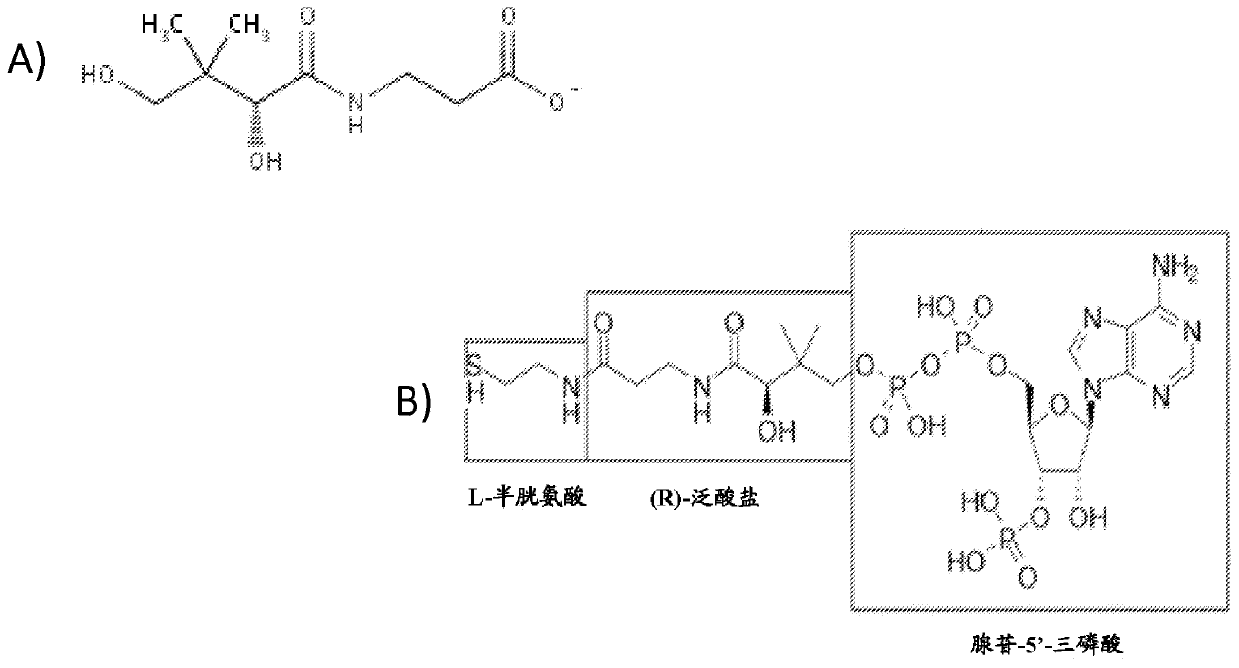
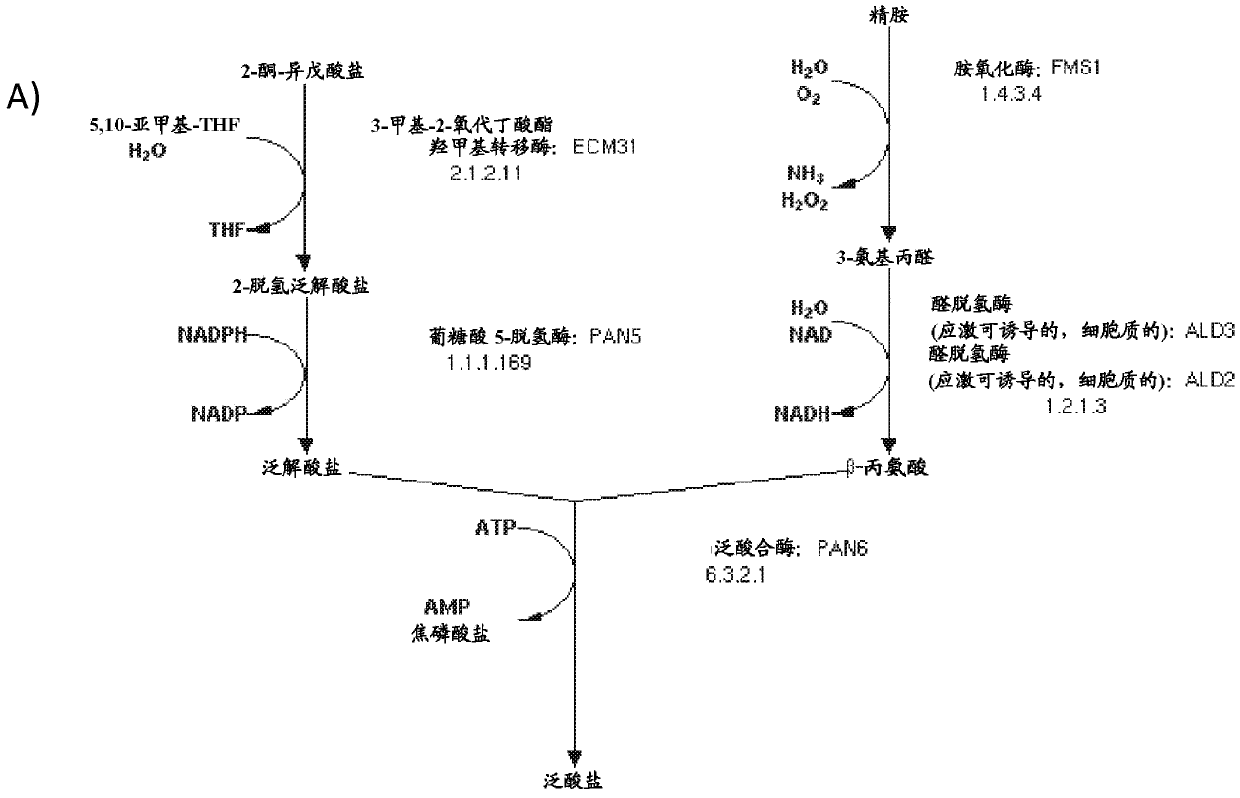
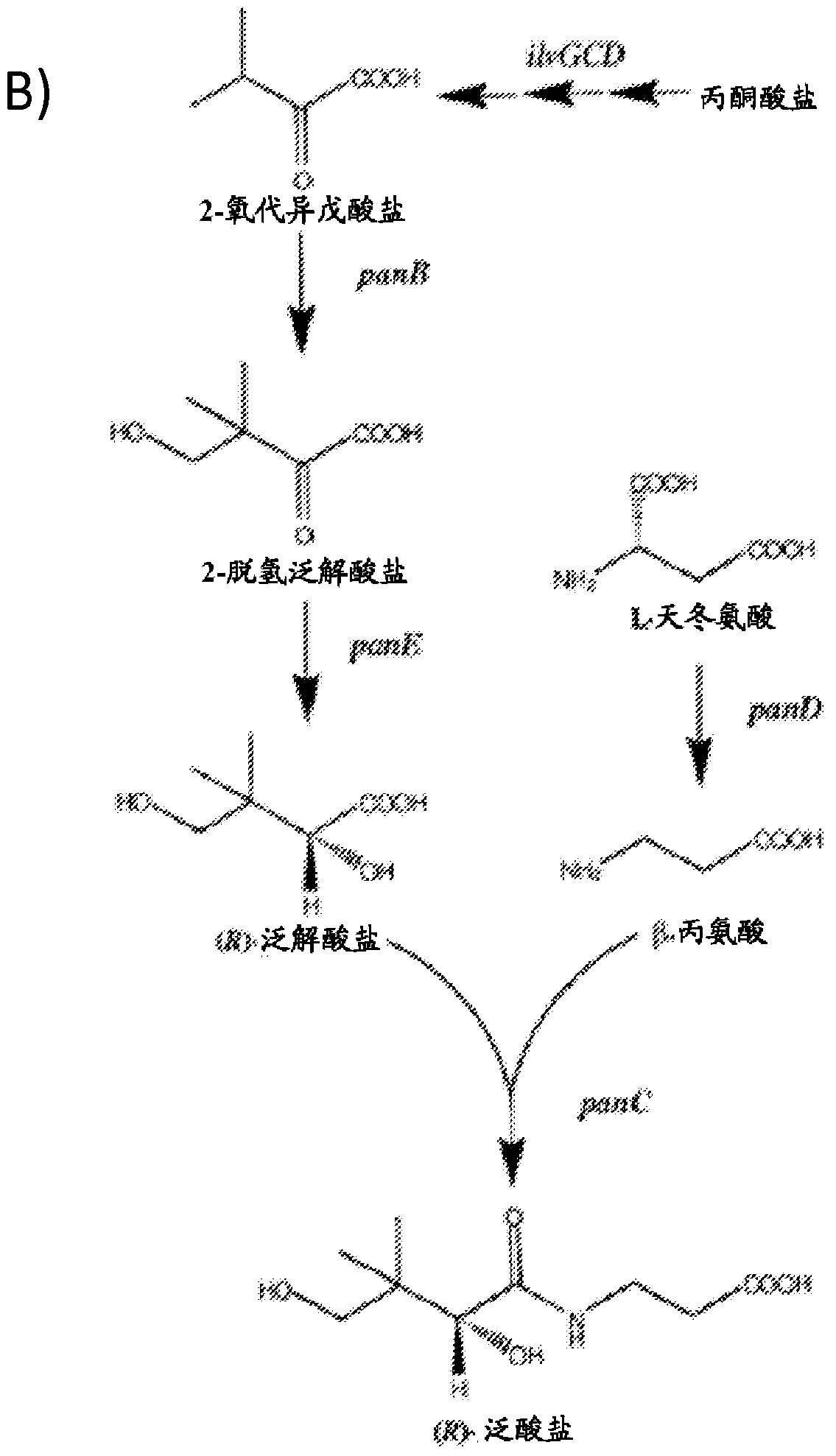
Production of compounds derived from acetyl-coenzyme A
An acetyl coenzyme, compound technology, applied in the direction of adding compounds to stimulate growth, DNA/RNA fragments, recombinant DNA technology, etc., can solve problems such as the lack of metabolic switches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
[0084] Example 5 (below) and image 3 A explains the effect of pantothenate compound concentration in the medium on the production of HACD compounds in genetically modified host cells with high metabolic flux through the acetyl-CoA biosynthetic pathway to produce isoprenoid Farnesene. At low pantothenate levels (0.2 mg / L, <1% of the maximum pantothenate concentration tested), HACD compound production was essentially absent. Increased levels of pantothenate compounds were associated with increased production of HACD compounds prior to the flat-topping effect. Further increases beyond 1 mg / L pantothenate (10% of the maximum pantothenate compound concentration tested) resulted in no further increase in HACD compound production.
[0085] Notably, the growth of these cells showed an opposite trend to the production of HACD compounds. image 3 B illustrates the growth of the same strain at the same pantothenate concentration levels tested for HACD compound production. No or low ...
Embodiment 1
[0229] This example describes a method for determining the cell density (OD 600 ) Exemplary method.
[0230] Mix 8 μL of cell culture samples and 92 μL of Triton OD diluent (20g / L Triton X-114, 200mL / L PEG200, 200mL / L 100% ethanol, balance water) in a clean 96-well plate and stir the solution at 1,000RPM 6 minutes and the OD was determined by measuring the absorbance at 600 nm on an M5 spectrophotometer (Molecular Devices, Sunnyvale, CA). 600 .
Embodiment 2
[0232] This example describes an exemplary Nile Red-based method that can be used to determine farnesene titers in yeast cell cultures.
[0233] 98 μL of cell culture samples were transferred into 96-well black polystyrene flat bottom assay plates, and 2 μL of Nile Red (Invitrogen, Carlsbad, CA) dissolved in DMSO at 100 μg / mL was added to each well. Fluorescence levels were immediately measured on an M5 spectrophotometer with excitation at 500 nm and emission at 550 nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com