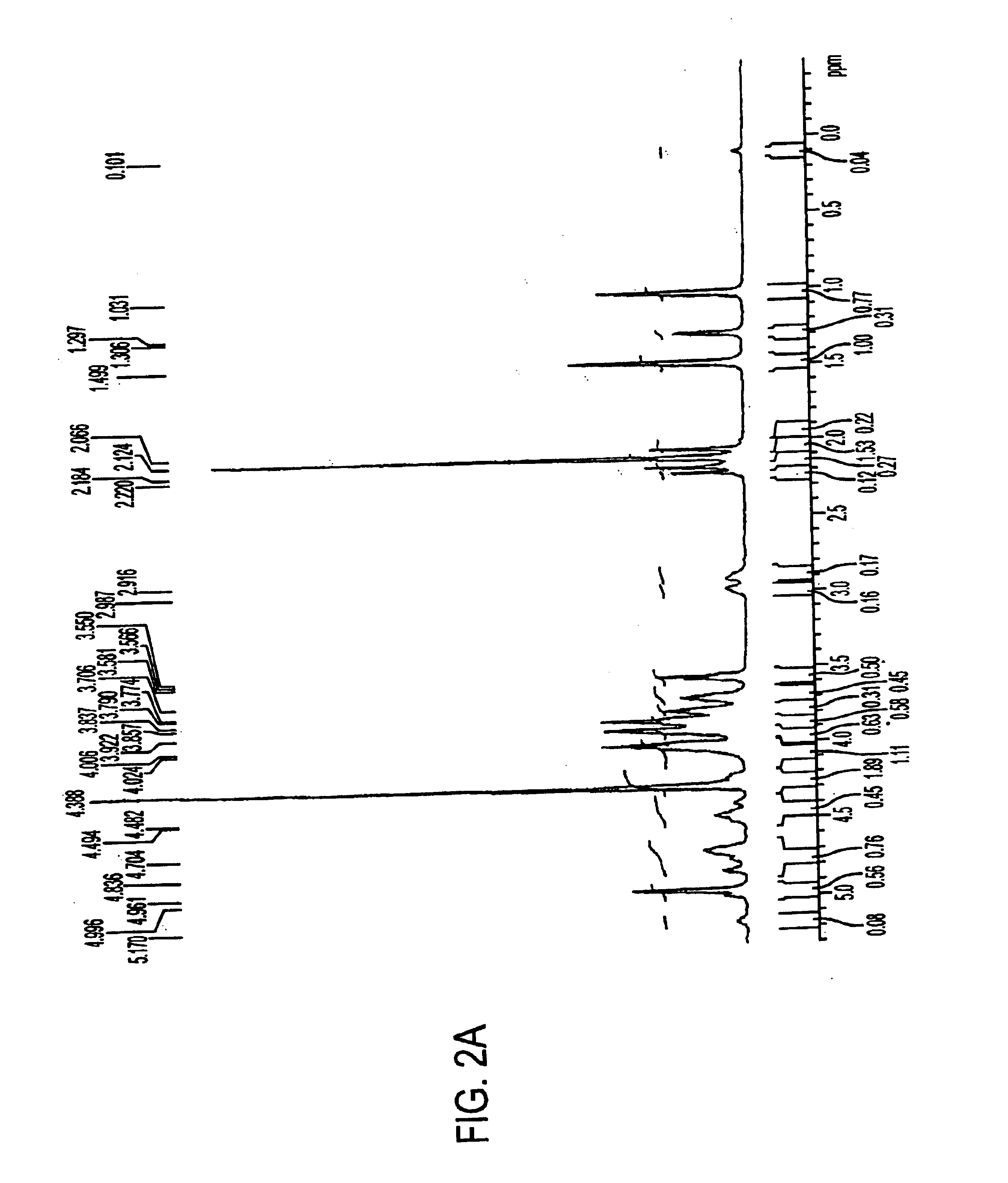
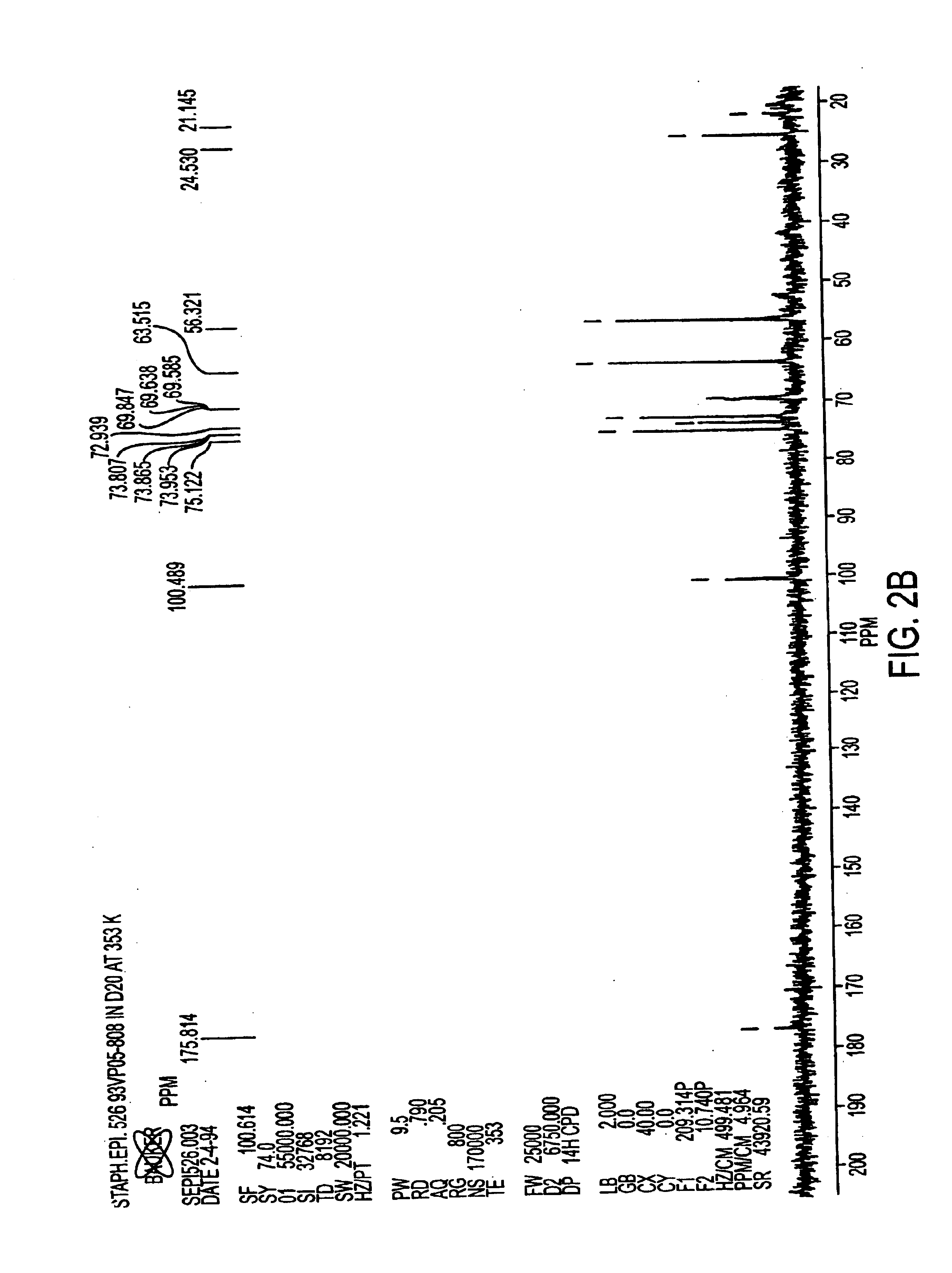
Patents
Literature
151 results about "Staphylococcal infections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Infection caused by specific round shaped bacteria called staphylococcus.
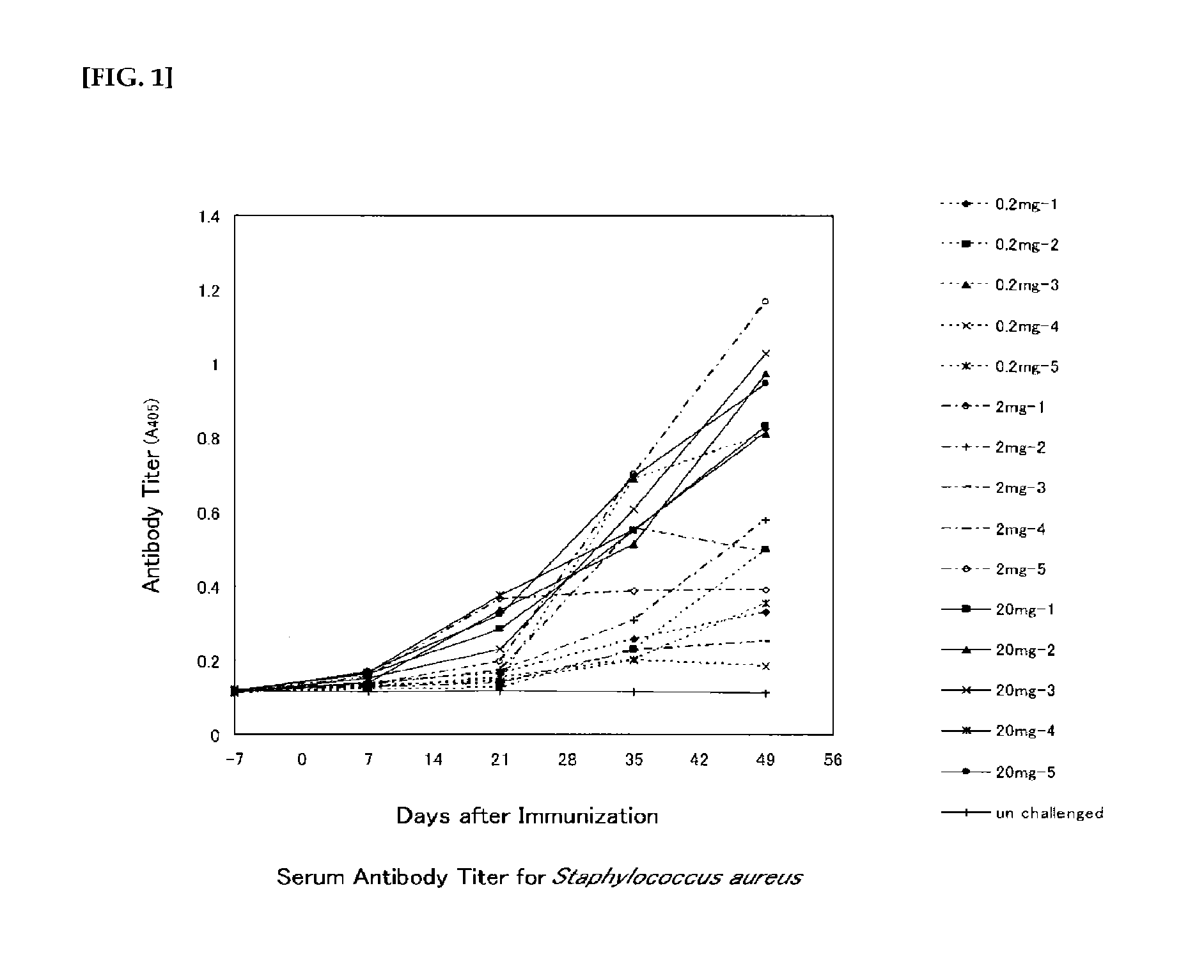
Polysaccharide vaccine for staphylococcal infections
ActiveUS20050118198A1Improving immunogenicityAntibacterial agentsOrganic active ingredientsNatural sourceIntracellular
The invention relates to compositions of a deacetylated poly N-acetylated glucosamine (dPNAG) of Staphylococci. The dPNAG may be isolated from natural sources or synthesized de novo. The invention also relates to the use of dPNAG as a vaccine for inducing active immunity to infections caused by Staphylococcus aureus, S. epidermidis, other related coagulase-negative or coagulase-positive Staphylococci, and other organisms carrying the ica (intracellular adhesion) locus. The invention further provides methods of use for antibodies directed to dPNAG, particularly for inducing passive immunity to the same class of infections.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Staphylococcus antigen and vaccine
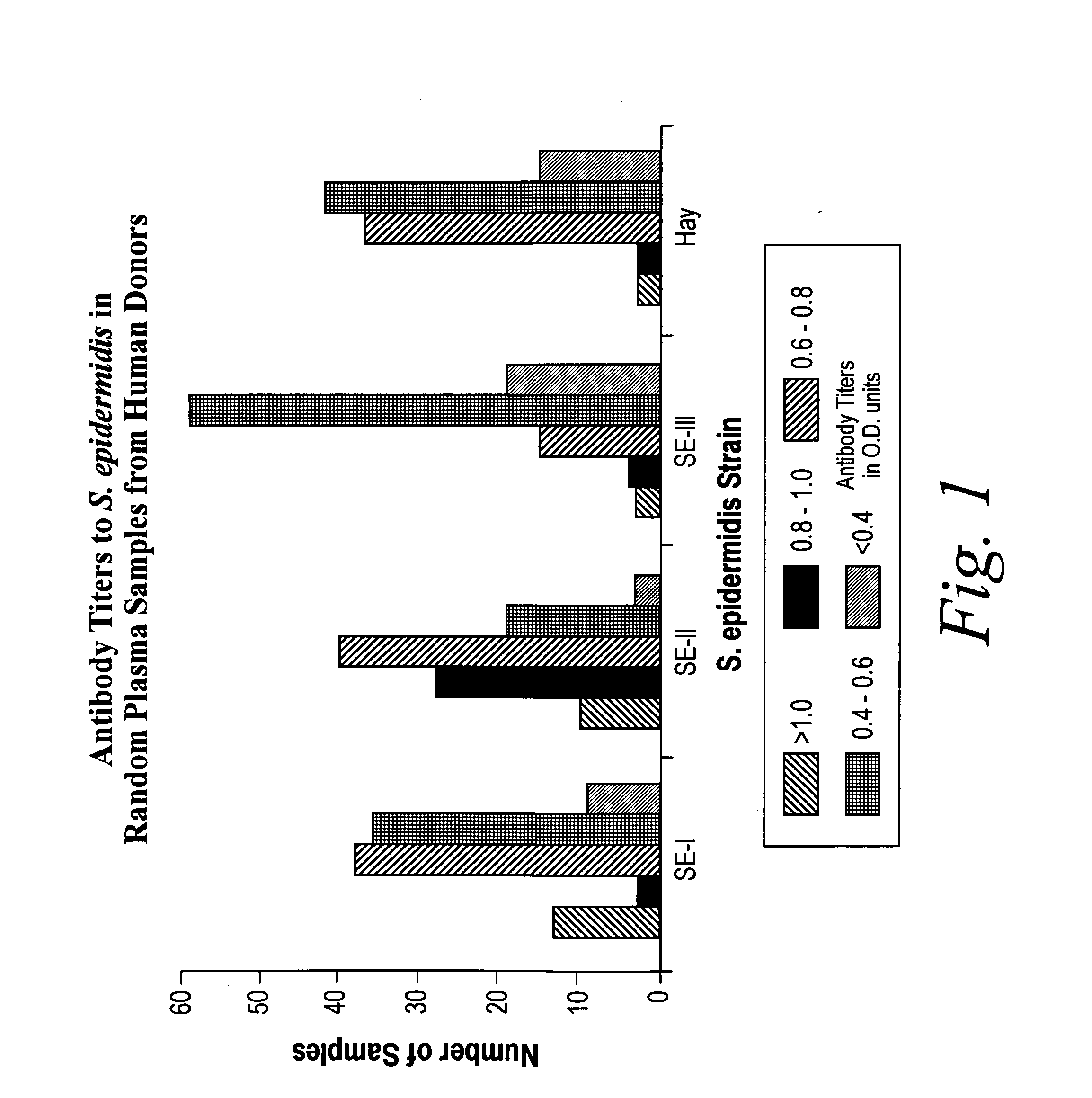
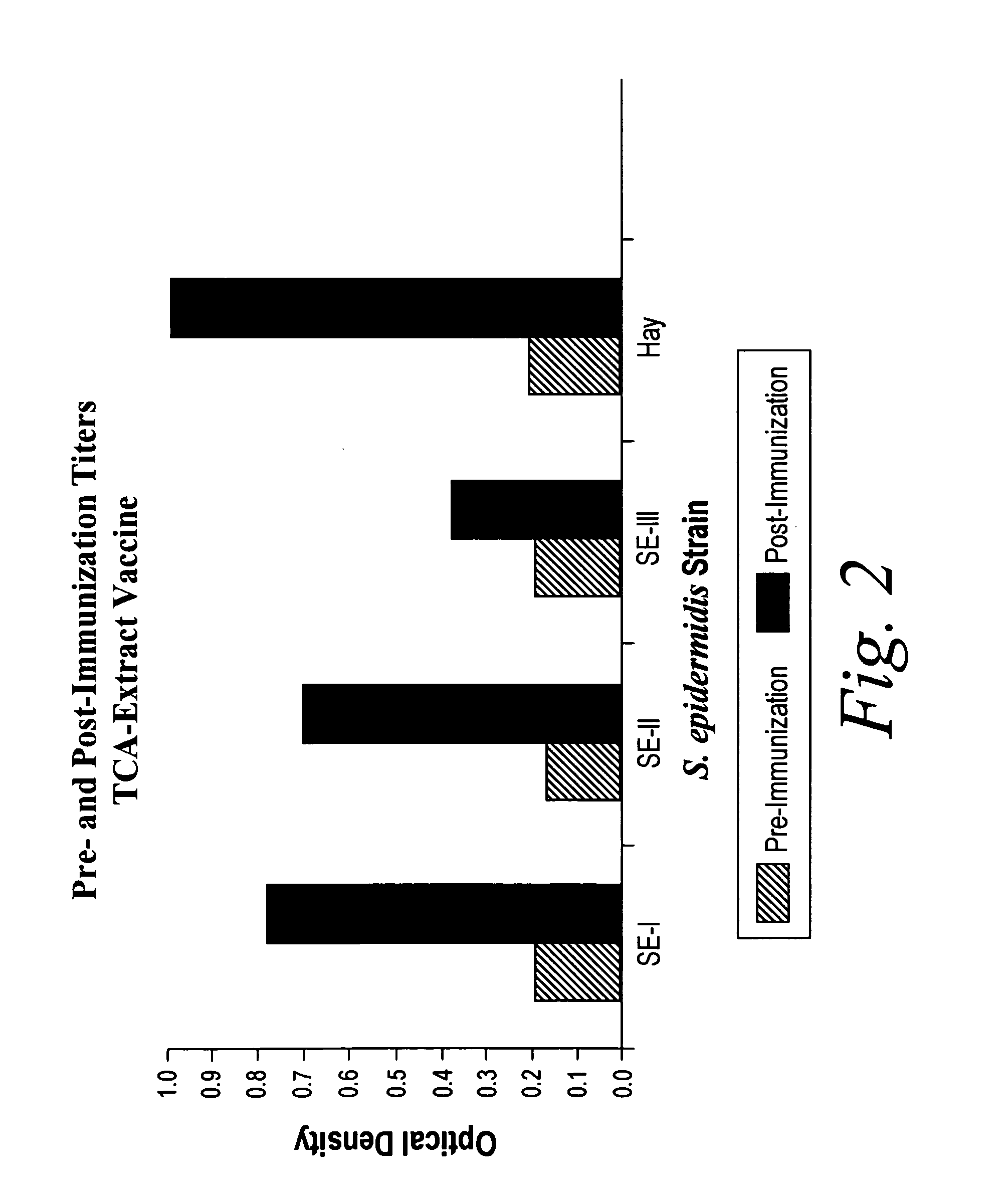
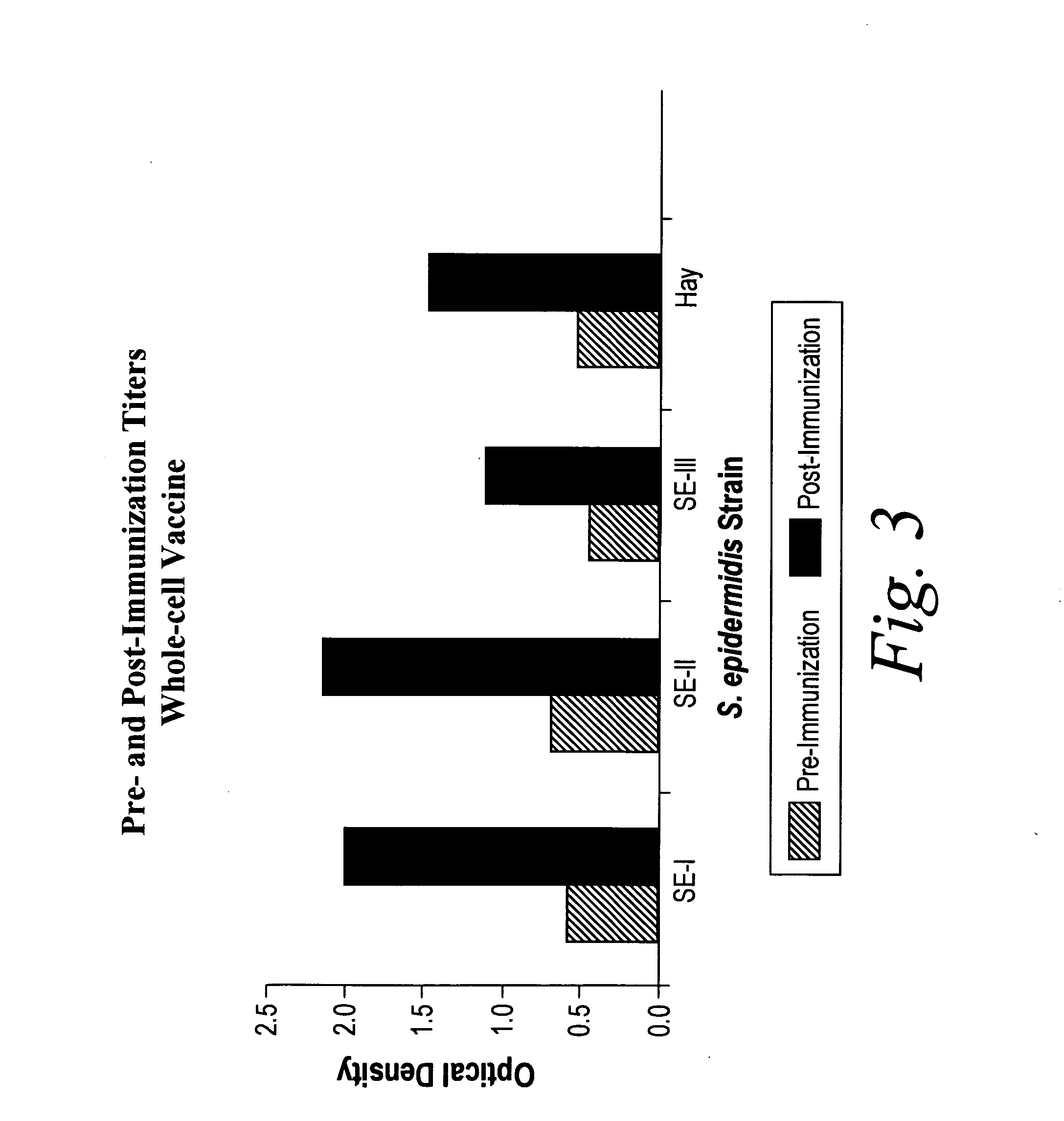
A negatively-charged Staphylococcus antigen contains amino acids and a N-acetylated hexosamine as a major carbohydrate component. The antigen is common to many coagulase-negative strains of Staphylococcus, including S. epidermidis, S. haemolyticus, and S. hominis. Staphylococcus strains that carry the antigen include many clinically significant strains of Staphylococcus. The antigen and antibodies to the antigen are useful in kits and assays for diagnosing Staphylococcus infection. Vaccines of the antigen and of whole cells that carry the antigen also are disclosed.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Use of alpha-toxin for treating and preventing staphylococcus infections
InactiveUS20090053235A1Low toxicityAntibacterial agentsBacterial antigen ingredientsBacteroidesAlpha-toxin
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Use of alpha-toxin for treating and preventing staphylococcus infections
InactiveUS20080131457A1Low toxicityAntibacterial agentsBacterial antigen ingredientsBacteroidesAlpha-toxin
Vaccines comprising an S. aureus alpha-toxin antigen and a pharmaceutically acceptable carrier are provided, and are useful for treating and preventing infections. The S. aureus alpha-toxin antigen may contain at least two alterations that reduce its toxicity and / or may be conjugated to or co-administered with another bacterial antigen. The vaccines may comprise one or more other bacterial antigens. Antibody compositions comprising antibodies to alpha-toxin and optionally one or more other bacterial antigens also are provided, and are useful for treating and preventing infections.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Staphylococcus aureus leukocidins, therapeutic compositions, and uses thereof
ActiveUS20110274693A1Reduce in quantityReduce severityAntibacterial agentsPeptide/protein ingredientsPhagocyteWhite blood cell
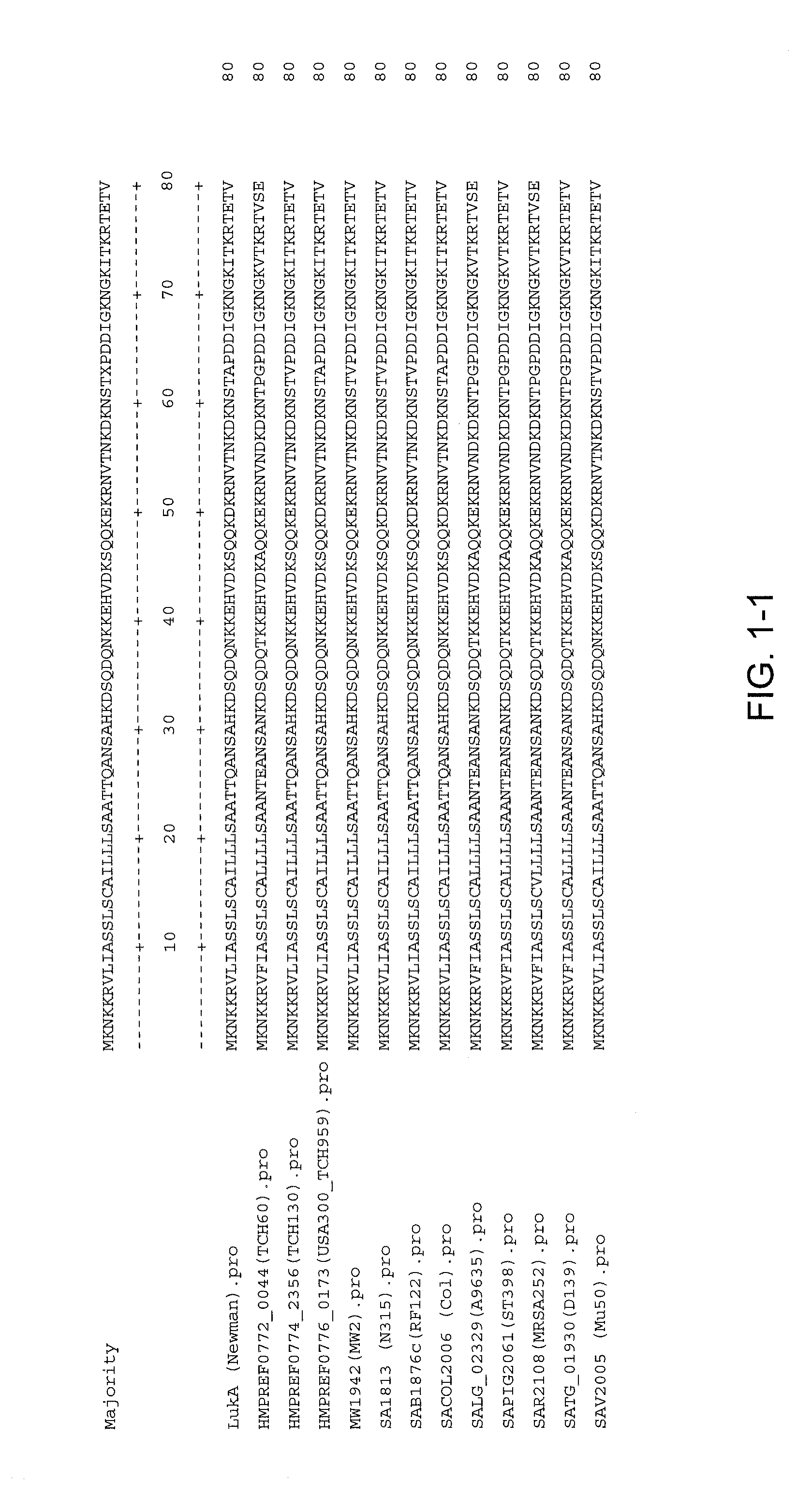
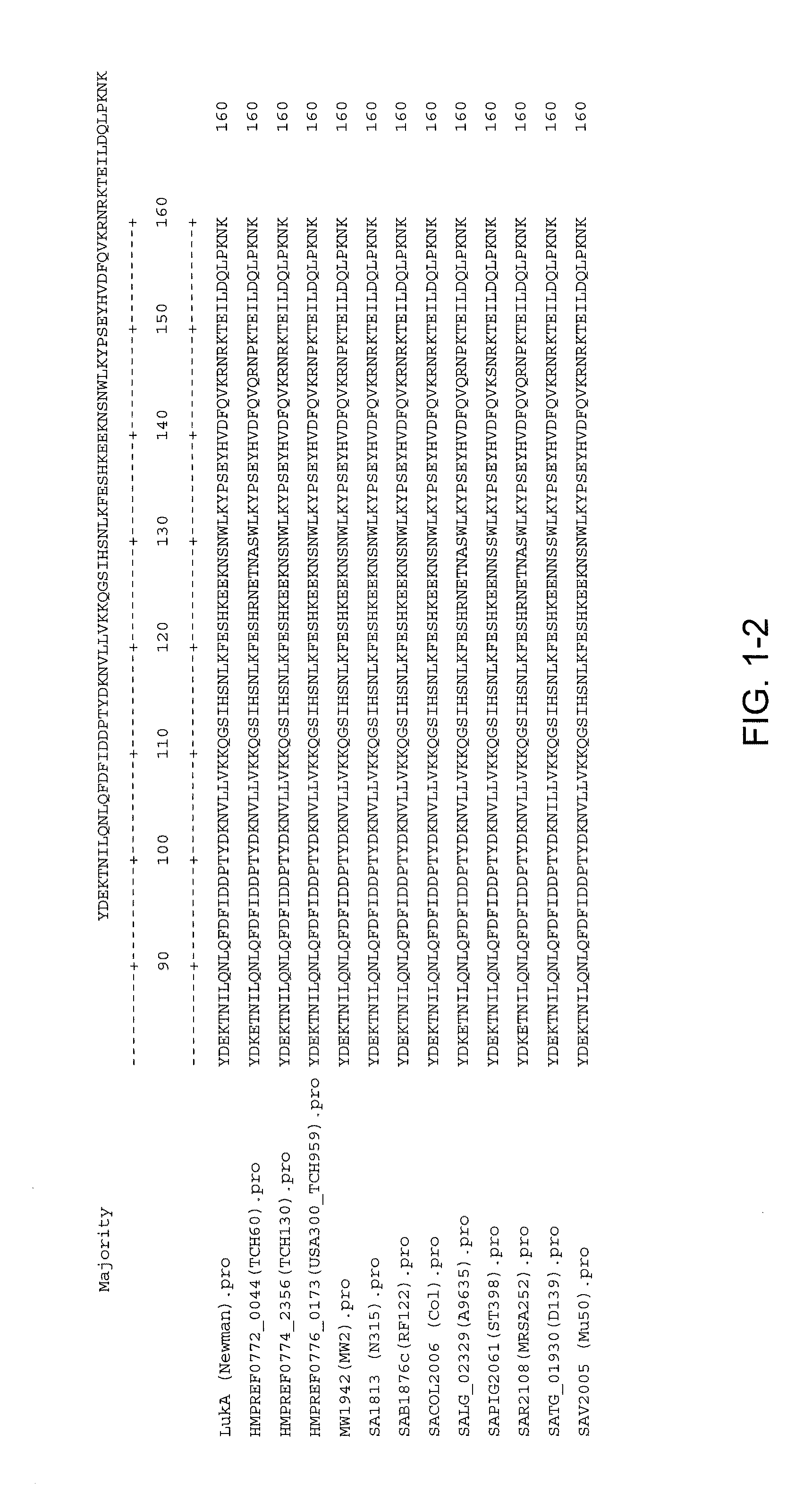
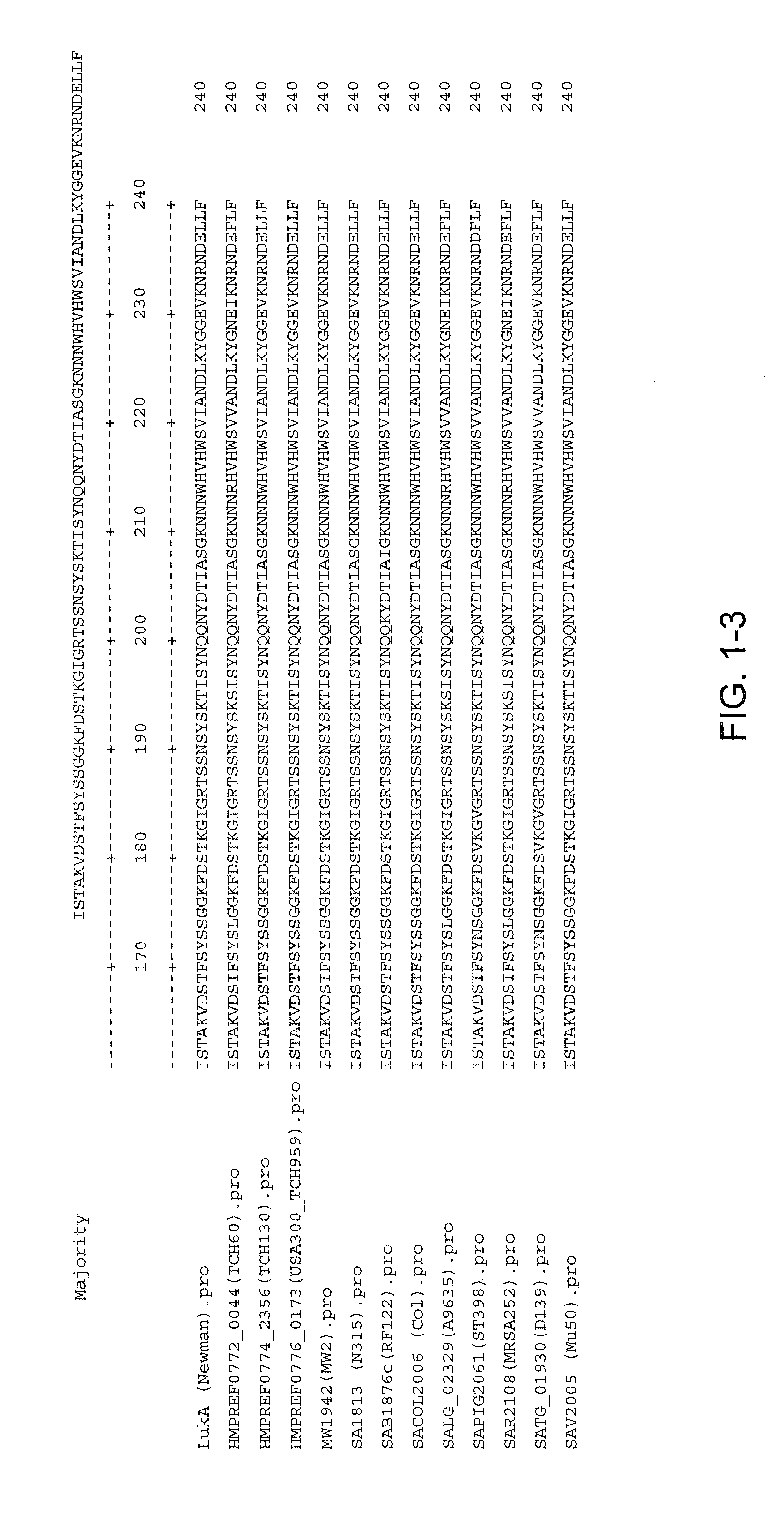
Disclosed herein are isolated and purified Staphylococcus aureus bi-component leukocidin, referred to herein as LukAB, and its components LukA and LukB, antibodies specific to LukA, antibodies specific to LukB, therapeutic compositions containing LukA and / or LukB, or anti-LukA and / or anti-LukB antibodies, uses of the compositions to treat acute inflammatory conditions or S. aureus infection, methods for identifying inhibitors of LukAB-mediated cytotoxicity of human phagocytes, and methods for using LukAB as a marker to predict severity of S. aureus infection.
Owner:NEW YORK UNIV
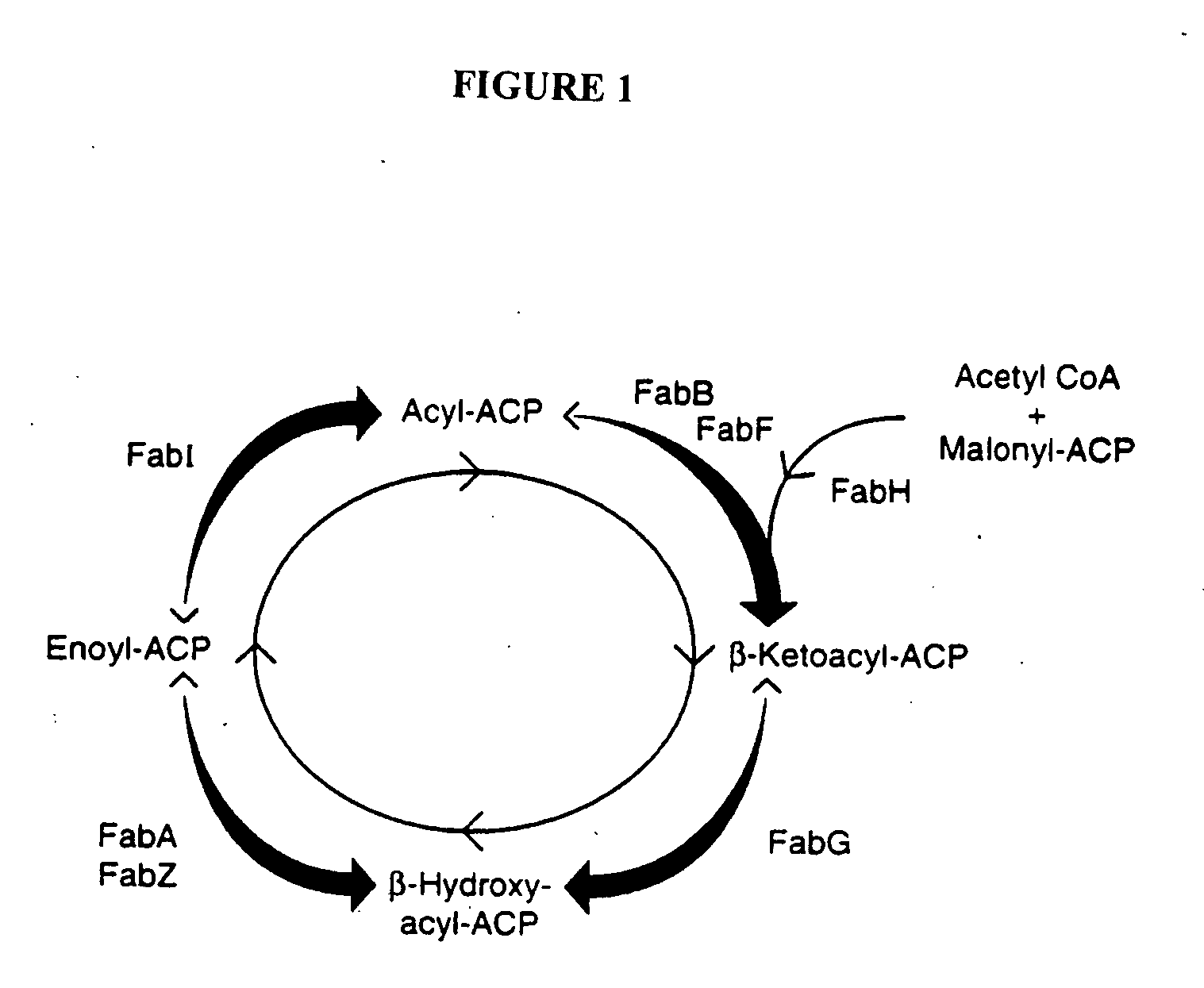
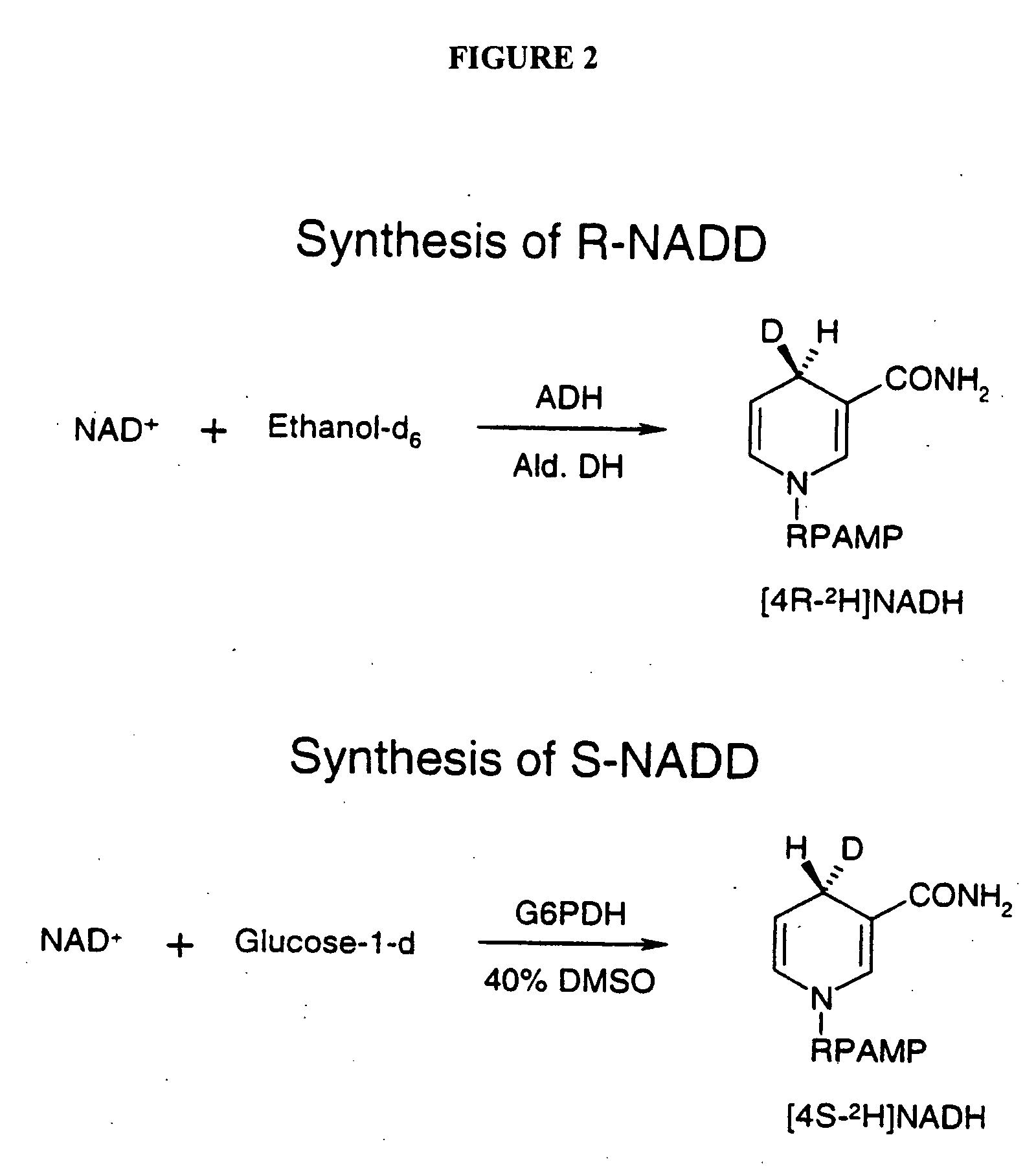
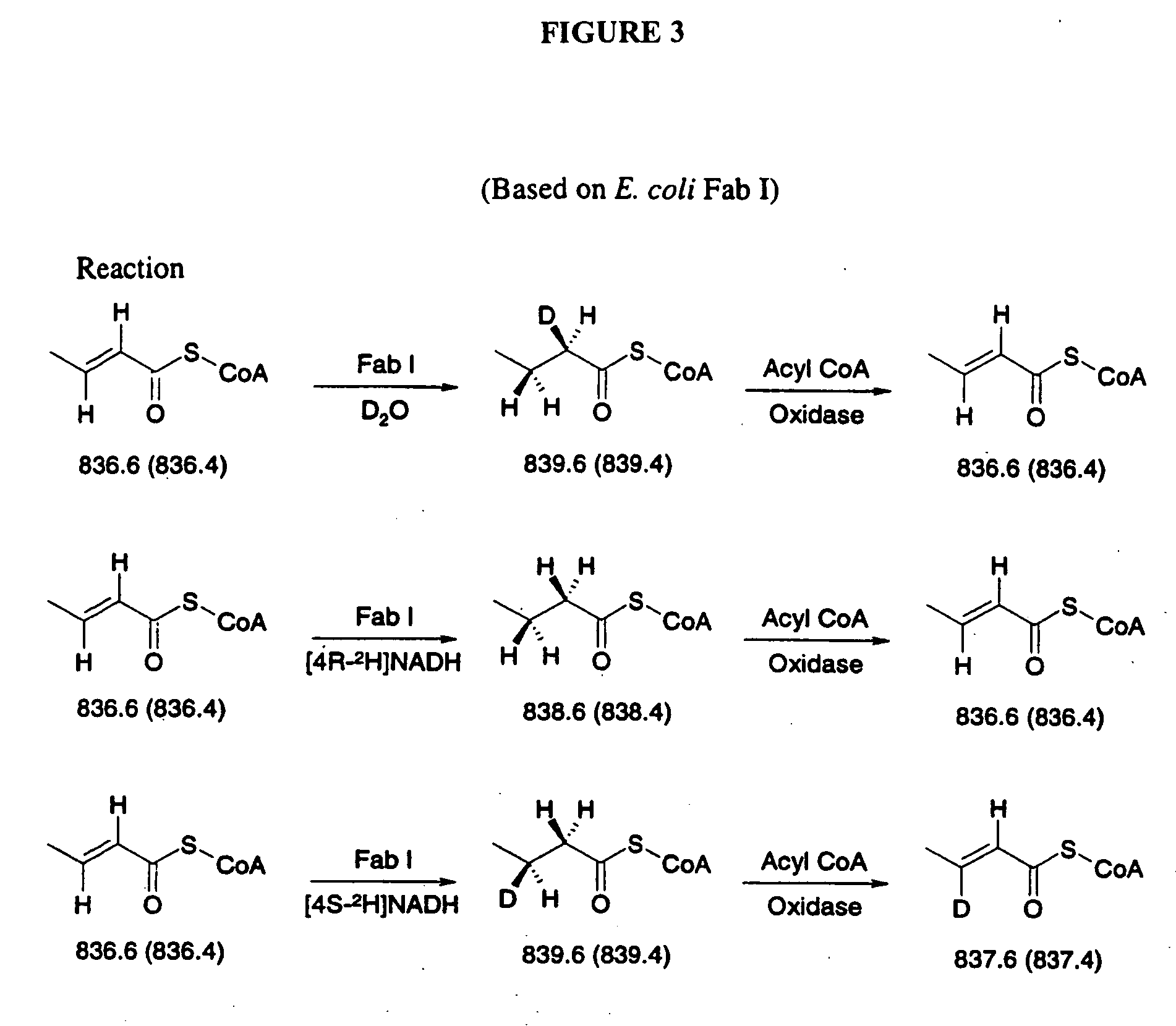
Polynucleotide encoding the enoyl-acyl carrier protein reductase of Staphylococcus aureus, FAB I
InactiveUS6995254B1Inhibition effectInhibit expressionBacteriaSugar derivativesEnoyl-acyl carrier protein reductaseStaphylococcus cohnii
Prokayrotic FAB I polypeptides and DNA (RNA) encoding such FAB I and a procedure for producing such polypeptides by recombinant techniques is disclosed. Also disclosed are methods for utilizing such FAB I for the treatment of infection, such as bacterial infections. Antagonists against such FAB I and their use as a therapeutic to treat infections, such as staphylococcal infections are also disclosed. Also disclosed are diagnostic assays for detecting diseases related to the presence of FAB I nucleic acid sequences and the polypeptides in a host. Also disclosed are diagnostic assays for detecting polynucleotides encoding FAB I and for detecting the polypeptide in a host.
Owner:DEBIOPHARM INTERNATIONAL SA
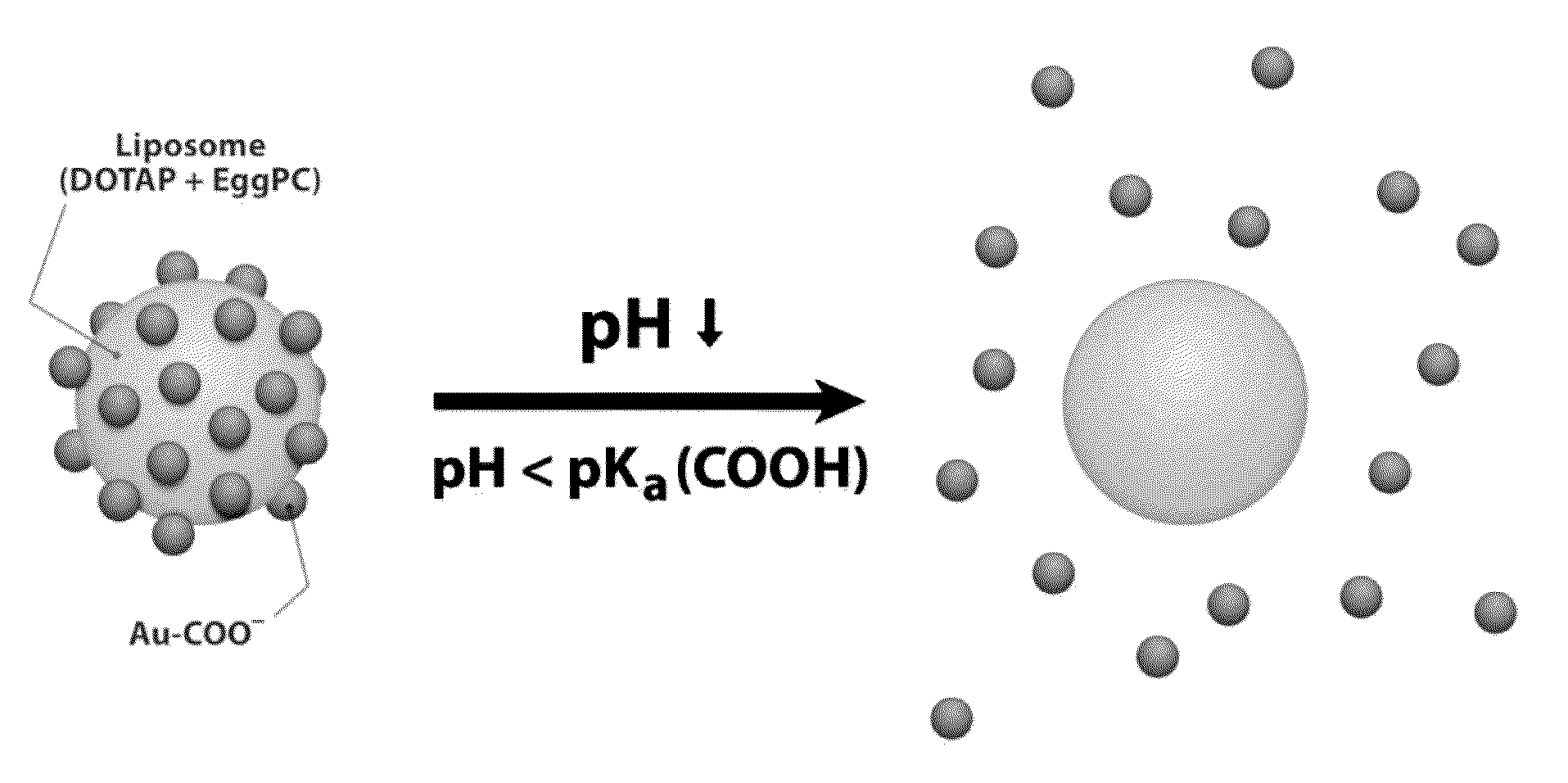
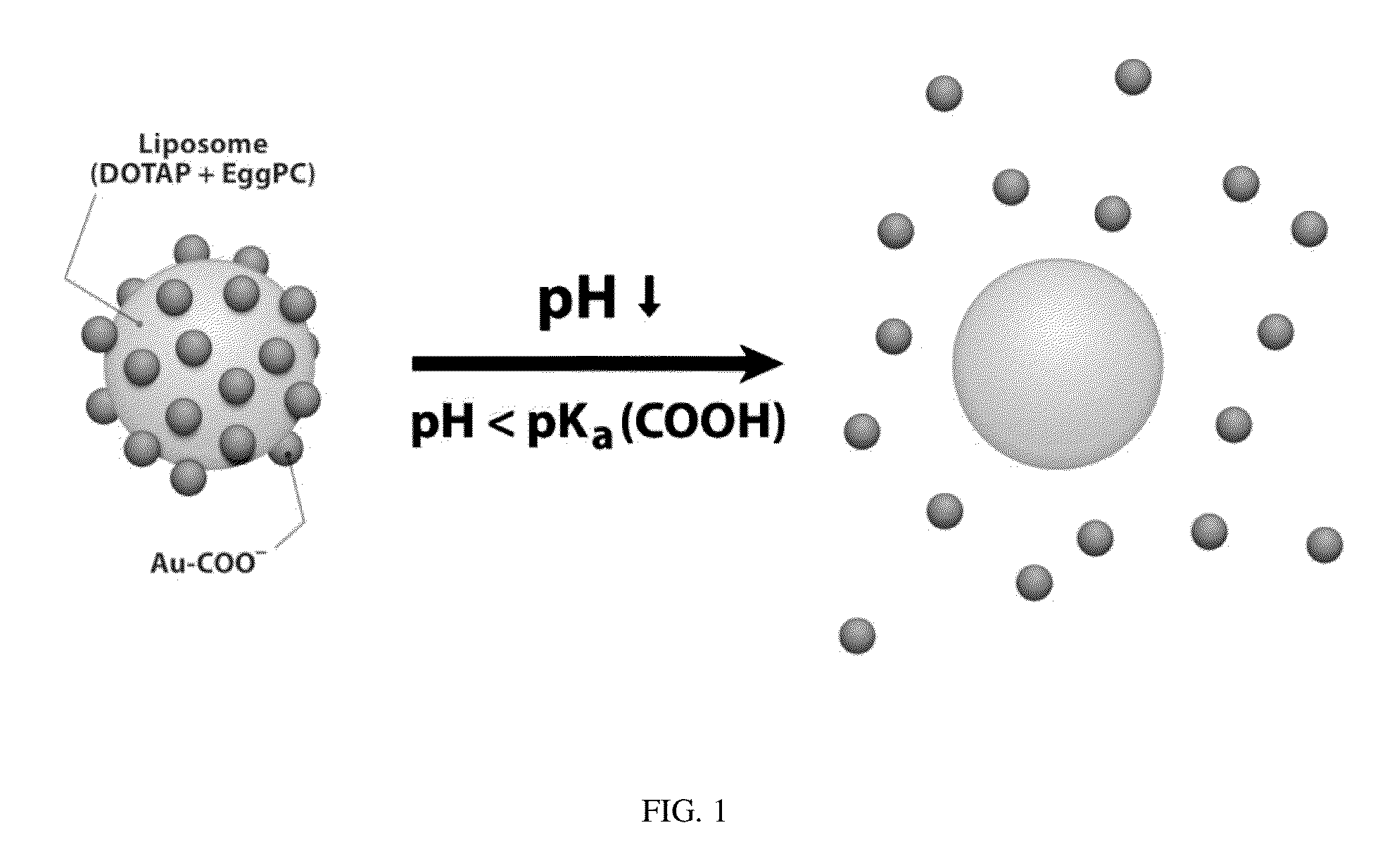
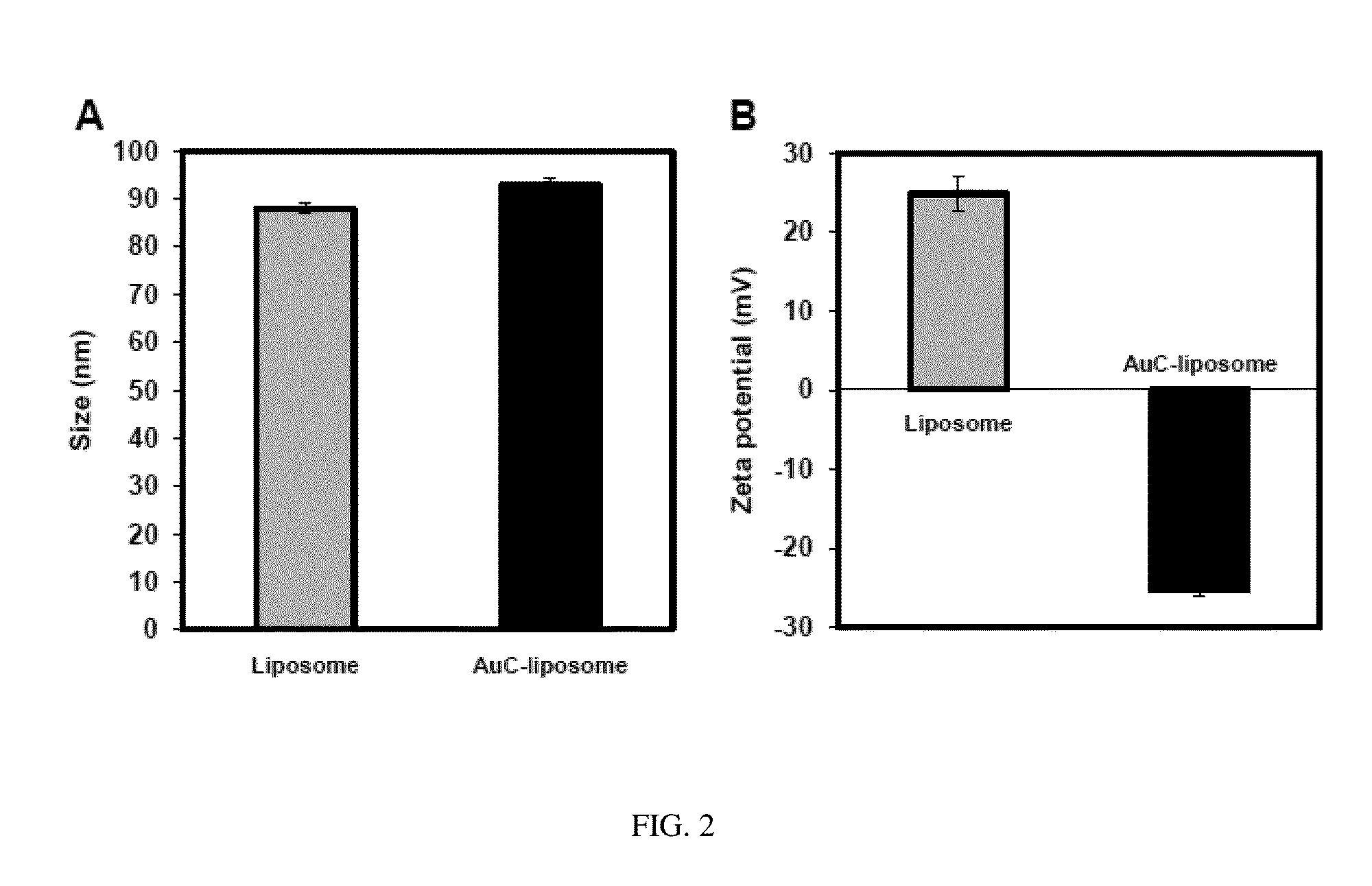
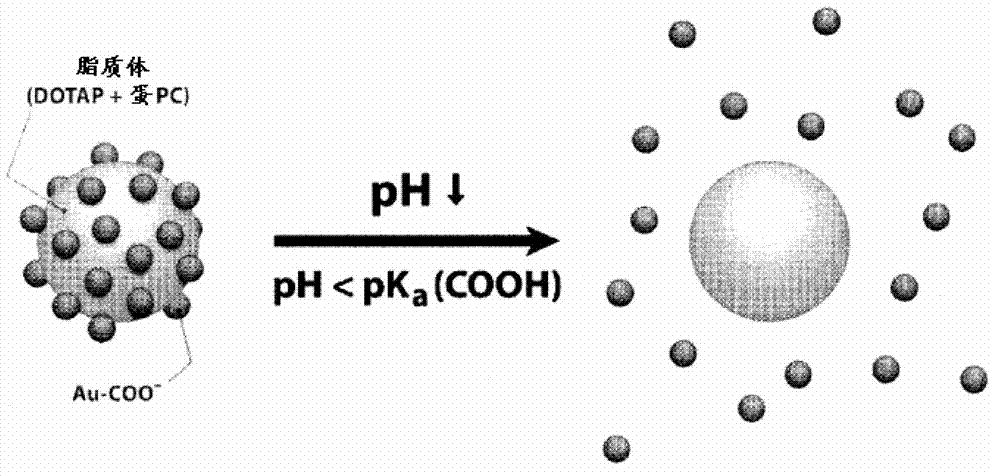
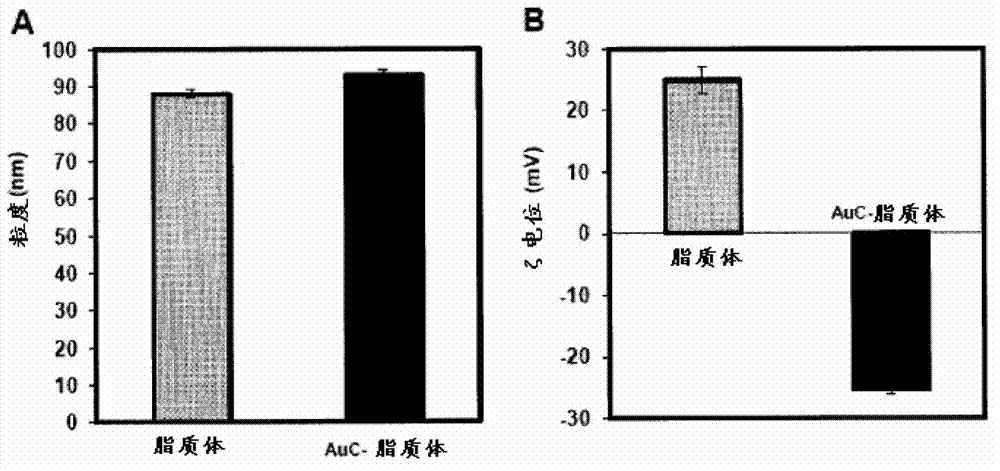
Triggered Cargo Release from Nanoparticle Stabilized Liposomes
Control of the fusion activity of liposomes by adsorbing biocompatible nanoparticles to the outer surface of phospholipid liposomes is disclosed. The biocompatible nanoparticles effectively prevent liposomes from fusing with one another. Release of cargo from the liposome is accomplished via trigger mechanisms that include pH triggers, pore forming toxing triggers and photosensitive triggers. Dermal drug delivery to treat a variety of skin diseases such as acne vulgaris and staph infections is contemplated.
Owner:RGT UNIV OF CALIFORNIA
Method of treating a staphylococcus infection in a patient having a low-level pathogenic pseudomonas aeruginosa infection
InactiveUS20110165172A1Lower Level RequirementsLow levelAntibacterial agentsAntibody ingredientsStaphylococcal infectionsPseudomonas
This invention provides methods of treating patients having a Staphylococcus infection where the patient also has a low level of Pseudomonas aeruginosa. The methods comprise administering an antagonist of the Pseudomonas Type III Secretion System, e.g., an anti-PcrV antibody antagonist.
Owner:KALOBIOS PHARMA
Compositions and methods for treatment of staphylococcal infection while suppressing formation of antibiotic-resistant strains
Co-administration of a lysostaphin or other anti-staphylococcal agent which cleaves cross-links of peptidoglycans of staphylococci cell walls such as lysostaphin and an antibiotic effective against staphylococci due to antibiotic activity mediated by cell-wall activity is effective against staphylococcal infection, even staphylococci that may be resistant to one or other of lysostaphin or the cell-wall active antibiotic. Co-administration simultaneously suppresses the generation of antibiotic-resistant mutant strains. Effective cell-wall active antibiotics include β-lactams and glycopeptides.
Owner:NUTRITION 21 INC
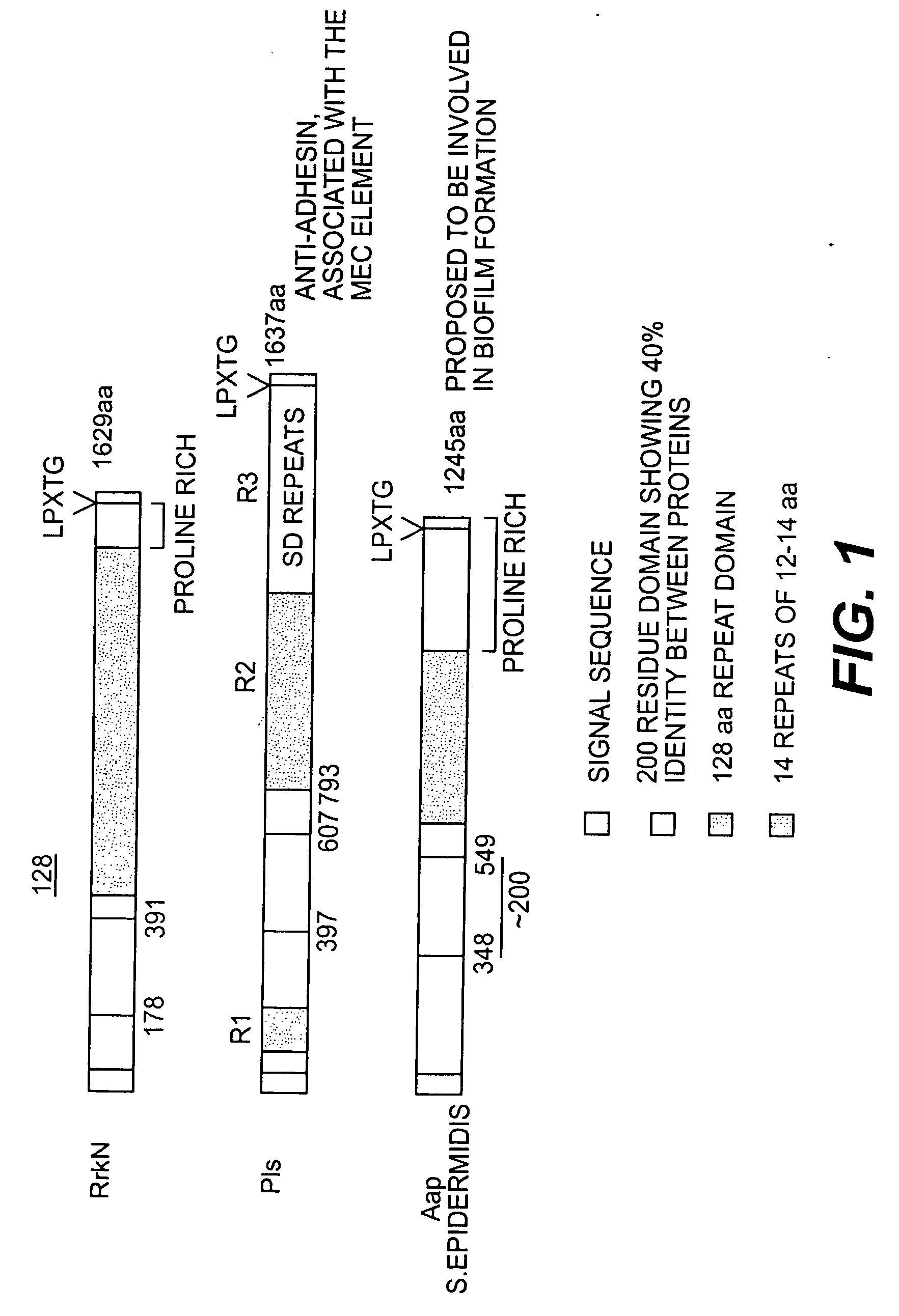
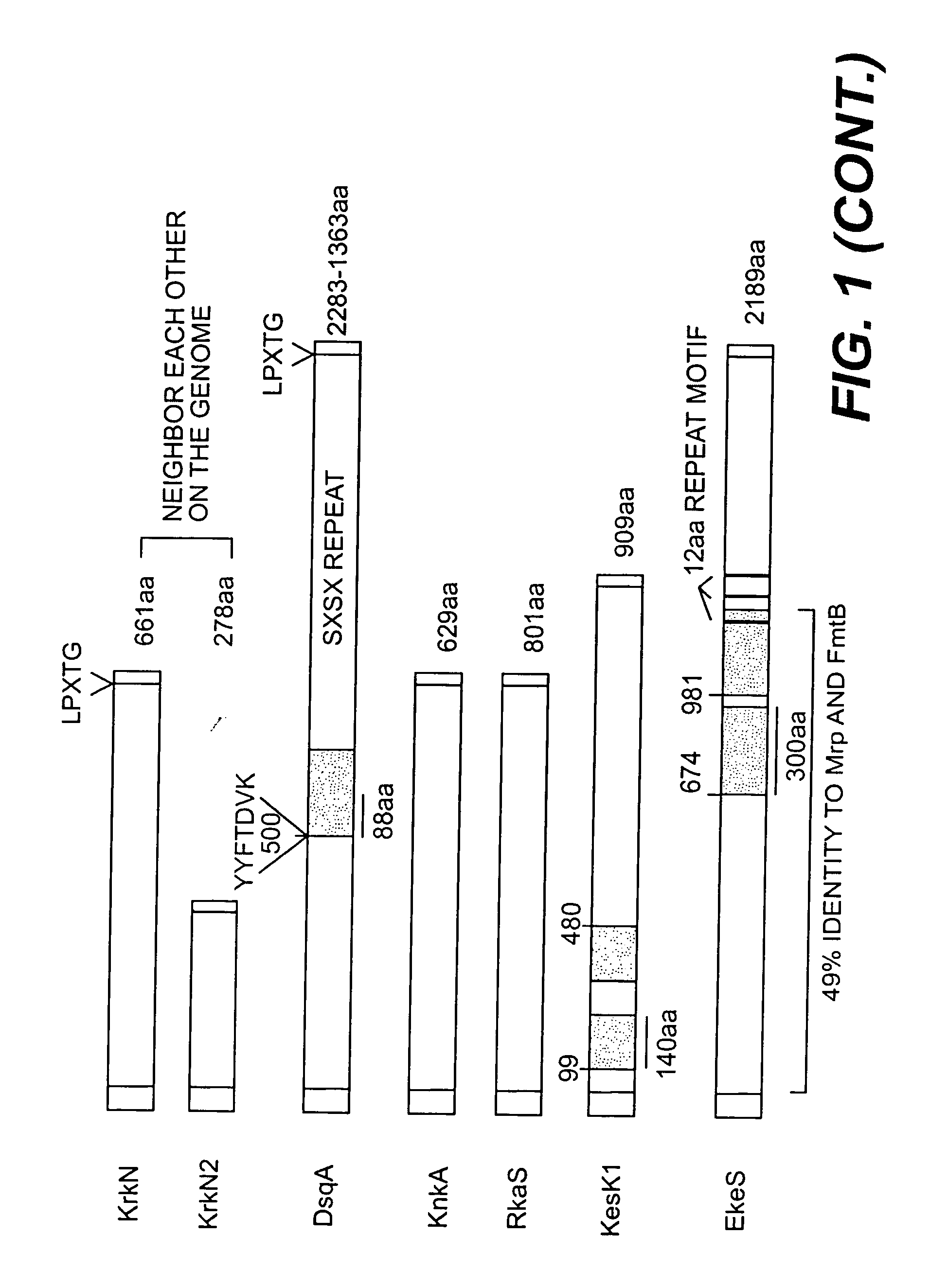
Proteins and polypeptides from coagulase-negative staphylococci
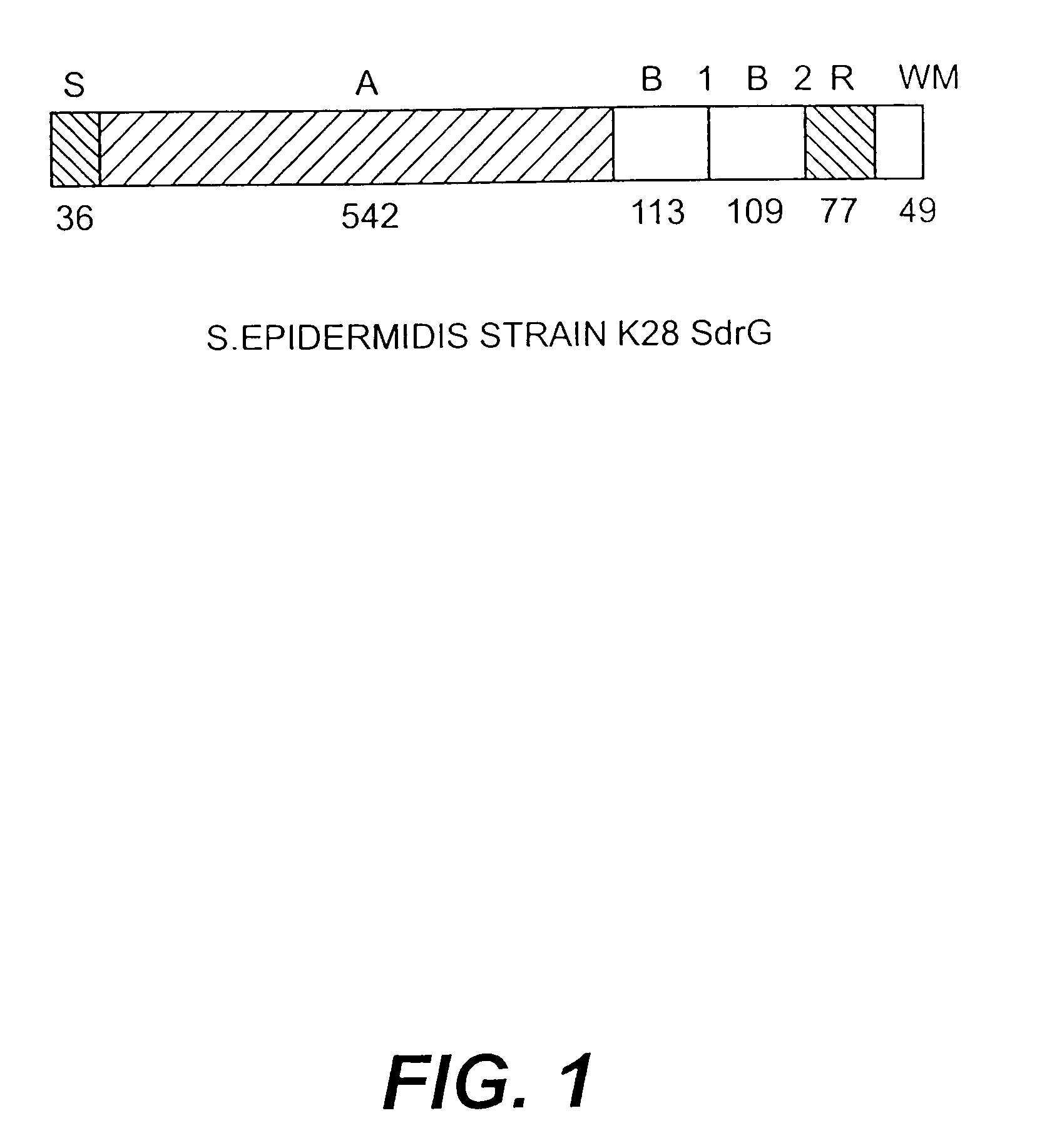
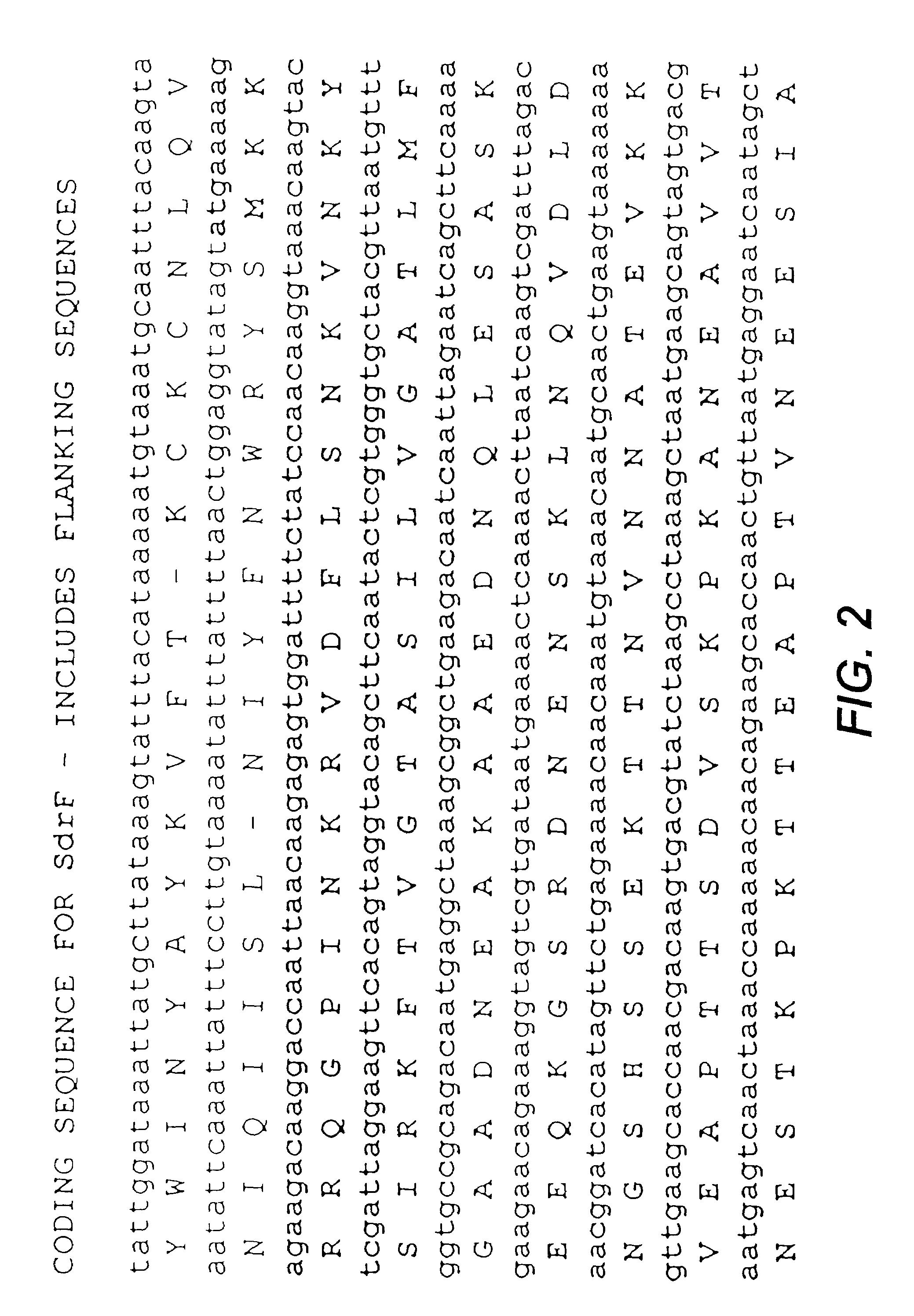
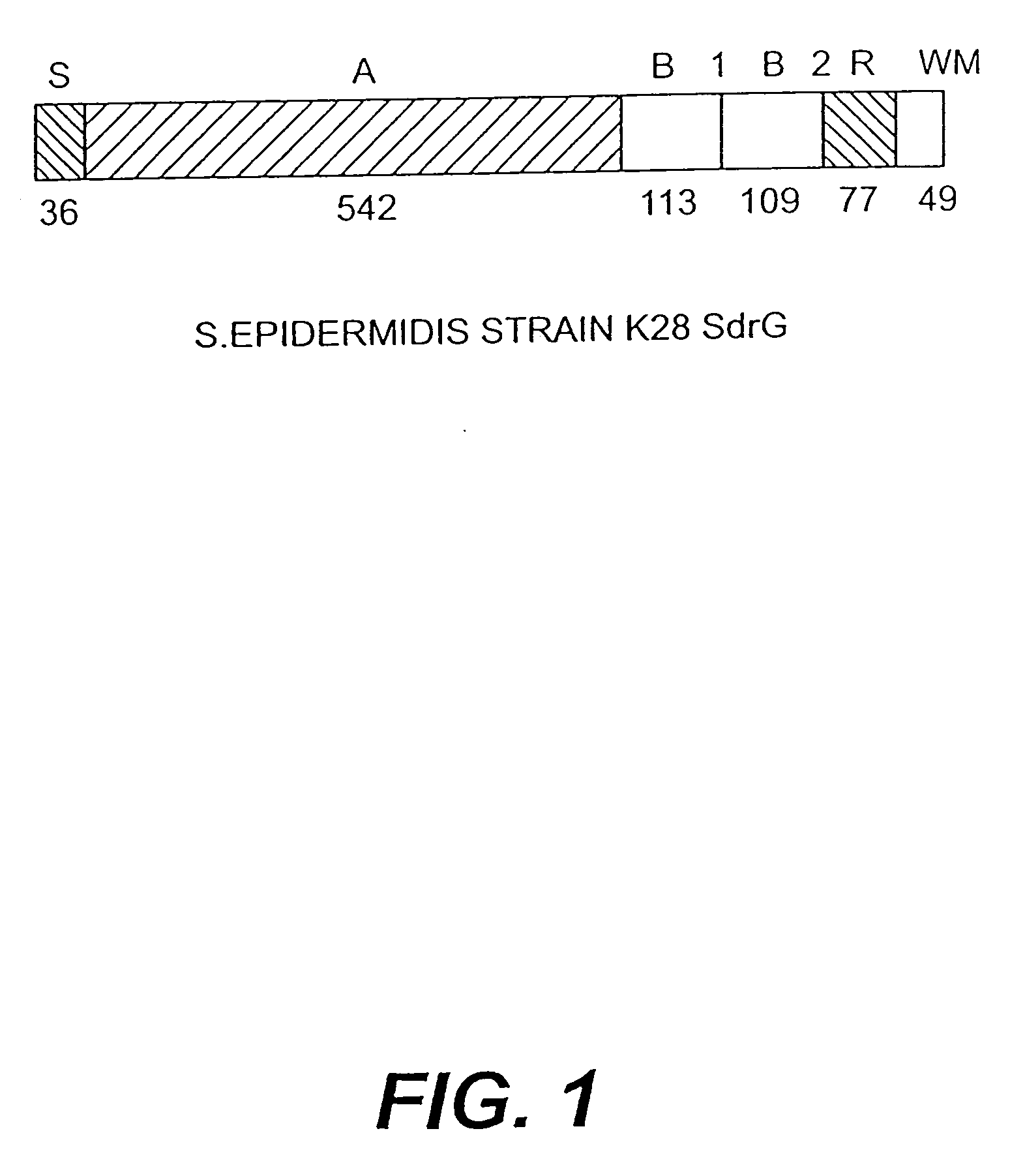
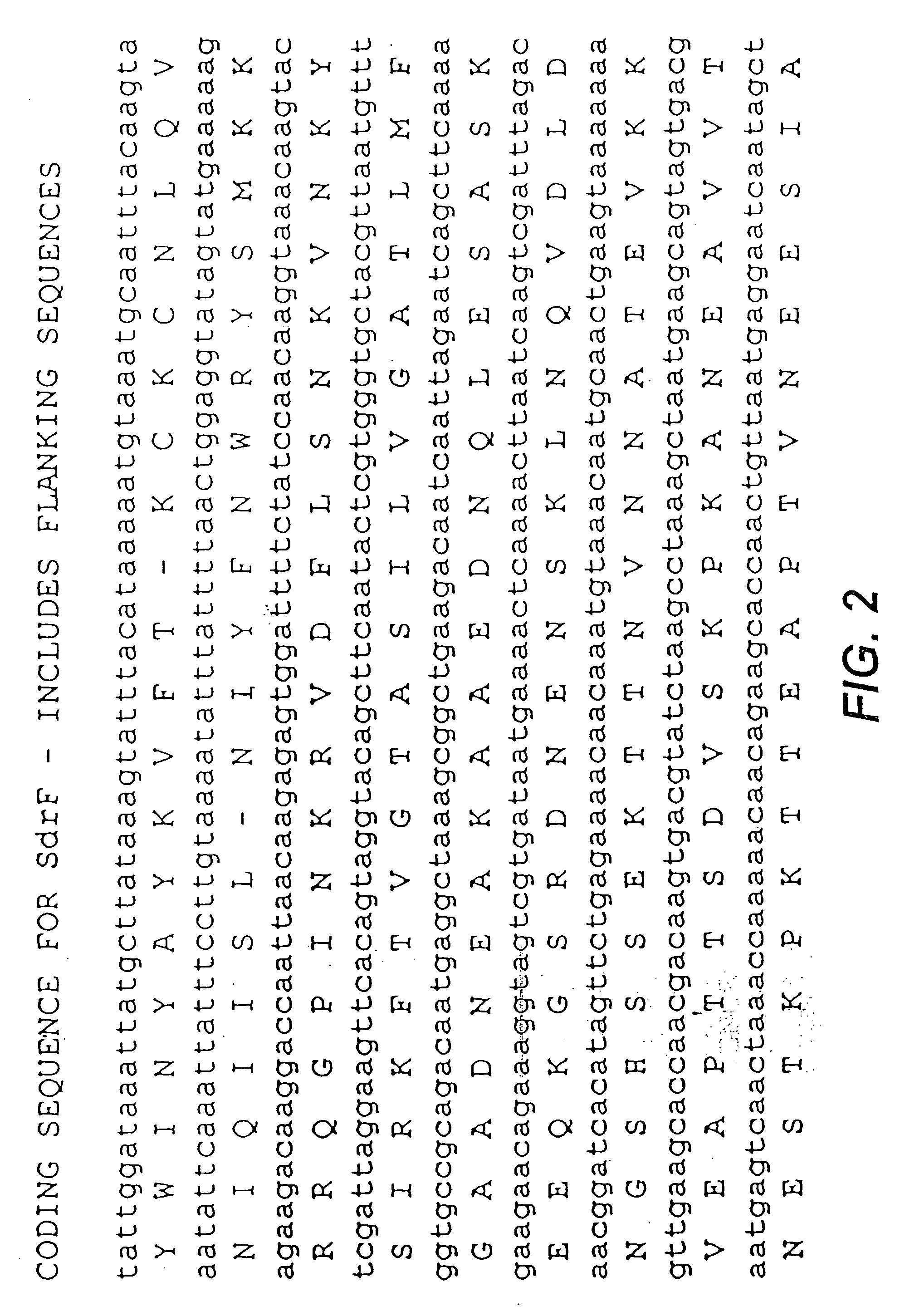
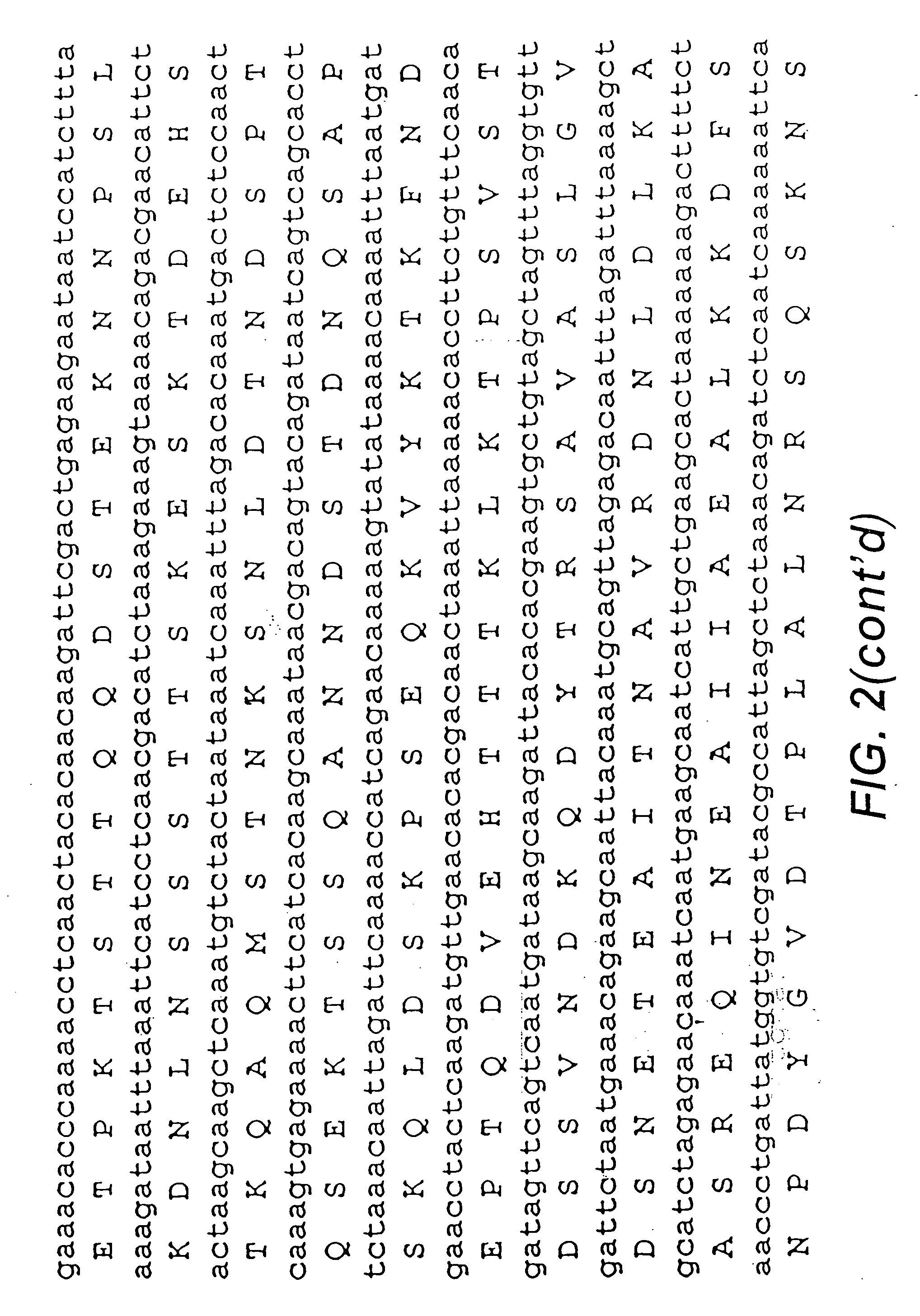
Proteins and polypeptides from coagulase-negative staphylococcal bacteria such as S. epidermidis, including proteins designated SdrF, SdrG and SdrH, and their effective fragments such as their respective A domains, are provided which are useful in the prevention and treatment of infection caused by coagulase-negative staphylococcal bacteria such as S. epidermidis. The SdrF, SdrG and SdrH proteins are cell-wall associated proteins that specifically bind host proteins and which each have a highly conserved motif of which the consensus sequence is TYTFTDYVD (SEQ ID NO: 16). The proteins and polypetides may be useful in generating antibodies for the diagnosis and treatment of coagulase-negative staphylococcal infections.
Owner:TRINITY COLLEGE DUBLIN +1
Isolated Broadly Reactive Opsonic Immunoglobulin for Treating a Pathogenic Coagulase-Negative Staphylococcus Infection
InactiveUS20080139789A1Immunoglobulins against animals/humansAntibody ingredientsAntigenImmunglobulin e
The invention describes the identification, making, and isolation of immunoglobulin and antigen useful for preventing, diagnosing, and treating staphylococcal infections. The invention further describes an in vivo animal model useful for testing the efficacy of pharmaceutical compositions, including pharmaceutical compositions of immunoglobulin and isolated antigen.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Compositions and methods for treatment of staphylococcal infection while suppressing formation of antibiotic-resistant strains
Co-administration of a lysostaphin or other anti-staphylococcal agent which cleaves cross-links of peptidoglycans of staphylococci cell walls such as lysostaphin and an antibiotic effective against staphylococci due to antibiotic activity mediated by cell-wall activity is effective against staphylococcal infection, even staphylococci that may be resistant to one or other of lysostaphin or the cell-wall active antibiotic. Co-administration simultaneously suppresses the generation of antibiotic-resistant mutant strains. Effective cell-wall active antibiotics include β-lactams and glycopeptides.
Owner:NUTRITION 21 INC
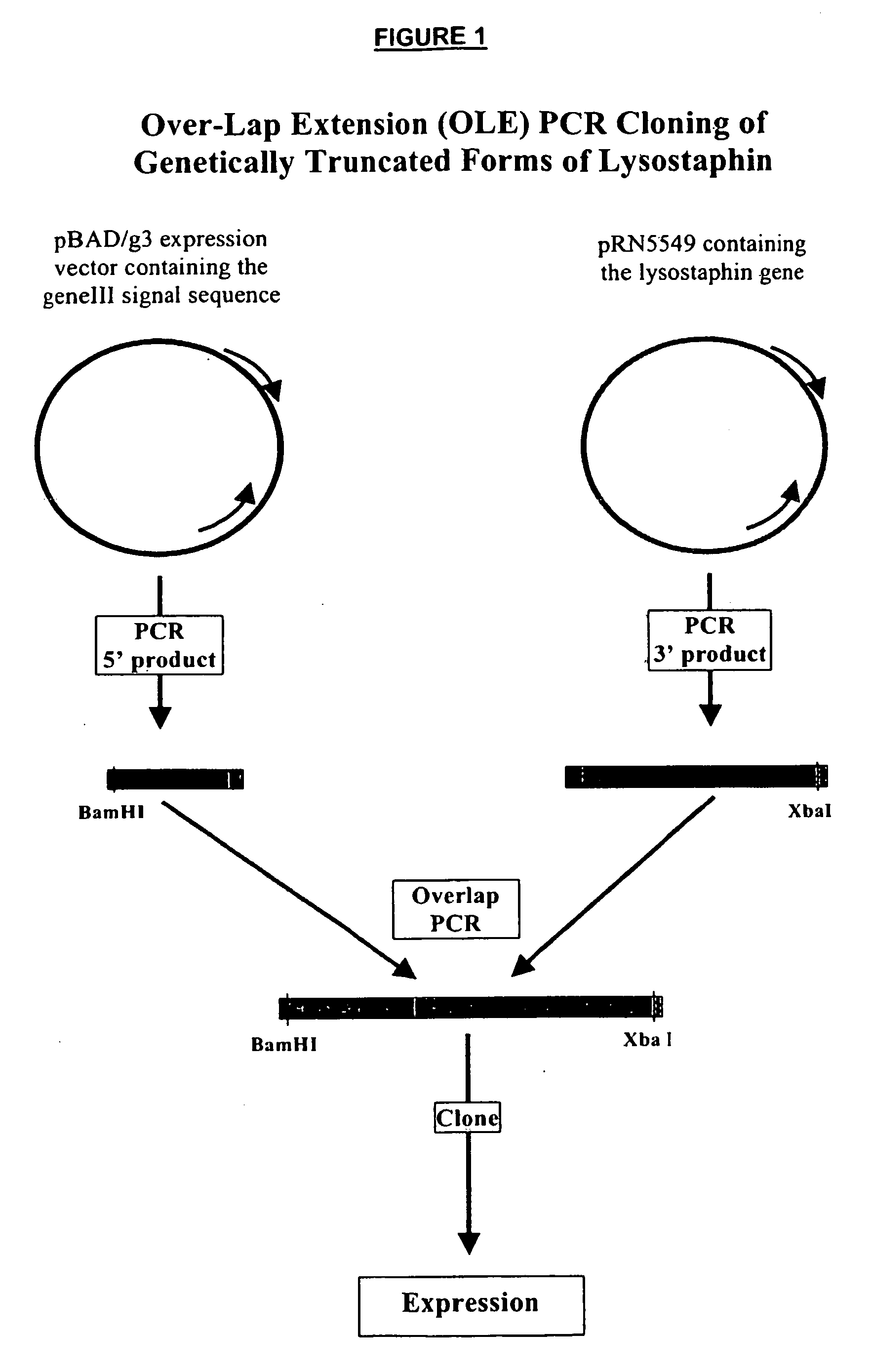
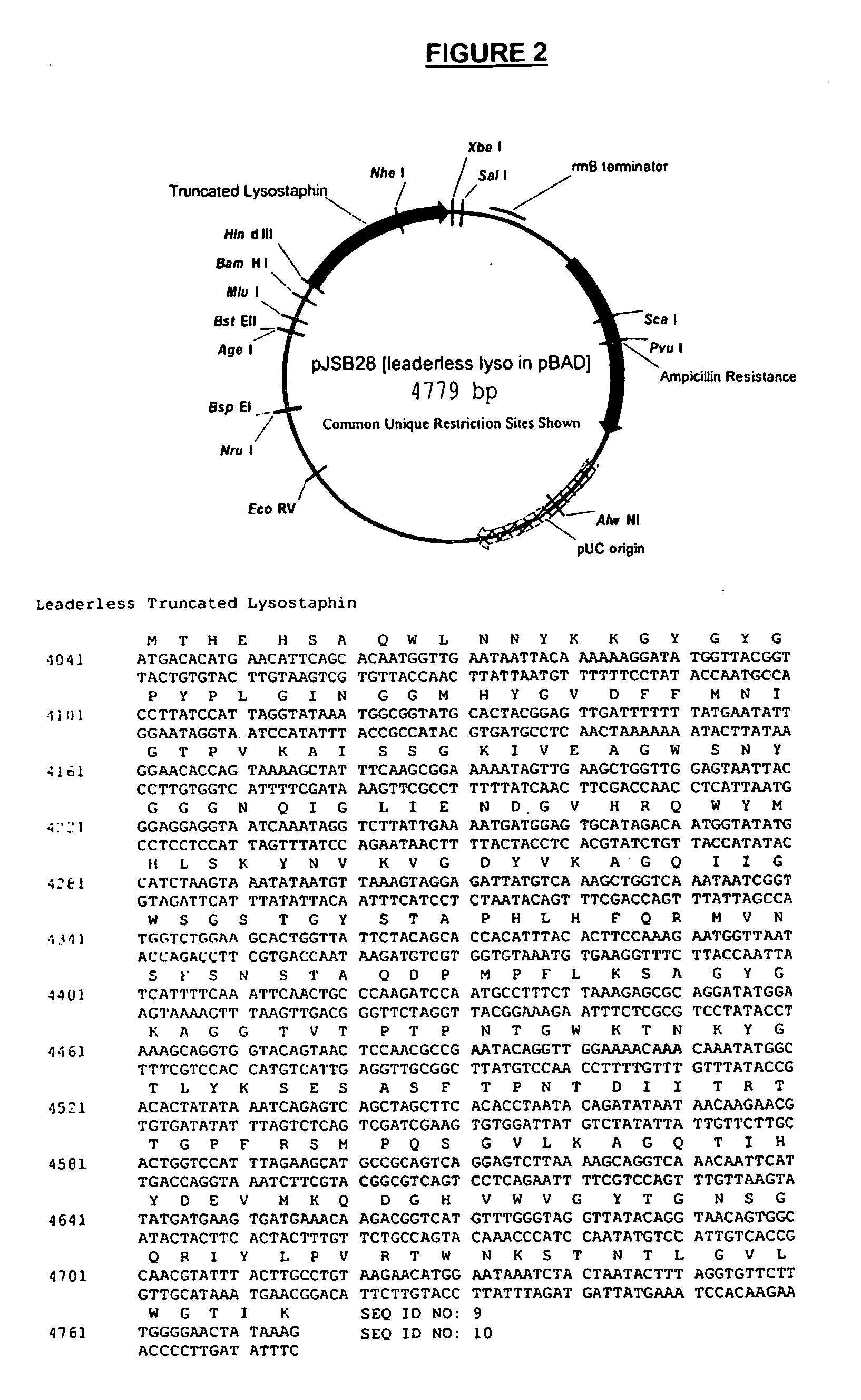
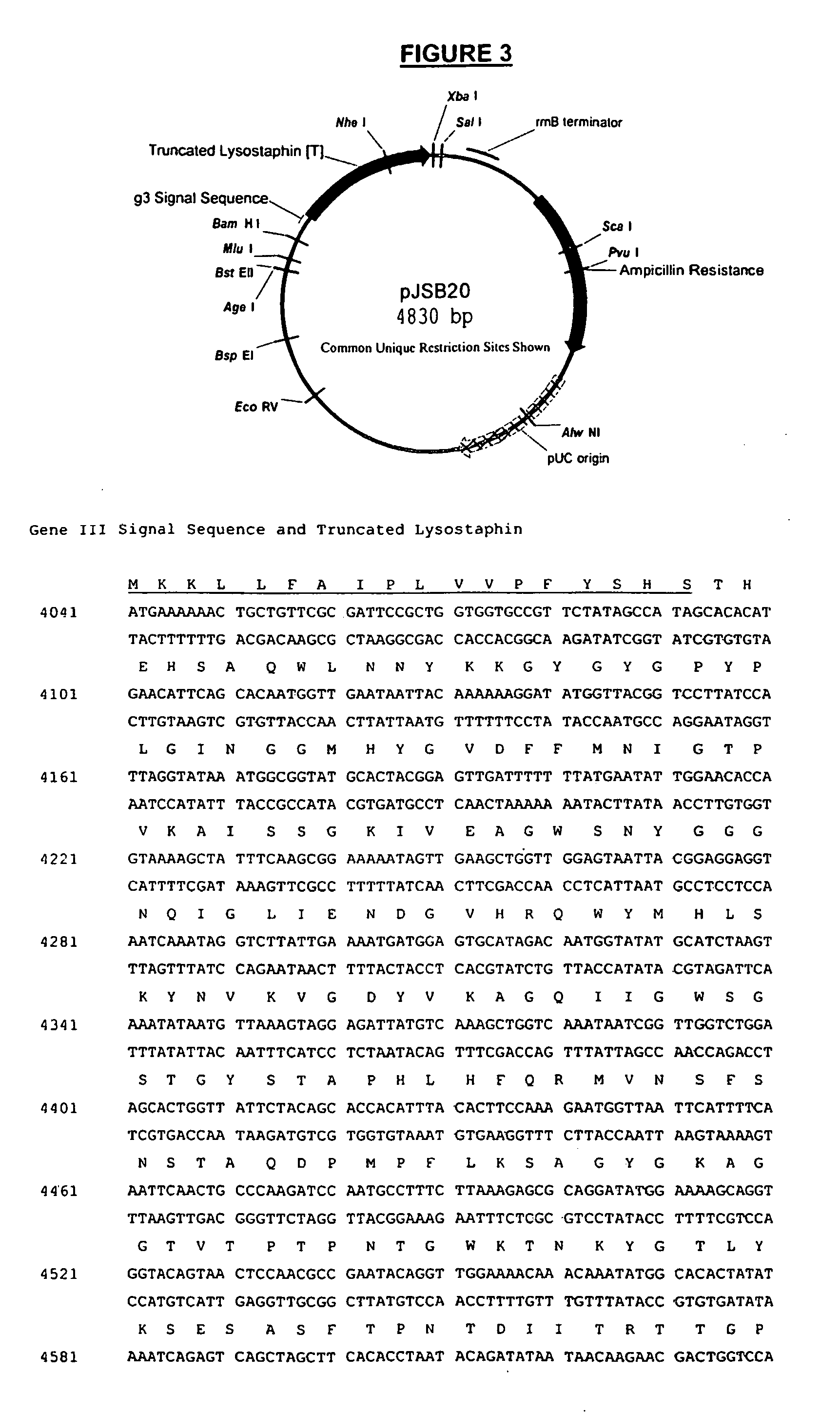
Truncated lysostaphin molecule with enhanced staphylolytic activity
InactiveUS20050118159A1Reduce individual infectionAvoid spreadingAntibacterial agentsSugar derivativesStaphylococcal infectionsStaphylococcus saprophyticus
This invention relates to the production of recombinant lysostaphin in a homogenous form through the use of recombinant DNA molecules that express homogenous lysostaphin and host cells transformed with these DNA molecules. This invention also relates to the production of truncated forms of lysostaphin. The resulting lysostaphin preparations can be administered to those at infected or risk for infection by staphylococcal bacteria.
Owner:BIOSYNEXUS INC
Lyase for killing staphylococcus and application of lyase
ActiveCN103122347ARapid lysisIncrease enzyme activityAntibacterial agentsBiocideMethicillin resistanceAdenosine
The invention discloses a lyase for killing staphylococcus and an application of the lyase and also discloses an amino acid sequence and an encoding gene sequence of the lyase. A recombined lyase constructed by using the disclosed encoding gene can realize soluble expression in Escherichia coli BL21 (DE3); the lyase can be used for efficiently killing various types of staphylococcus including clinically-separated methicillin-sensitive staphylococcus aureus (MSSA) and methicillin-resistant staphylococcus aureus (MRSA) in vitro and can also be used as an antibiotic for treating staphylococcus infection in vivo; and the disclosed lyase can also be used for rapidly cracking the cell wall of the staphylococcus and releasing substances in cells, such as adenosine triphosphate (ATP), DNA (Deoxyribonucleic Acid) and the like, and various detections on the staphylococcus can be realized through detecting released substances.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Anti-glucosaminidase passive immunization for staphylococcus aureus infections
ActiveUS20130110249A1Reduced enzymatic activityPromote cell-independent lysisAntibacterial agentsAnimal cellsAntiendomysial antibodiesOrthopedic department
The present invention is directed to a monoclonal antibody that binds specifically to a Staphylococcus aureus glucosaminidase and inhibits in vivo growth of S. aureus. Also disclosed are monoclonal antibody binding portions, recombinant or hybridoma cell lines, pharmaceutical compositions containing the monoclonal antibody or binding portions thereof, and methods of treating 5*. aureus infection and osteomyelitis, and methods for introducing an orthopedic implant into a patient using the monoclonal antibody, binding portion, or pharmaceutical composition of the present invention.
Owner:UNIVERSITY OF ROCHESTER
Method of protecting against staphylococcal infection
InactiveUS20060228368A1Easy to produceAntibody ingredientsImmunoglobulinsAntigenStaphylococcus cohnii
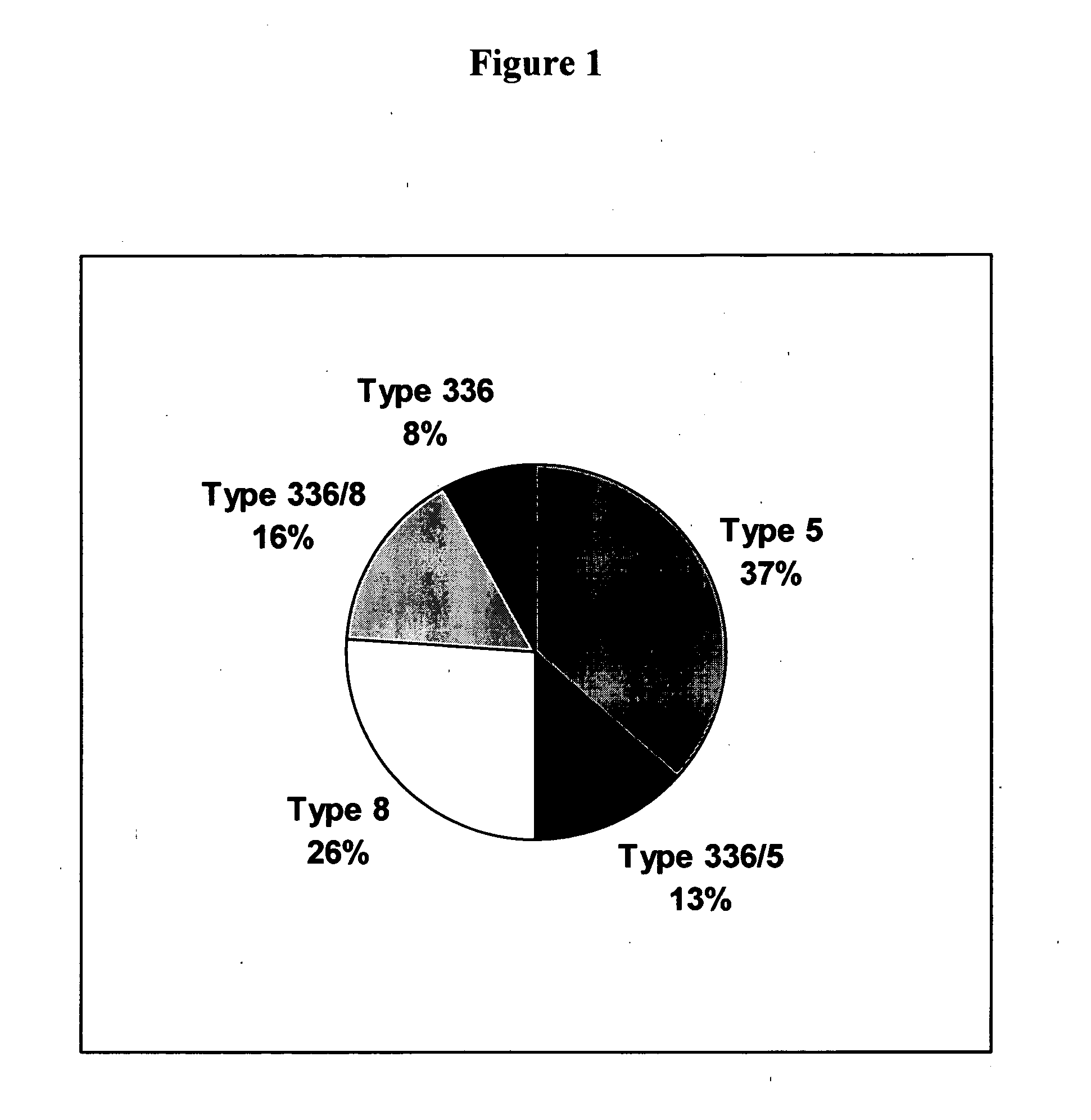
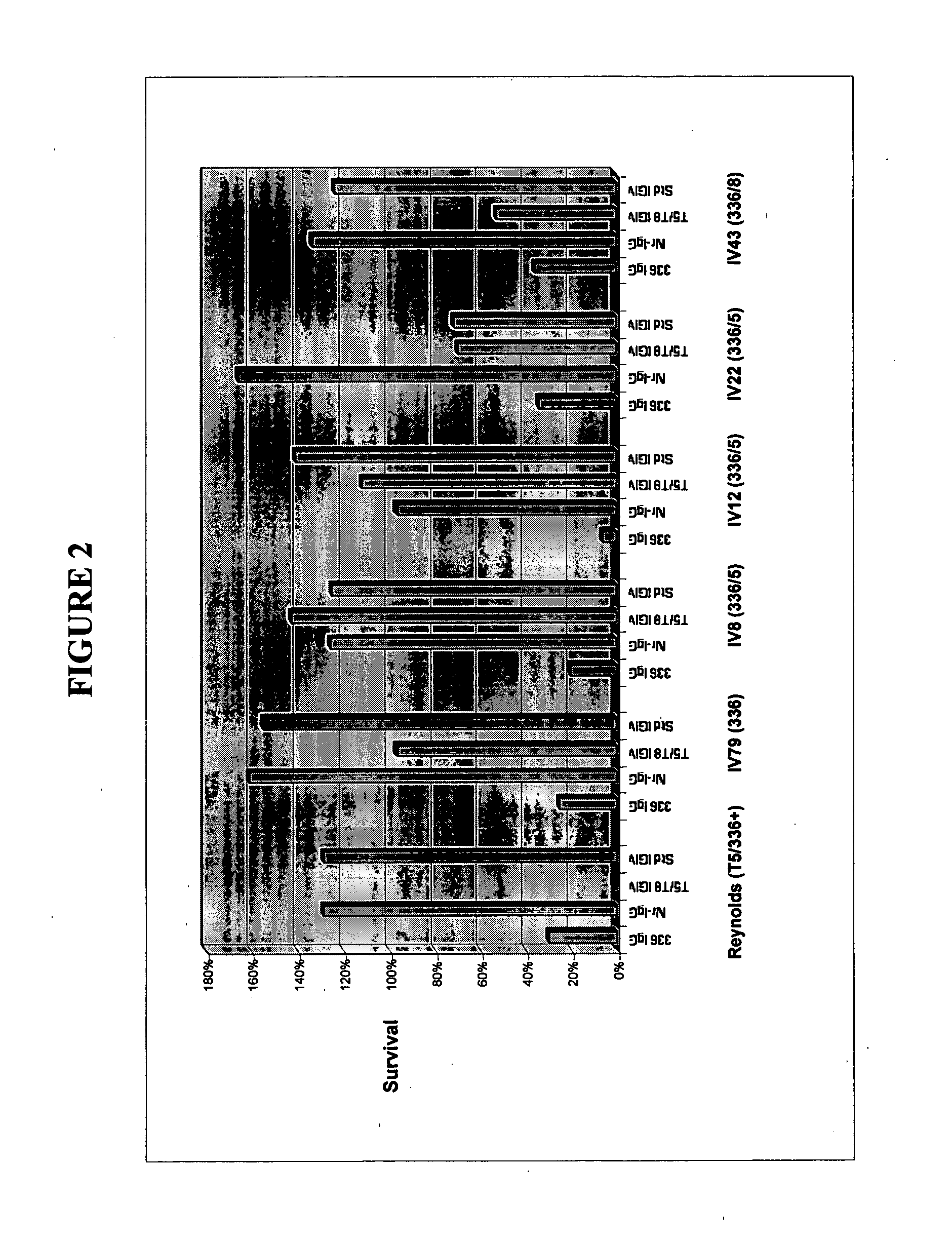
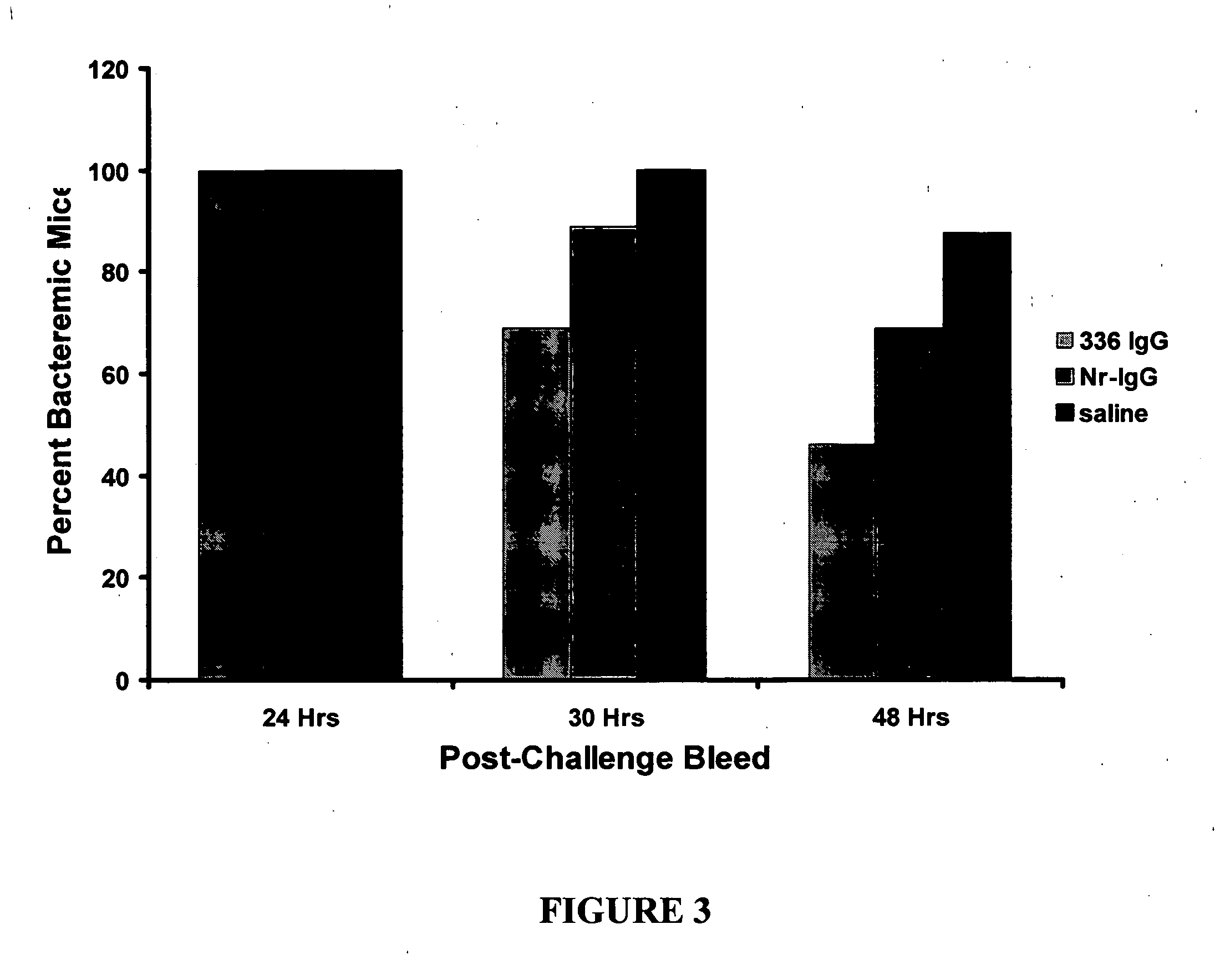
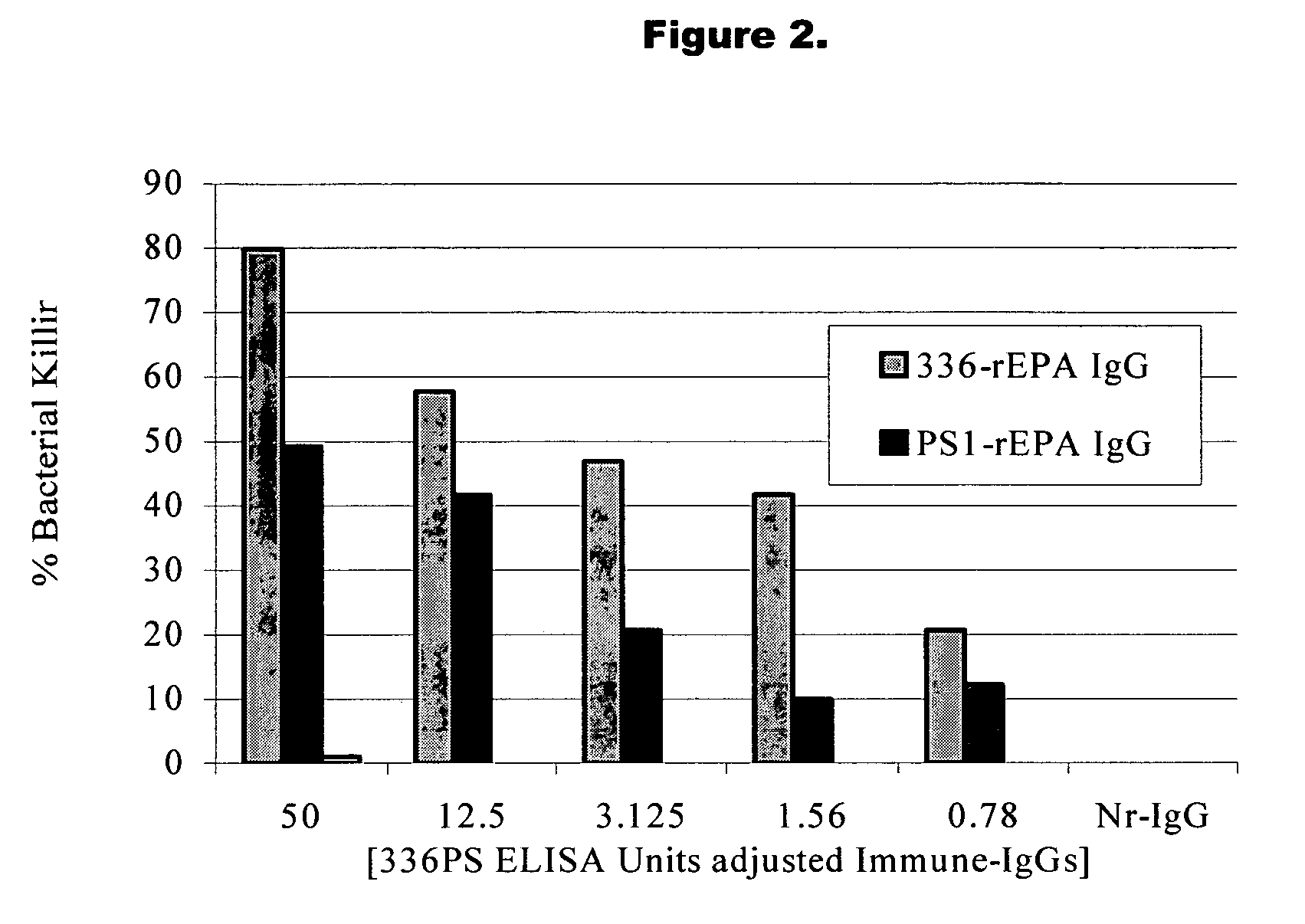
A method of preventing or treating staphylococcal bacterial infection in an individual is disclosed. A vaccine based on a conjugate the 336 polysaccharide antigen can be used for active protection in individuals who are to be subjected to conditions that place them at immediate risk of developing a bacterial infection, as would be case in the context of a catheterization or a surgical procedure. Alternatively, antibodies raised in response to the antigen can be used to treat or to provide passive protection to individuals. The method can be used in a population of patients at risk for infection by various species of Staphylococcus or various types of Staphylococcus aureus.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Polypeptides and polynucleotides from coagulase-negative staphylococci
InactiveUS20060171964A1Readily apparentAntibacterial agentsBacteriaNucleotideStaphylococcal infections
Methods for treating or preventing infections from coagulase-negative staphylococci using proteins and polypeptides from coagulase-negative staphylococcal bacteria such as S. epidermidis, including proteins designated SdrF, SdrG and SdrH, and their effective fragments such as their respective A domains, are provided. Methods are also provided wherein antibodies that recognize the SdrG protein or its ligand binding A region are used to treat or prevent staphylococcal infection, and these methods can also be utilized to prevent the formation of infections on indwelling medical devices.
Owner:TRINITY COLLEGE DUBLIN +1
Multicomponent vaccines
InactiveUS20100150956A1Convenient treatmentEnhance phagocytosisAntibacterial agentsBacterial antigen ingredientsBacteroidesBacterial strain
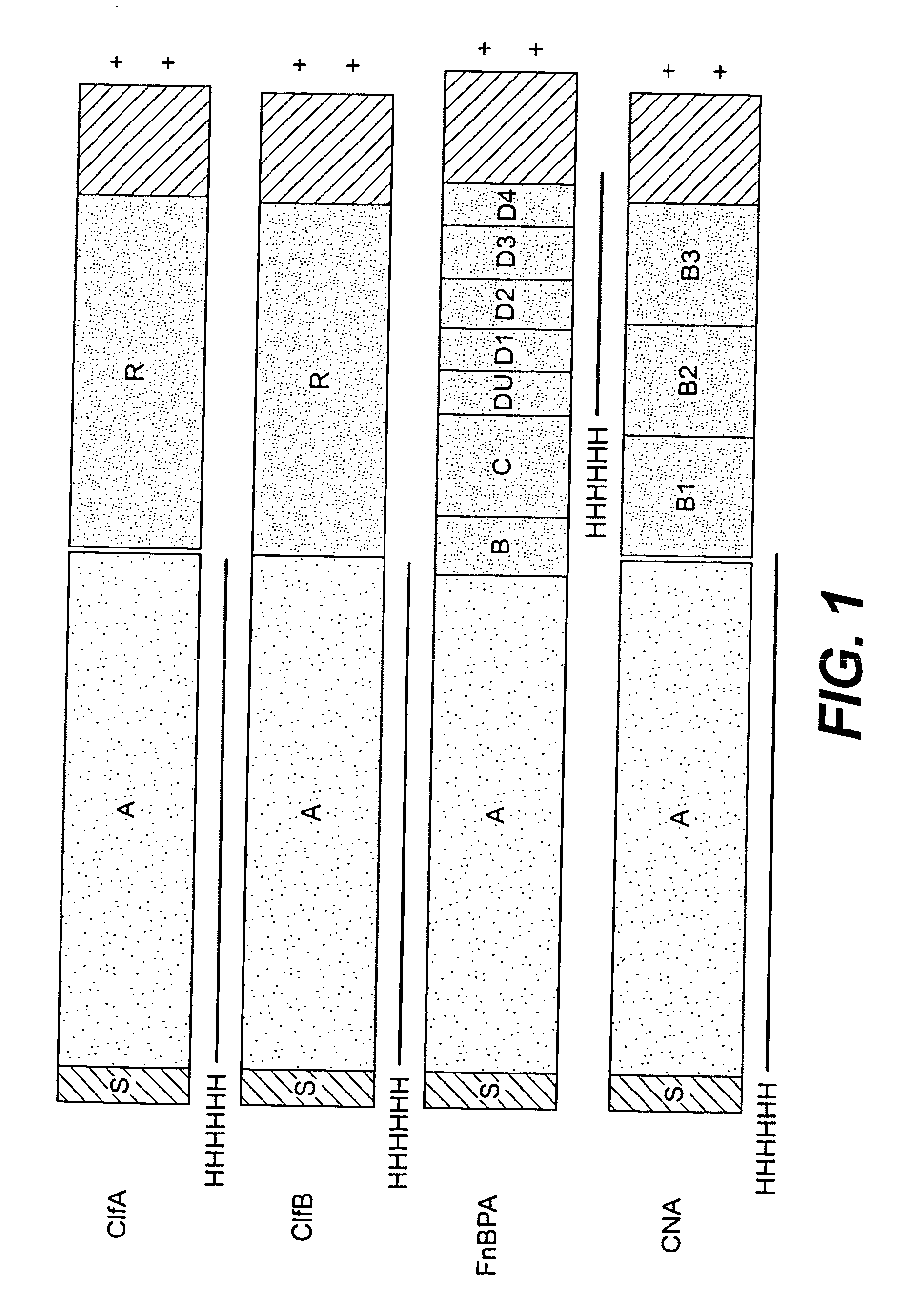
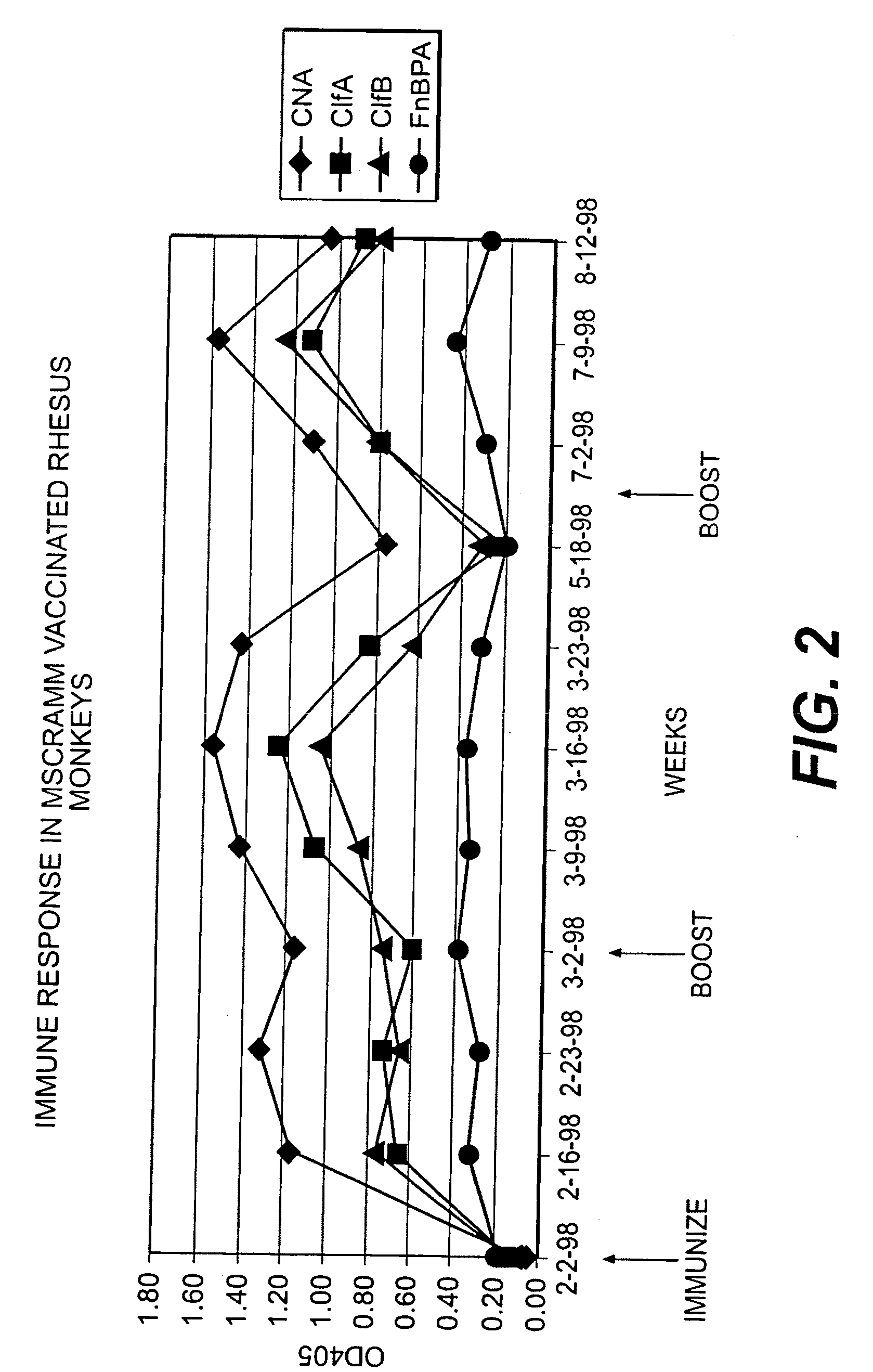
Multicomponent vaccines are provided which aid in the prevention and treatment of staphylococcal infections and which include certain selected combinations of bacterial binding proteins or fragments thereof, or antibodies to those proteins or fragments. By careful selection of the proteins, fragments, or antibodies, a vaccine is provided that imparts protection against a broad spectrum of Staphylococcus and other bacterial strains and against proteins that are expressed at different stages of the logarithmic growth curve. In one embodiment of the invention, a composition is provided that includes a fibrinogen binding domain of a fibrinogen binding protein and a bacterial component such as a capsular polysaccharide, and both active and passive vaccines based on these components are also provided, along with methods of treating infection using these compositions and vaccines.
Owner:INHIBITEX INC +2
Cross-reactive monoclonal and polyclonal antibodies which recognize surface proteins from coagulase-negative staphylococci and staphylococcus aureus
InactiveUS20050106648A1Preventing and treating staphylococcal infectionWide rangeAntibacterial agentsImmunoglobulins against bacteriaStaphylococcus cohniiStaphylococcal infections
Polyclonal and monoclonal antibodies which are cross-reactive to both coagulase-positive staphylococcus bacteria, such as S. aureus and to coagulase-negative bacteria, such as S. epidermidis and S. hemolyticus, are provided which can recognize surface proteins from both coagulase-positive and coagulase negative staph bacteria. The antibodies may be generated from surface proteins that have been isolated on the basis of characteristics that may be common between S. aureus and coagulase-negative staphylococci, and these recombinant surface proteins are used to generate the antibodies of the invention. There is also provided vaccines and methods which utilize these proteins and antibodies for the treatment or protection against a wide variety of staphylococcal infections.
Owner:FOSTER TIMOTHY +5
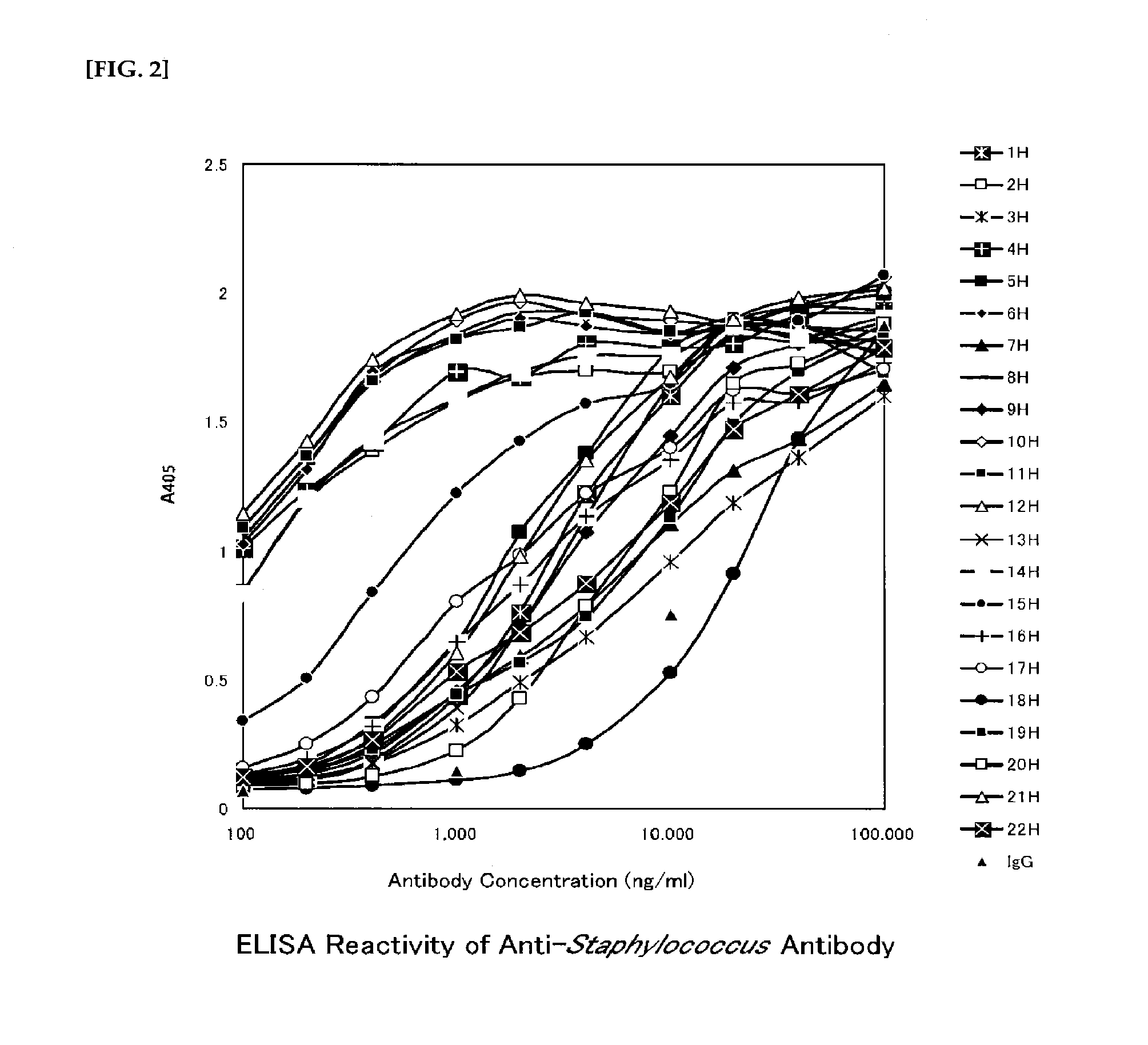
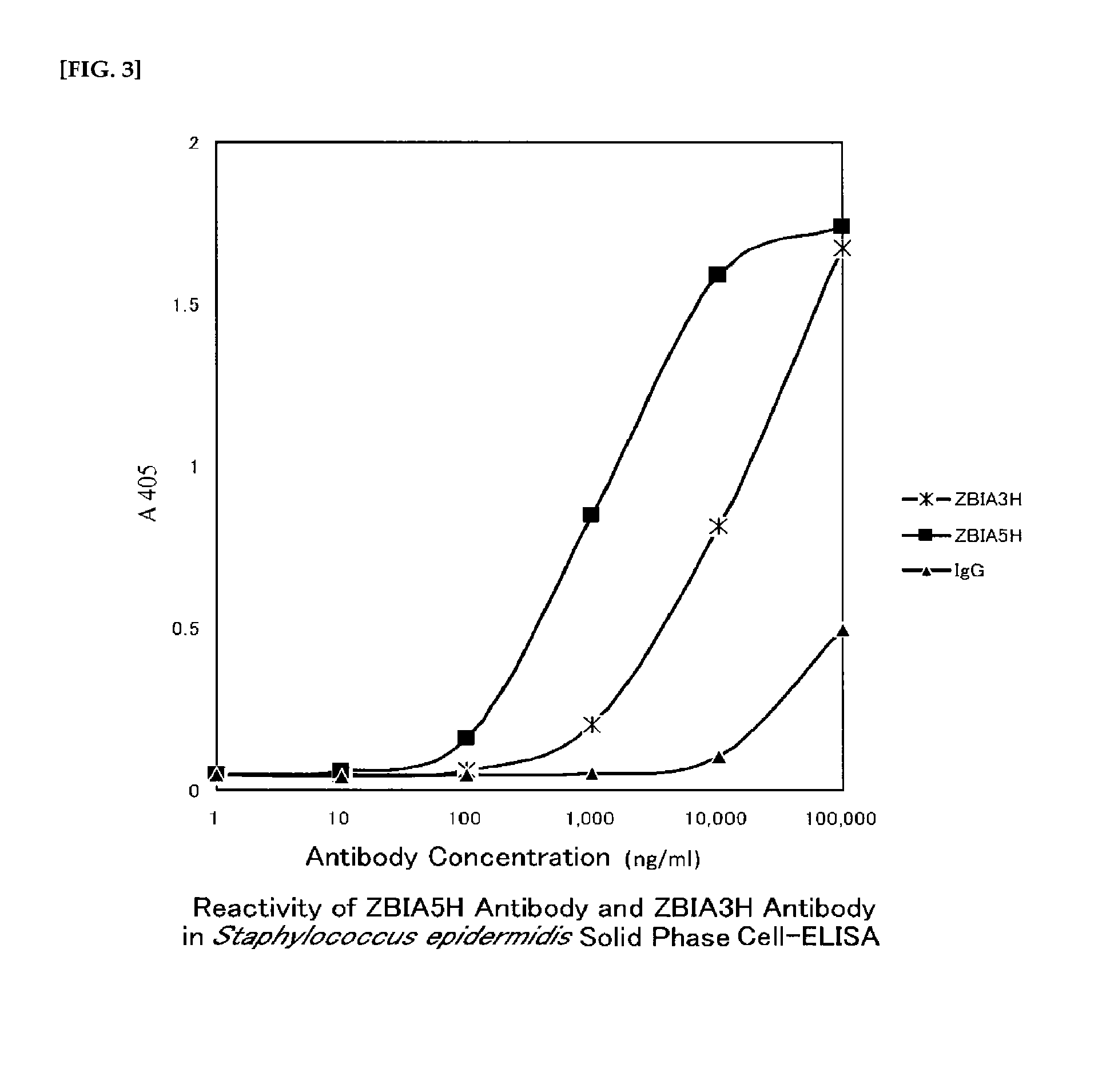
Anti-staphylococcus antibody, method for manufacturing same, and usage of same
[Problem] The present invention addresses the problem of providing an anti-Staphylococcus antibody having preventive or therapeutic effects on staphylococcal infections. [Solution] Provided is an anti-Staphylococcus antibody having preventive or therapeutic effects on staphylococcal infections and a method for manufacturing said antibody, as well as a composition, a product, and a drug containing said antibody. The antibody is obtained by using deacetylated Staphylococcus for immunization.
Owner:ZENYAKU KOGYO KK +1
Lipopeptid and derivative thereof, and preparation methods and application of lipopeptid and derivative of lipopeptid
ActiveCN102659932APrevent or reduce infectionReduced activityAntibacterial agentsCosmetic preparationsLipopeptideStereochemistry
The invention provides lipopeptid which comprises a peptide chain and an aliphatic chain which are connected through peptide bonds, wherein the molecular weight of the lipopeptid is 2848Da, and the lipopeptid is linear, and can obviously introduce the expression of phylaxin, and effectively inhibit staphylococcus aureus infection so as to prevent or reduce skin infection. The invention also discloses a derivative of the lipopeptid and preparation methods and application of the lipopeptid and the derivative of the lipopeptid.
Owner:EAST CHINA NORMAL UNIV
Multicomponent vaccines
InactiveUS8017133B2Convenient treatmentEnhance phagocytosisAntibacterial agentsBacterial antigen ingredientsADAMTS ProteinsBacterial strain
Multicomponent vaccines are provided which aid in the prevention and treatment of staphylococcal infections and which include certain selected combinations of bacterial binding proteins or fragments thereof, or antibodies to those proteins or fragments. By careful selection of the proteins, fragments, or antibodies, a vaccine is provided that imparts protection against a broad spectrum of Staphylococcus and other bacterial strains and against proteins that are expressed at different stages of the logarithmic growth curve. In one embodiment of the invention, a composition is provided that includes a fibrinogen binding domain of a fibrinogen binding protein and a bacterial component such as a capsular polysaccharide, and both active and passive vaccines based on these components are also provided, along with methods of treating infection using these compositions and vaccines.
Owner:INHIBITEX INC +2
Method of protecting against staphylococcal infection
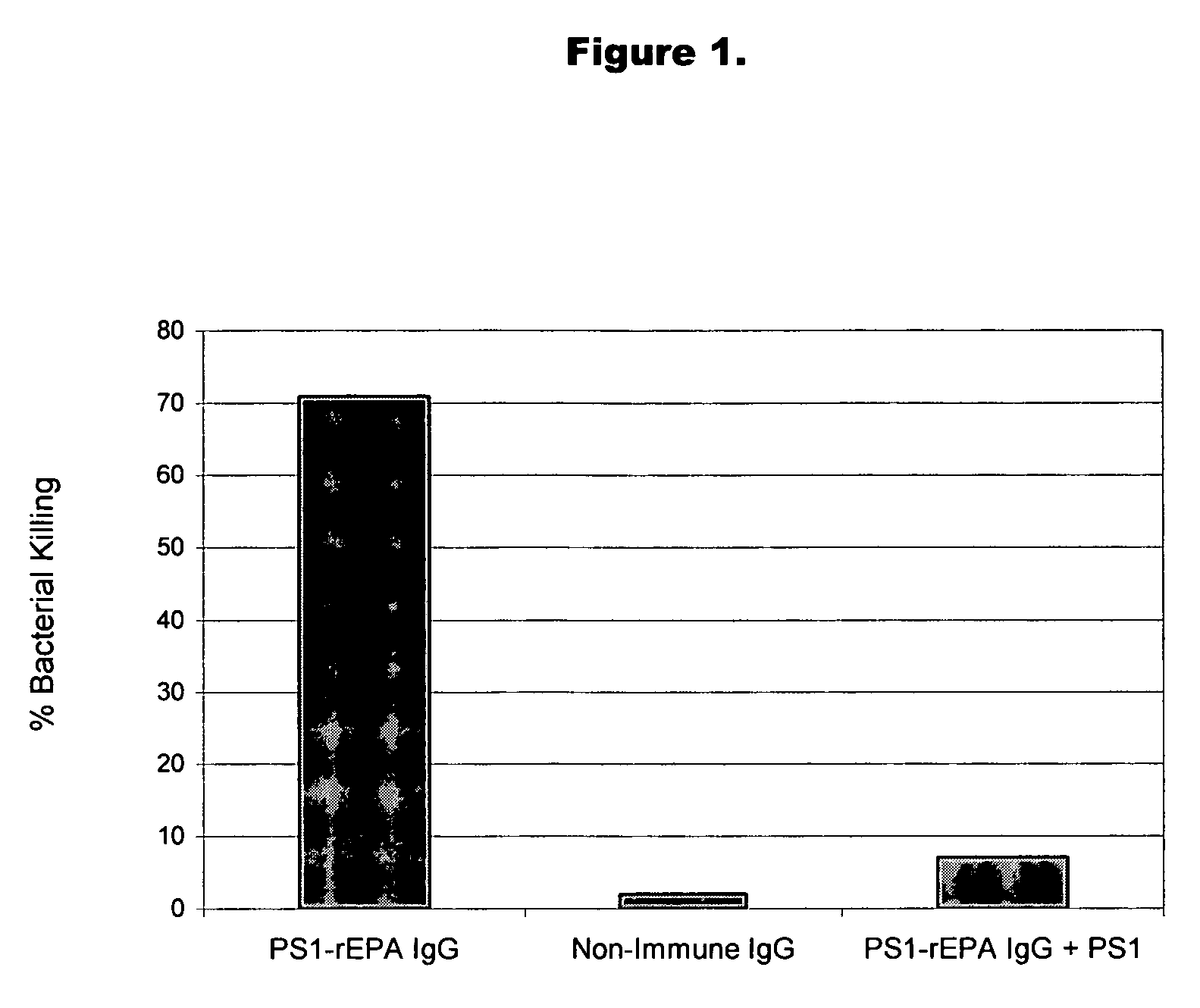
A method of preventing or treating staphylococcal bacterial infection in an individual is disclosed. A vaccine based on a conjugate of PS1 polysaccharide antigen can be used for active protection in individuals who are to be subjected to conditions that place them at immediate risk of developing a bacterial infection, as would be case in the context of a catheterization or a surgical procedure. Alternatively, antibodies raised in response to the antigen can be used to treat or to provide passive protection to individuals. The method can be used in a population of patients at risk for infection by various species of Staphylococcus or various types of Staphylococcus epidermidis.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Composition of sodium fusidafe as injection and preparing method thereof
ActiveCN1817340AEasy to useImprove toleranceAntibacterial agentsPowder deliveryHydrogen phosphateFreeze-drying
A freeze-dried powder injection of sodium fusidate composition for treating the serious Staphylococcus infection contains sodium fusidate, bisodium hydrogen phosphate and citric acid in weight ratio of 500: (78-113): (4-5.7). The water for injection is used as its solvent.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Miniaturized Staphylococcus aureus polypeptide of against drug resistance and its uses and preparation method
ActiveCN1766110AToxic reductionStrong bactericidal effectAntibacterial agentsPeptide/protein ingredientsBacteroidesEscherichia coli
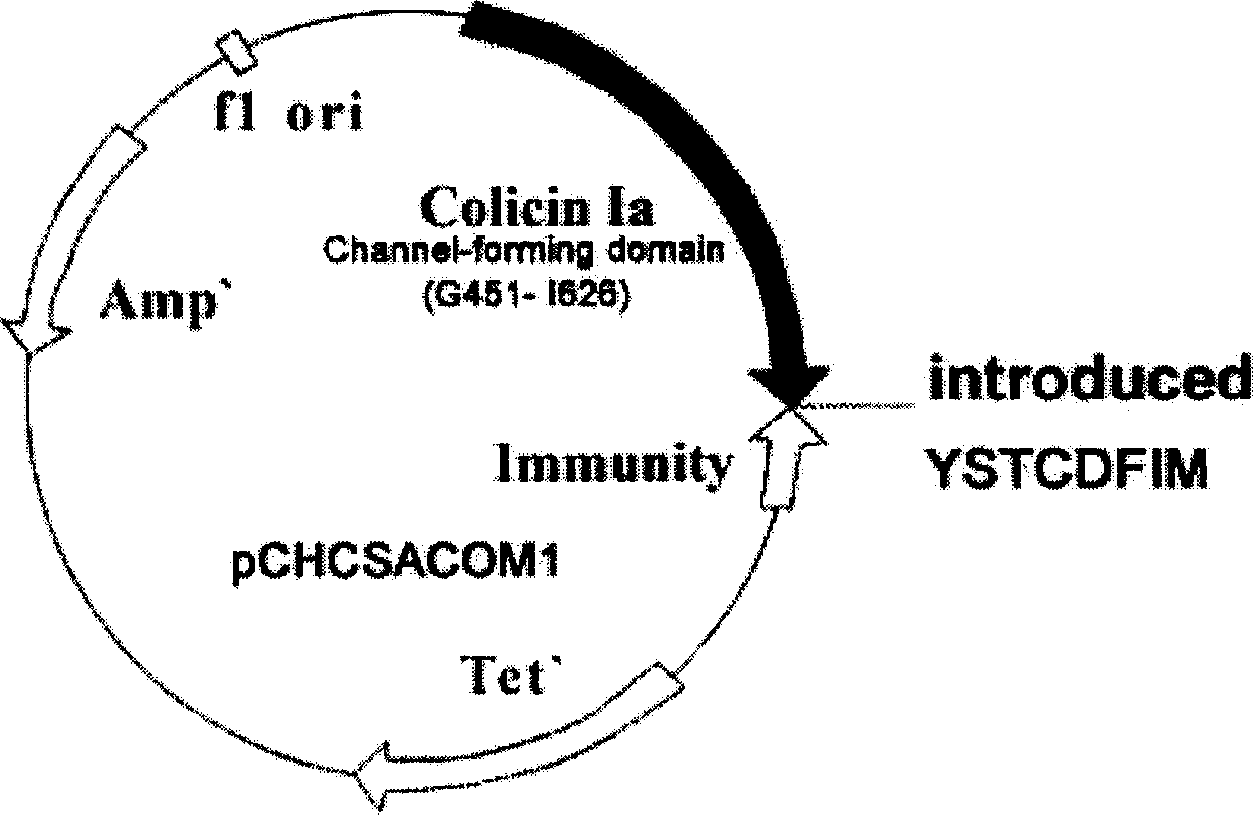
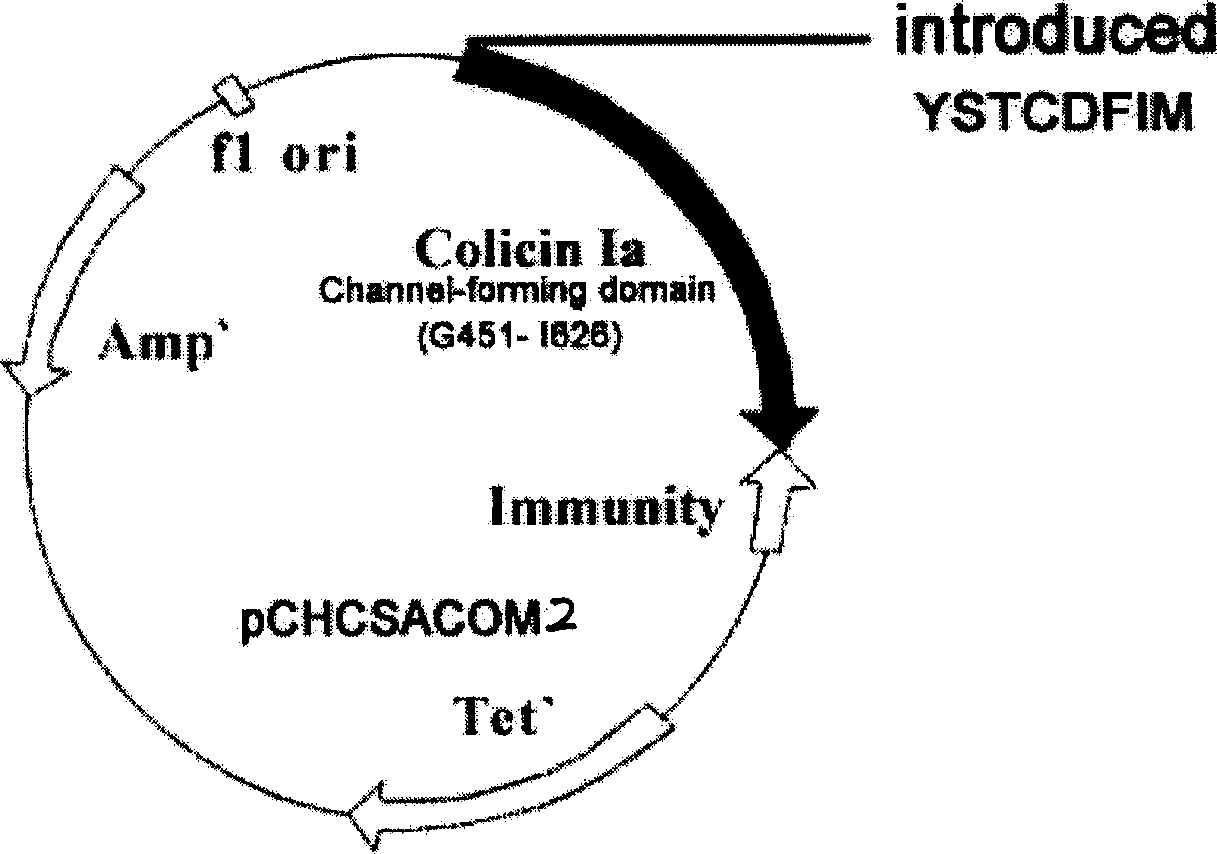
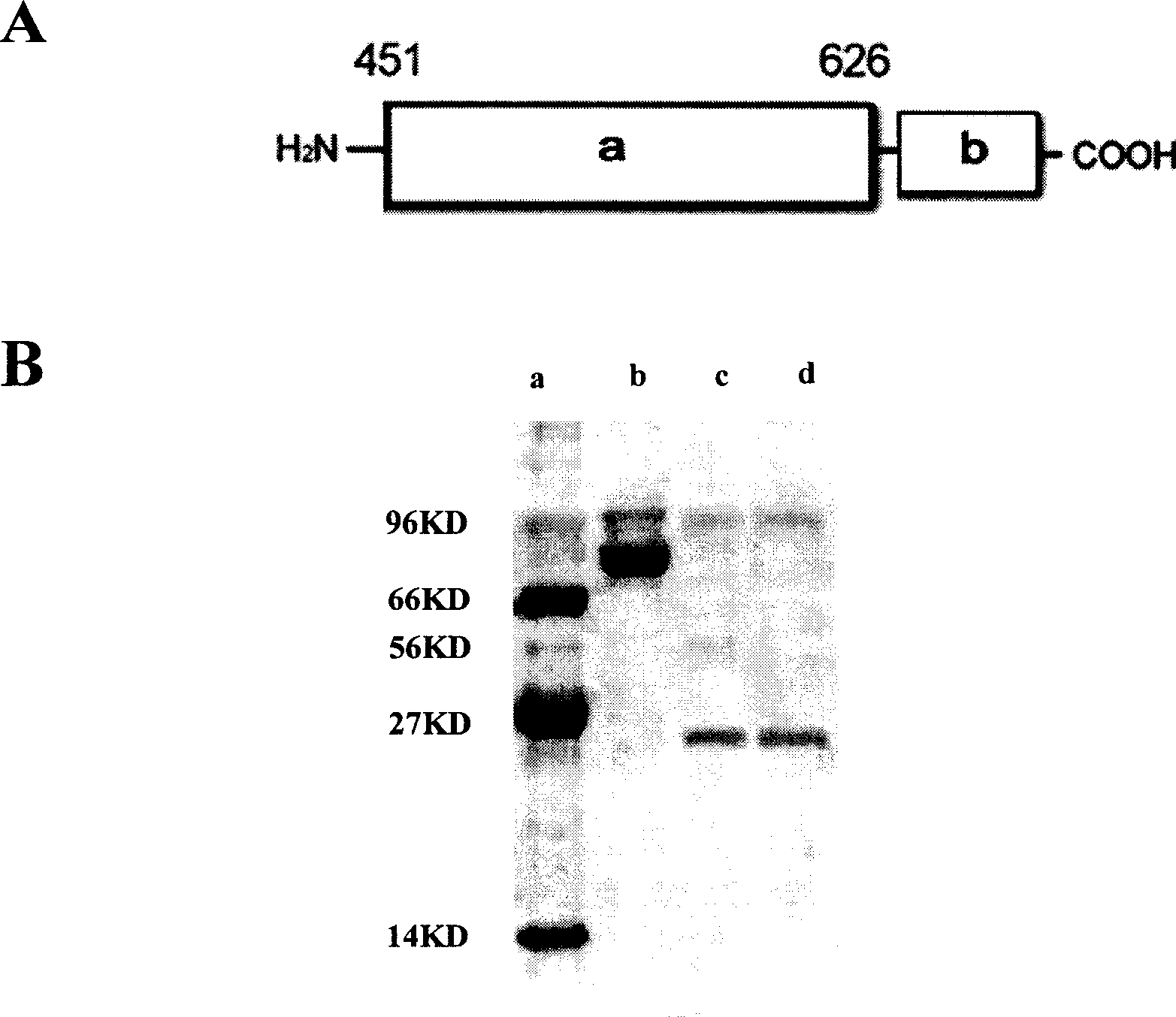
The invention discloses a midget anti drug-tolerance staphylococcus aureus polypeptide comprised of passage structure domain to form ion channel colicine and staphylococcus aureu pheromone, as well as opposite coded nucleotide sequence and recombination plasmid contained said sequence. Wherein, the plupeptide can not induce bacteria to generate trsditional drug-tolerance by the mechnism that it forms directly ion channel on cell film of target cell to kill bacteria. This invention has more single antimicrobial spectrum without effect to Gram-negative bacteria of escherichia coli, shows at least 500 times effect to pheneazonecillin, and can be used as synergist to beta-lactam antibiotics.
Owner:畿晋庆堂国际生物技术有限公司
Methods and compositions for the treatment and prevention of staphylococcal infections
Owner:CENTEGEN INC
Methods of using Fab I and compounds modulating Fab I activity
InactiveUS20050112713A1Inhibition effectInhibit expressionPeptide/protein ingredientsMicrobiological testing/measurementStaphylococcal infectionsNucleic acid sequencing
Prokayrotic FAB I polypeptides and DNA (RNA) encoding such FAB I and a procedure for producing such polypeptides by recombinant techniques is disclosed. Also disclosed are methods for utilizing such FAB I for the treatment of infection, such as bacterial infections. Antagonists against such FAB I and their use as a therapeutic to treat infections, such as staphylococcal infections are also disclosed. Also disclosed are diagnostic assays for detecting diseases related to the presence of FAB I nucleic acid sequences and the polypeptides in a host. Also disclosed are diagnostic assays for detecting polynucleotides encoding FAB I and for detecting the polypeptide in a host.
Owner:AFFINIUM PHARMA
Triggered cargo release from nanoparticle stabilized liposomes
Owner:RGT UNIV OF CALIFORNIA
Nosocomial infection multiple causative agent parallel detection gene chip and preparation method and application thereof
ActiveCN103255216AMicrobiological testing/measurementMicroorganism based processesAerobacter cloacaeEnterococcus faecalis
The invention belongs to the technical fields of molecular biological techniques and clinical tests, and particularly discloses a nosocomial infection multiple causative agent parallel detection gene chip and a preparation method and application thereof. The gene chip can be used for rapidly and sensitively detecting fourteen medical treatment infection typical causative agents such as acinetobacter baumannii, enterobacter cloacae, enterococcus faecalis and the like by using one reaction, and the detection data of coinfection of yeast and bacterium which cannot be provided by a traditional amplification method is offered. Furthermore, the gene chip also comprises a methicillin resistance target spot, thus realizing further detection of staphy lococcus infection, therefore, a clinician can realize which causative agent a patient is infected in one or two hours, and realize whether the patient has drug resistance, so as to decide whether the patient needs antibiotics or which antibiotics is needed, so that the gene chip has a greater clinical guiding significance.
Owner:SHANDONG ACV BIOTECH CO LTD
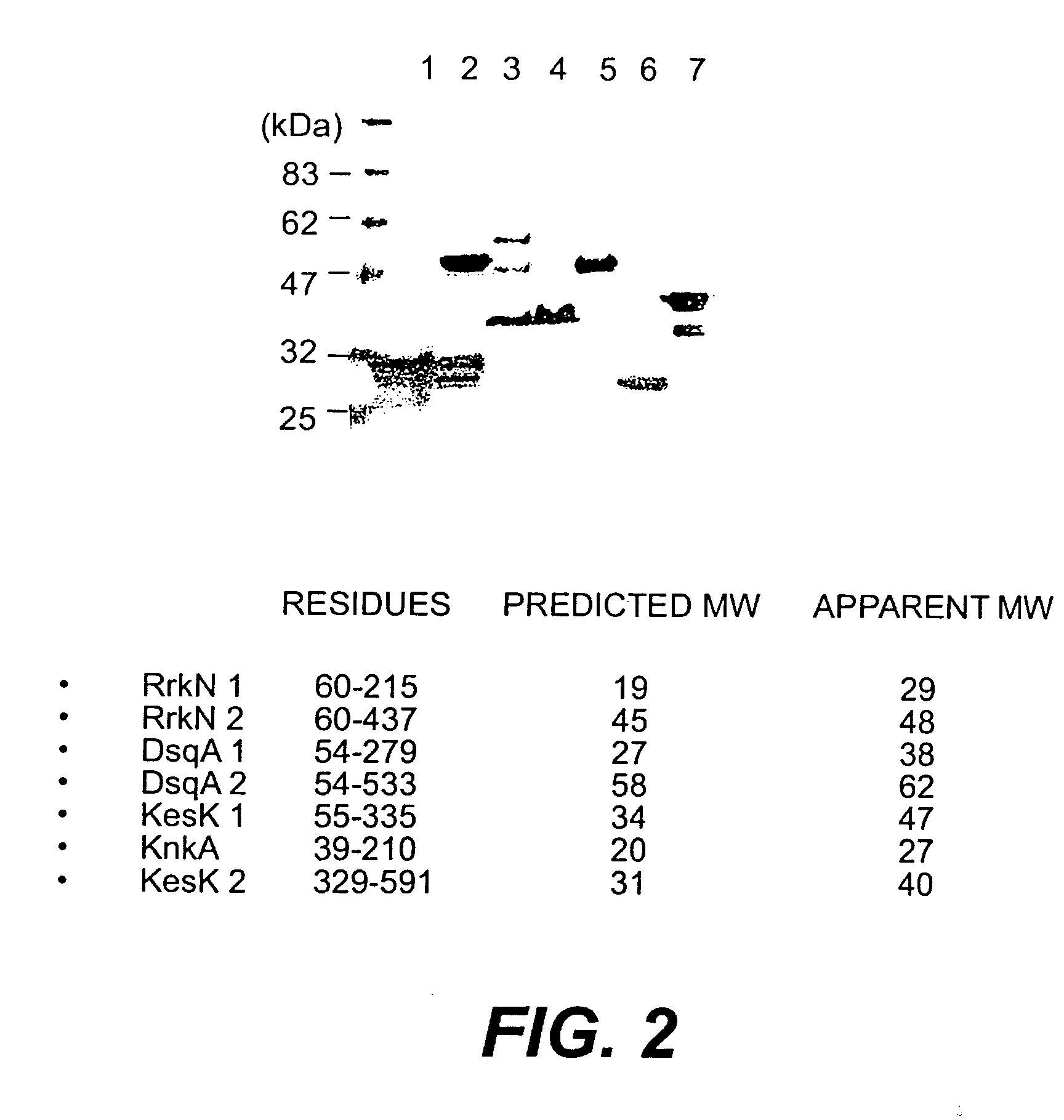
52 kda protein from coagulase negative staphylococci and fragments thereof
InactiveUS20030082200A1Inhibit bindingAntibacterial agentsBacteriaPassive ImmunizationsProtein isolate
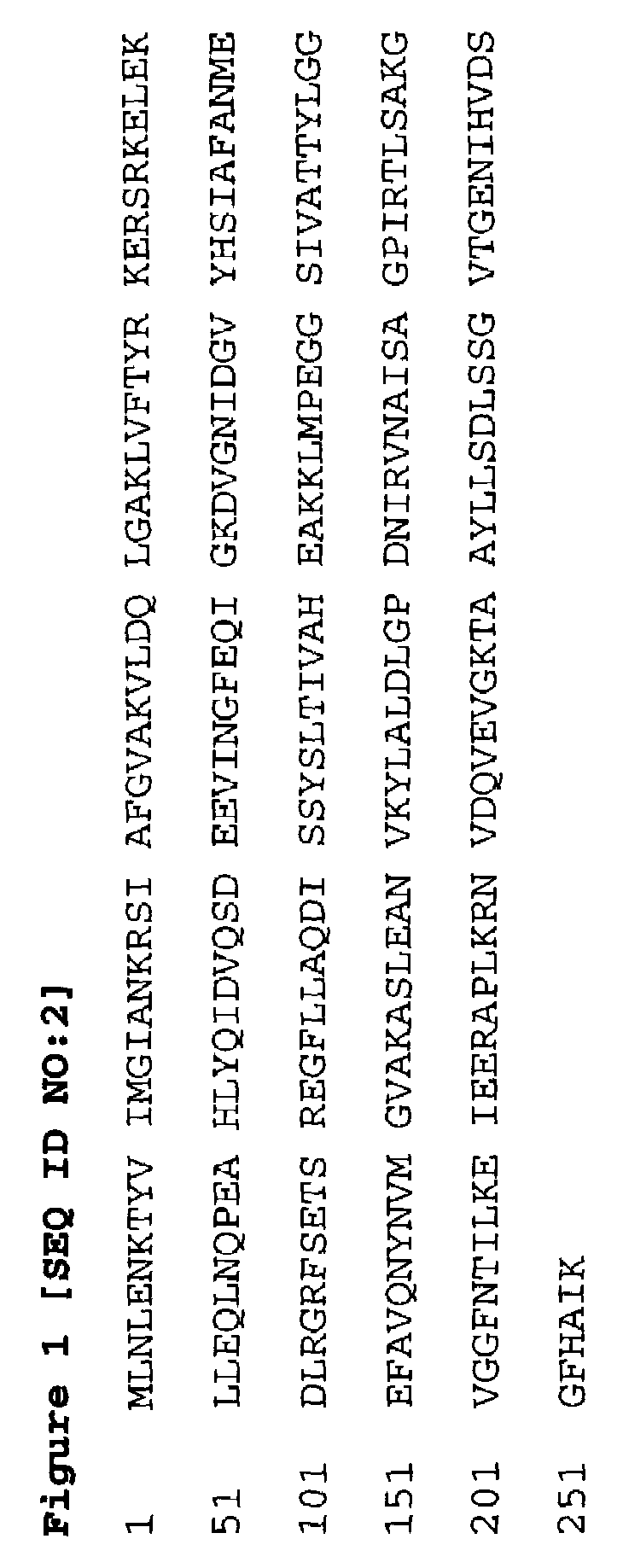
A protein isolated from Staphylococcus epidermidis having an approximate MW of 52 kD determined by SDS-PAGE and an N-terminal amino acid sequence (SEQ ID NO:1), and antigenic determinant-containing fragments of the protein, optionally coupled to an inert carrier or matrix, are disclosed. Disclosed are also a recombinant DNA molecule coding for the protein or the protein fragments; a vector comprising the DNA molecule or the corresponding RNA molecule; antibodies or antigen-binding peptides recognizing and specifically binding to the protein or protein fragment; use of the protein or protein fragment, or the vector, for the production of vaccines against Staphylococcal infections; use of the antibodies or antigen-binding peptides for the production of a medicament for passive immunization; a vaccine against Staphylococcal infections comprising the protein or protein fragment, or the vector, a medicament for passive immunization comprising the antibodies or antigen-binding peptides; and a method of prophylactic and / or therapeutic treatment of Staphylococcal infections.
Owner:BIOSTAPRO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com