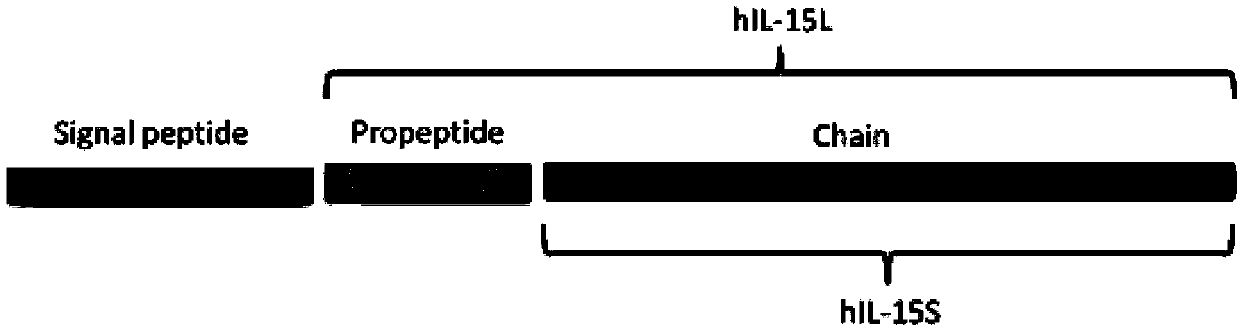
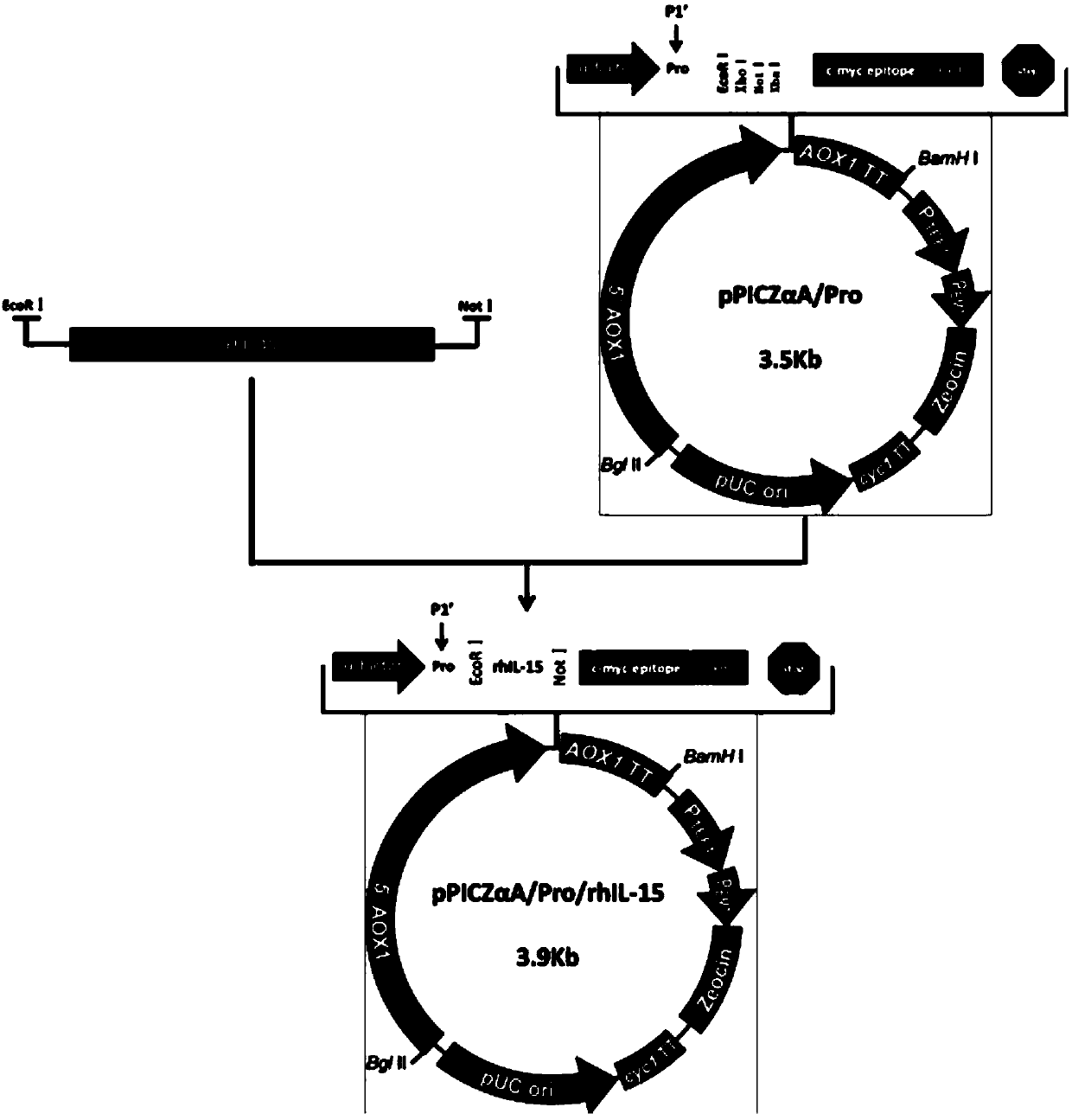
Recombinant human interleukin 15 long peptide fragment and production method thereof
A production method and a technology for human interleukin, which are applied in the field of high-efficiency recombinant human interleukin 15 long peptide segment and its production, can solve the problems of high price of rhIL-15, limit the yield of active protein, decrease the yield of protein, etc. Biological activity, a large number of efficient and stable expression, the effect of good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] In this embodiment, a high-efficiency rhIL-15L expression and purification method mainly includes the following steps:
[0057] 1. Clone the whole gene of rhIL-15L:
[0058] Design a pair of primers to specifically amplify the complete human recombinant IL-15L gene fragment using artificially synthesized IL-15L cDNA as a template, and the 5' end of IL-15L introduces the EcoR1 restriction site and the 3' end introduces the Not1 restriction site point (the base sequence of IL-15L introduced into the restriction site is shown in SEQ ID NO.1) so that subsequent IL-15 fragments can be inserted into the expression vector; the PCR conditions are: 95°C pre-denaturation, 5min, a thermal cycle; 95 Thermal denaturation at ℃ for 30s, annealing at 55℃ for 30s, extension at 72℃ for 45s, 30 thermal cycles; annealing at 72℃ for 10min. The amplified fragment recovered by agarose electrophoresis was connected to the pMD-20T vector (purchased from Guangzhou TAKARA Company) to obtain the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com