Freeze-dried powder preparation for curing bovine mastitis
A technology for mastitis and freeze-dried powder, which is applied in freeze-dried transportation, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems of reduced bactericidal activity of lysostaphylococcus enzyme, affecting milk quality, etc. , to achieve a significant therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1. Formula of recombinant lysostaphin freeze-dried powder preparation
[0019] Recipe 1:
[0020] Lysostaphin 0.04% (0.1mg), lysozyme content 72.5% (181.25mg), acacia gum 10.87% (27.175mg), potassium dihydrogen phosphate 5.76% (14.4mg), disodium hydrogen phosphate 10.83% (27.075mg ).
[0021] Recipe 2:
[0022] Lysostaphin 0.23% (0.23mg), lysozyme content 56.77% (56.77mg), soluble soybean polysaccharide 17.03% (17.03mg), potassium dihydrogen phosphate 9.01% (9.01mg), disodium hydrogen phosphate 16.96% (16.96 mg).
[0023] Recipe 3:
[0024] Lysostaphin content 1.09% (1mg), lysozyme content 10.9% (10mg), polyvinylpyrrolidone 38.14% (34.99mg), potassium dihydrogen phosphate 17.31% (15.88mg), disodium hydrogen phosphate 32.56% (29.87mg ).
[0025] Recipe 4
[0026] Lysostaphin 0.23% (0.23mg), lysozyme content 56.77% (56.77mg), pectin 17.03% (17.03mg), potassium dihydrogen phosphate 9.01% (9.01mg), disodium hydrogen phosphate 16.96% (16.96mg ).
[0027] Recipe 5
...
Embodiment 2
[0034] The screening experiment of embodiment 2 formula
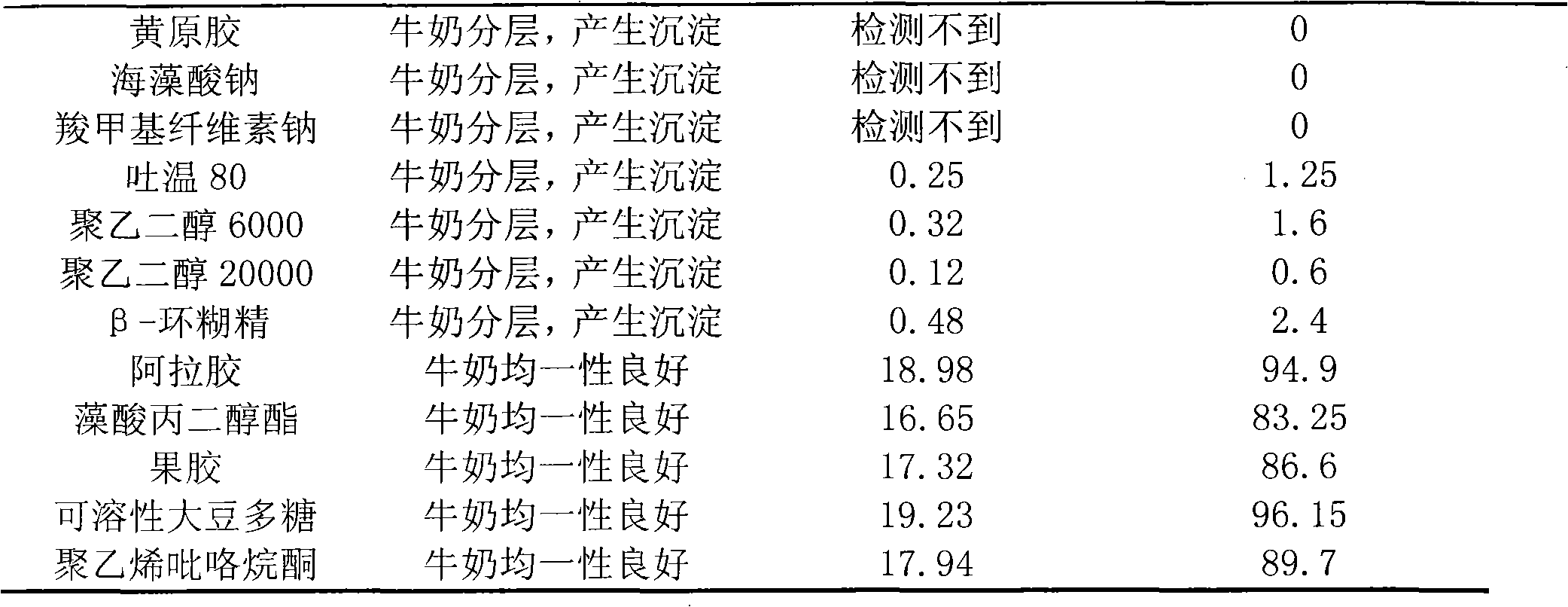
[0035] Prepare 100ml of solution with 0.2mol / L phosphate buffer solution of pH7.0, contain recombinant lysostaphin 2mg (enzyme activity is 2000U), this solution is divided into 20 parts, every part is 5ml. Then add the following auxiliary materials in a weight ratio of 1:1. After mixing evenly, add 20ml of freshly collected normal milk (pasteurized), incubate at 39°C for 20 hours, and then observe the changes in the properties of the milk after adding samples with different serial numbers. Preservation of enzyme bioactivity in milk. The specific results are shown in Table 1 below. The result of table 1 shows: arabic gum, propylene glycol alginate, pectin, soluble soybean polysaccharide, polyvinylpyrrolidone can well stabilize the activity of lysostaphin in milk, so our 5 formulas in embodiment 1 The stabilizers mentioned above were used.
[0036] Table 1 Pre-experimental results of prescription screening
[0037] ...
Embodiment 3
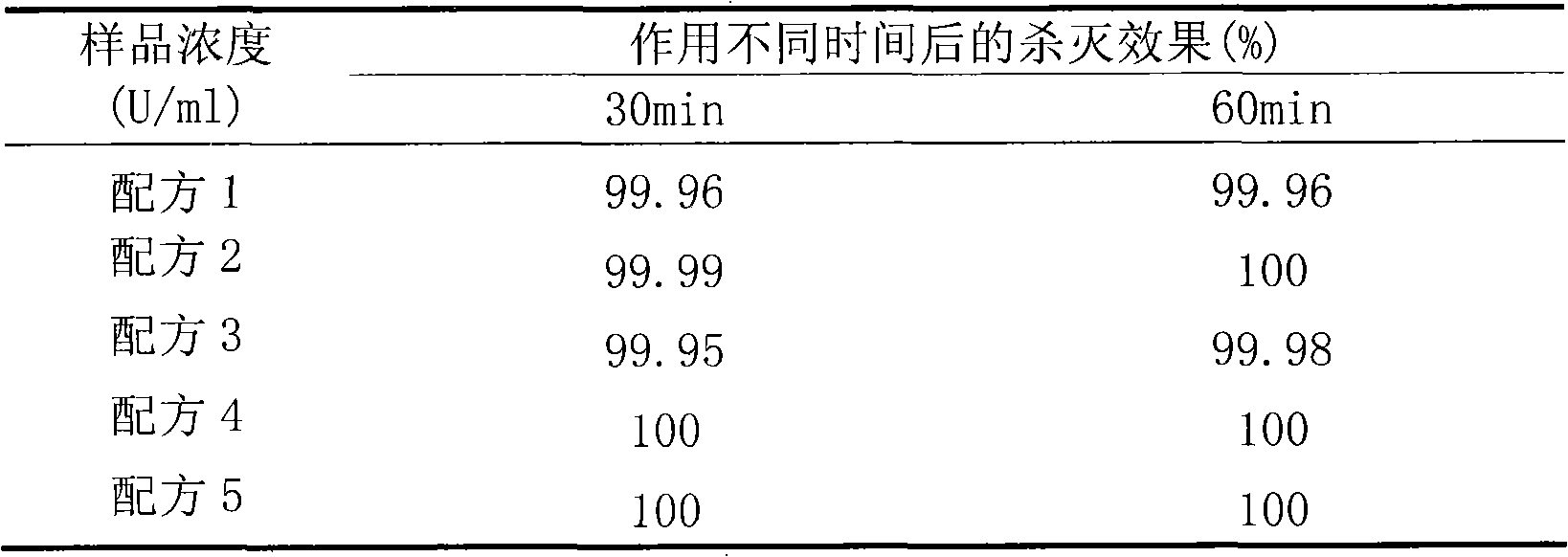
[0039] Example 3 Recombinant lysostaphin freeze-dried powder can effectively kill Staphylococcus aureus in milk
[0040] Refer to the 2002 edition of "Disinfection Technical Specifications". Get 1 bottle of freeze-dried powder prepared in Example 1 and dissolve it with 10ml of fresh milk and finally dilute it into 50ml of milk for subsequent use. Arrange the test tubes on the test tube rack and mark them well, and divide the prepared samples into the test tubes, the filling volume is 5ml / tube. Take the freshly cultured Staphylococcus aureus thallus, dilute it to a concentration of about 108cfu / ml with a diluent of physiological saline, and use it on the same day. Add 100 μl of bacterial solution into the sample tube, mix quickly, and start timing. After acting for 30 minutes and 60 minutes, take 0.5ml of the bacteria-drug mixture into a test tube containing 4.5ml of neutralizing agent, shake and mix well. After acting for 10 minutes, dilute the liquid appropriately, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com