Hydroxycamptothecine long-circulating liposome
A long-circulation liposome and hydroxycamptothecin technology, which is applied in liposome delivery, medical preparations of non-active ingredients, organic active ingredients, etc., can solve the problem of no long-circulation materials, unpublished models, difficult applications, etc. problems, to achieve the effect of expanding the scope of application, increasing the drug loading dose, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
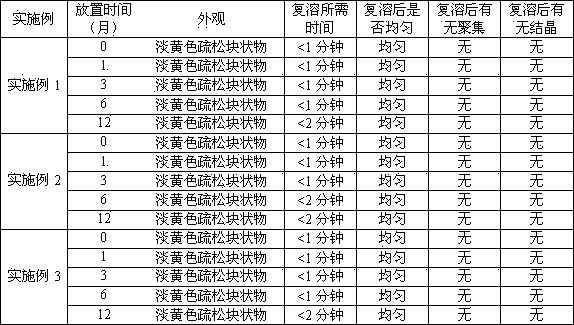
Embodiment 1
[0028] Embodiment 1: Preparation of hydroxycamptothecin long-circulating liposomes
[0029] The prescription consisted of: hydroxycamptothecin 0.4 g, soybean lecithin 7.5 g, cholesterol 1.0 g, TPGS 1.5 g, and poloxamer 188 1.0 g.
[0030] Preparation Process
[0031] (1) Formation of lipid film: Weigh 0.4 g of hydroxycamptothecin, 7.5 g of soybean lecithin, 1.0 g of cholesterol, 1.5 g of TPGS, and 1.0 g of poloxamer 188 in a small beaker, add 40 ml of absolute ethanol , add a magnet and gently stir until completely dissolved to obtain a clear solution. Transfer the above clarified mixed solution into a 500 ml round-bottomed flask, add a little glass beads, keep the temperature of the water bath at 55°C, slowly rotate and evaporate under reduced pressure on a rotary evaporator, and continue vacuum evaporation for 2 h until the solvent evaporates to dryness. The organic solvent was removed, at which point a uniform, clear lipid film formed on the flask walls.
[0032] (2) Hyd...
Embodiment 2
[0038] Embodiment 2: Preparation of hydroxycamptothecin long-circulating liposomes
[0039] The prescription consisted of: hydroxycamptothecin 0.3 g, soybean lecithin 3.0 g, hydrogenated soybean lecithin 0.3 g, cholesterol 1.5 g, TPGS 1.5 g, and poloxamer 188 2.0 g.
[0040] Preparation Process
[0041] (1) Formation of lipid film: Weigh 0.3 g of hydroxycamptothecin, 3.0 g of soybean lecithin, 0.3 g of hydrogenated soybean lecithin, 1.5 g of cholesterol, 1.5 g of TPGS, and 2.0 g of poloxamer 188 in a small beaker , add 40 ml of chloroform, add a magnet and stir gently until completely dissolved to obtain a clear solution. Transfer the above clarified mixed solution into a 500 ml round bottom flask, add a little glass beads, keep the water melting temperature at 60°C, slowly rotate and evaporate under reduced pressure on a rotary evaporator, and continue vacuum evaporation for 3 h after the solvent evaporates to dryness. The flask was removed from the rotary evaporator and pl...
Embodiment 3
[0045] Embodiment 3: the preparation of hydroxycamptothecin long circulation liposome
[0046] The prescription consisted of: hydroxycamptothecin 1.0 g, egg yolk lecithin 19.0 g, hydrogenated soybean lecithin 1.0 g, cholesterol 3.0 g, TPGS 5.0 g, and poloxamer 188 2.5 g.
[0047] Preparation Process
[0048] (1) Formation of lipid film: Weigh 1.0 g of hydroxycamptothecin, 19.0 g of egg yolk lecithin, 1.0 g of hydrogenated soybean lecithin, 3.0 g of cholesterol, 5.0 g of TPGS, and 2.5 g of poloxamer 188 in a beaker, Add 100 ml of absolute ethanol, add a magnet and stir gently until completely dissolved to obtain a clear solution. The above-mentioned clarified mixed solution was transferred to a 1000 ml round-bottomed flask, and the following steps were the same as in the "formation of lipid film" in Example 2.
[0049] (2) Hydration: 100 ml of phosphate buffer solution was added to the above-mentioned flask where the lipid film was formed, and the following steps were the sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com