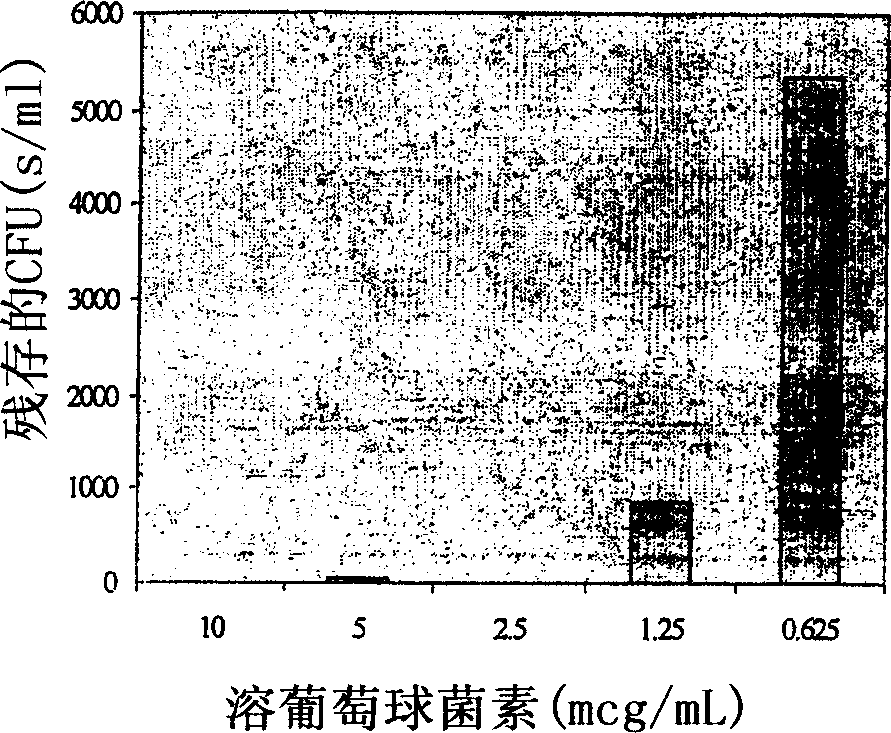
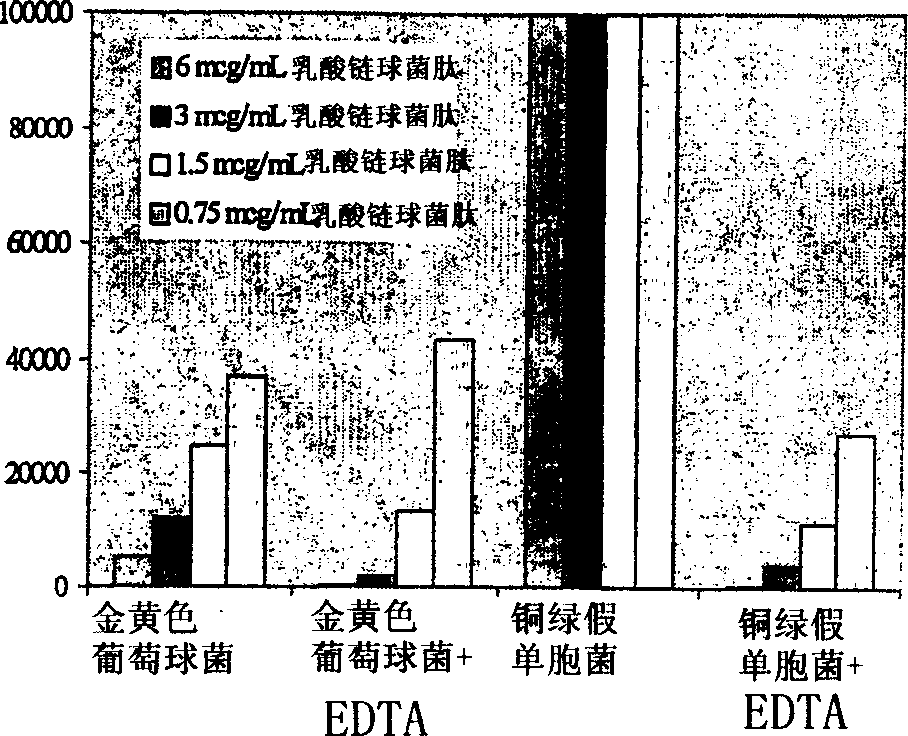
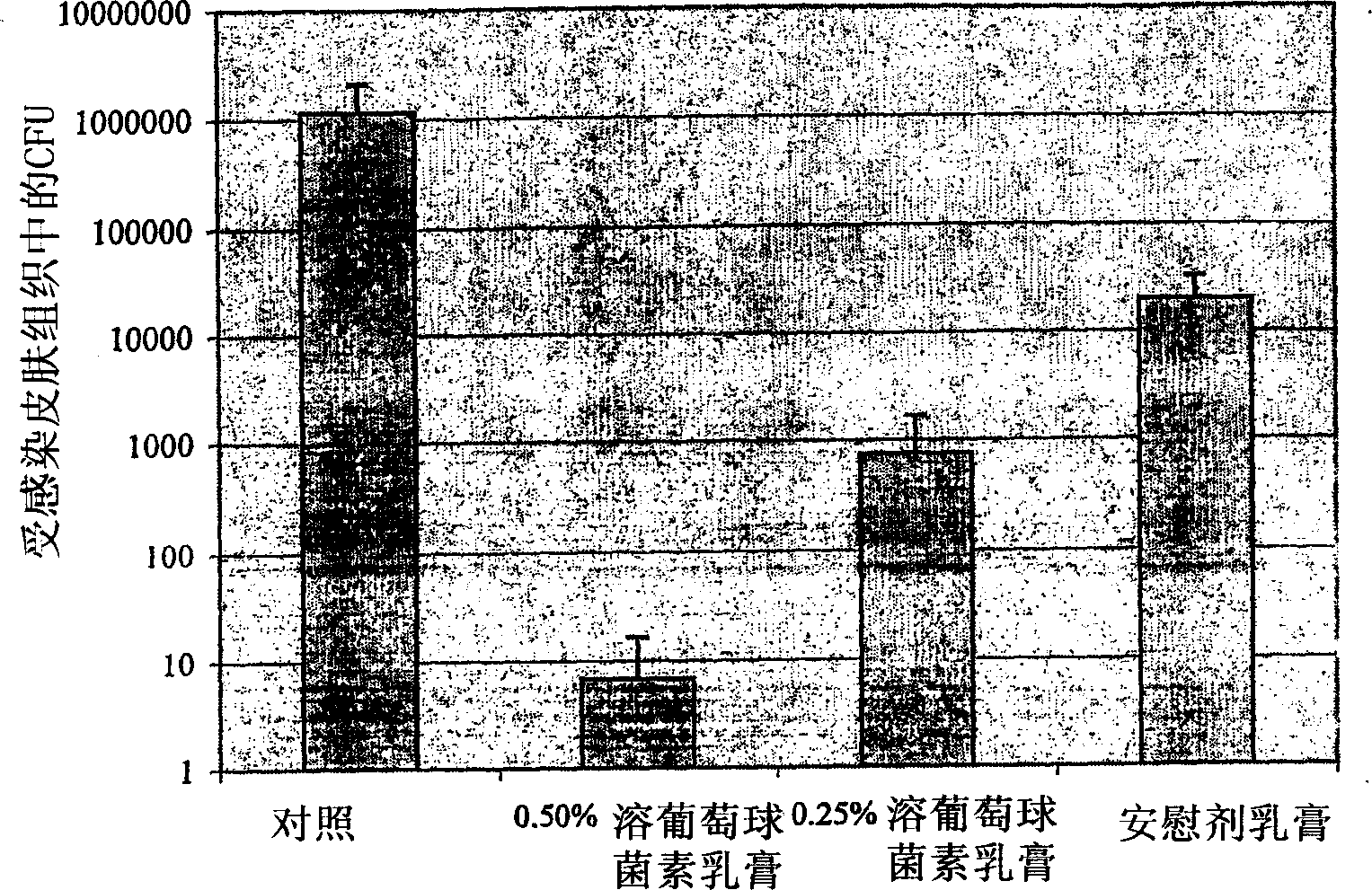
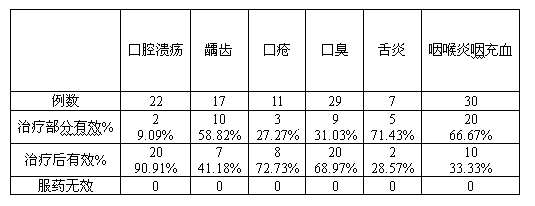
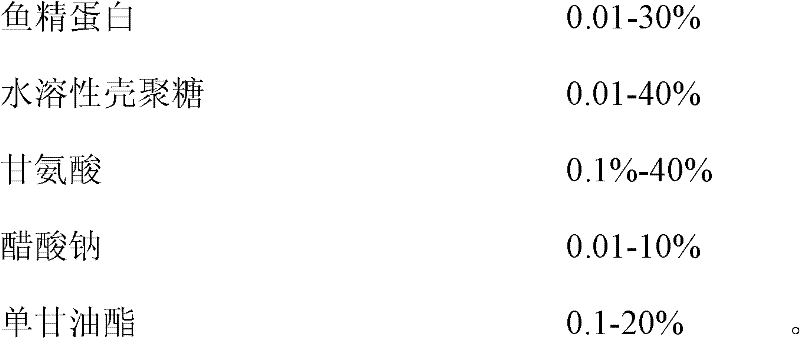Patents
Literature
70 results about "Lysostaphin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
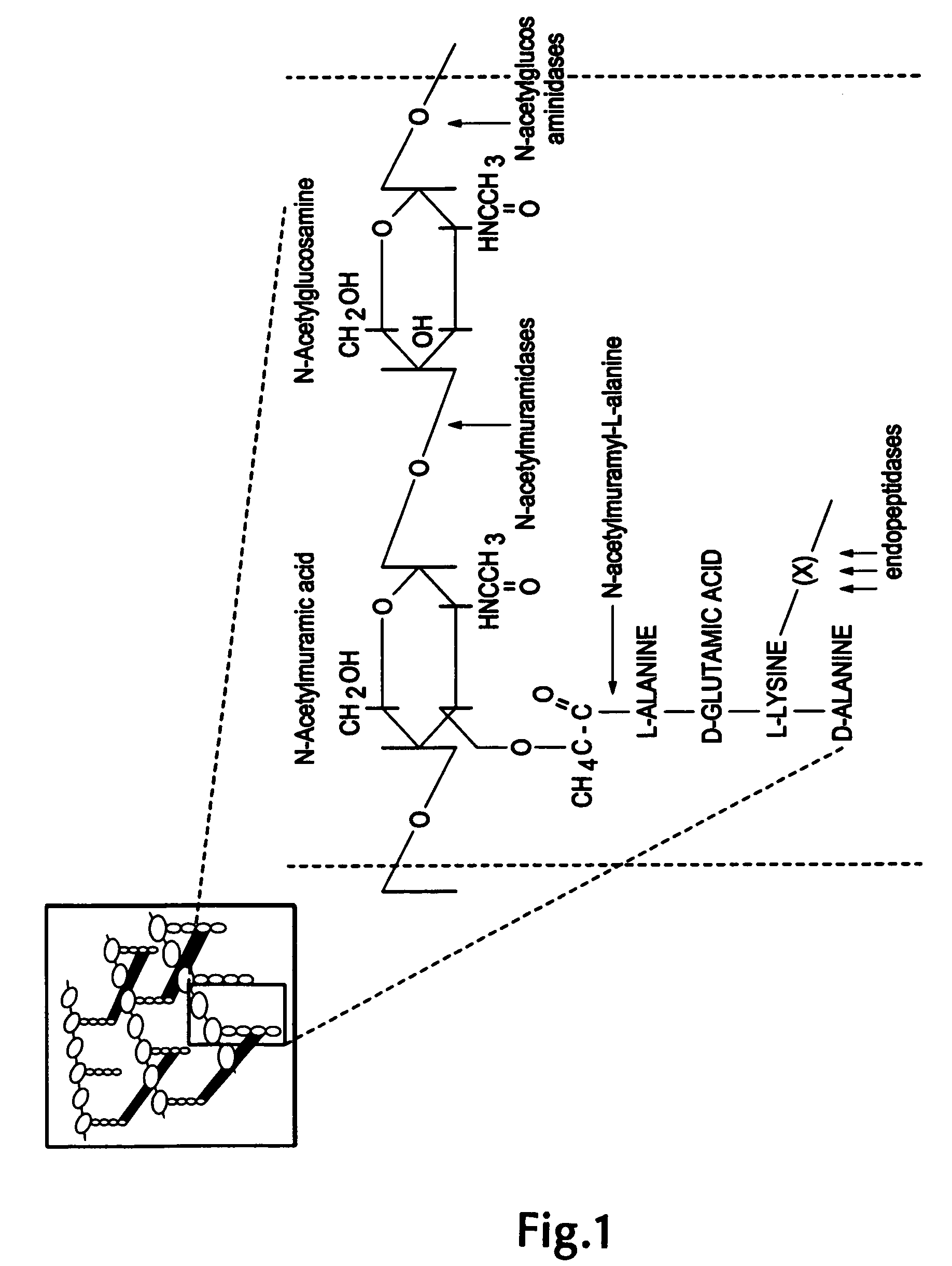
Lysostaphin (EC 3.4.24.75, glycyl-glycine endopeptidase) is a Staphylococcus simulans metalloendopeptidase (crystal structure of lysostaphin). It can function as an antimicrobial against Staphylococcus aureus.
Nucleic acid encoding endolysin fusion protein
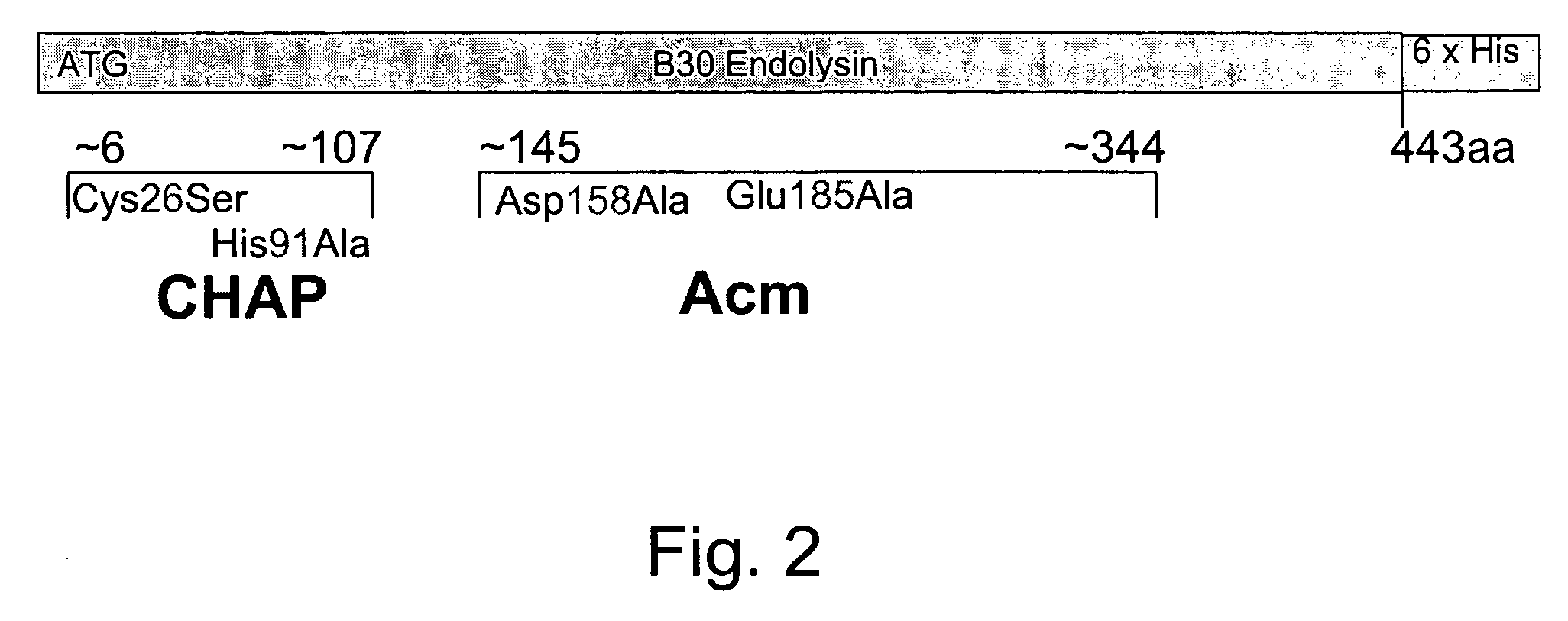
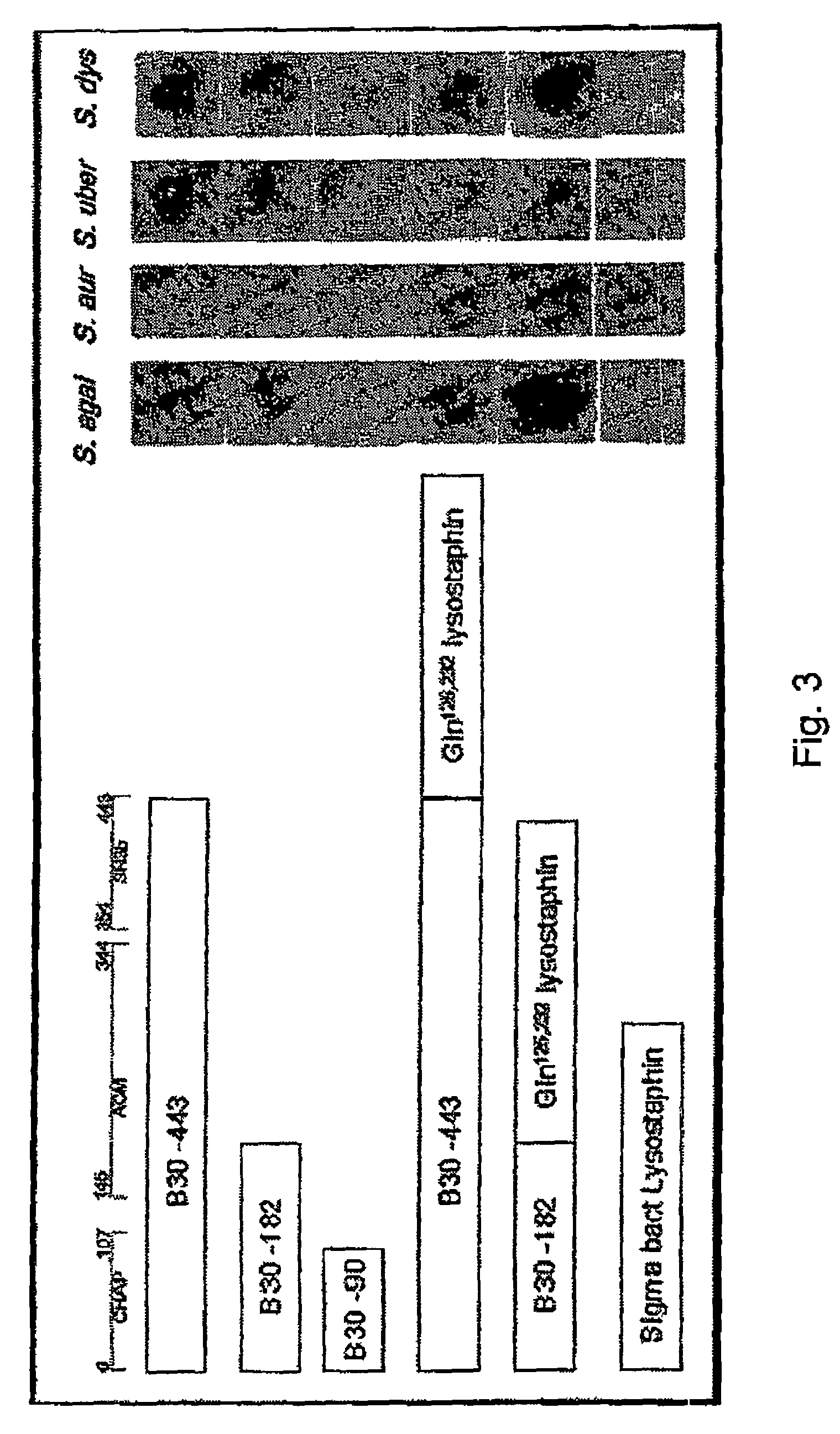
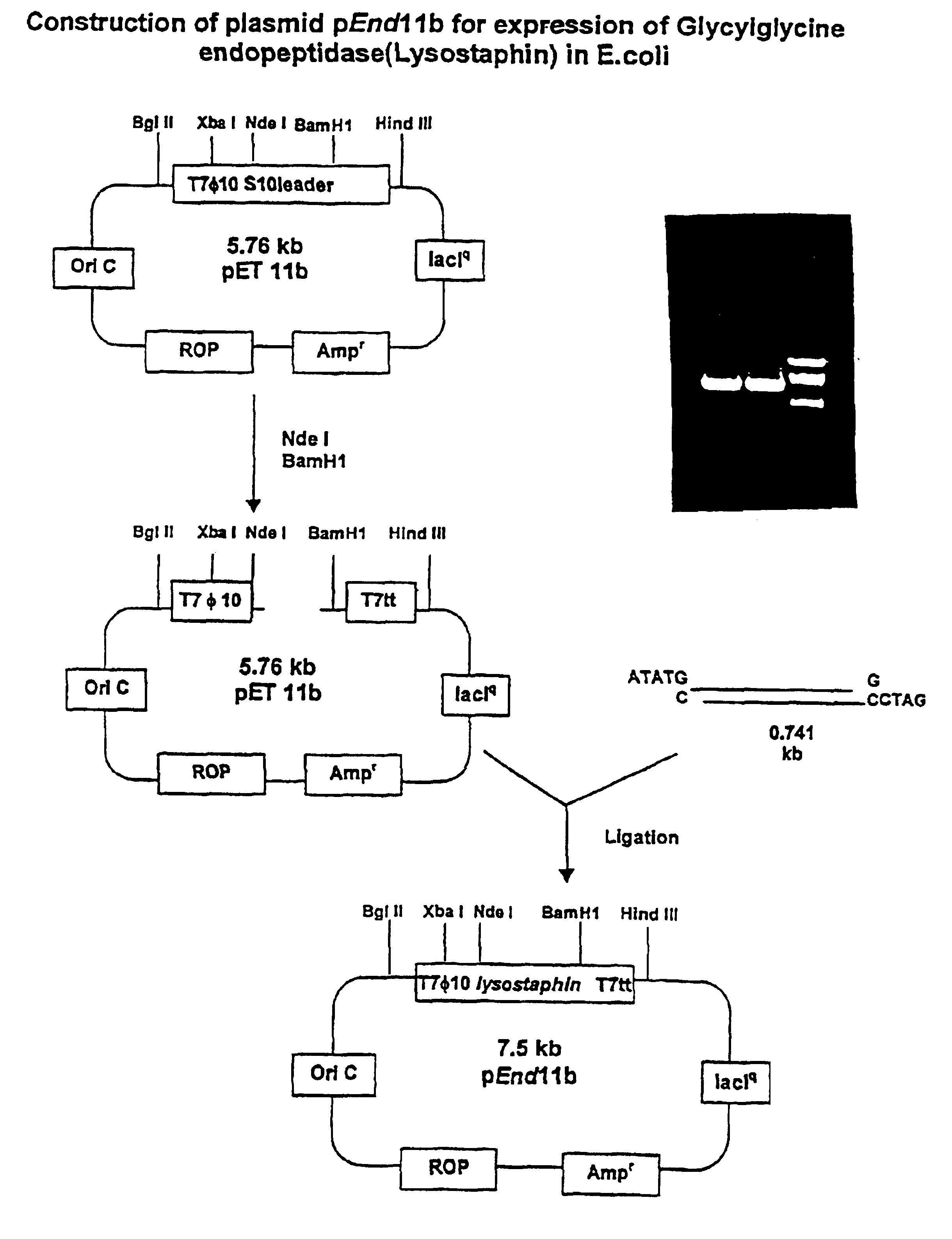
The invention concerns a recombinant nucleic acid molecule encoding an antimicrobial fusion peptidoglycan endopeptidase. The recombinant nucleic acid molecule according to the invention is formed from a nucleic acid encoding a bacterial endopeptidase (lysostaphin) from Staphylococcus simulans and a nucleic acid encoding a second endopeptidase (endolysin) module from Group B streptococcal bacteriophage B30. The encoded fusion endopeptidase has antimicrobial activity and kills both Staphylococcus bacteria and Streptococcus bacteria.
Owner:US SEC AGRI
Compositions and methods for treatment of staphylococcal infection while suppressing formation of antibiotic-resistant strains
Co-administration of a lysostaphin or other anti-staphylococcal agent which cleaves cross-links of peptidoglycans of staphylococci cell walls such as lysostaphin and an antibiotic effective against staphylococci due to antibiotic activity mediated by cell-wall activity is effective against staphylococcal infection, even staphylococci that may be resistant to one or other of lysostaphin or the cell-wall active antibiotic. Co-administration simultaneously suppresses the generation of antibiotic-resistant mutant strains. Effective cell-wall active antibiotics include β-lactams and glycopeptides.
Owner:NUTRITION 21 INC
Compositions and methods for treatment of staphylococcal infection while suppressing formation of antibiotic-resistant strains
Co-administration of a lysostaphin or other anti-staphylococcal agent which cleaves cross-links of peptidoglycans of staphylococci cell walls such as lysostaphin and an antibiotic effective against staphylococci due to antibiotic activity mediated by cell-wall activity is effective against staphylococcal infection, even staphylococci that may be resistant to one or other of lysostaphin or the cell-wall active antibiotic. Co-administration simultaneously suppresses the generation of antibiotic-resistant mutant strains. Effective cell-wall active antibiotics include β-lactams and glycopeptides.
Owner:NUTRITION 21 INC
Method of secretory expression of lysostaphin in Escherichia coli at high level
ActiveUS8241901B2Economical and social benefitSpeed up the processBacteriaPeptide/protein ingredientsEscherichia coliInclusion bodies
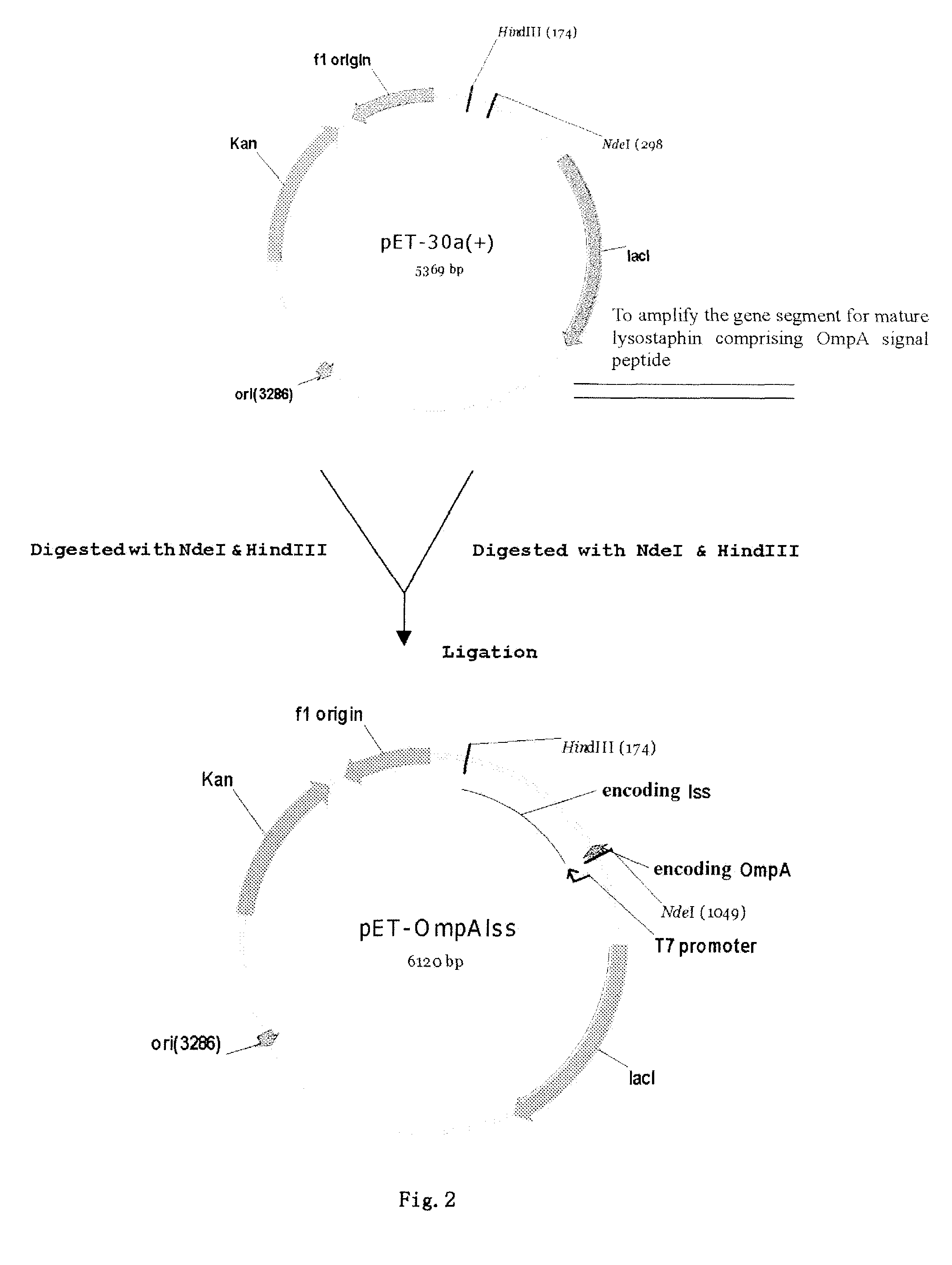
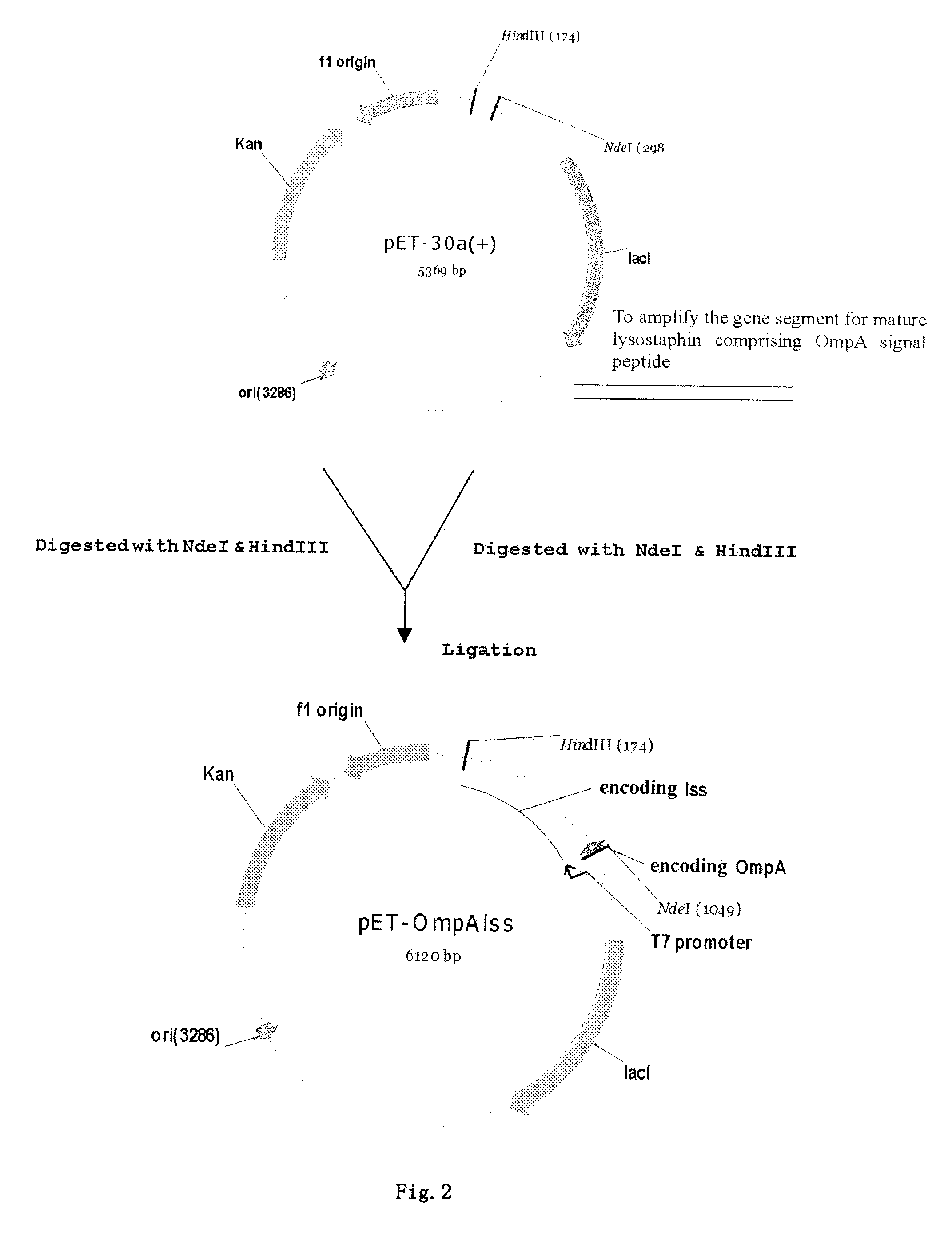
A method of secretory expression of lysostaphin in Escherichia coli at high level, which comprises constructing a expression vector by cloning a sequence encoding a signal peptide which is suitable for secretory expression in Escherichia coli before part or whole gene sequence which encodes mature lysostaphin, and ligating the cloned sequence with a promoter; and transforming Escherichia coli with the expression vector, culturing and fermenting, and then isolating lysostaphin from the supernatant of the fermentation broth. The advantage of secretory expression is that the expression product can exist in the medium in an active form, and thus does not need the process for renaturation of the inclusion body; it is more easily to purify from the supernatant of the fermentation broth with high rate of recovery; and there is less contamination from the host's proteins.
Owner:SHANGHAI HI TECH UNITED BIO TECHCAL RES
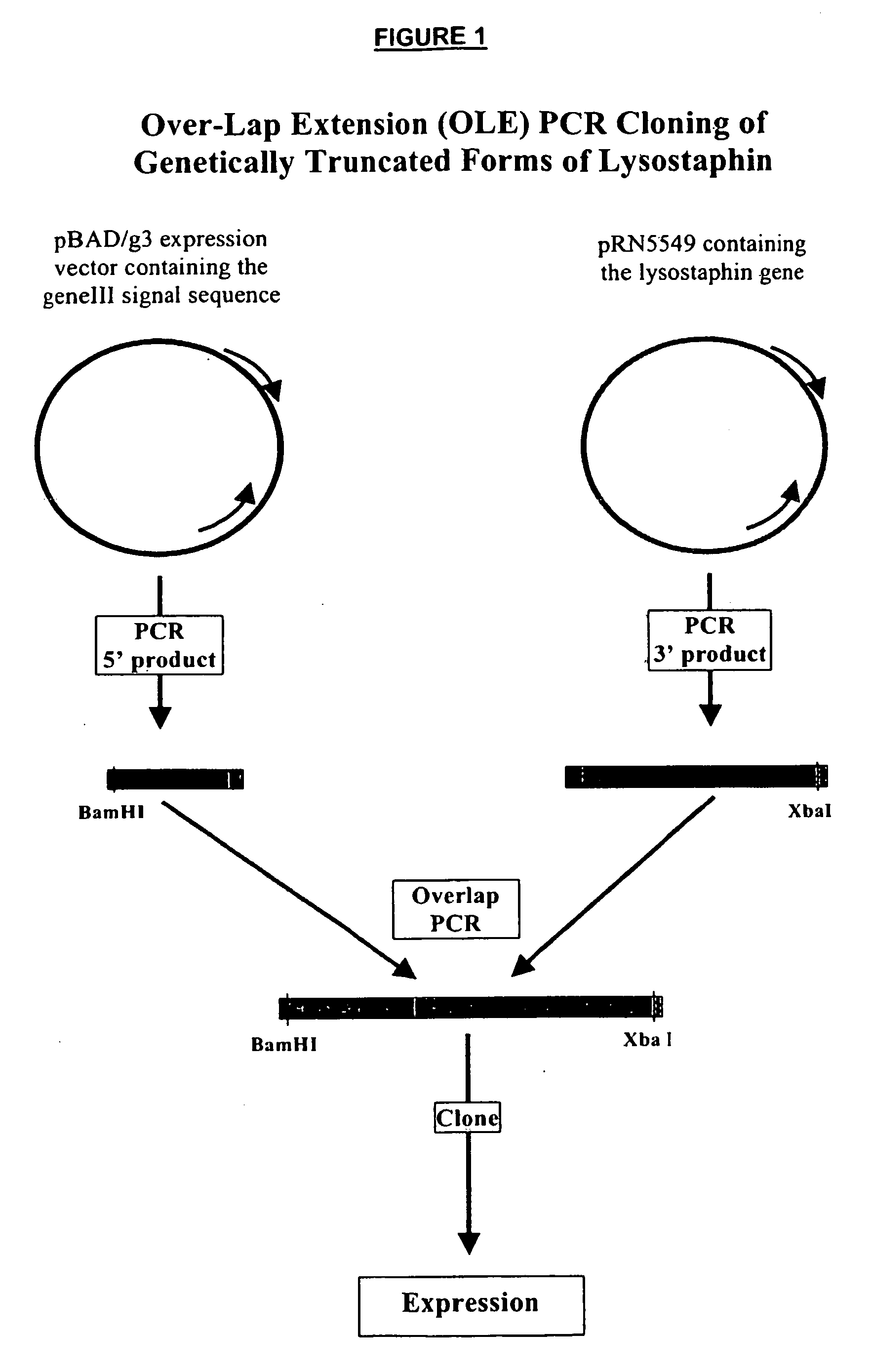
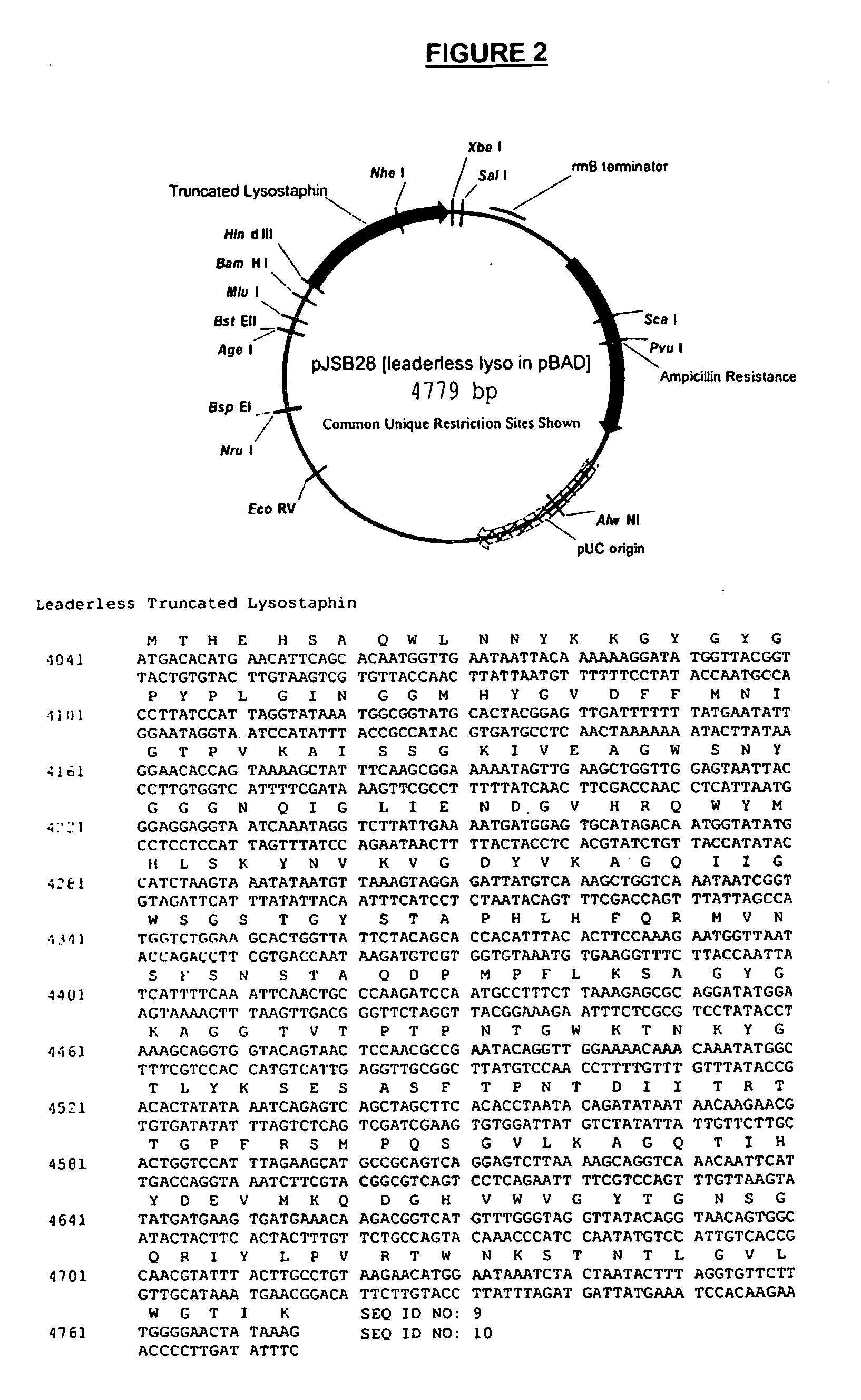
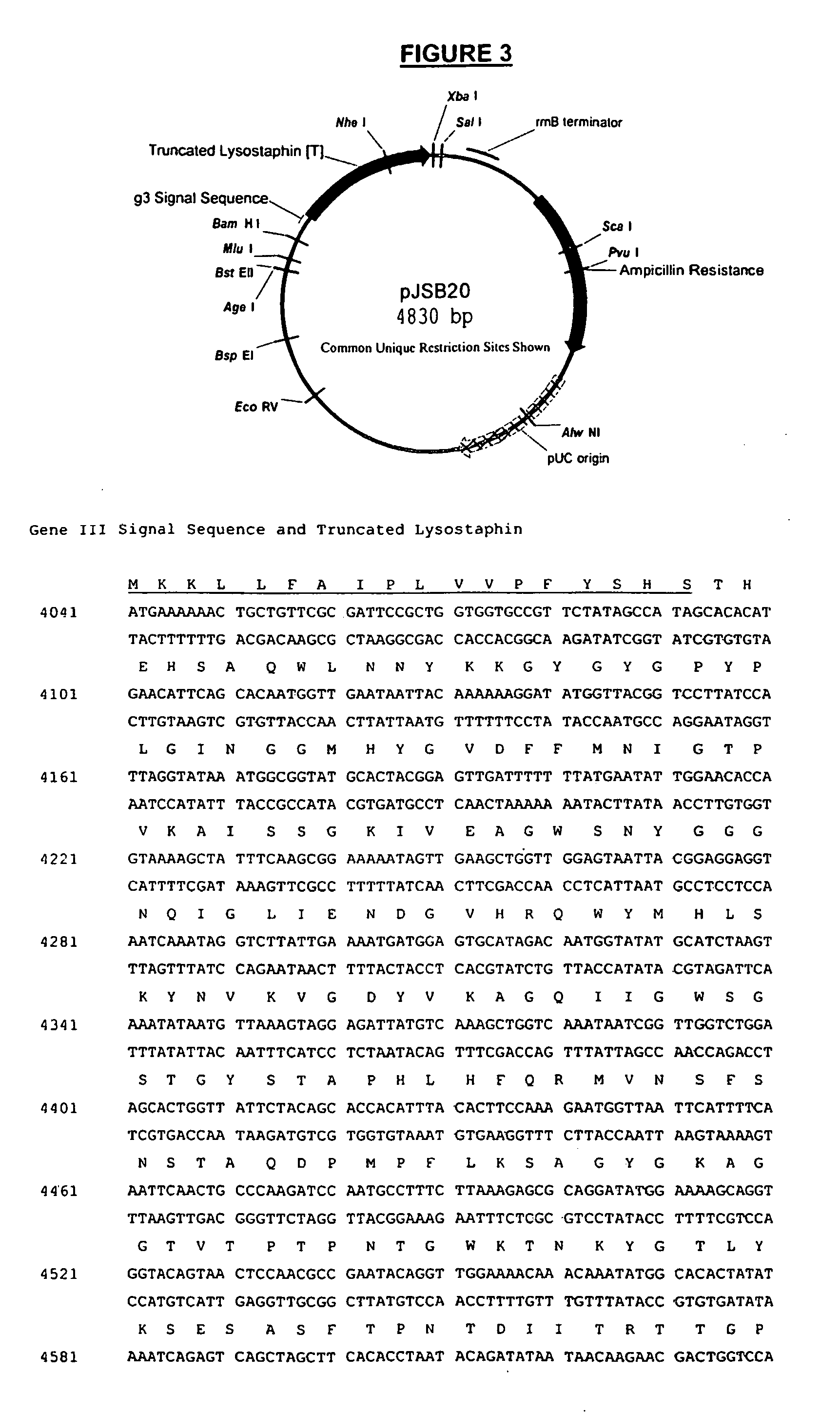
Truncated lysostaphin molecule with enhanced staphylolytic activity
InactiveUS20050118159A1Reduce individual infectionAvoid spreadingAntibacterial agentsSugar derivativesStaphylococcal infectionsStaphylococcus saprophyticus
This invention relates to the production of recombinant lysostaphin in a homogenous form through the use of recombinant DNA molecules that express homogenous lysostaphin and host cells transformed with these DNA molecules. This invention also relates to the production of truncated forms of lysostaphin. The resulting lysostaphin preparations can be administered to those at infected or risk for infection by staphylococcal bacteria.
Owner:BIOSYNEXUS INC
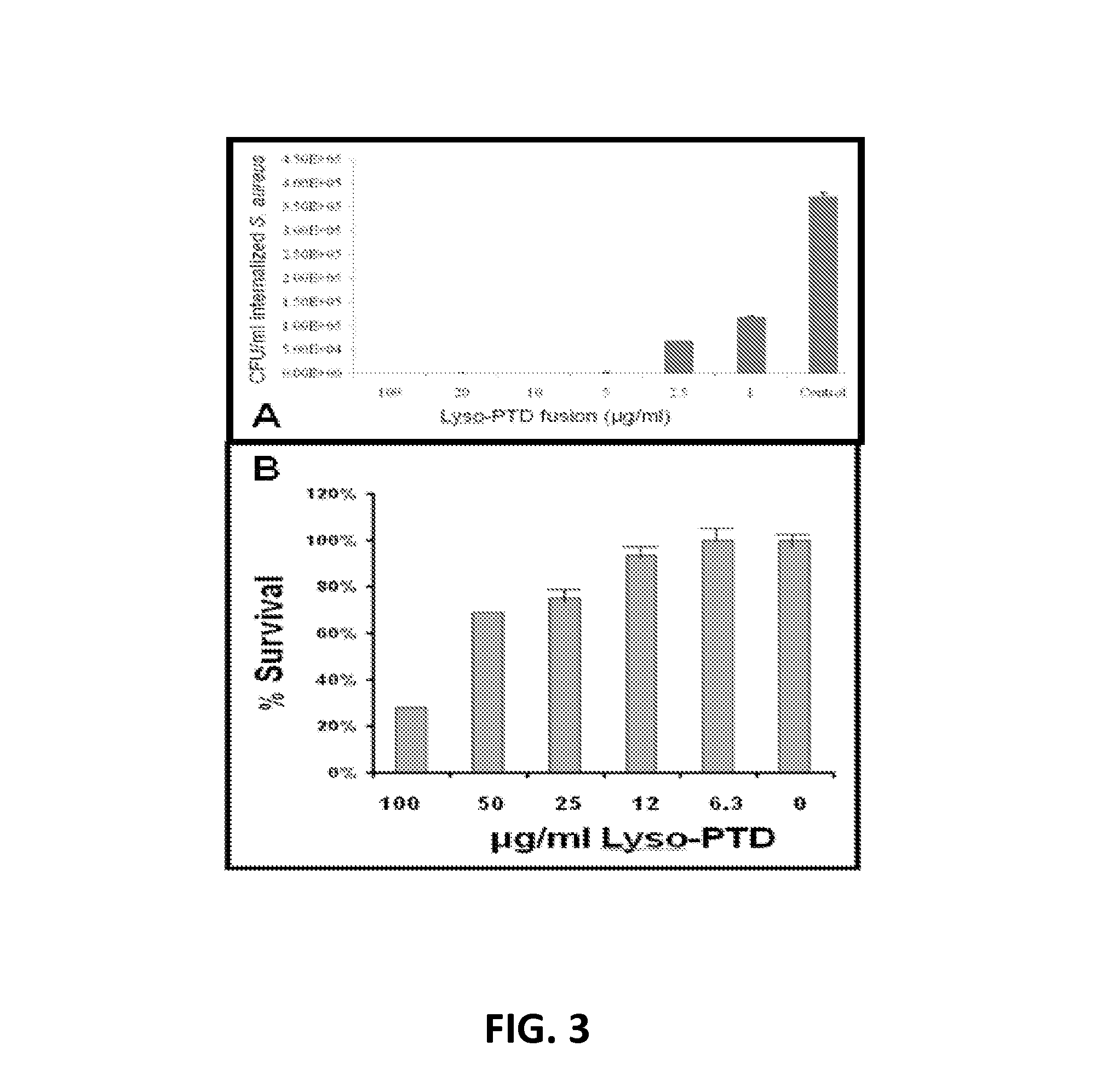
Fusion of peptidoglycan hydrolase enzymes to a protein transduction domain allows eradication of both extracellular and intracellular gram positive pathogens
InactiveUS8383102B2Facilitate its translocationEffective treatmentPolypeptide with localisation/targeting motifAntibacterial agentsPeptidoglycan HydrolaseGram
Lysostaphin is a bacteriocin secreted by S. simulans to kill S. aureus, and has been shown to also be a potent antimicrobial for many antibiotic-resistant strains of S. aureus. By adding a ˜13 amino acid protein transduction domain (PTD) from the HIV-TAT protein to lysostaphin to form lysostaphin-PTD, both extracellular and intracellular forms of S. aureus and MRSA are killed in all (multiple) cell types examined.
Owner:UNITED STATES OF AMERICA
Compositions Comprising Lysostaphin Variants And Methods Of Using The Same
InactiveUS20080095756A1Antibacterial agentsPeptide/protein ingredientsBiologyPolymicrobial Infections
The present invention relates to compositions comprising lysostaphin variants and methods of using the same. In particular, the present invention provides de-immunized lysostaphin variants and methods of using the same (e.g., to treat microbial infection in or on a subject).
Owner:BIOSYNEXUS INC
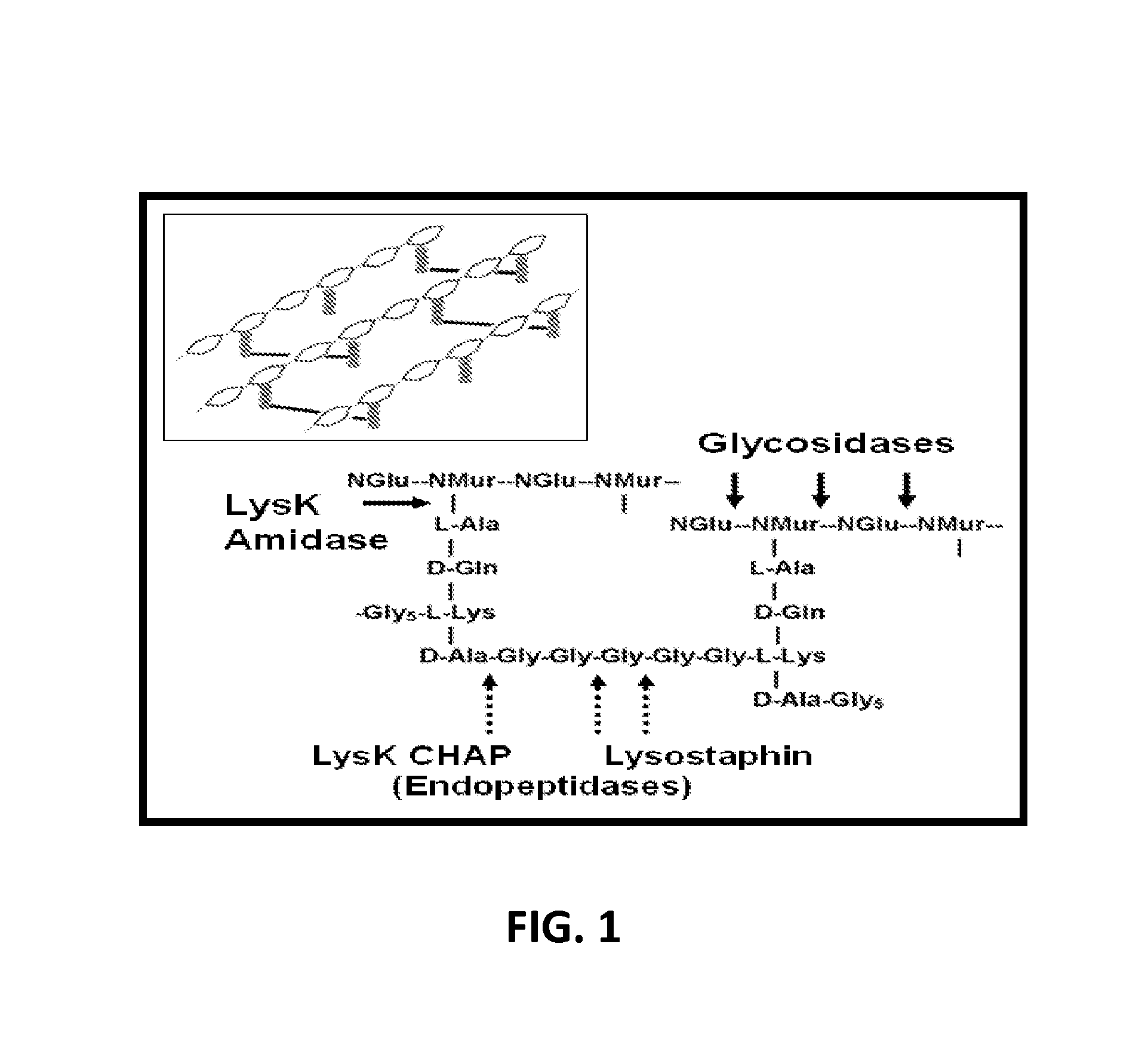
Triple acting antimicrobials that are refractory to resistance development
InactiveUS8481289B2Effectively lysingPeptide/protein ingredientsHydrolasesGrowth phaseGlycyl-Glycine
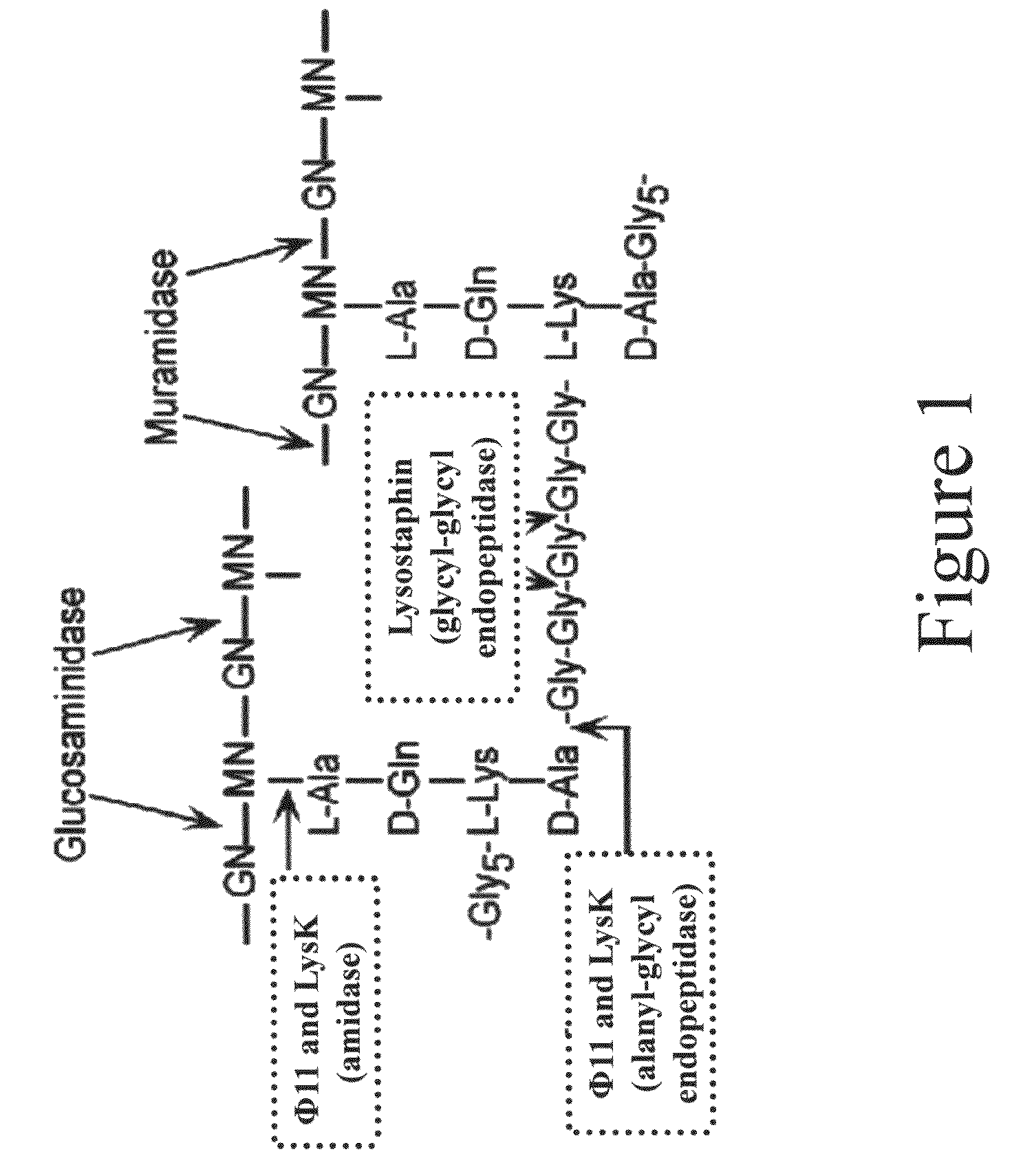
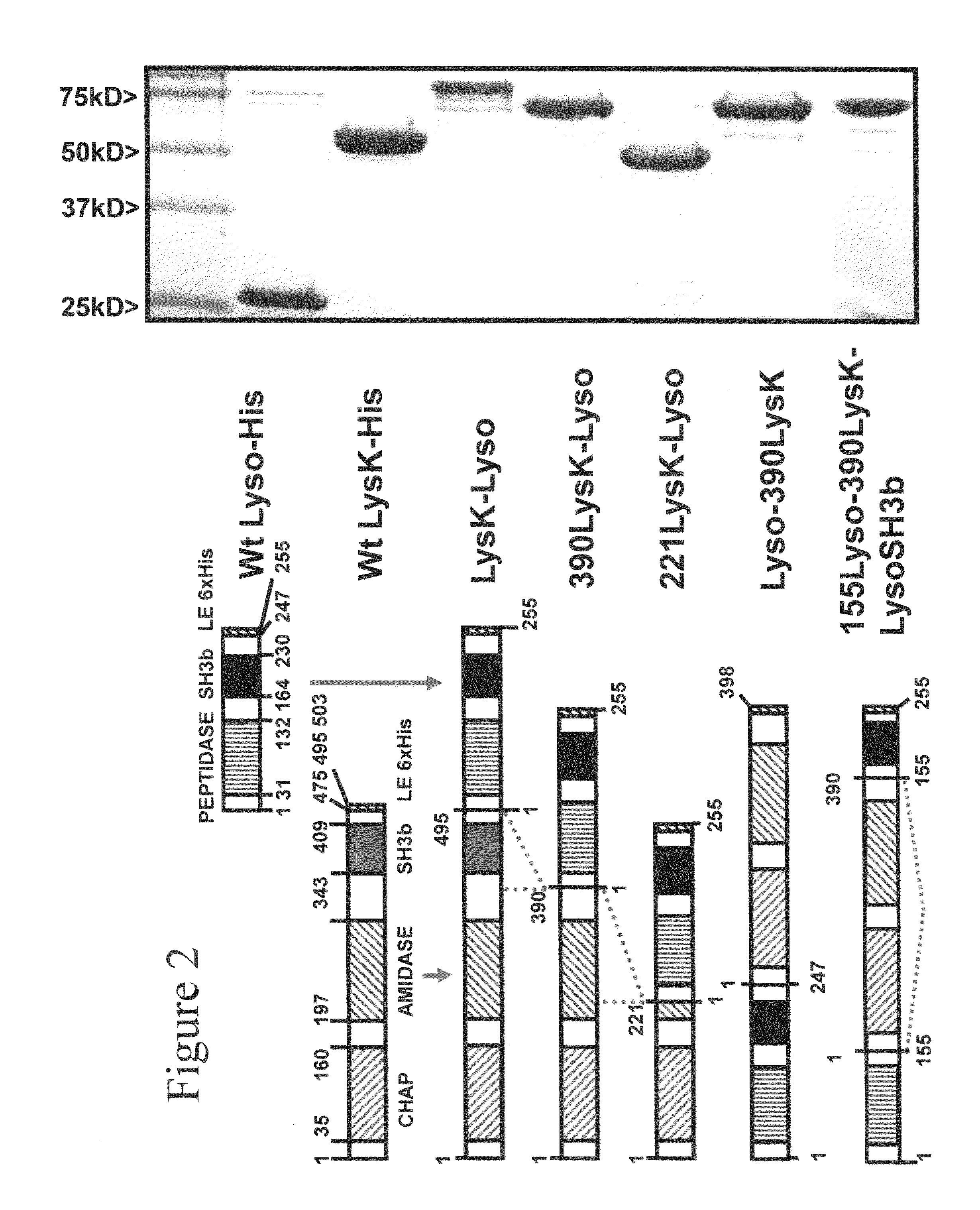
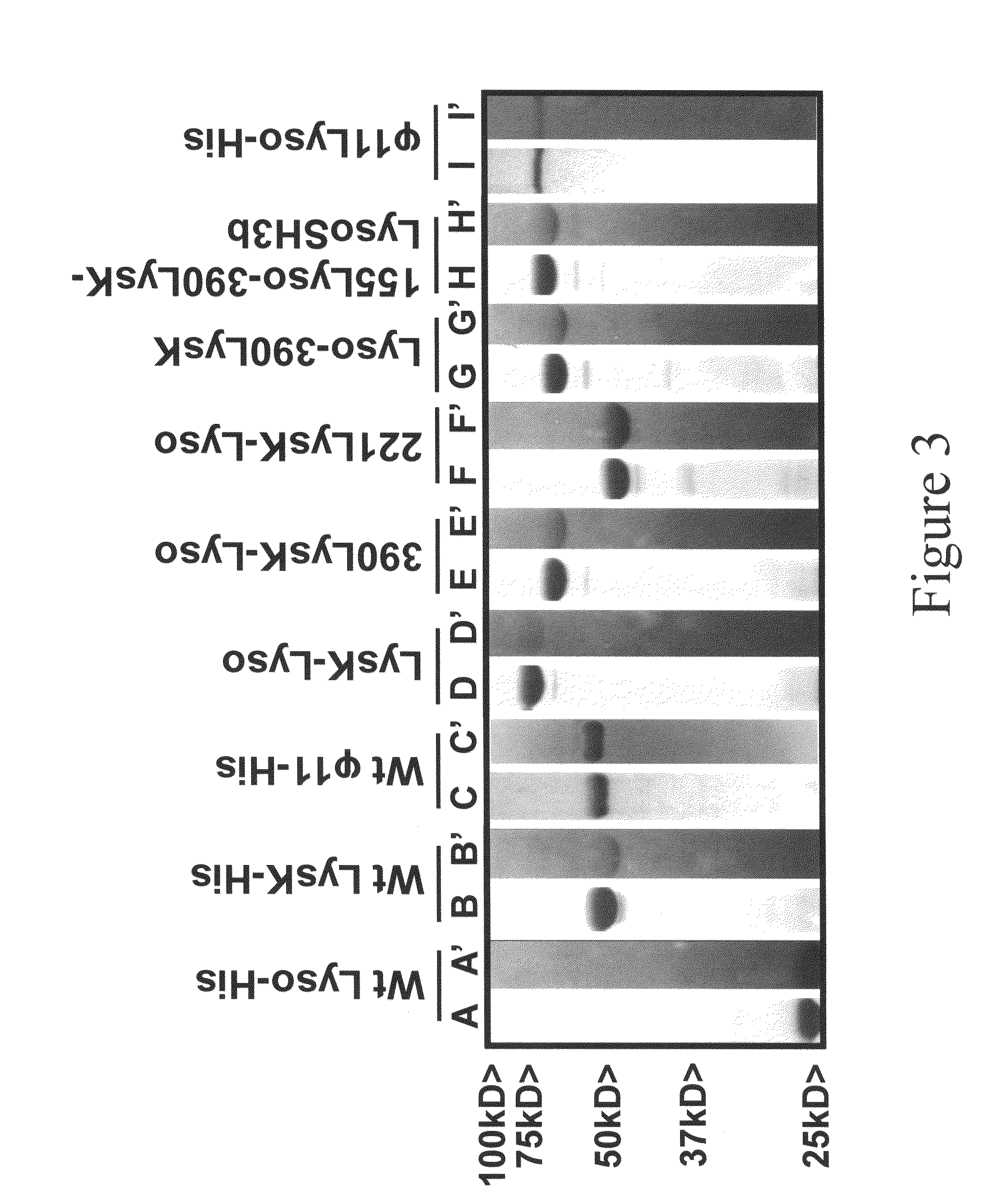
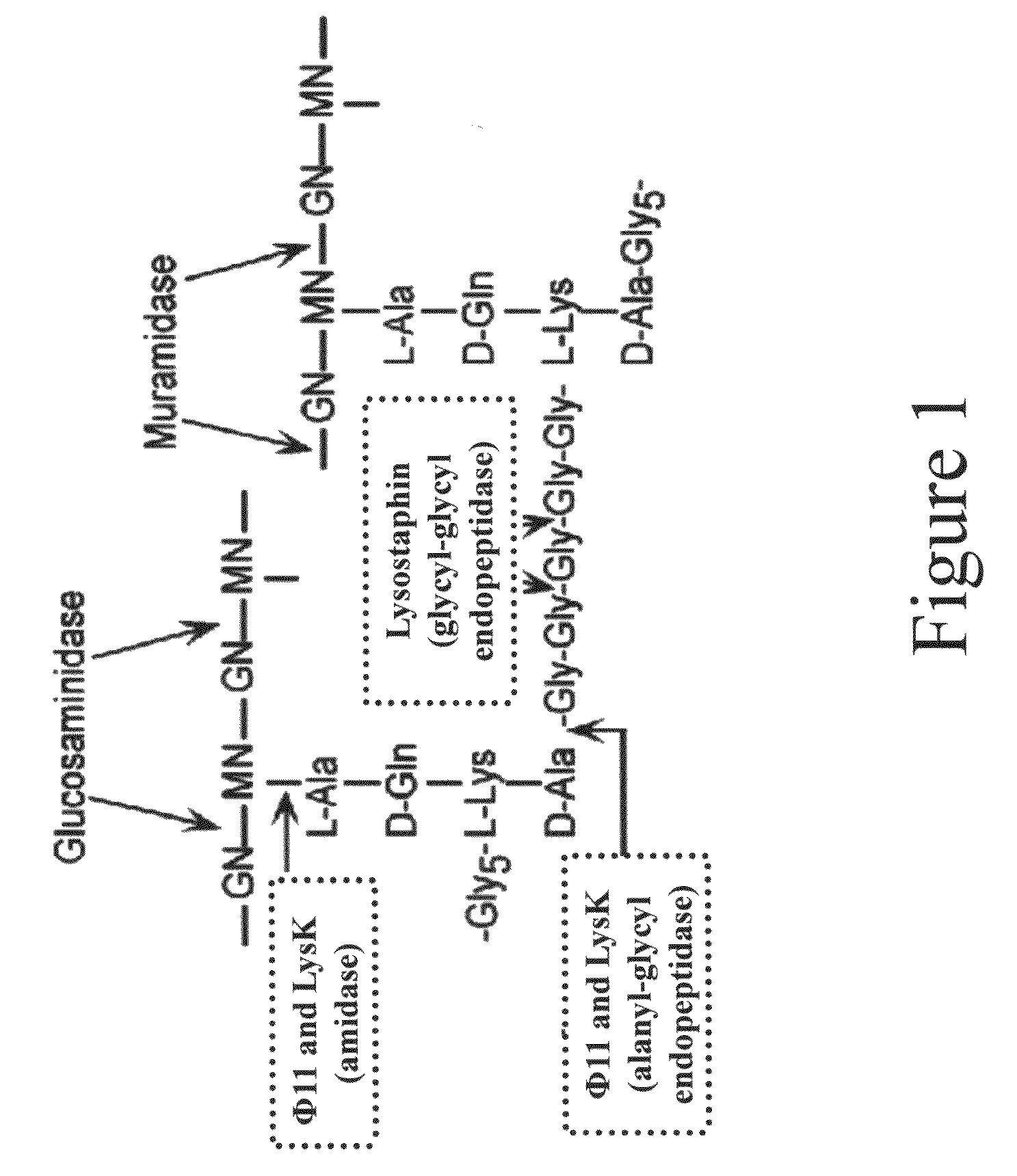
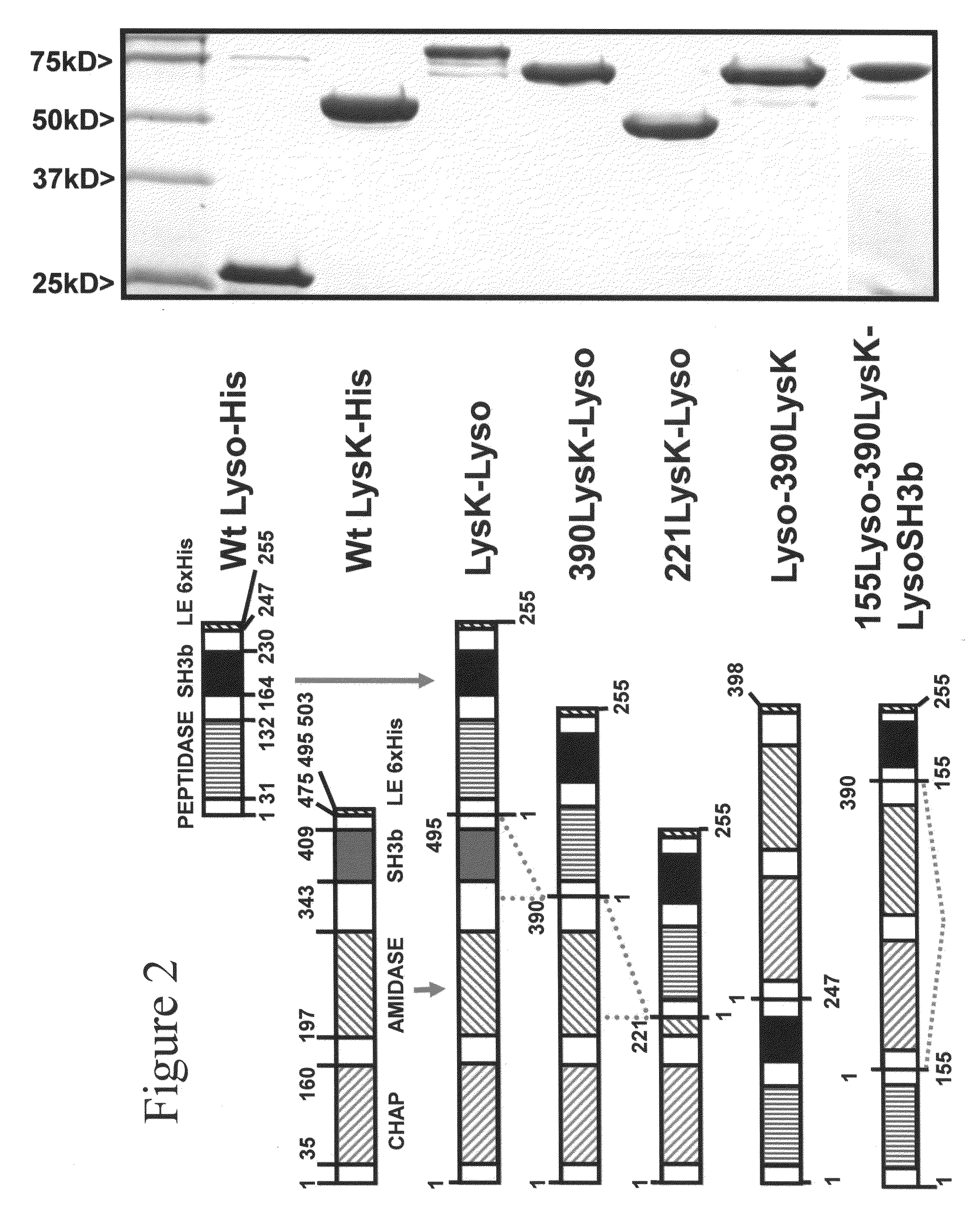
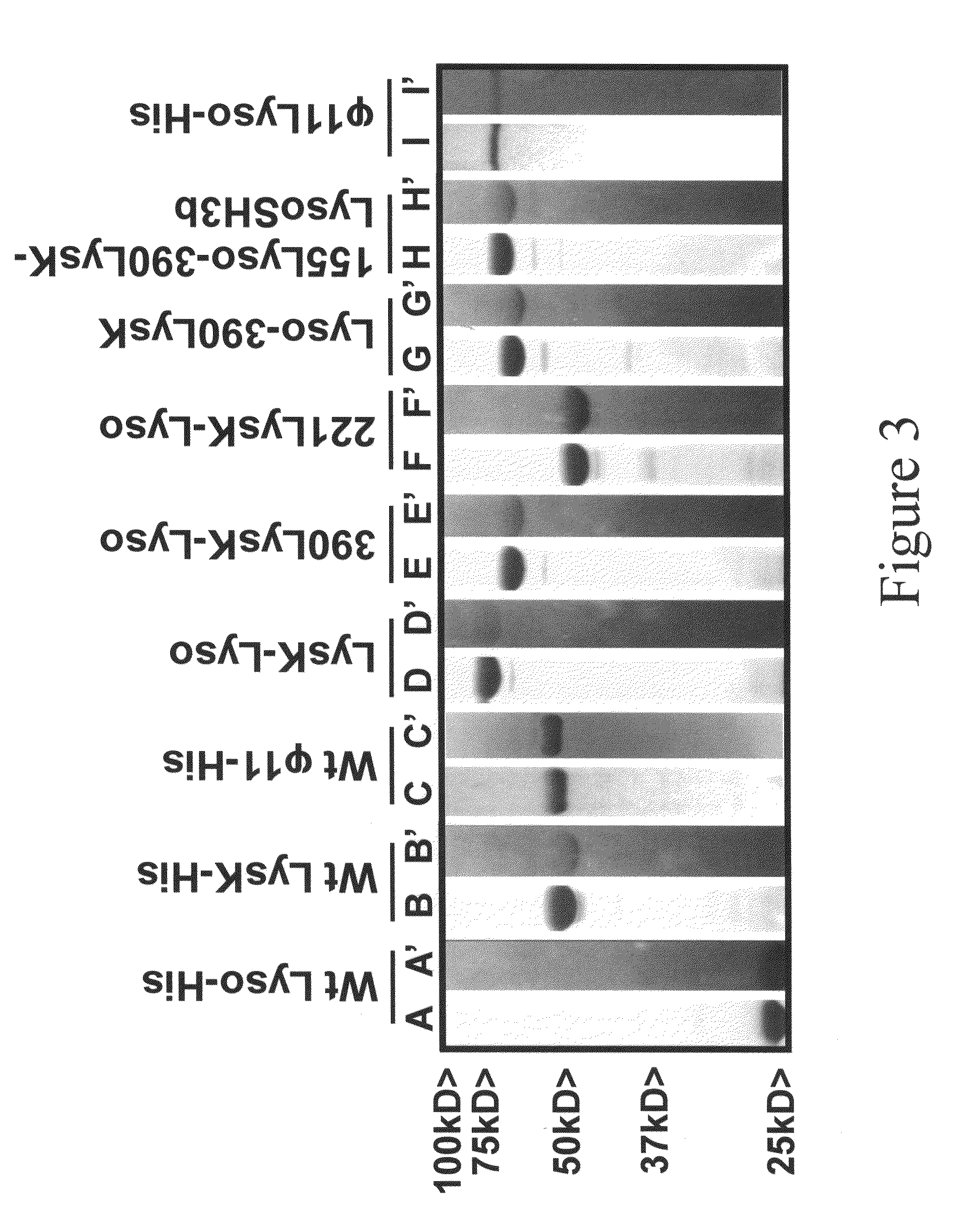
Multi-drug resistant superbugs are a persistent problem in modern health care. This invention provides an antimicrobial endolysin-Lysostaphin triple fusion protein, comprising (1) an endolysin CHAP endopeptidase domain, (2) an endolysin amidase domain, and (3) a Lysostaphin glycyl-glycine endopeptidase domain. The domains are derived from two proteins that show antimicrobial synergy when used in combination. The protein has specificity and exolytic activity for the peptidoglycan cell wall of untreated, live Staphylococcus aureus from many growth phases i.e. stationary, logarithmic and biofilm growth. The recombinant triple fusion protein comprising the three functional antimicrobial domains is designed to be refractory to resistance development.
Owner:US SEC AGRI
Triple acting antimicrobials that are refractory to resistance development
Multi-drug resistant superbugs are a persistent problem in modern health care. This invention provides an antimicrobial endolysin-Lysostaphin triple fusion protein, comprising (1) an endolysin CHAP endopeptidase domain, (2) an endolysin amidase domain, and (3) a Lysostaphin glycyl-glycine endopeptidase domain. The domains are derived from two proteins that show antimicrobial synergy when used in combination. The protein has specificity and exolytic activity for the peptidoglycan cell wall of untreated, live Staphylococcus aureus from many growth phases i.e. stationary, logarithmic and biofilm growth. The recombinant triple fusion protein comprising the three functional antimicrobial domains is designed to be refractory to resistance development.
Owner:US SEC AGRI
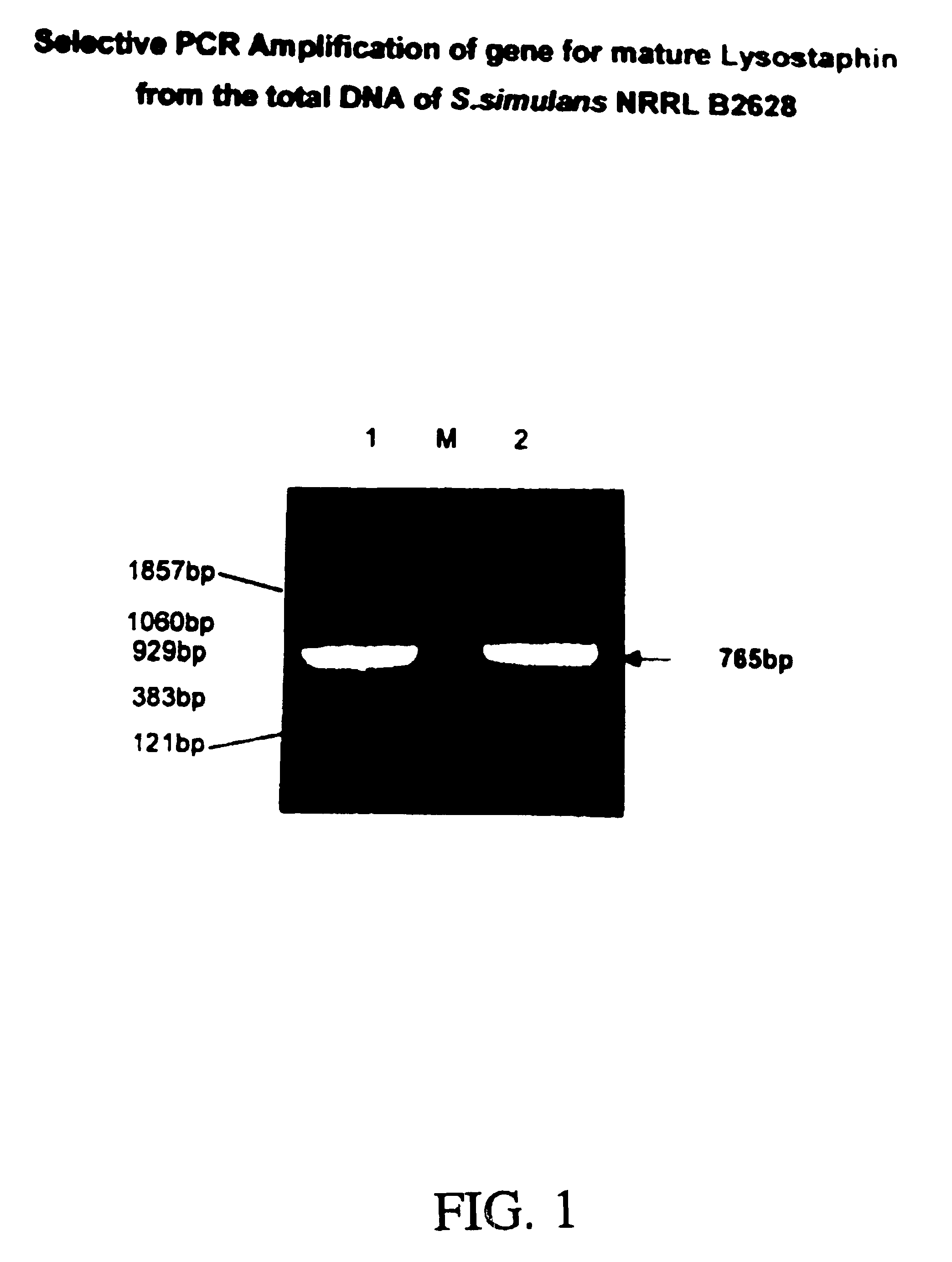
Expression of recombinant mature lysostaphin
A portion of the lysostaphin gene of Staphylococcus simulans has been cloned and overexpressed in the cytoplasm of E. coli to yield lysostaphin, in the absence of preprolysostaphin and prolysostaphin, under the transcriptional control of an IPTG-inducible promoter and a ribosome binding site. IPTG induction of the transformed host cells produces intracellular, soluble, mature lysostaphin (27 kDa), in the complete absence of preprolysostaphin and prolysostaphin. The mature lysostaphin so formed dose not require post-translational modification. The mature lysostaphin so formed can be used treat and prevent staphylococcal infections.
Owner:COUNCIL OF SCI & IND RES +1
Antimicrobial polymer conjugate containing lysostaphin and polyethylene glycol
InactiveUS7452533B2Extended half-lifeMaintain antimicrobial activityAntibacterial agentsPeptide/protein ingredientsMicrobial agentPolyethylene glycol
Water-soluble polymer conjugates of antimicrobial agents retaining at least a portion of the antimicrobial activity of the agent, pharmaceutical compositions containing the polymer conjugates, and methods for treating microbial infections with the pharmaceutical compositions.
Owner:BIOSYNEXUS INC
Method for preparing dry-cured goose through Lysostaphin co-fermentation of goose meat
The invention belongs to the field of the food processing technology and in particular relates to a method for preparing dry-cured goose through Lysostaphin co-fermentation of goose meat. The method comprises the following steps: I. curing goose meat preliminarily; II. inoculating, fermenting and airing; and III. packaging. According to the method for preparing dry-cured goose through Lysostaphin co-fermentation of goose meat, the defects that the existing goose meat is rough in meat quality and has poor mouthfeel and fragrance are overcome, the method disclosed by the invention has short fermentation period, and the prepared dry-cured goose has fine mouthfeel and is delicious in taste; and furthermore, the fermentation of the goose meat and airing are carried out simultaneously, the fermenting time and the airing time of the dry-cured goose are shortened, the goose meat has low oil oxidative rancidity and high protein decomposition in a low-temperature lysostaphin co-fermentation process; and besides, the lactobacillus fermentation quantity is low, the fermentation sour is reduced, the delicate flavor generated by amino acid is improved and the quality and the safety of the dry-cured goose product are improved.
Owner:CHONGQING QINGSHUIWAN FOOD
Compound preparation for dissolving staphyloase and preparation method
InactiveCN1438032AImprove the bactericidal effectExpanded bactericidal spectrumAntibacterial agentsPeptide/protein ingredientsSide effectMonilinia laxa
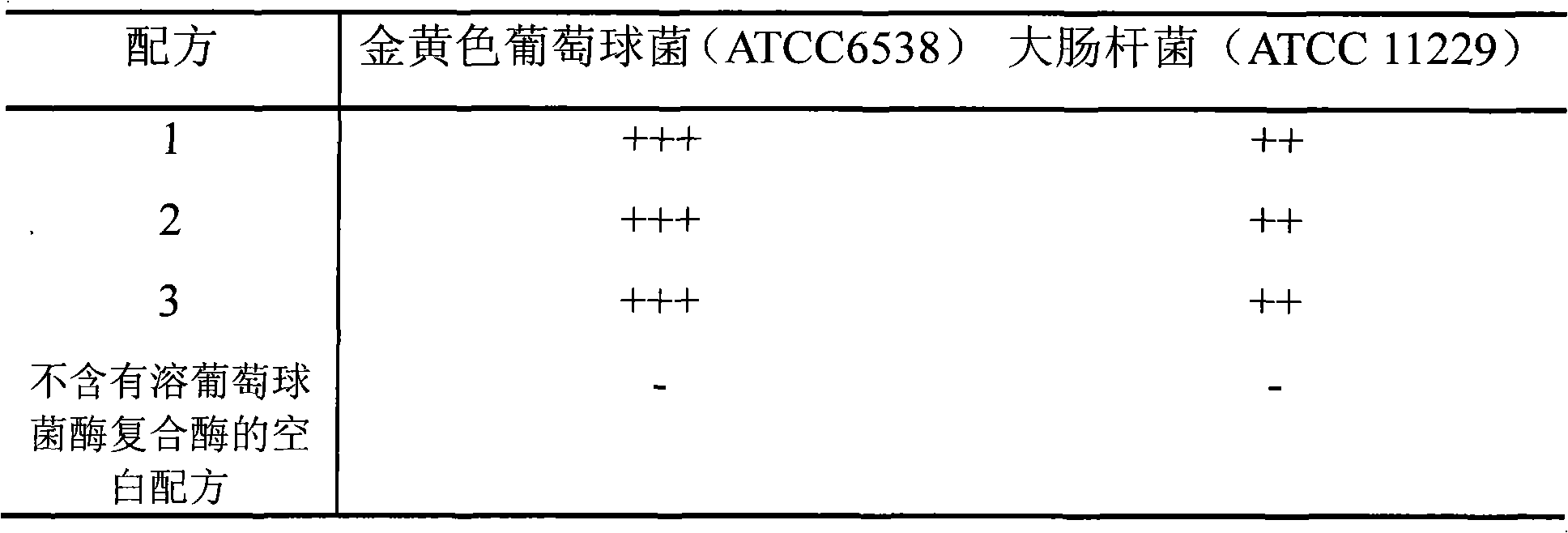
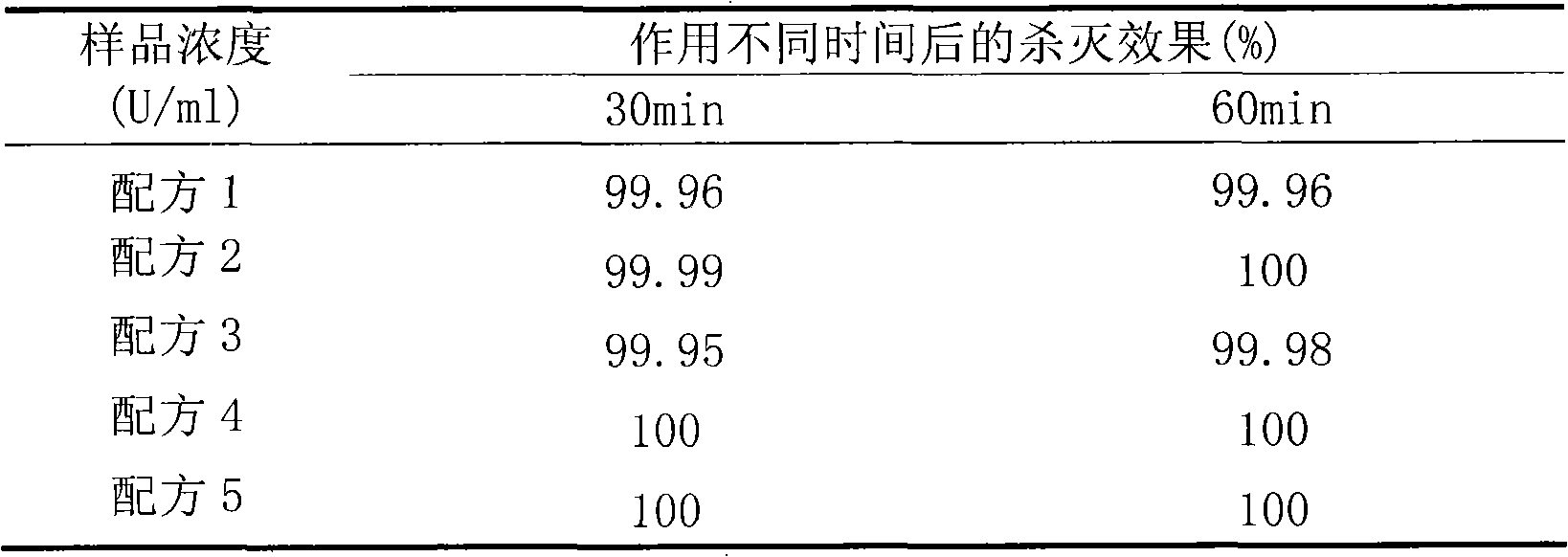
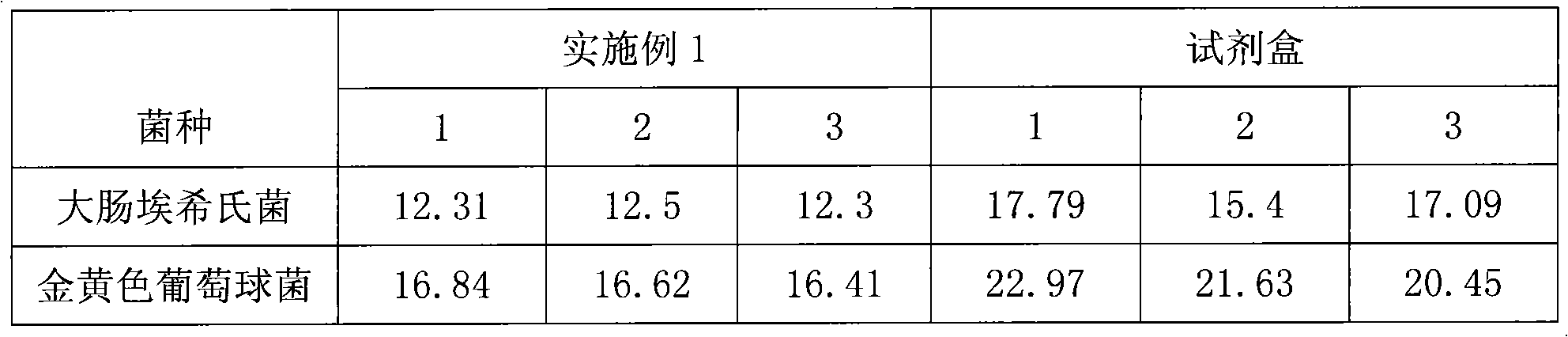
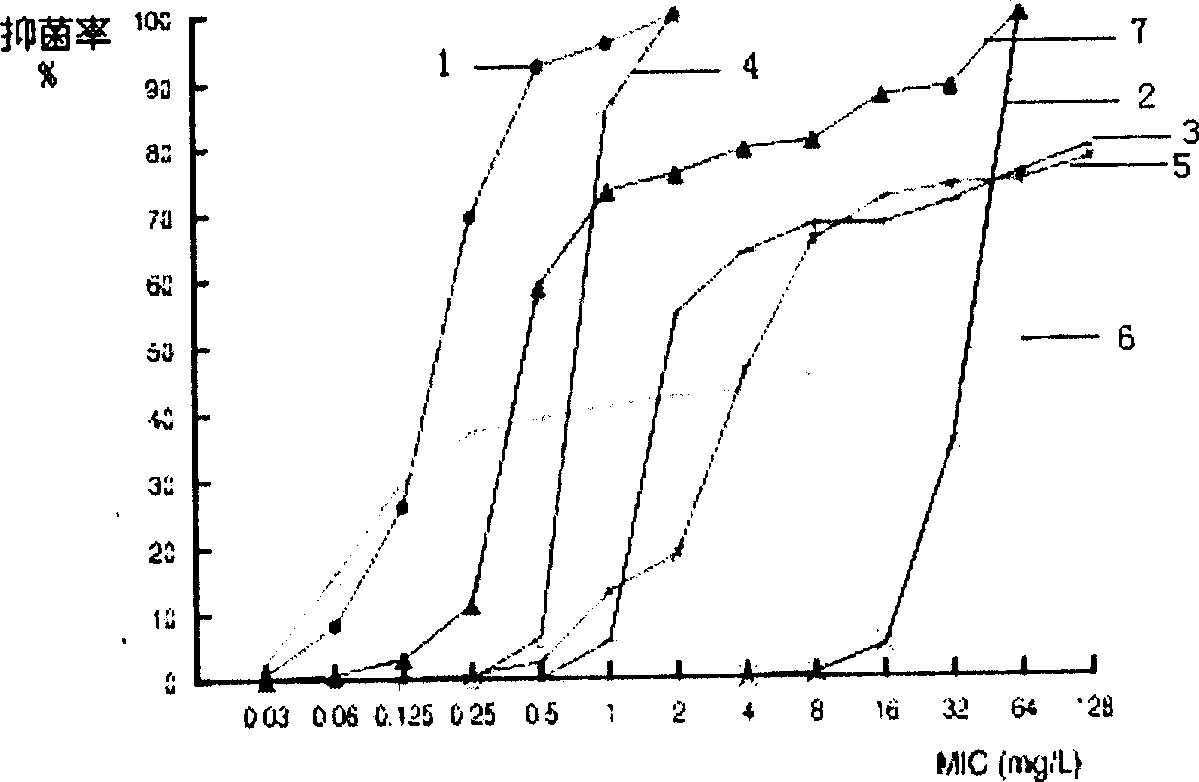
The present invention discloses a lysostaphin compounded preparation. Said preparation comprises bacteria-killing effective quantity of lysostaphin and lysozyme. Said lysostaphin compounded preparation has higher bacteria-killing effect as compared with single lysostaphin, not only has the strong bacteria-killing action for Gram-positive bacterium and Gram-negative bacterium, but also has the good effect for killing the fungi of Candida albicans and red trichophyton, etc. It has high bacteria-killing activity and stability, does not produce drug resistance, and has no toxic side effect.
Owner:SHANGHAI HI TECH BIOENG
Fusion of Peptidoglycan Hydrolase Enzymes to a Protein Transduction Domain Allows Eradication of both Extracellular and Intracellular Gram Positive Pathogens
InactiveUS20110027249A1Effective antimicrobial treatmentFacilitate its translocationPolypeptide with localisation/targeting motifAntibacterial agentsPeptidoglycan HydrolaseADAMTS Proteins
Lysostaphin is a bacteriocin secreted by S. simulans to kill S. aureus, and has been shown to also be a potent antimicrobial for many antibiotic-resistant strains of S. aureus. By adding a ˜13 amino acid protein transduction domain (PTD) from the HIV-TAT protein to lysostaphin to form lysostaphin-PTD, both extracellular and intracellular forms of S. aureus and MRSA are killed in all (multiple) cell types examined.
Owner:US SEC AGRI
Lysostaphin freeze dried powder used for preventing and treating cattle edometritis
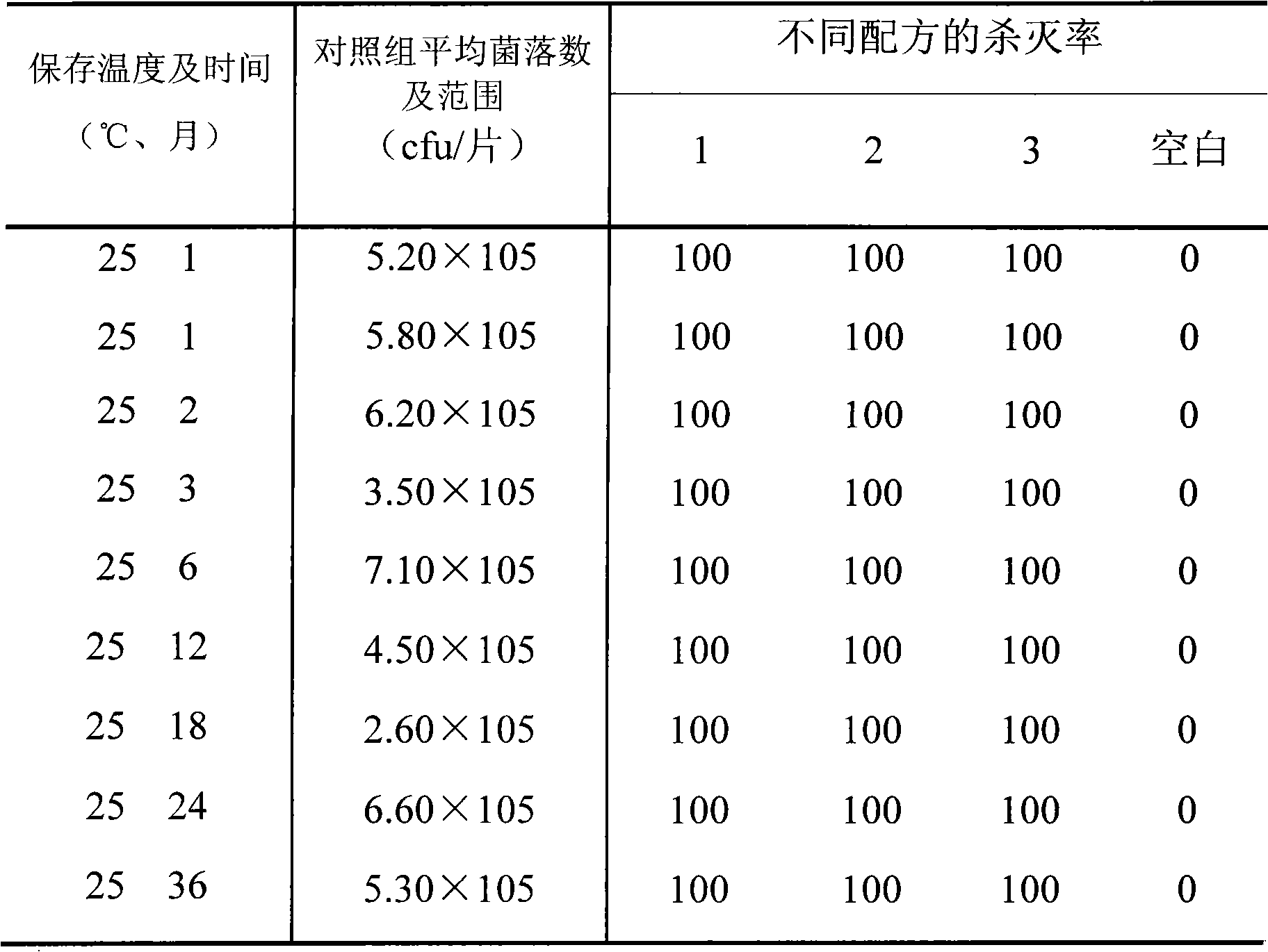
ActiveCN1911441AImprove the bactericidal effectImprove stabilityPowder deliveryPeptide/protein ingredientsPhosphateFreeze-drying
A freeze-dried powder-injection of staphylococcus lysozyme for preventing and treating the endometritis of cow and the skin mucosa infection of other animals, and cleaning and restoring the postpartum uterus of cow is composed of staphylococcus lysozyme, bovine serum albumin, glycine, mannitol, and phosphate. Its preparing process is also disclosed.
Owner:昆山博青生物科技有限公司
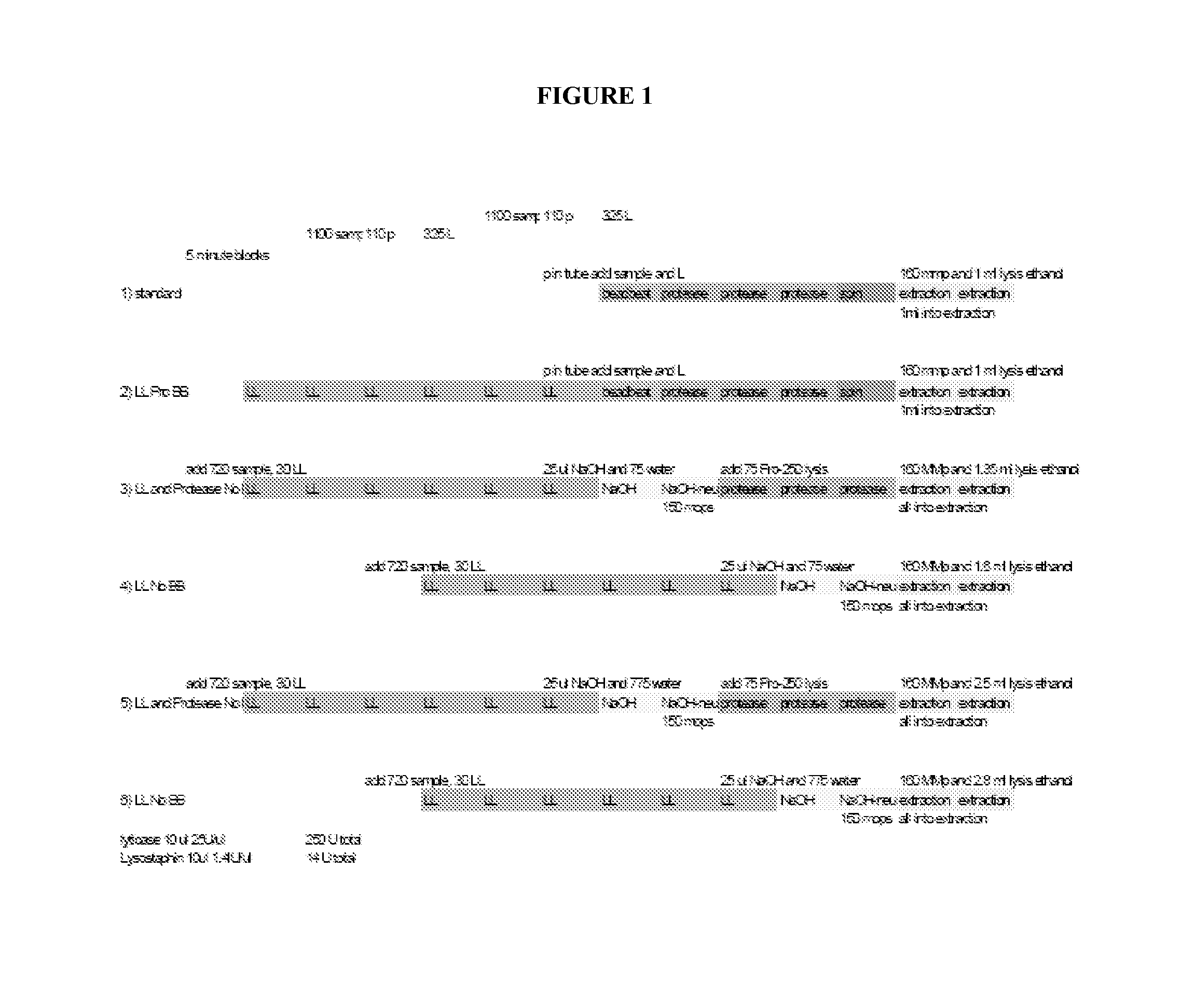
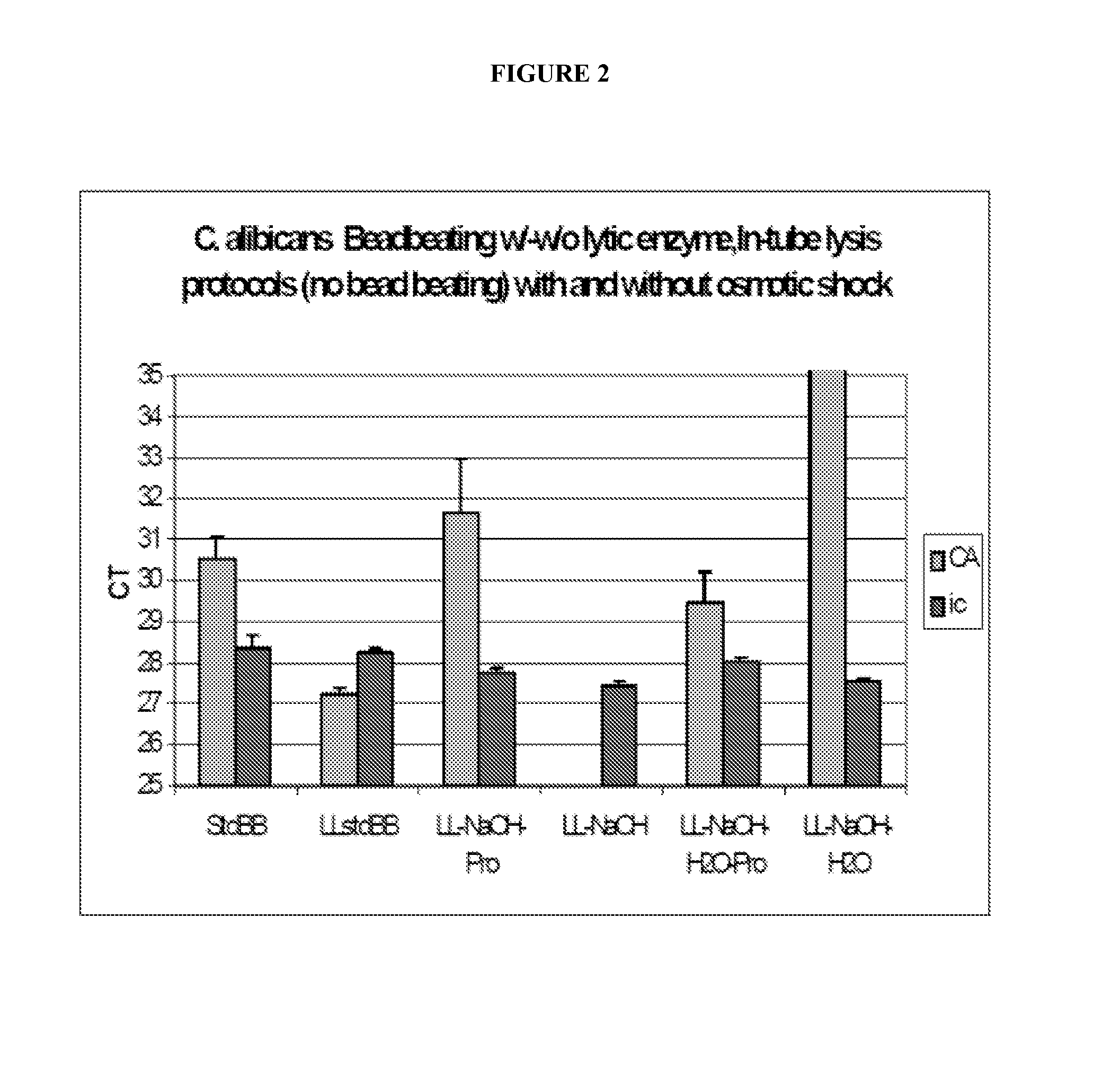
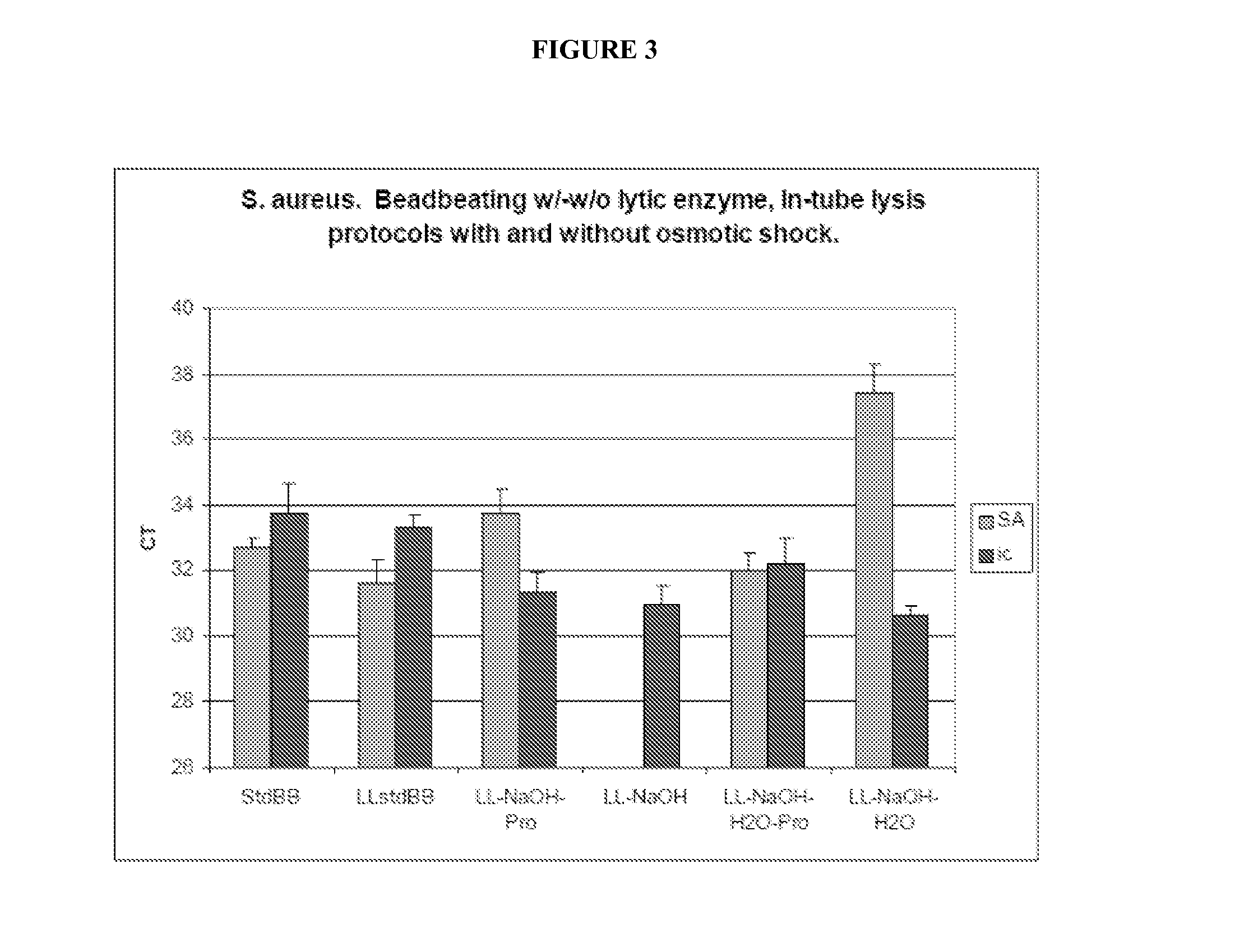
Fungal nucleic acid extraction
InactiveUS20130323815A1Facilitates sample homogenizationBioreactor/fermenter combinationsBiological substance pretreatmentsYeastBiotechnology
The invention provides methods for extraction of fungal (e.g., yeast spp., filamentous fungal spp.) nucleic acid (e.g., DNA, RNA) from a sample (e.g., be human or veterinary clinical or research samples, agricultural samples, agricultural commodity samples, food products, or environmental samples). In some embodiments, the present invention provides enhanced nucleic acid extraction from samples comprising fungal cell(s) wherein enzymatic (e.g., lysostaphin treatment, lyticase treatment) sample treatment is performed in combination with mechanical (e.g., bead beating) sample treatment.
Owner:IBIS BIOSCI
Suppository for treating mammalian endometritis and preparation method thereof
InactiveCN101766813AKeep aliveEfficient killingOrganic active ingredientsPeptide/protein ingredientsAscorbyl stearateTreatment effect
The invention discloses a suppository for treating mammalian endometritis and a preparation method thereof; the suppository comprises the following ingredients by counting total weight of the preparation: 0.001-0.1 percent of lysostaphin, 0.1-10 percent of lysozyme, 0.1-10 percent of sodium alginate tech grade, and 80.0-99.6 percent of polyoxyethylene ascorbyl stearate; by adopting the local administration mode, the mammalian endometritis can be effectively treated, the sterilizing effect is strong, the stability is good; in addition, in the processing process, as the lysozyme and the lysostaphin are packaged by the sodium alginate tech grade, so as to keep the activity of the enzymes in the suppository preparation process, lead the enzymes to be released in utero slowly and achieve better curative effect.
Owner:SHANGHAI HI TECH UNITED BIO TECHCAL RES
Method of secretory expression of lysostaphin in escherichia coli at high level
ActiveUS20090186380A1Easy to purifyLess protein contaminantBacteriaHydrolasesEscherichia coliInclusion bodies
A method of secretory expression of lysostaphin in Escherichia coli at high level, which comprises constructing a expression vector by cloning a sequence encoding a signal peptide which is suitable for secretory expression in Escherichia coli before part or whole gene sequence which encodes mature lysostaphin, and ligating the cloned sequence with a promoter; and transforming Escherichia coli with the expression vector, culturing and fermenting, and then isolating lysostaphin from the supernatant of the fermentation broth. The advantage of secretory expression is that the expression product can exist in the medium in an active form, and thus does not need the process for renaturation of the inclusion body; it is more easily to purify from the supernatant of the fermentation broth with high rate of recovery; and there is less contamination from the host's proteins.
Owner:SHANGHAI HI TECH UNITED BIO TECHCAL RES
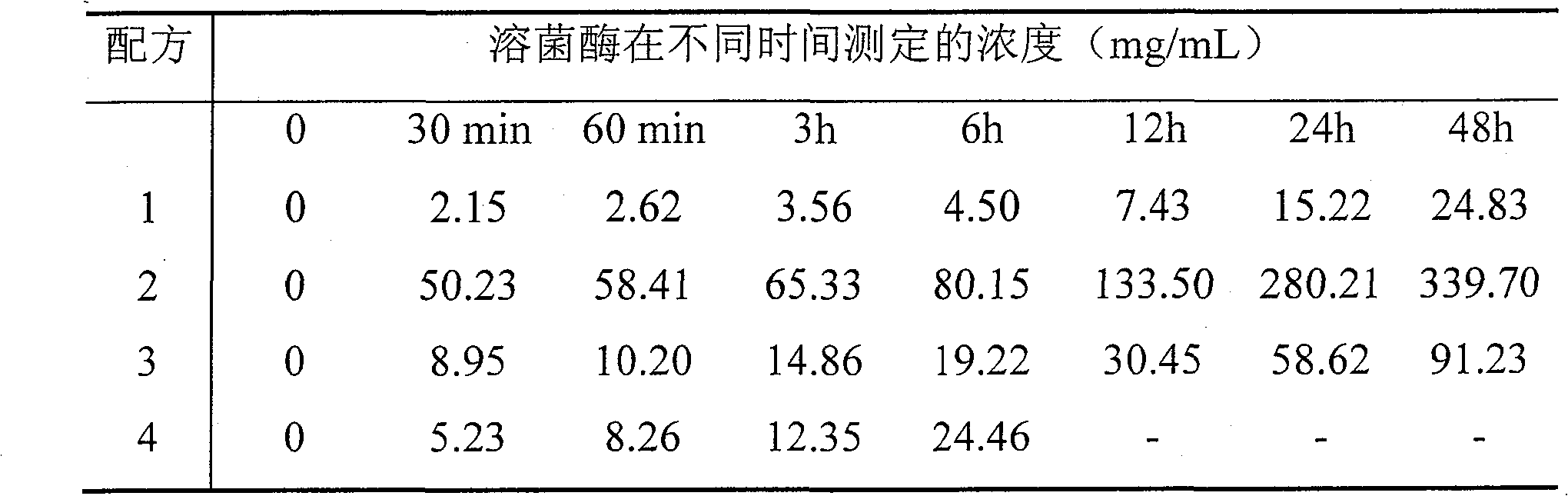
Lysostaphin industrial purifying process
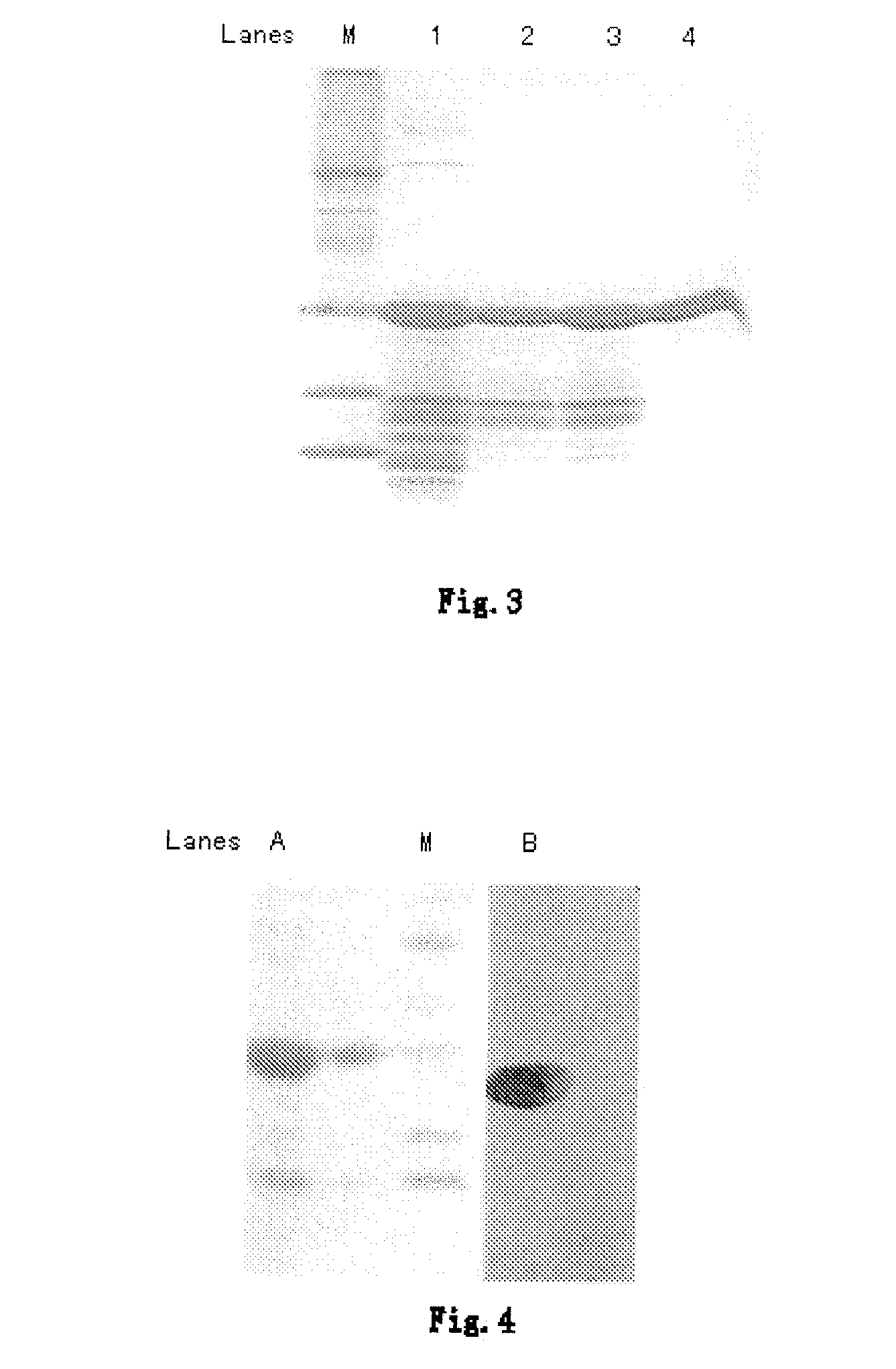
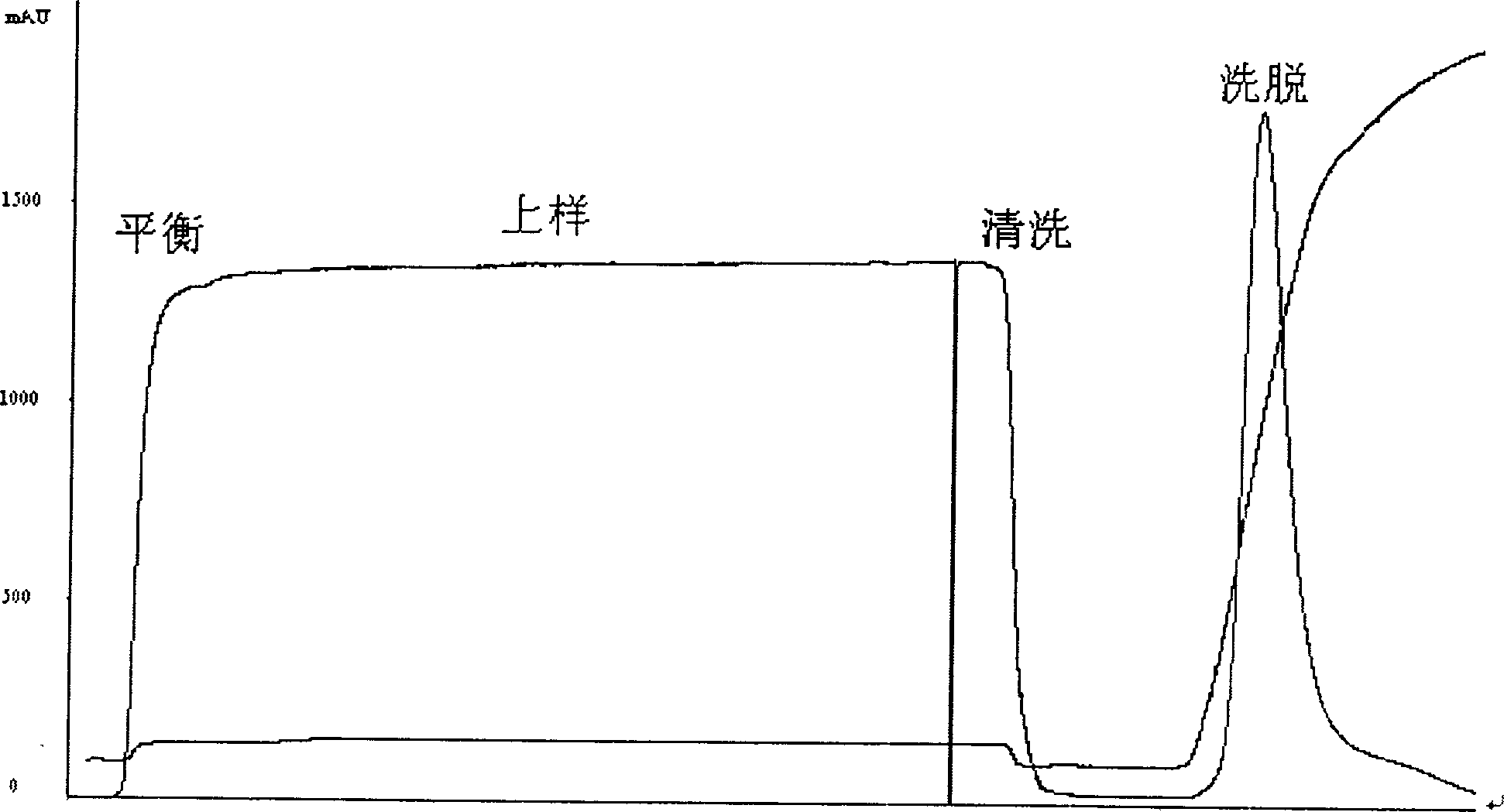
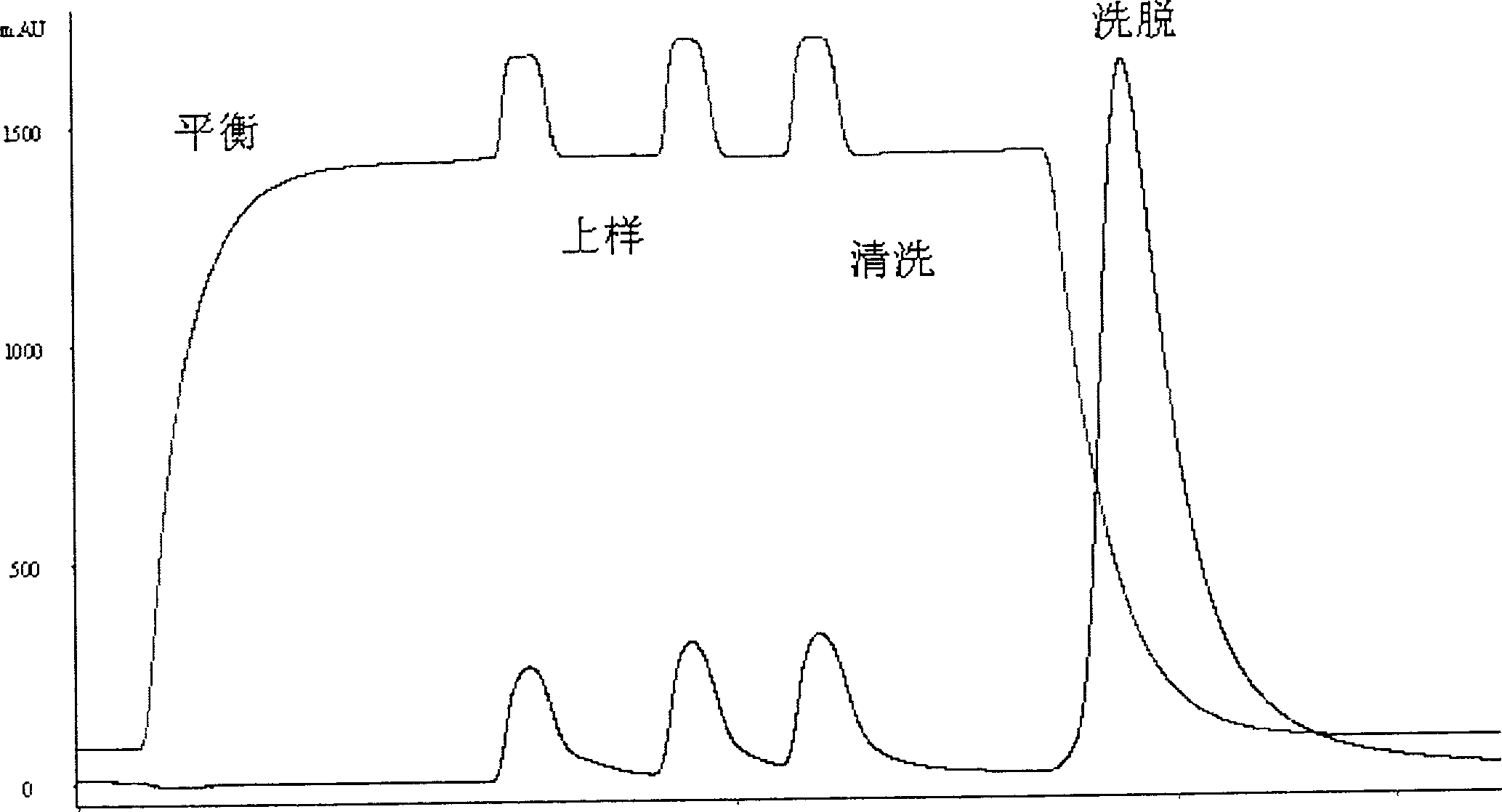
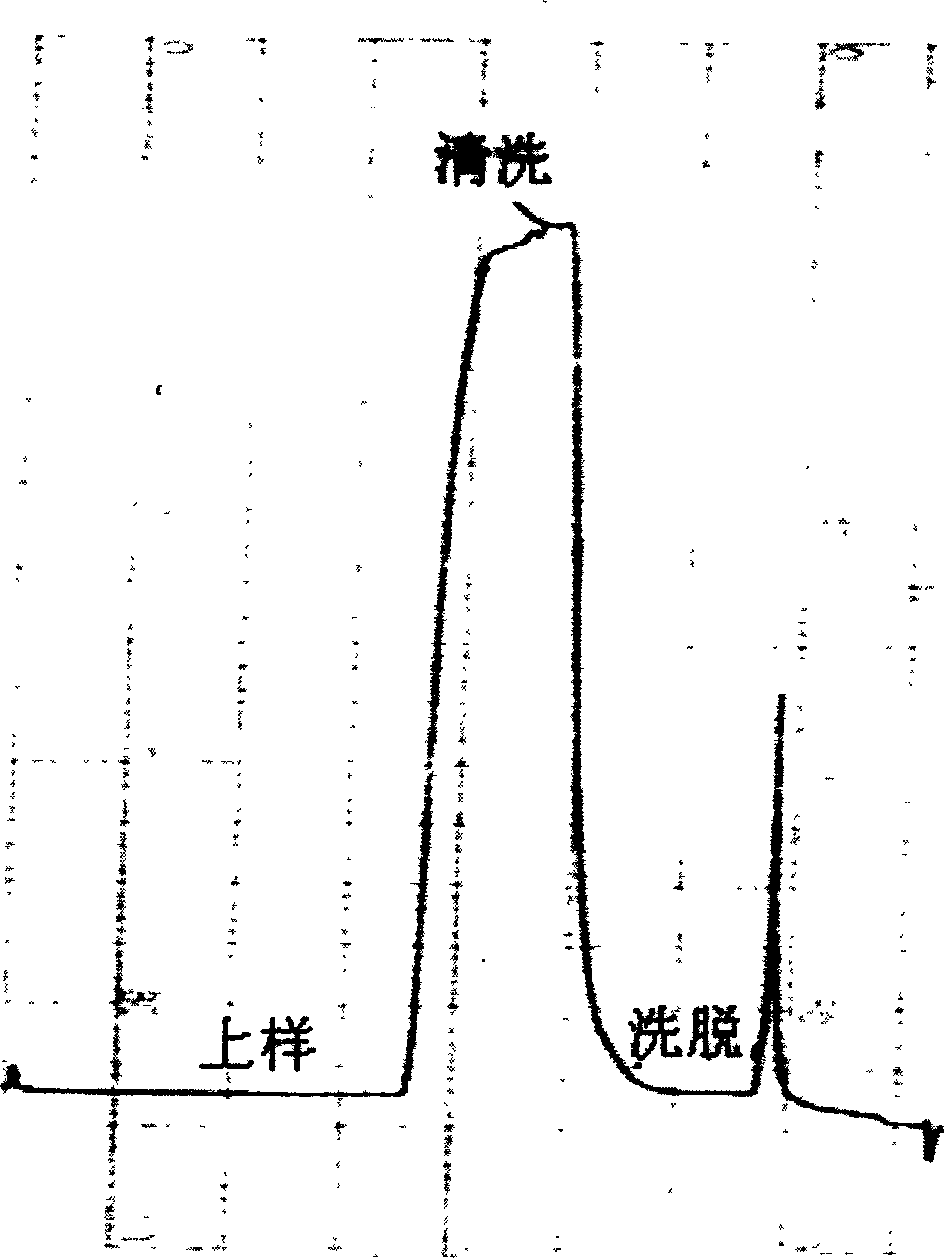
This invention discloses a purification technology for lysostaphin industry, which cultures supernatant purified lysostaphins from sphere bacilli containing the lysostaphin gene. Said method includes ionic exchange and drain chromatograph and needs two days only to process 45L sample, the total recovery rate is 50%, the specific activity is greater than 1000V / mg proteins and the purity is over 90% displayed on the non-recovery SDS-PAGE electrophoresis result.
Owner:昆山博青生物科技有限公司
Topical anti-infective formulations
InactiveCN1744905AHas bactericidal activityPromote healingAntibacterial agentsBiocideSurgical incisionLantibiotics
Topical compositions containing an effective amount of lysostaphin and / or one or more lantibiotics in a pharmaceutically acceptable carrier for topical application. Methods for treating skin or wound infections, including, but not limited to infected abrasions, skin or surface cuts, burns or surgical incisions or decubiti, with the topical compositions are also disclosed.
Owner:БИОСИНЕКСУС ИНКОРПОРЕЙТЕД
Composite lysozyme oral spray and preparation method thereof
ActiveCN103705912AUnique bactericidal mechanismLower resistanceOrganic active ingredientsPeptide/protein ingredientsOral diseaseDisease
The invention relates to a composite lysozyme oral spray. The oral spray is characterized by comprising the following components in percentage by weight: 3-7 percent of lysostaphin, 20-25 percent of lysozyme, 0.02-0.4 percent of tea polyphenol, 1.5-5 percent of olive oil, 1-10 percent of vitamin C, 1-3 percent of sorbitol, 0.8-2 percent of lecithin, 0.01-0.07 percent of xanthan gum, and the balance of purified water. The oral spray is remarkable in effects and convenient to use, and does not contain alcohol; all components are extracted from natural products, and enable fresh breath and no toxic or side effect in the action of compatibility of composite lysozyme, tea polyphenol and vitamin C. The composite lysozyme oral spray has good preventing and treating effects on dental ulcer, decayed teeth, aphtha, halitosis, glossitis, sphagitis and other oral diseases; the preparation method is concise and effective and low in cost, and the prepared composite lysozyme oral spray is good in stability.
Owner:JIANGSU XUE BAO DAILY CHEM CO
Method for detecting viable bacteria in staphylococcus aureus
InactiveCN108504753AImprove permeabilityThe right balance of damageMicrobiological testing/measurementMicroorganism based processesSulfonatePropidium monoazide
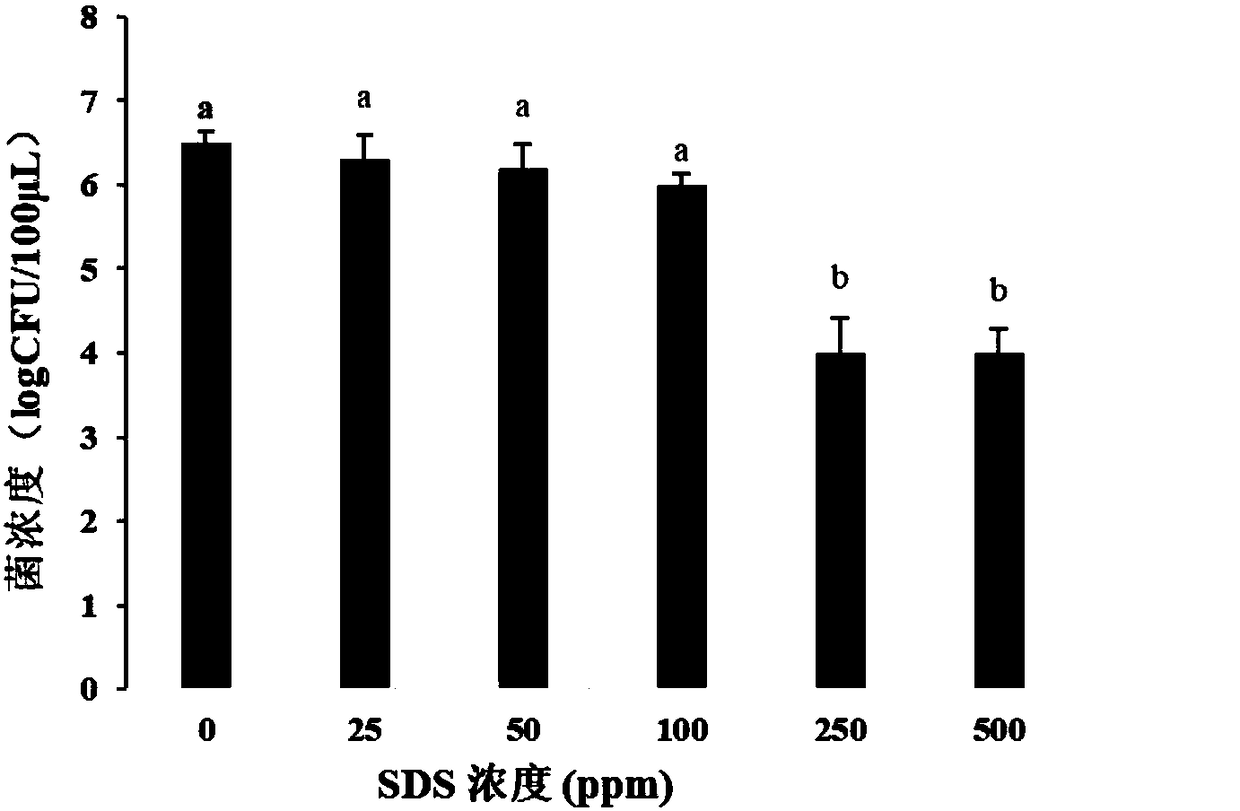
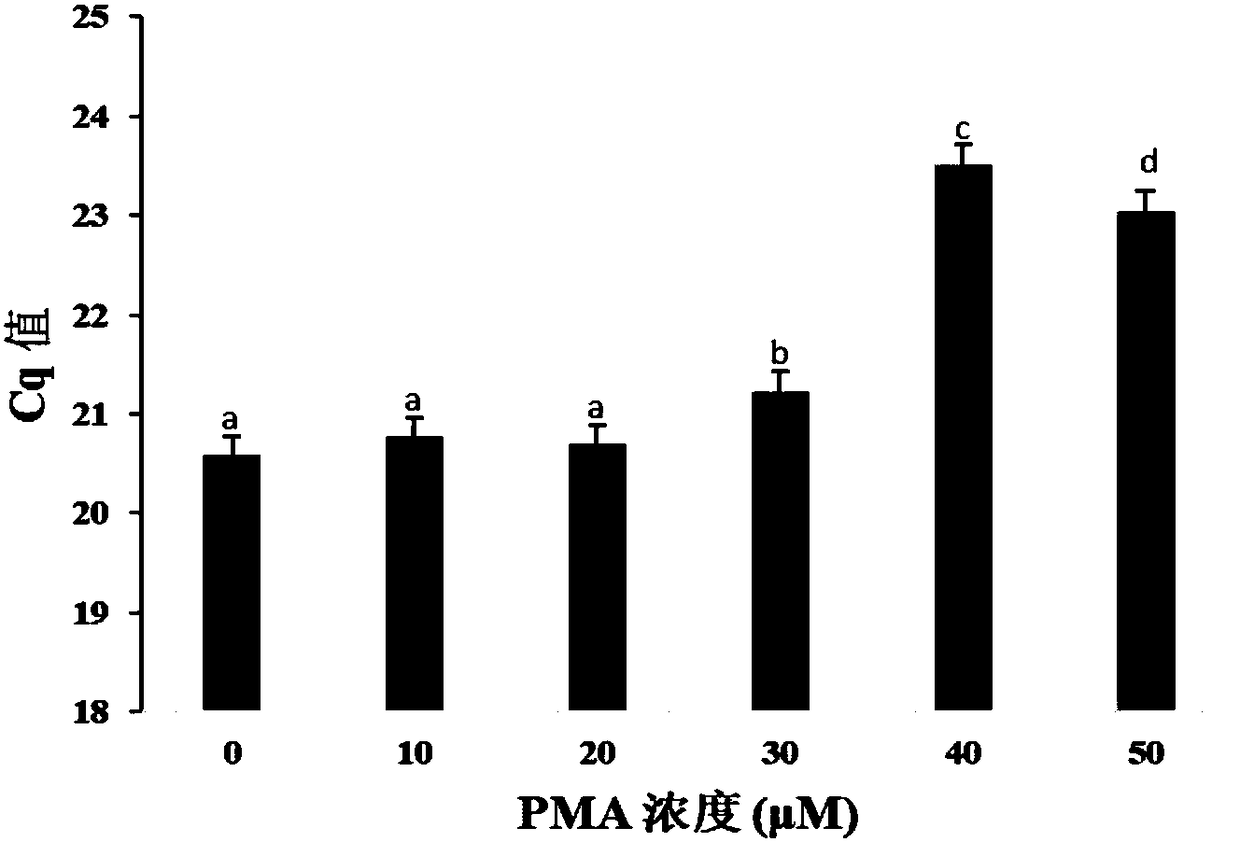
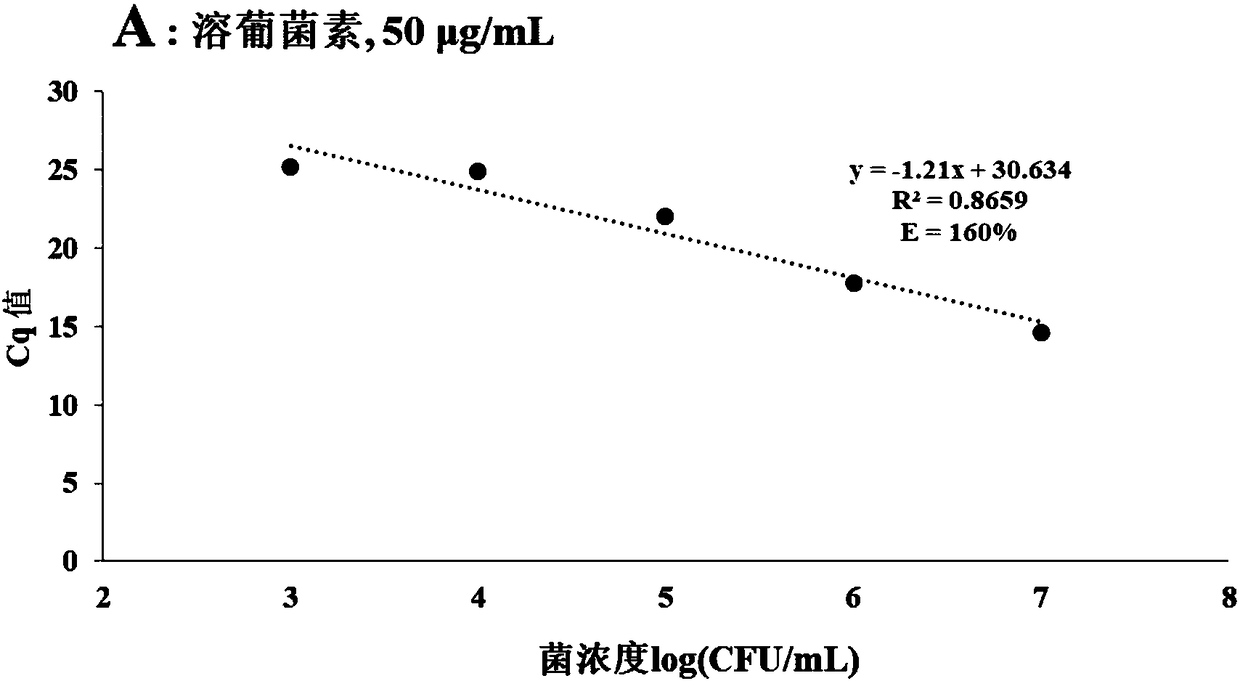
The invention provides a method for detecting viable bacteria in staphylococcus aureus in order to solve the problems of false positive and false negative when a PMA (Propidium Monoazide)-qPCR (quantitative Polymerase Chain Reaction) method is adopted for detecting the viable bacteria in the prior art. According to the method provided by the invention, an SDS (Sodium Dodecyl Sulfonate) treatment step is added before PMA treatment on the basis of the PMA-qPCR method, a lysostaphin treatment step is added before DNA (Deoxyribonucleic Acid) extracting, and bacillus cereus DNA is added as an amplification internal reference during a qPCR process, so that the problems of false positive and false negative in an original method are well solved, and the sensitivity and the specificity of the detecting method are improved.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Protamine compounded preparation, preparation method and application thereof
ActiveCN102405935ADoes not affect environmental protectionUnique effectAntibacterial agentsBiocideSodium acetateMonoglyceride
The invention discloses protamine compounded preparation, a preparation method and application thereof. The protamine compounded preparation of the invention can be singly prepared with the protamine and chitosan, or prepared by compounding one or mixture of over two of glycine, sodium acetate, undecynol monoglyceride, acetic acid, absolute alcohol, lysostaphin, lysozyme, anti-bacterial peptide and sterilized distilled water. The method for preparing the protamine compounded preparation comprises the following steps: accurately weighting each component according to rate in aseptic or clean workshops; putting in clean glass or stainless steel, and uniformly stirring; and sealing in sterile containers. The product not only effectively kills the gram-positive cocci, such as staphyloccocus aureus rosenbach, monilia albican, neisseria gonorrhoeae, streptococcus, anaerobic bacteria, pseudomonas aeruginosa, escherichia coli, pneumococcus, D enterococcus, staphylococcus tetragenus, listeria monocytogenes, stenotrophomonas maltophilia and stomach helicobacter pylori, but also kills the gram-negative bacteria.
Owner:广东康乃欣生物医疗科技有限公司
Lysostaphic complex enzyme oral cavity disinfectant and preparation method thereof
InactiveCN104524559AQuick killWon't hurtAntibacterial agentsOrganic active ingredientsOral diseaseChlorhexidine Acetate
The invention discloses a lysostaphic complex enzyme oral cavity disinfectant and a preparation method thereof. The lysostaphic complex enzyme oral cavity disinfectant comprises the following components in parts by mass: 20-40 parts of lysostaphin, 21-41 parts of antibacterial peptide, 21-41 parts of chlorhexidine acetate, 31-42 parts of sorbitol, 21-31 parts of sodium dihydrogen phosphate and 21-30 parts of disodium hydrogen phosphate. The oral cavity disinfectant can be used for treating oral cavity diseases, has the advantages of remarkable curative effect and no alcohol and drug resistance. The invention further discloses a preparation method of the oral cavity disinfectant. The preparation method comprises the following steps: 1) dissolving the lysostaphin, the ntibacterial peptide and the hlorhexidine acetate into distilled water, stirring to completely dissolve the lysostaphin, the ntibacterial peptide and the hlorhexidine acetate; 2) dissolving sorbitol into distilled water, and stirring to completely dissolve the sorbitol; 3) dissolving the sodium dihydrogen phosphate and the disodium hydrogen phosphate into distilled water, and stirring to completely dissolve the sodium dihydrogen phosphate and the disodium hydrogen phosphate; and 4) uniformly mixing solutions obtained in the steps 1), 2) and 3), and then filling the mixed solution. The preparation method has the advantages of simple process and strong operability.
Owner:杨陈
Lysostaphin compound disinfection gel
InactiveCN104524557ABroad spectrum of pathogenic bacteriaImprove the immunityAntibacterial agentsOrganic active ingredientsBiotechnologyChlorhexidine Acetate
The invention discloses a lysostaphin compound disinfection gel which comprises the following components in parts by weight: 10-20 parts of lysostaphin, 10-20 parts of antibacterial peptides, 10-20 parts of chlorhexidine acetate, 20-30 parts of sorbitol, 10-20 parts of sodium dihydrogen phosphate, 10-20 parts of disodium hydrogen phosphate and 2-5 parts of thickening agents. Through the synergistic interaction of the components, the antibacterial component in the disinfection gel can keep the disinfection capacity for a long time and relatively high disinfection efficiency is brought to the bacteria on the skin; the staphylococcus compound disinfection gel has the advantages of being stable in antibacterial performance, long in bacteriostasis ageing and free of stimulation to the skin.
Owner:杨陈
Freeze-dried powder preparation for curing bovine mastitis
InactiveCN102008455AGood treatment effectKeep alivePowder deliveryPeptide/protein ingredientsFreeze-dryingPhosphoric acid
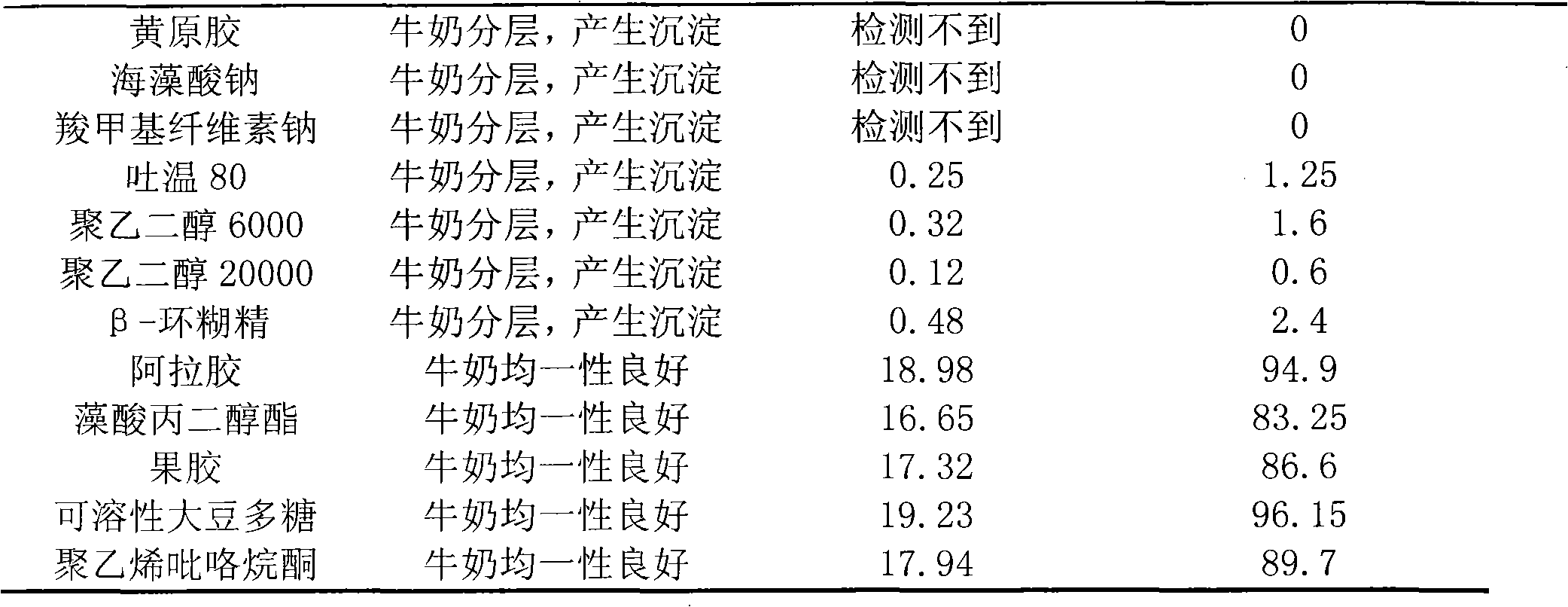
The invention belongs to the technical field of medicine, in particular relating to a freeze-dried powder preparation for curing bovine mastitis. The preparation disclosed in the invention comprises the following components by weigh percent: 0.04-1.5wt% of lysostaphin, 10-72.5wt% of lysozyme, 10-40wt% of retardant, 5-20% of monopotassium phosphate and 10-35% of disodium hydrogen phosphate. When the preparation is poured into the bovine breast, the lysostaphin and lysozyme can maintain favorable stability and sterilization vigor in milk, and flocculent precipitates generated by polymerization of lysostaphin or lysozyme with casein never appear in milk.
Owner:SHANGHAI HI TECH UNITED BIO TECHCAL RES +2
Bacteria genome DNA extraction liquid, preparation and application thereof
ActiveCN101319213AEasy to makeEasy to storeFermentationPlant genotype modificationChelex 100DNA extraction
The invention discloses a bacteria genome DNA extracting solution in a blood and platelet product and a preparation method and an application method thereof. The bacteria genome DNA extracting solution consists of chelex-100, tween, NP-40, SDS, lysostaphin, a wall-breaking enzyme, a protease K and water. The extracting solution can be effectively used for extracting a bacteria genome DNA with high extraction efficiency. The preparation method and the application method are simple and convenient for operation.
Owner:SHANGHAI BLOOD CENT
Fragrant disinfectant
InactiveCN106420503ACause damageGood disinfection and cleaning effectCosmetic preparationsToilet preparationsFacial skinAlcohol
The invention discloses a fragrant disinfectant. The fragrant disinfectant is prepared from the following components in parts by mass: 5-7 parts of citric acid, 3-5 parts of hydrogen peroxide, 5-6 parts of licorice extract, 70-90 parts of distilled water, 4-6 parts of basswood bark, 5-7 parts of parachlorometaxylenol, 4-6 parts of lysostaphin, 2-4 parts of ethyl alcohol, 3-5 parts of chamomile and 1-2 parts of essence. The fragrant disinfectant is suitable for cleaning facial skin, has fragrant smell, has excellent disinfecting and cleaning effects and is free of damage to the facial skin and subcutaneous tissue.
Owner:QINGDAO YUANZHILIN AGRI SCI & TECH DEV
Thermostable Composition Having Antiviral and Antibacterial Activity and the Use Thereof
InactiveUS20200254064A1Improve wettabilitySolve low adhesionAntibacterial agentsBiocideToothpasteAntibacterial activity
A thermostable composition with antiviral and antimicrobial activity is used as a biologically active component for the production of sanitary and hygienic products, as well as for protection against bacterial and viral infections. In particular, the thermostable composition is a lyophilized powder containing the following components per 1 g of the composition: recombinant interferon gamma human with the activity from 500,000 to 2,000,000 IU / g 0.03 to 0.12 mg; lysostaphin with the activity from 50 to 200 U / g 0.17 to 0.68 mg; methoxy polyethylene glycol 30-150 mg; sodium alginate 10-25 mg; sodium dihydrophosphate dihydrate 120-250 mg; citric acid 35-110 mg and remaining dextran. The thermostable composition can be used as a component of oral hygiene products, toothpastes, sanitary and hygiene articles, a clothes conditioner and condom lubricant and in a number of medicinal products and drugs.
Owner:CHUMBURIDZE GEORGY G
Lysostaphin freeze dried powder used for preventing and treating trauma surface infestation
A freeze-dried powder-injection of staphylococcus lysozyme for preventing and the infection of straphylococcus, especially the Staphylococcus aureus, to the wound caused by burn, scald, etc and the skin mucosa of eye and nose is composed of staphylococcus lysozyme, bovine serum albumin, glycine, mannitol, and phosphate. Its preparing process is also disclosed.
Owner:SHANGHAI HI TECH UNITED BIO TECHCAL RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com