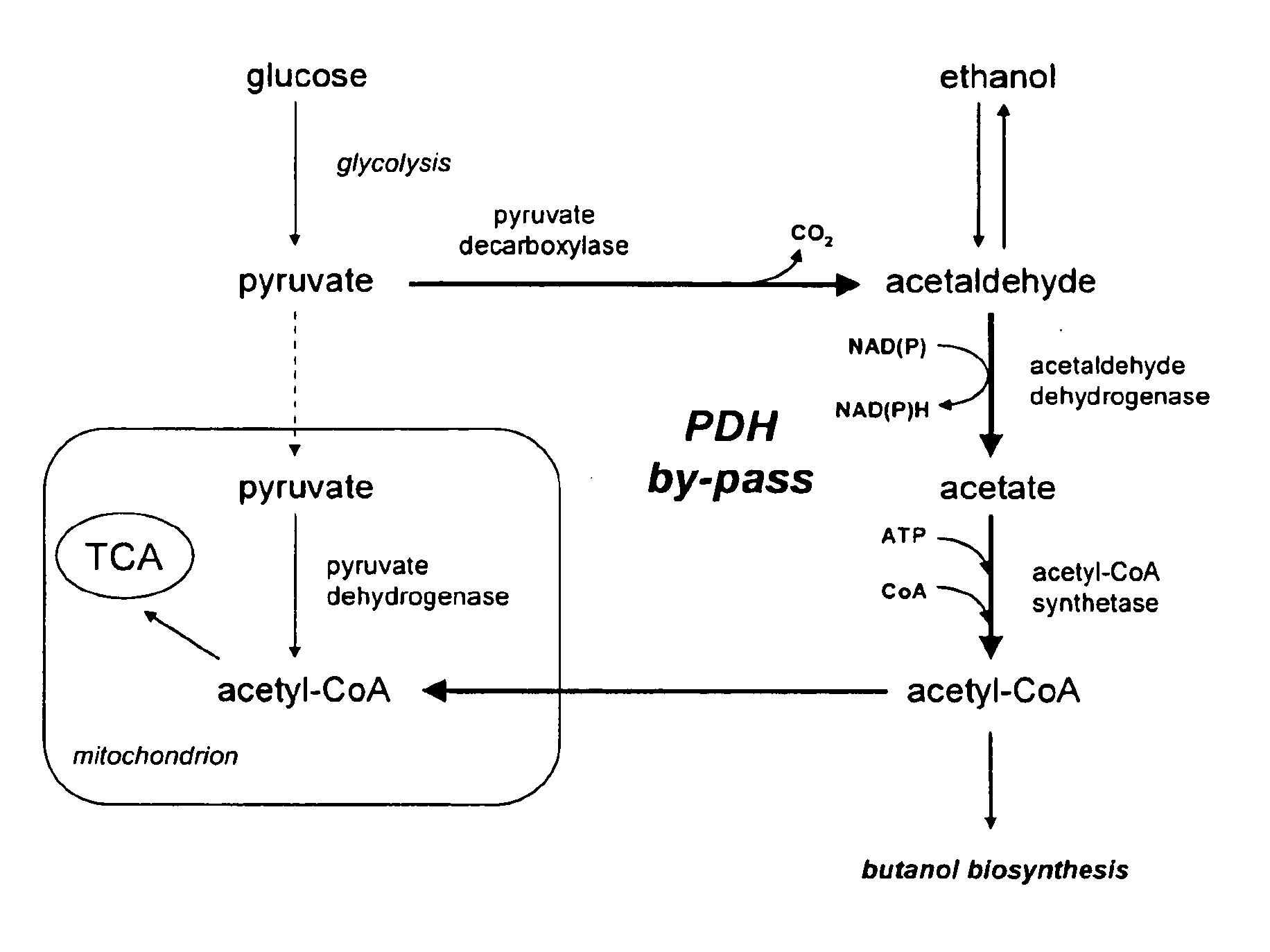
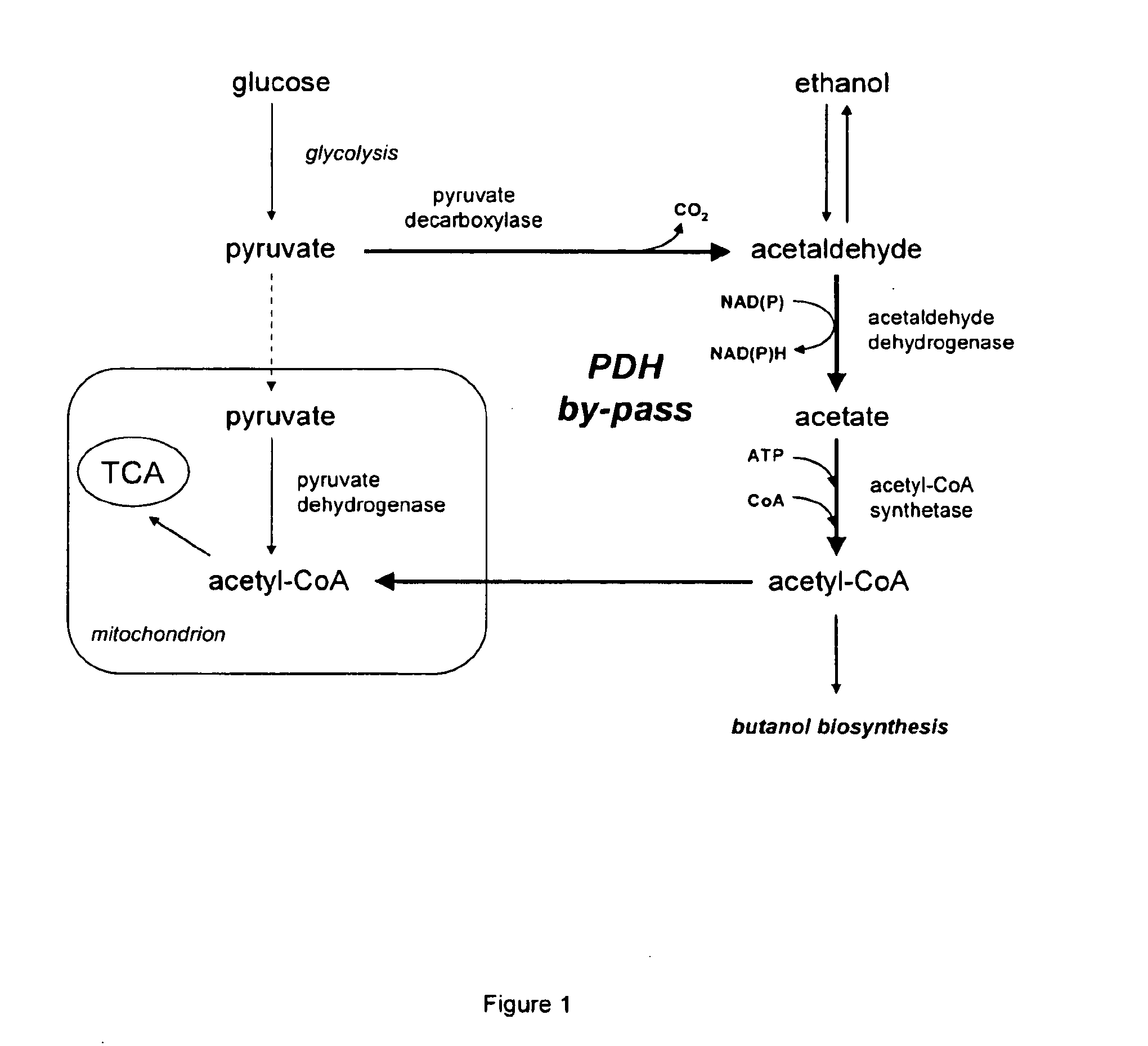
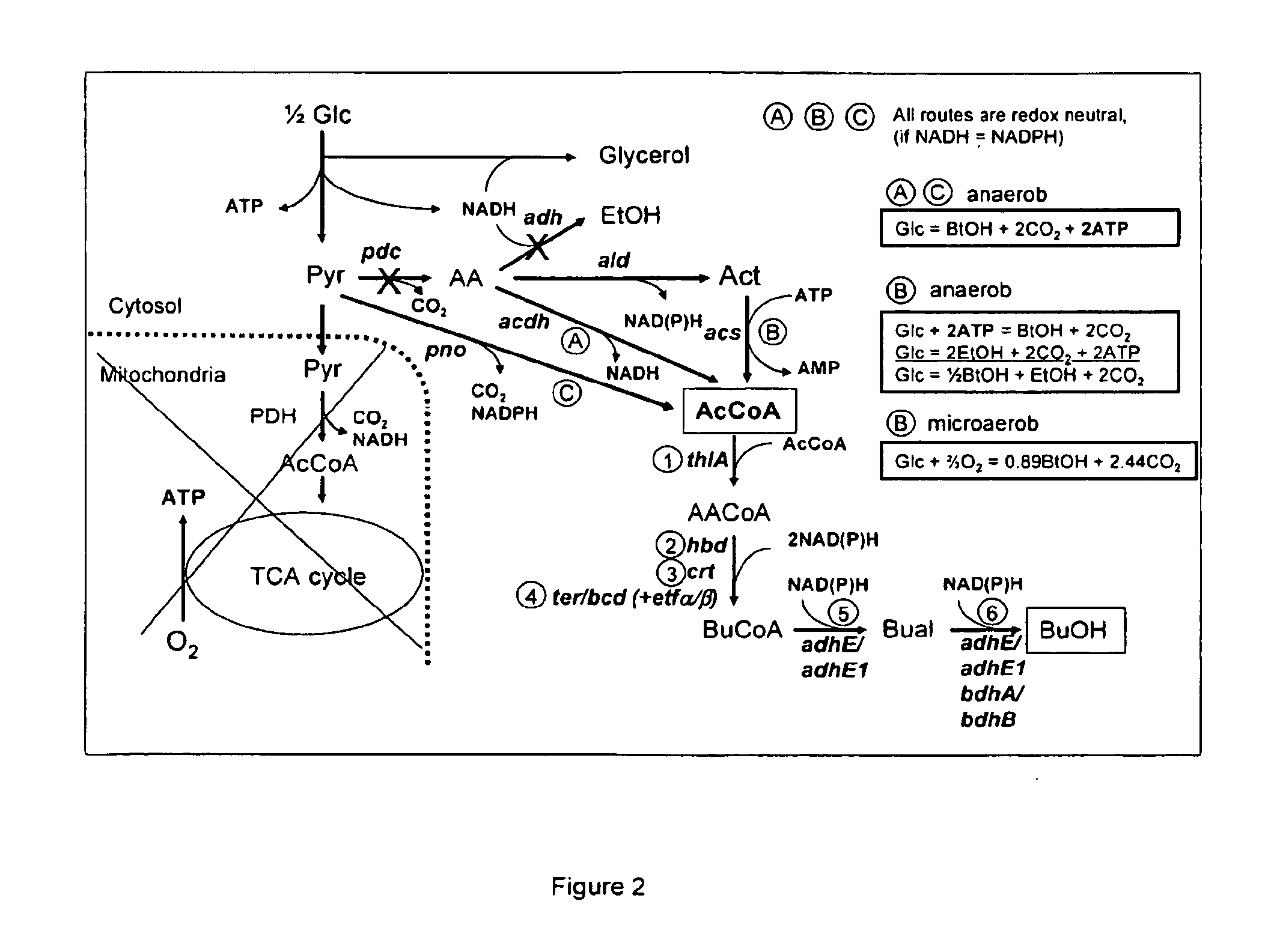
Acetyl-coa producing enzymes in yeast
a technology of acetylcoa and producing enzymes, which is applied in the direction of biofuels, microorganisms, peptides, etc., can solve the problems of reducing the overall yield of the product on carbon, reducing the production efficiency of pdh by-pass in yeast, and unable to achieve maximum theoretical yield, etc., to achieve the effect of increasing the production of cytosolic acetyl-coa
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Delta acs2 Strain
[0113]The S. cerevisiae acs2 deleted strain (acs2Δ strain) was produced by first performing a PCR on plasmid pUG6 (Güldener et al., 1996, supra) with the following oligonucleotides:
5′acs2::Kanlox5′-tacacaaacagaatacaggaaagtaaatcaatacaataataaaacagctgaagcttcgtacgc-3′3′acs2::Kanlox5′-tctcattacgaaatttttctcatttaagttatttctttttttgaggcataggccactagtggatctg-3′.
[0114]The resulting 1.4 kb fragment, containing the KanMX marker which confers resistance to G418, was used to transform S. cerevisiae CEN.PK113-3C (MATA trp1-289). After transformation the strain was plated on YPD (10 g I−1 yeast extract (BD Difco), 20 g I−1 peptone (BD Difco)), 10 g I−1 glucose) with 200 mg / ml Geneticin (G418). In resistant transformants, correct integration was verified by PCR using oligonucleotides:
5′ACS2:5′-gatattcggtagccgattcc-3′3′ACS2:5′-ccgtaaccttctcgtaatgc-3′ACS2internal:5′-cggattcgtcatcagcttca-3′KanA:5′-cgcacgtcaagactgtcaag-3′KanB:5′-tcgtatgtgaatgctggtcg-3′
[0115]The phenotype wa...
example 2
In Silico Identification of Putative Acetylating Acetaldehyde Dehydrogenases for Direct Conversion of Acetaldehyde to Acetyl-CoA
[0117]Enzymes described for the conversion of acetaldehyde to acetyl-CoA are the so-called acetylating acetaldehyde dehydrogenases (ACDH) (E.C. 1.2.1.10) catalysing the following reaction:
Acetaldehyde(AA)+NAD++CoAAcetyl-CoA+NADH+H+
[0118]From literature four types of proteins have been described that have this activity:
[0119]1) Bifunctional proteins that catalyze the reversible conversion of acetyl-CoA to acetaldehyde, and the subsequent reversible conversion of acetaldehyde to ethanol. An example of this type of proteins is the AdhE protein in E. coli (GenBank No: NP—415757). AdhE appears to be the evolutionary product of a gene fusion. The NH2-terminal region of the AdhE protein is highly homologous to aldehyde:NAD+ oxidoreductases, whereas the COOH-terminal region is homologous to a family of Fe2+ dependent ethanol:NAD+ oxidoreductases (Membrillo-Hernande...
example 3
Construction of Expression Plasmids and Complementation Test
[0125]To test whether acetylating acetaldehyde dehydrogenases (ACDH) could complement the deletion of ACS2 in S. cerevisiae, several genes coding for a (putative) ACDH were chosen from a variety of databases as described above.
[0126]To achieve optimal expression in yeast, the codon usage of all genes was adapted by codon pair optimization. These sequences were synthesized at Geneart AG (Regensburg, Germany).
[0127]The optimized sequences were cloned into the high copy yeast expression plasmid YEplac112PtdhTadh (SEQ ID NO:40; based on YEplac112 (2μ TRP1) (Gietz & Sugino [1988] Gene 74(2):527-34), allowing constitutive expression from the TDH3 promoter.
[0128]YEplac112PtdhTadh was made by cloning a KpnI-SacI fragment from p426GPD (Mumberg et al. [1995] Gene. 156(1):119-22), containing the TDH3 promoter and CYC1 terminator, into YEplac112 cut with KpnI-SacI. The resulting plasmid was cut with KpnI and SphI and the ends were made...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com