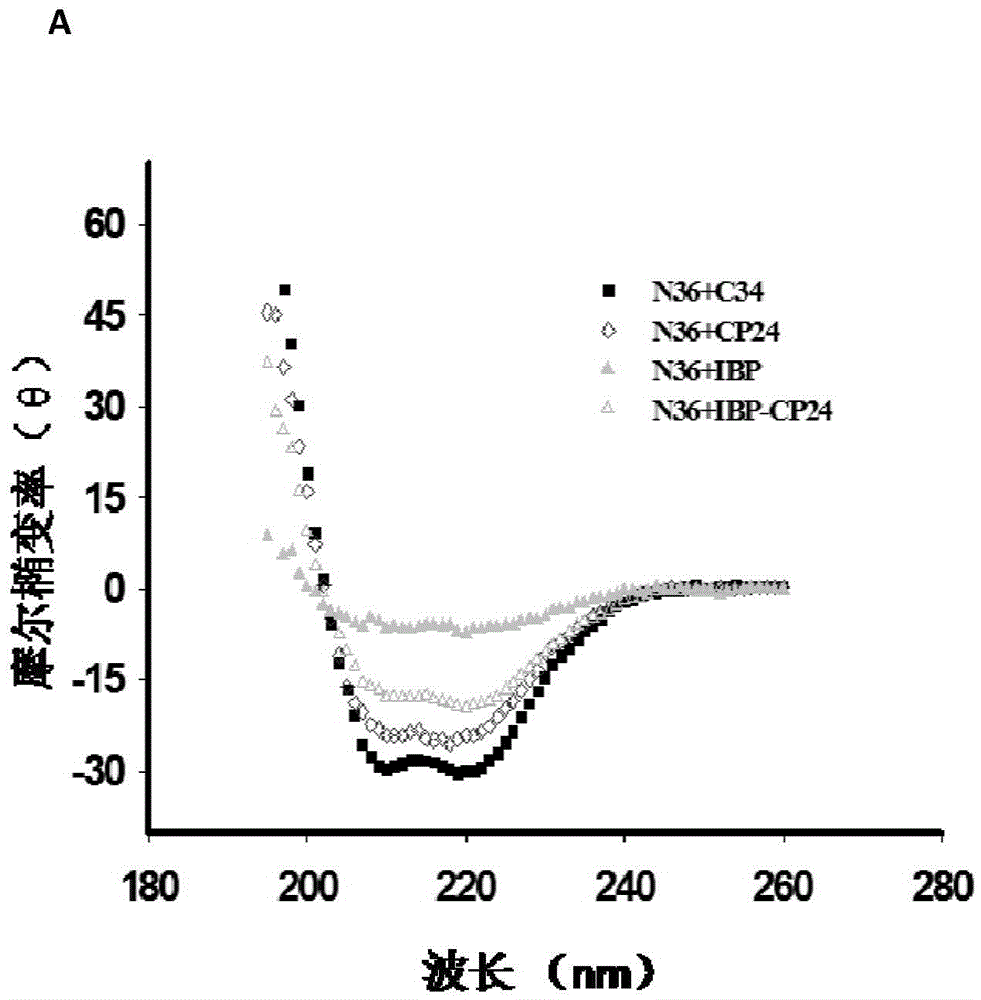
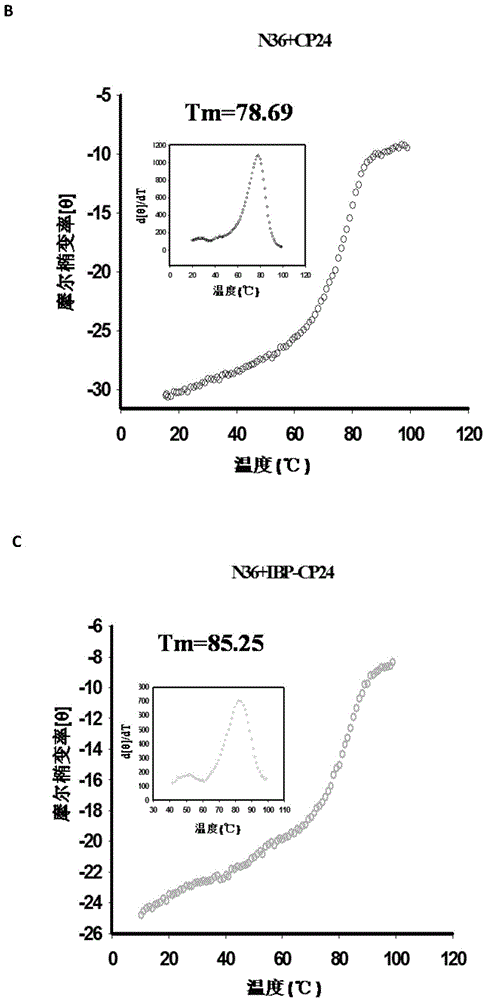
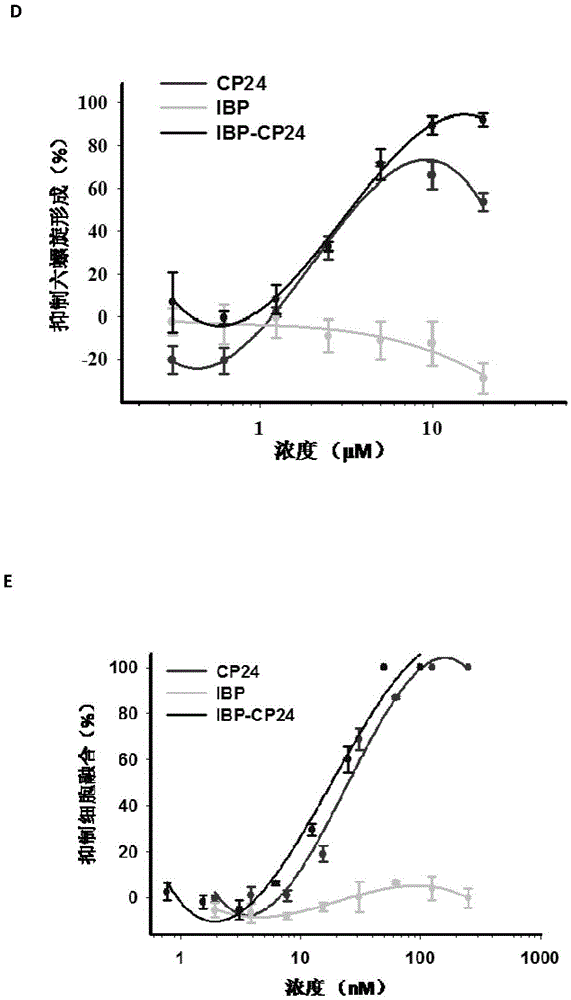
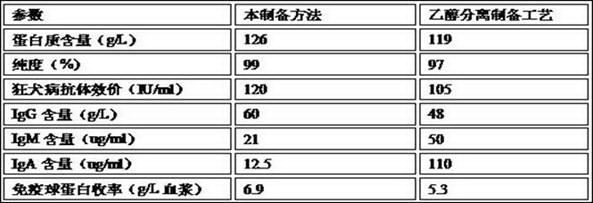
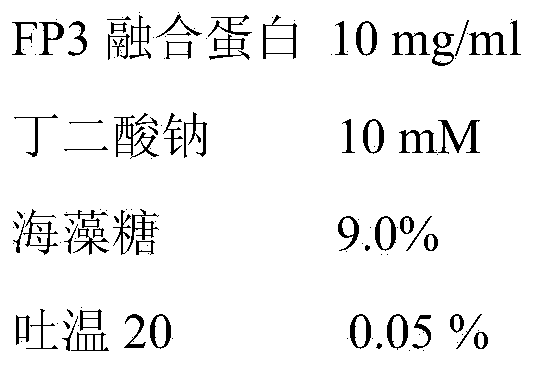
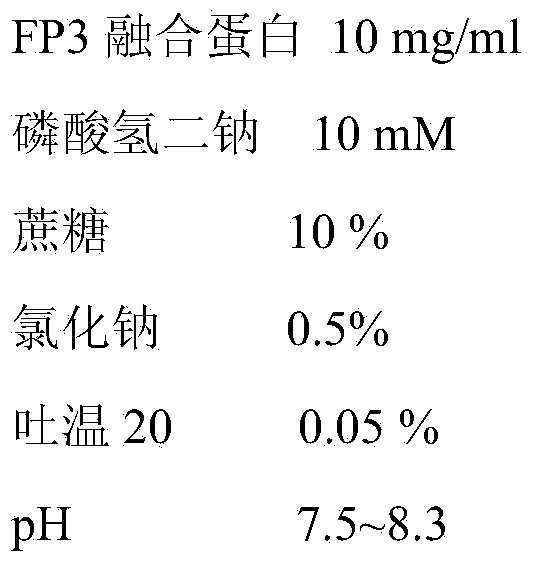
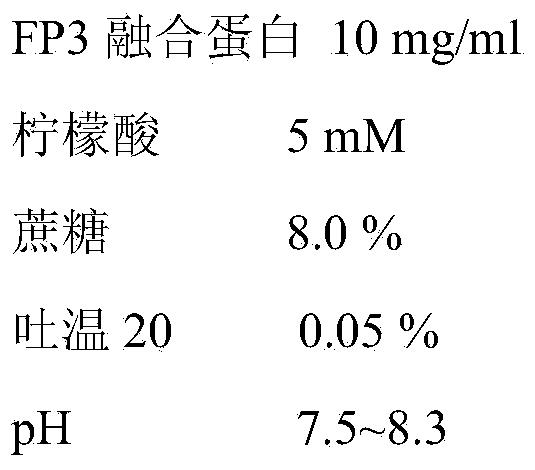
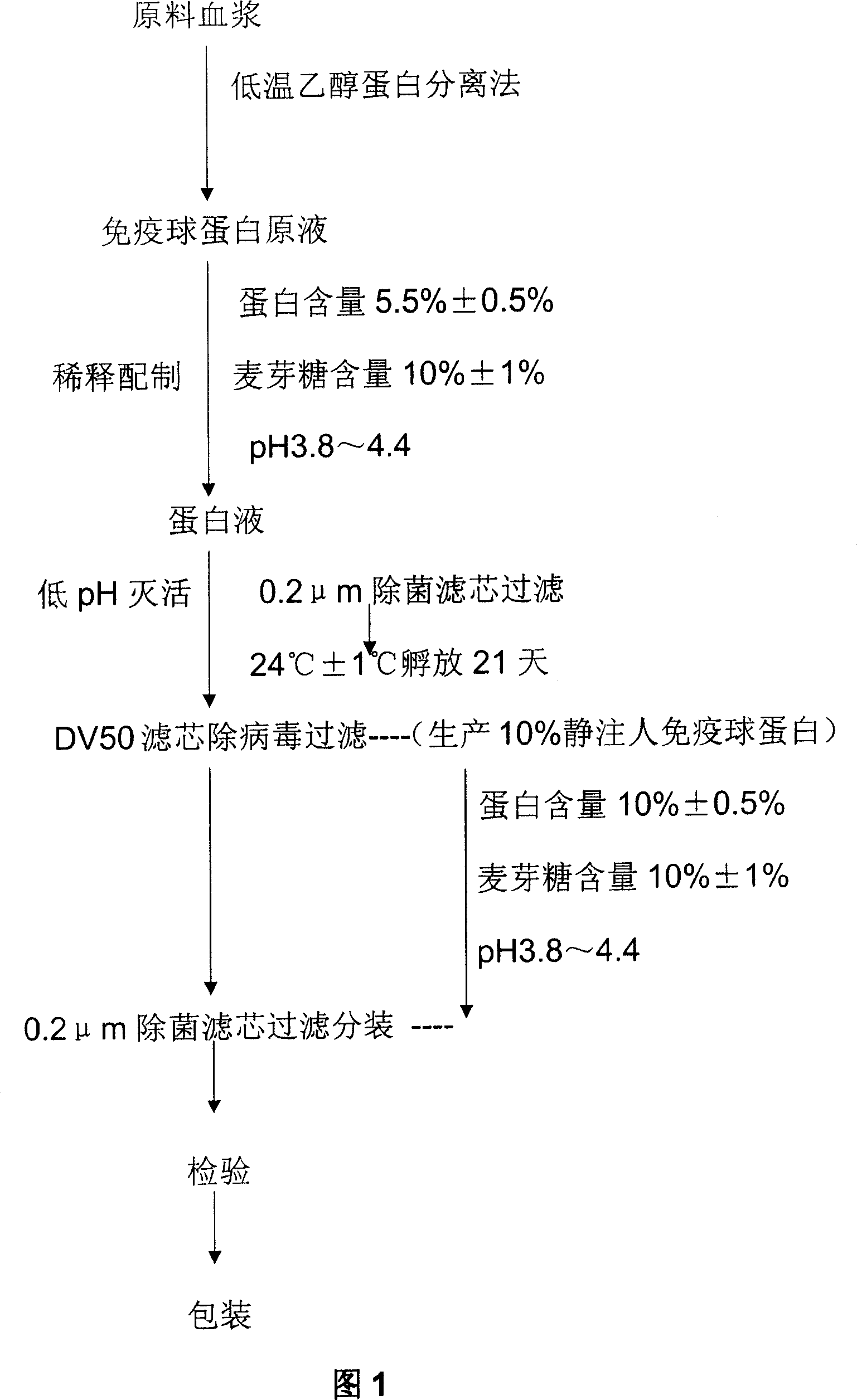
Patents
Literature
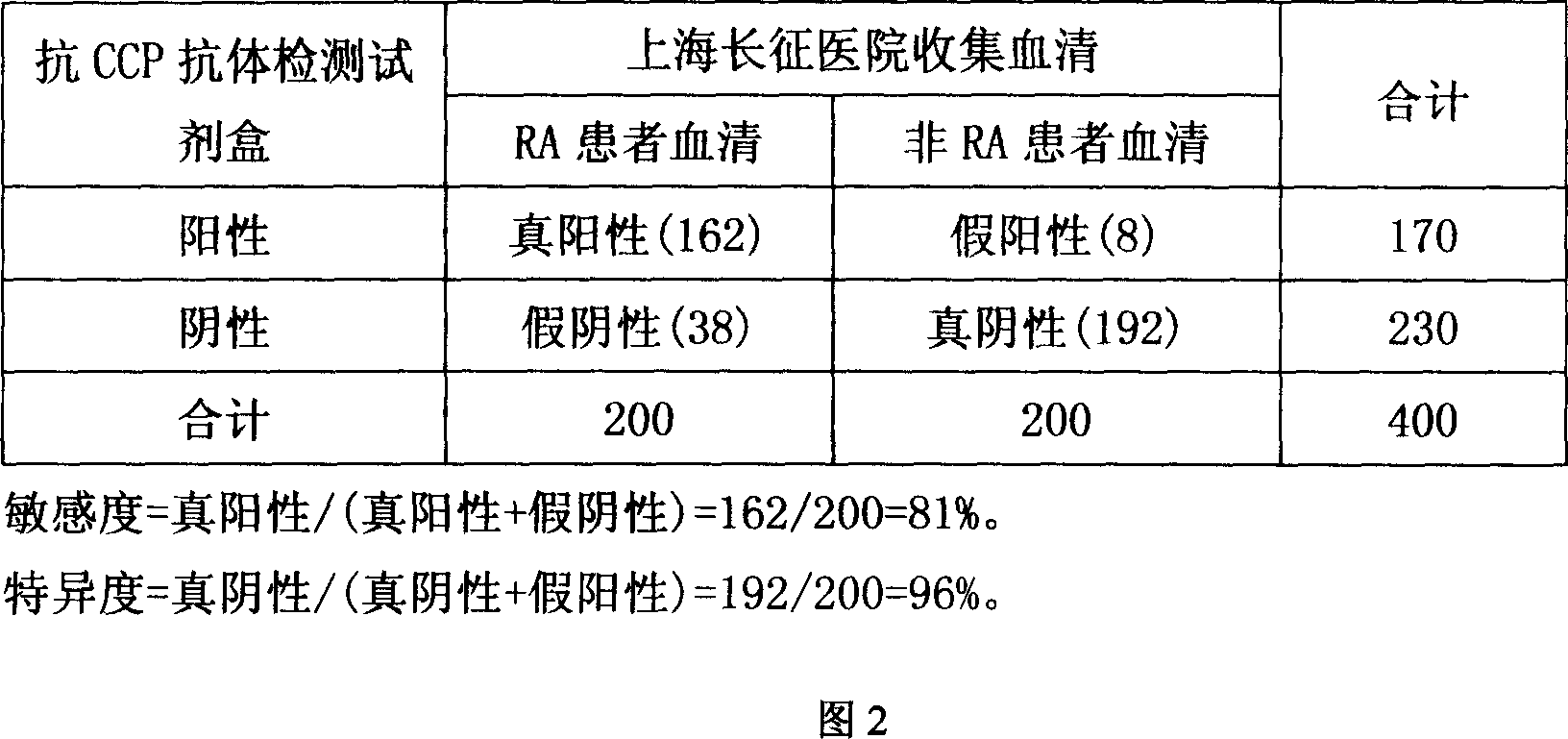
73 results about "Human immune globulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
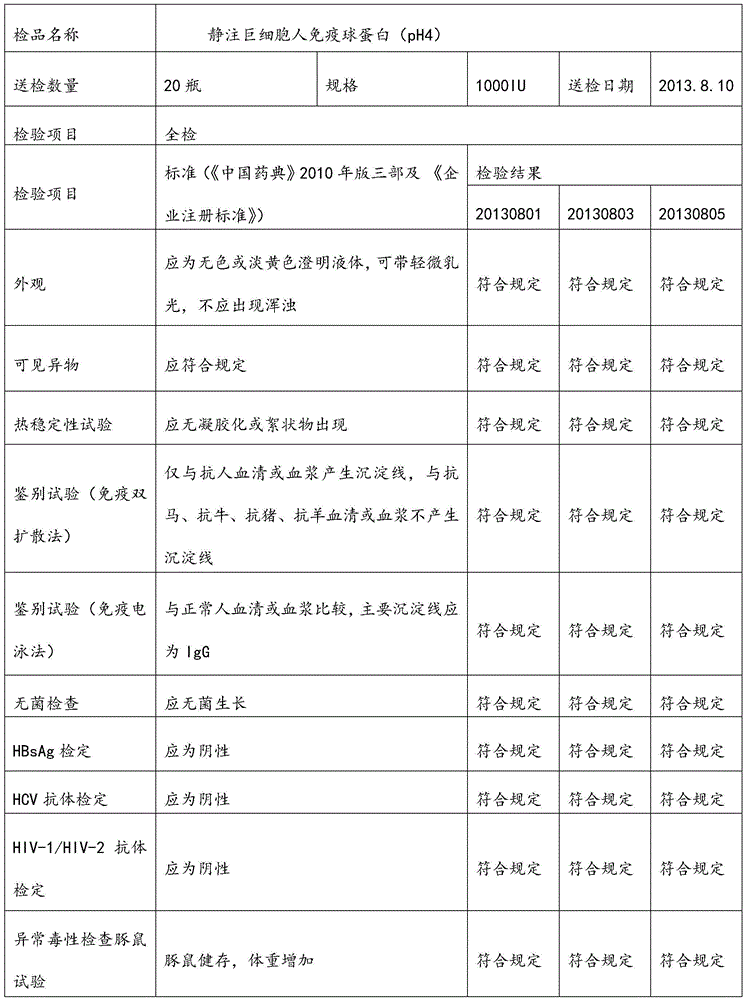
GAMUNEX (immune globulin intravenous human 10%) consists of 9%–11% protein in 0.16–0.24 M glycine. Not less than 98% of the protein has the electrophoretic mobility of gamma globulin. GAMUNEX (immune globulin intravenous human 10%) contains trace levels of fragments, IgA (average 0.046 mg/mL), and IgM.
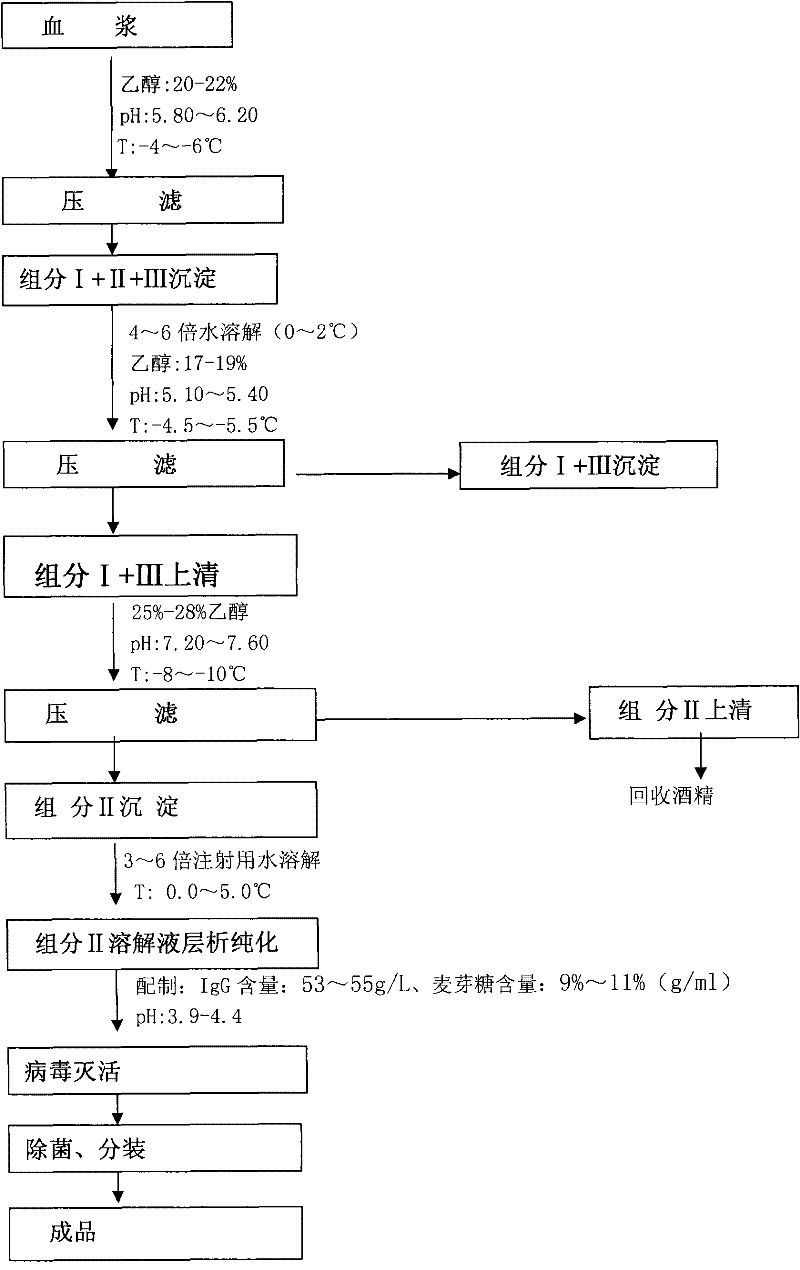
Method for producing intravenous injection human immune globulin
ActiveCN102178951AEasy temperature controlFast separationSerum immunoglobulinsAntibody ingredientsIon exchangeEngineering
The invention relates to a preparation method of human plasma protein, in particular a method for producing intravenous injection human immune globulin by using filter press technique for separation in combination with chromatograph for purification. The method is characterized in that a filter press is adopted as a main separation device to replace a centrifugal machine for producing intravenous injection human immune globulin, so that the temperature and other conditions for separation are easy to control, the separation speed is quick, and the safety is high without a high-speed operating device. Meanwhile, ion-exchange column chromatography is adopted to further purify the product and acquire the intravenous injection human immune globulin with the purity as high as 98.5-100%. The sum of monomer and dipolymer reaches 99-99.5%. PKA (proteinkinase A) is no more than 3IU / ml and ACA (Anti Cardiolipin Antibodies) is no more than 9%. Besides, the process is high in recovery rate, and for each ton of blood plasma, 5600-6300g of intravenous injection human immune globulin can be harvested.
Owner:哈尔滨派斯菲科生物制药有限公司
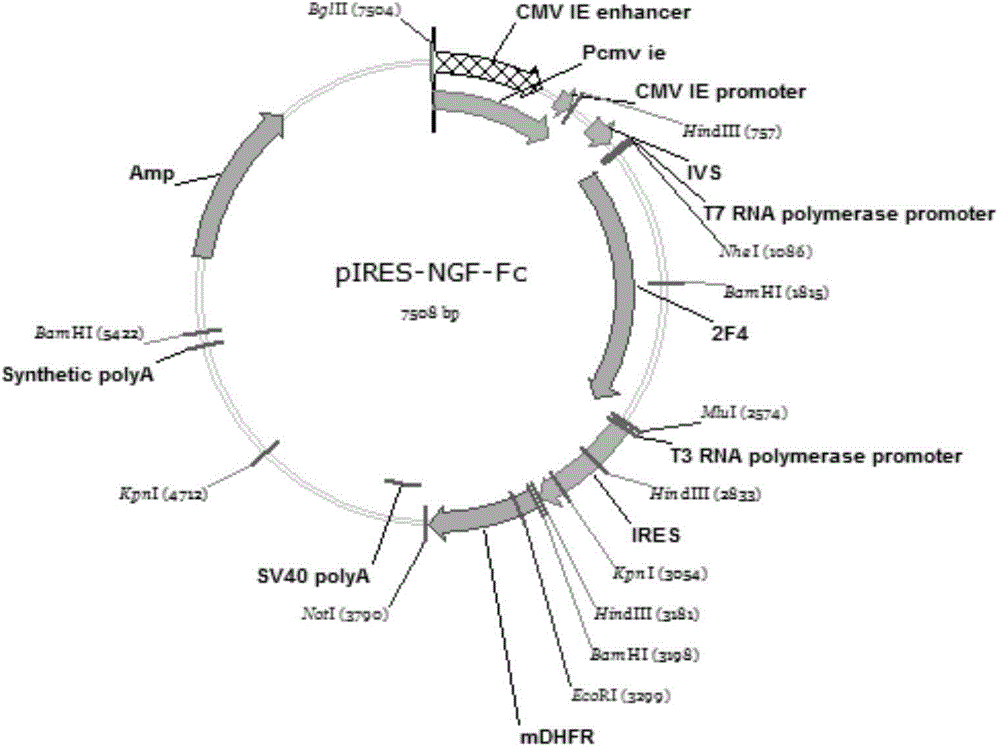
NGF-Fc fusion protein and preparation method thereof
InactiveCN105273087AImprove stabilityExtended half-lifeNervous disorderPeptide/protein ingredientsDiseaseNervous system
The invention belongs to the technical field of biological engineering, and relates to an NGF-Fc fusion protein and a preparation method thereof. The method comprises the following steps: constructing a human immune globulin IgG Fc and nerve growth factor (NGF) fusion gene expression vector through a genetic engineering means, transferring to a mammal cell to make the transferred mammal cell highly express and produce NGF-Fc fusion proteins, purifying, identifying and carrying out biological activity detection. The expression vector transferred mammal cell constructed in the invention can express bioactive NGF-Fc fusion proteins, so the expression level can be 150mg / L or above, and the obtained protein has good stability and long half life, and can be used to prepare drugs for treating Alzheimer's disease, diabetic peripheral neuropathy, Parkinson's disease, facial neuritis, craniocerebral trauma, trauma of spinal cord, acute cerebrovascular disease, encephalatrophy and other neurological diseases, and peripheral nerve injury acute cerebral vascular central nerve injuries induced by chemical drugs.
Owner:FUDAN UNIV
Cross-linked composite used as standard diagnosing reagent replacing positive serum and method for use as standard reagent
InactiveCN1924579AAvoid reactionUniform characteristicsImmunoglobulins against animals/humansDisease diagnosisCross-linkSerum ige
This invention relates to one positive serum as diagnose agent standard compound, which is processed through single clone antigen technique or antigen immune animal and is the crossed compound of antigen and human immune globulin. This invention also discloses one use method of the compound, which comprises the following steps: a, using antigen immune globulin to get the specific antigen; b, crossing the specific antigen and human immune globulin by the crossing agent; c, diluting the said cross antigen gradient to draw standard curve; d, using the said antigen as standard product for quantitative diagnose test method.
Owner:SHANGHAI FUCHUN ZHONGNAN BIOTECH
Method for extracting human immune globulin from component I+III or component III
ActiveCN101648998AAlleviate the tightness of plasma supplyAvoid pollutionImmunoglobulins against animals/humansPeptide preparation methodsSocial benefitsImmunglobulin e
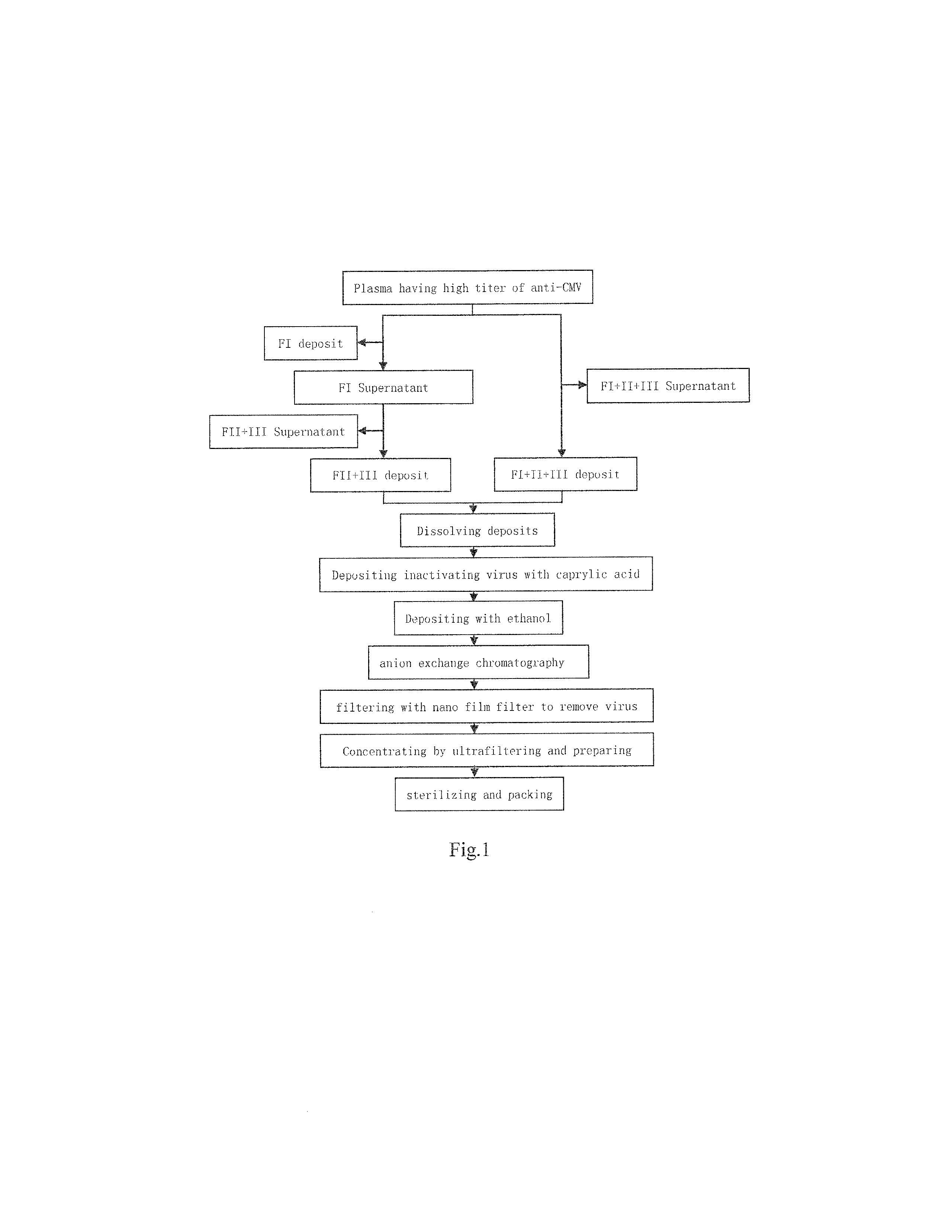
The invention relates to a method for extracting human immune globulin from a component I+III or a component III, which can effectively extract human immune globulin from a component I+III or a component III so as to meet the demands of human immune globulin for people, and comprises the following steps: (1) dissolving the deposits of component I+III or component III; (2) removing the component I;(3) separating the component III; (4) separating a component II; (5) dissolving and filtering the component II; (6) ultrafiltering, dialyzing and concentrating; (7) purifying the component II; (8) purifying; and (9) ultrafiltering and dialyzing. The method is advanced, has simple process and can effectively separate the component II from the component I+III or the component III so as to further make the component II into a finished product of human immune globulin, and has very important realistic significance of relieving the short supply situation of domestic source plasma, not only sufficiently utilize raw materials but also prevent environmental pollution; therefore, the method has huge economic and social benefits.
Owner:BANGHE PHARMA CO LTD
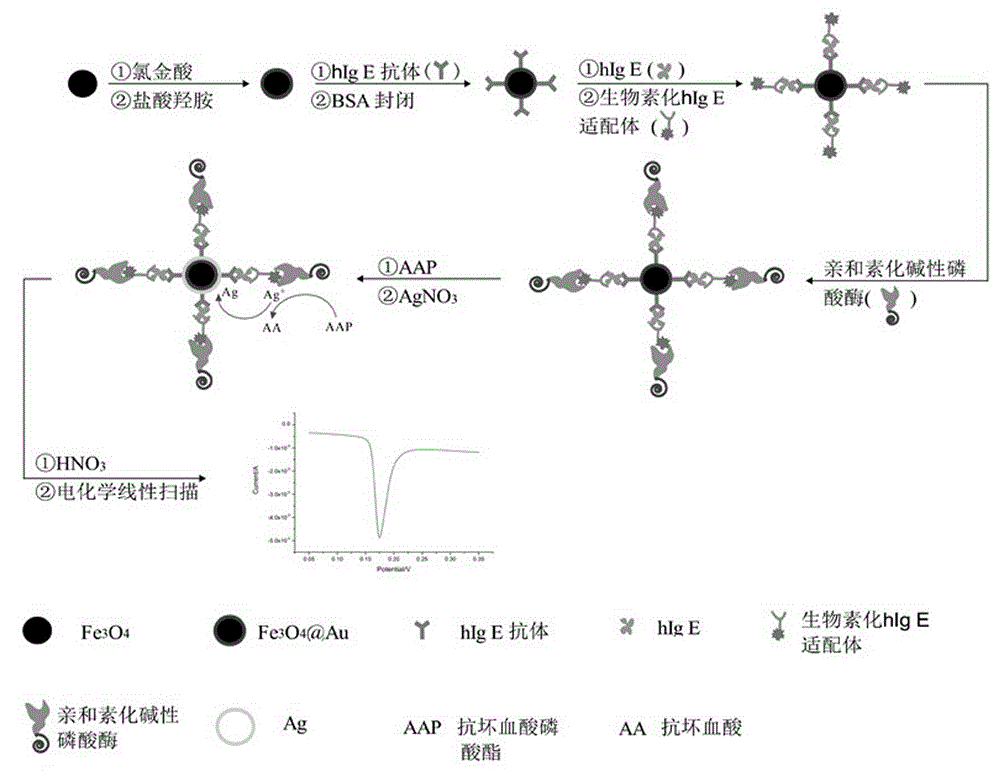
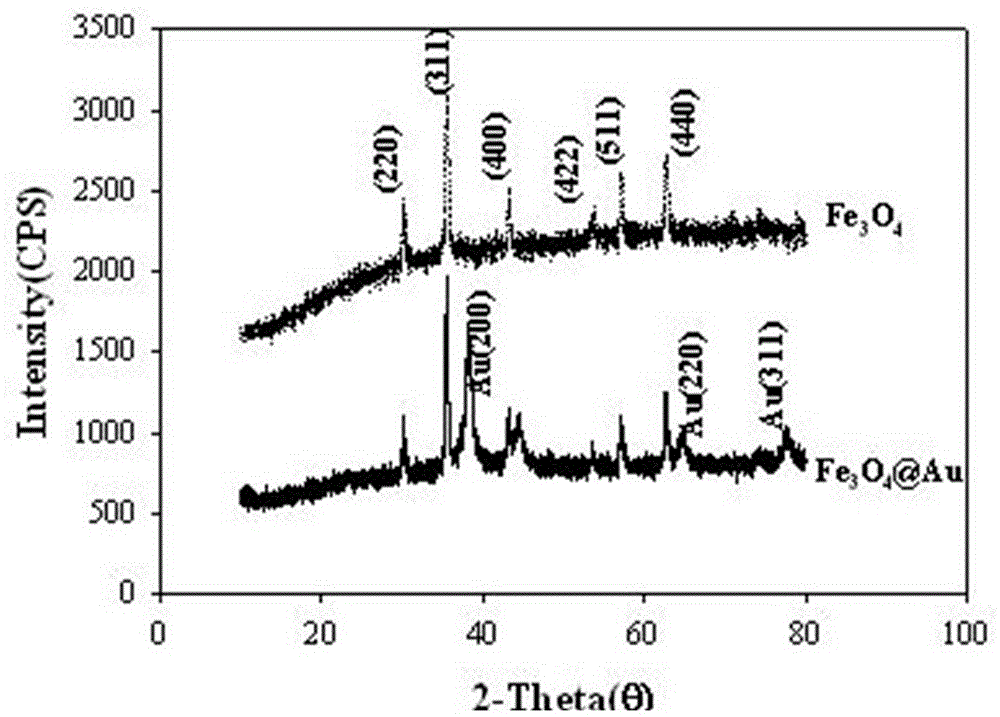
Method for electrochemical detection of human immune globulin E (hIgE)on the basis of magnetic Fe3O4 @ Au nano composite material
ActiveCN104133067ASuperparamagneticEasy to separateBiological testingIntravenous gammaglobulinSuperparamagnetism
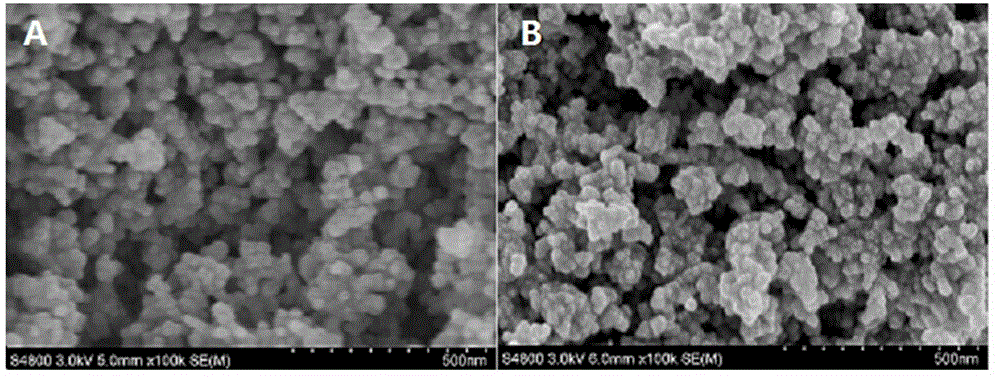
A method for electrochemical detection of human immune globulin E (hIgE) on the basis of magnetic Fe3O4 @ Au nano composite material is as follows: Fe3O4 @ Au nano composite material is prepared by one-step reduction method, Fe3O4 @ Au nano composite material is labeled with hIgE antibody; the Fe3O4 @ Au nano composite material is mixed with the hIgE and a biotinylated hIgE aptamer to form a hIgE antibody-hIgE-hIgE aptamer complex; avidin alkaline phosphatase is adsorbed to the hIgE antibody-hIgE-hIgE aptamer complex, and silver ions are catalytically reduced on the surface of the magnetic Fe3O4 @ Au composite nano material into elemental silver and deposited onto the surface of the nano composite material by biological catalysis deposition reaction of the alkaline phosphatase. By detection of dissolving-out voltammetric current value of the elemental silver, the detection of the hIgE can be achieved. The magnetic Fe3O4 @ Au nano composite material has the particle size of 35-45nm, and has the advantages of uniform particle size distribution and superparamagnetism.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Preparation method of transgenic animal capable of expressing human antibody
ActiveCN103571872AVector-based foreign material introductionAnimal husbandryGenomic DNAGene conversion
The invention relates to a preparation method of a mouse capable of producing a human antibody. The preparation method of the mouse capable of producing the human antibody comprises the step of carrying out hybridization on a transgenic mouse carrying a fragment containing a human antibody heavy chain gene locus part and a mouse carrying a fragment which is inserted into mouse genomic DNA (deoxyribonucleic acid) and contains an non-rearranged human antibody light chain gene locus part, wherein gene rearrangement and gene conversion can be carried out in the transgenic mouse by virtue of the human antibody heavy chain gene locus and the human antibody light chain gene locus, so as to produce various human immune globulins, and a mouse endogenous antibody heavy chain gene locus, a mouse endogenous antibody kappa light chain gene locus and a Lamda light chain gene locus are inactivated.
Owner:SHANDONG BIOANTY BIOLOGICAL TECH CO LTD
Liquid chip kit for diagnosing lung cancer
InactiveCN103389375AImplement diagnosticsNo cross-reactivityMaterial analysisBiotin-streptavidin complexMicrosphere
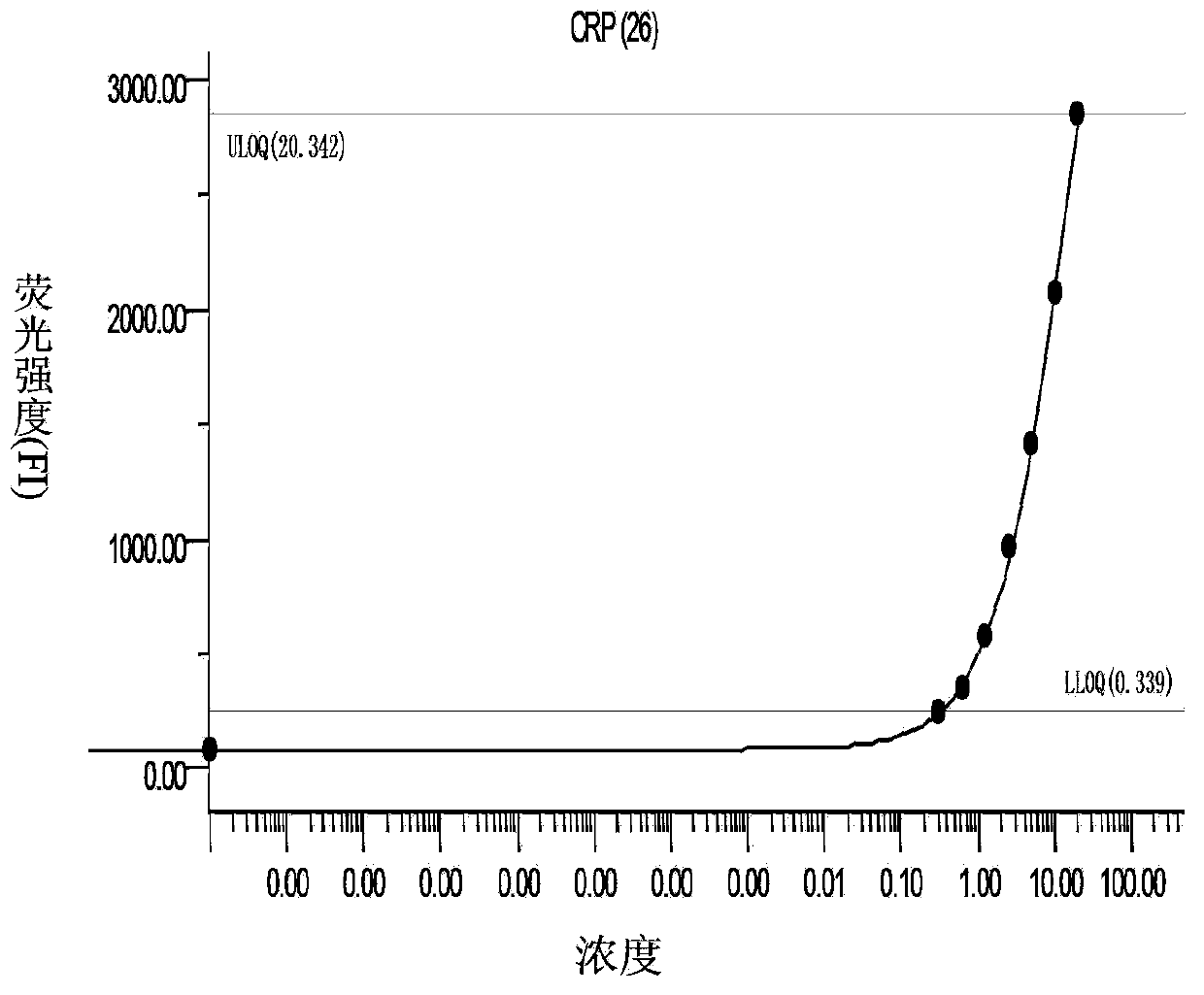
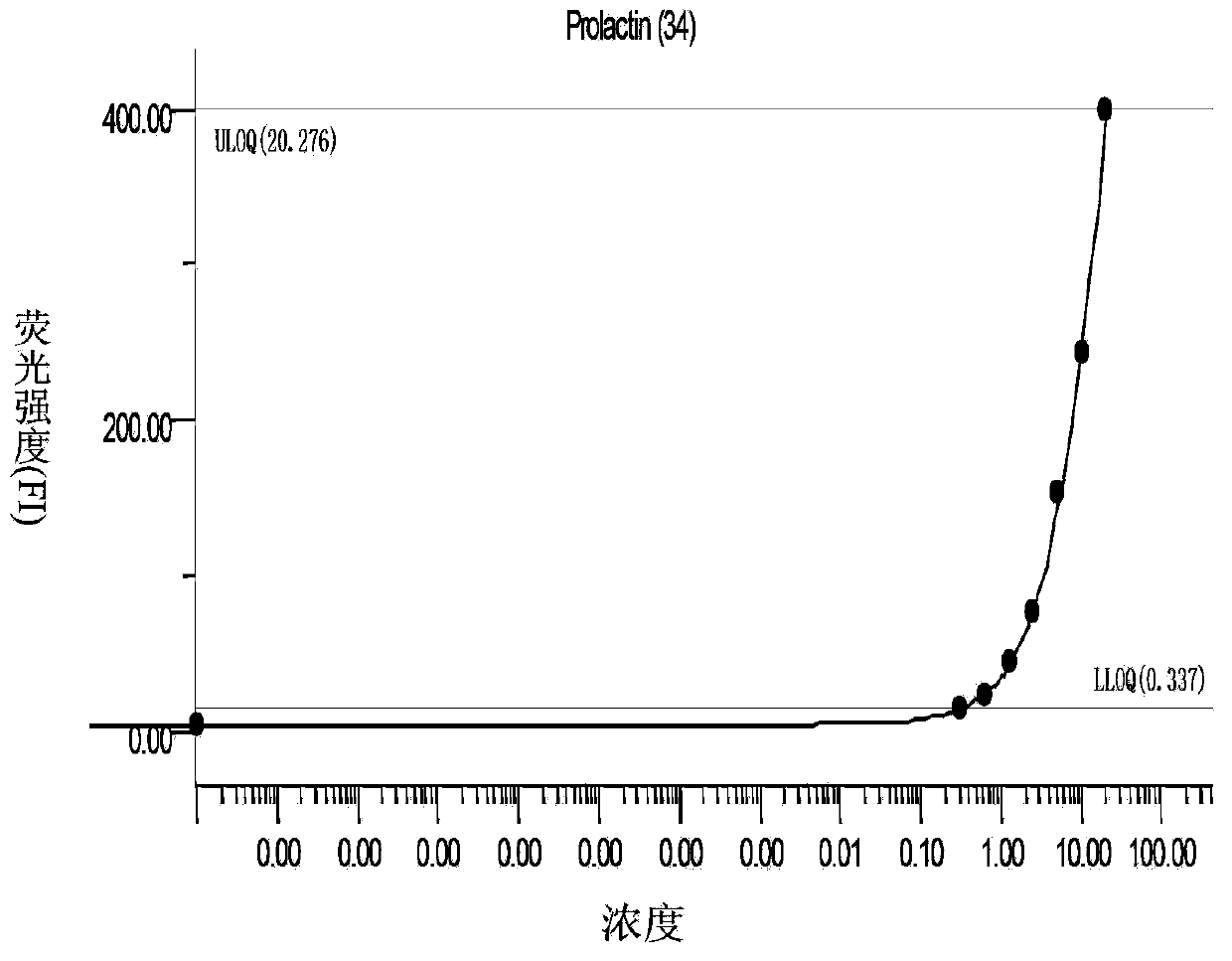
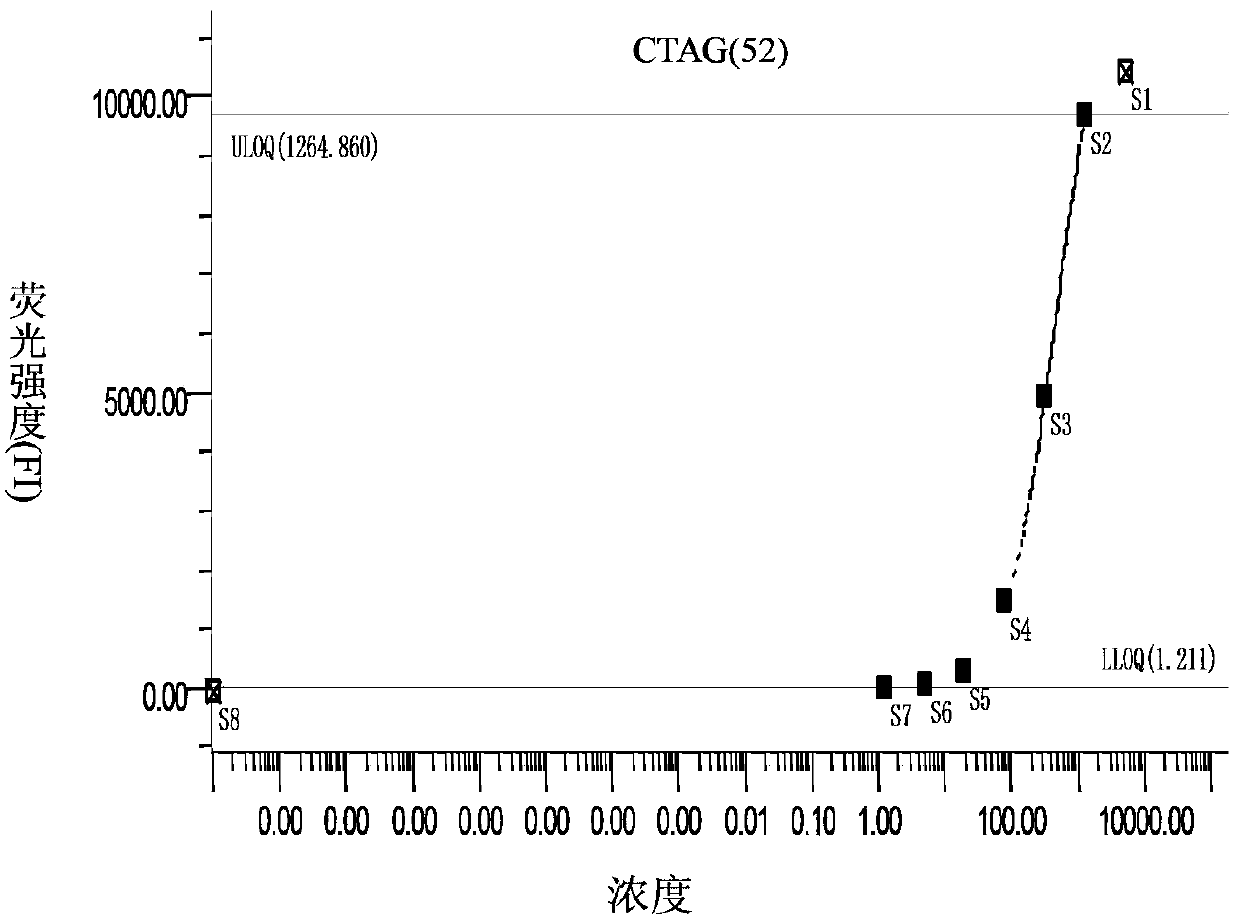
The invention discloses a liquid chip kit for diagnosing lung cancer. The kit comprises microballs coated with capturing antibodies, detecting antibodies labeled by biotin, microballs coupled with Halotag Amine (O2) ligand, antigen proteins, streptavidin-phycoerythrin and anti-human immune globulin G labeled by biotin; wherein the capturing antibodies contain at least one component, which is selected from the following capturing antibodies: CRP, Prolactin, CEA, NSE, CK19, Axl and ADAM8; the detecting antibodies contain at least one component, which is selected from the following detecting antibodies: CRP, Prolactin, CEA, NSE, CK19, Axl and ADAM8, and are corresponding to the capturing antibodies; the antigen proteins contain at least one component, which is selected from following components: p62, CTAG, p53 and CAGE. Microballs with different antibodies or antigen proteins have different color codes. The liquid chip kit carries out detections of antigen and autoantibody at the same time, and the precision rate is high.
Owner:HENGDIAN GRP JIAYUAN CHEM +2
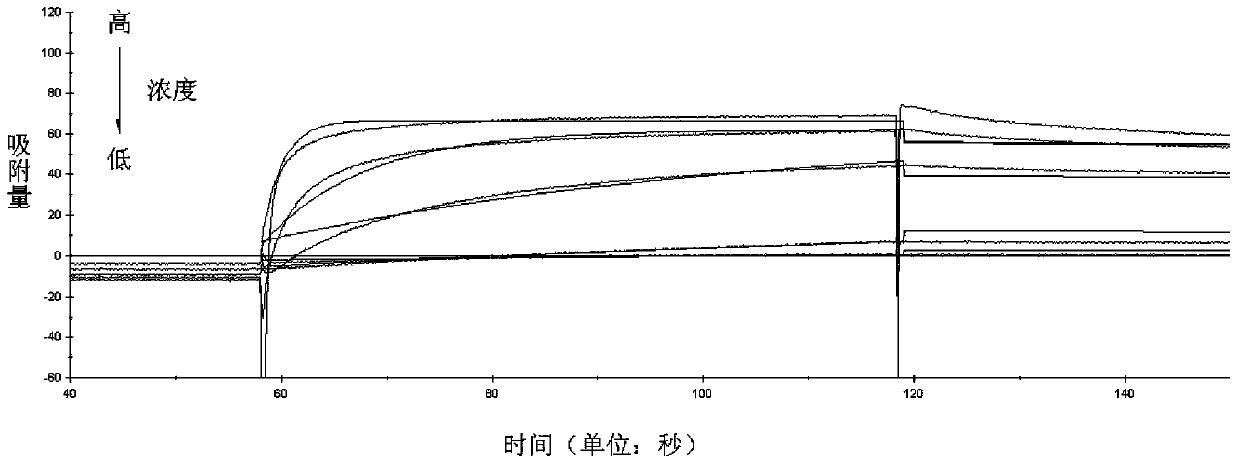
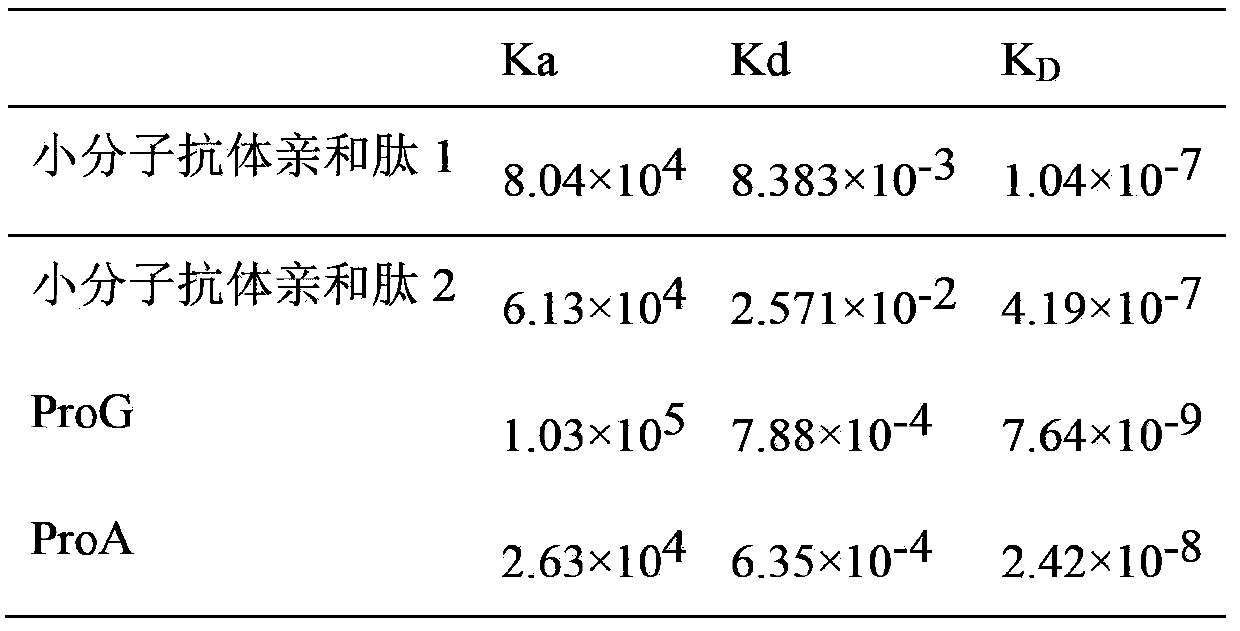
Micromolecule antibody affinity peptide and applications thereof
InactiveCN104211769ASmall molecular weightNon-immunogenicHaemofiltrationPeptide preparation methodsAntibody AffinitiesHuman immune globulin
The invention discloses a micromolecule antibody affinity peptide and applications thereof. The amino acid sequence of the peptide is represented by any one of SEQ ID No. 1-112, and the peptide can combine with the Fc section of human immune globulin IgG, and does not combine with human serum albumin HAS. The provided micromolecule antibody affinity peptide can be taken as the affinity ligand to achieve the high efficient, safe, and economic IgG separation and purification.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
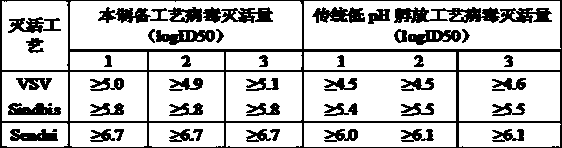
Double virus inactivating/removing method for venous injection human immune globulin
InactiveCN1457884AEfficient removalNo loss of biological activityImmunoglobulins against animals/humansPeptide preparation methodsHuman immune globulinCOMPONENT II
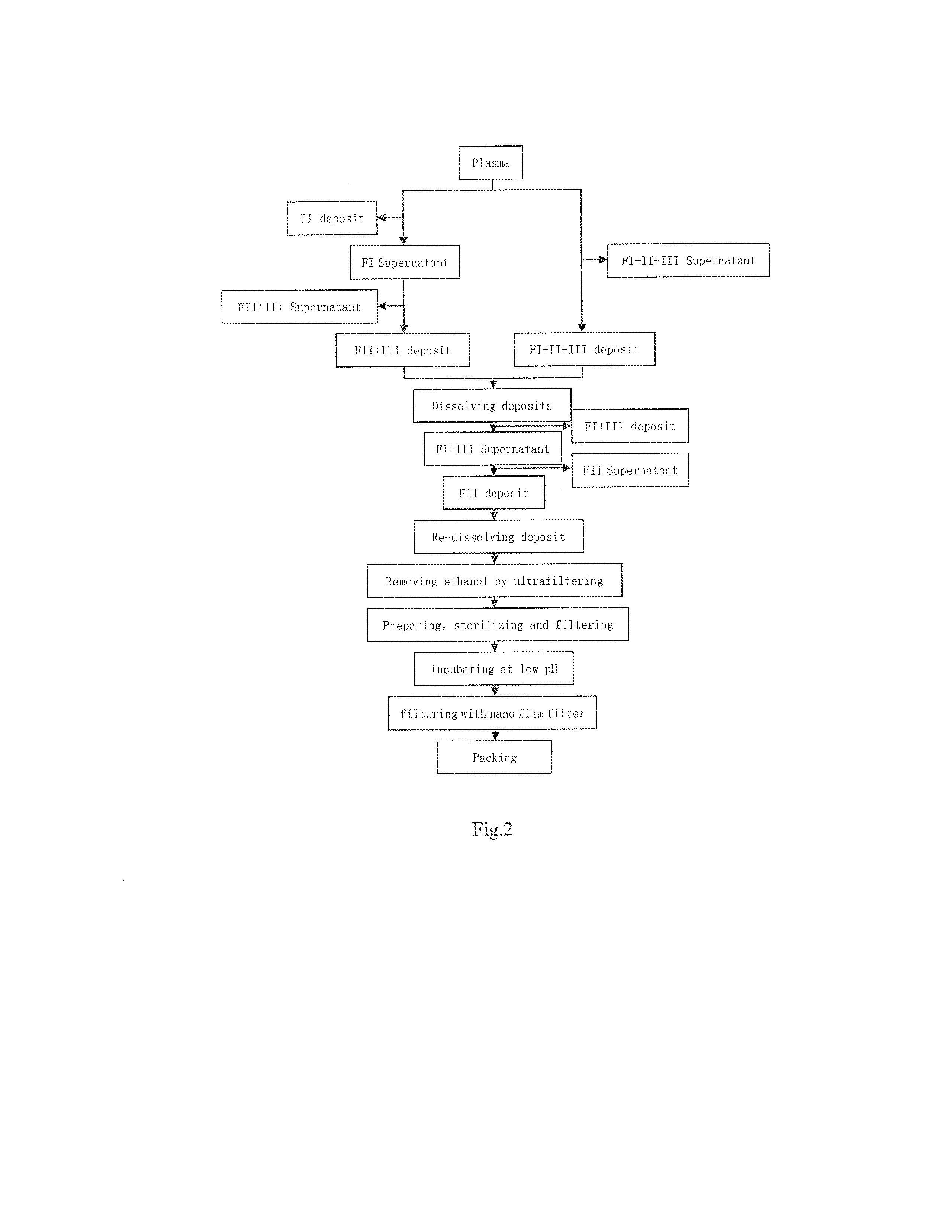
The present invention relates to the preparation process of human plasma protein, and is especially the double virus inactivating / removing process for preparing venous injected human immune globulin. The preparation process of human plasma immune globulin includes low temperature alcohol separation to obtain precipitated component II, low pH treatment to inactivate virus, and nanometer membrane filtering to eliminate virus. The present invention has no loss of IgG bioactivity while eliminating virus, so that the venous injected human immune globulin is even safe for clinical application.
Owner:CHENGDU RONGSHENG PHARMA
Immune globulin G4 subtype IgG4 detection kit with consideration to both specificity and sensitivity
ActiveCN106771251ASimple and fast operationEasy to industrializeBiological testingMicrosphereMonoclonal antibody
The invention belongs to the technical field of biological detection and particularly relates to an immune globulin G4 subtype IgG4 detection kit with consideration to both specificity and sensitivity. In order to solve technical problems of poor specificity and low sensitivity in the prior art, the invention provides the immune globulin G4 subtype IgG4 detection kit with consideration to both specificity and sensitivity. The immune globulin G4 subtype IgG4 detection kit with consideration to both specificity and sensitivity comprises a first reagent; and the first reagent is a latex microsphere solution labeled with glycosylated mouse anti-human immune globulin G4 monoclonal antibody. As the detection kit adopts the glycosylated mouse anti-human immune globulin G4 monoclonal antibody, the immune globulin G4 subtype IgG4 detection kit with consideration to both specificity and sensitivity, provided by the invention, is simple and convenient to operate, can treat antibodies on a mass scale and is beneficial for industrialization; a problem that no commercial HBR is available is solved; therefore, compared with Siemens reagent, the reagent has the advantage that the false positive rate of the reagent is greatly reduced during detecting HAMA and RF disturbance samples; a problem of low sensitivity caused by a method for cutting off an FC segment to eliminate RF is solved; and the linear range of the immune globulin G4 subtype IgG4 detection kit with consideration to both specificity and sensitivity can reach 0.040-4.000 g / L.
Owner:BYRON DIAGNOSTICS SHANGHAI
Type-B encephalitis virus antibody titer standard serum and preparation method thereof
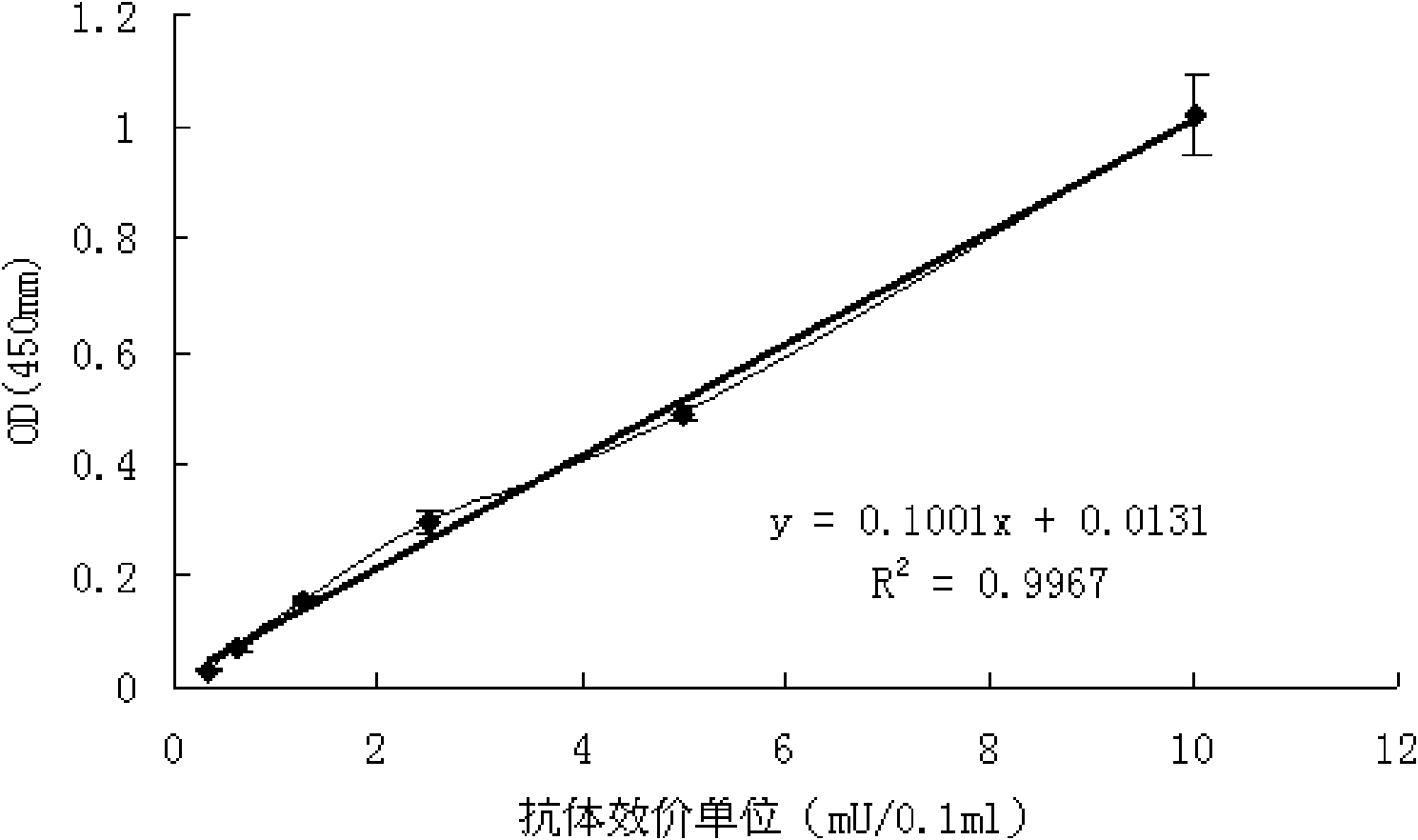
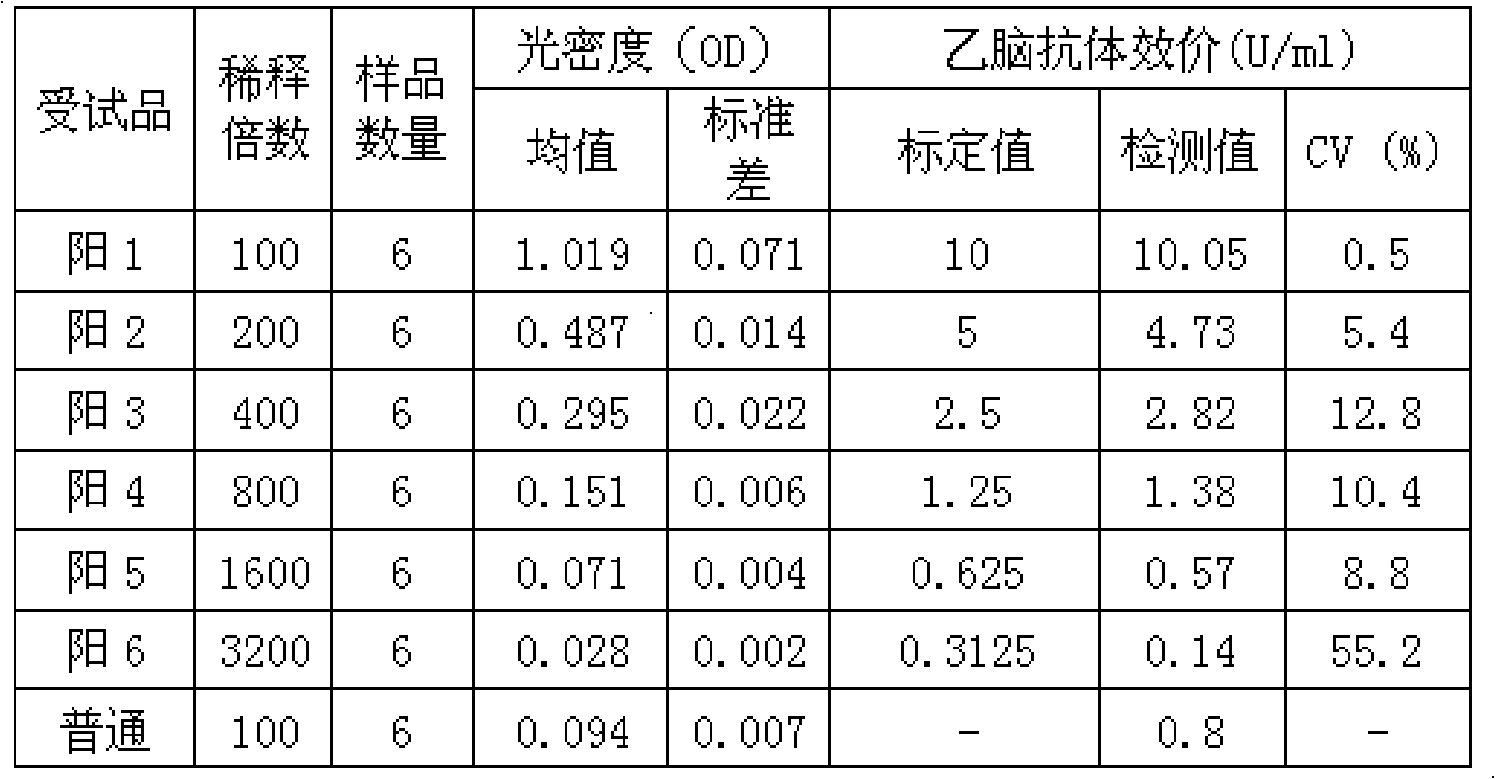
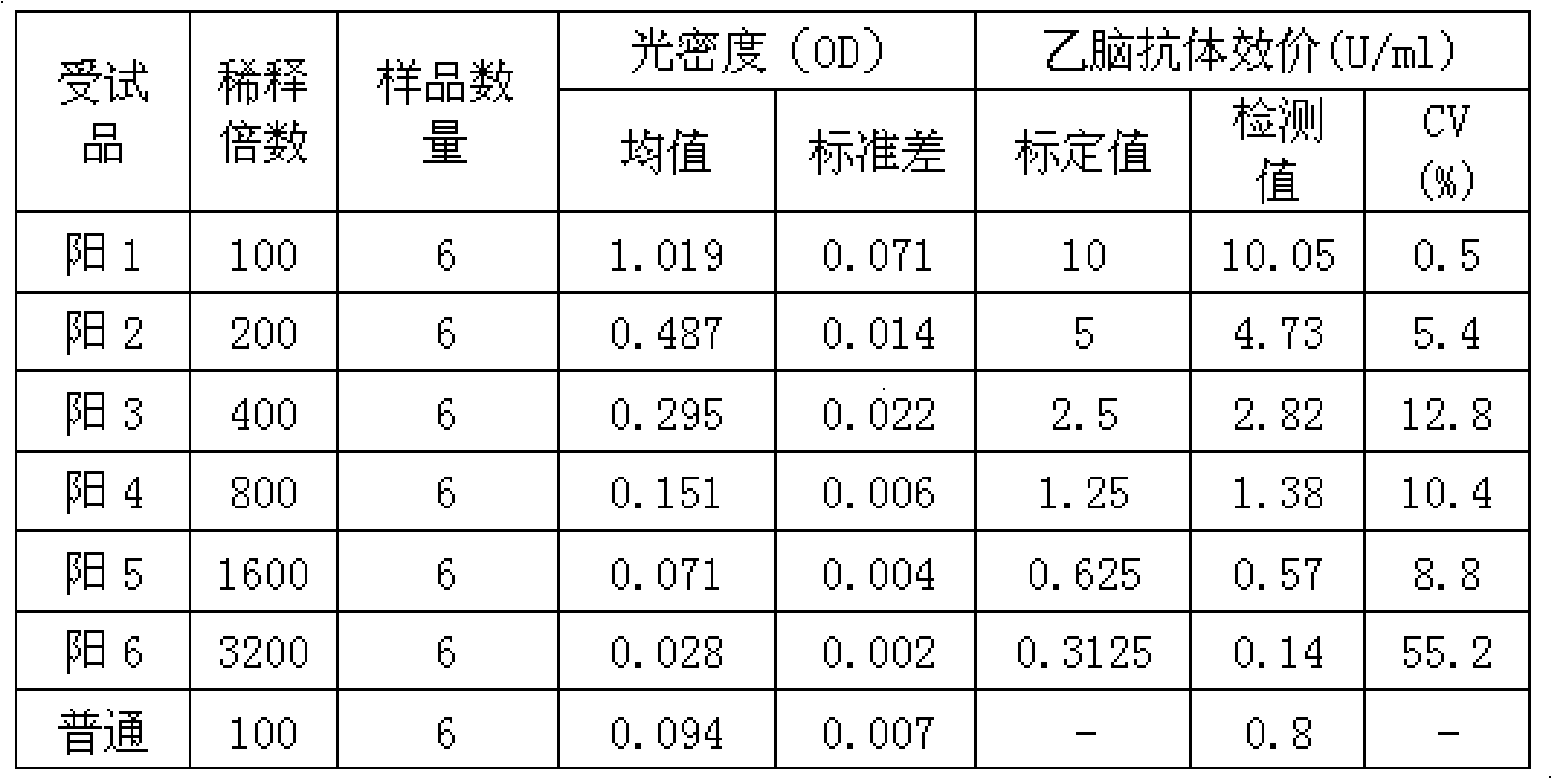
ActiveCN101839914AQuantitative Judgment PreventionQuantitative judgment of therapeuticMaterial analysisHuman immune globulinTreatment effect
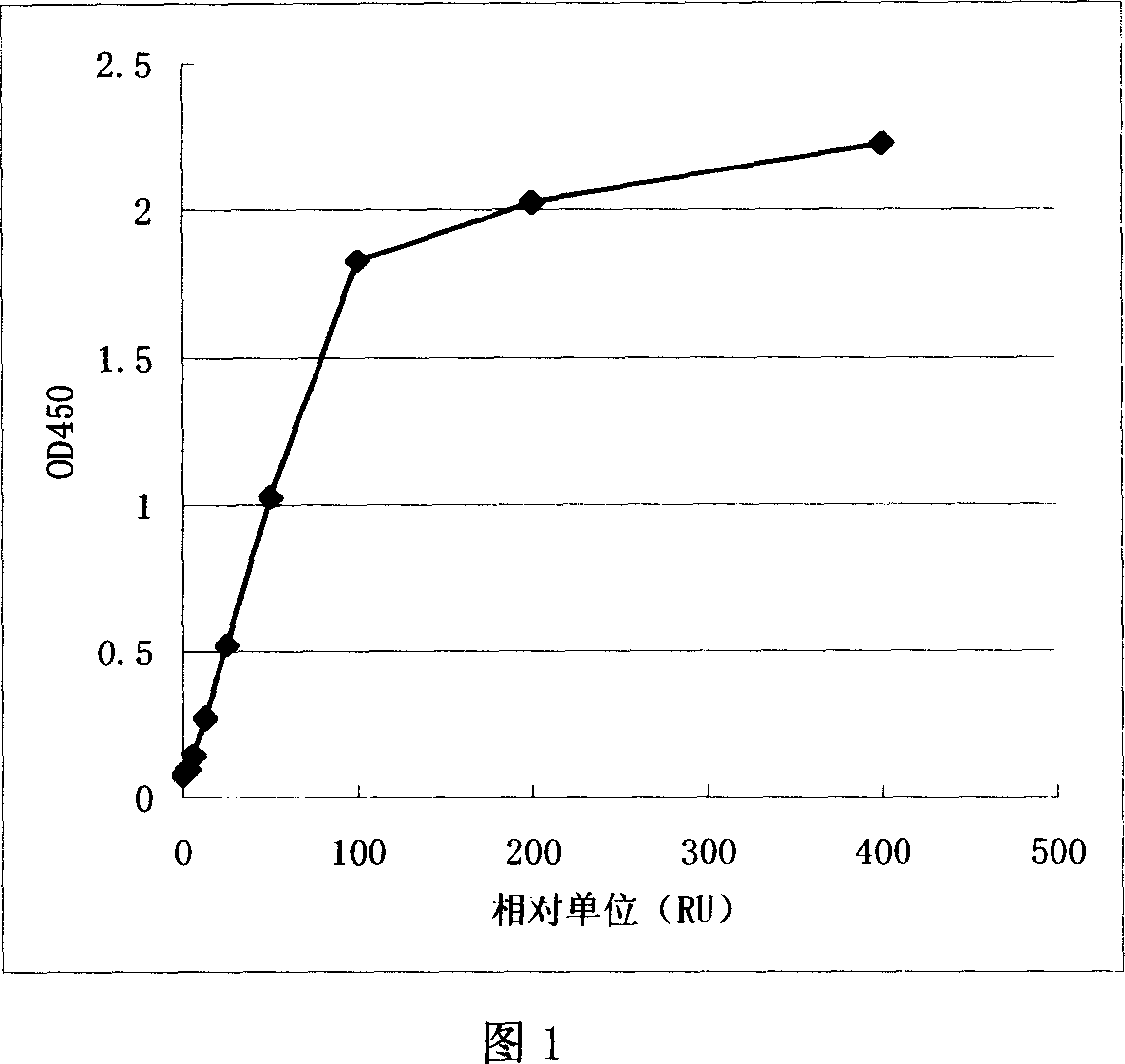
The invention relates to a type-B encephalitis virus antibody titer standard serum and a preparation method thereof, which belong to the field of standard substances of biological products. The invention solves the technical problem of providing the type-B encephalitis virus antibody titer standard serum which serves as a reference standard for measuring a type-B encephalitis human immune globulin titer value. The type-B encephalitis virus antibody titer standard serum is characterized in that the OD450nm value of the type-B encephalitis virus antibody titer standard serum is 1.019+ / -0.051 by detecting the serum with a type-B encephalitis virus IgG antibody diagnostic reagent kit. The type-B encephalitis virus antibody titer standard serum provides a reference for pharmaceutical research on type-B encephalitis human immune globulin, can be used for quantitatively judging the preventing and treating effect of the type-B encephalitis human immune globulin, and has a wide application prospect.
Owner:SICHUAN YUANDASHUYANG PHARM CO LTD
Method for detection of biological activity of Fc region of human immune globulin through ultraviolet spectrophotometry
InactiveCN102854160AShorten detection timeImprove throughputColor/spectral properties measurementsAntigenWhole blood product
The invention discloses a method for detection of biological activity of the Fc region of human immune globulin through ultraviolet spectrophotometry. According to the method, an adsorbed diphtheria-acellular pertussis-tetanus vaccine is used to substitute high-cost hardly-available labelled antigen for sensitization erythrocytes; an Ultrospec 6300pro ultraviolet / visible light spectrophotometer produced by GE Company is used for detection of a sample, and software of the spectrophotometer carries out data processing. Experimental results of the invention prove that standard deviation is in a range of 0.06 to 15.92% and variance is in a range of 0.00 to 1.69%. Results of comparison between the results of an ultraviolet spectrophotometer and the mean value of three detection results of the method provided by the invention are as follows: average data deviation is -1.37%, standard deviation of data difference values is -1.28%, and variance of data difference values is 0.02%. The detection results of the invention is in a reasonable theoretical scope, so the method provided by the invention is indeed a cheap method for micro-scale detection of biological activity of the Fc region of human immune globulin in a blood product.
Owner:GUIZHOU TAIBANG BIOLOGICAL PROD +1
Medicine for treating chicken pox and preparation method thereof
InactiveCN101361973AEffective treatmentImprove securityAntiviralsAntibody ingredientsWhole blood productChicken Pox
The invention relates to a drug for treating chicken pox and a preparation method thereof, pertaining to the field of blood products. The technical problem solved by the invention is that: a drug which can effectively treat chicken pox is provided. The drug for treating chicken pox is a preparation which is prepared by adopting a chicken pox human immune globulin as a main active ingredient and adding with accessories acceptable in pharmacy, wherein, the chicken pox human immune globulin is prepared by adopting blood plasma obtained after a varicella vaccine is used for immunizing human bodies as raw material. The drug for treating chicken pox can be used for treating or preventing cytomegalovirus diseases and has wide application prospect.
Owner:SICHUAN YUANDASHUYANG PHARM CO LTD +1
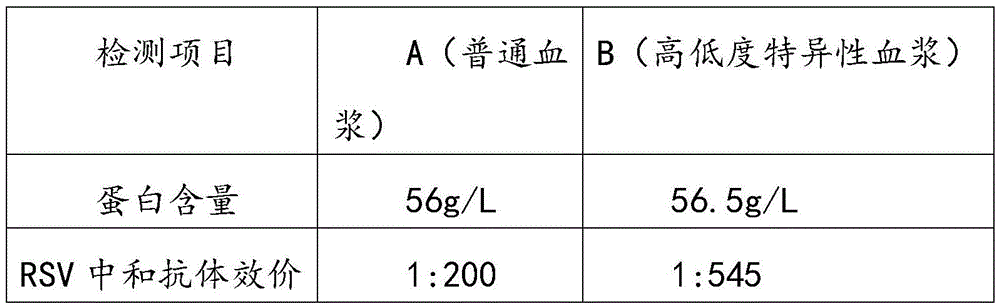
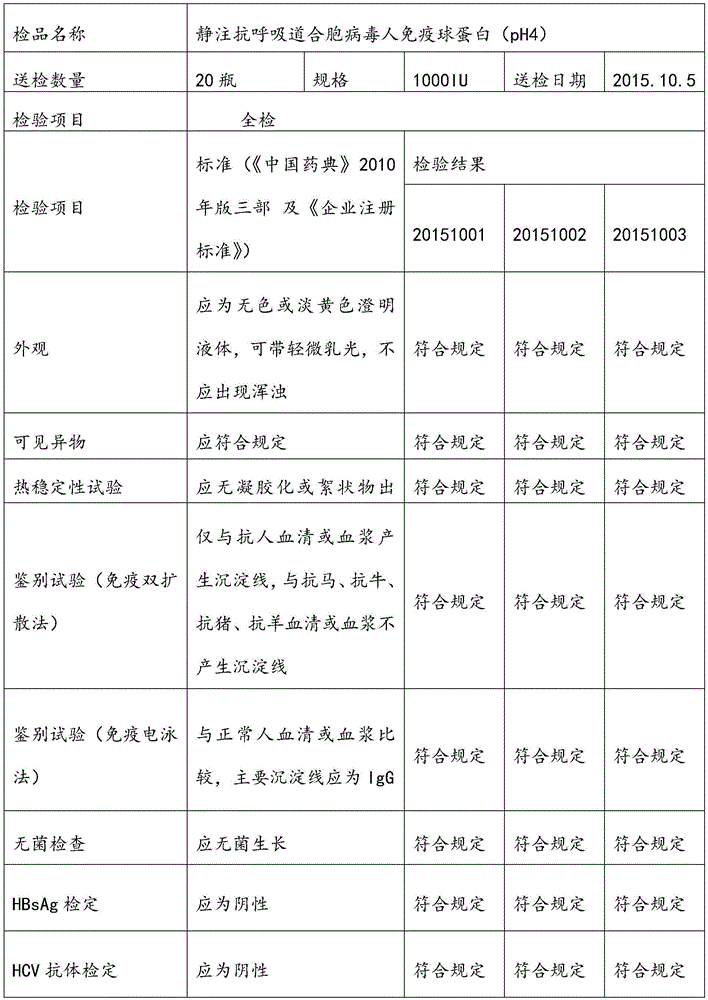
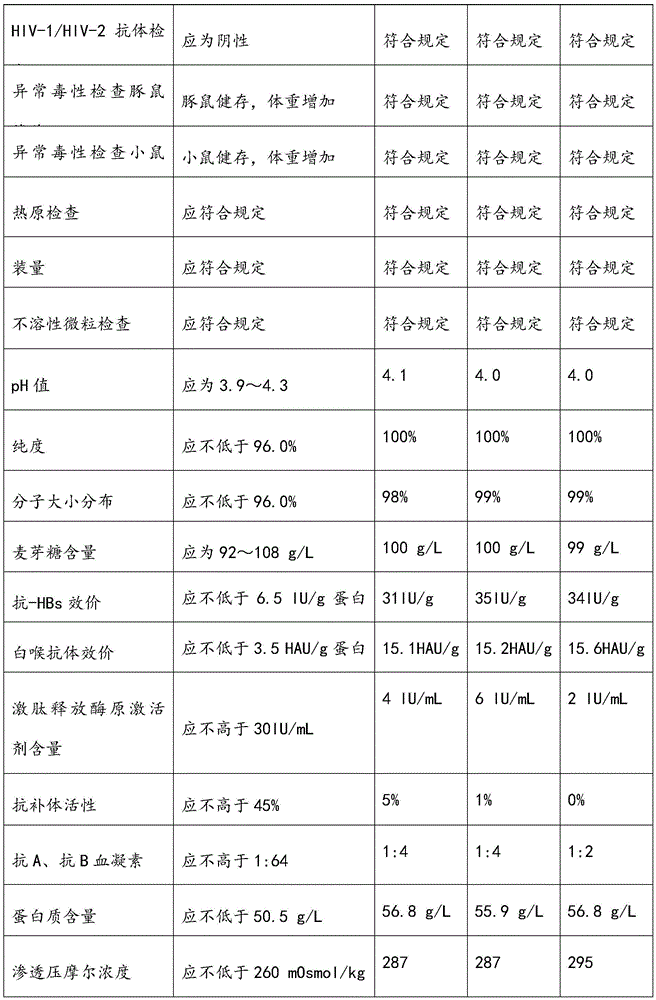
Respiratory syncytial virus resistance human immune globulin and preparation method thereof
ActiveCN105601736AStrong specificityExtended shelf lifeImmunoglobulins against virusesPeptide preparation methodsUltrafiltrationIon exchange
The invention relates to respiratory syncytial virus resistance human immune globulin and a preparation method thereof. The titer of the respiratory syncytial virus neutralizing antibody is larger than or equal to 1:900. The preparation method includes the steps of firstly, screening out privilege plasma with titer of the respiratory syncytial virus neutralizing antibody not lower than 1:200; secondly, mixing screened-out efficient privilege plasma; thirdly, separating the mixed plasma through a low-temperature ethyl alcohol filter press method, purifying and separating out a immune globulin component II through the combination with the ion-exchange column chromatography method, and obtaining immune globulin with the purity of 98-100% through filtration, chromatography, ultrafiltration, preparation, low-pH incubation virus inactivation, nano-membrane filtration virus removal and subpackaging. The titer, purity and recycling rate of the respiratory syncytial virus resistance human immune globulin obtained through the preparation and production method are high, treatment can be conducted for the respiratory syncytial virus, and the respiratory syncytial virus resistance human immune globulin and the preparation method have advantages of being safe, effective and the like.
Owner:哈尔滨派斯菲科生物制药有限公司
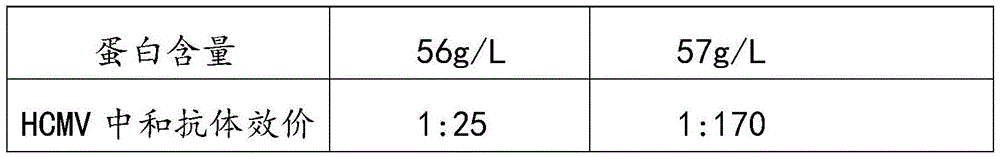
Intravenously injected cytomegalovirus human immune globulin and preparation method thereof
ActiveCN105601735AEffectively treat severe infectionsSuitable for industrial productionImmunoglobulins against virusesPeptide preparation methodsFiltrationUltrafiltration
The invention relates to intravenously injected cytomegalovirus human immune globulin and a preparation method thereof, and the titer of a cytomegalovirus neutralizing antibody of the human immune globulin is larger than or equal to 1:500. The preparation method comprises the steps that 1, positive plasma with the titer of the cytomegalovirus neutralizing antibody larger than or equal to 1:20 is screened out; 2, the screened-out efficient positive plasma is mixed; 3, the mixed plasma is separated through a low-temperature ethanol filter-pressing method, an immune globulin component II is separated and purified by combining an ion-exchange column chromatography method, viruses are removed through filtration, chromatography, ultrafiltration, preparation, low-pH incubation virus inactivation and nanofilm filtration, the immune globulin with the purity of 98.5%-100% is obtained through subpackaging, and the antibody titer is not lower than 1:500. The cytomegalovirus human immune globulin prepared through the preparation and production method is high in antibody titer, purity and recovery rate and capable of conducting targeted treatment on cytomegalovirus, is an effectively drug for treating recessive and dominant infection caused by the cytomegalovirus in people, is safe and reliable and has larger social benefits and economic benefits.
Owner:哈尔滨派斯菲科生物制药有限公司
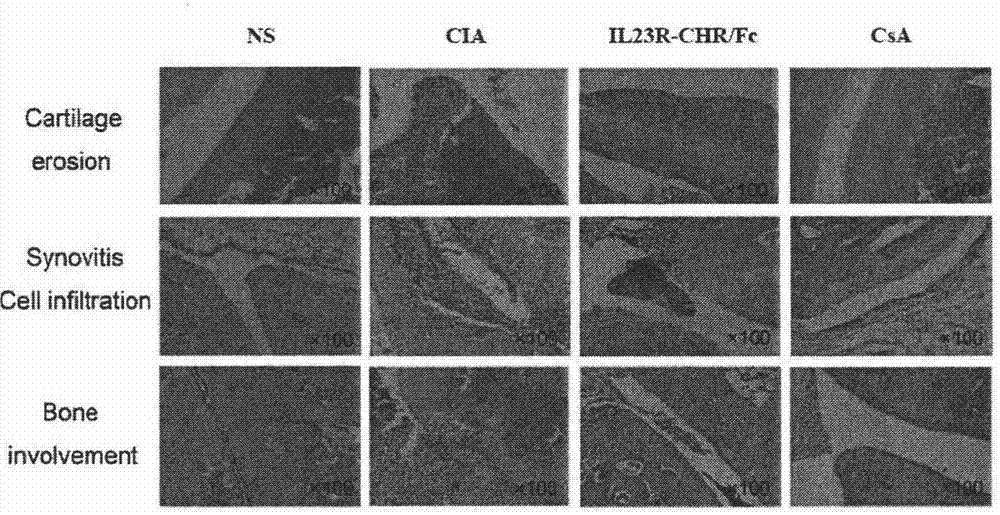
Novel IL23 antagonist
The invention relates to a recombinant human IL (Interleukin)-23 receptor extracellular region / Fc fusion protein capable of combining with IL-23 and antagonizing a function of IL-23. The fusion protein is characterized in that a fusion protein encoding gene is rich in mammalian cell preferred codons, and is highly expressed by a CHO (Chinese Hamster Ovary) stable transfection cell strain. The protein is formed by fusion of a gene optimized IL-23R-CHR active fragment and a human immune globulin molecule Fc fragment; the defects that the half-life period of the prokaryotic expression IL-23R-CHR fragment is short, the stability is poor and endotoxin is difficult to remove are overcome; the biological activity of IL-23-CHR is reserved; in addition, the fusion protein does not have ADCC (Antibody Dependent Cellular Cytotoxicity) and CDC (Complement Dependent Cytotoxicity) effects, so that the fusion protein is more suitable for treatment of chronic diseases such as an autoimmune disease and a chronic infection; and the contents of molecular design, expression, purification, construction of a stable expression cell strain and disease treatment and the like of the fusion protein are included.
Owner:CHINA PHARM UNIV
Immune globulin M detection kit and detection method
ActiveCN106771148AImprove accuracyStrong specificityMaterial analysisPolyethylene glycolMicroparticle
The invention belongs to the technical field of biology, and particularly relates to an immune globulin M detection kit and detection method. The immune globulin M detection kit comprises a reagent R1, a reagent R2 and an immune globulin M good merchantable quality, the reagent R1 comprises 20-300 mmol / L first buffering solution, 5-50 mmol / L of accelerant, 0.5-10 g / L of polyethylene glycol-400 and 5-50 g / L of sodium chloride; the reagent R2 comprises 20-300 mmol / L of a second buffering solution, 10-50 %w / v of magnetic particles wrapped by anti-human immune globulin M antibodies, 5-50 g / L of sodium chloride and 0.5-10 g / L of stabilizer. Compared with an existing immune globulin M kit, the immune globulin M detection kit has higher specificity, accuracy, precision and stability, and is suitable for large-scale application and popularization.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Intravenous Cytomegalovirus Human Immune Globulin and Manufacturing Method Thereof
InactiveUS20130172536A1Increase probabilityEffective maintenancePackage sterilisationImmunoglobulins against virusesMedicineEthanol precipitation
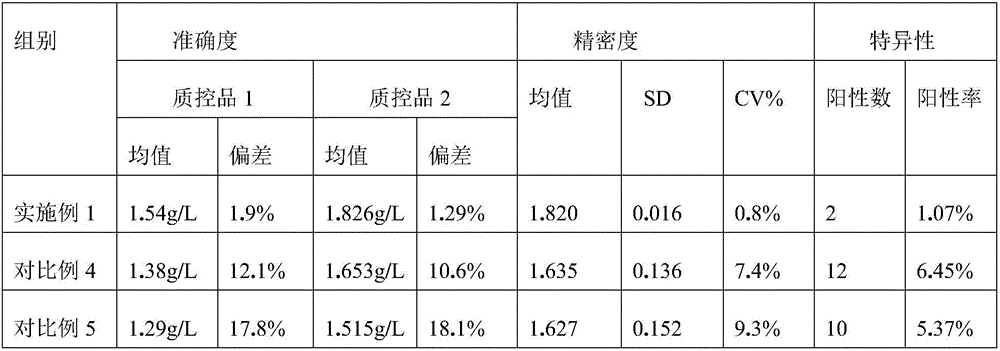
The present invention discloses an intravenous cytomegalovirus human immune globulin and a manufacturing method thereof, wherein the technical problem to be solved is to improve the purity, yield, and safety of the product. The intravenous cytomegalovirus human immune globulin of the present invention has a specific activity of no less than 2.5 PEI-U / mg, an anti-CMV titer of no less than 100 PEI-U / ml, a purity of greater than 98.2%, and a protein content of 51˜55 mg / ml. The present invention employs caprylic acid precipitation and anion exchange chromatography for replacing the step of ethanol precipitation in the conventional cold ethanol method to keep IgG in the supernatant and maintain the activity of the IgG; the present invention employing processes of caprylic acid inactivation of virus and nanometer film virus removal can effectively protect the safety of the product, and studies show that the preparing method of the present invention not only improves the purity, yield, and safety of the product; but also saves energy and reduces the cost of production.
Owner:SHENZHEN WEIGUANG BIOLOGICAL PROD
Long-acting HIV fusion inhibitor and application thereof
ActiveCN105646717APromote research and developmentExtended half-lifePeptide/protein ingredientsGenetic material ingredientsHalf-lifeTherapy HIV
Owner:FUDAN UNIV
Preparing method for human immune globulin
InactiveCN104004090AHigh purityHigh yieldSerum immunoglobulinsImmunoglobulins against virusesVirusGlobulin
The invention provides a preparing method for human immune globulin. According to the preparing method, a low-temperature ethanol extraction method and a chromatography are applied crossly to remove impure proteins in a combined mode and purify the immune globulin, the purity of the globulin product can be well improved, and an S / D inactivation method and a DV 50 nanometer film are adopted for removing viruses in a combined mode, so that the product is safer and more effective.
Owner:新疆德源生物工程有限公司
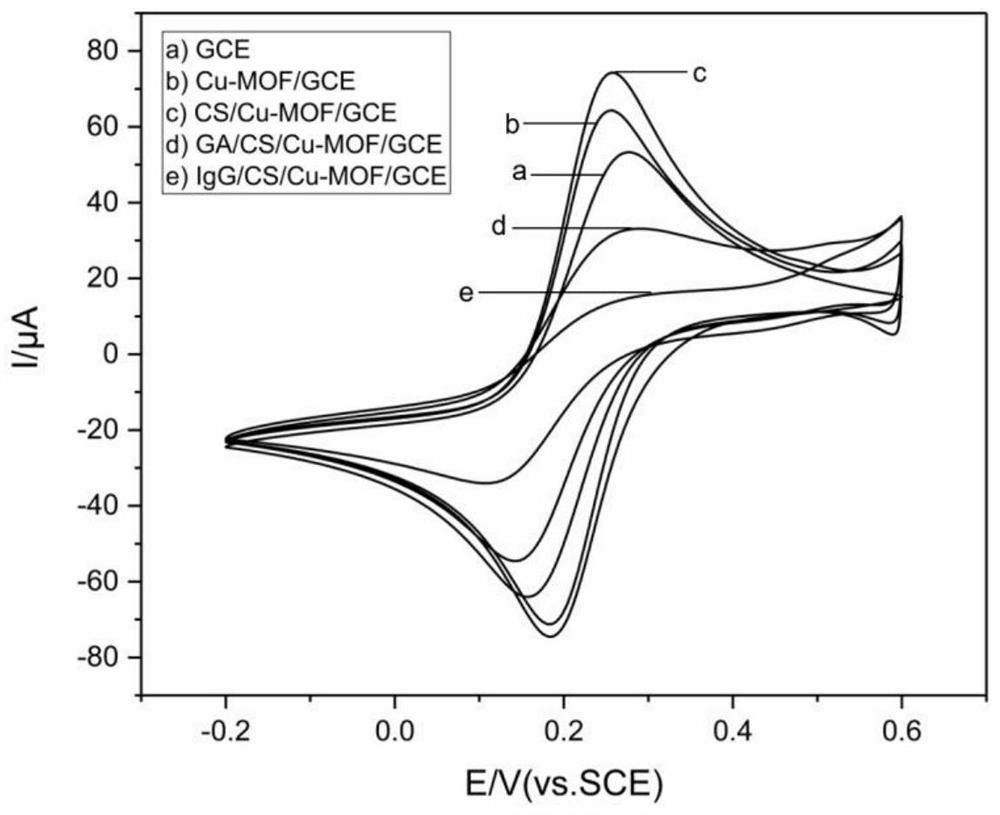
Molecularly imprinted electrochemical sensor of electric copper-based MOFs sensitive membrane modified electrode and preparation method and detection method thereof
ActiveCN112903781AHighly selective adsorptionFast electrical signal responseMaterial electrochemical variablesProtein targetElectrochemistry
The invention discloses a molecularly imprinted electrochemical sensor based on an electric copper-based MOFs sensitive membrane modified electrode and a preparation method and a detection method thereof, and belongs to the field of electrochemical sensing. The specific protein human immune globulin G (IgG) in serum is selected as a research object, and the novel high-sensitivity molecularly imprinted electrochemical sensor is successfully constructed by combining a nano composite material, a new imprinting method, a new template treatment thought and a new detection method. The preparation method comprises the following steps: synthesizing a copper-based MOFs modified electrode on the surface of a glassy carbon electrode by an electro-deposition method to improve the conductivity and increase the specific surface area, sequentially modifying chitosan and glutaraldehyde to provide attachment sites of IgG, directly forming a polymer film on the surface of the modified electrode through pyrrole electro-polymerization, and finally, eluting the template to obtain the molecularly imprinted polymer modified electrode. The molecularly imprinted electrochemical sensor is high in sensitivity and good in selectivity, and is successfully applied to detection of target protein in an actual sample.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Protein applied to culture of NK cells, culture medium component combination and preparation method
ActiveCN109627342AImprove efficiencyImprove stabilityAntibody mimetics/scaffoldsPeptide preparation methodsNatural Killer Cell Inhibitory ReceptorsMicrobiology
The invention provides protein applied to culture of NK cells, a culture medium component and a preparation method, and further relates to application of the protein. The protein and the culture medium component combination are used for culture of the NK cells to avoid exogenous cell line contamination of trophoblast cells and improve the efficiency and stability of NK culture, and the cultured NKcells are high in purity and adaptability. On one hand, the protein is applied to culture of the NK cells, and is MICA-Ig, and the MICA-Ig is a recombined MICA-Ig protein which is formed by fusing human MICA protein and the Fc segment of human immune globulin IgG1 of the surface of a tumor cell.
Owner:NOVOPROTEIN SCI INC
Preparing technology for human immune globulin
ActiveCN104004091AIncrease contentReduce loss of activitySerum immunoglobulinsImmunoglobulins against animals/humansImmunglobulin eAlcohol
The invention discloses a preparing technology for human immune globulin. The preparing technology includes the steps of (1) separating a component I, a component II and a component III from plasma, (2) primary chromatography, (3) primary separation, (4) secondary chromatography, (5) ultrafiltration, (6) preparing a semi-finished product and (7) preparing a finished product. One-step separation and two-step chromatography are adopted for extracting and purifying the globulin, compared with a traditional ethyl alcohol multi-level separation technology, the preparing technology is simple and convenient and rapid to carry out, and meanwhile the prepared human immune globulin is high in yield and purity and good in quality.
Owner:新疆德源生物工程有限公司
Pharmaceutical composition containing fusion protein capable of inhibiting blood vessel hyperplasia and application thereof
ActiveCN103816115AInhibition of Purity DeclinePowder deliverySenses disorderVascular endotheliumDrug biological activity
The invention discloses a pharmaceutical composition containing a fusion protein capable of inhibiting blood vessel hyperplasia and application thereof, specifically to the pharmaceutical composition containing the fusion protein of an extracellular structural domain 2 (Flt-2) of a vascular endothelial growth factor (VEGF) acceptor 1, extracellular structural domains 3 and 4 (KDR-3,4) of a VEGF acceptor 2 and human immune globulin 1 (IG1) Fc. The pharmaceutical composition enables the fusion protein to maintain stable and can effectively inhibit decrease of purity caused by generation of a fusion protein polymer, thereby maintaining biological activity of active components.
Owner:CHENGDU KANGHONG BIOTECH
Production process of human immune globulin for intravenous injection
InactiveCN101089015AMammal material medical ingredientsPeptide preparation methodsVirus inactivationMaltose
The present invention relates to production process of human immune globulin for intravenous injection with raw protein liquor. The raw protein liquor is first diluted and added with certain amount of maltose as the stabilizer, and then virus deactivated and eliminated to obtain two types of human immune globulin product for intravenous injection in the human immune globulin concentration of 5 % and 10 % separately. The production process has easy operation and saving in maltose, and the products has high safety.
Owner:陆荣政
Antibody molecule ATF-Fc fusion albumen of antiurokinase type fibrinolysin activator receptor and use thereof
InactiveCN1919874AHigh affinityBinding can't stopOrganic active ingredientsBacteriaHalf-lifePlasmin activator
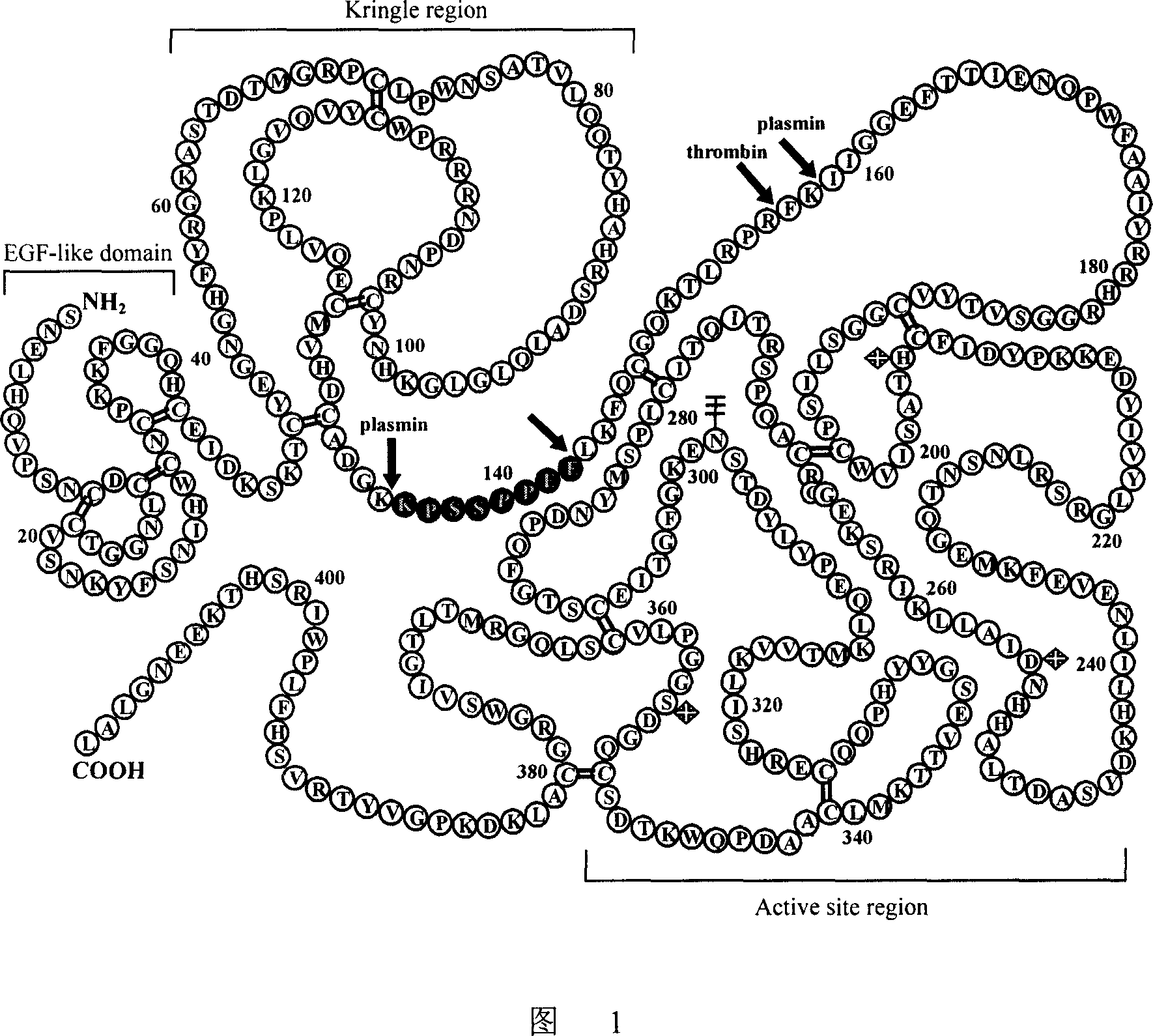
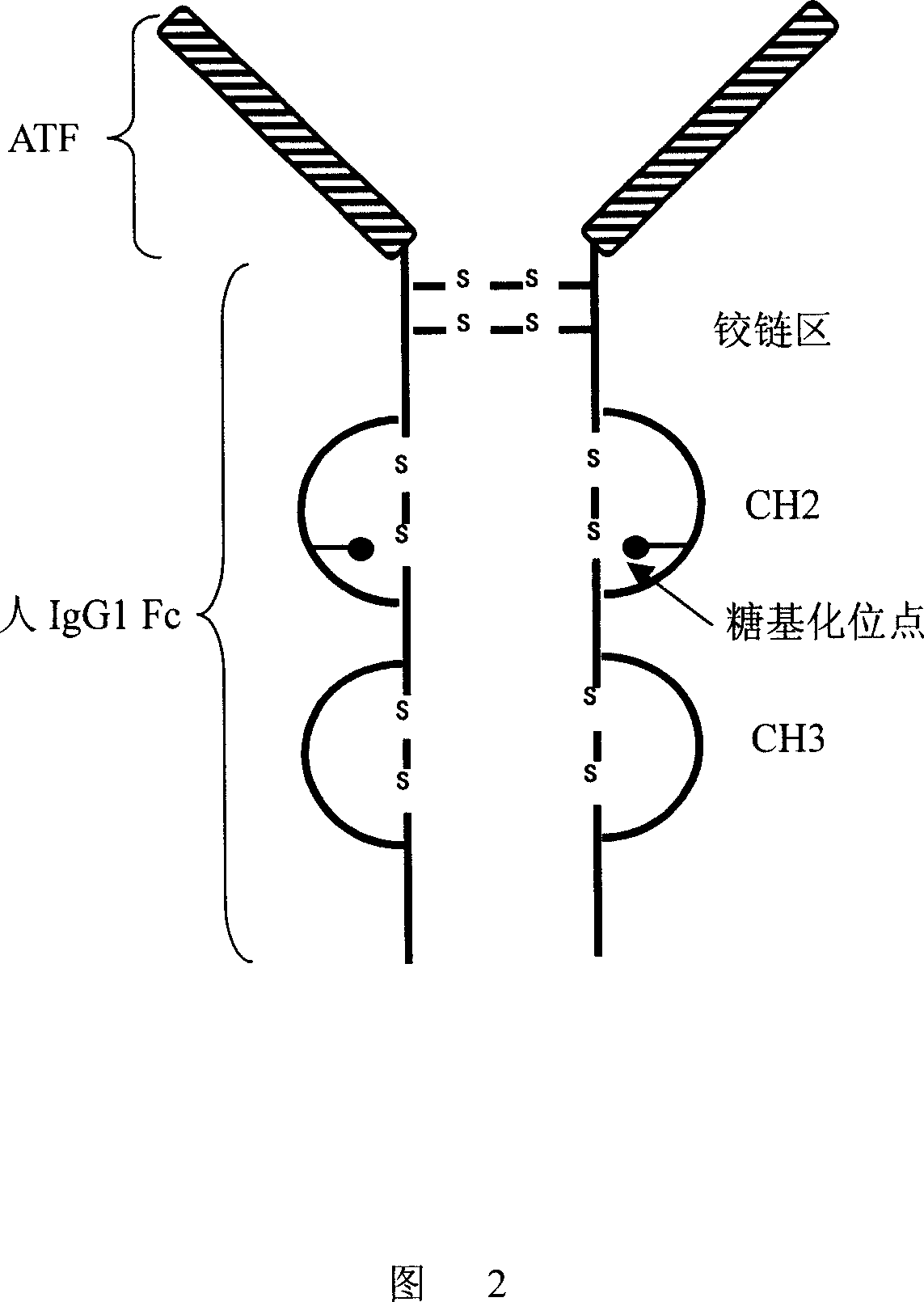
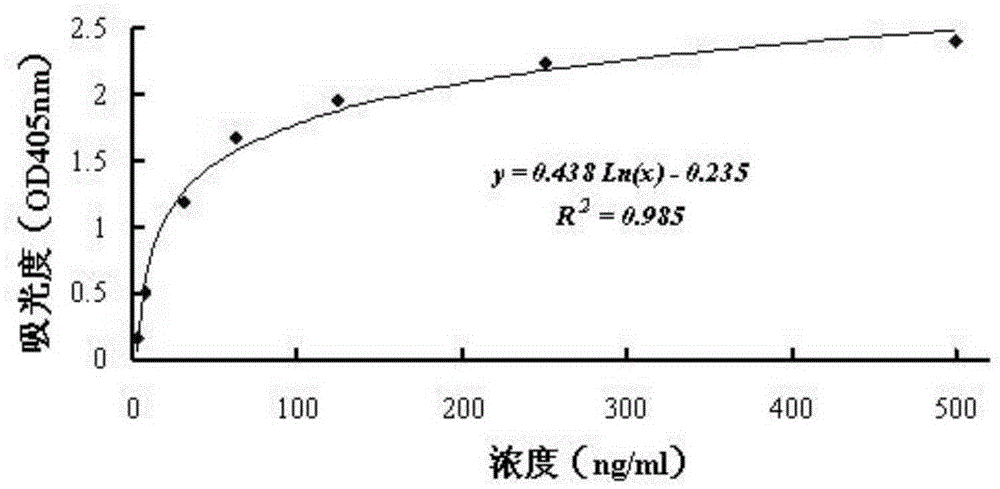
The invention discloses an antibody molecule ATF-Fc merge protein and application of antiurokinase type profibrinolytic activator acceptor in biological medicine area. which is characterized by the following: the ATF-Fc merge protein is ATF area of uPA and Fc of human immune globulin IgG1; heavy chain constants region 2 and heavy chain constants region 3 through Ser-Gly-Ser-Gly-Ser joint form to dimer merge protein; possessing 742 amino acids; single-chain possesses amino acids sequence of sequence table SEQ ID No.1, wherein the front 20 amino acids is uPA signal peptide.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
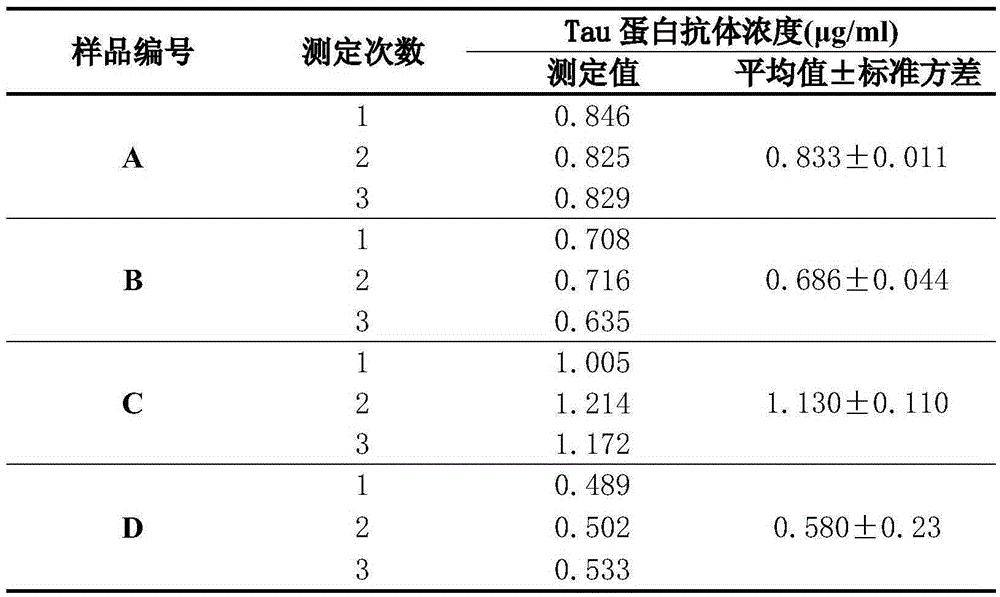
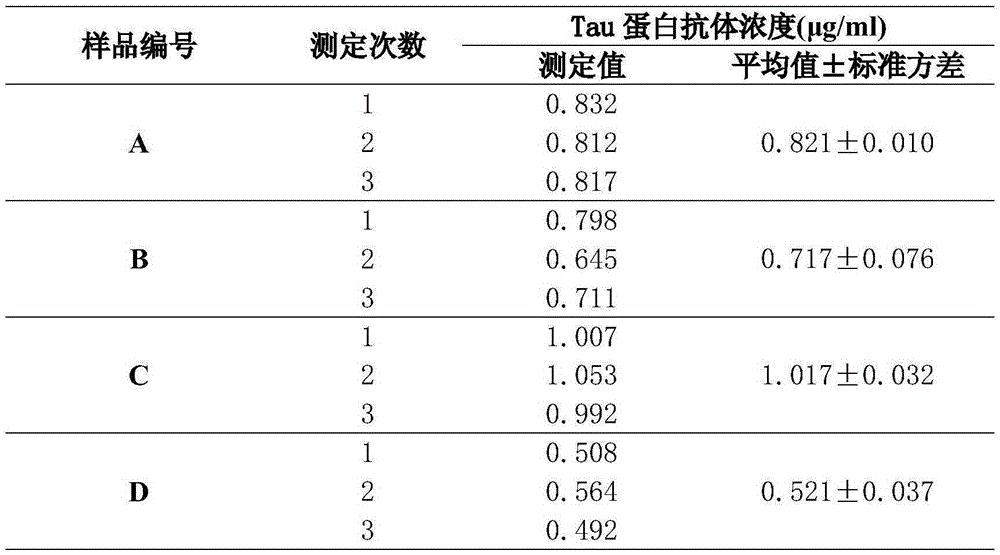
Detection method for content of Tau protein antibodies in human immune globulin product
InactiveCN105388302AAccurate measurementAvoid reactionBiological material analysisBiological testingProtein insertionBovine serum albumin
The invention provides a detection method for the content of Tau protein antibodies in a human immune globulin product, and belongs to the technical field of biomedical detection. The detection method includes the first step of antigen coating, the second step of confining, the third step of primary antibody incubation, the fourth step of secondary antibody incubation, the fifth step of enzyme-labeled tertiary antibody incubation and the sixth step of absorbancy detection and content calculation. According to the detection method, protein-free confining liquid is selected and used in the second step, so that the situation that protein in conventional confining liquid is in nonspecific binding with multivariate antibodies in a sample to be detected and consequently the detection result is influenced is avoided; bovine serum albumin with immune globulin removed is used in the third step and the fourth step for preparing sample diluent, so that influences generated when a cross reaction between residual IgG molecules in bovine serum albumin and a detection system occurs are avoided; detection signals of the Tau protein antibodies in the sample to be detected are amplified through a biotin amplification system in the third step, the fourth step and the fifth step so that the content of the Tau protein antibodies in the human immune globulin product can be detected more accurately through the detection method.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI
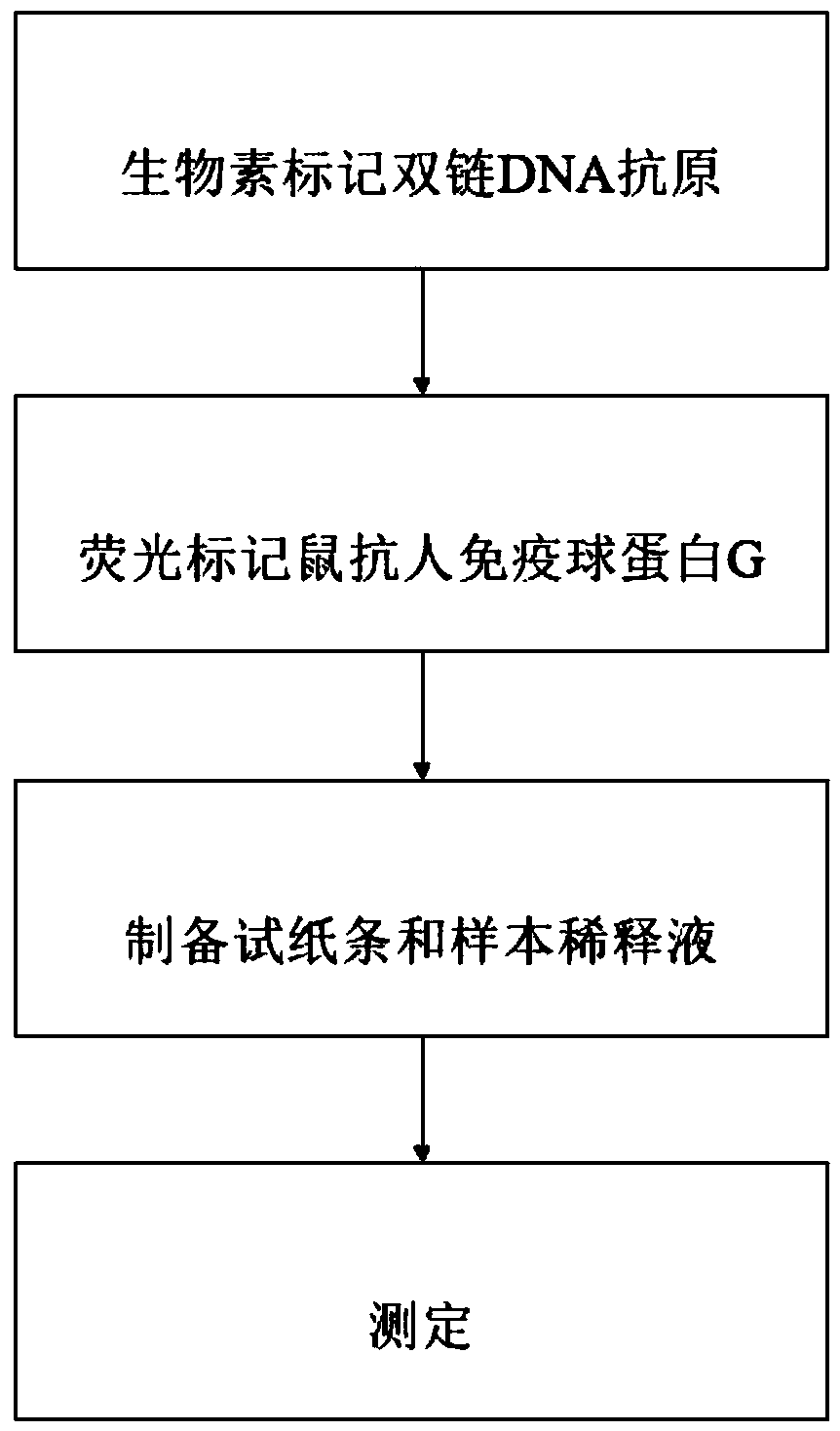
Rapid anti-double-stranded DNA (deoxyribonucleic acid) antibody detection method
The invention discloses a rapid anti-double-stranded DNA (deoxyribonucleic acid) antibody detection method. A DNA extraction method includes the steps: biotin labeling of double-stranded DNA antigens;fluorescence labeling of mouse anti-human immune globulin G; test strip and sample diluent preparation; rapid and accurate detection by the aid of a centrifuge tube, a centrifugal machine, a water bath box, an electronic pH (potential of hydrogen) meter, a fluorescence immunoassay instrument, a continuous point film spraying integrated machine and corresponding reagents and materials. According to the method, detection is implemented by a test strip, time is short, streptavidin protein is fixed by biotin labeled plasmid double-stranded DNA, so that sensitivity is improved, the test strip andsample diluent can be prepared in advance, so that the test strip and sample diluent is conveniently stored and taken in using, and the rapid anti-double-stranded DNA antibody detection method is simple and convenient to operate and high in detection accuracy and saves time.
Owner:江苏齐耀生物科技有限公司
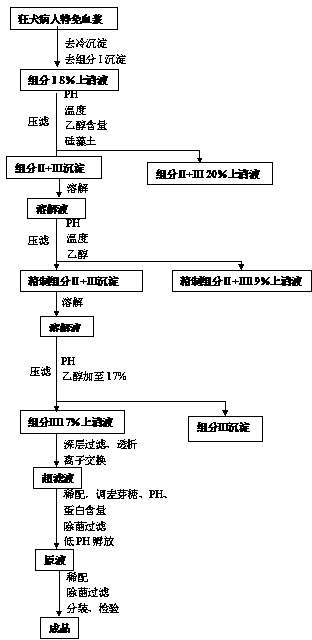
Process for preparing rabies human immune globulin
ActiveCN104193822AHigh yieldHigh puritySerum immunoglobulinsPeptide preparation methodsRabiesUltrafiltration
The invention discloses a process for preparing rabies human immune globulin. According to the process, immune globulin is extracted and separated by using a low-temperature ethanol filter press technique, a chromatography method, an ultrafiltration method, a nano filtering method, a secondary ultrafiltration method and a washing, baking and filling linked sub-packaging process technique. The process is characterized in that a one-step ion exchange chromatography method is adopted as the chromatography method, the component III supernate is firstly refined before chromatography, then the premasters of the refined component III supernate are adjusted so that the protein concentration of the refined component III supernate is 3-6%, the pH value of the refined component III supernate is 6.4-6.6, and the conductivity is 0.18-0.22s / m when the temperature is 19 DEG C, after the adjustment, column chromatography purification is performed by using an ion exchange column, DEAESepharoseFF packing is adopted in chromatography, and the pH value is adjusted to be 3.8-4.0 after chromatography is completed. Compared with a conventional process, the process has the advantages that the yield is increased by 19.2-25%, small dosage is needed, and the use of human immune globulin is reduced, so that the scarce plasma resource is saved.
Owner:华润博雅生物制药集团股份有限公司
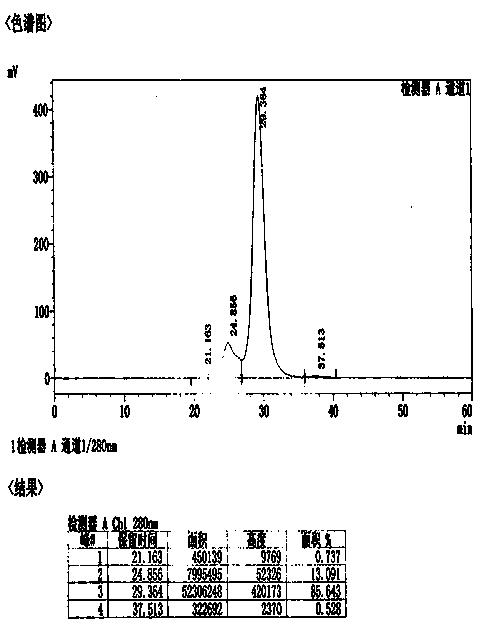
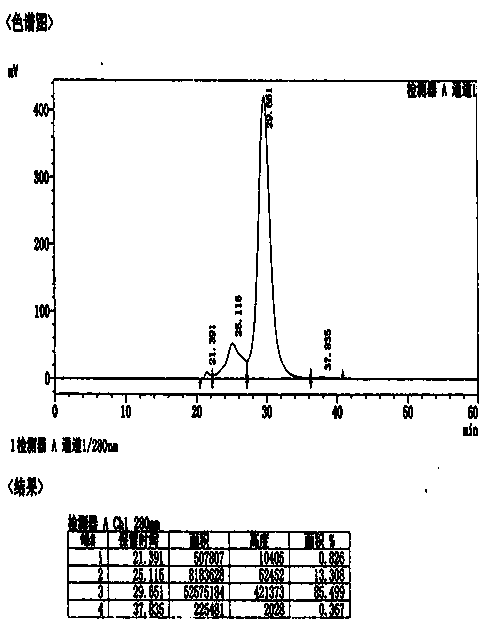
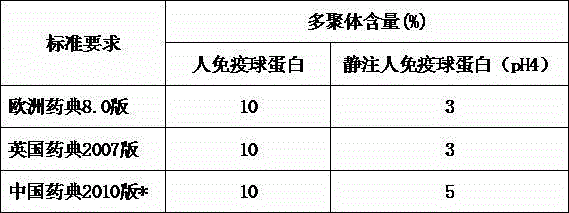
Method for effectively removing human immune globulin polymer
ActiveCN104356231AReduce energy consumptionMultimer reductionPeptide preparation methodsImmunoglobulinsPolyethylene glycolGlobulin
The invention discloses a method for effectively removing human immune globulin polymer. The operation of reducing polymer in an immune globulin purification process is necessary. A polyethylene glycol method and a column chromatography which can remove human immune globulin polymer are reported in public at present, but the two methods have problems of being difficult in removing additives, small in handling capacity and expensive in equipment, and the popularization and application are difficult. The method comprises the following steps: by using a sodium caprylate precipitation method, precipitating to remove IgG polymer under the condition that the solution pH value is 5.4 and the caprylate concentration is 6-8mmol / L, wherein more than 60% of polymer can be effectively removed so that the polymer can be reduced to 3% or below, the loss of the immune globulin is little, the polymer content is stable, and the polymer content can achieve requirement of Chinese pharmacopoeia and Foreign related pharmacopoeia after being placed for two years.
Owner:BEIHAI KAIYUAN BIOLOGICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com