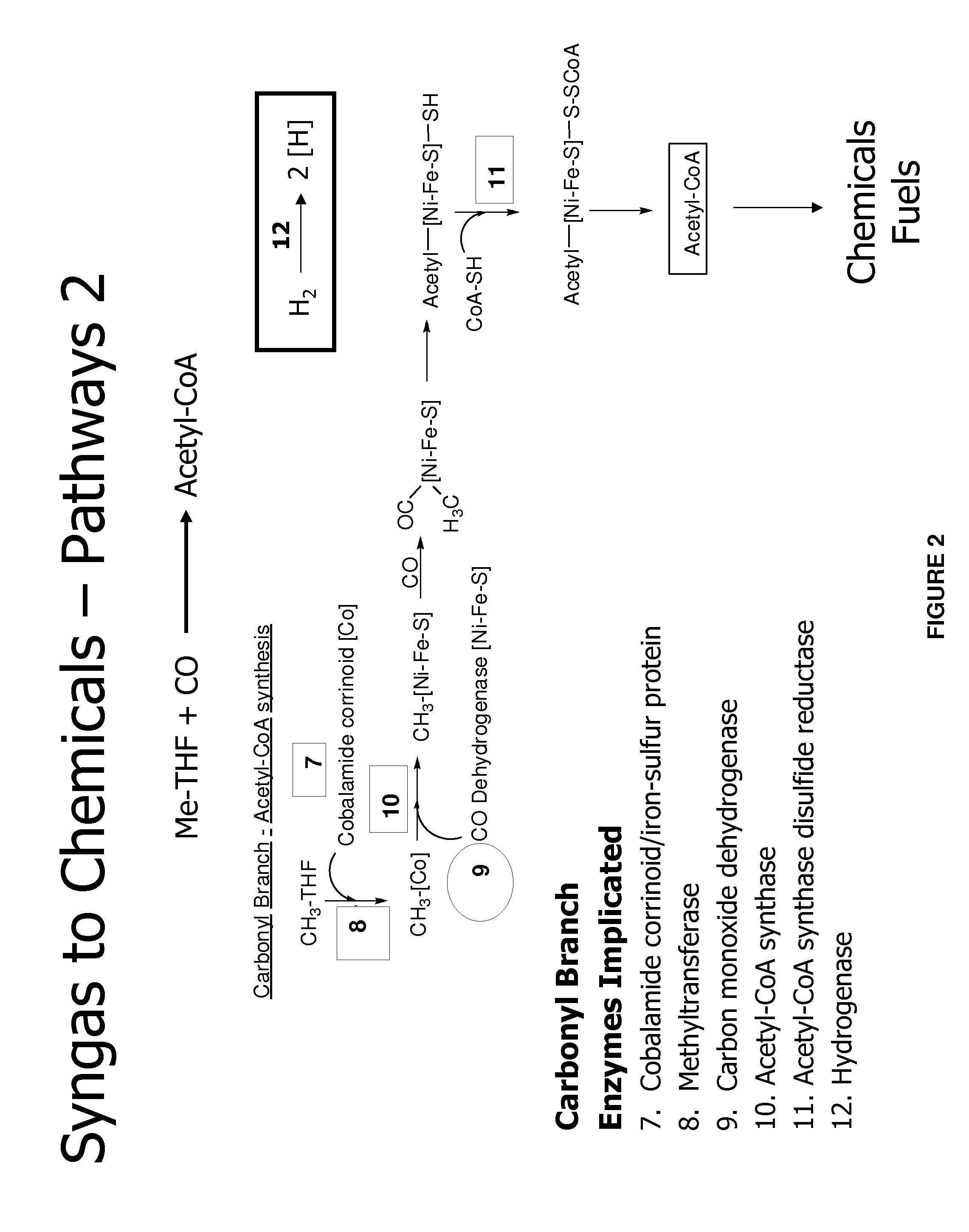
Methods and organisms for utilizing synthesis gas or other gaseous carbon sources and methanol
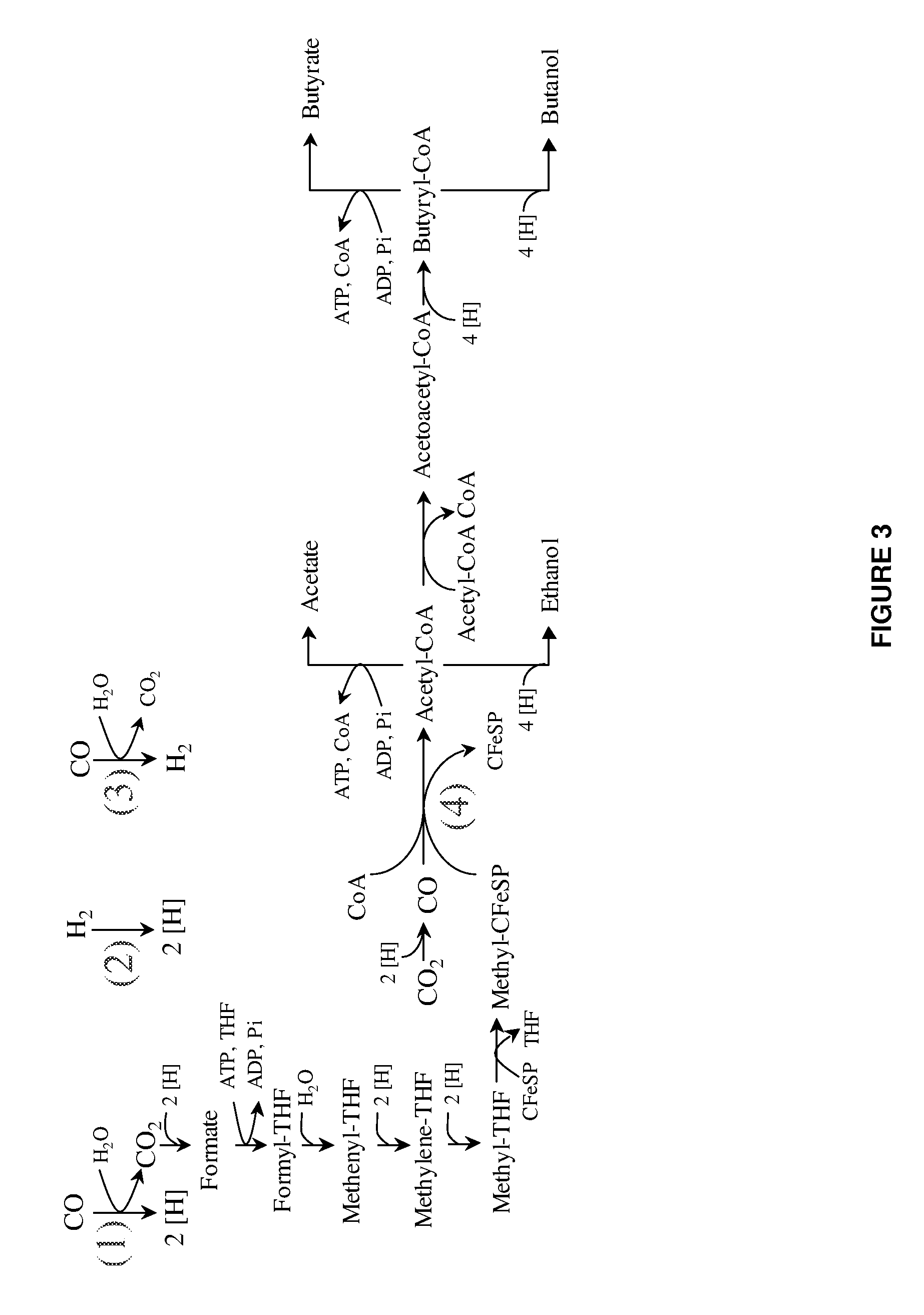
The invention provides a non-naturally occurring microbial organism having an acetyl-CoA pathway and the capability of utilizing syngas or syngas and methanol. In one embodiment, the invention provides a non-naturally occurring microorganism, comprising one or more exogenous proteins conferring to the microorganism a pathway to convert CO, CO2 and / or H2 to acetyl-coenzyme A (acetyl-CoA), methyl tetrahydrofolate (methyl-THF) or other desired products, wherein the microorganism lacks the ability to convert CO or CO2 and H2 to acetyl-CoA or methyl-THF in the absence of the one or more exogenous proteins. For example, the microbial organism can contain at least one exogenous nucleic acid encoding an enzyme or protein in an acetyl-CoA pathway. The microbial organism is capable of utilizing synthesis gases comprising CO, CO2 and / or H2, alone or in combination with methanol, to produce acetyl-CoA. The invention additionally provides a method for producing acetyl-CoA, for example, by culturing an acetyl-CoA producing microbial organism, where the microbial organism expresses at least one exogenous nucleic acid encoding an acetyl-CoA pathway enzyme or protein in a sufficient amount to produce acetyl-CoA, under conditions and for a sufficient period of time to produce acetyl-CoA.
Owner:GENOMATICA INC
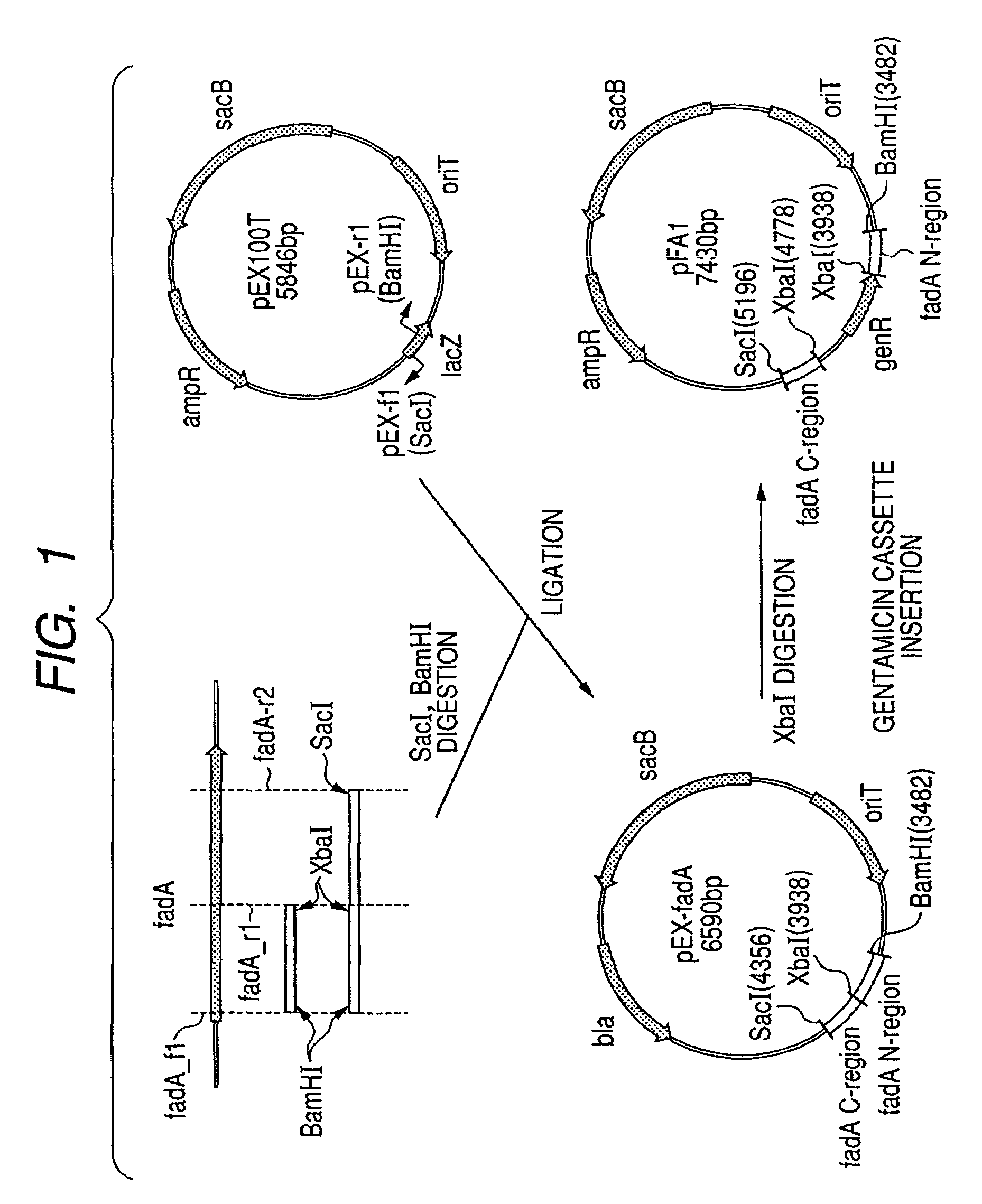
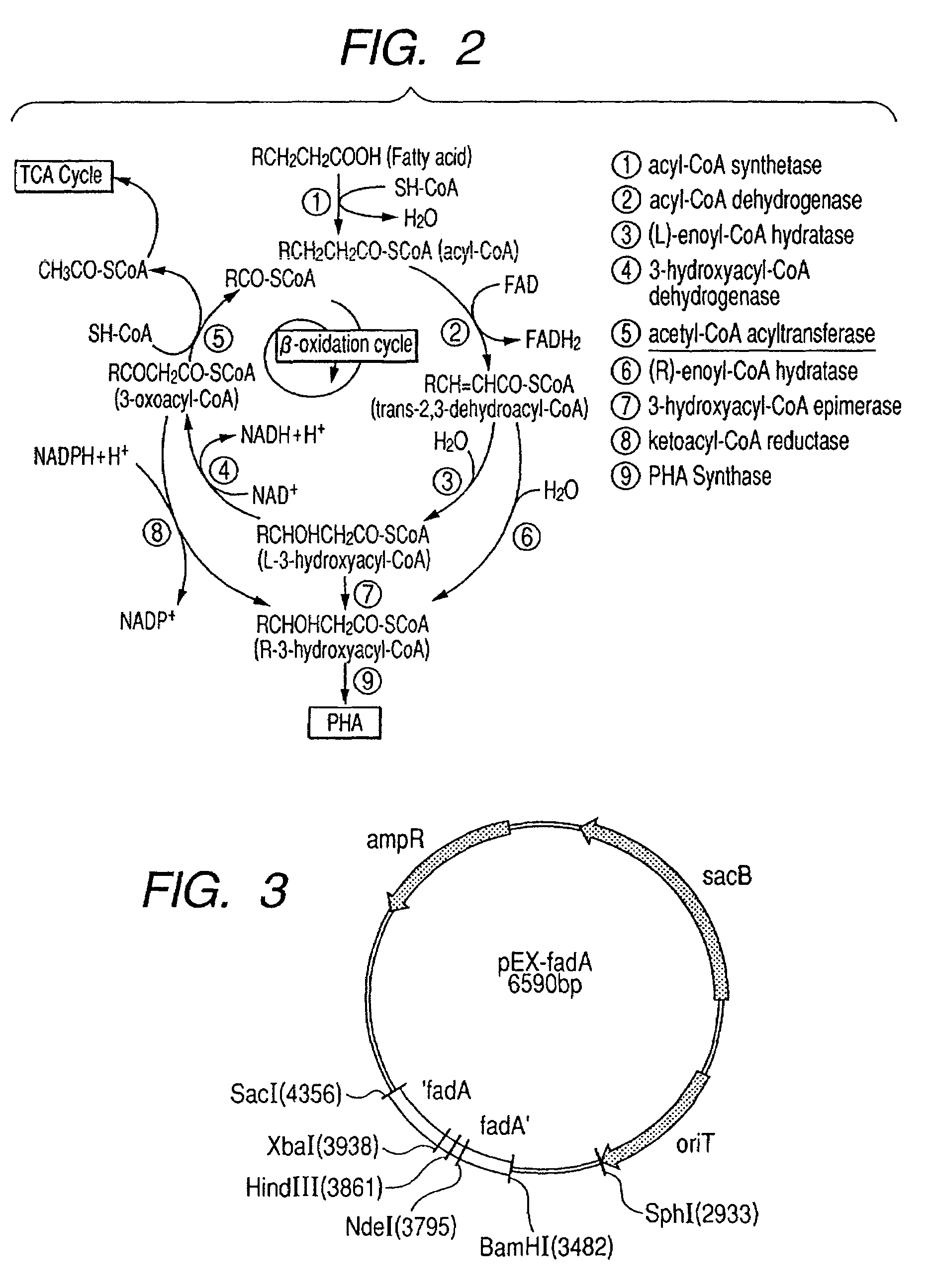
Acetyl-CoA acyltransferase gene disrupted bacterium for producing polyhydroxyalkanoate and method for producing polyhydroxyalkanoate using the same
A method for producing a polyhydroxyalkanoate with improved productivity and composition is provided. Polyhydroxyalkanoate is produced by a bacterium for producing polyhydroxyalkanoate in which a gene encoding acetyl-CoA acyltransferase is disrupted.
Owner:CANON KK
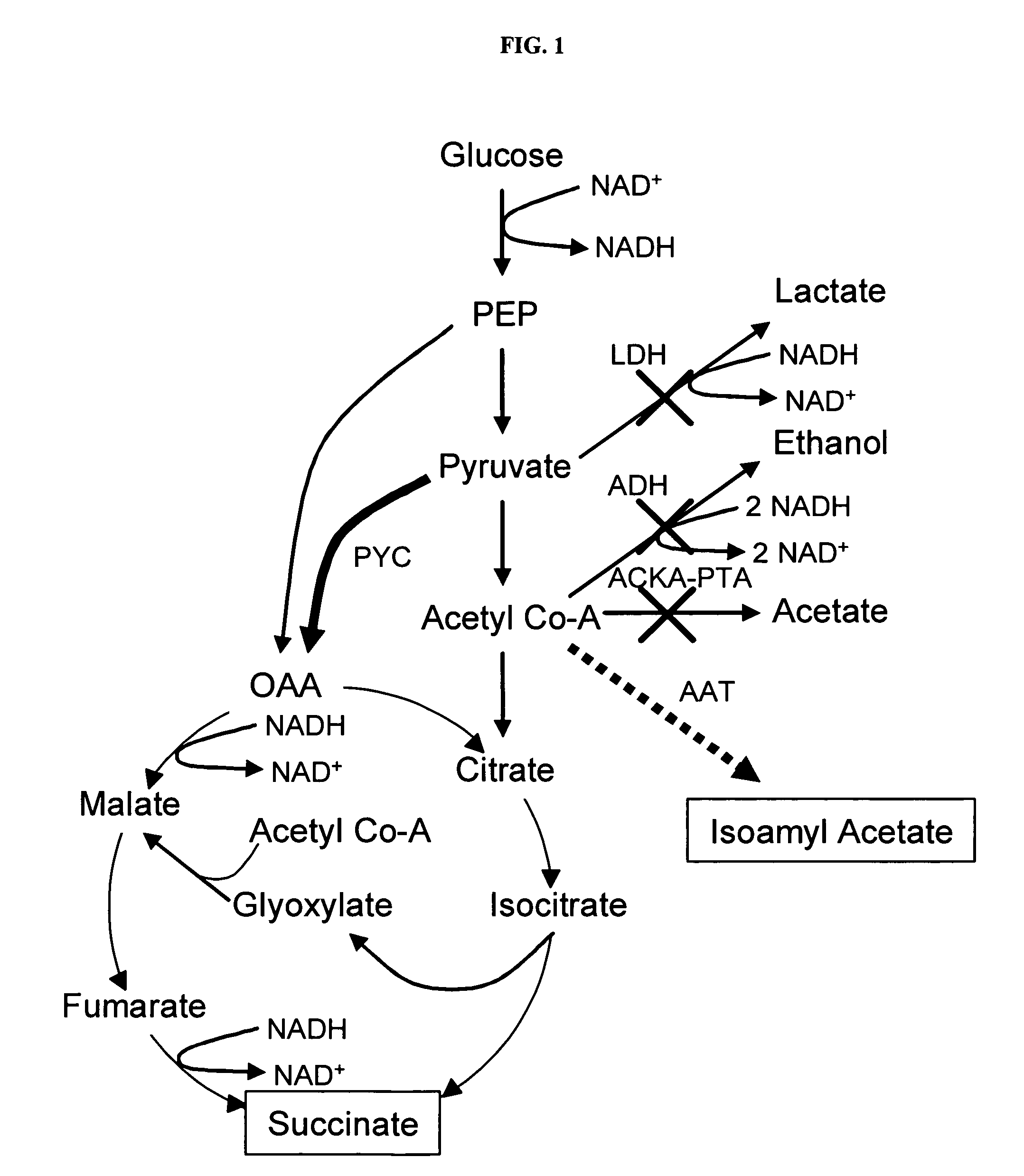
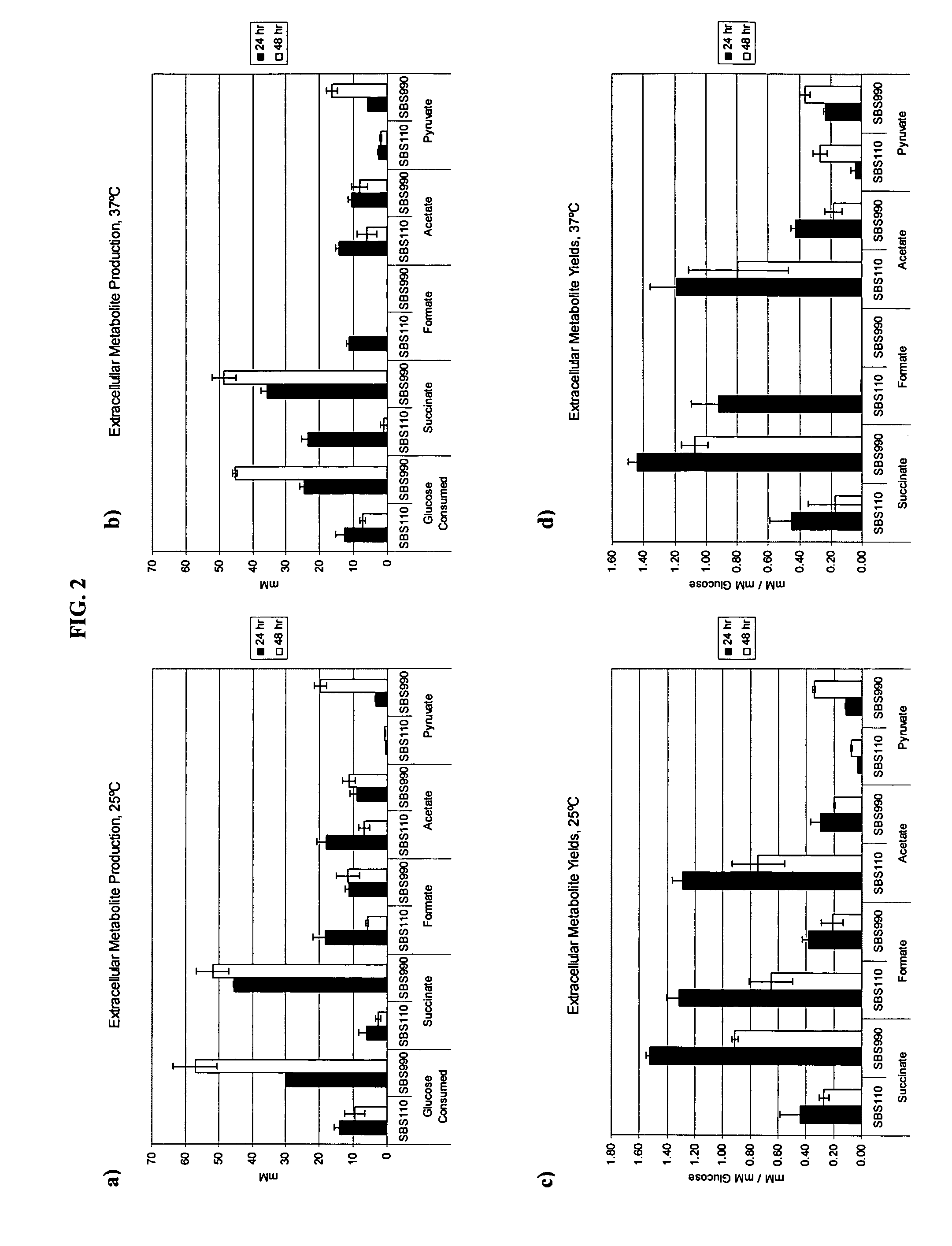
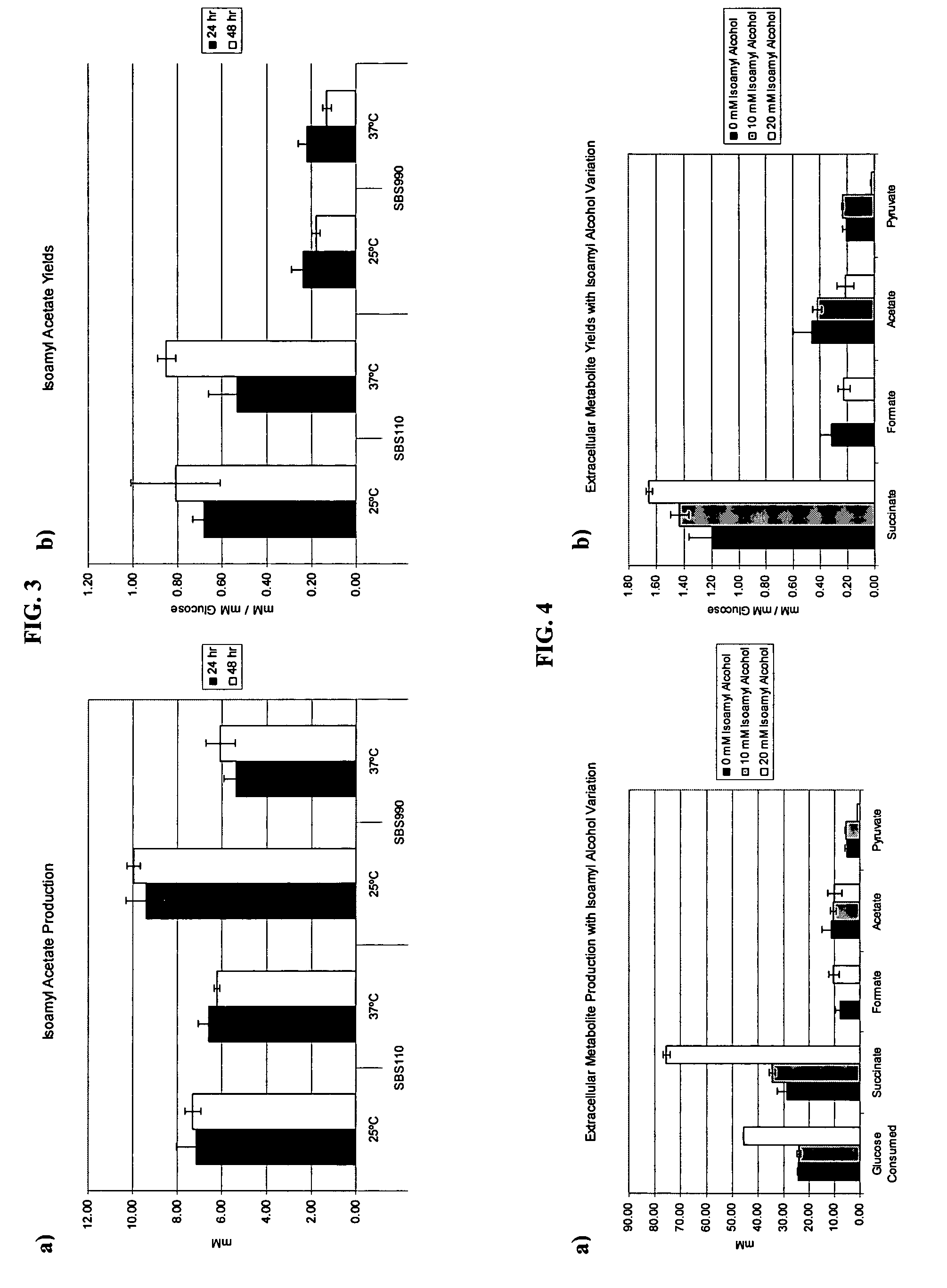
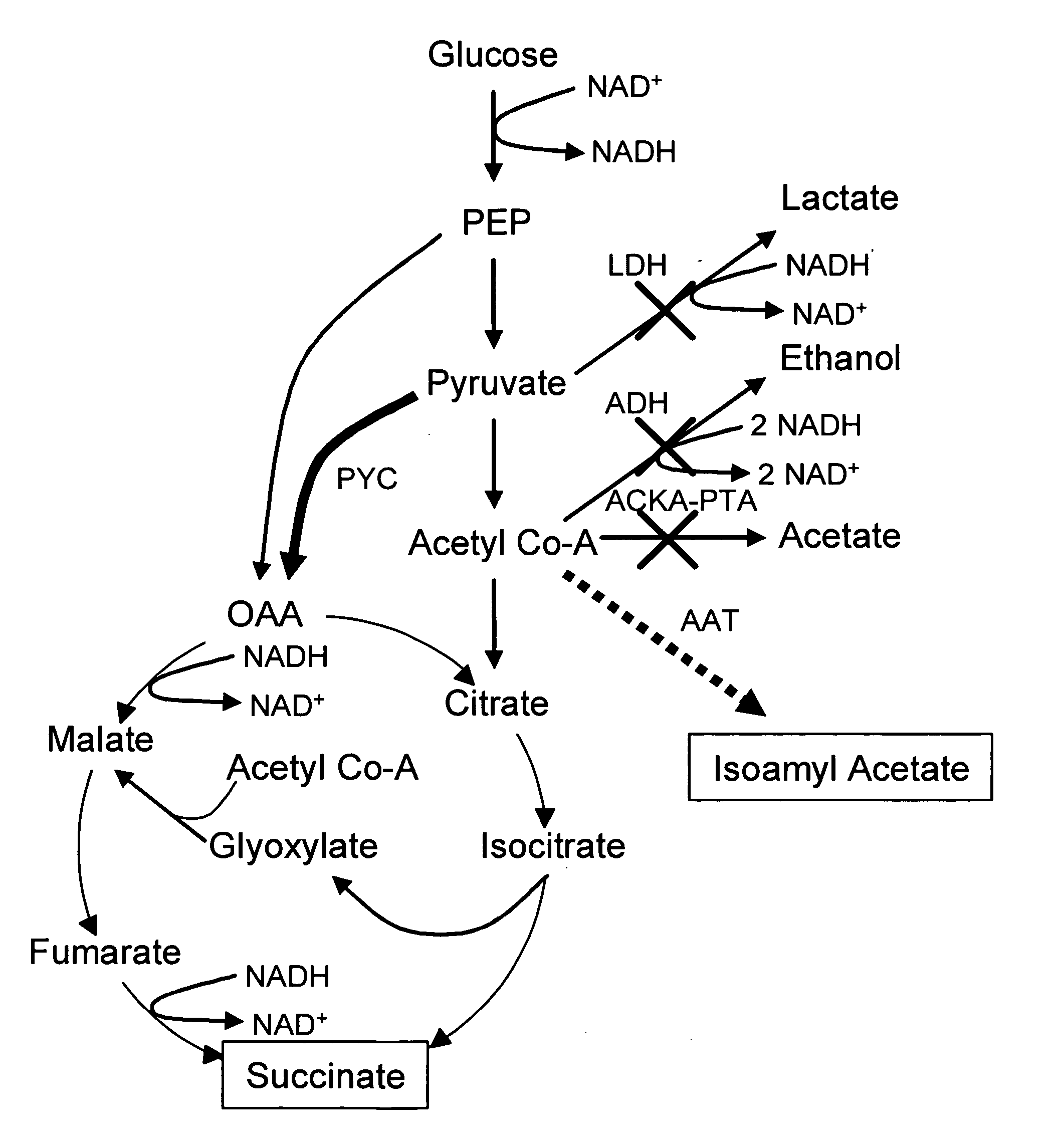
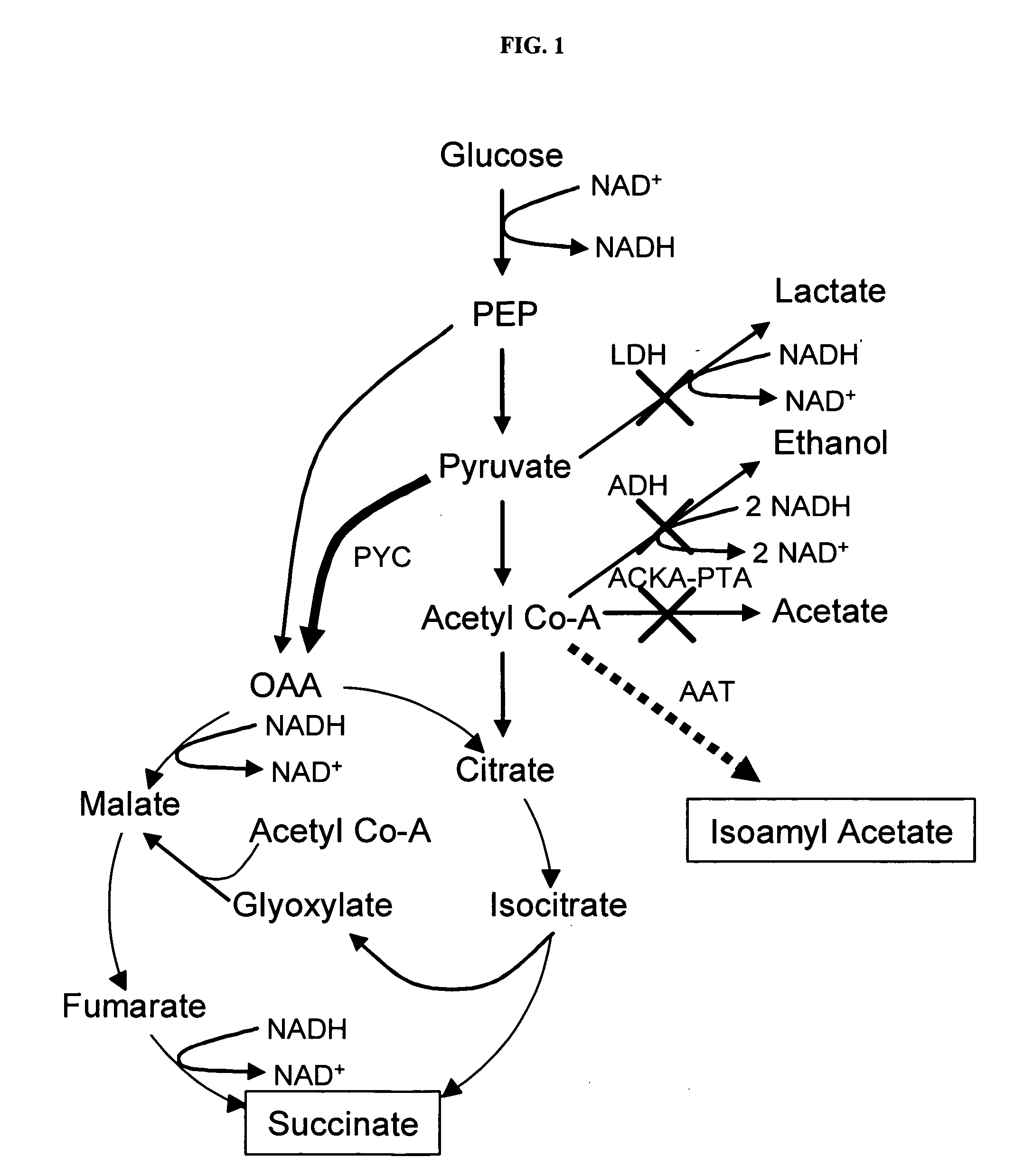
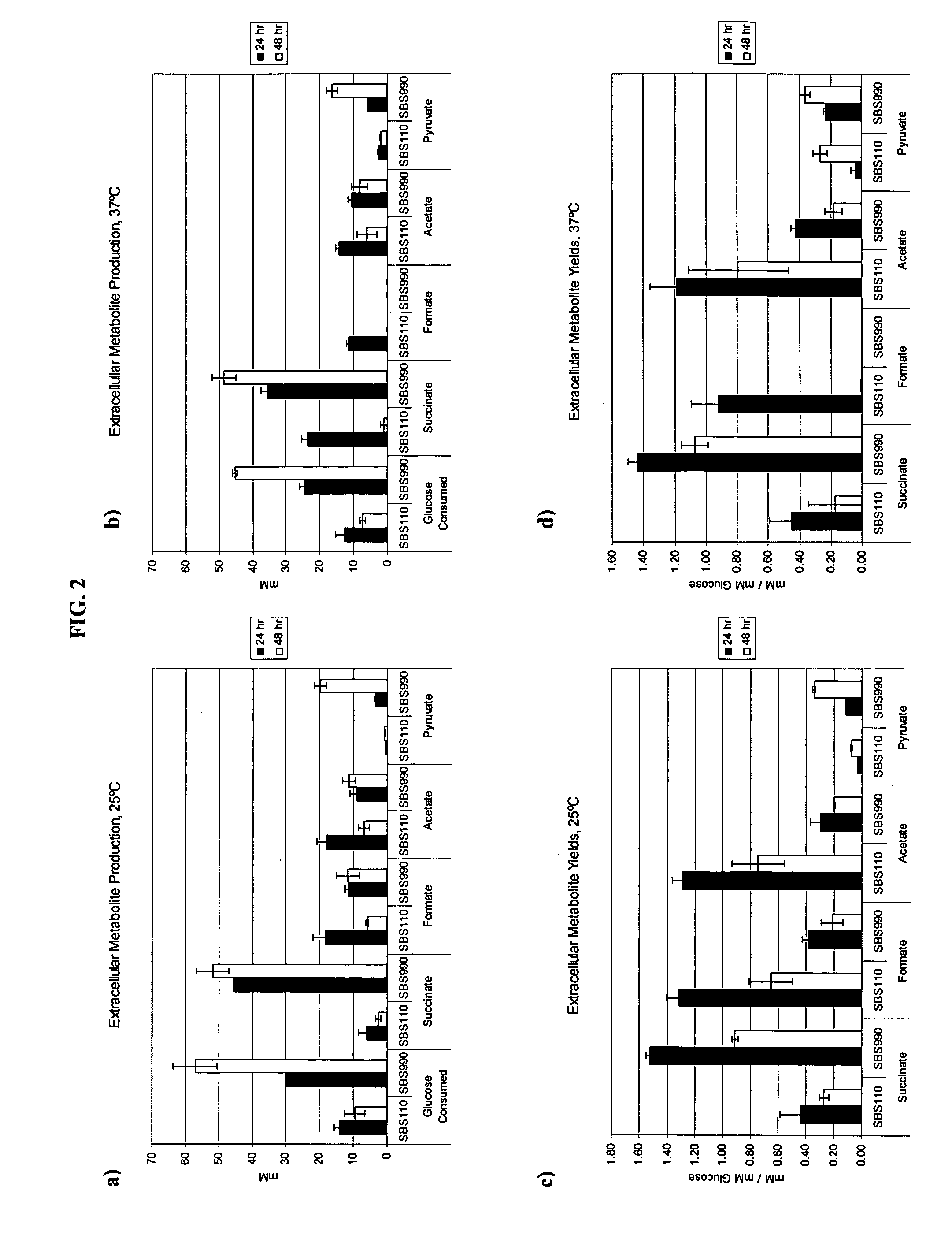
Simultaneous anaerobic production of isoamyl acetate and succinic acid
In vivo method of producing esters from acetyle coA, such as isoamyl acetate and succinate, has been developed by producing null mutants in pathways that use acetyl coA and by overexpressing products that use NADH and in order to maintain the proper redox balance between NADH and NAD+. The method is exemplified with null mutations in ldhA, adhE, ackA-pta and overexpression of pyruvate carboxylase and alcohol acetyltransferase. This strain produces higher levels of both isoamyl acetate and succinate.
Owner:RICE UNIV
Simultaneous anaerobic production of isoamyl acetate and succinic acid
InactiveUS20060141594A1Maximizing isoamyl acetate productionEasy to separateBacteriaTransferasesSuccinic acidPyruvate synthesis
In vivo method of producing esters from acetyle coA, such as isoamyl acetate and succinate, has been developed by producing null mutants in pathways that use acetyl coA and by overexpressing products that use NADH and in order to maintain the proper redox balance between NADH and NAD+. The method is exemplified with null mutations in ldhA, adhE, ackA-pta and overexpression of pyruvate carboxylase and alcohol acetyltransferase. This strain produces higher levels of both isoamyl acetate and succinate.
Owner:RICE UNIV
Methods of using crystal structure of carboxyltransferase domain of acetyl-CoA carboxylase, modulators thereof, and computer methods
InactiveUS20050009163A1Increase and decreases acetyl-CoA carboxylase activityTransferasesPeptide preparation methodsX-rayCrystal structure
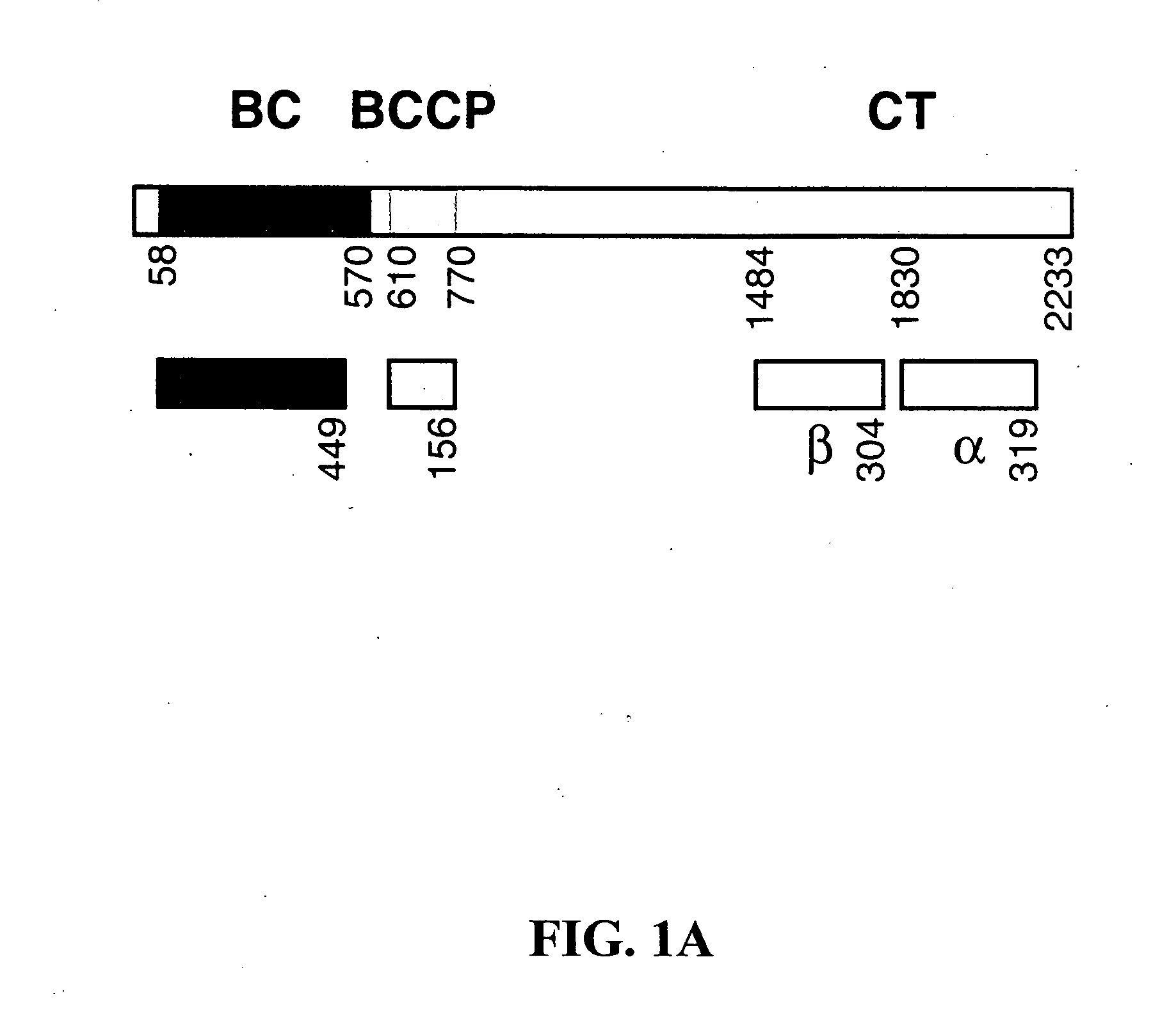
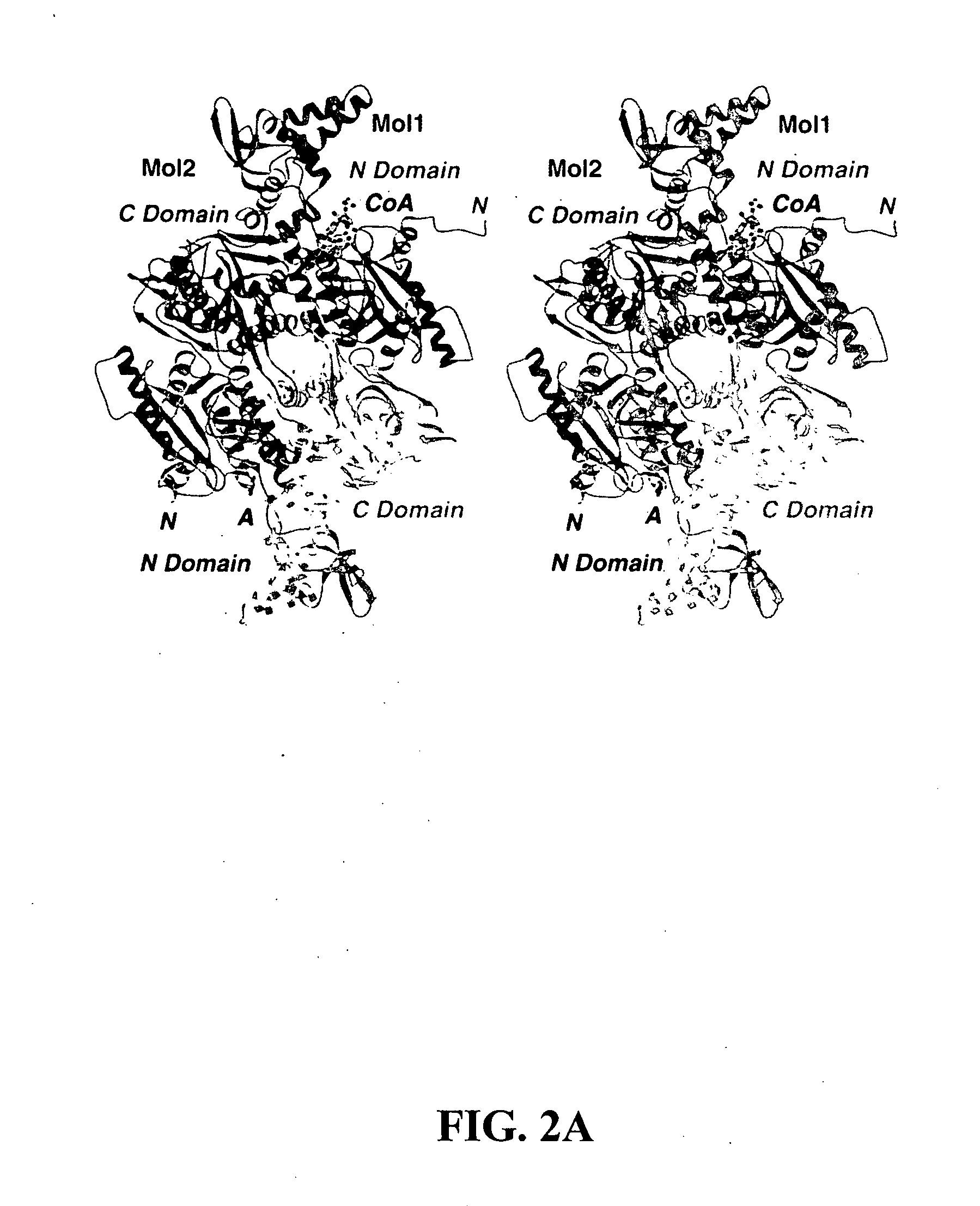
The present invention provides compositions and crystals of the carboxyltransferase (CT) domain (the C-terminal ˜90 kDa fragment) of various acetyl-CoA carboxylase (ACC) proteins, including yeast, mouse and human ACCs. Further, the present invention provides methods for identifying and designing compounds that can modulate ACC activity. These methods are based, in part, on the X-ray crystallographic structures of the CT domain of yeast ACC, either alone or bound to acetyl-CoA or a CT inhibitor, such as haloxyfop or diclofop or CP-640186. Thus, the present invention relates to the crystal structures of the carboxyltransferase (“CT”) domain of acetyl-CoA carboxylase (“ACC”), and to the use of these structures in the design of anti-obesity compounds, anti-diabetes compounds, antibiotic compounds, herbicide compounds, and in the design of herbicide resistant plants.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Acetyl-coa producing enzymes in yeast
InactiveUS20100248233A1Increase productionFungiMicrobiological testing/measurementHeterologousAcetyl Coenzyme A Synthetase
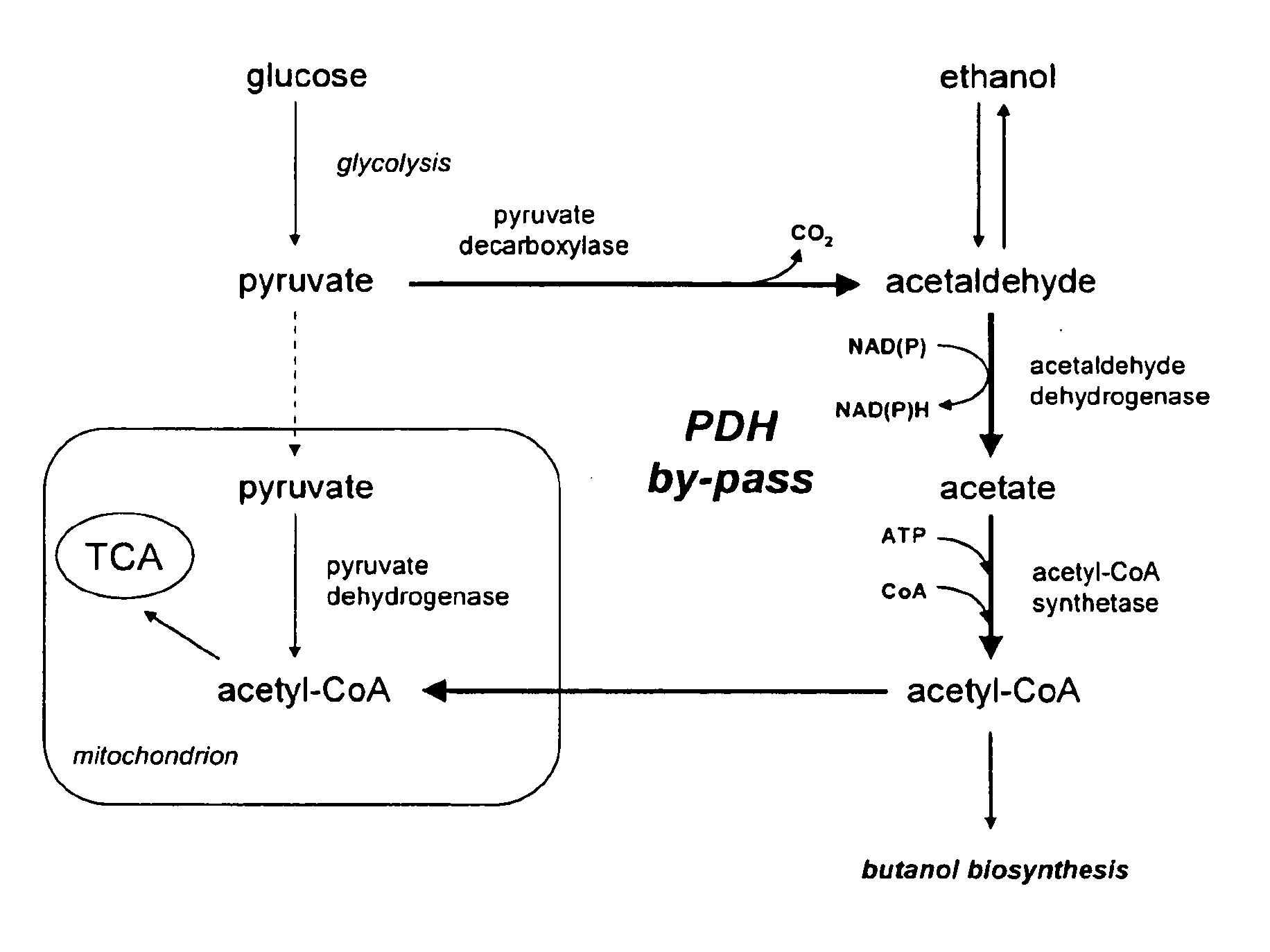
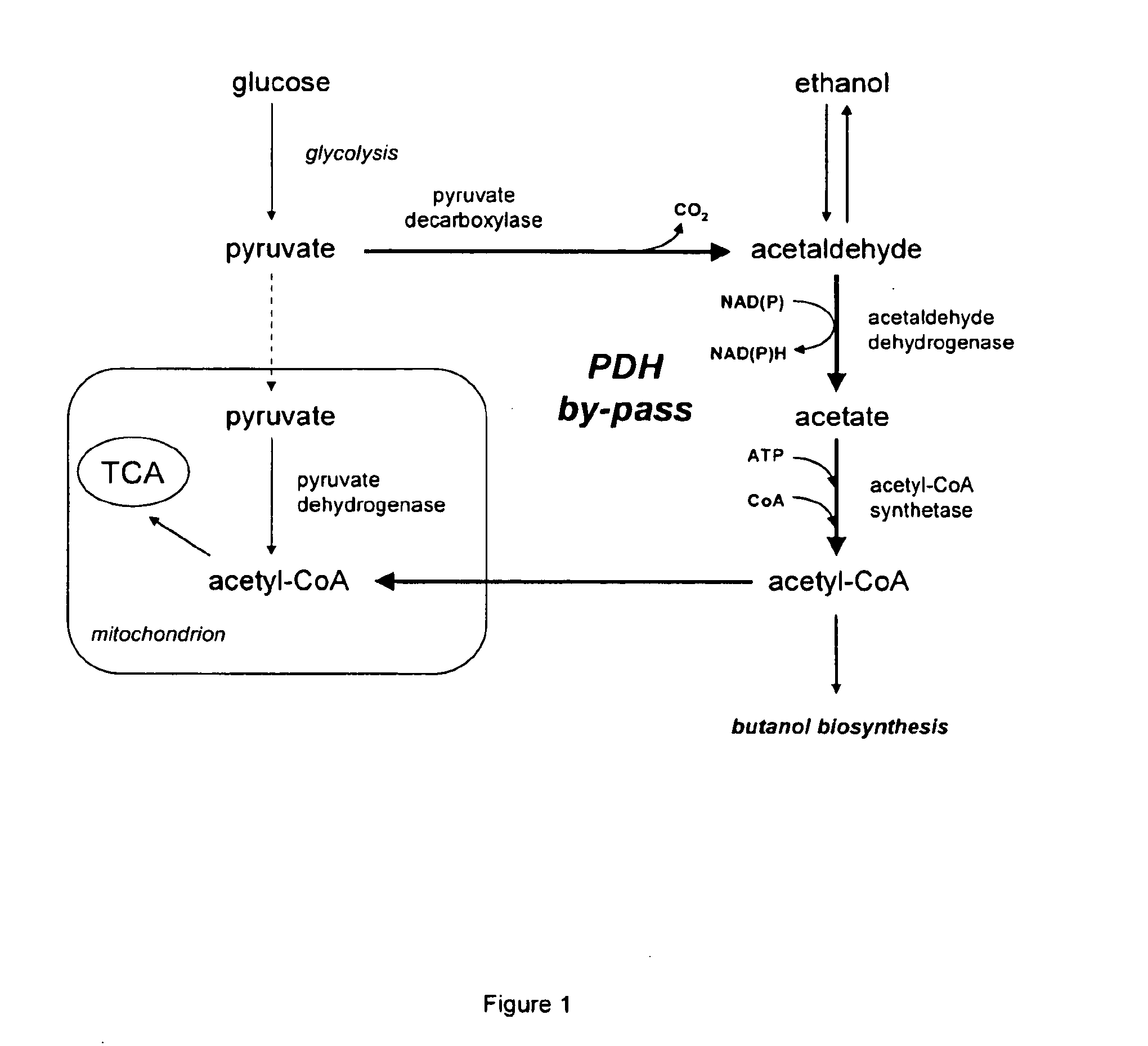
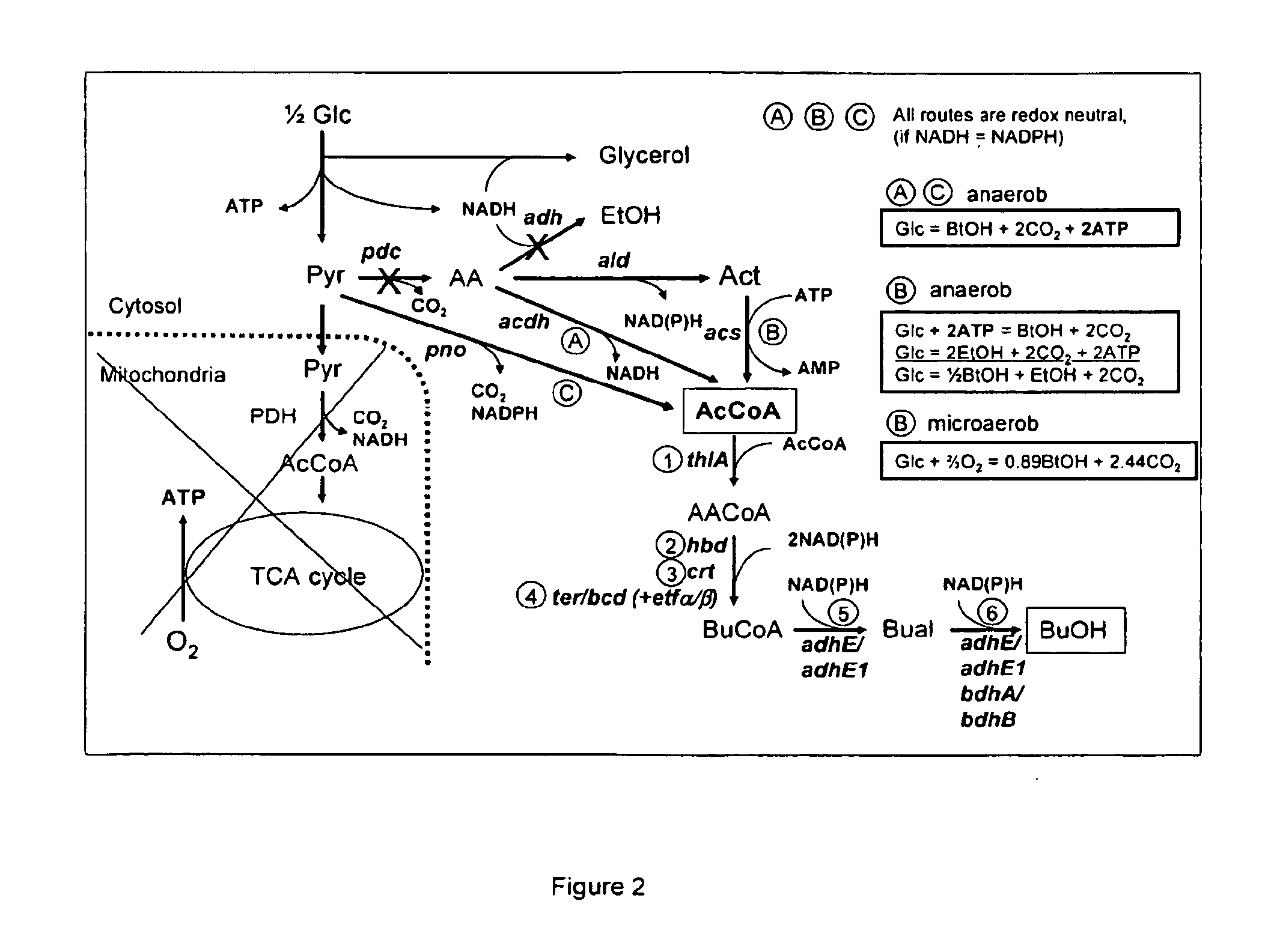
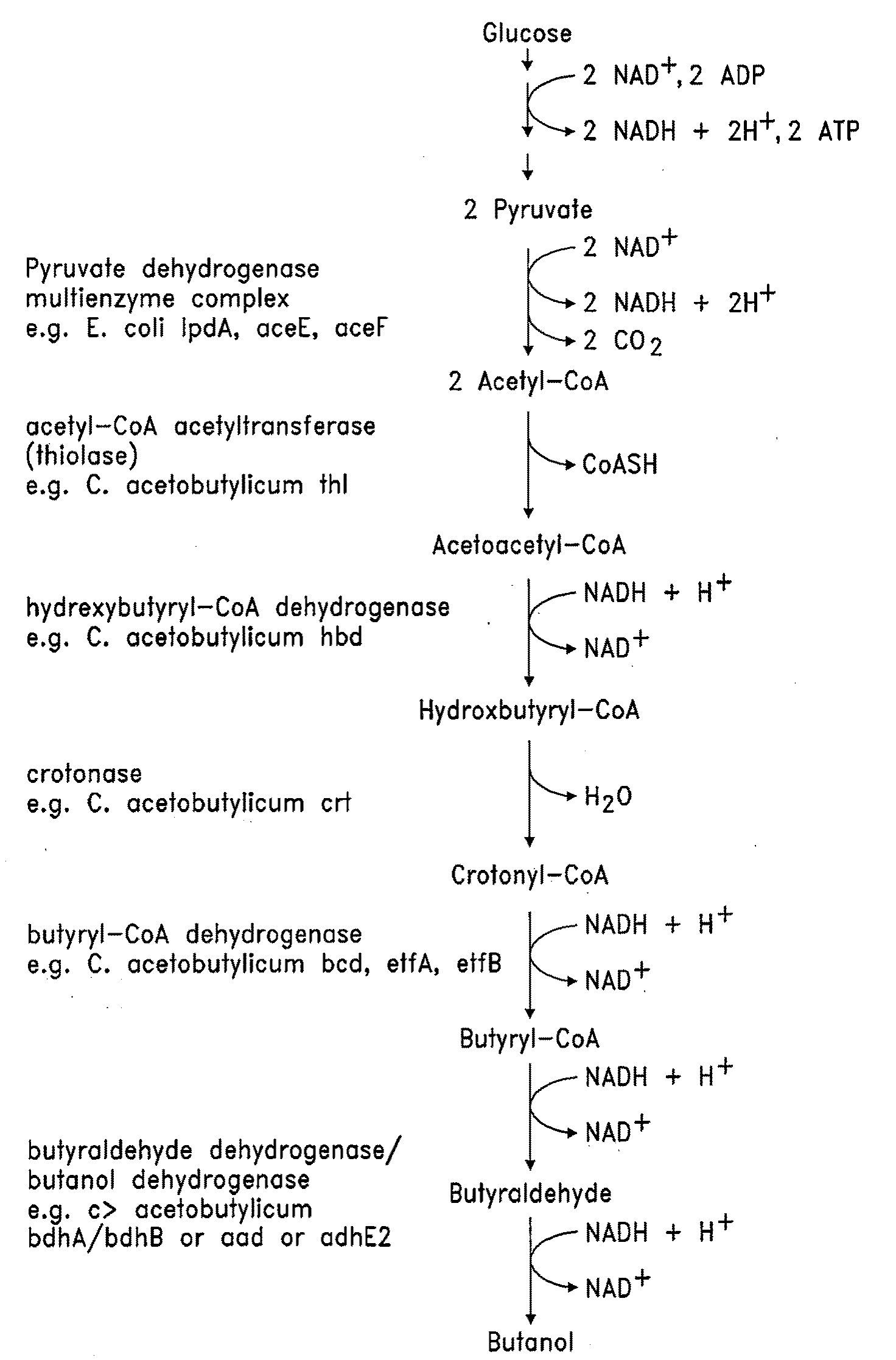
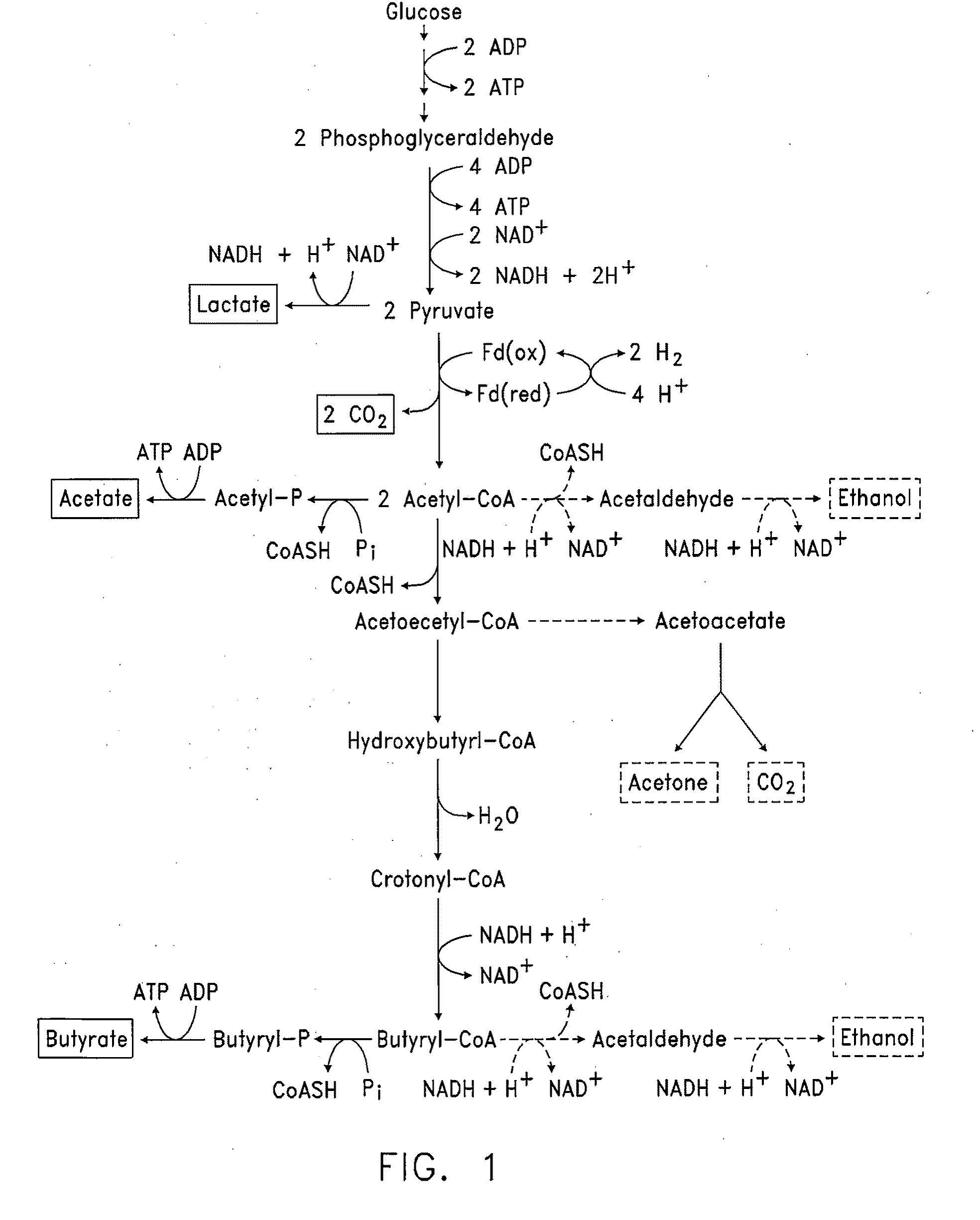
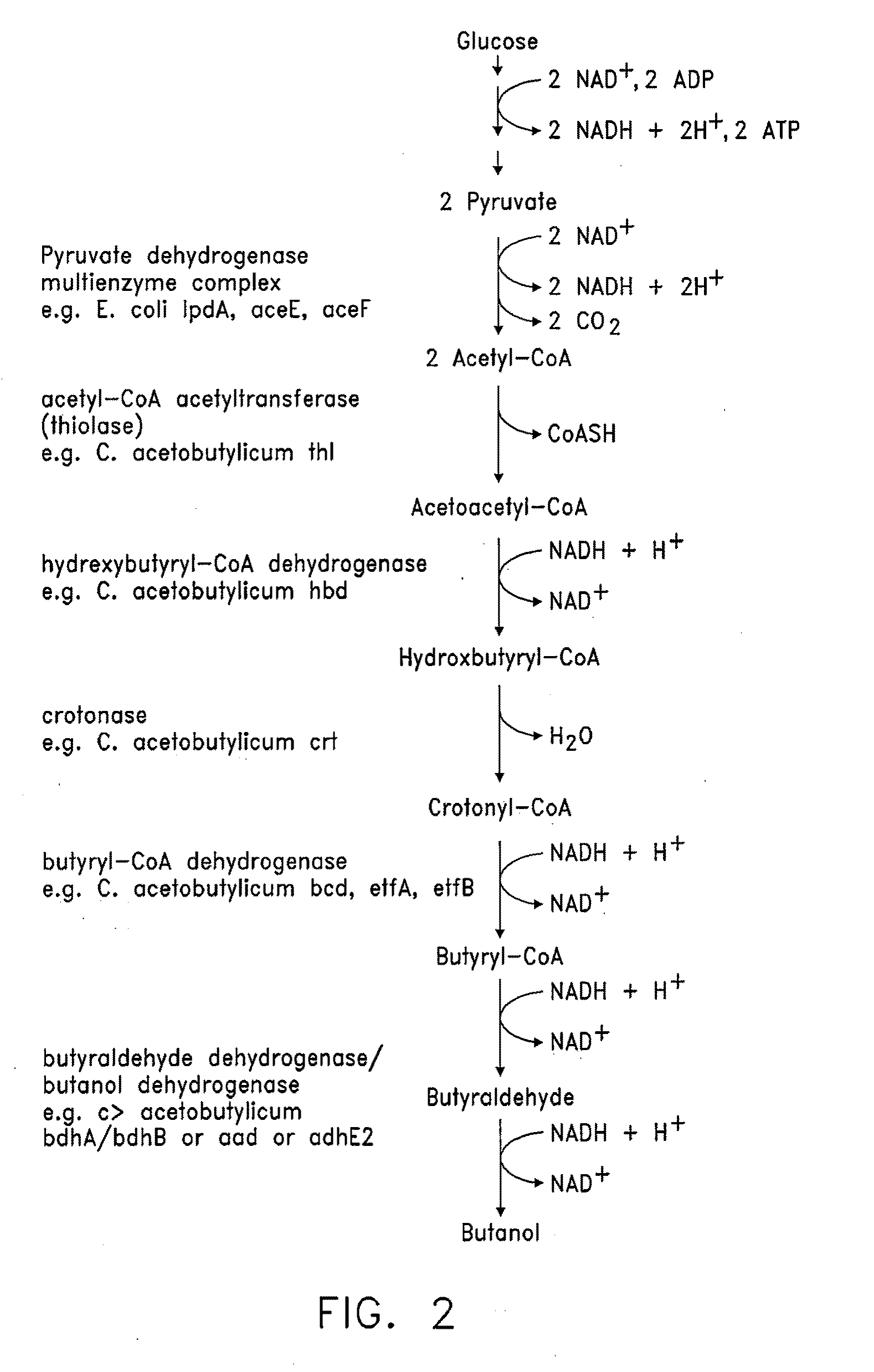
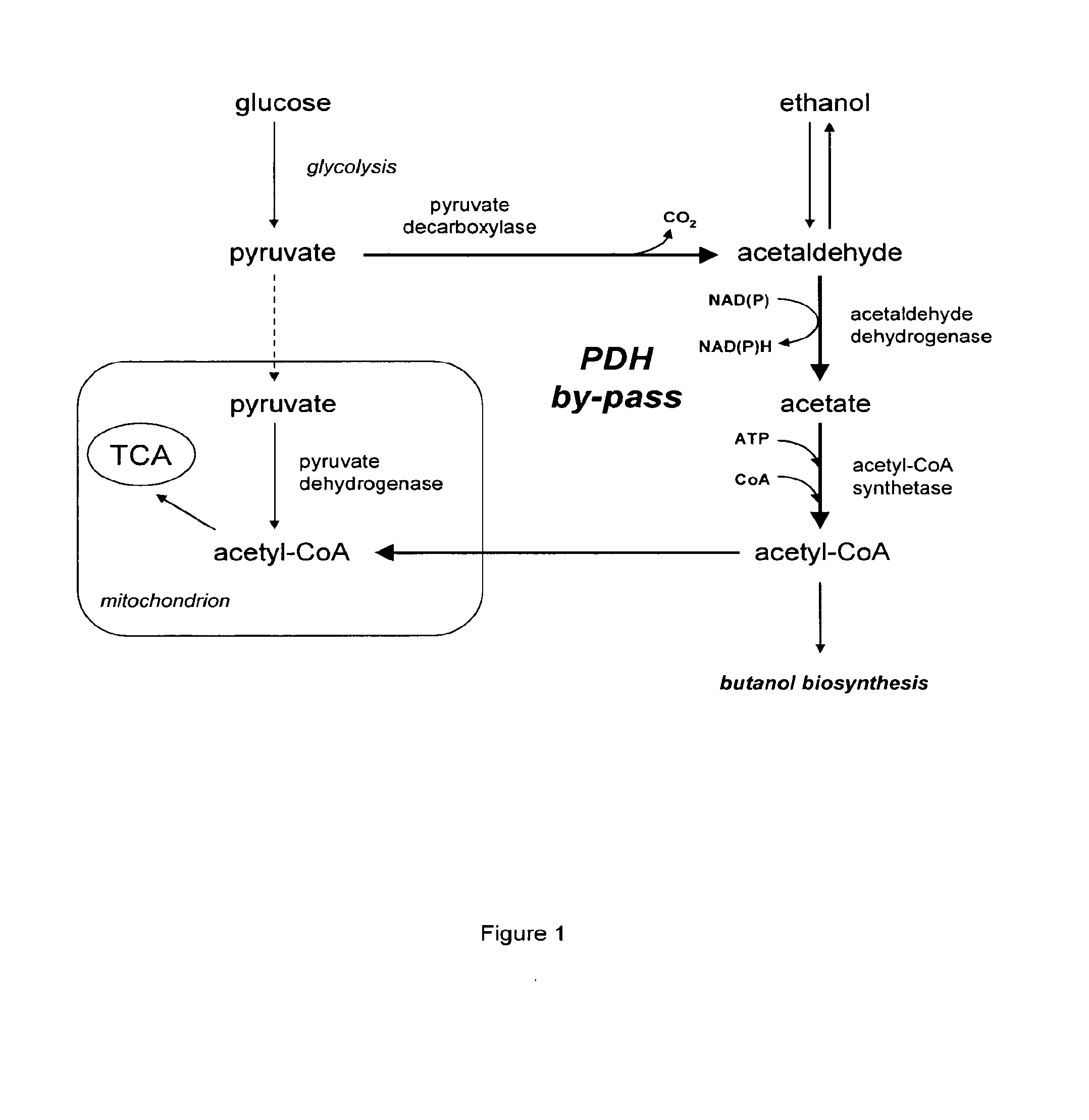
The present invention relates to a method of identifying a heterologous polypeptide having enzymatic activity for converting pyruvate, acetaldehyde or acetate into acetyl-CoA in (the cytosol of) a yeast cell comprising: a) providing a mutated yeast cell comprising a deletion of at least one gene of the (PDH) by-pass, selected from the genes encoding the enzymes pyruvate decarboxylase (PDC), acetaldehyde dehydrogenase (ALD), and acetyl-CoA synthetase (ACS); b) transforming said mutated yeast cell with an expression vector comprising a heterologous nucleotide sequence encoding a candidate polypeptide having potential enzymatic activity for converting pyruvate, acetaldehyde or acetate into acetyl-CoA; c) testing said recombinant mutated yeast cell for its ability to grow on minimal medium containing glucose as sole carbon source, and d) identifying said candidate polypeptide as a heterologous polypeptide having enzymatic activity for converting pyruvate, acetaldehyde or acetate into acetyl-CoA in (the cytosol of) said yeast cell when growth of said cell is observed. The invention further relates to a method of producing a fermentation production such as butanol.
Owner:DSM IP ASSETS BV
Butanol production by metabolically engineered yeast
InactiveUS20100062505A1Improve metabolic activityIncreased amount of acetyl-CoAFungiBiofuelsHeterologousYeast
There are disclosed metabolically-engineered yeast and methods of producing n-butanol. In an embodiment, metabolically-engineered yeast is capable of metabolizing a carbon source to produce n-butanol, at least one pathway produces increased cytosolic acetyl-CoA relative to cytosolic acetyl-CoA produced by a wild-type yeast, and at least one heterologous gene encodes and expresses at least one enzyme for a metabolic pathway capable of utilizing NADH to convert acetyl-CoA to n-butanol. In another embodiment, a method of producing n-butanol includes (a) providing metabolically-engineered yeast capable of metabolizing a carbon source to produce n-butanol, at least one pathway produces increased cytosolic acetyl-CoA relative to cytosolic acetyl-CoA produced by a wild-type yeast, and at least one heterologous gene encodes and expresses at least one enzyme for a metabolic pathway utilizing NADH to convert acetyl-CoA to n-butanol; and (b) culturing the yeast to produce n-butanol. Other embodiments are also disclosed.
Owner:GEVO INC
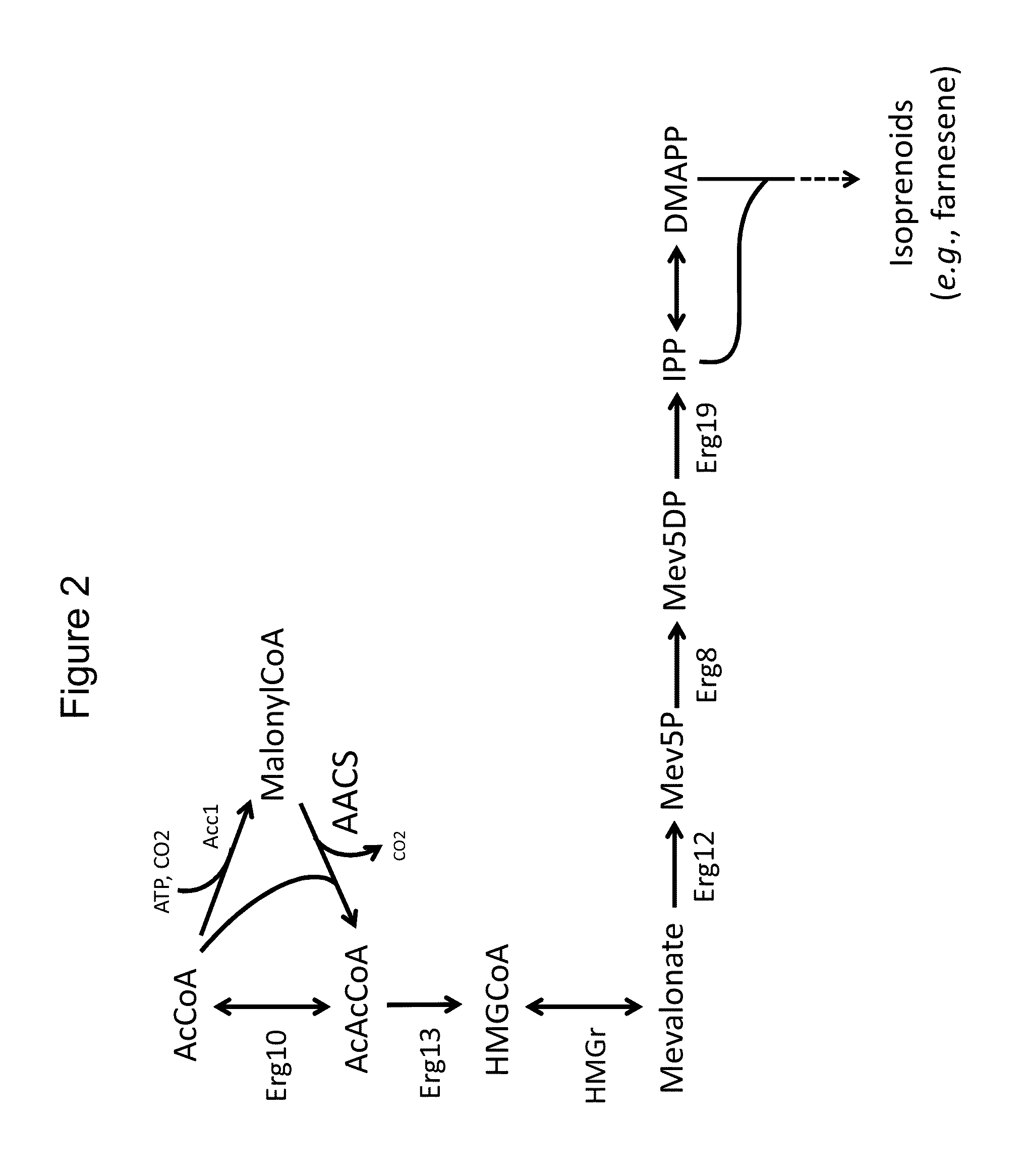
Genetically modified host cells and use of same for producing isoprenoid compounds
The present invention provides genetically modified eukaryotic host cells exhibiting increased activity levels of one or more enzymes that generate precursors to be utilized by the mevalonate pathway enzymes, increased activity levels of one or more mevalonate pathway enzymes, prenyl transferase, and / or decreased levels of squalene synthase activity; such cells are useful for producing isoprenoid compounds. The present invention provides genetically modified eukaryotic host cells that produce higher levels of acetyl-CoA than a control cell; such cells are useful for producing a variety of products, including isoprenoid compounds. Methods are provided for the production of an isoprenoid compound or an isoprenoid precursor in a subject genetically modified eukaryotic host cell. The methods generally involve culturing a subject genetically modified host cell under conditions that promote production of high levels of an isoprenoid or isoprenoid precursor compound.
Owner:RGT UNIV OF CALIFORNIA
Methods and organisms for utilizing synthesis gas or other gaseous carbon sources and methanol
The invention provides a non-naturally occurring microbial organism having an acetyl-CoA pathway and the capability of utilizing syngas or syngas and methanol. In one embodiment, the invention provides a non-naturally occurring microorganism, comprising one or more exogenous proteins conferring to the microorganism a pathway to convert CO, CO2 and / or H2 to acetyl-coenzyme A (acetyl-CoA), methyl tetrahydrofolate (methyl-THF) or other desired products, wherein the microorganism lacks the ability to convert CO or CO2 and H2 to acetyl-CoA or methyl-THF in the absence of the one or more exogenous proteins. For example, the microbial organism can contain at least one exogenous nucleic acid encoding an enzyme or protein in an acetyl-CoA pathway. The microbial organism is capable of utilizing synthesis gases comprising CO, CO2 and / or H2, alone or in combination with methanol, to produce acetyl-CoA. The invention additionally provides a method for producing acetyl-CoA, for example, by culturing an acetyl-CoA producing microbial organism, where the microbial organism expresses at least one exogenous nucleic acid encoding an acetyl-CoA pathway enzyme or protein in a sufficient amount to produce acetyl-CoA, under conditions and for a sufficient period of time to produce acetyl-CoA.
Owner:GENOMATICA INC
Acetyl-CoA acyltransferase gene disrupted bacterium for producing polyhydroxyalkanoate and method for producing polyhydroxyalkanoate using the same
A method for producing a polyhydroxyalkanoate with improved productivity and composition is provided. Polyhydroxyalkanoate is produced by a bacterium for producing polyhydroxyalkanoate in which a gene encoding acetyl-CoA acyltransferase is disrupted.
Owner:CANON KK
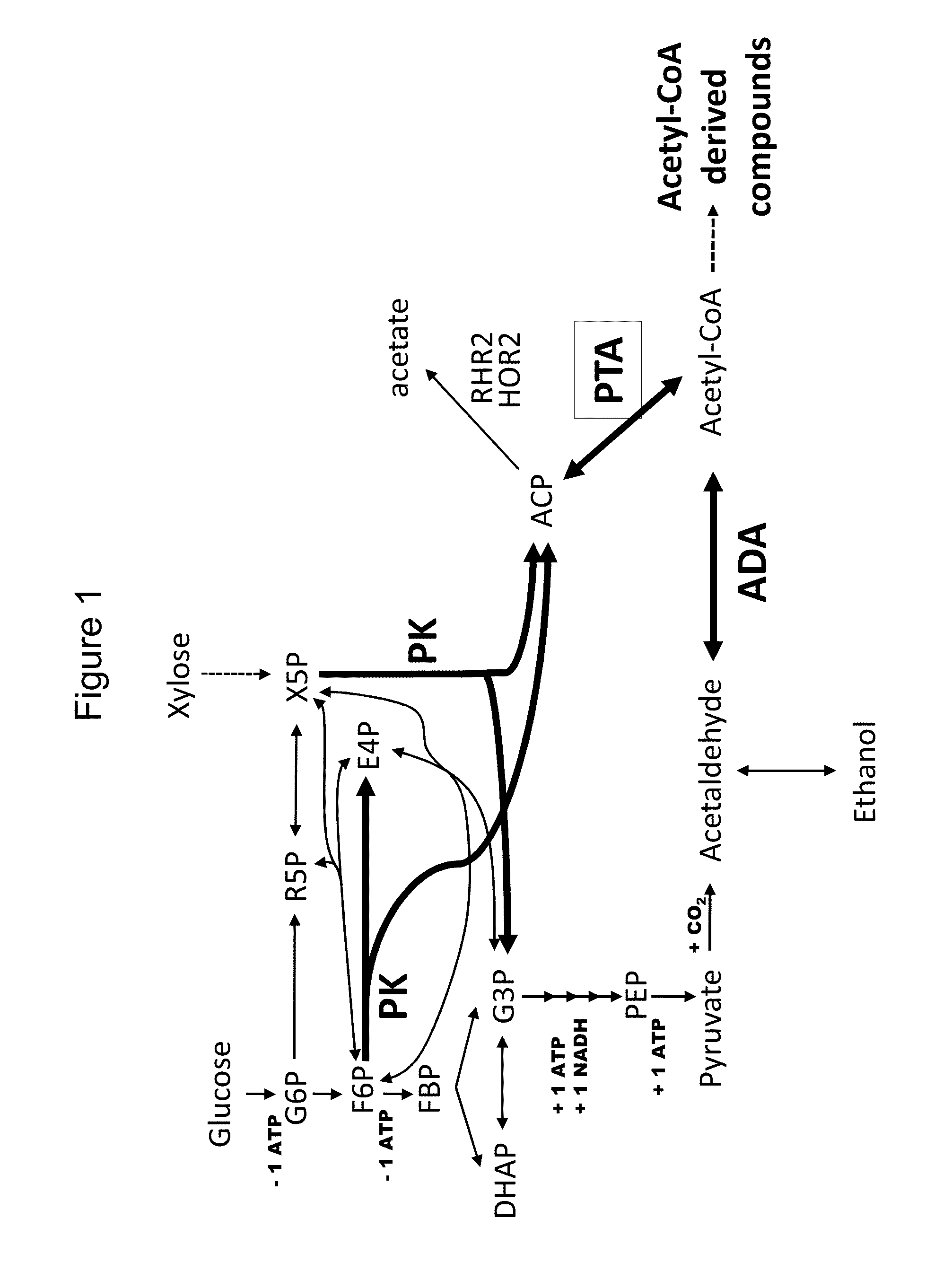
Use of phosphoketolase and phosphotransacetylase for production of acetyl-coenzyme a derived compounds
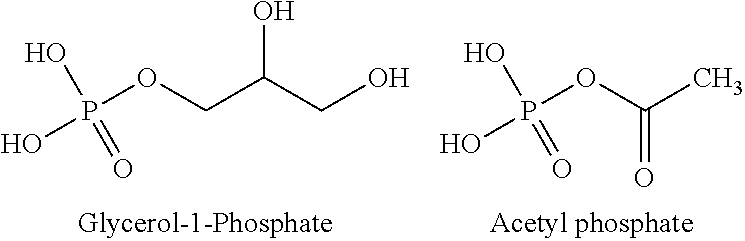
Provided herein are compositions and methods for improved production of acetyl-CoA and acetyl-CoA derived compounds in a host cell. In some embodiments, the host cell is genetically modified to comprise a heterologous nucleotide sequence encoding a phosphoketolase (PK), and a functional disruption of an endogenous enzyme that converts acetyl phosphate to acetate. In some embodiments, the host cell further comprises a heterologous nucleotide sequence encoding a phosphotransacetylase (PTA). In some embodiments, the enzyme that converts acetyl phosphate to acetate is a glycerol-1-phosphatase. In some embodiments, the glycerol-1-phosphatase is GPP1 / RHR2. In some embodiments, the glycerol-1-phosphatase is GPP2 / HOR2. The compositions and methods described herein provide an efficient route for the heterologous production of acetyl-CoA-derived compounds, including but not limited to, isoprenoids, polyketides, and fatty acids.
Owner:TOTALENERGIES ONETECH +1
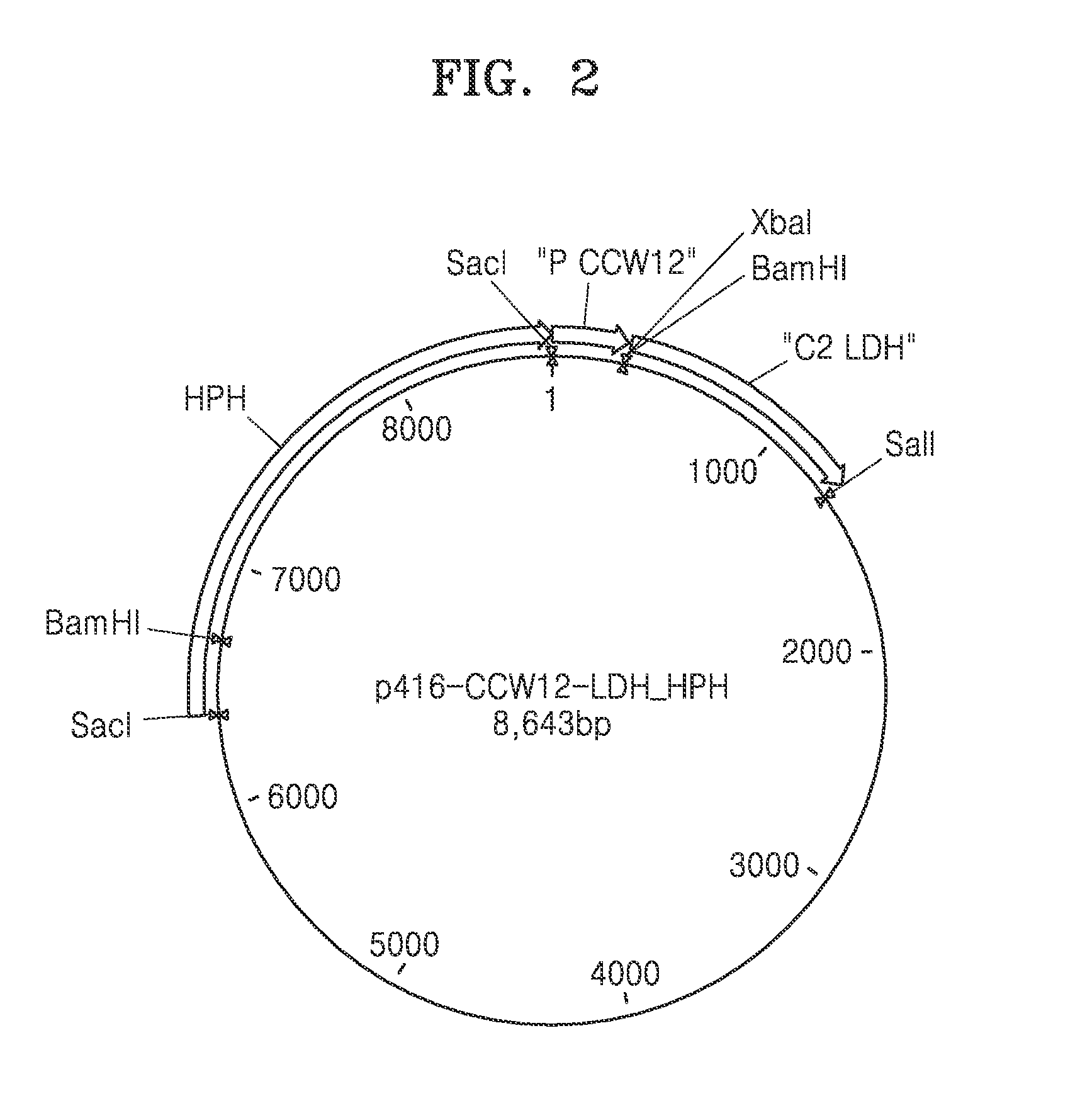
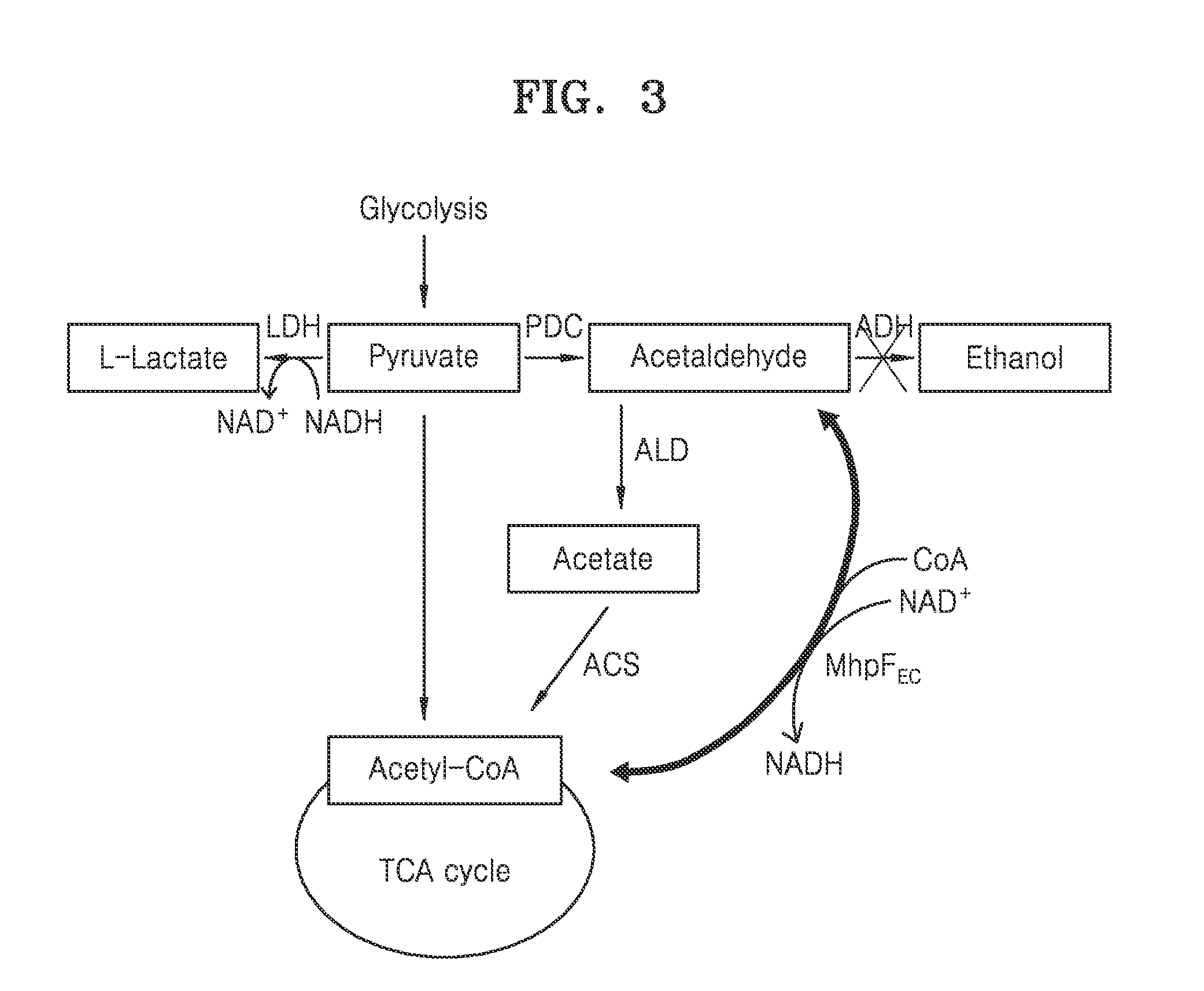
Genetically engineered yeast cell producing lactate including acetaldehyde dehydrogenase, method of producing yeast cell, and method of producing lactate using the same
Provided is a genetically engineered yeast cell with lactate production capacity, including an enzyme that catalyzes conversion of acetaldehyde to acetyl-CoA and an enzyme that catalyzes conversion of pyruvate to lactate, which activities are increased compared to a parent cell of the yeast cell, as well as a method of producing the genetically engineered yeast cell and method of producing lactate using the genetically engineered yeast cell.
Owner:SAMSUNG ELECTRONICS CO LTD
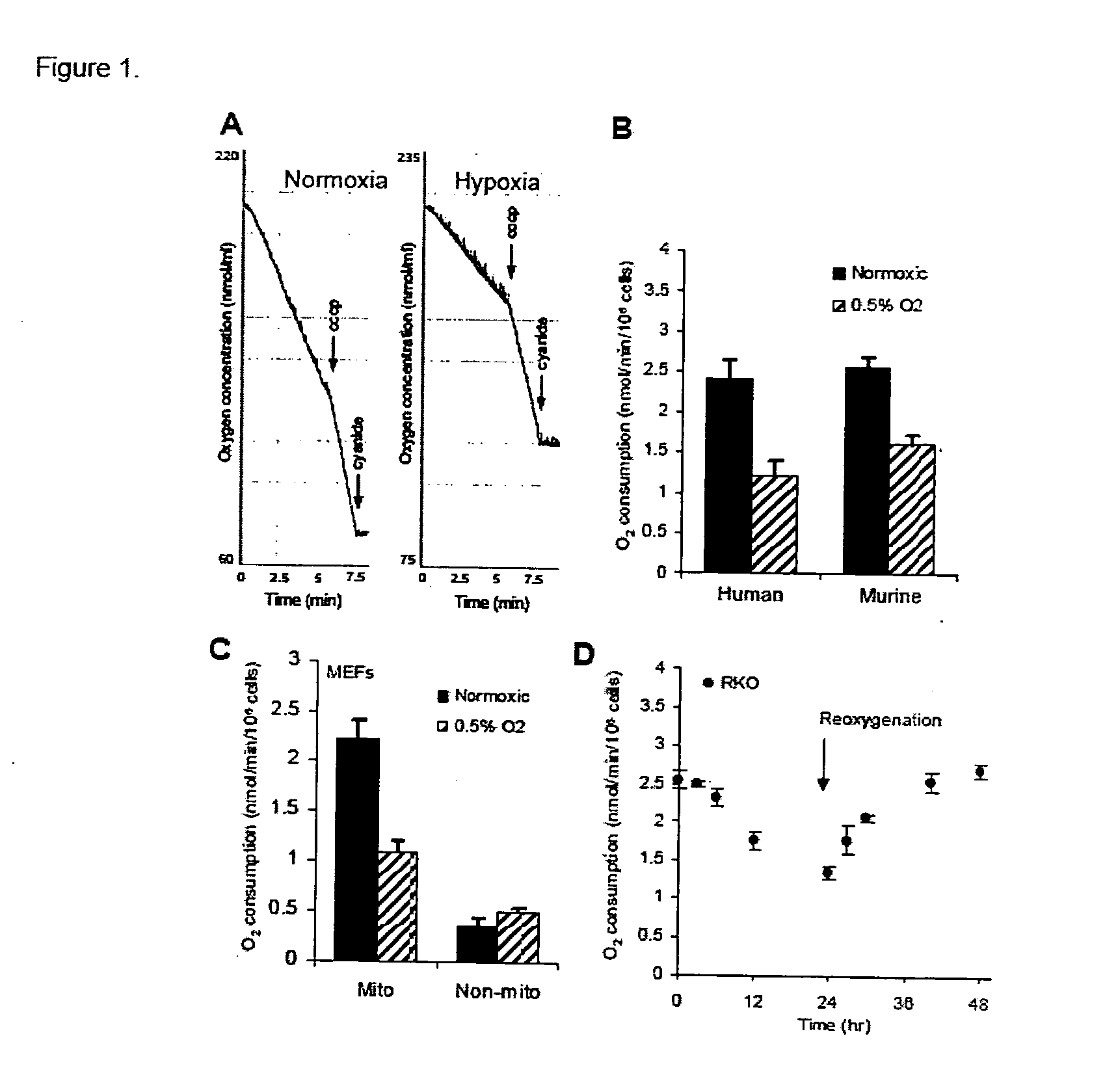
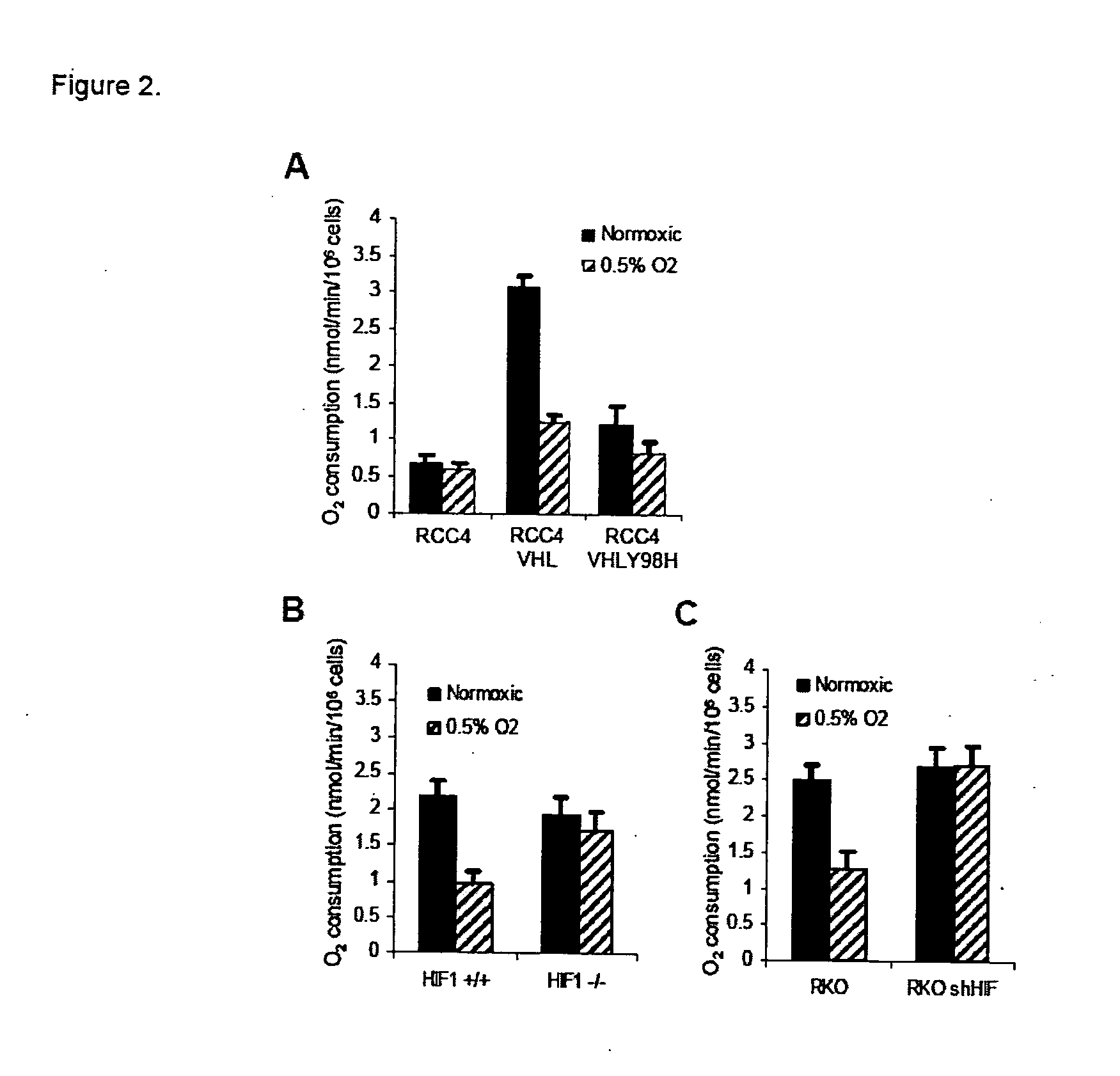
Modulation of mitochondrial oxygen consumption for therapeutic purposes
InactiveUS20070212360A1Increased blood vessel formationReduced activityBiocideAntibody ingredientsPhysiologyTherapeutic intent
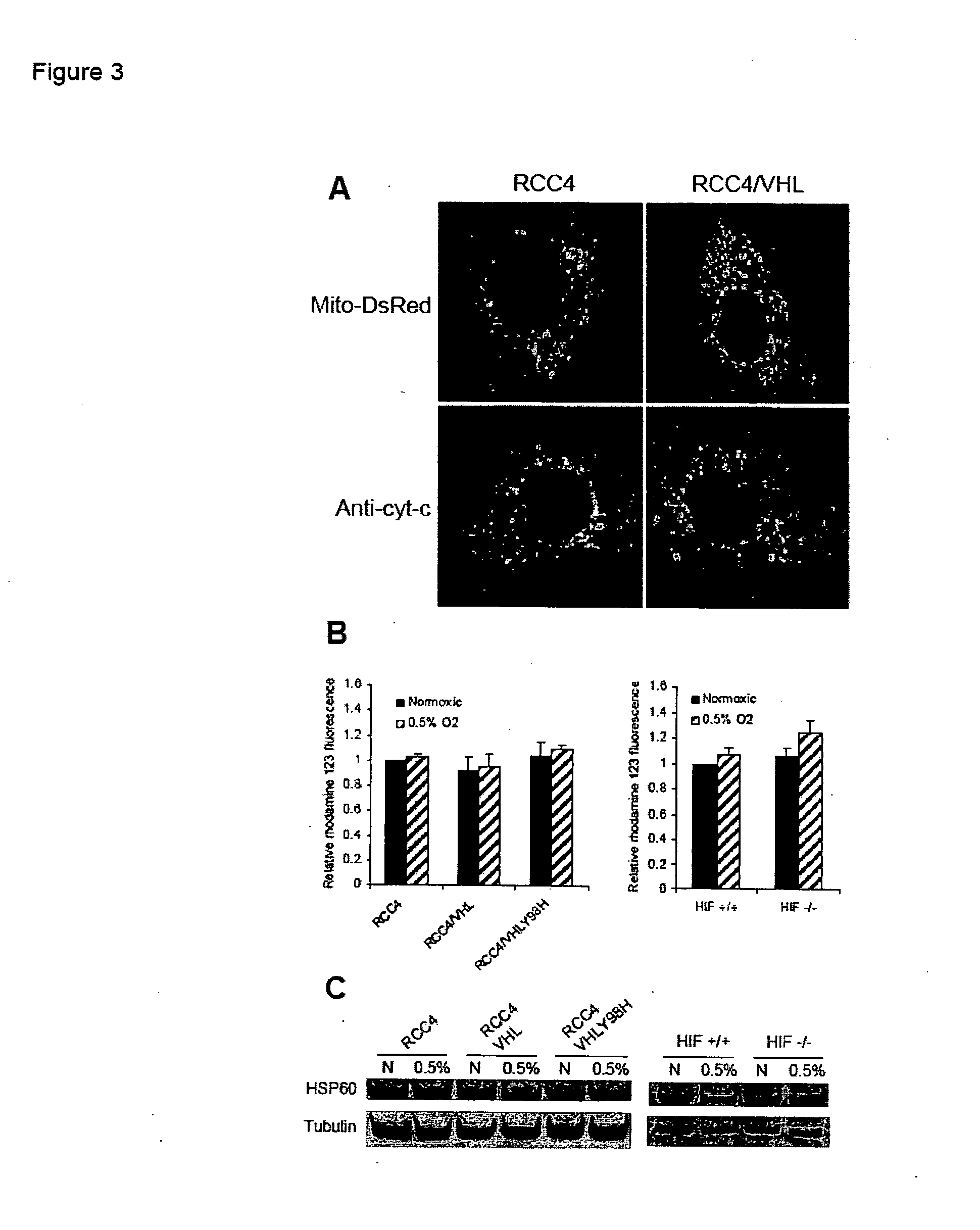
The HIF-1 transcription factor drives gene expression changes in hypoxia. While HIF-1 stimulates glycolysis, it also actively represses mitochondrial function and oxygen consumption by inducing pyruvate dehydrogenase kinase 1 (PDK1). PDK1 phosphorylates and inhibits pyruvate dehydrogenase from converting pyruvate to acetyl-CoA to fuel the mitochondrial TCA cycle. This causes a drop in mitochondrial oxygen utilization and results in a relative increase in intracellular oxygen tension.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Recombinant yeast and substance production method using the same
ActiveUS20130295616A1Reduced activityIncrease productivityMicroorganismsTransferasesBiotechnologyAcetic acid
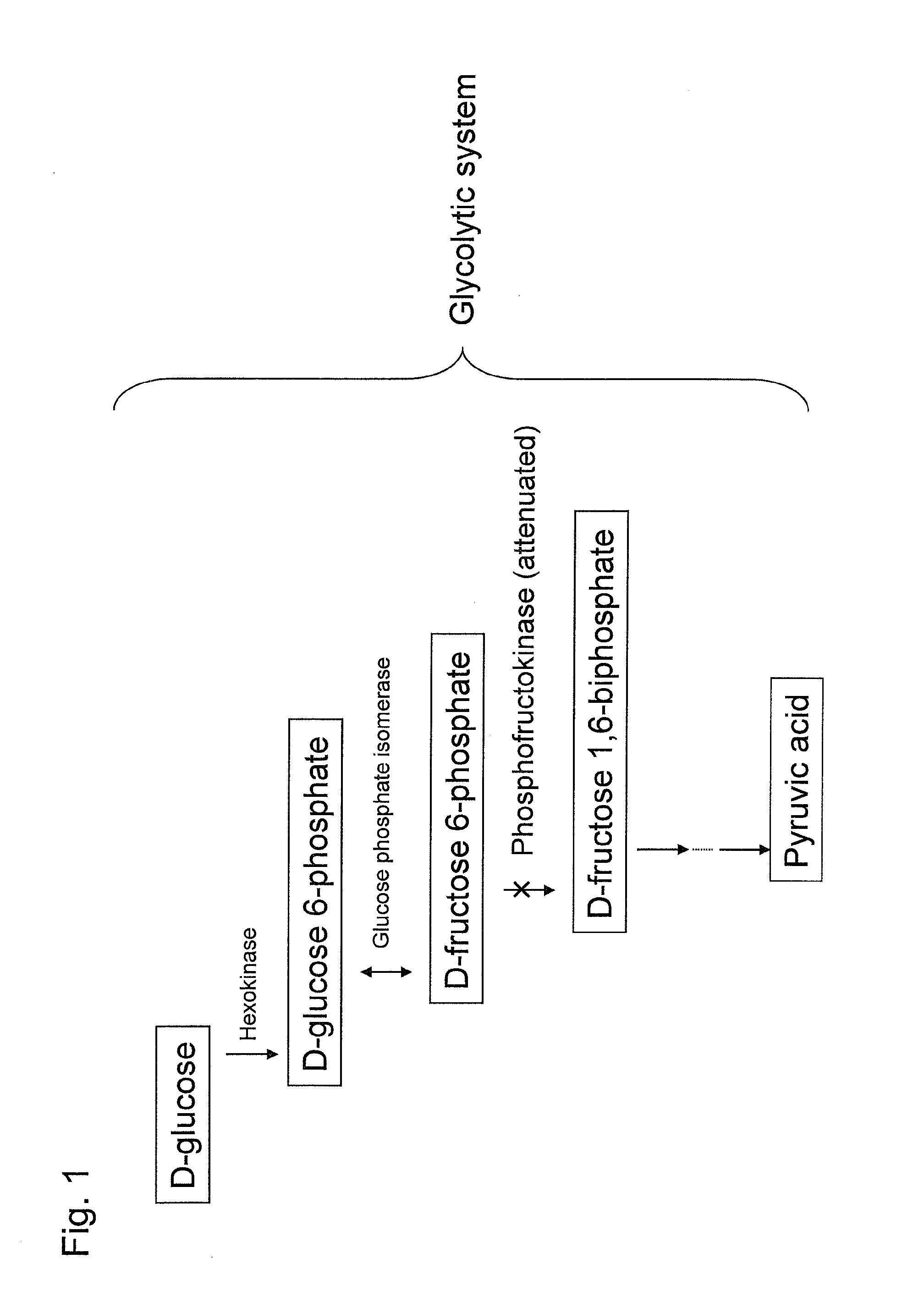
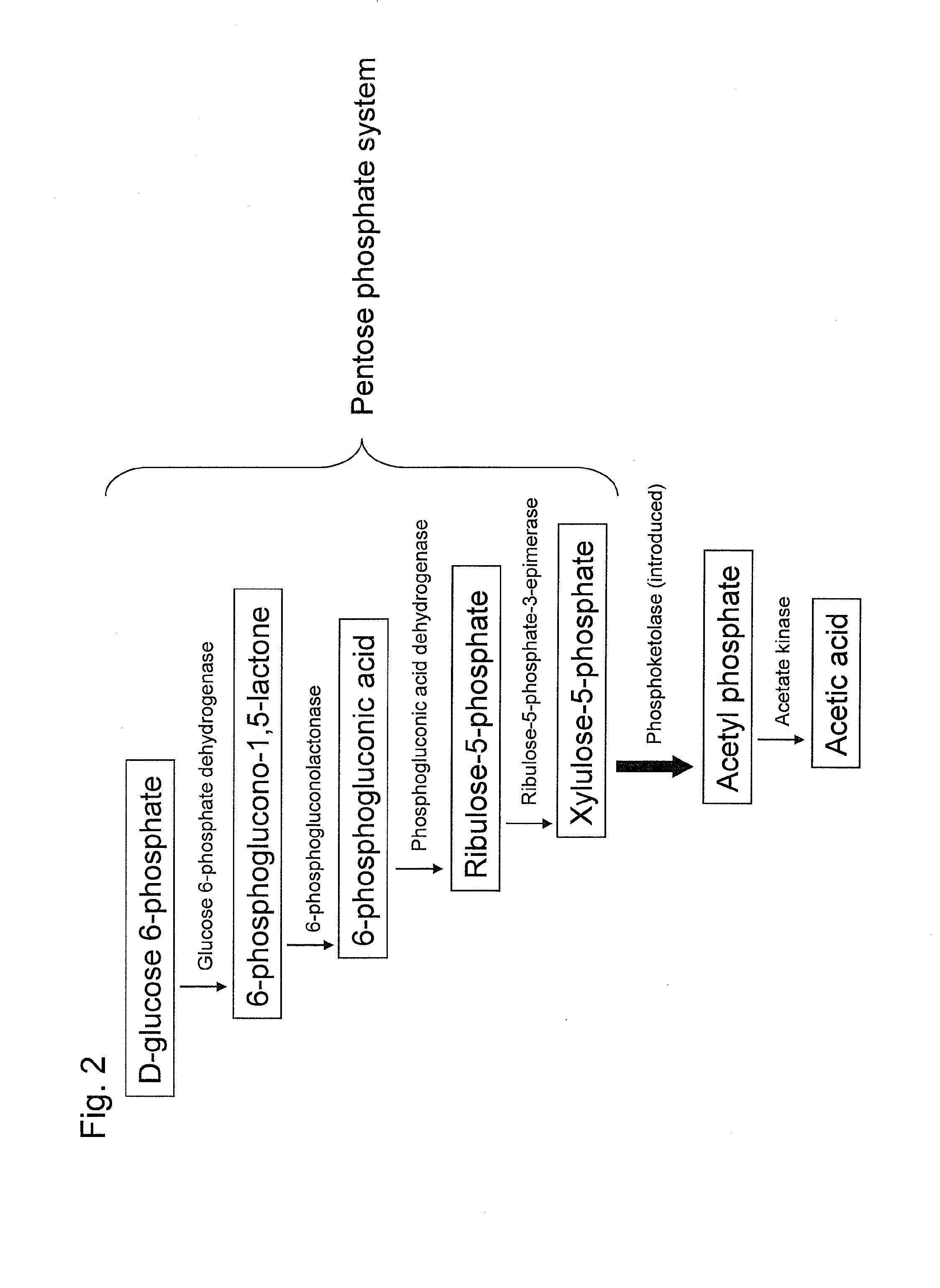
Substance productivity is improved by introducing a metabolic pathway for synthesis of acetyl-CoA or acetic acid from glucose-6-phosphate into yeast. Acetic acid productivity, acetyl-CoA productivity, and productivity of a substance made from acetyl-CoA-derived are improved by attenuating genes involved in the glycolytic system of yeast and introducing a phosphoketolase gene into the yeast.
Owner:TOYOTA JIDOSHA KK
Genetic engineering bacterium producing isoprene and application thereof
ActiveCN104031872AAvoid the influence of own metabolismShort reaction timeBacteriaMicroorganism based processesEscherichia coliBiotechnology
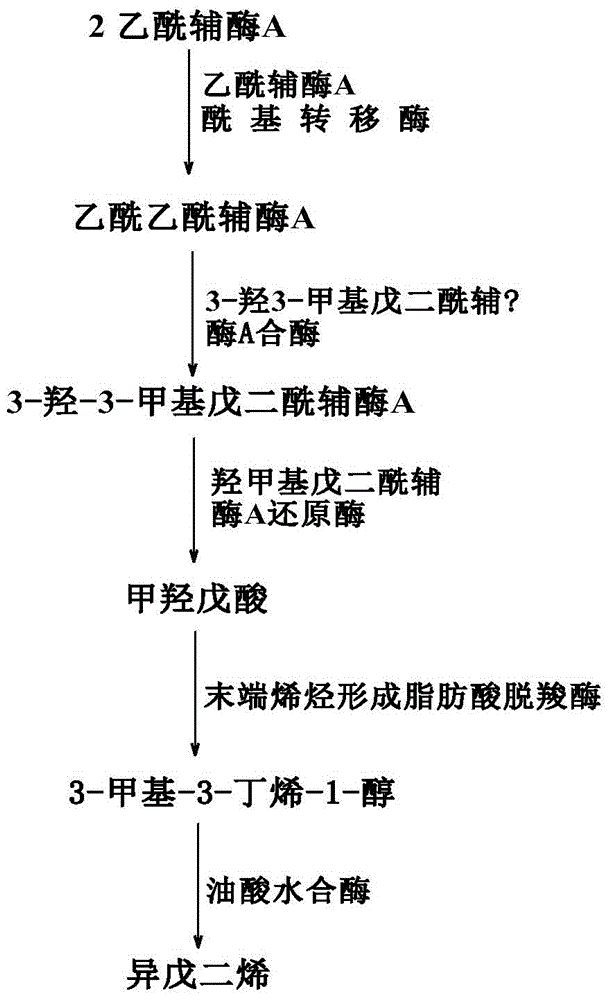
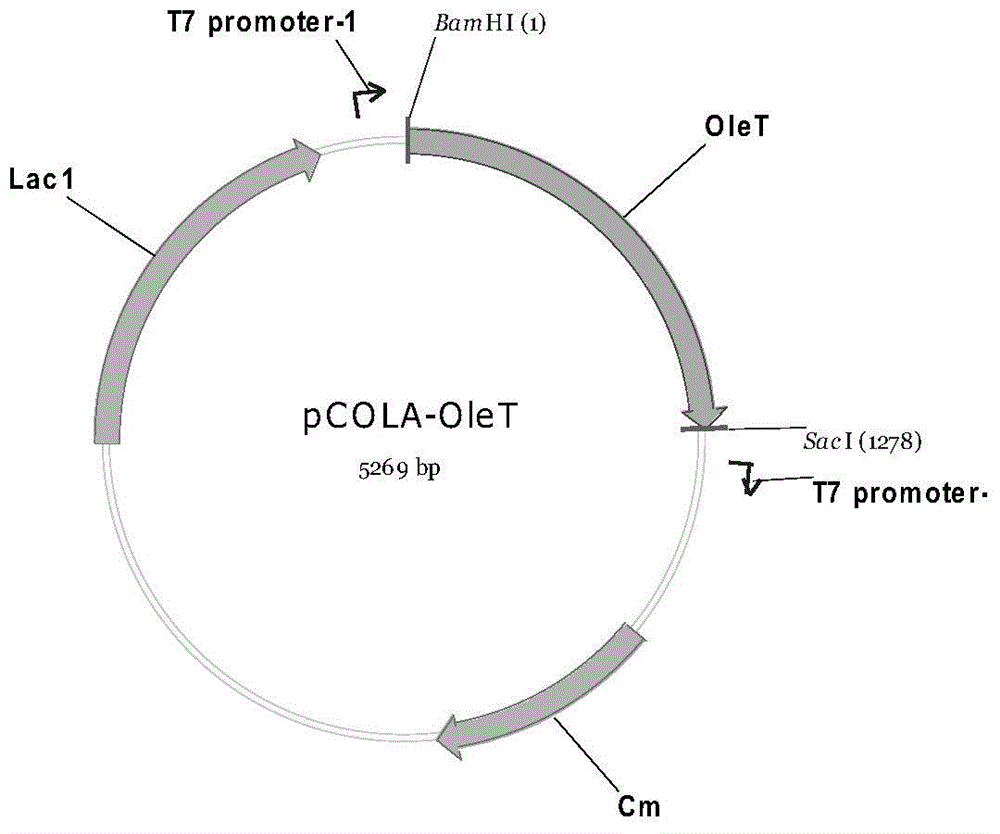
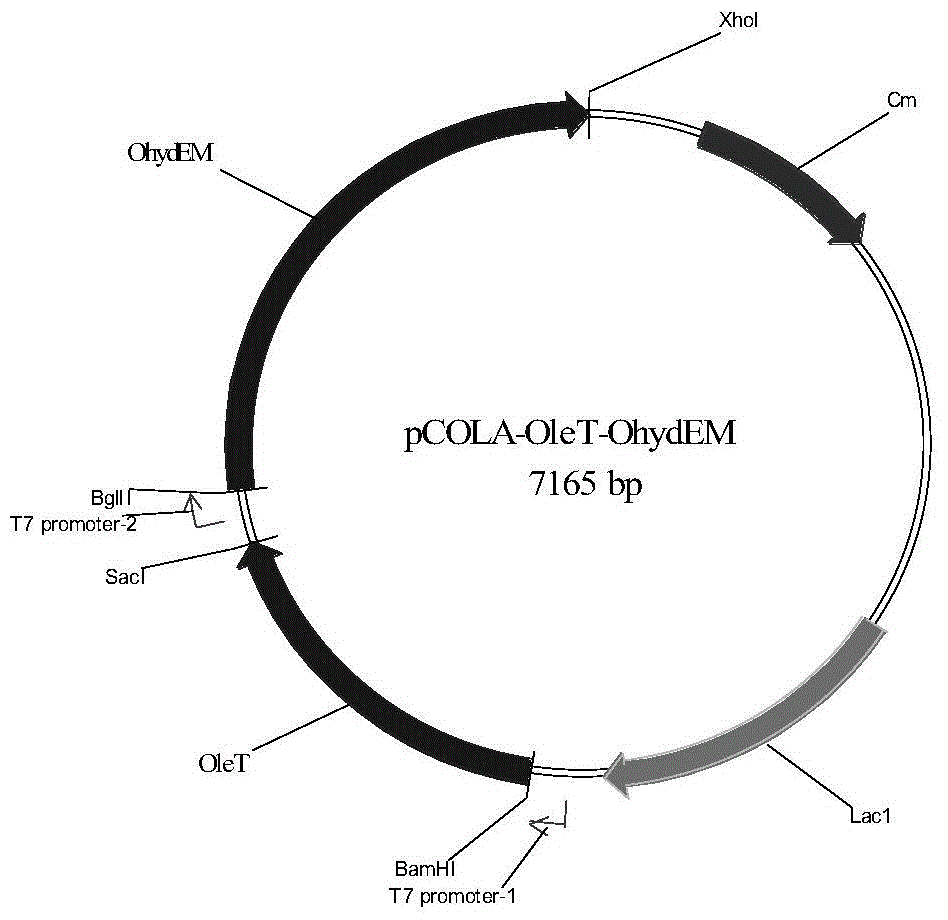
The invention discloses a genetic engineering bacterium producing isoprene and application thereof, belonging to the field of genetic engineering technology. According to the invention, a recombinant bacterium is obtained by transforming a gene containing acetyl-CoA acyltransferase, 3-hydroxy-3-methylglutaryl-CoA synthase, hydroxymethylglutaryl-CoA reductase, terminal alkene formed fatty acid decarboxylase and oleic acid hydratase into a host bacterium, and is used for fermentation production of isoprene. Such a method substantially shortens reaction process for synthesis of isoprene through an exogenous MVA approach, only five reaction steps are needed for synthesis of isoprene of acetyl-CoA, so influence of excess exogenous gene expression on metabolism of cells is avoided. Through optimization of fermentation conditions, the yield of the fermentation product isoprene can reach 39.49 mu g / L.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Constructing method for candida tropicalis gene engineering recombinant bacterium
InactiveCN1614004AIncrease the rate of acid productionIncrease productionFungiFermentationBiotechnologyCandida tropicalis
This invention relates to a construction method of tropic Candida gene engineering strain. Initial strain is product strain for industry, blocking one copy gene of carnitine acetyl CoA CAT and constructing a gene engineering recombination strain. At the same conditions, growth state of initial and recombination strain are same. CAT enzyme activity of recombination strain drops to half of that of initial strain. Speed rate of producing acid is improved. Crop of dibasic acid and percent conversion increases obviously.
Owner:TSINGHUA UNIV
Metabolically Enhanced Photoautotrophic Ethanol Producing Host Cells, Method For Producing The Host Cells, Constructs For The Transformation Of The Host Cells, And Method Of Producing Ethanol Using The Host Cells
InactiveUS20120142066A1Enhancing activity for formationIncrease the number ofBacteriaSugar derivativesAcetic acidWild type
One embodiment of the invention provides a metabolically enhanced photoautotrophic, ethanol producing host cell comprising:at least two first metabolic enhancements reducing the enzymatic activity or affinity of at least two endogenous host cell enzymes involved in acetate and lactate fermentation,the first metabolic enhancements resulting in an enhanced level of biosynthesis of acetaldehyde, pyruvate, acetyl-CoA or precursors thereof compared to the respective wild type host cell,at least one second metabolic enhancement different from the first metabolic enhancement comprising an overexpressed enzyme for the formation of ethanol.
Owner:ALGENOL BIOTECH
Construction method and application of metabolic engineering escherichia coli strain for producing hydracrylic acid from acetic acid
The invention discloses a construction method of a metabolic engineering escherichia coli strain for producing hydracrylic acid from acetic acid. Escherichia coli reformed by metabolic engineering isutilized for producing hydracrylic acid by fermenting acetic acid; and construction ways are as follows: constructing a metabolic way for producing hydracrylic acid from acetyl CoA, and / or over-expressing relevant genes of an acetic acid intake way so as to increase the transmission rate of acetic acid, and / or blocking or decreasing TCA circle so as to increase metabolic flow of acetyl CoA flowingtowards a target metabolite, and / or reducing decarboxylic reaction between malic acid or oxaloacetic acid so as to delete a byproduct generation way, and / or deleting key genes in an alcohol production way so as to regulate metabolic flow of an acetyl CoA node and / or regulating intracellular oxidation reduction balance through coenzyme engineering. According to the construction method, a production way for exogenously expressing hydracrylic acid is constructed, the metabolic ways, the adjustment and control are analyzed, and escherichia coli is reformed by virtue of a metabolic engineering measure, so that the prepared strain can be utilized for producing hydracrylic acid in a culture medium taking acetic acid as a carbon source.
Owner:EAST CHINA UNIV OF SCI & TECH
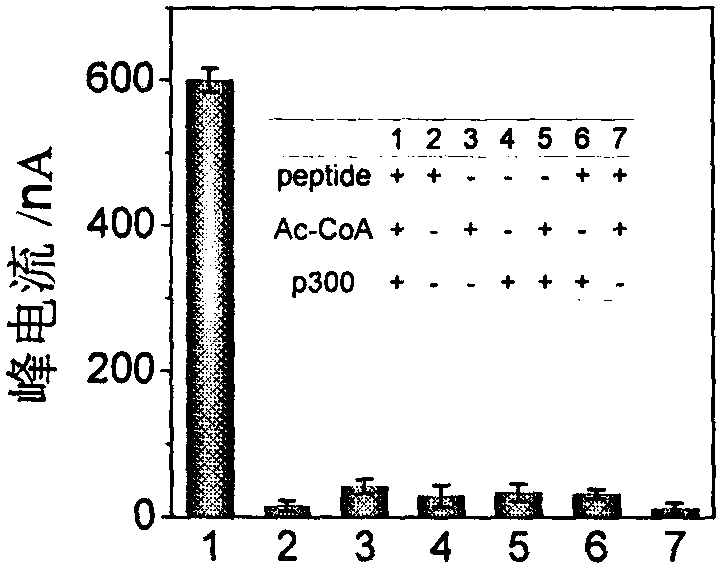
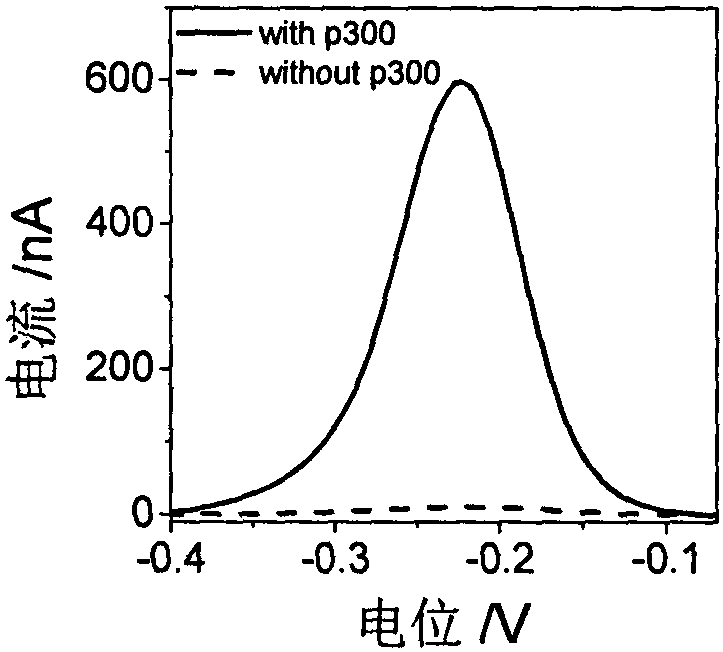
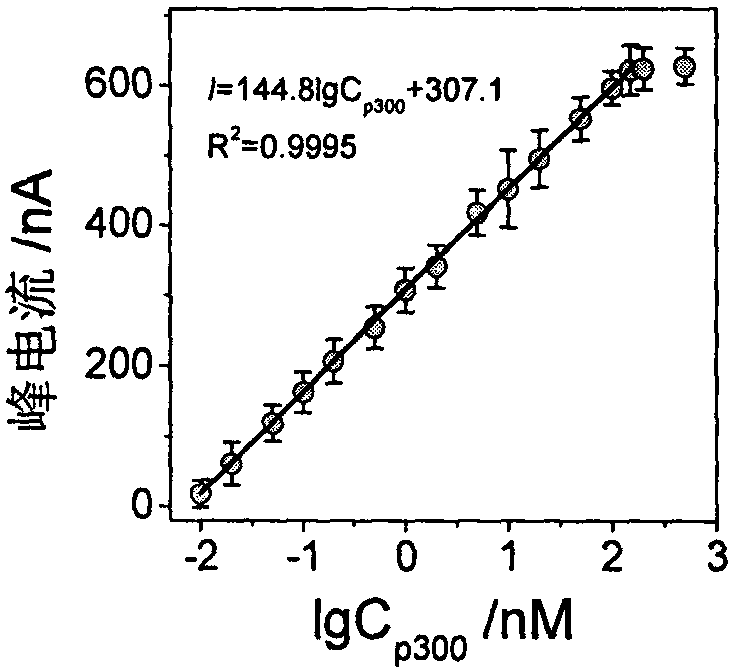
Construction method and application of electrochemical Faraday cage immunosensor for detecting activity of histone acetyltransferase
ActiveCN108593751AHigh sensitivityAchieving High Sensitivity DetectionMaterial electrochemical variablesWater bathsUltrasonic dispersion
The invention discloses a construction method and an application of an electrochemical Faraday cage immunosensor for detecting the activity of histone acetyltransferase. The construction method comprises the following steps: (1) preparing peptide / Au: respectively taking acetyltransferase p300, polypeptide and acetyl-CoA, fully mixing the taken substances in PBS (0.1 M, pH 7.0), incubating the obtained solution in a constant-temperature water bath, taking and dropwise applying a catalytic reaction to the surface of a gold electrode, and incubating the gold electrode in a 4 DEG C refrigerator; (2) preparing MB&AuNPs@GO-Ab: mixing HAuCl4 and CTAB with water, adding ascorbic acid to the obtained reaction mixture, adding NaOH to obtain CTAB covered AuNPs, centrifuging and purifying the CTAB covered AuNPs, dispersing the purified CTAB covered AuNPs in an equal amount of water, adding GO to the obtained solution, performing ultrasonic dispersion, standing the dispersed solution for later use,adding an acetyl antibody, performing incubation, adding MB, and performing vibration and uniform mixing; and (3) producing the electrochemical Faraday cage immunosensor: taking and dropwise applyingthe MB&AuNPs@GO-Ab to the surface of the peptide / Au, performing incubation at room temperature, and placing the produced sensor in the PBS (0.1 M, pH 7.0) to carry out electrochemical SWV test.
Owner:NINGBO UNIV
Acetyl-coa producing enzymes in yeast
ActiveUS20140030730A1Increase productionMicroorganismsMicrobiological testing/measurementAcetyl Coenzyme A SynthetaseFermentation
The present invention relates to a method of identifying a heterologous polypeptide having enzymatic activity for converting pyruvate, acetaldehyde or acetate into acetyl-CoA in (the cytosol of) a yeast cell comprising: a) providing a mutated yeast cell comprising a deletion of at least one gene of the (PDH) by-pass, selected from the genes encoding the enzymes pyruvate decarboxylase (PDC), acetaldehyde dehydrogenase (ALD), and acetyl-CoA synthetase (ACS); b) transforming said mutated yeast cell with an expression vector comprising a heterologous nucleotide sequence encoding a candidate polypeptide having potential enzymatic activity for converting pyruvate, acetaldehyde or acetate into acetyl-CoA; c) testing said recombinant mutated yeast cell for its ability to grow on minimal medium containing glucose as sole carbon source, and d) identifying said candidate polypeptide as a heterologous polypeptide having enzymatic activity for converting pyruvate, acetaldehyde or acetate into acetyl-CoA in (the cytosol of) said yeast cell when growth of said cell is observed. The invention further relates to a method of producing a fermentation production such as butanol.
Owner:DSM IP ASSETS BV
Fatty acid and derivatives production
At least one fatty acid and / or derivative thereof is produced from a gas containing H2, CO2, and O2 by providing a genetically modified hydrogen oxidizing bacterium in an aqueous medium; and contacting the aqueous medium with the gas containing H2, CO2 and O2 in a weight ratio of 20 to 70 (H2): 10 to 45 (CO2): 5 to 35 (O2); wherein the fatty acid contains at least 5 carbon atoms and wherein the hydrogen oxidizing bacterium is genetically modified relative to the wild type bacterium to increase the expression of enzyme E1 that is capable of catalyzing the conversion of acetyl CoA to acyl ACP via malonyl coA and to increase the expression of enzyme E2 that is capable of catalyzing the conversion of Acyl ACP to the fatty acid.
Owner:EVONIK DEGUSSA GMBH
Production of acetyl-coenzyme a derived compounds
ActiveUS20120288891A1Improve stabilityLonger sustained HACD compound productionBiofuelsFermentationBiotechnologyHeterologous
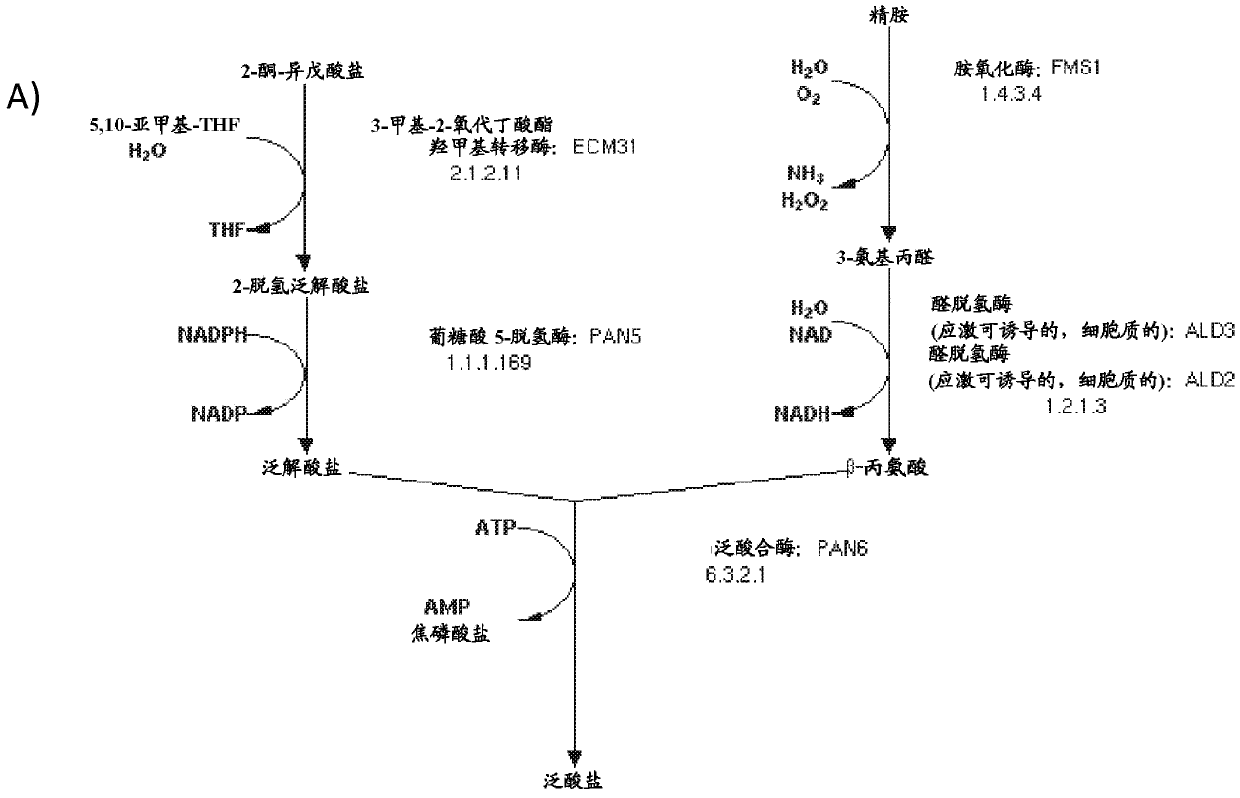
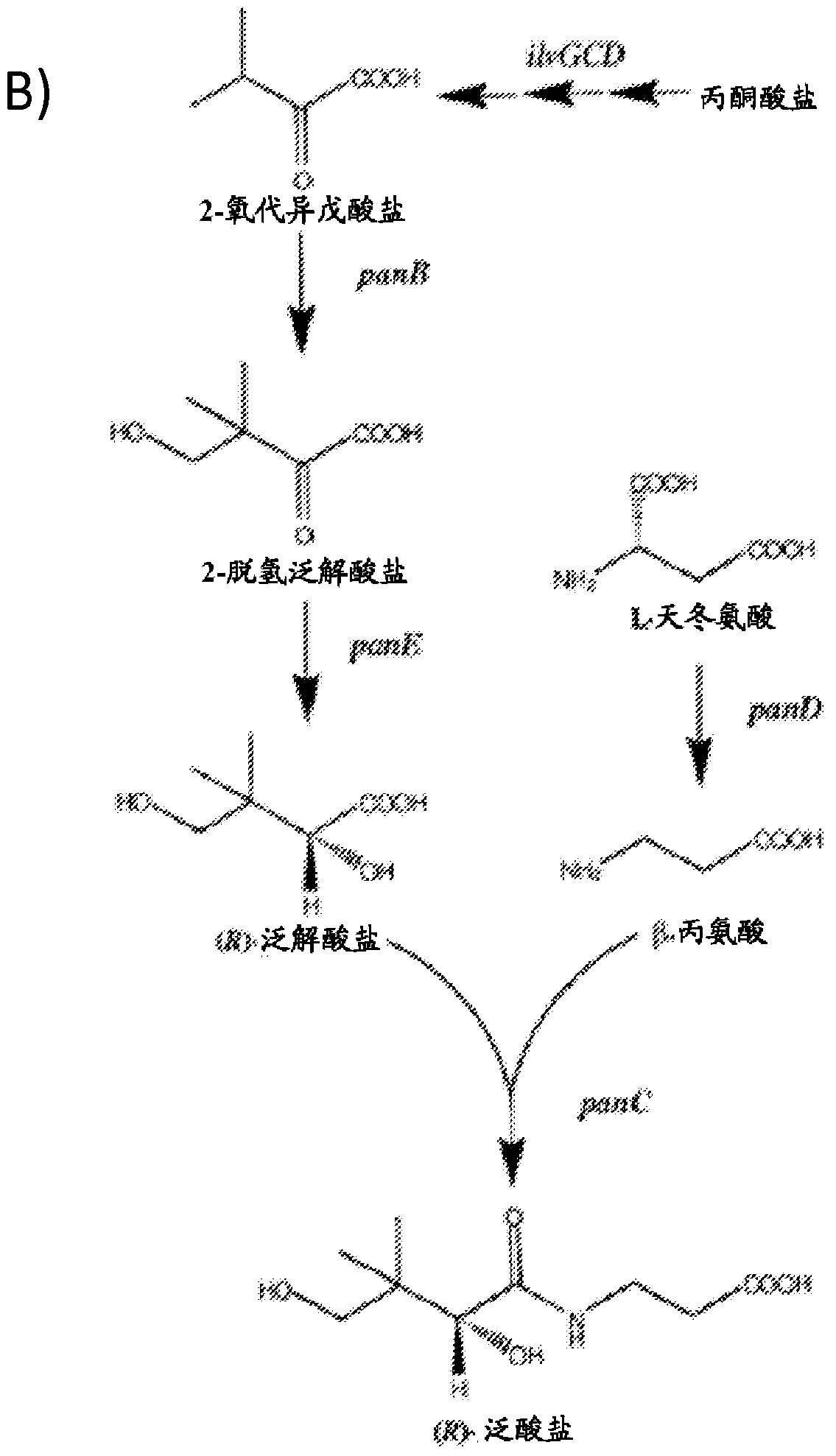
The present disclosure relates to the use of pantothenate compounds as a non-genetic switch for the production of heterologous acetyl-CoA derived (HACD) compounds in microbial host cells. The invention provides genetically modified microorganisms that are more stable when stored and initially cultured under reduced pantothenate concentrations, cell culture media having reduced concentrations of pantothenate compounds, and methods of producing HACD compounds using the cell culture media and the genetically engineered microorganisms of the invention.
Owner:AMYRIS INC
Method for producing polymer
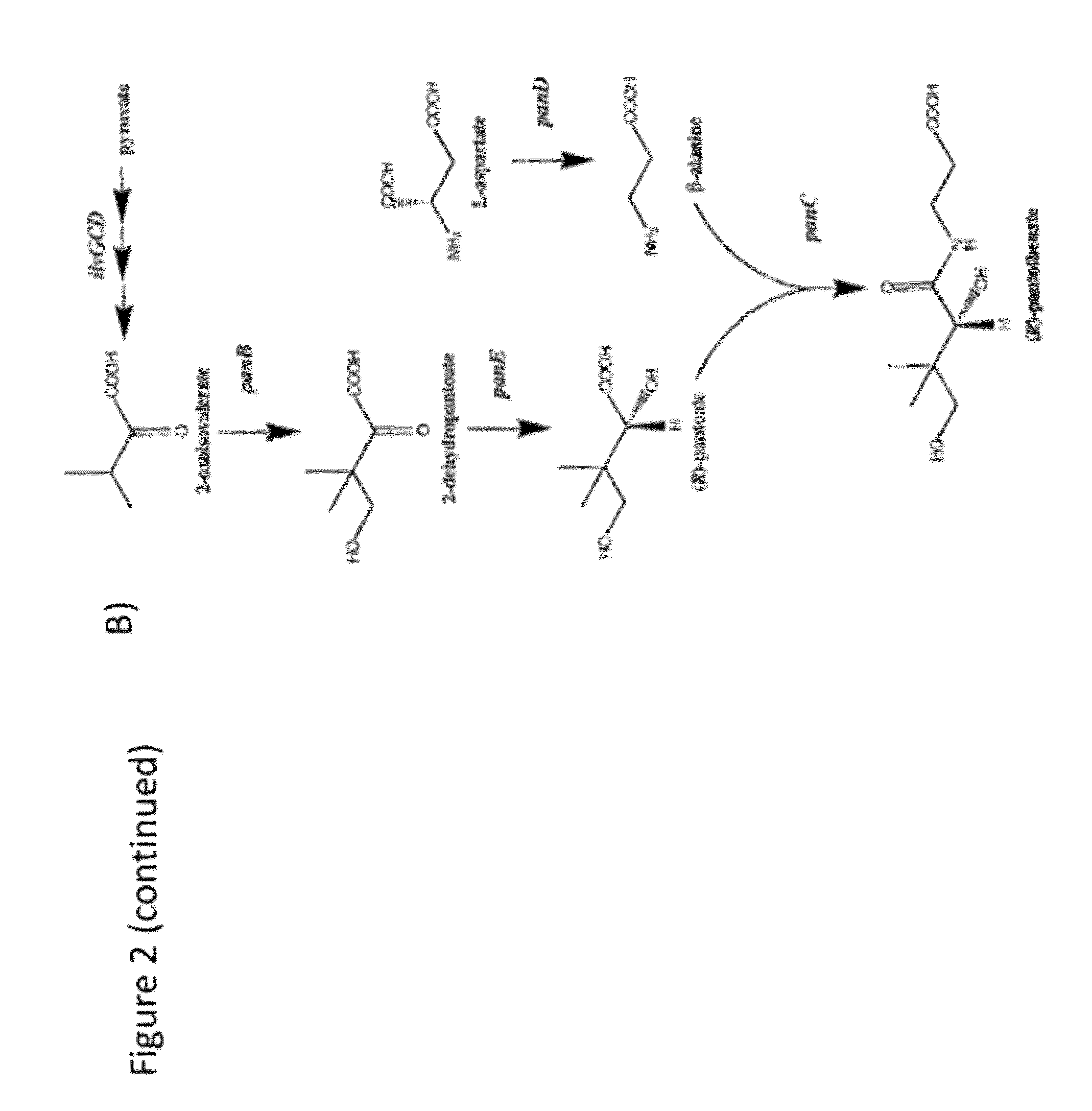
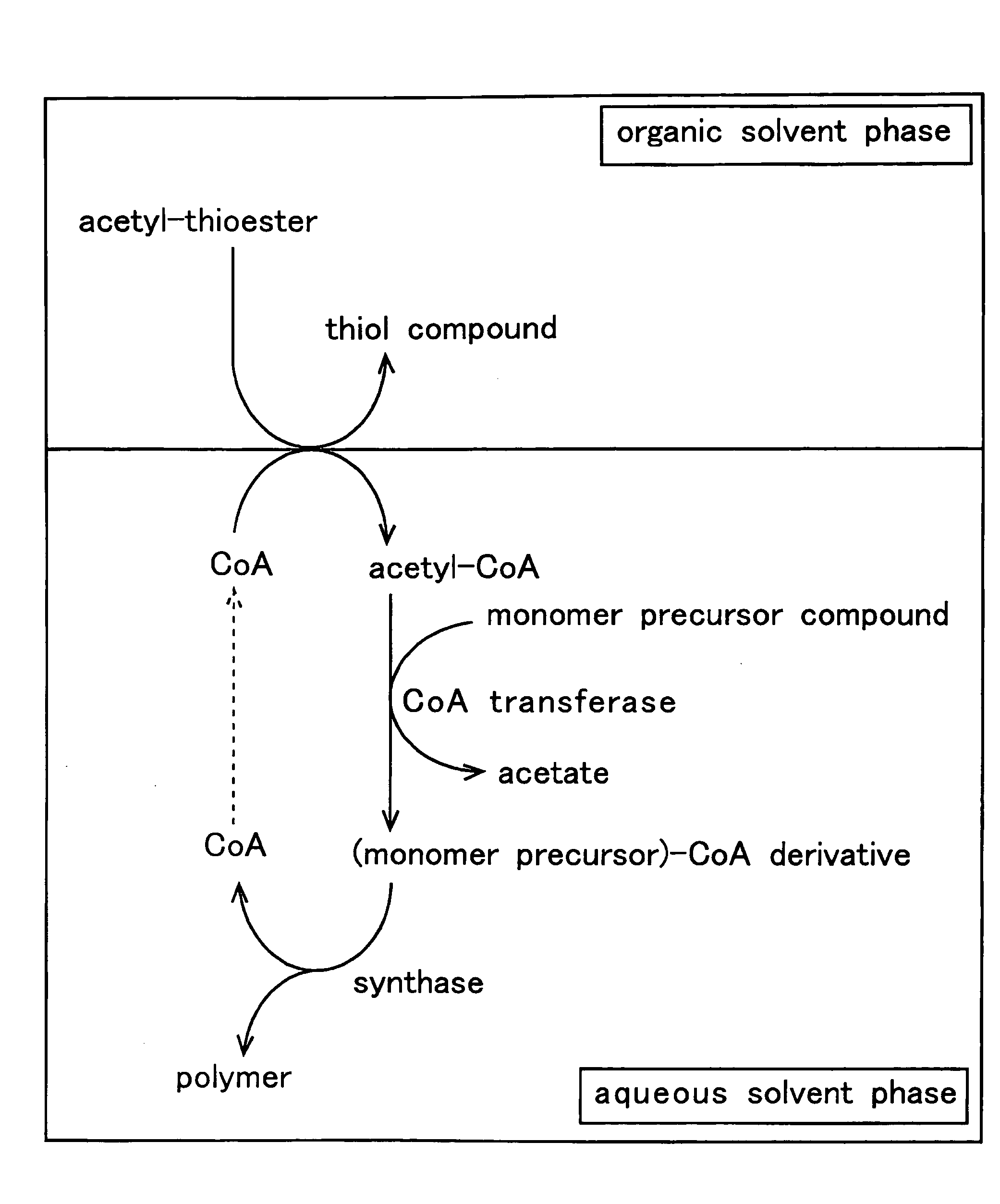
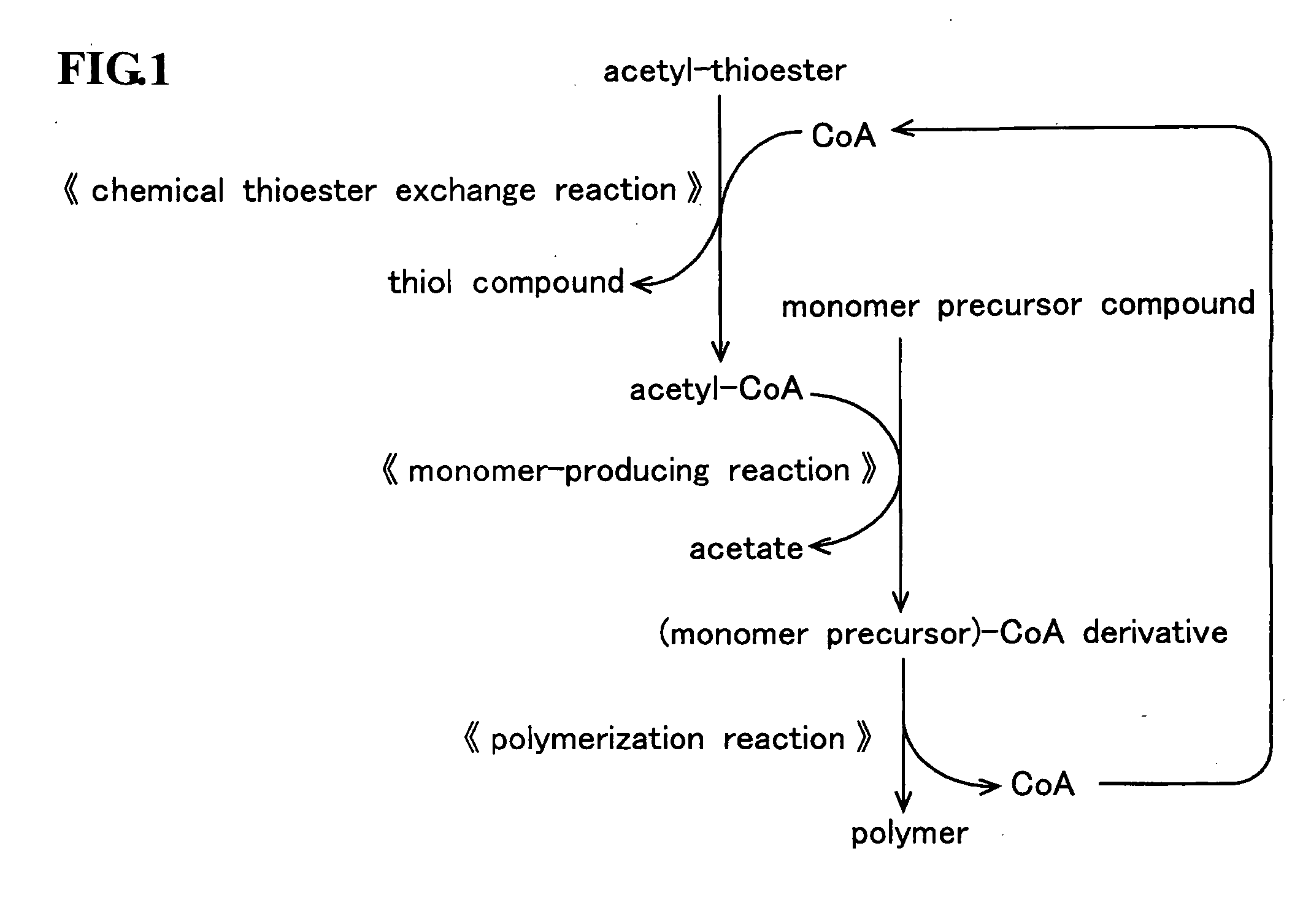
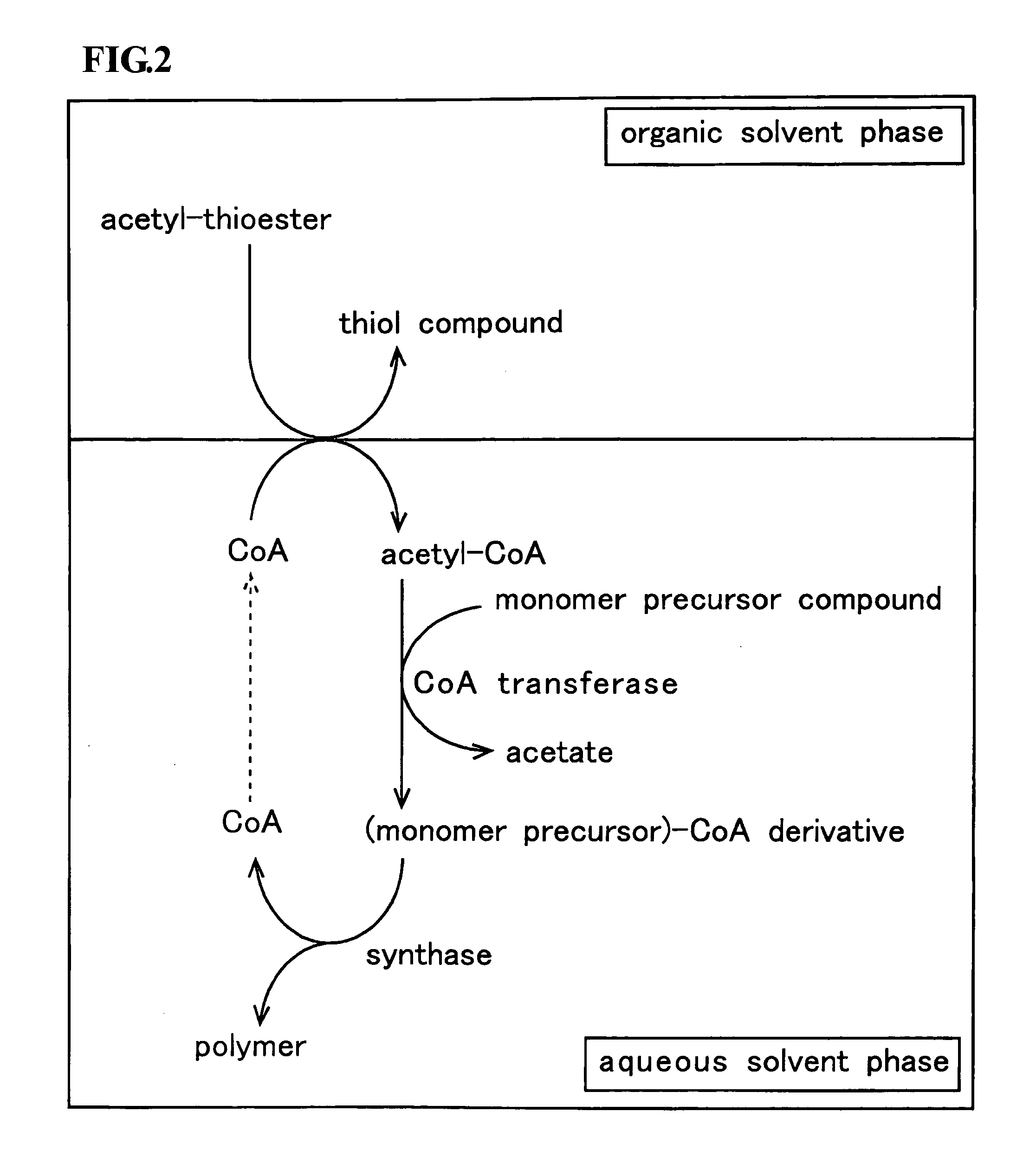
InactiveUS20090226988A1Ease of industrial applicationWide choiceFermentationOrganic chemistryThioester
A method for producing a polymer including a chemical thioester exchange reaction for forming an acetyl-CoA by reacting an acetyl-thioester with CoA, a monomer-producing reaction for forming a (monomer precursor)-CoA derivative by reacting at least one monomer precursor compound with the acetyl-CoA and a polymerization reaction for forming the polymer comprising units of the monomer by polymerizing the (monomer precursor)-CoA derivative.
Owner:HOKKAIDO UNIVERSITY +1
Fatty acid and derivatives production
The present invention relates to at least one method of producing at least one fatty acid and / or derivative thereof from a gas comprising H 2 , CO 2 and O 2 the method comprising the steps of: (a) providing a genetically modified hydrogen oxidising bacterium in an aqueous medium; and (b) contacting the aqueous medium with a gas comprising H2, CO2 and O2 in a weight ratio of 20 to 70: 10 to 45: 5 to 35; wherein the fatty acid comprises at least 5 carbon atoms and wherein the hydrogen oxidising bacterium is genetically modified relative to the wild type bacterium to increase the expression of enzyme E 1 that is capable of catalysing the conversion of acetyl CoA to acyl ACP via malonyl coA and to increase the expression of enzyme E 2 that is capable of catalysing the conversion of Acyl ACP to the fatty acid.
Owner:EVONIK DEGUSSA GMBH
Analysis and application of salvia miltiorrhiza acetyl-CoA acyltryansferase (SmAACT) gene
InactiveCN101613704AIncrease contentImprove qualityMicrobiological testing/measurementTransferasesSalvia miltiorrhizaNucleotide
The invention discloses a salvia miltiorrhiza acetyl-CoA acyltryansferase (SmAACT) gene, prolease encoded by the gene and application. The gene is obtained by cloning from salvia miltiorrhiza by utilizing cDNA chip technology for the first time, and therefore fills the gap of separating and cloning an acetyl-CoA acyltryansferase gene from the salvia miltiorrhiza, a traditional rare medicinal material in our country. The SmAACT gene has a nucleotide sequence shown in SEQ IDNO.1 or a homologous sequence which adds, substitutes, inserts or deletes one or more nucleotides or nucleotide sequences of an allelic gene and a derivative thereof. Protein encoded by the gene has a nucleotide sequence shown in SEQ ID No.2 or a homologous sequence which adds, substitutes, inserts or deletes one or more nucleotides. The salvia miltiorrhiza acetyl-CoA acyltryansferase catalyzes a first enzymatic reaction on an MVA pathway, ensures that acetyl-CoA with two molecules is condensed into acetyl acetyl-CoA, and is first key enzyme on a biosynthetic pathway of diterpenoid tanshinone compounds from the salvia miltiorrhiza; and the expression level of the gene is estimated to be closely related to the content of tanshinone components. The SmAACT gene can improve the content of diterpene active components, namely tanshinones in the salvia miltiorrhiza through biotechnology, can be used for the research and industrialization on improving the content of tanshinone substances by utilizing the biotechnology, is favorable for the quality improvement and breeding of salvia miltiorrhiza medicinal materials, and has good application prospect.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
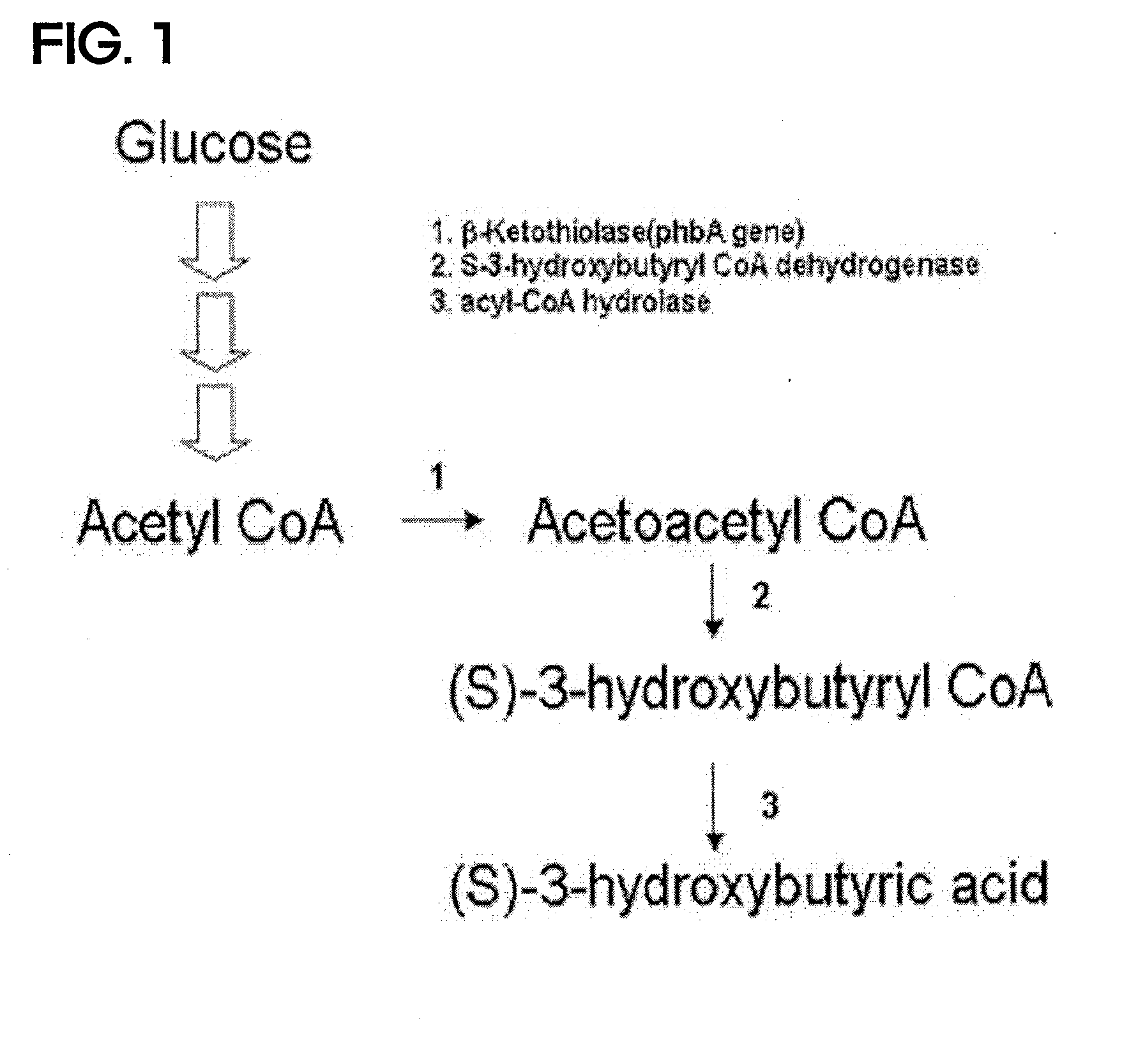
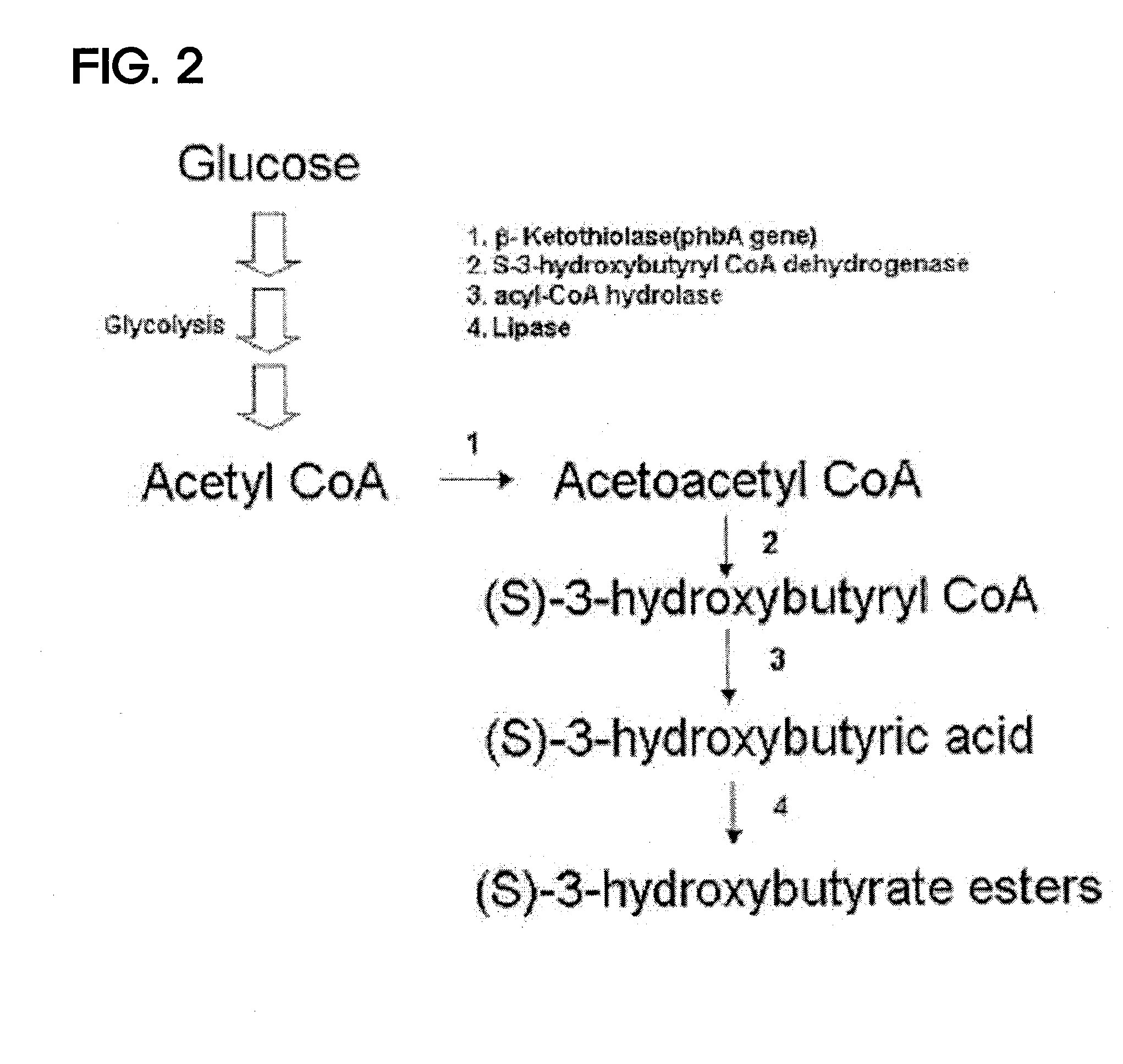
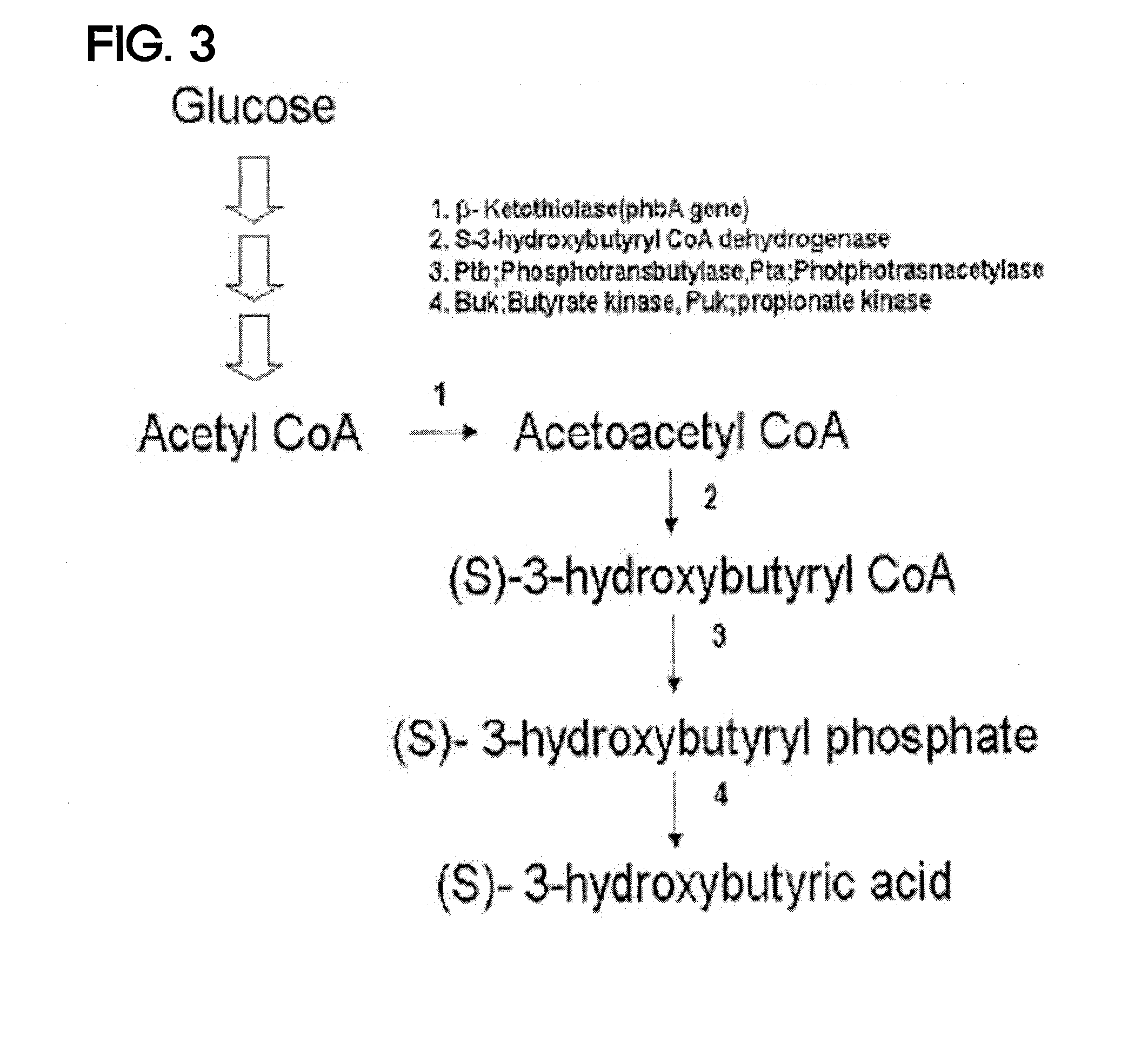
Preparing method for (s)-3hydroxybutyric acid and (s)-3 hydroxybutyrate ester using recombinant microorganism
ActiveUS20100209983A1High optical purityFermentationVector-based foreign material introductionBiologyHydroxybutyrates
A method of synthesizing optically-active (S)-3-hydroxybutyric acid and (S)-3-hydroxybutyrate ester using a mutated microorganism is provided. More particularly, a mutated microorganism for preparing (S)-3-hydroxybutyric acid transformed with a gene encoding b ketothiolase, a gene encoding (S)-3-hydroxybutyryl CoA dehydrogenase and a gene encoding acyl CoA hydrolase; a method of preparing (S)-3-hydroxybutyric acid using the mutated microorganism; a mutated microorganism for preparing (S)-3-hydroxybutyrate ester transformed with a gene encoding b ketothiolase, a gene encoding (S)-3-hydroxybutyryl CoA dehydrogenase, a gene encoding acyl CoA hydrolase and a gene encoding lipase; and a method of preparing (S)-3-hydroxybutyrate ester using the mutated microorganism are provided.Accordingly, (S)-3-hydroxybutyric acid with high optical purity may be produced from acetyl CoA produced in glycolysis of a microorganism by a simple process involving the manipulation of a metabolic pathway by a recombinant gene introduced into the microorganism without using a high-cost metal catalyst or a substrate. Further, (S)-3-hydroxybutyrate ester and lactone of (S)-3-hydroxybutyrate ester may be simply produced from (S)-3-hydroxybutyric acid produced by the above method using lipase.
Owner:LG CHEM LTD
Production of compounds derived from acetyl-coenzyme A
The present disclosure relates to the use of pantothenate compounds as a non-genetic switch for the production of heterologous acetyl-CoA derived (HACD) compounds in microbial host cells. The invention provides genetically modified microorganisms that are more stable when stored and initially cultured under reduced pantothenate concentrations, cell culture media having reduced concentrations of pantothenate compounds, and methods of producing HACD compounds using the cell culture media and the genetically engineered microorganisms of the invention.
Owner:AMYRIS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com