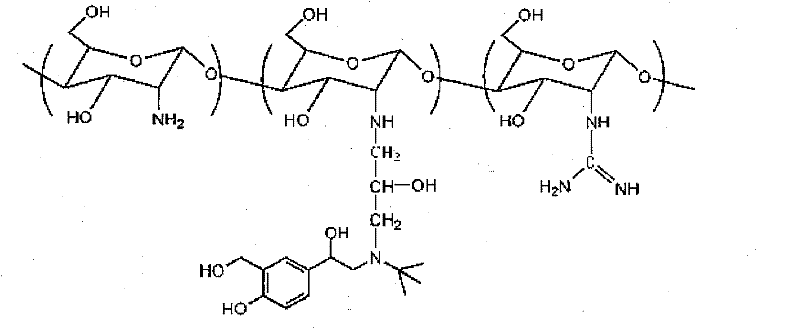
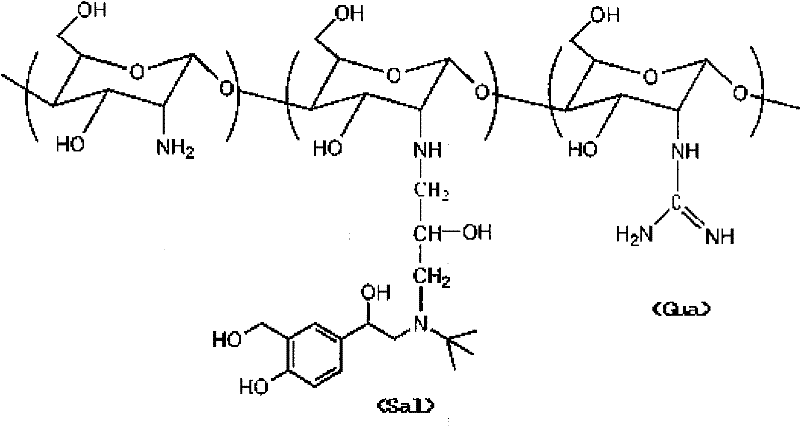
Salbutamol modified guanidinated chitosan and preparation method and application thereof
A technology of albuterol and chitosan, which is applied in gene therapy, pharmaceutical formulations, respiratory diseases, etc., can solve the problems of low transfection efficiency of chitosan and poor cell targeting, so as to reduce the stimulation of tissues and cells, Overcoming insoluble and improving transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 0.1M HCl (150ml) into the beaker, add chitosan (Mw=50kDa, 0.161g, 1mmol), then stir and heat to 80°C, stop heating after the chitosan is completely dissolved, and cool to room temperature. Add 2M NaOH solution (150ml, excess), immediately produce a large amount of white flocculent precipitate, centrifuge at 10000r / min for 5min, and pour off the supernatant. The chitosan white colloidal precipitate after lye treatment is obtained.
[0026] Dissolve albuterol sulfate (0.288g, 1mmol) in DMSO (20ml) into a three-neck flask, then add epichlorohydrin (78.3μl, 1mmol) dropwise and stir until uniform. 1M NaOH (20ml) was quickly added and reacted at room temperature for 4.5h.
[0027] Add chitosan treated with lye, and continue to react for 4.5h. The reaction solution was diluted with water and dialyzed in deionized water for 5 days (dialysis bag molecular weight cut-off 3500), changing the water every 8 hours, and then lyophilized to obtain albuterol-modified chitosan.
...
Embodiment 2
[0031] Weigh thiourea dioxide (10.8g, 0.1mol), take a small amount and add it to a three-necked flask, measure hydrogen peroxide (30%, 0.15mol, 17ml) and concentrated sulfuric acid (98%, 34ml) and mix them in proportion in a small beaker first, and prepare into hydrogen peroxide acidic solution. After cooling to room temperature, the mixed solution was added to a constant pressure dropping funnel. Control the temperature in the reactor at 50-60°C, slowly add hydrogen peroxide acidic solution dropwise while stirring, and at the same time add thiourea dioxide in small amounts, finish feeding within 1 hour, continue to react for 40 minutes, stop heating, pour the reaction solution into a beaker, Cool at 4°C for 2h. Excessive ethanol was added to the reaction solution to generate granular white crystals. The precipitate was collected by filtration, dissolved in a small amount of water, and recrystallized by adding excess ethanol. Repeat this three times. Finally, wash with abs...
Embodiment 3
[0037] Weigh thiourea dioxide (10.8g, 0.1mol), take a small amount and add it to a three-necked flask, measure hydrogen peroxide (30%, 0.15mol, 17ml) and concentrated sulfuric acid (98%, 34ml) and mix them in proportion in a small beaker first, and prepare into hydrogen peroxide acidic solution. After cooling to room temperature, the mixed solution was added to a constant pressure dropping funnel. Control the temperature in the reactor at 50-60°C, slowly add hydrogen peroxide acidic solution dropwise while stirring, and at the same time add thiourea dioxide in small amounts, finish feeding within 1 hour, continue to react for 40 minutes, stop heating, pour the reaction solution into a beaker, Cool at 4°C for 2h. Excessive ethanol was added to the reaction solution to generate granular white crystals. The precipitate was collected by filtration, dissolved in a small amount of water, and recrystallized by adding excess ethanol. Repeat this three times. Finally, wash with abs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com