Method for preparing recombinant human interleukin-11
A technology of interleukin and fusion protein, which is applied in the preparation method of peptides, chemical instruments and methods, organic chemistry, etc., to achieve the effects of avoiding the renaturation step of inclusion bodies, improving recovery rate and efficient purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
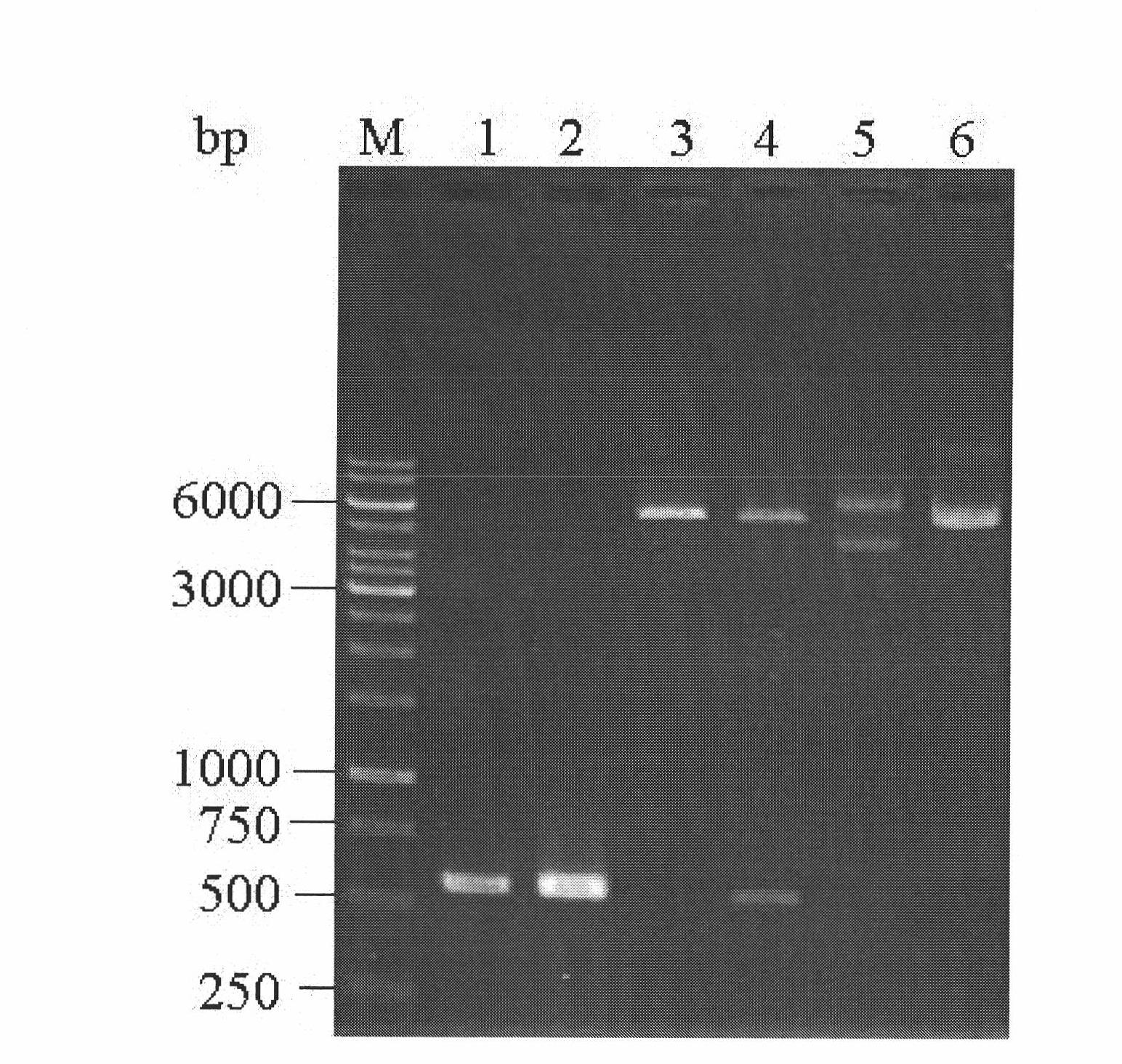
[0040] Design of DNA Sequence for Expressing rhIL-11
[0041] Compared with natural human interleukin 11, the amino acid sequence of human interleukin 11 expressed in the present invention lacks the first amino acid (proline) at the N-terminus, and its amino acid sequence is shown in SEQ ID NO:1. The nucleotide sequence encoding the rhIL-11 is shown in SEQ ID NO:2.
[0042] According to the IL-11 coding sequence listed in SEQ ID NO: 2, the stop codon TAA was introduced at the 3' end, and the DNA coding sequence GACGACGACGACAAG of the enterokinase digestion recognition site was introduced at the 5' end. Such a DNA sequence was named sequence 3 (see SEQID NO: 3), and Shanghai Sangon Bioengineering Technology Service Co., Ltd. was entrusted to artificially synthesize the sequence by means of whole gene synthesis. To facilitate subsequent subcloning, the synthetic gene was cloned into pUC18 (Fermentas Life Science Company) for preservation and further cloning of the synthetic DNA...
Embodiment 2
[0108] Embodiment 2 rhIL-11 freeze-dried powder injection
[0109] The rhIL-11 prepared above was taken, and freeze-dried powder was prepared according to the following formula.
[0110] Table 4 freeze-dried powder formula
[0111]
[0112] Ingredients Content
[0113]
[0114] rhIL-11 1.00g
[0115] NaCl 5.85g
[0116] Na 2 HPO 4 12H 2 O 2.187g
[0117] NaH 2 PO 4 2H 2 O 0.608g
[0118] Glycine 30g
[0119] Add water for injection to 1000ml
[0120]
[0121] The pH value was adjusted to 7.0, and after aseptic filtration, it was divided into 3ml / vials. Then freeze-dry to make a freeze-dried powder injection and store at 4°C.
Embodiment 3
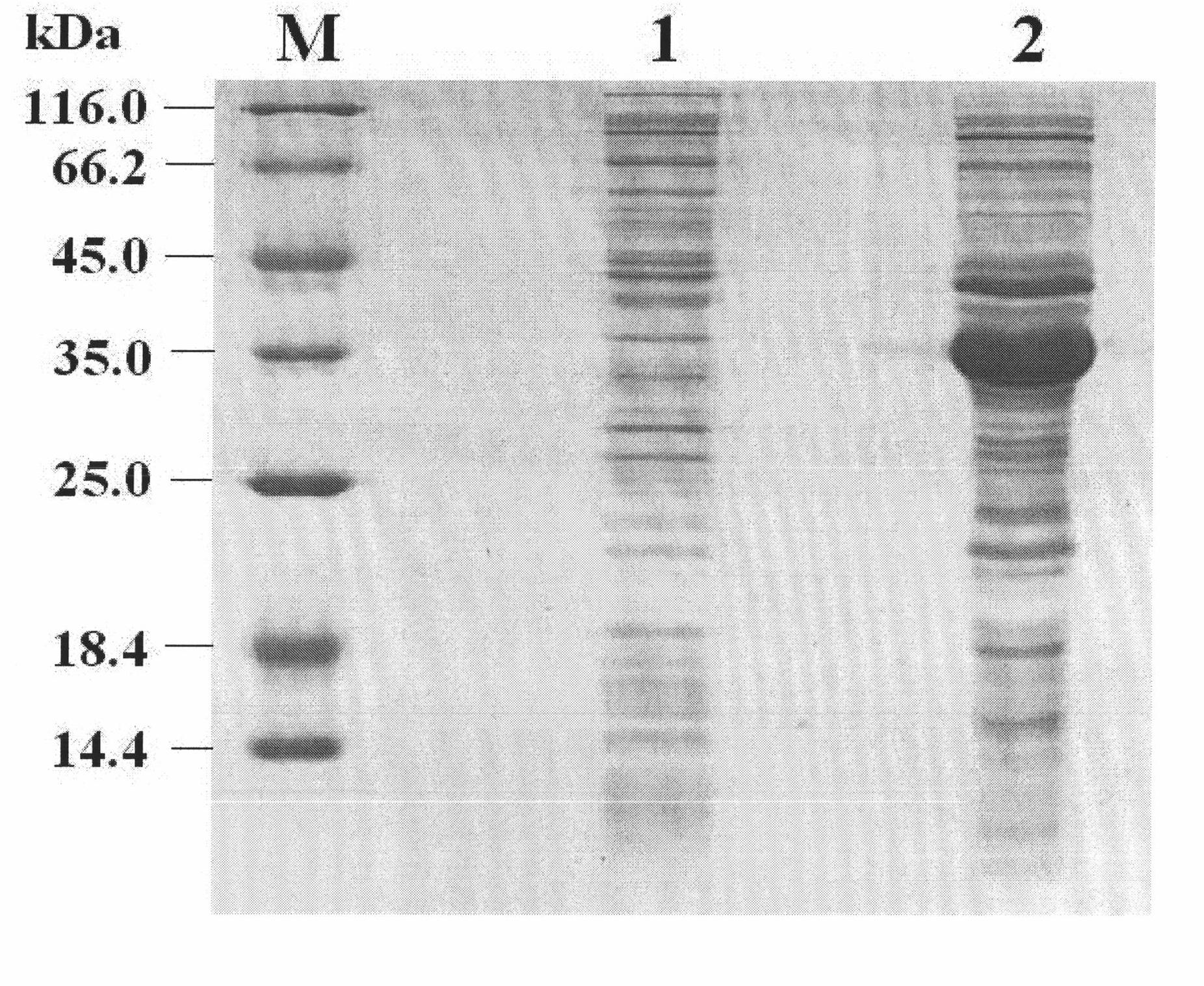
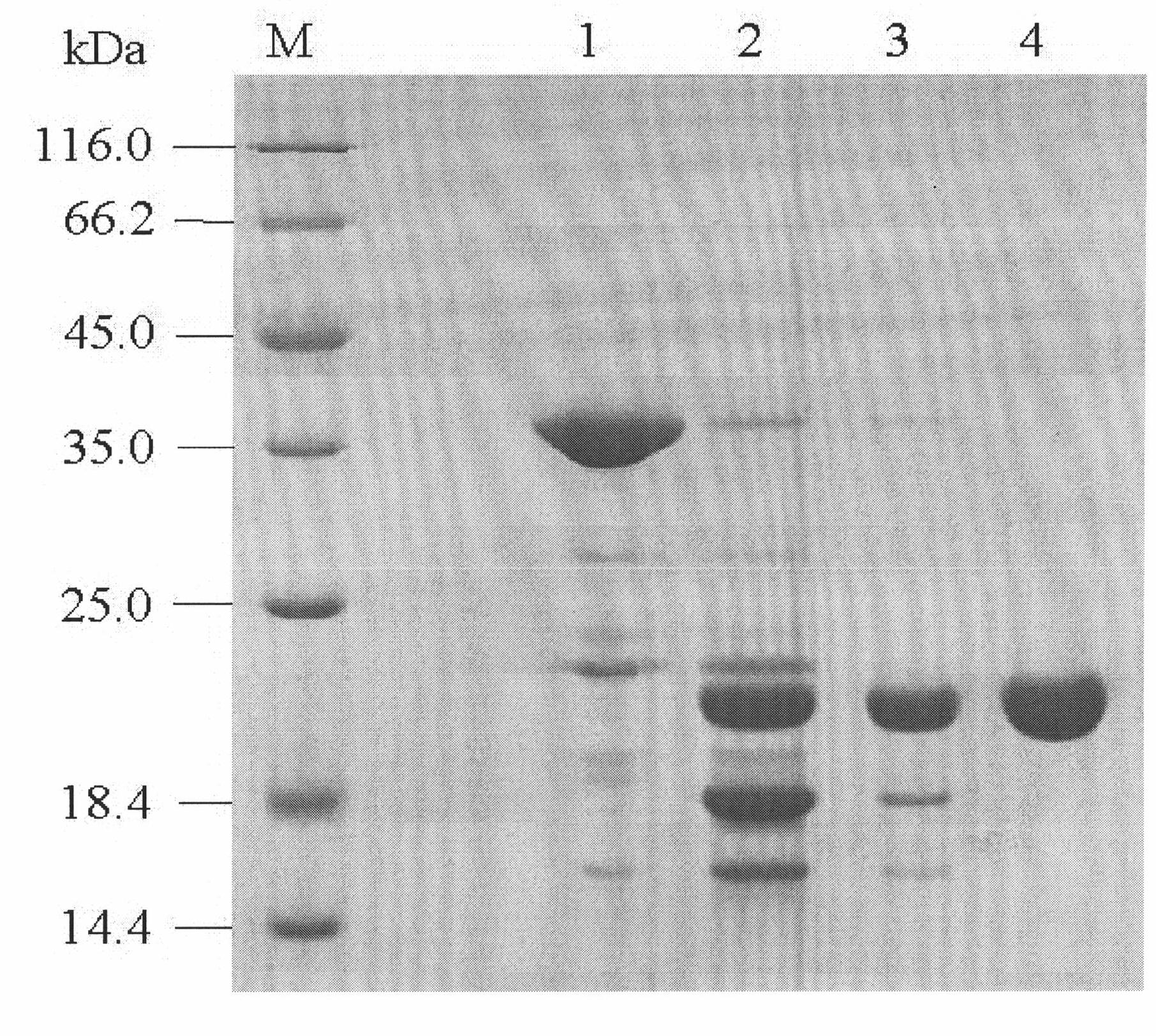
[0122] Example 3 Determination of rhIL-11
[0123] 1. SDS-PAGE purity analysis
[0124] Adopt SDS-PAGE system (Guo Yaojun, "Protein Electrophoresis Experimental Technology", Science Press, 1999) to carry out non-reducing electrophoresis, scan and measure with Bio-Rad Gel Doc 2000 gel imaging system, rhIL-11 purity is 98.90% (see image 3 in track 5).
[0125] 2. RP-HPLC purity analysis
[0126] The chromatographic column is Delta-Pak C18 5 μ m 3.9 × 150 (Waters Co.), from the eluent (at 95% by volume dH 2 O and 0.1 vol% trifluoroacetic acid (TFA) in 5 vol% acetonitrile) was changed to the eluent (in 95 vol% acetonitrile and 5 vol% dHO 2 0.1 vol% TFA in O) for linear gradient elution for 40 min, flow rate 1 ml / min, and UV detection at 220 nm.
[0127] Analysis results showed that the purity of rhIL-11 prepared by the above method was 99.28%. RP-HPLC analysis results see Figure 4 and Table 5.
[0128] table 5
[0129] serial number
Retention time (minutes) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com