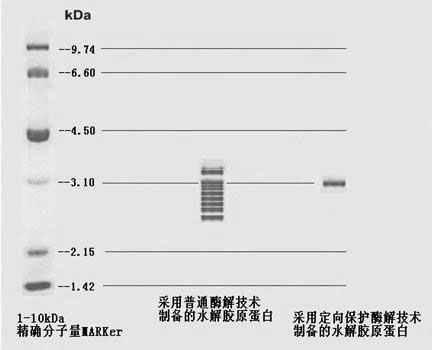
Patents
Literature
221results about How to "Guaranteed biological activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
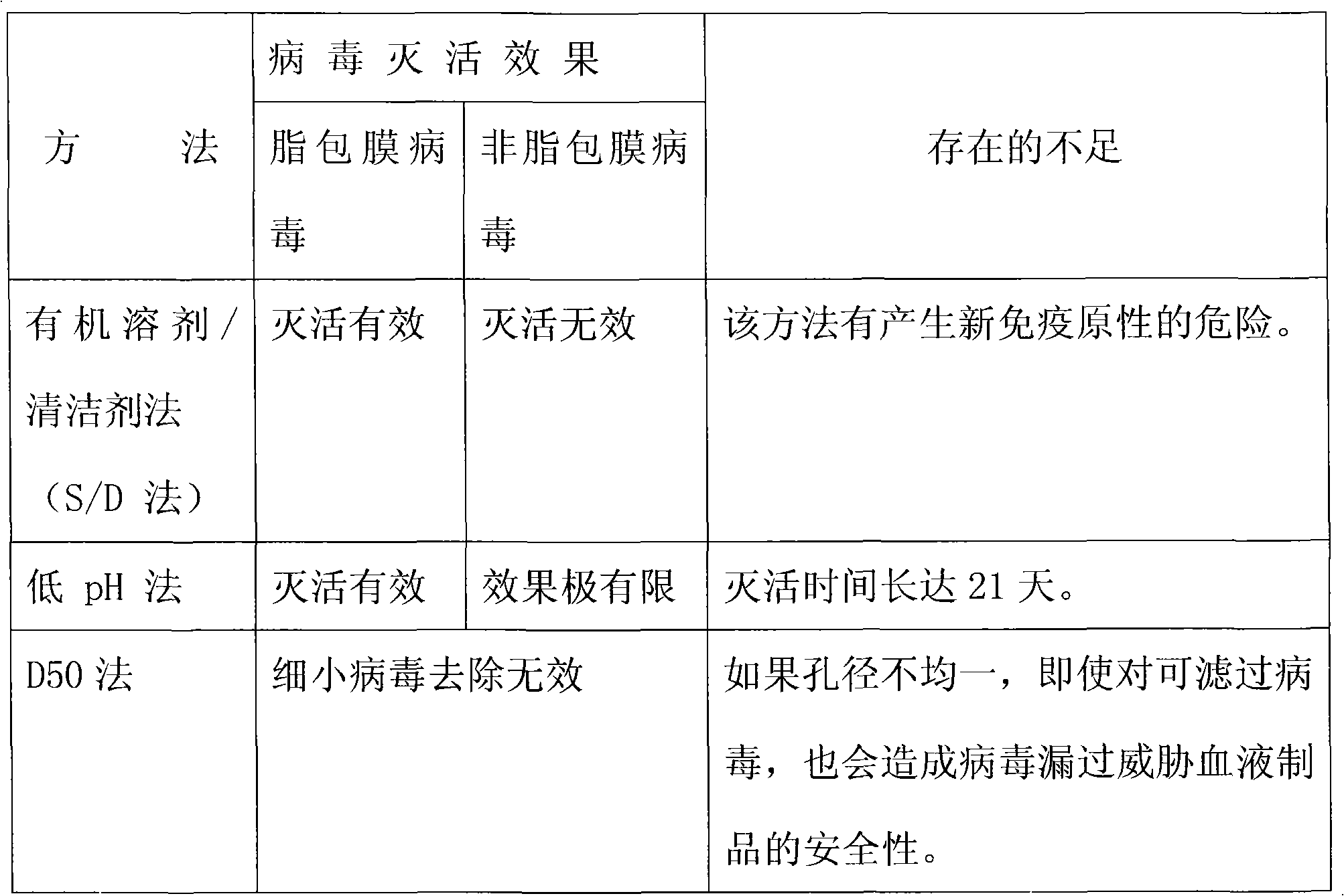
Method and device for treating high-concentration organic chemical-industrial sewage
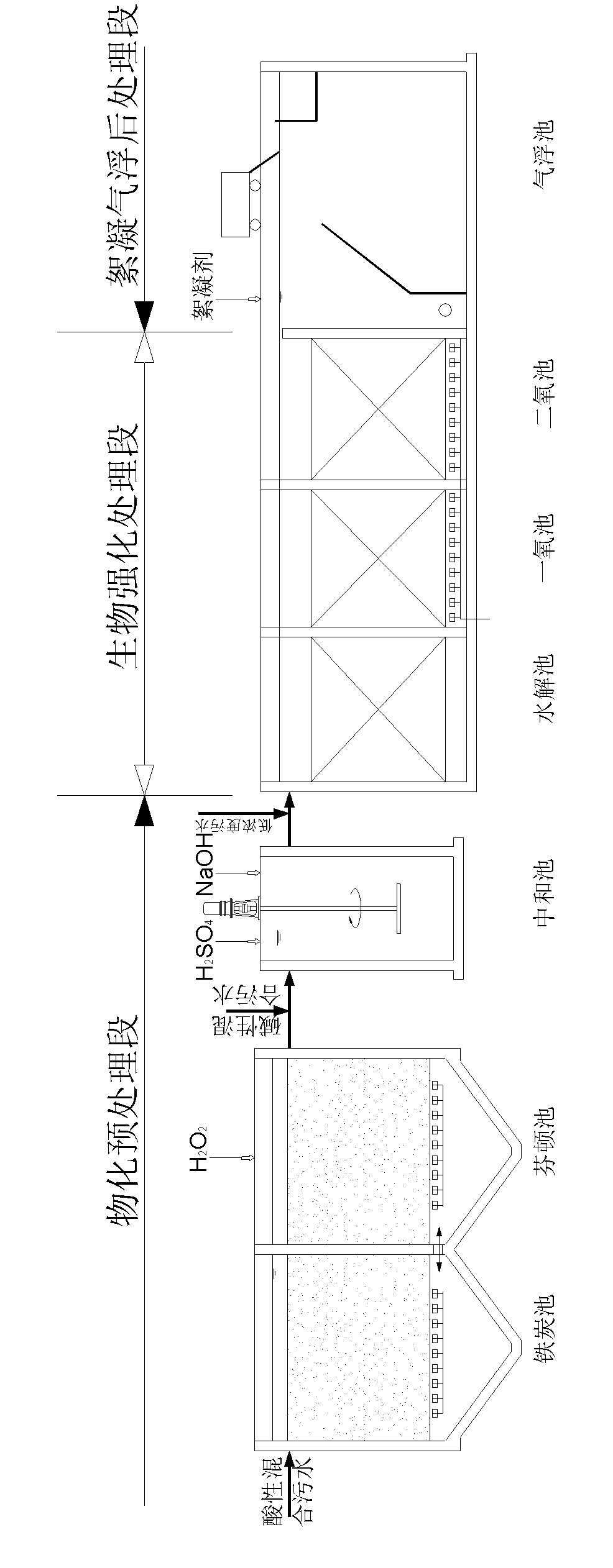
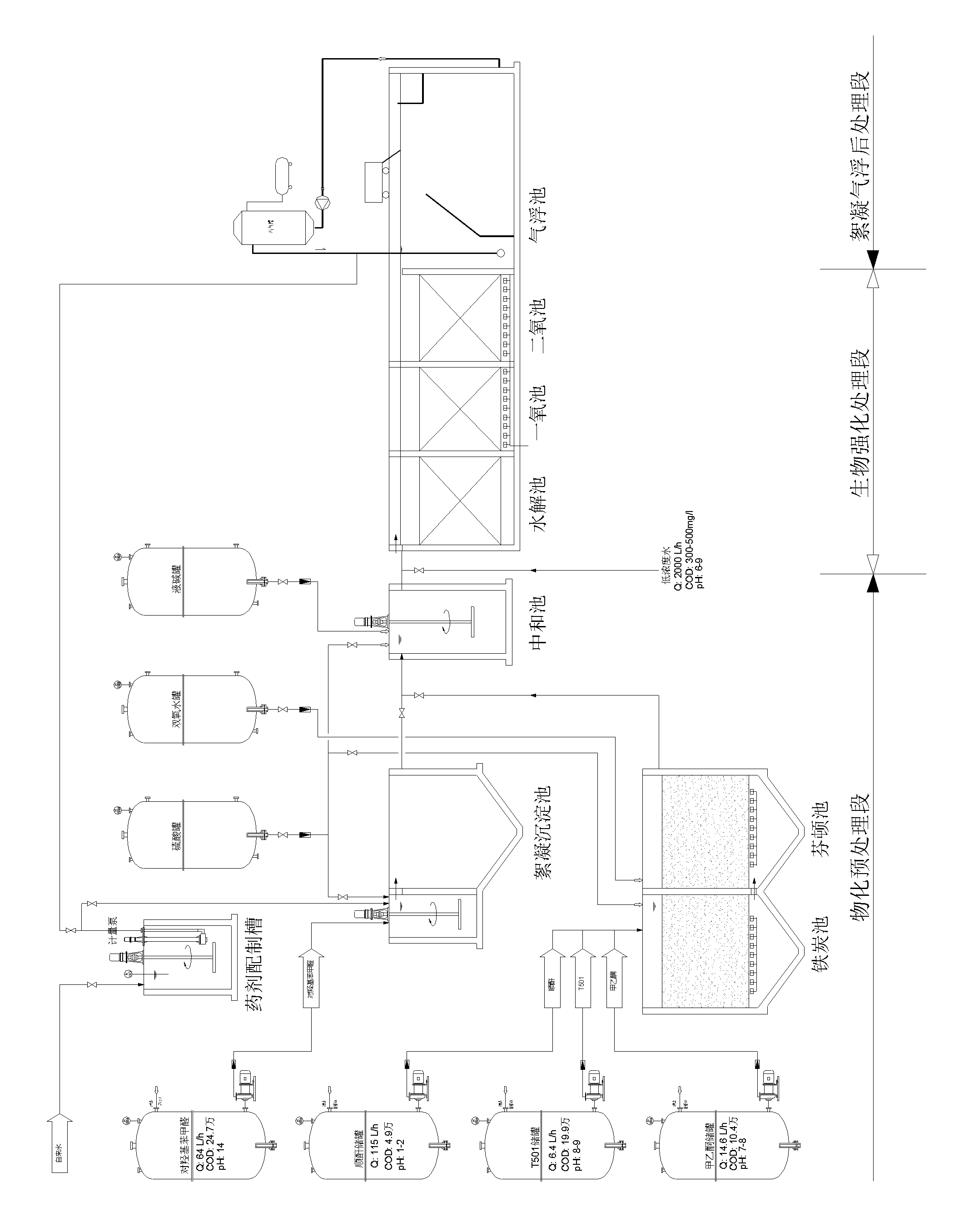
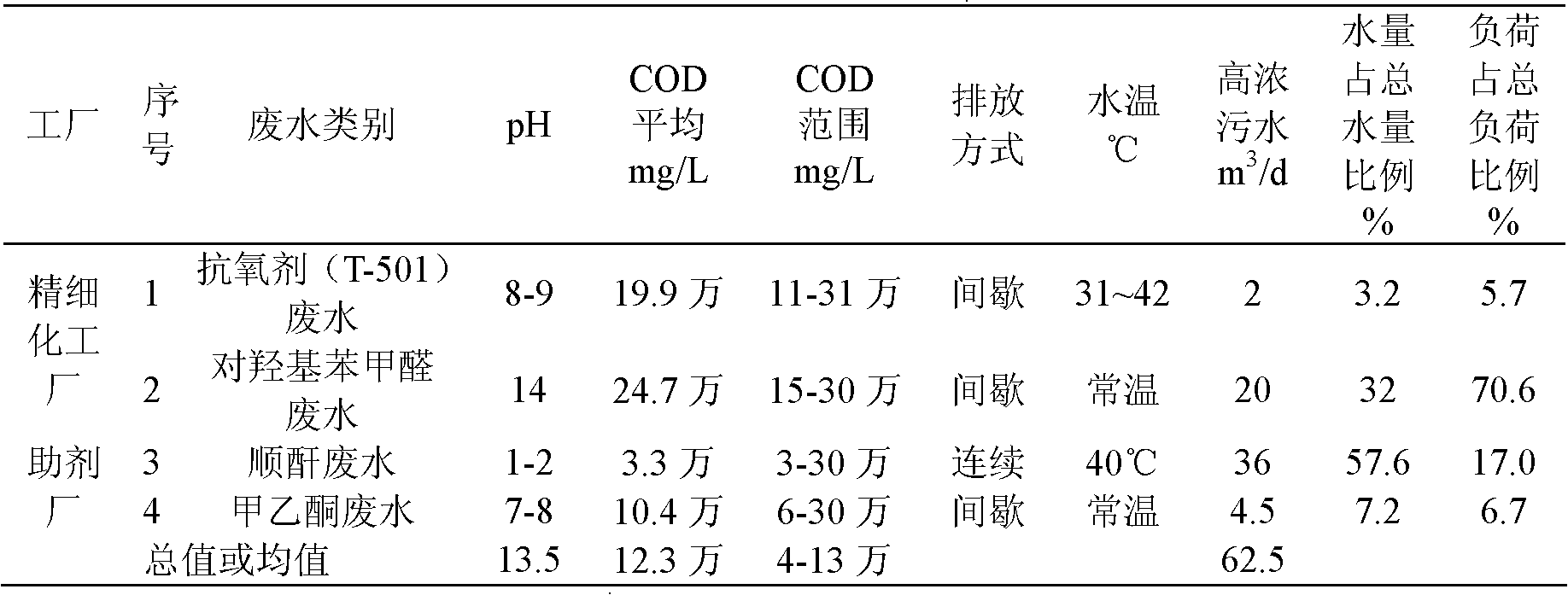
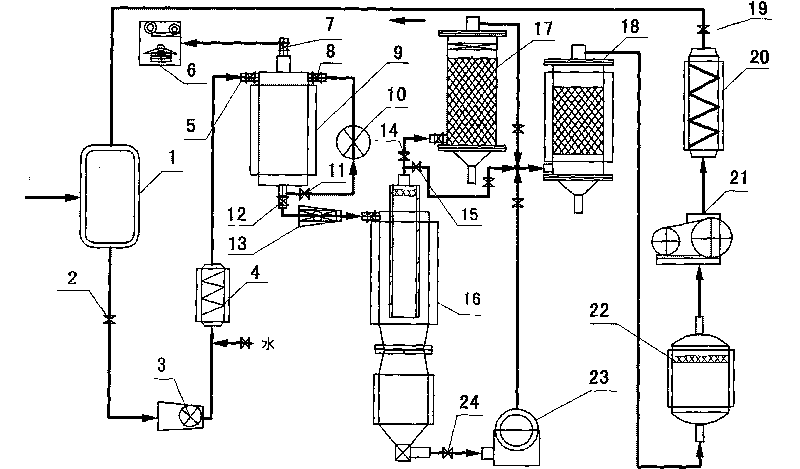
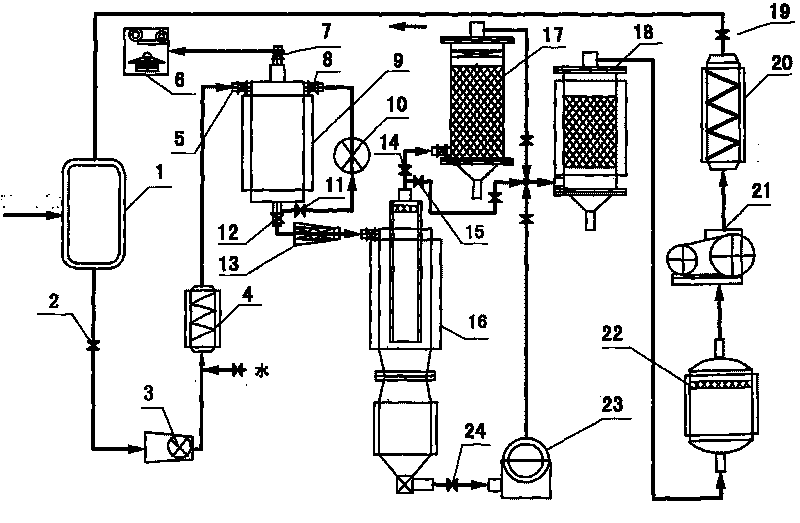
InactiveCN102153231AImprove purification effectReduce processing costsMultistage water/sewage treatmentWater/sewage treatment by oxidationHigh concentrationChemical oxygen demand
The invention provides a method and a device for treating high-concentration organic chemical-industrial sewage. Specifically, the invention provides an integrated sewage treatment method of 'materialized pretreatment' plus 'biological intensification treatment' plus 'flocculating air-flotation aftertreatment' as well as a sewage treatment device. The sewage treatment method and the sewage treatment device can realize high-efficient and low-cost treatment of chemical-industrial sewage which has high COD (Chemical Oxygen Demand) and is difficult to degrade.
Owner:BC P INC CHINA NAT PETROLEUM CORP +1
Method and device for extracting fat-soluble natural pigment
InactiveCN101712812AImprove antioxidant capacityGuaranteed biological activityNatural dyesVaporizationSolvent
The invention provides a method and a device for extracting a fat-soluble natural pigment. The method comprises the following steps of: (1) performing pretreatment on a material, and then performing pressure reduction treatment on the treated material in an extraction container; (2) introducing a solvent into the extraction container to mix the solvent and the material, and extracting the mixture to obtain extract liquor; (3) putting the extract liquor into a vaporization separation device to separate out a crude extract of the fat-soluble natural pigment, and simultaneously separating the solvent, compressing and cooling the solvent, and then reclaiming the solvent for cyclic utilization; and (4) putting the crude extract of the fat-soluble natural pigment into a refining system to perform refining treatment to prepare a refined extract. The extraction device comprises an extraction separation system, the refining system, a solvent purification system, a vacuum system and a heat exchange system. The method and the device can improve the oxidation resistance and the stability of a product, and ensure the biological and chemical activities of the extracts; the product has pure and stable colors; the extraction rate is high; the solvent residue is low; the product has no peculiar smells; and the solvent is easy to reclaim for cyclic utilization.
Owner:YUNNAN REASCEND TOBACCO TECH GRP
Preparation method of co-crosslinked double-network hydrogel support for promoting cartilage injury repair
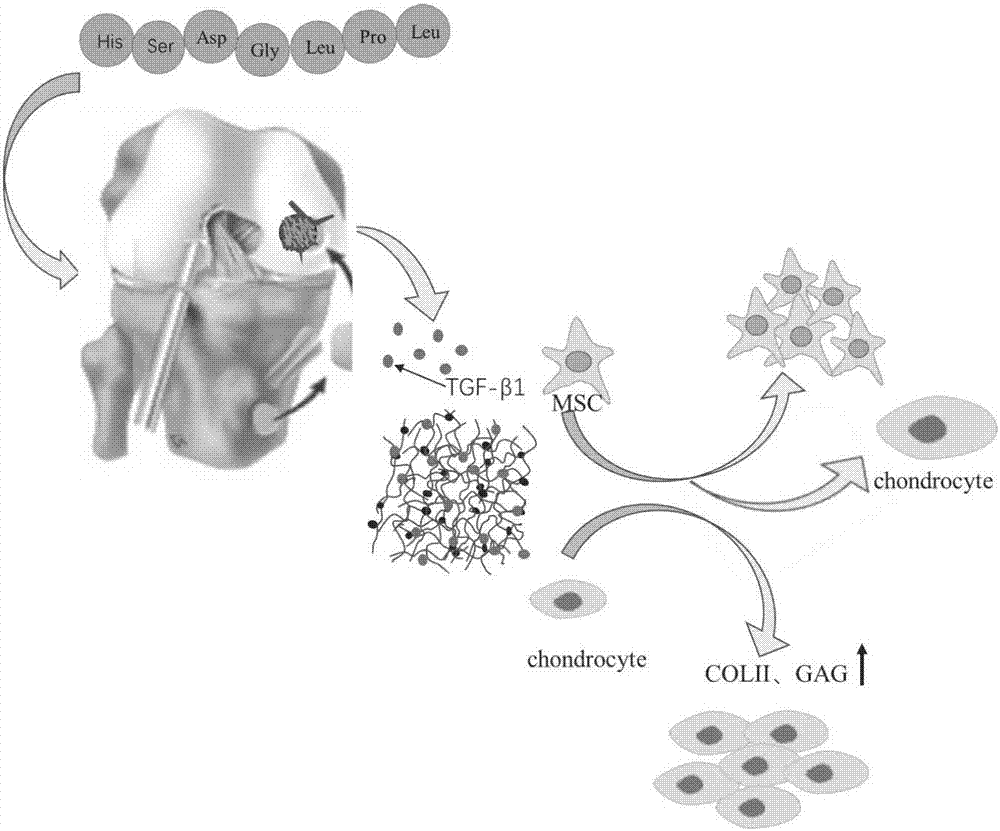
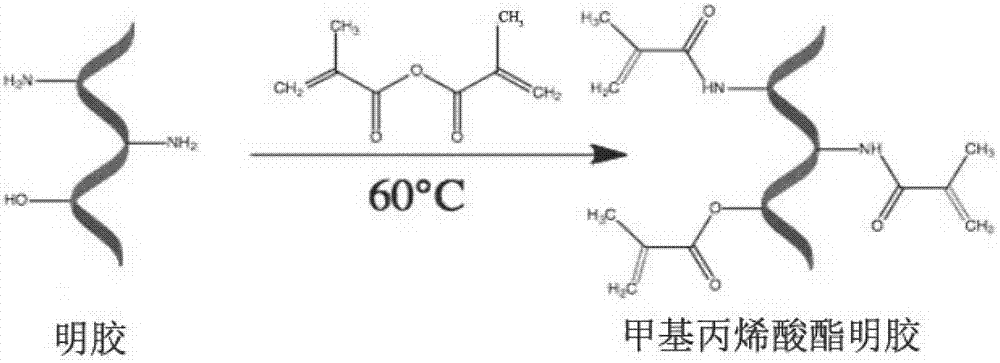
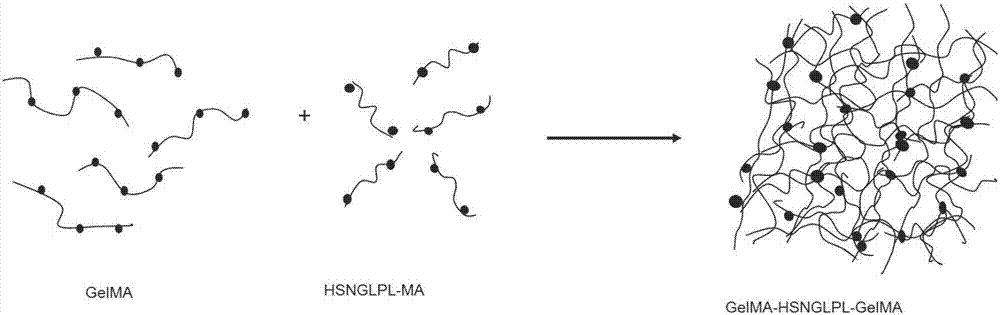
ActiveCN107281550AImprove adhesionThe effect of promoting defect healingTissue regenerationProsthesisCartilage injuryDouble network
The invention belongs to the field of biomedical materials and particularly relates to a preparation method of a co-crosslinked double-network hydrogel support for promoting cartilage repair and growth. The method comprises the following steps of (1) preparation of GelMA; (2) preparation of a photo-crosslinked polypeptide; and (3) preparation of a hydrogel support, namely preparing the hydrogel support from the GelMA and the photo-crosslinked HSNGLPL under the action of a photoinitiator. The prepared hydrogel support has an obvious effect of promoting new cartilage generation and cartilage defect healing, and is capable of regulating cartilage matrix secretion of normal chondrocytes around the injured tissue, mesenchymal stem cell migration to the injured part and cartilage differentiation; by virtue of a porous structure of the hydrogel support, the HSNGLPL can be crosslinked to the surface of a porous support, endogenous TFG-beta is effectively adsorbed, the concentration of local TFG-beta beta1 is improved and the effects of the biological support in the orthopedics fields, such as a cartilage defect and a cartilage injury are achieved.
Owner:SUZHOU UNIV
Method for extracting hydrolyzed collagen from bovine cartilage
ActiveCN101948900AAchieve hydrolysisGuaranteed HalalPeptide preparation methodsFermentationBovine CartilageCollagen VI
The invention provides a method for extracting hydrolyzed collagen from bovine cartilage. The method sequentially comprises the following steps of: processing fresh bovine cartilage which is obtained from a Muslim slaughter house and serves as a raw material with solution of sodium hydroxide; adding papain and alkali protease into the obtained product by stages and simultaneously adding a composite enzymolysis protecting agent into the obtained product; raising temperature for inactivating enzyme after two enzymolysis processes; decoloring by oxidation; filtering and performing ultra-filtering; collecting retained solution; and performing spray-drying on the solution to obtain the hydrolyzed collagen. In the method, the bovine cartilage of the Muslim slaughter house is used as a basic rawmaterial, and other animal derived enzyme preparations are not added during the entire process, so that the Muslim property and no pollution of the product can be ensured.
Owner:SHANGHAI AL AMIN BIOTECH
Frozen stock solution for nerve cells and freezing storage method
InactiveCN102578078AImprove survival rateFor long-term storageDead animal preservationNerve cellsDrug biological activity
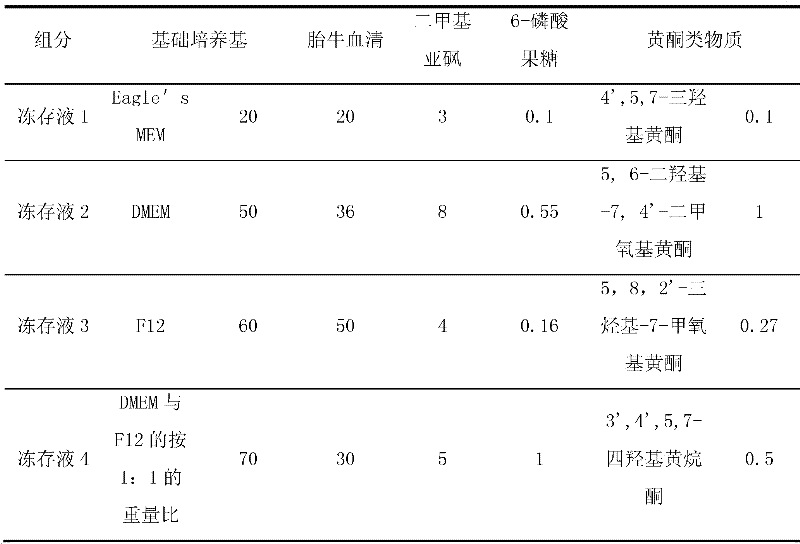
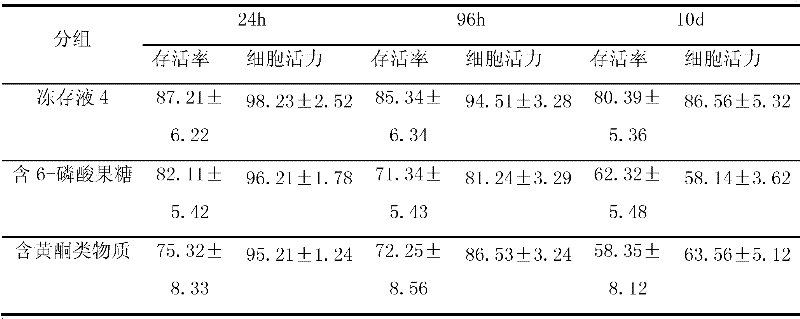
The invention discloses cell frozen stock solution for freezing and storing nerve cells and a freezing storage method, wherein, the cell frozen stock solution and the freezing storage method are used for freezing and storing the nerve cells, especially primary animal nerve cells that are isolated freshly, such as hippocampus nerve cells. The frozen stock solution is formed by adding basic medium,fetal bovine serum, dimethyl sulfoxide,6-fructose phosphate and flavonoid compounds to the basic medium; 6-fructose phosphate (w / w) achieves the function of improving cell viability (effective cell rate) when the frozen cells are recovered; and in addition, the flavonoid compounds achieve definite oxidation resistance and can prevent free radical from damaging the cells. The cell frozen stock solution and the freezing storage method can improve the survival rate and the cell function of the nerve cells through freezing storage, the survival rate of unfreezing cells can be increased to reach more than 95 percent, the frozen stock solution for the nerve cells can preserve the nerve cells for a long period, the biological activity of the nerve cells can be ensured, and very high utility values can be achieved.
Owner:温州医学院附属第二医院
Method for preparing powder rich in whey small peptides with molecular weight of 1000 daltons
InactiveCN101518295ASolve the problem of prominent bitterness after high degree of hydrolysisEnhance physical fitnessAnimal proteins working-upAdditive ingredientInfants milk
A method for preparing powder rich in whey small peptides with molecular weight of 1000 daltons belongs to the technical field of processing dairy products. The main production process thereof is as follows: enzymatic hydrolysis, embedding and homogenization are firstly carried out on concentrated powder (WPC80 powder) containing 80 percent of whey protein, then the powder is prepared by spray drying, and the product can be used as functional ingredients for infant milk powder. The method adopts trypsin for enzymatic hydrolysis, the degree of hydrolysis achieves 20-21 percent, and then hydrophilic-hydrophobic wall materials of 5-10 percent of beta-cyclodextrin and 5-8 percent of lecithin are adopted, so the method can not only bury the bitterness, but also maintain the biological activities of anti-protein allergy and improve the immunity of the whey small peptides with the molecular weight of 1000 daltons to the maximum extent. The method can be applied to the infant formula milk powder and have good effects of improving the ability of anti-protein allergy of the infant milk powder and improving the immunity.
Owner:ZHEJIANG UNIVERSITY OF SCIENCE AND TECHNOLOGY
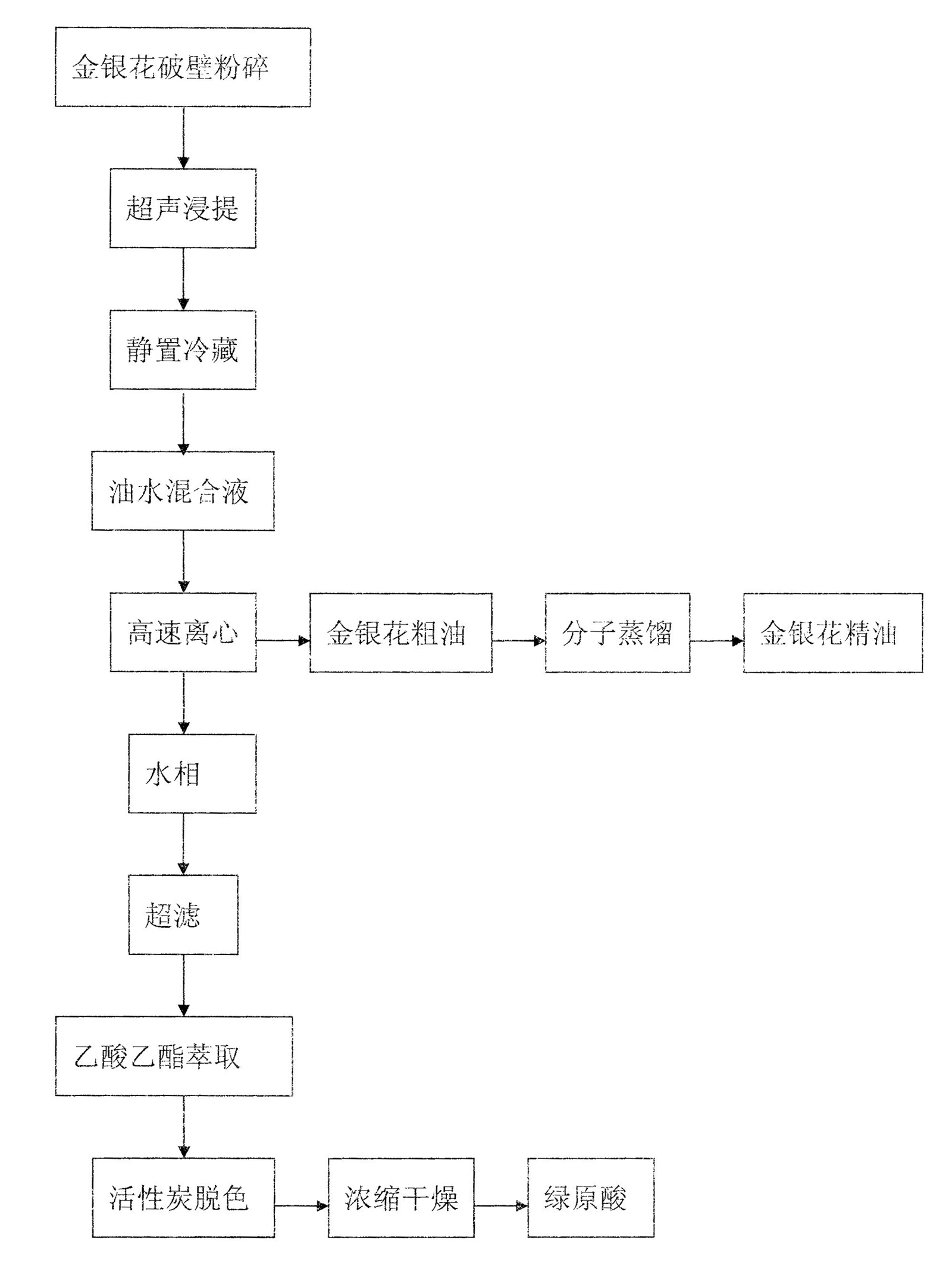
Novel method for simultaneously extracting and separating chlorogenic acid and honeysuckle flower essential oil from honeysuckle flower
ActiveCN102001946AReasonable purification processMild extraction conditionsChemical industryEssential-oils/perfumesChlorogenic acidUltrafiltration
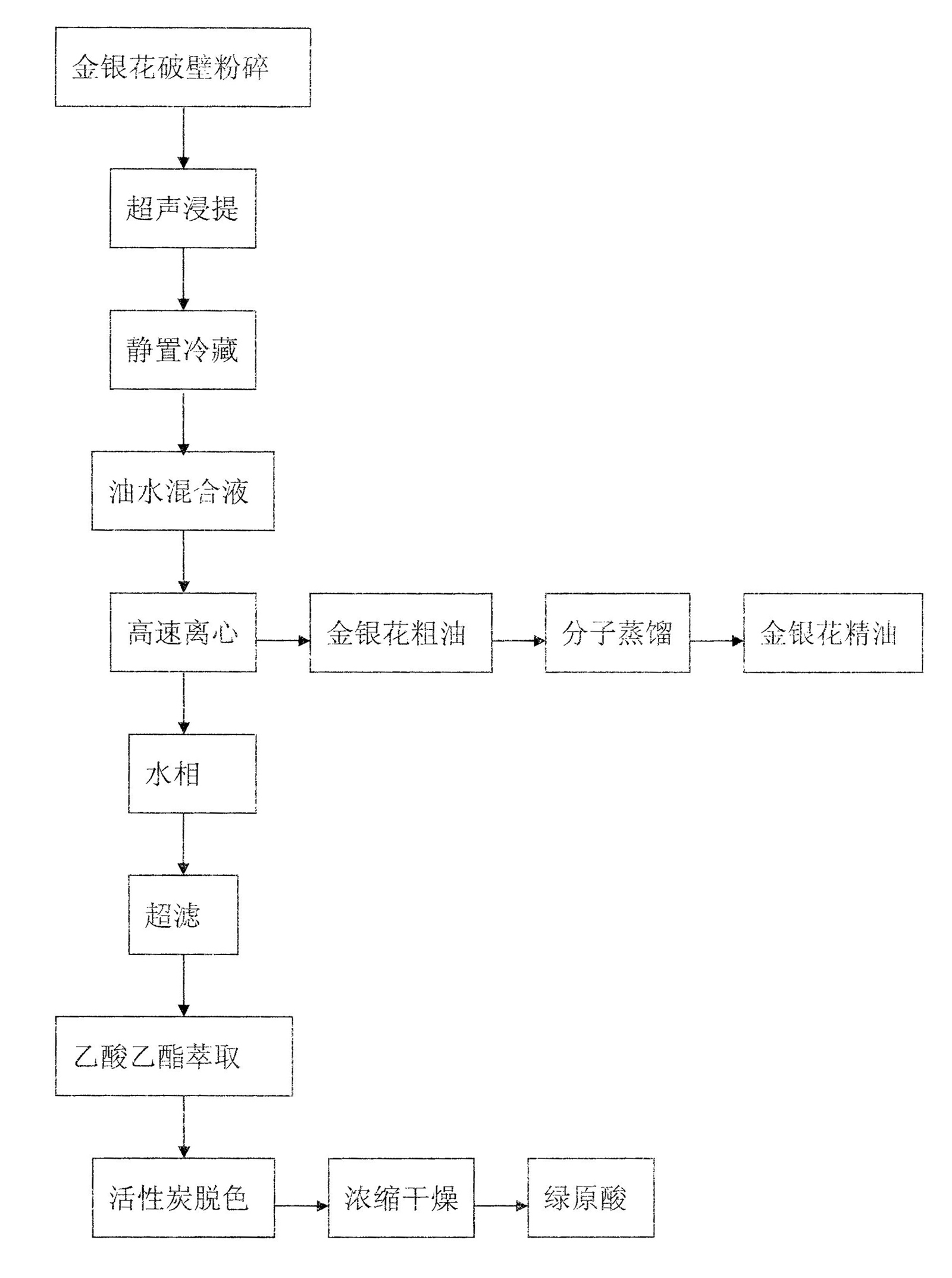
The invention relates to a novel method for simultaneously extracting and separating chlorogenic acid and honeysuckle flower essential oil from honeysuckle flower by wall-breaking ultrasonic extraction separation technology. The method comprises the following steps of: performing wall-breaking treatment by adopting a high-frequency vibration wall-breaking crusher, and performing ultrasonic water extraction filtration; standing and refrigerating filtrate, and centrifugally filtering to obtain an oil-water mixture; centrifuging the oil-water mixture at a high speed again; performing ultrafiltration on a water phase, extracting by using ethyl acetate, and separating, concentrating and drying to obtain high-purity chlorogenic acid; and performing molecular distillation on an oil phase to prepare high-quality honeysuckle flower essential oil. The method furthest ensures the bioactivity and original characteristics of active ingredients, greatly improves the utilization rate of a honeysuckle flower resource and can remarkably save energy and reduce pollution. The honeysuckle flower treated by unique methods such as wall-breaking ultrasonic extraction and the like can be used to simultaneously prepare high-purity and high-quality chlorogenic acid and honeysuckle flower essential oil, which are widely applied to food, medicines, cosmetics and commodities, so that the aim of fully utilizing resources is fulfilled and the method has good economic benefits.
Owner:HUNAN RHON PHARMA
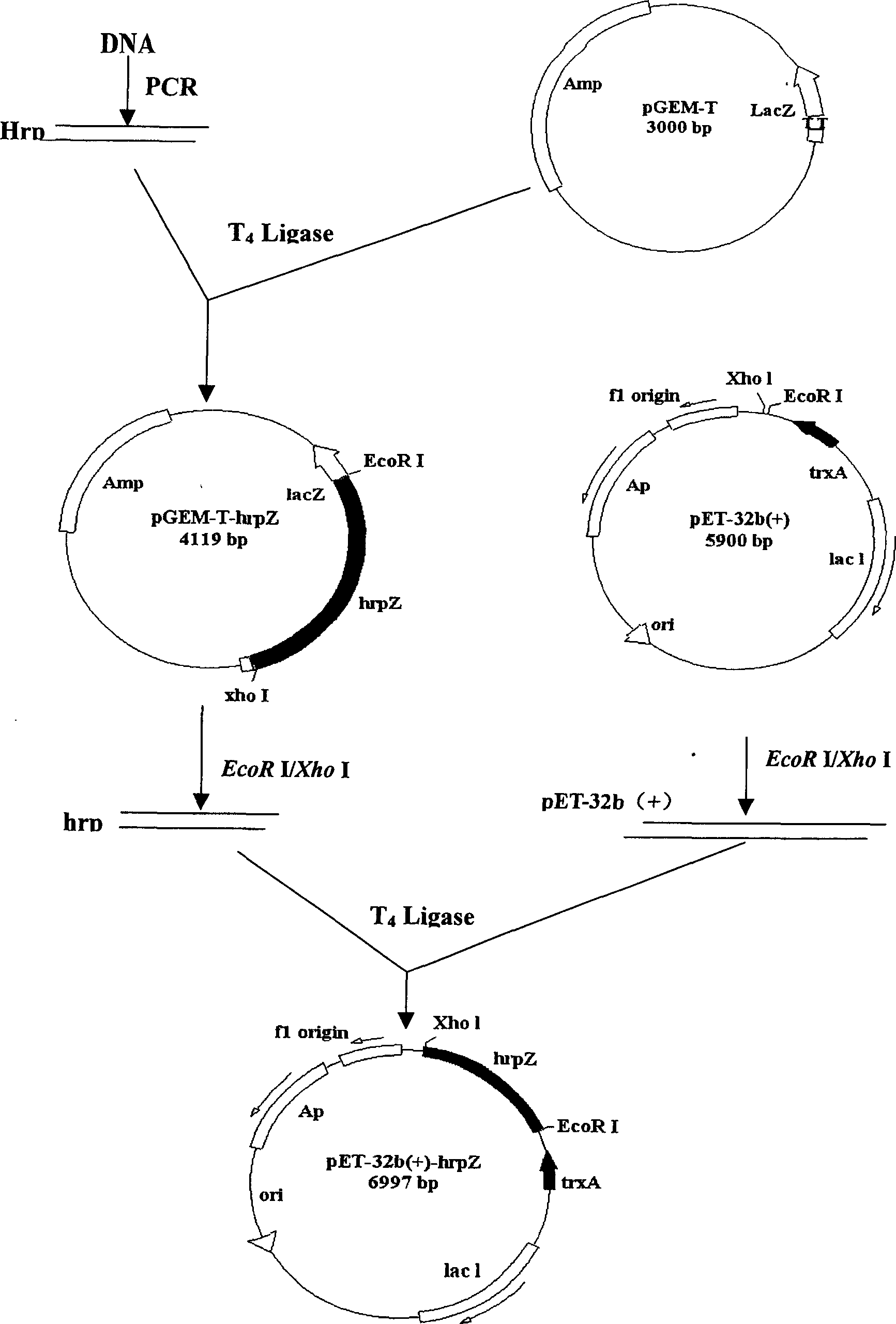
Non-inducing expressing gene engineering strain and structural process and application thereof
The invention discloses a non-induced expression genetic engineering strain and the constructing method and the application. The host cell of the strain is enteric bacilli E.coliBL21, CCTCC M205136, PCR expanding hrp Z gene section to clone to pGEM-T carrier, ferment cutting and bonding by EcoR I / Xho I, inserting the hrp Z gene into the enteric bacilli expression carrier pET-32b(+) lower reaches of thioredoxin, constructing rebuilding expression particle pET-32b-hrp, and transferring into E.coliBL21, filtering to gain positive clone. The non-induced expression rebuilt albumen HrpZ, SDS-PAGE shows that high efficiently expresses APDZ albumen. The rebuilt albumen has good prevention effect to plant disease and could improves the yield.
Owner:WUHAN UNIV
Cell freezing medium for human adipose-deprived stem cells
ActiveCN105494317ANo lossFor long-term storageDead animal preservationPeptidesLow-density lipoproteinMicrobiology
The invention provides a cell freezing medium for human adipose-deprived stem cells and a preparation method of the cell freezing medium. The cell freezing medium comprises low-density lipoprotein, trehalose, glycerin, lecithin, polypeptide, bFGF (fibroblast growth factor), vitamin E and reduced glutathione. The cell freezing medium is used for freezing the human adipose-deprived stem cells, especially can increase survival rate of frozen cells after recovery and has a good application prospect.
Owner:北京九思九如健康科技有限公司
Wettable powder for fungi spore
InactiveCN1907036AGuaranteed biological activityGuaranteed control effectBiocideAnimal repellantsBiotechnologySpore
The invention provides a fungus spore wettable powder which comprises diatomite 85-90%, lignin 1-30%, carboxymethyl cellulose 0.1-1%, sodium humate 0.01-0.2% and Gliocladium spp. spores powders having hyperparasitism actions against various plant pathogenic bacteria such as sclerotiorum, gray mold bacterium, sheath blight bacterium and root rot bacterium.
Owner:INST OF ENVIRONMENT & SUSTAINABLE DEV IN AGRI CHINESE ACADEMY OF AGRI SCI
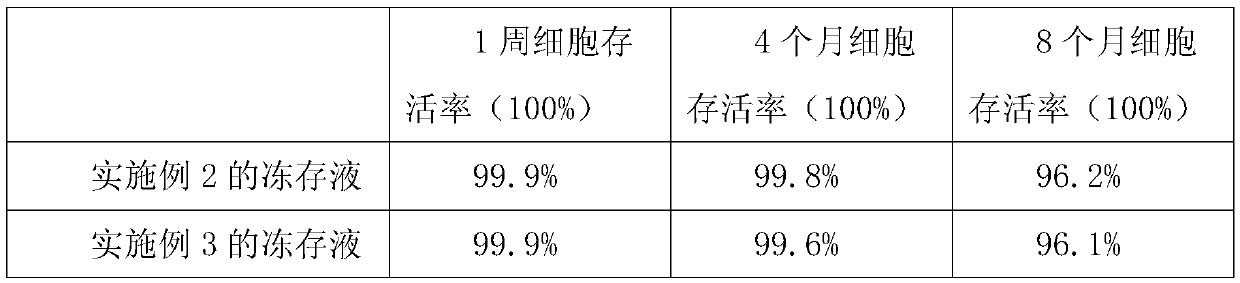
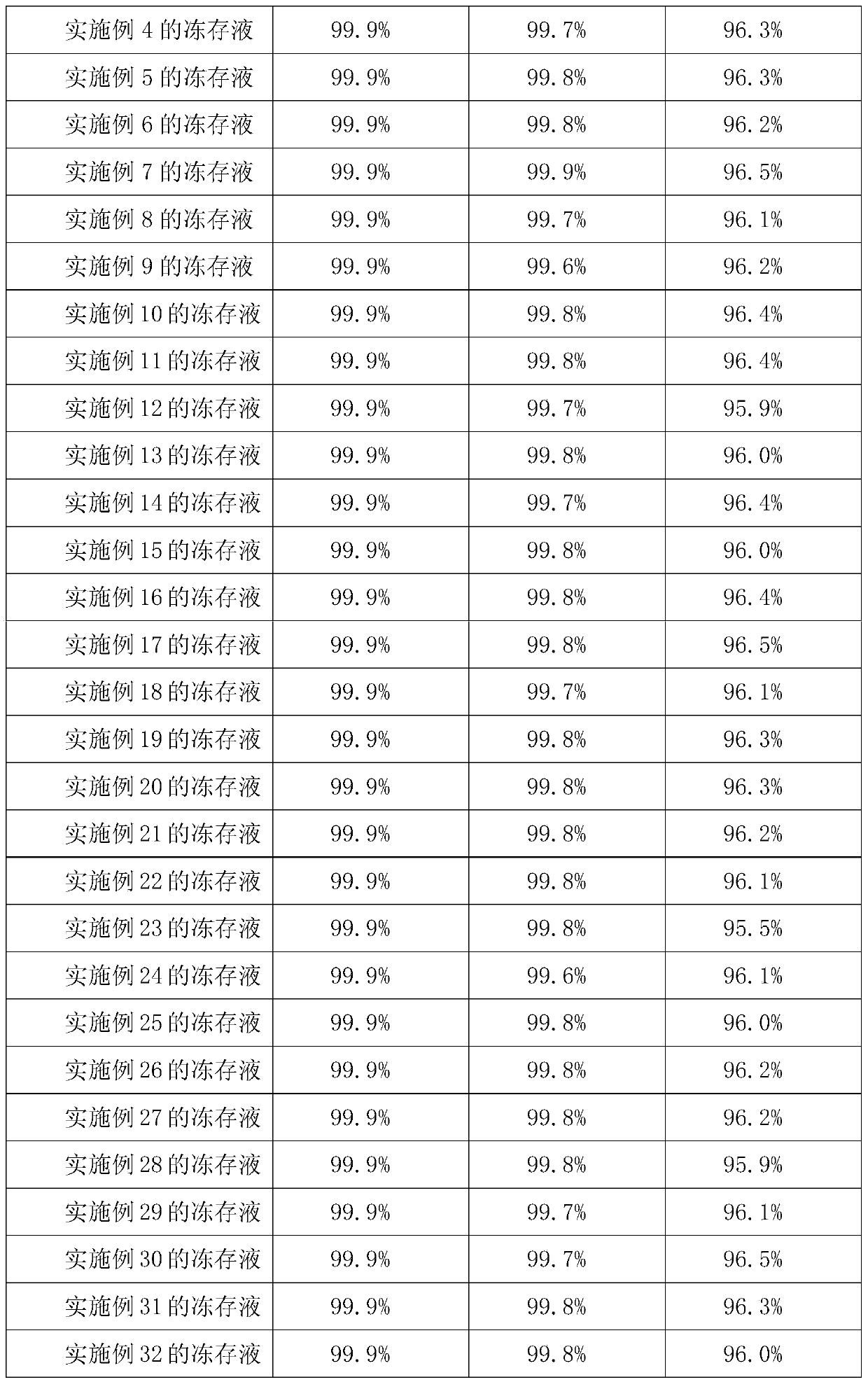
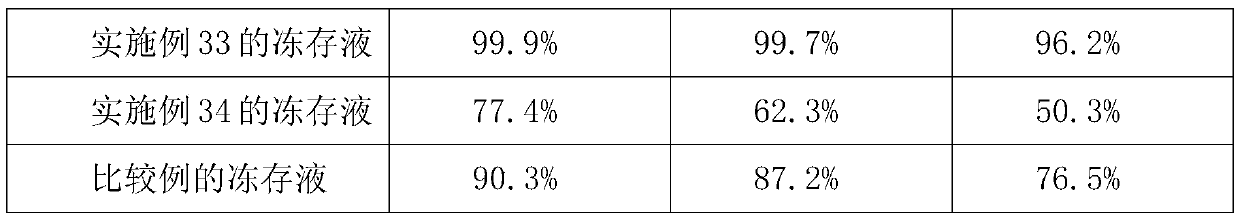
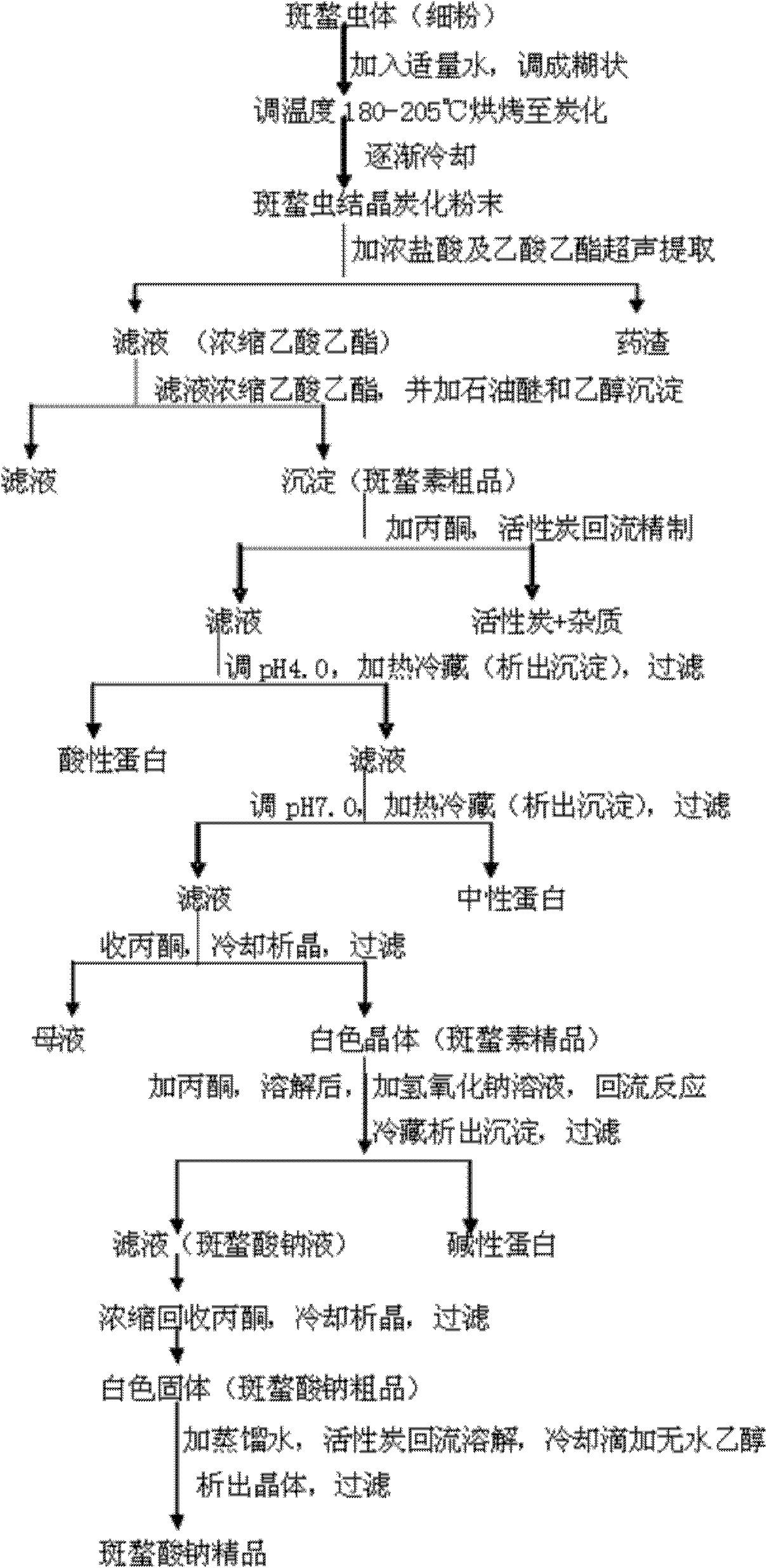
Preparation method of sodium cantharidate
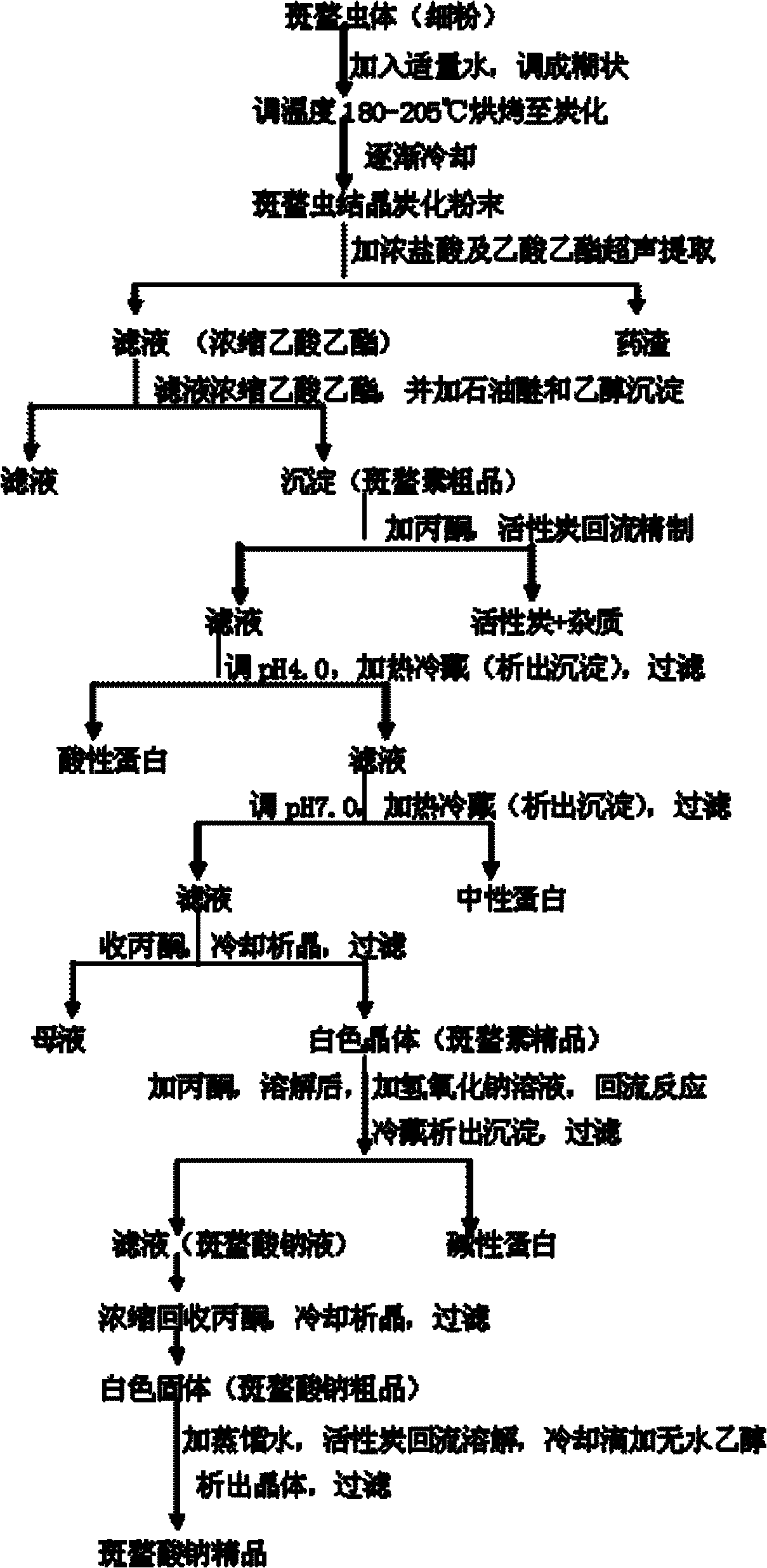
ActiveCN102146086ASimple extraction processSimple manufacturing processOrganic chemistryCarbonizationCantharidin
The invention discloses a preparation method of sodium cantharidate. The method comprises the following processing steps: performing high temperature carbonization of cantharis, extracting cantharidin, degreasing cantharidin and precipitating, refining cantharidin, synthetizing sodium cantharidate, refining sodium cantharidate, etc. By adopting the preparation method, the extracting time of cantharidin can be greatly shortened, lipidic and peptdic proteins and impurities in crude cantharidin and refined cantharidin can be completely removed, and the yield and purity of cantharidin can be increased. Therefore, sodium cantharidate with higher purity and yield can be obtained, the quality and safety of sodium cantharidate can be further increased and the quality reliability of sodium cantharidate can be increased when used in an injection.
Owner:贵州君之堂制药有限公司
Fine processing process and production equipment for organic rice
InactiveCN105170222AGuaranteed biological activityGuaranteed NutrientsGrain treatmentsAdditive ingredientEngineering
Provided is a fine processing process for organic rice. Processing time is selected to be from the middle ten days of December to the first ten days of February in the next year, and the outdoor temperature is minus 10 DEG C or lower. The processing process includes the following steps of fine selection, unshelling, whitening, classification, color sorting and polishing, packing, quality inspection, large packing and warehousing; the finished organic rice is stored in the environment with the temperature ranging from 0 DEG C to 5 DEG C, and the storage time is no longer than 240 days since the organic rice is warehoused. As for the organic rice obtained through the method, it can be effectively guaranteed that the water content of the finished rice is controlled to range from 12% to 16%, the biological activity and nutritional ingredients of the organic rice are guaranteed, and nutrient matter loss is reduced to the minimum. The organic rice processed through the fine processing process and production equipment for the organic rice can be stored under the cold storage environment of a refrigerator for 6-8 months without losing activity, and even the unsealed organic rice can still be stored for 2-4 months under the cold storage environment of the refrigerator without losing activity.
Owner:沈阳久兴粮谷加工有限公司
Expressing method of human interleukin 7 in eucaryon host
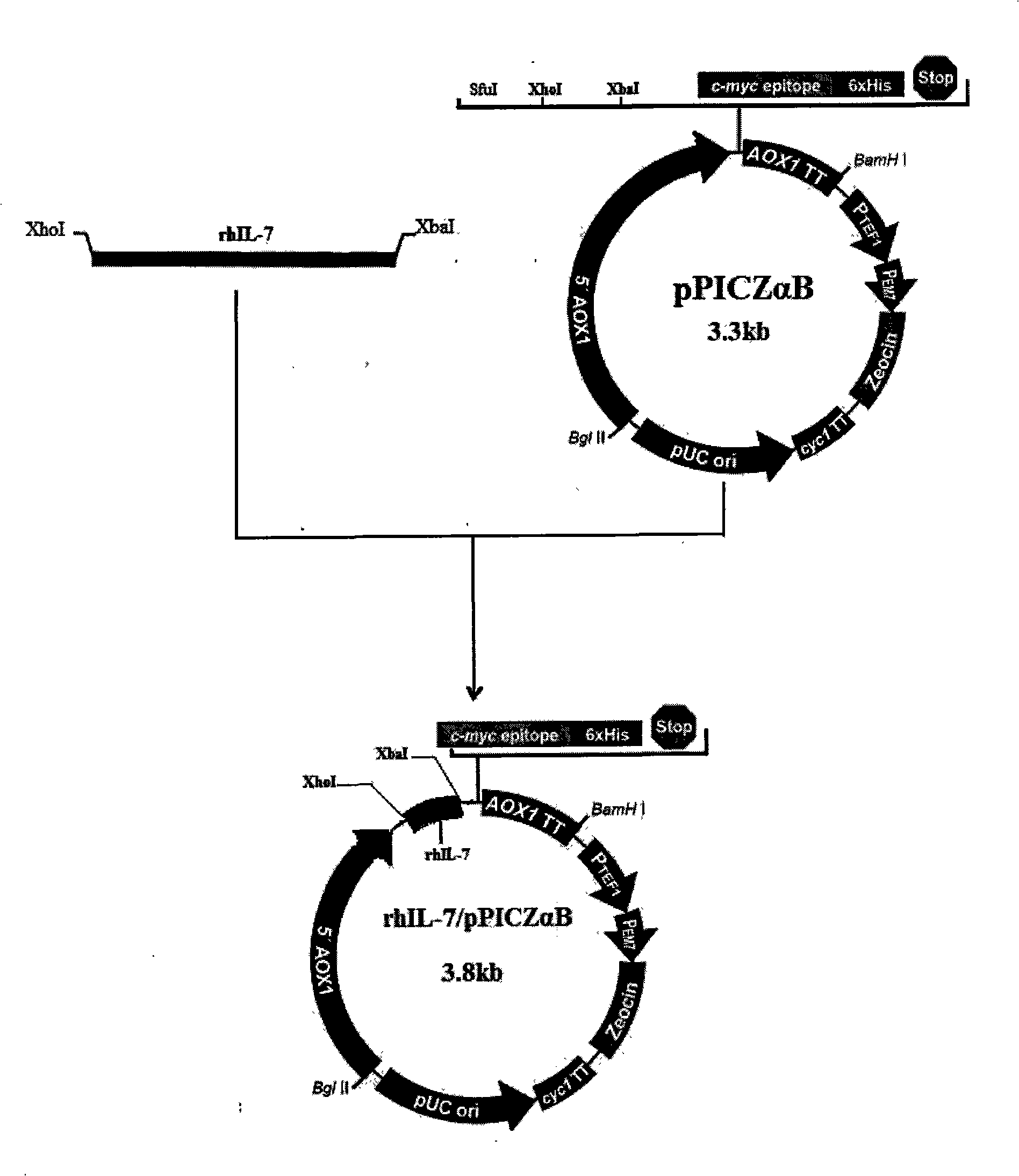
InactiveCN101302517AAvoid degradationReduce loadFermentationVector-based foreign material introductionPichiaInterleukin I
The invention discloses a method for expressing human interleukin 7 in eucaryotic host. When the eucaryotic host is pichia X-33, the method comprises the following steps of: (1) cloning an IL-7 gene of human; (2) building an eucaryotic expression vector; (3) transforming recombinant vector to the eucaryotic yeast host; and (4) expressing a rIL-7 protein in the yeast host. A prior method for expressing IL-7 by using prokaryotic host escherichia coli is changed, and a method for expressing IL-7 through eucaryotic host pichia is searched out, thereby ensuring an IL-7 biologic activity and obtaining a large amount of stable protein.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI +1
Method for promoting CD4+T proliferation and activation and suppressing Jurkat T cell by using red cassia tree lectin
InactiveCN102226170APromote proliferation and activationPrevent proliferationBlood/immune system cellsGrowth retardantAnti virus
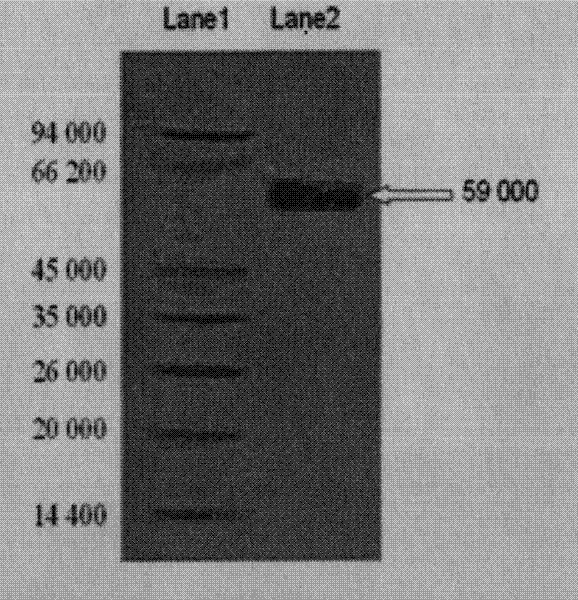
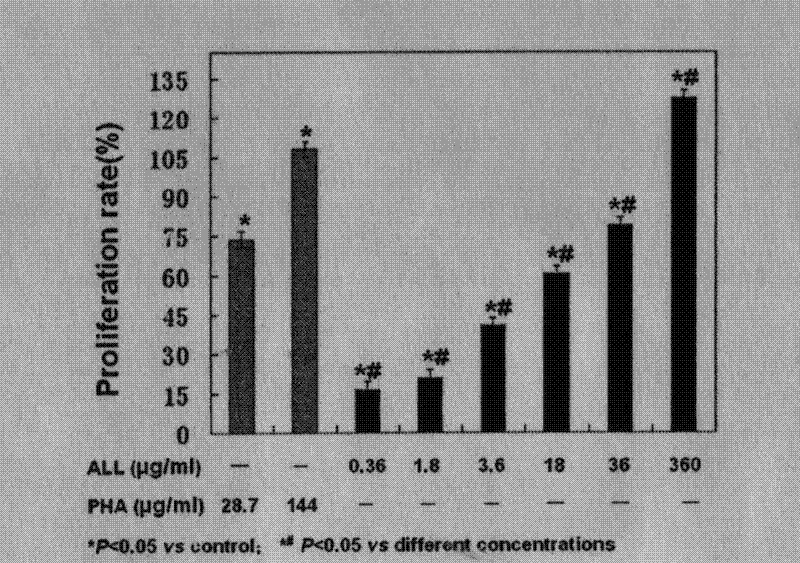
The invention discloses a method for promoting cell proliferation and activation of peripheral blood CD4+T lymphocyte and suppressing cell proliferation and migration of acute lymphocytic leukemia (ALL) Jurkat T cells by using red cassia tree lectin. The separation and purification of red cassia tree lectin from red cassia tree seeds can be carried out at the temperature of 1-5 DEG C during the entire process, the proliferation and activation of the peripheral blood T lymphocyte CD4 subgroup can be promoted by the red cassia tree lectin through the verifications of an MTT (3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide) experiment, a DNA (deoxyribonucleic acid) analysis and an FCM (flow cytometry), and the red cassia tree lectin is CD4+T lymphocyte mitogen more efficient than PHA (Phytohaemagglutinin) and provides a new and effective way for anti-virus infection and vaccine intensifier development; through the verifications of CCK-8 (Cell Counting Kit-8) experiment, DNA analysis, cell apoptosis analysis, ELISA (enzyme-linked immuno sorbent assay) experiments and migration assay, the red cassia tree lectin can obviously suppress the proliferation and migration of the Jurkat T lymphocyte, is a growth inhibitor more efficient for the ALL cells and provides a new and effective supplementary means for treating the hematological malignancies.
Owner:GUANGXI MEDICAL UNIVERSITY
Representation method for recombinant american cockroaches allergen protein
InactiveCN101220361AAvoid non-uniformity and standardization difficultiesGuaranteed biological activityPeptide/protein ingredientsImmunological disordersGenetic engineeringDisease
The invention relates to the field of biotechnology and discloses an expression method of a recombinant periplaneta Americana allergen protein. The invention aims at solving the shortcoming of the immune tolerance therapy of cockroach allergen leaching liquor, total RNA is extracted from common cockroach species periplaneta Americana tissues for obtaining the coding genes of major allergen genes of Per a 1.0101, Per a 1.0102, Per a 1.0104 and Per a 7.01 of the periplaneta Americana by amplification, the low-temperature induction expression of the recombinant cockroach allergen protein in escherichia coli by adopting genetic engineering means can be used for the diagnosis and the treatment of hypersensitivity diseases which are caused by cockroaches and can avoid the obstacles of the non-single property and the difficult standardization of natural extracts.
Owner:SHANTOU UNIV MEDICAL COLLEGE
Method for expressing and purifying human recombinant interleukin-3
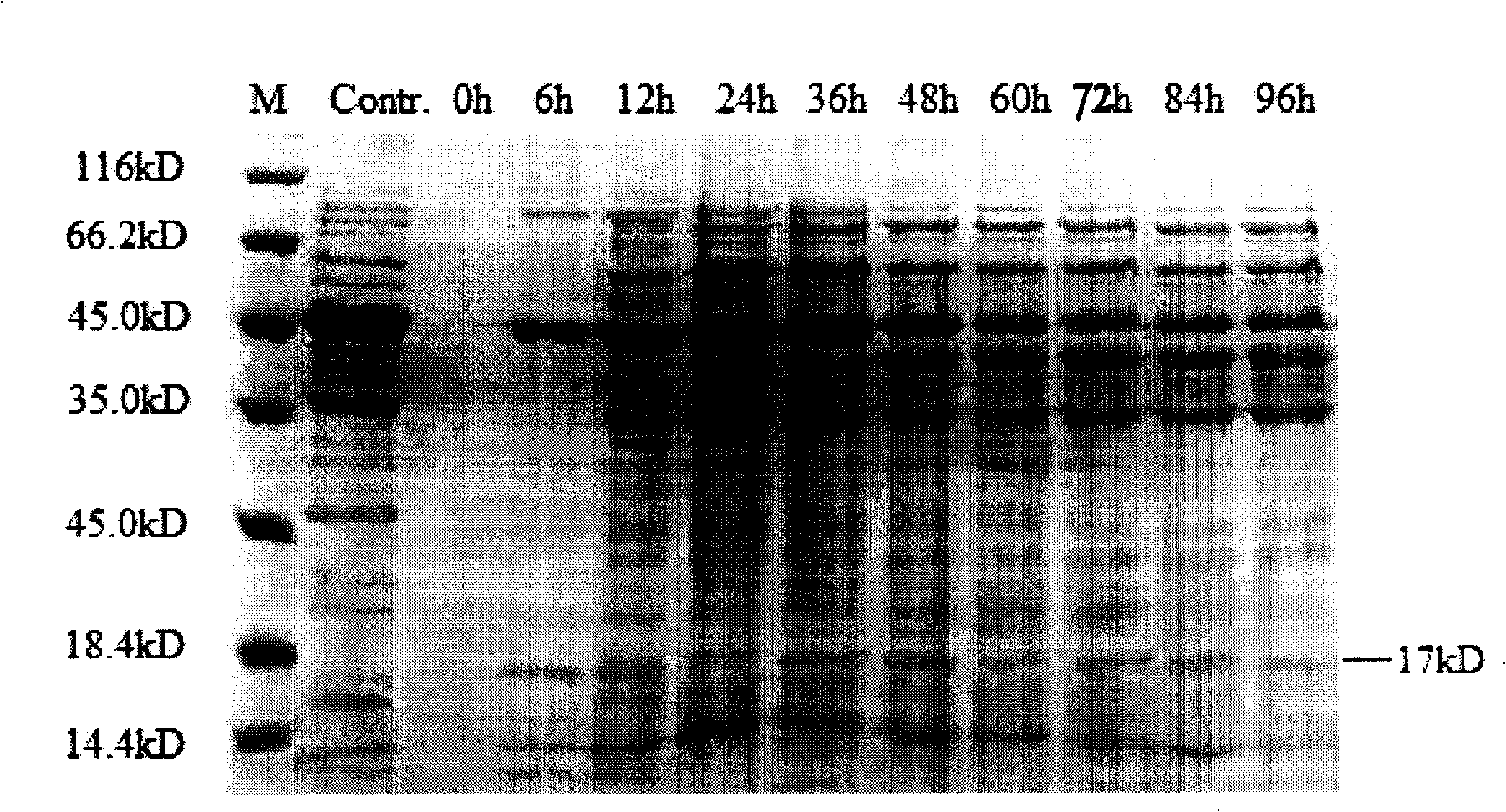
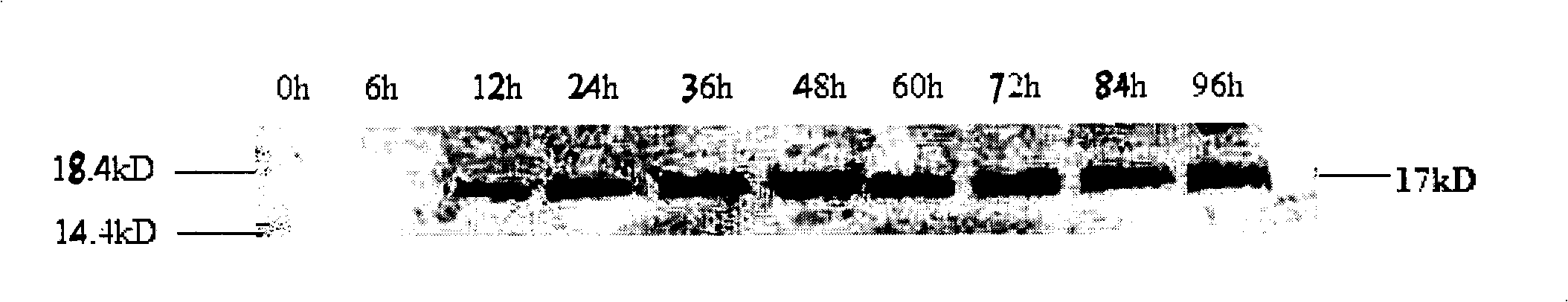
ActiveCN102146413AAvoid degradationLow toxicityPeptide preparation methodsFermentationPichia pastorisMass spectrometric
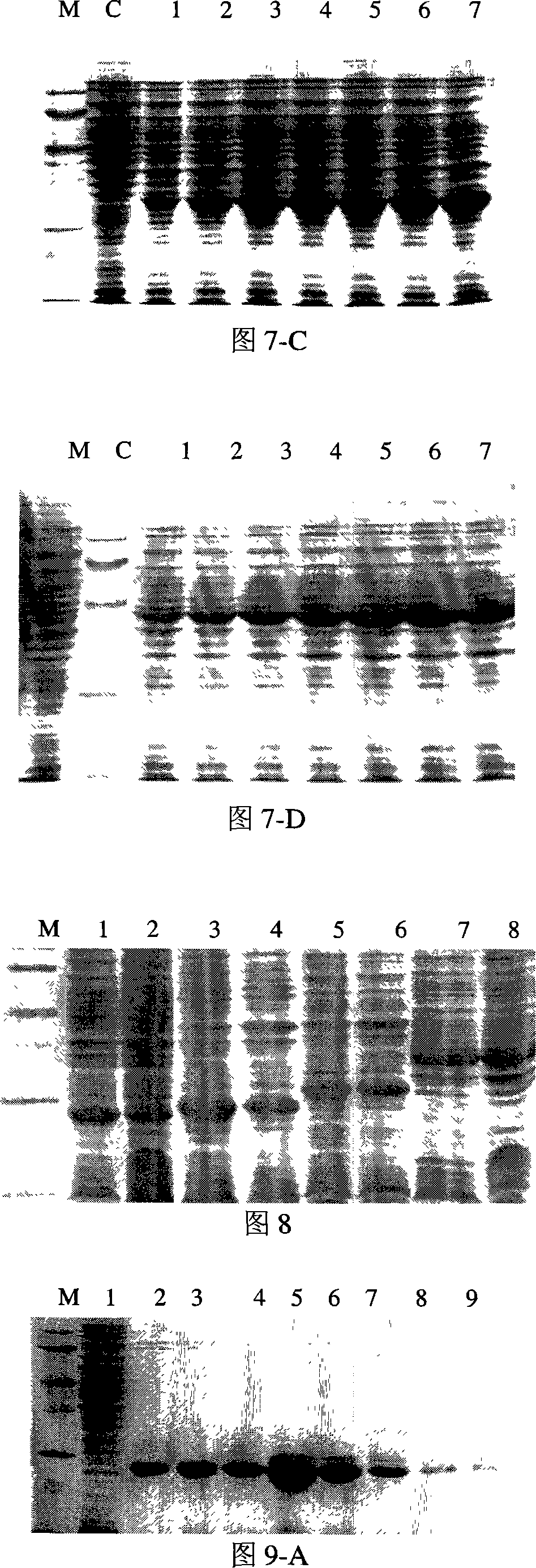
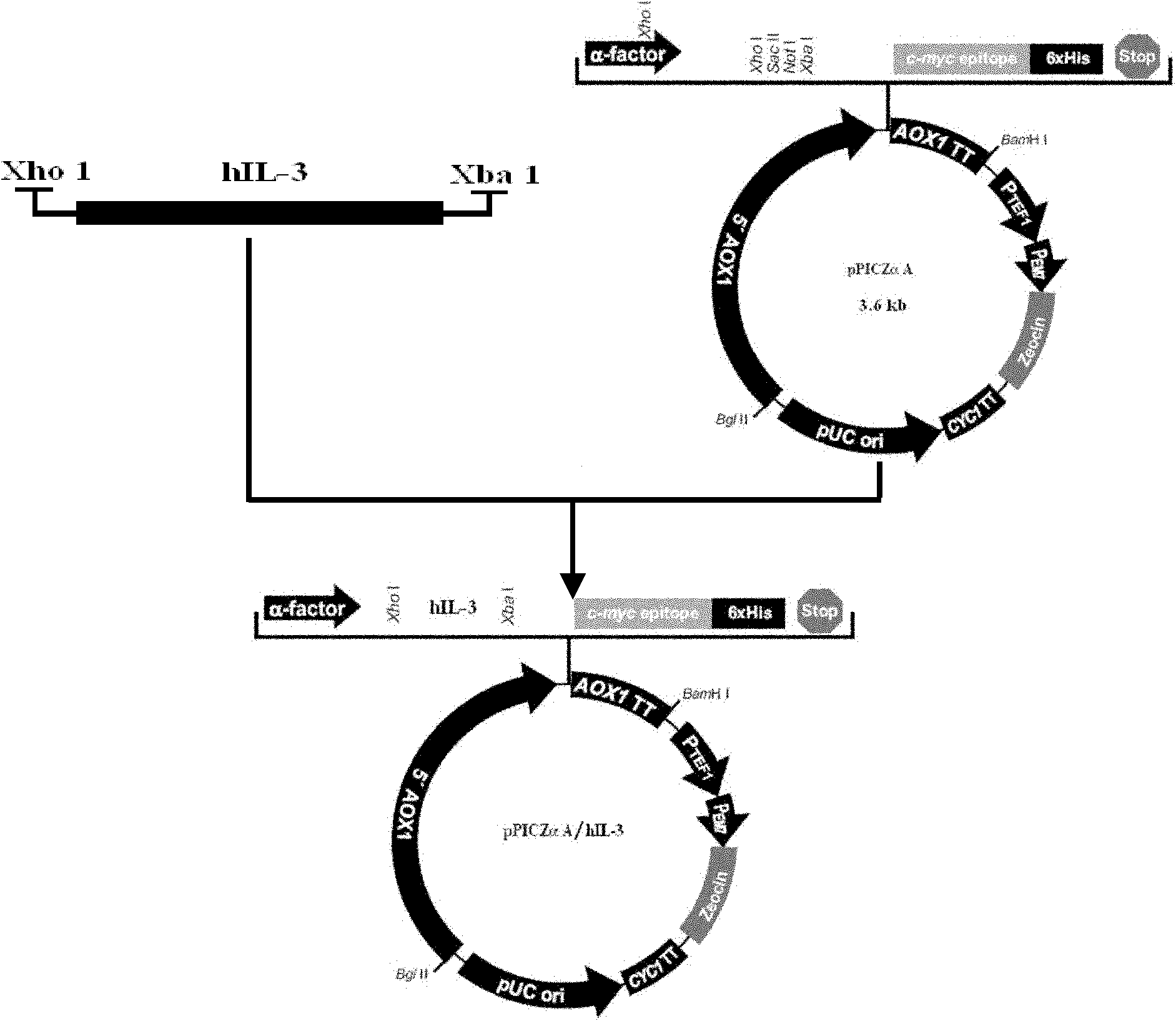
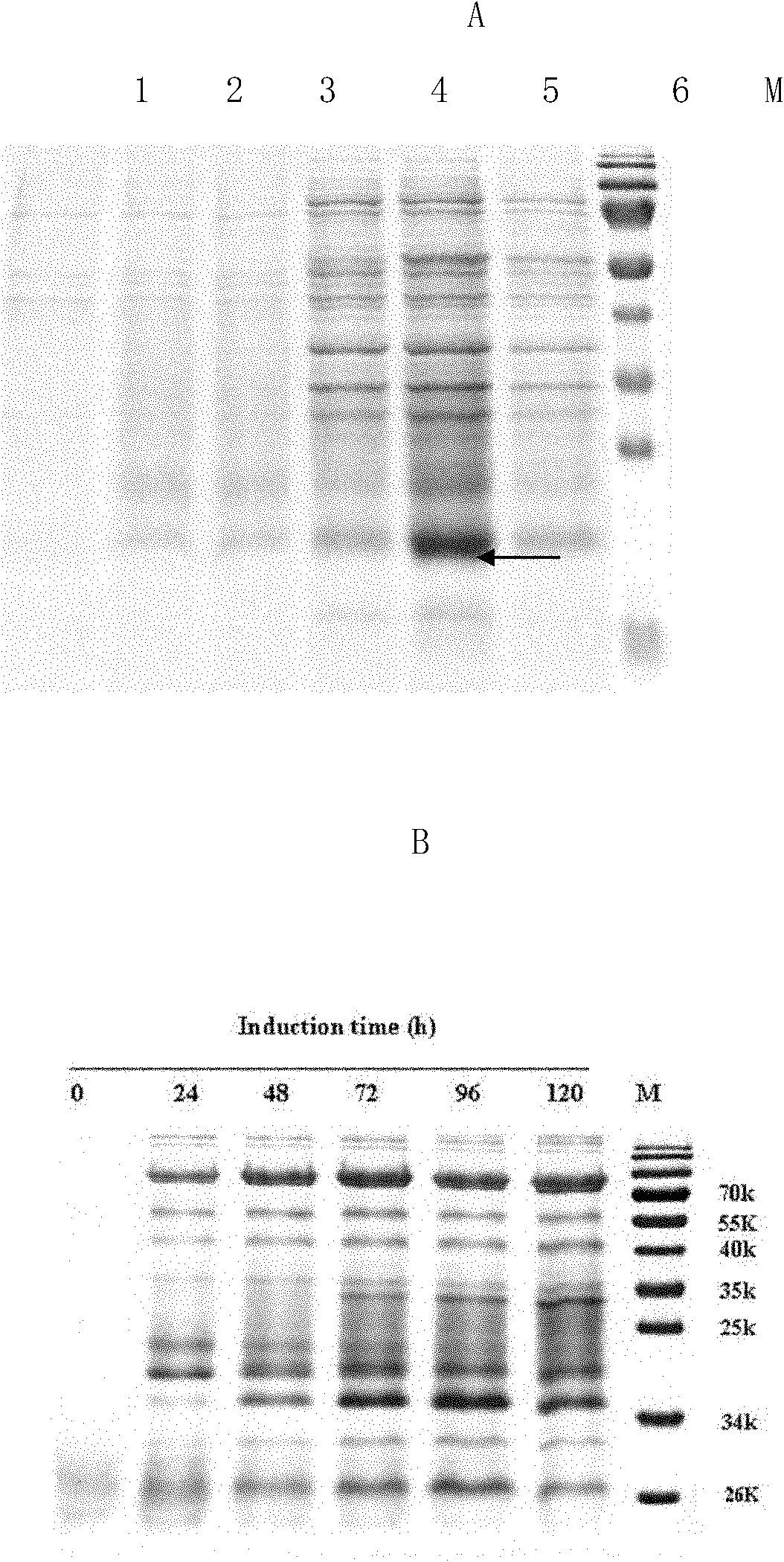
The invention discloses a Pichia pastoris transformant capable of expressing human recombinant interleukin-3 (rhIL-3) with high efficiency and a purification method thereof. In the method, the eukaryotic host is pichia pastorisX-33. The purification method comprises the following steps: cloning a human IL-3 gene; establishing a eukaryotic expression vector, and transforming the eukaryotic expression vector into the eukaryotic yeast host; obtaining a yeast transformant for high-level secretory expression by screening, wherein the IL-3 expressed by the yeasts are available in a glycosylated mode and a non-glycosylated module; and performing amplified culture by using a shake flask, and subjecting the supernate of the culture solution to dialysis, nickel affinity purification and further purification by diethylaminoethanol (DEAE) anion column. The purified product is subjected to mass spectrometric identification and analysis, and the result of the mass spectometric identification and analysis indicates that the expressed IL-3 is modified by different glycosyls and that the IL-3 has an his*6 tag and a C-MYC tag and is easy for purification and detection of expression product. In the invention, different from the conventional method using a prokaryotic host to express the rhIL-3, the method for expressing a large amount of rhIL-3 by using a Pichia pastoris expression system is adopted for the first time, quick purification is realized by using a His-tag protein, the purified rhIL-3 is high-activity rhIL-3 protein which is glycosylated to different extents and of which the molecular weight is 19kDa and 22kDa. The method ensures that the high-activity rhIL-3 recombinant protein is obtained quickly.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Method for large scale preparing Gliocladium chlamydospore
ActiveCN101451107AGuaranteed biological activityGuaranteed control effectFungiDipotassium hydrogen phosphateLiquid medium
The invention provides a method for preparing a large quantity of chlamydospore of Gliocladium virens, which comprises a culture medium formula and culture technology. A liquid medium comprises the following compositions: 20 to 25 grams per liter of glucose, 1 to 3 grams per liter of bean cake powder, 1 to 3 grams per liter of urea, 0.005 to 0.05 gram per liter of ferrous sulfate, 0.75 to 1.5 grams per liter of dipotassium hydrogen phosphate and distilled water. The liquid medium is used for fermentation culture of Gliocladium virens which have superparasitic function on various plant pathogenic fungi (such as sclerotinia, gray mold, banded sclerotial blight and pine root fungi). The culture time is calculated beginning from inoculation, and the chlamydospore of the Gliocladium virens begins to be greatly generated after 36 to 48 hours and is respectively and independently separated. The invention aims to provide the culture medium formula with lower price and rich sources of raw materials, wherein a large quantity of the chlamydospore of the Gliocladium virens can be prepared by utilization of the culture medium formula; and the repeatability is good and the cost is greatly reduced under the condition of liquid fermentation culture, so as to guarantee the biological activity and the prevention and treatment effect of the spores in production and application.
Owner:北京启高生物科技有限公司
Surface-modified fluorescent quantum dot/silica composite microspheres, preparation method thereof, and application thereof
InactiveCN102757790AOptically stableGood colloidal stabilityMicrobiological testing/measurementChemiluminescene/bioluminescenceBiological macromoleculeEmulsion
The invention relates to anti-bio-sorption surface-modified fluorescent quantum dot / silica composite microspheres, a preparation method thereof, and an application thereof. The method provided by the invention is characterized in that: fluorescent quantum dot / silica composite microspheres are first prepared with a reverse micro-emulsion method, and then biocompatible molecules and active functional groups are modified on the surfaces of the microspheres. The method is characterized by simple technology and convenient operation. The surface-modified fluorescent quantum dot / silica composite microspheres can overcome nonspecific interactions thereof upon biological molecules and cells, and can realize covalent coupling thereof with biological molecules. Therefore, surface-bio-functionalized molecular fluorescent probes are constructed. The fluorescent probes can be further applied in fields such as cell biology, bacteria detection, biological macromolecule detection, and immunofluorescence analysis.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Method for continuous hypothermal desiccation and sterilization of bony tissue, and equipment therefor
InactiveCN1861197AGuaranteed biological activityEfficient killingDrying solid materials with heatBone implantVacuum pressureBone tissue
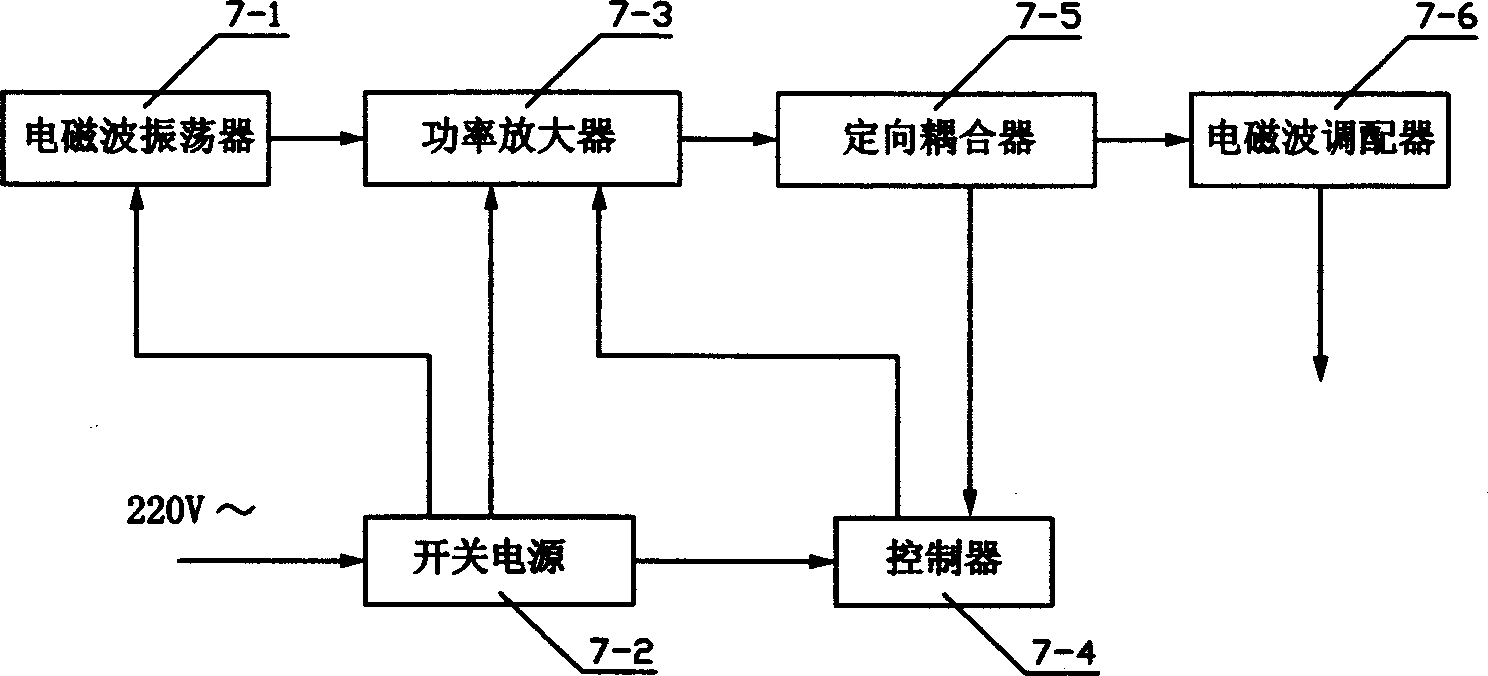
A method for continuous low-temp drying and sterilizing of the bone tissue used for transplanting or repairing purpose includes such steps as processing the bone tissue in cleaned operation room, putting it on a holder in a sealed container, vaccuumizing to 0.1-1000 Pa for 5-60 min, filling argon or oxygen or inertial gas or hydrogen peroxide vapor under said vacuum deg., and applying electromagnetic waves for 5-30 min.
Owner:王立飞
Preparing method of lycium barbarum polysaccharides
The invention relates to a preparing method of lycium barbarum polysaccharides. The method specifically includes the steps of lycium barbarum cleaning, water adding for soaking, smashing, purified water adding for extracting, lycium barbarum seed removing by a pulper, lycium barbarum pulp removing by a centrifugal machine, passing of filtrate through a ceramic film and an organic film system, andconcentrating and freeze-drying of an extraction solution for obtaining the product, namely the lycium barbarum polysaccharides. The most effective water temperature and ratio of purified water in theextraction stage are determined, the sufficient dissolving-out of the lycium barbarum polysaccharides is ensured, and the extraction rate of the lycium barbarum polysaccharides is greatly increased;the biological activity of the lycium barbarum polysaccharides is kept to the maximum extent, and the problem that effective components are lost when the inactivation and flocculation of polysaccharides and proteins are caused by heating through an alcohol deposition method is avoided.
Owner:宁夏天仁枸杞生物科技股份有限公司
Poultry antivirus composition, freeze-dried powder, preparation method and applications of the composition
InactiveCN104208673AImprove the body's immunityEnhance body adaptability and resistanceOrganic active ingredientsPowder deliveryAntibodyDisease
The invention discloses a poultry antivirus composition, freeze-dried powder, a preparation method and applications of the composition, and belongs to the technical field of feed additive for poultry and livestock. The poultry antivirus composition is mainly composed of the following components in parts by weight: 90 to 100 parts of poultry antivirus yolk antibody, 1 to 2 parts of poultry antivirus transfer factor, and 1 to 2 parts of alpha-interferon, and preferably also comprises the following components in parts by weight: 5 to 10 parts of astragalus polysaccharide, 5 to 10 parts of angelica polysaccharide, 10 to 20 parts of wolfberry polysaccharide, and 10 to 30 parts of licorice polysaccharide. The poultry antivirus yolk antibody, poultry antivirus transfer factor, alpha-interferon, and traditional Chinese herbal extracts (astragalus polysaccharide, angelica polysaccharide, wolfberry polysaccharide, and licorice polysaccharide) are compounded to prepare the composition, which can effectively improve the immunity and disease preventing performance of poultry organism and has a good preventing and treating effect on poultry viral diseases. The composition can be made into freeze-dried powder, which can be preserved at a normal temperature for a long time and has the advantages of high antibody titer and good bio-activity.
Owner:ZHENGZHOU HOUYI PHARMA
Novel process for extracting golden fungus polysaccharides by step enzyme method
The invention relates to a production process for an edible and medicinal fungus polysaccharide, belonging to the biological product production technical field, which is characterized in that a water lixiviation liquid of a golden fungus is processed step by step by the means of enzymolysis via a pectinase, a cellulose, and a neutral prolease, identifying the process conditions comprising the suitable amount, enzymolysis time, temperature, pH value and the other figures, enabling the polysaccharide component inside and outside of the golden fungus solid cell to be separated fully from the non-polysaccharide big molecules such as a pectin, a cellulose, a protein and the other members, the polysaccharide component is discharged and dissolved into the water, finally, the polysaccharide is separated from the solution by the means of ethanol sedimentation. The analysis result shows compared with the traditional water lixiviation the extraction rate of the polysaccharide is increased from 7.8% to 14.3%, the protein content in the polysaccharide is lowered from 13.3% to 1.6%, and the product yield and quality are improved drastically by utilizing the stepping enzymolysis to extract the golden fungus polysaccharide. The production process for an edible and medicinal fungus polysaccharide has the advantages of efficiently extracting the golden fungus polysaccharide with high purity, completely protecting the molecular structure and the conformation of the polysaccharide, and maintaining the biological activity under the moderate production conditions.
Owner:ZHEJIANG UNIV +1
Cell freezing medium for treating leukemia
ActiveCN105432599ANo lossFor long-term storageDead animal preservationPeptidesCulture cellLow-density lipoprotein
The invention provides a cell freezing medium and a preparation method thereof. The cell freezing medium is prepared from 5 percent w / v dimethyl sulfoxide, 0.5 percent w / v low density lipoprotein, 1 percent w / v trehalose, 1 percent w / v lecithin, 1 percent w / v polypeptide, 1 percent w / v bFGF (Basic Fibroblast Growth Factor), and 1 percent w / v vitamin E; finally, the volume of the cell freezing medium is fixed to 100 ml by using a DMEM culture medium and fetal calf serum in the proportion of 1:1. The cell freezing medium provided by the invention is used for freezing culture cells, and particularly, the survival rate of frozen cells after the frozen cells resuscitate is improved. The cell freezing medium has a good application prospect.
Owner:重庆斯德姆生物技术有限公司
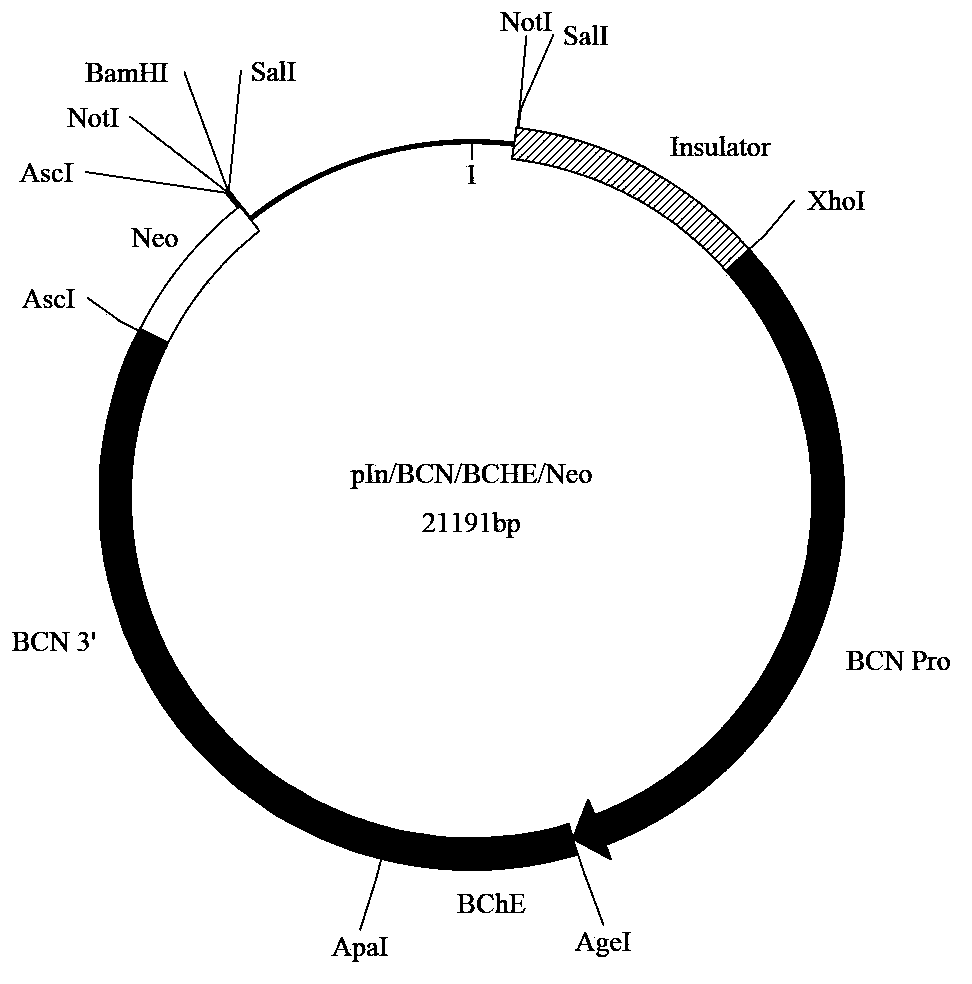
Method for producing recombinant human butyrylcholine esterase on large scale by utilizing biological platform of mammary gland of transgenic animal
ActiveCN103898101AHigh activityGuaranteed biological activityHydrolasesPeptide/protein ingredientsPhysiologyPesticide residue
The invention provides a method for producing recombinant human butyrylcholine esterase on large scale by utilizing a biological platform of the mammary gland of a transgenic animal. The method comprises the following steps: utilizing the biological platform of the mammary gland of the transgenic animal to efficiently produce holonomic and monomeric recombinant human butyrylcholine esterase on large scale for the first time, namely cut-off type enzyme without containing the last 40 amino acid residues of the holonomic butyrylcholine esterase, and a protein fused with monomeric butyrylcholine esterase and albumin. The produced recombinant protein can be used for preventing and treating apnea caused by poisoning of nerve gas, poisoning of organophosphorus pesticide, poisoning of cocaine and succinylcholine, and is used for detecting and removing residues of organophosphorus pesticide on vegetables, melons, fruits, other crops, surfaces of various articles and soil.
Owner:SHANGHAI JENOMED BIOTECH CO LTD
Preparation method of collagen diaphragm material
ActiveCN104098783AGuaranteed biological activityHigh strengthPeptide preparation methodsChemistryFreeze dry
The invention relates to a preparation method of a collagen diaphragm material. The method comprises the following steps: firstly, cleaning animal skin in an alkalescent solution, carrying out unhairing and cleaning treatment to the animal skin in a water solution containing Na2S and Ca(OH)2, then adjusting the pH value to be 6 to 8 by using an acidulous ammonium salt, after obtaining a collagen fiber layer, and drying after degreasing the collagen fiber layer repeatedly by adding acetone, so as to obtain a raw material; crushing, purifying, refining and freeze-drying the raw material so as to obtain the collagen diaphragm material. The collagen diaphragm material is pliable and tough in texture, excellent in stickiness, low in salinity content, and good in bioactivity and biosecurity, has a complete 3D structure, and is very suitable for clinical application.
Owner:CHANGZHOU INST OF MATERIA MEDICA
Mesenchymal stem cell cryopreservation agent
The invention discloses a mesenchymal stem cell cryopreservation agent which comprises 0.2%w / v cane sugar, 0.5%w / v low-density lipoprotein, 3%w / v trehalose, 2%w / v lecithin, 100ppm w / v polypeptide, 1.5%w / v bFGF, 1%w / v vitamin E, 5% v / v glycerin and the balance of a DMEM (Dulbecco's Modified Eagle Medium) and fetal calf serum in a ratio of 1:1 till 100ml. In the mesenchymal stem cell cryopreservation agent disclosed by the invention, the glycerin, the trehalose, the vitamin E and the polypeptide have definite functions of preventing external damage to cells, the polypeptide also has a function of preventing cells from damage of ice crystal generated in cryopreservation, and the other components are essential for maintaining survival of specific MSCs (Mesenchymal Stem Cells). The cell cryopreservation agent disclosed by the invention is applied to cryopreservation and culture of cells, is particularly capable of increasing the survival rate of revived cryopreservation cells, and has relatively good application prospects.
Owner:陈印平
Virus inactivation method for solution of globulin
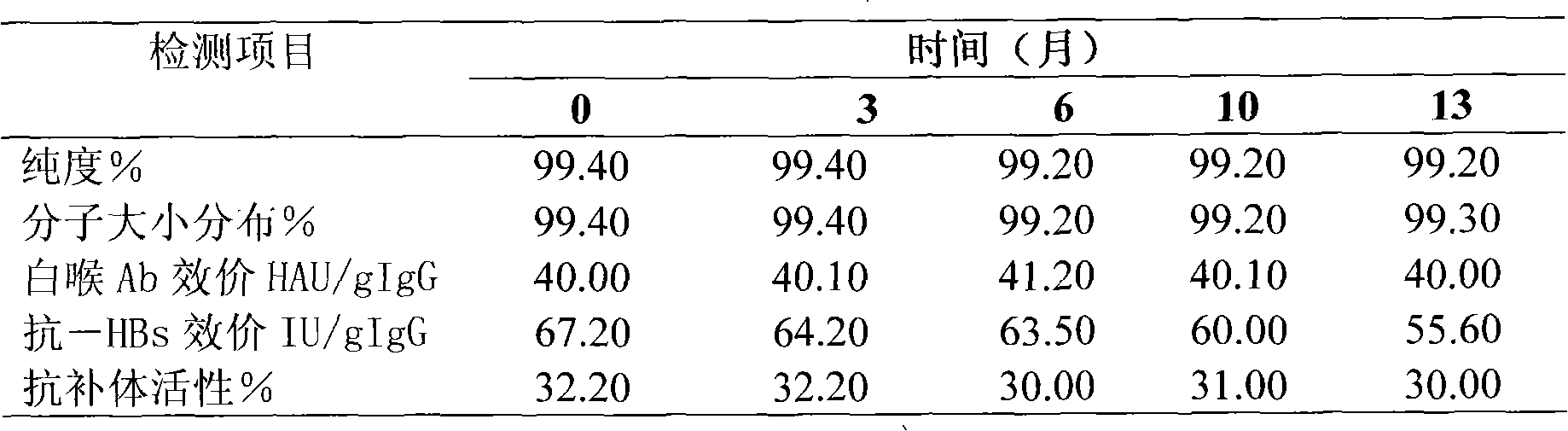
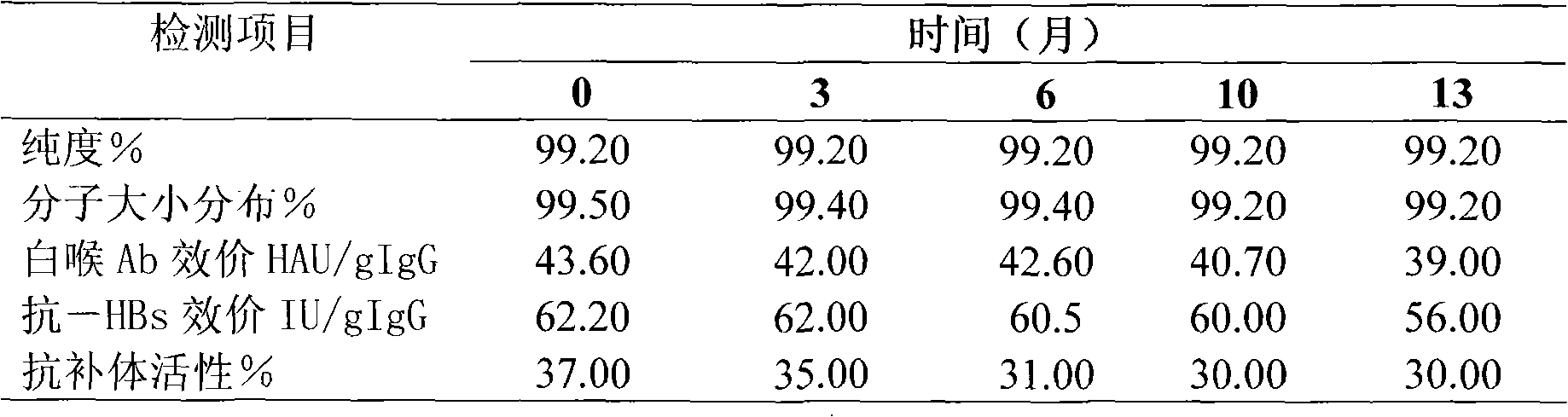
ActiveCN101927011AHigh recovery rateDoes not affect biological activityPeptide preparation methodsImmunoglobulinsGlobulinVirus inactivation
The invention provides a virus inactivation method for solution of globulin. An optimum proportion with a practical value of various parameters during virus inactivation with sodium caprylate is provided by a large number of researches on various conditions such as a temperature, a pH value, concentration of caprylate, concentration of protein and hatch time during the virus inactivation. Experiments prove that the virus inactivation method for the solution of the globulin has the advantages of simpleness, safety, rapidness, effectiveness, high protein recovery and no effect on the bioactivity of a target protein.
Owner:国药集团武汉血液制品有限公司
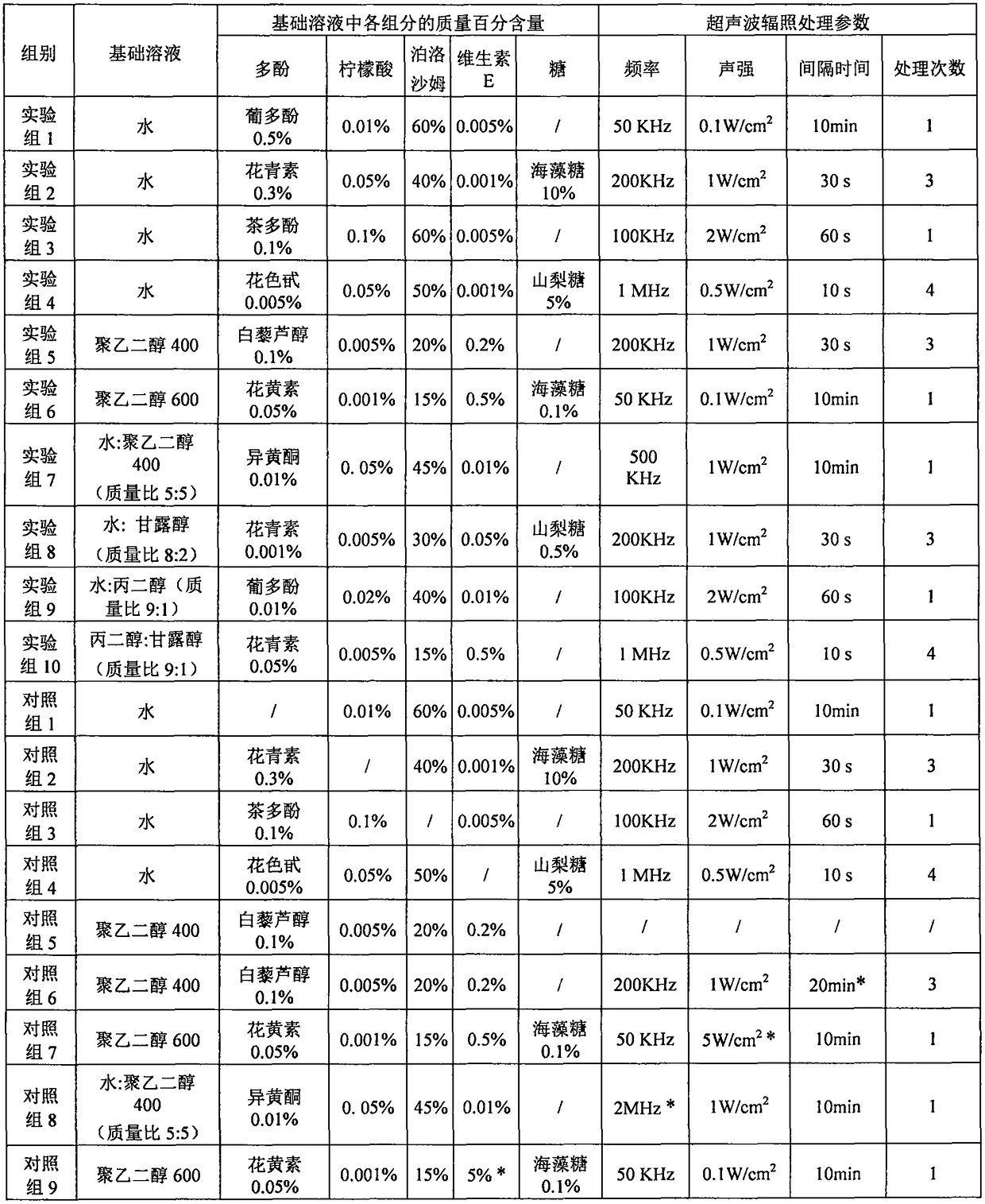
Solution system for promoting cryopreserved tissue organ and cell activity recovery
ActiveCN108753683AGuaranteed biological activityAntioxidantCulture processCell culture mediaAdditive ingredientWavelength
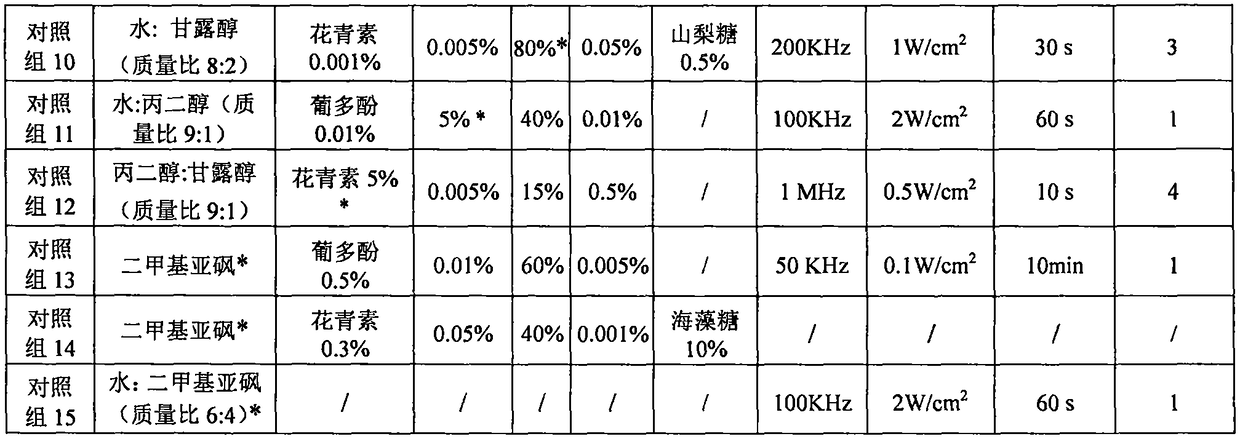
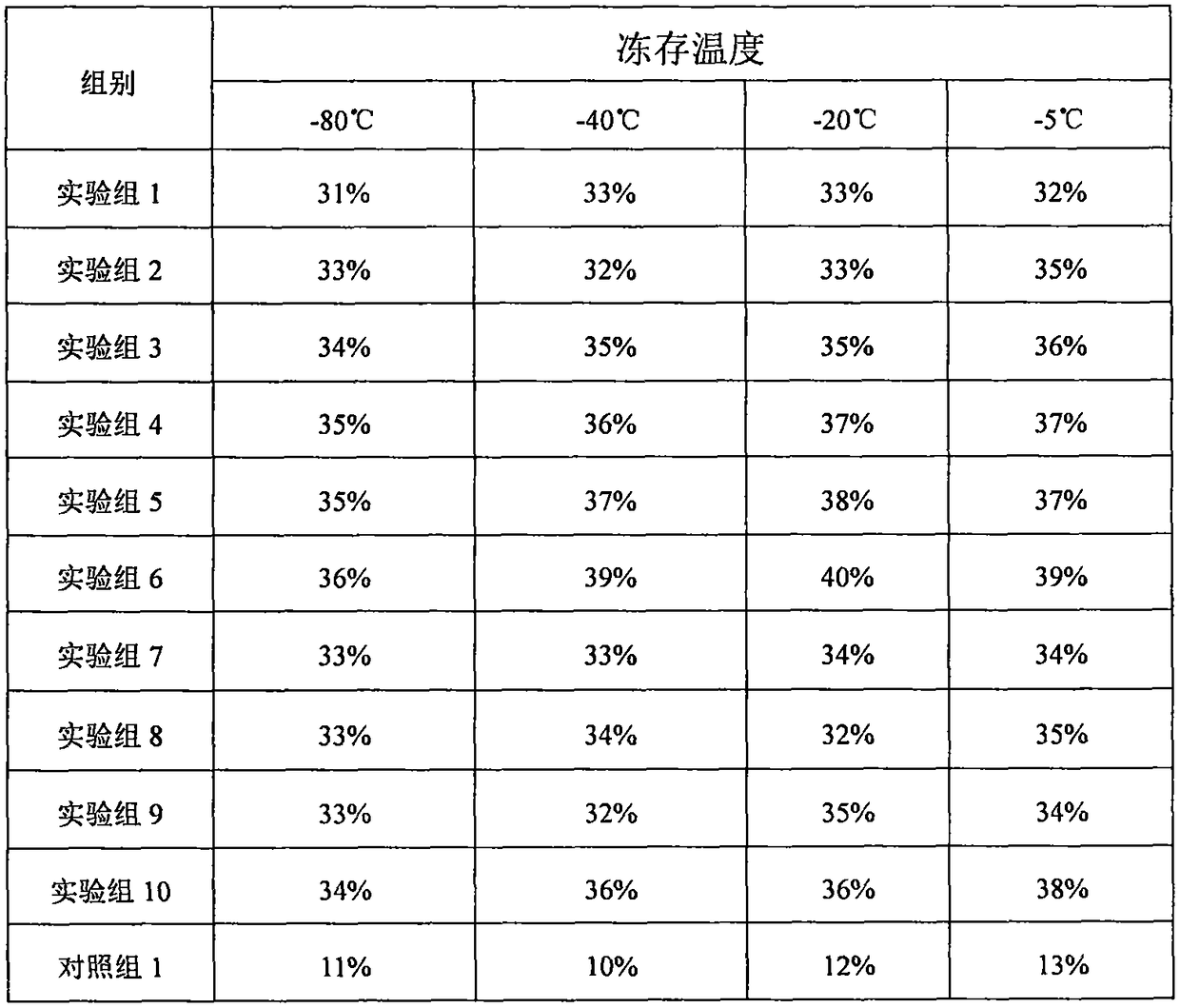
The invention relates to a solution system for promoting cryopreserved tissue organ and cell activity recovery. One or several kinds of materials from water and polyalcohol are used as basic solutions; polyphenol, citric acid, poloxamer and vitamin E are added into the basic solutions; tissue organs and cells are put into the solution system for cryopreserving; after the cryopreserving, ultrasonicwaves with the wavelength in the range of 20KHz to 1MHz and the sound intensity not greater than 3W / cm<2> are used for performing irradiation treatment on cells; by regulating the proportion of different ingredients in the solution and controlling the wavelength, sound intensity and irradiation treatment of the ultrasonic waves, the biological activity after the cryopreserved tissue organ and cell recovery is improved.
Owner:WENZHOU MEDICAL UNIV
Preparation technology of pig liver peptide powder
InactiveCN109957595AAvoid pollutionGuaranteed stabilityPeptide preparation methodsFermentationPig liverSeparation technology
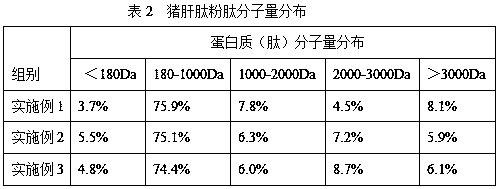
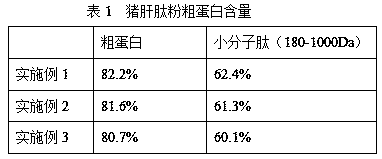
The present invention relates to a preparation technology of pig liver peptide powder. The preparation technology comprises the following steps: (1) pig liver homogenizing, lipase enzymatic degreasing, protease hydrolyzing and ultrafiltering are conducted to obtain liver peptide liquid, and the liver peptide liquid is spray-dried to obtain a finished product. The enzymatic hydrolysis method is combined with ultrafiltration membrane separation technology to extract and purify small molecular liver peptides. The preparation technology is simple in processing processes, short in production cycle,high in production efficiency, low in cost, and suitable for large-scale industrial production. The obtained pig liver peptide powder is high in quality and has a crude protein content of 80% or more, and the small molecular liver peptides (with a relative molecular weight between 180 and 1,000 Da) account for 70% or more of the protein.
Owner:陈石良
Plant seed expression system for single-chain antibody against CD20
InactiveCN103981191AAvoid pollutionHigh expressionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD20Molecular biology
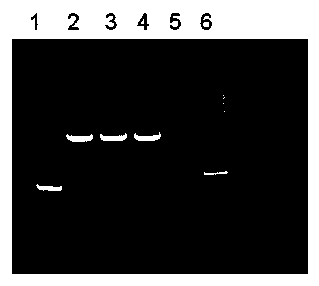
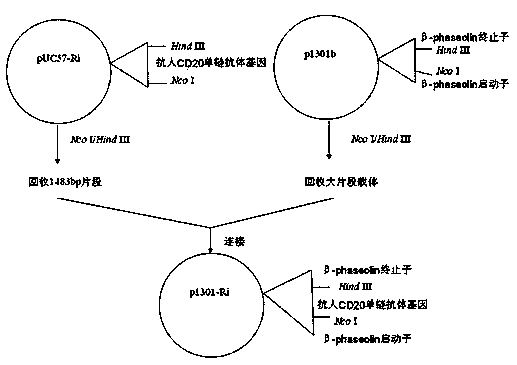
The invention discloses a plant seed expression system for a single-chain antibody against CD20. According to the invention, a single-chain antibody gene against CD20 is designed and artificially synthesized according to the sequence of VL-VH-Linker-hIgG1Fc by adding the nucleotide sequence of a signal peptide sequence coding barley alpha-Amylase at the 5' end of the gene, the gene is inserted into a plant seed specific expression vector p1301b, and an Agrobacterium-mediated method is employed for transformation of plants; an expression frame with a seed specific promoter, the single-chain antibody gene against CD20 and a terminator is integrated into the genomes of the plants; plants with the gene are screened and cultured, screening and propagation are further carried out so as to obtain a homozygous plant, and seeds produced by the homozygous plant contain the single-chain antibody against CD20; recombinant protein is directionally expressed in vacuole. The expressed single-chain antibody against CD20 has biological activity, and ADCC killing capability of the antibody on Daudi cells is not significantly different from that of Rituxan.
Owner:JILIN AGRICULTURAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com