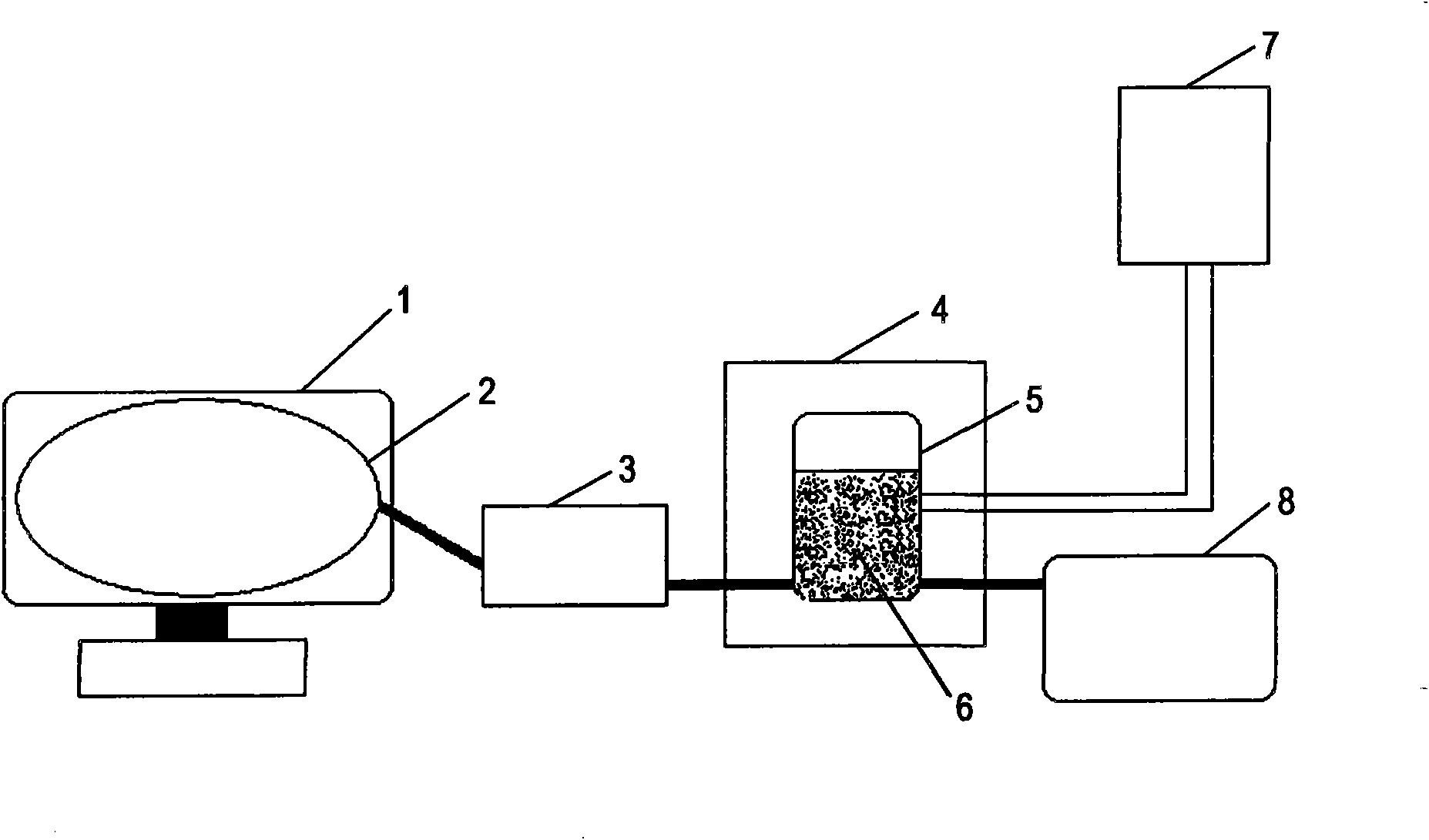
Patents
Literature
109 results about "Cell Maintenance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Processes that promote, sustain, and preserve cell viability. (NCI)
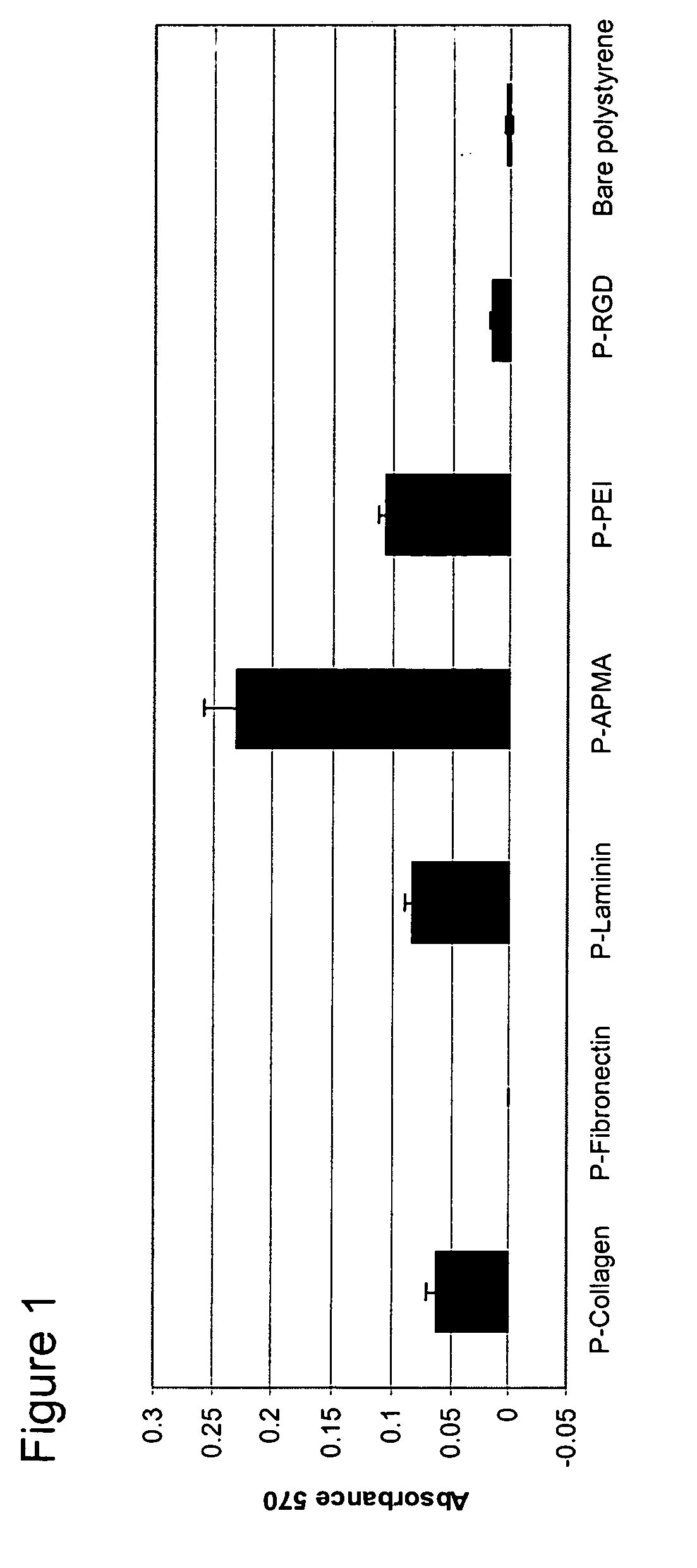
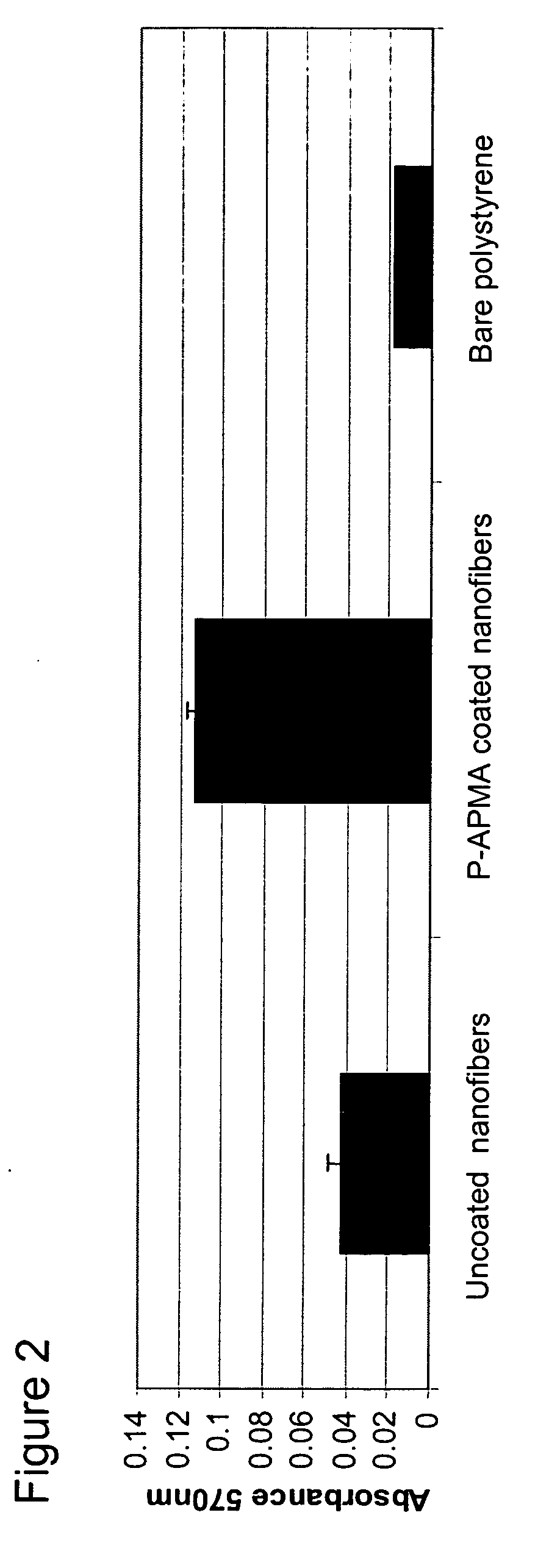
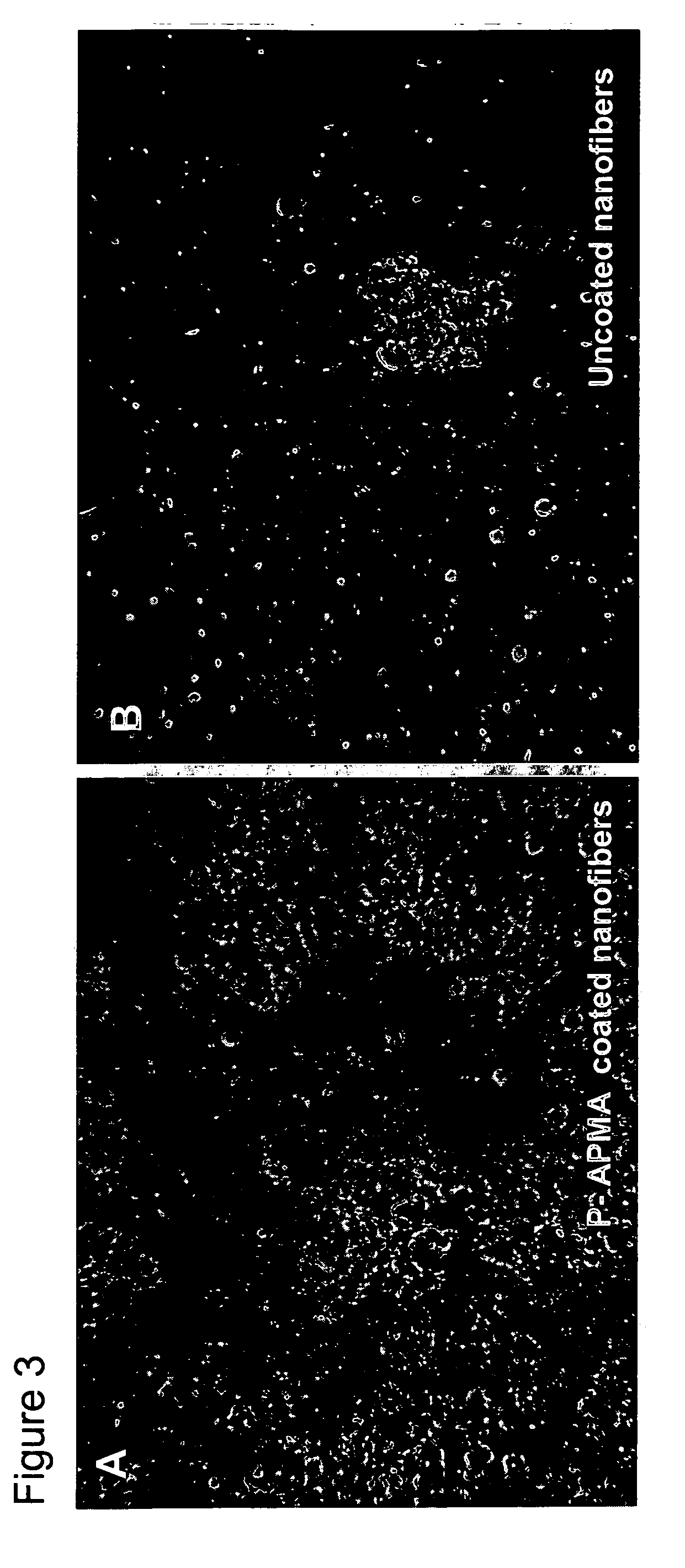
Polymer coated nanofibrillar structures and methods for cell maintenance and differentiation
InactiveUS20070082393A1Improve adhesionReduced metabolic activityBioreactor/fermenter combinationsBiological substance pretreatmentsCell adhesionPolymer chemistry
The invention provides cell-adherent polymeric coatings for articles having a nanofibrillar structure. The coatings include a synthetic, non-biodegradable polymer having at least one pendent amine group, wherein the polymer is covalently immobilized on the article via latent reactive groups. The invention also provides methods for the long term culturing of cells using the polymer coated nanofibrillar structures. The polymer coated nanofibrillar structures of the invention have been found to be particularly useful for the growth and differentiation of cells, including neural precursors.
Owner:LODHI MUHAMMAD +4
Method for separating and extracting stem cells from placenta, umbilical cord or adipose tissue
ActiveCN101693884AEasy accessSpecification acquisitionSkeletal/connective tissue cellsArtificially induced pluripotent cellsFicollDisease
The invention relates to a method for separating and extracting stem cells from a placenta, umbilical cord or adipose tissue, which comprises the following process steps: firstly mixing the placenta, umbilical cord or adipose tissue and a cell maintenance fluid according to the proportion by weight of 2.5-4:1, putting the mixture into a tissue crushing barrel, adding collagenase after crushing, uniformly mixing, hatching at the temperature of 37 DEG C, filtering, adding a precipitator, sucking a supernatant fluid after settling, centrifuging, removing the supernatant fluid, adding concentrated cells into a liquid of diatrizoate sodium-ficoll 400#, then centrifuging, collecting 10-15 ml of intermediate cell layer, washing with the cell maintenance fluid, counting the collected cells, and providing the cells for clinical use when the cell survival ratio is more than or equal to 95 percent. The invention not only realizes the separation and the extraction of all stem cells from the placenta, umbilical cord or adipose tissue, but also realizes industrialized production, and enables doctors to conveniently, safely and canonically obtain the adult stem cells in clinic and use the adult stem cells for treating the diseases of patients as using medicaments, thereby solving the bottleneck problem of difficult obtainment of adult stem cells in clinic and popularizing a cell treatment technology.
Owner:NINGXIA ZHONGLIANDA BIOPHYSICS
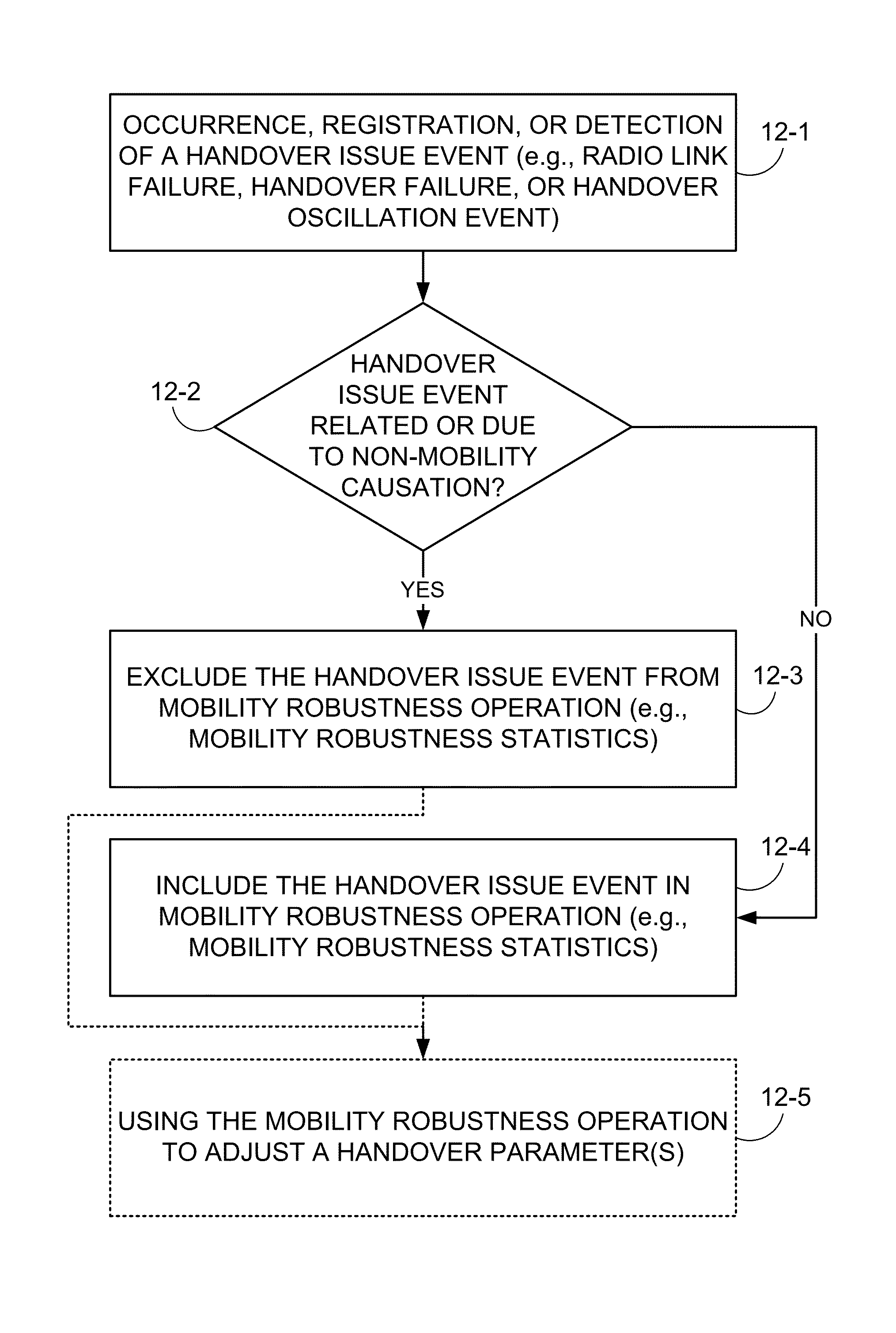
Method and apparatus for excluding non-mobility data from mobility key performance indicators
Handover (HO) statistics and handover issue events which are due to non-mobility causes are not included in the statistics fed into mobility robustness optimization (MRO). The non-mobility causes may include, e.g., load balancing, retracting users to prepare for cell maintenance or restart / reconfiguration and cell outage including compensation means. Radio link failure (RLF), handover failure (HOF), and handover oscillations (HOosc) that are due to non-mobility cause are excluded from the statistics upon which mobility robustness optimization is based. Non-mobility causes are also differentiated from mobility causes when reporting key performance indicators to an operator.
Owner:TELEFON AB LM ERICSSON (PUBL)
Rationally designed media for cell culture
ActiveUS20080108553A1Peptide/protein ingredientsMicrobiological testing/measurementCell culture mediaCell mass
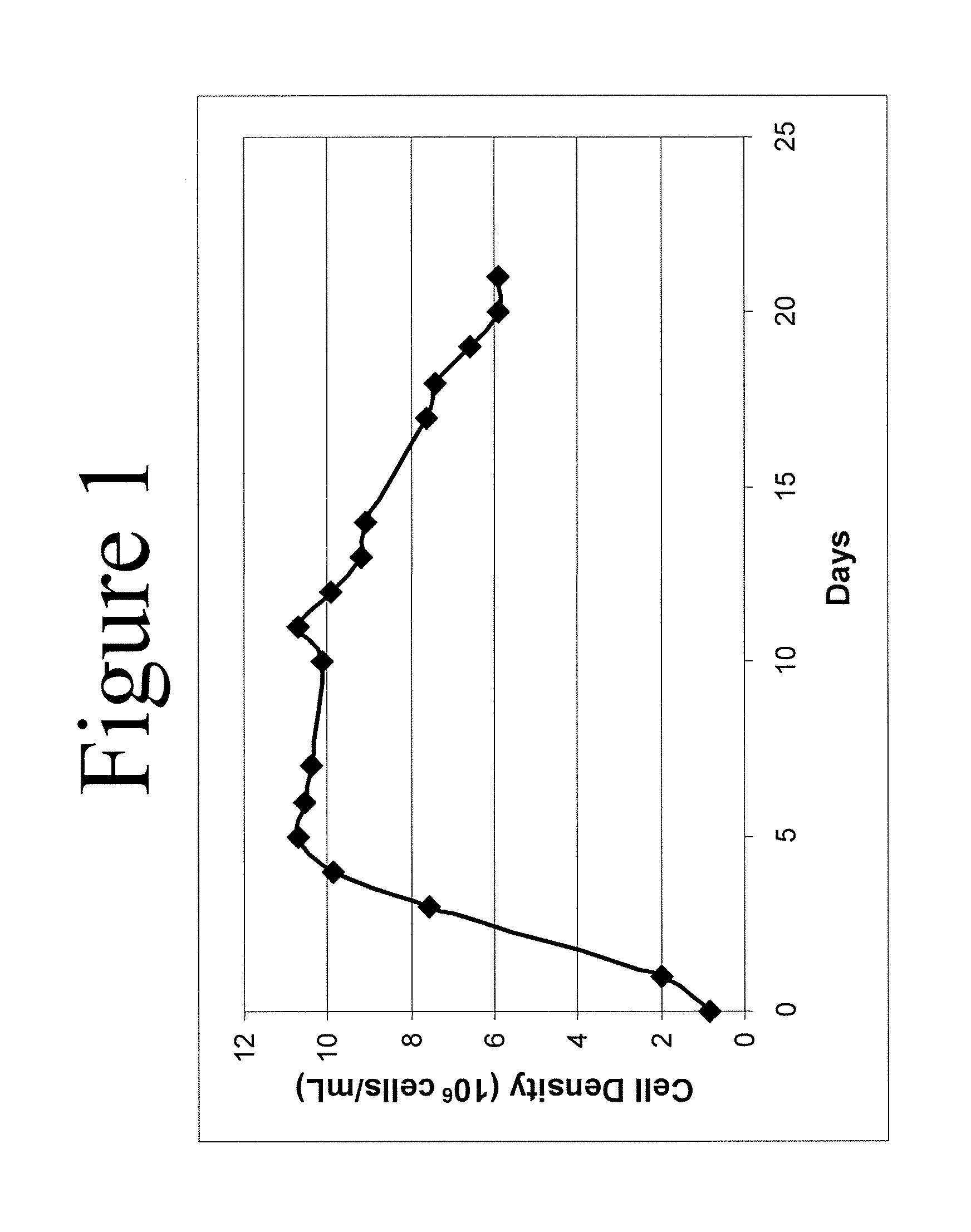
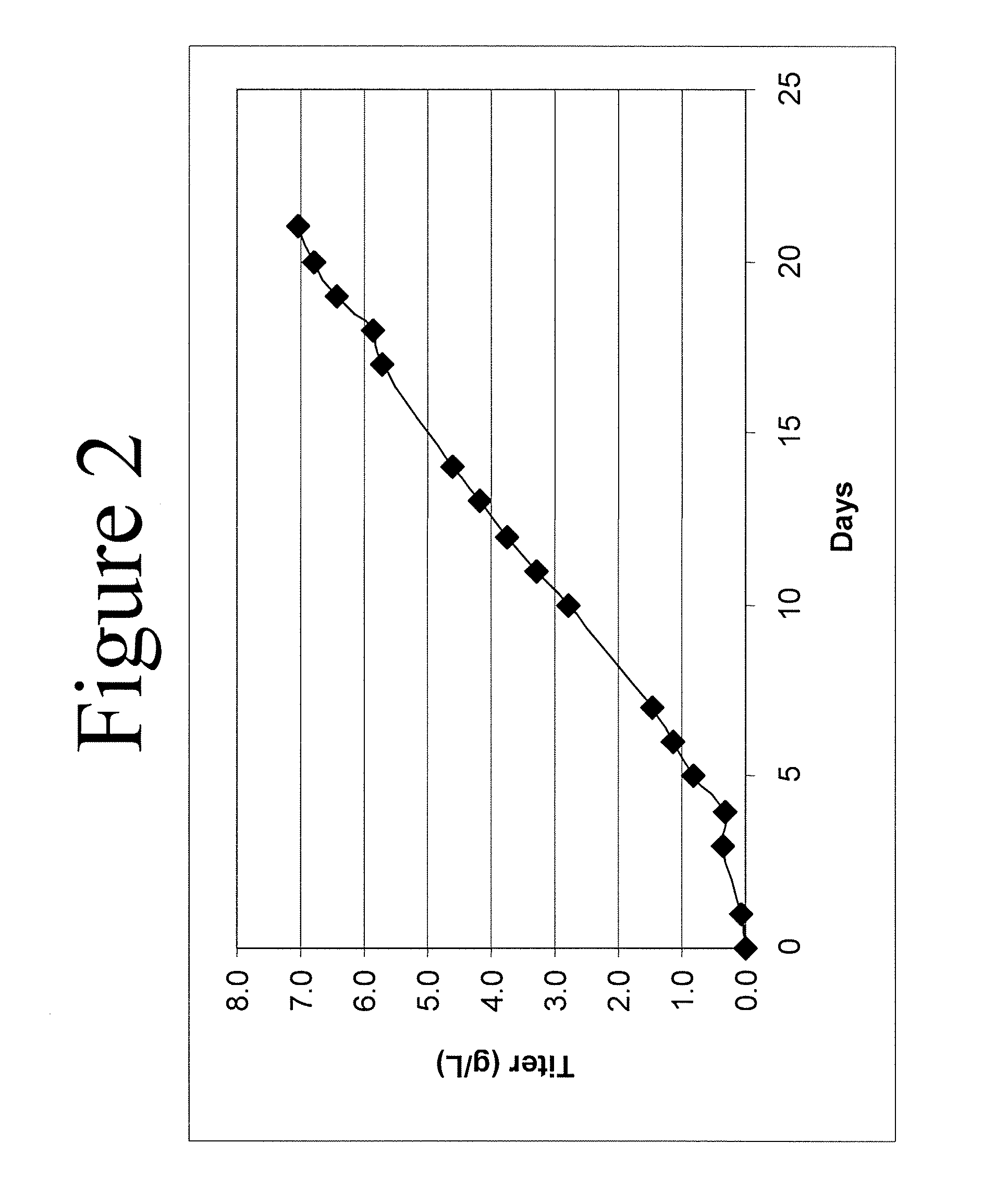
This invention relates to methods for rationally designing cell culture media for use in cell cultures, e.g., cell cultures employed in polypeptide production; cell culture media designed with the disclosed methods; methods of producing a polypeptide of interest, e.g., an antibody, using such media; polypeptides produced using the methods and media disclosed herein; and pharmaceuticals compositions containing such polypeptides. The rationally designed media contain a concentration of an amino acid that is calculated for use in cell mass, a concentration of the amino acid that is calculated for use in cell maintenance, and a concentration of the amino acid that is calculated for incorporation into the polypeptide of interest. The rationally designed media may contain a baseline-adjusted concentration, A, of at least one amino acid that is calculated according to the formula A=[(M*X)+(N*P)+(Y*M*X)]*F, wherein X is the concentration of the amino acid that is used per unit of cell mass, P is the concentration of the amino acid that is used for incorporation into the polypeptide of interest per unit of polypeptide titer, M is the multiplier for the desired peak cell density of the cell culture, N is the multiplier for the desired concentration of the polypeptide of interest, Y is the cell maintenance factor; and F is the baseline factor. This rationally designed media may also be used to produce a starting cell culture medium comprising a concentration, B, of at least one amino acid according to the formula B=[A−(Z*V)] / (1−V), wherein Z is a concentration of the amino acid in the feeding cell culture medium, and V is a volume of the feeding culture medium as a proportion of the desired cell culture medium volume.
Owner:WYETH LLC
Reagent box for separating karyocyte in vitro and application method thereof
ActiveCN1920012ANo change in biological activityConvenient treatmentBlood/immune system cellsFicollPaleontology
The invention relates the separating agent box and application. The agent box comprises erythrocyte precipitating agent, hypaque sodium- ficoll 400# or HISTOPAQUE1077 and cell maintenance liquid. The agent box can separate bone marrow and umbilical cord blood, on the surface of cell there is no any mark, and it doesn't change biological activity of cell. The invention can commercially manufacture, and the application is wide.
Owner:NINGXIA ZHONGLIANDA BIOPHYSICS
Triple vaccine of pig transmissible gastroenteritis, pig epidemic diarrhea and pig rotavirus
InactiveCN101491673AAvoid pollutionDoes not destroy nutrientsViral antigen ingredientsDigestive systemDiseaseCytopathic effect
The invention provides a method for preparing triple vaccine for preventing porcine transmissible gastroenteritis, porcine epidemic diarrhea and porcine rotavirus. The method comprises the following steps: inoculating a host-cell line with a 90 percent grown monostratum against a porcine transmissible gastroenteritis virus, a porcine epidemic diarrhea virus and a porcine rotavirus respectively, and adding a cell maintenance media into the host-cell lines respectively to be cultured at 37 DEG C; after cytopathic effect reaches over 75 percent, collecting viruses to be stored at 20 DEG C below zero for standby; mixing the viruses according to 10 TCID50 in 1:1:1, and simultaneously adding Freund's complete adjuvant and immunopotentiator into the mixture to inactivate the mixture by formaldehyde at 37 DEG C for 24 hours; and adding an oil adjuvant into the mixture to prepare a vaccine of water-oil-water preparation. The method can be used for preparing the triple vaccine for preventing the porcine transmissible gastroenteritis, the porcine epidemic diarrhea and the porcine rotavirus so as to solve the problem that the diseases do not have an effective medicine to treat currently.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
Preparation method of swine pseudorabies vaccine
The invention relates to a preparation method of a swine pseudorabies vaccine, which comprises the following steps of: culturing a virus by using a swine testicle cell, and when one layer of cells grows, inoculating a swine pseudorabies virus; then adding into a cell maintenance medium, statically or rotatably culturing, when the cell suffers from more than 80 percent of pathological changes, harvesting a cell culture, repeatedly freeze-thawing to obtain a cell venom containing supernate, and mixing the cell venom qualified in toxic valence detection with formaldehyde for inactivating; and mixing with an emulsifying agent for emulsifying to obtain the swine pseudorabies vaccine. Compared with the prior art, a strain used for preparing the vaccine has the advantages of stronger toxicity and high virus valence; the swine pseudorabies vaccine has good immunogenicity and long immunization period; and the preparation method has the advantages of reasonable process and lower cost, thereby greatly lowering the cost load of the fish breeding and poultry raising industry.
Owner:SHANGHAI ELITE AGRI SCI TECH GROUP +1
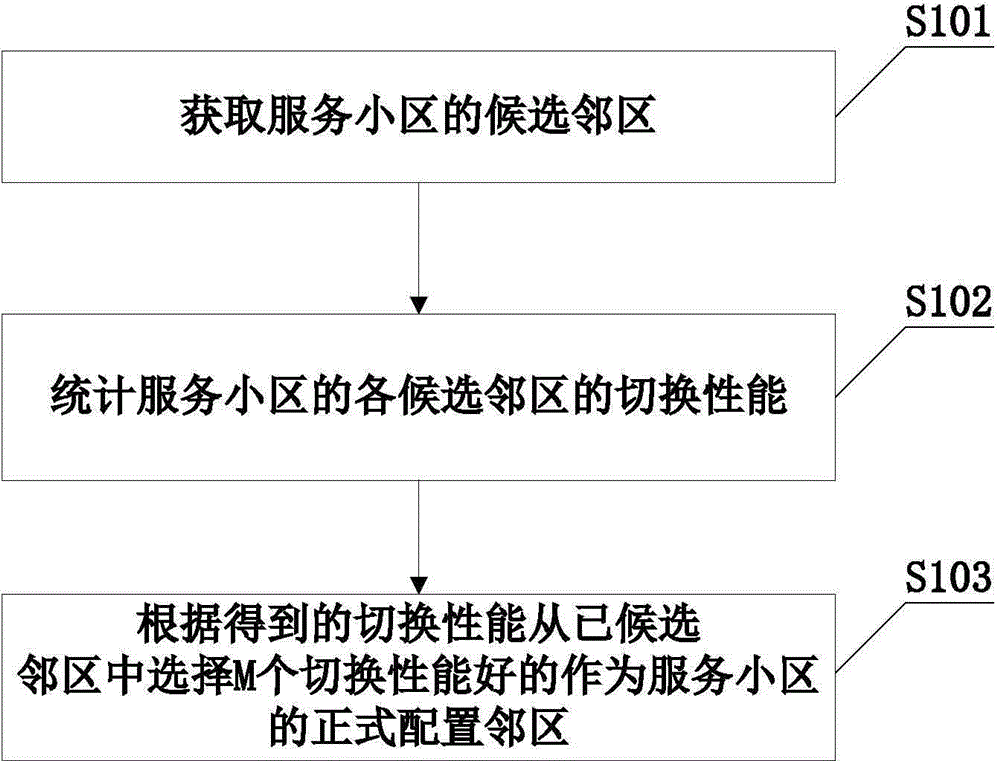
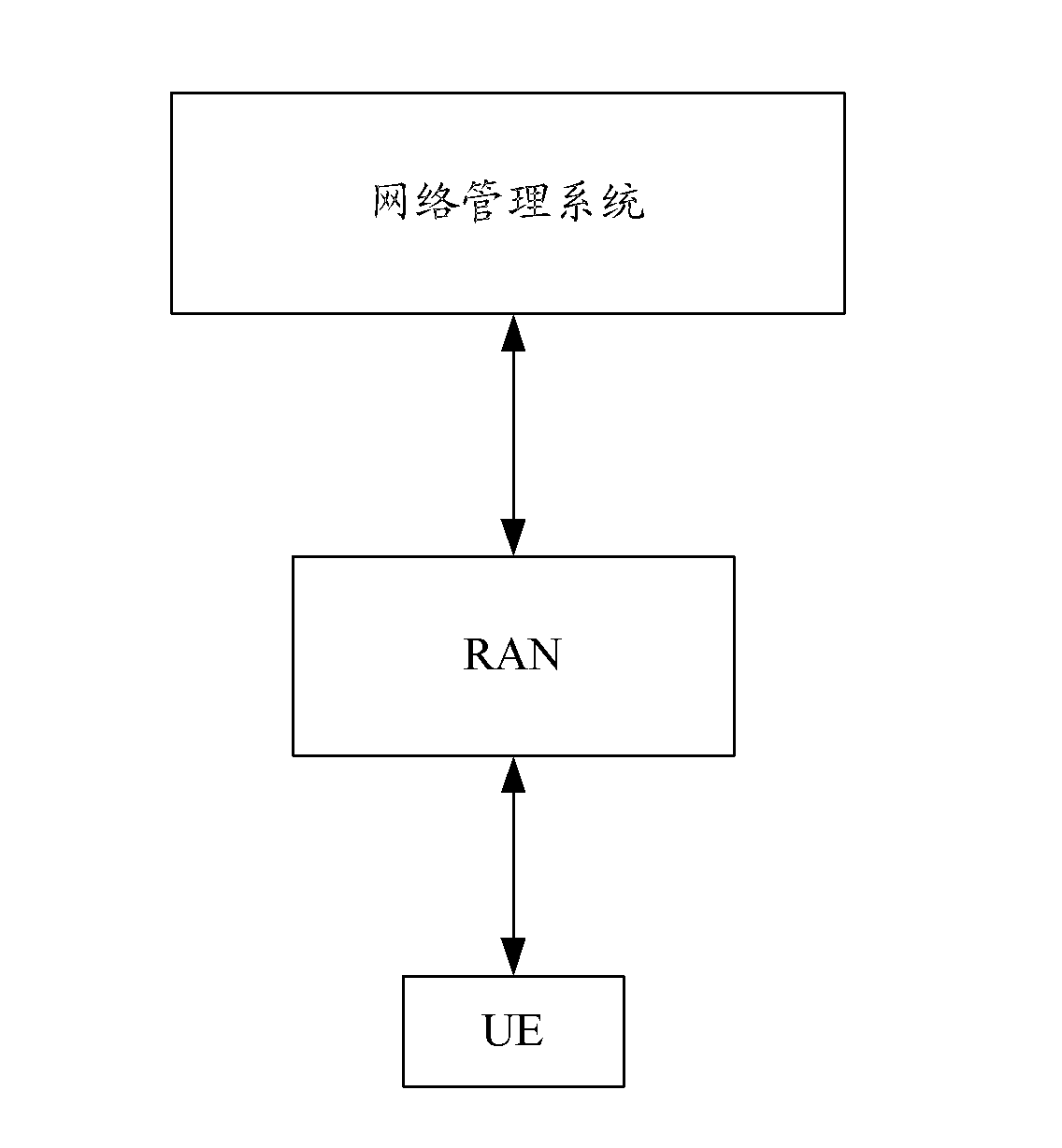
Neighbor cell maintenance method and neighbor cell maintenance device
InactiveCN105578443AGood switching effectNetwork data managementComputer architectureStatistical analysis
The invention discloses a neighbor cell maintenance method and a neighbor cell maintenance device. The neighbor cell maintenance process comprises the following steps: after acquiring candidate neighbor cells of a service cell (including configured neighbor cells in a configured neighbor cell list of the service cell and discovered unknown neighbor cells of the service cell), making statistical analysis of the switching performance of each neighbor cell; and selecting M neighbor cells of good switching performance as formal configured neighbor cells of the service cell according to the switching performance of each neighbor cell. According to the invention, after unknown neighbor cells are detected, whether or not to add the unknown neighbor cells is not judged immediately based on the current remaining space of the service cell. The switching performance of configured and to-be-configured neighbor cells is acquired first, and then, M neighbor cells of good switching performance are selected as formal configured neighbor cells of the service cell. Previously-configured neighbor cells of poorer performance than the selected M neighbor cells are deleted from the configured neighbor cell list. Thus, the added neighbor cells are neighbor cells of best switching performance.
Owner:ZTE CORP
Animal rabies virus and vaccine and production method thereof
ActiveCN101979515AHigh infection efficiencyHigh titerInactivation/attenuationAntiviralsFreeze thawingAdjuvant
Owner:PULIKE BIOLOGICAL ENG INC
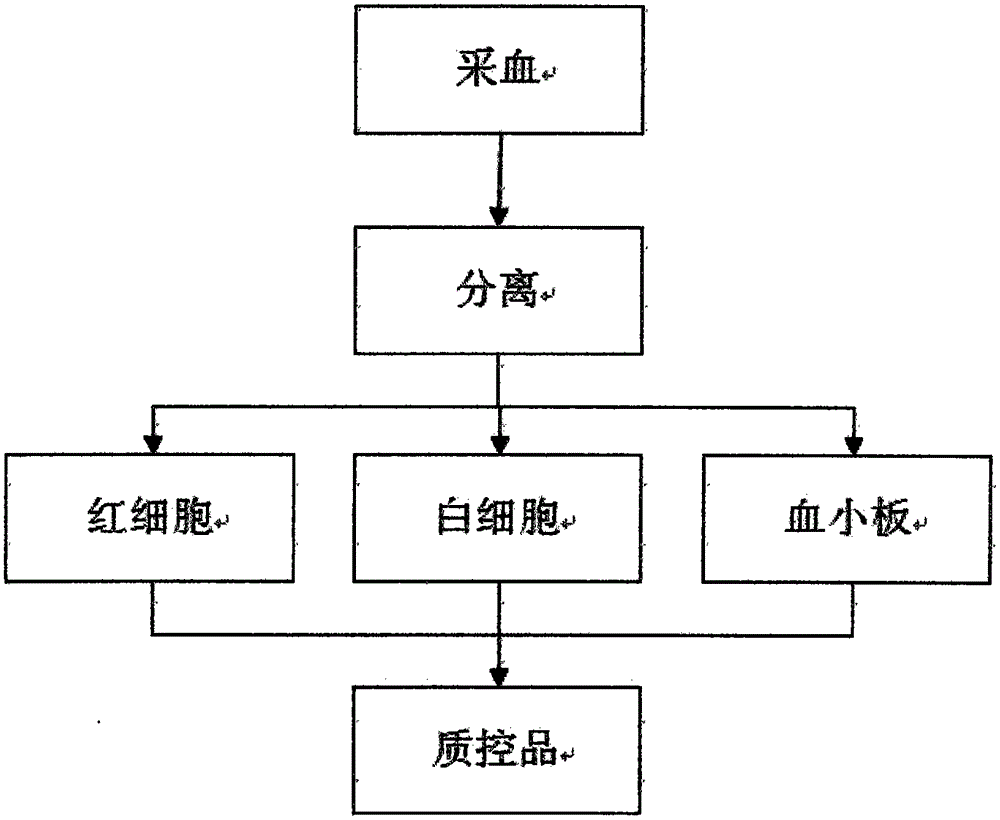
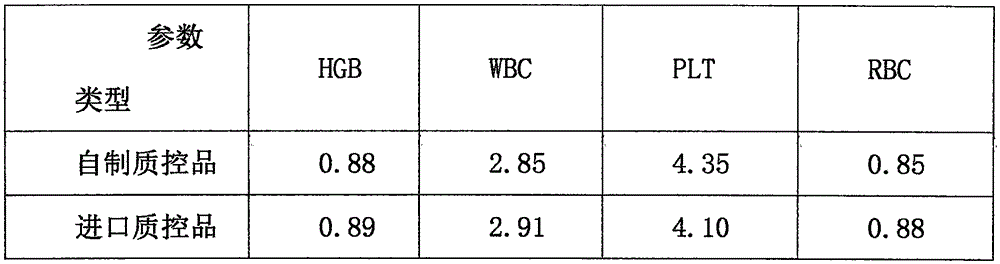
Preparation method of control material for blood cell analyzer
InactiveCN105651568AKeep the original shapeImprove stabilityPreparing sample for investigationBiological testingWhite blood cellBottle
A preparation method of a control material for a blood cell analyzer belongs to the field of clinical medical supplies. The method is characterized by comprising the steps of: separating leukocytes and platelets of healthy human whole blood by using an albuginea method; fixing the isolated erythrocytes by a fixative, adding a fixative into the isolated leukocytes and platelets, and washing the fixture; adding a cell maintenance liquid to the fixture after washing; after stabilization, subpackaging the quality control material in sterile small glass bottles for storage, so as to complete preparation of quality control materials. The method uses fresh whole blood as the raw material, and applies formaldehyde and glutaraldehyde with different concentrations as fixatives; the added maintenance fluid maintains the original cell morphology; the control material has high stability, and advantages of low price, convenience in transportation compared to imported original control products, and shows great marketing value.
Owner:THE FIRST AFFILIATED HOSPITAL OF XIAN JIAOTONG UNIV
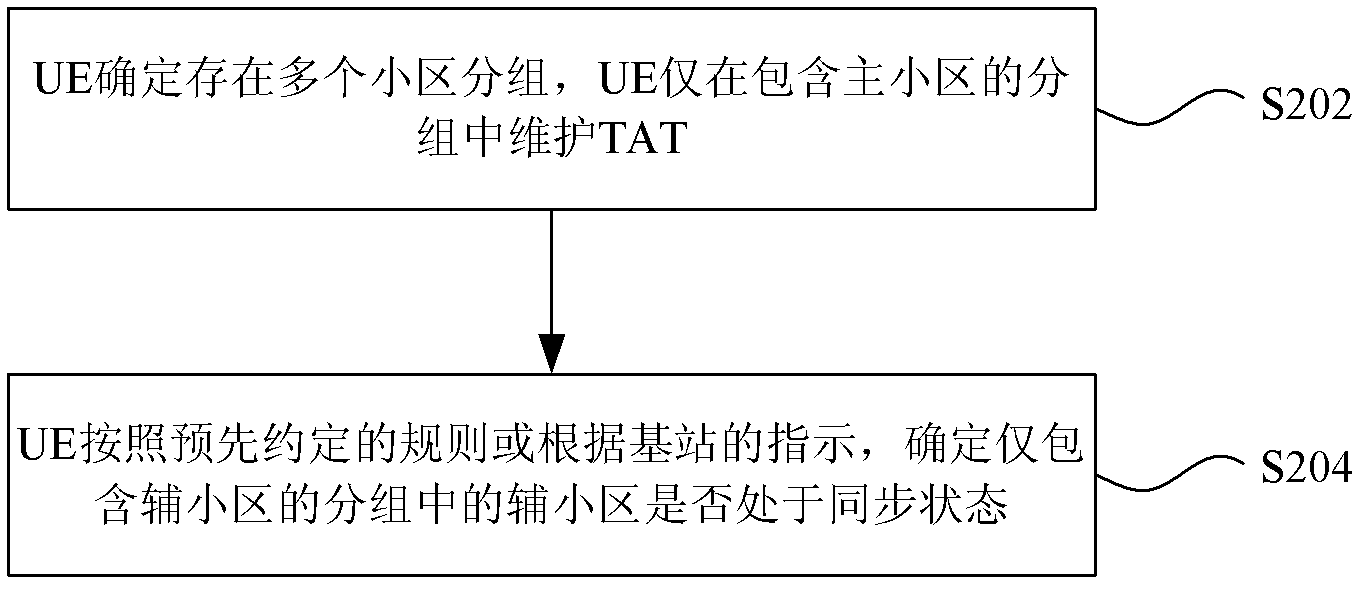
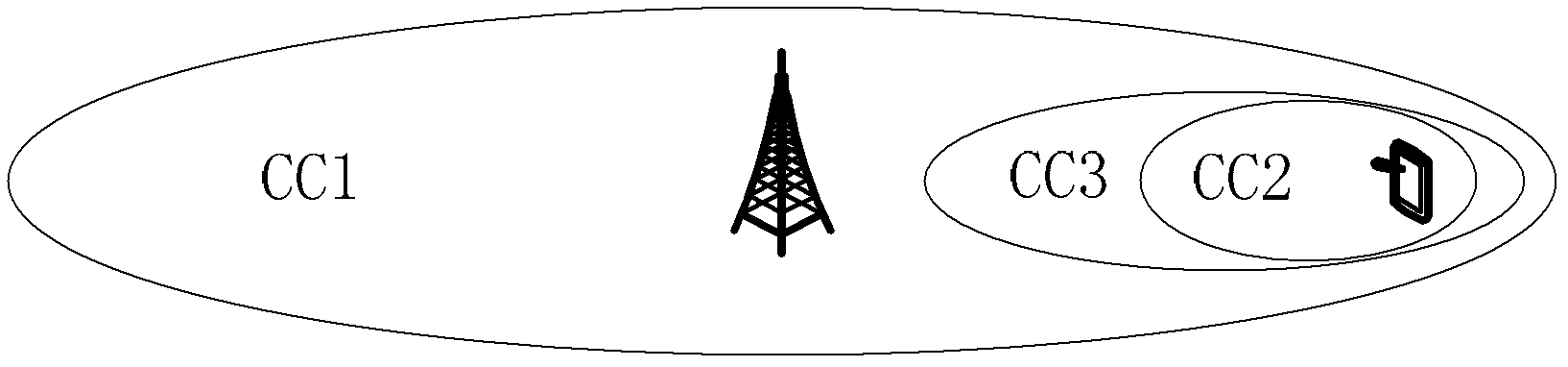
Method and device for synchronous cell maintenance of multi-carrier communication system
InactiveCN102932901AReduce complexityBackward Compatibility GuaranteedSynchronisation arrangementCommunications systemTime alignment
The invention discloses a method and a device for synchronous cell maintenance of a multi-carrier communication system. The method comprises the steps: under the condition that a plurality of cell subgroups exist, when user equipment maintains a time alignment timer (TAT) only in a subgroup containing a main cell, the user equipment determines whether an auxiliary cell in a subgroups containing the auxiliary cell only in a synchronization state according to a predetermined rule or an indication of a base station. The method and the device disclosed by the invention have the advantage of solving the problems that because the user equipment maintains the TAT only in the subgroup of the main cell, the UE cannot acquire the synchronization condition of a cell in the subgroup only containing the auxiliary cell and thus is likely to carry out uplink signal transmission when the cell in the auxiliary cell subgroup is in a step-out state and a base station side cannot receive uplink signals transmitted by the cells correctly.
Owner:ZTE CORP
Method for producing porcine reproductive and respiratory syndrome viruses (PRRSV) in large scale
InactiveCN101831412ASolve outputSmall difference between batchesInactivation/attenuationMicroorganism based processesFreeze thawingCulture fluid
The invention discloses a method for producing porcine reproductive and respiratory syndrome viruses (PRRSV) in a large scale. The method comprises the following steps of: producing PRRSV by using a cell microcarrier suspension culture system by utilizing a bioreactor; inoculating host cells used for preparing the viruses to a carrier pot containing a culture solution and microcarriers and uniformly mixing the cells and the microcarriers to ensure that the cells are attached on the microcarriers; under a proper culture environment, providing sufficient nutrients and an appropriate gas environment for the cells to ensure that the cells grow on the microcarriers to reach a concentration 5-40 times of an inoculating concentration; changing to use a cell maintenance culture solution and preparing the PRRSV into a viral suspension to ensure that the viral suspension is adsorbed on the cells; culturing the viruses under an appropriate environment; obtaining a viral solution after continuously culturing for 2-3 days; after the viral solution is inspected to be qualified, freeze thawing the obtained viral solution twice at -20 DEG C; and inactivating and purifying to obtain a PRRSV solution. The method has large production scale, high single-batch output and relatively lower production cost.
Owner:PU LIKE BIO ENG
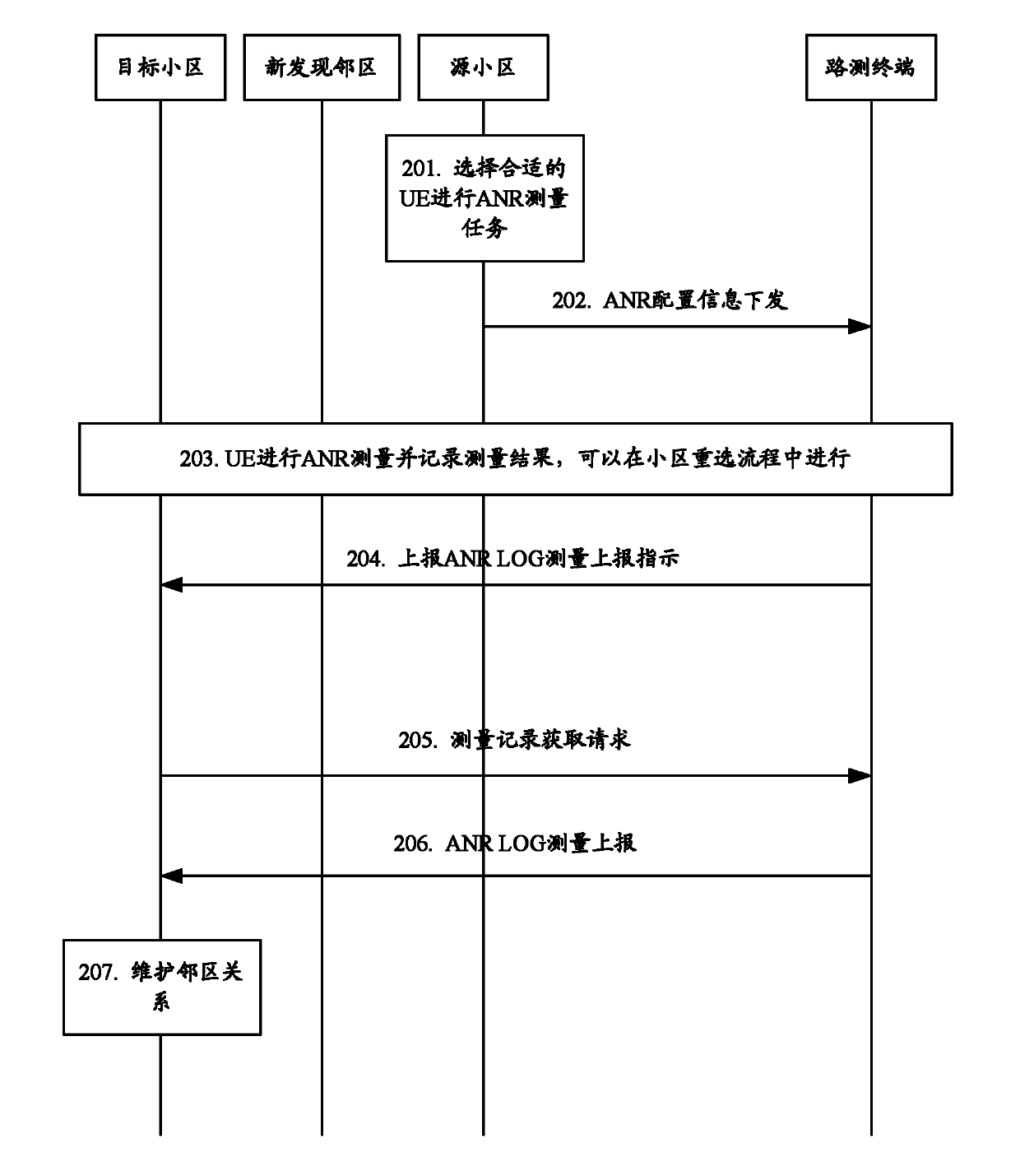
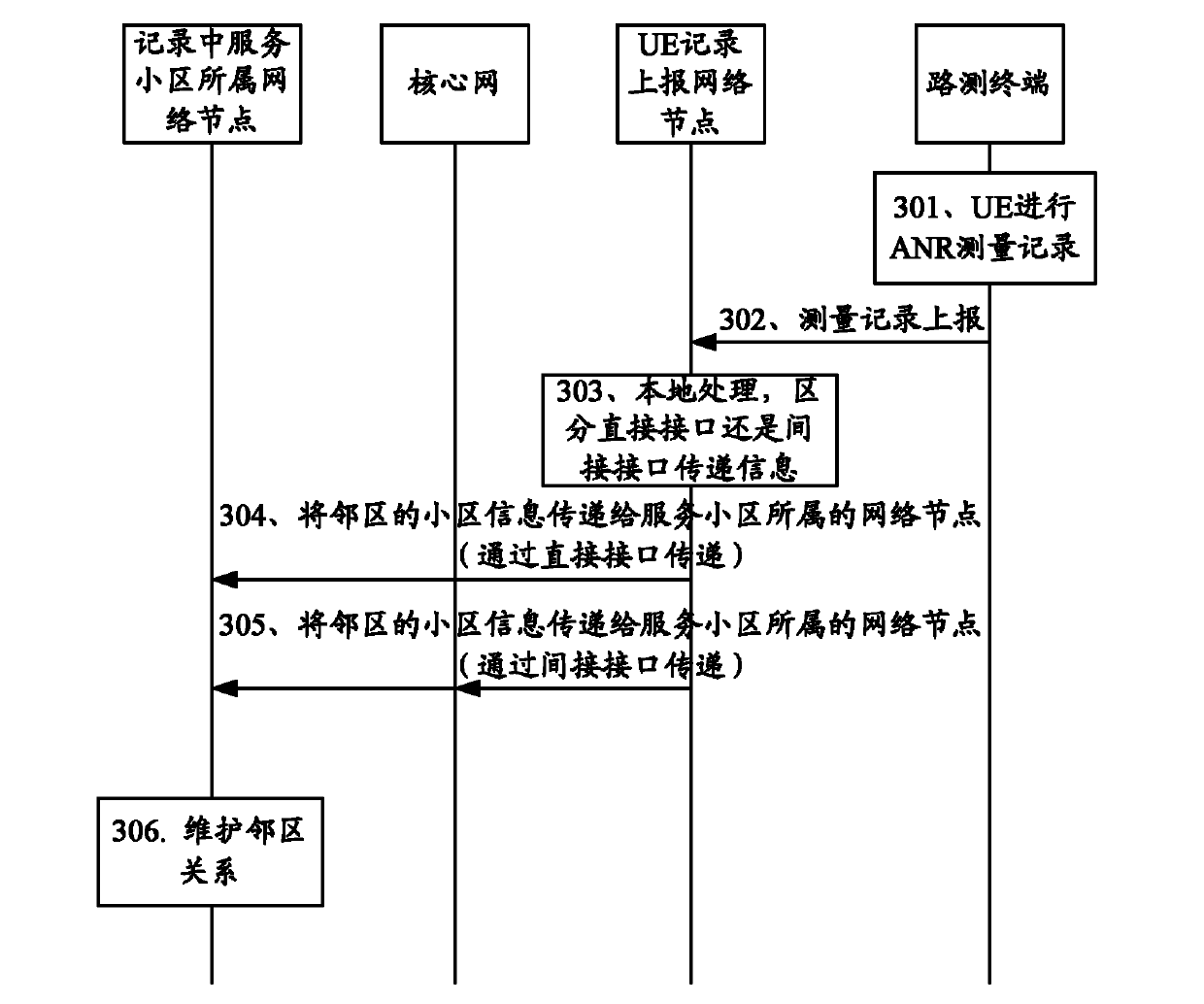
Automatic neighbor cell list maintenance method and system
ActiveCN102572769AImprove accuracyEasy to switchEnergy efficient ICTHigh level techniquesTelecommunicationsUser equipment
The invention discloses an automatic neighbor cell list maintenance method which comprises the following steps that: user equipment (UE) detects cell information of a UE serving cell and / or neighbor cells of the serving cell according to a drive test signaling, and reports the detection result to a network side; the network side sends the cell information of the neighbor cells of the serving cell to a network node to which the serving cell belongs; or, the network side sends the cell information of the serving cell to network nodes to which the neighbor cells of the reported serving cell belong; and the network nodes receiving the serving cell information or the neighbor cell information carries out local neighbor cell maintenance. Meanwhile, the invention discloses an automatic neighbor cell list maintenance system for realizing the method. According to the invention, the manpower is saved, and the cost for neighbor cell maintenance is saved as well. In addition, the cell information detection realized through automatic drive test enables the cell information acquisition to be accurate and timely, and neighbor cell lists maintained according to the cell information are rather high in accuracy rate.
Owner:ZTE CORP
Method and apparatus for excluding non-mobility data from mobility key performance indicators
Handover (HO) statistics and handover issue events which are due to non-mobility causes are not included in the statistics fed into mobility robustness optimization (MRO). The non-mobility causes may include, e.g., load balancing, retracting users to prepare for cell maintenance or restart / reconfiguration and cell outage including compensation means. Radio link failure (RLF), handover failure (HOF), and handover oscillations (HOosc) that are due to non-mobility cause are excluded from the statistics upon which mobility robustness optimization is based. Non-mobility causes are also differentiated from mobility causes when reporting key performance indicators to an operator.
Owner:TELEFON AB LM ERICSSON (PUBL)
Rationally designed media for cell culture
ActiveUS8232075B2Peptide/protein ingredientsMicrobiological testing/measurement3D cell cultureCell culture media
This invention relates to methods for rationally designing cell culture media for use in cell cultures, e.g., cell cultures employed in polypeptide production; cell culture media designed with the disclosed methods; methods of producing a polypeptide of interest, e.g., an antibody, using such media; polypeptides produced using the methods and media disclosed herein; and pharmaceuticals compositions containing such polypeptides. The rationally designed media contain a concentration of an amino acid that is calculated for use in cell mass, a concentration of the amino acid that is calculated for use in cell maintenance, and a concentration of the amino acid that is calculated for incorporation into the polypeptide of interest.
Owner:WYETH LLC
Production of infectious bronchitis virus and vaccine from cell line
ActiveCN102965344AEasy to adaptHigh titerAntiviralsRecovery/purificationInfectious bronchitis virusVaccine Production
The invention provides a method for production of an infectious bronchitis virus from a cell line. The employed cell line can be a Vero cell line, a BHK-21 cell line, a PK-15 cell line, a Marc145 cell line, a ST cell line, and an IBRS-2 cell line. The method includes: when the cell line forms a well-grown cell monolayer, inoculating an infectious bronchitis virus and making it adsorbed to the cell line; using a cell maintenance medium to conduct culture, adding an incubation agent to incubate the cell for 20-40min, and performing infectious bronchitis virus proliferation; and when CPE reaches over 75%, harvesting the infectious bronchitis virus. The method for production of an infectious bronchitis virus vaccine from a cell line provided in the invention can achieve the purpose of more secure and more effective infectious bronchitis virus and vaccine production.
Owner:PU LIKE BIO ENG
Virus and vaccine of porcine reproductive and respiratory syndrome and preparation method of same
ActiveCN101979514AHigh viral titerHigh poison priceViral antigen ingredientsAntiviralsFreeze-dryingCells/microL
The invention discloses a method for preparing virus of porcine reproductive and respiratory syndrome on a large scale. In the method, the virus of the porcine reproductive and respiratory syndrome is prepared in a cell microcarrier suspension culture system by a bioreactor. The method comprises the following steps of: inoculating host cells for preparing the virus to a carrier tank containing culture solution and a microcarrier, and mixing the cells and the microcarrier uniformly to ensure that the cells are attached to the microcarrier; providing sufficient nutrients and appropriate gas environment for the cells under the appropriate culture environment to ensure that the cells are grown until the cells are in an amount which are 10 to 20 times of the inoculation concentration on the microcarrier; preparing virus suspension from the virus of the porcine reproductive and respiratory syndrome by using cell maintenance culture solution to ensure that the suspension is adsorbed to the cells; culturing the virus under the appropriate culture environment; culturing continuously for 2 to 3 days to obtain virus solution; and after the virus solution passes inspection, performing freeze thawing on the virus solution twice at the temperature of -20 DEG C, and inactivating and purifying to prepare an inactivated vaccine of the porcine reproductive and respiratory syndrome or adding a freeze-drying protective agent for freeze drying to prepare a live vaccine of the porcine reproductive and respiratory syndrome. The method has large production scale, high yield of single batch and low production cost.
Owner:PU LIKE BIO ENG
Production method of pseudorabies virus vaccine
ActiveCN103550772AWill not be subject to supplyReduce manufacturing costInactivation/attenuationMicroorganism based processesInfected cellFreeze and thaw
The invention discloses a production method of a pseudorabies virus vaccine. The production method comprises the following steps: (1) adopting a cell of a continuous cell line, subjecting the cell to digestive passage, and continuously culturing the cell in a cell culture flask by using a cell growth medium; (2) diluting a virus seed into 10 times by using a cell maintenance medium, and inoculating a cell monolayer to obtain an infected cell virus liquid, that is, a virus seed for production; (3) preparing the cell monolayer formed in the step (1) into a cell suspension through digestion, inoculating the cell suspension into a bioreactor, and adding a microcarrier into the bioreactor; (4) performing virus-introduction operation on the cell, wherein the introduced virus seed is the virus seed produced in the step (2); and (5) harvesting liquid in the reactor together with the microcarrier until all of the cells on the microcarrier fall off and a dissolved oxygen value increases obviously, placing the liquid and the microcarrier under conditions of minus 20 DEG C, repeatedly freezing and thawing the liquid and the microcarrier twice, and removing the microcarrier and cell fragments to prepare the pseudorabies virus vaccine. The production method of the pseudorabies virus vaccine is short in production cycle and large in yield.
Owner:成都史纪生物制药有限公司
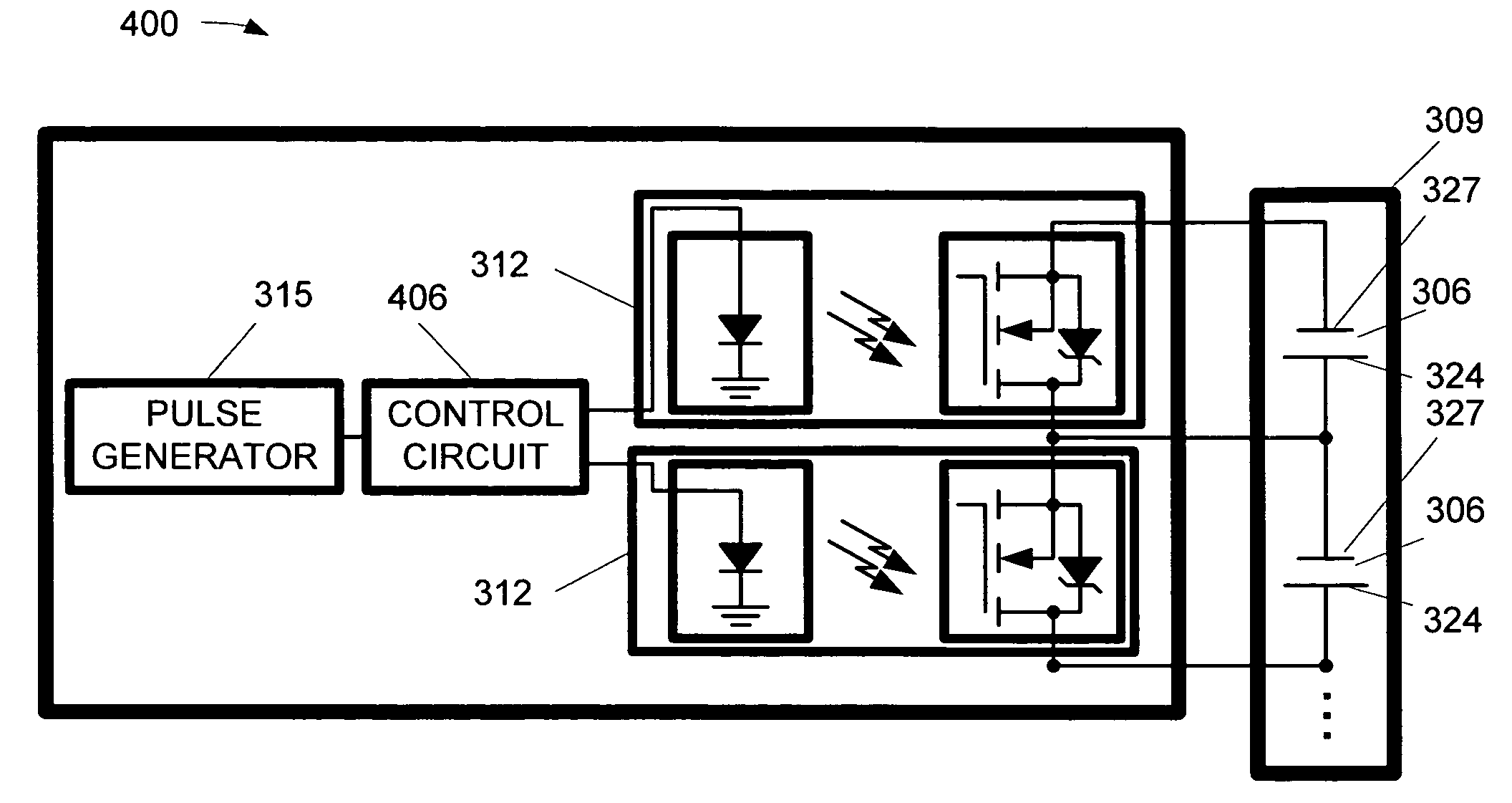
Cell maintenance device for fuel cell stacks
InactiveUS7474078B2Lower impedanceBatteries circuit arrangementsFuel cells groupingFuel cellsCell Maintenance
A method and apparatus for maintaining the cells of a fuel cell stack are disclosed. The apparatus includes a fuel cell maintenance device including means for imposing a low impedance across at least one cell of a fuel cell stack, e.g., a switch, and a pulse generator. The pulse generator is capable of pulsing a cathode of the at least one cell of through the low impedance imposing means, e.g., when the switch is closed. The method transparently maintains the cells of a fuel cell stack, and includes sequentially pulsing the cathodes of a plurality of cells in a fuel cell stack, and maintaining a consistent number of the cells providing power to a load of the fuel cell stack while sequentially pulsing the cathodes of the cell.
Owner:TEXACO INC & TEXACO DEV CORP
Method for separating and extracting stem cells from placenta, umbilical cord or adipose tissue
ActiveCN101693884BEasy accessSpecification acquisitionSkeletal/connective tissue cellsArtificially induced pluripotent cellsDiseaseFicoll
The invention relates to a method for separating and extracting stem cells from a placenta, umbilical cord or adipose tissue, which comprises the following process steps: firstly mixing the placenta, umbilical cord or adipose tissue and a cell maintenance fluid according to the proportion by weight of 2.5-4:1, putting the mixture into a tissue crushing barrel, adding collagenase after crushing, uniformly mixing, hatching at the temperature of 37 DEG C, filtering, adding a precipitator, sucking a supernatant fluid after settling, centrifuging, removing the supernatant fluid, adding concentrated cells into a liquid of diatrizoate sodium-ficoll 400#, then centrifuging, collecting 10-15 ml of intermediate cell layer, washing with the cell maintenance fluid, counting the collected cells, and providing the cells for clinical use when the cell survival ratio is more than or equal to 95 percent. The invention not only realizes the separation and the extraction of all stem cells from the placenta, umbilical cord or adipose tissue, but also realizes industrialized production, and enables doctors to conveniently, safely and canonically obtain the adult stem cells in clinic and use the adult stem cells for treating the diseases of patients as using medicaments, thereby solving the bottleneck problem of difficult obtainment of adult stem cells in clinic and popularizing a cell treatment technology.
Owner:NINGXIA ZHONGLIANDA BIOPHYSICS
Method for producing rabies virus antigens for animals at a large scale
The invention discloses a method for producing rabies virus antigens for animals at a large scale, which produces the rabies virus antigens at a large scale by utilizing a bioreactor by a cell microcarrier suspension culture system. The method comprises the following steps: inoculating cells for preparing the antigens into a carrier tank containing a culture solution and microcarriers to enable the cells to be attached to the microcarriers; in a proper culture environment, enabling the cells to grow on the microcarriers until the quantity of the cells is 5-40 times more than inoculum density; making rabies viruses into a virus suspension, and enabling the virus suspension to be adsorbed on the cells; culturing the viruses in the proper culture environment by using a cell maintenance culture solution; continuously culturing for 3-5 days and then harvesting a virus solution for the first time, wherein a semicontinuous process is adopted and the ratio of a changed solution is 50 percent; continue culturing for 9-11 days, and harvesting the changed solution once every 24 hours; mixing the harvested virus solution with the virus solution of the bioreactor; and carrying out freeze thawing at the temperature of -20 DEG C and inactivation purification to obtain the rabies virus antigens. The method has large production scale, high single-scale yield and relatively low production cost.
Owner:PU LIKE BIO ENG
Method for large scale cultivation in bioreactor
ActiveCN102732487AEasy to operateReduce workloadMicroorganism based processesViruses/bacteriophagesVaccine ProductionFixed bed
The invention discloses a method for large scale cultivation in a bioreactor, comprising the following steps: (1) making a recombinant H5N1 avian influenza virus adapt to MDCK cells; (2) preparing a seed lot by using the adapted virus strain as a reactor large-scale culture virus, wherein the seed lot virus generation is no less than F6, the hemagglutination titer is no less than 28, and EID50 is no less than 107.5; (3) culturing the MDCK cells by using polyester sheets as a carrier and the reactor as a basket fixed bed, and pouring the cells on every gram of the carrier, wherein the culture medium is newborn calf serum DMEM; (4) inoculating the virus on the cultured MDCK cells, washing the cells by using a virus diluent, conducting virus inoculation, after absorption, and replacing a cell maintenance media; (5) maintaining the MDCK cells and conducting virus propagation, stirring, and replacing the cell maintenance media; and (6) monitoring the hemagglutination titer and harvesting the virus, and harvesting the reactor to culture a volume virus liquid. According to the invention, the method is easy to operate and the operation is simple, the HA titer of the virus after adaption is higher than 2<7>, and the problem that the culture virus titer cannot satisfy the demand of produce vaccine in the existing culture of the recombinant H5 subtype avian influenza virus on the MDCK cells is solved.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
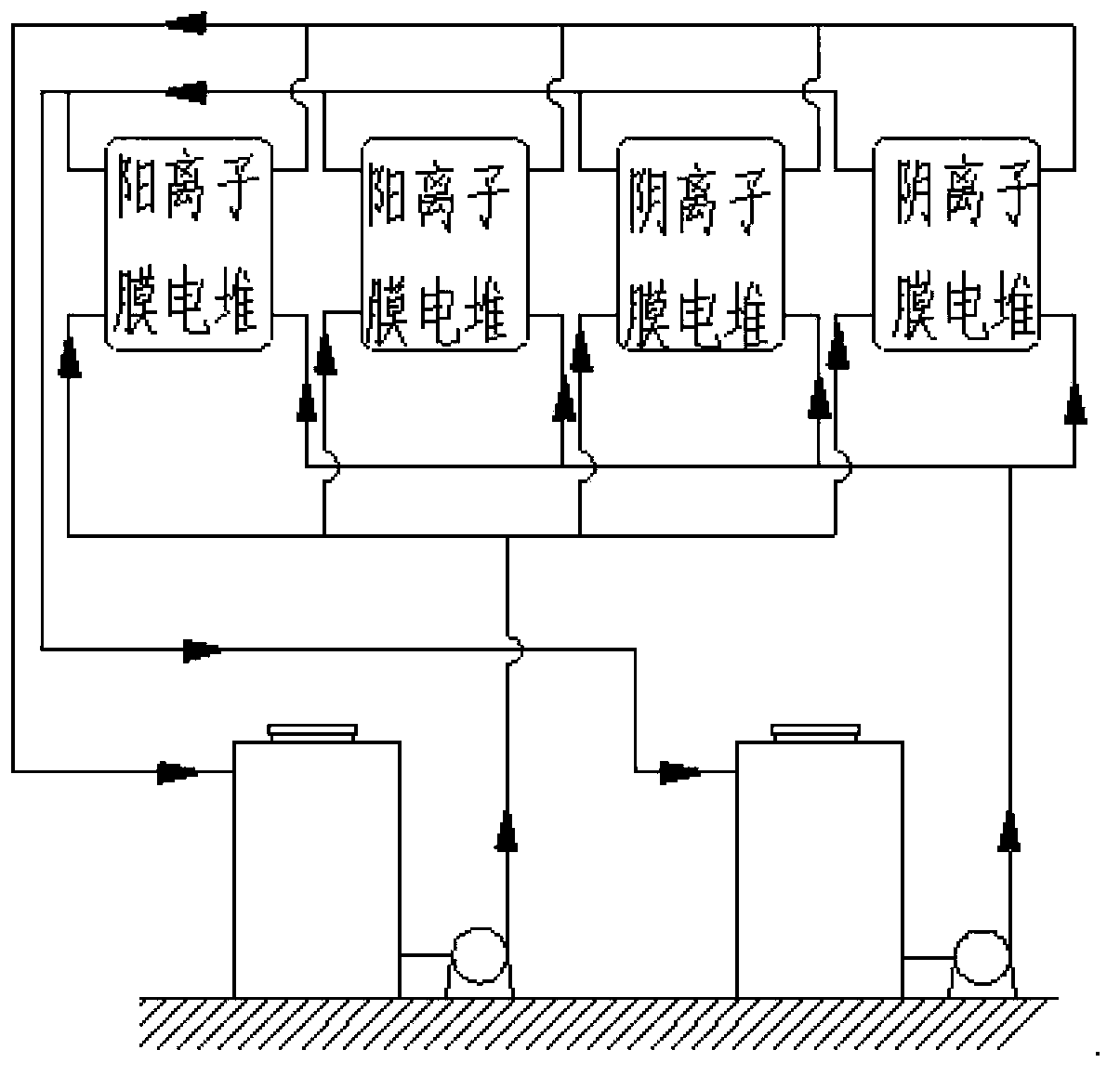
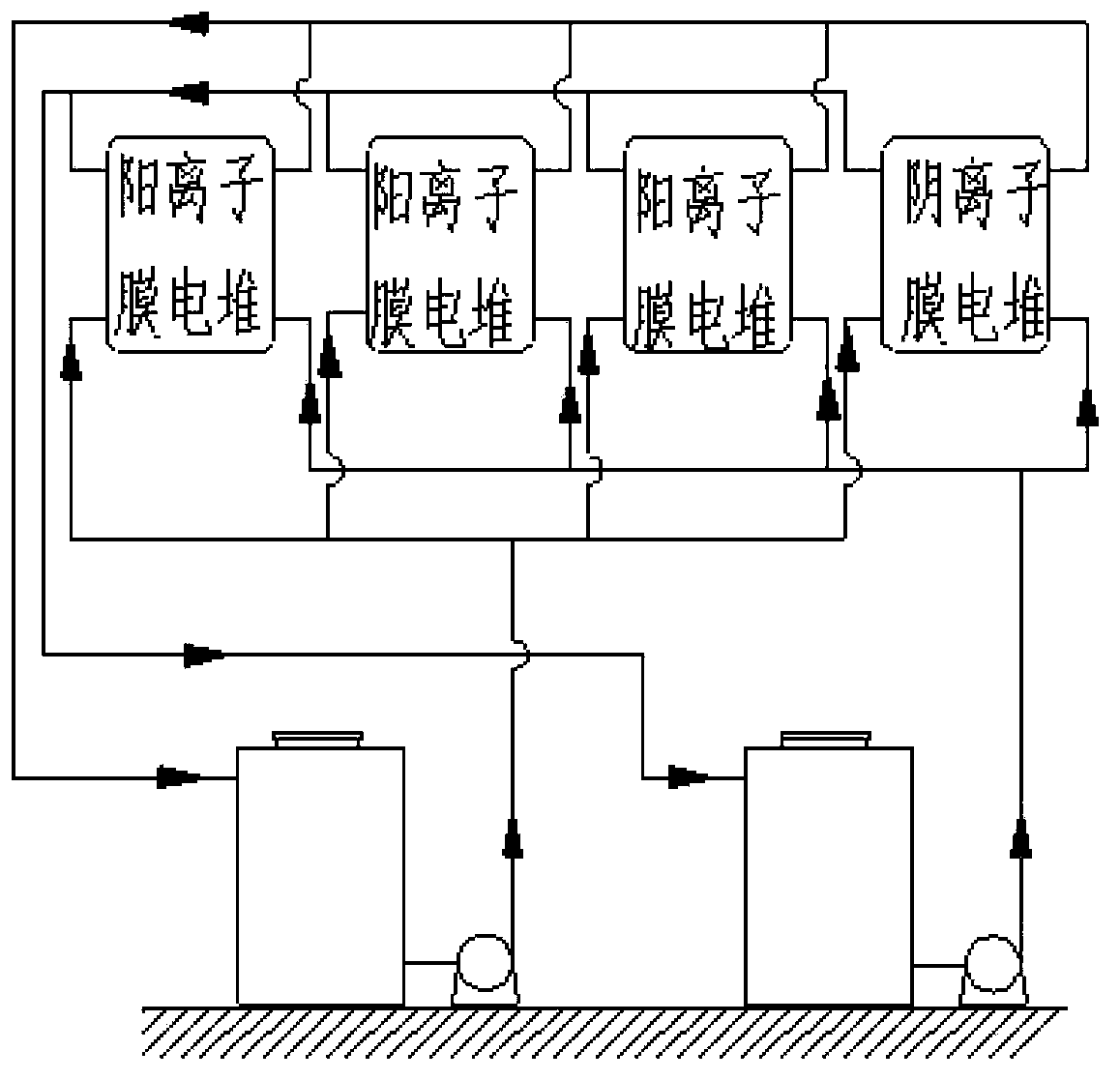
Redox flow cell system
InactiveCN104143651AImprove efficiencyIncrease profitIndirect fuel cellsFuel cell detailsFlow cellRedox
The invention relates to a redox flow cell system. The redox flow cell system comprises a cationic membrane pile, an anionic membrane pile, a positive electrode circulating pump, a positive electrode pipeline, a negative electrode circulating pump, a negative electrode pipeline, a positive electrode electrolyte storage tank filled with an electrolyte, and a negative electrode electrolyte storage tank filled with an electrolyte. The redox flow cell system formed by simultaneously using the cationic membrane pile and the anionic membrane pile can effectively reduce the change of the concentrations and the volumes of positive electrode and negative electrode electrolyte solutions in order to improve the efficiency of the cell system during long-term charge and discharge circulation and the utilization rates of the electrolyte solutions and prolong the service lives of the electrolyte solutions of the cell system. The system saves the cell maintenance time, avoids the consumption of extra electric energy and manpower and reduces the maintenance cost.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Monocyte separating method
The invention relates to a separating method for monocytes. The separating method comprises the following steps sequentially: a. injecting a monocyte separating reagent into a vessel, wherein the monocyte separating reagent comprises separating liquid, separating glue and cell maintenance liquid; b. collecting blood into the vessel and causing the blood to be uniformly mixed with the cell maintenance liquid; c. exposing the vessel to the influence of a centrifugal force so that differently-hierarchical zones are formed in the solution in the vessel; and d. sucking out a monocyte layer. The separating method has the advantages that the monocyte separation method is simplified, the blood is directly collected into a test tube, in which the separating reagent is positioned in advance, no anti-freezing blood needs to be prepared or diluted, the cell maintenance liquid prolongs the sample storage time before analysis, the separating glue effectively isolates erythrocytes after the centrifugation, and the mass control is easy.
Owner:CHENGDU RICH SCI IND
Inactivation inspection method suitable for BVDV, IBRV, PIV3 and BRSV inactivated vaccines
InactiveCN108152498AGuaranteed infection timeImprove the detection rateMaterial analysisFreeze thawingAntigen
The invention discloses an inactivation inspection method suitable for BVDV, IBRV, PIV3 and BRSV inactivated vaccines, and relates to the field of biological medicines, in particular to an inactivation inspection method for BVDV, IBRV, PIV3 and BRSV inactivated vaccines. The inactivation inspection method comprises the following steps: (1) adsorbing an inactivated virus liquor on an appropriate cell for 40-60 min, and shaking for 2-3 times in the period; (2) after adsorption, adding a certain volume of a cell maintenance fluid in a cell spinner bottle, continuously performing culture, observing cytopathy, as for the virus fluid free from cytopathy, harvesting a cell culture fluid, and performing freeze thawing for 2-3 times; and (3) taking 10-15 ml of the frozen and thawed cell culture fluid, inoculating the taken cell culture fluid on the appropriate cell for adsorption, and continuously performing culture. Observation and fluorescence detection are performed, and if no specific fluorescence appears, complete inactivation is manifested. According to the technical scheme disclosed by the prevention, the anti-gen concentration and the virus infection time are guaranteed. The virus detection rate of the inactivation inspection method is high, and the bio-safety of the inactivated vaccines can be effectively ensured.
Owner:华威特(江苏)生物制药有限公司
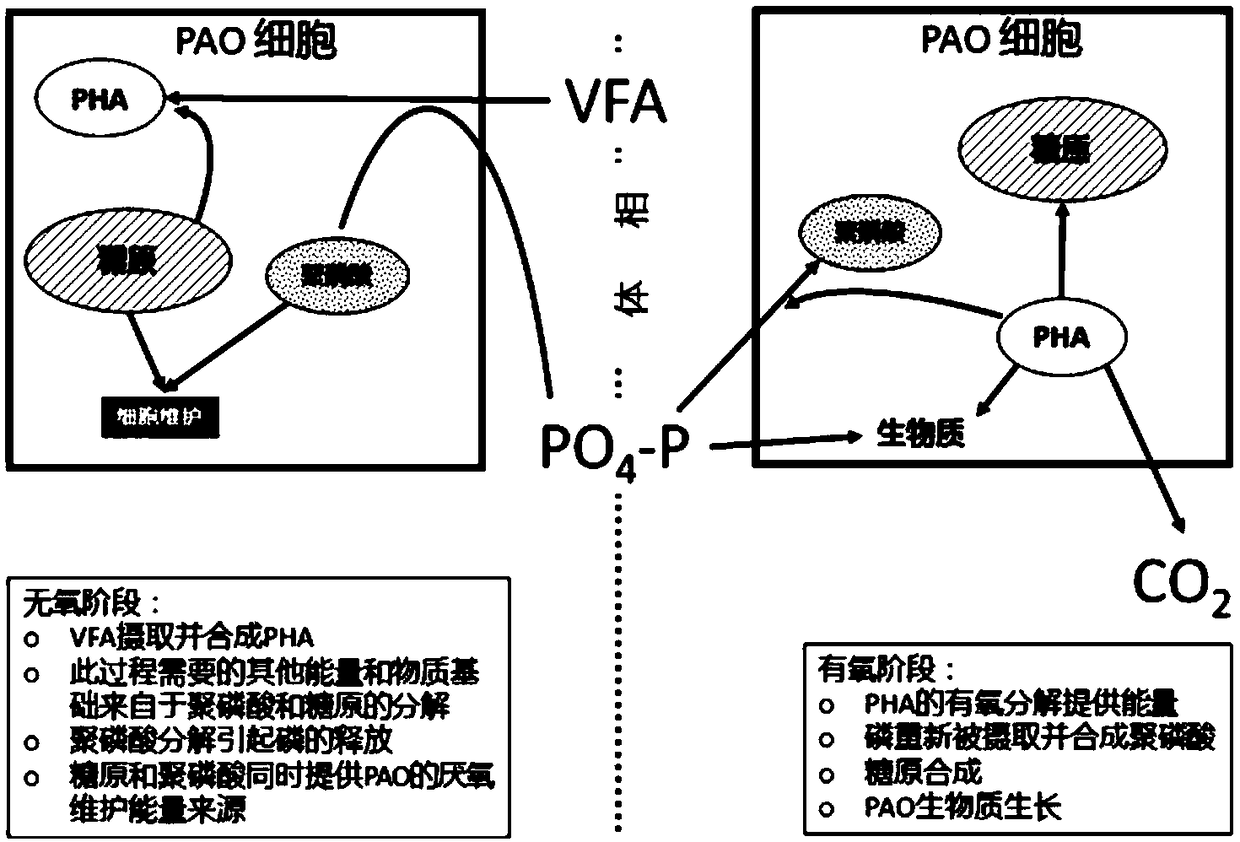
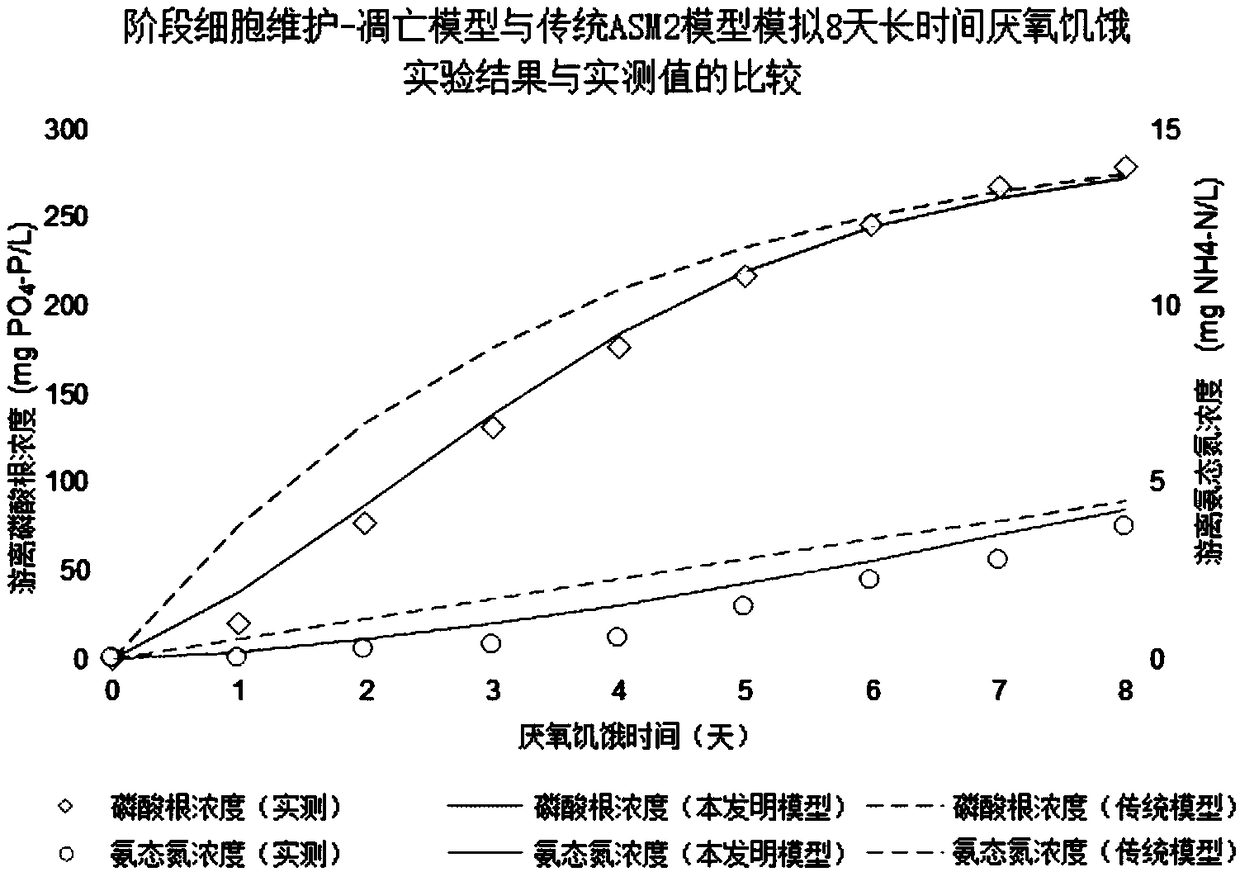
Method for optimizing phosphate-accumulating organism enrichment in sewage strengthened biological phosphorus removal course and method for improving properties of sewage strengthened biological phosphorus removal course
InactiveCN108996684AImprove processing stabilityReduce usageWater contaminantsTreatment with aerobic and anaerobic processesSludgeApoptosis
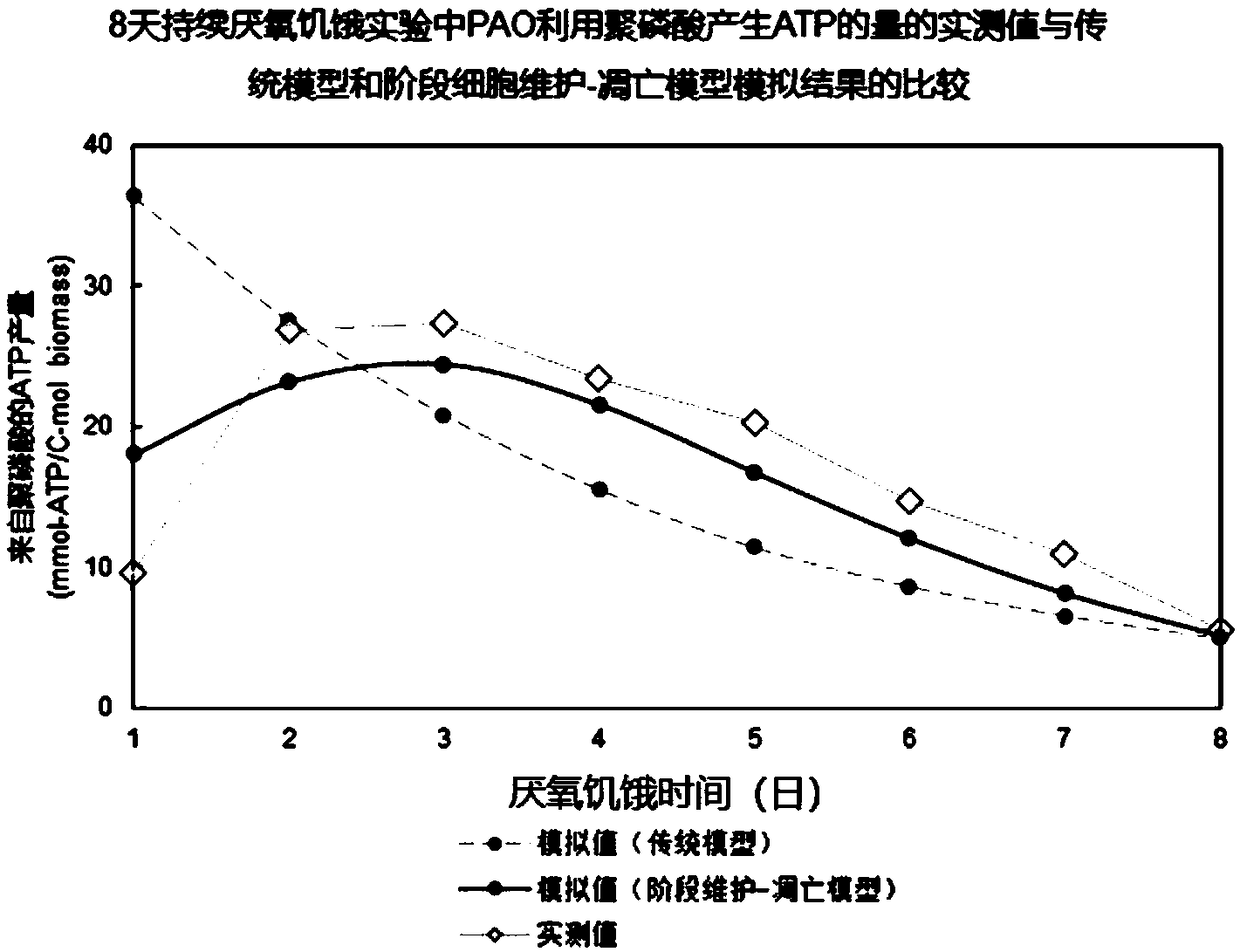
The invention provides a method for optimizing design and / or operation of phosphate-accumulating organism enrichment in a sewage strengthened biological phosphorus removal course. According to the method, an improved activated sludge model is used and comprises a phosphate-accumulating organism-glycogen accumulating organism competition module and staged cell maintenance-apoptosis. In addition, the invention further provides a method for improving the properties of the sewage strengthened biological phosphorus removal course. Through adoption of the method, the dependence degree on a carbon source in influent water in a sewage organism strengthened phosphorus removal course is reduced, and besides, phosphate-accumulating organisms are enriched.
Owner:谷中春
Online middle repairing method for electrolytic bath side part inner lining
An electrolytic bath sideliner on-line second line maintenance method relates to aluminium cell maintenance method. It includes 1, adjusting electrolyte aluminum liquid horizontal total height to 35-15cm; 2, adjusting bath voltage to 1.8-5.5 v; 3, keeping cell temperature at 850-1100 degree centigrade; 4, pressing one barrier layer between electrolyte and furnace side; 5, using pneumatic tools to clean electrolytic bath side covered material, electrolyte furnace side, residuary side liner material, depth reaching 20-45cm, whole side being cleaned; 6, laying single-layer carbonaceous paste on cleaned interface with thickness of 1-10cm; 7, using charcoal block or carborundum block laying side of carbonaceous paste; 8, recovering production according to managing method after starting up electrolytic bath. Said invented method can replace whole sideliner material without electrolytic bath stopping operation.
Owner:GUIZHOU BRANCH CHINA ALUMINUM IND
Collection and enrichment system for microbial aerosol
PendingCN112725160AEffective filteringSpeed up filteringBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismEnvironmental engineering
The invention provides a microorganism aerosol collecting and enriching system, which comprises an aerosol collecting assembly, an aerosol enriching assembly and a sample collecting assembly, wherein the aerosol collecting assembly is connected to the input end of the aerosol enriching assembly, the sample collecting assembly is arranged on the outer side of the aerosol enriching assembly in a sleeving mode, the aerosol enriching assembly comprises an aerosol enriching device and a collecting tube cover, the aerosol enriching device is filled with a cell maintenance solution, the bottom end of the aerosol enriching device is open, a filter plate is packaged on the outer side of the opening, an air inlet pipe and an air outlet pipe are arranged on the collecting pipe cover in a penetrating manner, the air inlet pipe comprises a first end and a second end, the air outlet pipe comprises a third end and a fourth end, the first end and the third end are located above the collecting pipe cover, the second end and the fourth end are both located in the aerosol enriching device, the second end is fixedly connected with a porous spheroid located in the cell maintenance solution, the first end is connected with a first sucking pump, the fourth end is located above the liquid level of the cell maintenance solution, and a plug is detachably inserted into the third end.
Owner:JIANGSU PROVINCE HOSPITAL THE FIRST AFFILIATED HOSPITAL WITH NANJING MEDICAL UNIV
Communication method and system applied to sharing economy
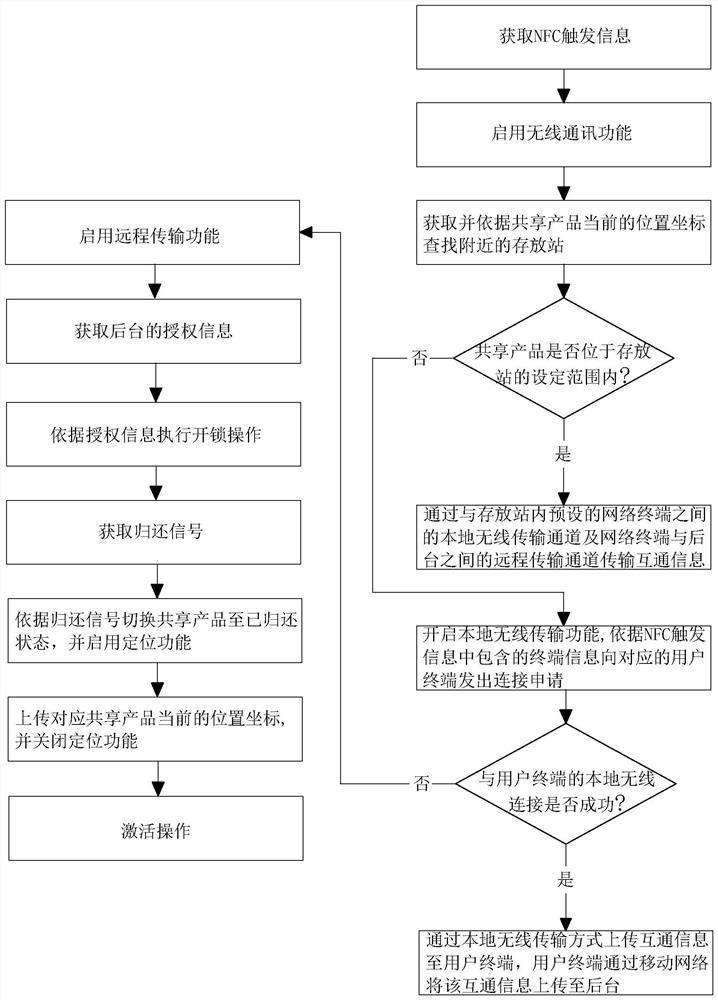
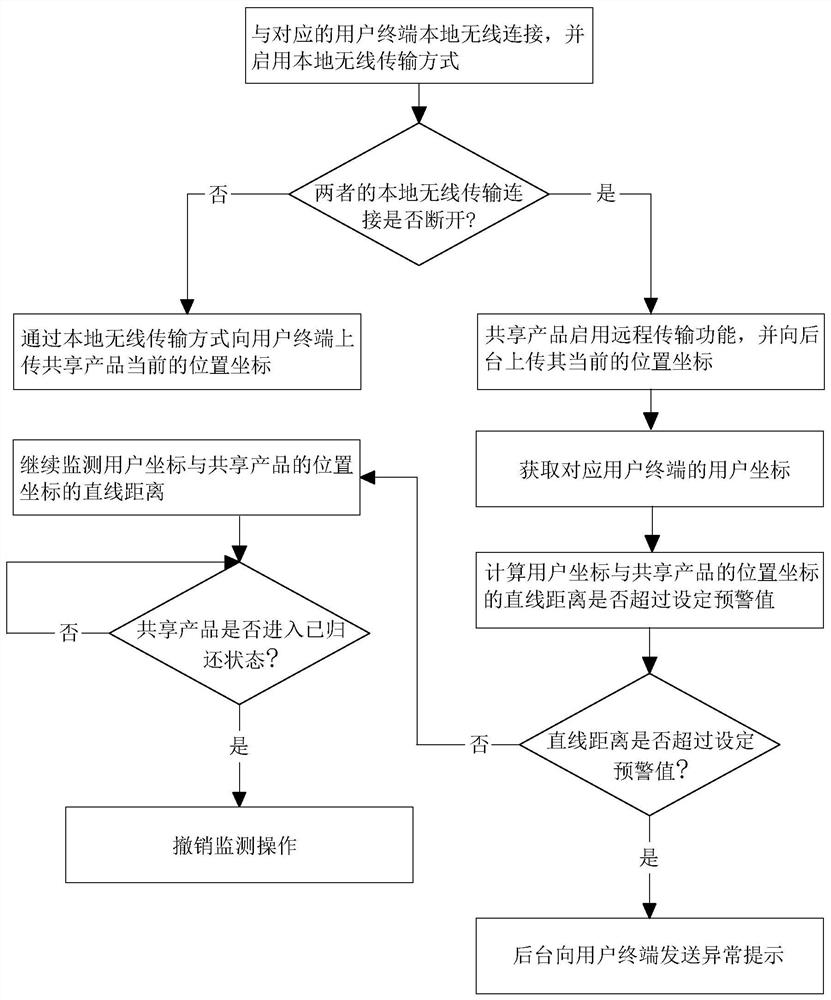
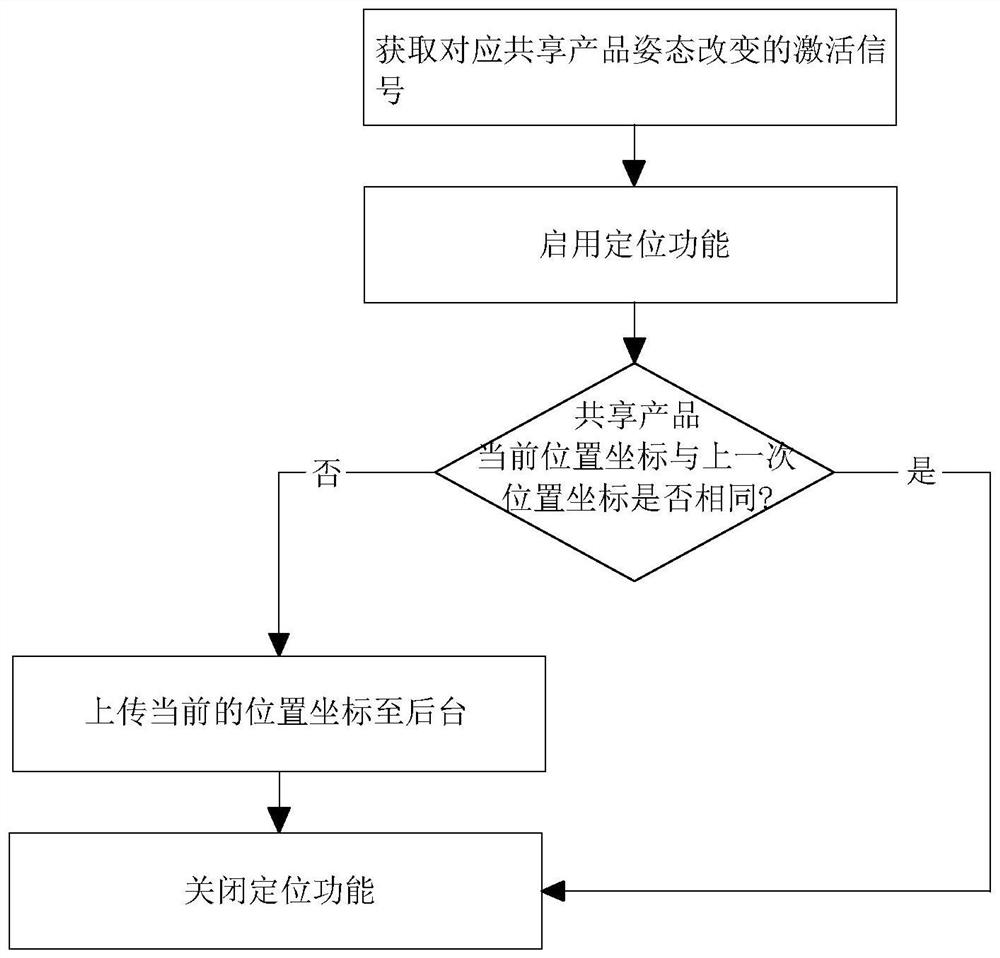
InactiveCN113554819AReduce turn-on timeReduce consumptionApparatus for meter-controlled dispensingShort range communication serviceInformation interoperabilityEngineering
The invention relates to a communication method and system applied to sharing economy. The method comprises the steps of: obtaining NFC trigger information obtained through NFC induction with a user terminal; starting a wireless communication function according to the NFC trigger information, and transmitting intercommunication information between the shared product and a background through the wireless communication function, the intercommunication information including authorization information and position coordinates of the shared product; executing unlocking operation according to the authorization information; obtaining a return signal; switching the shared product to a returned state according to the return signal, starting a positioning function, and uploading a current position coordinate of the corresponding shared product; and closing the positioning function. The method has the effects of reducing the battery maintenance frequency of the shared product and reducing the maintenance cost.
Owner:杨迎卯
Removal method of residual disinfectant in virus inactivation test
InactiveCN110643742AShorten test timeImprove detection efficiencyMicrobiological testing/measurementVirus inactivationDisinfectant
The invention belongs to the technical field of disinfection, relates to a virus inactivation test, and particularly relates to a removal method of residual disinfectant in the virus inactivation test. The removal method comprises the steps of that (1) a preparation step is performed, specifically, a virus suspension is prepared; (2) a removal step is performed, specifically, first, disinfectant is sucked into a test tube, disinfectant removal treatment is performed after water bath, then ultracentrifugation is performed after 30-50 times dilution, then supernatant is removed, finally a cell maintenance solution is added for dilution and mixing again,, and the residual disinfectant is removed; and (3) a detection step is performed, specifically, virus suspension is sucked and added and mixed well, and a final sample is sucked for virus titer determination. According to the removal method of the residual disinfectant, the test time for disinfectant removal in the whole process can be shortened, the test efficiency of the virus inactivation test can be greatly improved, and the effects of disinfectants and neutralizers on cells and viruses in chemical neutralization methods are avoided.
Owner:GUANGDONG PROVINCIAL INST OF BIOLOGICAL PROD & MATERIA MEDICA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com