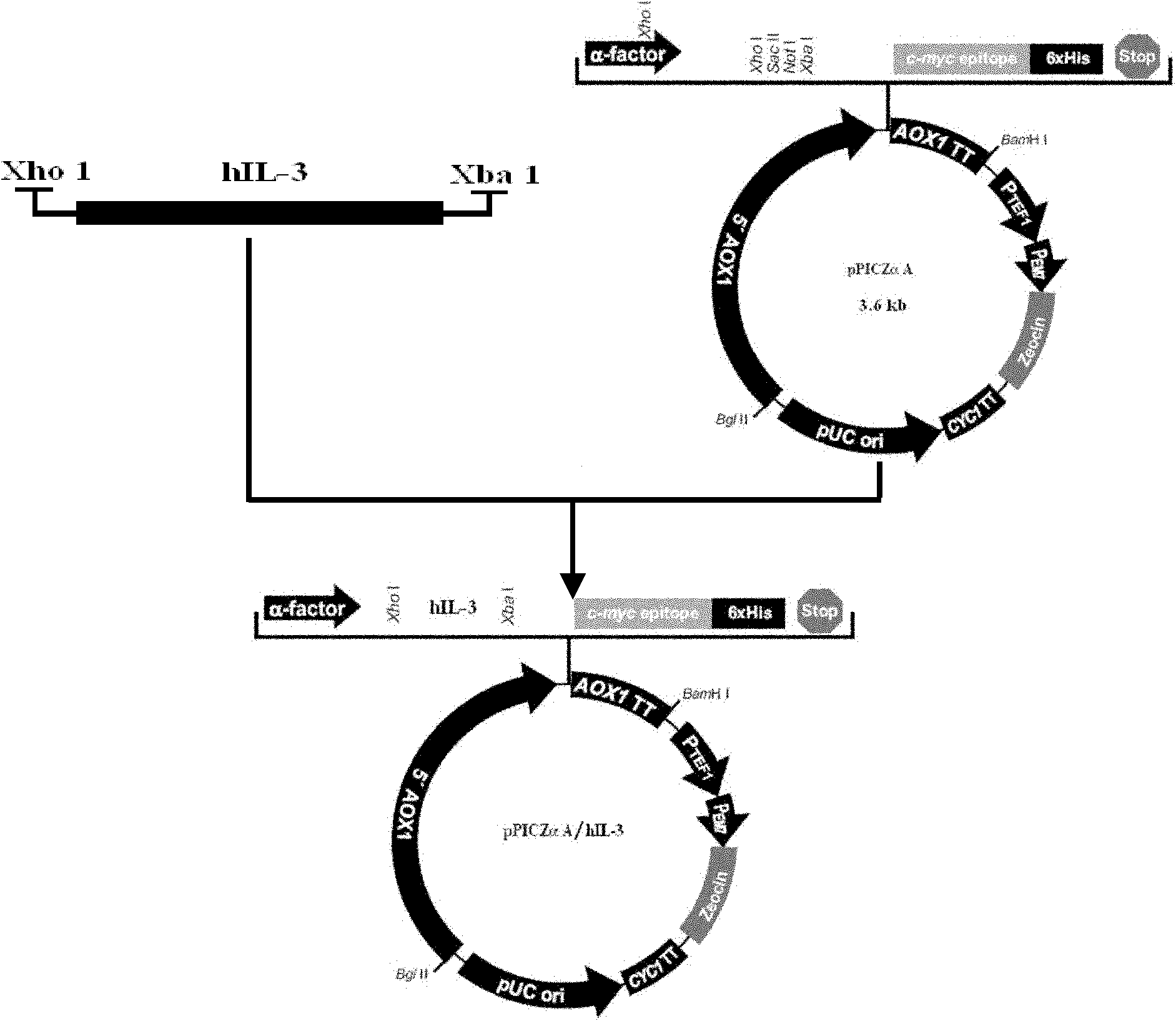
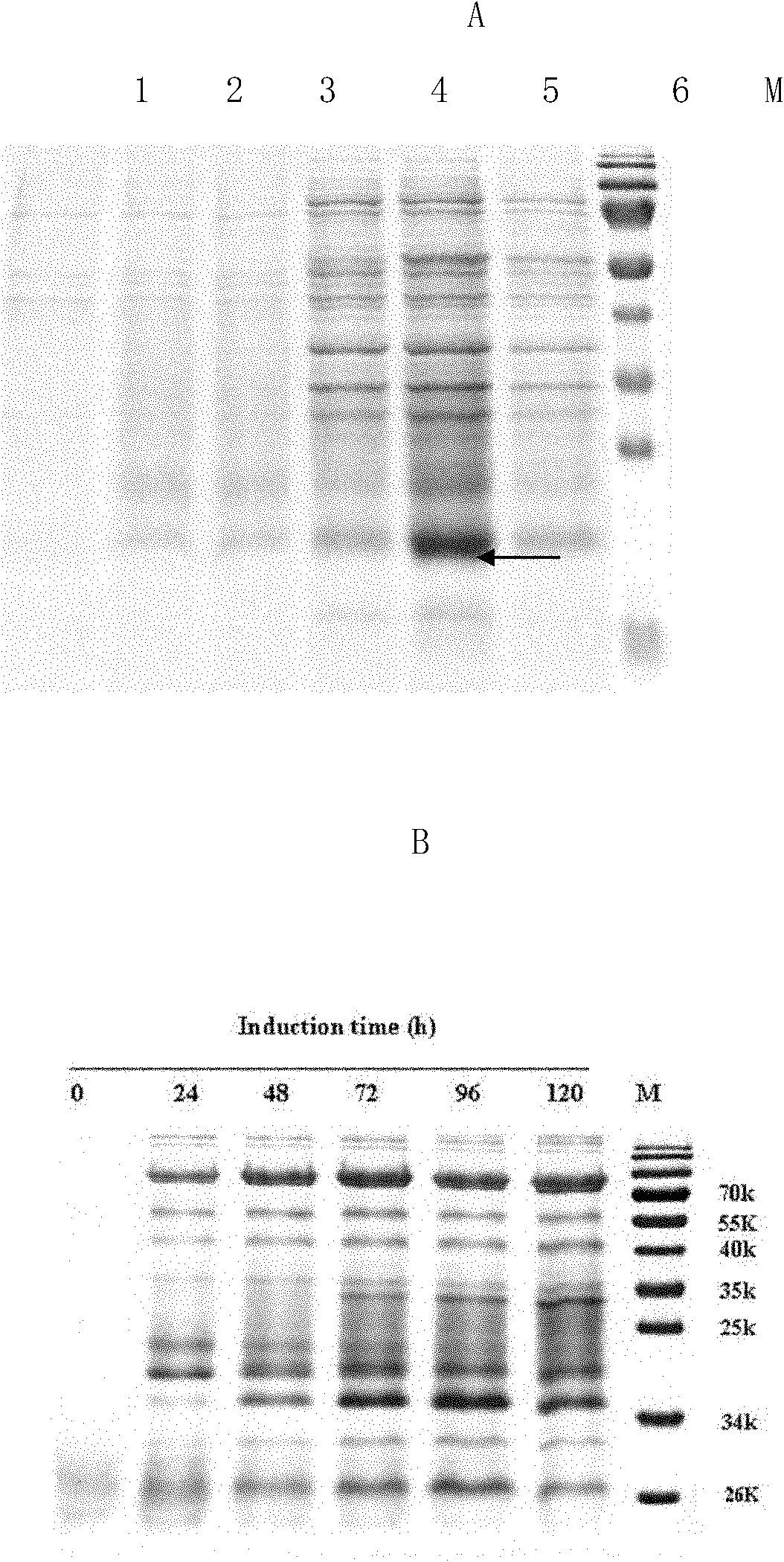
Method for expressing and purifying human recombinant interleukin-3
An expression and purification, interleukin technology, applied in the field of expression and purification of human recombinant interleukin-3, can solve the problems of high price, high cost, low yield, etc., and achieve the effect of preventing degradation and reducing load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The Pichia pastoris X-33 strain is selected in the present invention, and the integrated expression plasmid pPICZαA vector is purchased from Invritrogen, USA.
[0030] The medium formula used is as follows:
[0031] 1) Yeast Growth Medium (BMGY):
[0032] Completely dissolve 10g yeast extract, 20g peptone, and dilute to 700mL. Steam autoclave at 121°C for 15-20min, cool to room temperature, add 100mL 1M potassium phosphate solution, 100mL YNB, 2mL 500*B, 100mL 10*GY;
[0033] 2) Yeast induction medium (BMMY)
[0034] Completely dissolve 10g yeast extract, 20g peptone, and dilute to 700mL. Steam autoclave at 121°C for 15-20min, cool to room temperature, add 100mL 1M potassium phosphate solution, 100mL YNB, 2mL500*B, 100mL10*M;
[0035] 3) YPD liquid medium
[0036] Completely dissolve 10g yeast extract, 20g peptone, 10g glucose, dilute to 1000mL, and autoclave at 121℃ for 15-20min. (Solid YPD medium: add 18g agar to YPD liquid medium to obtain YPD solid medium, which is used to p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com