Stable liquid preparation containing GLP-1 analogue fusion protein and preparation thereof
A technology for liquid preparations and fusion proteins, which can be used in medical preparations containing active ingredients, medical preparations without active ingredients, and drug combinations, and can solve problems such as safety, problems, and inconvenience for patients to use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The influence of embodiment 1 different pH on preparation stability
[0019] 1.1pH preliminary screening
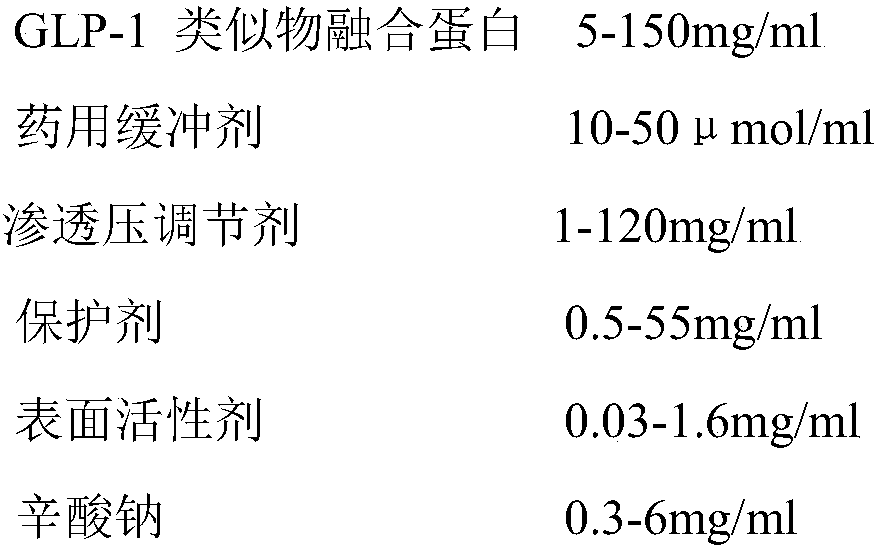
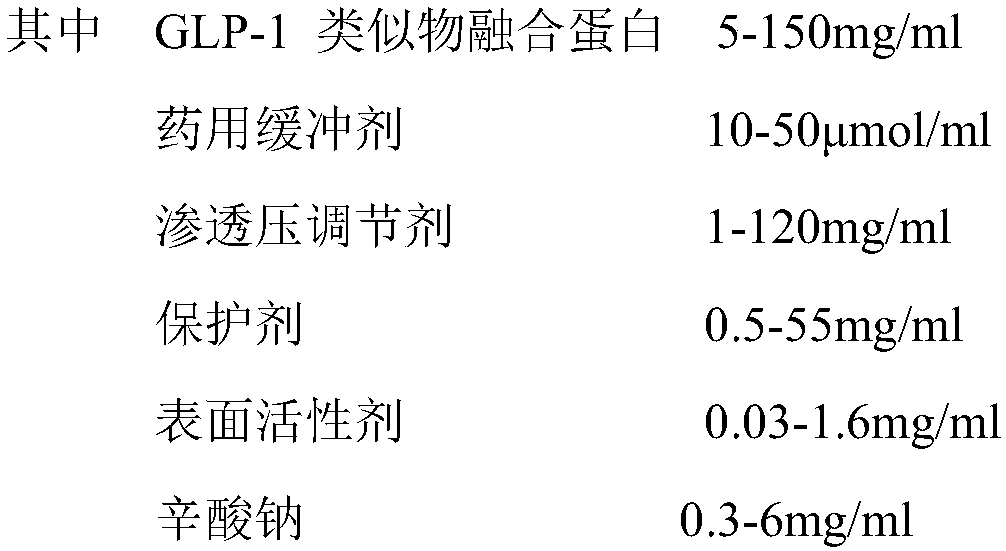
[0020] Each group contained 5.0 mg / ml GLP-1 analogue fusion protein, 20 μmol / ml acetic acid / sodium acetate buffer or Tris-HCl buffer at different pH, pH 4.0-10.0, 13 groups were set up, as shown in Table 1. After sterile filtration, the samples of each group were divided into sterile non-pyrogenic vials, and placed in a 37°C incubator after capping and capping. Samples were taken on the 15th and 30th days, and detected by 10% non-reducing SDS-PAGE For the samples, see Table 2 for details.
[0021] Table 1pH preliminary screening form
[0022] group
1
2
3
4
5
6
7
8
9
10
11
12
13
pH
4.0
4.4
5.0
5.4
6.0
6.4
7.0
7.4
8.0
8.4
9.0
9.4
10.0
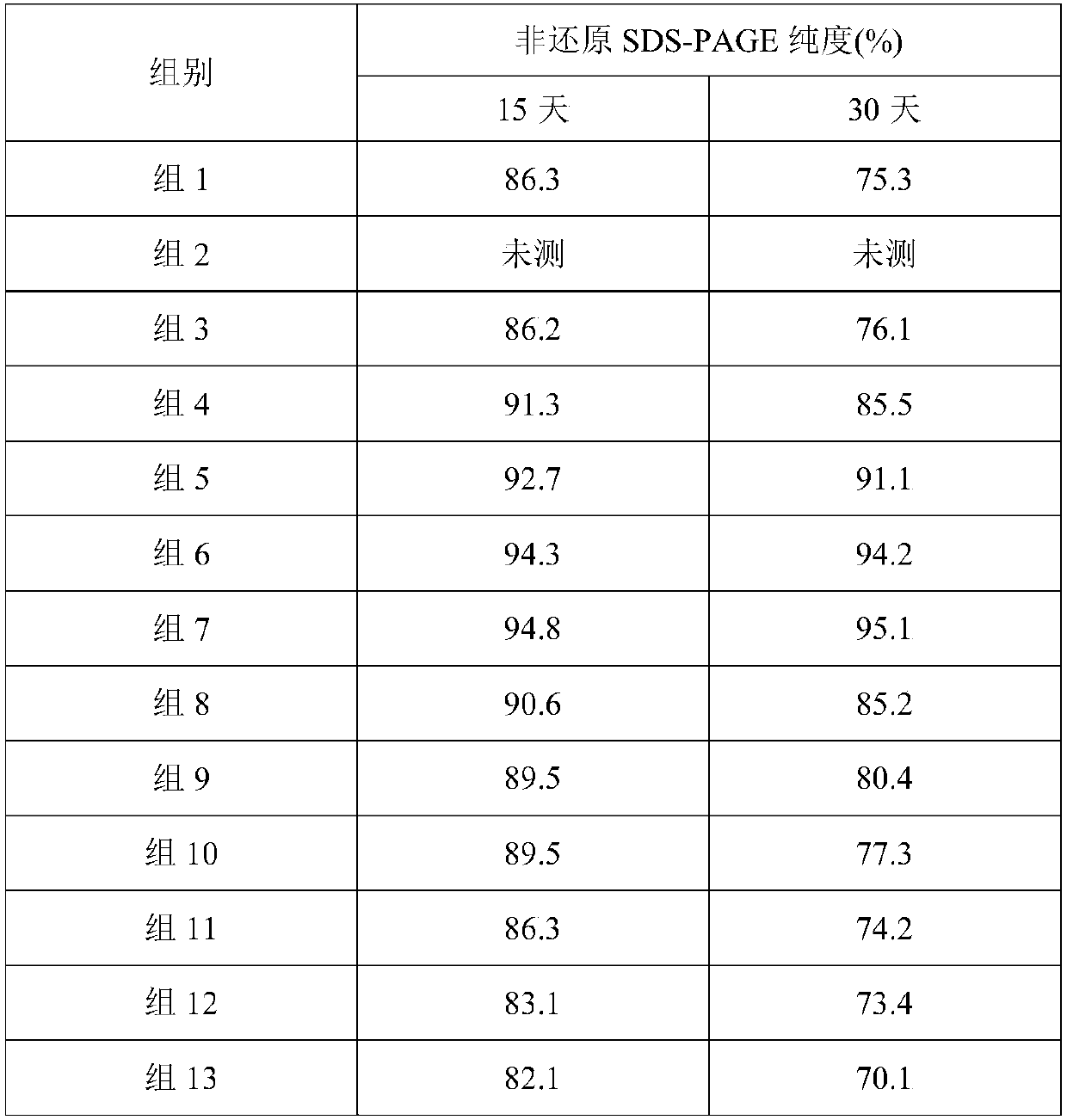
[0023] Table 2 10% non-reducing SDS-PAGE results at different pH
[0024]
[0025] The results showed t...
Embodiment 2
[0031] The influence of embodiment 2 sodium octanoate on the stability of GLP-1 analog fusion protein
[0032] The effect of sodium octanoate on the stability of GLP-1 analog fusion protein was investigated. The GLP-1 analogue fusion protein was substituted into 30 μmol / ml phosphate buffer (pH 6.5), with a final concentration of 25 mg / ml, and processed according to Table 4. After the treatment, the samples were aseptically filtered and packed into sterile pyrogen-free vials, and then placed in a constant temperature incubator at 37°C for accelerated investigation. Samples were taken on the 15th and 30th days, and 10% non-reducing SDS was used. -PAGE method detection, the results are shown in Table 5.
[0033] Table 4 Addition of sodium octanoate and heat treatment of each group of samples
[0034] group
processing method
1
Without adding sodium caprylate and heat treatment at 50℃ for 1h
2
Add sodium octanoate (0.3mg / ml) and heat treatment a...
Embodiment 3
[0038] Embodiment 3 Different protective agents (amino acids) affect the stability of GLP-1 analog fusion protein
[0039]Amino acids are beneficial to inhibit protein degradation and aggregate formation. This example examines the effects of various amino acids on the stability of GLP-1 analog fusion proteins. The fusion protein containing GLP-1 analogs in each group was substituted into 30 μmol / ml phosphate buffer (pH6.5) to a final concentration of 5 mg / ml, and the amino acid single component of each group was added and the pH was appropriately adjusted to 6.5, type and concentration See Table 6 for details. All groups were aseptically dispensed into vials, capped and placed in a constant temperature incubator at 37°C for accelerated investigation. Samples were taken on the 15th day and 30th day respectively, and detected by 10% non-reducing SDS-PAGE and SEC-HPLC methods. The results are shown in Table 7.
[0040] Table 6 Adding concentration of different amino acids
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com