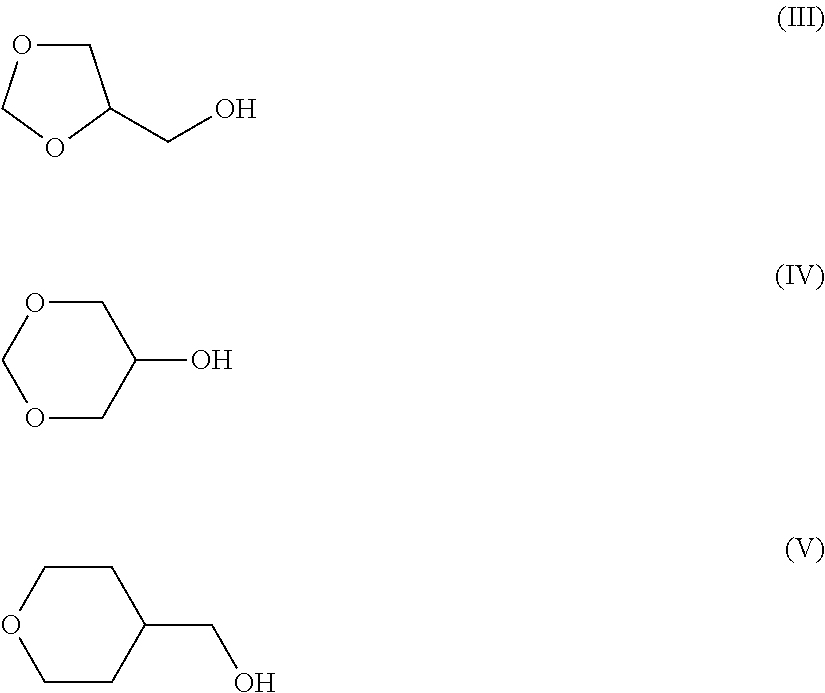
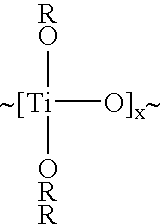
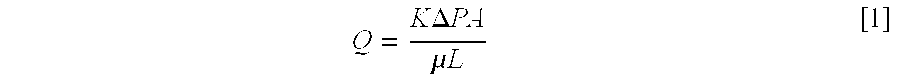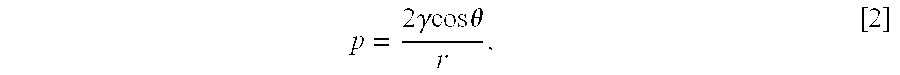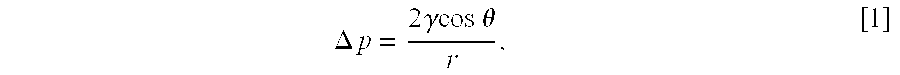Patents
Literature
481 results about "Animal origin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Animals such as the fruit fly Drosophila melanogaster serve a major role in science as experimental models. Animals have been used to create vaccines since their discovery in the 18th century. Some medicines such as the cancer drug Yondelis are based on toxins or other molecules of animal origin.
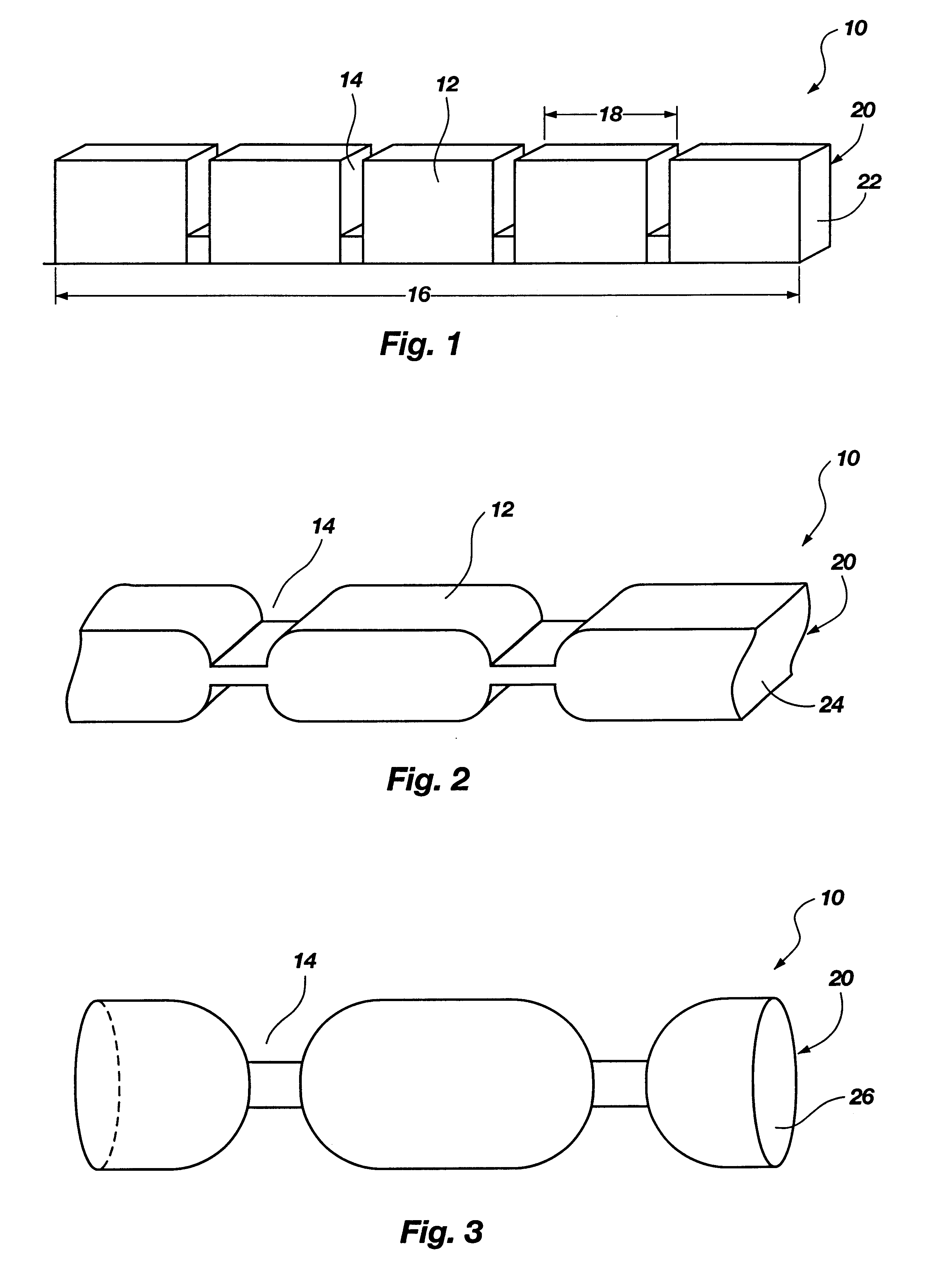
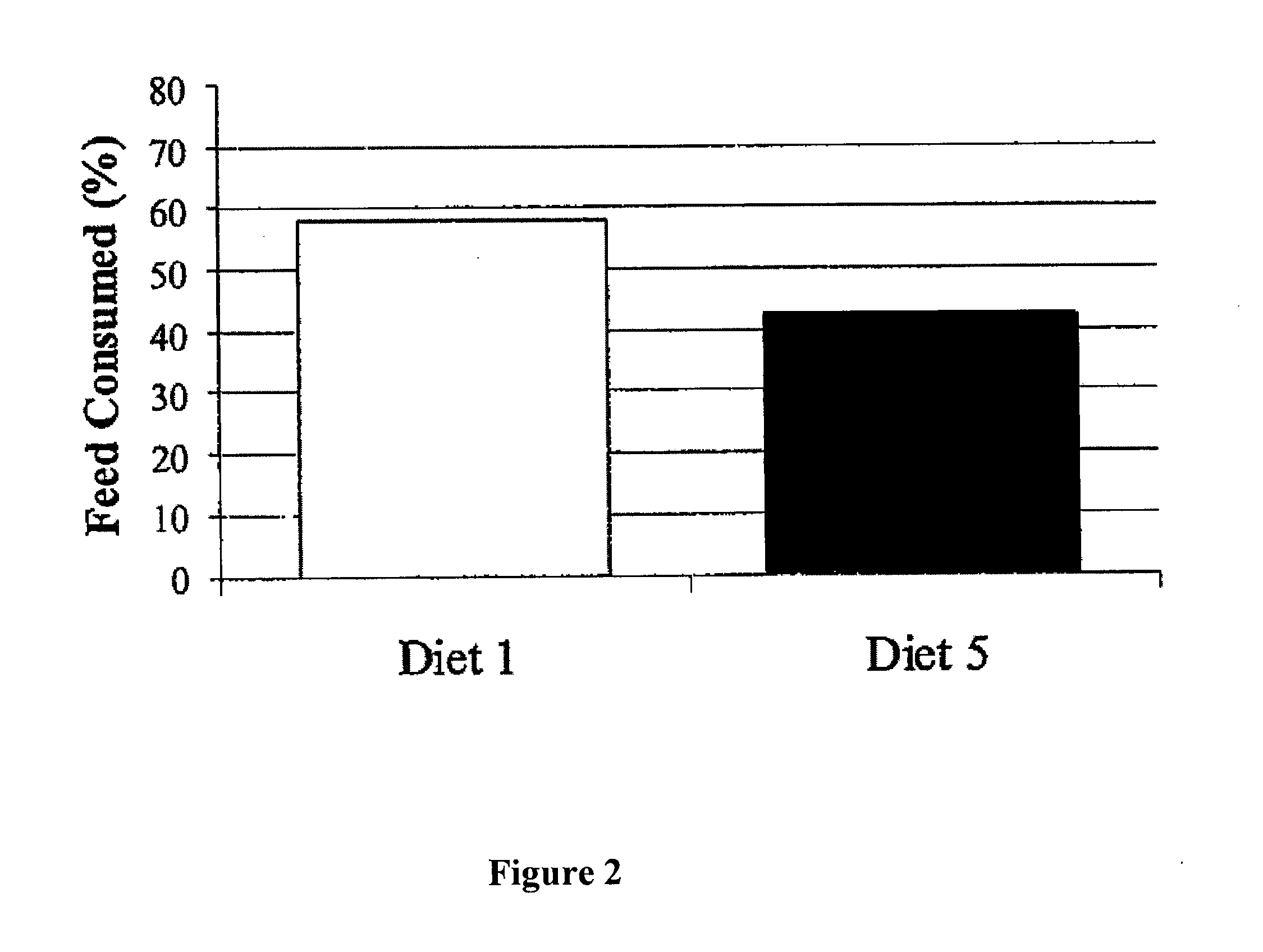
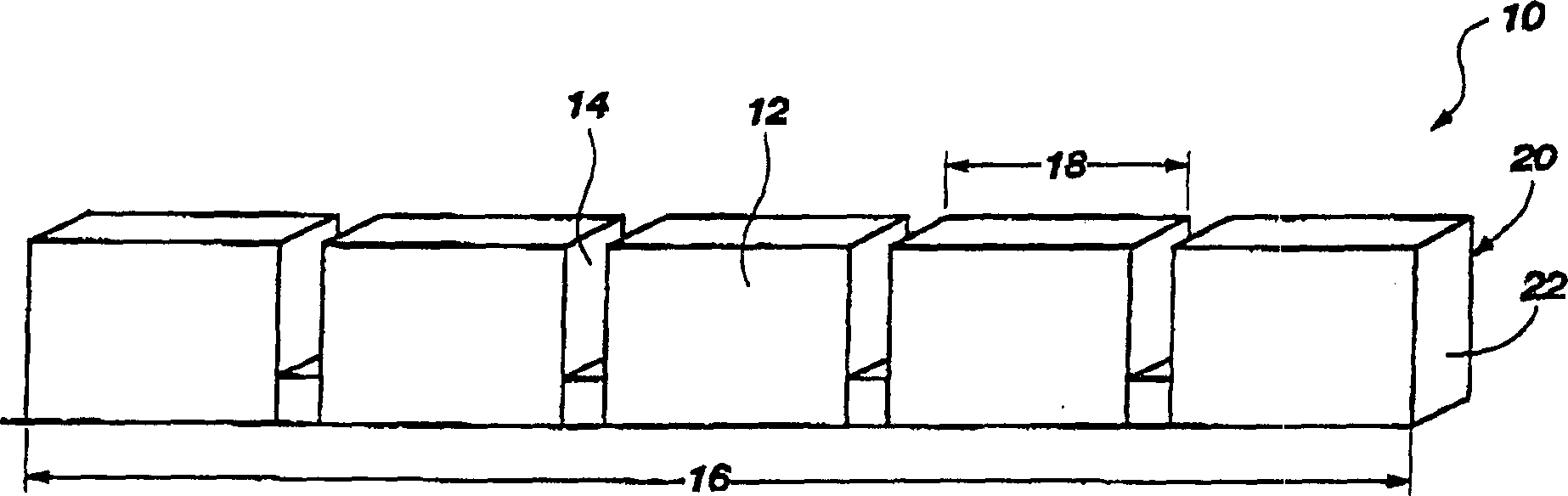
Edible thermoplastic and nutritious pet chew
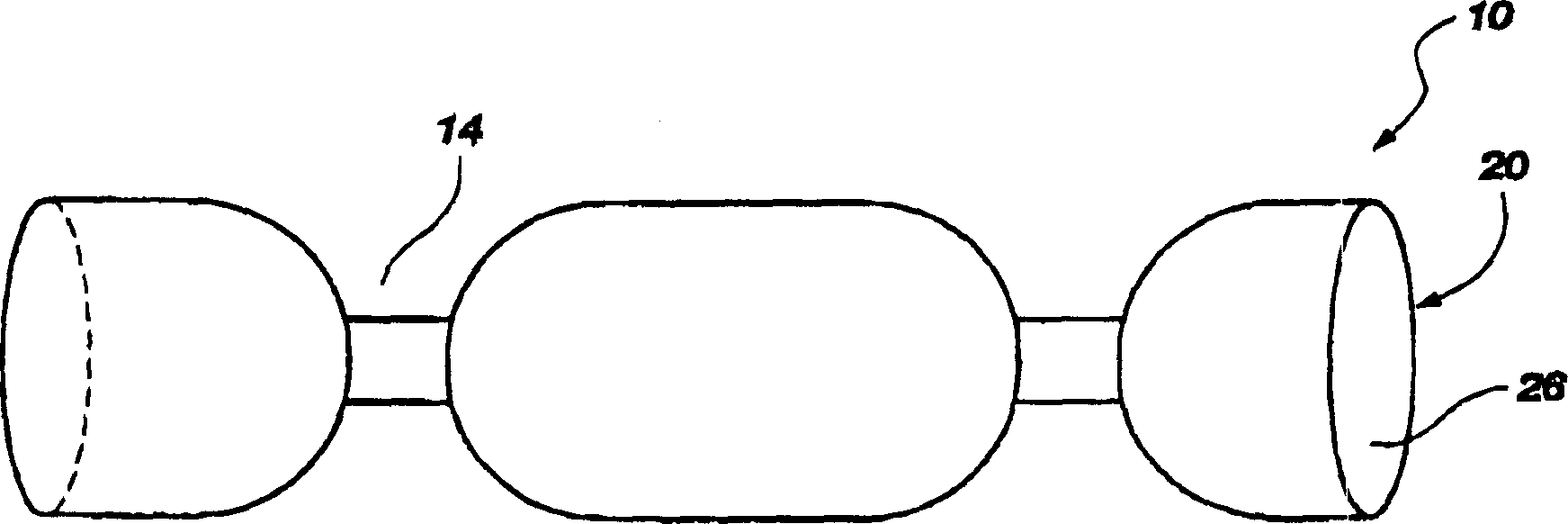
The present invention relates to an edible thermoplastic made from about 30 to 50 wt. % protein comprising a mixture of plant and animal derived protein, about 20 to 50 wt. % starch about 10 to 20 wt. % water, about 1 to 10 wt. % edible fiber, and about 0.5 to 3 wt. % metallic salt hydrate. When molded, the thermoplastic has good strength and stiffness and other physical properties. The edible thermoplastic may be molded in a variety of shapes including a segmented nutritional pet chew with a plurality of segments separated by a plurality of scores. The scores serve to structurally weaken the pet chew so that it may be broken into smaller pieces. When molded the edible thermoplastic has a density of about 1.2 to 1.5 g / cubic centimeters.
Owner:BISMUTH INVESTMENTS
Preparation of recombinant factor VIII in a protein free medium
InactiveUS6171825B1Eliminate and at least greatly reduce riskImprove productivityFactor VIICulture processFactor iiManganese
Recombinant Factor VIII can be produced in relatively large quantities on a continuous basis from mammalian cells in the absence of any animal-derived proteins such as albumin by culturing the cells in a protein free medium supplemented with polyol copolymers, preferably in the presence of trace metals such as copper. In very preferred embodiments, the medium includes a polyglycol known as Pluronic F-68, copper sulfate, ferrous sulfate / EDTA complex, and salts of trace metals such as manganese, molybdenum, silicon, lithium and chromium. With an alternative medium which included trace copper ions alone (without polyol copolymers) we were also able to enhance the productivity of Factor VIII in recombinant cells such as BHK cells that are genetically engineered to express Factor VIII.
Owner:BAYER HEALTHCARE LLC +1
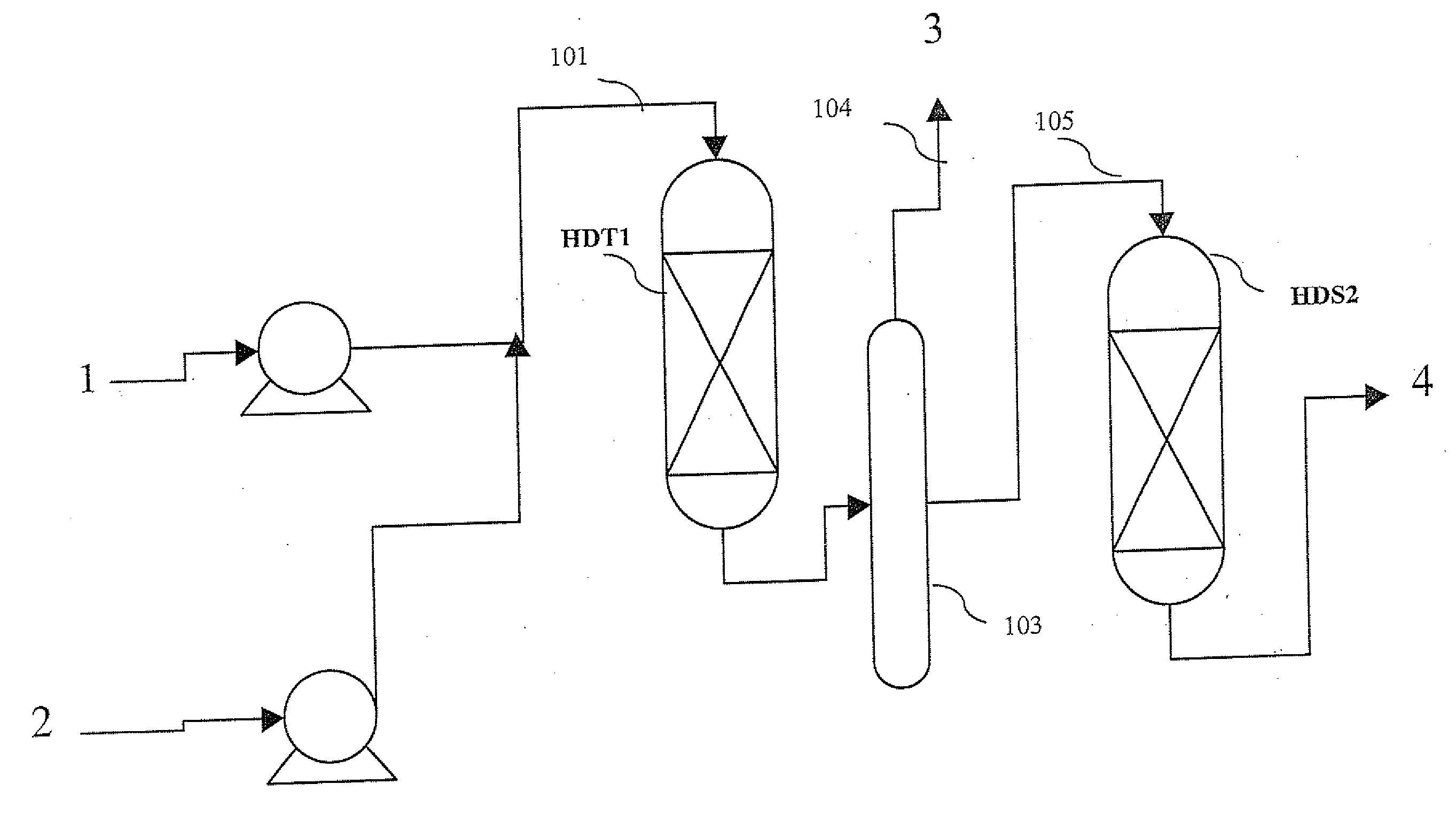
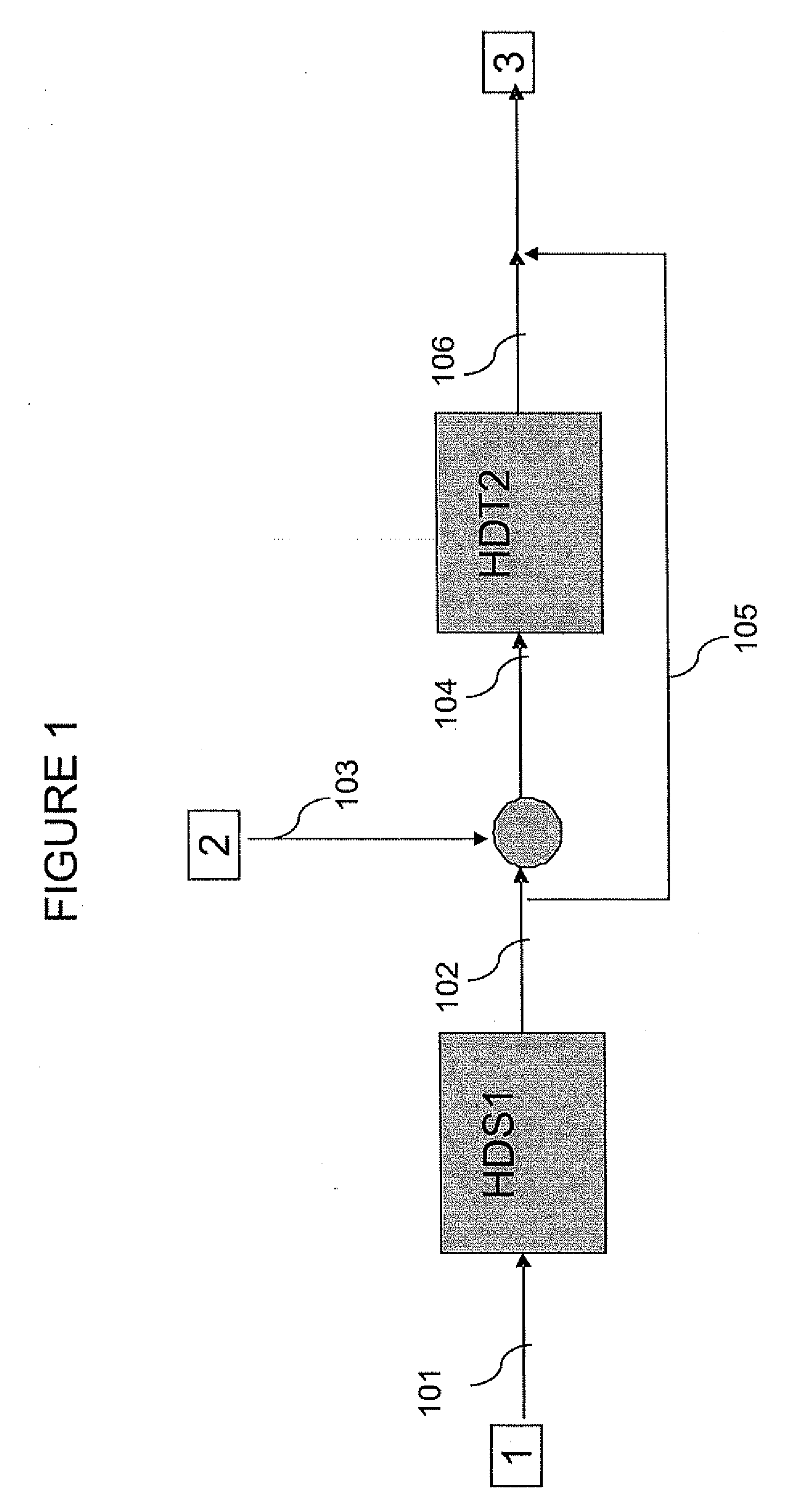
Methods of hydrotreating a mixture made up of oils of animal or vegetable origin and of petroleum cuts with intermediate stripping
ActiveUS20080161614A1Low costLimit consumption of hydrogenThermal non-catalytic crackingCatalytic crackingVegetable oilVolumetric Mass Density
The invention relates to a hydrotreating method (HDT) using two plants working under different operating conditions with an intermediate stripping for co-treating a mixture made up of oils of vegetable or animal origin and petroleum cuts (gas oil cuts (GO) and middle distillates) in order to produce gas oil fuel bases meeting specifications. The first plant (HDT1) is more particularly dedicated to the reactions concerning oils of vegetable or animal origin in comixture while pretreating the hydrocarbon feed, whereas the second plant (HDS2) works under more severe conditions to obtain diesel fuel according to standards, in particular in terms of effluent sulfur content, density and cold properties. The process economy, the activity and the stability of the catalyst of the second plant are greatly improved by the intermediate stripping.
Owner:INST FR DU PETROLE
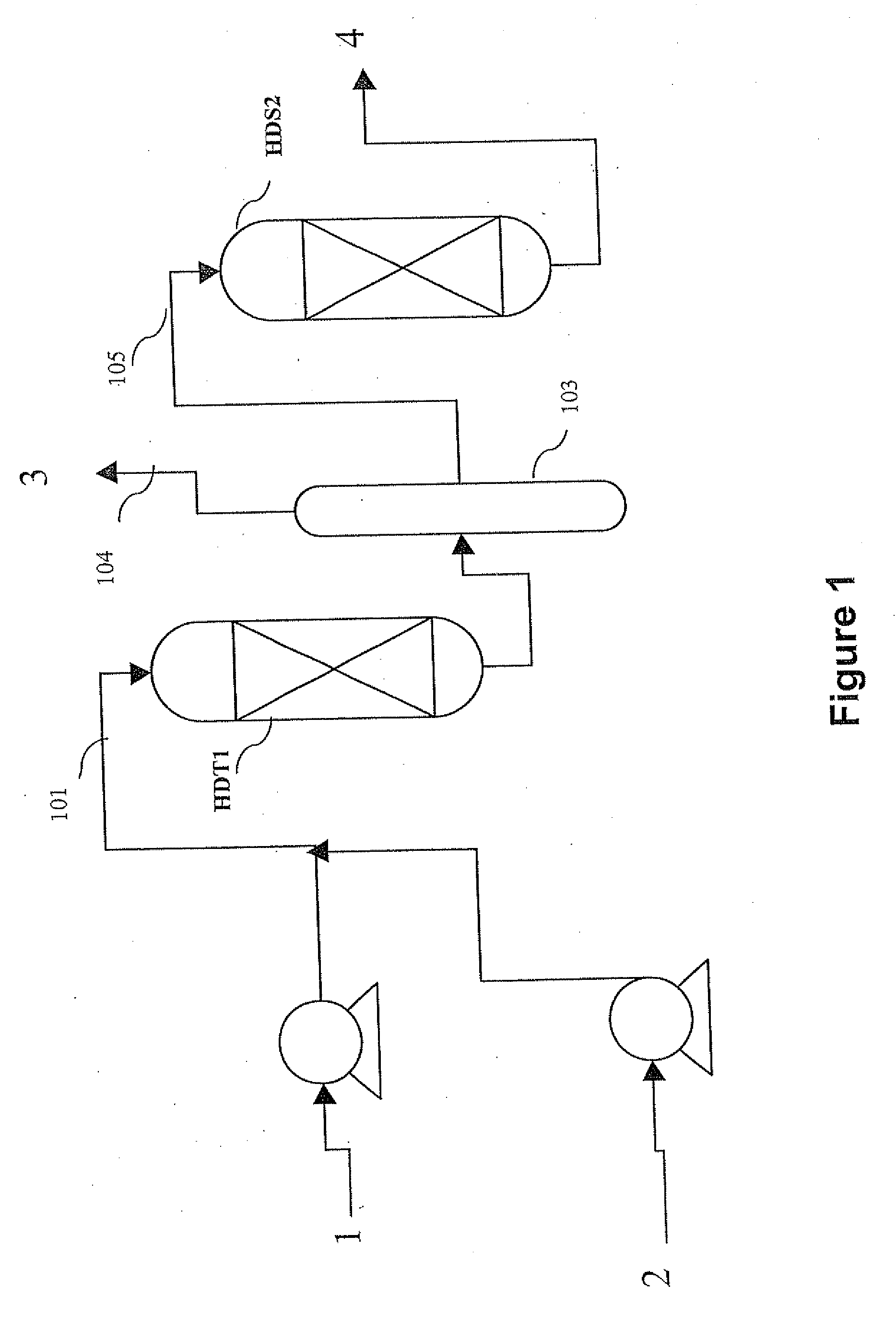
Methods of hydrotreating a mixture made up of oils of animal or vegetable origin and of petroleum cuts with quench injection of the oils on the last catalyst bed
ActiveUS20080173570A1Low costReduce use costCatalytic crackingHydrocarbon oil crackingDistillates petroleumVegetable oil
A hydrotreating method uses two catalyst beds with the introduction, on the last catalyst bed, of oils of animal or vegetable origin for co-treating a mixture made up of oils of vegetable or animal origin and of petroleum cuts (gas oil cuts (GO) and middle distillates) in order to produce gas oil effluents meeting specifications with an improved cetane number. The first catalyst bed is dedicated to only the deep desulfurization reactions (HDS1) of a petroleum type feed. The effluents of the first catalyst bed having an effluent sulfur content below or equal to 50 mg / kg are separated into two streams. The first stream, which is predominant, is sent to the gas oil pool. The second stream is mixed with oils of vegetable or animal origin. The resultant oil-petroleum cut mixture is then subjected to a milder hydrotreatment (HDT2). The effluents obtained at the outlet of the second catalyst bed can optionally be mixed with the predominant stream from the first bed. The process economy, the tolerance to the specifications relative to oils of animal or vegetable origin and the quality of the products obtained are thus greatly improved.
Owner:INST FR DU PETROLE
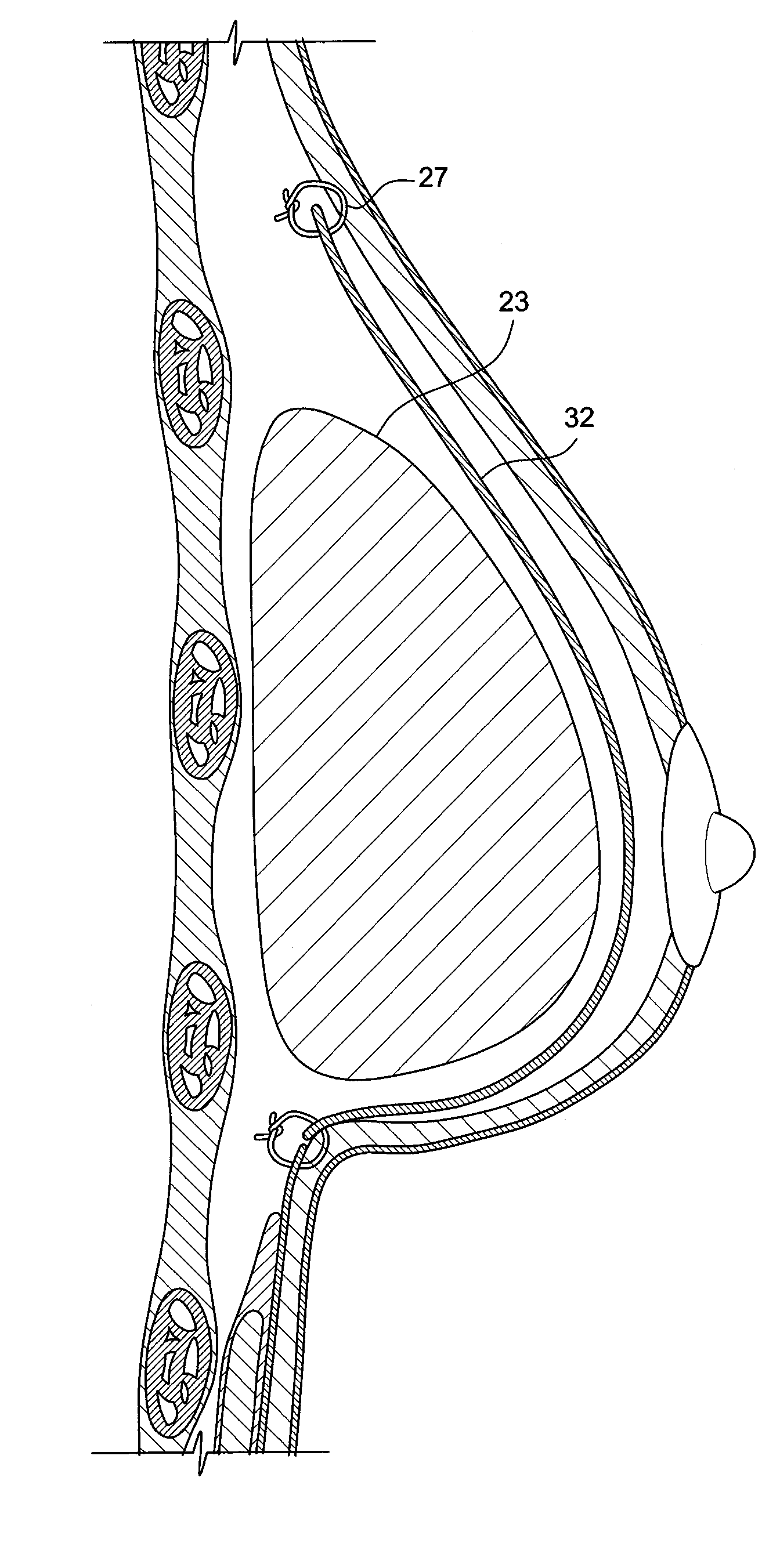
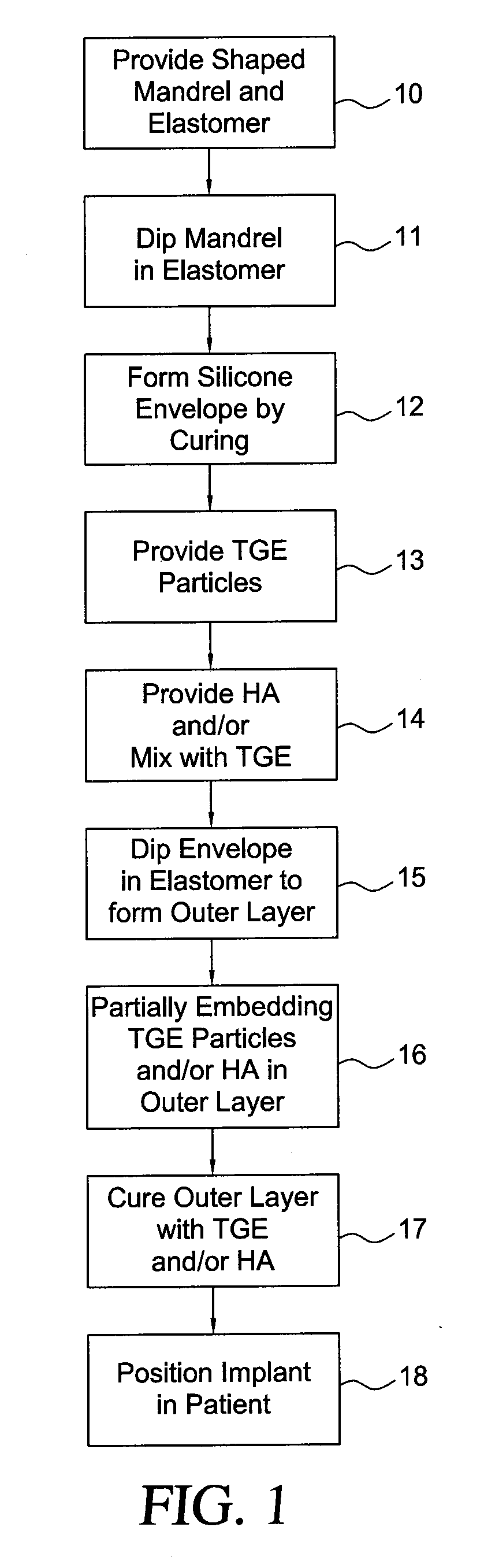
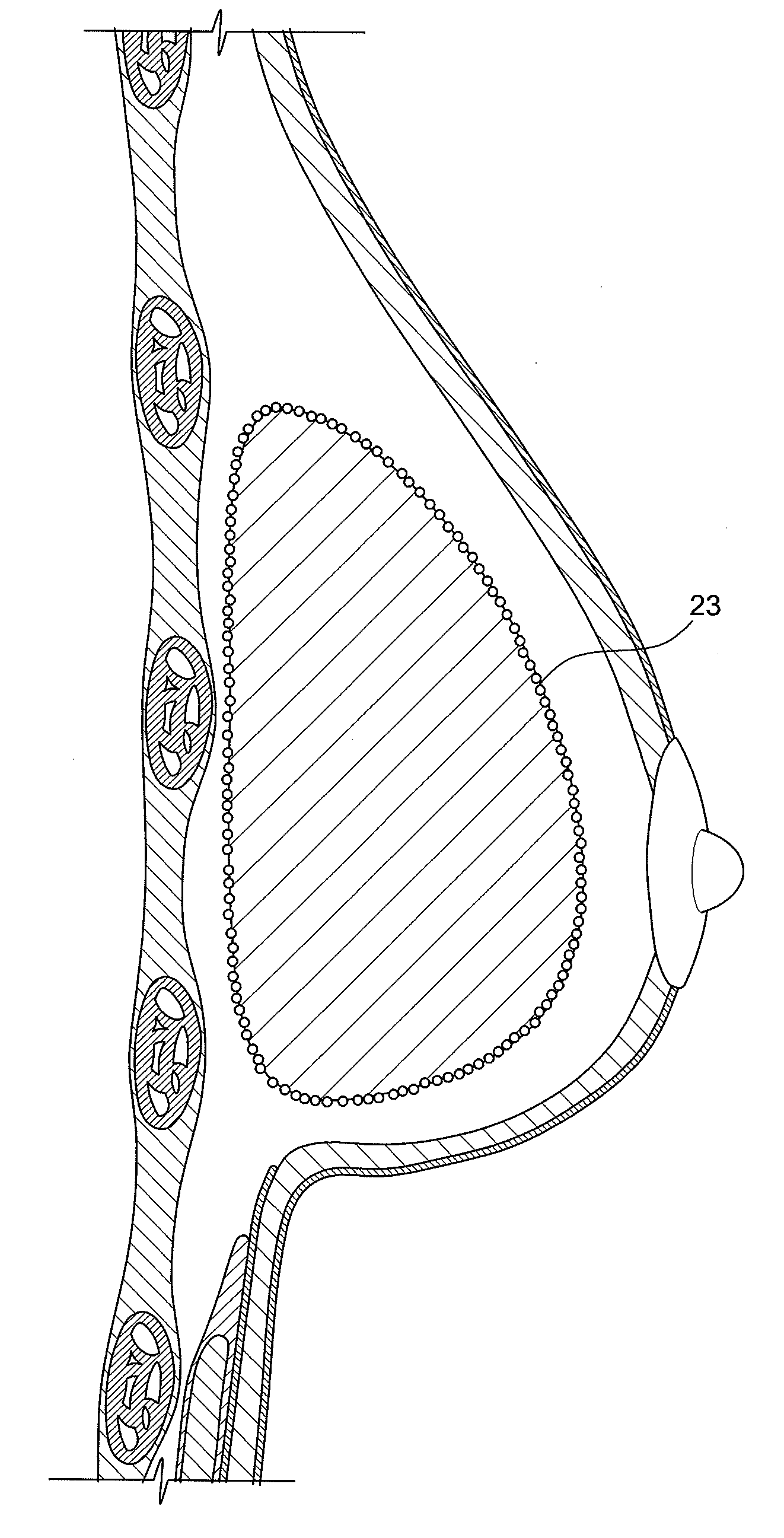
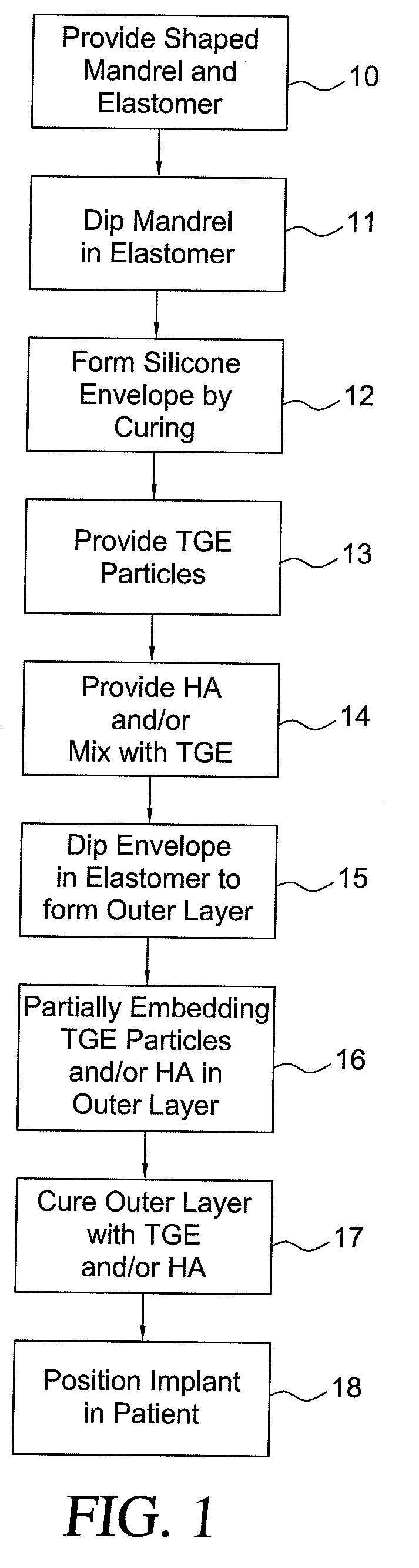
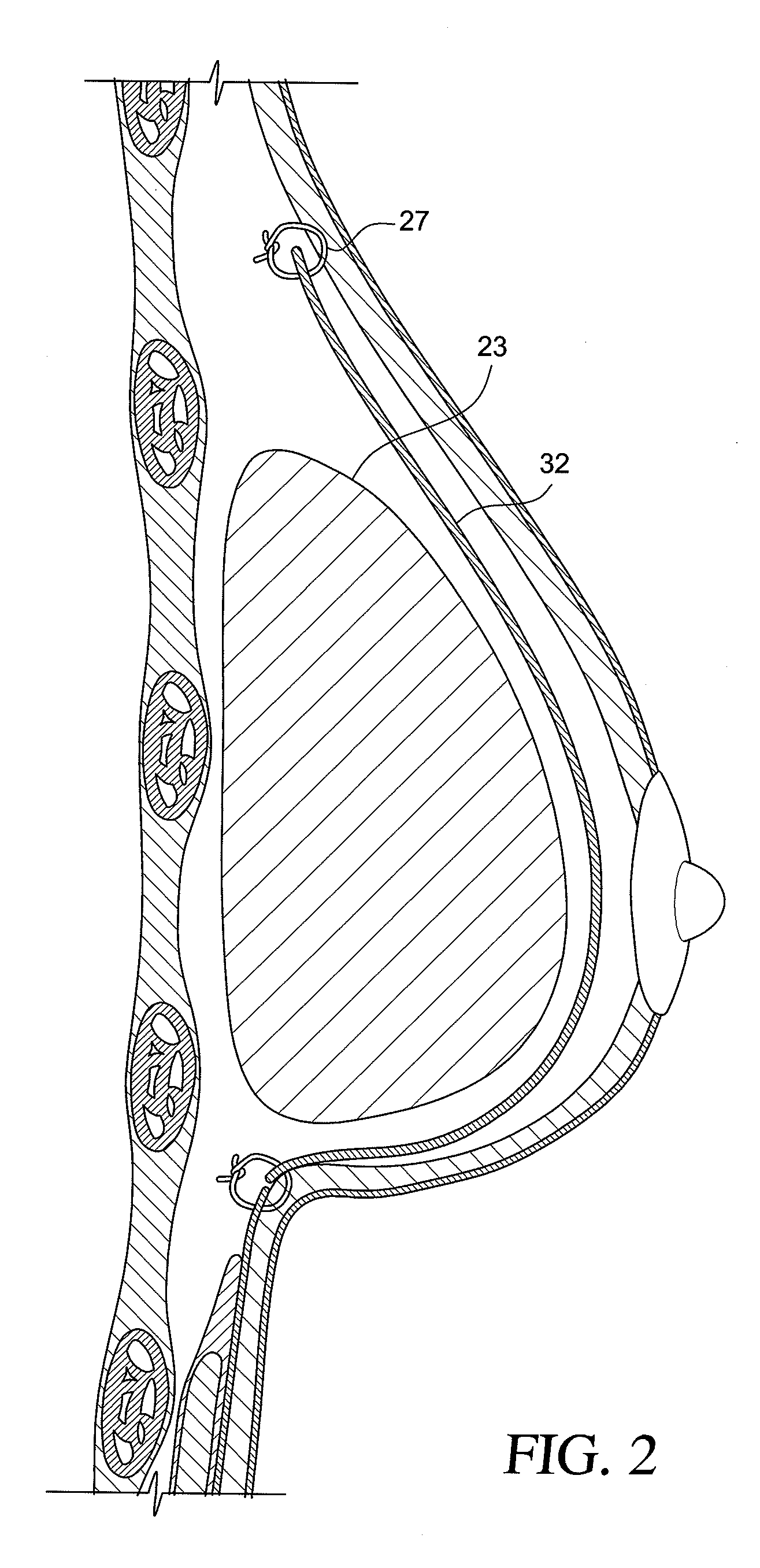
Method for texturing the surface of a synthetic implant
InactiveUS20090198333A1Increase heightReducing capsular contractionMammary implantsDiagnosticsBreast implantAcellular Dermis
A method for texturing the surface of a breast implant includes the step of partially impregnating a silicone outer surface of the implant with particles of a biologically active material such as acellular dermis of human or animal origin impregnated with hyaluronic acid. The biologically active material promotes tissue ingrowth into a plurality of cavities filled with a biologically active material.
Owner:BECKER HILTON
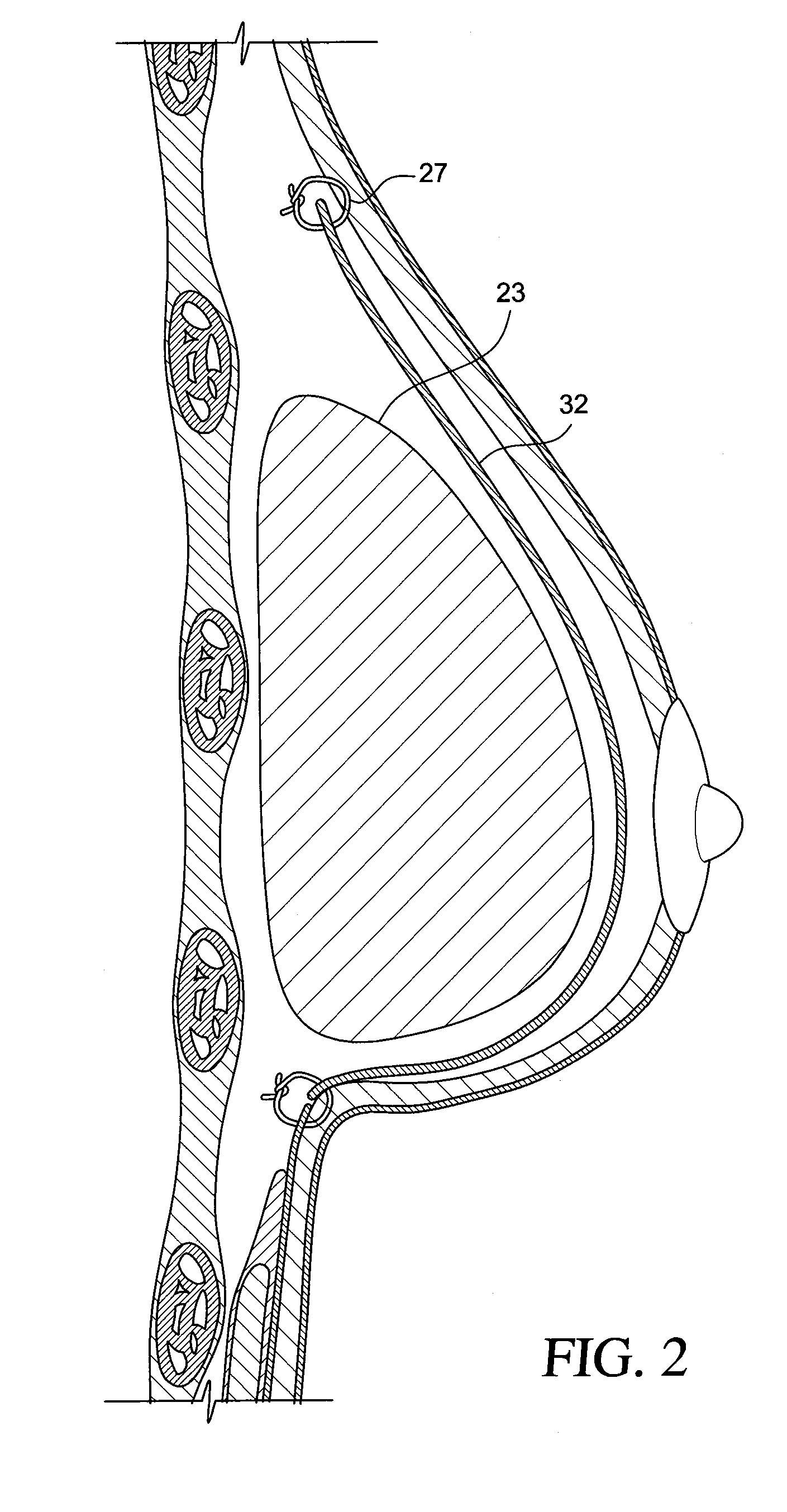
Method for texturing the surface of a synthetic implant
InactiveUS20090198332A1Increase heightReducing capsular contractionMammary implantsCoatingsBreast implantAcellular Dermis
A method for texturing the surface of a breast implant includes the step of partially impregnating a silicone outer surface of the implant with particles of a biologically active material such as acellular dermis of human or animal origin impregnated with hyaluronic acid. The biologically active material promotes tissue ingrowth into a plurality of cavities filled with a biologically active material.
Owner:BECKER HILTON
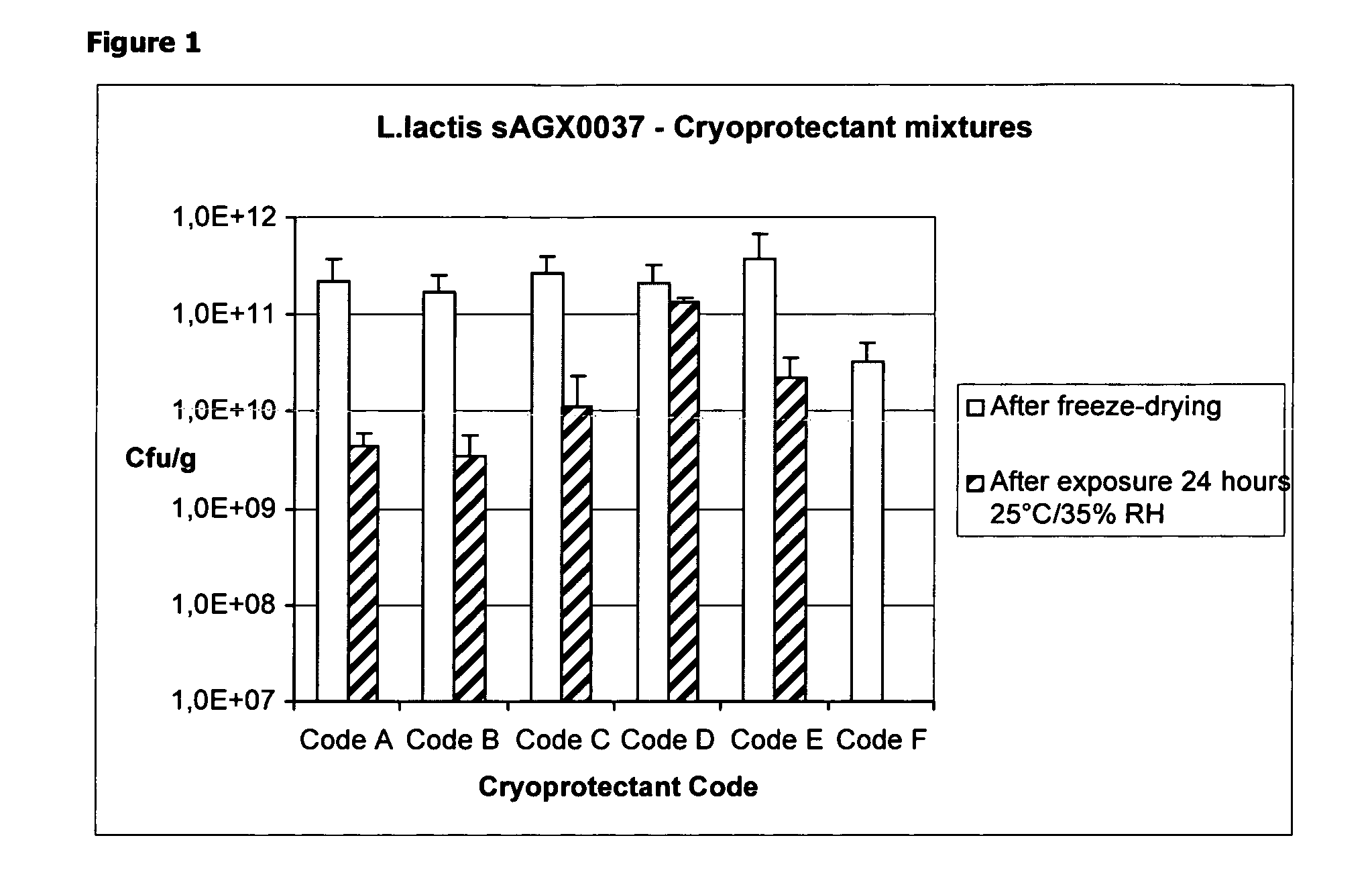
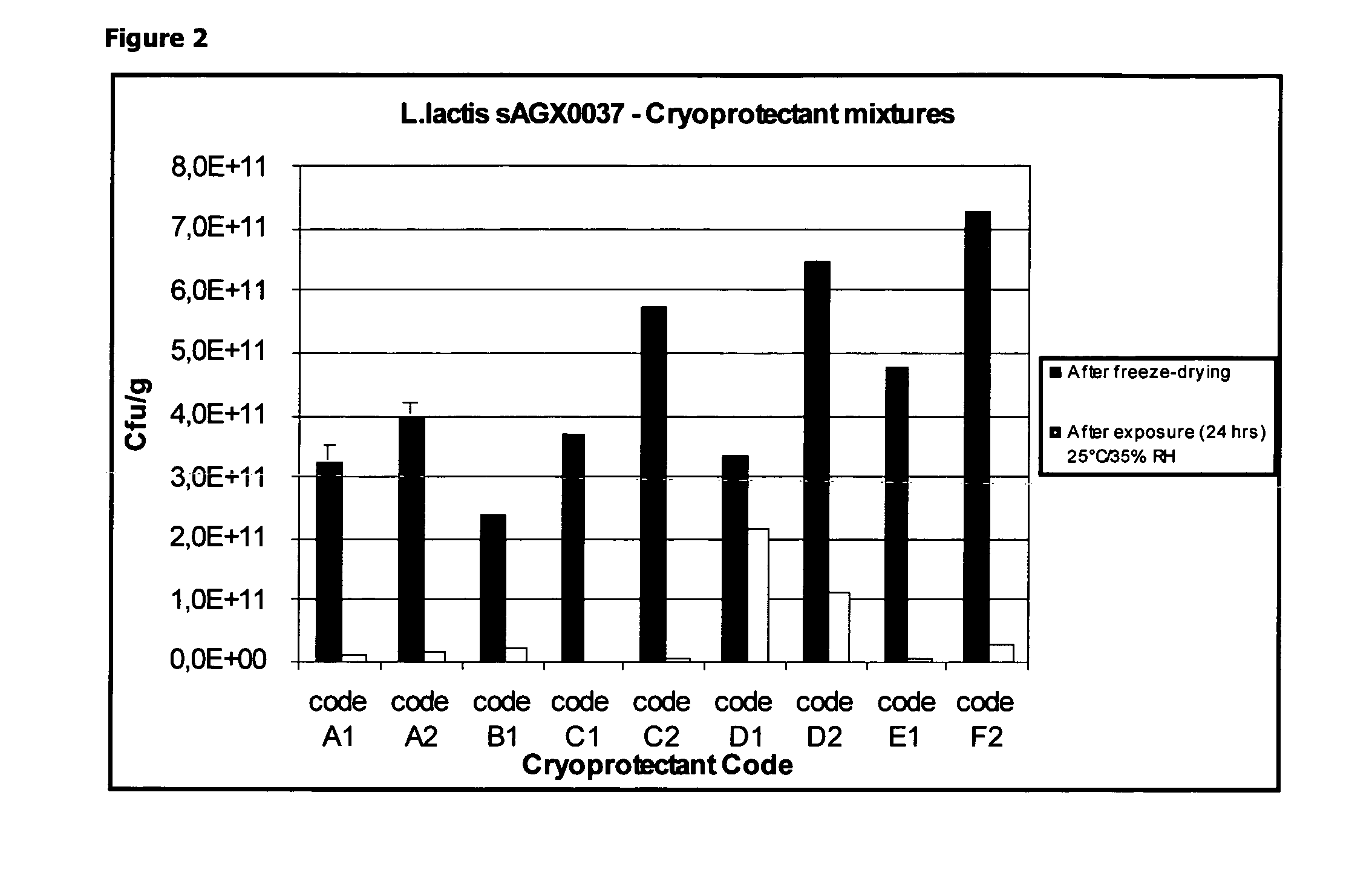
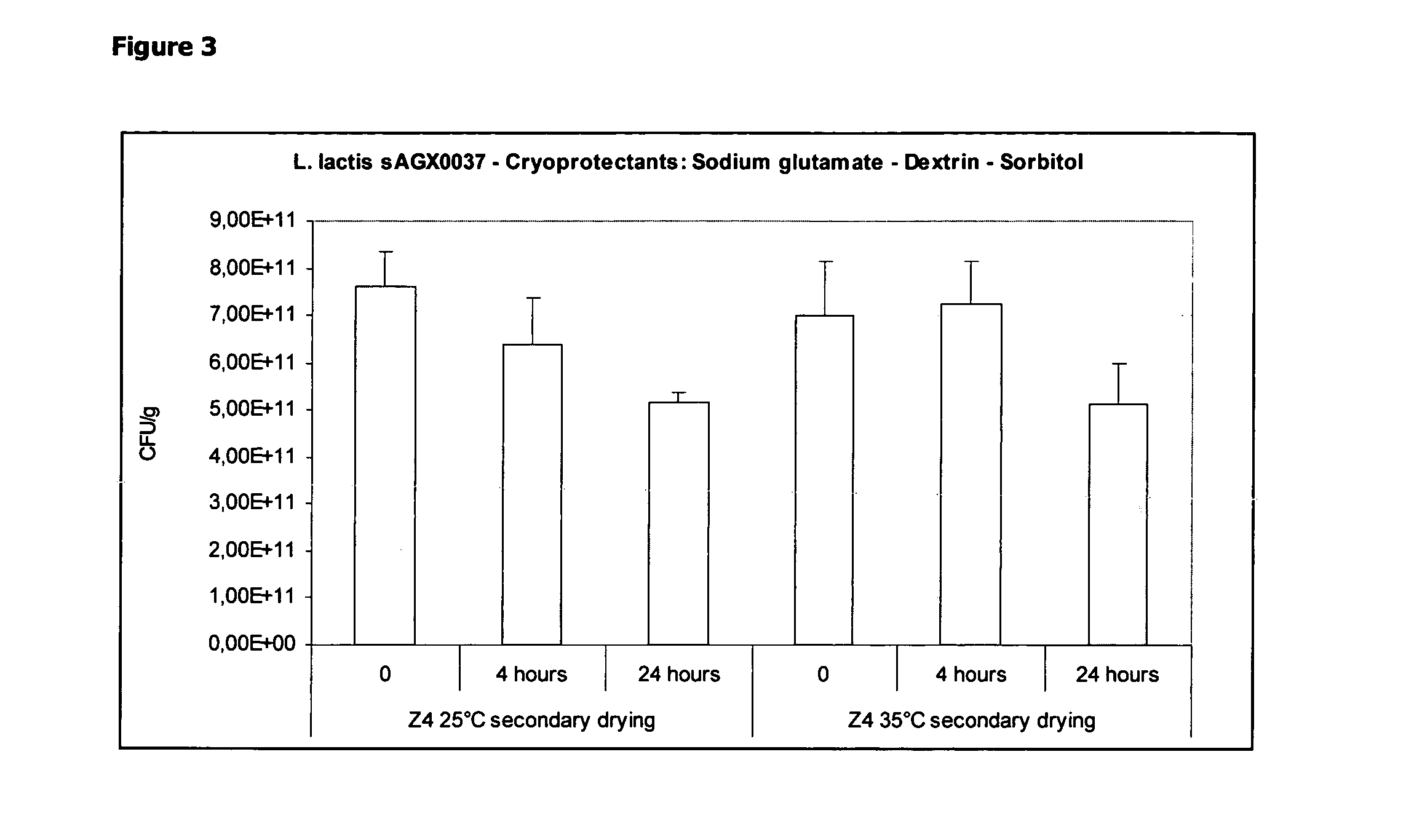
Cryoprotectants for freeze drying of lactic acid bacteria
ActiveUS20120039853A1Improve survivabilityImprove textureBiocideMilk preparationBacteroidesVaccination
The present invention comprises the discovery and development of an effective cryoprotectant composition, without containing skim milk or any other animal-derived compounds, to achieve long-term stability of freeze-dried lactic acid bacteria (LAB), at different temperatures, whereby the retention of viability of the freeze-dried LAB after 6 months of storage, preferably after 9 months of storage, more preferably after 12 months of storage is more than 50%. The invention is in the field of producing freeze dried bacteria, in particular Lactic acid bacteria. More in particular, the invention relates to the use of a novel combination of cryoprotectants for increasing the viability of bacteria after freeze drying, improving the texture of the lyofilized cake for easy grinding and improving the long term stability of the freeze dried bacteria at different temperature conditions. The invention further relates to such freeze dried bacteria for use in food industry or in human or animal health applications. More in particular, the invention relates to the increased viability and long-term storage of recombinant bacteria capable of expressing heterologous proteins or peptides and administered to humans or animals for therapeutic or vaccination purposes.
Owner:INTREXON ACTOBIOTICS NV
Process for manufacturing chewable dosage forms for drug delivery and products thereof
ActiveUS7955632B2Without risk of cross-contaminatingParticularly palatable to pet animalsOrganic active ingredientsPowder deliveryFood gradeAdditive ingredient
A palatable, edible soft chewable medication vehicle for delivery of a pharmaceutically acceptable active ingredient, such as a drug, to an animal or human subject. The edible soft chews contain only food grade or better inactive ingredients, and preferably do not contain ingredients of animal origin. Processes for manufacturing the edible soft chews do not require the use of heat or the addition of water during mixing of active and inactive ingredients, provide stable concentrations of the active ingredient, and produce chews of consistent weight and texture.
Owner:ELANCO US INC
Method for preparing biological scaffold materials
InactiveUS20080027562A1Maintain mechanical strengthLess degree of cross-linkingHeart valvesDead animal preservationProsthesisElastin tissue
A method for preparing a scaffold material for use in the prosthesis therapy is disclosed. The method comprises (a) lyophilizing a segment of native soft tissue of mammalian origin, heating the lyophilized tissue at a temperature of 100-200° C., and incubating the tissue with elastase to selectively remove elastin leaving the extracellular components mainly comprised of collagen.
Owner:NAT CEREBRAL & CARDIOVASCULAR CENT
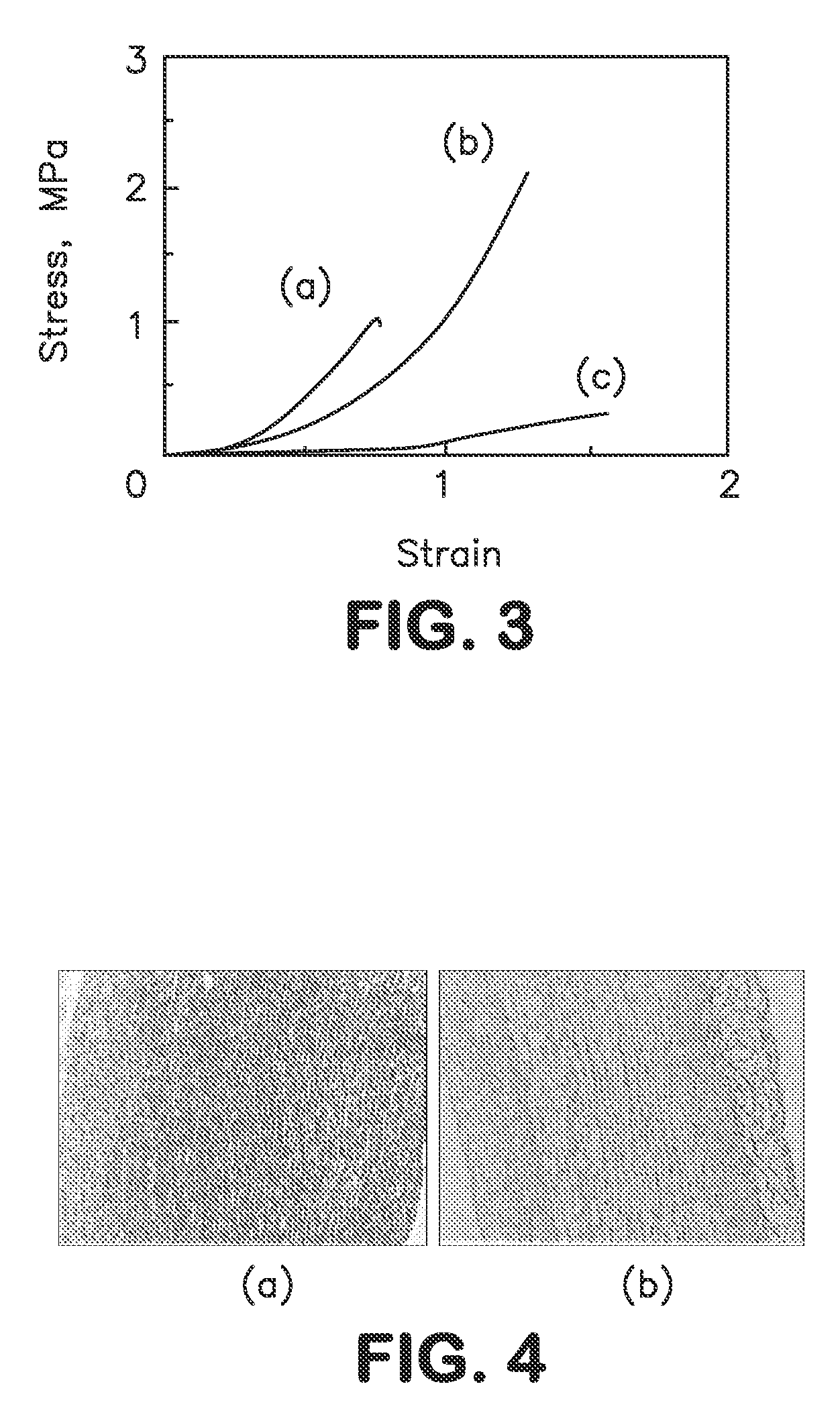
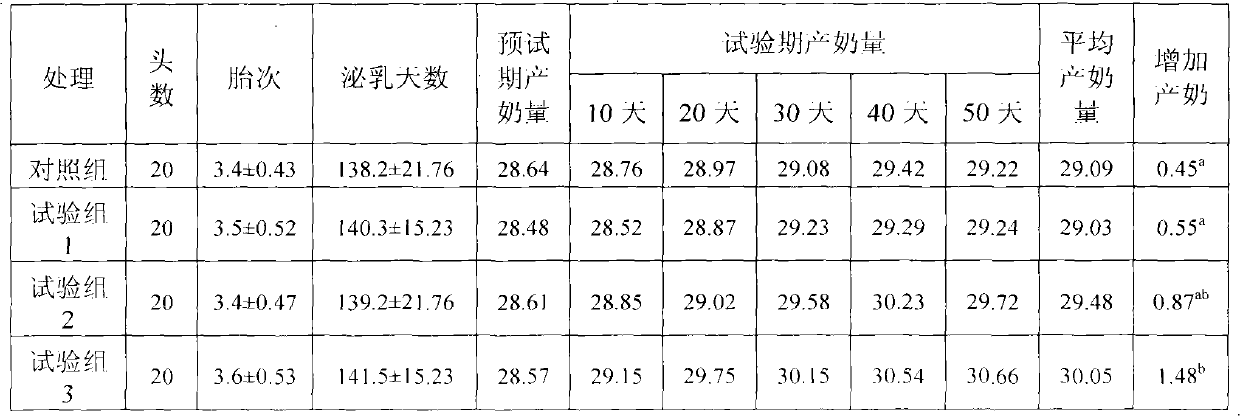
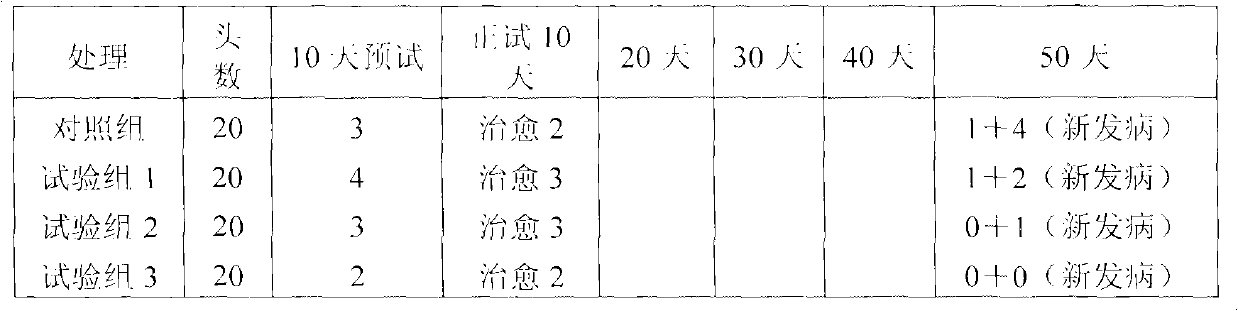
Physiological regulator for maternal animals, and preparation method thereof
ActiveCN102232487AProtected from vandalismGood curative effectFood processingAnimal feeding stuffDiseaseFood additive
The invention discloses a special physiological regulator for maternal animals, and a preparation method thereof, and belongs to the field of feed additive. A technical scheme of the physiological regulator comprises: carrying out crushing and screening for dandelion, leonurus, licorice root and angelica; adding soybean meal and / or wheat bran; adding water to carry out immersing; adjusting pH value of the resulting mixture; carrying out a sterilization; inoculating probiotics to carry out a fermentation after cooling, followed by stoving after completing the fermentation to obtain the physiological regulator. With the prepared physiological regulator for the maternal animals, immunity and disease resistance of the maternal animals are raised, incidence rate of genital system infection is effectively reduced, feed inversion rate is increased, mammogenesis is improved, milk yield is raised, incidence rate of mammitis is reduced, and milk quality is improved. The physiological regulator has characteristics of quick onset, stable and reliable effect, no toxic and side-effect, no pollution, no residue, and no drug resistance generating, and meets requirements of safe feed additive and assurance of foods of animal origin. In addition, the physiological regulator for the maternal animals can be adopted as a veterinary drug through raising using dosage of the physiological regulator, and provides a substantial curative effect for prevention and cure of the mammitis.
Owner:BEIJING KEEPYOUNG TECH
Liquid composition containing phosphoric or thiophosphoric triamide derivative and use thereof
ActiveUS20110233474A1Improve stabilityIncrease concentrationCosmetic preparationsAmmonium nitratesPhosphoric acidSolvent
The invention relates to a liquid composition containing phosphoric or thiophosphoric triamide derivatives and suitable solvents selected from the group comprising esters of hydroxyacids, heterocyclic alcohols and their derivatives, cyclic carbonic acid esters and dicarboxyacid esters, optionally the composition may also contain glycol ethers and auxiliary substances. The invention further includes the use of this liquid composition as urease inhibitor in urea-containing fertilizers, in fertilizers and wastes of animal origin or in sprays masking animal urine odours.
Owner:AGRA GROUP AS
Methods of using modified natural products as dewatering aids for fine particles
InactiveUS6375853B1Reduce moistureHigh degreeDrying solid materials without heatSolid fuelsNatural productTransesterification
Naturally occurring lipids of vegetable and animal origin are broken into smaller molecules, and used as dewatering aids. The process of breaking the molecules include transesterification, interesterification, and saponification followed by acidulation. The modified lipid molecules can adsorb on the surface of the particles to be dewatered and greatly enhance their hydrophobicity, which will help increase the rate of dewatering and hence reduce cake moisture. The modified lipids are more effective dewatering aids than the naturally occurring unmodified lipids, possibly because they can more readily form close-packed monolayers of hydrophobes on the surface of the particles.
Owner:YOON ROE HOAN
Medicaments for healing skin conditions in humans
InactiveUS20040214750A1Short timeQuick cureBiocideCosmetic preparationsOral medicationAdditive ingredient
The present invention is directed to a novel progression of topical and oral administration or use of lactoferrin or lactoferrin and other biological ingredients to prevent and treat dermatological conditions or disorders in humans. More specifically, the present invention is directed to the administration of lactoferrin in a topical material such as an ointment or cream to the skin of humans and also including a oral administration, together the topical and oral administration has a synergetic effect in the treatment of the skin. The progression of medicaments of the present invention are: 1) lactoferrin; 2) lactoferrin and conjugated linoleic acids; 3) lactoferrin, a plant or animal derived lipid and oblepicha; 4) lactoferrin, a plant or animal derived lipid, oblepicha, and an omega fatty acid; and 5) lactoferrin, a plant or animal derived lipid, oblepicha, an omega fatty acid and a whey protein.
Owner:GEORGIADES IZOLDA M
Therapeutic Agent For Ophthalmic Diseases
A therapeutic agent for ophthalmic diseases containing Laennec (trade name) as an active ingredient. Laennec, the active ingredient, exhibits a therapeutic effect on a wide variety of ophthalmic diseases by increasing tears and the like and is highly safe even though it is an animal-derived component. Therefore, the therapeutic agent is applicable to the prevention and / or treatment of various types of ophthalmic diseases, particularly corneal disorders, dry eye, asthenopia, inflammatorily ophthalmic diseases (e.g., meibomian gland dysfunction, Stevens-Johnson syndrome, Sjogren syndrome, uveitis) and ophthalmic diseases caused by active oxygen (e.g., cataract, glaucoma, age-related macular degeneration, optic disc atrophy).
Owner:HIBINO SAWAKO
Rubber compositions with non-petroleum oils
ActiveUS7211611B2Facilitate the processGood physical propertiesOrganic chemistrySolesPolymer sciencePlasticizer
Owner:NIKE INTERNATIONAL LTD
Methods of using natural products as dewatering aids for fine particles
InactiveUS6526675B1Reduce moistureImprove hydrophobicityPigmenting treatmentDrying using combination processesLipid formationSlurry
A method of dewatering fine particulate materials is disclosed. In this method, an aqueous slurry of fine particles is treated with appropriate hydrophobizing reagents so that the particulate material becomes moderately hydrophobic. A lipid of vegetable or animal origin is then added to the slurry in solutions of light hydrocarbon oils and short-chain alcohols, so that the hydrophobic lipid molecules adsorb on the moderately hydrophobic surface and, thereby, greatly enhance its hydrophobicity. By virtue of the enhanced hydrophobicty, the water molecules adhering to the surface are destabilized and more readily removed during the process of mechanical dewatering. The moisture reduction can be further improved using appropriate electrolytes in conjunction with the lipids, spraying surface tension lowering reagents onto the filter cake, subjecting the cake to a suitable vibratory means, and using combinations thereof.
Owner:YOON ROE HOAN
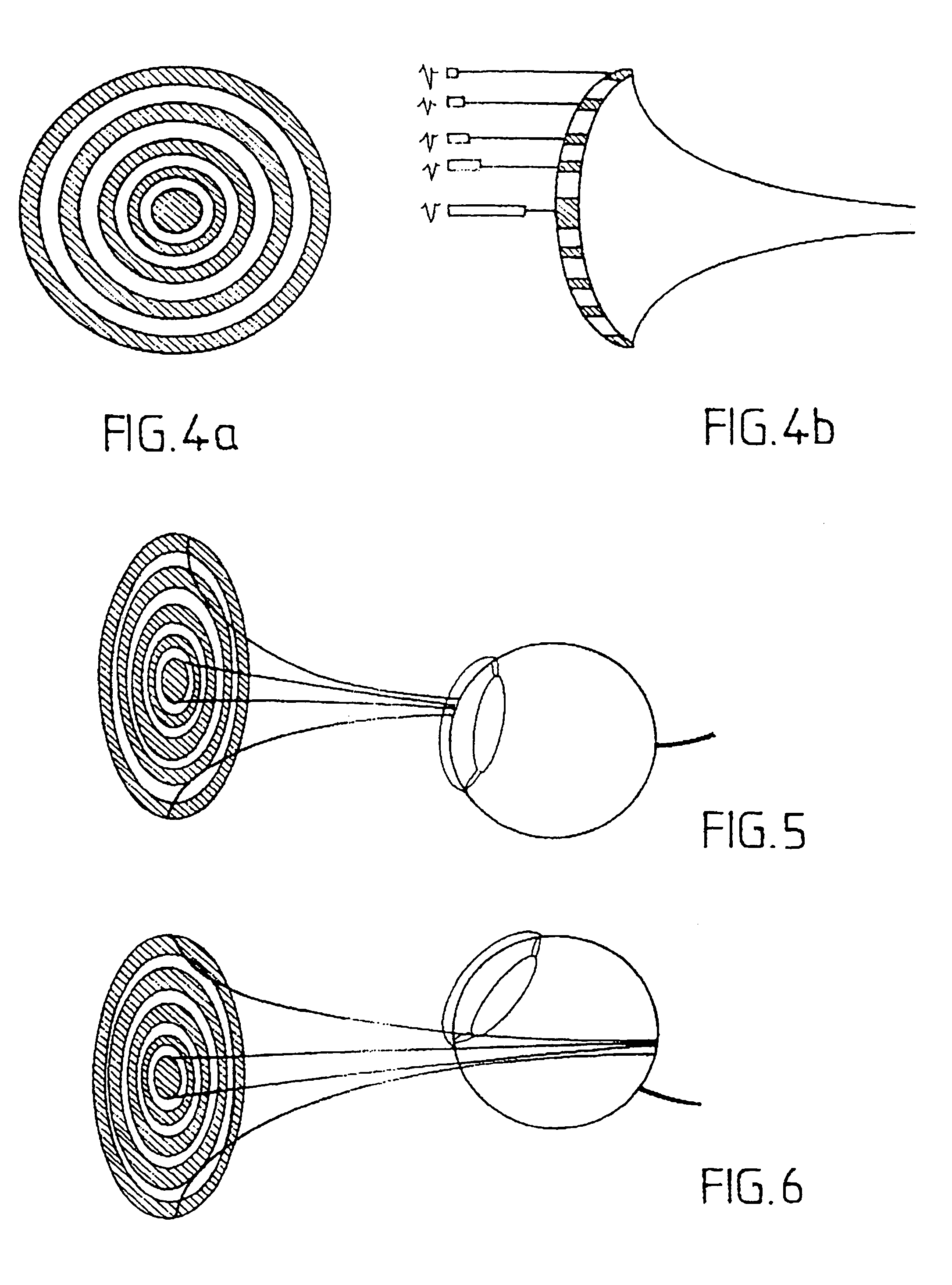
Method for exploring and displaying tissue of human or animal origin from a high frequency ultrasound probe
InactiveUS6949071B1Promote reproductionEasy to explainInfrasonic diagnosticsEye diagnosticsProximateUltrasonic transmission
A method for displaying scanned ultrasound images of tissue employs an apparatus including an ultrasound probe mounted to a mechanical head. A three-dimensional positioning system mounts the head for positioning the probe in proximate orthogonal relation to the tissue. A computer controls the three-dimensional positioning system thereby moving the probe during a scan. The probe transmits high frequency ultrasound waves whose nominal frequency is included within the range from 30 to 100 MHz and with a large pass band, adapted to frequencies reflected by the tissue. The beams of ultrasound transmission are focused in a given zone of the tissue over a vertical penetration distance of between 20 and 30 mm. Reflected signals are acquired and processed for display.
Owner:CENT NAT DE LA RECHERCHE SCI
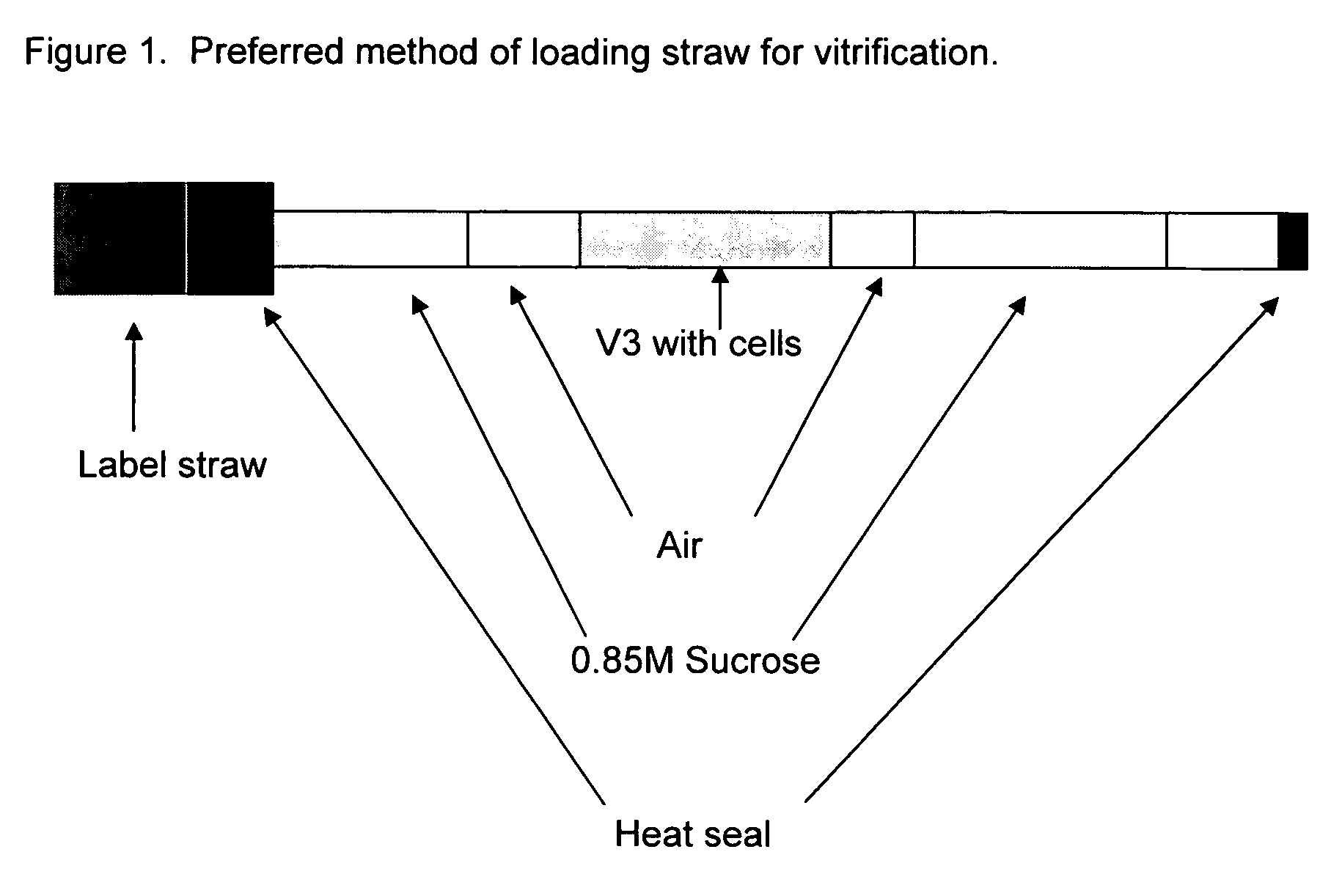
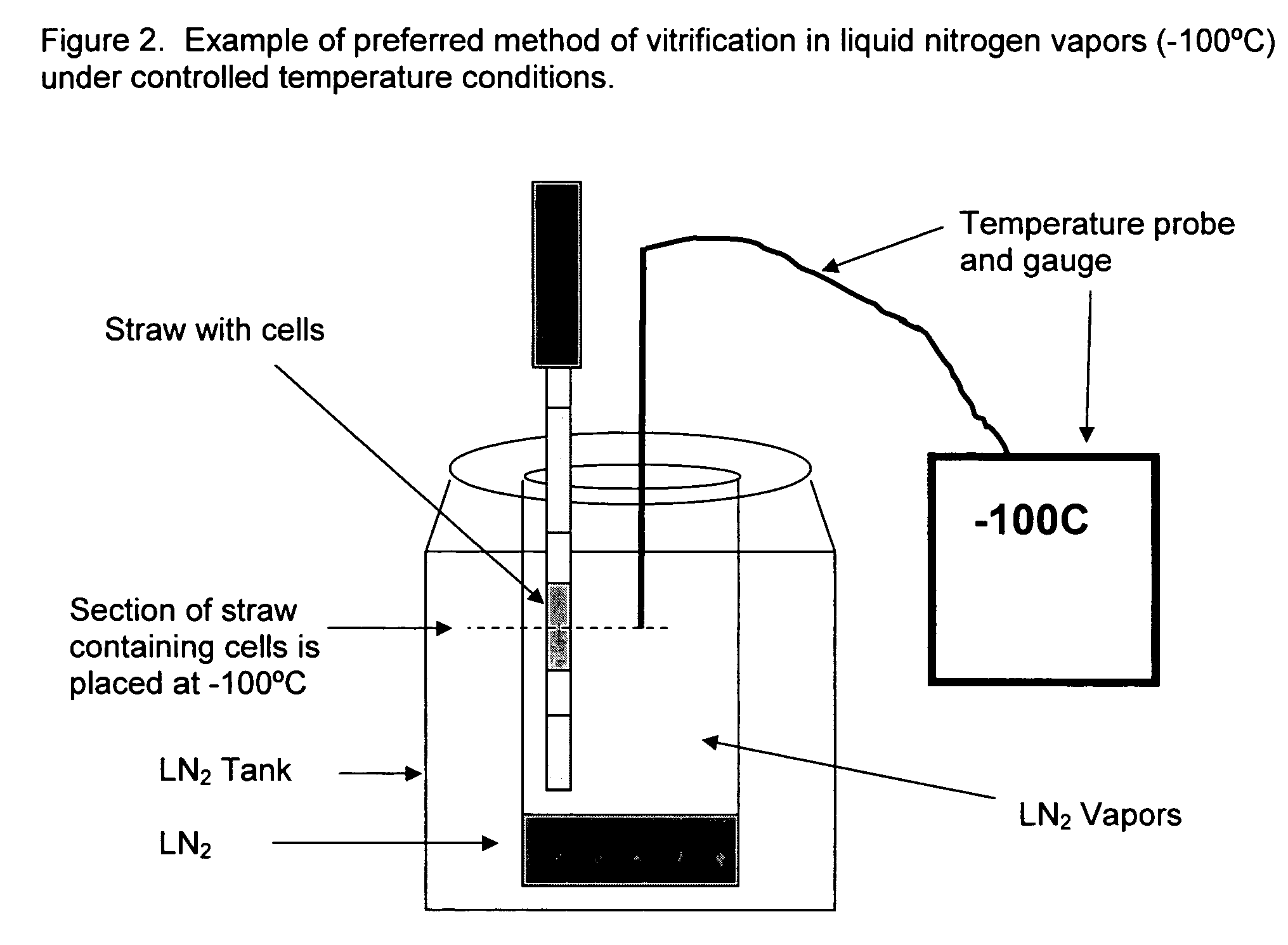
Method for vitrification of mammalian cells
InactiveUS20060046243A1Enough timeAffects successDead animal preservationVitrificationBiological cell
A method of vitrifying mammalian cells. According to the method of the present invention, biological cells of mammalian origin are frozen quickly by a vitrification method. Upon exposure to a coolant, the biological cells undergo vitrification. The biological cells which have undergone vitrification may be stored for a period of time and then devitrified at a later date. The devitrified biological cells remain viable. Preferred biological cells according to the present invention are developmental cells including blastocysts, embryos, and oocytes.
Owner:TYHO GALILEO RES LAB
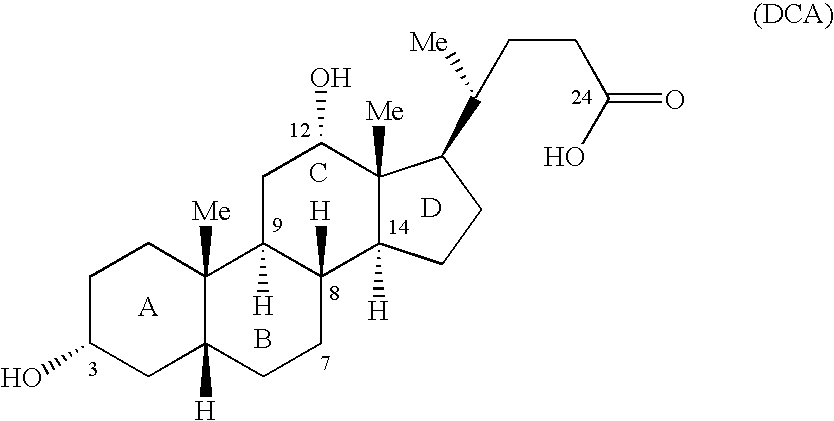
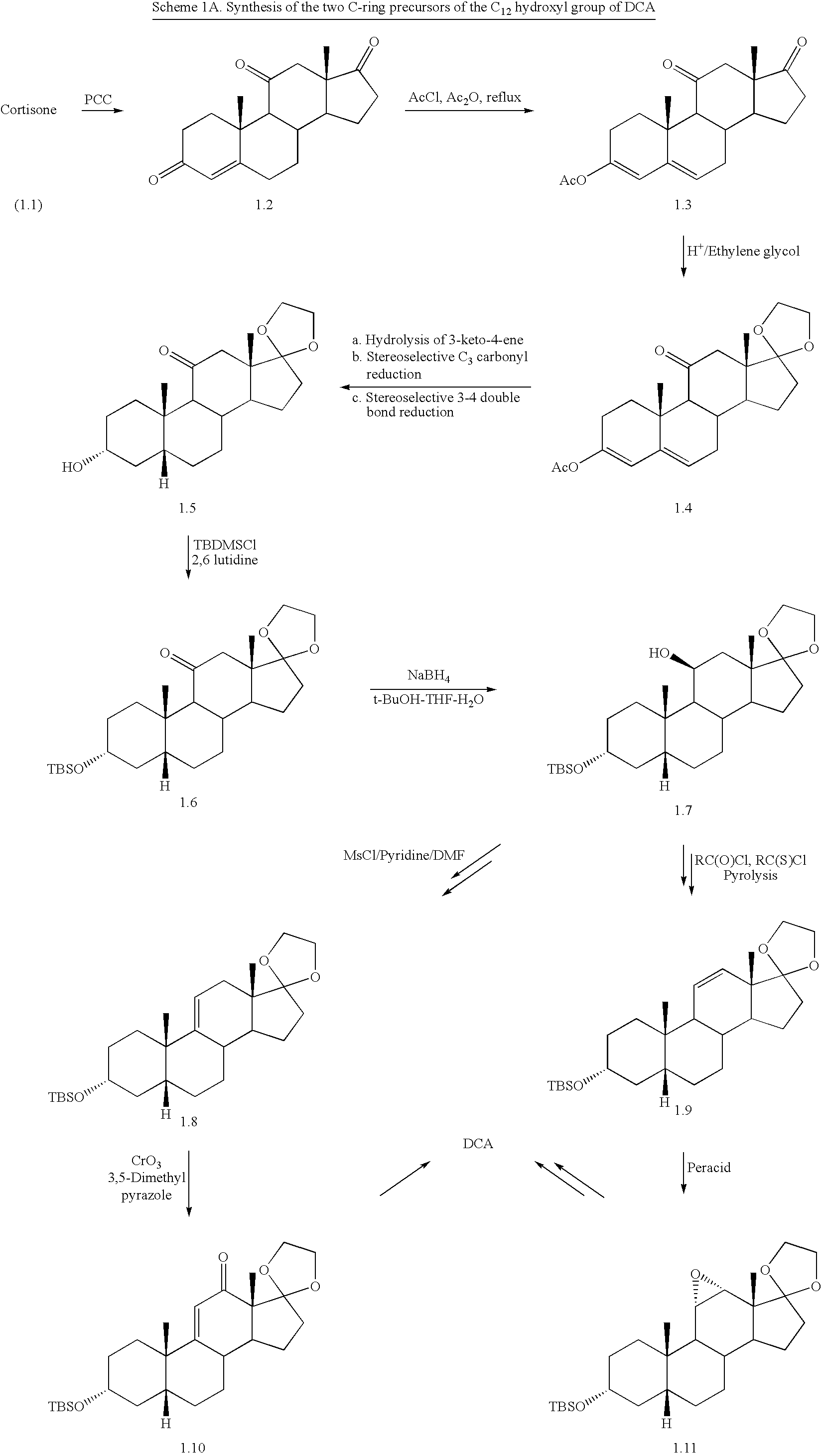
Synthetic bile acid compositions and methods
Bile acids and related compositions and methods of synthesis and use. More specifically, deoxycholic acid and related compositions, said compositions being free of all moieties of animal origin and free of pyrogenic moieties.
Owner:ALLERGAN SALES LLC
Animal origin free flocks and herds frozen semen diluent and production method of flocks and herds frozen semen
InactiveCN101310729AReduce wasteEliminate the potential risk of spreading various diseasesMammal material medical ingredientsDead animal preservationAdditive ingredientSemen
A cattle and sheep non-animal-derived frozen semen diluent comprises at least one or the combination of the following ingredients: trehalose, propylene glycol, soybean lecithin, soluble soybean protein, nitrogen-trishydroxymethyl aminoethanesulfonic acid and dodecanoyl sodium sulfate. A production method of a cattle and sheet frozen semen by using the cattle and sheep non-animal-derived frozen semen diluent fully mixes the cattle or sheet semen and the dilutent, lowers the temperature and carries out the preservation. Compared with the prior art, the animal semen dilutent of the invention eliminates the animal-derived ingredients; the preservation method is more practical, the technical effects are better, thus thoroughly eliminating the potential risks of spreading various epidemic diseases between the animals and the people by the animal-derived ingredients in the dilutent. The cattle and sheep non-animal-derived frozen semen diluent can promote the popularization of animal artificial insemination technology to the utmost extent and improve the labor productivity and the economic benefits, thus having great economic and social values.
Owner:辽宁省重大动物疫病应急中心 +1
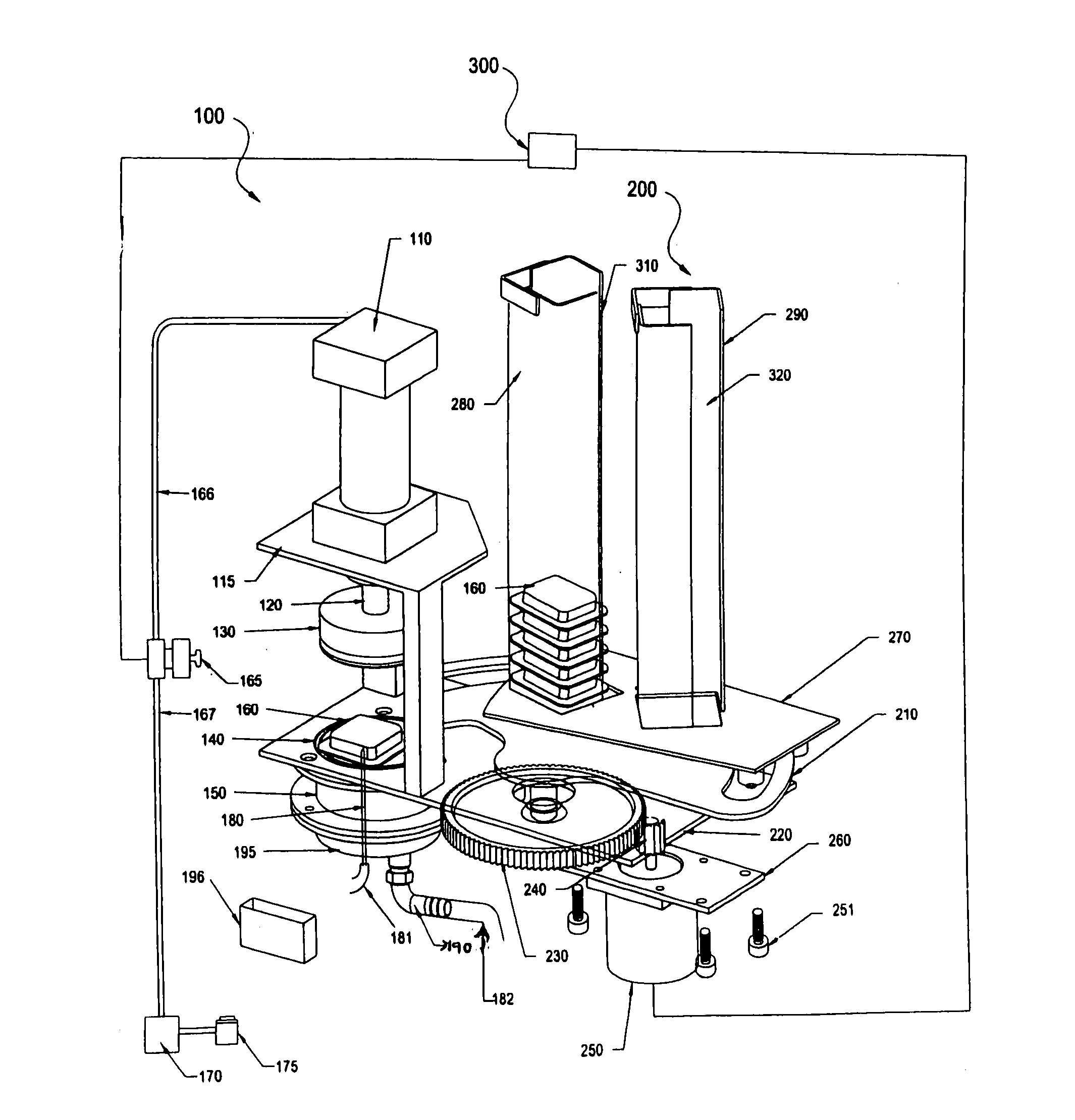
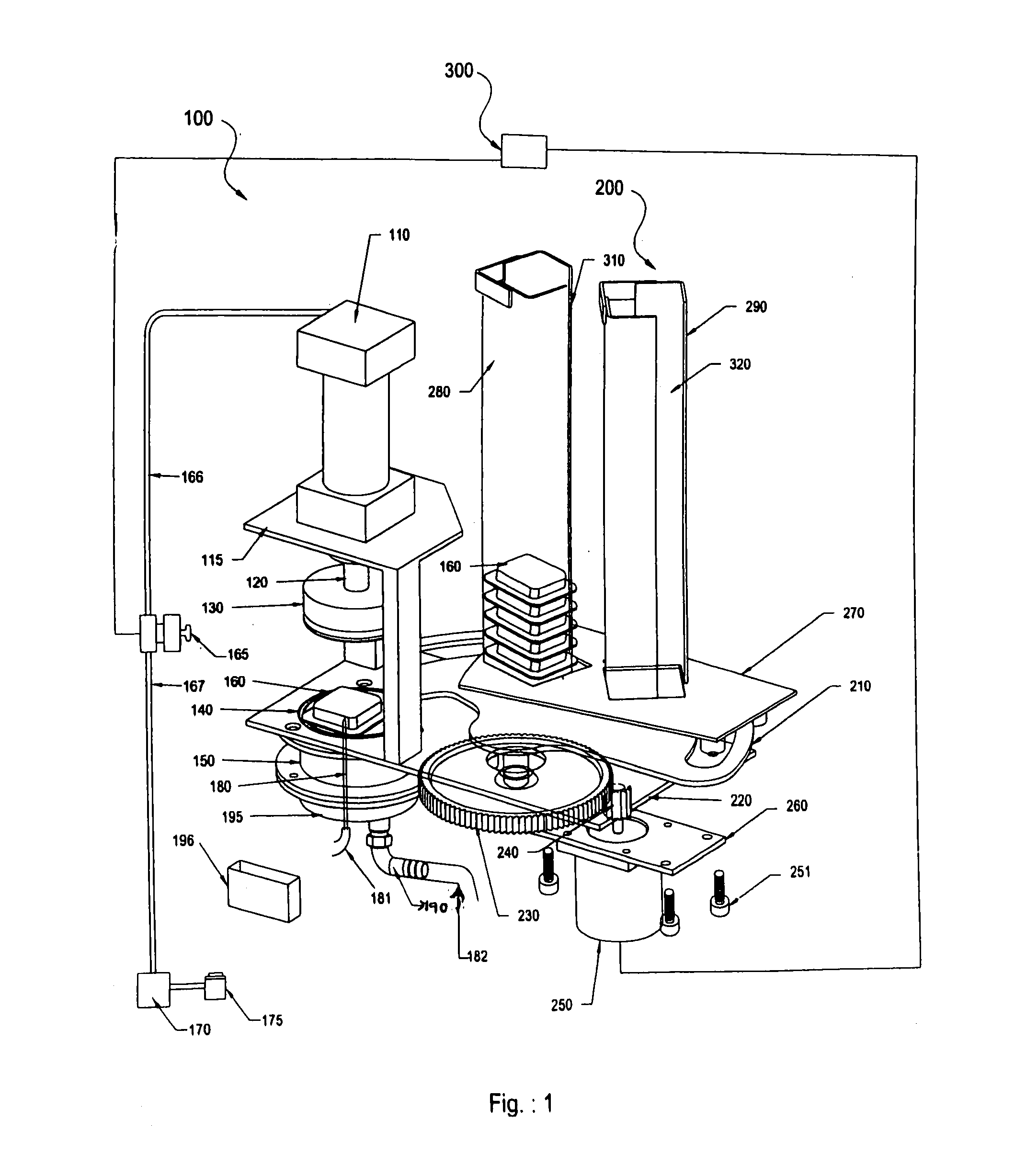
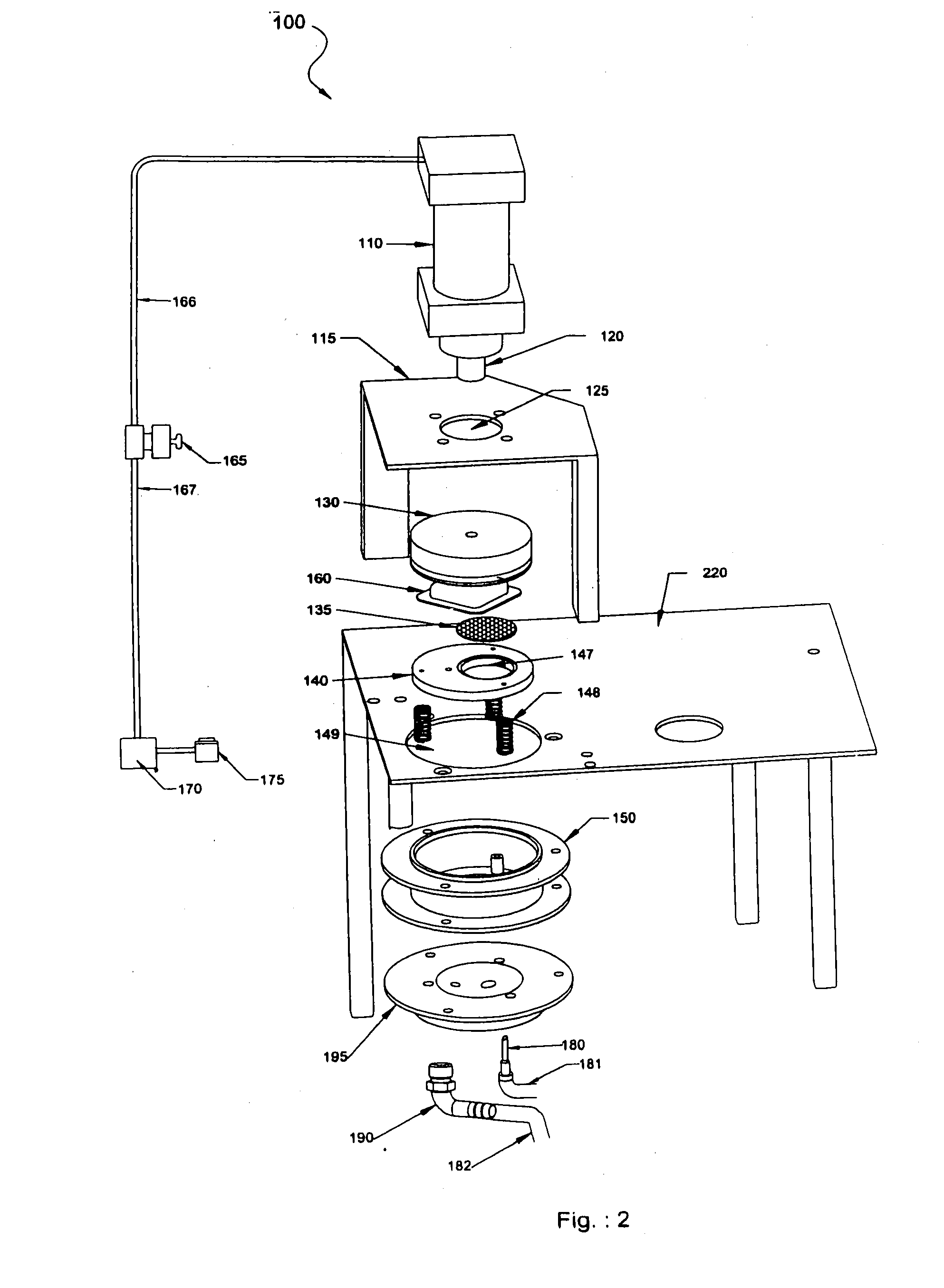
Automatic pod conveyor and brewer assembly for fresh hot beverage
This invention relates to an automatic pod-conveyor and brewer assembly for fresh hot beverage. The brewer sub-assembly (100) includes a pneumatic cylinder (110) with its piston rod (120) fitted to a brewer plunger (130). An injection nozzle (180) is detachably fixed from underneath to the brewer base (195) with its conical tip protruding into the brewer chamber (150). The single serving pod (160) is a receptacle (510) containing the brewing, infusible or other material (520), covered and sealed with a porous membrane (530). The injection nozzle (180) injects hot pressurized water, received through its interior from a suitable water heating system, into a single serving pod (160) placed within the brewer sub-assembly (100), and the brewed beverage flows out to a dispensing cup through a liquor outlet port (190) in the brewer base (195). The pneumatic cylinder (110) is operated by air solenoid valve (165) with compressed air from a compressed air storage tank (170). The pod-conveyor sub-assembly (200) includes a pod conveyor (210) which, by its rotational movement in a horizontal plane, conveys the pod (160) to the brewer sub-assembly (100) from chutes (280, 290). The pod-conveyor (210) is operated by a pusher motor (250) in co-operation with a pusher gear (230) fitted to the integral shaft (215) of the pod-conveyor (210). The assembly can be used to prepare hot beverage of a wide variety of brewing, infusible or other materials like tea, coffee, materials of plant origin such as floral pieces like Jasmine flowers, vegetable pieces like carrots, onions, materials of animal origin such as honey or other suitable beverage powder / material which can be placed in the single serving pod (160) of the assembly.
Owner:TATA GLOBAL BEVERAGES
Animal origin-free low-protein culture medium suitable for animal cell product production
ActiveCN101603026AEasy to separate and purifySuitable for productionTissue cultureFermentationLipid formationAntioxidant
The invention relates to an animal origin-free low-protein culture medium suitable for animal cell product production, comprising 24 basic metabolism nutrients, 11 vitamins, 3 transferrin substitute compounds, 5 lipid compounds, 2 nucleic acid compounds, 4 hormones and growth factors, 3 antioxidants, 1 shear-resistant protective agent, 1 pH indicator, 2 pH buffers, 9 other inorganic salts, soy hydrolysates adopted to substitute animal origin component, and composition of ferrous sulfate, ferric nitrate and EDTA-2Na adopted to substitute transferrin. The culture medium can be made by dissolving the aforementioned components in triply distilled water. The positive effects of the culture medium are as follows: the culture medium contains no animal origin component, the total protein content is lower than 10mg / L, which helps separate and purify products and is suitable for production of recombinant protein medicaments; the culture medium supports normal growth and long-term subculturing of animal cells; the culture medium can be used without adaptation, is easily prepared and is suitable for massive production of animal cell products.
Owner:EAST CHINA UNIV OF SCI & TECH
Use of one or more natural or modified oxygen carriers, devoid of plasma and cellular membrane constiuents, for externally treating open, in particular chronic wounds
The present invention relates to the use of one or more natural or modified oxygen carriers, devoid of plasma or cellular membrane constituents, for the production of an agent for the external treatment of open wounds, particularly chronic wounds. Hemoglobin or myoglobin of human or animal origin are suitable as oxygen carriers. The oxygen carriers can also preferably be modified. Suitable modifications are cross-linking, reaction with polyalkylene oxides, chemically reactive or chemically non-reactive effectors, or combinations. The agent is applied to the wound area particularly by means of spraying on an aqueous solution containing the oxygen carrier(s). The oxygen carriers can be used in particularly effective manner in the case of chronic wounds resulting from tissue degeneration, particularly diabetic tissue degeneration.
Owner:SANGUIBIOTECH
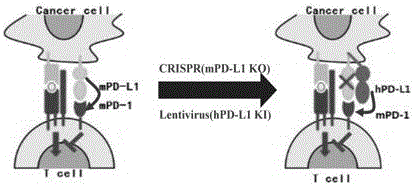
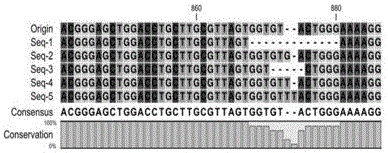
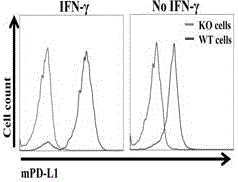
Humanized PD-L1 tumor cell line, animal model with same and application of humanized PD-L1 tumor cell line and animal model
ActiveCN105950560ASpeed up the processLethalCompounds screening/testingCell receptors/surface-antigens/surface-determinantsPD-L1Wilms' tumor
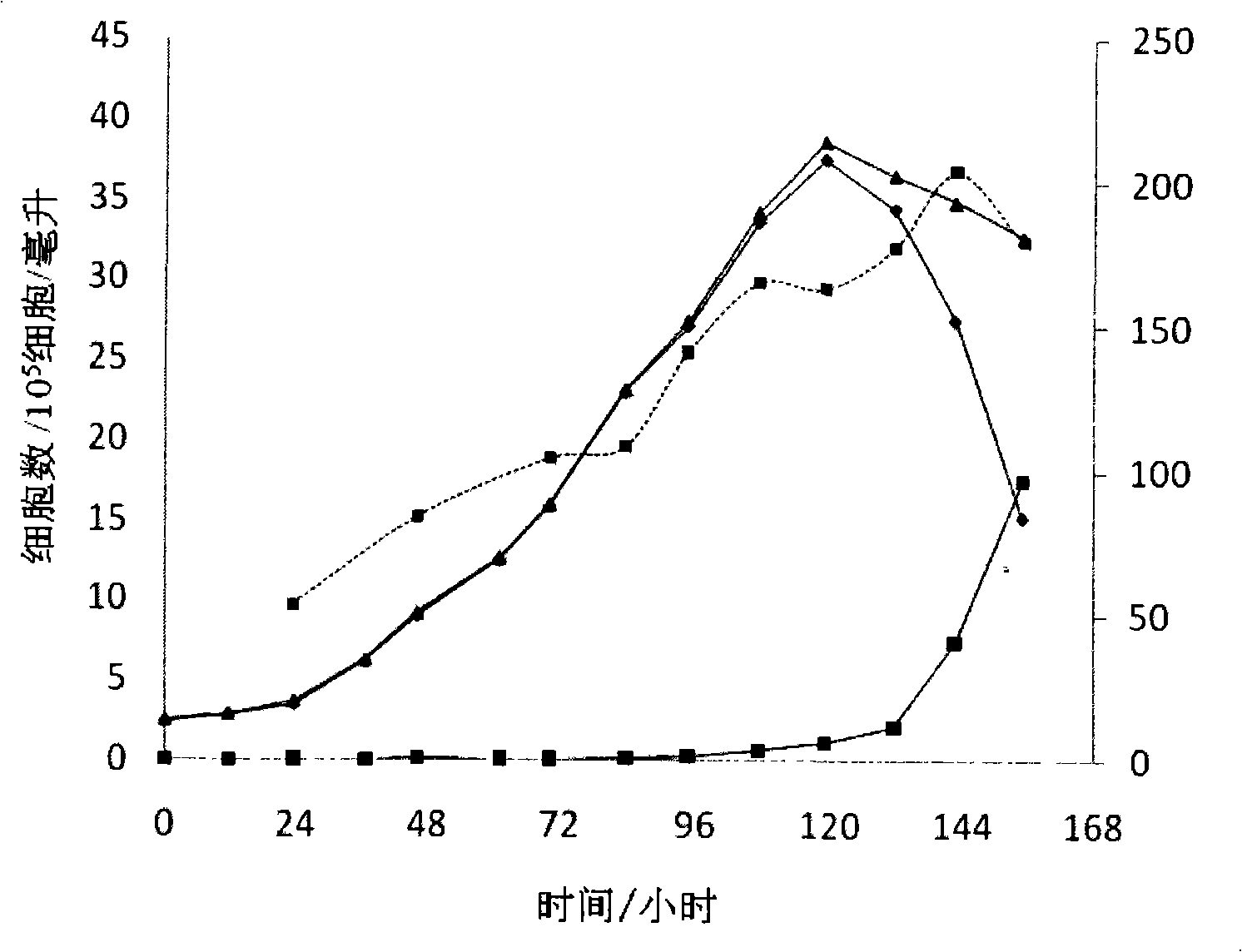
The invention provides a humanized PD-L1 tumor cell line MC-38-hPD-L1, a builtanimal tumor model with the same and a method for constructing the humanized PD-L1 tumor cell line. The method particularly includes knocking out animal-origin PD-L1 by the aid of CRISPR-CAS9; carrying out amplification and cultivation to obtain knocked-out cell banks; extracting DNA (deoxyribonucleic acid) and carrying out PCR (polymerase chain reaction) amplification; recycling and cloning amplification products; carrying out over-expression on human-origin PD-L1 in MC-38 cell lines of mPD-L1 KO by the aid of lentivirus systems; packaging lentivirus and screening Puromycin to obtain the humanized MC-38 cell line of PD-L1. The humanized PD-L1 tumor cell line, the animal tumor model and the method have the advantages that as shown by results, high killing efficiency and multiplication capacity are obviously presented by tumor infiltration CD8 T lymphocytes after antibody treatment is carried out, tumor infiltration Treg cells can be obviously inhibited after antibody treatment is carried out, and accordingly the method is proved to be effective and feasible from the aspect of molecular mechanisms.
Owner:SUZHOU INST OF SYST MEDICINE
Process for manufacturing chewable dosage forms for drug delivery and products thereof
ActiveUS20090280159A1Without risk of cross-contaminatingParticularly palatable to pet animalsAntibacterial agentsNervous disorderPharmacyFood grade
A palatable, soft chewable medication vehicle for delivery of a pharmaceutically acceptable active ingredient, such as a drug, to an animal or human subject. The soft chews contain only food grade or better inactive ingredients, and preferably do not contain ingredients of animal origin. Processes for manufacturing the soft chews do not require the generation of heat during mixing of active and inactive ingredients, provide stable concentrations of the active ingredient, and produce chews of consistent weight and texture.
Owner:ELANCO US INC
Process for manufacturing chewable dosage forms for drug delivery and products thereof
ActiveUS20080075759A1Soft textureOpportunities decreasePowder deliveryOrganic active ingredientsFood gradeAdditive ingredient
A palatable, edible soft chewable medication vehicle for delivery of a pharmaceutically acceptable active ingredient, such as a drug, to an animal or human subject. The edible soft chews contain only food grade or better inactive ingredients, and preferably do not contain ingredients of animal origin. Processes for manufacturing the edible soft chews do not require the use of heat or the addition of water during mixing of active and inactive ingredients, provide stable concentrations of the active ingredient, and produce chews of consistent weight and texture.
Owner:ELANCO US INC
Use of Emu Oil and its various fractions as a carrier for antifungal, antibacterial, and antiviral medications and preparations
InactiveUS7048950B2Mitigated and preventedStay healthyBiocidePowder deliveryLipid formationAdditive ingredient
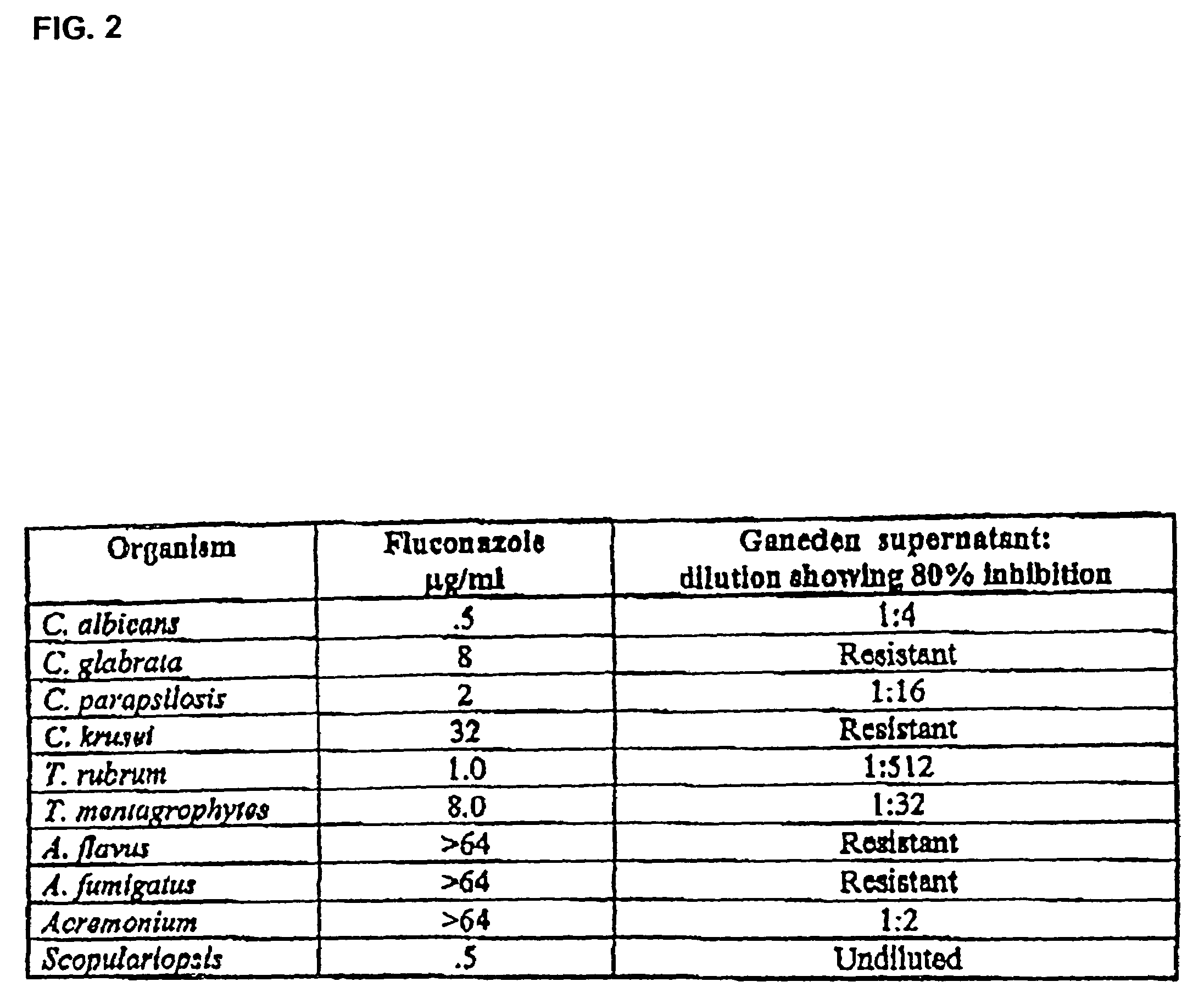
An animal-derived lipid is disclosed that is useful as a carrying agent for anti-microbial formulations. Pharmaceutical and other preparations including Emu Oil are also described as profoundly useful components in anti-bacterial, anti-fungal, and anti-viral treatments. This lipid material is extracted from the Emu (Dromais Novae-Hollandiae), an indigenous bird of Australia and New Zealand. The present invention also discloses therapeutic compositions comprising Emu Oil in combination with an extracellular product of Bacillus coagulans or Pseudomonas lindbergii strain, comprising a supernatant or filtrate of said culture suitable for topical application to the skin or mucosal membranes of a mammal, which are utilized to inhibit the growth of bacterium, yeast, fungi, virus, and combinations thereof. Additionally, the aforementioned therapeutic composition may also include an anti-microbial, anti-mycotic, and / or anti-viral agent. The present invention also discloses methods of treatment and therapeutic systems for inhibiting the growth of bacterium, yeast, fungi, virus, and combinations thereof, by topical application of therapeutic compositions comprising Emu Oil in combination with an extracellular product of Bacillus coagulans or Pseudomonas lindbergii strain suitable for topical application to the skin or mucosal membranes of a mammal. Similarly, the aforementioned method may also employ a therapeutic composition additionally containing an anti-microbial, anti-mycotic, and / or anti-viral agent.
Owner:GANEDEN BIOTECH
Feed formulations containing docosahexaenoic acid
InactiveUS20100086638A1Optimal neurological developmentImprove developmentAlgae medical ingredientsAnimal feeding stuffDocosahexaenoic acidMicroorganism
The disclosure relates to an animal feed or feed ingredient containing from about 0.01% to 1.0% DHA, wherein all, or substantially all of the DHA comes from material that is of non-animal origin and the use of microbially-derived DHA at these low levels provides sufficient DHA for the optimal neurological development of the animal.
Owner:ADVANCED BIONUTRITION CORP
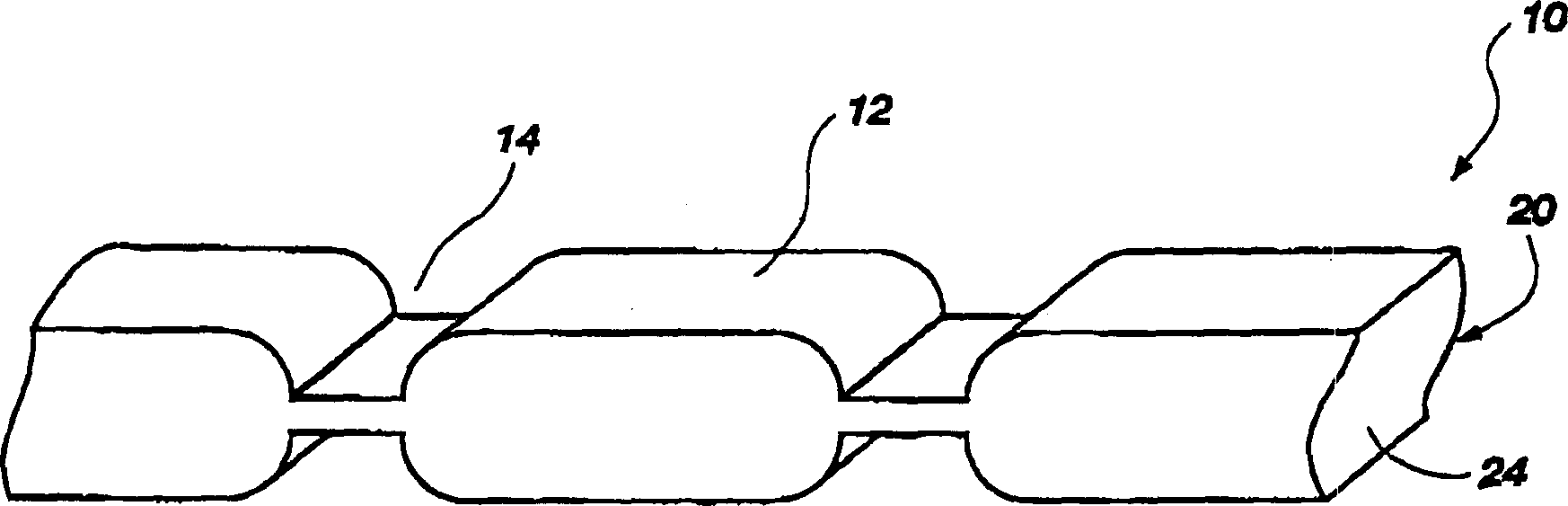
Edible thermoplastic and nutritious segmented pet chew
The present invention relates to an edible thermoplastic made from about 30 to 50 wt. % protein comprising a mixture of plant and animal derived protein, about 20 to 50 wt. % starch about 10 to 20 wt. % water, about 1 to 10 wt. % edible fiber, and about 0.5 to 3 wt. % metallic salt hydrate. When molded, the thermoplastic has good strength and stiffness and other physical properties. The edible thermoplastic may be molded in a variety of shapes including a segmented nutritional pet chew with a plurality of segments separated by a plurality of scores. The scores serve to structurally weaken the pet chew so that it may be broken into smaller pieces. When molded the edible thermoplastic has a density of about 1.2 to 1.5 g / cubic centimeters.
Owner:NATURAL POLYMER INT CORP
Concept for slurry separation and biogas production
InactiveUS7883884B2High yieldNot subsidisedBio-organic fraction processingAnimal corpse fertilisersEngineeringBiogas production
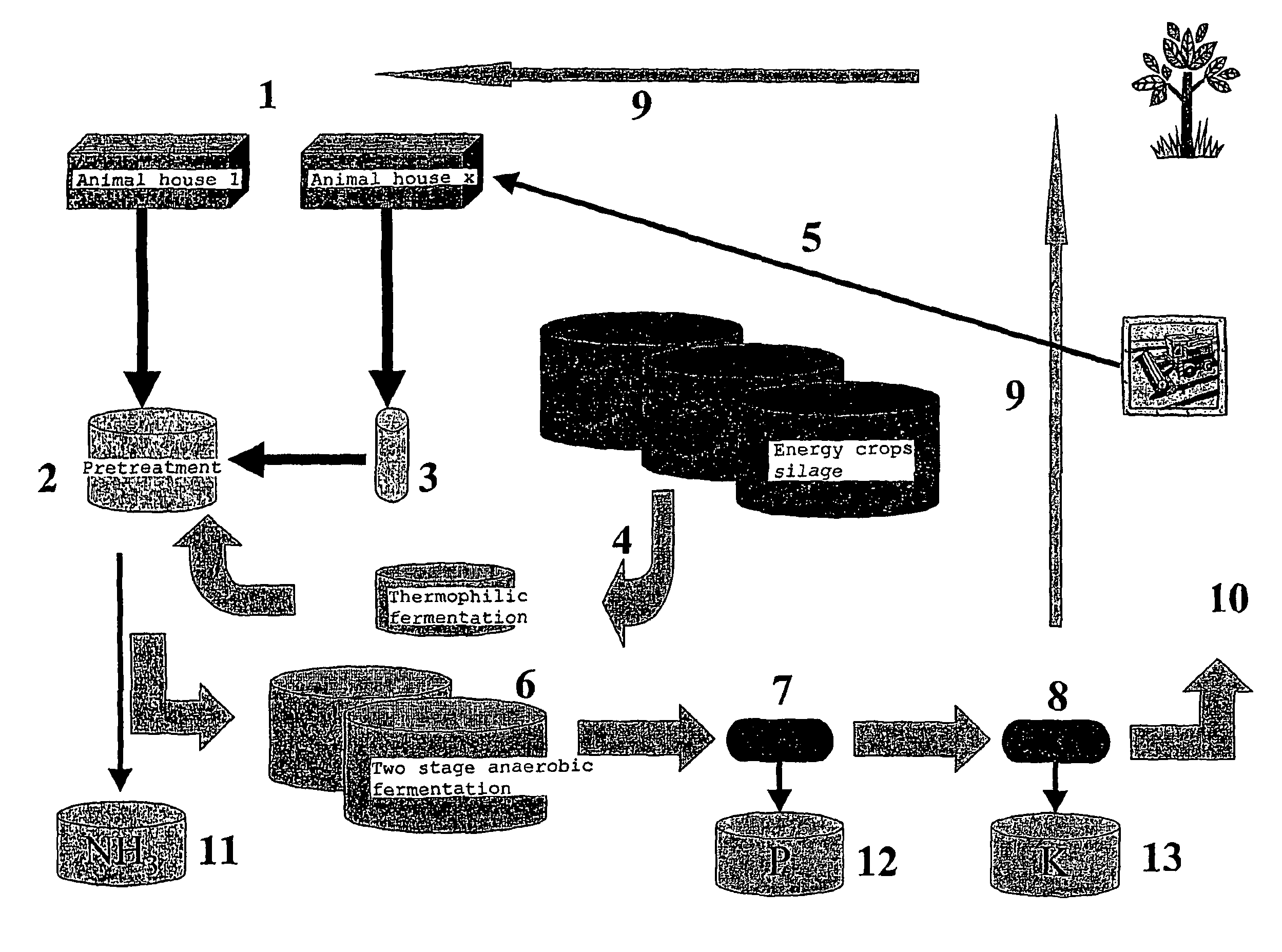
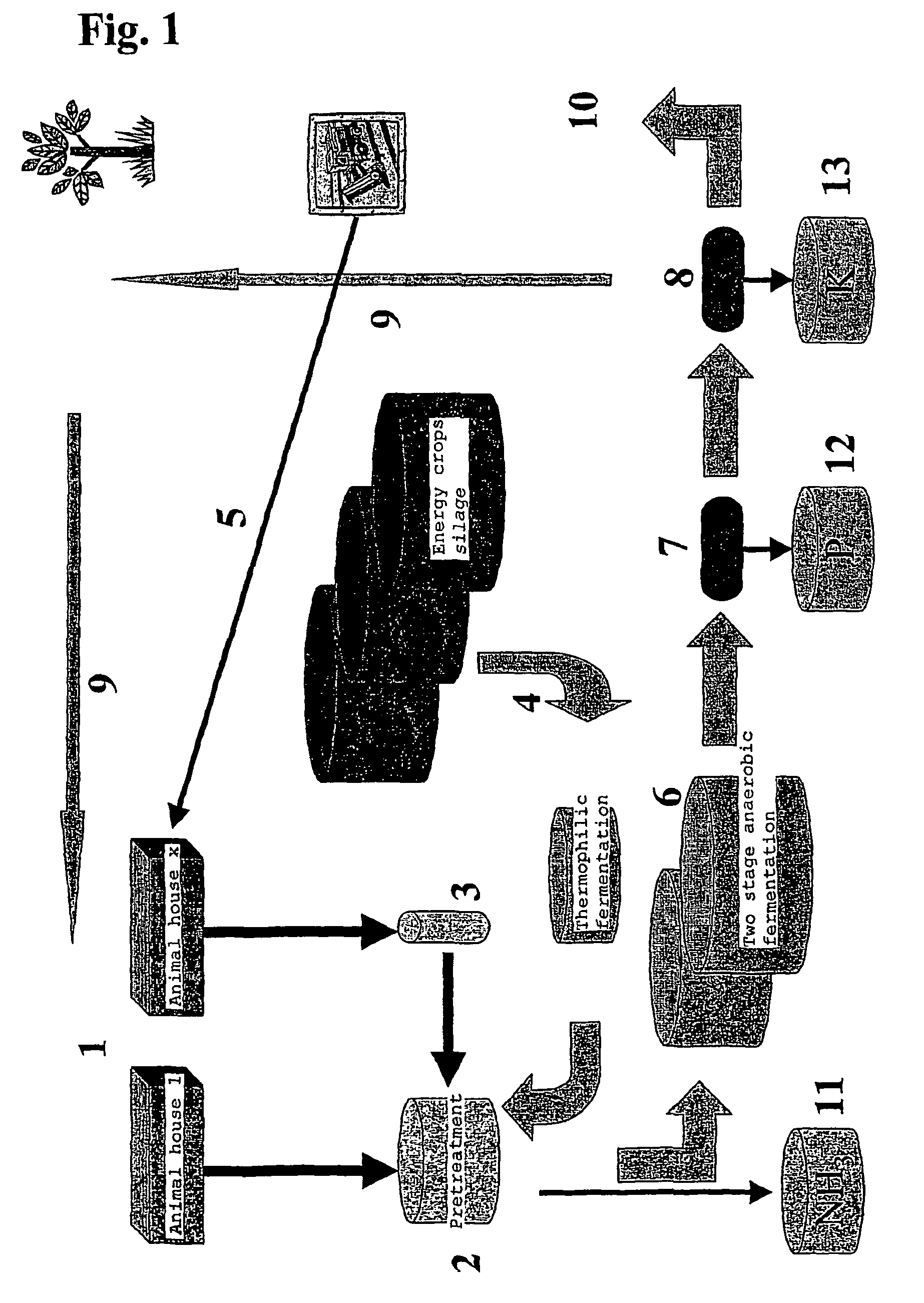
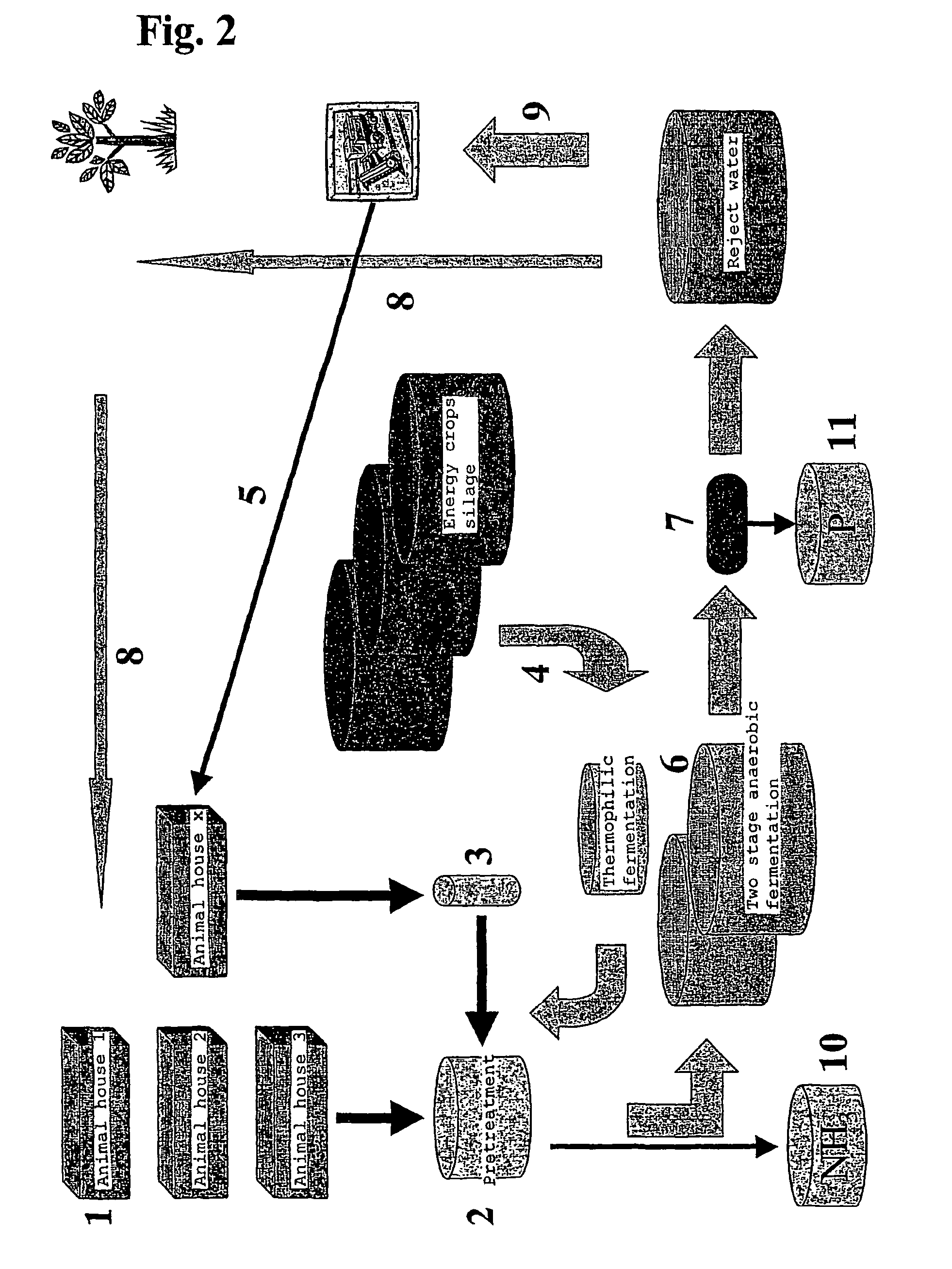
The present invention concerns an anaerobic digestion of animal manures, energy crops and similar organic substrates. The process is capable of refining nutrients comprised in the digested biomass to fertilizers of commercial quality. The invention also provides a method for oprocessing animal carcasses or fractions thereof including meat and bone meal etc., with the objective of providing an alternative means for processing the organic waste material of animal origin while at the same time facilitating the production of fertilizers. The risk of spreading BSE prions or any other prions to animals or humans is thus substantially reduced if not eliminated. The biogas and slurry separation system according to the present invention is preferably integrated with the operations of animal husbandries into a total concept in which the internal and external performances of animal husbandries are optimised. The internal performances concern quality aspects related to the management of the animal houses and include industrial hygiene, animal welfare, gaseous and dust emissions and food safety. The external performances concern mainly energy production and emissions to the environment of nutrients and greenhouse gases and the sale of high quality food product.
Owner:GFE PATENT AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com