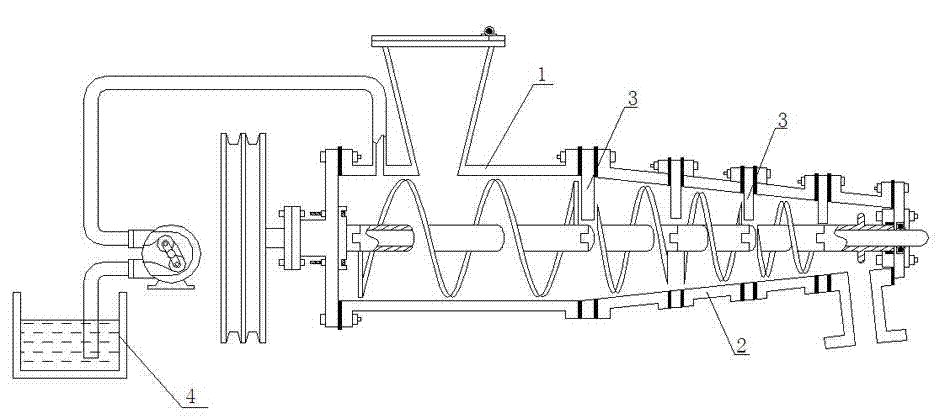
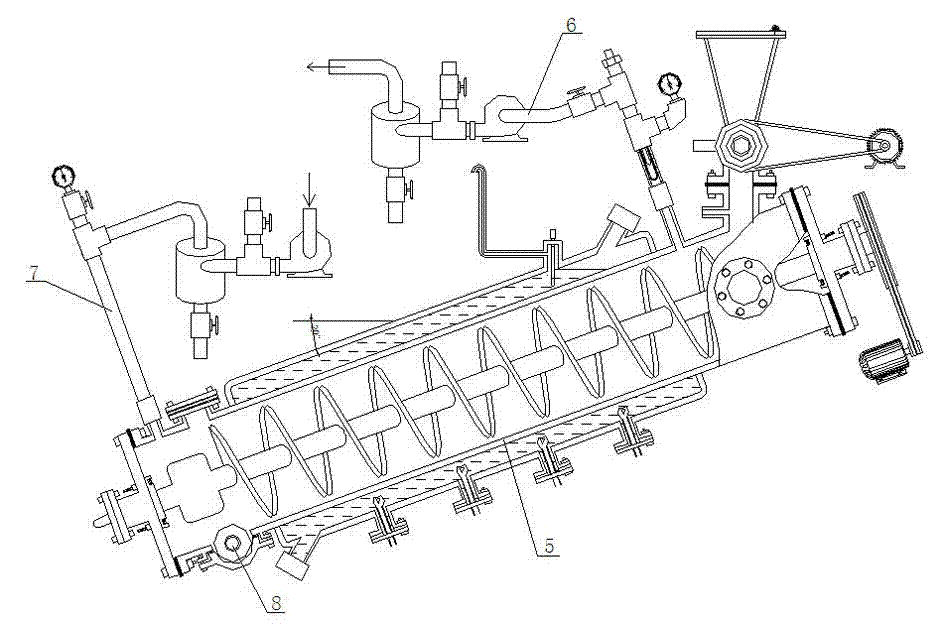
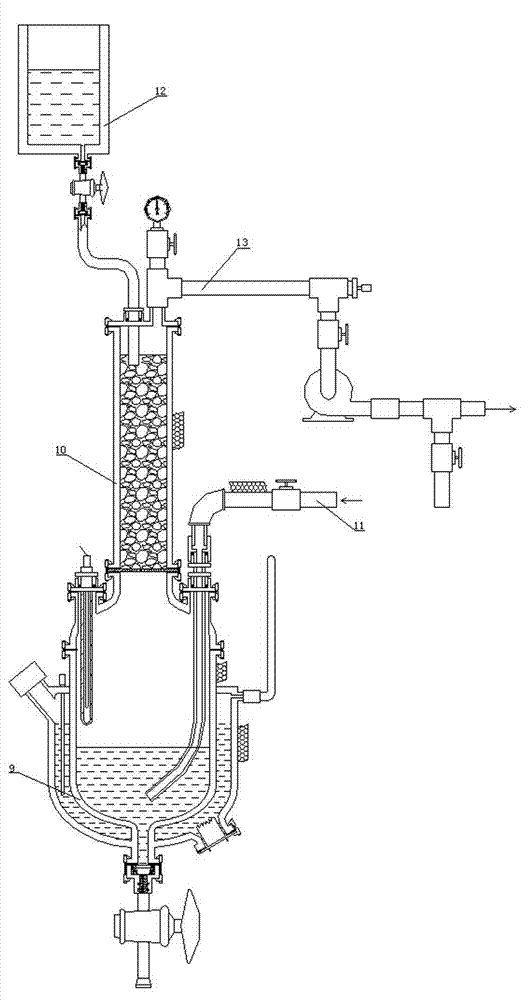
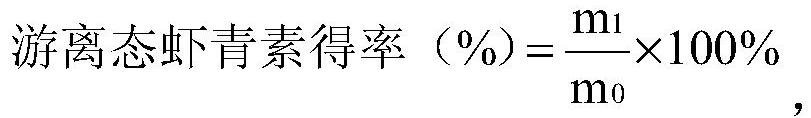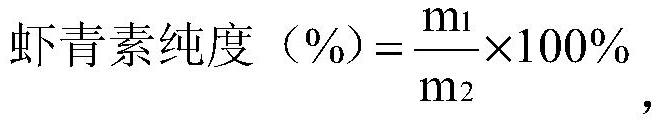Patents
Literature
41results about How to "No change in biological activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Reagent box for separating karyocyte in vitro and application method thereof
ActiveCN1920012ANo change in biological activityConvenient treatmentBlood/immune system cellsFicollPaleontology
The invention relates the separating agent box and application. The agent box comprises erythrocyte precipitating agent, hypaque sodium- ficoll 400# or HISTOPAQUE1077 and cell maintenance liquid. The agent box can separate bone marrow and umbilical cord blood, on the surface of cell there is no any mark, and it doesn't change biological activity of cell. The invention can commercially manufacture, and the application is wide.
Owner:NINGXIA ZHONGLIANDA BIOPHYSICS
Method for preparing microcapsule coated microvesicle ultrasonic contrast medium
InactiveCN101219224AImprove acoustic propertiesGood contrast effectEchographic/ultrasound-imaging preparationsMicro bubbleMedicine
A preparation method of a micro bubble ultrasound contrast agent coated by a microcapsule relates to a preparation method of a micro bubble ultrasound contrast agent. The invention solves the problems of the instability of a micro bubble contrast agent and the disappearance of acoustic characteristic of a modified micro bubble. The preparation method of the micro bubble ultrasound contrast agent is that: absorbing, standing or centrifuging, cleaning, absorbing once again, standing or centrifuging and cleaning once more; finally the micro bubble ultrasound contrast agent is available. The invention has simple process, mild reaction condition, simple operation, good reproducibility, wild range of application and environment-friendliness, and is applicable to the surface modification of various ultrasound contrast agents and can be easily implemented.
Owner:HARBIN INST OF TECH
Method for cultivating hepatitis e virus
InactiveCN105062979AImprove replication efficiencyNo change in biological activityViruses/bacteriophagesDrugEpidemiology
The invention discloses a method for cultivating hepatitis e virus. The method comprises the steps that a serum of a later pregnant woman is added into an HEB strain obtained through separation of an epidemic disease investigation for in-vitro culture, the virus can be replicated massively, and immunofluorescence and Western Blot analysis are utilized for revealing that the serum of the later pregnant woman can promote massive replication of HEV progeny virus. According to the method, great breakthrough is made in the aspect of HEV in-vitro culture, and a good foundation is laid for further researching the biological and immunological characteristics of HEV and the HEV infection and pathogenic mechanism and screening and identifying anti-HEV drugs.
Owner:KUNMING UNIV OF SCI & TECH
Method for preserving bone tissue materials
InactiveCN110402918AKeep Inorganic Matter IntactSimple structureDead animal preservationRinger's solutionOrganic component
The invention discloses a method for preserving bone tissue materials. The method for preserving the bone tissue materials comprises the steps that (1) the bone tissue materials are cleaned; (2) the bone tissue materials are placed into a povidone iodine preservation solution with mass concentration of 0.01%-0.5%, the mixture is stored in refrigeration equipment, and the storage temperature is lower than 0 DEG C; and (3) the bone tissue materials stored in a refrigerated mode and the immersion liquid are transferred to the refrigeration, and the mixture is taken out after thawing, the mixtureis soaked by 0.9% physiological saline, a ringer's solution or phosphate buffer and then cleaned, and the mixture can be used as a bone graft tissue. The preservation solution uses sodium bicarbonateor the phosphate buffer as a pH adjuster such that a pH value of the preservation solution is between 5.8 and 8.0. According to a cryopreservation bag, the side edge of a part of the bag body is openthe bag body is connected with a flow guiding hose, and the outer end of the hose is closed. According to the method for preserving the bone tissue materials, the integrity of a bone tissue structureis maintained, and the mechanical properties are kept unchanged; and organic components and inorganic components of the bone tissue materials are not lost, so that the suffering and economic burden ofthe patient are alleviated.
Owner:庄明华
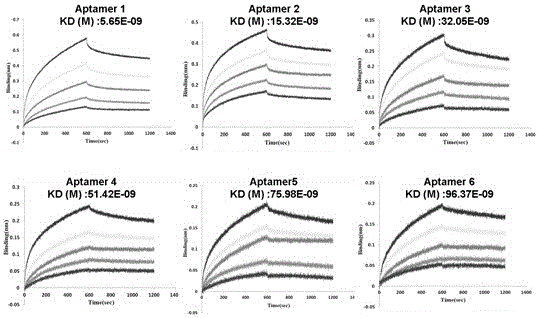
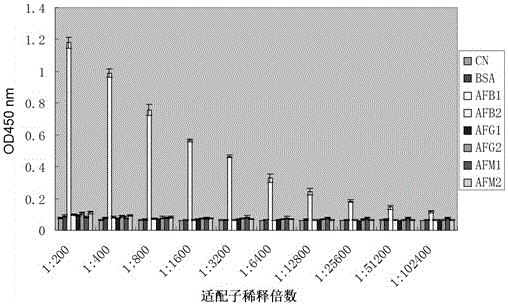
Single chain DNA oligonucleotide aptamer capable of specifically recognizing aflatoxin B1
ActiveCN104911186AEasy to distinguishStrong specificityDNA preparationDNA/RNA fragmentationAptamerFluorescence
The invention discloses a single chain DNA oligonucleotide aptamer capable of specifically recognizing aflatoxin B1. The invention provides the single chain DNA oligonucleotide aptamer capable of highly and specifically recognize aflatoxin B1, and aflatoxin B1 in food can be rapidly and accurately detected through a labeling method. In the invention, aflatoxin B1 in food and grain can be taken as a target substance, a nucleic acid aptamer sequence specifically bind to aflatoxin B1 can be obtained by using a SELEX technology, the aptamer can be converted to a report nucleic acid aptamer by labeling report molecule, rapid detection of aflatoxin B1 in food can be realized by routine detection means such as colloidal-gold test paper, fluorescence report detection and an ELISA method, massive synthesis is carried out, chemical modification reconstruction is easily carried out, stability is high, and the single chain DNA oligonucleotide aptamer can be widely used on food detection, base research, clinical diagnosis and medicine research.
Owner:ZHEJIANG PUZHENG DETECTING TECH
Method for analyzing carbohydrate-binding protein by functional sugar chip and flight mass spectrometer
ActiveCN101210928AStable activitySimple and efficient operationComponent separationBiological testingIonChemistry
A method for analyzing carbohydrate-binding protein with a functional carbohydrate chip and a time-of-flight mass spectrometer comprises the following steps of: preparing a coupling agent solution, incubating solid carrier, cleaning, drying, dissolving reducing carbohydrate in water, adjusting to required concentration, spotting, and incubating to obtain a functional carbohydrate chip; directly adding a biological sample on the functional carbohydrate chip, incubating, removing impurities, forming crystal, dissociating crystal, detecting the flight time of charging ions in a vacuum electric field with a time-of-flight mass spectrometer, obtaining a protein map that directly shows molecular weight, abundance and other information of proteins in the detected biological sample, and identifying protein differential expression; and comparing the protein map information of the detected biological sample with a sample map information to obtain the information of carbohydrate-binding protein with differential expression. The invention solves the technical problems in the prior art, including low detection throughput, complex detection procedures, and long detection period. The invention has the advantages of simple operation, high sensitivity and good repeatability; and is suitable for rapid analysis in medical clinics.
Owner:SHANXI LIFEGEN
Method for extracting nicotine through steam distillation and acid absorption
InactiveCN104761538AEfficient separationNo change in biological activityOrganic chemistryChemistryProcess engineering
The invention discloses a method for extracting nicotine through steam distillation and acid absorption. The method comprises the following steps: crushing a tobacco raw material, adding the crushed tobacco raw material into a feeder together with an alkali liquid, and mixing so as to obtain an alkali-containing material; conveying the alkali-containing material into a steam distillation tank, wherein the alkali-containing material and steam inversely moves to be mixed, so as to obtain steam with nicotine; introducing steam with nicotine into an acid absorbing liquid in a jacket type enamel kettle so that the acid absorbing liquid absorbs nicotine till the acid absorbing liquid is saturated, discharging out, separating, and purifying, thereby obtaining a nicotine extract; reabsorbing the gas generated inside the jacket enamel kettle by using a columnar enamel cylinder, and discharging the gas into a steam distillation tank for recycling. By adopting the method, nicotine can be effectively separated from other components in tobacco, the biological activity of solanesol in residues can be kept unchanged, and industrial production of a nicotine product can be achieved.
Owner:NANYANG NORMAL UNIV
Preparation method of biological sterilization preparation and application thereof
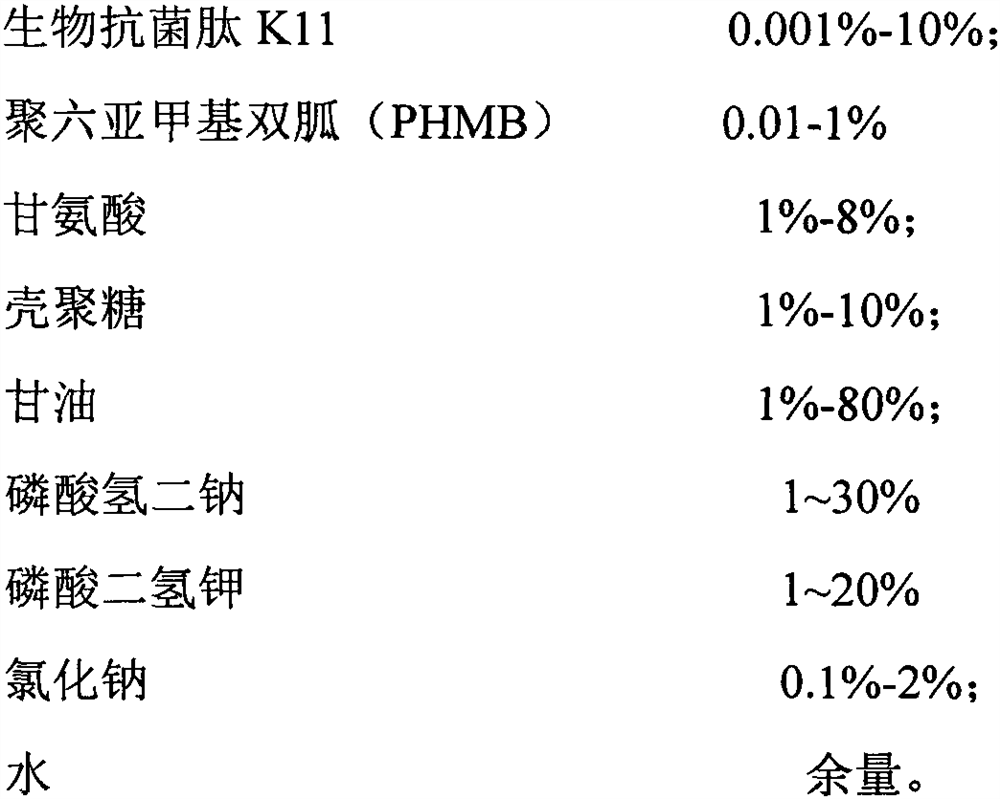
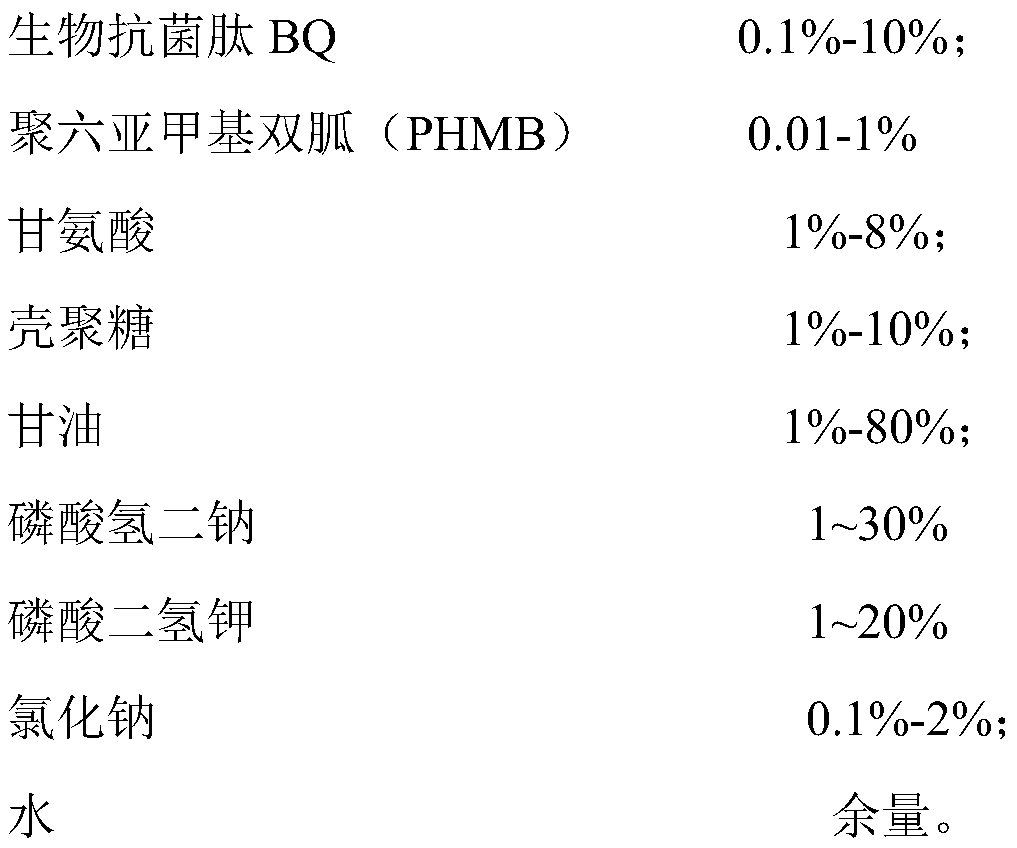
PendingCN112999331AImprove the bactericidal effectImprove securityAntibacterial agentsBiocideStaphyloccocus aureusEnterobacter
The invention discloses a biological sterilization preparation, a preparation method and an application thereof, the biological sterilization preparation takes water as a carrier, and comprises the following components by weight percent: 0.001-10% of biological antibacterial peptide K11; and 0.01 to 1% of polyhexamethylene biguanide (PHMB). The product has a good sterilizing effect. A core sterilization component of the preparation is novel antibacterial peptide which has a very strong sterilization effect on staphylococcus aureus, escherichia coli, candida albicans and viruses. The sterilization rate can reach 99.9% after 2-5 minutes of in-vitro experiment. The product is high in safety, free of toxicity or residues to human bodies, high in stability and capable of being used as a preventive drug. The main acting component is a biological agent, and the defects that a traditional chemical preparation has irritation to mucosa, and bacteria easily generate drug resistance are overcome. The composition is easily accepted by organisms and has no toxic or side effect. The product is suitable for long-term use or preventive medication.
Owner:昆山博青生物科技有限公司
Staphylococcus lysozyme local sustained release preparation, preparation method and application thereof
InactiveCN101618210AImprove the bactericidal effectImprove release abilityAntibacterial agentsPeptide/protein ingredientsMicrosphereMicroparticle
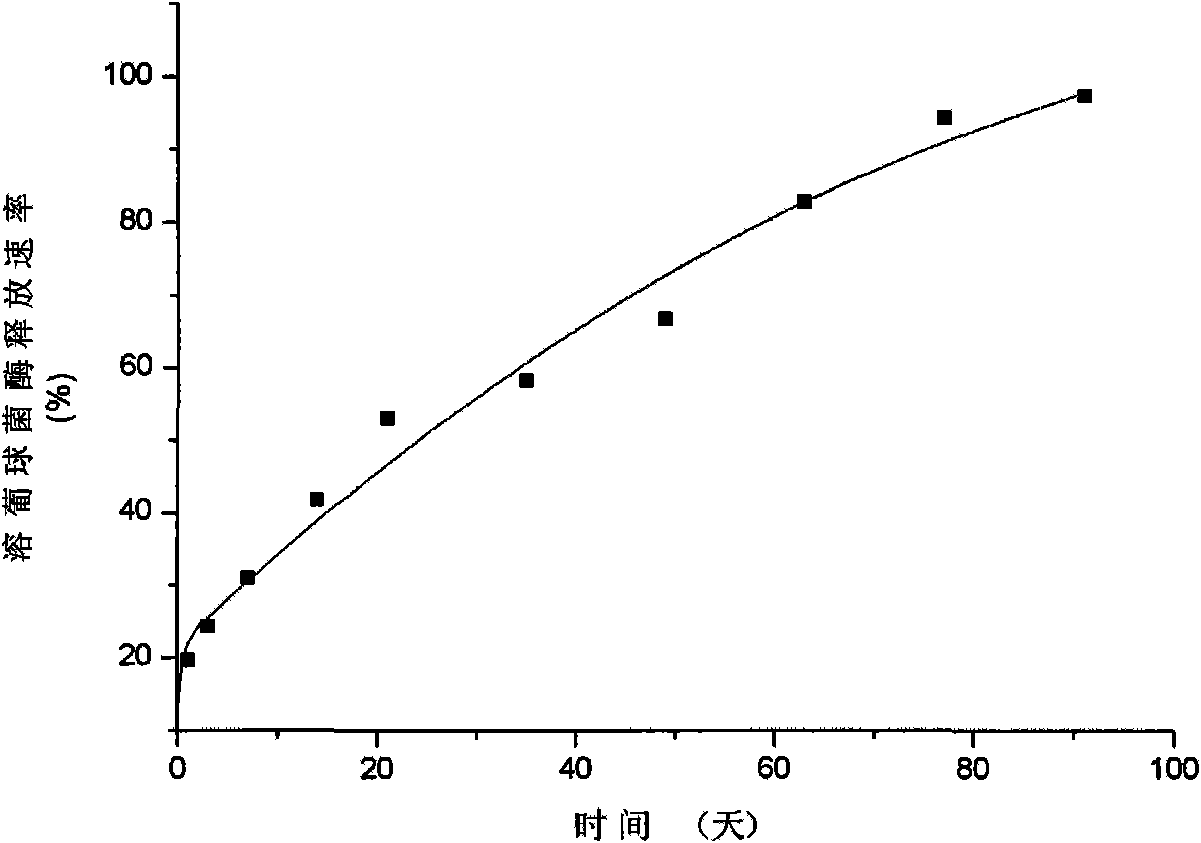
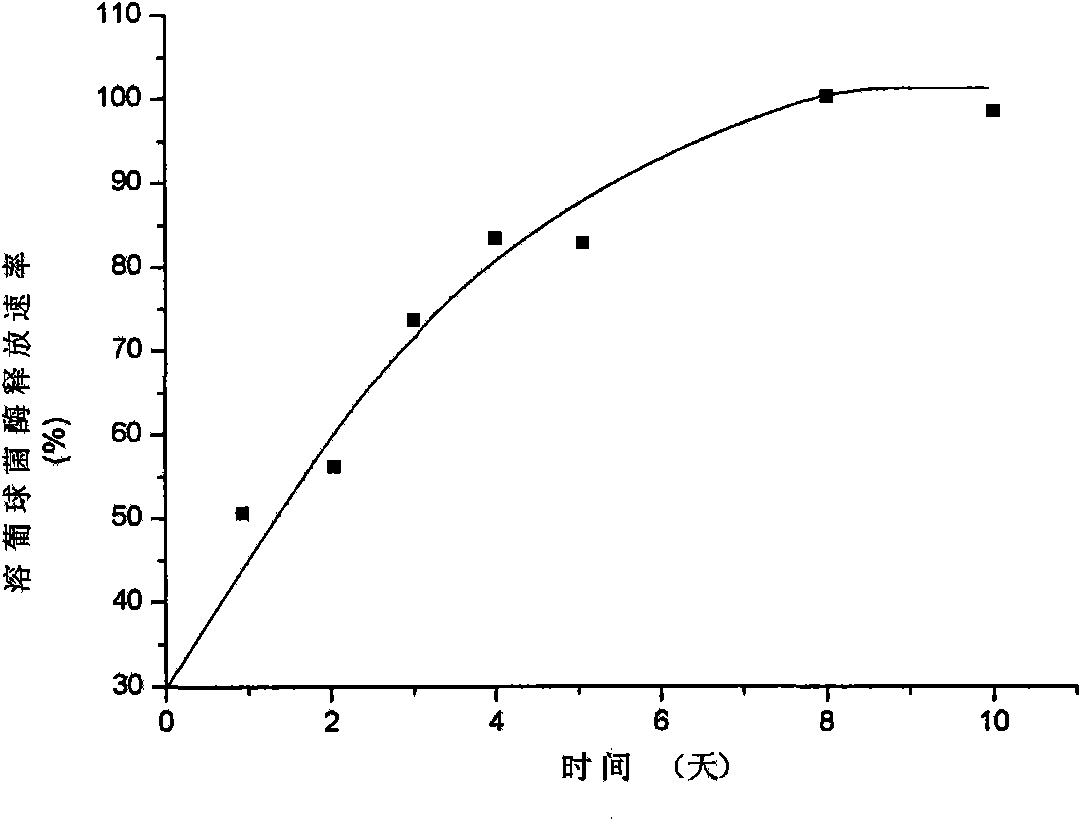
The invention discloses a staphylococcus lysozyme local sustained release preparation, a preparation method and application thereof. The preparation comprises the following components in percentage by weight: 1 to 10 percent of staphylococcus lysozyme, 1 to 10 percent of mannite, 0 to 90 percent of polylactic acid (PLA), and 5 to 95 percent of polylactide-co-glycolide (PLGA). The preparation takes the staphylococcus lysozyme as a bactericidal active component to obtain polylactide-co-glycolide (PLA-PLGA) polymer microspheres containing the staphylococcus lysozyme through supercritical fluid microparticle preparation technology. The preparation has good physical and chemical properties and strong sterilization effect, has adjustable time during the in vitro medicament release, ensures that the medicament release process can reach three days to three months, and has steady release rate.
Owner:SHANGHAI HI TECH UNITED BIO TECHCAL RES
Suppository for treating mammal endometritis
ActiveCN102552888AIncrease contactProlong the action timePeptide/protein ingredientsSuppositories deliverySodium bicarbonateDecomposition
The invention belongs to the technical field of medicines, and specifically relates to a suppository for treating mammal endometritis. The preparation disclosed in the invention comprises the following components of: by weight, 0.004-0.01% of staphylococcus lysozyme, 0.1-4% of lysozyme, 10-80% of semisynthetic fatty glyceride, 1-20% of poloxamer 188(F68), 1-20% of HPMC, 5-50% of sodium bicarbonate and 5-50% of sodium dihydrogen phosphate. After the preparation provided by the invention enters into mammal uterus through administration, the preparation can undergo effervescence decomposition in a body fluid. The medicine is mixed in foams and rapidly released. Contact area between the medicine and mucosal of uterus becomes larger and the medicine can penetrate into deep portions of mucosa wrinkles so as to prolong the action time between the medicine and the mucosa, raise the concentration of the medicine on local tissues, further enhance the curative effect and effectively kill various pathogens which can cause endometritis.
Owner:昆山博青生物科技有限公司
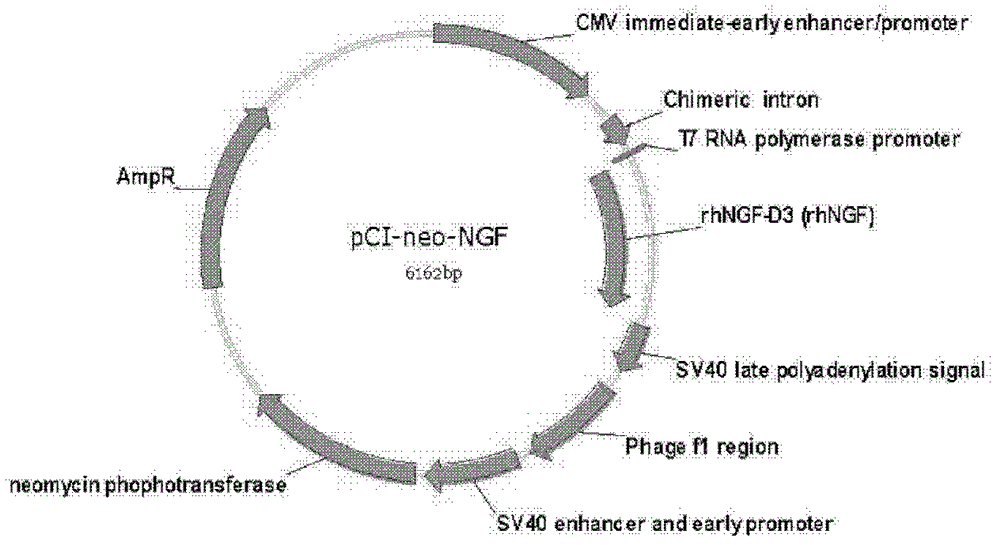
Recombinant human nerve growth factor deletion mutant, its preparation method and application
ActiveCN102898514BIncrease protein expression levelBiological activity remains unchangedFermentationAnimals/human peptidesDeletion mutantEukaryotic cell
Disclosed is a deletion mutant of a recombinant human nerve growth factor. The mutant is a peptide chain of the complete human nerve growth factor, with 3 amino-acids at C-terminal deleted, wherein the amino-acid sequence of the mutant is as shown in sequence list SEQ ID No.1, or the amino-acid sequence of the mutant is an amino-acid sequence that shares over 95% homology with that which is shown in SEQ ID No.1 and has the biological activity of a nerve growth factor. The mutant in the invention retains the C-terminal integrity in rhNGF by the strategy of expressing rhNGF with 3 amino-acids deleted at C-terminal (rhNGF-D3), so the expression level of rhNGF-D3 protein in eukaryotic cells is increased five- to ten-fold by this modification compared to rhNGF, with the same bioactivity and uniform protein C-terminal.
Owner:军事科学院军事医学研究院生物工程研究所 +1
Effervescent suppository for treating mammal vaginitis
ActiveCN103784948ARapid effectFacilitated releasePeptide/protein ingredientsSuppositories deliverySodium bicarbonateTherapeutic effect
The invention belongs to the technical field of medicines, and particularly relates to an effervescent suppository for treating mammal vaginitis. A preparation disclosed by the invention consists of the following components in percentage by weight: 0.01-0.04 percent of recombinant lysostaphin, 10-50 percent of lactose, 5-30 percent of microcrystalline cellulose, 1-20 percent of polyoxyl[40]stearate, 1-10 percent of povidone, 5-40 percent of sodium bicarbonate, 5-40 percent of sodium dihydrogen phosphate and 0.1-5 percent of superfine silica powder. The preparation can disintegrate in an effervescent manner in the presence of body fluid after entering the vagina of a mammal, and medicaments are released rapidly by being mixed in foam, so that the contact area between the medicaments and vaginal mucosa is increased, the medicaments can permeate deep into mucosa folds, the acting time of the medicaments on mucosa is prolonged, the local tissue medicament concentration is increased, the treatment effect is enhanced, and various pathogenic bacteria causing vaginitis are killed effectively.
Owner:昆山博青生物科技有限公司
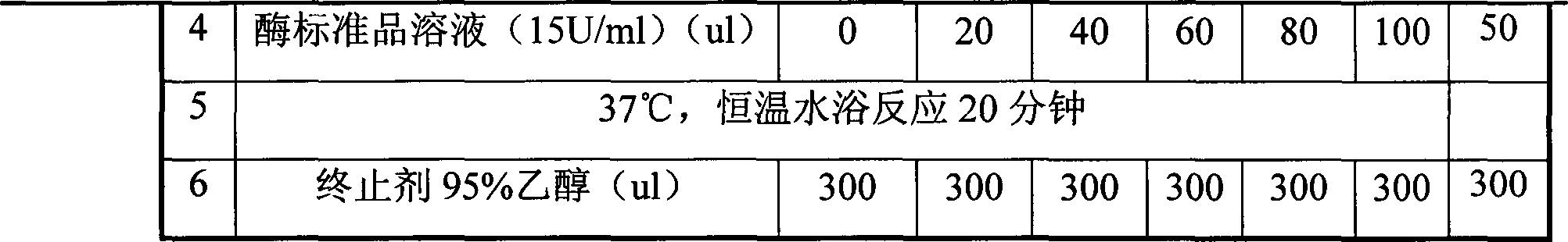
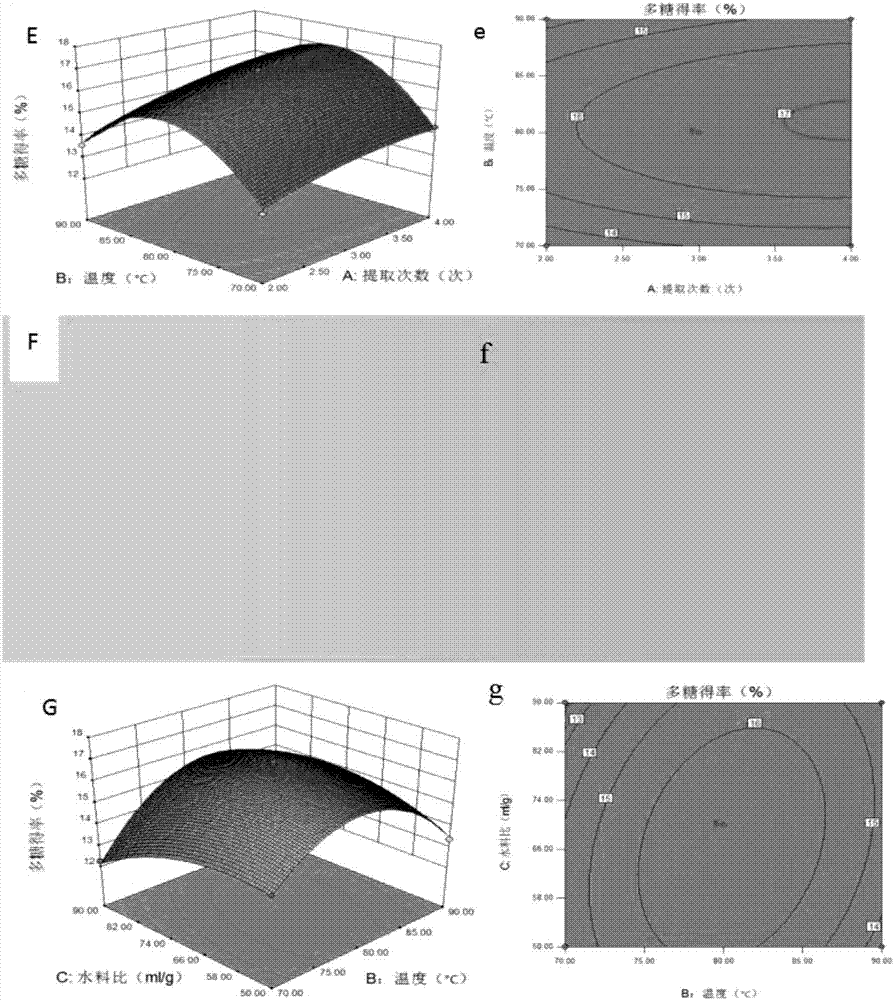
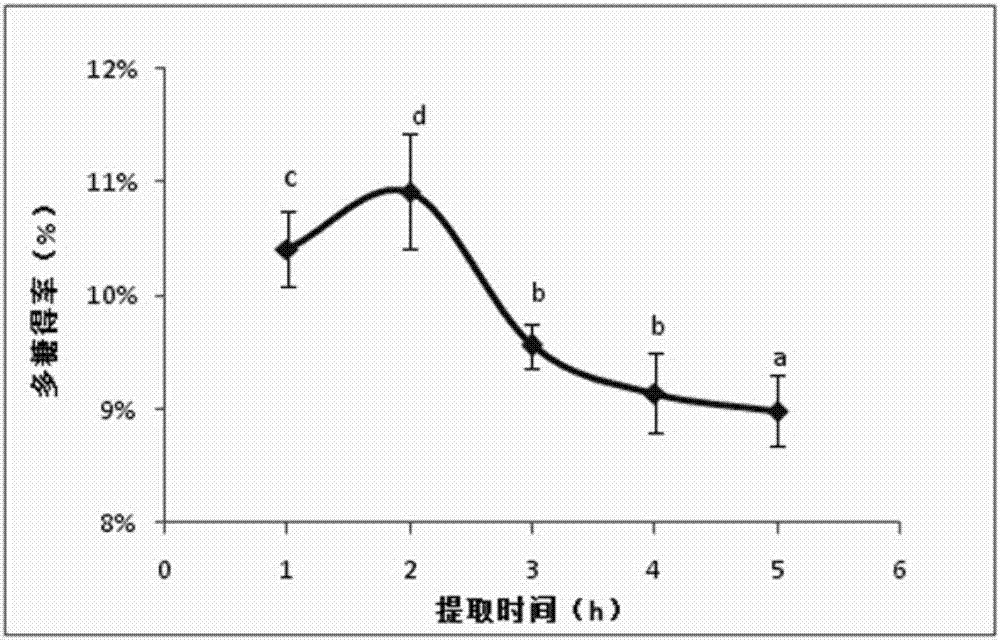
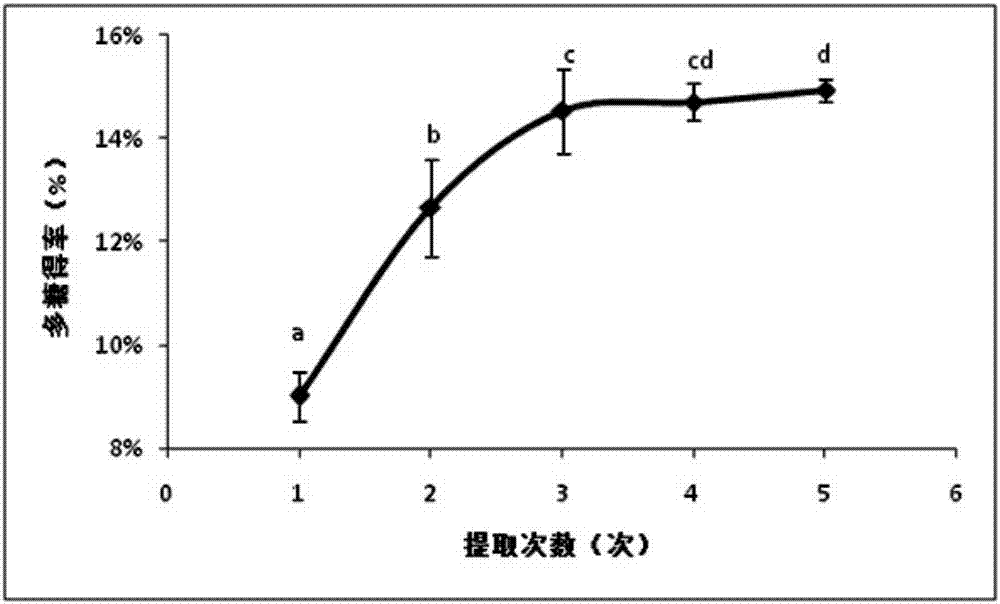
Angelica anomala polysaccharide extracted on basis of response surface optimization and preparation method and application thereof
ActiveCN107090051AHigh antibacterial activityImprove antioxidant capacityAntibacterial agentsAntinoxious agentsChromatographic separationAlcohol
The invention discloses an angelica anomala polysaccharide extracted on the basis of response surface optimization and a preparation method and an application thereof. The molecular weight of the angelica anomala polysaccharide is 1.17kDa; after angelica anomala as a medicinal material undergoes water extraction, alcohol precipitation, deproteinization and desalination, chromatographic separation is then carried out by a DEAE-agarose gel FF column and an agarose gel 6FF gel column, and thereby the angelica anomala polysaccharide is obtained; and the conditions of water extraction are as follows: extraction is carried out under 81 DEG C for three times, extraction is carried out for one hour each time, and the dosage ratio of water to angelica anomala powder is 68mL / g. The angelica anomala polysaccharide prepared by the invention has good bacteriostatic activity, oxidation resistance and whitening activity. The method disclosed by the invention has the advantages of high extraction rate and good purification effect, and does not change the structure of the angelica anomala polysaccharide and affect the bioactivity of the angelica anomala polysaccharide.
Owner:SICHUAN AGRI UNIV
Preparation method of health-care food raw-material
InactiveCN101507510BQuality unchangedConstant volumeFood preparationNatural productGamma-Aminobutyric acid
The invention discloses a method for preparing gamma-aminobutyric acid as raw material of health foods. The method comprises the steps of selecting extract which is rich in gamma-aminobutyric acid after green-tea extraction as raw material, using natural capsule material, coating gamma-aminobutyric acid extract to be microcapsule core substances, forming capsule membrane with the diameter of between 200 and 400 microns, using natural colloid as embedding wall material and utilizing an embedding-method preparation technique for sustained-release microcapsules to produce powdered gamma-aminobutyric acid. The method has the advantages that all components used in the method are natural products, safe and reliable; the gamma-aminobutyric acid is sustained in the acting time of efficacy, balanced in release, little in the loss of functional components, insipid, colorless, free from interference in the own characteristics of foods and medicines, easy to dissolve, good in compatibility and capable of dissolving in water-soluble and fat-soluble products; and the method is simple in preparation process, extensive in raw materials and easy to popularize and apply.
Owner:安徽来福高科股份有限公司
Recombinant polypeptide and its application
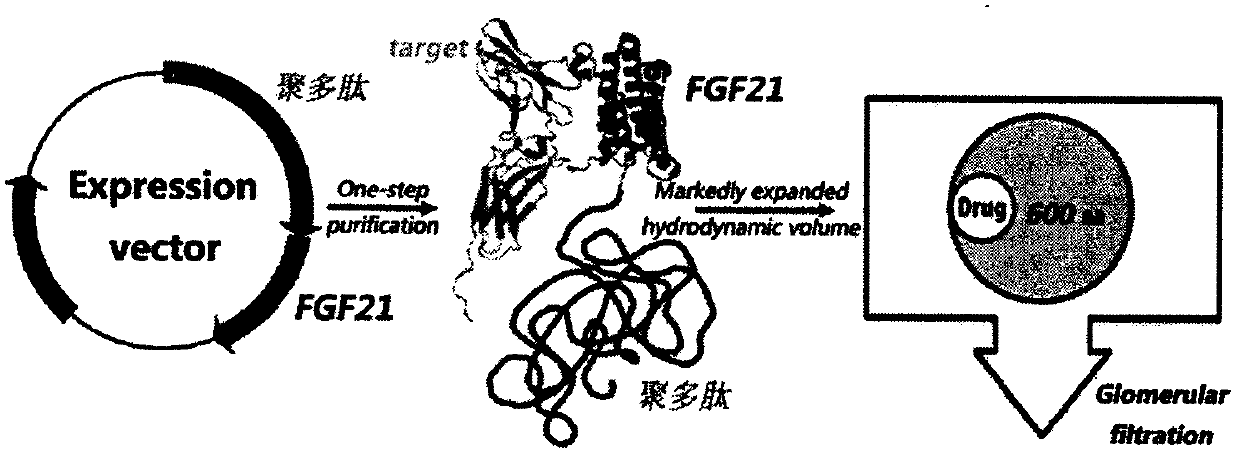
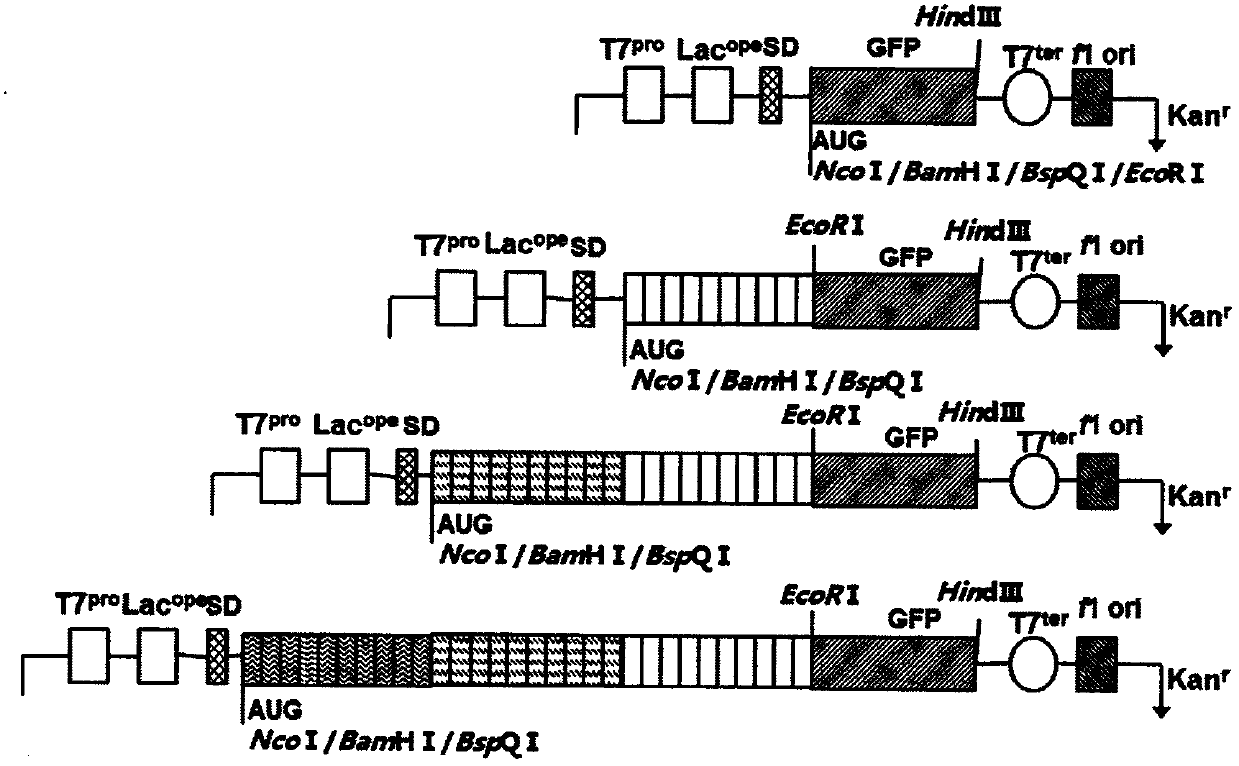
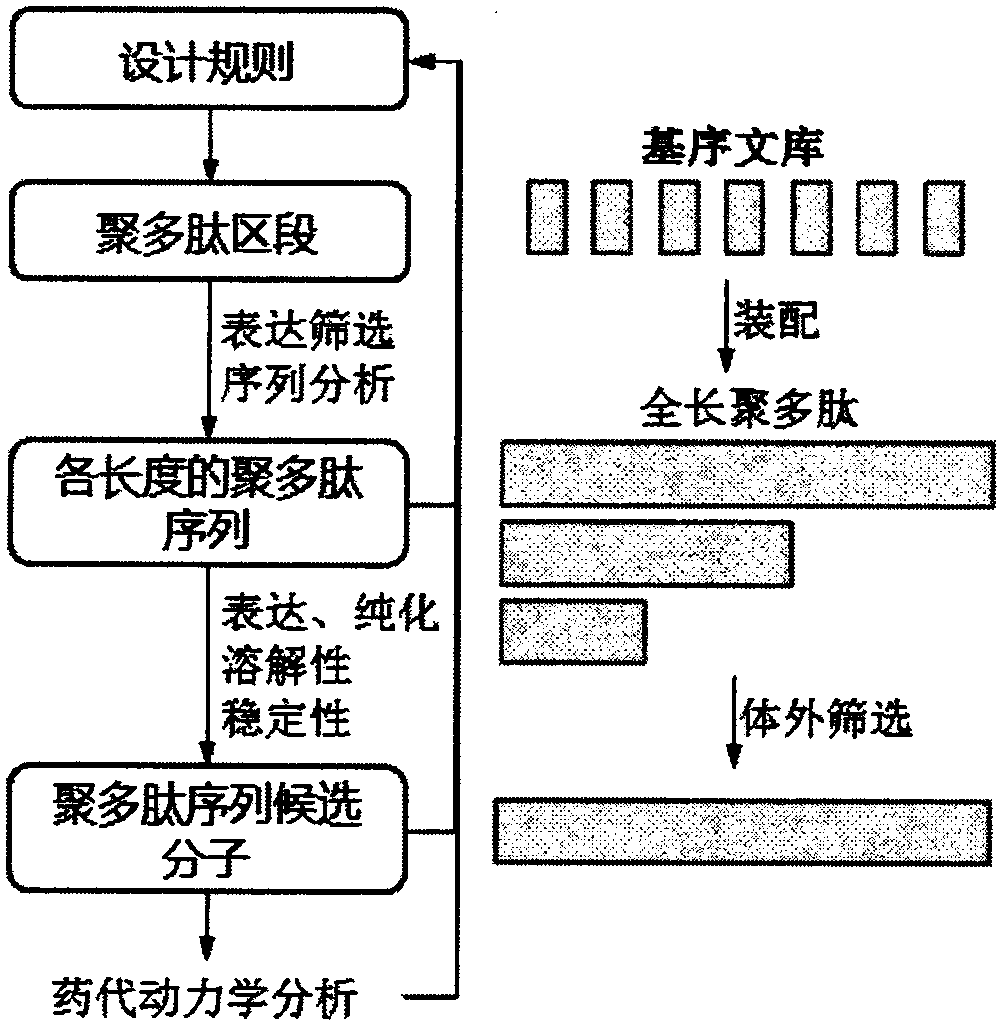
ActiveCN105524147BGood natureQuick filterDepsipeptidesFusions for plasma life prolongingGlycineThreonine
The invention relates to a composition of recombinant polypeptides with different lengths based on screening and construction. The invention discloses a stable and non-structure polypeptide molecule without immunogenicity. The polypeptide molecule comprises 3-6 types of amino acids selected from glycine, alanine, serine, threonine, proline and lysine is designed through a complete artificial method. The polypeptide molecule and a bio-active protein are subjected to fusion expression so that the problem that the original biological activate protein has poor solubility, high immunogenicity or a short half life.
Owner:CHINA PHARM UNIV
Corn noodles prepared by utilizing corn in dough stage and production method thereof
The invention discloses corn noodles prepared by utilizing corn in a dough stage and a production method thereof. The corn noodles comprise the following ingredient and auxiliary ingredient constituents in a mass ratio: corn flour in the dough stage 15%-25%, flour 60%-70%, salt 0.5%-2%, vital wheat gluten flour 5%-10% and soybean powder 5%-8%. The corn noodles are prepared through the operation steps of preparing corn juice and corn the corn flour with the corn in the dough stage, performing mixing, performing dough kneading, performing fermentation, , performing rolling, performing slicing, performing drying, performing cutting, etc. According to the invention, through utilizing the characteristic of complementary physical properties and nutrients of the corn in the dough stage, soybeans, etc., the adopted production method provided by the invention guarantees that the biological activity of nutritional health care components is not damaged to the greatest extent; and the corn noodles have certain health care efficacy.
Owner:湖北十星杂粮食品有限公司
Multi-grain noodles containing kudzu vine roots
PendingCN110810716AIncrease added valueNutritious and comprehensiveNatural extract food ingredientsFood dryingHorticultureHuman health
The invention discloses multi-grain noodles containing kudzu vine roots. The noodles are prepared from the following raw materials in parts by mass: 46-50 parts of wheat flour, 13-15 parts of kudzu vine root flour, 2-4 parts of konjac powder, 6-8 parts of corn flour, 8-10 parts of red long bean flour, 5-10 parts of vital gluten and 1-2 parts of edible salt. The preparation method comprises the steps of preparation of partial raw materials, kneading, curing, mixing, rolling, slitting, drying, cutting off, packaging and the like. According to the preparation method, the biological activity of nutritional ingredients in the raw materials can be maintained by selecting local specialty, such as kudzu vine root flour, konjac powder, red long bean flour, corn flour and the like, as raw materialsand a corresponding preparation method, no essence or other chemical substance is added, the taste and flavor of noodles are improved by adding the kudzu vine root flour and the konjac powder, the noodles are chewable and flexible due to the reasonable prescription, the nutritional value and edible therapy effect of the noodles are improved, and human health is favored.
Owner:湖北十星杂粮食品有限公司
Kit for separating karyocyte in vitro and application method thereof
ActiveCN100485029CNo change in biological activityConvenient treatmentBlood/immune system cellsFicollDrug biological activity
The invention relates the separating agent box and application. The agent box comprises erythrocyte precipitating agent, hypaque sodium- ficoll 400# or HISTOPAQUE1077 and cell maintenance liquid. The agent box can separate bone marrow and umbilical cord blood, on the surface of cell there is no any mark, and it doesn't change biological activity of cell. The invention can commercially manufacture, and the application is wide.
Owner:NINGXIA ZHONGLIANDA BIOPHYSICS
Process of enriching nutritional components of sea cucumber processing liquid
InactiveCN107616439AReduce the temperatureMaintain biological activityFood scienceWater vaporMembrane bioreactor
The invention discloses a process of enriching nutritional components of sea cucumber processing liquid. The process comprises the following steps: filtering and centrifuging the sea cucumber processing liquid, adding NaHCO3, Na2CO3 and a mixed solution, adding a HCl solution of 0.2 to 0.5 moL / L, putting into a PDMS membrane bioreactor, and performing pervaporization to obtain concentrated liquid;and sterilizing the concentrated liquid by a pasteurization method and performing cold storage at 4 DEG C for later use. The sea cucumber processing liquid is treated by the process provided by the invention, so that the nutritional components of the sea cucumber processing liquid can be enriched and recycled, the discharged liquid of the treated sea cucumber processing liquid is condensed watervapor, and pollution to the surrounding environment is reduced.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Salsola collina liquid milk and preparation method thereof
InactiveCN103392809BPromote absorptionImprove physiological activityMilk preparationBiotechnologyNutritive values
The invention relates to the technical field of food processing, and comprises the following steps: cleaning, drying and pulverizing the slaw liquid milk, extracting flavonoids and alkaloids in the slaw by ultrasonic extraction, and then The vetch extract is mixed with milk, and finally the mixed vetch extract and fresh milk are homogenized, sterilized at ultra-high temperature, cooled, and filled and sealed by a packaging machine. The liquid milk of pigskin is composed of pigskin extract and fresh milk at a volume ratio of 1:30, and the pigskin extract is composed of purified flavonoids, purified alkaloids and water, adding 1 gram of pigskin per liter of water. It is made by mixing the purified flavonoids and 1.5 grams of purified alkaloids. The invention organically combines the flavonoids and alkaloids in the slaw with milk, and scientifically matches them, so that the obtained slaw liquid milk has good taste and has both nutritional value and health care function.
Owner:HENAN UNIV OF SCI & TECH
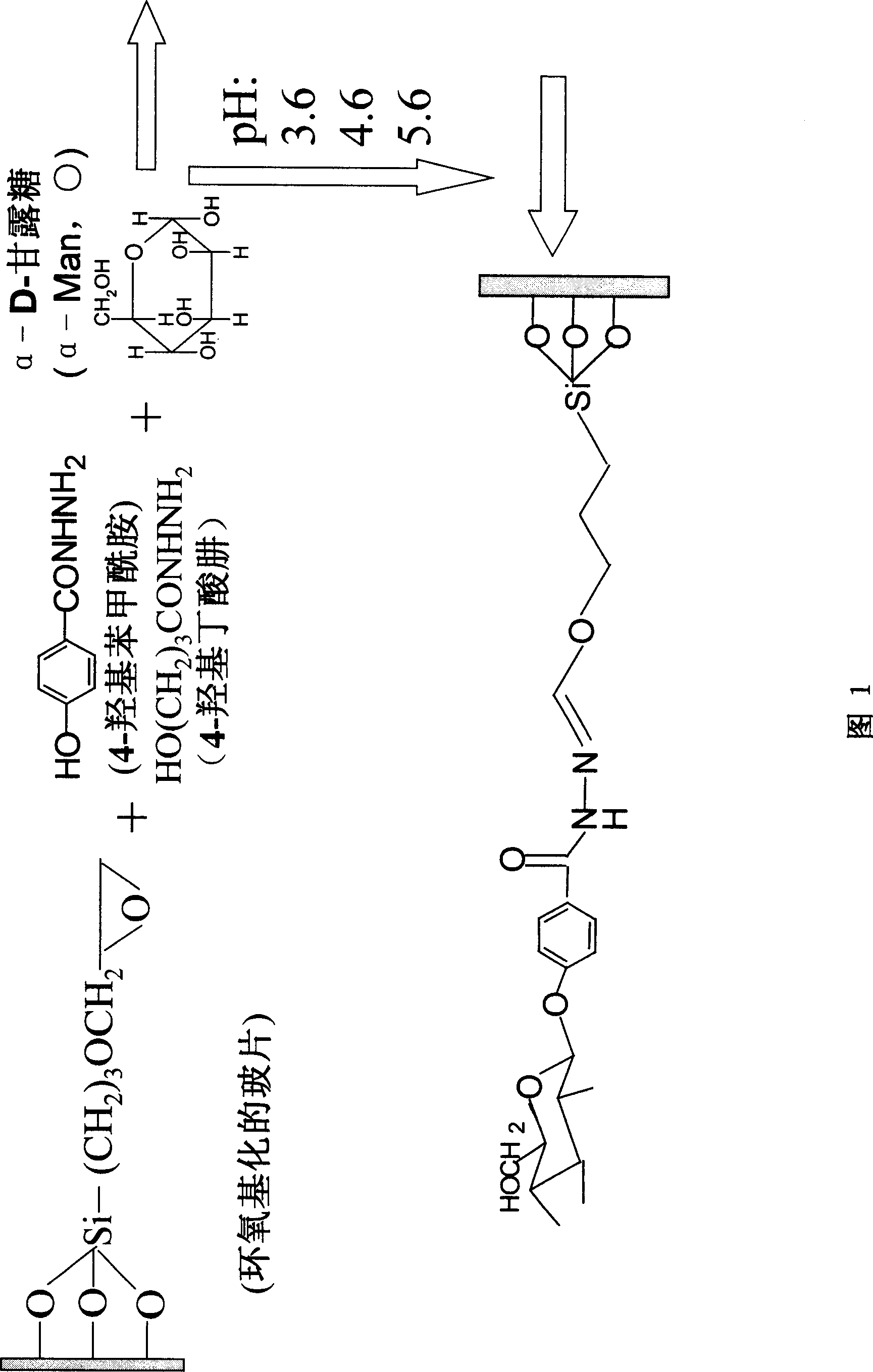
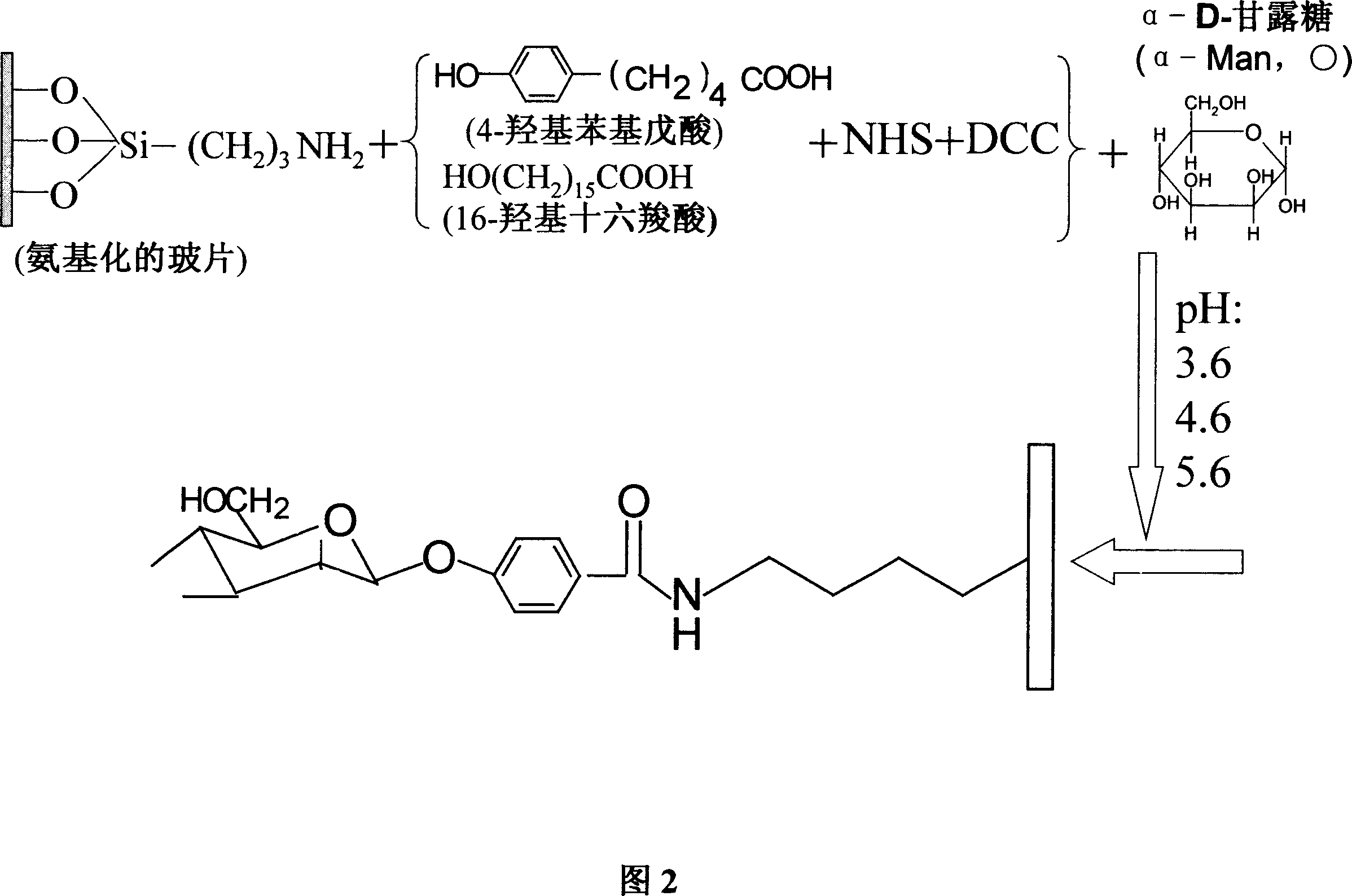
Process of sugar bio-chip
ActiveCN1952658BEasy to connectFast connectionMicrobiological testing/measurementBiological testingDerivatizationAcid group
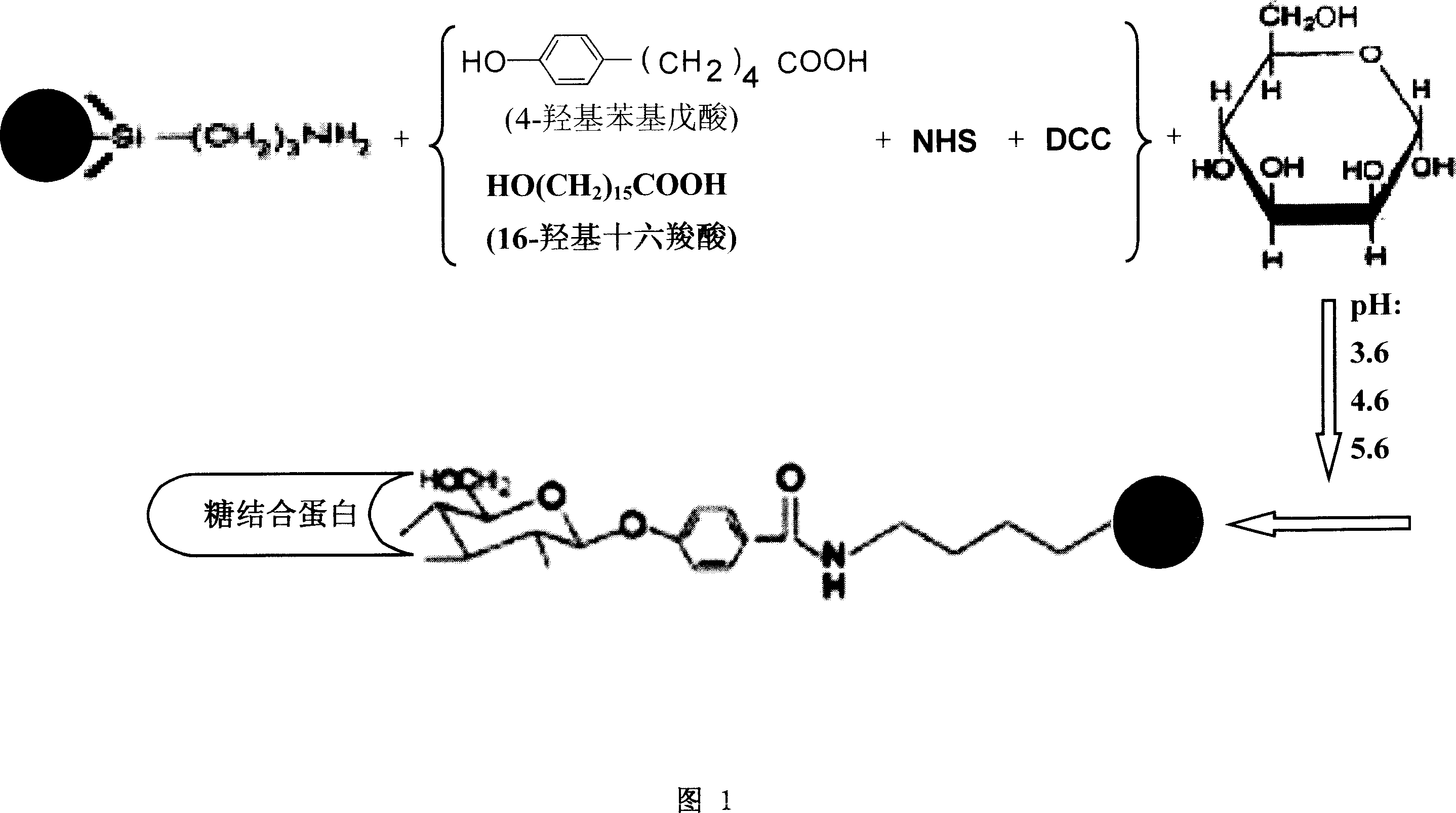
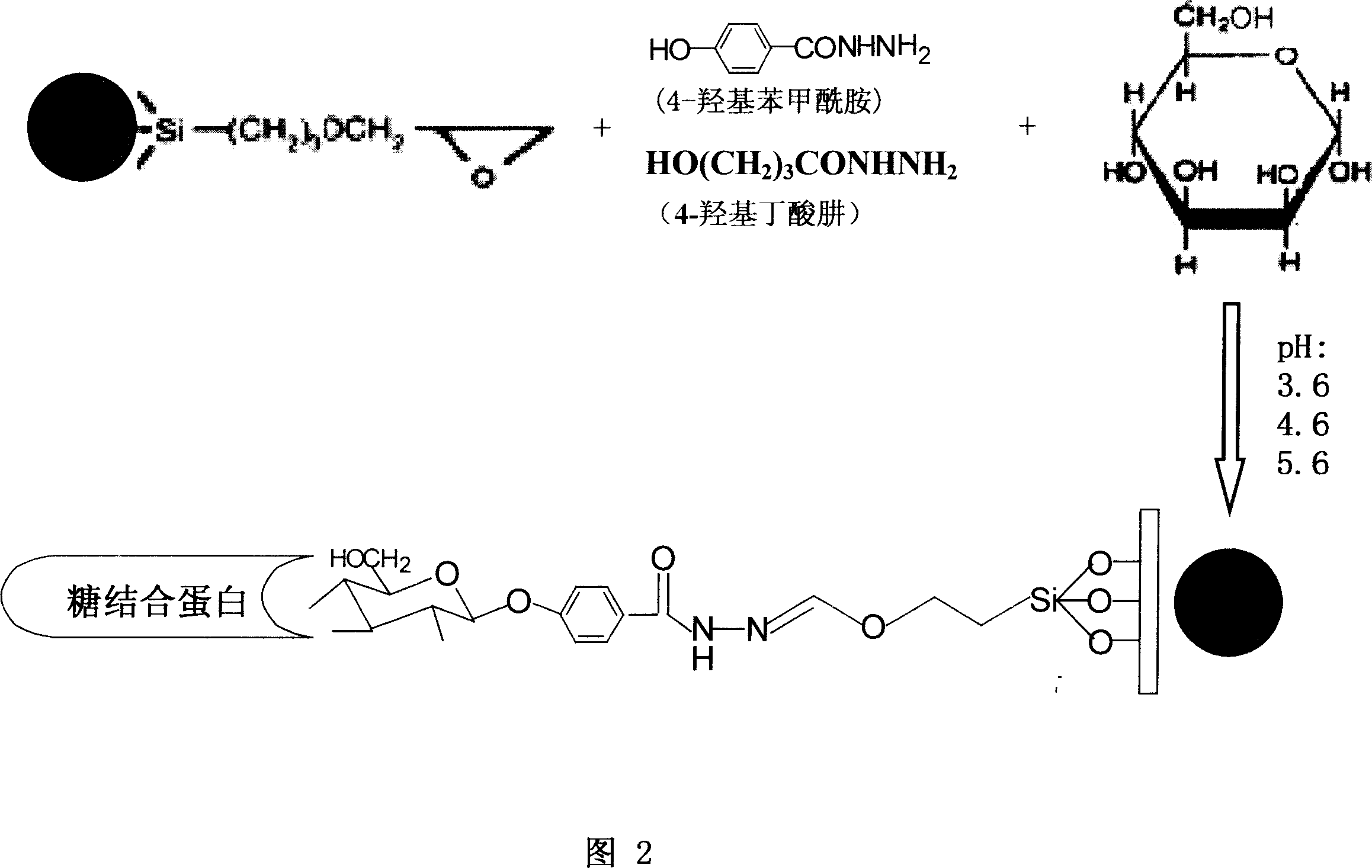
A Method for preparation of sugar biological chip is disclosed that the epoxyethane radical derived solid carrier is covalence coupled with the amidol of coupling agents by the epoxyethane radical contained on the surface, the aldolisation condensation reaction occurred between the hydroxyl which is in the other side of the coupling agents and the deacidizing end of the deacidizing sugar to form the glycosidic bond, the deacidizing sugar is coupled by covalent formula to the solid carrier to form the sugar chip. Or the amidol radical derived solid carrier is covalence coupled with the acid group which is activated by the N-hydroxy succinimide and the dicyclo hexylcar bodiimide of the coupling agents by the amidol radical contained on the surface, the aldolisation condensation reaction occurred between the hydroxy which is in the other side of the coupling agents and the deacidizing end of the deacidizing sugar to form the glycosidic bond, the deacidizing sugar is coupled by covalent formula to the solid carrier to form the sugar chip. The invention resolves the technical matters of that the deacidizing sugar is friable, the Method for preparation is complex, and the cost is high. The invention is simple and quick; the bioactivity of the sugar chip is stable, and easy to apply dimensionally.
Owner:SHANXI LIFEGEN
Suppository for treating mammal endometritis
ActiveCN102552888BIncrease contactProlong the action timePeptide/protein ingredientsSuppositories deliveryWrinkle skinSodium bicarbonate
The invention belongs to the technical field of medicines, and specifically relates to a suppository for treating mammal endometritis. The preparation disclosed in the invention comprises the following components of: by weight, 0.004-0.01% of staphylococcus lysozyme, 0.1-4% of lysozyme, 10-80% of semisynthetic fatty glyceride, 1-20% of poloxamer 188(F68), 1-20% of HPMC, 5-50% of sodium bicarbonate and 5-50% of sodium dihydrogen phosphate. After the preparation provided by the invention enters into mammal uterus through administration, the preparation can undergo effervescence decomposition in a body fluid. The medicine is mixed in foams and rapidly released. Contact area between the medicine and mucosal of uterus becomes larger and the medicine can penetrate into deep portions of mucosa wrinkles so as to prolong the action time between the medicine and the mucosa, raise the concentration of the medicine on local tissues, further enhance the curative effect and effectively kill various pathogens which can cause endometritis.
Owner:昆山博青生物科技有限公司
Biological antibacterial polypeptide preparation, preparation method and application thereof
ActiveCN106818837BImprove the bactericidal effectImprove securityOrganic active ingredientsBiocideSide effectIrritation
The invention discloses a biological antibacterial polypeptide preparation as well as a preparation method and application of the biological antibacterial polypeptide preparation. The biological antibacterial polypeptide preparation takes water as a carrier and is prepared from the following components in percentage by weight: 0.1 percent to 10 percent of a biological antibacterial peptide BQ and 0.01 percent to 1 percent of polyhexamethylene biguanidine. The biological antibacterial polypeptide preparation has good sterilization effect. A core sterilization component of the preparation is a novel antibacterial peptide and has very high sterilization effect on staphylococcus aureus, escherichia coli, candida albicans and viruses. When an in-vitro experiment is carried out for 2min to 5min, 99.9 percent of sterilization rate can be realized. The biological antibacterial polypeptide preparation has high safety, has no toxicity to or residues to human bodies, has high stability and can be used as a prevention medicine. The main acting component is a biological preparation and the disadvantages that a traditional chemical preparation has irritation on mucosa and bacteria easily have drug resistance are overcome. The biological antibacterial polypeptide preparation is easily accepted by organisms, has no toxic side effect and is suitable for long-time utilization or used as the prevention medicine.
Owner:昆山博青生物科技有限公司
A single-stranded DNA oligonucleotide aptamer that specifically recognizes aflatoxin b1
ActiveCN104911186BEasy to distinguishStrong specificityDNA preparationDNA/RNA fragmentationAptamerFluorescence
The invention discloses a single-stranded DNA oligonucleotide aptamer that specifically recognizes aflatoxin B1; the purpose of the invention is to provide a single-stranded DNA oligonucleotide that can highly specifically recognize aflatoxin B1 The acid aptamer can quickly and accurately detect aflatoxin B1 in food through the labeling method. In the present invention, the common aflatoxin B1 in foods and grains is used as a target substance, and a nucleic acid aptamer sequence specifically binding to aflatoxin B1 is obtained by using SELEX technology, and the aptamer can be transformed by labeling a reporter molecule In order to report nucleic acid aptamers, combined with conventional detection methods such as gold standard test paper, fluorescent reporter detection, and enzyme-linked immunoassay technology (ELISA method), the rapid detection of aflatoxin B1 in food can be realized, and it can be synthesized in large quantities and is easy It is chemically modified and has high stability, and is widely used in food testing, basic research, clinical diagnosis, and drug research.
Owner:ZHEJIANG PUZHENG DETECTING TECH
Preparation method of weikang capsule
InactiveCN103961545AEasy to crushReduce volatile lossDigestive systemMammal material medical ingredientsMyrrhRadix Astragali seu Hedysari
A preparation method of a weikang capsule comprises processing steps as follows: (1), 64 g of rhizoma bletillae, 63 g of cuttlebone, 38 g of endothelium corneum gigeriae galli, 1 g of stir-fried egg shells and 13 g of plant soot are taken and subjected to ultrafine grinding for standby; (2), 63 g of frankincense and 15 g of myrrh are taken and ground by an ultralow-temperature grinder for standby; (3) 64 g of pseudo-ginseng and 64 g of rhizoma cyperi are taken and ground into fine powder, ethyl alcohol which accounts for 3-4 times of the quantity of the and has the mass concentration of 65%-85% are added into the fine powder, the fine powder is soaked for 3-4 hours and subjected to ultrasonic continuous countercurrent extraction for 30-60 min, and an extraction liquid is filtered for standby; (4), 63 g of radix astragali and 64 g of radix paeoniae alba are taken and put into an extraction tank, water accounting for 8 times of the quantity of the mixture is added for decoction extraction for two hours, and the extraction liquid is filtered for standby; and (5), the step (3) and the step (4) are combined, thick paste is obtained, the ultrafine powder of the step (1) and the step (2) is added and uniformly mixed, and particles are prepared. According to the method, bioavailability of a human body can be improved, and the production cycle can be shortened while effective ingredients are extracted to the greatest extent.
Owner:沈阳神龙药业有限公司
Protein drug solid preparation and preparation method thereof
ActiveCN113248724ANo change in biological activityGood package efficiencyPowder deliveryPeptide/protein ingredientsPolymer scienceHydrophobe
The invention discloses a protein drug solid preparation and a preparation method thereof, and discloses a polymer which is formed by polymerizing a hydrophilic high-molecular material and a hydrophobic high-molecular material. The polymer has good protein wrapping efficiency, and the biological activity of freeze-dried protein is kept unchanged. Therefore, the polymer can be used for preparing a protein solid preparation which is easy to preserve at normal temperature. The invention also provides a preparation method and application of the polymer, and provides a protein drug solid preparation prepared from the polymer, and a protein drug solution can be freeze-dried into a solid preparation and maintain the activity of the protein drug, and can be preserved at normal temperature and even high temperature. The protein drug solid preparation can be re-dissolved into a protein drug solution with undamaged activity, and the activity of the protein drug is maintained.
Owner:康汉医药(广州)有限公司
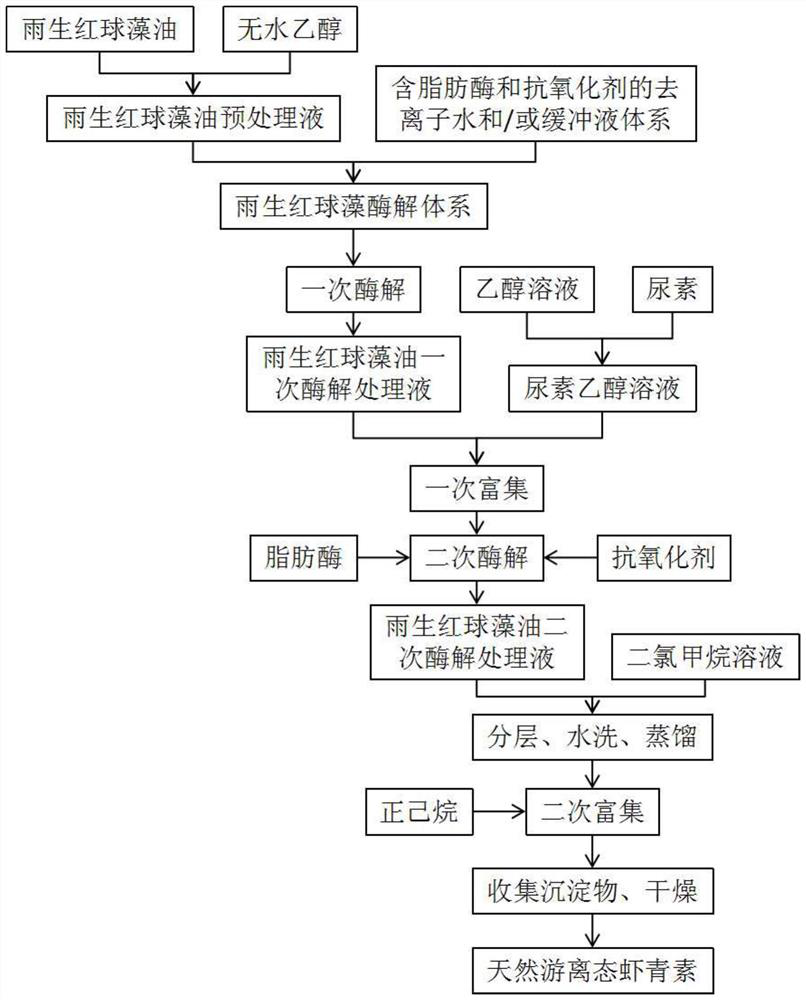
Preparation method of astaxanthin-rich haematococcus pluvialis oil
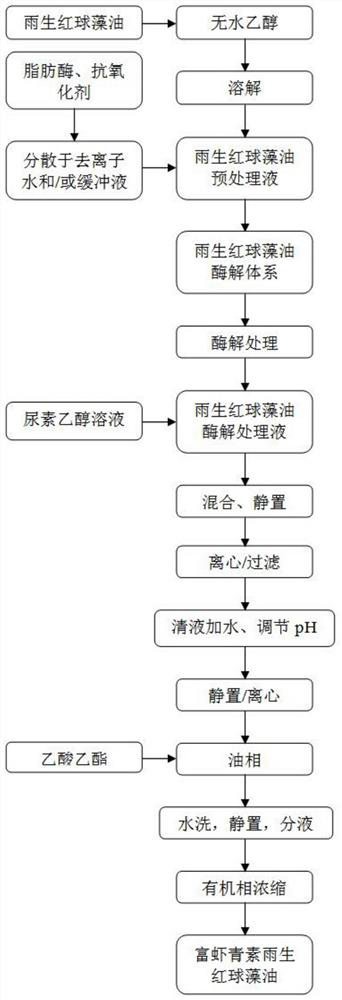
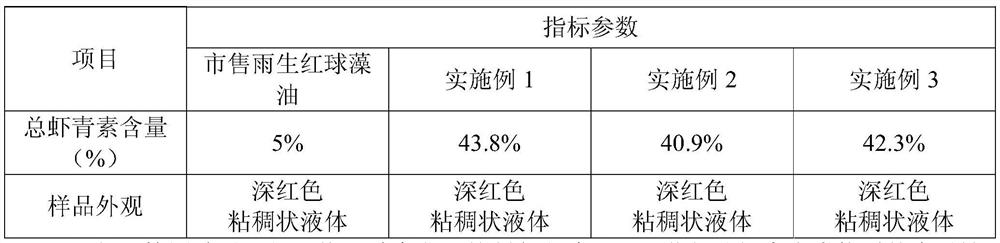
InactiveCN113604533AAchieve enrichmentMeeting Dosage NeedsOrganic chemistryFermentationBiotechnologyChemical compound
The invention relates to a preparation method of astaxanthin-rich haematococcus pluvialis oil, and belongs to the technical field of compound extraction. The method comprises the following steps of (1) pretreating raw materials; (2) preparing an enzymolysis system; (3) performing enzymolysis treatment; and (4) performing enrichment process. According to the method, the enrichment of astaxanthin in haematococcus pluvialis oil is efficiently realized by adopting a method of combining enzymolysis with heterogeneous purification, and the content of total astaxanthin substances in a finally prepared product is more than 40%, so that the dosage requirement in the application process of the product is well met.
Owner:RIZHAO POLYTECHNIC
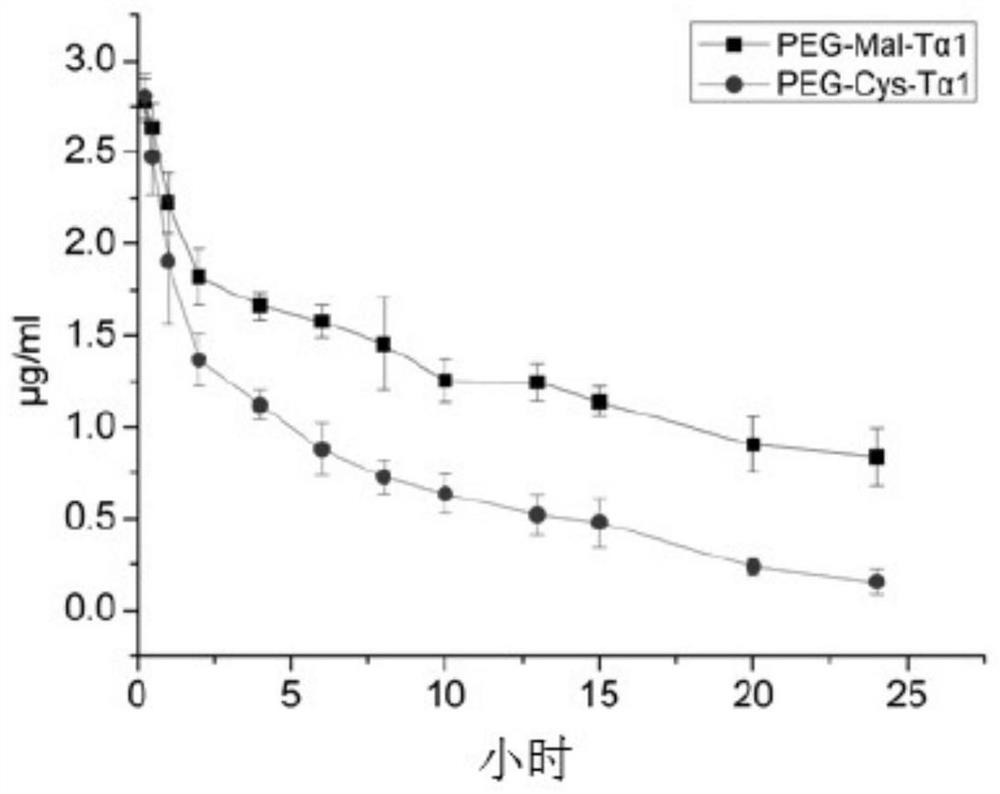
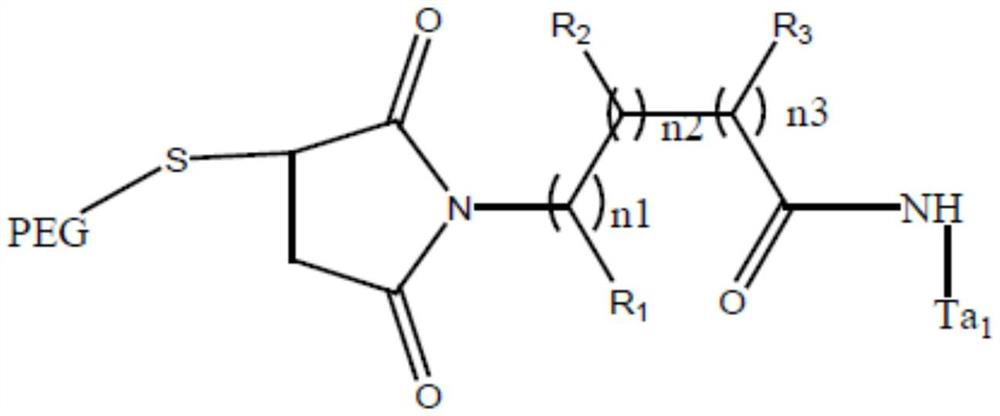
A kind of Thymofaxin polyethylene glycol polymer, its pharmaceutical composition and application
ActiveCN107163134BSimple processEasy to industrializeThymosin peptidesPeptide/protein ingredientsImidePolymer science
The invention discloses a thymalfasin (T[alpha]1) polyethylene glycol polymer, a medicine composition including same, and an application thereof. The T[alpha]1 polyethylene glycol polymer is represented as the following general formula. In the invention, the T[alpha]1 is synthesized through a regular solid phase synthesis method, wherein the last one step, acetylation, is replaced by introducing a maleimide active group, which can be specifically coupled with monosulfydryl polyethylene glycol at room temperature to prepare a polymer. The T[alpha]1 polyethylene glycol polymer not changes the biostructure of T[alpha]1 and also not changes the bioactivity of the T[alpha]1, and significantly increases the half-life period of the polymer. The method has simple process, is easy to industrialize, has gentle reaction conditions, is more than 95% in molar yield and has excellent market prospect.
Owner:广州维奥康药业科技有限公司
Efficient preparation and purification method of natural free astaxanthin
ActiveCN112626158AAchieve esterolysis conversionMeeting Dosage NeedsOrganic chemistryFermentationBiochemical engineeringHaematococcus
The invention relates to the technical field of astaxanthin, in particular to an efficient preparation and purification method of natural free astaxanthin. The preparation and purification method comprises the following steps of (1) performing raw material selection and pretreatment; (2) constructing a haematococcus pluvialis enzymolysis system: dispersing lipase and an antioxidant into deionized water and / or a buffer solution, and then adding the deionized water and / or the buffer solution system containing the lipase and the antioxidant into a haematococcus pluvialis pretreatment solution obtained in the step (1) to obtain a haematococcus pluvialis oil enzymolysis system; (3) performing primary enzymolysis; (4) performing primary enrichment; (5) performing secondary enzymolysis; and (6) performing secondary enrichment. The method is simple and easy to operate, low in cost, mild in condition and environmentally friendly; the content of the free astaxanthin in a finally prepared product reaches 60% or above; and the generation of byproducts in the enzymolysis and enrichment processes of the astaxanthin can be effectively avoided.
Owner:RIZHAO POLYTECHNIC
Method for enriching and purifying saccharide binding protein
This invention discloses a method for enriching and purifying glycoprotein. The coupling of sugar with magnetic micro- and nano-particles comprises: activating the coupling agent and coupling sugar onto amino-derived magnetic micro- and nano-particles or ethylene oxide derived magnetic micro- and nano-particles. The separation, enriching and purification of glycoprotein comprises: adding pretreated glycoprotein into sugar-coupled magnetic micro- and nano-particles for connection, washing and eluting to obtain enriched and purified glycoprotein. This invention solves the problems of complex process, inconvenient usage and low recovery rate faced by background technology. The method has such advantages as simple process, high speed, stable glycoprotein bioactivity, and high efficiency in separating, enriching and purifying glycoprotein.
Owner:SHANXI LIFEGEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com