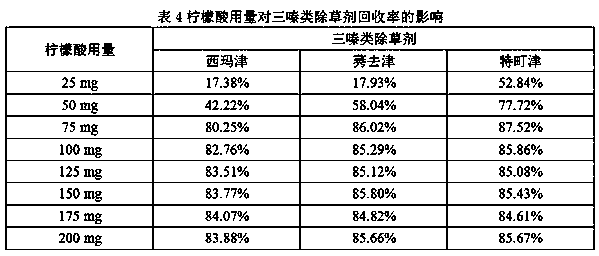
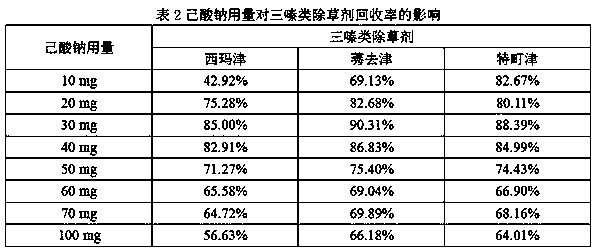
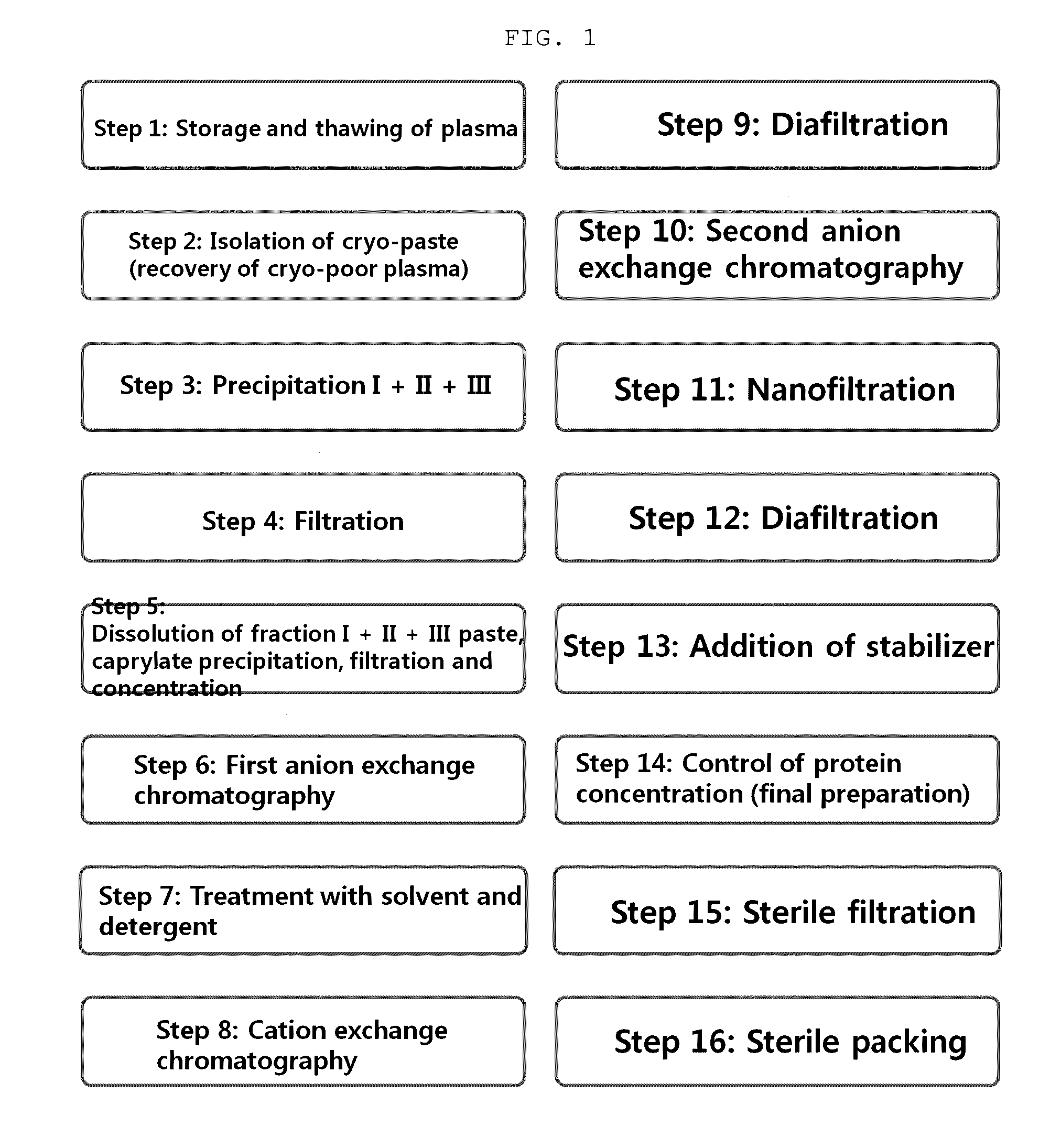
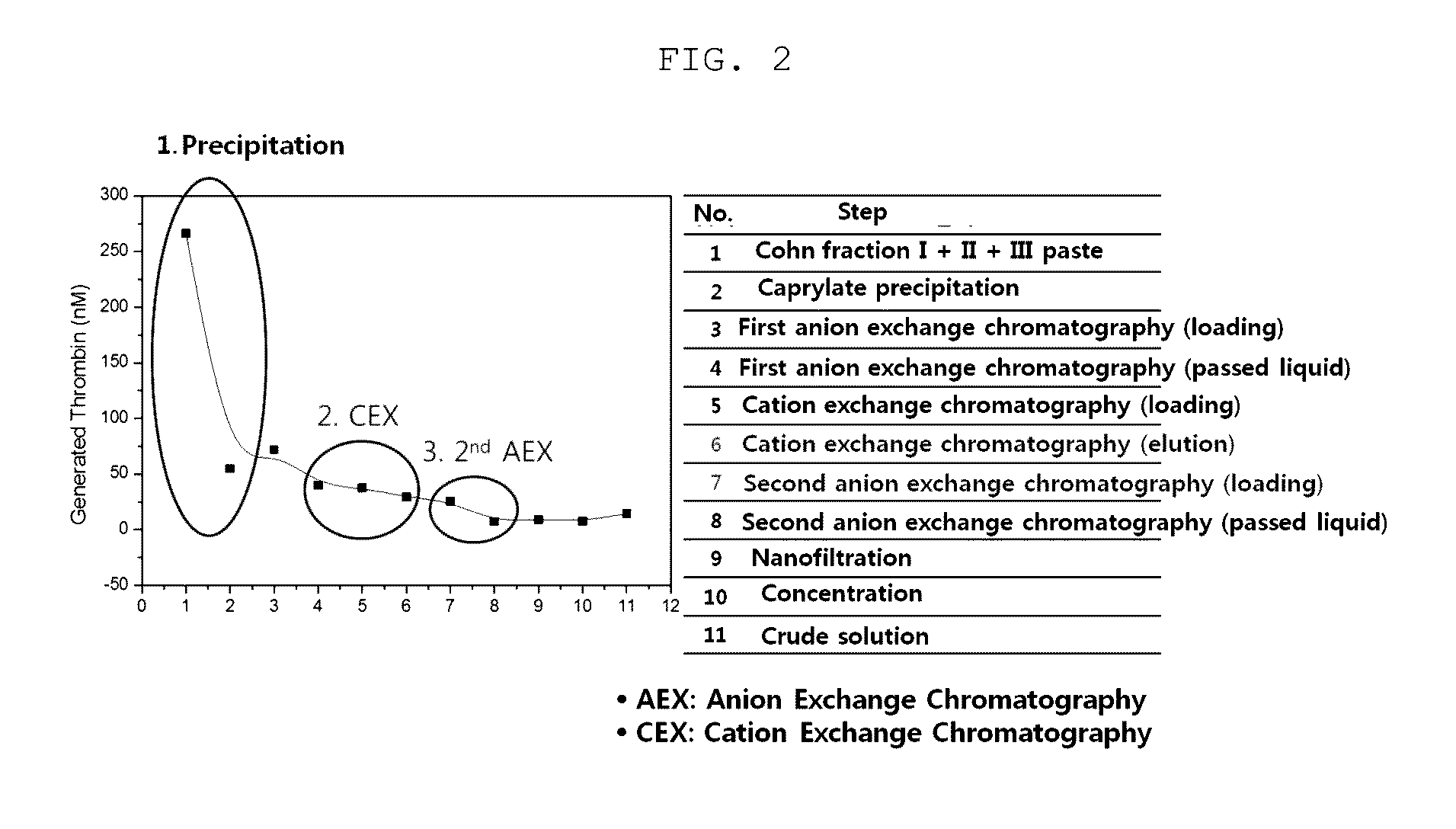
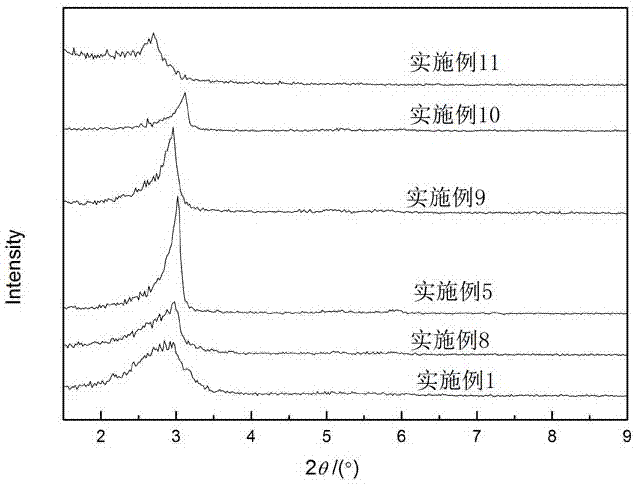
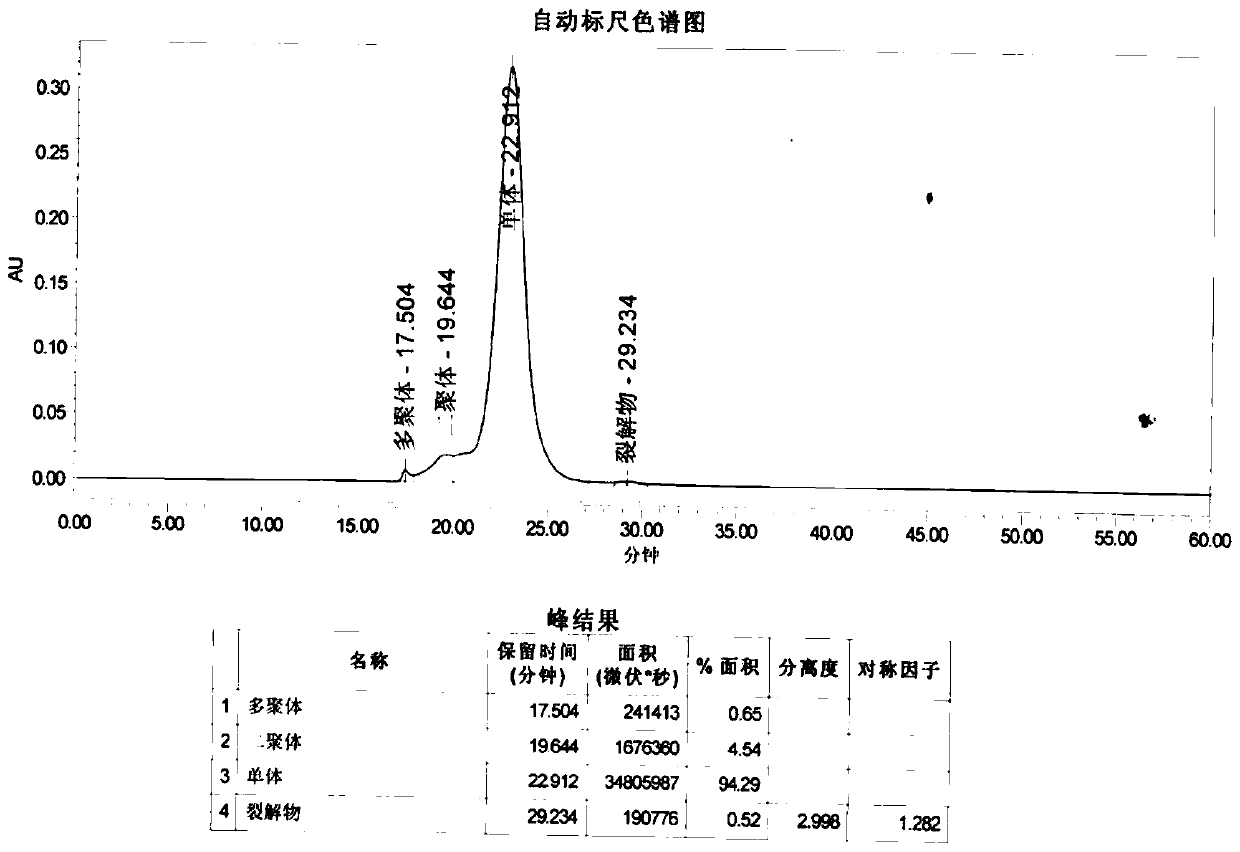
Patents
Literature
88 results about "Sodium caprylate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical Definition of sodium caprylate. : the sodium salt C8H15O2Na of caprylic acid used especially in the topical treatment of fungal infections.
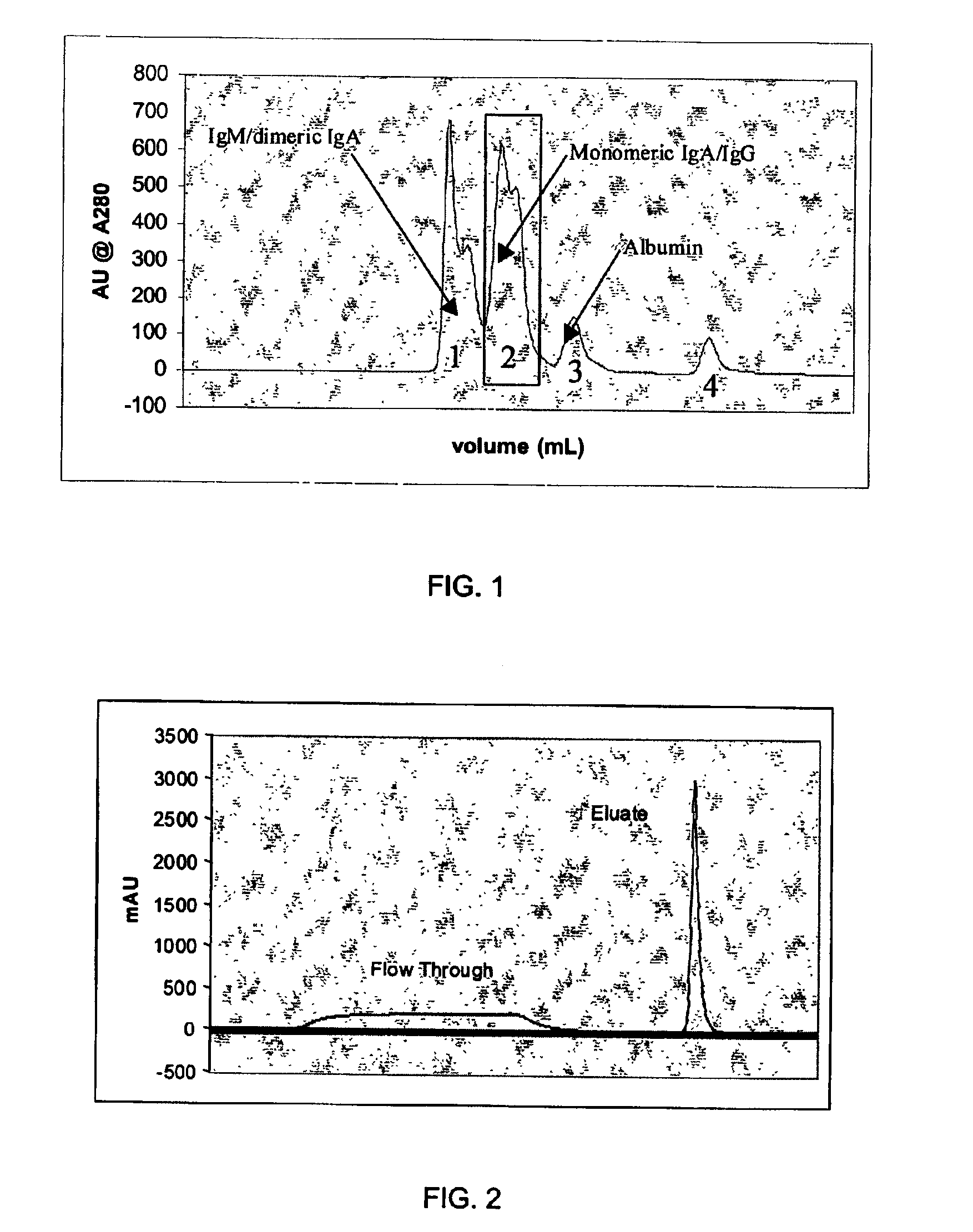
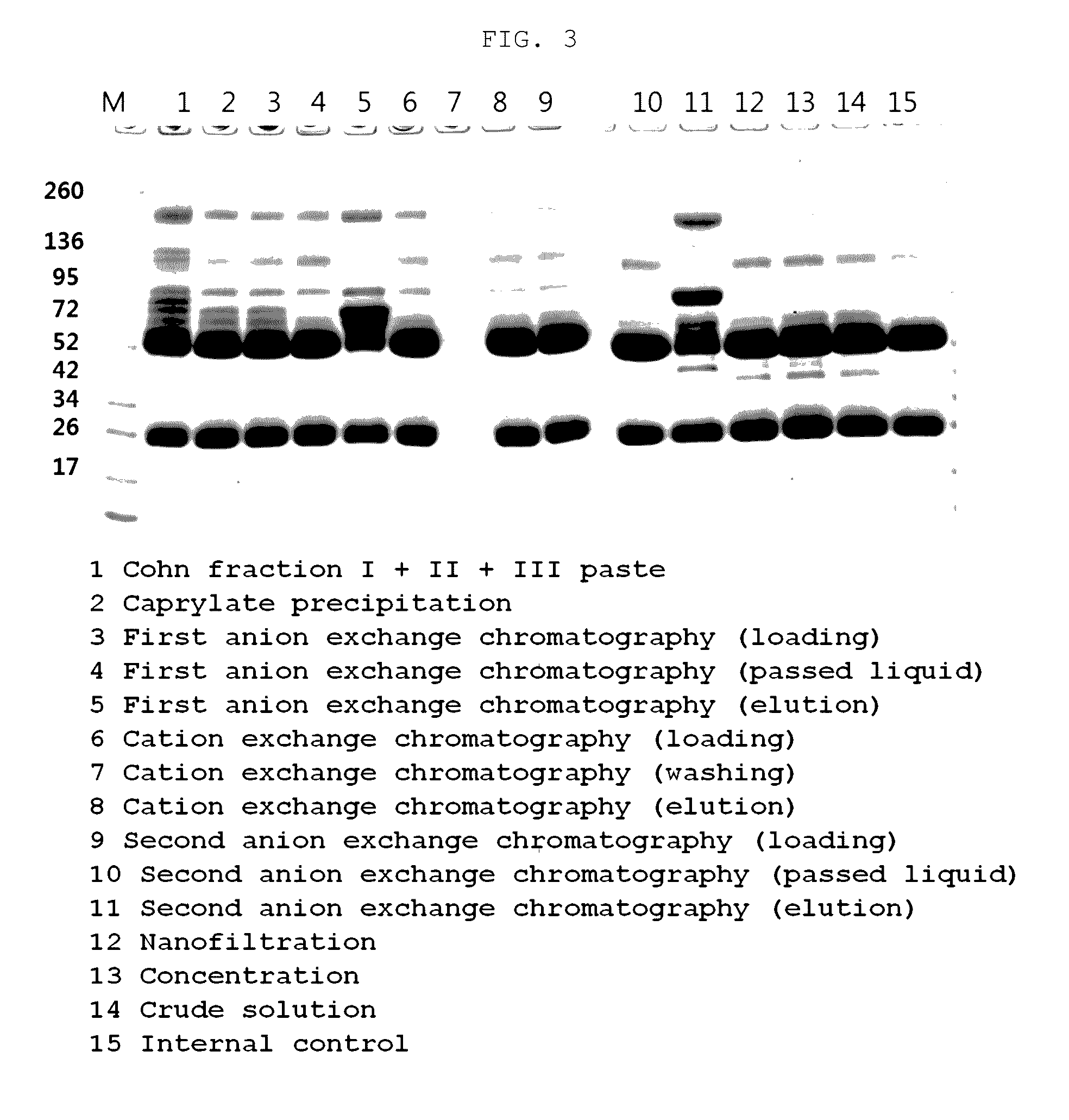
Chromatographic method for high yield purification and viral inactivation of antibodies
InactiveUS6955917B2Minimizes post virus treatment manipulationYield maximizationPeptide/protein ingredientsSerum immunoglobulinsLipid formationLow ionic strength
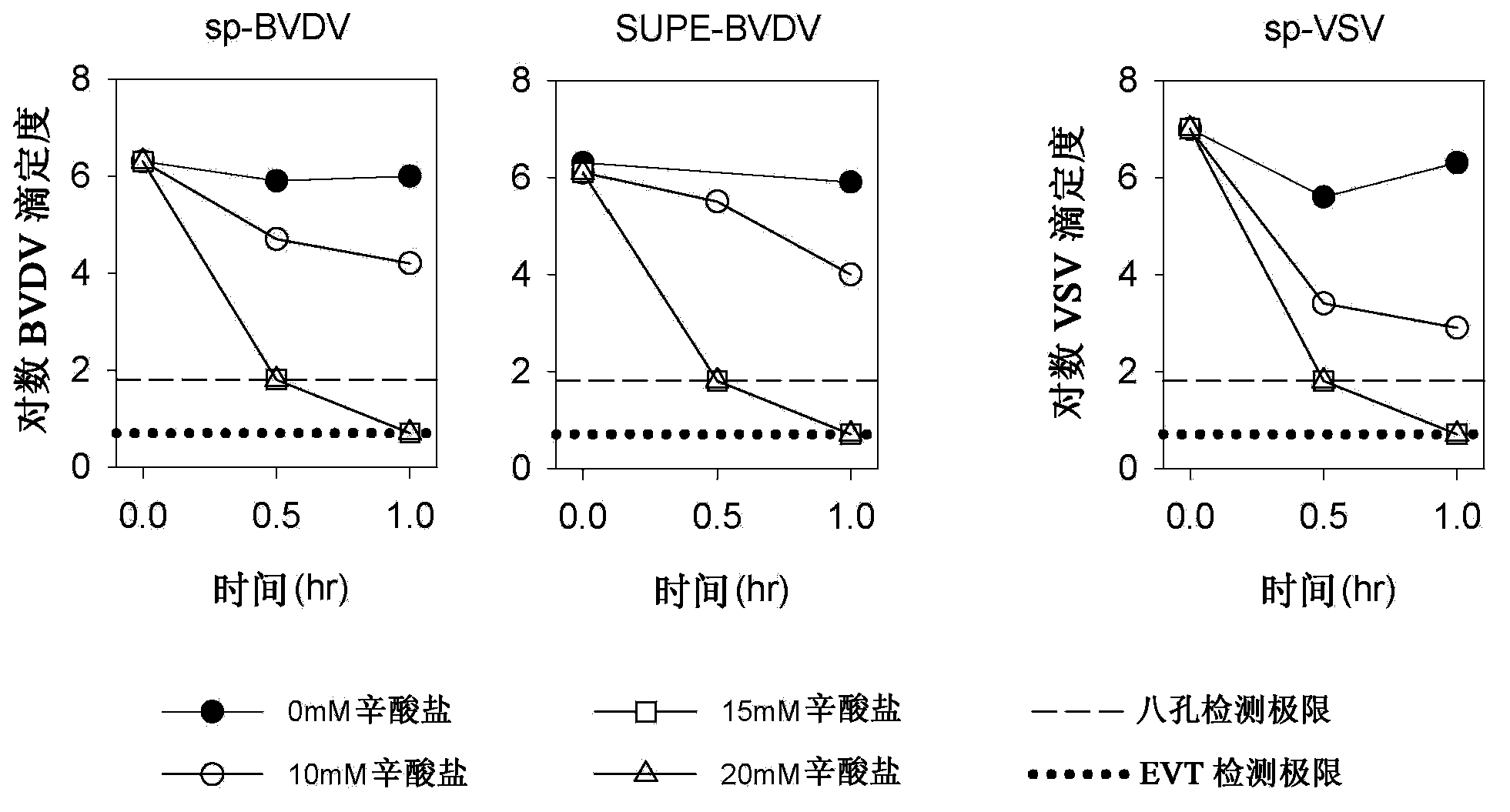
An improved process for the purification of antibodies from human plasma or other sources is disclosed. The process involves suspension of the antibodies at pH 3.8 to 4.5 followed by addition of caprylic acid and a pH shift to pH 5.0 to 5.2. A precipitate of contaminating proteins, lipids and caprylate forms and is removed, while the majority of the antibodies remain in solution. Sodium caprylate is again added to a final concentration of not less than about 15 mM. This solution is incubated for 1 hour at 25° C. to effect viral inactivation. A precipitate (mainly caprylate) is removed and the clear solution is diluted with purified water to reduce ionic strength. Anion exchange chromatography using two different resins is utilized to obtain an exceptionally pure IgG with subclass distribution similar to the starting distribution. The method maximizes yield and produces a gamma globulin with greater than 99% purity. The resin columns used to obtain a high yield of IgG retain IgM and IgA. IgA and IgM may be eluted from these resins in high yield and purity.
Owner:BAYER HEALTHCARE LLC
Method for preparing alpha-lipoic acid
ActiveCN103058989ANo pollution in the processLipoic Acid High PurityOrganic chemistryEthyl esterHydrolysis
The invention discloses a method for preparing alpha-lipoic acid, comprising the following steps: preparing the sodium sulphide liquor, synthesizing lipoic acid ethyl ester, hydrolyzing the lipoic acid ethyl ester, acidizing the sodium thioctate, and purifying the crude product of the lipoic acid. The liquor adopted by the method during the preparation process has no pollution for human bodies, is safe and easy to operate, and has the performances of high lipoic acid purity, high reaction yield and gentle reaction conditions, so that the method is suitable for industrial production on large scales.
Owner:SHANDONG QIDU PHARMA
Preparing method of human serum albumin
ActiveCN105037487AReduce yield lossReduce processing timePeptide preparation methodsWhole blood productUltrafiltration
The invention relates to the technical field of biological product and blood product production, and mainly relates to a separation and purification method of human serum albumin in the blood product production, in particular to a preparing method of human serum albumin. According to the method, healthy plasma supernatant is used as raw materials; a Kistler-Nitchmann low-temperature ethanol method is adopted for precipitating and separating ingredients FI+II+III and ingredients FIV; supernatant after the ingredient FIV separation is subjected to dealcoholization treatment; then, one-step ion exchange chromatography and ultrafiltration are further performed; a proper amount of sodium caprylate is added as a stabilizer; after the Pasteur virus inactivation treatment is performed, the human serum albumin finished product is obtained. Through efficient liquid chromatography detection, the purity of the human serum albumin prepared by the process is higher than 99 percent; the polymer content is lower than or equal to 1 percent; the yield of the plasma reaches 28 to 30g / L. The purity of the product is higher; the impurity protein content is lower, so that the clinical medication is safer.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Treatment of osteoporosis
PendingUS20180028622A1Peptide/protein ingredientsHydroxy compound active ingredientsOral medicationPharmaceutical drug
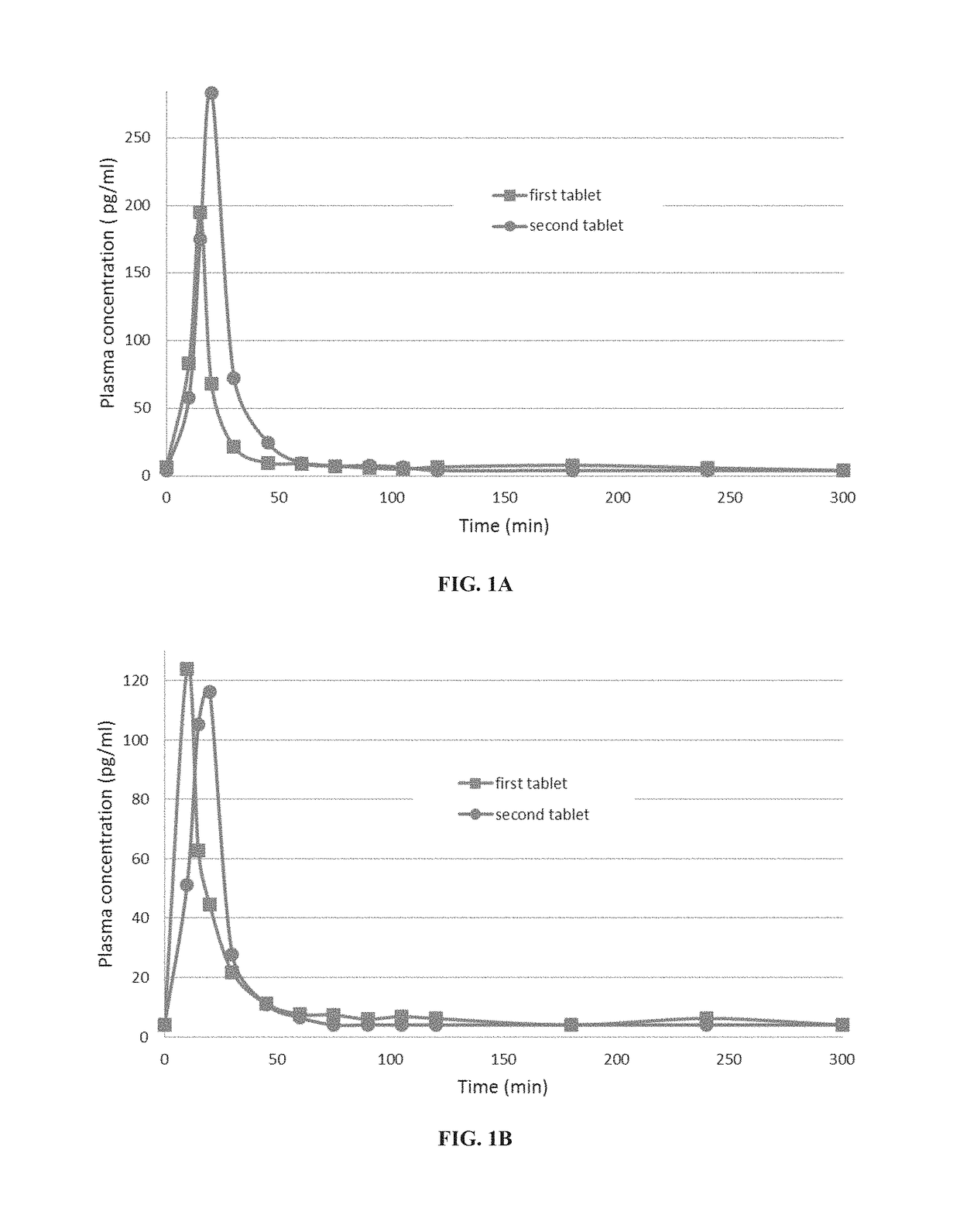
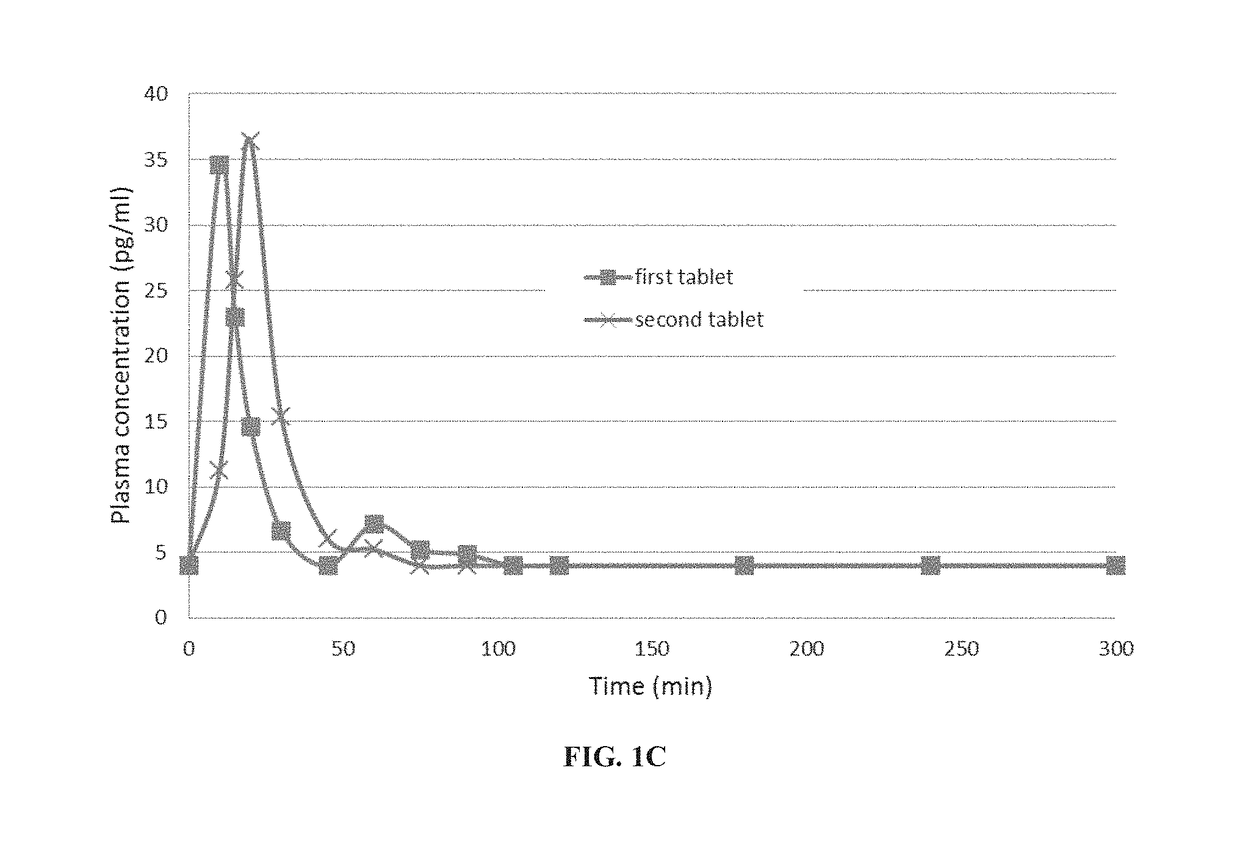
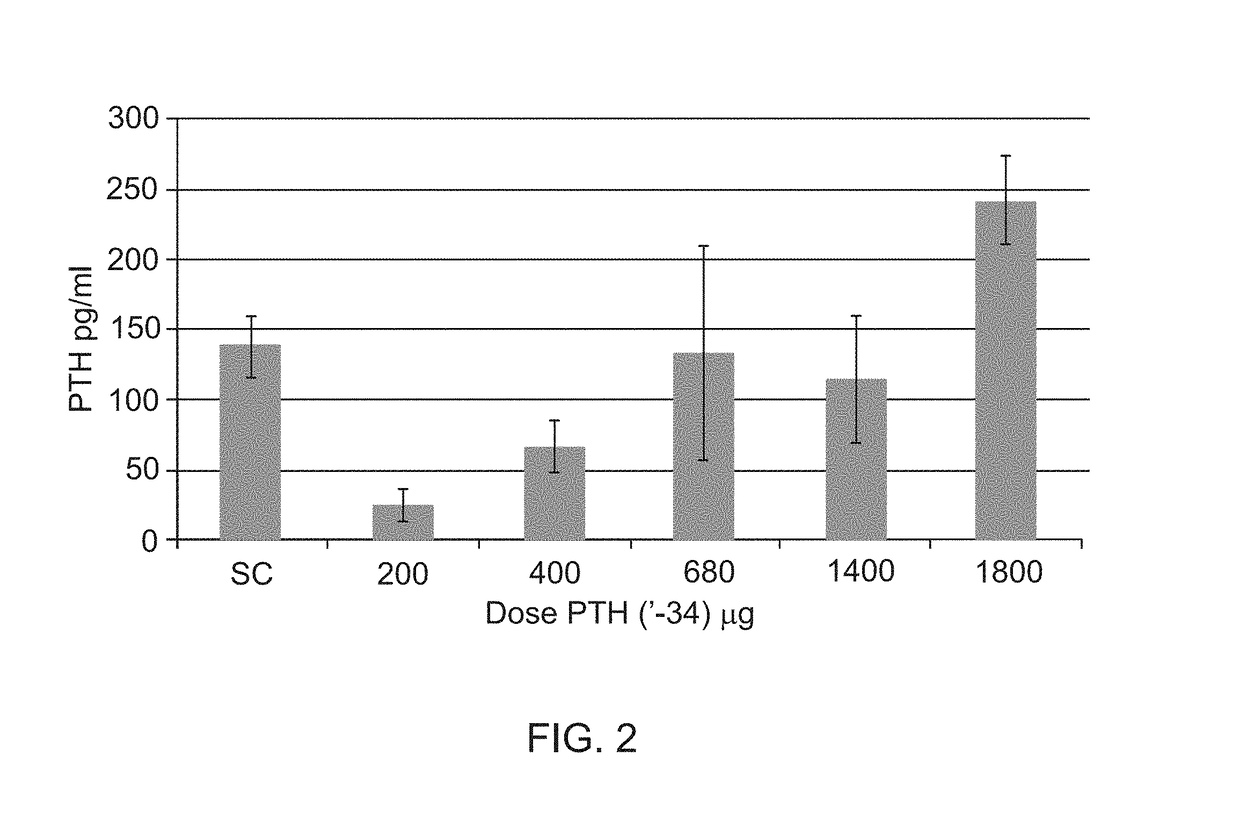
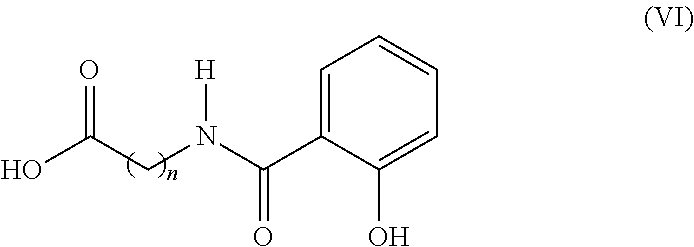
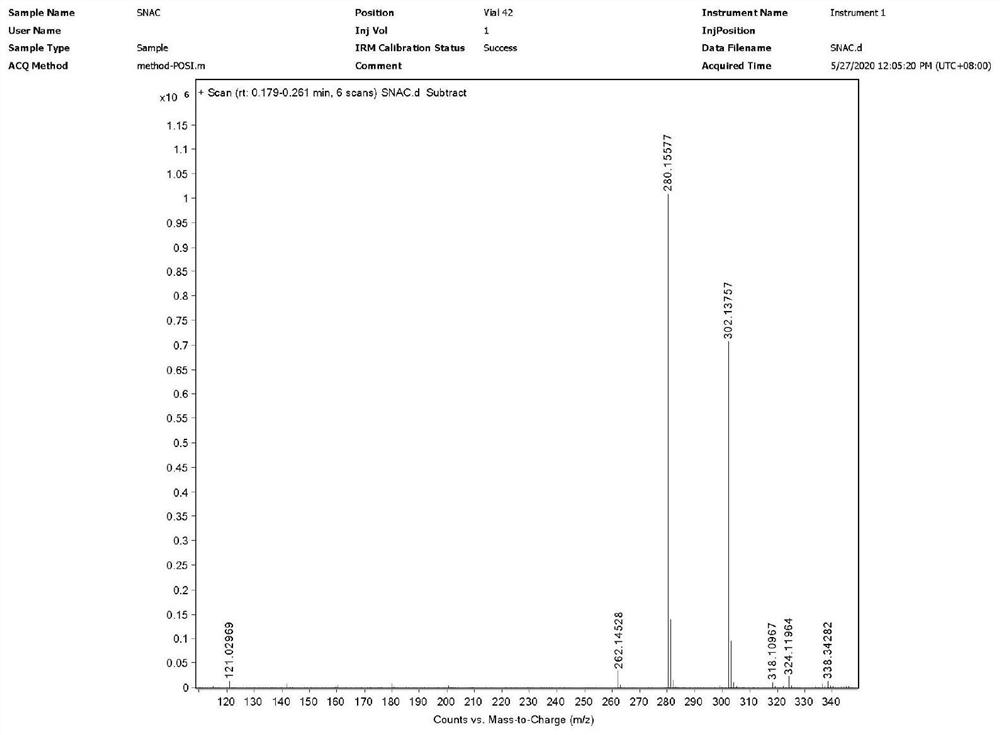
A pharmaceutical composition for use in the treatment of osteoporosis by oral administration of the composition is provided herein. The composition comprises parathyroid hormone or a fragment thereof; and SNAC (sodium 8-N-(2-hydroxybenzoyl)aminocaprylate). Further disclosed are uses of the composition in the preparation of a medicament and methods of treating osteoporosis utilizing the composition.
Owner:ENTERA BIO LTD
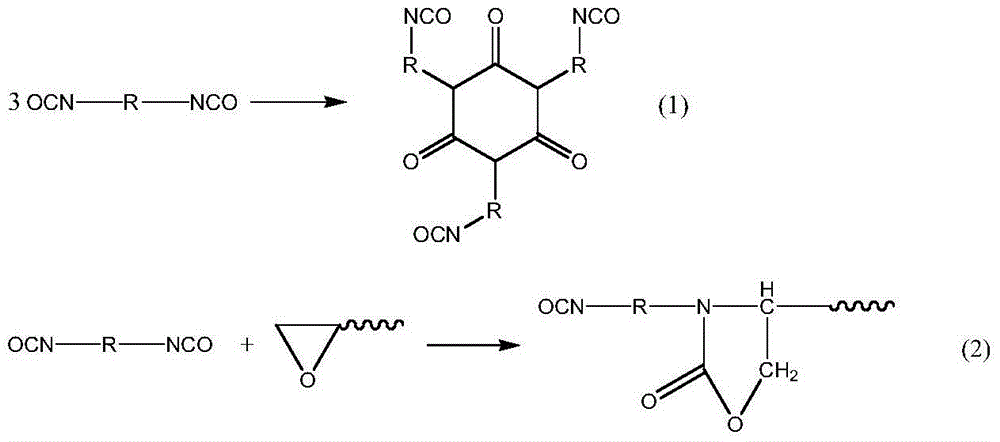
Crosslinking polyvinyl chloride foam and preparation method thereof
The invention discloses crosslinking polyvinyl chloride foam and a preparation method thereof, and belongs to the technical field of foamed materials. The problem of long time consumption of preparation of a crosslinking polyvinyl chloride foamed material in the prior art is solved, so that the quality and heat-resistant performance of the foamed material can be improved. The crosslinking polyvinyl chloride foam comprises the components in parts by weight: 100 parts of PVC resins, 30-150 parts of isocyanate, 1-15 parts of foaming agents, 0-80 parts of anhydride, 0-20 parts of epoxy compounds and 0.02-4 parts of catalysts, wherein the catalyst is one or more of N',N'',N'''-tri(dimethyl aminopropyl) symmetrical hexahydro-triazine, N-(alpha-ethoxyl) dimethylenimine, indole, sodium caprylate, isocaprylic acid, potassium oleate and potassium stearate. The foamed material is high in quality and heat-resistant performance, and high in production efficiency.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Production process for high purity porcine blood albumin and uses thereof
ActiveCN101367865ASimple preparation processEasy to scaleSerum albuminPeptide preparation methodsFibrin glueFiltration
The present invention discloses a technique for extracting high-purity porcine serum albumin. The technique includes the following steps: sodium citrate and sodium chloride are added into porcine blood to separate plasma and erythrocytes; after being centrifugated, filtered by a membrane and extracted, the plasma is put into an interlayered reactor; alcohol, sodium caprylate and sodium chloride are added into the plasma and stirred slowly after the interlayered reactor is heated; hydrochloric acid is added to adjust the pH value for two times; after centrifugation, supernatant is added into precipitating reagent, ground into paste and stirred; after being refrigerated overnight, the mixed solution is centrifugated and left for precipitation; the precipitate is resolved in water and, after the pH is adjusted by sodium hydroxide solution, kept still; supernatant is separated out by centrifugation; and after membrane filtration and virus inactivation, the filtrate is frozen out into the finished product. The technique has the advantages of little material usage, short process flow, high production efficiency, easy operation, low production cost and high product quality and is suitable for mass production. The product can be used as an auxiliary material for the preparation of fibrin glue sealant, a biochemical material and a substitute for other albumins.
Owner:GUANGZHOU BIOSEAL BIOTECH
Method for low-temperature extraction of human serum albumin employing ethanol
InactiveCN103709245AHigh yieldAvoid sex changeSerum albuminPeptide preparation methodsUltrafiltrationBlood plasma
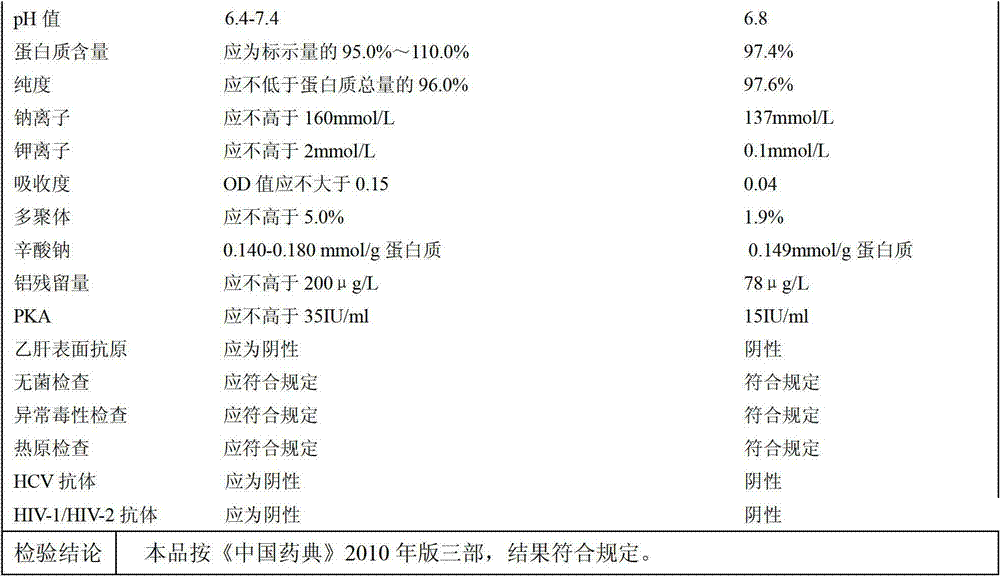
The invention discloses a method for low-temperature extraction of human serum albumin employing ethanol. The method comprises the following steps: orderly separating out FI, FII+III and FIV from human plasma as a raw material, so as to obtain an FV sediment; dissolving the FV sediment, adjusting the protein content of the solution to 20-60g / L, the pH value to 4.50-4.70, and the ethanol concentration to 9.0-13.0; filtering by 3-5 stages, carrying out ultrafiltration dialysis on the solution and concentrating into required concentration, and then adding sodium caprylate, so that the sodium caprylate content in the solution is 0.140-0.180mmol / g of protein; adding sodium chloride, so that the osmotic pressure of the solution is 210-400 mOsmol / kg; adjusting the pH value of the solution to 6.40-7.40 and diluting into specified packaging concentration. By adopting the method disclosed by the invention, the extraction rate of the FV sediment is improved, and the yield of the human serum albumin is greatly improved.
Owner:HUALAN BIOLOGICAL ENG CHONGQING
Preparation method of human serum albumin
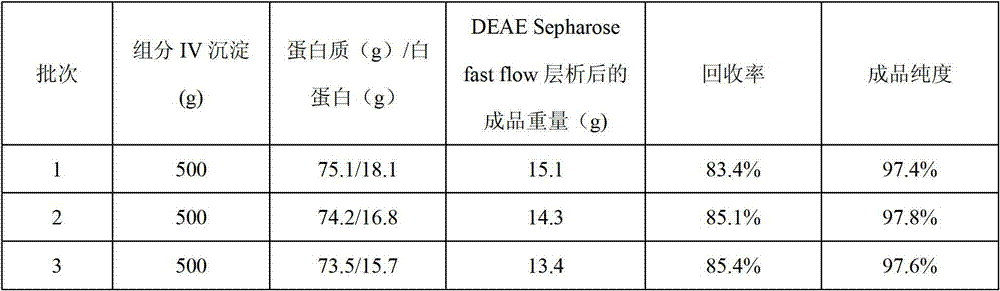
ActiveCN102816230AImprove utilizationReduce demandSerum albuminPeptide preparation methodsSerum protein albuminDEAE-Sepharose
The invention relates to a preparation method of a human serum albumin. The human serum albumin is extracted and purified from a waste component IV precipitate separated from human plasma, and thus the comprehensive utilization of plasma can be improved. The preparation method comprises the following steps of: 1, dissolving the component IV precipitate and then press-filtering and separating to obtain a filtrate A; 2, adding polyethylene glycol while stirring the filtrate A, and press-filtering and separating to obtain a filtrate B; 3, adjusting the pH of the filtrate B, controlling the temperature of the filtrate B, and press-filtering and separating to obtain a filtrate C; 4, adding the polyethylene glycol while stirring the filtrate C to obtain a reaction solution C, press-filtering and separating to obtain a precipitate; 5, dissolving the precipitate, carrying out DEAE Sepharose fast flow weak anion exchange chromatography, and ultrafiltering; and 6, adding sodium caprylate after the concentration of protein in an ultra-filtrate is diluted, adjusting the pH to 6.8 to 7.0, sterilizing and filtering, and carrying out pasteurized inactivation to obtain a human serum albumin finished product.
Owner:TONROL BIOLOGICAL PHARM CO LTD
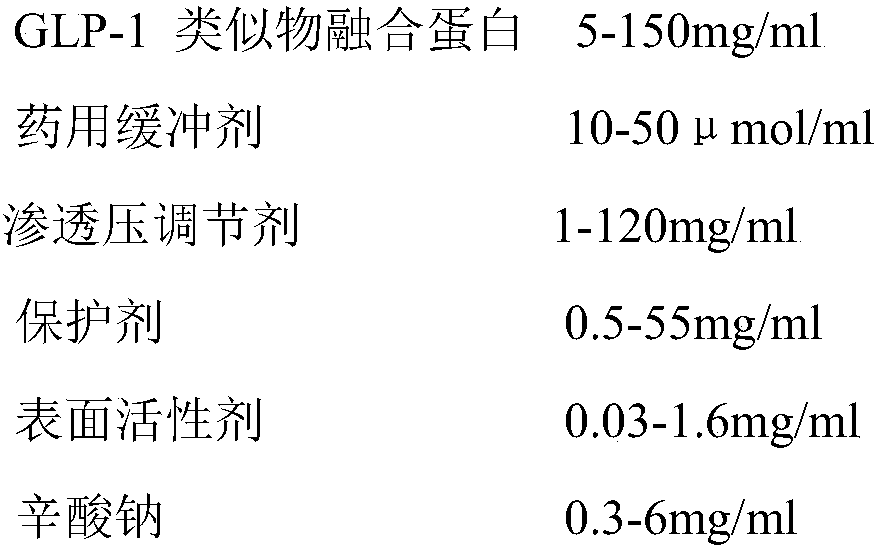
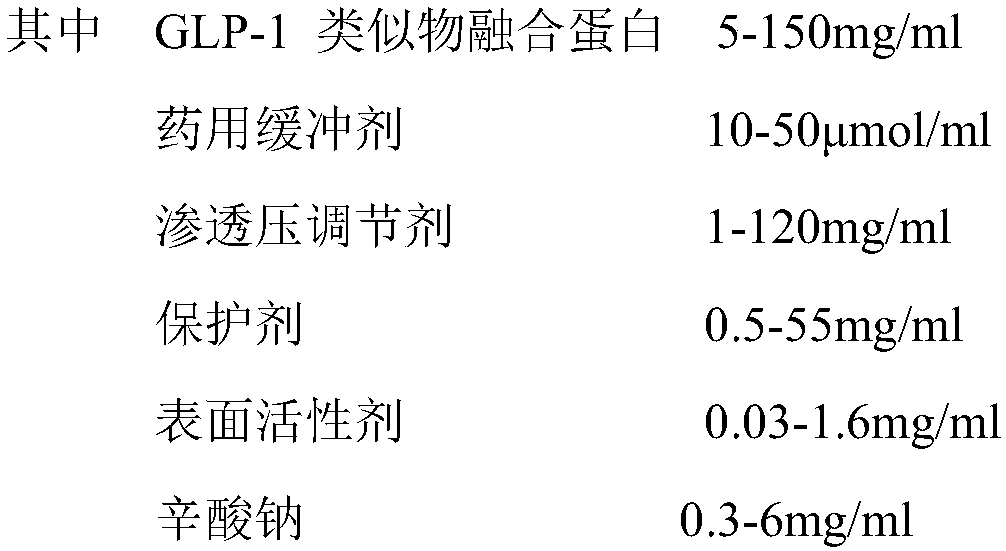
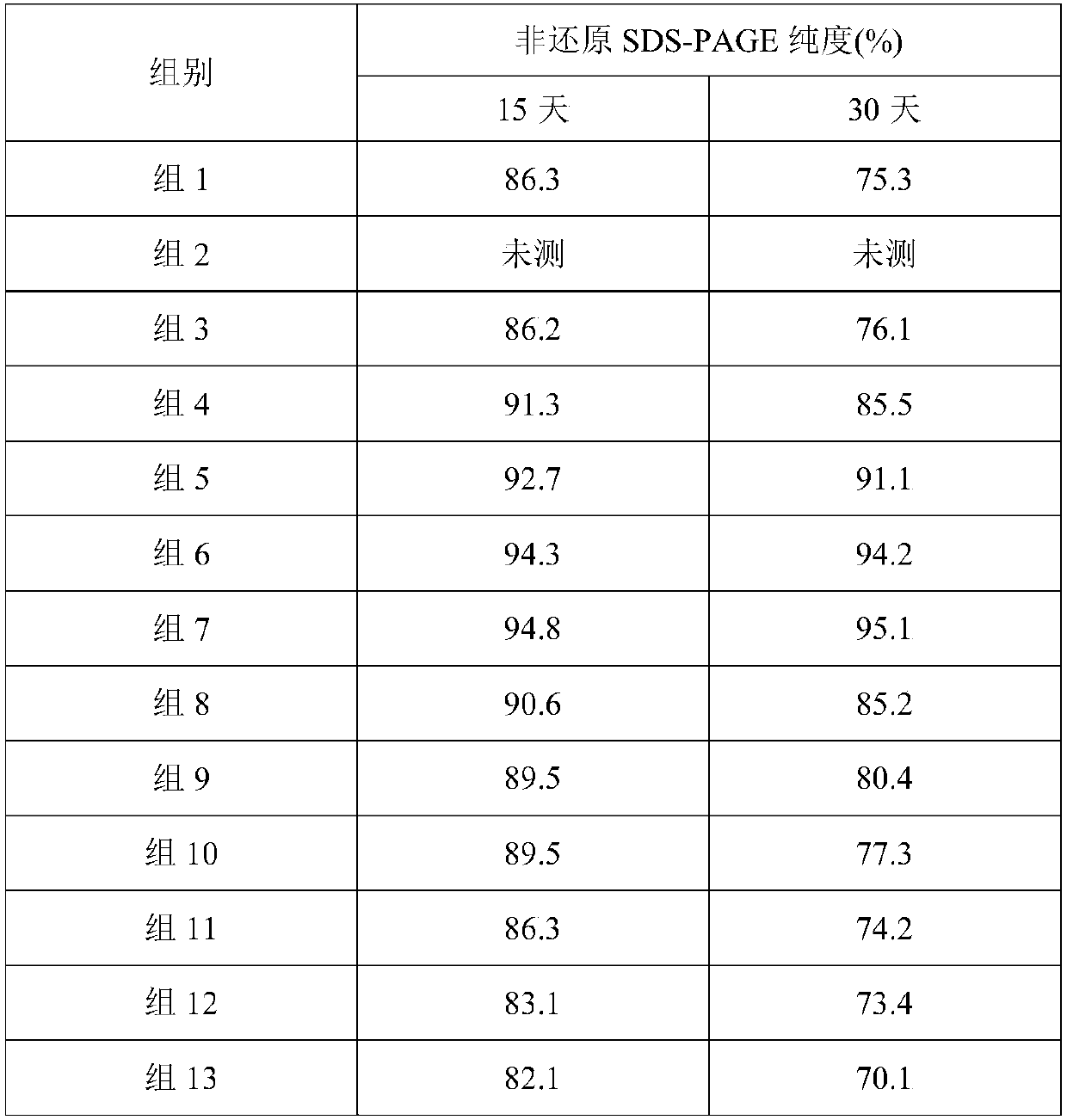
Stable liquid preparation containing GLP-1 analogue fusion protein and preparation thereof
InactiveCN107661288APeptide/protein ingredientsMetabolism disorderSurface-active agentsBuffering agent
The invention discloses a stable liquid preparation containing GLP-1 analogue fusion protein and preparation thereof. The stable liquid preparation is prepared from the effective components of: the GLP-1 analogue fusion protein, a pharmaceutical buffering agent, an osmotic-pressure regulating agent, a protective agent, a surface active agent, sodium caprylate and the like. The stable liquid preparation can be used for treating the diabetes and relevant diseases thereof.
Owner:JIANGSU T MAB BIOPHARMA
Preparation method of thickness controllable WO3 nano sheet for trimethylamine gas sensor
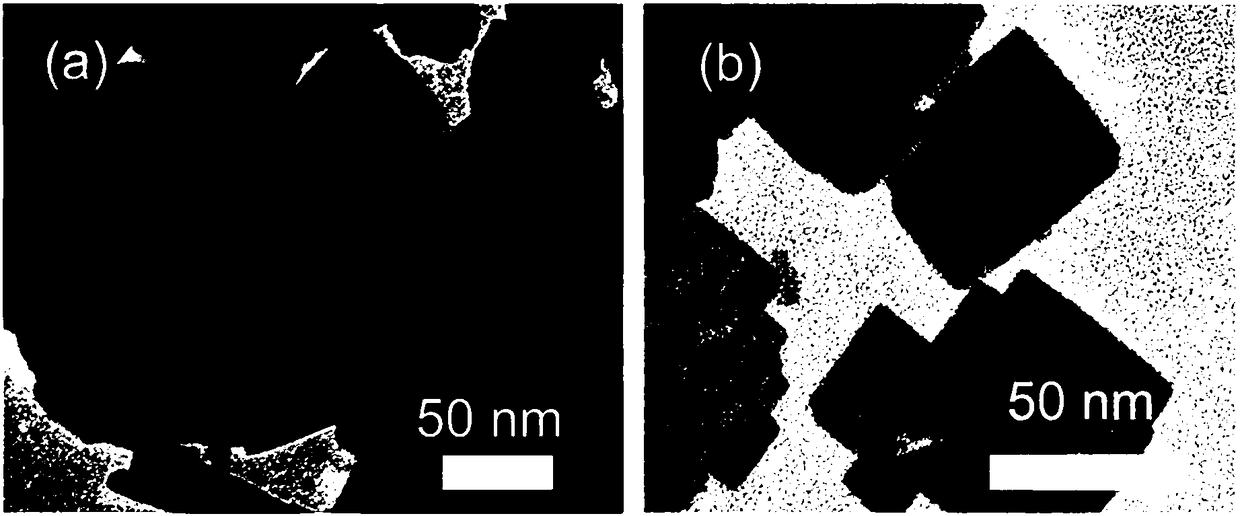
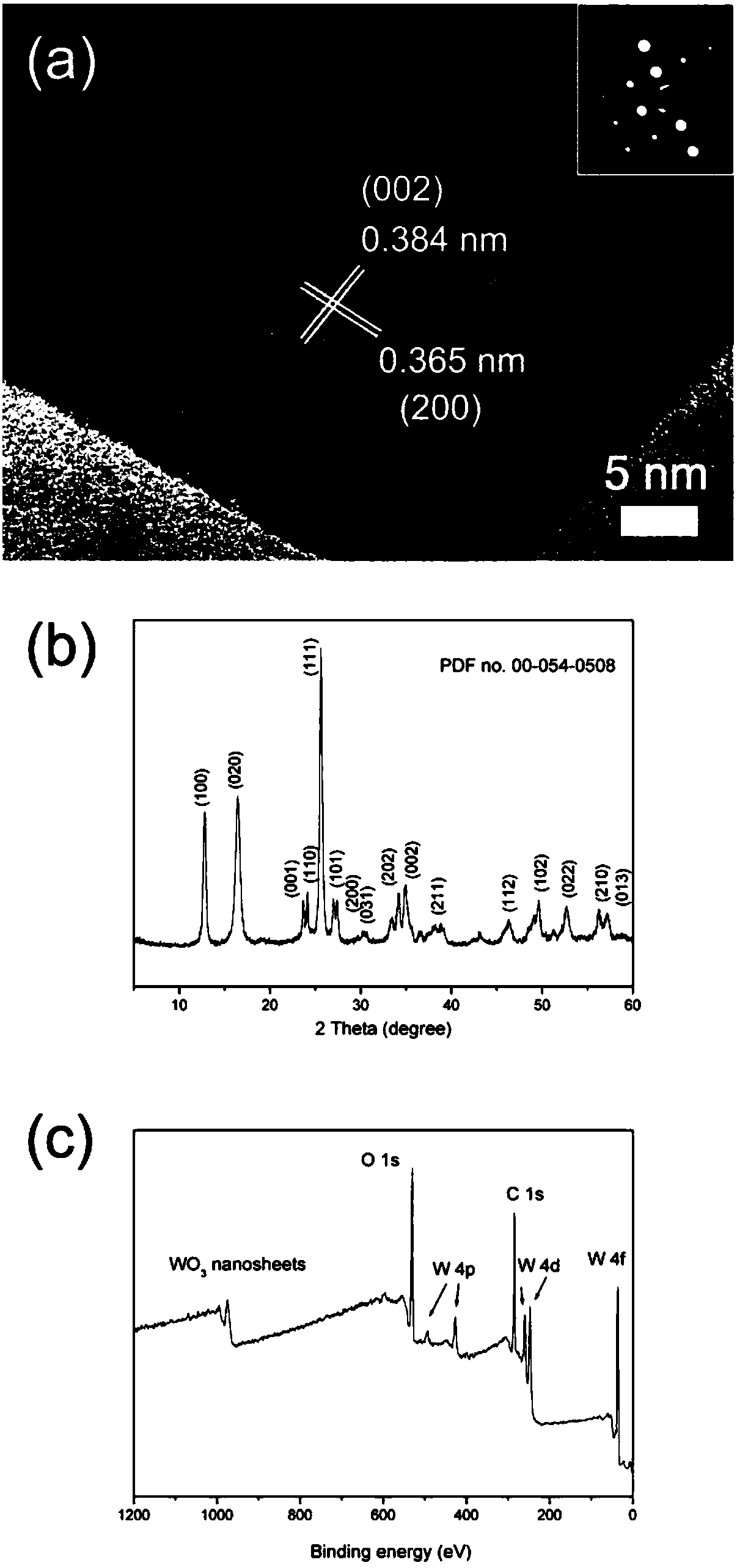
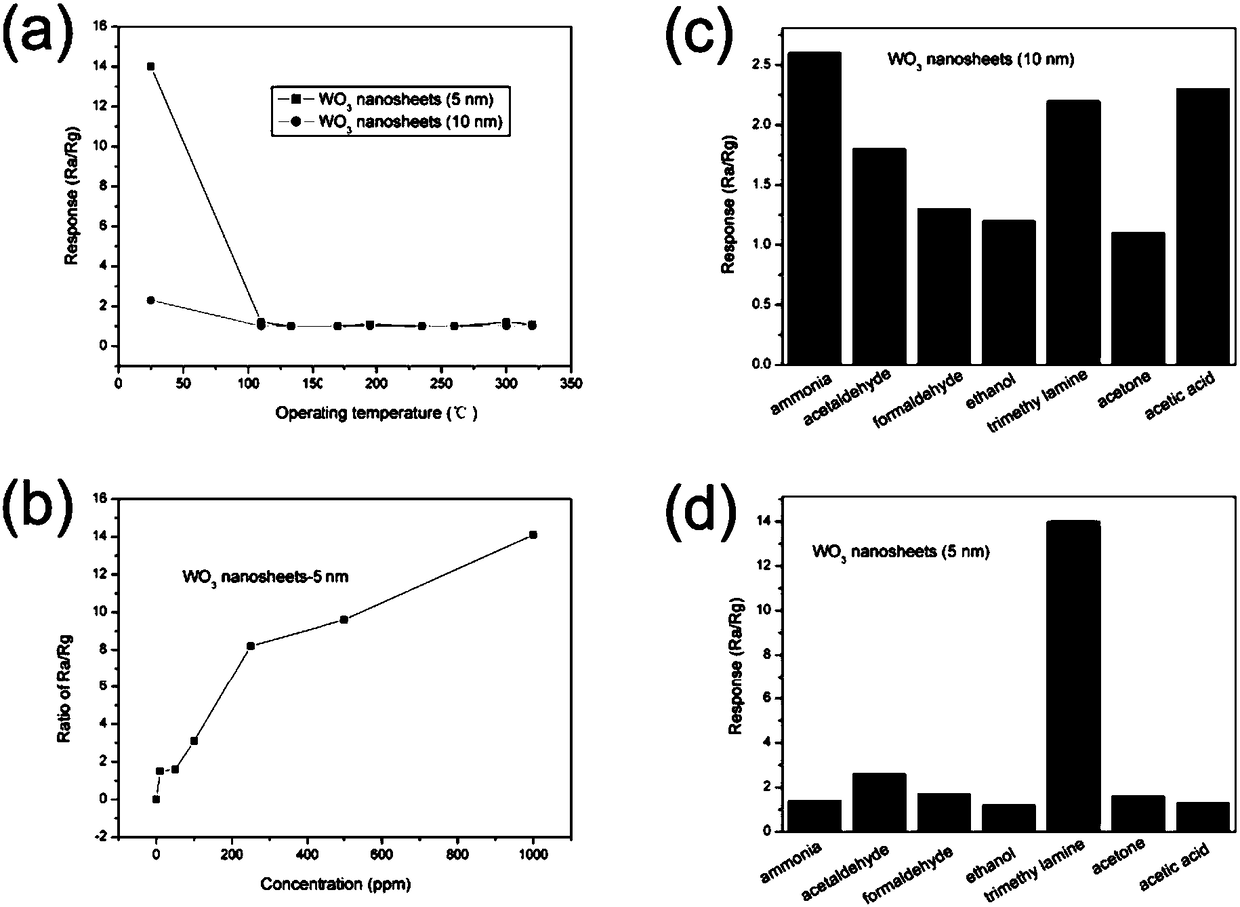
ActiveCN108483498AEasy to makeIncrease working temperatureMaterial nanotechnologyTungsten oxides/hydroxidesSodium tungstate dihydrateReaction temperature
The invention discloses a preparation method of a thickness controllable WO3 nano sheet for a trimethylamine gas sensor, and belongs to the field of gas sensors. The thickness of the WO3 nano sheet can be regulated from about 10 nm to about 5nm. According to the invention, a method of adopting dual surfactants is carried out, and the thickness of the WO3 nano sheet can be controlled through the regulation of preparation conditions such as the initial reaction temperature, the usage amounts of sodium oleate and sodium caprylate, the concentration and usage amount of nitric acid and sodium tungstate, the temperature preservation time, the stirring time and the like. The thickness controllable WO3 nano sheet prepared by the method is used as a coating material of a gas sensor and achieves a good effect in detecting trimethylamine. When the thickness of the nano sheet is 5 nm, the response to trimethylamine (TEA) is good at a temperature of 25 DEG C, and when the TEA content is 250 ppm, the response value is 8.2, and the selectivity is good.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Magnetic effervescent tablet, preparation method thereof, and application of magnetic effervescent tablet in extraction of triazine herbicides in water
ActiveCN110227425AEasy to makeGood repeatabilityComponent separationOther chemical processesSodium bicarbonateSodium hexanoate
The invention provides a magnetic effervescent tablet, a preparation method thereof, and an application of the magnetic effervescent tablet in the extraction of triazine herbicides in water, and belongs to the technical field of sample detection. The magnetic effervescent tablet comprises a magnetic material, sodium aliphatate, a proton donor, a salt and an alkaline salt; the magnetic material isnanometer ferroferric oxide; the sodium aliphatate is any one of sodium caprylate, sodium hexanoate and sodium decanoate; the proton donor is any one of citric acid, oxalic acid and sodium dihydrogenphosphate; the salt is sodium chloride; and the alkaline salt is sodium bicarbonate or sodium carbonate. The application has the advantages of simplicity and quickness in operation, simple and easy operation of two-phase separation, high extraction efficiency, no need of an extraction device, minimal consumption of organic solvent products, greenness and environmental protection.
Owner:SHANXI AGRI UNIV
Method for purifying immunoglobulin
ActiveUS20170015732A1Reduce impurityRemove substanceSerum immunoglobulinsPeptide preparation methodsPurification methodsPolyethylene glycol
The present invention relates to a method for purifying an immunoglobulin, and more particularly, to a method for purifying an immunoglobulin, which comprises: dissolving immunoglobulin-containing plasma protein fraction I+II+III or fraction II+III; adding caprylate to the solution to cause precipitation; performing dialysis and concentration after removal of the precipitate; performing anion exchange resin and ceramic cation exchange resin purification processes to effectively remove a solvent and detergent added to inactivate viruses; and performing elution while maintaining salt concentration at a constant level to maintain the immunoglobulin polymer content at a low level. According to the method for preparing the intravenous immunoglobulin according to the present invention, a precipitation step of preparing fraction II from fraction I+II+III or fraction II+III as a starting material can be omitted, and problems, including a complicated process and a low yield, which occur in the conventional preparation process employing the polyethylene glycol treatment process, can be solved by use of first sodium caprylate precipitation, anion exchange chromatography and cation exchange chromatography. In addition, when the immunoglobulin purification method according to the present invention is used, the efficiency with which impurities and thrombotic substances are removed can be increased and the immunoglobulin polymer content can be maintained, and thus a stable immunoglobulin with increased quality can be produced.
Owner:GREEN CROSS HLDG
Use of oral heparin preparations to treat urinary tract diseases and conditions
An improved method of treating lower urinary dysfunctional epithelium (LUDE) or a disease, condition, or syndrome associated with LUDE, including interstitial cystitis, comprises the step of administering orally a pharmaceutically effective quantity of heparin to a patient in need of treatment for LUDE or a disease, condition, or syndrome associated with LUDE in order to treat LUDE or a disease, condition, or syndrome associated with LUDE. The heparin can be administered together with a quantity of a penetration enhancer that is sufficient to result in a tissue concentration of heparin that is sufficient to treat LUDE or a disease, condition, or syndrome associated with LUDE. A suitable penetration enhancer is sodium N-[8-(2-hydroxybenzoyl)amino]caprylate. The method can further comprise the administration of at least one additional pharmaceutical composition to treat LUDE or a disease, condition, or syndrome associated with LUDE The invention further includes a pharmaceutical composition comprising: (1) a quantity of heparin that is pharmaceutically sufficient for the treatment of LUDE or a disease, condition, or syndrome associated with LUDE; and (b) at least one filler, excipient, or carrier; wherein the pharmaceutical composition is formulated for the treatment of LUDE or a disease, condition, or syndrome associated with LUDE.
Owner:URIGEN PHARMA INC
Method of preparing albumin from a solution comprising albumin and a method for inactivating viruses using caprylate in solutions containing albumin
Described herein are a method of preparing albumin from a solution comprising albumin and a method for inactivating viruses using caprylate in solutions containing albumin. The method of preparing albumin from a solution comprising albumin comprising: adjusting the protein concentration of the solution to less than about 5%; adjusting pH of the solution to about pH 5 or less; adding caprylic acid (octanoic acid) or sodium caprylate; raising the solution temperature greater than about 20 DEG C; and incubating the solution.
Owner:GRIFOLS
Method for synthesizing highly ordered super-microporous silicon dioxide
InactiveCN107188186ALow costMild reaction conditionsSilicaDecyltrimethylammonium bromideRoom temperature
The invention relates to a method for synthesizing super-microporous silicon dioxide by using a short-chain cationic surfactant (decyltrimethylammonium bromide, namely C10TAB) as a template and sodium caprylate as a co-surfactant. The method comprises the following steps that the short-chain cationic surfactant, sodium octanoate, a silicon source and water are mixed at room temperature in the molar ratio of 1:X:5:800 and stirred for 0.5 h, then the pH value is adjusted to 9-10 by using 2M sulfuric acid, then a hydrothermal reaction is conducted for 72 hours at the temperature of 80 DEG C, and the product is filtered, washed with water, dried in the air and roasted for 4-5 hours at the temperature of 560 DEG C to obtain the product. The silicon source used in the method is sodium silicate, wherein X ranges from 0.25 to 0.35. The method is mild in reaction condition and low in cost, the product is high in pore order, and a novel method for large-scale development and utilization of the super-microporous silicon dioxide in the future is provided.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Preparation method of rhodium caprylate
The invention discloses a preparation method of rhodium caprylate. The preparation method comprises the following steps of: adding rhodium sodium chloride into excess caprylic acid-sodium caprylate buffer aqueous solution with pH value of 4.5-7.0; heating to 80-100 DEG C; reacting for 2-5 hours; cooling; neutralizing; filtering; washing; and performing vacuum drying to obtain rhodium caprylate emerald crystal. According to the preparation method, the rhodium sodium chloride is used as a rhodium source in place of rhodium chloride, the reaction speed is high, the yield is high (greater than 95 percent), the purity is high (greater than 99 percent), and large-scale production is facilitated.
Owner:陕西瑞科新材料股份有限公司
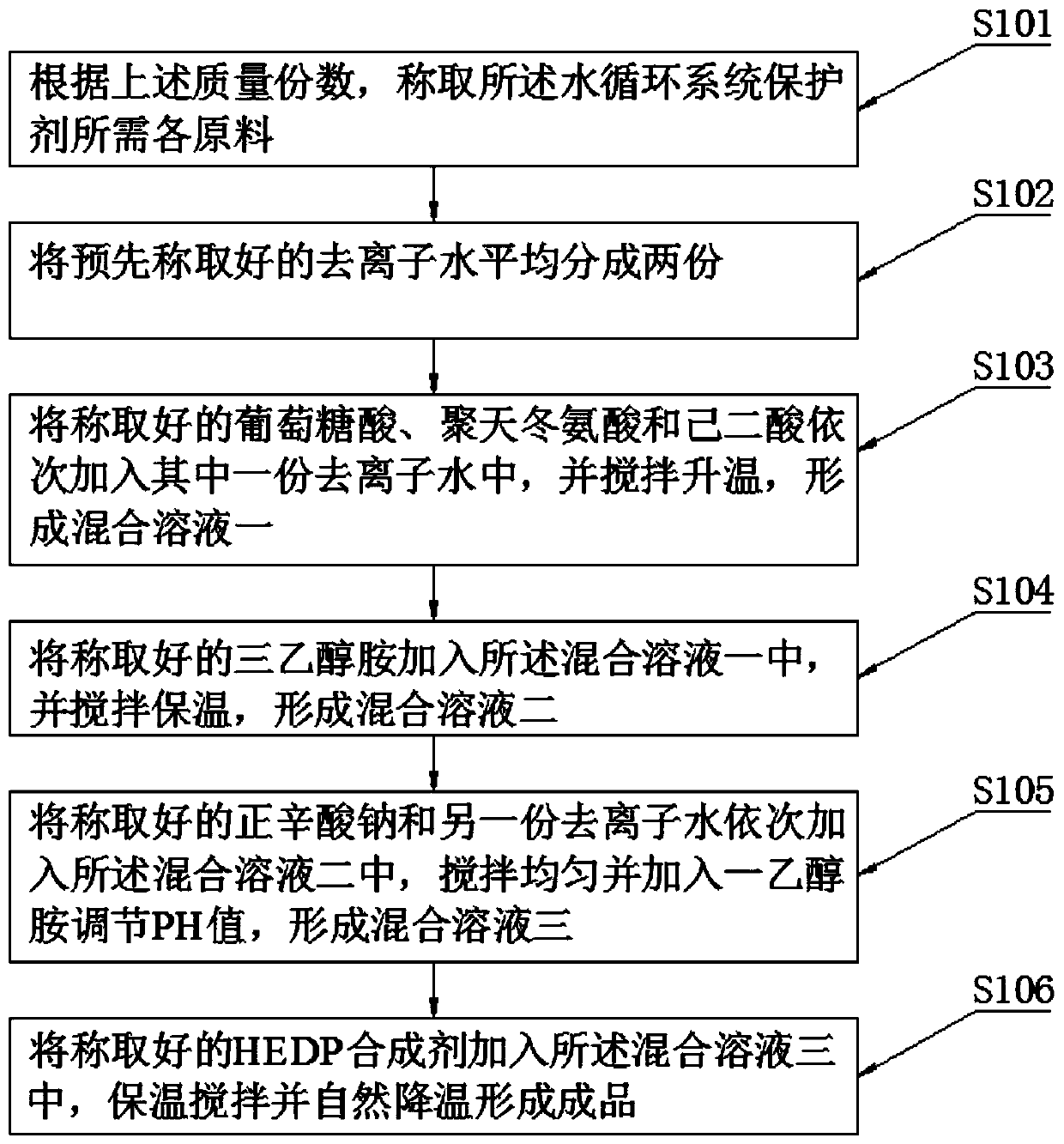
Protective agent for water circulation system, and preparation method thereof
InactiveCN110055541AImprove sealingAvoid destructionScale removal and water softeningHigh resistanceWater cycling
The invention discloses a protective agent for a water circulation system, and a preparation method thereof. The protective agent for the water circulation system comprises the following raw materialcomponents: 8-12 parts of gluconic acid, 15-25 parts of HEDP synthetic agents, 8-12 parts of poly-aspartic acid, 3-7 parts of adipic acid, 8-12 parts of sodium caprylate, 10-20 parts of triethanolamine, 25-35 parts of deionized water and 5-7 parts of monoethanolamine. The protective agent has the beneficial effects that a firm and elastic protective film is formed on a metal surface, and used forisolating water from the metal surface; damage to the metal surface by water flow is avoided; the protective agent has the advantages of long life, resistance to acid and alkali, extremely high resistance to high temperature, a certain repair function for the metal surface damage and excellent osmosis prevention, and the airtightness of a water system is improved; slight leakage of equipment and pipelines is avoided; the protective agent has the high hard water resistance, and calcium and magnesium in water can be decomposed quickly; the protective agent can be chelated with various metals, and no scaling is generated; and the water quality remains stable.
Owner:北京欧源化工环保科技有限公司
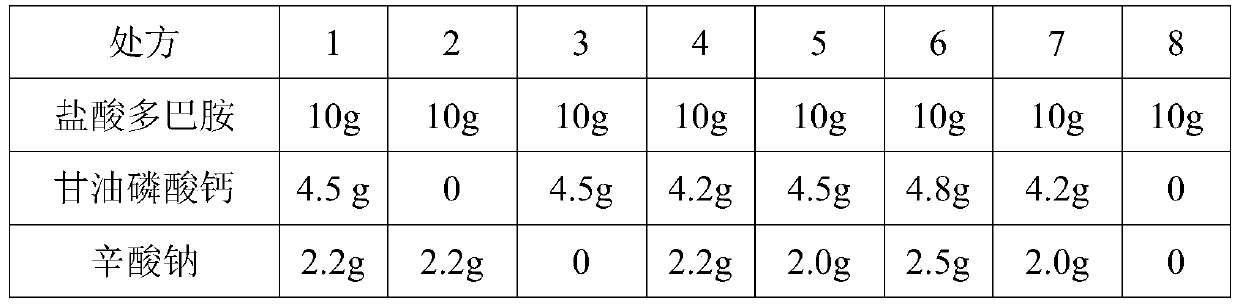
Dopamine hydrochloride injection and preparation method thereof
ActiveCN111494311AImprove stabilitySpecific ratios work wellOrganic active ingredientsPharmaceutical delivery mechanismUse medicationGlycerol
The invention relates to a dopamine hydrochloride injection and a preparation method thereof. Every 1000 ml of the injection contains 10-40 g of dopamine hydrochloride, and also contains sodium caprylate and calcium glycerophosphate. The dopamine hydrochloride injection prepared according to the method disclosed by the invention has extremely high quality stability, the medication safety is improved, the dopamine hydrochloride injection is obviously superior to the prior art and a reference preparation, and the injection consistency evaluation requirement is met.
Owner:WUHAN UNIV
Manufacturing method of nano cerium oxide
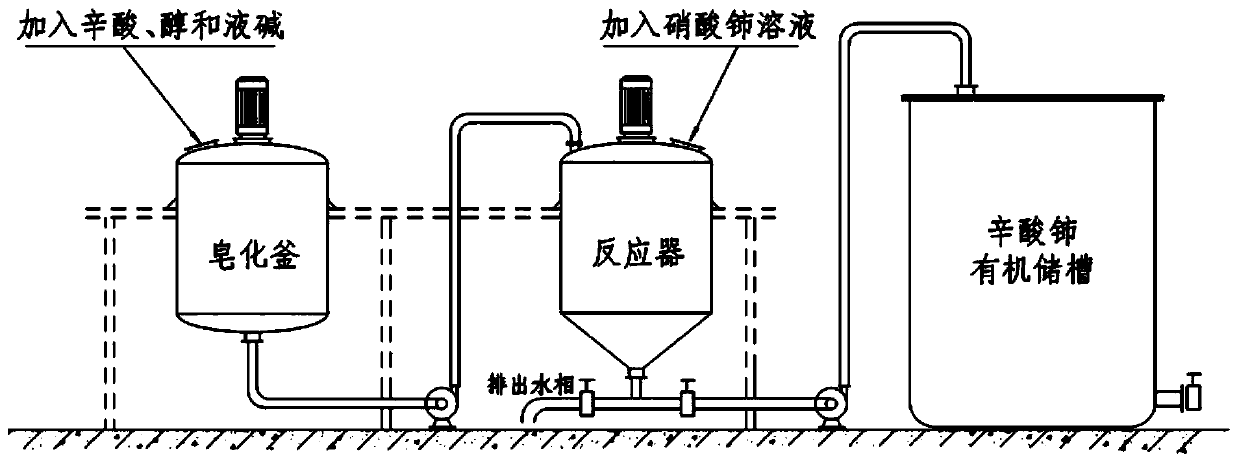
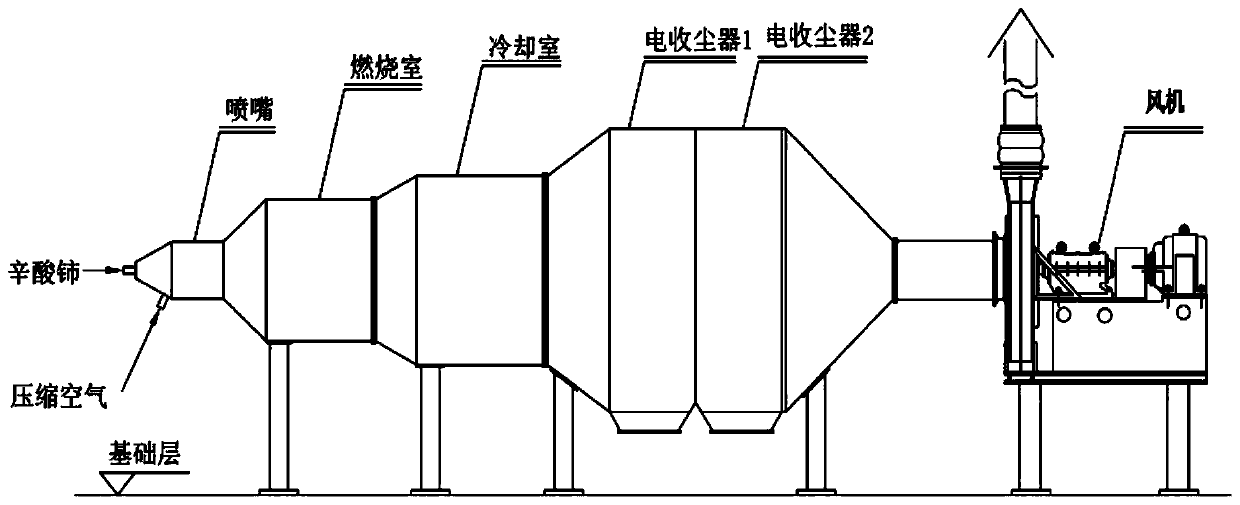
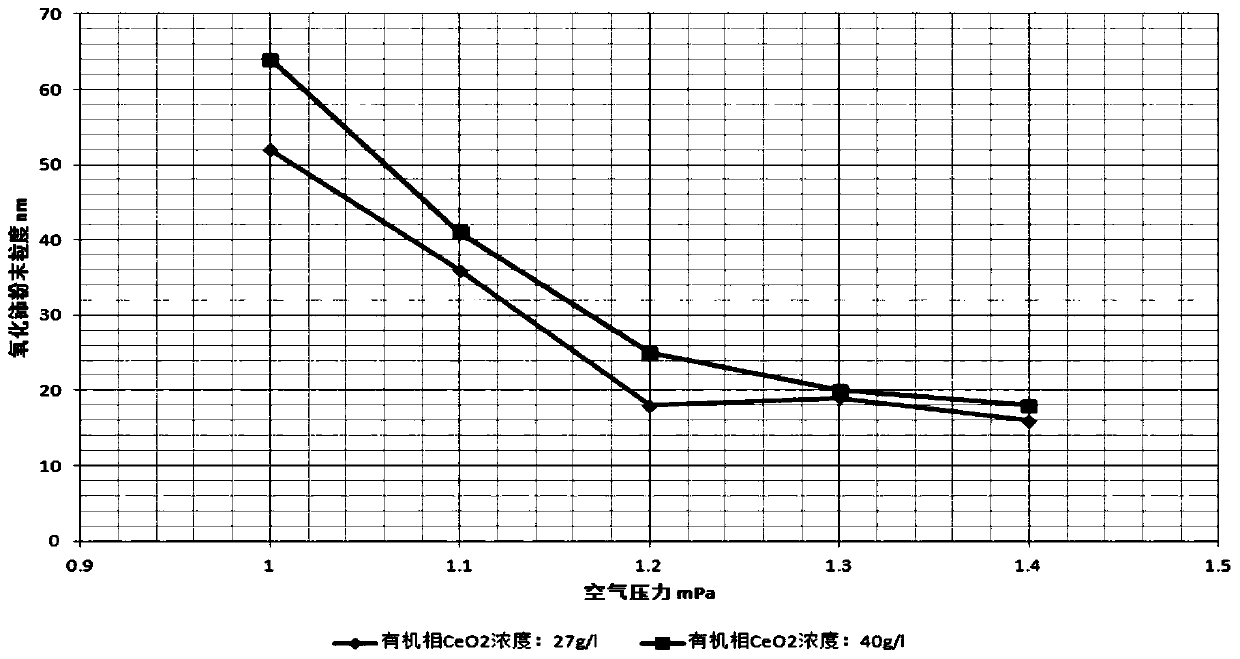
The invention discloses a manufacturing method of nano cerium oxide. The method comprises the following steps: firstly, octanoic acid and sodium hydroxide are subjected to saponification to obtain a low carbon alcohol-aqueous solution of sodium caprylate; cerate is added for reaction to obtain a cerium octoate solution; the cerium octoate solution is then treated by atomizing combustion under highpressure and collected by an electrostatic dust collector to obtain nano cerium oxide dry powder with high dispersibility, wherein the particle size of the nano cerium oxide dry powder is controlledwithin the range of 15-100 nm; and the octanoic acid can be replaced by isocaprylic acid and nonylic acid. The manufacturing method has the advantages that the liquid cerium octoate is directly changed into the nano cerium oxide solid particles in nanometer size, thereby not only reducing the steps of separating and drying an intermediate, but also generating nanometer particles with good dispersibility.
Owner:WEIHAI PIDC NEW MATERIALS
Quick-drying leather restoration agent
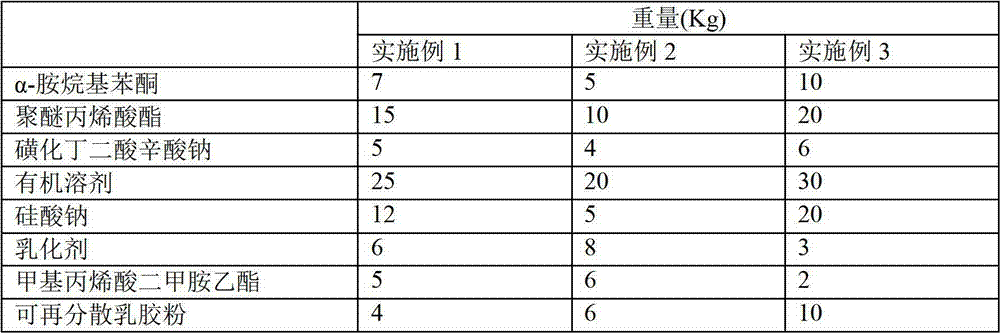
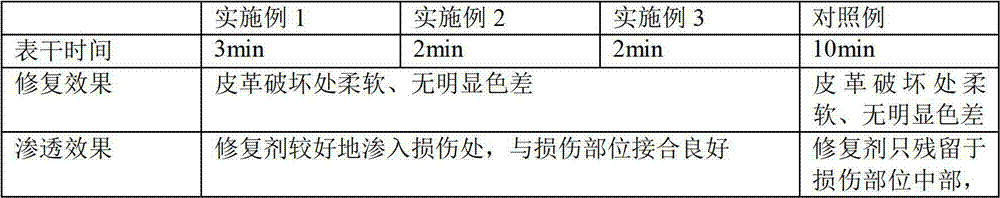
InactiveCN102965456AImprove bindingImprove permeabilityLeather surface finishingOrganic solventEthyl ester
The invention provides a leather restoration agent, which belongs to the technical field of a leather aid. The quick-drying leather restoration agent comprises the components in parts by weight as follows: 5-10 parts of alpha-amine alkyl benzophenone, 10-20 parts of polyether acrylic ester, 4-6 parts of sulfonated butanedioic sodium caprylate, 20-30 parts of organic solvent, 5-20 parts of sodium silicate, 3-8 parts of emulsifier, 2-6 parts of methylacrylic acid dimethylamine ethyl ester, and 4-10 parts of redispersible powder. The leather restoration agent provided by the invention has the advantages of firm combination, good penetrating quality and great curing speed, and can be cured within 3 minutes by illumination at normal temperature.
Owner:WUJIANG CITY LI DA LUSTRE FINISHED PROD
Sodium 8-(2-hydroxylbenzamido)caprylate and preparation method therefor
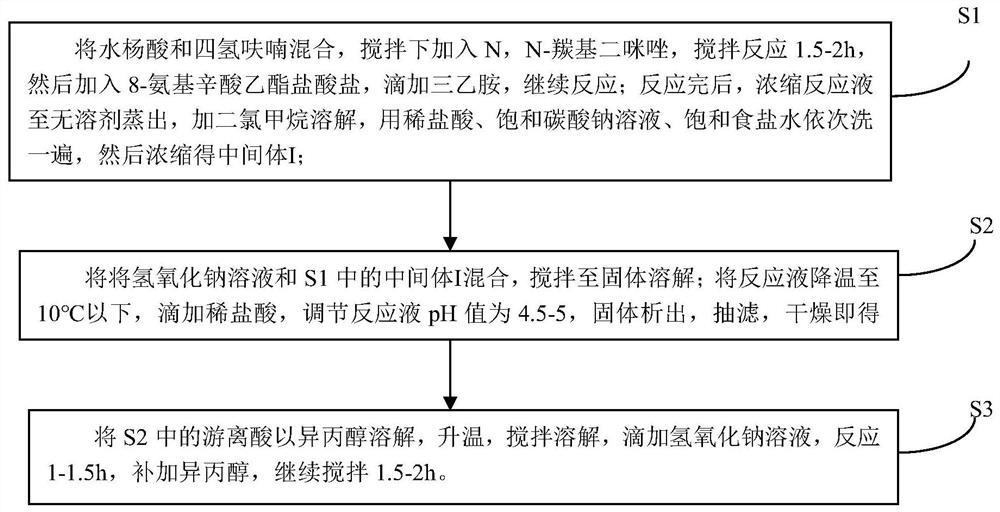
InactiveCN111978193ADoes not affect yieldGood for stirring reactionOrganic compound preparationCarboxylic acid amide separation/purificationSalicylic acidAmidogen
The invention discloses sodium 8-(2-hydroxylbenzamido)caprylate and a preparation method therefor and belongs to the field of preparation of compounds. A key of the technical scheme of the invention is as follows: the preparation method comprises the steps: mixing salicylic acid with tetrahydrofuran, adding N,N-carbonyl diimidazole, adding 8-amino ethyl caprylate hydrochloride, and dropwise addingtriethylamine; carrying out a concentrating reaction solution until no solvent is distilled off, adding dichloromethane for dissolving, and carrying out washing once separately with diluted hydrochloric acid, a saturated sodium carbonate solution and a saturated saline solution, so as to obtain an intermediate I; mixing a sodium hydroxide solution with the intermediate I, and carrying out stirring until solids are dissolved; cooling the temperature of a reaction solution to 10 DEG C or below, dropwise adding diluted hydrochloric acid, adjusting a pH value of a reaction solution to 4.5 to 5, and carrying out solid precipitation, so as to obtain free acids; and dissolving the free acids with isopropanol, dropwise adding a sodium hydroxide solution, carrying out a reaction for 1 to 1.5 hours, supplementing isopropanol, and continuing to carry out stirring for 1.5 to 2 hours. The method has the advantages that steps are few, the yield of each step is high, the product purity is good, impurities are more easily controlled, and raw materials are more readily available.
Owner:无锡紫杉药业股份有限公司
Method for extracting recombinant human serum albumin from transgenic rice grain
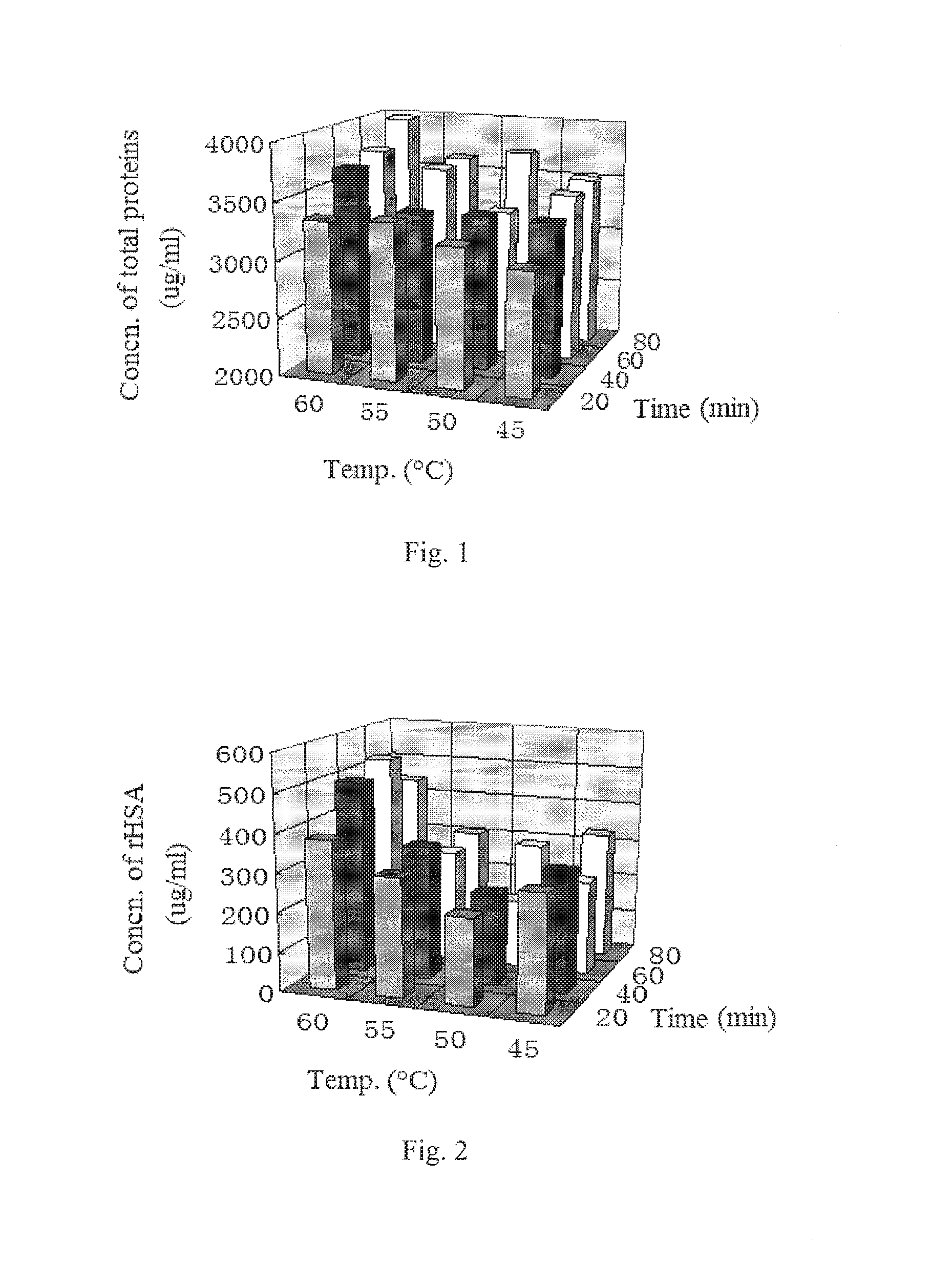
ActiveUS20160122414A1Low extraction rateHigh extraction rateSerum albuminDigestive systemHigh concentrationSodium acetate
A method for extracting recombinant human serum albumin (rHSA) from transgenic rice grain is provided, comprising the steps of: 1) grinding dehusked rice containing rHSA into milled rice grain with a fineness of 80˜120 mesh, which is mixed with a extraction buffer in a wily ratio of 1:5, then extracting at 5560° C. for 1˜3 hours to obtain mixture 1; said extraction buffer comprises 10˜30 mM phosphate buffer, 10˜20 mM sodium acetate, 1530 mM ammonium sulfate and 5˜20 mM sodium caprylate and has a pH of 6.5˜8; 2) adjusting the pH of mixture I to 4.0˜4.5, followed by precipitating at room temperature for 3˜12 hours to obtain mixture 3) filtering the mixture 11 and collecting the filtrate, to obtain a solution containing high concentration of rHSA. The concentration of rHSA in the resultant solution is 650˜660 μg / mL, which increases by 1.15 times comparing to the extraction amount before improvement, and the amount of non-target protein is reduced by 2.46 times. The method provides a basis for subsequent purification of rHSA.
Owner:WUHAN HEALTHGEN BIOTECHNOLOGY CORP
Method for extracting recombinant human serum albumin from transgenic rice grain
ActiveUS9255138B2Low extraction rateHigh extraction rateSerum albuminDigestive systemSodium acetateHigh concentration
A method for extracting recombinant human serum albumin (rHSA) from transgenic rice grain is provided, comprising the steps of: 1) grinding dehusked rice containing rHSA into milled rice grain with a fineness of 80˜120 mesh, which is mixed with a extraction buffer in a w / v ratio of 1:5, then extracting at 55˜60° C. for 1˜3 hours to obtain mixture I; said extraction buffer comprises 10˜30 mM phosphate buffer, 10˜20 mM sodium acetate, 15˜30 mM ammonium sulfate and 5˜20 mM sodium caprylate and has a pH of 6.5˜8; 2) adjusting the pH of mixture I to 4.0˜4.5, followed by precipitating at room temperature for 3˜12 hours to obtain mixture II; 3) filtering the mixture II and collecting the filtrate, to obtain a solution containing high concentration of rHSA. The concentration of rHSA in the resultant solution is 650˜660 μg / mL, which increases by 1.15 times comparing to the extraction amount before improvement, and the amount of non-target protein is reduced by 2.46 times. The method provides a basis for subsequent purification of rHSA.
Owner:WUHAN HEALTHGEN BIOTECHNOLOGY CORP
Extraction process for bovine serum albumin by graduated heat shock method
InactiveCN107344965AEffective inactivationEnsure safetySerum albuminPeptide preparation methodsFiltrationFreeze-drying
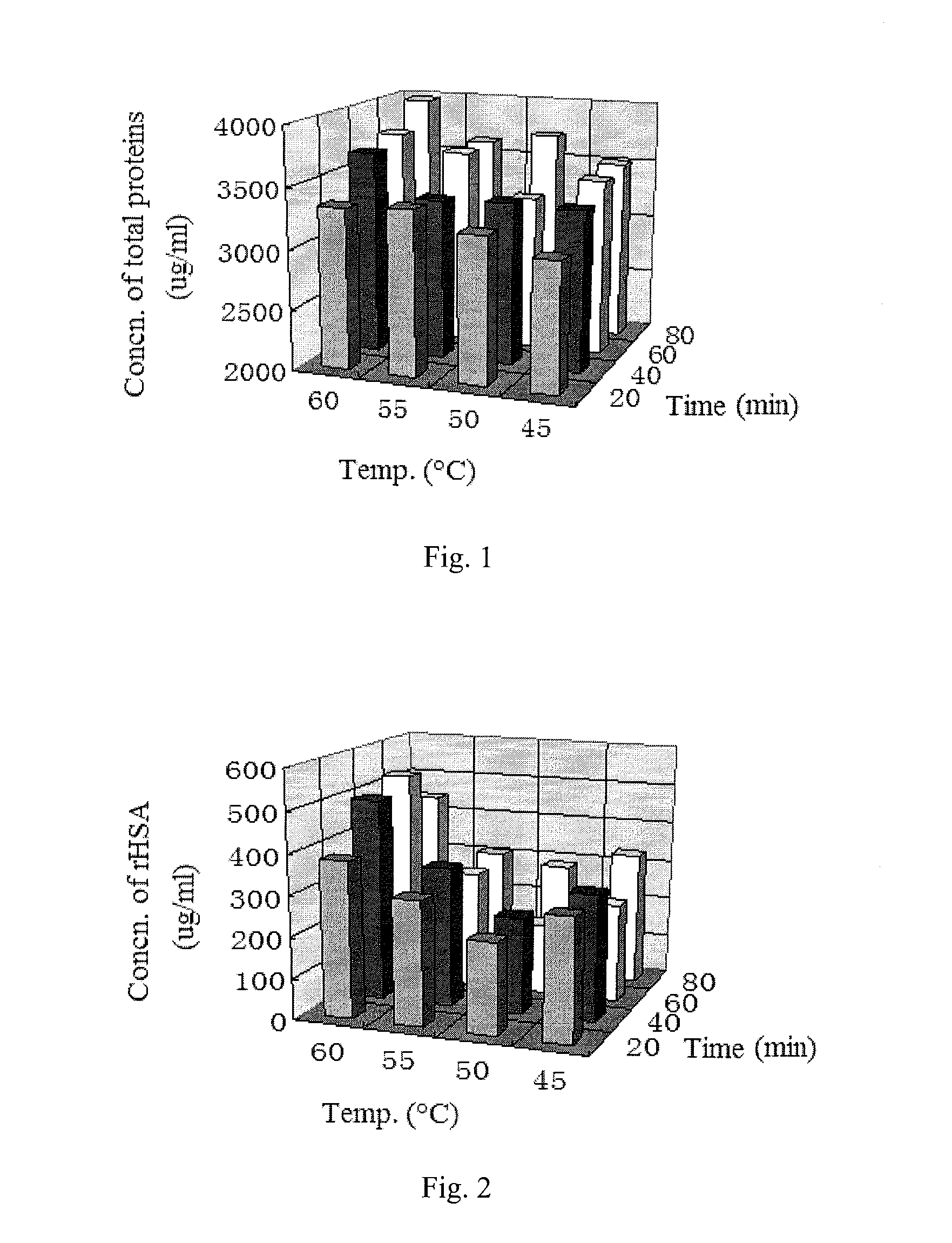
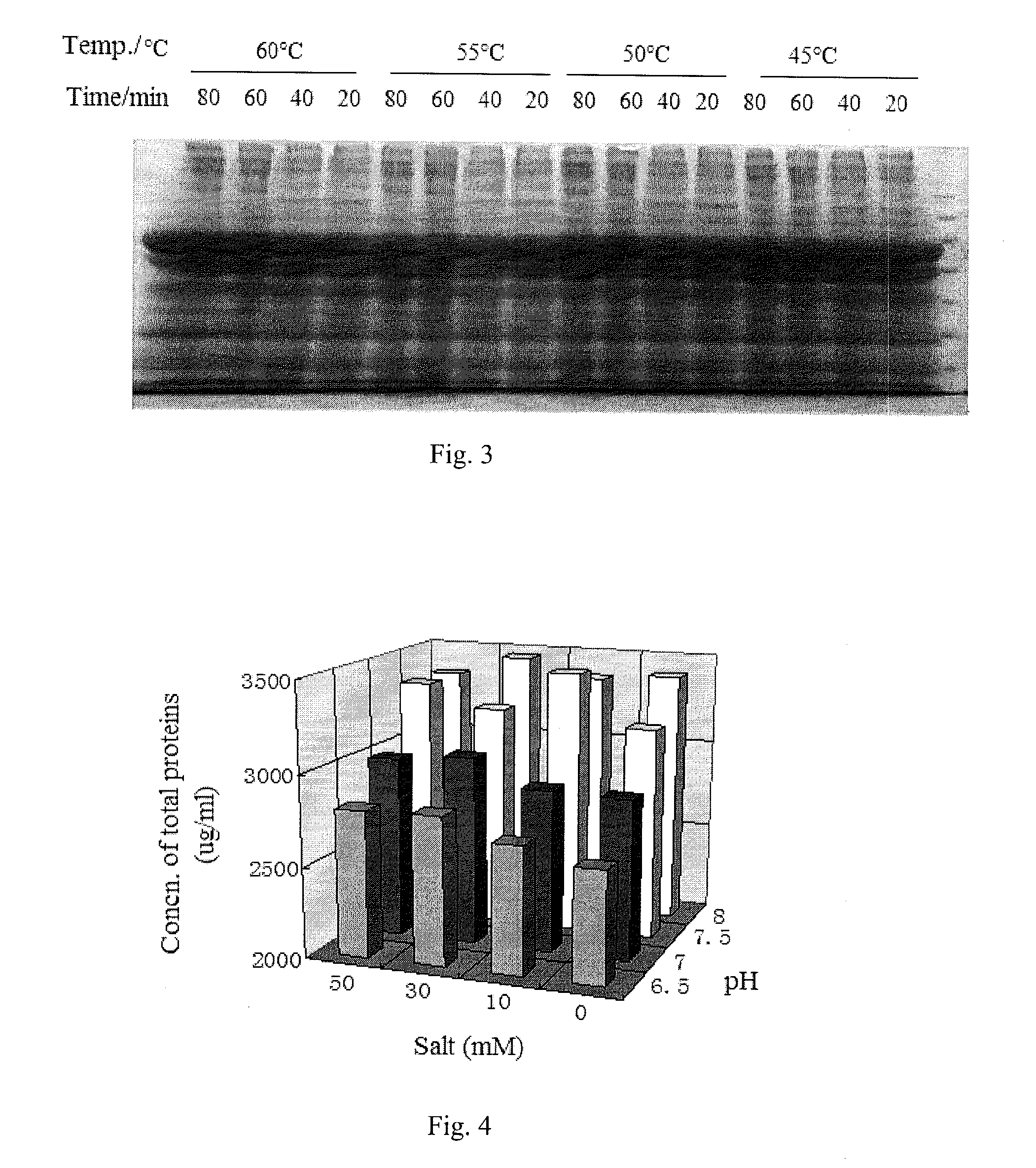
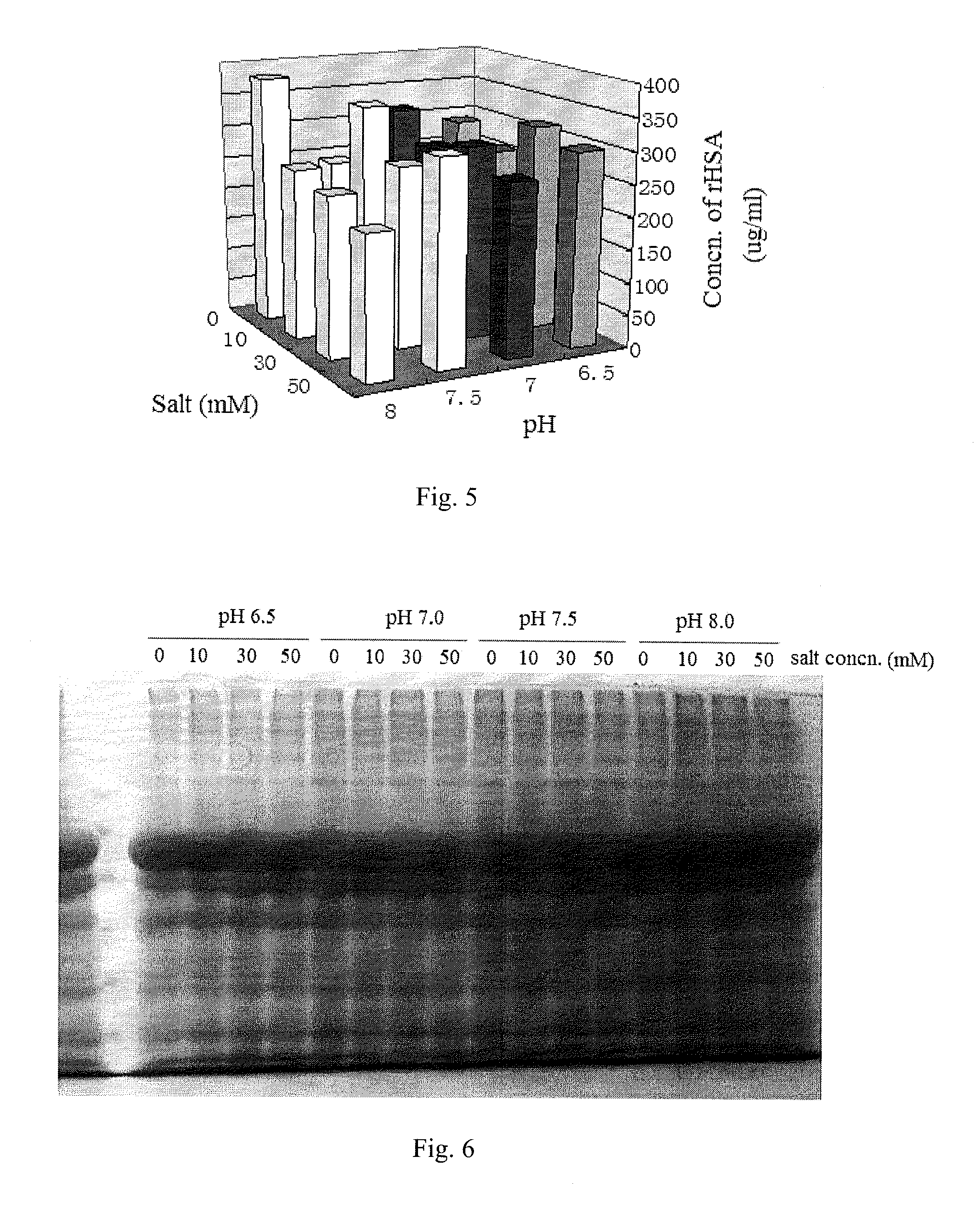
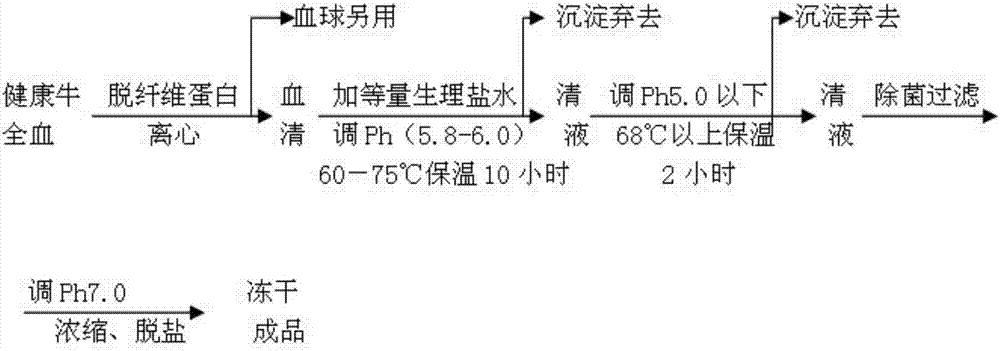
The invention relates to an extraction process for bovine serum albumin by a graduated heat shock method. The process comprises the following steps: 1. serum separation: removing fibrin from healthy bovine fresh whole blood, carrying out continuous centrifugation to collect serum and erythrocytes for other use; 2. extraction: adding the serum and an equivalent volume of normal saline into a reaction tank, carrying out uniform mixing at the stirring rate of 20rpm to 40rpm, adding 0.32% to 0.38% of sodium caprylate with stirring, so as to enable the sodium caprylate to be thoroughly bonded to albumin; adding 0.1M dilute hydrochloric acid, adjusting the pH to 5.8 to 6.0, heating to the temperature of 60 DEG C to 75 DEG C, carrying out heat preservation for 10 hours, carrying out filtering to remove precipitates, adjusting the pH of supernatant to 4.5 to 5.0, carrying out heat preservation, carrying out filtering to remove precipitates, and collecting clear liquid; 3. concentrating, demineralizing and on-site sterilization filtration: adjusting the pH of the clear liquid to 6.8 to 7.2, carrying out cyclic sterilization, carrying out ultrafiltration concentrating by an ultrafiltration membrane with the molecular weight of 10,000, and carrying out saline matter eluting, wherein the final concentration of a protein concentrate does not exceed 15%; 4. carrying out freeze drying to obtain crystalline dry powder. According to the process, the used materials are simple, the cost is reduced, the process is simple and convenient, and the operation is easy.
Owner:天津康源生物技术有限公司
Method for purification of albumin
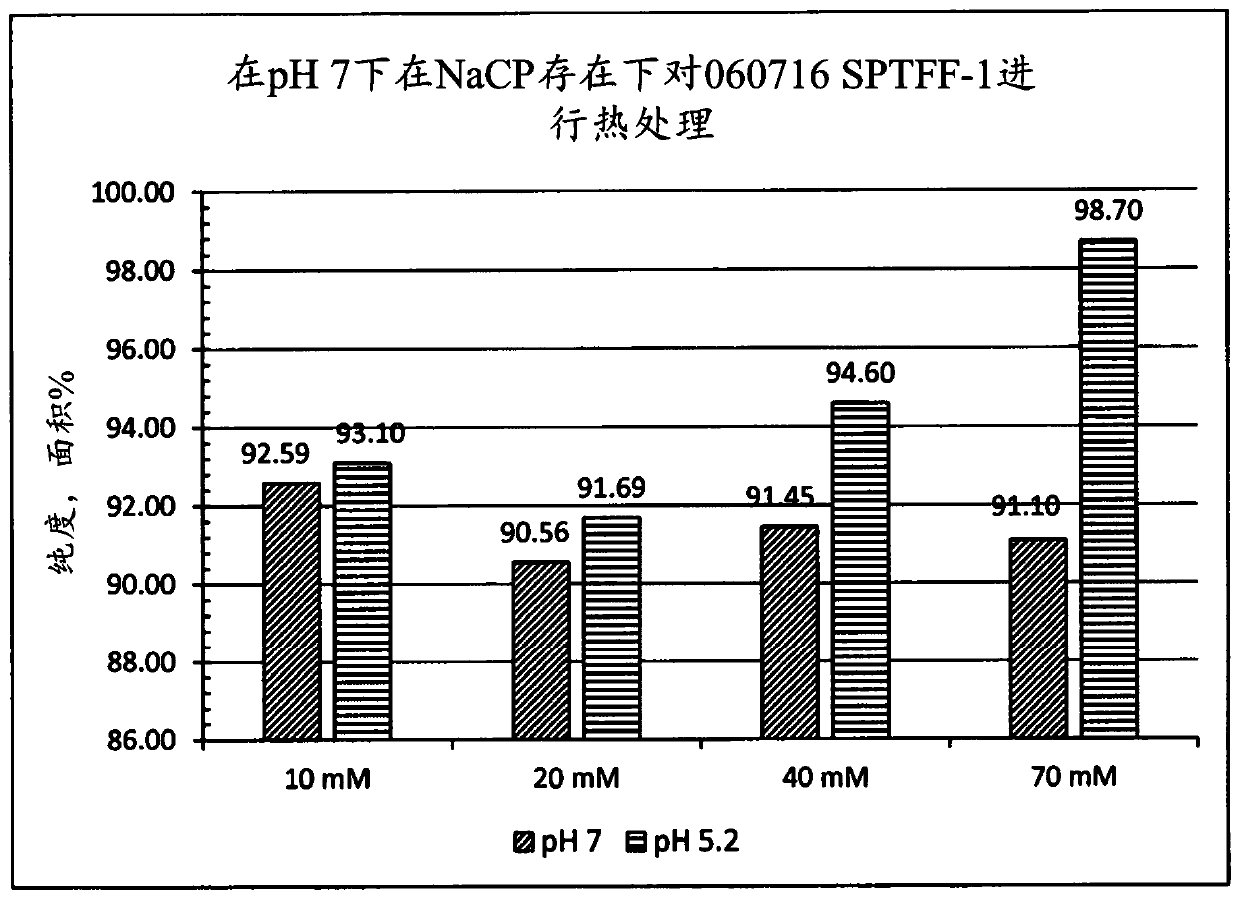
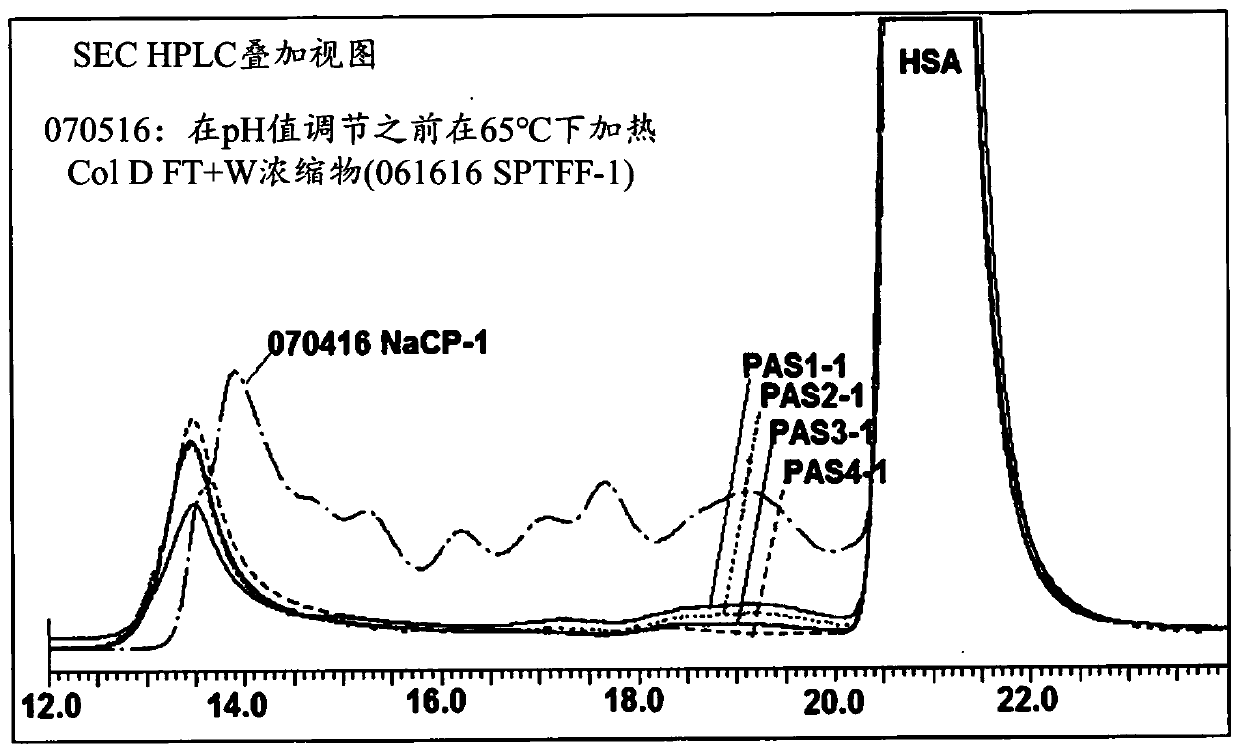
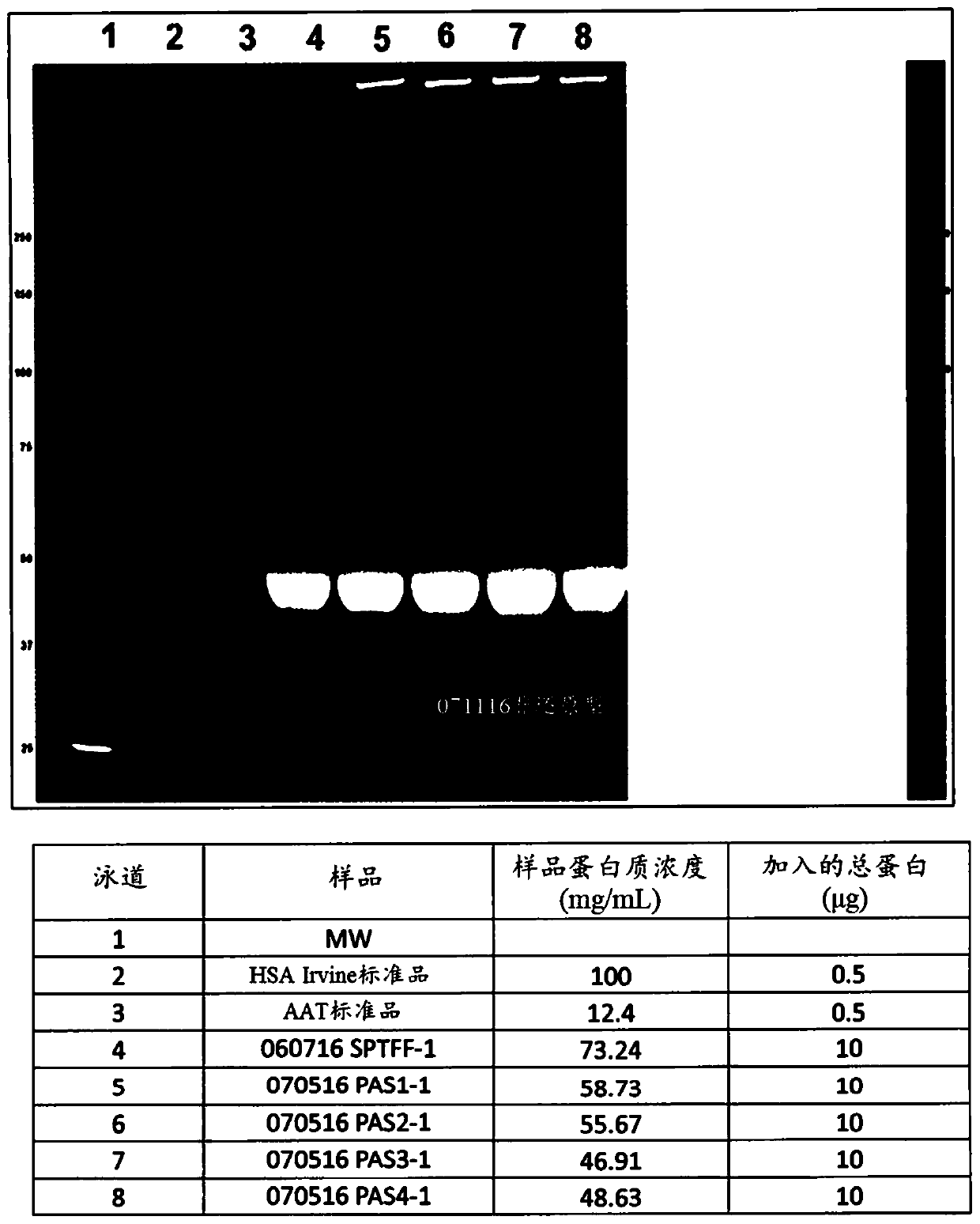
A method for the purification of albumin from plasma is described. The method comprises (a) contacting the plasma with sodium caprylate (NaCP) at an amount dependent on total protein concentration andthe ratio of NaCP to total protein in the plasma, (b) heating the plasma at a near neutral pH range, and (c) separating the albumin from non-albumin-phase. The said method provides for a high yield and purity albumin solution.
Owner:伊沃芙生物制品公司
Fen-flavor detergent special for leather
InactiveCN104877794AIncrease the fragranceGood removal effectOrganic detergent compounding agentsSurface-active detergent compositionsBiotechnologyMeth-
The invention discloses fen-flavor detergent special for leather. The fen-flavor detergent is prepared by, by weight, 4-6 parts of linalyl acetate, 5-8 parts of sulfonated succinic acid sodium caprylate, 6-9 parts of alpha-amine alkyl benzene kecone, 4-7 parts of jojoba oil, 6-8 parts of palm oil, 5-7 parts of acrylate, 15-26 parts of difluorotetrachloroethane, 5-7 parts of ethyl acrylate, 12-15 parts of triolein, 5-8 parts of mildew-proof agent, 2-4 parts of lavender essential oil, 5-9 parts of octadecyl trimethyl ammonium chloride, 3-6 parts of sandalwood and 5-9 parts of polyoxypropylene triol. The fen-flavor detergent has the advantages that the fen-flavor detergent can remove stain on the surface of the leather well, mildew breeding is reduced, hand feeling is improved, and the leather can have good fen-flavor after being treated by the detergent.
Owner:QINGDAO GUOHANG XIANGYU TECH SERVICE
Method for preparing alpha-lipoic acid
ActiveCN103058989BNo pollution in the processLipoic Acid High PurityOrganic chemistryEthyl esterHydrolysis
The invention discloses a method for preparing alpha-lipoic acid, comprising the following steps: preparing the sodium sulphide liquor, synthesizing lipoic acid ethyl ester, hydrolyzing the lipoic acid ethyl ester, acidizing the sodium thioctate, and purifying the crude product of the lipoic acid. The liquor adopted by the method during the preparation process has no pollution for human bodies, is safe and easy to operate, and has the performances of high lipoic acid purity, high reaction yield and gentle reaction conditions, so that the method is suitable for industrial production on large scales.
Owner:SHANDONG QIDU PHARMA
Method for extracting intravenous injection human immunoglobulin from plasma separation component I and plasma separation component III
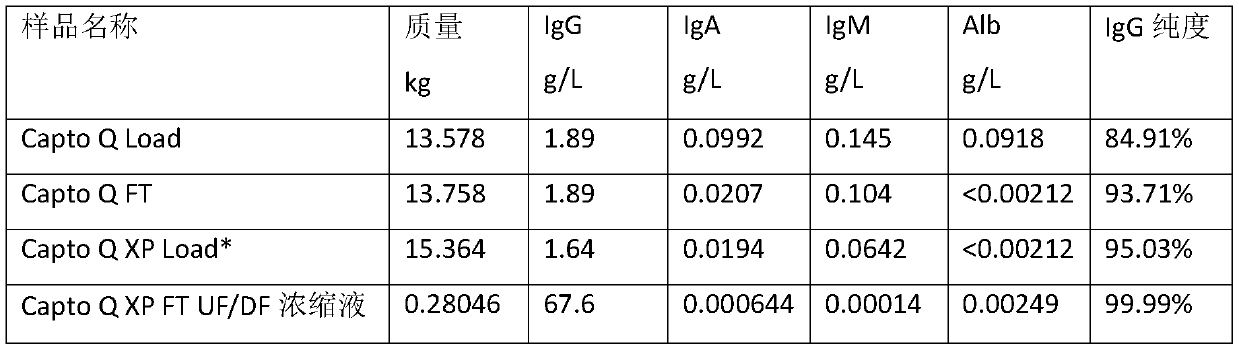
The invention relates to a method for extracting intravenous injection human immunoglobulin from a plasma separation component I and a plasma separation component III. The method comprises the steps of (1) precipitating F I+III components: dissolving F I+III precipitate in water for injection according to the concentration of 20%(wt), and precipitating impurity protein in F I+III components underthe condition of 50mM sodium caprylate and pH being 4.9; and (2) further refining IgG components by using ion exchange chromatography, wherein the step of further refining IgG components by using ionexchange chromatography comprises substeps of (a): performing Capto Q chromatography pretreatment on an IgG solution obtained in the step (1) to obtain an IgG solution 1; (b) performing Capto Q XP chromatography treatment on the IgG solution 1 so as to obtain an IgG solution 2; and (c) performing ultrafiltration and concentration on the IgG solution 2 so as to obtain IgG refined liquid.
Owner:国药集团武汉血液制品有限公司
Method for effectively removing human immune globulin polymer
ActiveCN104356231AReduce energy consumptionMultimer reductionPeptide preparation methodsImmunoglobulinsPolyethylene glycolGlobulin
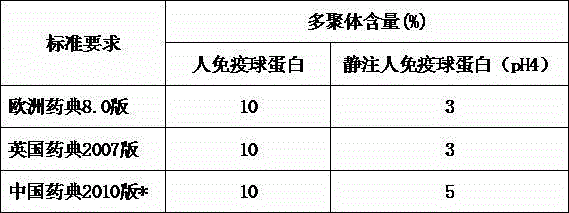
The invention discloses a method for effectively removing human immune globulin polymer. The operation of reducing polymer in an immune globulin purification process is necessary. A polyethylene glycol method and a column chromatography which can remove human immune globulin polymer are reported in public at present, but the two methods have problems of being difficult in removing additives, small in handling capacity and expensive in equipment, and the popularization and application are difficult. The method comprises the following steps: by using a sodium caprylate precipitation method, precipitating to remove IgG polymer under the condition that the solution pH value is 5.4 and the caprylate concentration is 6-8mmol / L, wherein more than 60% of polymer can be effectively removed so that the polymer can be reduced to 3% or below, the loss of the immune globulin is little, the polymer content is stable, and the polymer content can achieve requirement of Chinese pharmacopoeia and Foreign related pharmacopoeia after being placed for two years.
Owner:BEIHAI KAIYUAN BIOLOGICAL TECH
Composition containing chloroquine phosphate
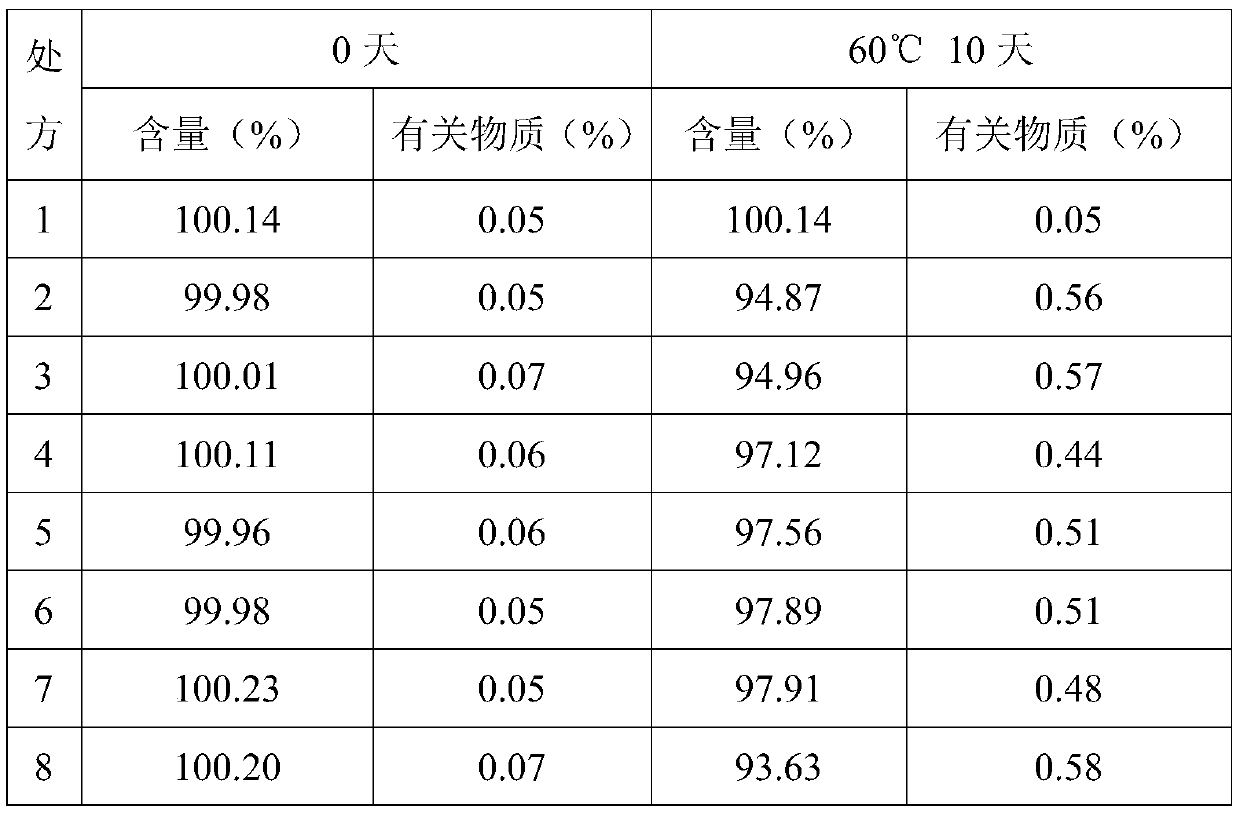
InactiveCN103191113AImprove stabilityStrong specificityOrganic active ingredientsPharmaceutical non-active ingredientsHistidineXylitol
The invention discloses a composition containing chloroquine phosphate. The composition also contains the following components: 5-20wt% of histidine sodium, 10-20wt% of xylitol, 30-50wt% of alkyl trimethyl ammonium bromide and 10-20wt% of sodium caprylate. The composition has the beneficial effect of good stability.
Owner:天津市嘉凡生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com