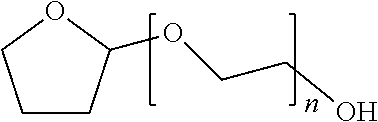
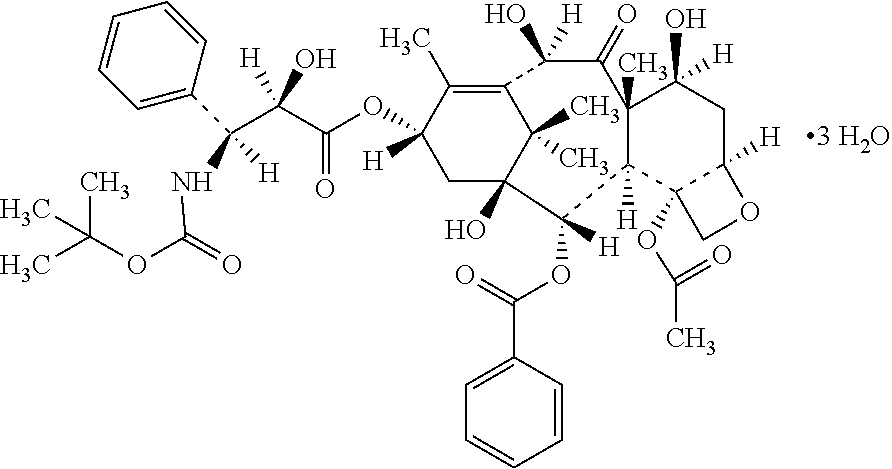
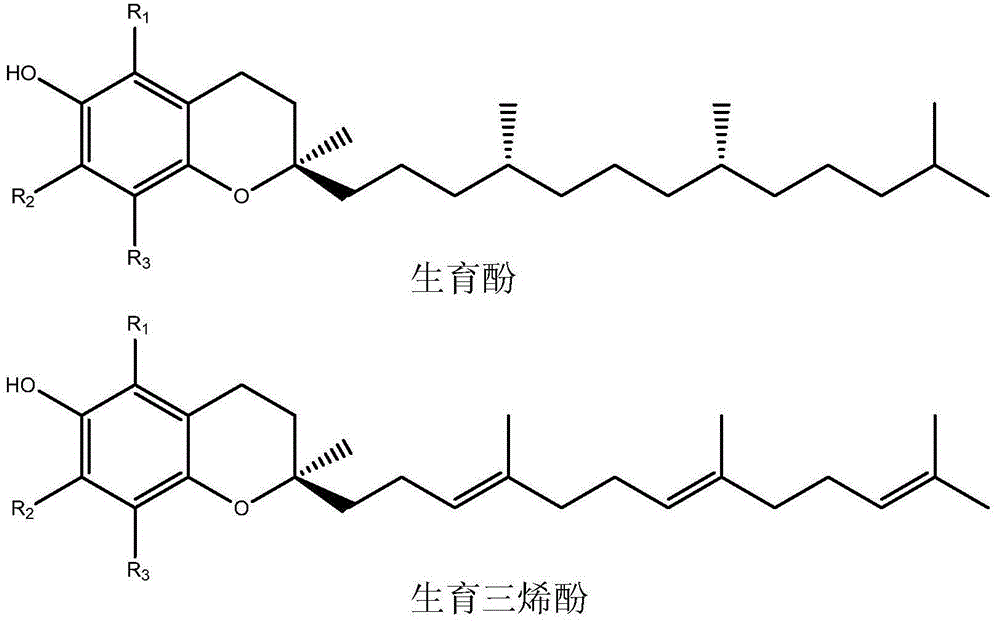
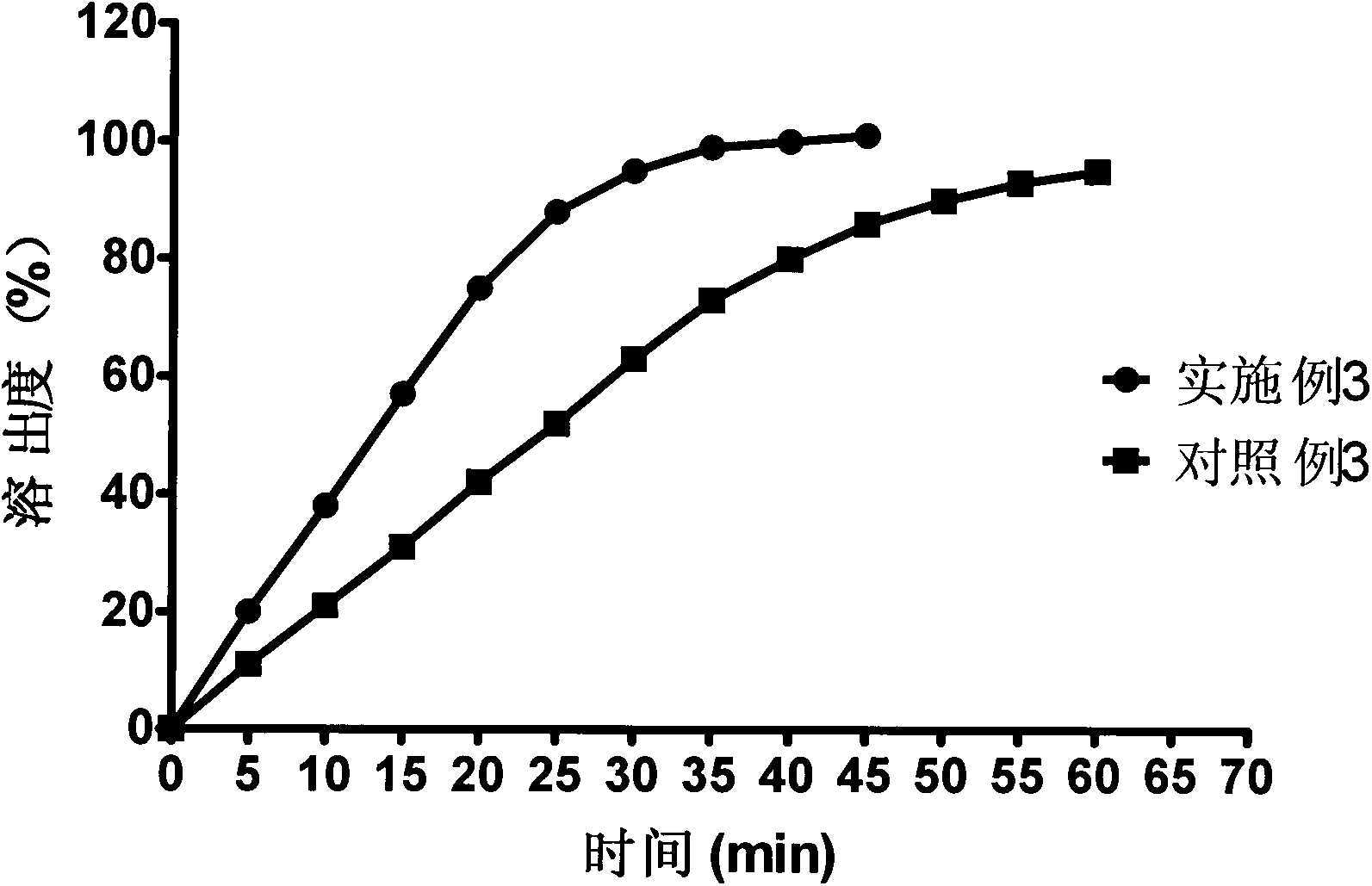
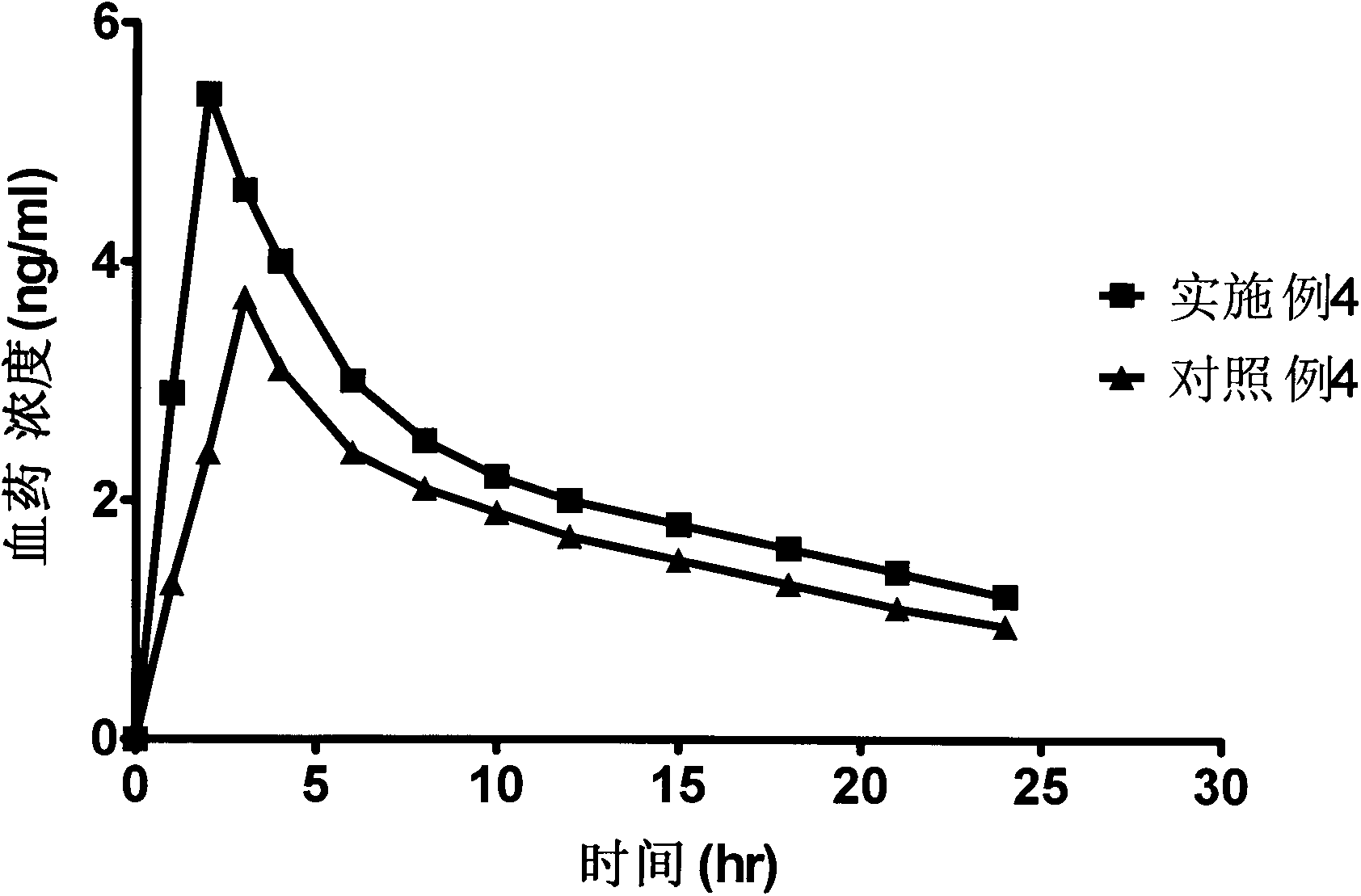
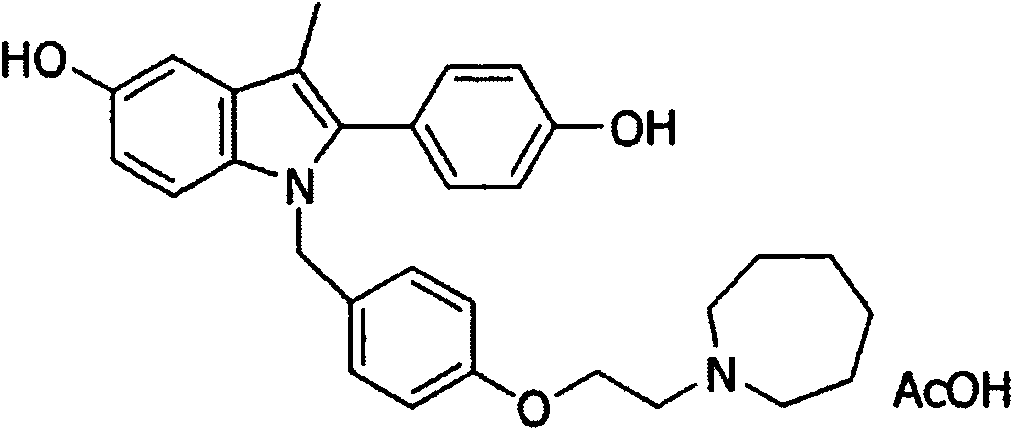
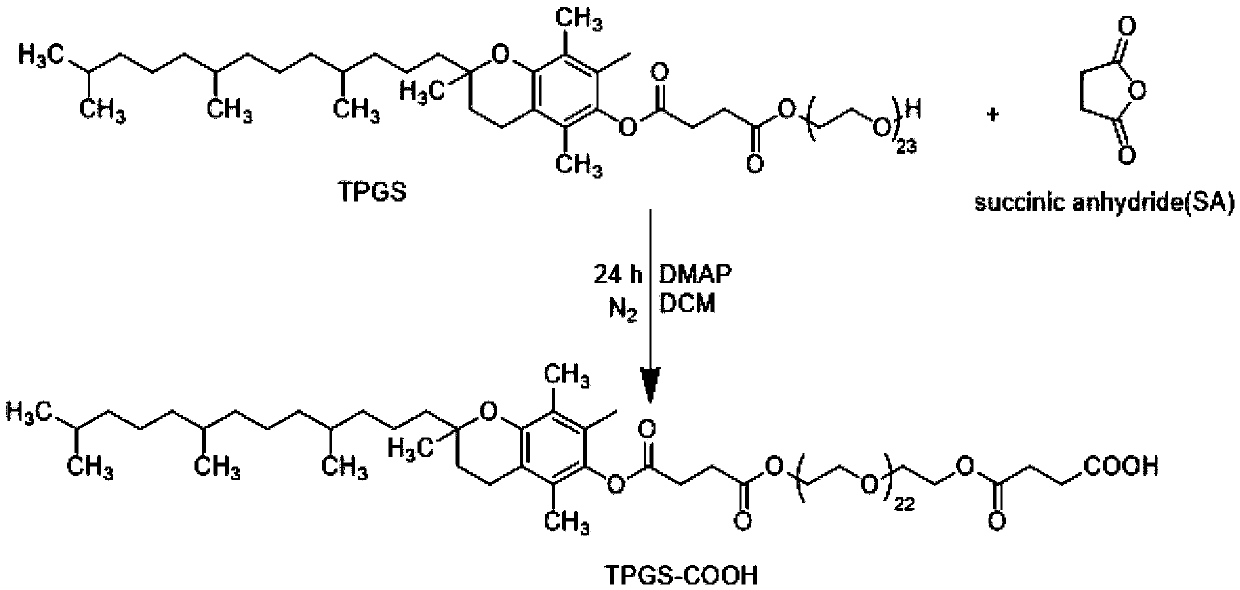
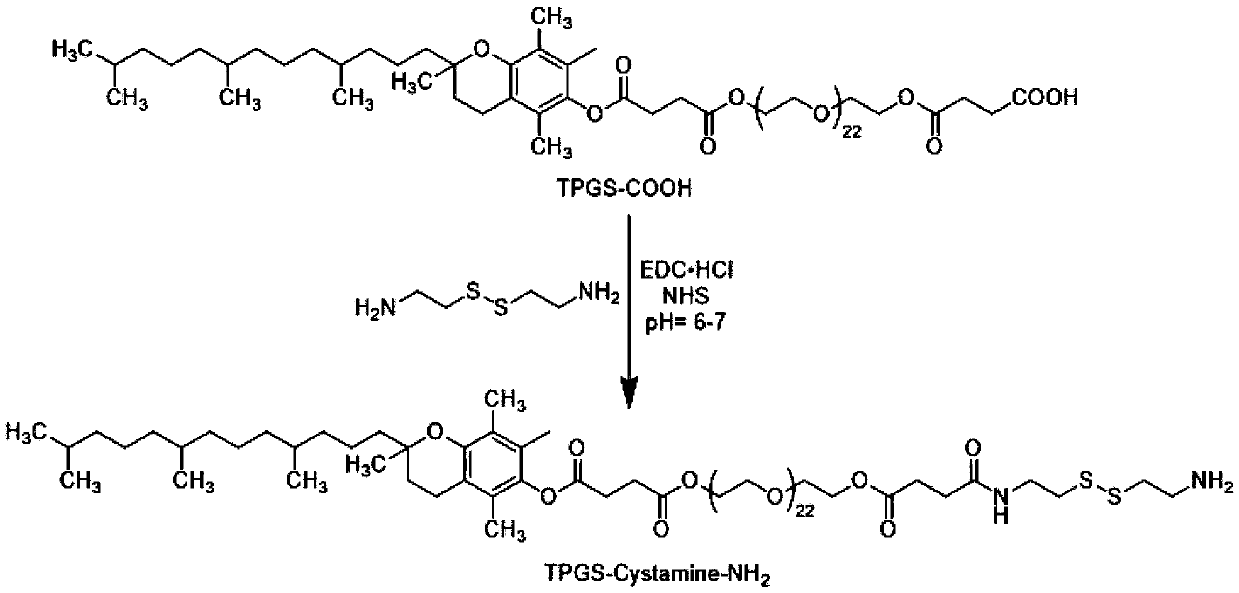
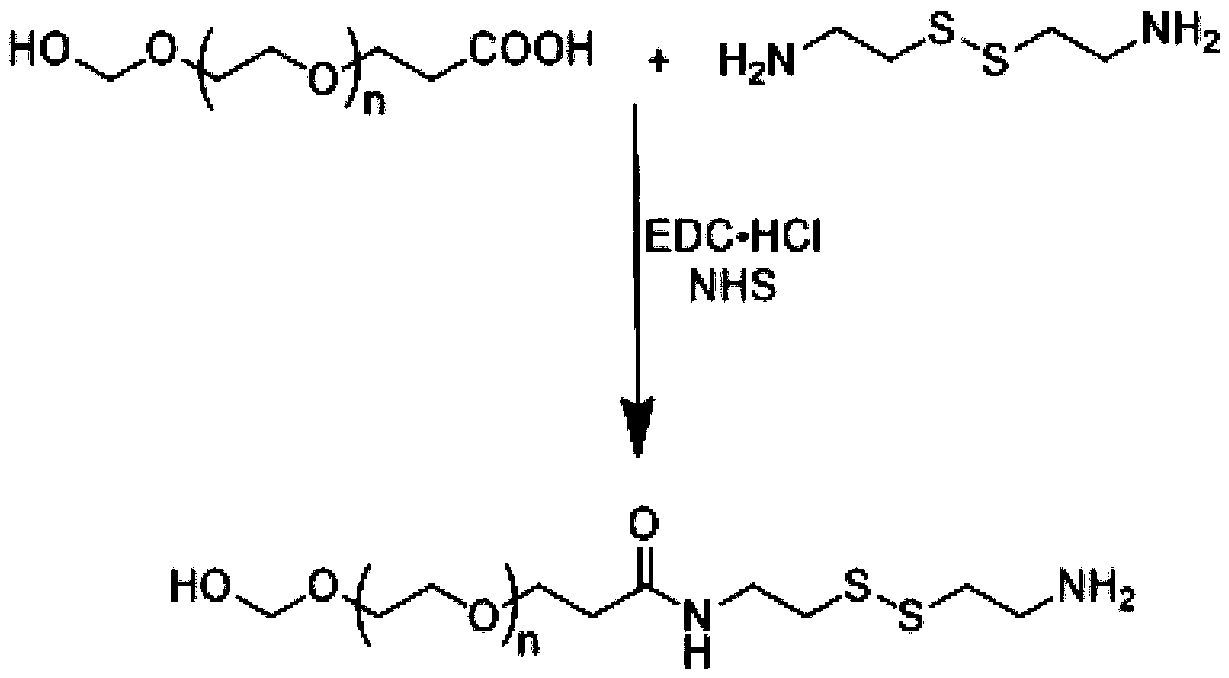
Patents
Literature
40 results about "Tocopherol polyethylene glycol succinate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tocopheryl polyethylene glycol succinate powder and process for preparing same
InactiveUS20070184117A1Without compromising handling characteristicPowder deliveryOrganic active ingredientsPolymer scienceTocopherol polyethylene glycol succinate
A powdered tocopheryl polyethylene glycol succinate (TPGS™) having an average particle size of less than about 1000 microns. In one embodiment, the powdered tocopheryl polyethylene glycol succinate is prepared by a process that includes atomizing a fluidic tocopheryl polyethylene glycol succinate into an environment suitable for solidifying the atomized tocopheryl polyethylene glycol succinate. In another embodiment, the powdered tocopheryl polyethylene glycol succinate is prepared by a process of applying a force to a solid tocopheryl polyethylene glycol succinate starting material that is sufficient to produce a powdered product.
Owner:EASTMAN CHEM CO
Pharmaceutical compositions comprising cyclosporins
A liquid comprising a therapeutically effective concentration of a cyclosporin and a vitamin E tocopherol polyethylene glycol succinate, wherein said liquid is an aqueous solution, and wherein no hydrophilic organic solvent is present at a concentration greater than half of that of the cyclosporin is also disclosed herein. A composition comprising a therapeutically effective concentration of cyclosporin A and an effective amount of a vitamin E tocopherol polyethylene glycol succinate, wherein said composition is an aqueous liquid solution which is intended for ophthalmic use, and wherein no hydrophilic organic solvent is present at a mass concentration greater than or equal to that of the cyclosporin, is disclosed herein. A composition comprising a therapeutically effective concentration of cyclosporin A and an effective amount of a vitamin E tocopherol polyethylene glycol succinate, wherein said composition is an aqueous liquid solution which is intended for parenteral use, and wherein no hydrophilic organic solvent is present at a mass concentration greater than or equal to that of the cyclosporin, is disclosed herein. Methods of treating diseases or conditions using said compositions, and medicaments related thereto, are also disclosed herein.
Owner:ALLERGAN INC
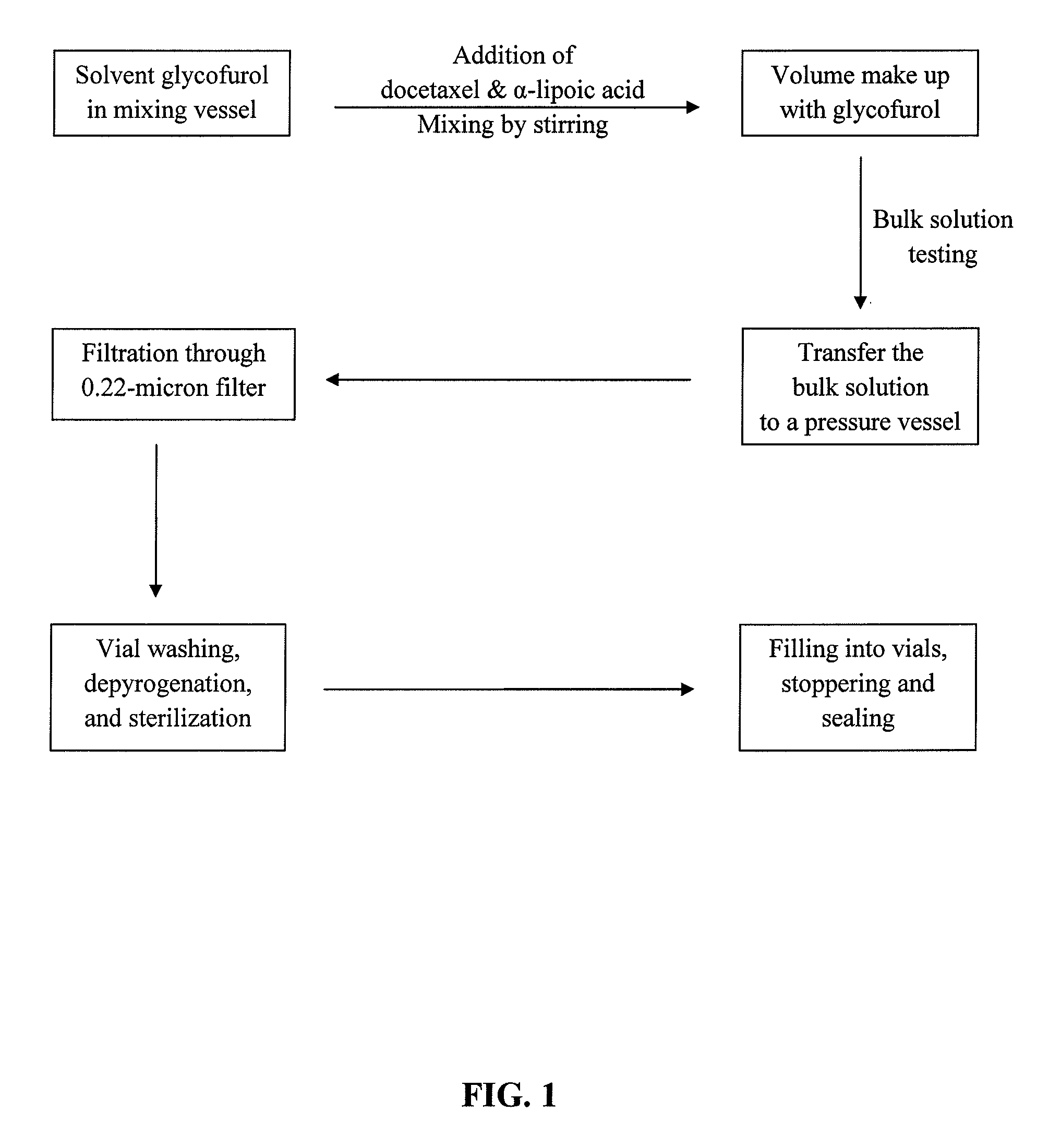
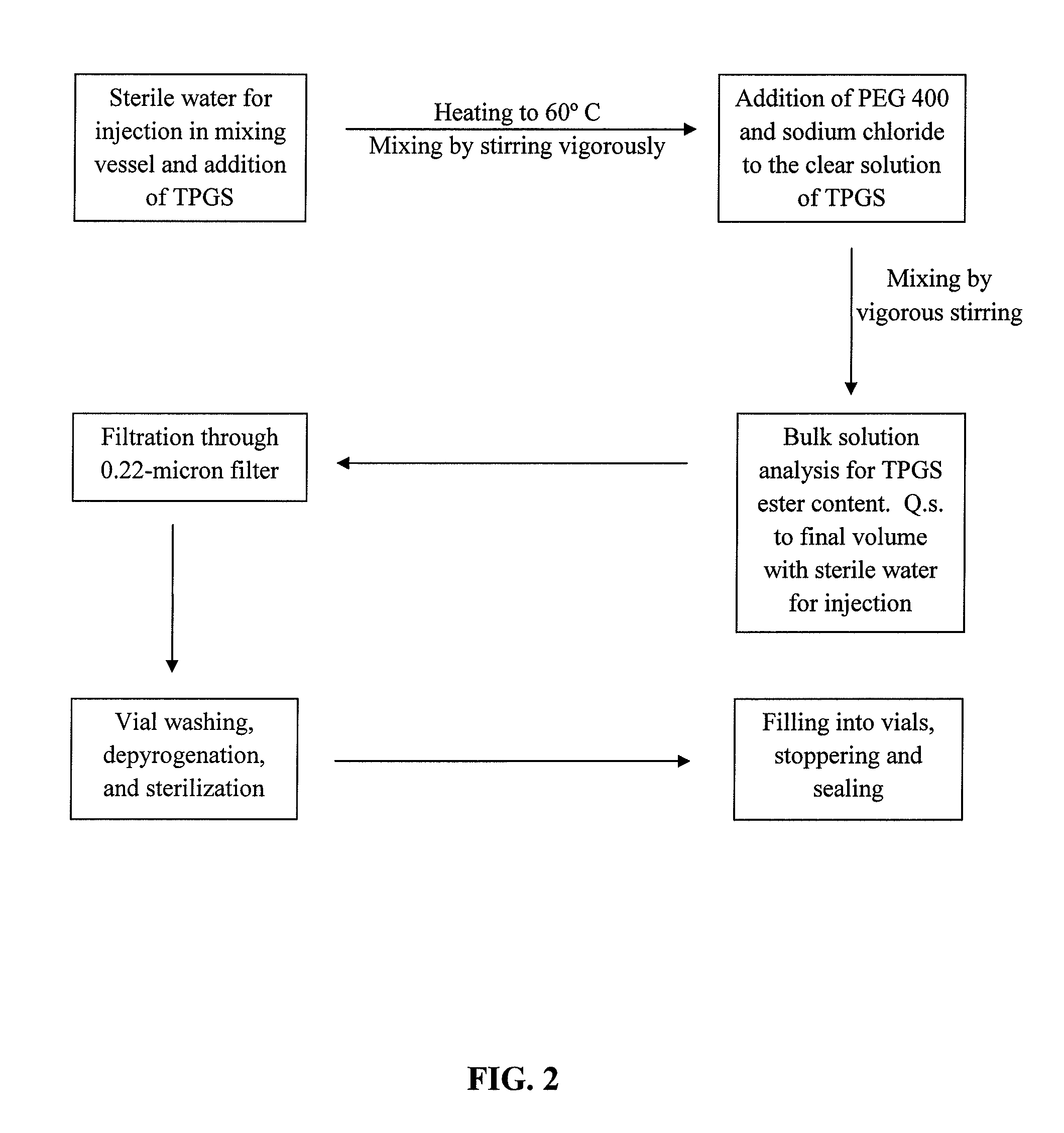
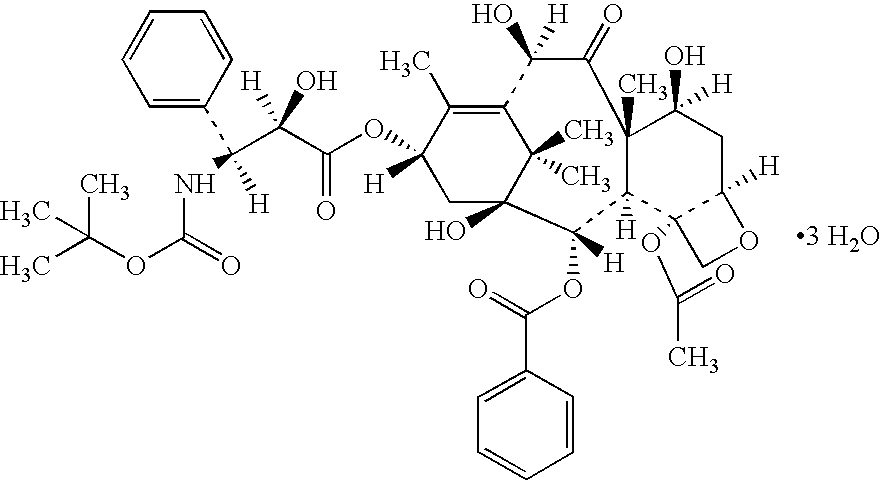
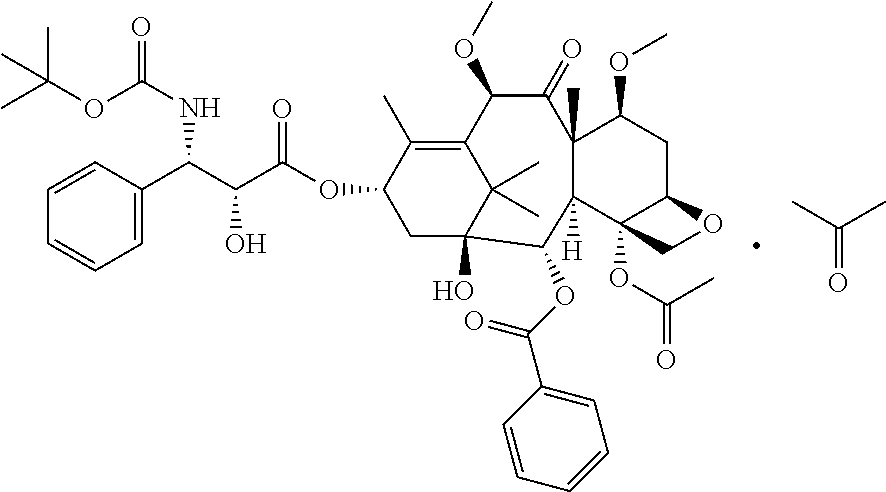
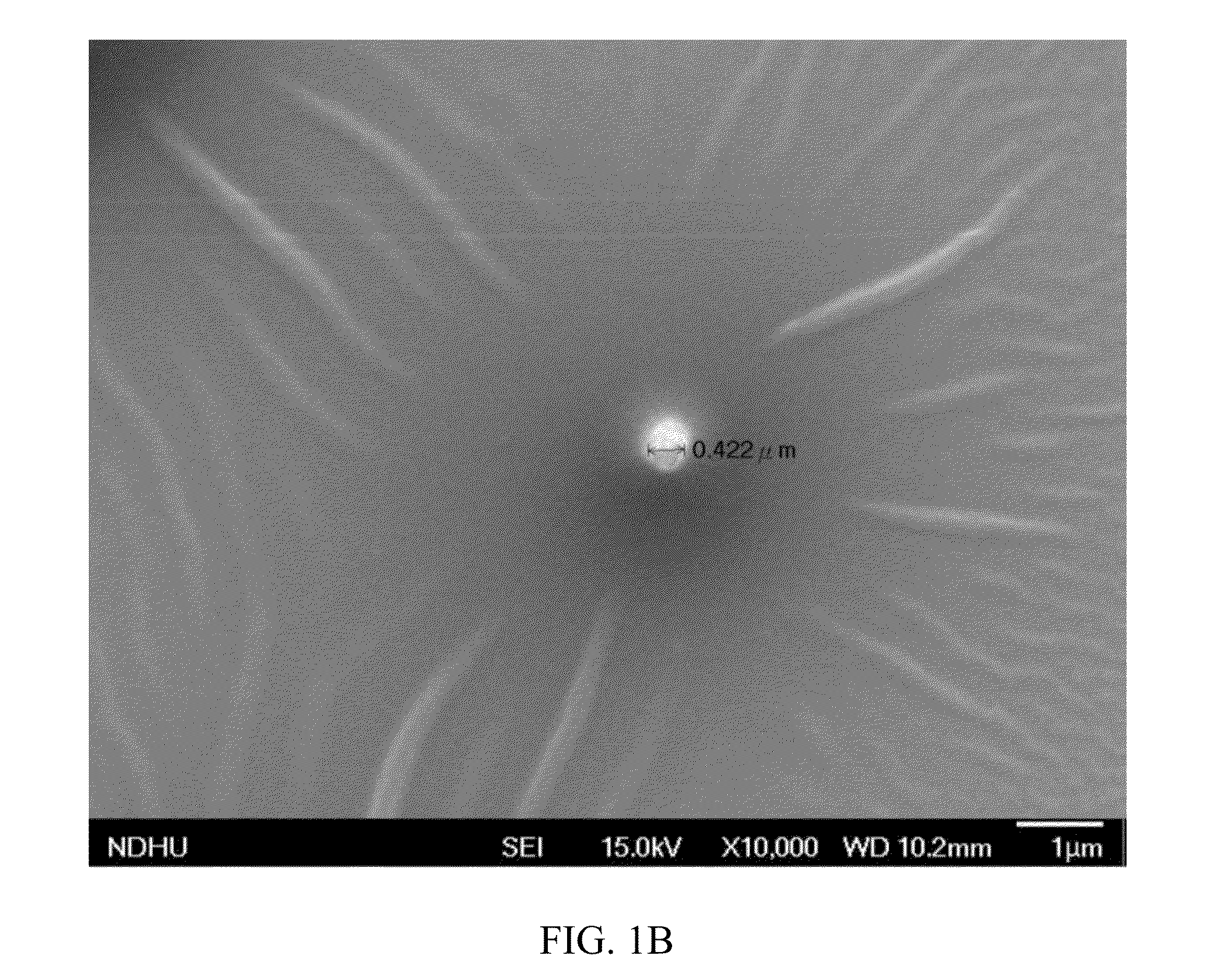
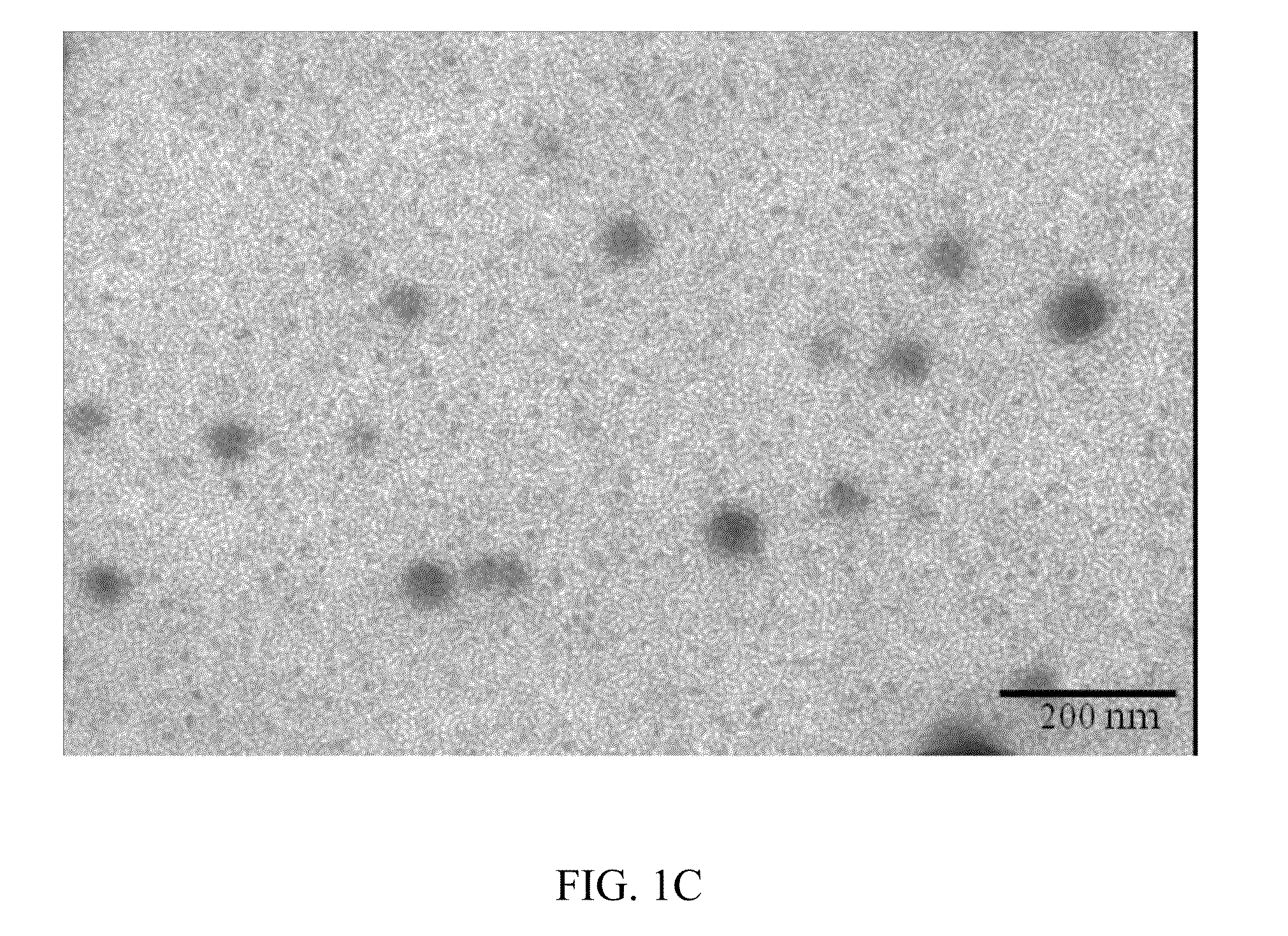
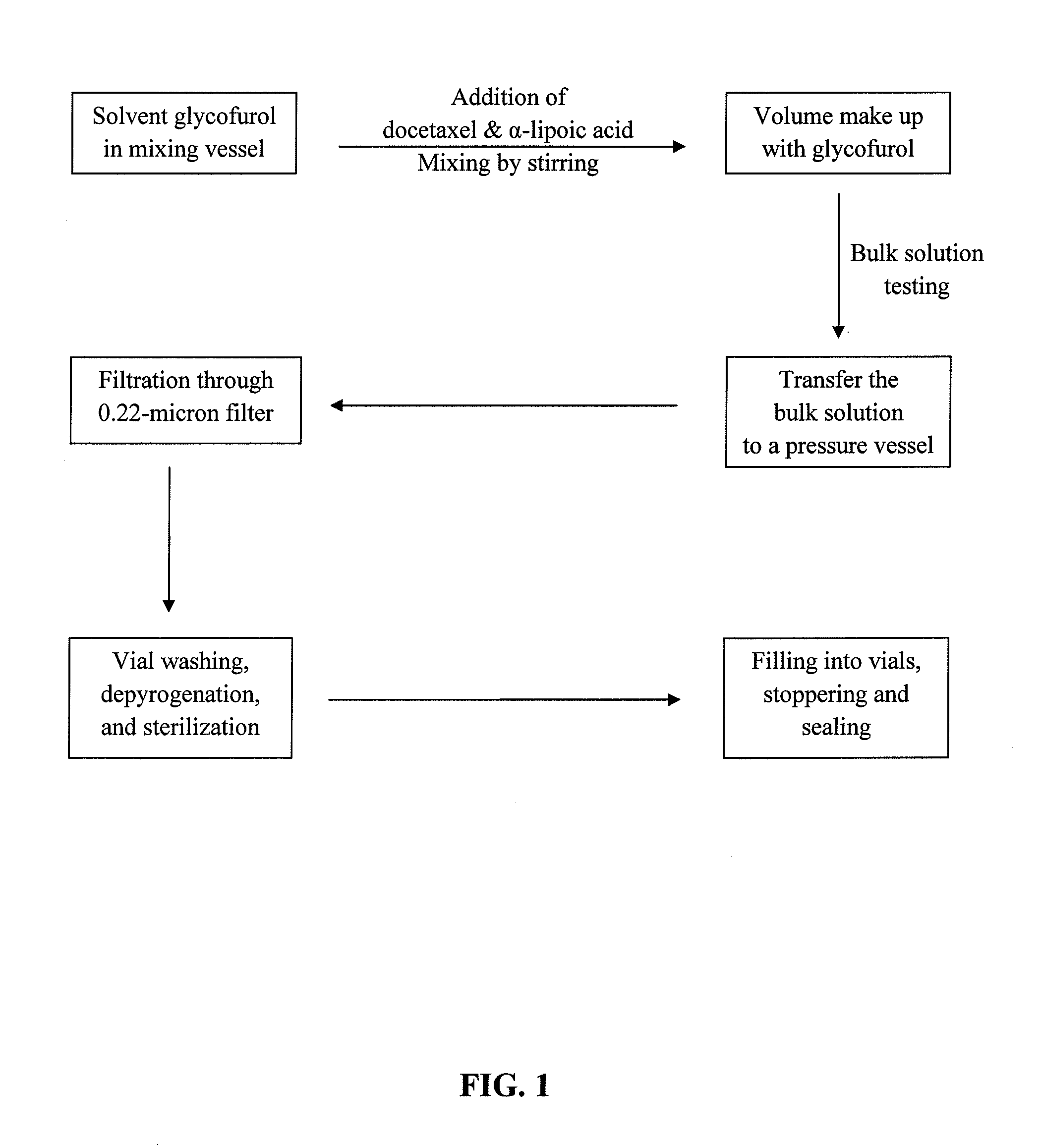
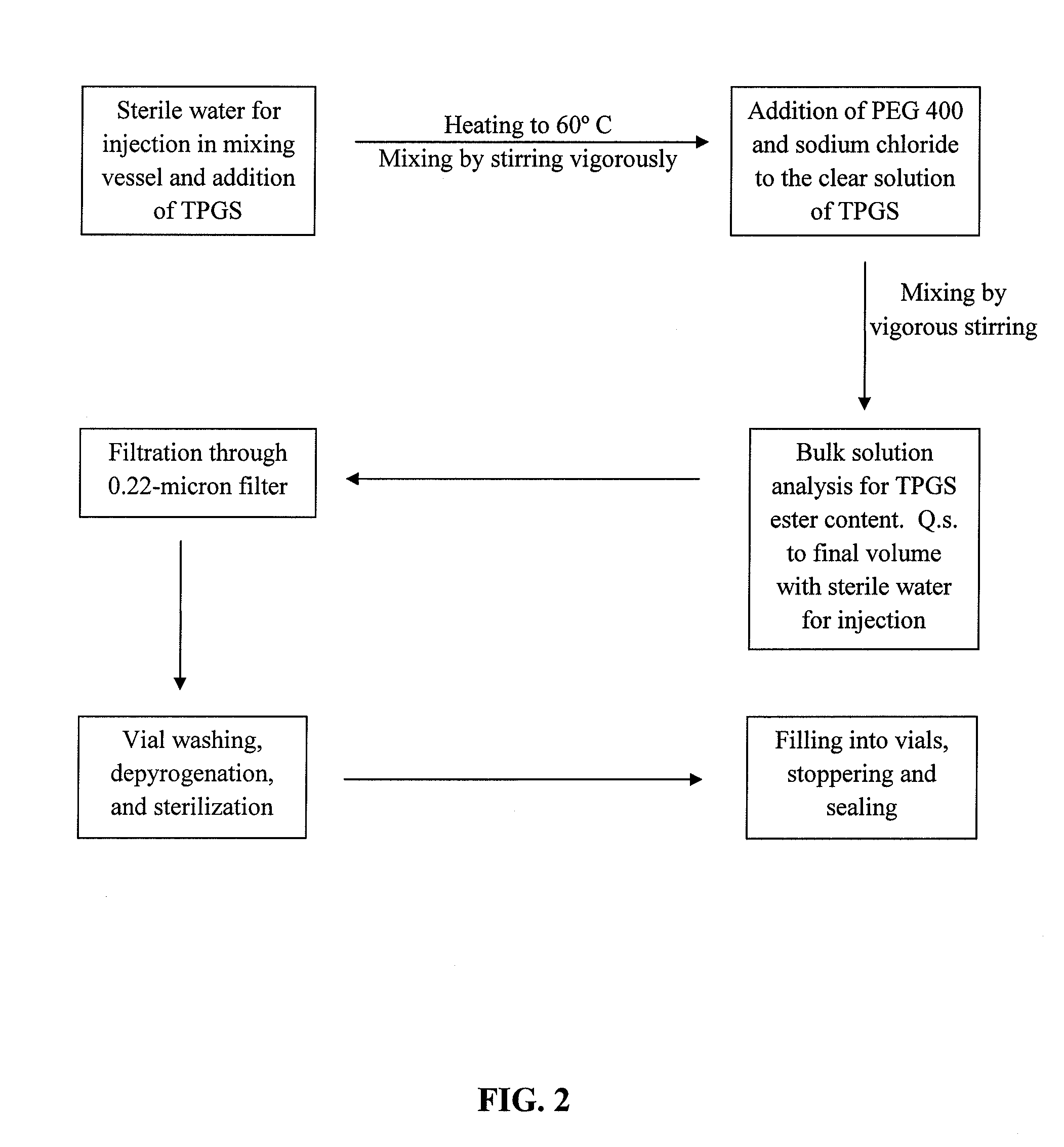
Docetaxel formulations with lipoic acid
Pharmaceutical formulations comprising docetaxel, solubilizer, and α-lipoic acid, wherein the formulation is substantially free of polysorbates and polyethoxylated castor oil. The solubilizer may comprise glycofurol, acetic acid, benzyl alcohol, or ethanol. The α-lipoic acid, at certain concentrations, may impart stability and prevent degradation of docetaxel while the formulations are in storage. The formulations may be combined with a diluent, which comprises one or more hydrotropes such as tocopherol polyethylene glycol succinate and polyethylene glycol. The formulations combined with the diluent also exhibit stability after storage. Methods of administering docetaxel comprise preparing the formulation comprising docetaxel, solubilizer, and α-lipoic acid; mixing the formulation with a diluent; diluting the resulting formulation in saline, water for injection, or the like; and then injecting the formulations into patients in need thereof.
Owner:SCIDOSE
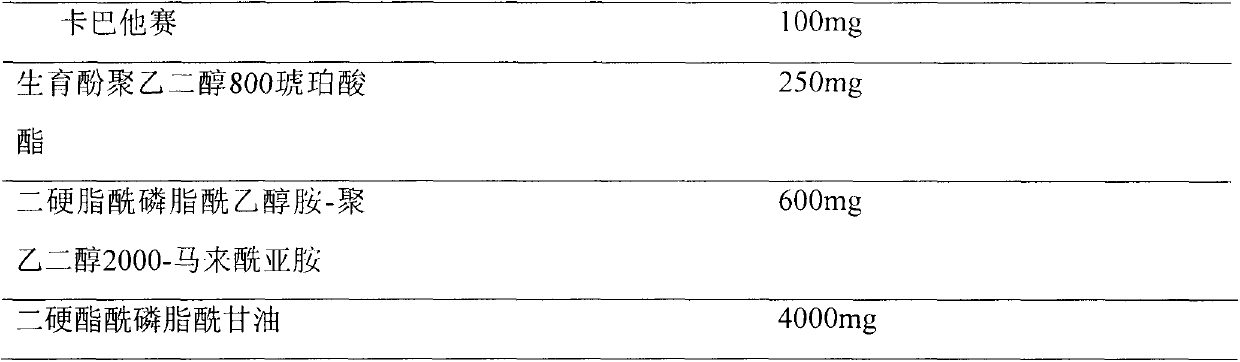
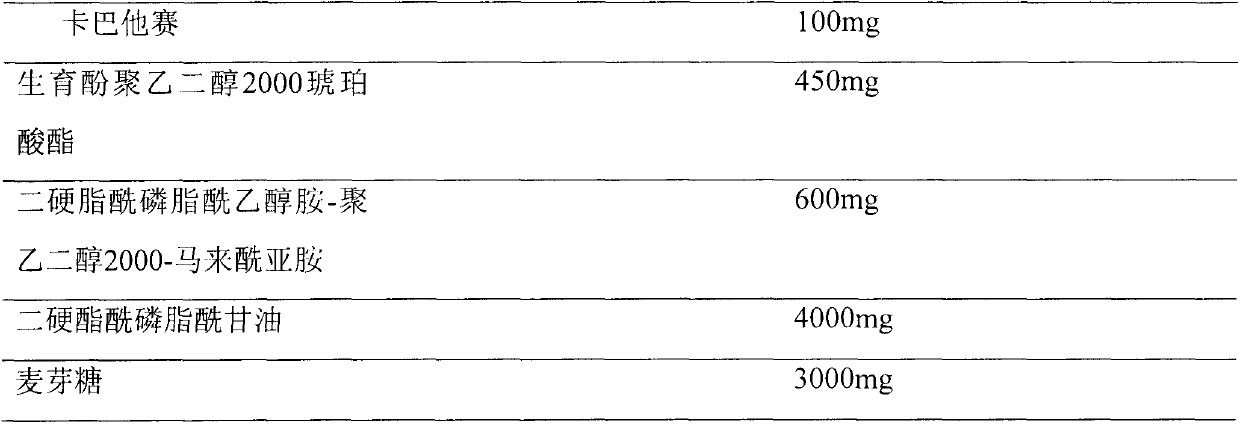
Cabazitaxel formulations and methods of preparing thereof
InactiveUS20120065255A1Organic active ingredientsBiocideHydrotropeTocopherol polyethylene glycol succinate
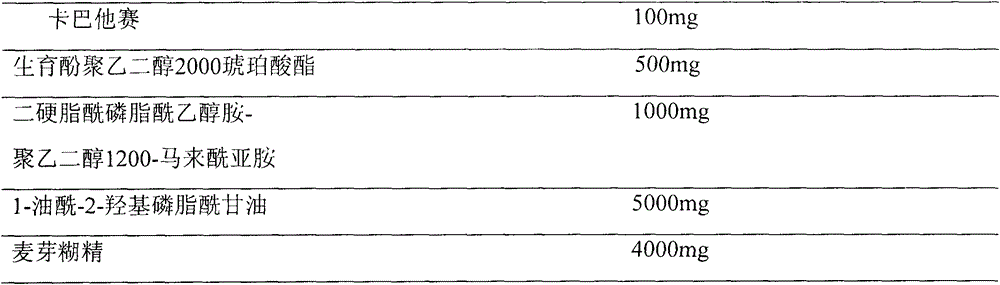
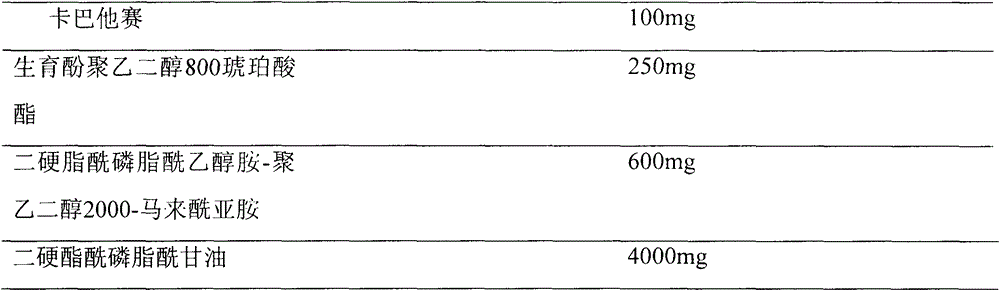
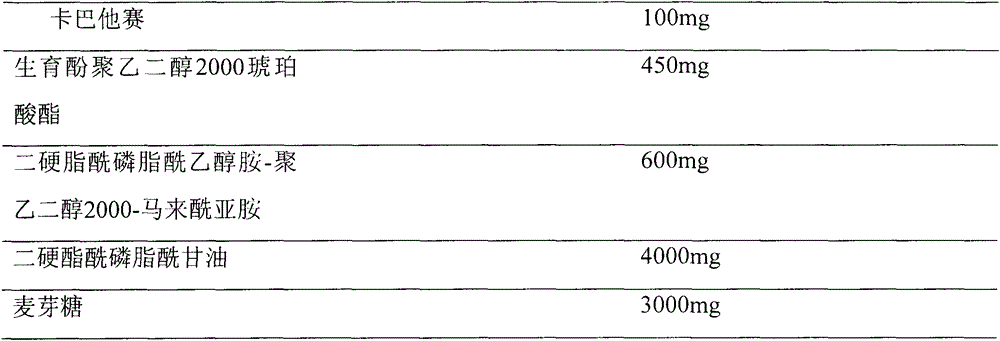
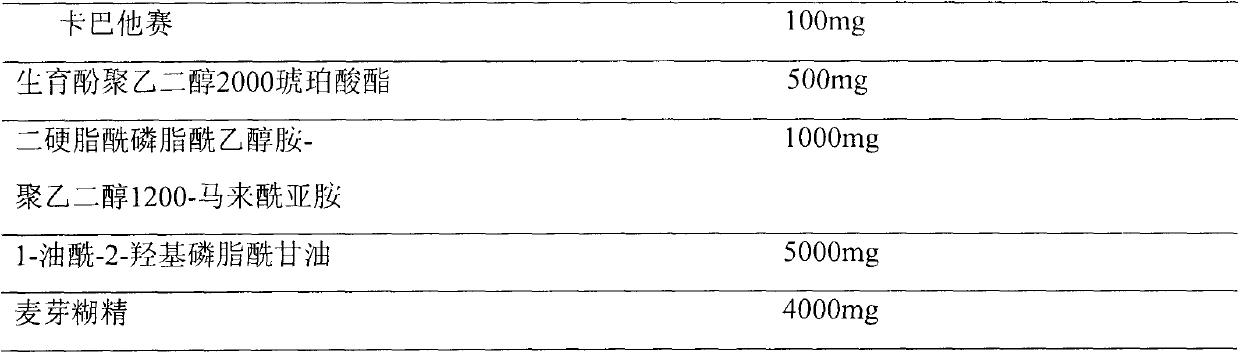
Pharmaceutical formulations comprising cabazitaxel, solubilizer, tocopherol polyethylene glycol succinate (TPGS), one or more hydrotropes, optionally one or more agents having a pKa of about 3 to about 6, and optionally one or more antioxidizing agents, wherein the formulations are substantially free of polysorbates and polyethoxylated castor oil. The solubilizer may comprise glycofurol or ethanol. Pharmaceutical formulations may alternatively comprise cabazitaxel, solubilizer, optionally one or more agents having a pKa of about 3 to about 6, and optionally one or more antioxidizing agents, wherein the formulations are substantially free of polysorbates and polyethoxylated castor oil. These formulations may be combined with a diluent, which comprises TPGS and one or more hydrotropes. Methods of administering the cabazitaxel formulations include combining the formulations with an infusion solution.
Owner:SCIDOSE
Pharmaceutical compositions comprising cyclosporins
A liquid comprising a therapeutically effective concentration of a cyclosporin and a vitamin E tocopherol polyethylene glycol succinate, wherein said liquid is an emulsion. is disclosed herein. Methods of treating diseases or conditions using said compositions, and medicaments related thereto, are also disclosed herein.
Owner:ALLERGAN INC
Paclitaxel mixed micelle preparation, and preparation method thereof
InactiveCN102198084AReduce toxic and side effectsImprove securityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityPolyoxyethylene castor oil
The invention discloses a paclitaxel mixed micelle preparation, comprising 100 to 300 milligram of tocopherol polyethylene glycol succinate 1000 (TPGS), 0 to 50 milligram of phosphatide, 0.5 milliliter of anhydrous ethanol and 6 milligram of paclitaxel. The preparation method is as follows: the TPGS is dissolved in the anhydrous ethanol, or the TPGS and the phosphatide are dissolved in the anhydrous ethanol; the paclitaxel is added and dissolved under stirring; the mixture is filtered with a millipore filtration of 0.22 micrometer so as to obtain the paclitaxel mixed micelle preparation. In the invention, the TPGS and the phosphatide are used to form mixed micelles which have good stability and little toxic and side effects; the preparation method is simple and practicable, having a good application prospect. Compared to the prior art, polyoxyethylene castor oil in conventional prescription is substituted in the invention, thereby reducing toxic side effects of paclitaxel injections and greatly enhancing the safety of the injections on condition that solubility is guaranteed.
Owner:SHANDONG UNIV
Tripterine nano structure lipid carrier and preparation method and application thereof
InactiveCN102225205AReduce systemic side effectsImprove bioavailabilityOrganic active ingredientsAntipyreticNano structuringTreatment effect
The invention relates to the field of Chinese medicine preparation, in particular to a method for preparing tripterine nano structure lipid carrier containing traditional Chinese medicine monomer and application of the tripterine nano structure lipid carrier in preparation of transdermal drugs used for treating psoriasis, rheumatoid arthritis, skin cancer and breast cancer. The tripterine nano structure lipid carrier is characterized by comprising the following components in parts by weight: 1 part of tripterine, 5-100 parts of mixed lipid, 0.5-10 parts of phospholipid, 0.1-15 parts of poloxamer-188 and 0.5-10 parts of vitamin E and tocopherol polyethylene glycol succinate, wherein the mixed lipid is composed of solid lipid monoglycerine and liquid lipid octylic acid / caprin according to the weight ratio of 1: 0.1-10: 1. Tripterine is prepared into the nano structure lipid carrier, the tripterine nano structure lipid carrier in a semi-solid or liquid preparation form is applied in a transdermal way, bioavailability of the tripterine can be improved, toxic response of tripterine can be reduced, and the nano structure lipid carrier provided by the invention has great clinical application value in the improvement of the treatment effect of tripterine.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Tocopheryl polyethylene glycol succinate articles and process for preparing TPGS articles
InactiveUS20050163828A1Improved surface tackinessExcellent hardness propertiesOrganic active ingredientsOrganic chemistryPolymer scienceGram
The present invention relates to a TPGS article having a weight no greater than 1 gram and a tackiness no greater than about 1550 grams. In addition, the present invention relates to a process for producing said TPGS articles.
Owner:EASTMAN CHEM CO
Cabazitaxel long-circulation liposome injection and preparation method thereof
ActiveCN104473873AHigh encapsulation efficiencyImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsMedicinePhospholipid
The invention provides cabazitaxel long-circulation liposome injection and a preparation method thereof. The cabazitaxel long-circulation liposome injection comprises cabazitaxel or a pharmaceutically acceptable salt thereof, tocopherol polyethylene glycol succinate, distearoyl-phosphatidylethanolamine-polyethylene glycol-maleimide and phospholipid. The cabazitaxel long-circulation liposome injection is prepared by a film method. The liposome not only has the advantages of reduced toxicity, simple and convenient clinical use and improved bioavailability, but also has good stability of storage and use.
Owner:南京西默思博检测技术有限公司
Stable pharmaceutical composition
A pharmaceutical composition comprising:(a) a medium system, comprising a first component, a second component and a third component, wherein the first component is a phosphate buffered saline, the second component is selected from the group consisting of vegetable oils, animal oils, fatty acids and combinations thereof, and the third component is selected from the group consisting of polyethylene glycol, dimethyl sulfoxide (DMSO), ethanol, polypropylene glycol, polysorbate, polyoxyethylated vegetable oil, ethyl acetate, 2-hydroxyethyl 12-hydroxyoctadecanoate, tocopheryl polyethylene glycol succinate and combinations thereof; and(b) n-butylidenephthalide (BP).
Owner:EVERFRONT BIOTECH
Tocopheryl polyethylene glycol succinate powder and process for preparing same
InactiveCN101232871APowder deliveryOrganic active ingredientsPolymer scienceTocopherol polyethylene glycol succinate
The invention relates to a powdered tocopheryl polyethylene glycol succinate (TPGS) having an average particle size of less than about 1000 microns. In one embodiment, the powdered tocopheryl polyethylene glycol succinate is prepared by a process that includes atomizing a fluidic tocopheryl polyethylene glycol succinate into an environment suitable for solidifying the atomized tocopheryl polyethylene glycol succinate. In another embodiment, the powdered tocopheryl polyethylene glycol succinate is prepared by a process of applying a force to a solid tocopheryl polyethylene glycol succinate starting material that is sufficient to produce a powdered product.
Owner:EASTMAN CHEM CO
Docetaxel Formulations with Lipoic Acid
Pharmaceutical formulations comprising docetaxel, solubilizer, and α-lipoic acid, wherein the formulation is substantially free of polysorbates and polyethoxylated castor oil. The solubilizer may comprise glycofurol, acetic acid, benzyl alcohol, or ethanol. The α-lipoic acid, at certain concentrations, may impart stability and prevent degradation of docetaxel while the formulations are in storage. The formulations may be combined with a diluent, which comprises one or more hydrotropes such as tocopherol polyethylene glycol succinate and polyethylene glycol. The formulations combined with the diluent also exhibit stability after storage. Methods of administering docetaxel comprise preparing the formulation comprising docetaxel, solubilizer, and α-lipoic acid; mixing the formulation with a diluent; diluting the resulting formulation in saline, water for injection, or the like; and then injecting the formulations into patients in need thereof.
Owner:SCIDOSE
Oleanolic acid nanometer suspension and preparation method thereof
InactiveCN103356480ARapid dissolutionOral bioavailability is lowOrganic active ingredientsDigestive systemCurative effectTocopherol polyethylene glycol succinate
The invention belongs to the technical field of medicines, and discloses an oleanolic acid nanometer suspension and a preparation method thereof. The curative effect of oleanolic acid is restricted because oleanolic acid has small solubility in water and low oral bioavailability. The oleanolic acid nanometer suspension of the invention is characterized by comprising oleanolic acid, tocopherol polyethylene glycol succinate 1000 and polyethylene glycol 4000 with a mass ratio of 10-5:1-2:1-2. The preparation method of the nanometer suspension is mild in condition, simple and controllable; and oleanolic acid in the prepared nanometer suspension has higher oral bioavailability.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Hydroxycamptothecine long-circulating liposome
InactiveCN103690556AOvercoming insoluble defectsImprove stabilityPowder deliveryPharmaceutical non-active ingredientsCholesterolPhospholipid
The invention discloses a hydroxycamptothecine long-circulating liposome and a preparation method thereof. The hydroxycamptothecine long-circulating liposome is prepared from the following components in parts by weight: 1 part of hydroxycamptothecine, 5-30 parts of phospholipid, 1-5 parts of cholesterol, 1-20 parts of TPGS (tocopherol polyethylene glycol succinate), 1-8 parts of poloxamer188. The hydroxycamptothecine long-circulating liposome prepared by the method is proper in grain diameter, high in encapsulation, low in raw material price and simple in process, and is suitable for industrial production.
Owner:UNIV OF JINAN
Formulations of water-soluble derivatives of vitamin E and compositions containing same
InactiveCN105228470AOrganic active ingredientsNervous disorderWater solubleTocopherol polyethylene glycol succinate
Provided herein are compositions that contain water-soluble vitamin E derivative mixtures (compositions), such as tocopheryl polyethylene glycol succinate (TPGS), TPGS analogs, TPGS homo logs and TPGS derivatives. The water-soluble vitamin E mixtures contain mixtures of dimers and monomers of the vitamin E derivative. Also provided are products containing the water-soluble vitamin E derivative mixtures, including concentrates for dilution into aqueous beverages and compositions for direct ingestion.
Owner:VIRUN INC
Bazedoxifene acetate composition with excellent property
ActiveCN103845336AGood chemical stabilityFast drug releaseSkeletal disorderPharmaceutical non-active ingredientsSolid solutionTocopherol polyethylene glycol succinate
The invention discloses a bazedoxifene acetate composition with excellent properties. The composition comprises bazedoxifene acetate, polyethylene glycol (PEG) 6000-8000 and vitamin E tocopherol polyethylene glycol succinate (TPGS), wherein a solid solution and / or solid sol is formed by polyethylene glycol (PEG) 6000-8000 and vitamin E TPGS, and bazedoxifene acetate is dispersed into the solid solution and / or solid sol. The composition is high in chemical stability and medicine release speed.
Owner:JIANGSU SEMPOLL PHARMA
Anti-tumor magnetic drug-loading hybridized nanocapsules and preparation method thereof
ActiveCN109674764APromote decompositionGood light-to-heat conversion propertiesOrganic active ingredientsDrug photocleavageUltrasonic emulsificationBiocompatibility Testing
The invention discloses anti-tumor magnetic drug-loading hybridized nanocapsules and a preparation method thereof. The preparation method comprises the following steps: firstly, preparing oleic acid coated magnetic ferroferric oxide nanoparticles (Fe3O4-OA); after modifying heparin sodium by TPGS (Tocopherol Polyethylene Glycol Succinate) and PEG (Polyethylene Glycol) through condensation reaction, carrying out an ultrasonic emulsification method to prepare Fe3O4-OA and adriamycin coated magnetic nanocapsules; then modifying the peripheries of the capsules with tumor cell-penetrating peptidesiRGD to provide a tumor cell targeting function; finally, treating through ammonium bicarbonate to form the magnetic drug-loading hybridized nanocapsules. The magnetic drug-loading hybridized nanocapsules prepared by the invention have strong targeting performance and have good stimulation response performance under the illumination of near infrared laser; carbon dioxide gas can be produced and high-selectivity rapid drug release of the nanocapsules on a tumor part is realized; meanwhile, the nanocapsules have good biocompatibility and high safety and also have certain magnetic response behaviors.
Owner:SICHUAN UNIV
Cardioprotective nano-pharmaceutical formulation
ActiveUS10653666B1Increased and improved bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsCardiac dysfunctionSuccinic acid
A nano-pharmaceutical formulation, comprising pumpkin seed oil; D-α-tocopheryl polyethylene glycol succinate (TPGS); and polyethylene glycol (PEG) 200 is provided. Additional cardioprotective therapies such as quercetin may be included in the formulation. Methods of treating cardiac dysfunction by administering the formulation are also provided.
Owner:KING ABDULAZIZ UNIV
Tripterine nano structure lipid carrier and preparation method and application thereof
InactiveCN102225205BReduce systemic side effectsImprove bioavailabilityOrganic active ingredientsAntipyreticNano structuringPhospholipid
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
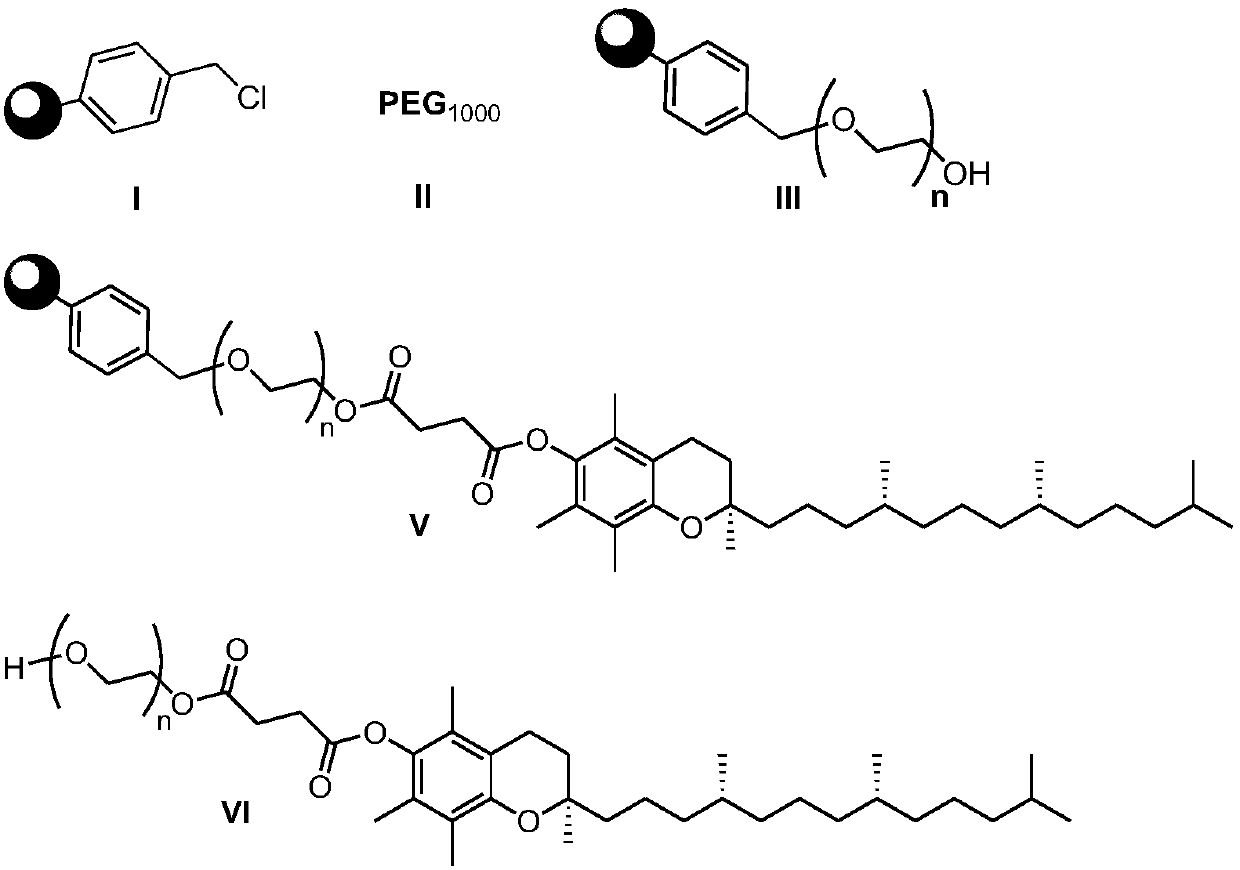
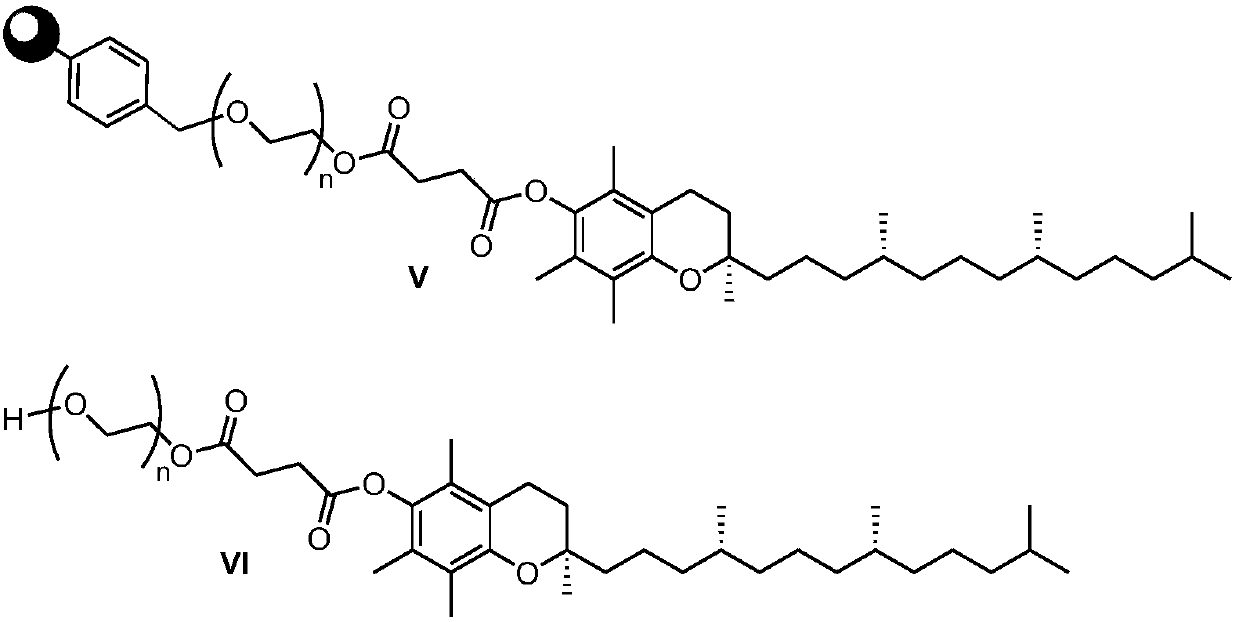
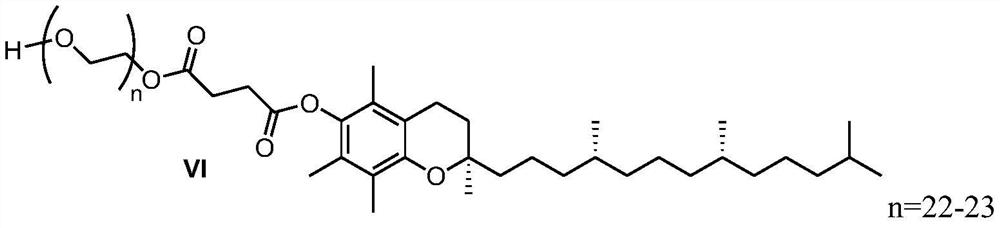
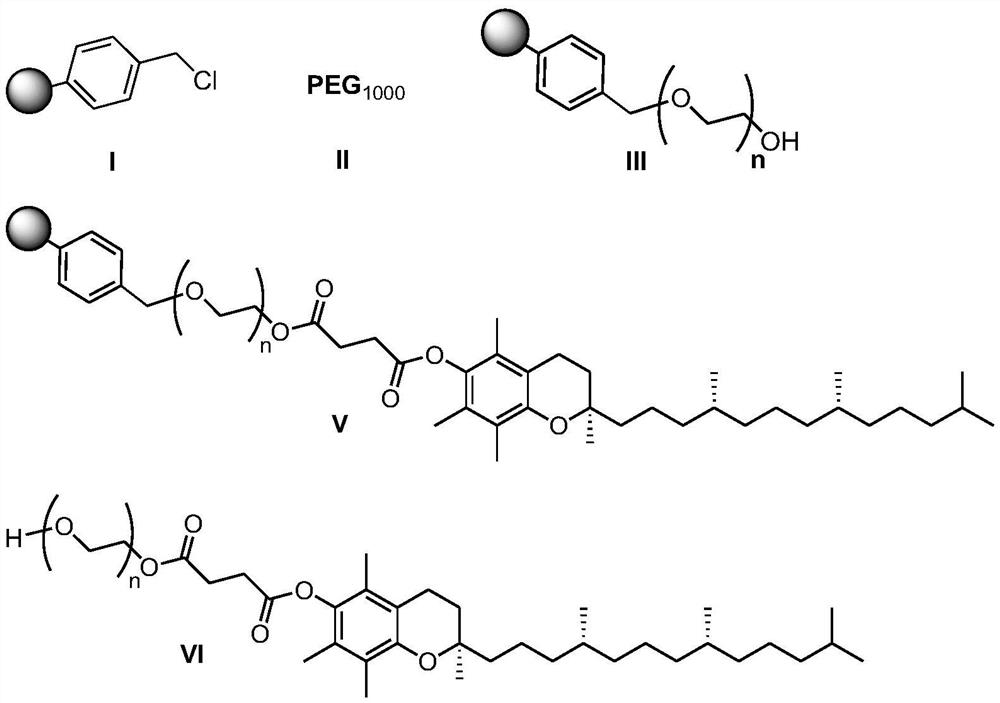
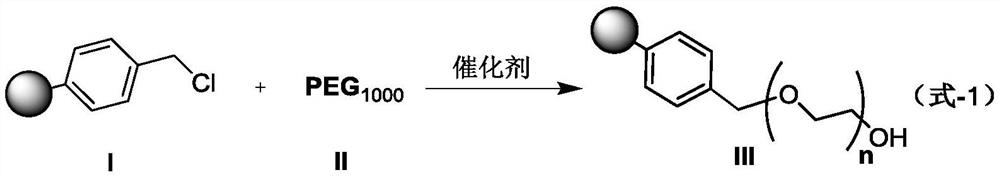
Preparation method of d-alpha-tocopherol polyethylene glycol succinate
ActiveCN108676157AEliminate the purification processOmit separabilityPalladium on carbonTocopherol succinate
The invention discloses a preparation method of d-alpha-tocopherol polyethylene glycol succinate. The preparation method comprises the following steps: by taking Merrifield resin and PEG1,000 as raw materials and alkali as a catalyst, performing reaction at 45-135 DEG C in an organic solvent to obtain resin loaded with PEG1,000; under the action of the catalyst, enabling the resin loaded with PEG1,000 and d-alpha-tocopherol succinate to be subjected to water diversion reflux reaction to obtain resin loaded with TPGS; and soaking the resin loaded with TPGS in toluene, then adding ethanol, palladium on carbon and phosphopyridoxal, and performing stirring reaction at 100-110 DEG C for 24 hours in hydrogen gas of 0.8-1.5MPa to obtain the d-alpha-tocopherol polyethylene glycol succinate. By adopting the synthetic method disclosed by the invention, the yield of TPGS is as high as 99%, the monoester content of TPGS is 99%, and the diester content of TPGS is 0.
Owner:NINGBO WANGLONG TECH +1
Tablets comprising a poorly compressible active agent and tocopherol polyethyleneglycol succinate (tpgs)
InactiveCN101043876AMeet the requirements of the pharmaceutical processPowder deliveryGranular deliveryActive agentSuccinic acid
A solid composition suitable for forming into a tablet includes a pharmaceutically active substance in an amount sufficient to provide a therapeutic effect when administered; from about 0.2 to about 15 weight %, based on the total weight of the composition, of a water-soluble preparation of a fat-soluble vitamin; and from about 10 to 80 weight %, based on the total weight of the composition, of an excipient. Another aspect of the present invention is method for making the solid composition.
Owner:EASTMAN CHEM CO
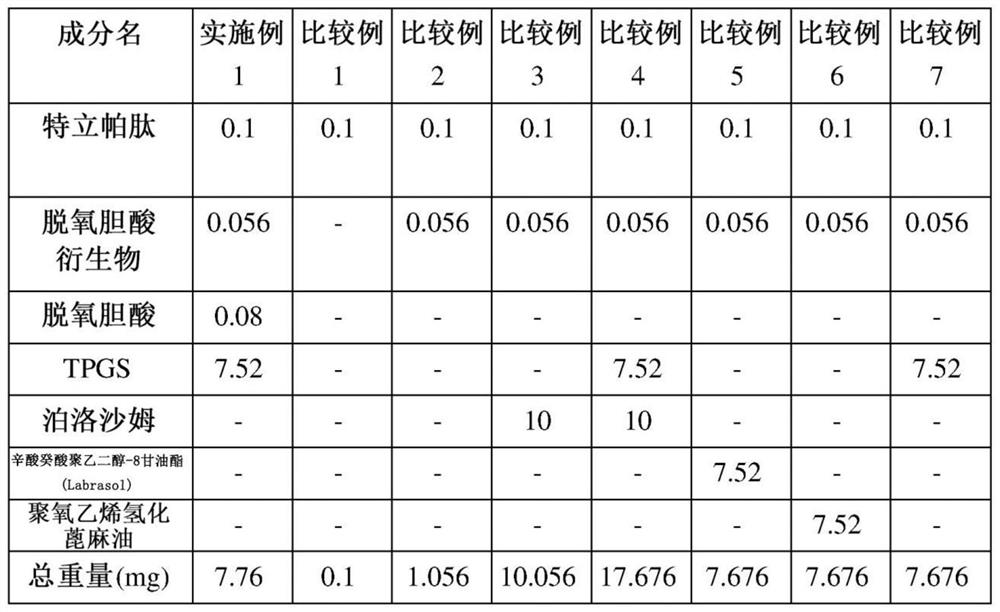
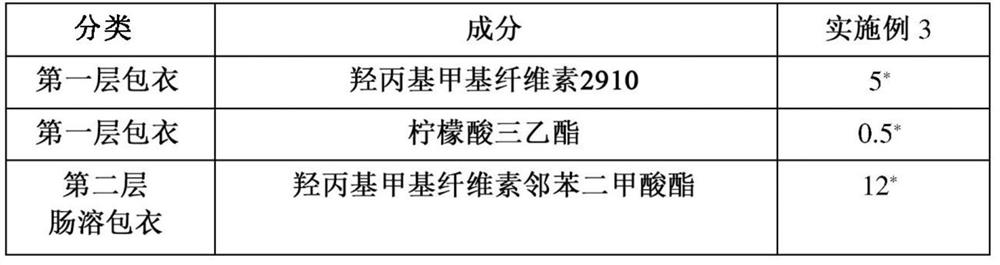
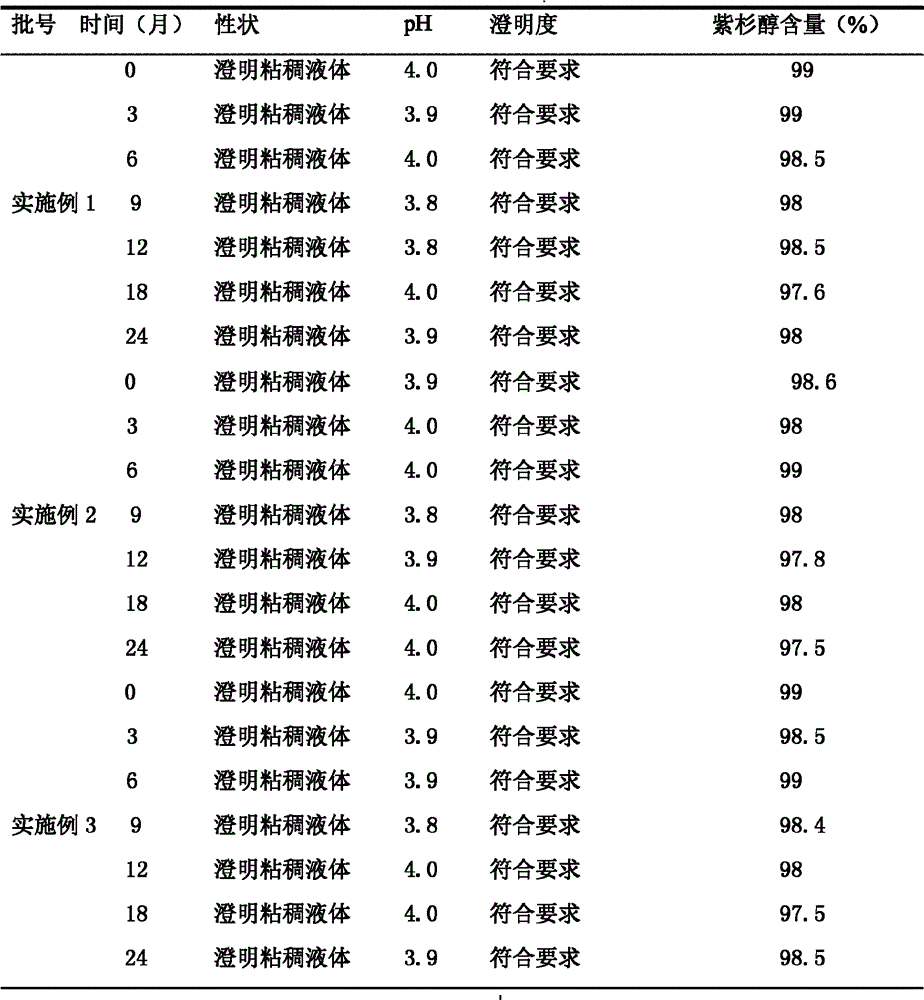
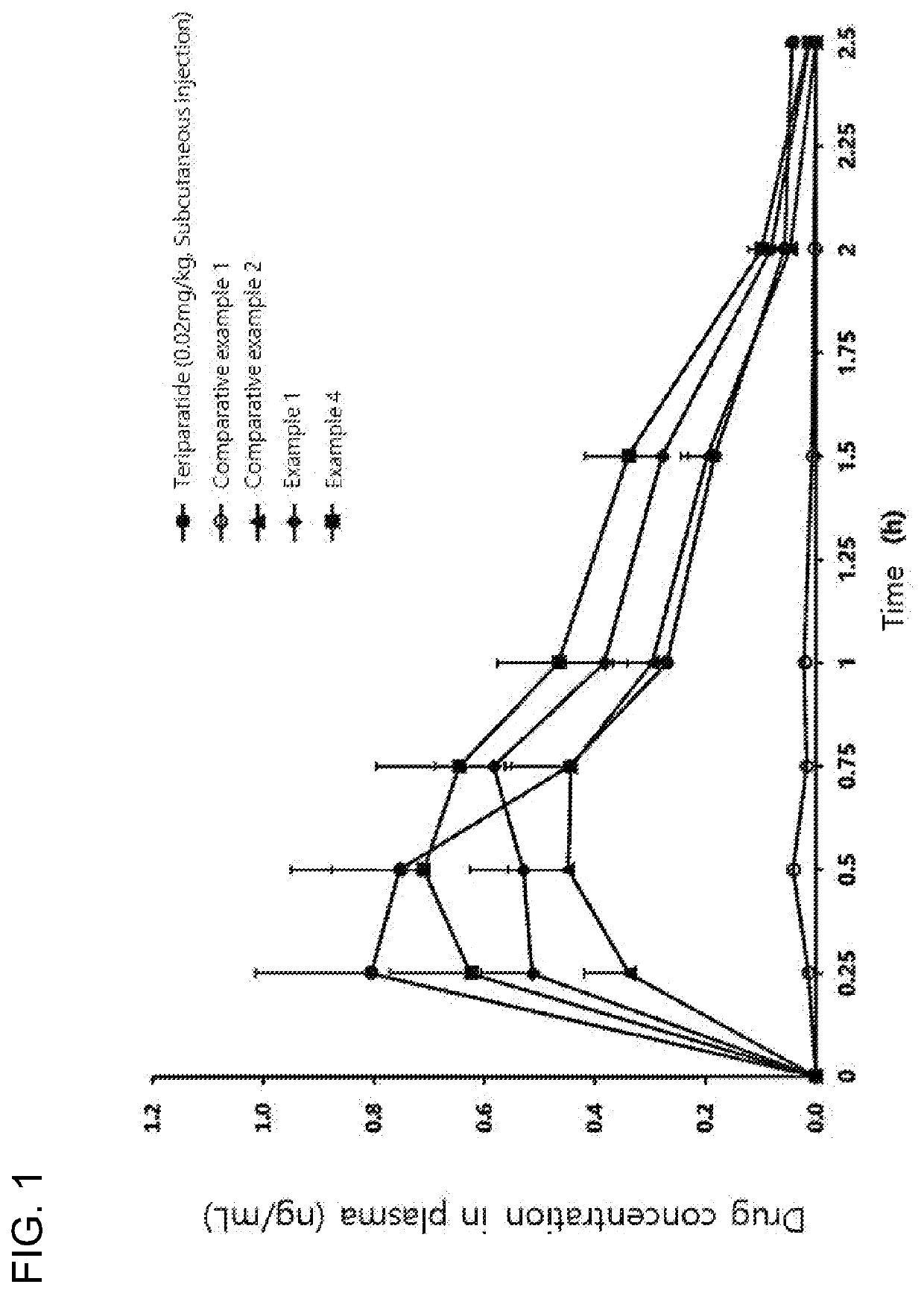
Oral pharmaceutical composition containing teriparatide and preparation method thereof
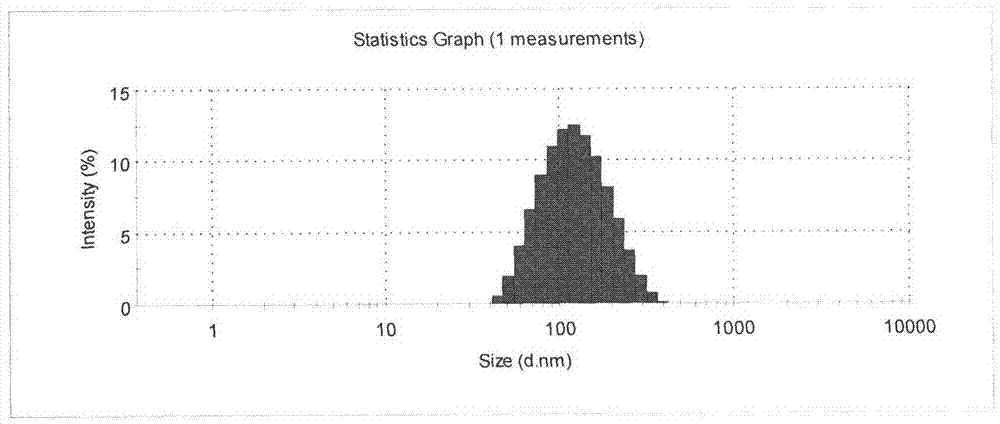
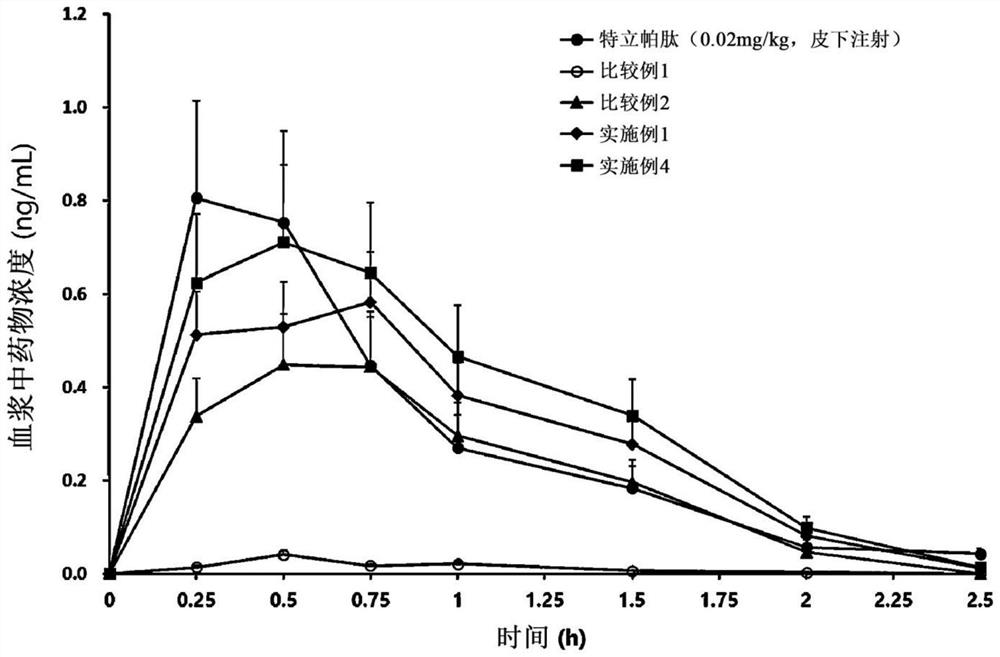
PendingCN114599388AImprove permeabilityImprove bioavailabilityPeptide/protein ingredientsSkeletal disorderCholic acidAcyl group
The present invention relates to an oral pharmaceutical composition and a preparation method therefor, the oral pharmaceutical composition comprising an ionic bond complex consisting of teriparatide, deoxycholic acid, N [alpha]-deoxycholyl-L-lysyl methyl ester (N [alpha]-deoxycholyl-L-lysyl methyl ester, DCK), and D-[alpha]-tocopherol polyethylene glycol 1000 succinate (D-[alpha]-tocopherol polyethylene glycol 1000 succinate), the ionic bond complex comprising at least one compound selected from the group consisting of N [alpha]-deoxycholyl-L-lysyl methyl ester, N [alpha]-tocopherol polyethylene glycol 1000 succinate, N [alpha]-tocopherol polyethylene glycol 1000 succinate, N [alpha]-tocopherol polyethylene glycol 1000 succinate, N [alpha]-tocopherol polyethylene glycol 1000 succinate, N [alpha]- The oral pharmaceutical composition provided by the invention can improve the intestinal mucosa permeability and oral administration bioavailability of teriparatide and improve patient compliance, and can be used for treating osteoporosis.
Owner:ICURE BNP CO LTD
Paclitaxel mixed micelle preparation, and preparation method thereof
InactiveCN102198084BReduce toxic and side effectsImprove securityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityPolyoxyethylene castor oil
The invention discloses a paclitaxel mixed micelle preparation, comprising 100 to 300 milligram of tocopherol polyethylene glycol succinate 1000 (TPGS), 0 to 50 milligram of phosphatide, 0.5 milliliter of anhydrous ethanol and 6 milligram of paclitaxel. The preparation method is as follows: the TPGS is dissolved in the anhydrous ethanol, or the TPGS and the phosphatide are dissolved in the anhydrous ethanol; the paclitaxel is added and dissolved under stirring; the mixture is filtered with a millipore filtration of 0.22 micrometer so as to obtain the paclitaxel mixed micelle preparation. In the invention, the TPGS and the phosphatide are used to form mixed micelles which have good stability and little toxic and side effects; the preparation method is simple and practicable, having a good application prospect. Compared to the prior art, polyoxyethylene castor oil in conventional prescription is substituted in the invention, thereby reducing toxic side effects of paclitaxel injections and greatly enhancing the safety of the injections on condition that solubility is guaranteed.
Owner:SHANDONG UNIV
Stable pharmaceutical composition
A pharmaceutical composition comprising:(a) a medium system, comprising a first component, a second component and a third component, wherein the first component is a phosphate buffered saline, the second component is selected from the group consisting of vegetable oils, animal oils, fatty acids and combinations thereof, and the third component is selected from the group consisting of polyethylene glycol, dimethyl sulfoxide (DMSO), ethanol, polypropylene glycol, polysorbate, polyoxyethylated vegetable oil, ethyl acetate, 2-hydroxyethyl 12-hydroxyoctadecanoate, tocopheryl polyethylene glycol succinate and combinations thereof; and(b) n-butylidenephthalide (BP).
Owner:EVERFRONT BIOTECH
Oleanolic acid nanometer suspension and preparation method thereof
InactiveCN103356480BRapid dissolutionOral bioavailability is lowOrganic active ingredientsDigestive systemSolubilityCurative effect
The invention belongs to the technical field of medicines, and discloses an oleanolic acid nanometer suspension and a preparation method thereof. The curative effect of oleanolic acid is restricted because oleanolic acid has small solubility in water and low oral bioavailability. The oleanolic acid nanometer suspension of the invention is characterized by comprising oleanolic acid, tocopherol polyethylene glycol succinate 1000 and polyethylene glycol 4000 with a mass ratio of 10-5:1-2:1-2. The preparation method of the nanometer suspension is mild in condition, simple and controllable; and oleanolic acid in the prepared nanometer suspension has higher oral bioavailability.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
A kind of cabazitaxel long circulation liposome injection and preparation method thereof
ActiveCN104473873BImprove bioavailabilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsMedicinePhospholipid
The invention provides cabazitaxel long-circulation liposome injection and a preparation method thereof. The cabazitaxel long-circulation liposome injection comprises cabazitaxel or a pharmaceutically acceptable salt thereof, tocopherol polyethylene glycol succinate, distearoyl-phosphatidylethanolamine-polyethylene glycol-maleimide and phospholipid. The cabazitaxel long-circulation liposome injection is prepared by a film method. The liposome not only has the advantages of reduced toxicity, simple and convenient clinical use and improved bioavailability, but also has good stability of storage and use.
Owner:南京西默思博检测技术有限公司
A kind of preparation method of water-soluble natural vitamin E
The invention discloses a preparation method for water-soluble natural vitamin E. the method comprises the steps that chloromethylated polystyrene resin and PEG1000 serve as raw materials, alkali serves as a catalyst, and reaction is carried out in an organic solvent at 45-135 DEG C so as to obtain PEG1000-loaded resin; under the action of the catalyst, 4-dimethylaminopyridine (DMAP) and tetrahydrofuran, water division reflux reaction is carried out on the PEG1000-loaded resin and d-alpha-tocopherol succinate so as to obtain TPGS-loaded resin; and after the TPGS-loaded resin is soaked by usingmethylbenzene, ethanol, palladium carbon and pyridoxal phosphate are added, and under the atmosphere of 0.8-1.5 MPa of hydrogen , stirring is carried out for reaction for 24 hours at the temperatureof 100-110 DEG C so as to obtain d-alpha-tocopherol polyethylene glycol succinate. According to the synthetic method, the yield of TPGS can reach 97.8%, the TPGS monoester content is 98.6%, and the TPGS diester content is 0.
Owner:ZHEJIANG UNIV OF TECH
Oral pharmaceutical composition including teriparatide and method for preparing same
PendingUS20220339261A1Improve bioavailabilityGood membrane permeabilityPeptide/protein ingredientsSkeletal disorderCholic acidPatient compliance
Proposed is a pharmaceutical composition for oral administration, the composition including an ionic bond complex composed of teriparatide, deoxycholic acid, Nα-deoxycholyl-L-lysyl-methylester (DCK), and di-alpha-tocopherol polyethylene glycol 1000 succinate, and a method for preparing the same is also proposed. The oral pharmaceutical composition is useful for the treatment of osteoporosis because the pharmaceutical composition can increase intestinal membrane permeability and oral administration bioavailability of teriparatide and improve patient compliance.
Owner:IPHAMA CO LTD
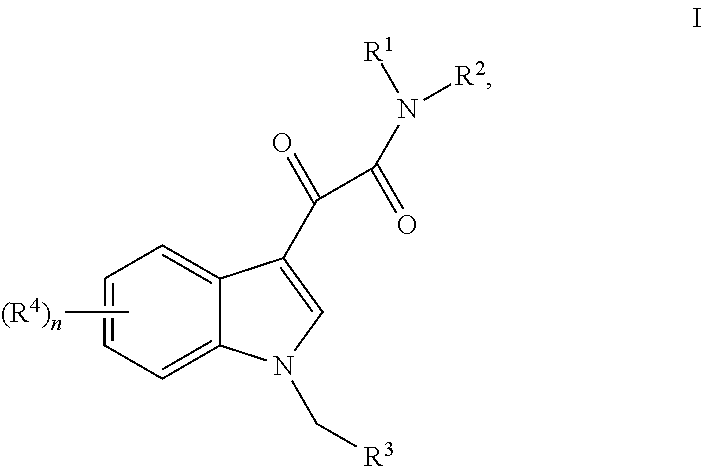
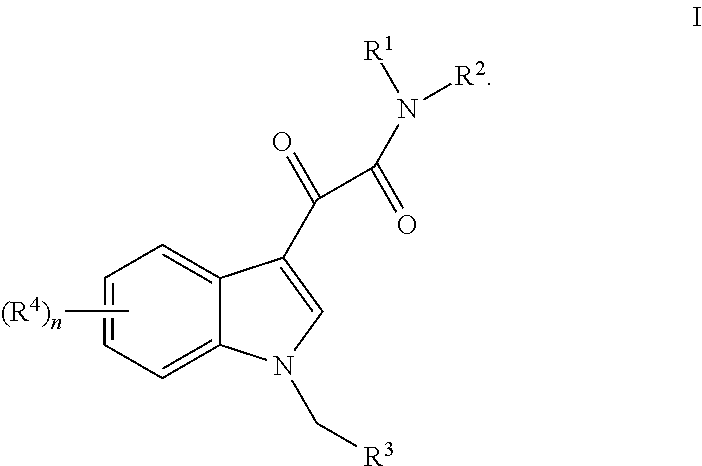
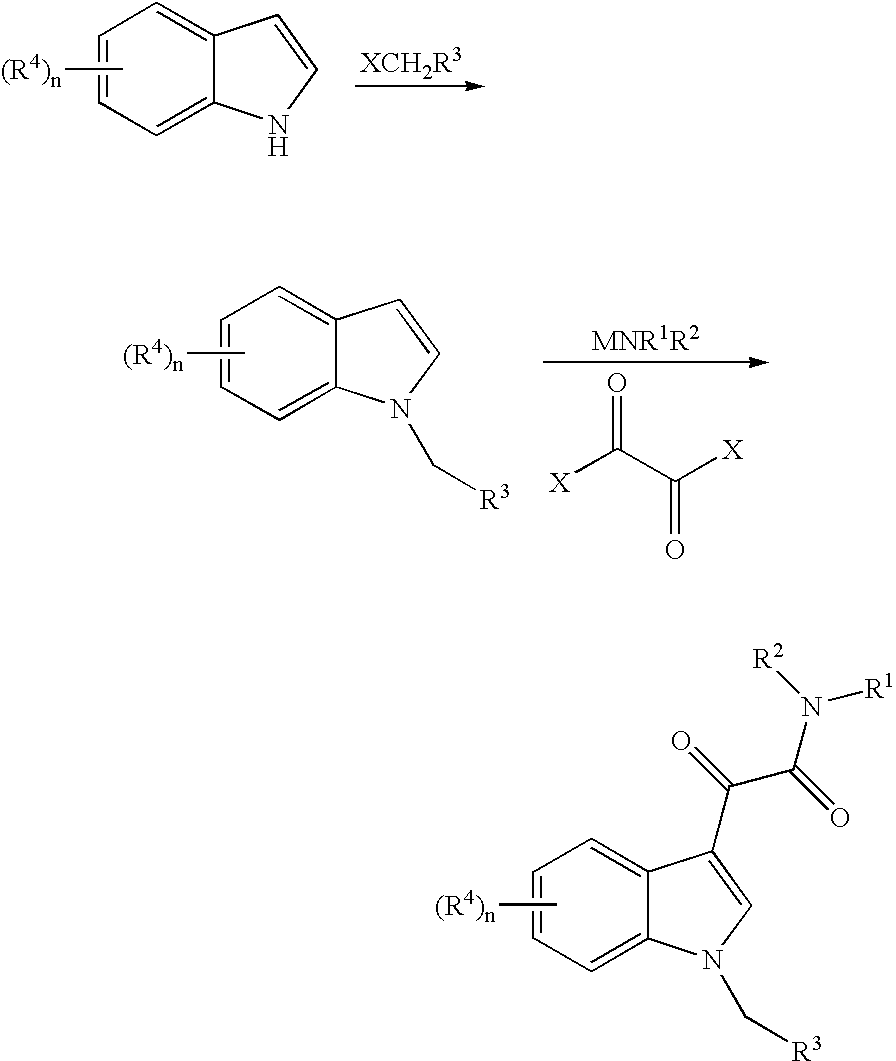
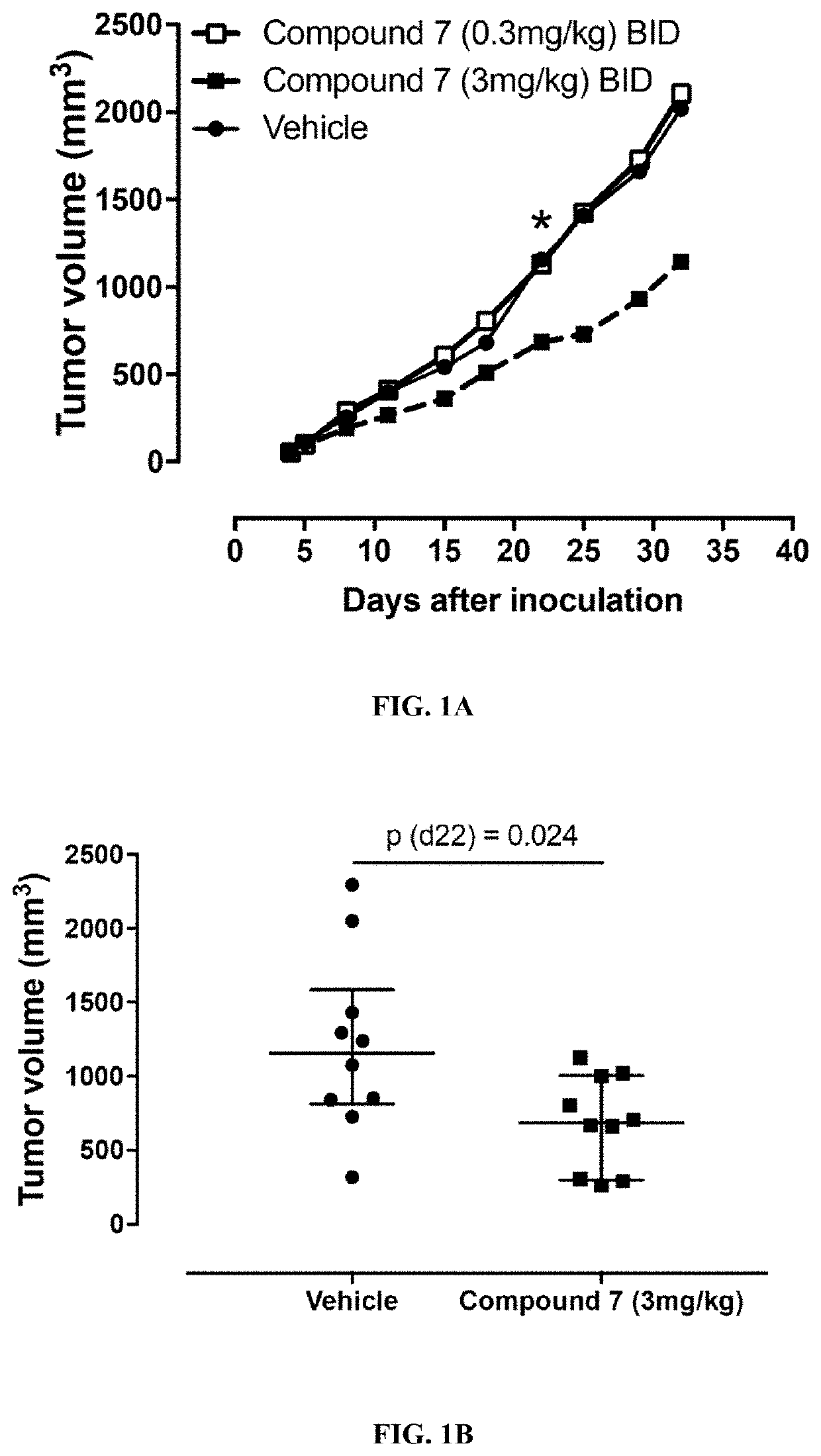
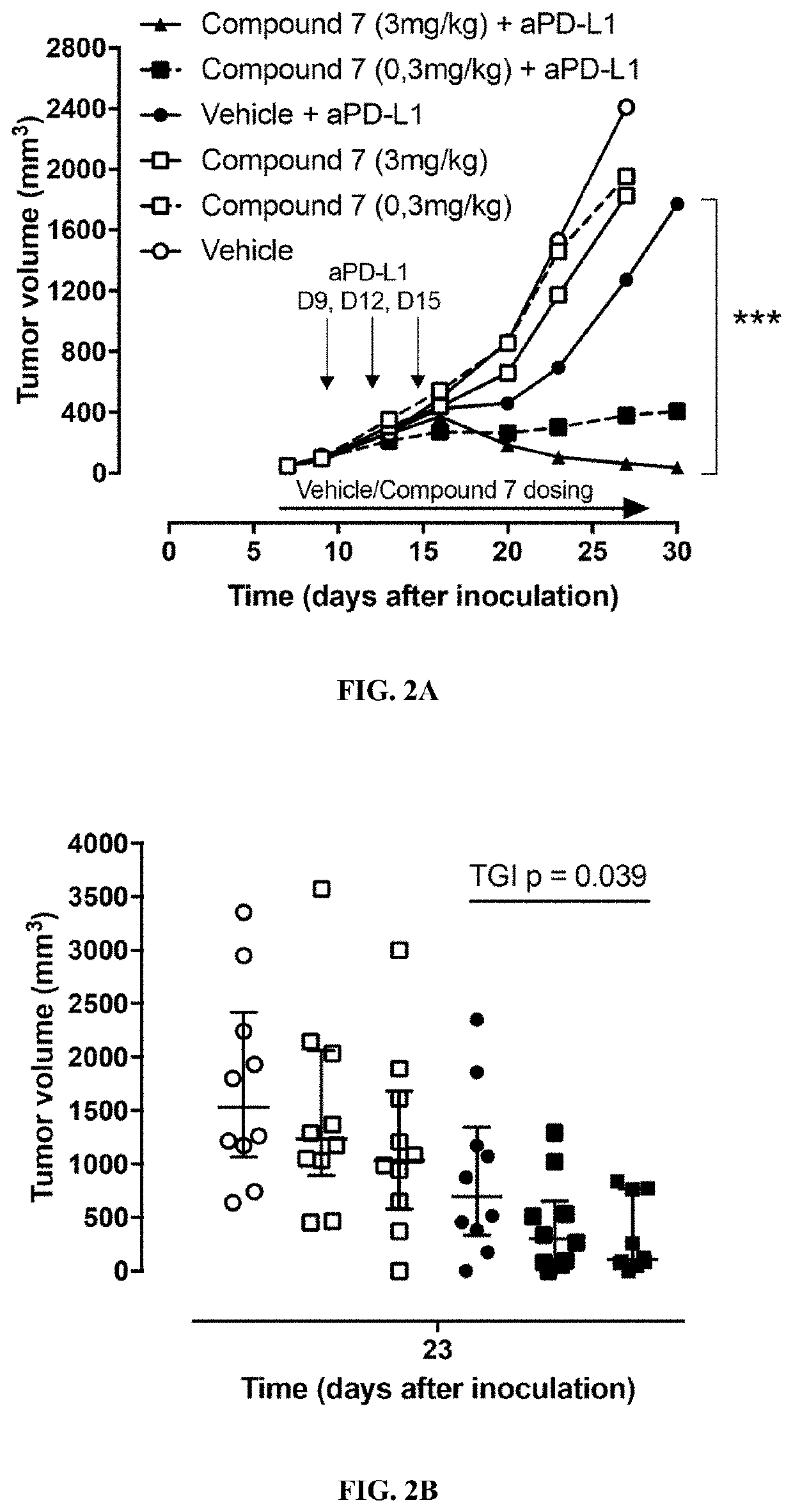
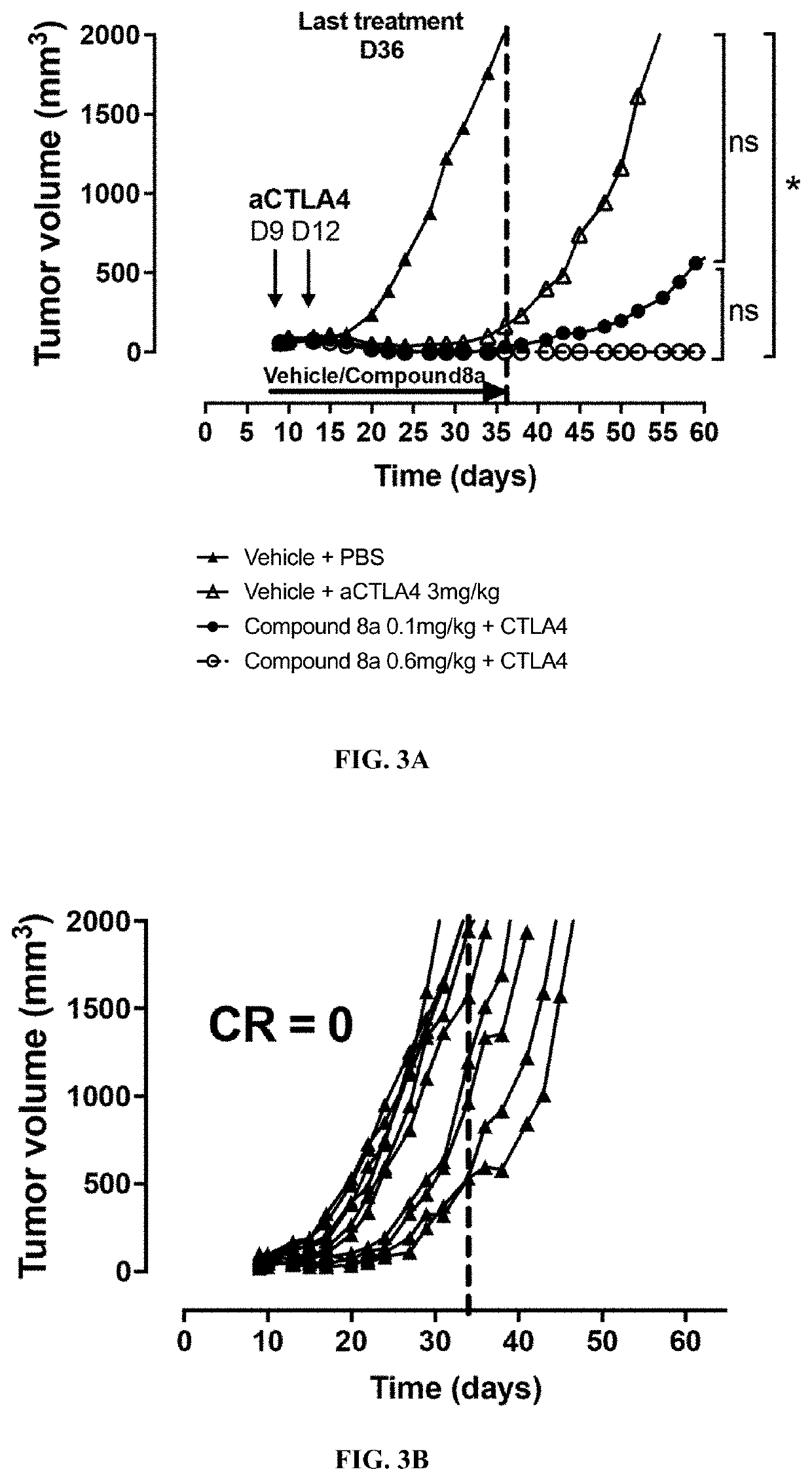
Formulations of indol-3-yl-2-oxoacetamide compounds
InactiveUS8026271B2Improve oral bioavailabilityReduce the possibilityBiocideDispersion deliveryTocopherol polyethylene glycol succinateSuccinates
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
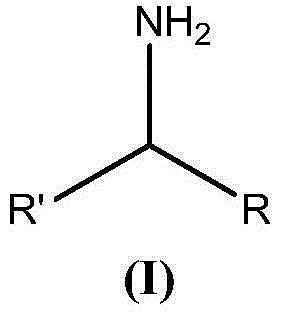
Thiocarbamate derivatives as A2A inhibitors, pharmaceutical composition thereof and combinations with anticancer agents
ActiveUS11376255B2Reduce riskRapid and sustained and delayed releaseOrganic active ingredientsInorganic active ingredientsThiocarbamateAnticarcinogen
The present invention relates to thiocarbamate derivatives of Formula (I) which are useful as A2A adenosine receptor (A2AR) inhibitorsEspecially, the present invention relates to a pharmaceutical composition comprising an A2A inhibitor of Formula (I) and a lipid carrier such as lauroyl macrogol-32 glycerides, D-α-tocopherol-polyethylene glycol-1000 succinate or a mixture thereof. The pharmaceutical composition of the invention is particularly useful for oral dosing in the treatment of cancers. The present invention also relates to a combination comprising an A2A receptor inhibitor of Formula (I) and an anticancer agent. The anticancer agent is for example an immunotherapeutic agent, such as a checkpoint inhibitor. The invention further relates to a pharmaceutical composition and a kit of parts comprising such combination. Additionally, the combination of the invention is particularly useful for the treatment and / or prevention of cancers.
Owner:ITEOS BELGIUM SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com