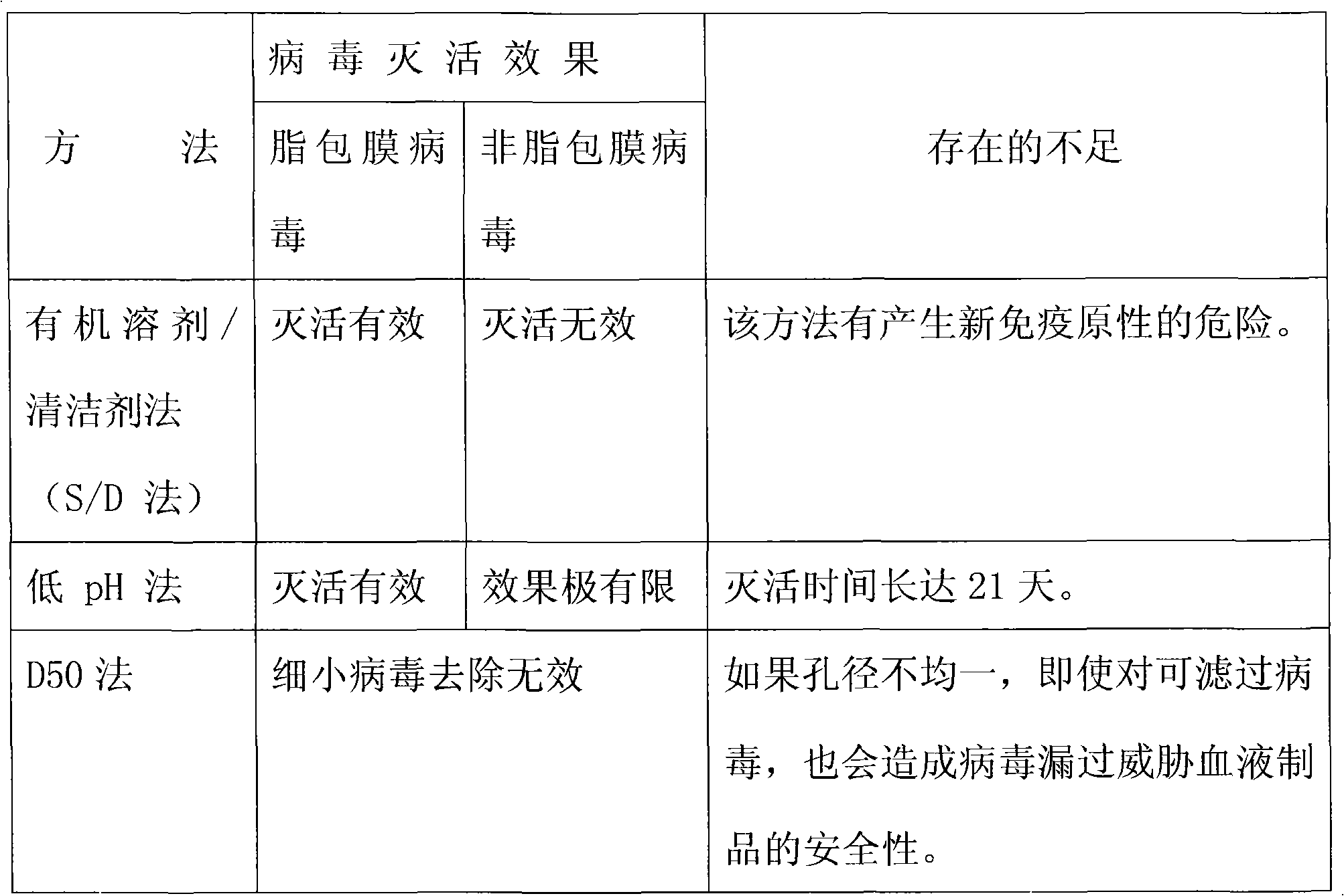
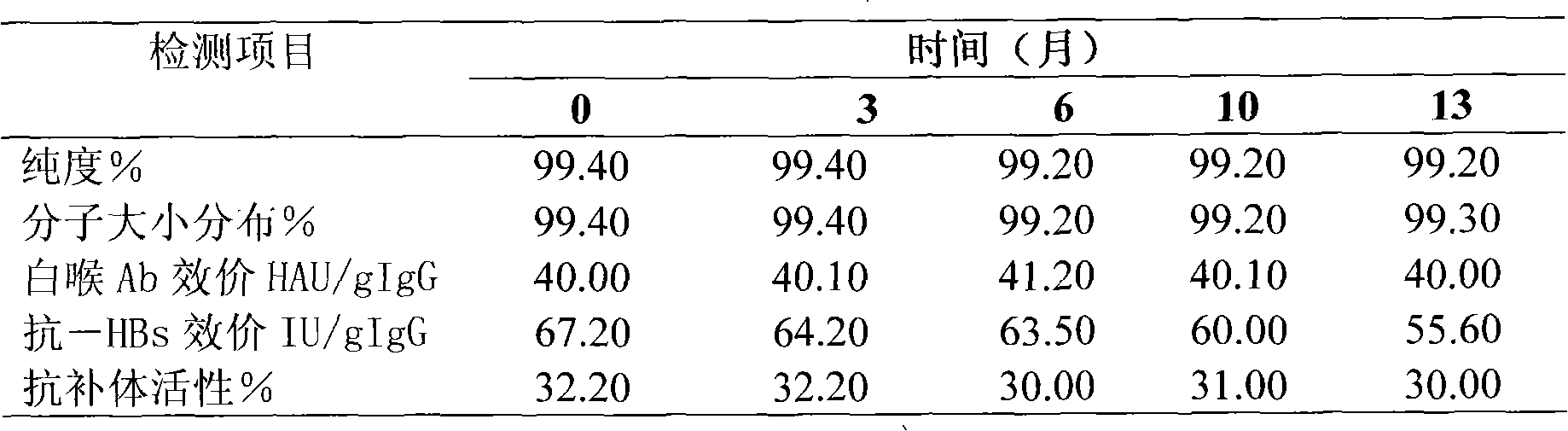
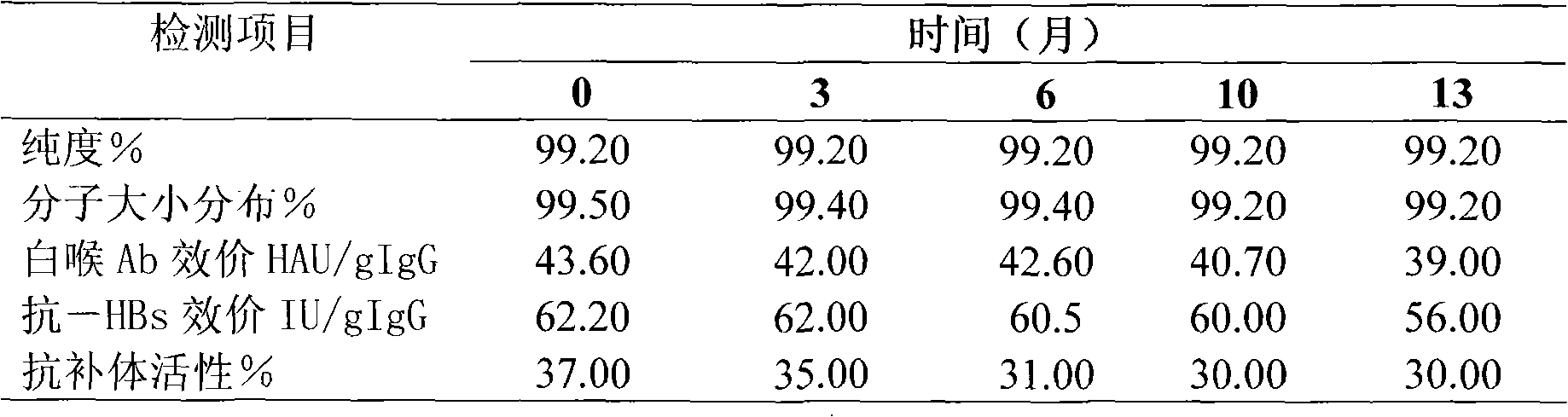
Virus inactivation method for solution of globulin
A virus inactivation and globulin technology, applied in the biological field, can solve the problems of no practical value and low protein recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Take 400 ml of intravenous human immunoglobulin provided by Wuhan Institute of Biological Products, and determine the protein content with reference to the three parts of the "Pharmacopia of the People's Republic of China" 2005 edition Kjeldahl method. Test result: the protein content is 11.8%.
[0136] Inactivation parameters: sodium octanoate concentration 16mmol, protein solution to be inactivated 100ml, inactivation time 1.5 hours, inactivation temperature 30±1°C, sodium octanoate solution pH 8.5, pH 4.9 during inactivation, diluent 0.9% NaCl solution.
[0137] Inactivation step:
[0138] a. Calculate the consumption of sodium octanoate: the consumption of sodium octanoate is calculated according to the ratio of 16: 1 by the weight ratio (mmol / g) of protein in sodium octanoate and the protein in the virus inactivation globulin solution: 100ml * 11.8% * 166 * 16 / 1000=31.3 grams, take by weighing 31.3 grams of sodium caprylate; Add the sodium caprylate that takes by...
Embodiment 2
[0142] Take 400 ml of intravenous human immunoglobulin provided by Wuhan Institute of Biological Products, and determine the protein content with reference to the three parts of the "Pharmacopia of the People's Republic of China" 2005 edition Kjeldahl method. Test result: the protein content is 11.8%.
[0143] Inactivation parameters: sodium octanoate concentration 16mmol, protein solution to be inactivated 100ml, inactivation time 1.5 hours, inactivation temperature 30±1°C, sodium octanoate solution pH 5.5, pH 4.7 during inactivation, diluent 0.9% NaCl solution.
[0144] Inactivation step:
[0145] a. Calculate the consumption of sodium octanoate: the consumption of sodium octanoate is calculated according to the ratio of 16: 1 by the weight ratio (mmol / g) of protein in sodium octanoate and the protein in the virus inactivation globulin solution: 100ml * 11.8% * 166 * 16 / 1000=31.3 grams, take by weighing 31.3 grams of sodium octanoate, add the sodium octanoate that takes by...
Embodiment 3
[0149] Take 400 ml of intravenous human immunoglobulin provided by Wuhan Institute of Biological Products, and determine the protein content with reference to the three parts of the "Pharmacopia of the People's Republic of China" 2005 edition Kjeldahl method. Test result: the protein content is 11.8%.
[0150] Inactivation parameters: sodium octanoate concentration 16mmol, protein solution to be inactivated 100ml, inactivation time 1.5 hours, inactivation temperature 30±1°C, sodium octanoate solution pH 5.6, pH 4.8 during inactivation, diluent 0.9% NaCl solution.
[0151] Inactivation step:
[0152] a. Calculate the consumption of sodium octanoate: the consumption of sodium octanoate is calculated according to the ratio of 16: 1 by the weight ratio (mmol / g) of protein in sodium octanoate and the protein in the virus inactivation globulin solution: 100ml * 11.8% * 166 * 16 / 1000=31.3 grams, take by weighing 31.3 grams of sodium octanoate, add the sodium octanoate that takes by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com