Patents
Literature
2522results about "Glue/gelatin preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
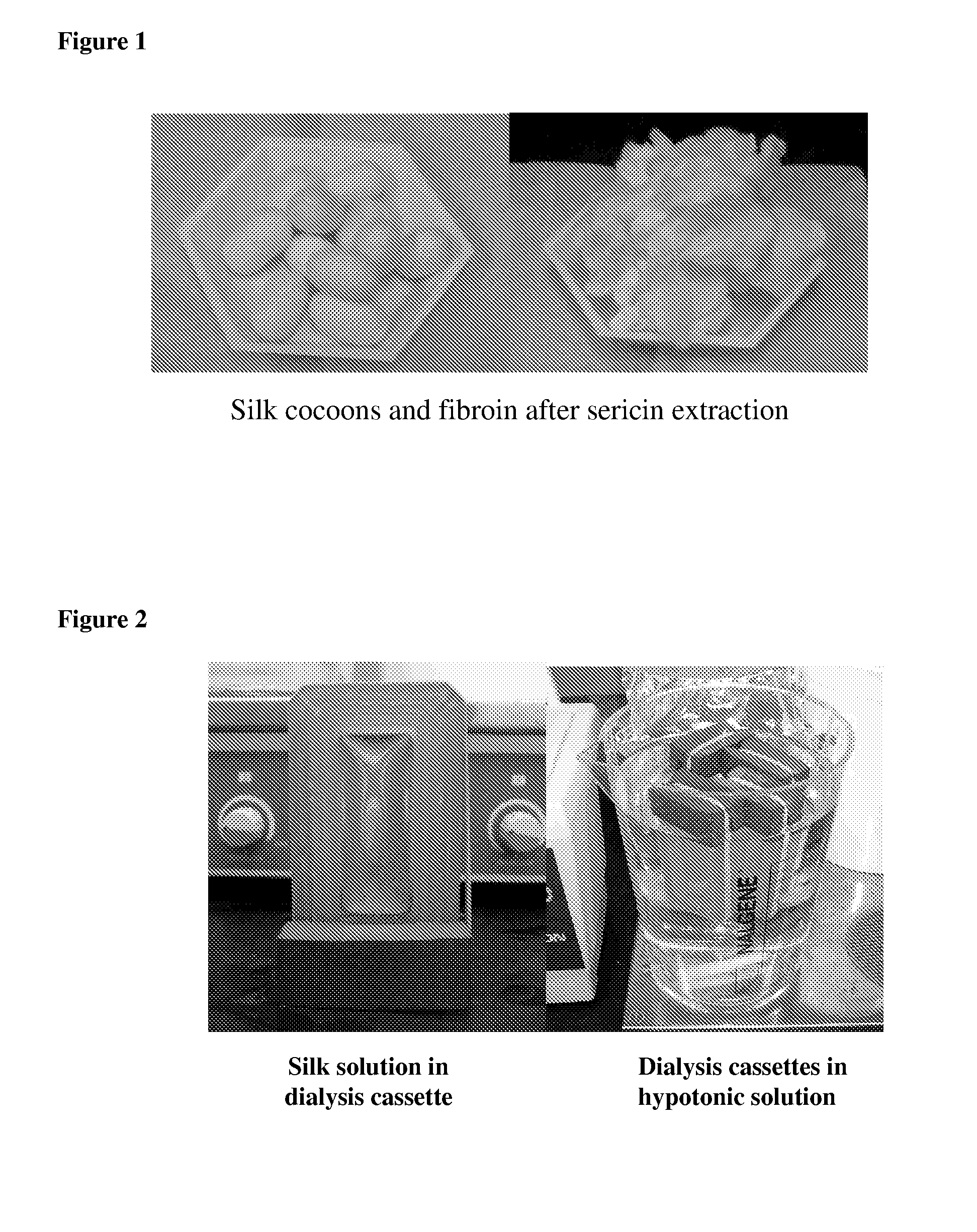
Method for preparing two-layer bicomposite collagen material for preventing post-operative adhesions
InactiveUS6596304B1Improve propertiesAvoid stickingPeptide/protein ingredientsSurgerySurgical operationPost operative
A bicomposite material based on collagen is prepared which has two closely bound layers and is biocompatible, non-toxic, hemostatic and biodegradable in less than a month, and can be used in surgery to achieve hemostasis and prevent post-surgical adhesion. To prepare the material, a solution of collagen or gelatin, which may contain glycerine and a hydrophilic additive such as polyethylene glycol or a polysaccharide, is poured onto an inert support to form a layer 30 .mu.m to less than 100 .mu.m thick. Then a polymeric porous fibrous layer is applied during gelling of the collagen or gelatin, and the resultant material is dried. The polymeric porous fibrous layer may be made of collagen or a polysaccharide, and have a density of not more than 75 mg / cm.sup.2, a pore size from 30 .mu.m to 300 .mu.m and a thickness of 0.2 cm to 1.5 cm.
Owner:IMEDEX BIOMATERIAUX CHAPONOST
Thermoplastic starch compositions incorporating a particulate filler component
InactiveUS6231970B1Reduce molecular weightAvoid hydrolysisProtein adhesivesPaper coatingParticulatesCross-link
Thermoplastic starch compositions that include a particulate filler, e.g. an inorganic filler component, and optional fibrous component The compositions include a thermoplastic phase comprising a thermoplastic starch melt that contains, at a minimum, starch blended with an appropriate plasticizing agent under conditions in order for the starch to form a thermoplastic melt. The thermoplastic phase may also include one or more additional thermoplastic polymers and other optional reactants, liquids or cross-linking agents to improve the water-resistance, strength, and / or other mechanical properties of the thermoplastic melt, particularly upon solidification. The inorganic filler component may affect the mechanical properties but will mainly be added to reduce the cost of the thermoplastic starch compositions by displacing a significant portion of the more expensive starch or starch / polymer melt. Fibers may optionally be included in order to improve the mechanical properties of the thermoplastic starch compositions. The thermoplastic starch compositions may be shaped into a wide variety of useful articles, such as sheets, films, containers, and packaging materials. Because the thermoplastic starch compositions will typically include a thermoplastic phase that is biodegradable, and because the other components will either constitute a naturally occurring mineral and optionally a natural fiber, the overall composition will typically be more environmentally friendly compared to conventional thermoplastic materials.
Owner:BIO TEC BIOLOGISCHE NATURVERPACKUNGEN
RF active compositions for use in adhesion, bonding and coating
A susceptor composition that can bond two or more layers or substrates to one another and that can be used to coat or cut a substrate. The susceptor composition is activated in the presence of radio frequency (RF) energy. In one embodiment, the susceptor composition of the present invention comprises a susceptor and a carrier. The carrier and susceptor are blended with one another and form a mixture, preferably a uniform mixture. The susceptor is present in an amount effective to allow the susceptor composition to be heated by RF energy. In a preferred embodiment, the susceptor also functions as an adhesive. The susceptor is an ionic or polar compound and acts as either a charge-carrying or an oscillating / vibrating component of the susceptor composition. The susceptor generates thermal energy in the presence of an RF electromagnetic or electrical field (hereafter RF field).
Owner:AMBRELL CORP
Medical devices with triggerable bioadhesive material
Described herein are implantable medical devices comprising a biocompatible polymer comprising a triggerable bioadhesive property that allows the device to adhere to body tissue. The triggerable bioadhesive property of the polymer can be triggered or activated by exposure to a stimulus. Also, the present invention pertains to methods of making an implantable medical device comprising a biocompatible polymer comprising a triggerable bioadhesive property that allows the device to adhere to body tissue.
Owner:BOSTON SCI SCIMED INC
Methods and compositions for improved articular surgery using collagen
InactiveUS7119062B1Alleviate patient painShorten recovery timePeptide/protein ingredientsSurgerySide effectIncisional pain
The invention provides methods for treating post-surgical articular or incisional pain and / or discomfort in a patient. The invention further provides improved surgical methods and controlled release formulations for treating an articular injury of a joint in a patient, in which a collagen formulation is used in conjunction with a surgical procedure to treat the articular injury or the side effects of the surgical procedure. Collagen formulations of the invention and their use, in addition to the surgical procedure, may provide at least one or more of the following benefits: reduced patient pain, shortened recovery time and / or improved joint condition (including treatment of the underlying articular injury). Moreover, the methods and compositions of the invention can be used in conjunction with essentially any surgical procedure used to treat an articular injury. The invention further provides compositions and methods for treating post-surgical articular or incisional pain in a patient as well as a catheter for use in the methods of the invention.
Owner:SURGICAL SPECIALTIES CORP LTD
High density fibrous polymers suitable for implant
InactiveUS6974862B2Easy to shapeEvenly dispersedFinger jointsInternal osteosythesisFiberHigh density
This invention includes malleable, biodegradable, fibrous compositions for application to a tissue site in order to promote or facilitate new tissue growth. One aspect of this invention is a fibrous component (e.g., collagen, chitosan, alginate, hyaluronic acid, poly-lactic acid, poly-capralactone, and polyurethane) that provides unique mechanical and physical properties. The invention may be created by providing a vessel containing a slurry, said slurry comprising a plurality of natural or synthetic polymer fibers and at least one suspension fluid, wherein the polymer fibers are substantially evenly dispersed and randomly oriented throughout the volume of the suspension fluid; applying a force, e.g., centrifugal, to said vessel containing said slurry, whereupon said force serves to cause said polymer fibers to migrate through the suspension fluid and amass at a furthest extent of the vessel, forming a polymer material, with said polymer material comprising polymer fibers of sufficient length and sufficiently viscous, interlaced, or interlocked to retard dissociation of said polymer fibers.
Owner:DSM IP ASSETS BV
Microstructure synthesis by flow lithography and polymerization
In a method for synthesizing polymeric microstructures, a monomer stream is flowed, at a selected flow rate, through a fluidic channel. At least one shaped pulse of illumination is projected to the monomer stream, defining in the monomer stream a shape of at least one microstructure corresponding to the illumination pulse shape while polymerizing that microstructure shape in the monomer stream by the illumination pulse.
Owner:MASSACHUSETTS INST OF TECH
Sealants for Skin and Other Tissues
InactiveUS20070225631A1Prevent and reduce and eliminate flow of fluidSurgical adhesivesPeptide/protein ingredientsSealantTissue skin
The present invention relates to sealants for skin and other tissues. The sealants include an electroprocessed material. The sealants may contain more than one electroprocessed materials and may contain additional substances. The invention further relates to methods of making and using such sealants.
Owner:VIRGINIA COMMONWEALTH UNIV INTPROP FOUND INC +1
Electrospun blends of natural and synthetic polymer fibers as tissue engineering scaffolds
InactiveUS20060263417A1Facilitate cell penetrationFacilitate proliferationBiocidePeptide/protein ingredientsFiberPolymer science
Non-woven fibrous scaffolds made by electrospinning from the synthetic biodegradable polymer such as, for example, poly(lactic-co-glycolic acid) (PLGA) and natural proteins, such as, for example, gelatin (denatured collagen) and elastin and a method of making thereof.
Owner:DREXEL UNIV
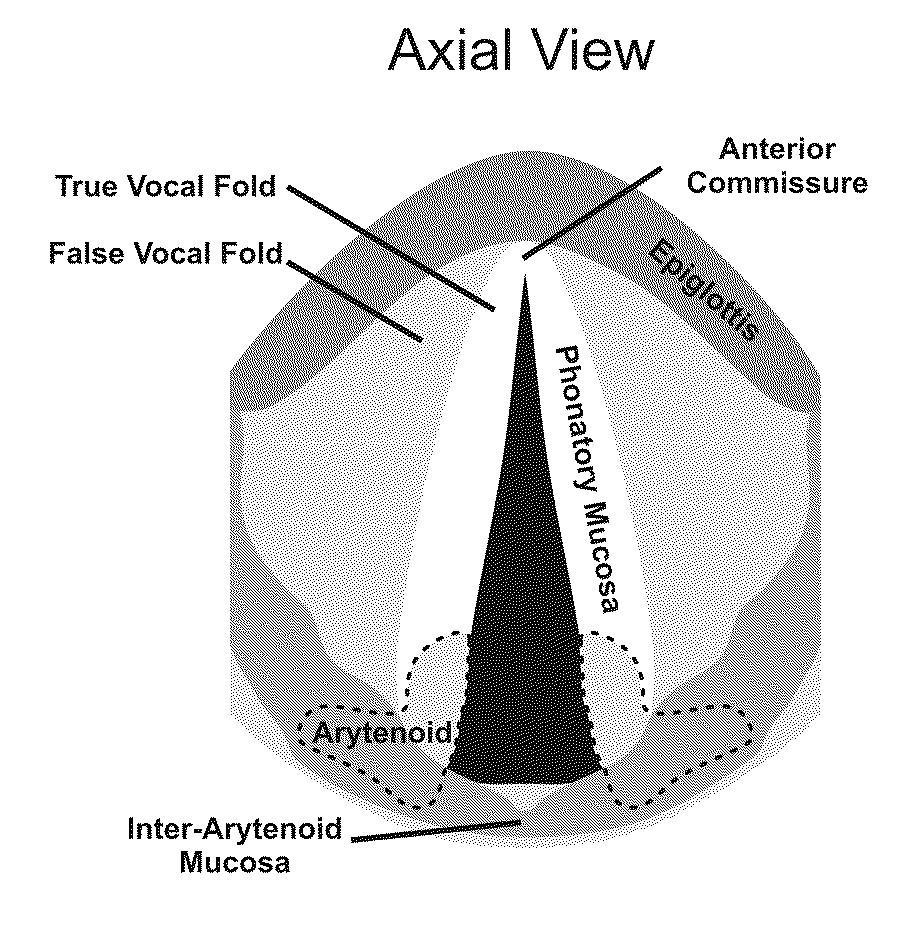
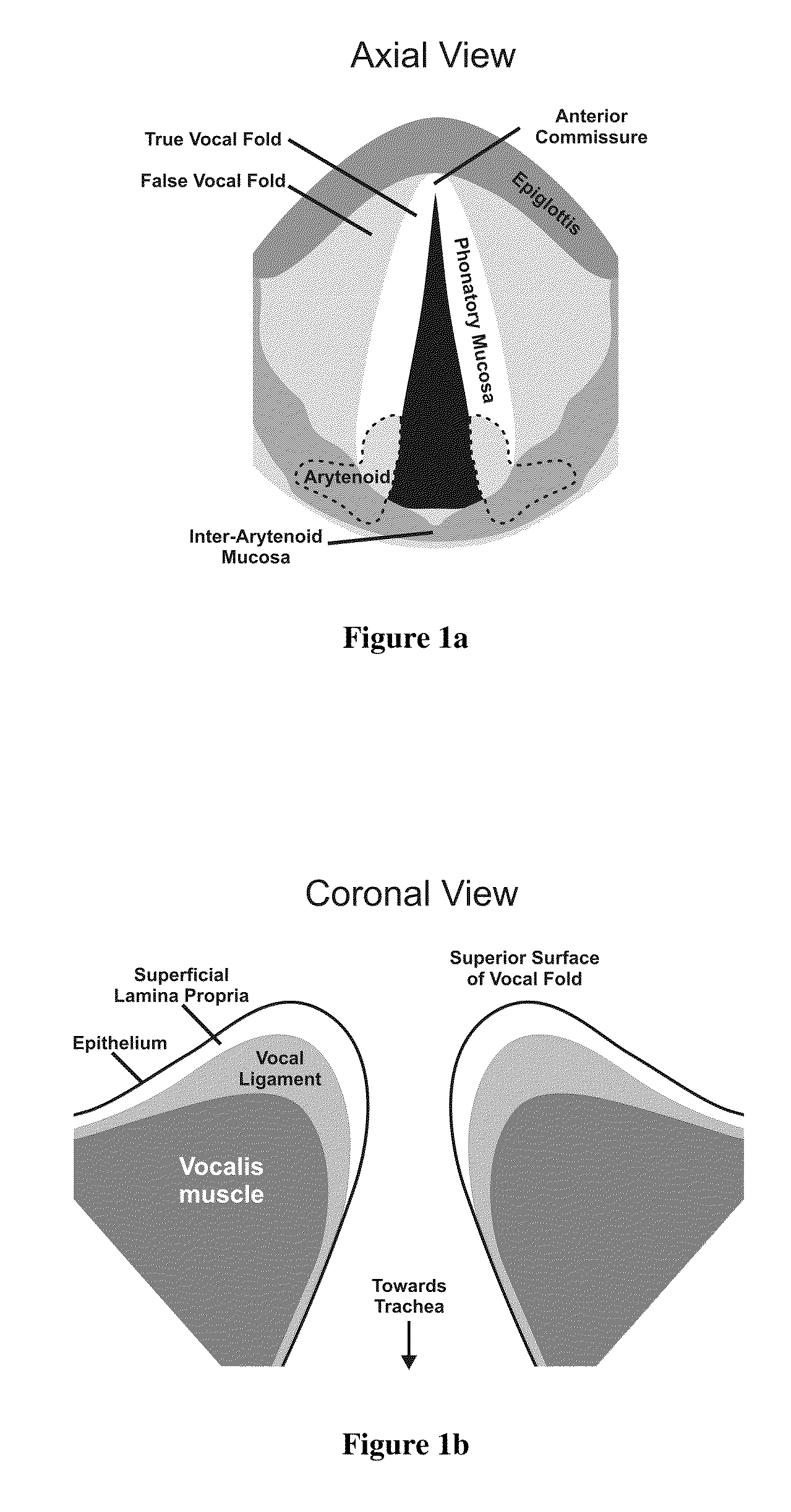
Hydrogels for vocal cord and soft tissue augmentation and repair
ActiveUS20100055184A1Repairing pliabilityDiminished functional vibratory capacityBiocideOrganic active ingredientsEpitheliumBreast implant
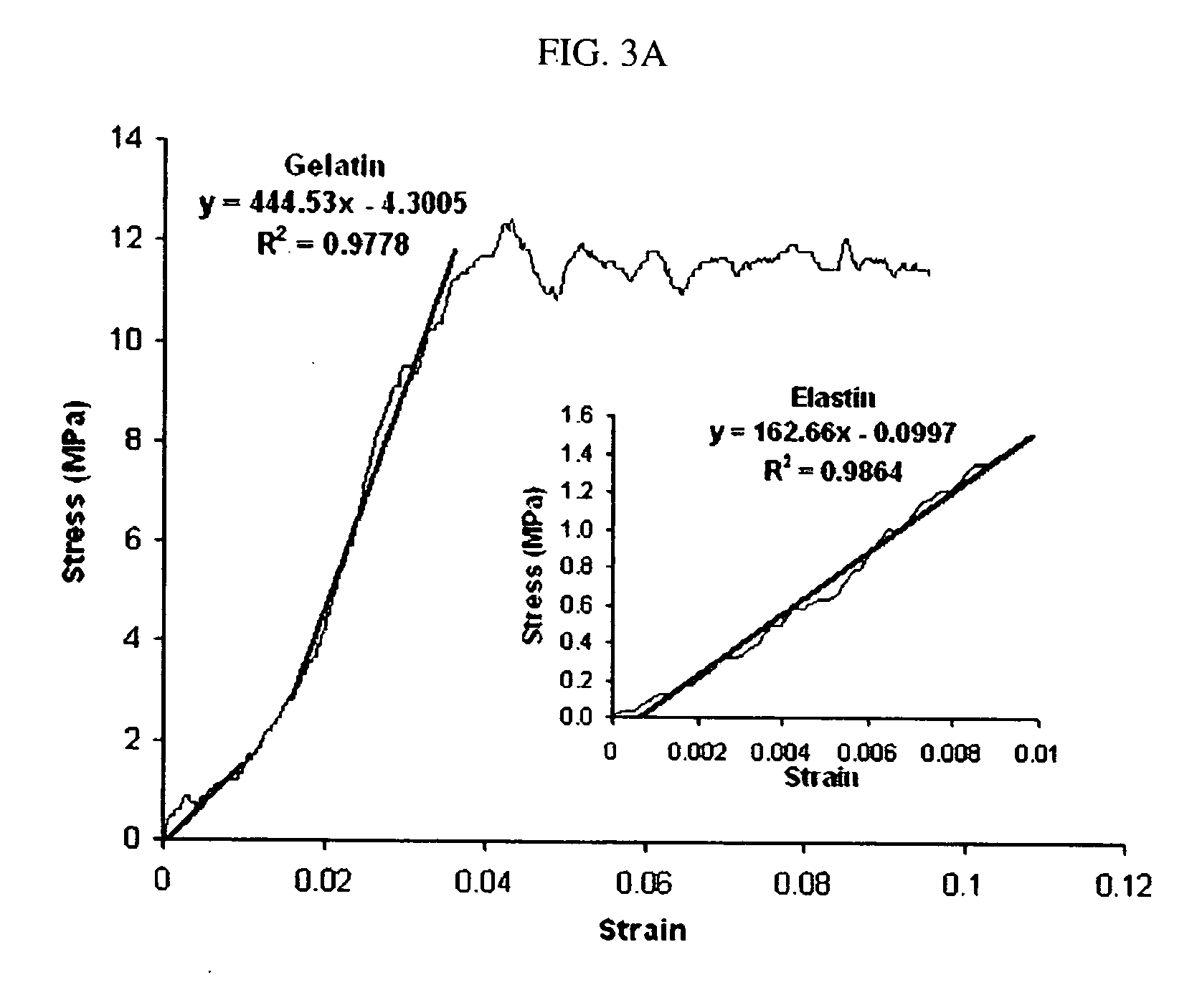
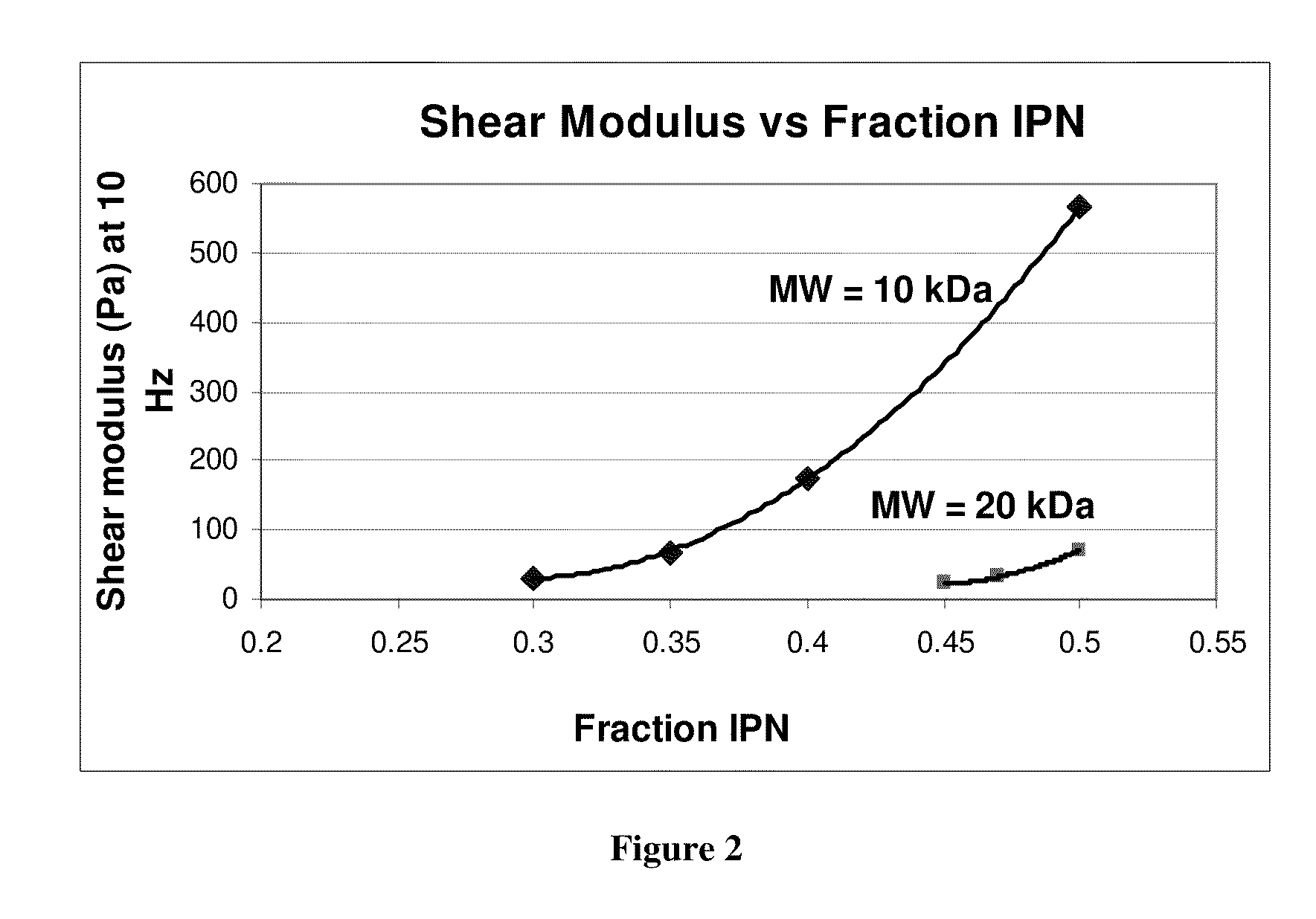
The present invention provides hydrogels and compositions thereof for vocal cord repair or augmentation, as well as other soft tissue repair or augmentation (e.g., bladder neck augmentation, dermal fillers, breast implants, intervertebral disks, muscle-mass). The hydrogels or compositions thereof are injected into the superficial lamina propria or phonatory epithelium to restore the phonatory mucosa of the vocal cords, thereby restoring a patient's voice. In particular, it has been discovered that hydrogels with an elastic shear modulus of approximately 25 Pa are useful in restoring the pliability of the phonatory mucosa. The invention also provides methods of preparing and using the inventive hydrogels.
Owner:MASSACHUSETTS INST OF TECH
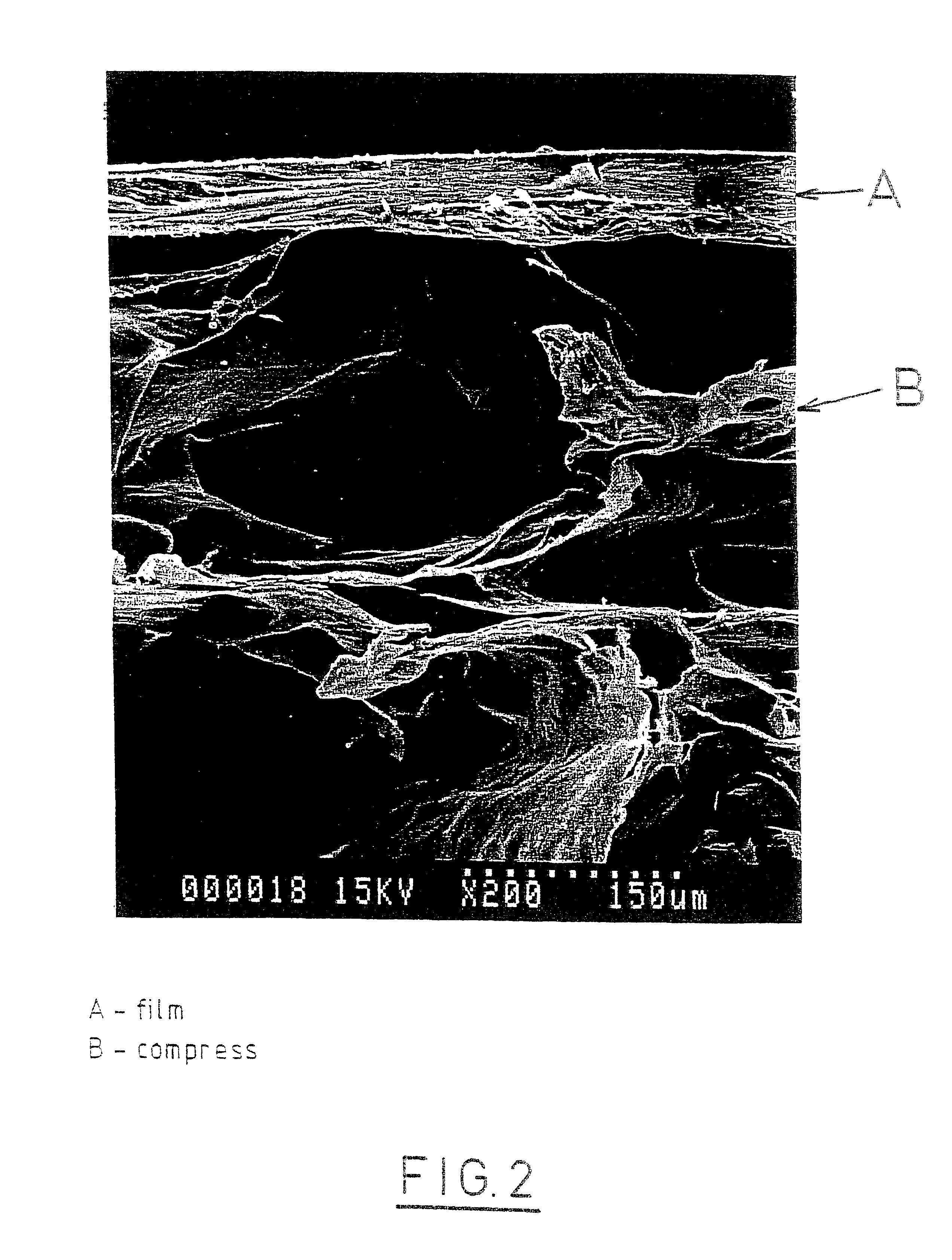
Processes for the preparation of novel collagen-based supports for tissue engineering, and biomaterials obtained
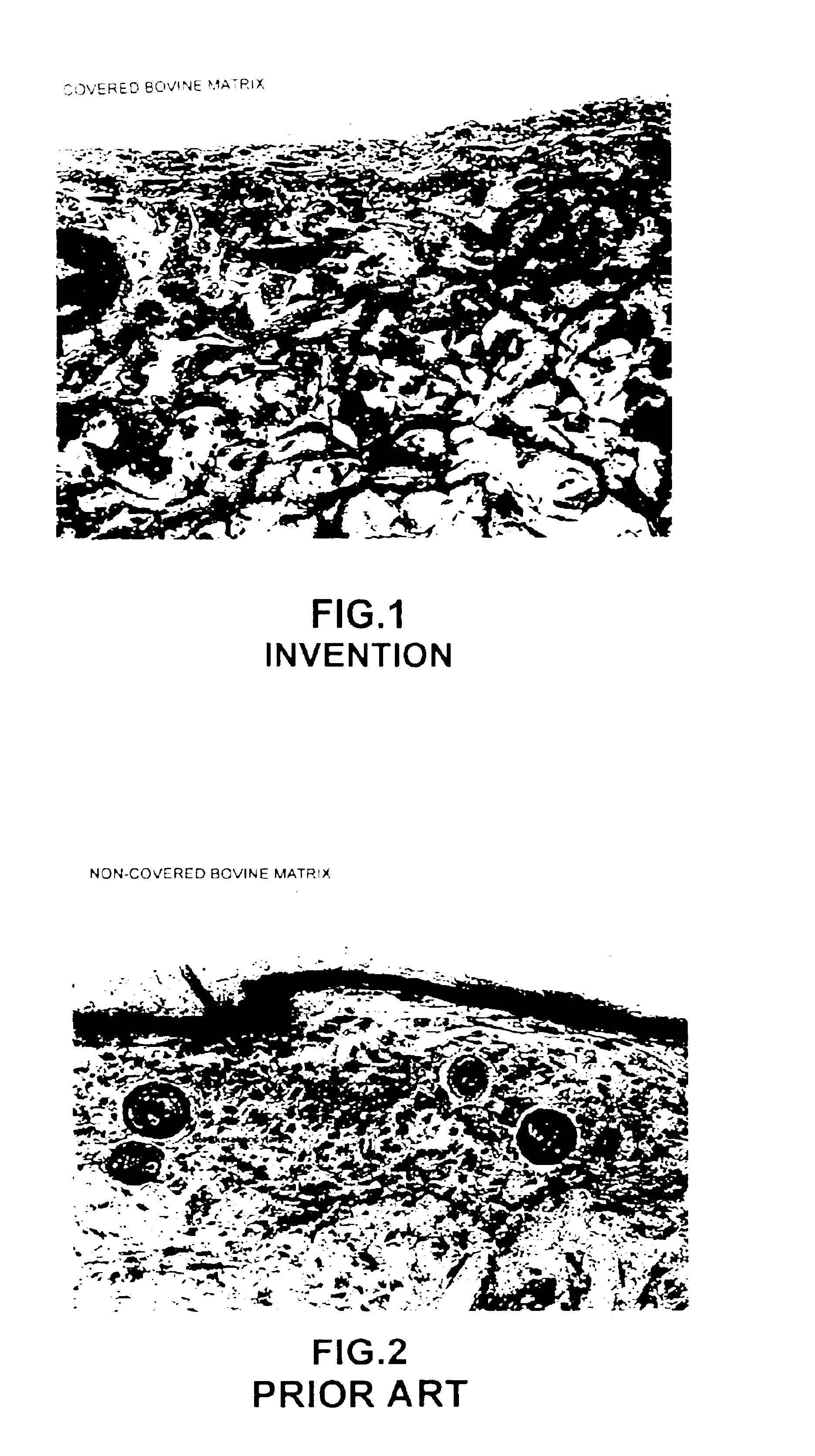
A composite product is disclosed as a collagen support comprising at least one porous collagen layer covered on at least one side with an essentially compact collagen membrane consisting either of a collagen film prepared by drying a collagen gel, preferably in air or a gaseous fluid, or of a very highly compressed collagen sponge. At least one of the two layers, i.e. the porous layer and the essentially compact membrane, may comprise normal, genetically modified or malignant living cells originating particularly from young or elderly subjects. This composite product is used as a collagen support for the manufacture of artificial skin intended especially for performing in vitro tests on the efficacy of potentially active substances or for reconstructing damaged areas of skin in vivo.
Owner:BASF BEAUTY CARE SOLUTIONS FRANCE SAS
Methods for dispersing fibers within aqueous compositions
InactiveUS6379446B1Quantity maximizationMinimize interstitial spaceNon-fibrous pulp additionProtein adhesivesFiberSolid component
Compositions and methods in which dry-committed fibers are substantially homogeneously dispersed throughout a fibrous composition. The fibrous composition is characterized as having sufficient yield stress and viscosity such that the shearing forces from the mixing apparatus are effectively transferred down to the fiber level. This is accomplished by means of an appropriate thickening agent, e.g, gelatinized starch. The dry-committed fibers are exemplified by flash dry fibers or fibrous sheets that have been cut or torn into fragments less than 2 cm across. Providing fibers that have been dry-committed greatly reduces the time that it takes to obtain substantially homogeneous dispersion of the fibers throughout the fibrous composition. This, in turn, reduces the risk of mixture spoilage and mechanical or chemical damage to the solid components within the fibrous composition.
Owner:E KHASHOGGI INDS
Compositions and methods for soft tissue augmentation
The present invention provides compositions comprising isolated human collagen, isolated human elastin and a pharmaceutically acceptable carrier wherein the human elastin is substantially insoluble in water with a molecular weight greater than 100 kDa. The present invention further provides methods and kits for soft tissue augmentation.
Owner:HUMACYTE INC
Osteogenic devices and methods of use thereof for repair of endochondral bone and osteochondral defects
InactiveUS7041641B2Avoid undesirable formationOvercome problemsPowder deliveryImpression capsRepair tissueNon union
Disclosed herein are improved osteogenic devices and methods of use thereof for repair of bone and cartilage defects. The devices and methods promote accelerated formation of repair tissue with enhanced stability using less osteogenic protein than devices in the art. Defects susceptible to repair with the instant invention include, but are not limited to: critical size defects, non-critical size defects, non-union fractures, fractures, osteochondral defects, subchondral defects, and defects resulting from degenerative diseases such as osteochondritis dessicans.
Owner:MARIEL THERAPEUTICS
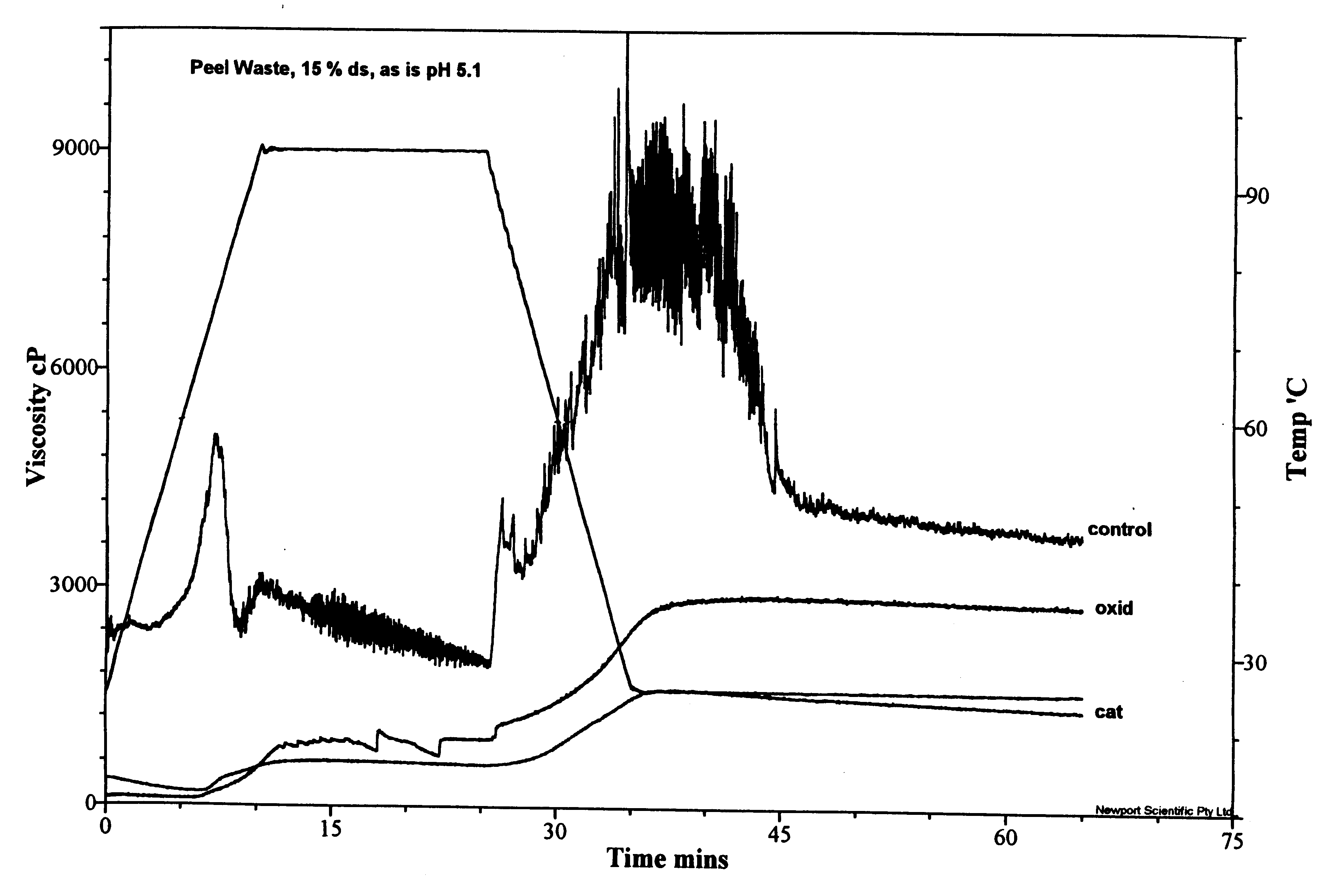
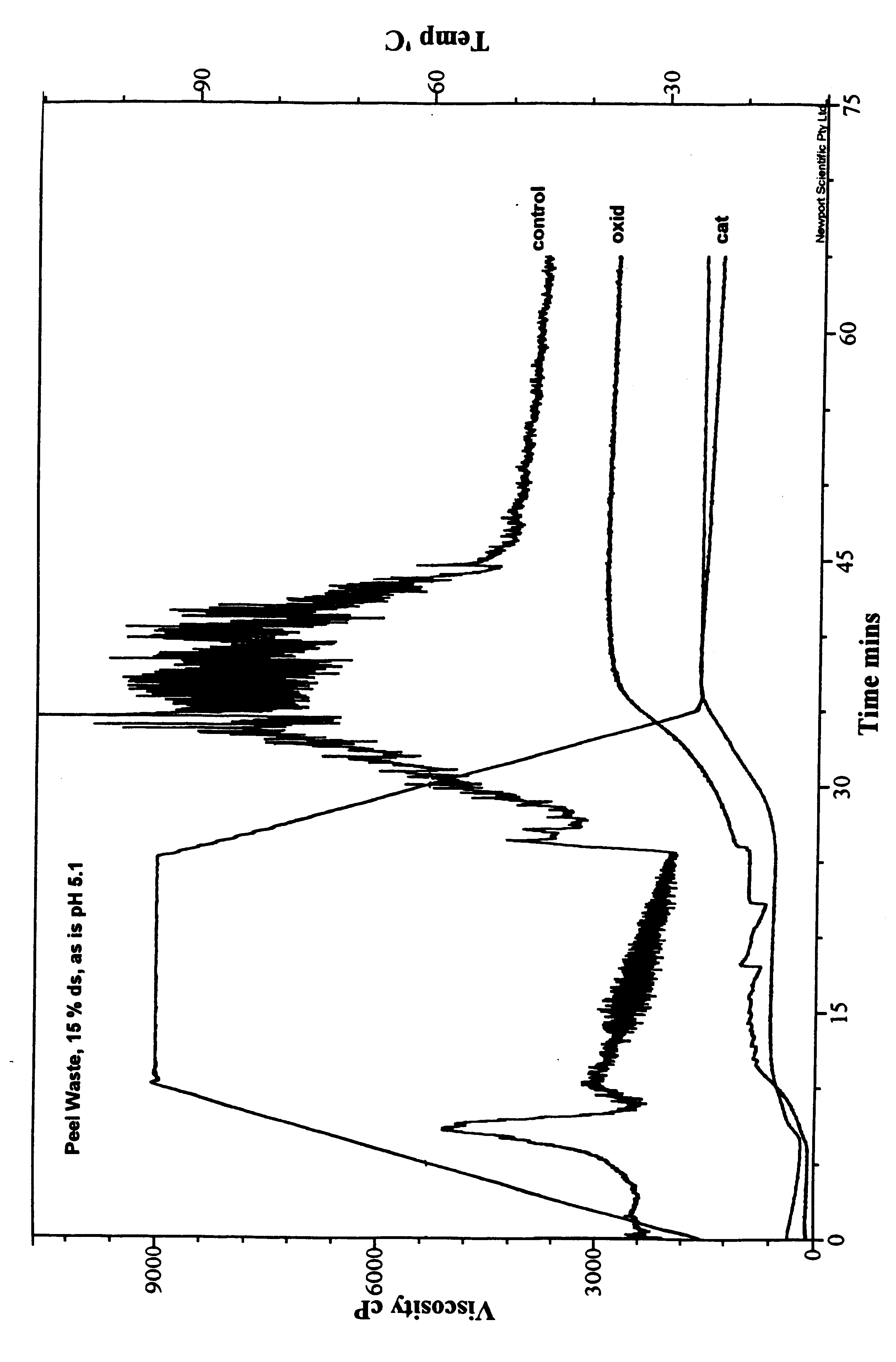
Packaging and structural materials comprising potato peel waste
InactiveUS6440204B1Inhibit migrationImprove stabilityProtein waste adhesivesLayered productsFiberAdhesive
Owner:HARRIS TRUST & SAVINGS BANK AS ADM AGENT +1
Wood adhesive and method of preparing thereof
InactiveUS20100258033A1Drying rate of adhesiveLow pour pointProtein waste adhesivesStarch adhesivesHigh densityAdhesive
A wood adhesive having a) 100 weight parts of water; b) between 3 and 45 weight parts of a proteinaceous material; c) between 0.01 and 15 weight parts of an acidity regulator; d) between 0.01 and 15 weight parts of an aromatic compound; e) between 0.01 and 15 weight parts of a curing agent; f) between 0.01 and 15 weight parts of a preservative; g) between 0 and 15 weight parts of a viscosity modifier; h) between 0 and 10 weight parts of a filler; and i) between 0 and 15 weight parts of a drier. The wood adhesive can be used for preparation of plywood, blockboard, oriented strand board (OSB), flakeboard, fiberboard, veneer plywood, middle density fibreboard, high density fibreboard, hardboard, flooring substrate, LVL, and so on. A method for preparing the wood adhesive is also provided.
Owner:YANG GUANG +1
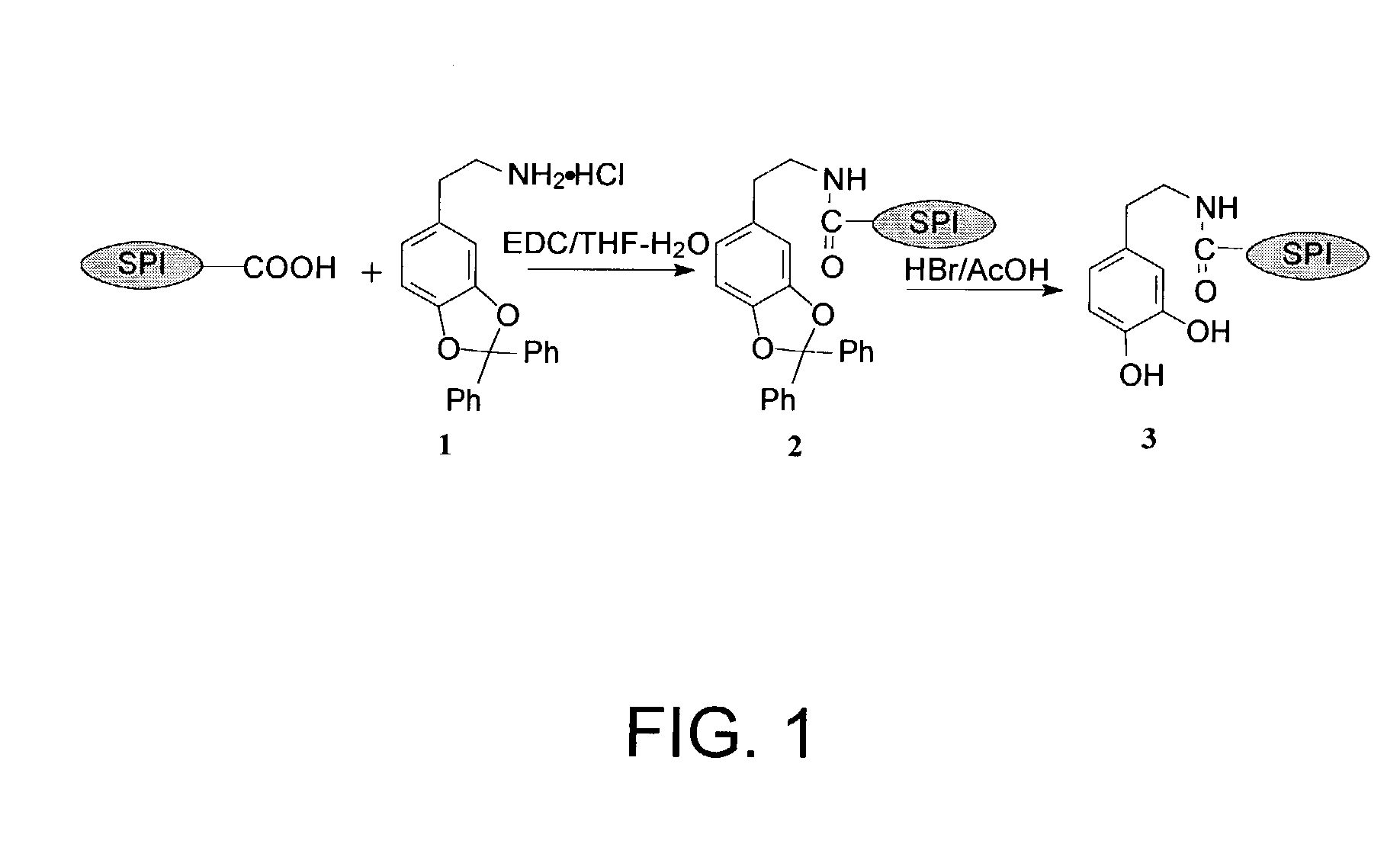
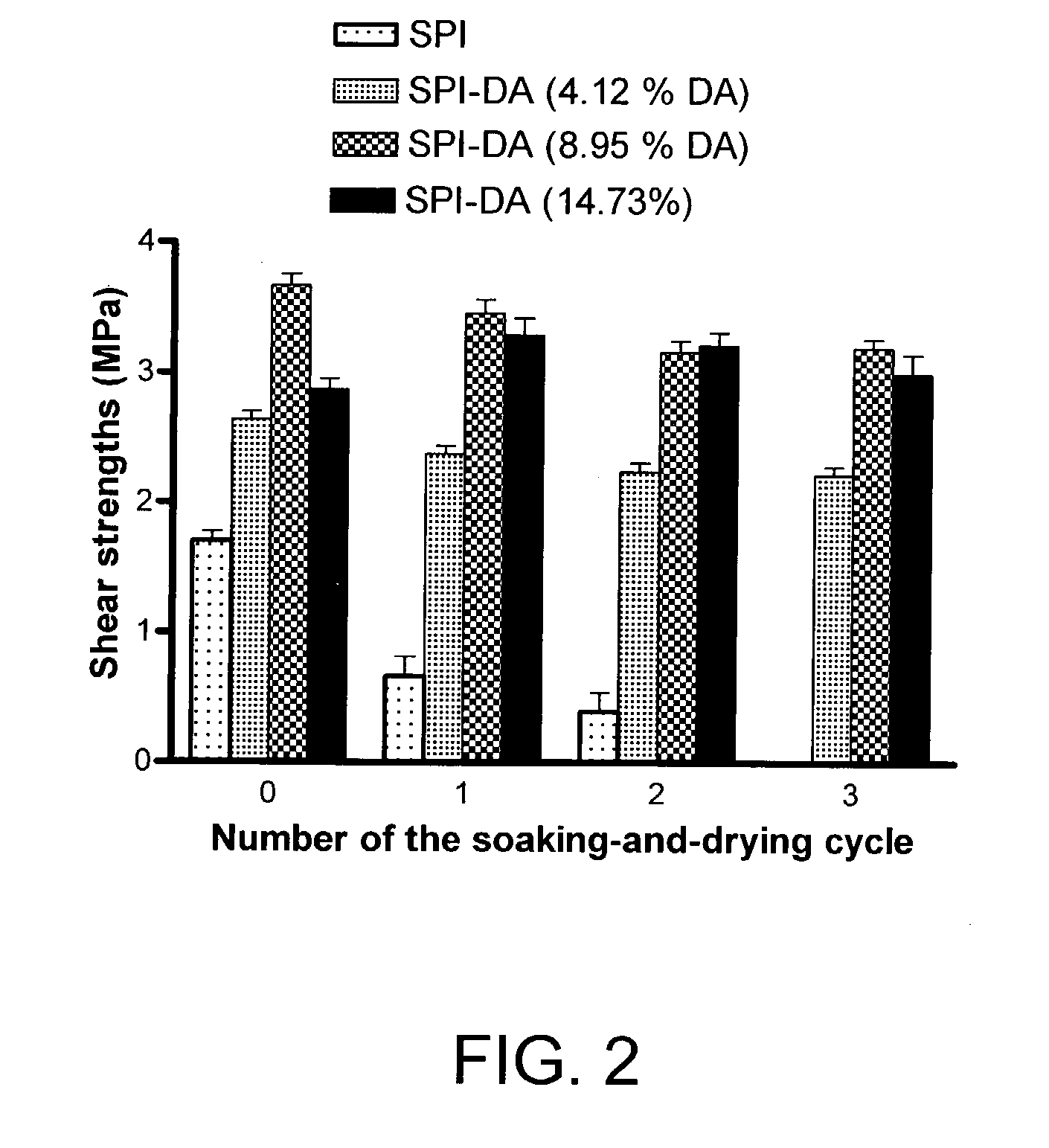
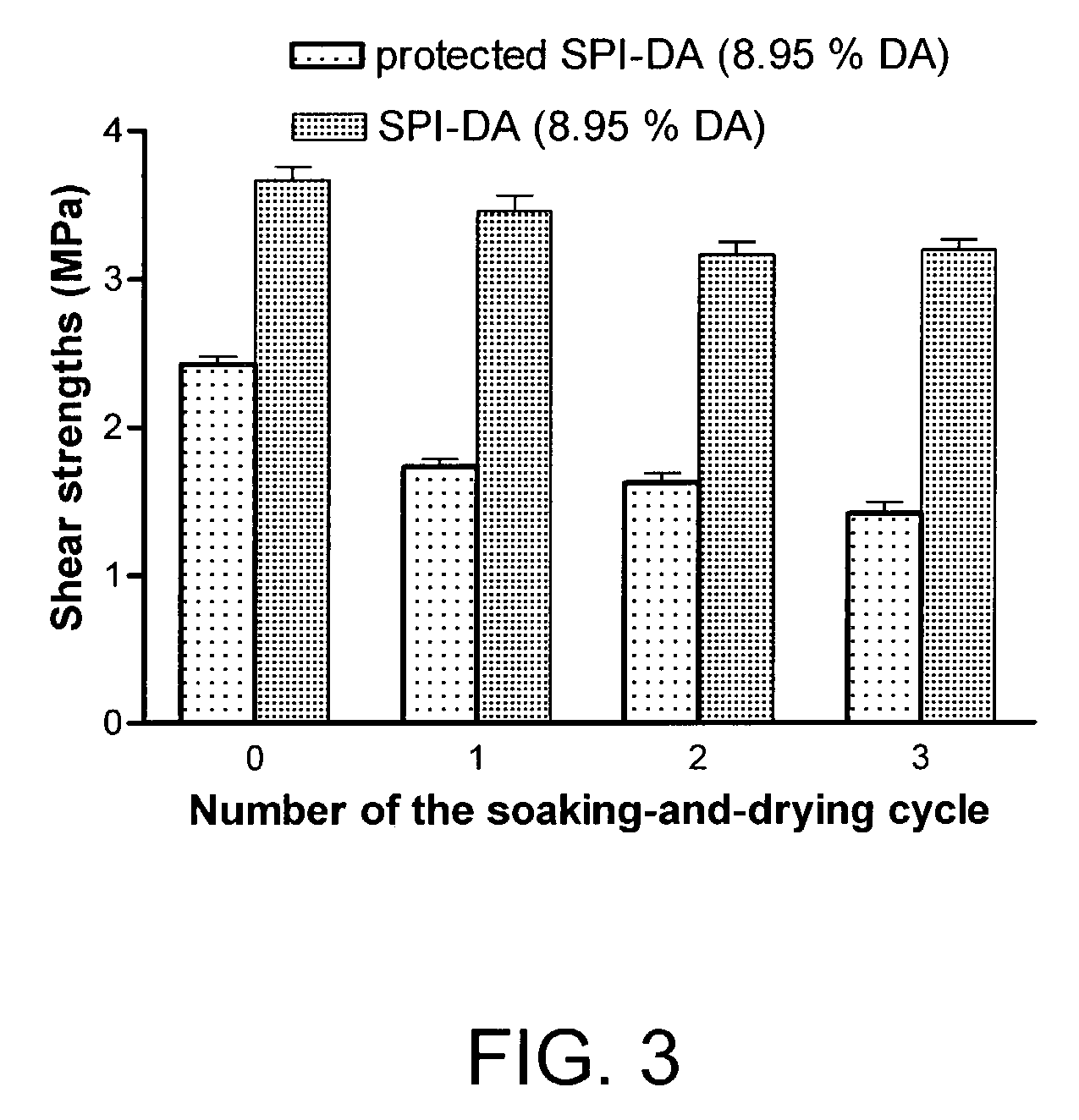
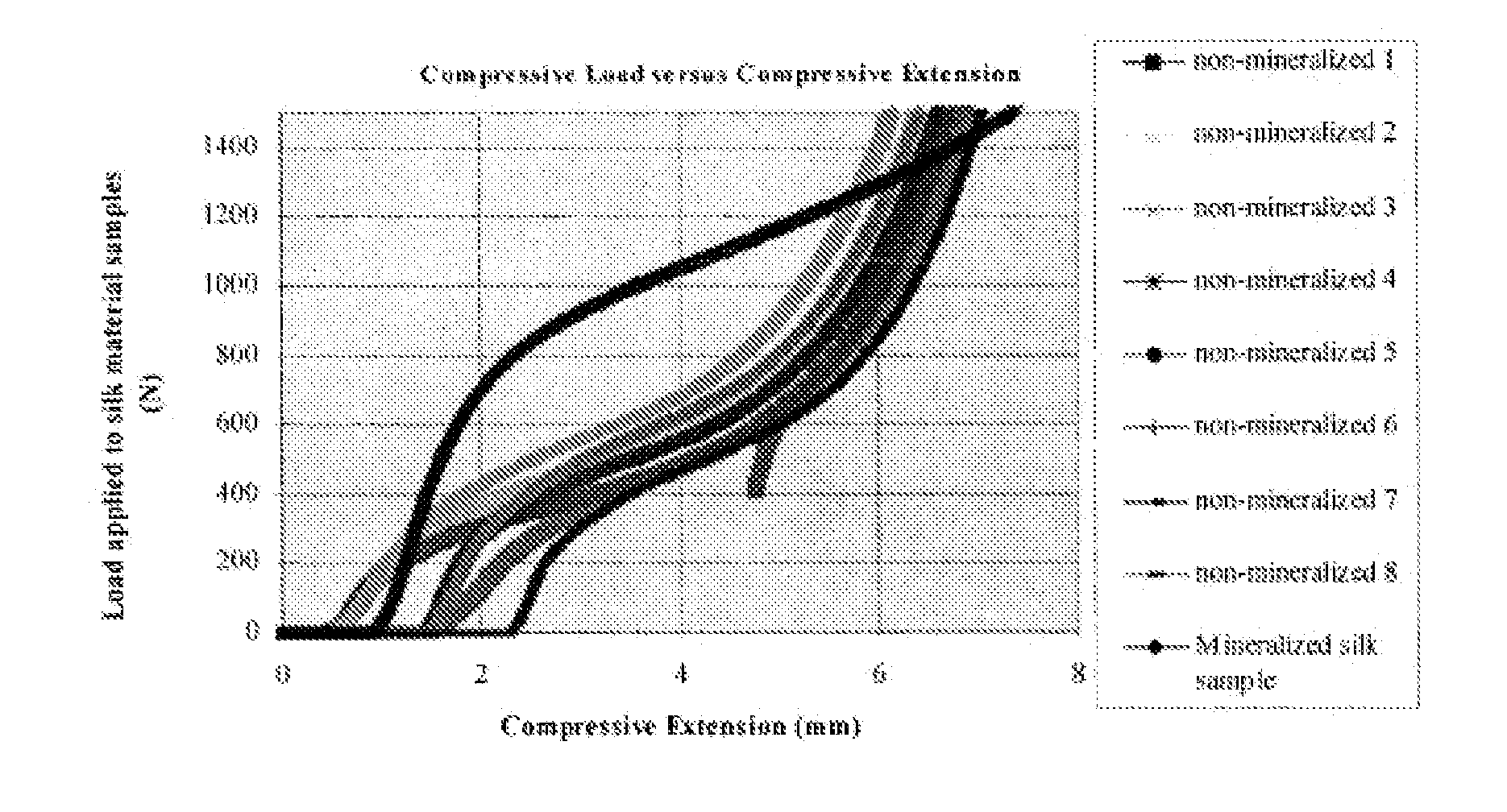
Fibrous Protein Fusions and Use Thereof in the Formation of Advanced Organic/Inorganic Composite Materials
The claimed invention provides a fusion polypeptide comprising a fibrous protein domain and a mineralization domain. The fusion is used to form an organic-inorganic composite. These organic-inorganic composites can be constructed from the nano- to the macro-scale depending on the size of the fibrous protein fusion domain used. In one embodiment, the composites can also be loaded with other compounds (e.g., dyes, drugs, enzymes) depending on the goal for the materials, to further enhance function. This can be achieved during assembly of the material or during the mineralization step in materials formation.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV +1
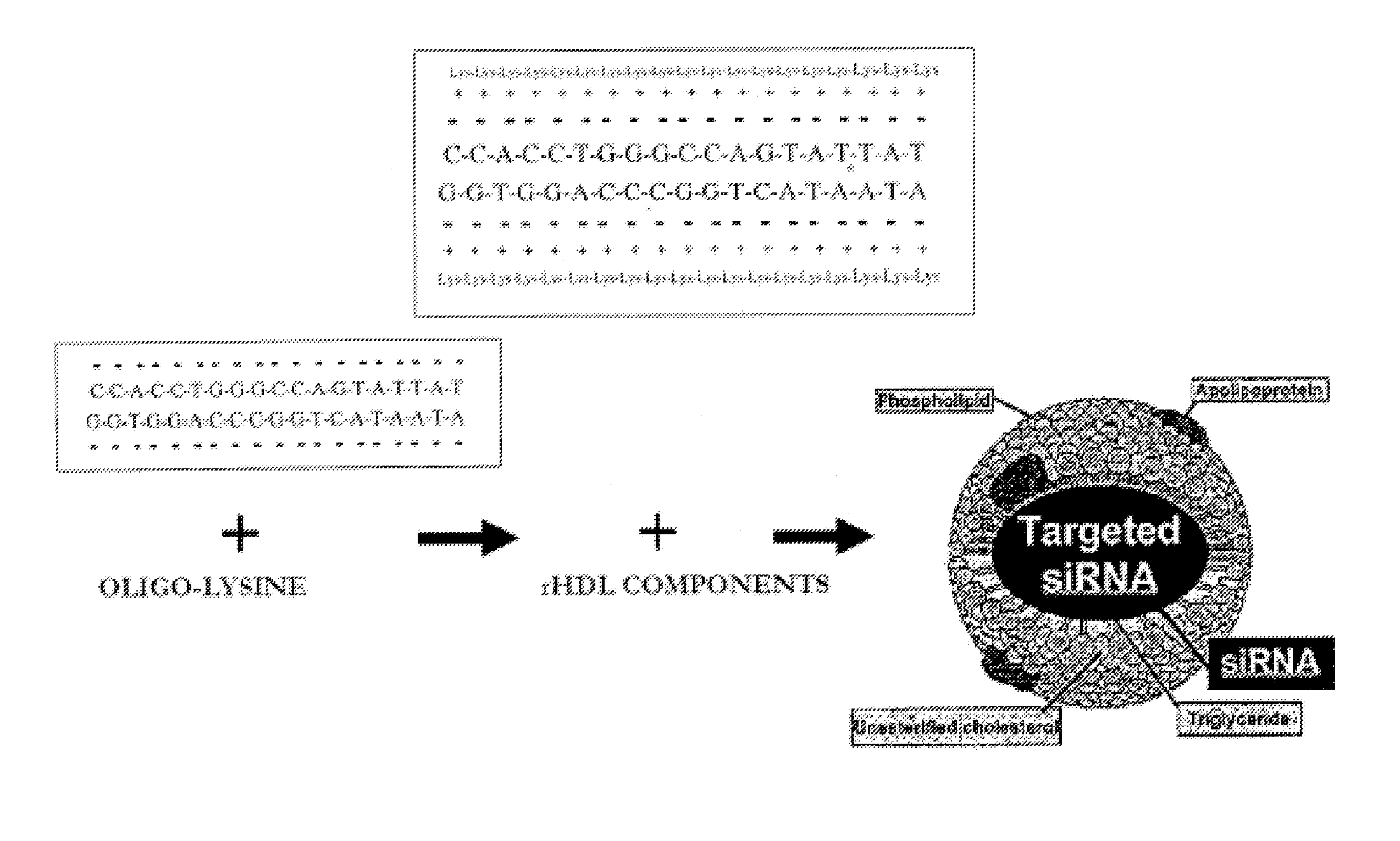
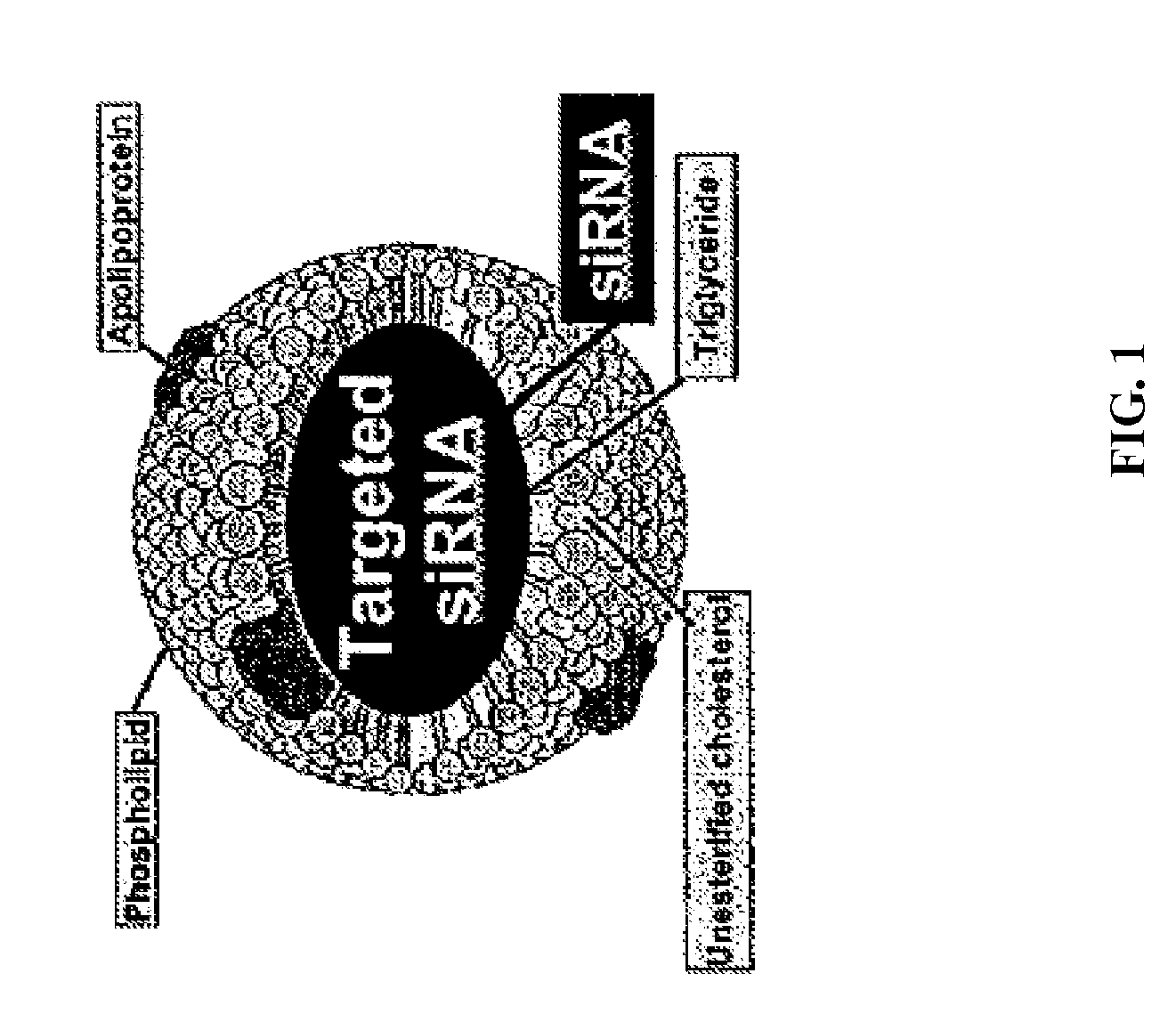
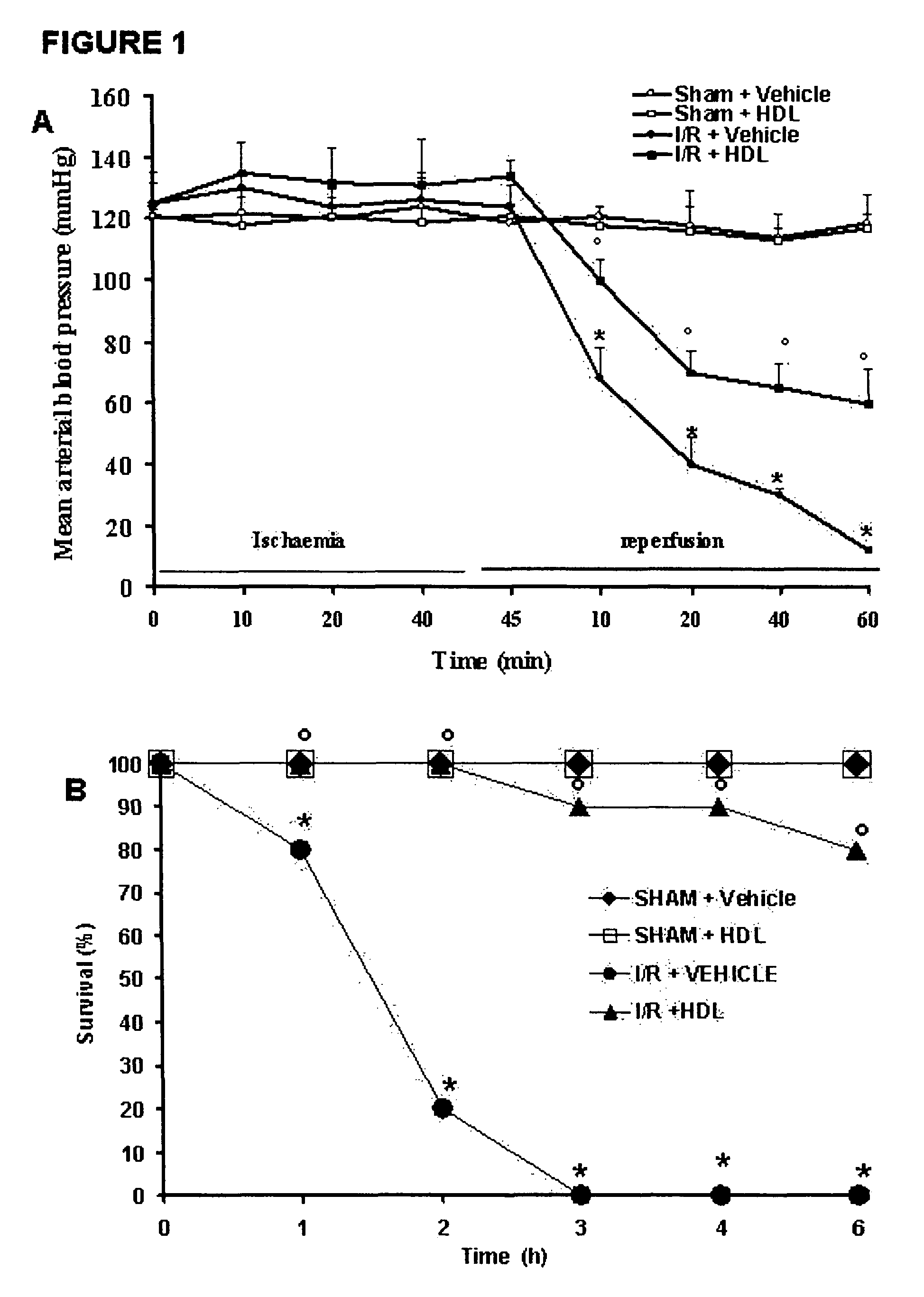
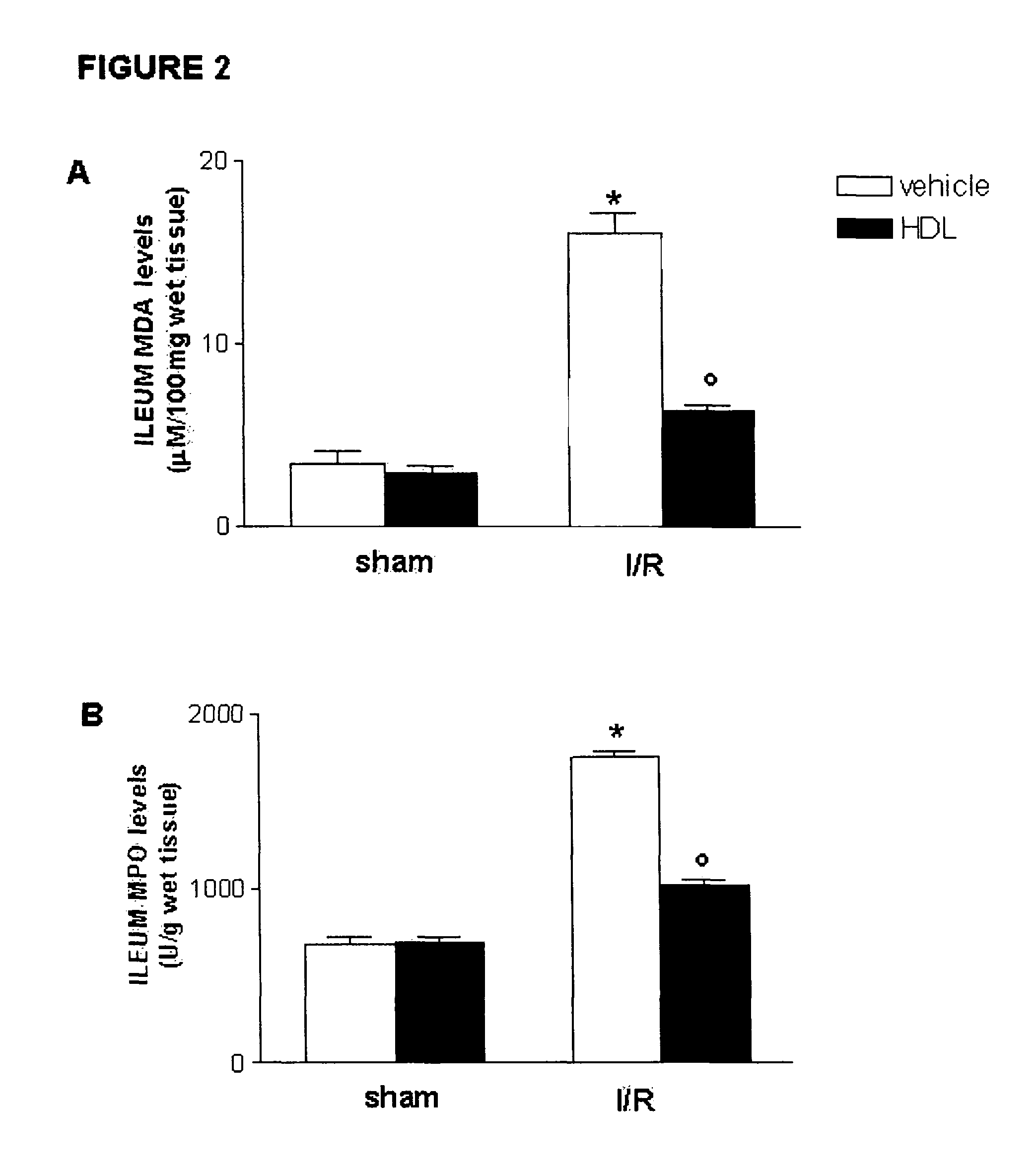
HDL particles for delivery of nucleic acids
InactiveUS8734853B2Efficient deliveryHigh densityPowder deliveryOrganic active ingredientsApolipoproteins EHDL particle
Disclosed are high density lipoprotein-nucleic acid particles, wherein the particles include (a) an apolipoprotein; (b) a nucleic acid component comprising a therapeutic nucleic acid segment; and (c) a polypeptide comprising a positively charged region, wherein the positively-charged region of the polypeptide associates with the nucleic acid component. Also disclosed are pharmaceutical compositions that include a) an apolipoprotein; (b) a nucleic acid component comprising a therapeutic nucleic acid segment; and (c) a polypeptide comprising a positively charged region. Methods that concern the particles and pharmaceutical compositions of the present invention are also set forth, as well as kits.
Owner:UNIV OF NORTH TEXAS HEALTH SCI CENT +1
Pressure Sensitive Adhesive Composition
A pressure sensitive adhesive composition suitable for medical purposes comprising a rubbery elastomeric base and one or more water soluble or water swellable hydrocolloids, the adhesive composition comprising a substantially homogeneous mixture of 35-50% of one or more polybutenes, 5-20% of one or more styrene copolymers, and 20-60% of one or more hydrocolloids has very good properties as an adhesive for ostomy appliances.
Owner:COLOPLAST AS
Collagen device and method of preparing the same
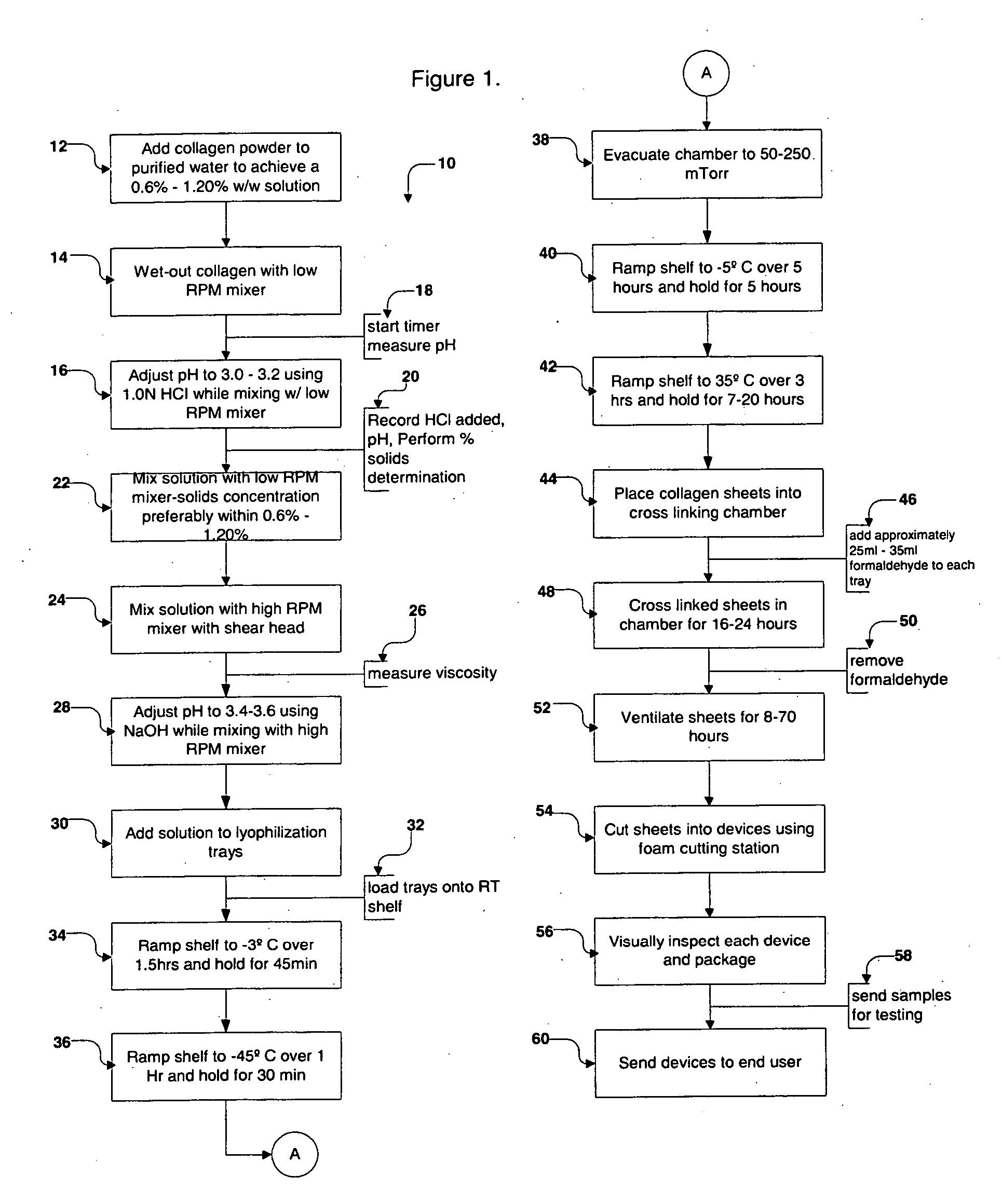
InactiveUS20050175659A1Easy to operateEasy to handlePeptide/protein ingredientsSurgeryBody tissuePurified water
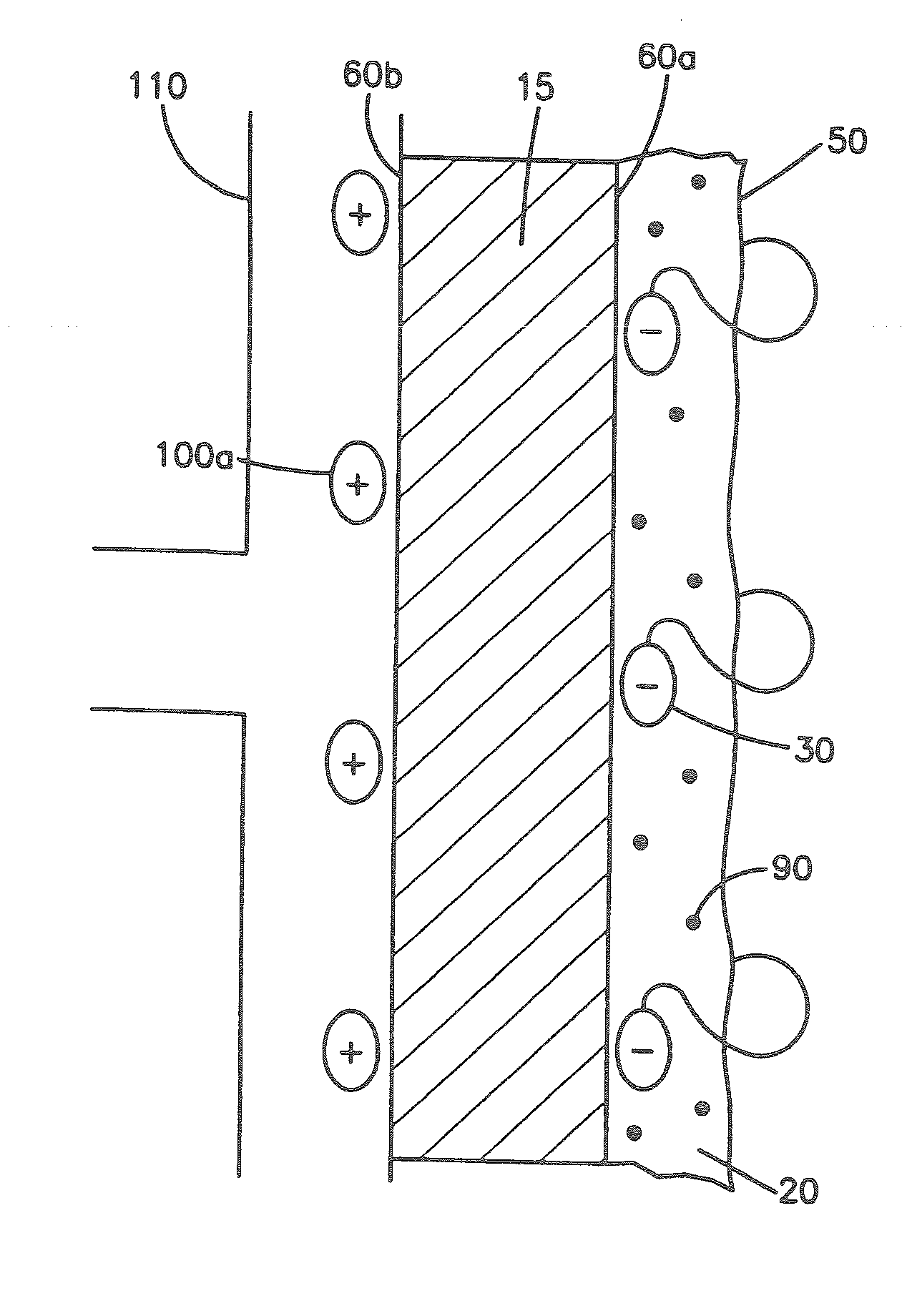
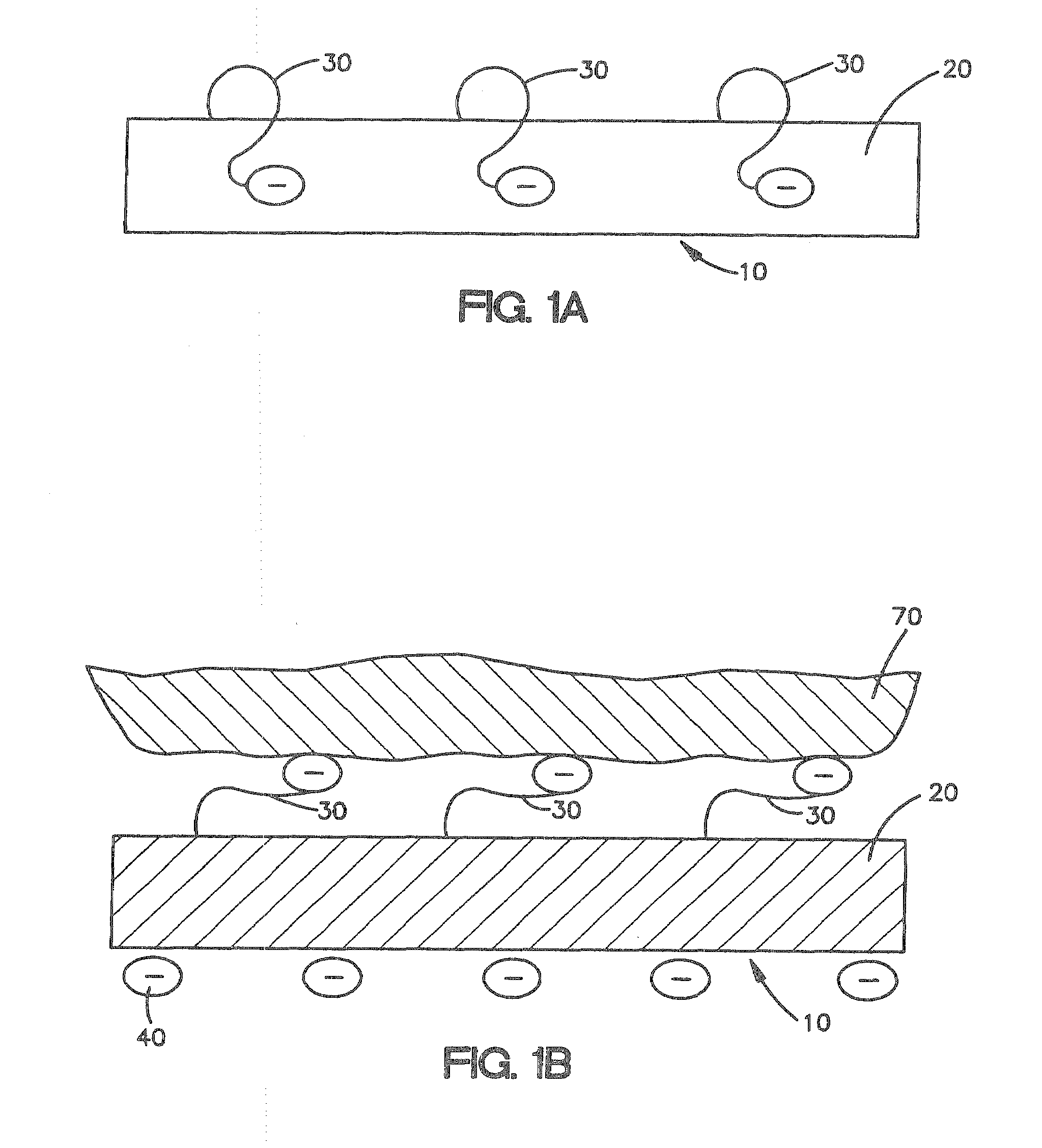
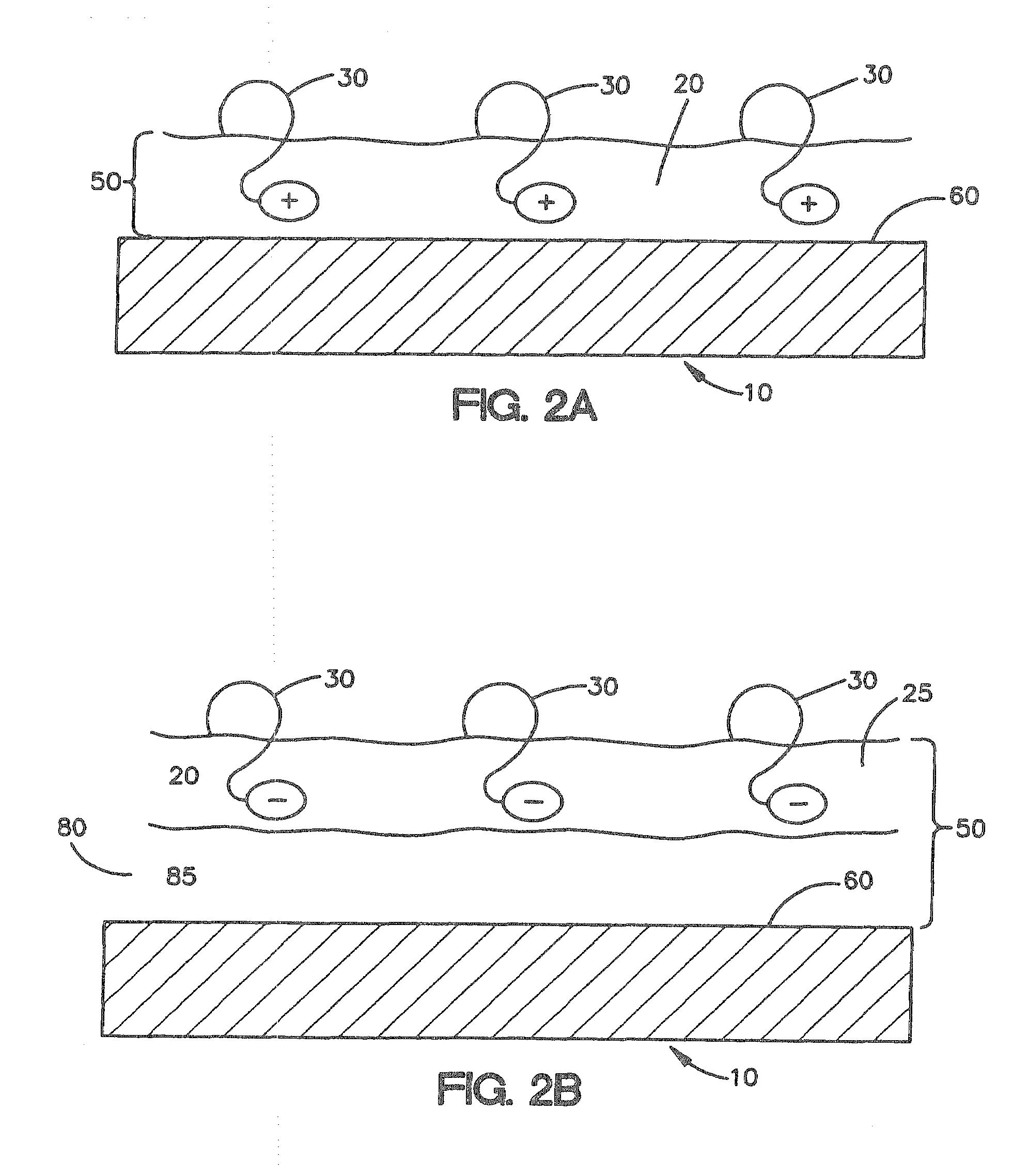
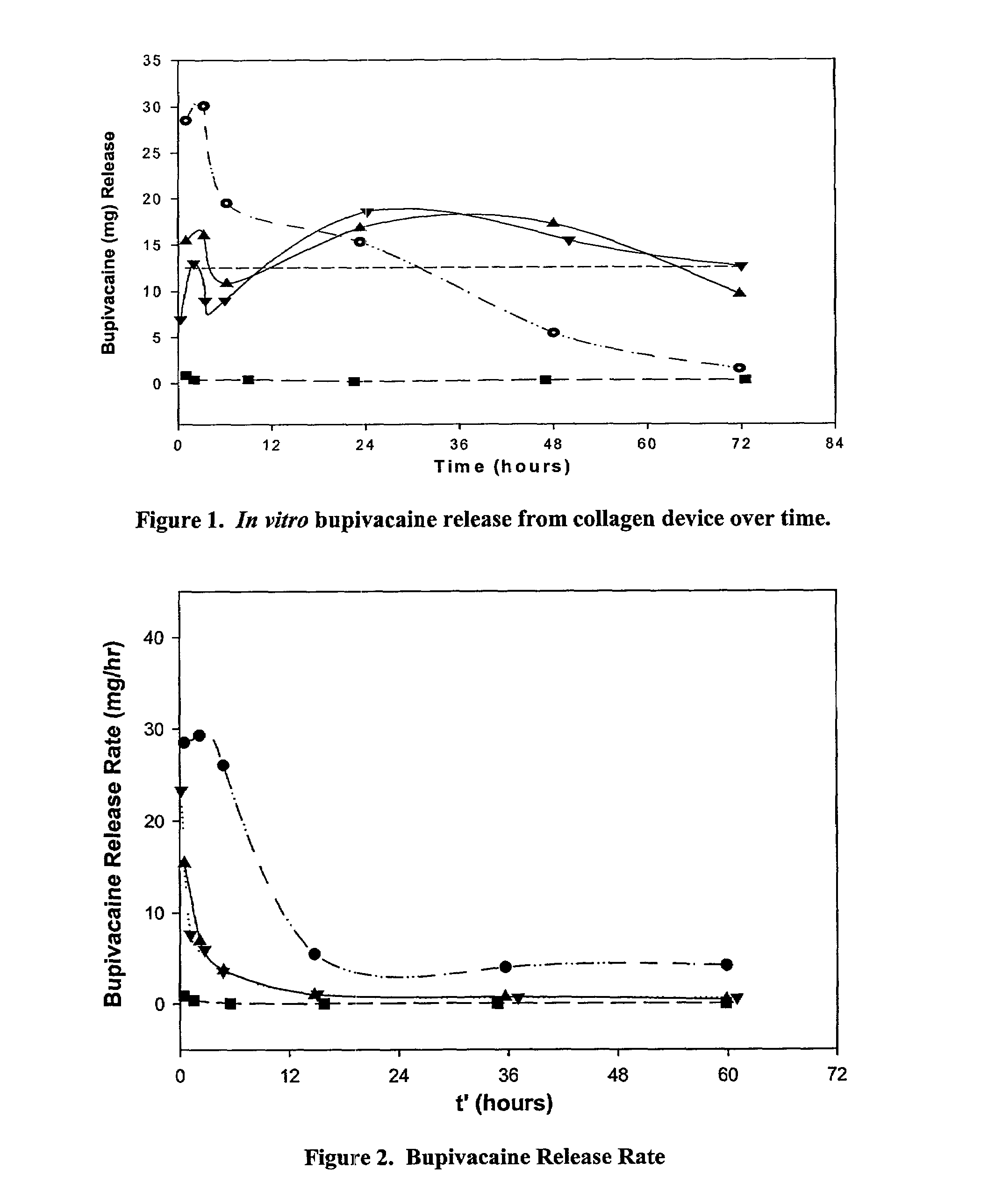
A collagen device is prepared by mixing collagen with purified water for a period of time sufficient to form a mixture. The pH of the mixture is adjusted to a pH level sufficient to substantially solubilize the collagen. A first predetermined amount of the mixture is placed into a container. The mixture is subject to a lyophilizing process and formed into a collagen device. The collagen device is also cross-linked. The collagen device has a plurality of pores wherein a majority of the pores have a diameter of less than 10 μm. To use the collagen device as an implant to replace, reinforce or strengthen bodily tissue, or to act as an adhesion barrier, the collagen device is placed in contact with bodily tissue and that contact is maintained until the collagen device is substantially resorbed within the bodily tissue.
Owner:CODMAN & SHURTLEFF INC
Microstructure synthesis by flow lithography and polymerization
In a method for synthesizing polymeric microstructures, a monomer stream is flowed, at a selected flow rate, through a fluidic channel. At least one shaped pulse of illumination is projected to the monomer stream, defining in the monomer stream a shape of at least one microstructure corresponding to the illumination pulse shape while polymerizing that microstructure shape in the monomer stream by the illumination pulse.
Owner:MASSACHUSETTS INST OF TECH
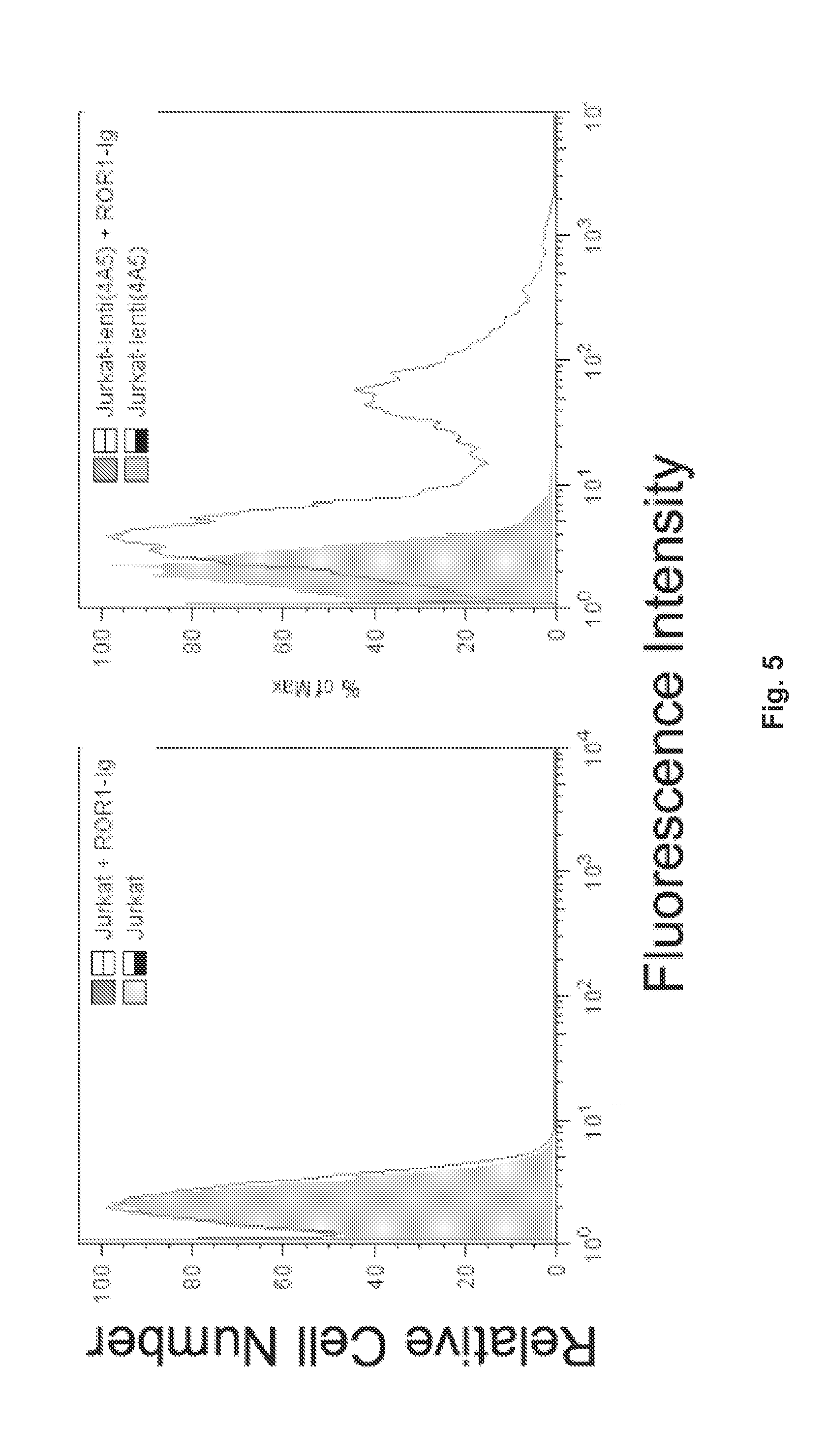
Receptor Tyrosine Kinase-Like Orphan Receptor 1 (ROR1) Single Chain FV Antibody Fragment Conjugates and Methods of Use Thereof
ActiveUS20130101607A1Peptide/protein ingredientsAntibody mimetics/scaffoldsCancer cellScFv Antibodies
Compositions including an antibody single-chain variable fragment (scFv) conjugate that specifically binds to ROR1 tumor-associated antigen are provided. The anti-ROR1 scFv antibody and conjugates may include a biologically-active molecule. Such conjugates may comprise a chimeric receptor to direct T cells to respond to ROR1 cancer cells, Methods to use the scFV conjugates to target cells expressing ROR1 for therapeutic and diagnostic purposes are also provided.
Owner:RGT UNIV OF CALIFORNIA
Prion-free collagen and collagen-derived products and implants for multiple biomedical applications; methods of making thereof
InactiveUS6197935B1Preserve integrityPeptide/protein ingredientsImmunoglobulinsIn vivo biocompatibilityCollagen VI
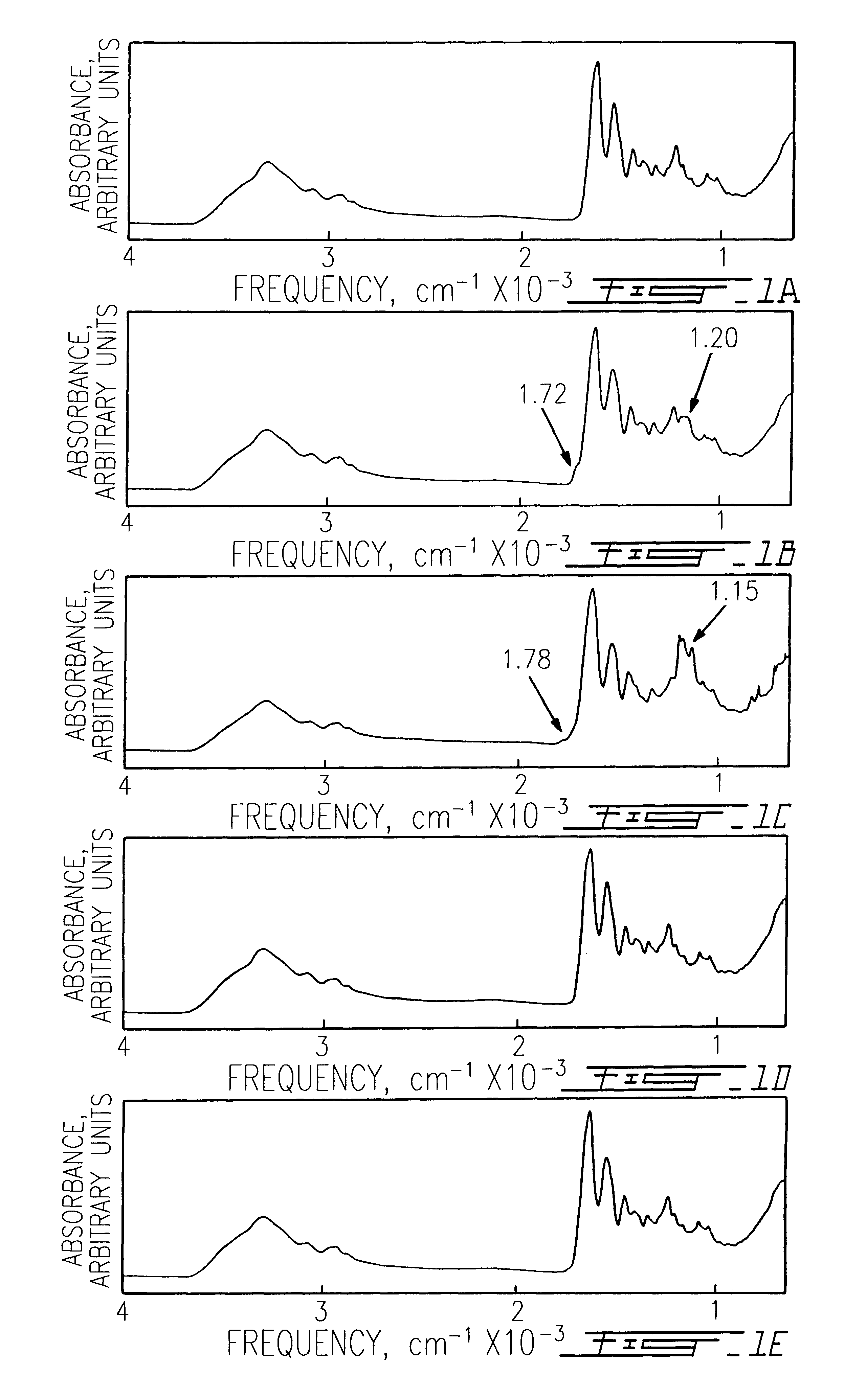
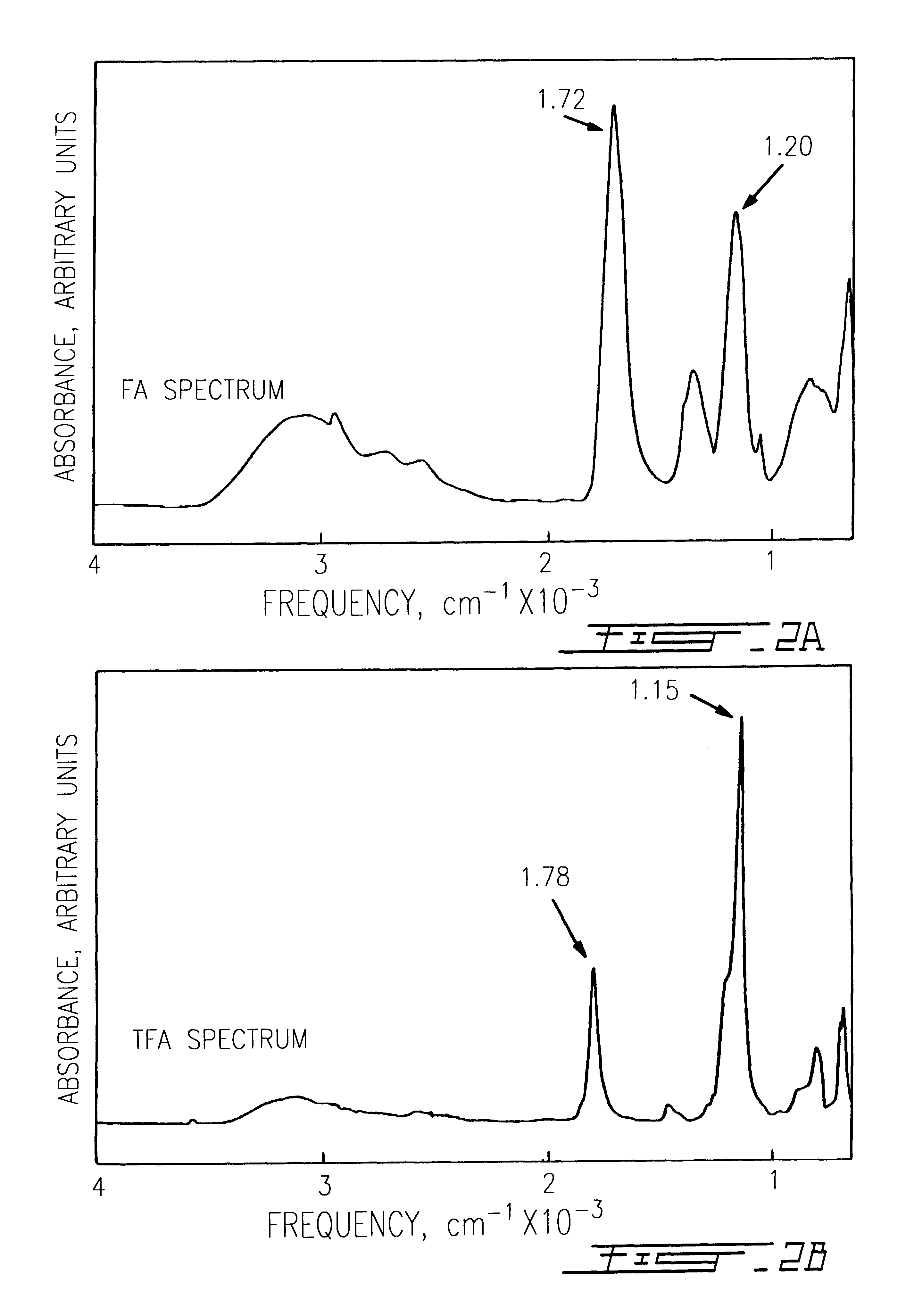
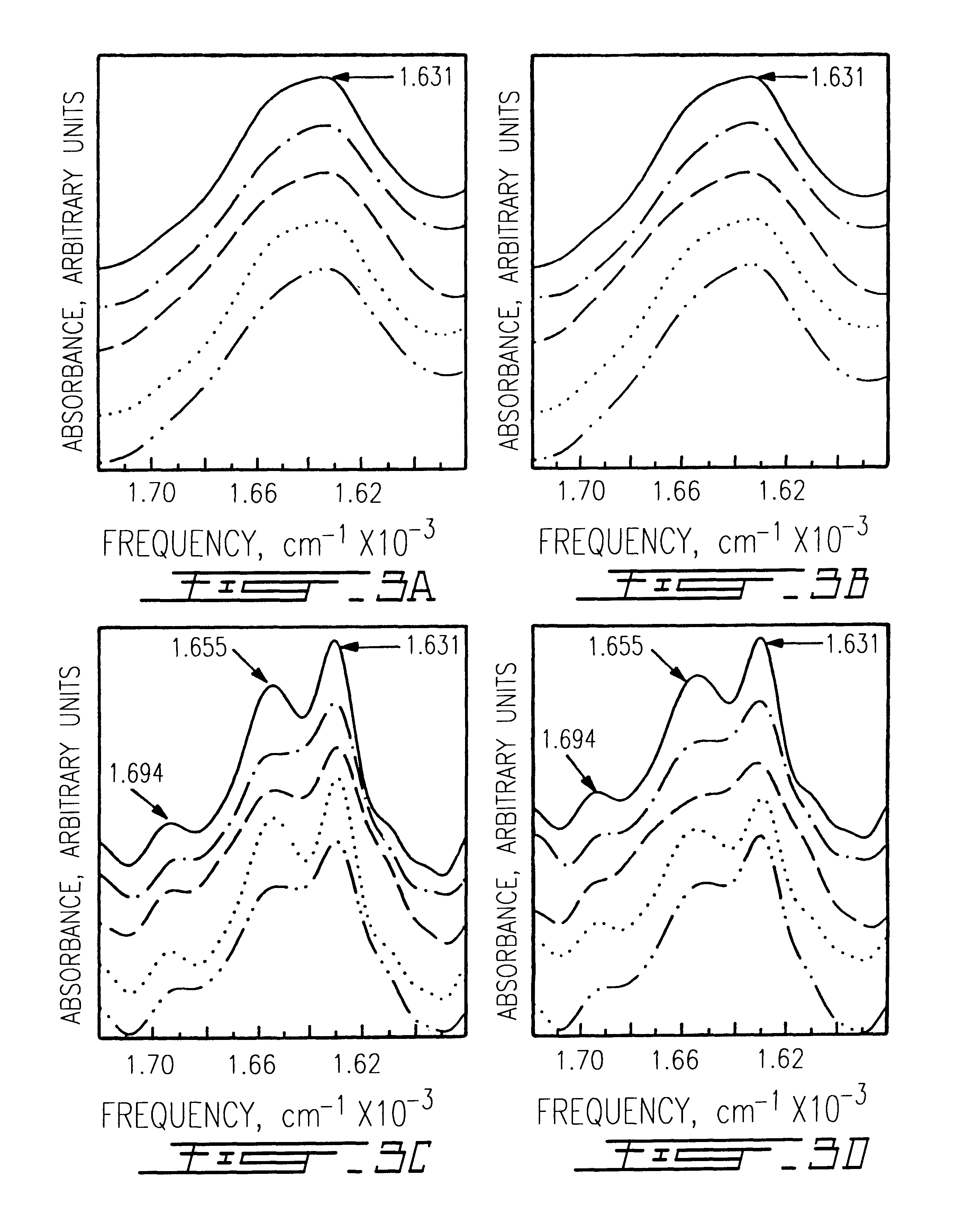
The use of collagen as a biomedical implant raises safety issues towards viruses and prions. The physicochemical changes and the in vitro and in vivo biocompatibility of collagen treated with heat, and by formic acid (FA), trifluoroacetic acid (TFA), tetrafluoroethanol (TFE) and hexafluoroiso-propanol (HFIP) were investigated. FA and TFA resulted in extensive depurination of nucleic acids while HFIP and TFE did so to a lesser degree. The molecules of FA, and most importantly of TFA, remained within collagen. Although these two acids induced modification in the secondary structure of collagen, resistance to collagenase was not affected and, in vitro, cell growth was not impaired. Severe dehydrothermal treatment, for example 110° C. for 1-3 days under high vacuum, also succeeded in removing completely nucleic acids. Since this treatment also leads to slight cross-linking, it could be advantageously used to eliminate prion and to stabilize gelatin products. Finally, prolonged treatment with TFA provides a transparent collagen, which transparency is further enhanced by adding glycosaminoglycans or proteoglycans, particularly hyaluronic acid. All the above treatments could offer a safe and biocompatible collagen-derived material for diverse biomedical uses, by providing a virus or prion-free product.
Owner:UNIV LAVAL
Extended binder compositions
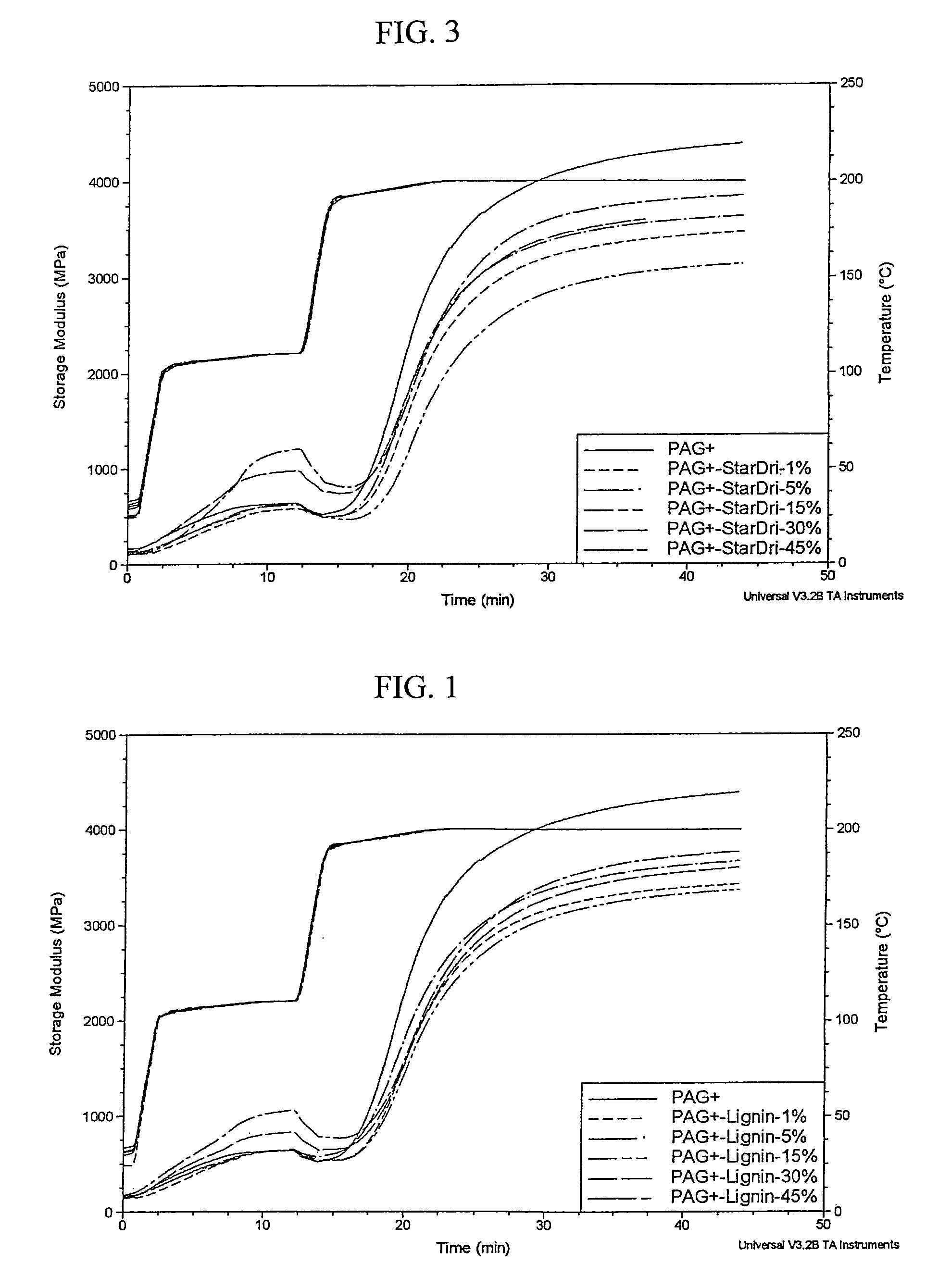
The present invention provides a variety of extended polyacrylic acid based binder compositions comprising a low molecular weight polyacrylic acid (typically hypophosphite or sulfite terminated), a crosslinking agent (such as triethanolamine or glycerol) and one or more water soluble materials, such as lignin, low molecular weight starch and soybean protein. The extended binder composition of the present invention provides a lower cost binder composition without degrading the performance and may be selected to alter one or more characteristics of the basic binder composition such binder wetting, emulsion compatibility, dust suppression and wash water flow properties.
Owner:OWENS CORNING INTELLECTUAL CAPITAL LLC
Collagen type I and type III compositions for use as an adhesive and sealant
InactiveUS20030032143A1Reduce riskInherently hemostatic propertiesFibrinogenSurgical adhesivesWound healingCollagen i
Polymerized type I and / or III collagen based compositions for medical use as adhesives and sealants and preparation thereof are described. Prior to polymerization, the collagen monomers are prepared recombinantly whereby chemical modifications of the collagen are not needed to form such monomers. The type I and / or III collagen compositions are useful as medical adhesives for bonding soft tissues or in a sealant film for a variety of medical uses. In a further aspect of the present invention, the polymerized type I and / or III collagen composition includes agents which induce wound healing or provide for additional beneficial characteristics desired in a tissue adhesive and sealant.
Owner:NEFF THOMAS B +1
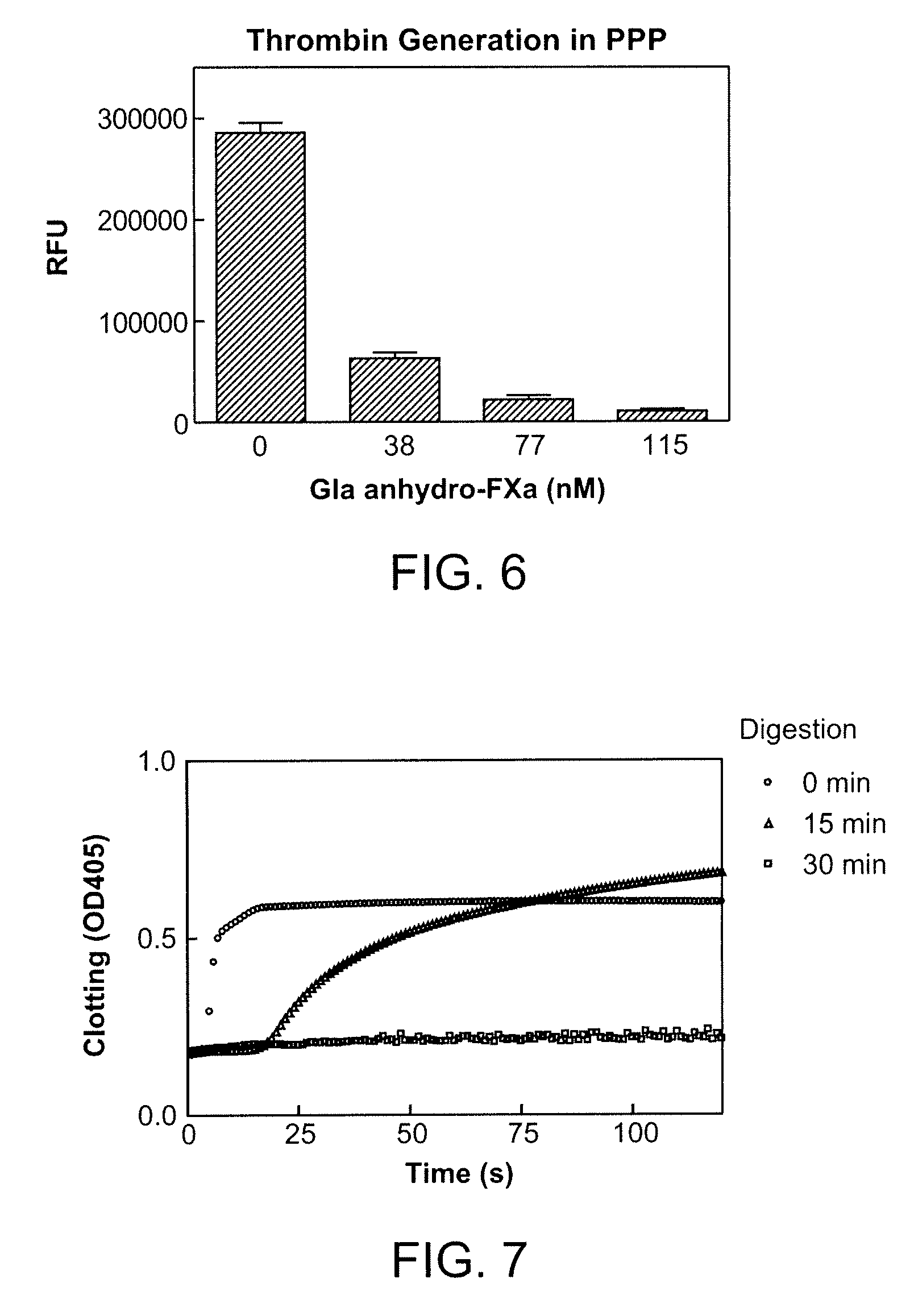
Antidotes for factor xa inhibitors and methods of using the same
ActiveUS20090098119A1Reduce and remove intrinsic procoagulantReduce and remove and anticoagulant activityOrganic active ingredientsHydrolasesMedicineAntidote
The present invention relates antidotes to anticoagulants targeting factor Xa. The antidotes are factor Xa protein derivatives that bind to the factor Xa inhibitors thereby substantially neutralizing them but do not assemble into the prothrombinase complex. The derivatives describe herein lack or have reduced intrinsic coagulant activity. Disclosed herein are methods of stopping or preventing bleeding in a patient that is currently undergoing anticoagulant therapy with a factor Xa inhibitor.
Owner:ALEXION PHARMA INC
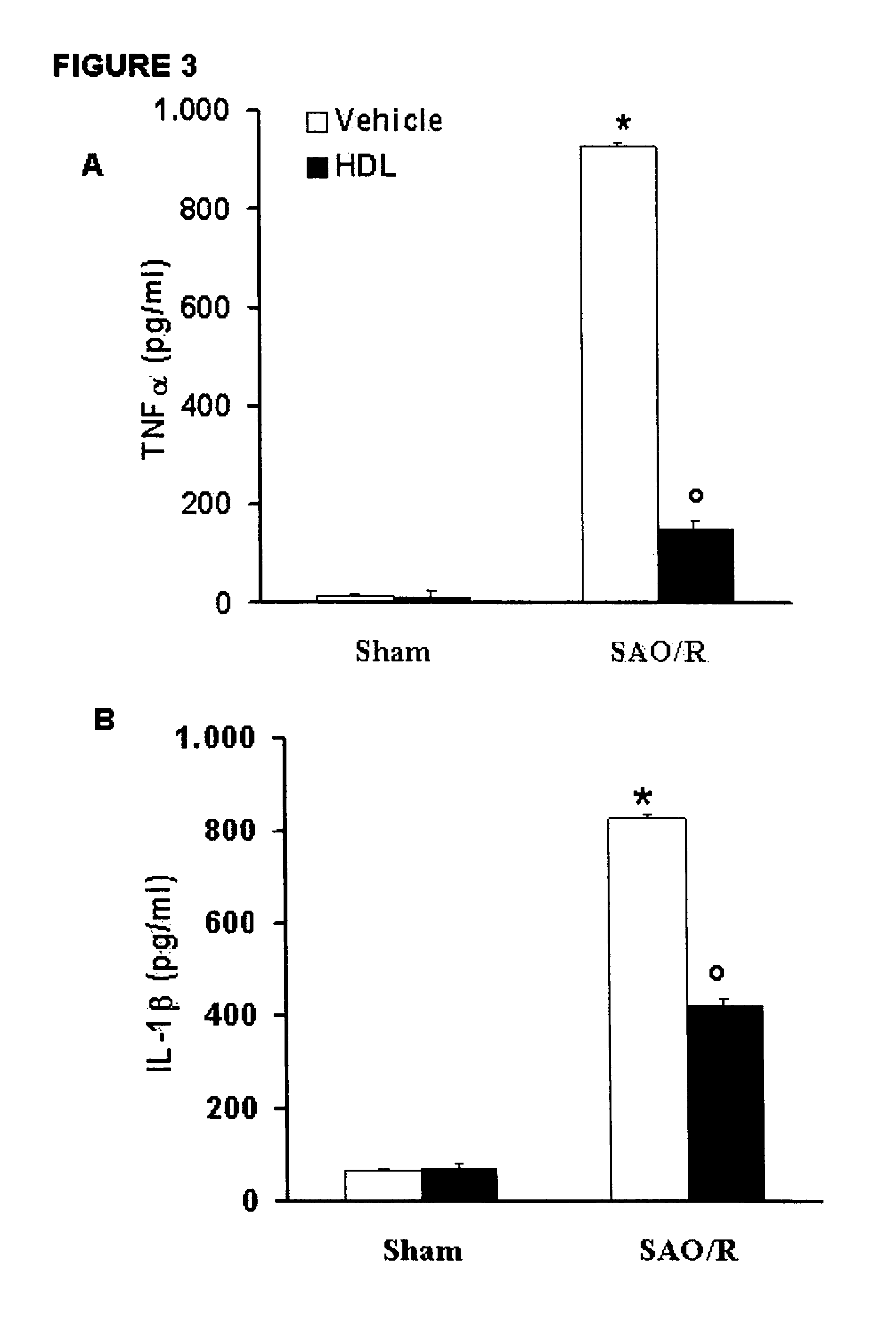
Treatment of inflammatory conditions of the intestine
Owner:CSL BEHRING AG
Modified protein adhesives and lignocellulosic composites made from the adhesives
An adhesive composition made by reacting a soy protein with at least one compound under conditions sufficient for introducing additional phenolic hydroxyl functional groups, amine functional groups, and / or thiol functional groups into the soy protein structure.
Owner:THE STATE OF OREGON ACTING BY & THROUGH THE OREGON STATE BOARD OF HIGHER EDUCATION ON BEHALF OF OREGON STATE UNIV
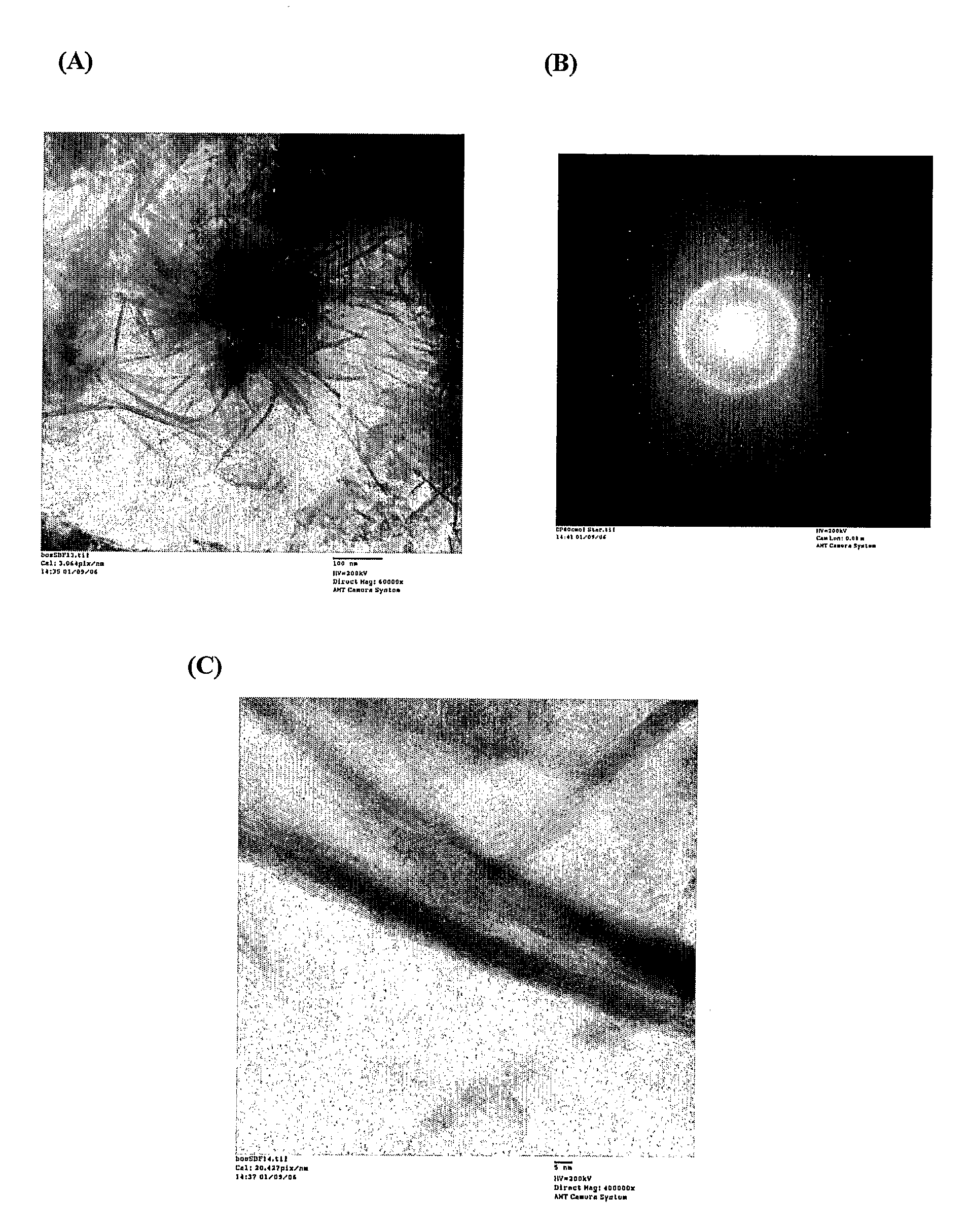
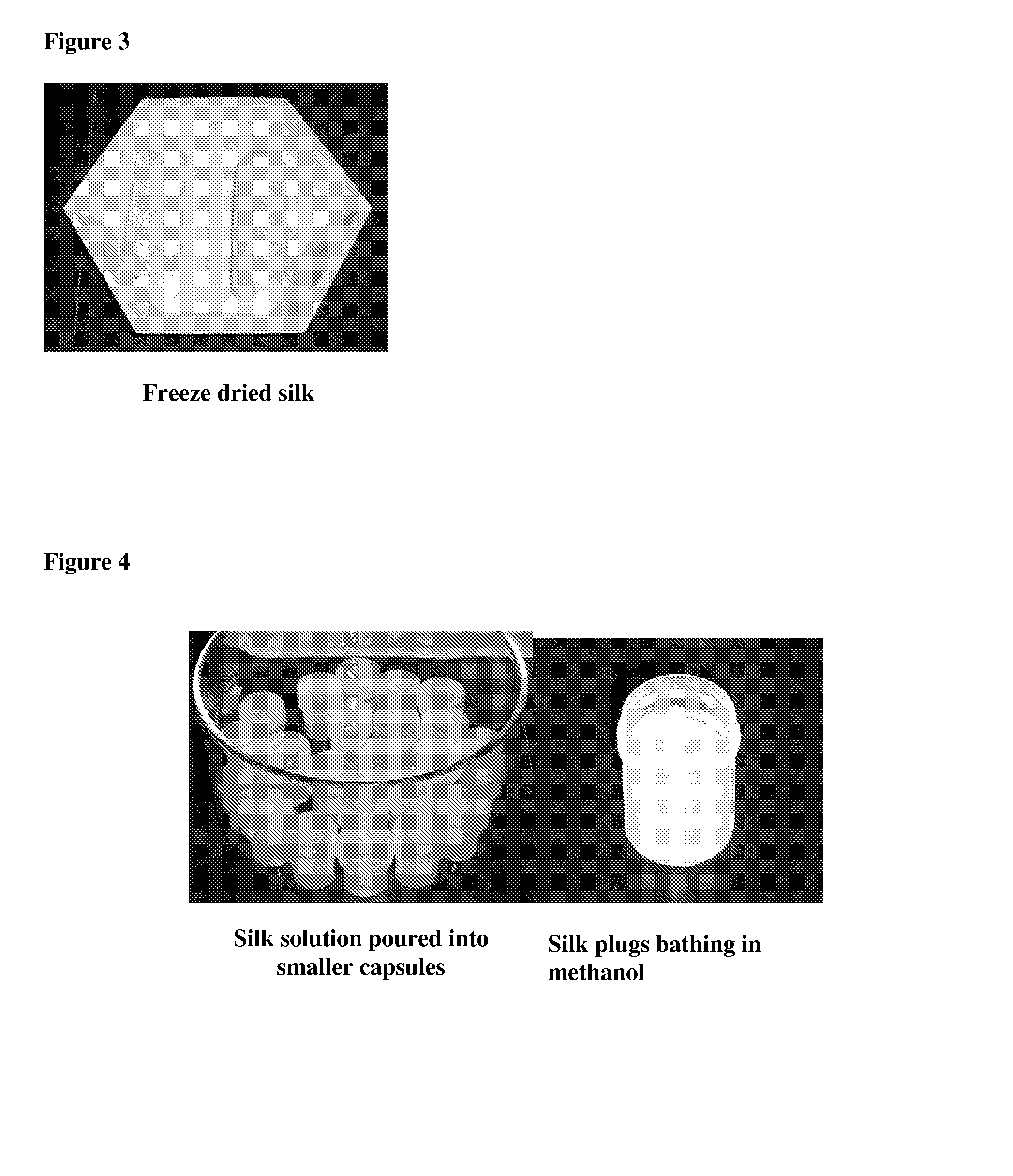
3-dimensional silk hydroxyapatite compositions
ActiveUS20110046686A1Facilitate bone healingFacilitate tooth structureBiocideImpression capsMedicineBone healing
Described herein are methods and compositions comprising a mixture of silk polymer and hydroxyapatite. The methods described herein can be used to prepare a mixture of silk polymer and hydroxyapatite and further provide mixtures that can be molded into a desired shape. Also encompassed herein are compositions comprising a mixture of silk polymer and hydroxyapatite having a desired shape, which can further be implanted, for example, to facilitate bone healing or tooth structure or support. Such compositions can also include agents, such as therapeutic agents, or cells.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Apolipoprotein analogues
InactiveUS20020156007A1Antibody mimetics/scaffoldsGenetic material ingredientsCubilinLipid formation
The invention relates to a pharmaceutical composition comprising an apolipoprotein construct, to an apolipoprotein construct, a nucleic acid sequence encoding the apolipoprotein construct, a vector comprising the nucleic acid sequence, a method for producing the apolipoprotein construct, and a method of treatment comprising administering the apolipoprotein construct. The presented data document that the constructs according to the invention are capable of binding lipids, are capable of binding cubilin, which is a strong Apo Al receptor, stronger than native Apo A-I and that the plasma half life of the constructs is at least tripled compared to native Apo A-I. Together these data document that the constructs according to the invention are strong candidates for treatment of cardiovascular diseases.
Owner:F HOFFMANN LA ROCHE & CO AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com