Respiratory syncytial virus resistance human immune globulin and preparation method thereof
A technology of human immunoglobulin and immunoglobulin, which is applied in the direction of antiviral immunoglobulin, immunoglobulin, peptide preparation methods, etc., can solve the problems of unstable properties and low efficiency of human immunoglobulin, and achieve long shelf life , large social and economic benefits, good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1. Screening and collection of plasma from donors:
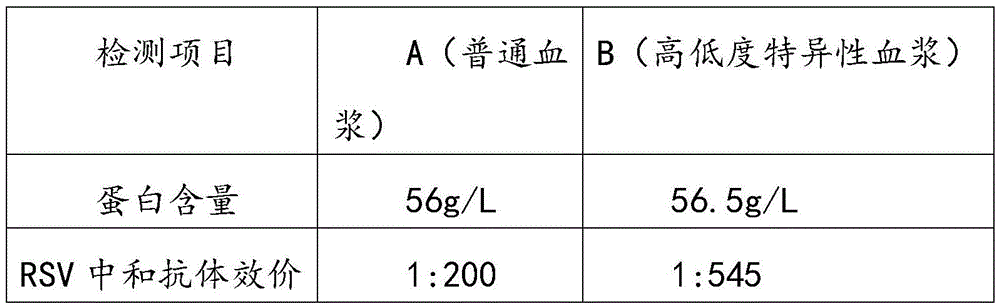
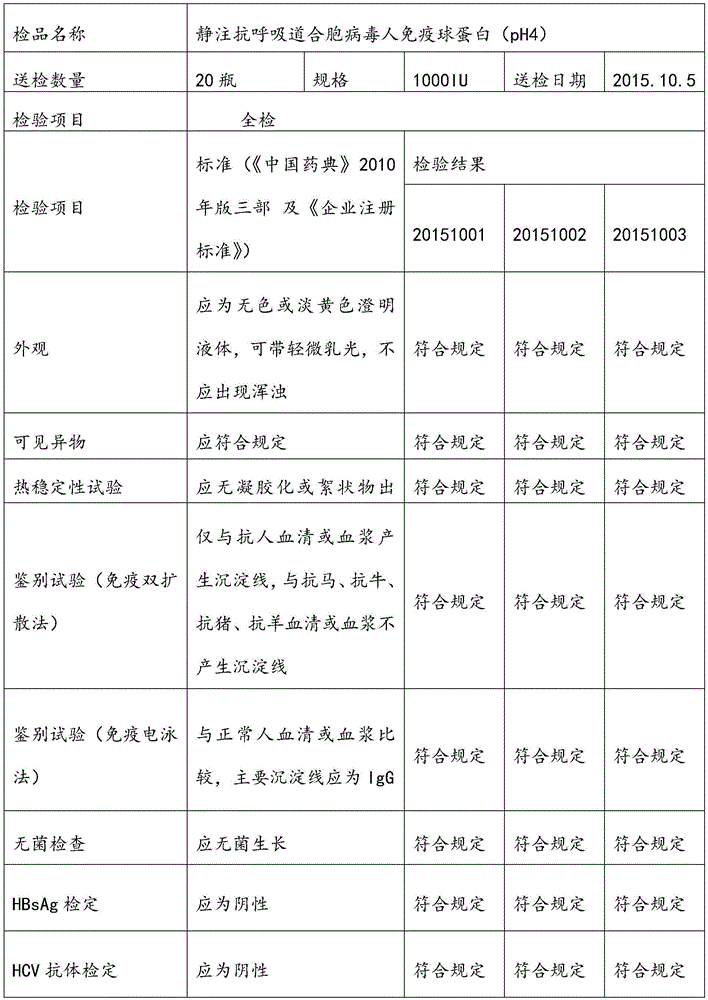
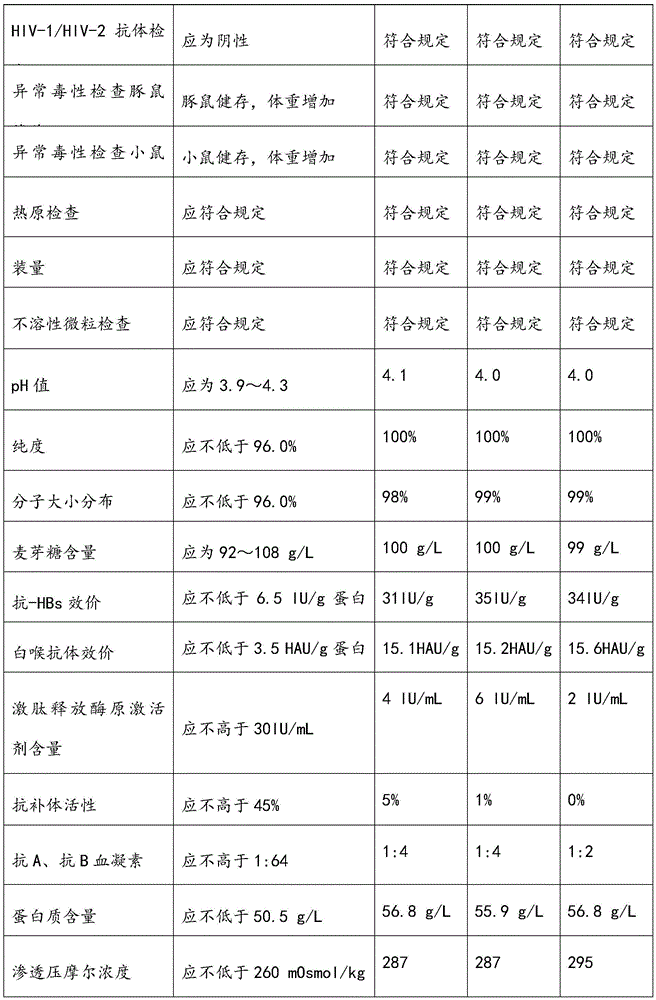
[0055] Select the plasma, and use the neutralization test method to detect the neutralization titer of the respiratory syncytial virus antibody in the raw plasma should meet the following conditions: the neutralization titer is not less than 1:200; the protein content (detected by the biuret method) is greater than or equal to 56g / L; alanine aminotransferase (monitored by Lai's method) was not higher than 35 units; ELISA method was negative for syphilis, hepatitis B surface antigen, HIV-1 / HIV-2 antibody, and HCV antibody. Store in a freezer below -20°C, and the shelf life should not exceed 2 years.
[0056] 2. Determination of antibody titer in raw plasma by neutralization test:
[0057] (A) MEM cell culture medium (purchased from Gibco) of the plasma sample to be tested was pre-diluted 1:4 with cell maintenance medium (3% fetal bovine serum by volume, purchased from Gibco), and then inactivated at 56°C for 30 Minut...
Embodiment 2
[0087] 1. Screening and collection of plasma from donors:
[0088] Select the plasma, and use the neutralization test method to detect the neutralization titer of the respiratory syncytial virus antibody in the raw plasma should meet the following conditions: the neutralization titer is not less than 1:200; the protein content (detected by the biuret method) is greater than or equal to 56g / L; alanine aminotransferase (monitored by Lai's method) was not higher than 35 units; ELISA method was negative for syphilis, hepatitis B surface antigen, HIV-1 / HIV-2 antibody, and HCV antibody. Store in a freezer below -20°C, and the shelf life should not exceed 2 years.
[0089] 2. Determination of antibody titer in raw plasma by neutralization test:
[0090] (A) MEM cell culture medium (purchased from Gibco) of the plasma sample to be tested was pre-diluted 1:4 with cell maintenance medium (3% fetal bovine serum by volume, purchased from Gibco), and then inactivated at 56°C for 30 Minut...
Embodiment 3
[0112] 1. Screening and collection of plasma from donors:
[0113] Select the plasma, and use the neutralization test method to detect the neutralization titer of the respiratory syncytial virus antibody in the raw plasma should meet the following conditions: the neutralization titer is not less than 1:200; the protein content (detected by the biuret method) is greater than or equal to 56g / L; alanine aminotransferase (monitored by Lai's method) was not higher than 35 units; ELISA method was negative for syphilis, hepatitis B surface antigen, HIV-1 / HIV-2 antibody, and HCV antibody. Store in a freezer below -20°C, and the shelf life should not exceed 2 years.
[0114] 2. Determination of antibody titer in raw plasma by neutralization test:
[0115] (A) MEM cell culture medium (purchased from Gibco) of the plasma sample to be tested was pre-diluted 1:4 with cell maintenance medium (3% fetal bovine serum by volume, purchased from Gibco), and then inactivated at 56°C for 30 Minut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com