A long-circulating liposome of hydroxycamptothecin
A long-circulation liposome and hydroxycamptothecin technology, which is applied in liposome delivery, medical preparations of non-active ingredients, organic active ingredients, etc., can solve the problem of no long-circulation materials, unpublished models, difficult applications, etc. problems, to achieve the effect of expanding the scope of application, increasing the drug loading dose, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
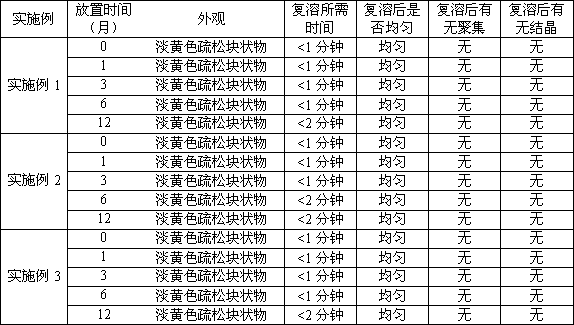
[0028] Embodiment 1: Preparation of hydroxycamptothecin long-circulating liposomes
[0029] The prescription is composed of: hydroxycamptothecin 0.4g, soybean lecithin 7.5g, cholesterol 1.0g, TPGS 1.5g, poloxamer 1881.0g.
[0030] Preparation Process
[0031] (1) Formation of lipid film: Weigh 0.4g of hydroxycamptothecin, 7.5g of soybean lecithin, 1.0g of cholesterol, 1.5g of TPGS, and 1881.0g of poloxamer in a small beaker, add 40ml of absolute ethanol, add Gently stir with a magnet until completely dissolved to obtain a clear solution. Transfer the above clarified mixed solution into a 500ml round bottom flask, add a little glass beads, keep the temperature of the water bath at 55°C, and slowly rotate and evaporate under reduced pressure on a rotary evaporator. After the solvent evaporates, continue vacuum evaporation for 2h until the organic Solvent, at this time a uniform transparent lipid film was formed on the wall of the flask.
[0032] (2) Hydration: Add 40ml of pho...
Embodiment 2
[0038] Embodiment 2: Preparation of hydroxycamptothecin long-circulating liposomes
[0039] The prescription is composed of: hydroxycamptothecin 0.3g, soybean lecithin 3.0g, hydrogenated soybean lecithin 0.3g, cholesterol 1.5g, TPGS 1.5g, poloxamer 1882.0g.
[0040] Preparation Process
[0041] (1) Formation of lipid film: Weigh 0.3g of hydroxycamptothecin, 3.0g of soybean lecithin, 0.3g of hydrogenated soybean lecithin, 1.5g of cholesterol, 1.5g of TPGS, and 1882.0g of poloxamer in a small beaker, Add 40ml of chloroform, add a magnet and stir gently until completely dissolved to obtain a clear solution. Transfer the above clarified mixed solution into a 500ml round-bottom flask, add a little glass beads, keep the water melting temperature at 60°C, and slowly rotate and evaporate under reduced pressure on a rotary evaporator. After the solvent evaporates, continue vacuum evaporation for 3h, and remove the flask from Remove it from the rotary evaporator and put it in a vacuum...
Embodiment 3
[0045] Embodiment 3: the preparation of hydroxycamptothecin long circulation liposome
[0046] The prescription consists of: hydroxycamptothecin 1.0g, egg yolk lecithin 19.0g, hydrogenated soybean lecithin 1.0g, cholesterol 3.0g, TPGS 5.0g, poloxamer 1882.5g.
[0047] Preparation Process
[0048] (1) Formation of lipid film: Weigh 1.0g of hydroxycamptothecin, 19.0g of egg yolk lecithin, 1.0g of hydrogenated soybean lecithin, 3.0g of cholesterol, 5.0g of TPGS, and 1882.5g of poloxamer in a beaker, add Add 100ml of absolute ethanol, add a magnet and stir gently until completely dissolved to obtain a clear solution. The above-mentioned clarification mixed solution is transferred in the 1000ml round-bottomed flask, and the following steps are the same as the "formation of lipid film" in Example 2.
[0049] (2) Hydration: 100 ml of phosphate buffer solution was added to the above-mentioned flask where the lipid film was formed, and the following steps were the same as "hydration"...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com