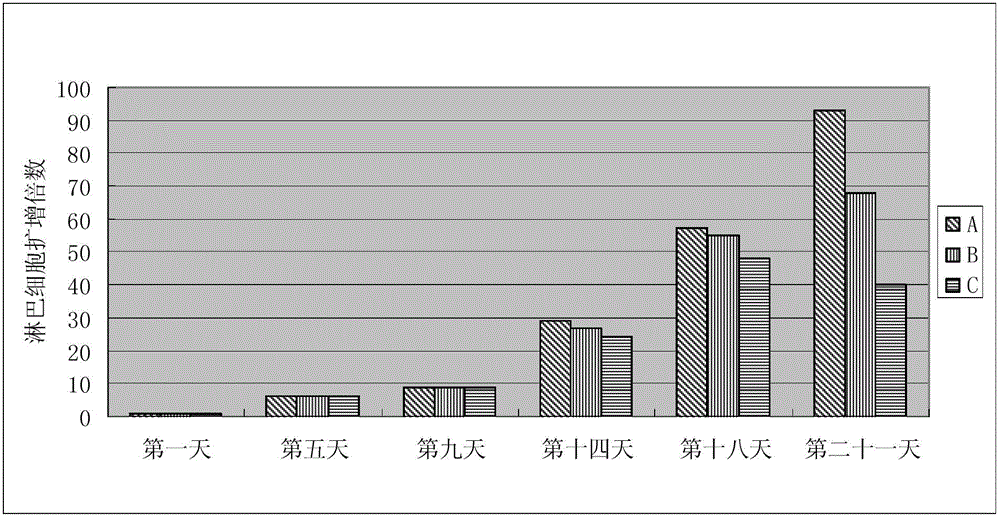
Animal source-free and serum-free culture medium of lymphocyte
A technology of serum-free medium and human serum albumin, which is applied in the field of serum-free medium for lymphocytes, can solve the problems of high price, unfavorable promotion, and increased cost of immune cell therapy, and achieve the effect of ensuring consistency and clear properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the preparation of culture medium
[0020] Preparation of recombinant human insulin stock solution: Weigh 100mg of recombinant human insulin and dissolve it in 40mL of water for injection, add dropwise 2M HCl until the recombinant human insulin is completely dissolved, dilute to 50mL, prepare a 20mg / mL stock solution, and store at 4°C.
[0021] Preparation of human transferrin stock solution: Weigh 100 mg of human transferrin and dissolve it in 40 mL of water for injection, and dilute to 50 mL after complete dissolution, prepare a 20 mg / mL stock solution, and store at 4°C.
[0022] The medium composition of the present invention is as follows:
[0023] Component
Amount added
IMDM
17.7g / L
1-5mM
3.024g / L
recombinant human insulin
1-10mg / L
human transferrin
5-20mg / L
1-10g / L
2-Mercaptoethanol
55uM
N-acetyl-cystein...
Embodiment 2
[0031] Example 2: Umbilical cord blood separation
[0032] Source of umbilical cord blood: The umbilical cord blood comes from the obstetrics department of Beijing Armed Police Hospital. , with an average volume of 80-130 mL. The separation process is as follows:
[0033] 1: After diluting the fresh cord blood and the buffer solution PBS at a volume ratio of 1:1, slowly add to the upper layer of the separation solution Ficoll along the wall of the test tube. Pay attention to keep the two interfaces clear and prevent blood from mixing into the separation solution.
[0034] 2: Centrifuge at 1800rpm for 15min, gently insert the white mist layer (the second layer from top to bottom) with a capillary pipette, gently suck out the mononuclear cells in this layer along the tube wall, and put it into another centrifuge tube.
[0035] 3: The resulting mononuclear cell suspension was washed with one volume of PBS, centrifuged at 1500 rpm for 10 min, and washed 3 times.
[0036] 4: Co...
Embodiment 3
[0037] Example 3: Peripheral Blood Separation
[0038] Peripheral blood source: Peripheral blood comes from Beijing Armed Police Hospital, which is qualified according to the "Blood Donation Physical Examination Standard" promulgated by the Ministry of Health. After intravenous disinfection, the peripheral blood is collected in a fully closed manner, anticoagulated with heparin, and the average volume is 50mL. The separation process is as follows:
[0039] 1: After diluting the fresh peripheral blood and the buffer solution PBS at a volume ratio of 1:1, slowly add to the upper layer of the separation solution Ficoll along the wall of the test tube. Pay attention to keep the two interfaces clear and prevent blood from mixing into the separation solution.
[0040]2: Centrifuge at 1800rpm for 15min, gently insert the white mist layer (the second layer from top to bottom) with a capillary pipette, gently suck out the mononuclear cells in this layer along the tube wall, and put it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com