Treatment of osteoporosis
a technology for osteoporosis and treatment, applied in the field of treatment, can solve problems such as problems in the oral administration of peptide pharmaceuticals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
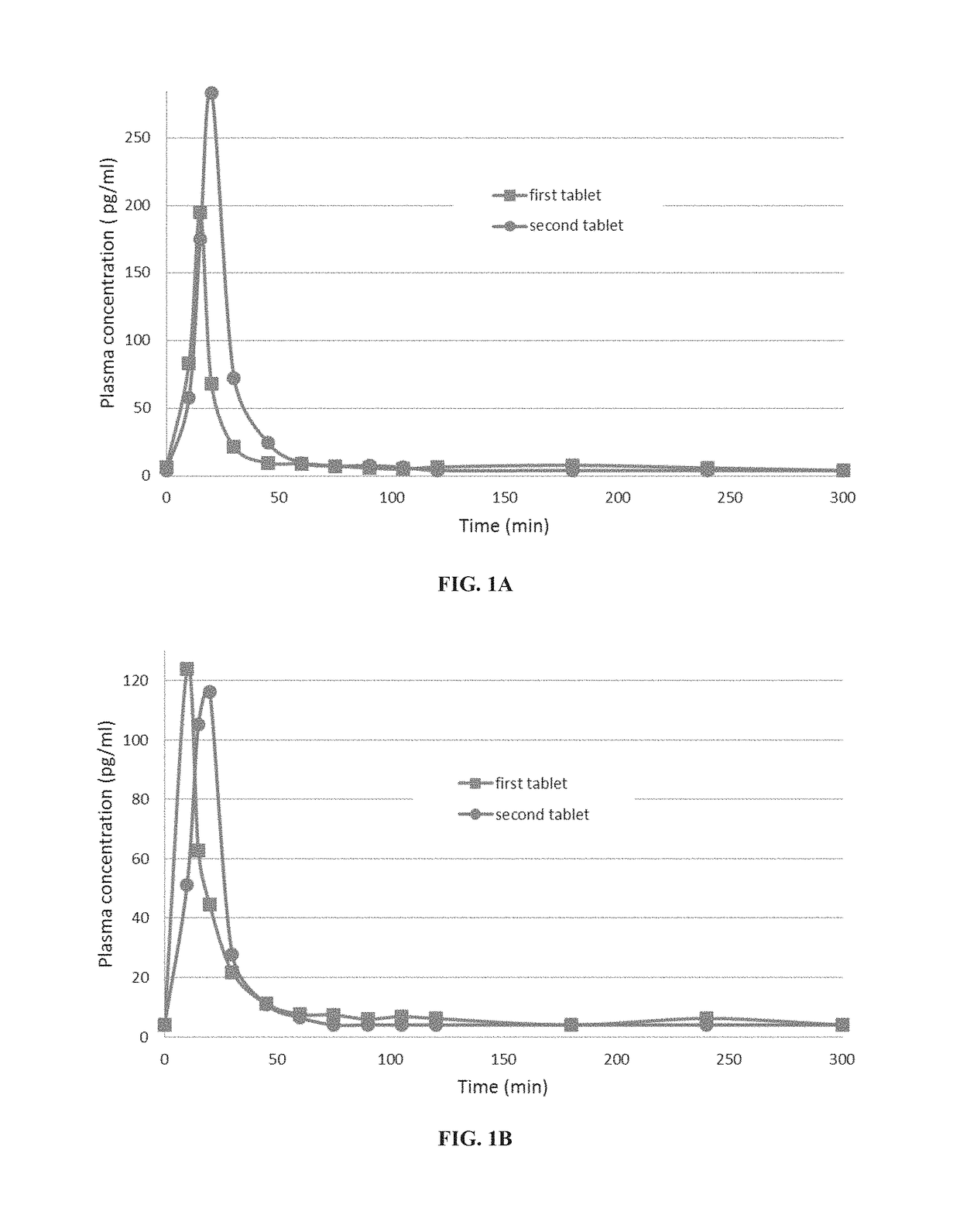
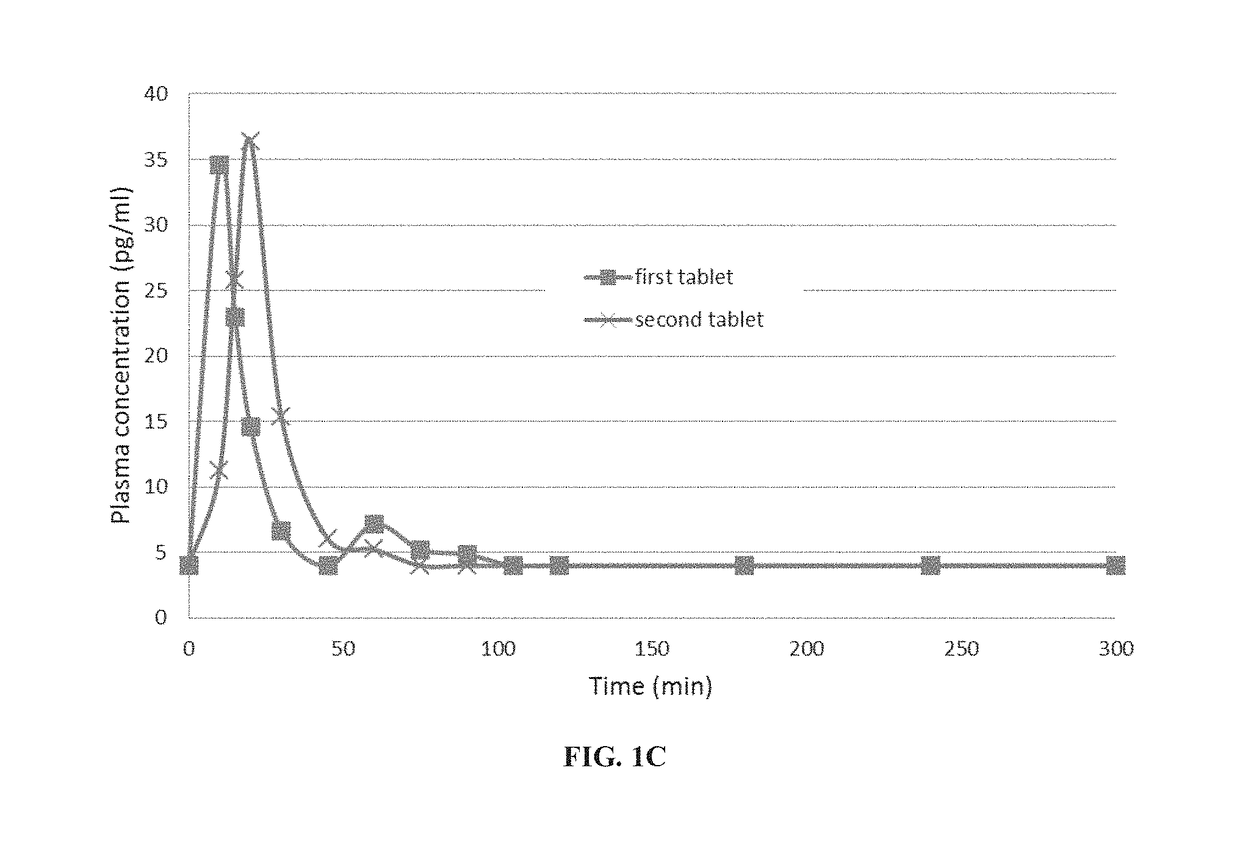
Pharmacokinetic Profile of Orally Administered Parathyroid Hormone (PTH)
[0545]Pharmacokinetic Study Design:
[0546]An open label comparative pharmacokinetic study was performed in healthy volunteers over the course of 3 months. Each volunteer received—in each of two visits—the same oral tablet containing 0.75 mg of teriparatide, a recombinant form of parathyroid hormone (1-34).
[0547]The formulation was composed of teriparatide (0.75 mg), SNAC (sodium 8-N-(2-hydroxybenzoyl)aminocaprylate), soybean trypsin inhibitor (SBTI) and a small amount of magnesium stearate.
[0548]Tablets were administered in the morning after an 8-hour overnight fast and immediately followed by 150 ml of water. At each visit a standard meal was provided 3 hours after drug administration. Patients did not eat or drink alcoholic or caffeinated beverages. There was a two weeks period between the visits.
[0549]To determine parathyroid hormone(1-34) (PTH(1-34)) concentrations, blood samples (4 ml each) were drawn via an...
example 2
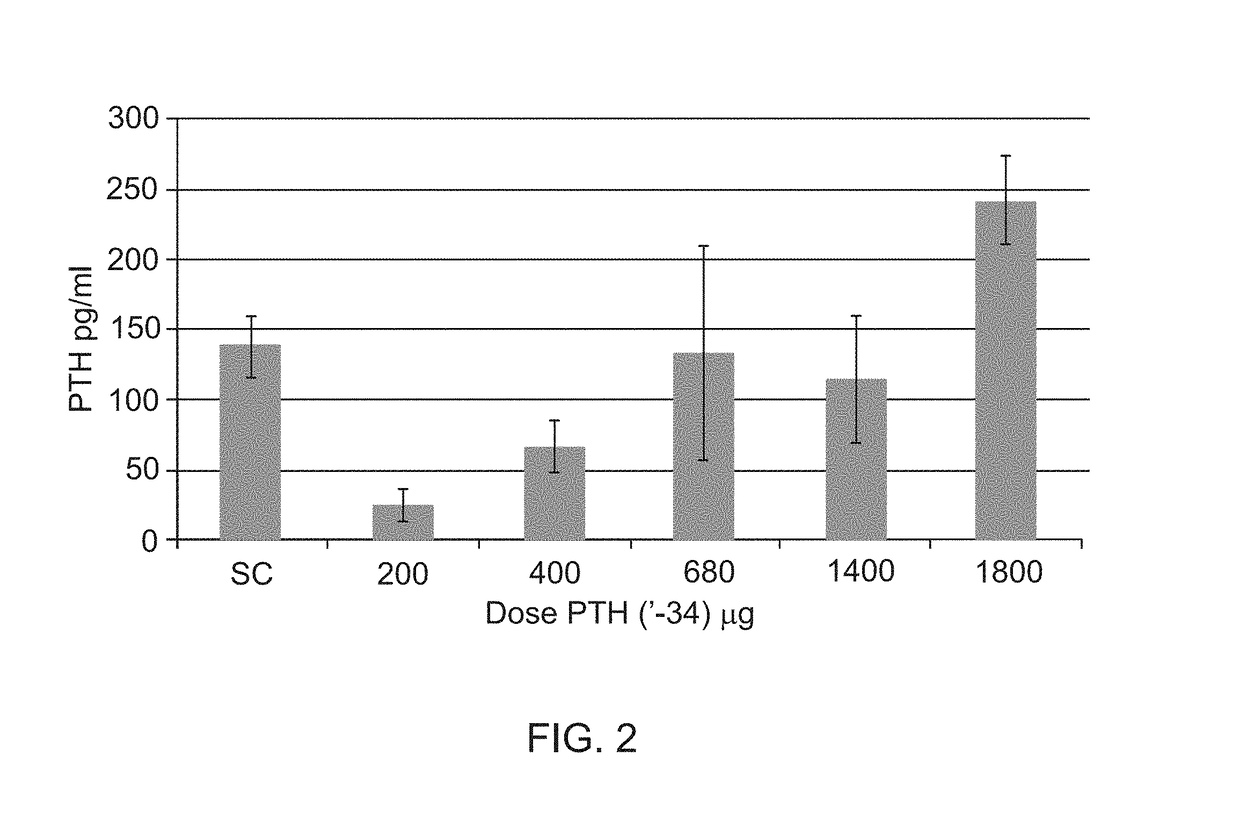
Phase I Clinical Trial of Orally Administered Parathyroid Hormone (PTH)
[0554]A Phase I clinical study of exemplary oral formulations comprising teriparatide (parathyroid hormone (1-34)) was conducted at the Hadassah Clinical Research Center. 42 healthy volunteers were included throughout the study.
[0555]The formulation was composed of teriparatide (200, 400, 680, 1400 or 1800 μg), SNAC (sodium 8-N-(2-hydroxybenzoyl) aminocaprylate), soybean trypsin inhibitor and magnesium stearate.
[0556]Tablets were administered in the morning after an 8-hour overnight fast and immediately followed by 150 ml of water. At each visit a standard meal was provided 3 hours after drug administration. Patients did not eat or drink alcoholic or caffeinated beverages.
[0557]To determine parathyroid hormone concentrations, blood samples (4 ml each) were drawn via an indwelling catheter from the forearm vein at predetermined time points. The cannula was flushed with 1.5 ml normal saline after each sampling. In ...
example 3
Casing Containing Parathyroid Hormone and Swellable Substance
[0565]A drug delivery system comprising a casing encapsulating active ingredients, parathyroid hormone and SNAC (sodium 8-N-(2-hydroxybenzoyl)aminocaprylate) (per se, or formulated as a pharmaceutical composition according to any of the respective embodiments described herein) according to some embodiments of the invention is optionally assembled as depicted in FIG. 12.
[0566]A first casing component (A) is filled with substance which swells upon contact with water (B), followed by a barrier (C), and active ingredients (parathyroid hormone and SNAC) (D), optionally in granular form. A second casing component (E) is then placed in contact with first casing component (A), thereby encapsulating (B), (C) and (D). A layer of enteric polymer (not shown) as described herein in any of the respective embodiments, is then formed over at least a portion of an external surface of casing components (A) and (E). Adhesion of the layer to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com