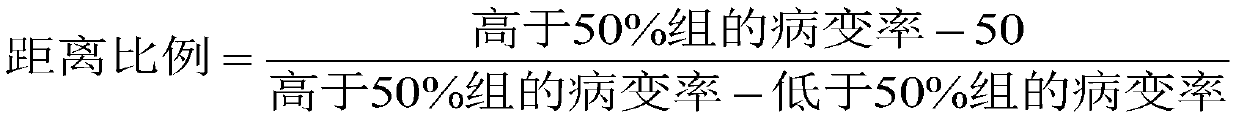Removal method of residual disinfectant in virus inactivation test
A virus inactivation and disinfectant technology, applied in the field of disinfection, can solve the problems that the neutralizer components cannot be harmful to microorganisms or cells, the chemical neutralizer is difficult to find, and the cells are affected, so as to eliminate adverse effects, reduce test time, The effect of improving detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0023] A method for removing residual disinfectant in a virus inactivation test, characterized in that said method for removing comprises the following steps:
[0024] 1) Preparation steps: prepare virus suspension; dilute the disinfectant to be tested with sterilized hard water to 1.25 times the concentration used;
[0025] 2) Removal steps: first absorb the antiseptic into the test tube, perform antiseptic treatment after water bath, then perform 30-50 times dilution and then ultracentrifuge, then discard the supernatant, and finally add cell maintenance solution to dilute and mix to remove residual Disinfectant; in this embodiment, add cell maintenance solution and dilute and mix the volume to 1mL~2mL, the volume ratio of virus suspension to disinfectant is 1:4, and the virus suspension is 0.2mL~0.4mL; where the water bath temperature The temperature is 20°C±1°C, the time is 5min, the centrifugation conditions are: speed 10000rpm-25000rpm, centrifugation time 1h-2.5h, tempe...
specific Embodiment
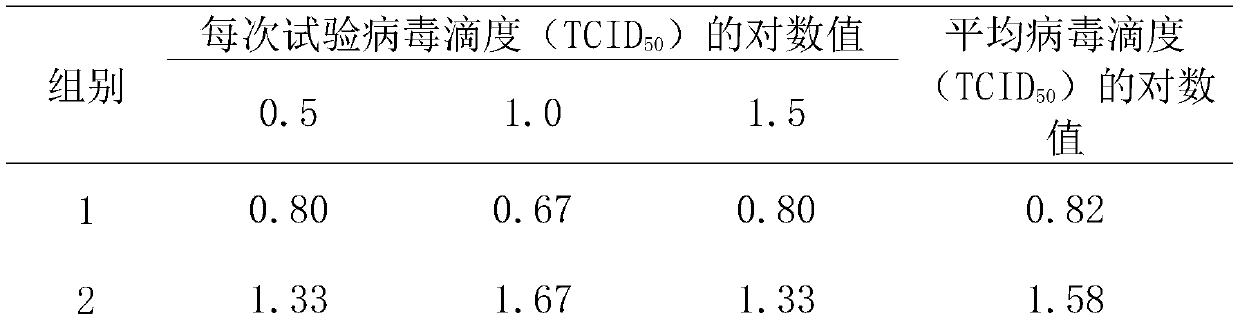
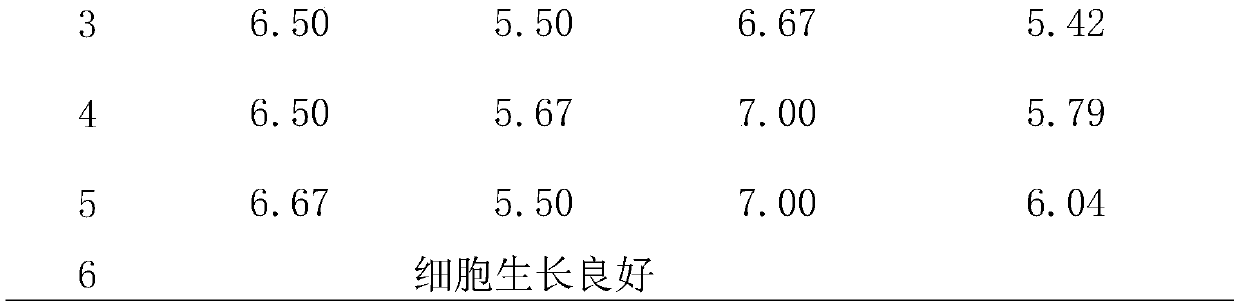
[0034] 1. Scope of application
[0035] This embodiment is mainly applicable to disinfection product identification or daily monitoring, with a certain representative, live virus and its cell infection technology, to evaluate the killing effect of various disinfection factors on the test virus, to the inactivation of disinfection factors Important aspects of viral competence were validated.
[0036] 2. Test equipment
[0037] (1) Virus strains used in the test: poliovirus type Ⅰ (poliovirus-Ⅰ, PV-Ⅰ) vaccine strain, enterovirus type 71 (EV71) provided by the Guangdong Provincial Center for Disease Control and Prevention;
[0038] (2) Host cells: Hep-2 cells, as test cells for PV-1; VERO cell lines, as test cells for EV71; both cells were provided by Guangdong Provincial Center for Disease Control and Prevention;
[0039] (3) Cell culture flasks and 96-well culture plates;
[0040] (4) Test equipment: constant temperature water bath; carbon dioxide incubator; laminar flow ult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com