Staphylococcus lysozyme local sustained release preparation, preparation method and application thereof
A technology for lysostaphin and sustained-release preparations, which are applied in the directions of antibacterial drugs, pharmaceutical formulations, and medical preparations with inactive ingredients, etc. , to achieve the effect of strong bactericidal effect, strong bactericidal effect, and strong drug release ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 lysostaphin-PLA-PLGA microspheres
[0038] 1. Preparation of lysostaphin solution: Weigh 10 mg of lysostaphin freeze-dried powder, dissolve in 10 ml of dimethyl sulfoxide (DMSO) containing 10 mg of mannitol, and prepare 1 mg / ml of lysostaphin solution.
[0039] 2. Preparation of PLA-PLGA organic solution: weigh 0.4 gram of polylactic acid polymer with a molecular weight of 30,000-50,000, 0.1 gram of polylactic acid-glycolic acid with a molecular weight of 30,000-50,000 (wherein the ratio of lactic acid and glycolic acid is 75:25 ) polymer, dissolved in 10 milliliters of dichloromethane to prepare a 5% PLA-PLGA solution.
[0040] 3. Slowly add 5% PLA-PLGA solution to the prepared lysostaphin-DMSO solution drop by drop, set aside.
[0041] 4. Equilibrium supercritical CO 2 Fluid, process temperature 37°C, pressure 13.5MPa, CO 2 The flow rate is 20 g / min, and the above prepared lysostaphin solution is pumped in, and the lysostaphin-PLA-P...
Embodiment 2
[0043] The preparation of embodiment 2 lysostaphin-PLGA microspheres
[0044] 1. Preparation of lysostaphin solution: Weigh 20 mg of lysostaphin freeze-dried powder, dissolve in 10 ml of dimethyl sulfoxide (DMSO) containing 10 mg of mannitol, and prepare 1 mg / ml of lysostaphin solution.
[0045] 2. Preparation of PLGA organic solution: Weigh 0.5 grams of polylactic acid-glycolic acid (wherein the ratio of lactic acid and glycolic acid is 50:50) polymer with a molecular weight of 30,000-50,000, dissolve it in 10 milliliters of dichloromethane, and prepare 5% PLGA solution.
[0046] 3. Slowly add the 5% PLGA solution dropwise to the prepared lysostaphin-DMSO solution for later use.
[0047] 4. Equilibrium supercritical CO 2 Fluid, process temperature 37°C, pressure 13.5MPa, CO 2 The flow rate was 20 g / min, and the lysostaphin solution prepared above was pumped in, and the lysostaphin-PLGA microspheres were prepared by the supercritical anti-solvent method.
[0048] 5. After t...
Embodiment 3
[0049] The in vitro sustained release curve of embodiment 3 lysostaphin-PLA-PLGA microspheres
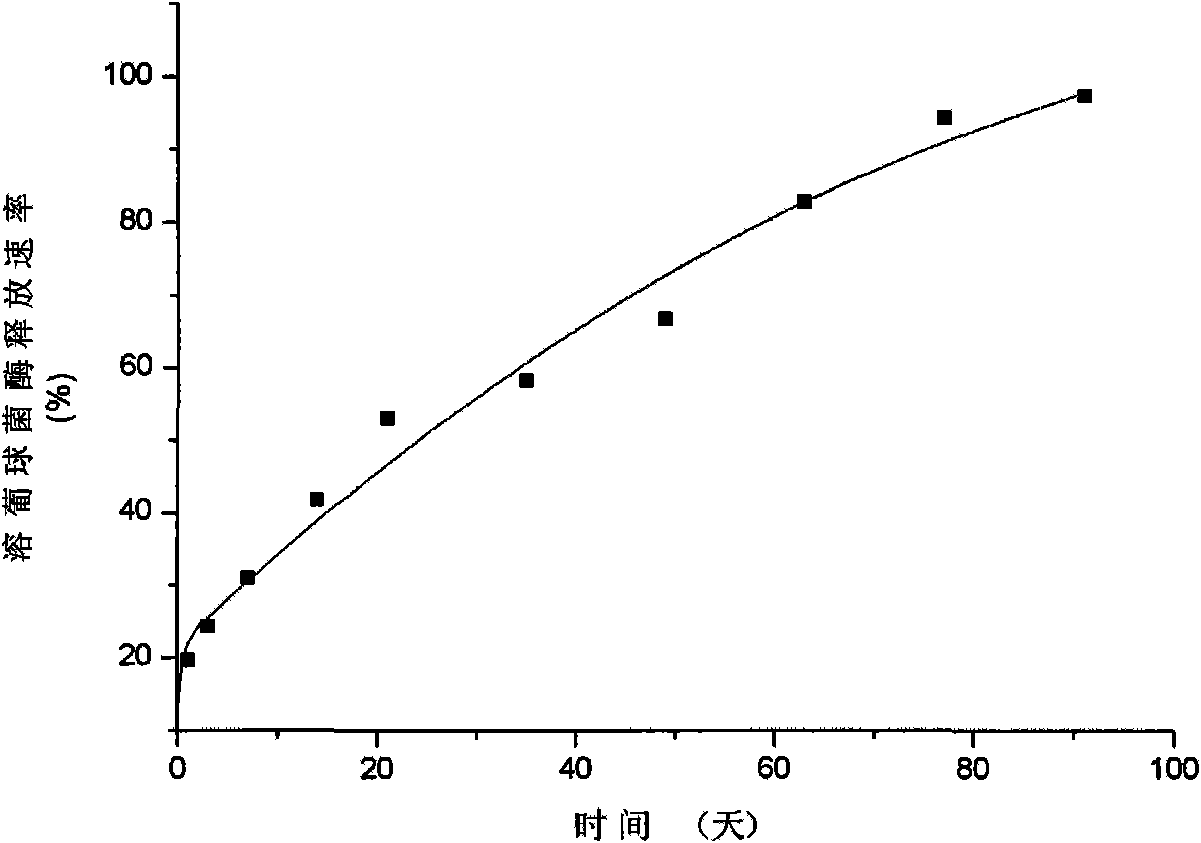
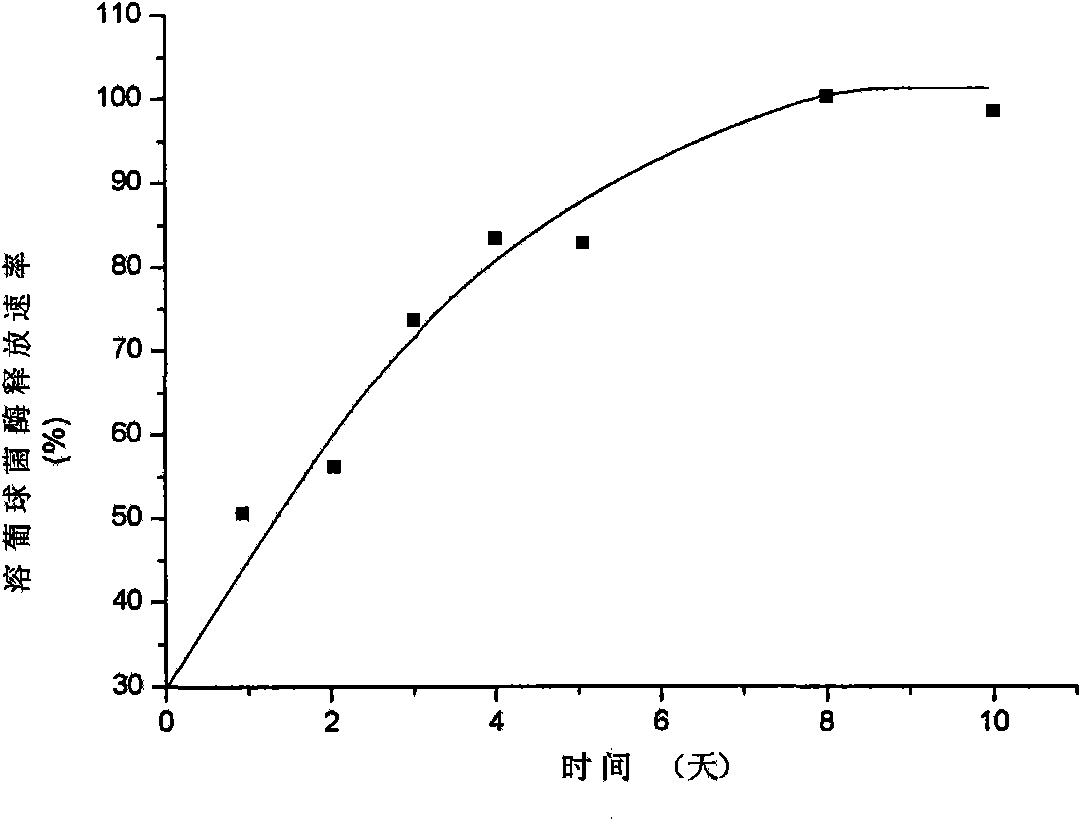
[0050] Weigh 5 mg of the microparticles prepared in Example 1, place in 10 ml of PBS buffer solution (pH 7.4), seal, shake at a constant temperature and speed (100 r·min −1 ) at 4° C. The buffer solution was taken regularly within three months, the enzyme activity of lysostaphin was detected, and the cumulative release percentage of lysostaphin was calculated. The in vitro drug release results of sustained-release lysostaphin microspheres are as follows: figure 1 Shown:
[0051] The results show that the release process of lysostaphin in vitro lasts up to three months, and the release rate is relatively stable, which can maintain the stable and long-term release of lysostaphin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com