Cross-reactive monoclonal and polyclonal antibodies which recognize surface proteins from coagulase-negative staphylococci and staphylococcus aureus
a staphylococcus, coagulase-negative technology, applied in the field of staphylococcus aureus surface proteins, can solve the problems of remained a significant problem in developing antibodies that exhibit cross-reactivity across the different types, and dramatically alter the physiology of the staphylococcus, so as to prevent or treat the infection of the staphylococcus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation and Sequencing of MSCRAMM's from S. Aureus
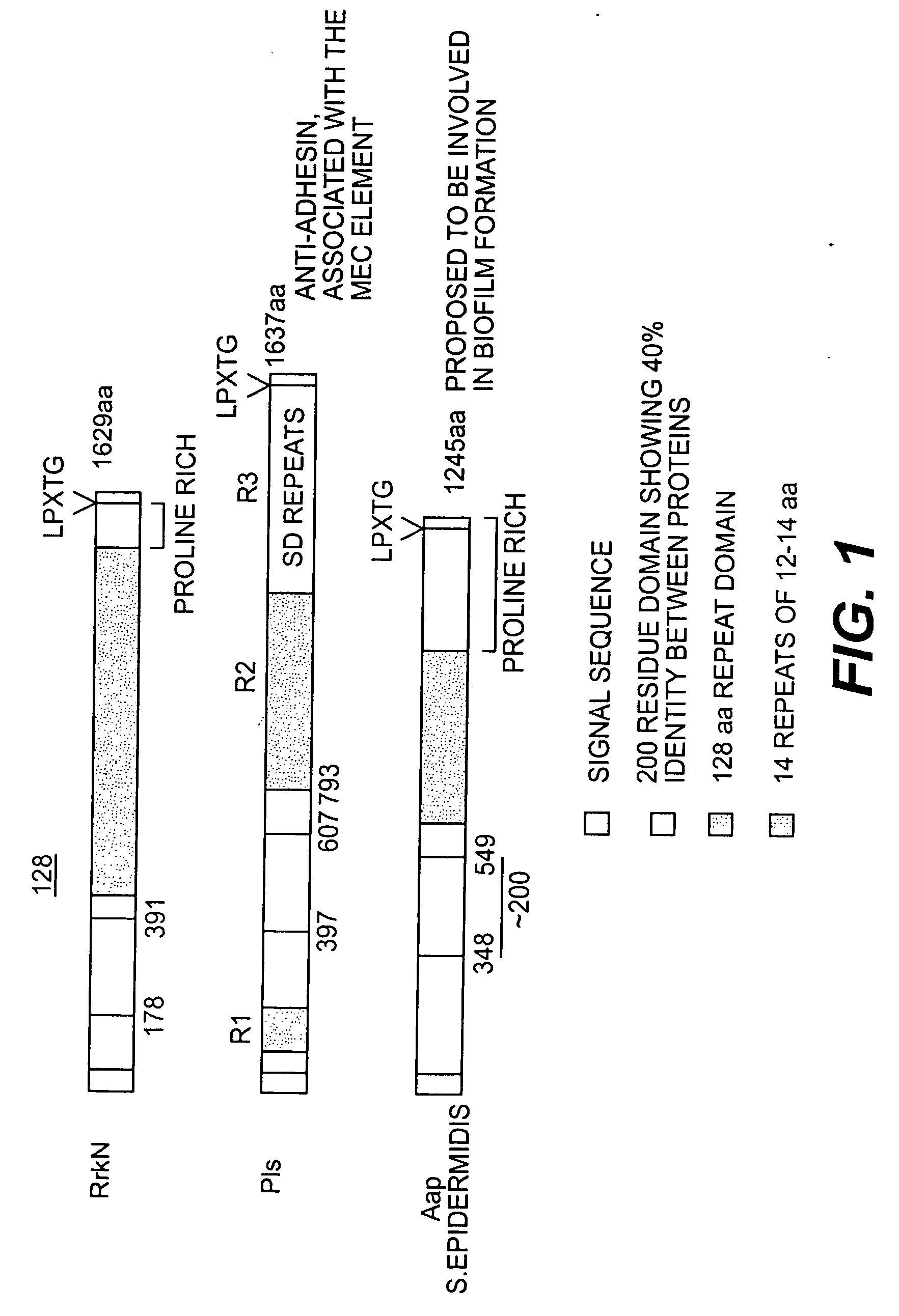
[0052]Staphylococcus aureus is known to express a class of surface-associated proteins which play important roles in pathogenicity by allowing bacteria to avoid host defenses and by acting as adhesins. These proteins are known as MSCRAMMs (Microbial Surface Components Recognizing Adhesive Matrix Molecules) and in most cases are covalently anchored to the cell wall peptidoglycan. They have several common features: (i) an N-terminal signal peptide (approximately 40 residues in length) required for Sec-dependent secretion, (ii) a wall spanning domain either rich in proline and glycine residues or composed of serine and aspartate dipeptide repeats, (iii) an LPXTG motif required for covalent anchoring of the protein to the pentaglycine crossbridge in peptidoglycan, (iv) a hydrophobic membrane-spanning domain followed by (v) several positively charged residues.
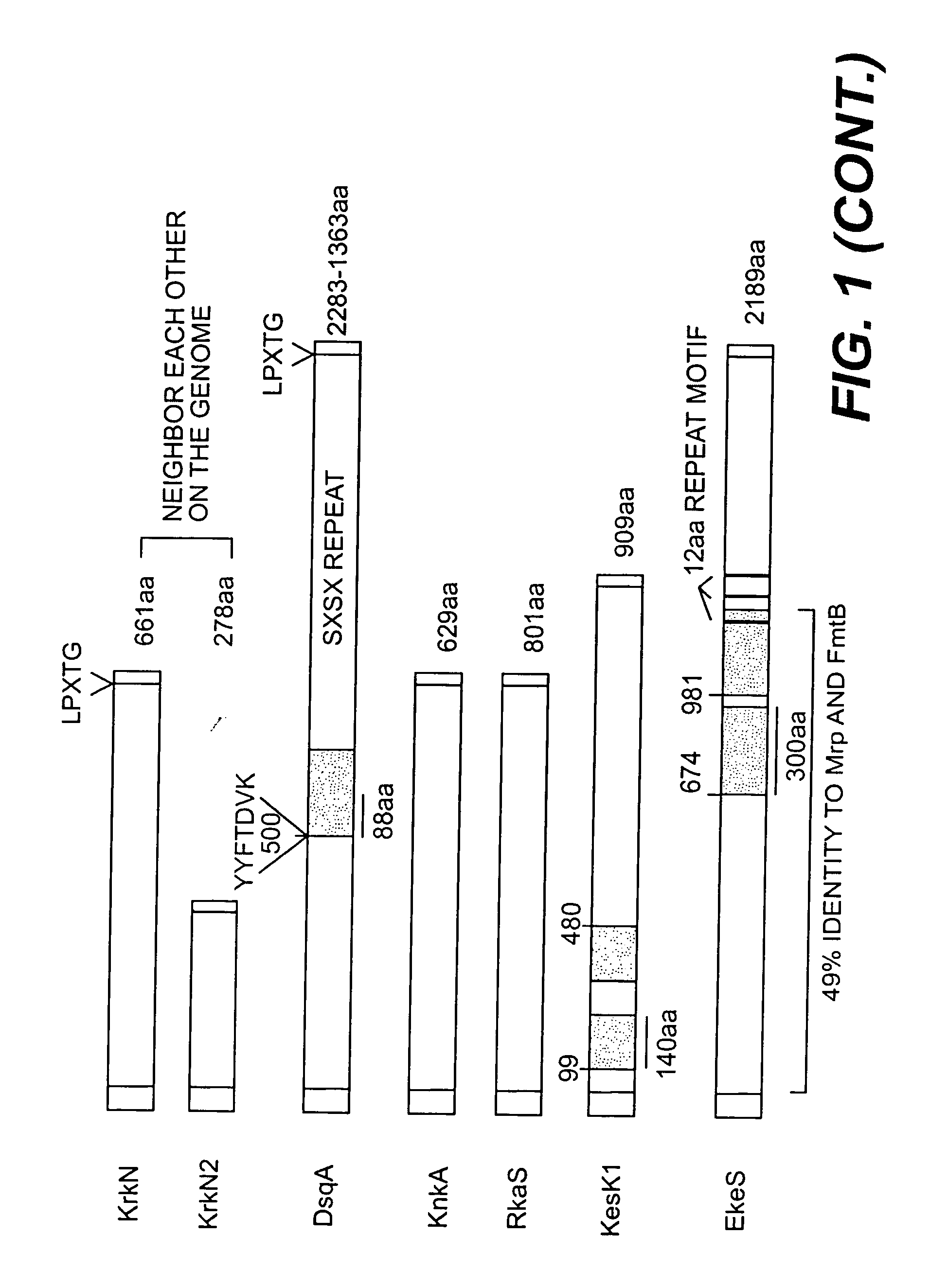
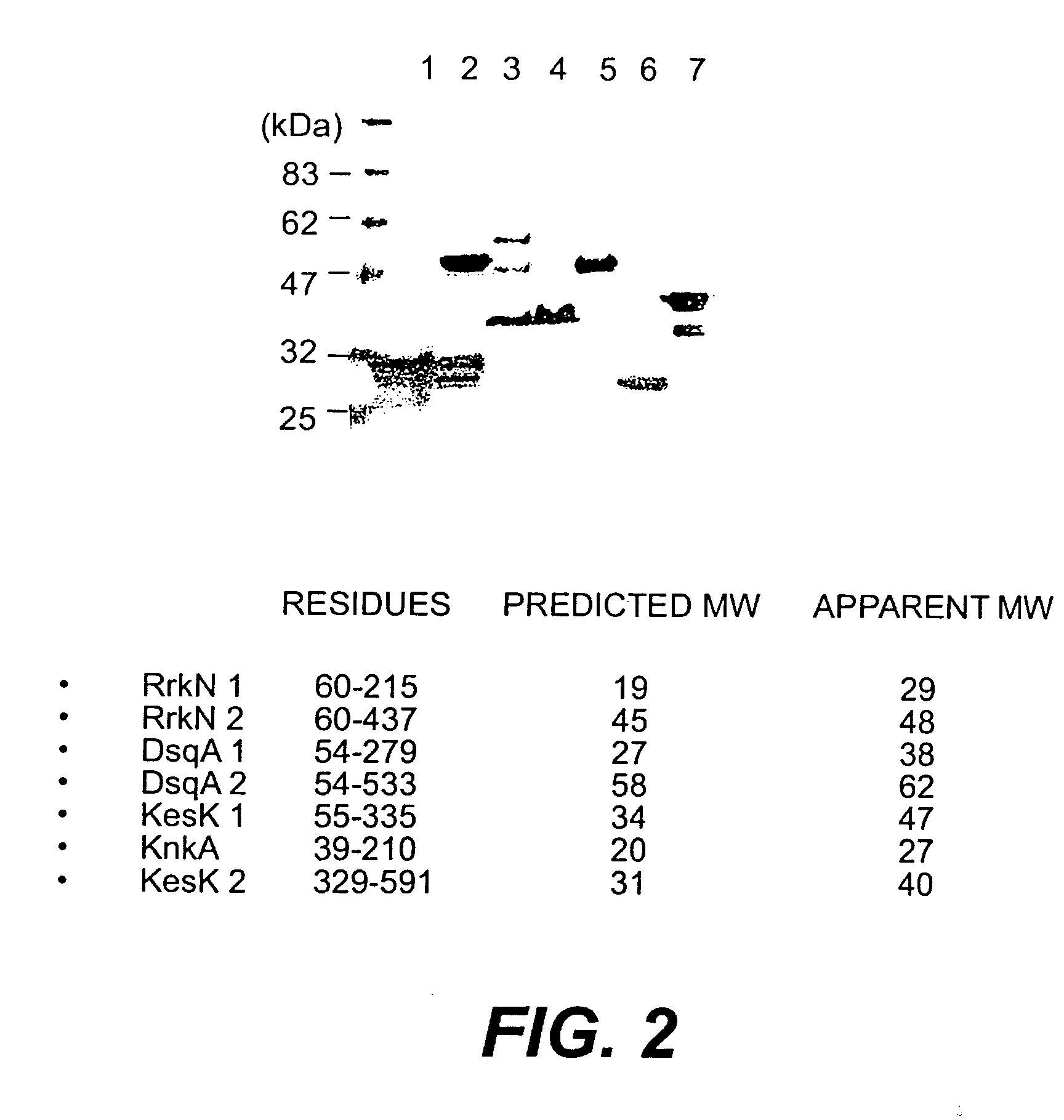
[0053] By exploiting the whole genome sequences of S. aureus, eight novel op...
example 2
Isolation and Sequencing of Cross-Reactive Proteins from S. Aureus and from Coagulase-Negative Staphylococci
[0072] It has been recently shown that S. epidermidis contains surface proteins structurally related to S. aureus MSCRAMM® proteins (U.S. Ser. No. 09 / 386,962). One protein from S. aureus is of particular interest since it has a close homologue in S. epidermidis. The protein is called DsqA or SasA (S. aureus) and DgsK (S. epidermidis). They are characterized by a typical “A” domain of approximately 500 amino acid residues, followed by two B repeats of 88 residues that are ˜40% identical, and a unique SXSX dipeptide repeat that can vary in length depending on the strain. Contained within the A domain of the S. aureus DsqA / SasA is a 180 residue region that has ˜40% identity to a similar sized domain within region A of S. aureus proteins RrkN, Pls and S. epidermidis protein Aap The A regions of the DsqA / SasA and DgsK proteins are 46% identical at the amino acid level, the BB repe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical density | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com