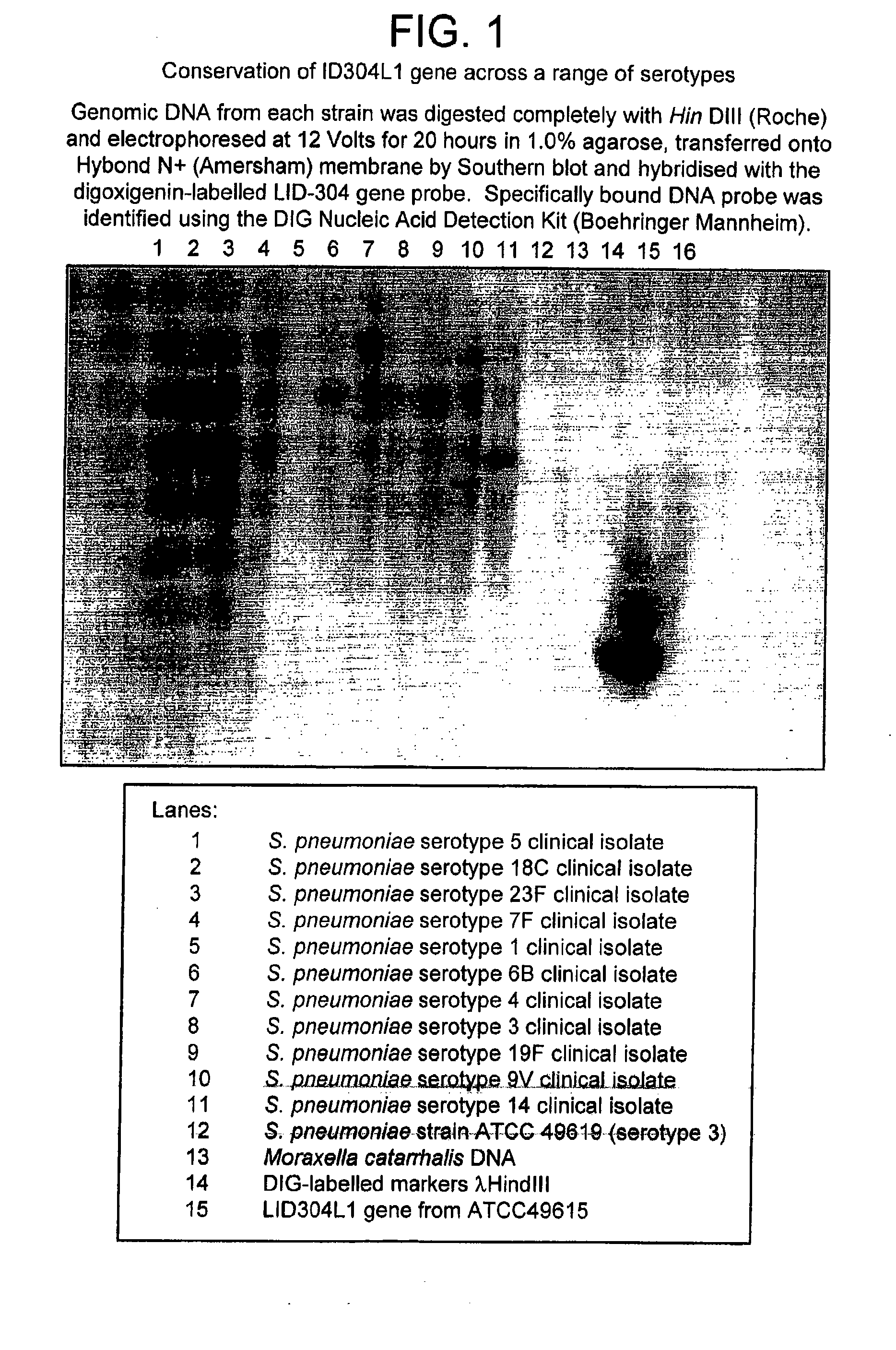
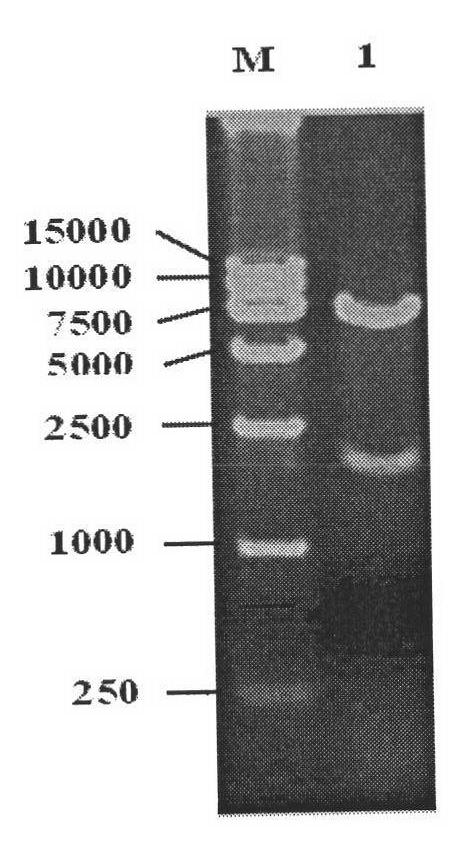
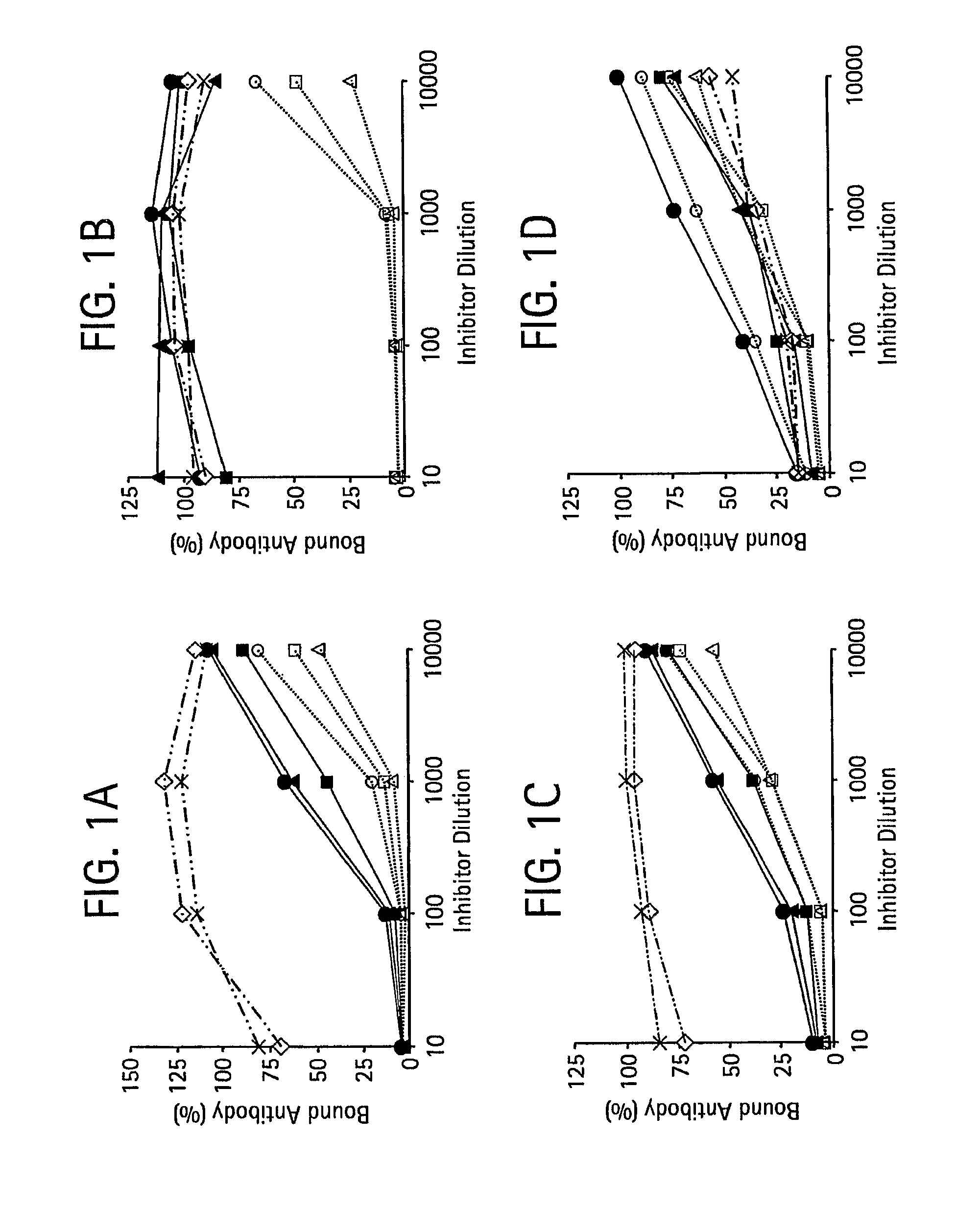
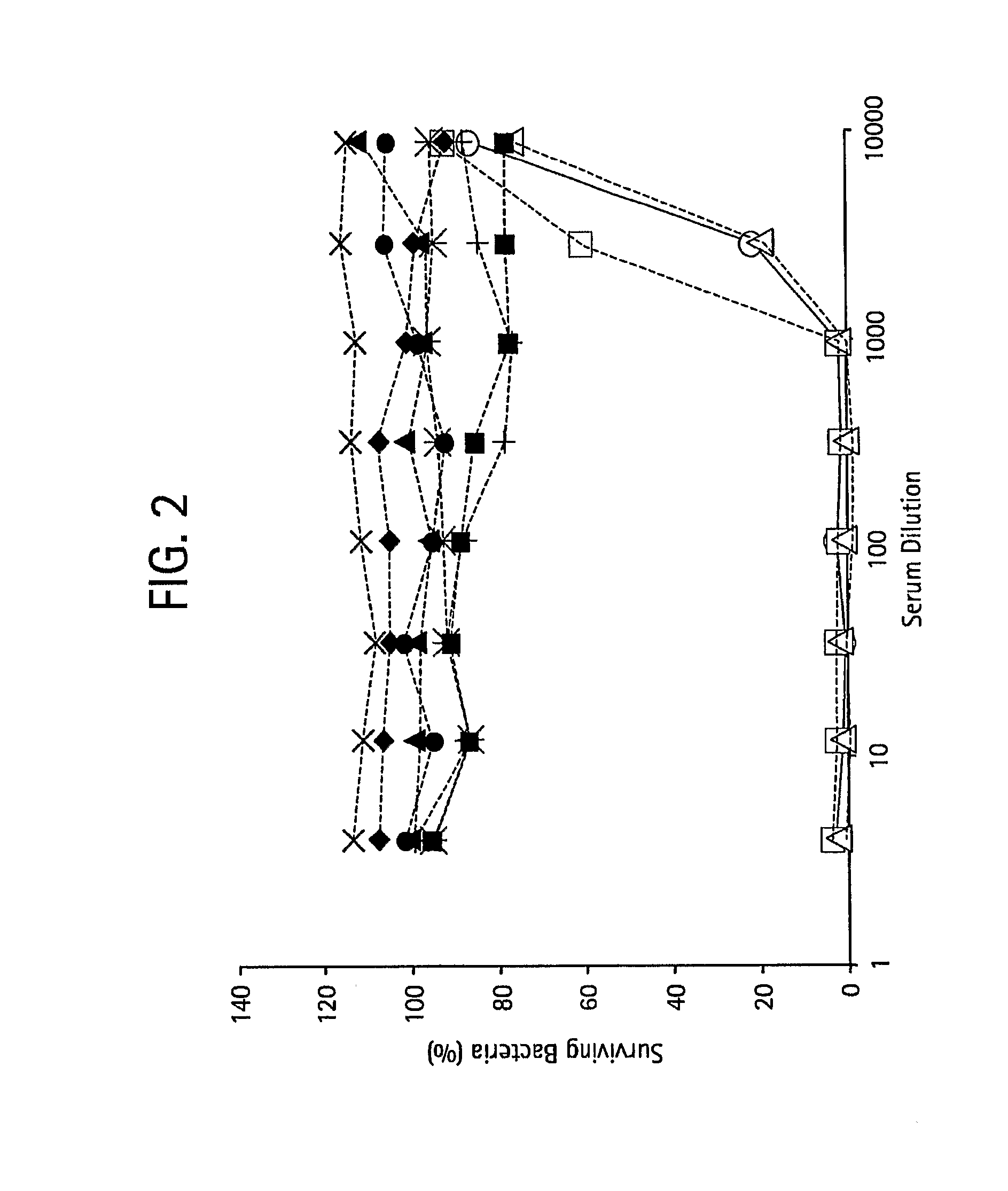
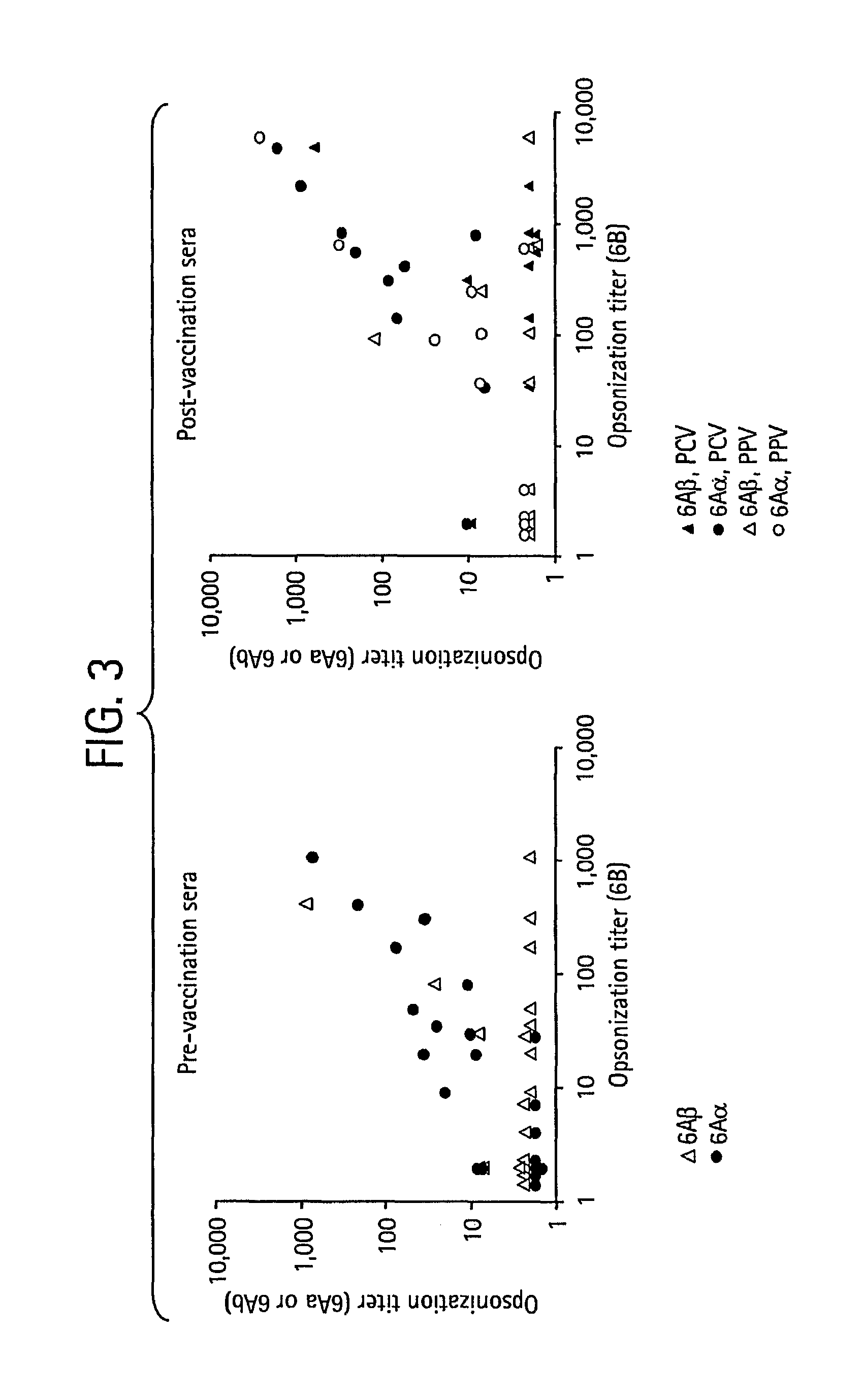
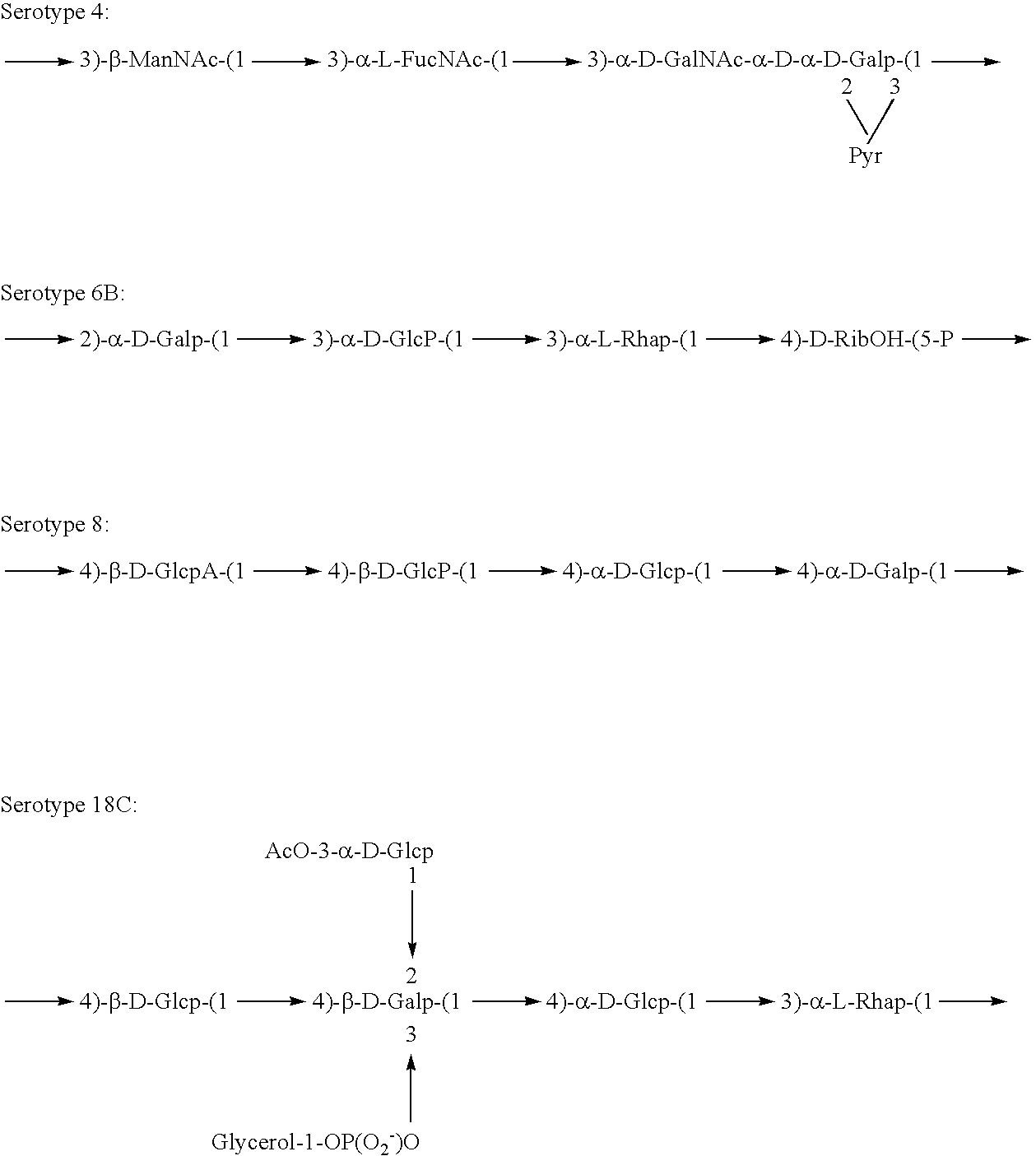Patents
Literature
152 results about "Streptococcus sobrinus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
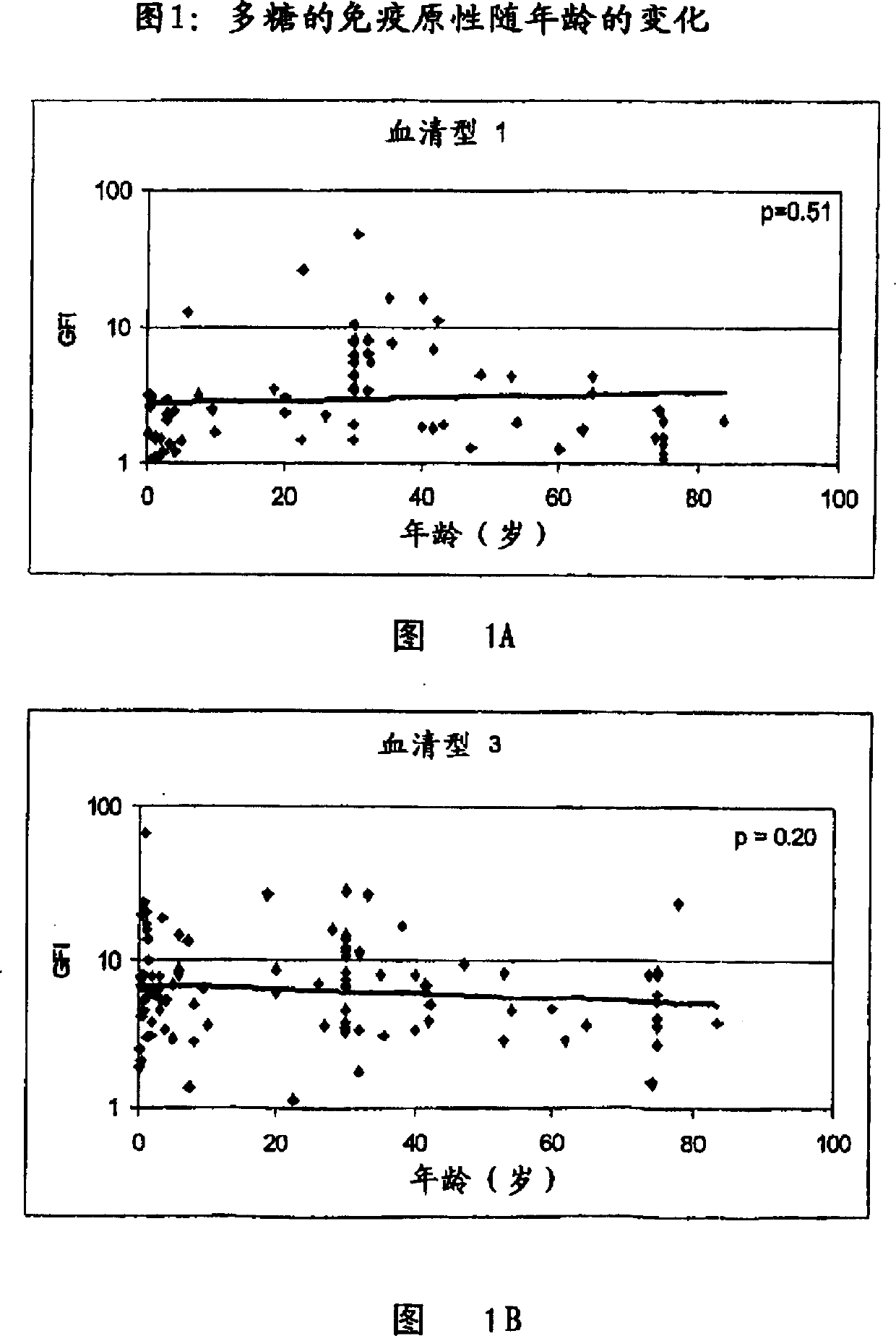
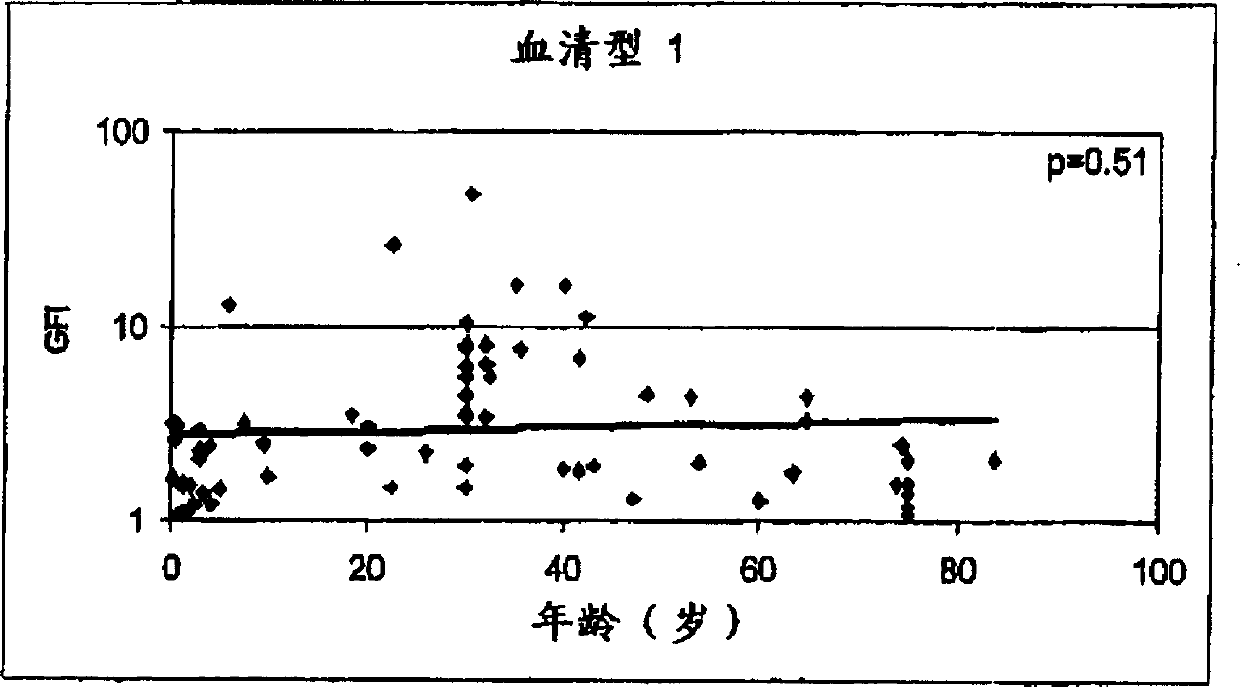
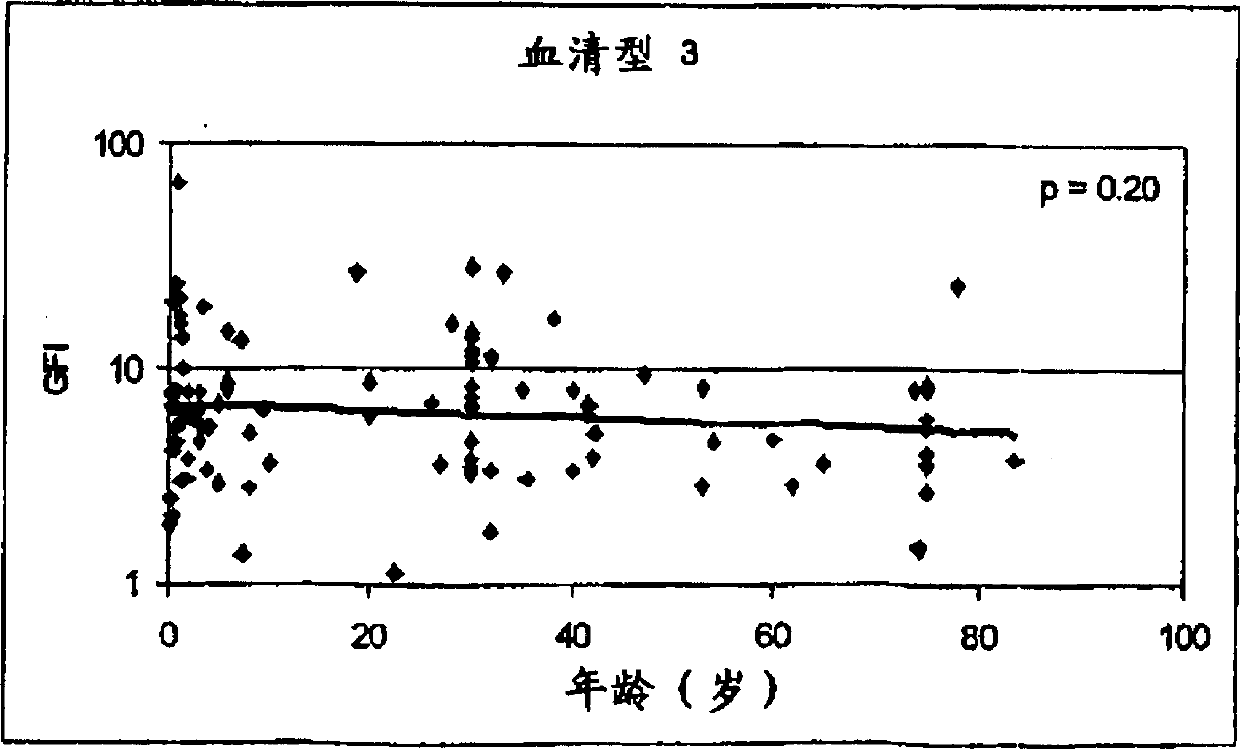
Streptococcus sobrinus is a Gram-positive, catalase-negative, non-motile, and anaerobic member of the genus Streptococcus.
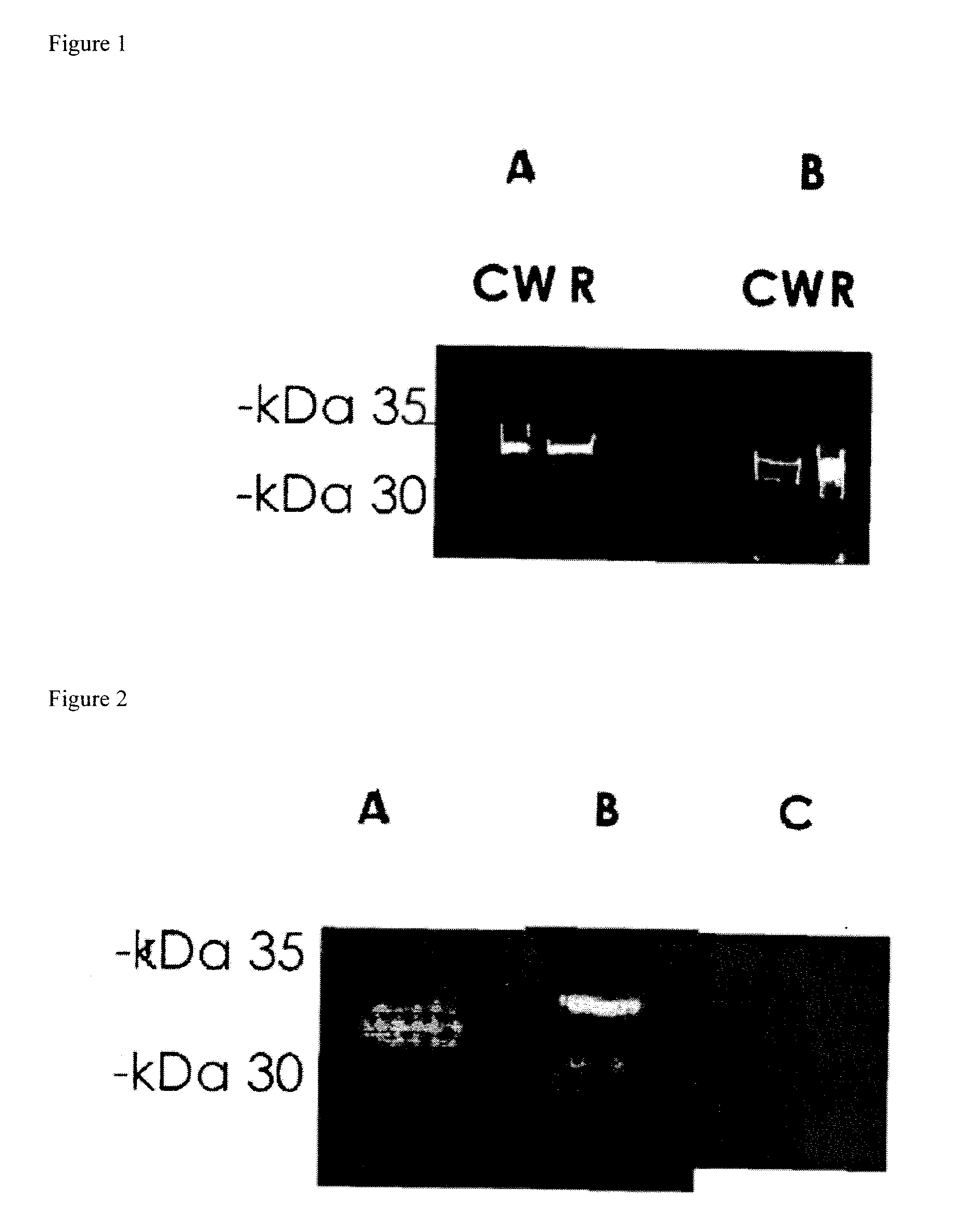
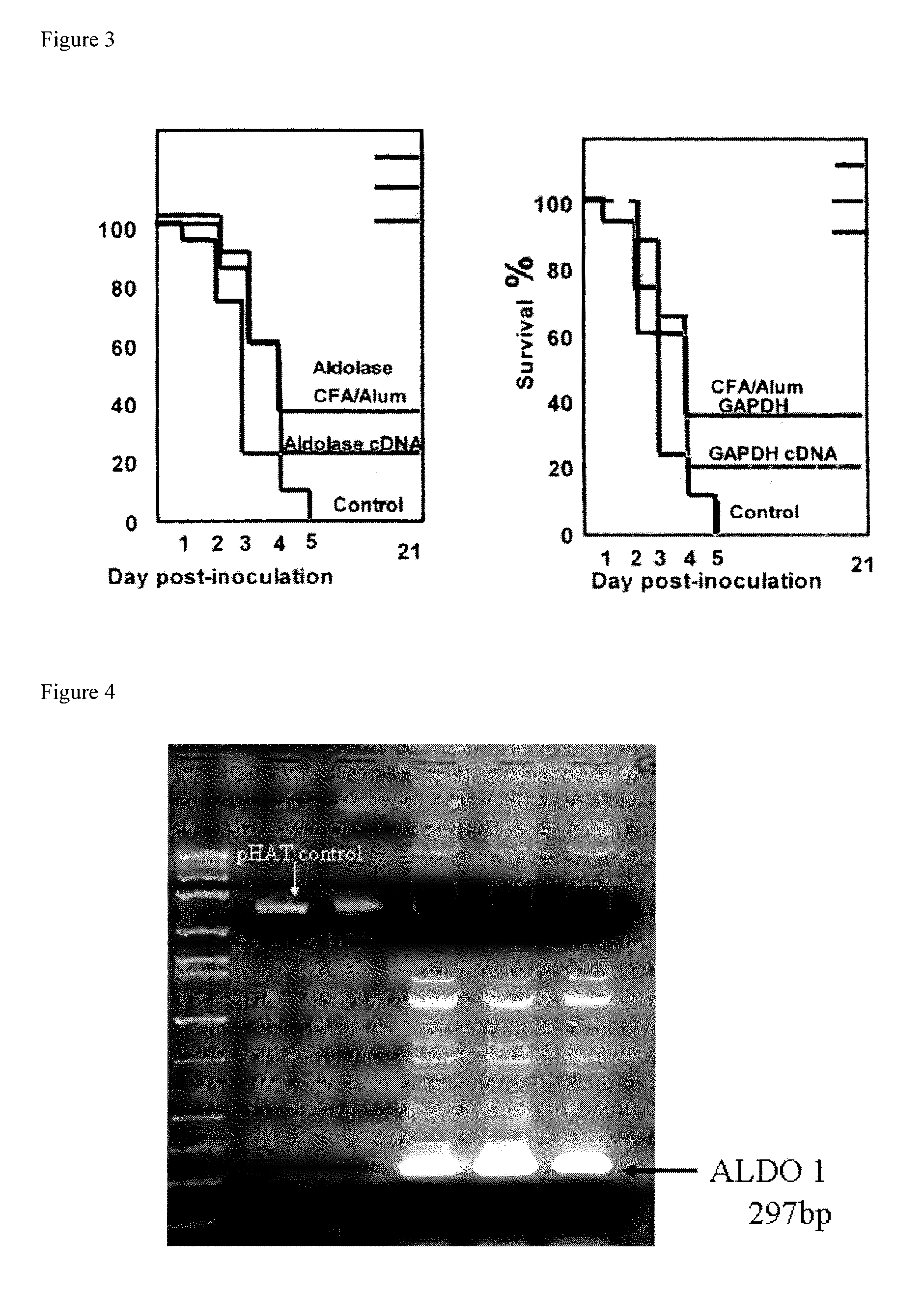
Streptococcus pneumoniae 37-kDa surface adhesin a protein
The invention provides a nucleic acid encoding the 37-kDa protein from Streptococcus pneumoniae. Also provided are isolated nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. The invention also provides purified polypeptides encoded by the nucleic acid encoding the 37-kDa protein from and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Also provided are antibodies which selectively binds the polypeptides encoded by the nucleic acid encoding the 37-kDa protein and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Also provided are vaccines comprising immunogenic polypeptides encoded by the nucleic acid encoding the 37-kDa protein and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Further provided is a method of detecting the presence of Streptococcus pneumoniae in a sample comprising the steps of contacting a sample suspected of containing Streptococcus pneumoniae with nucleic acid primers capable of hybridizing to a nucleic acid comprising a portion of the nucleic acid encoding the 37-kDa protein, amplifying the nucleic acid and detecting the presence of an amplification product, the presence of the amplification product indicating the presence of Streptococcus pneumoniae in the sample. Further provided are methods of detecting the presence of Streptococcus pneumoniae in a sample using antibodies or antigens, methods of preventing and treating Streptococcus pneumoniae infection in a subject.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
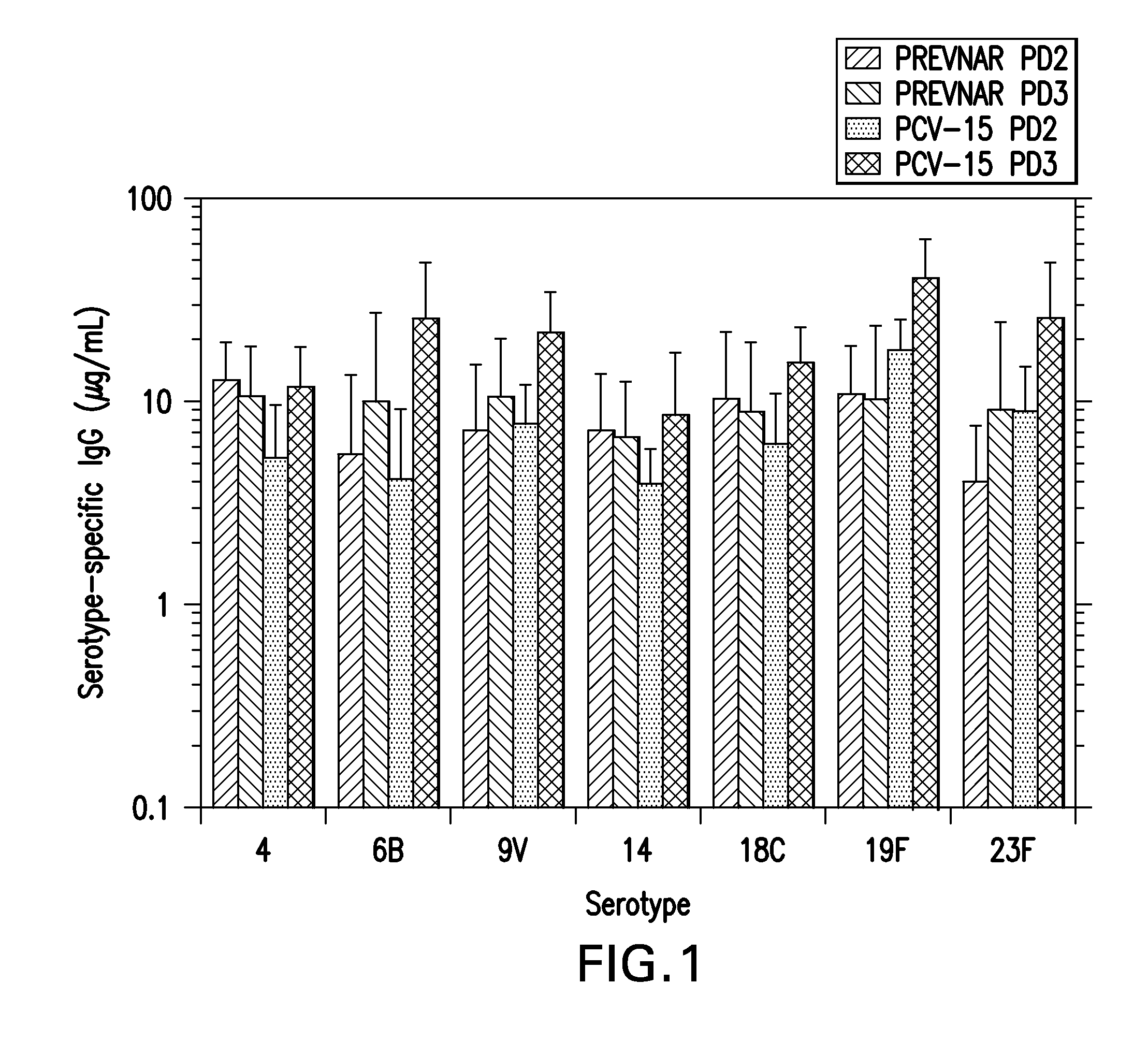
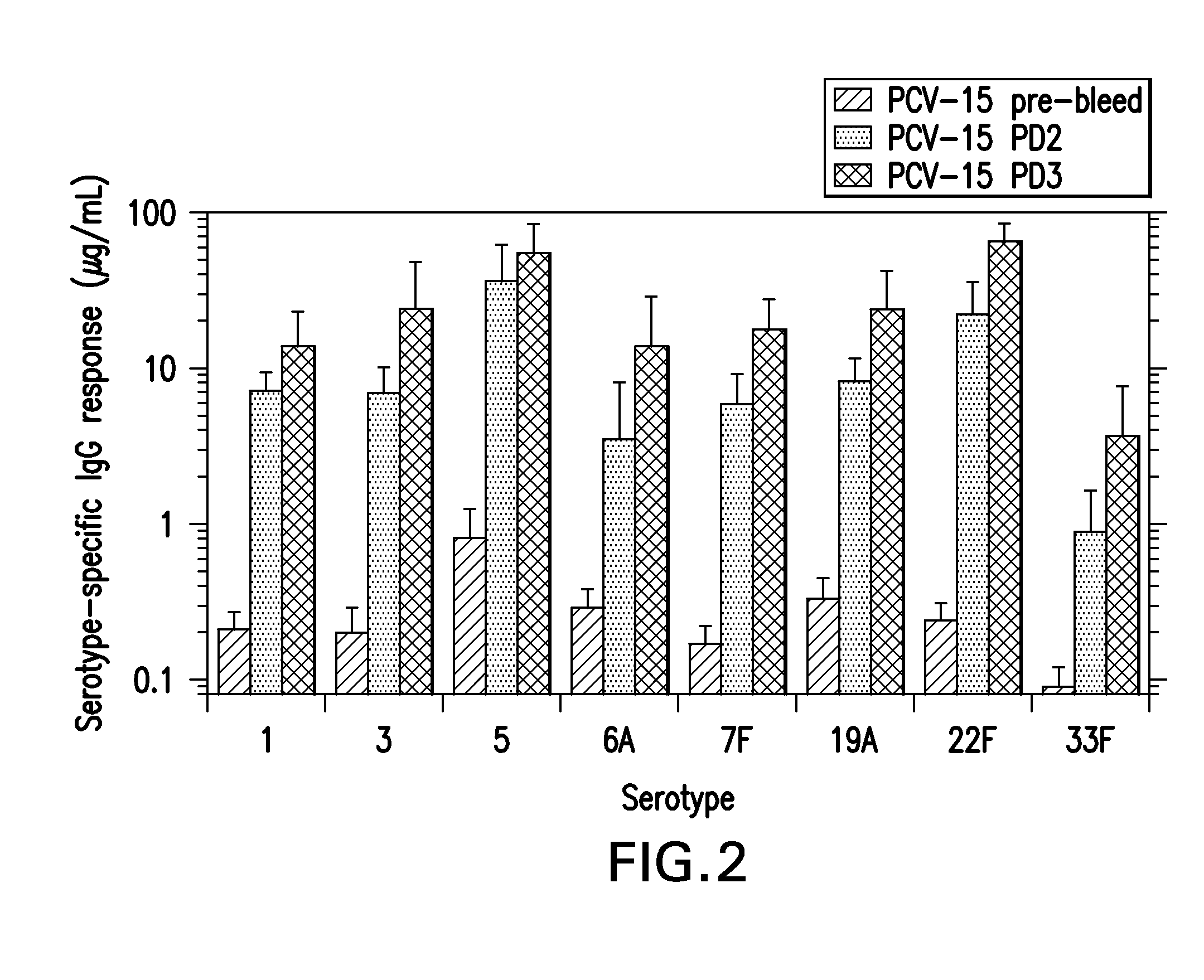
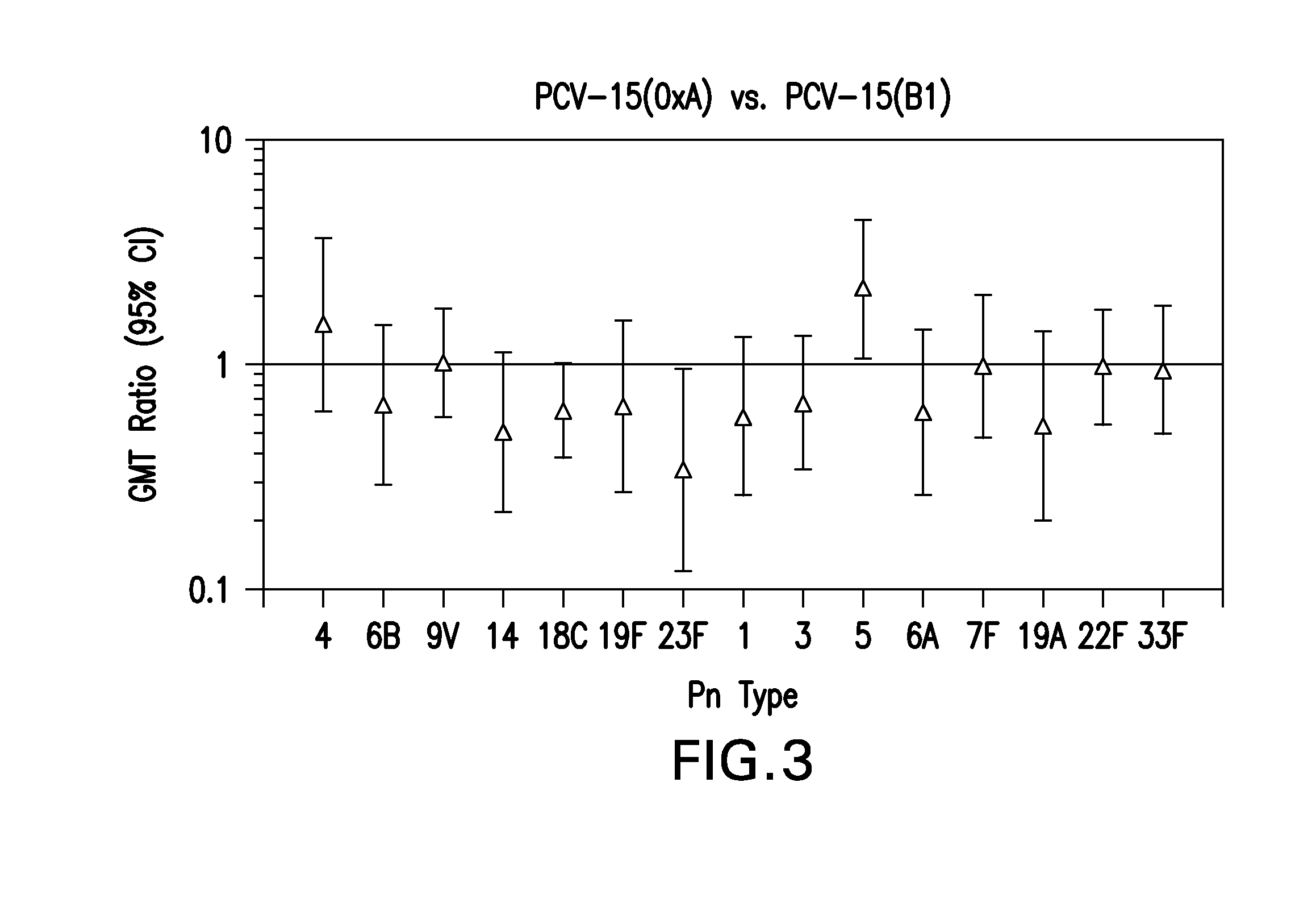
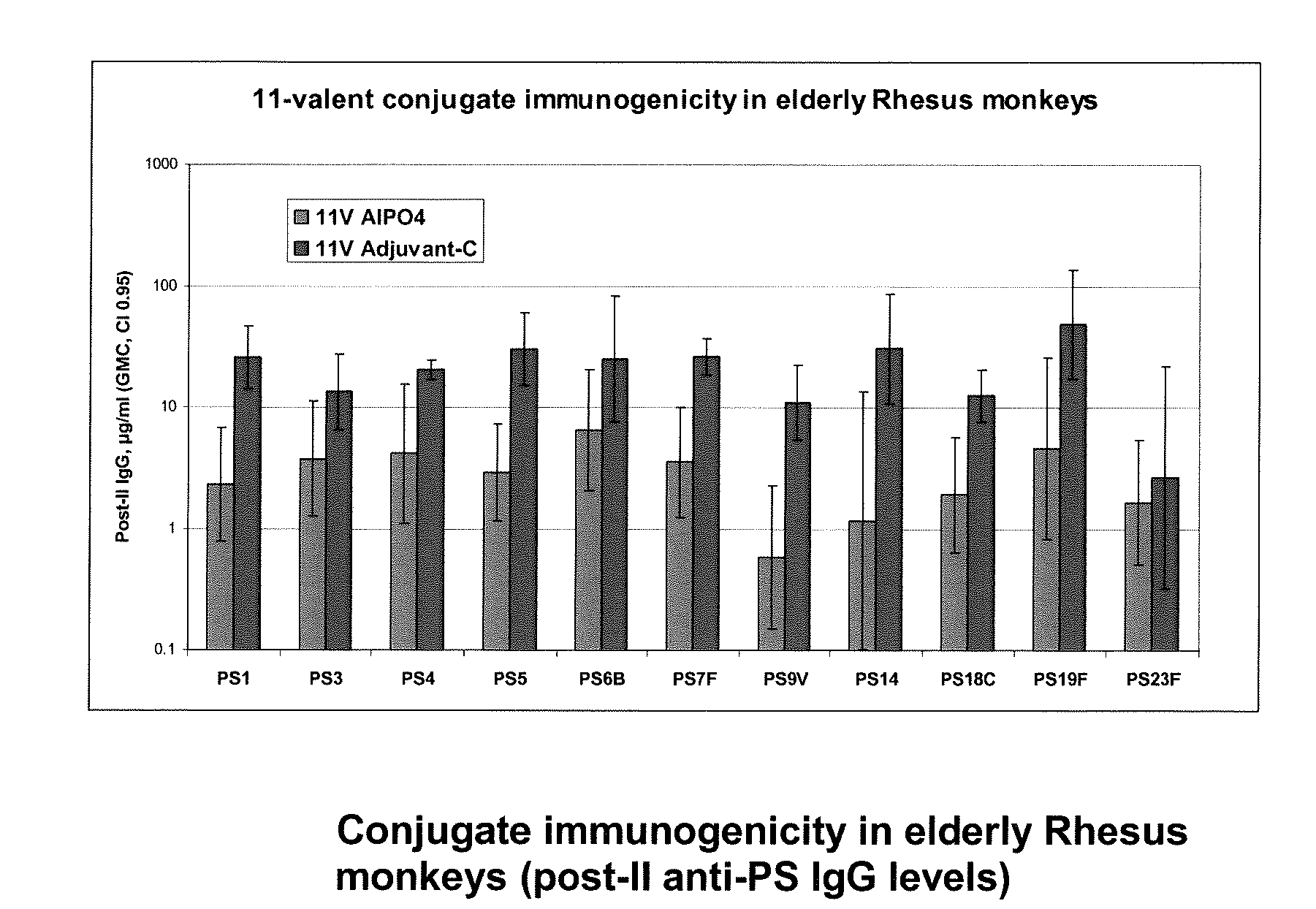
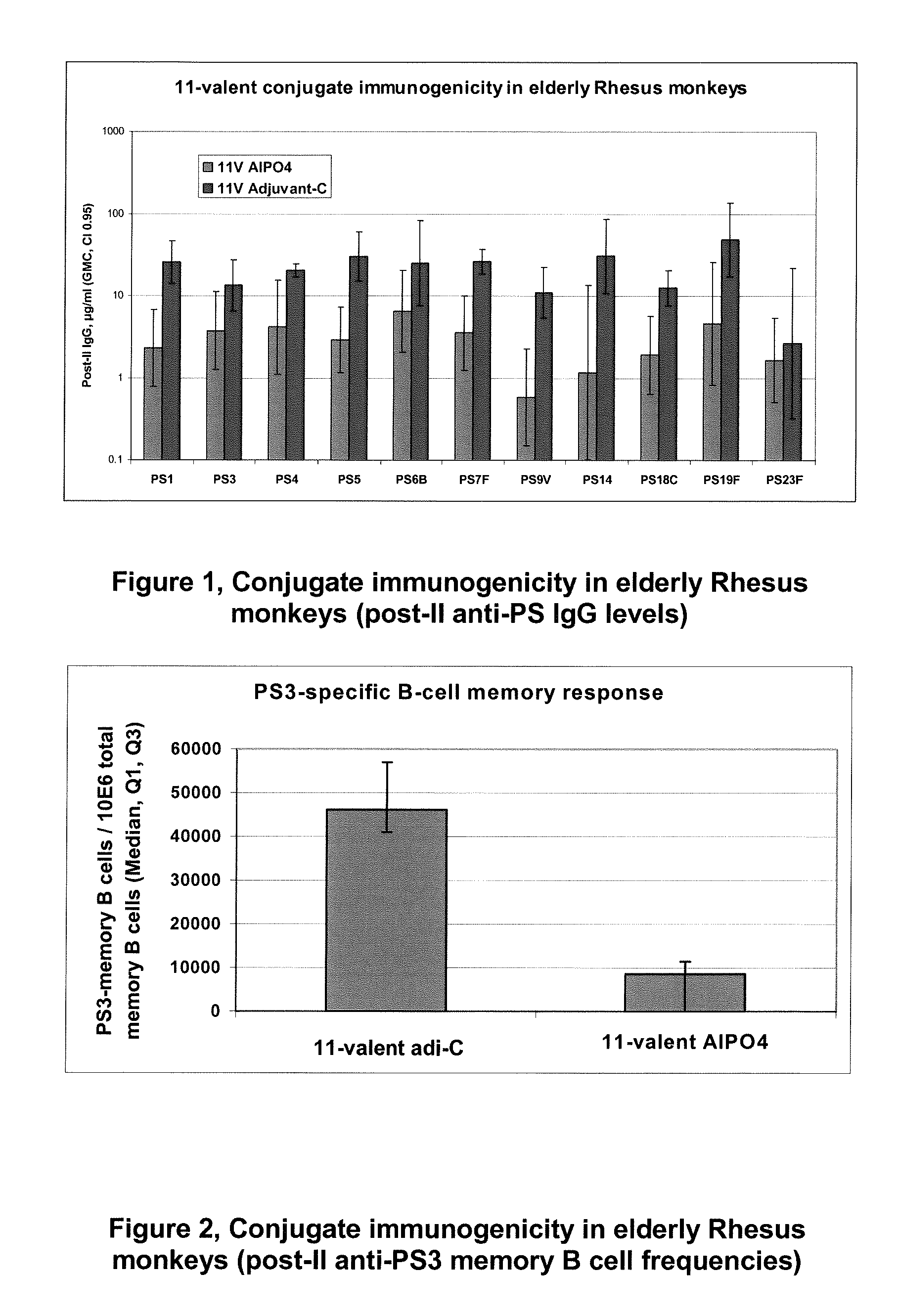
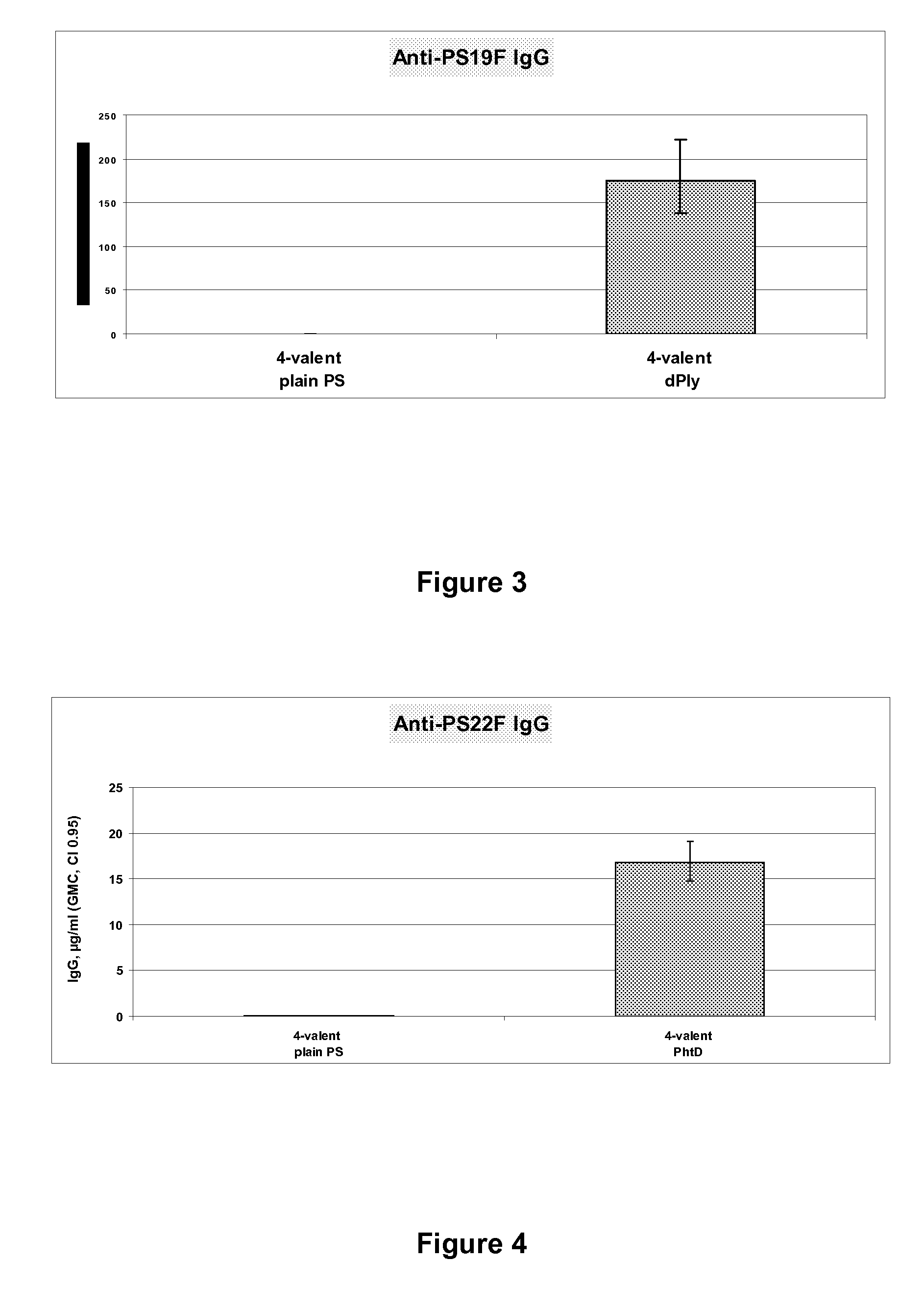
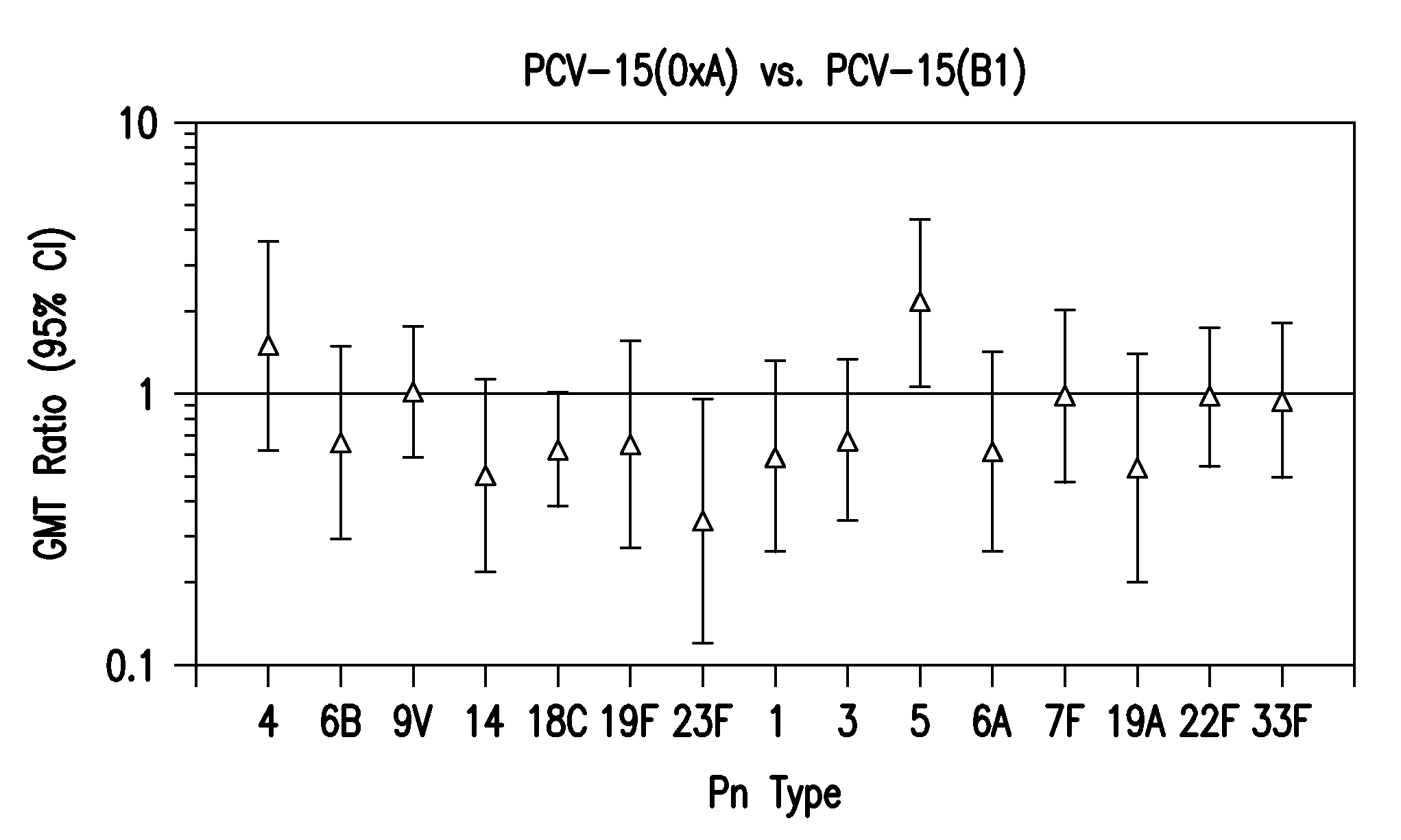
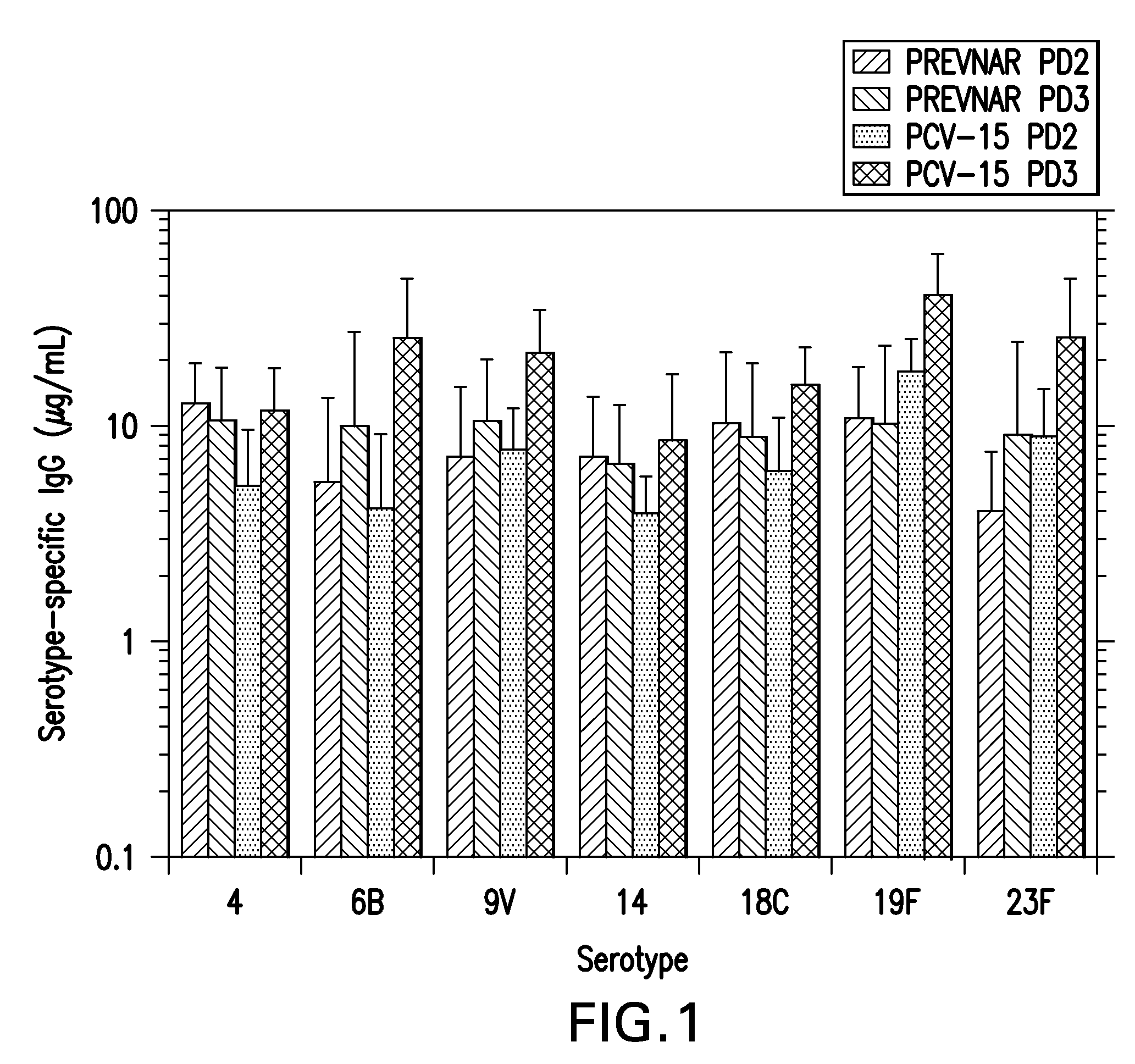
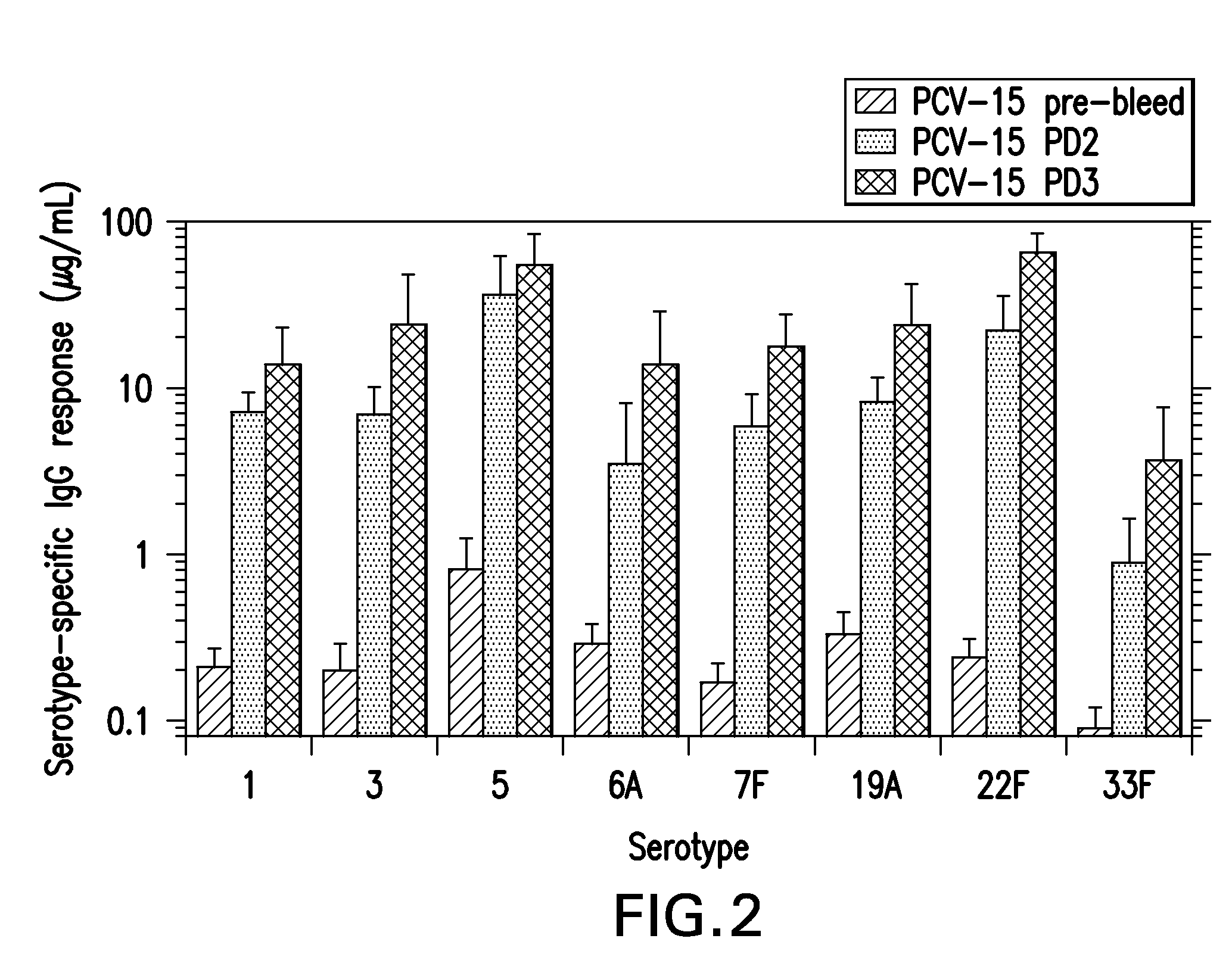
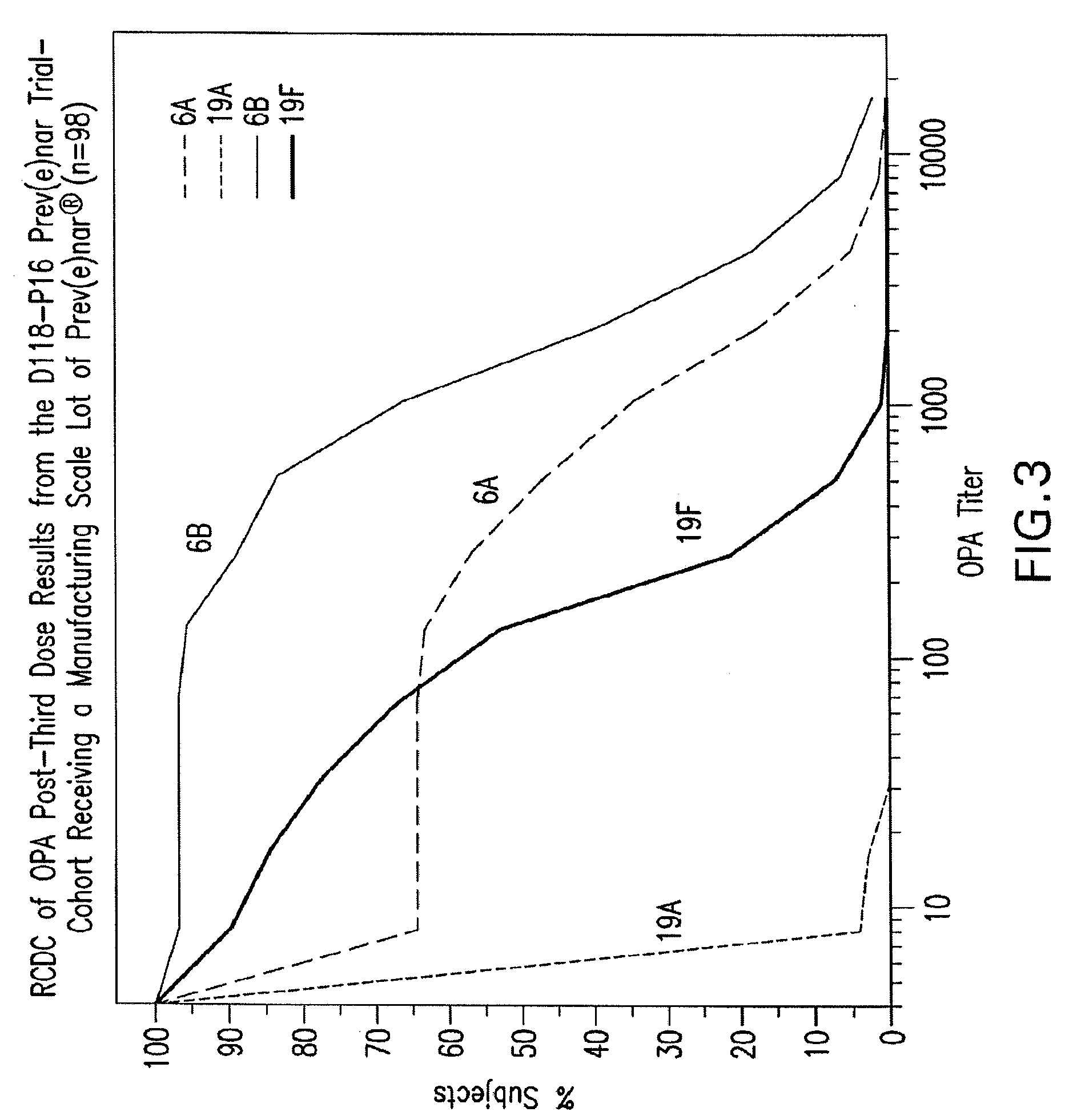
15-valent pneumococcal polysaccharide-protein conjugate vaccine composition
ActiveUS20110195086A1Antibacterial agentsBacteria material medical ingredientsConjugate vaccineDisease
The present invention provides a multivalent immunogenic composition having 15 distinct polysaccharide-protein conjugates. Each conjugate consists of a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F or 33F) conjugated to a carrier protein, preferably CRM197. The immunogenic composition, preferably formulated as a vaccine on an aluminum-based adjuvant, provides broad coverage against pneumococcal disease, particularly in infants and young children.
Owner:MERCK SHARP & DOHME LLC
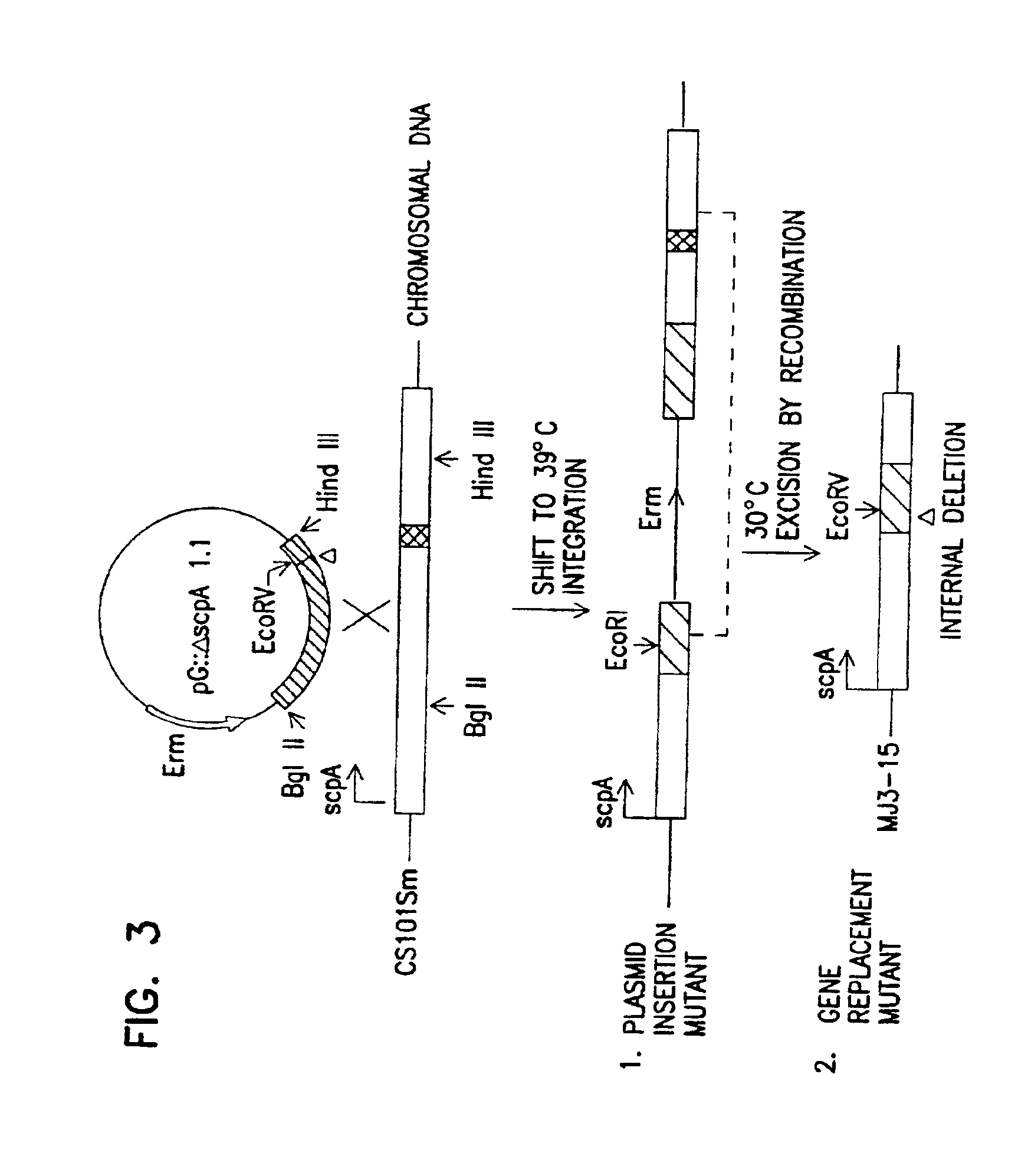
Streptococcal C5a peptidase vaccine
Novel vaccines for use against β-hemolytic Streptococcus colonization or infection are disclosed. The vaccines contain an immunogenic amount of a variant of streptococcal C5a peptidase (SCP). Also disclosed is a method of protecting a susceptible mammal against β-hemolytic Streptococcus colonization or infection by administering such a vaccine. Enzymatically inactive SCP, and polynucleotides encoding these SCP proteins are further disclosed.
Owner:RGT UNIV OF MINNESOTA
Pneumococcal Polysaccharide Conjugate Vaccine
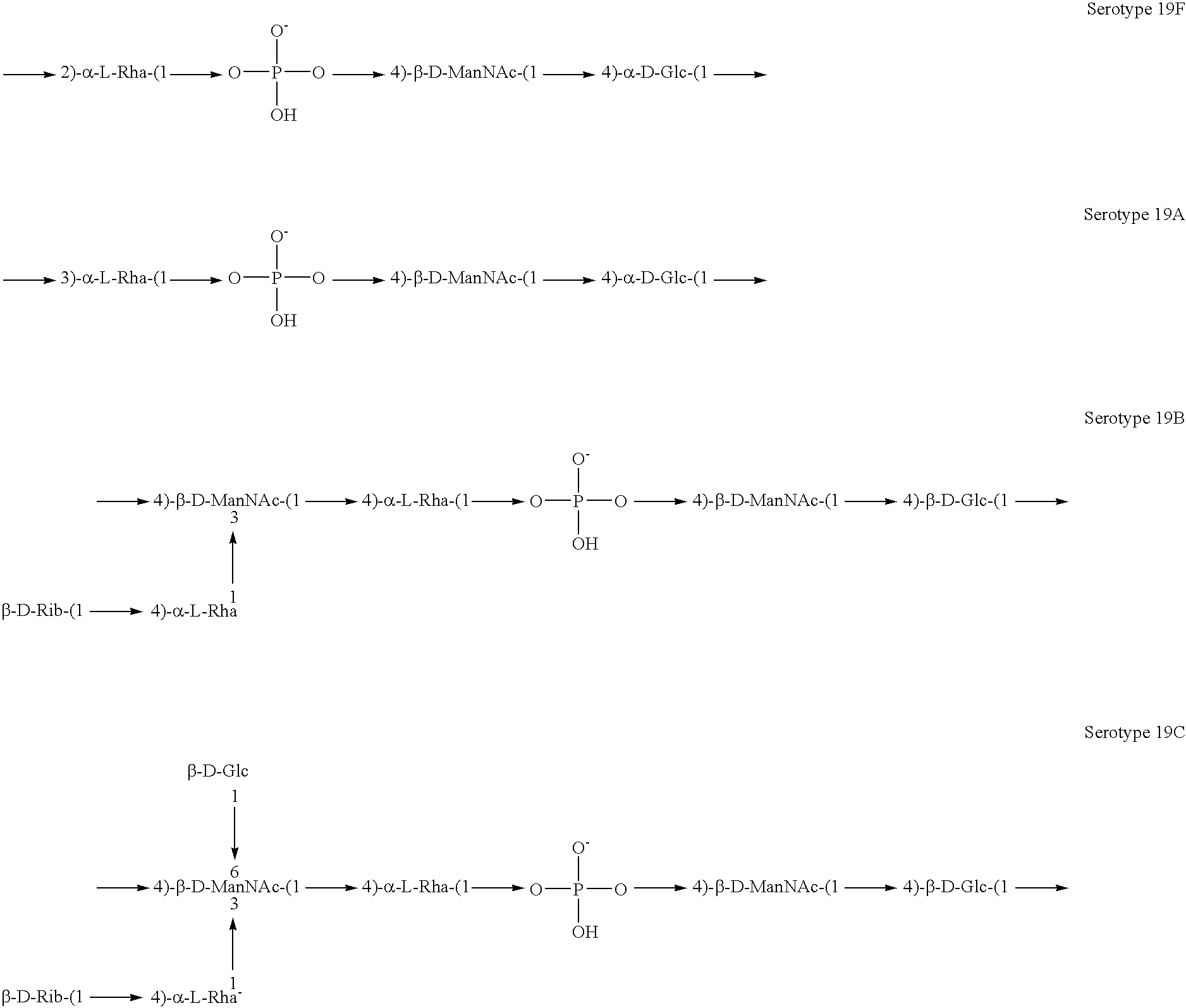
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various conjugated capsular saccharides from different S. pneumoniae serotypes conjugated to 2 or more different carrier proteins, where the composition comprises serotype 19F capsular saccharide conjugated to diphtheria toxoid (DT) or CRM197, optionally wherein 19F is the only saccharide in the composition conjugated to diphtheria toxoid (DT) or CRM197.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Multivalent pneumococcal polysaccharide-protein conjugate composition
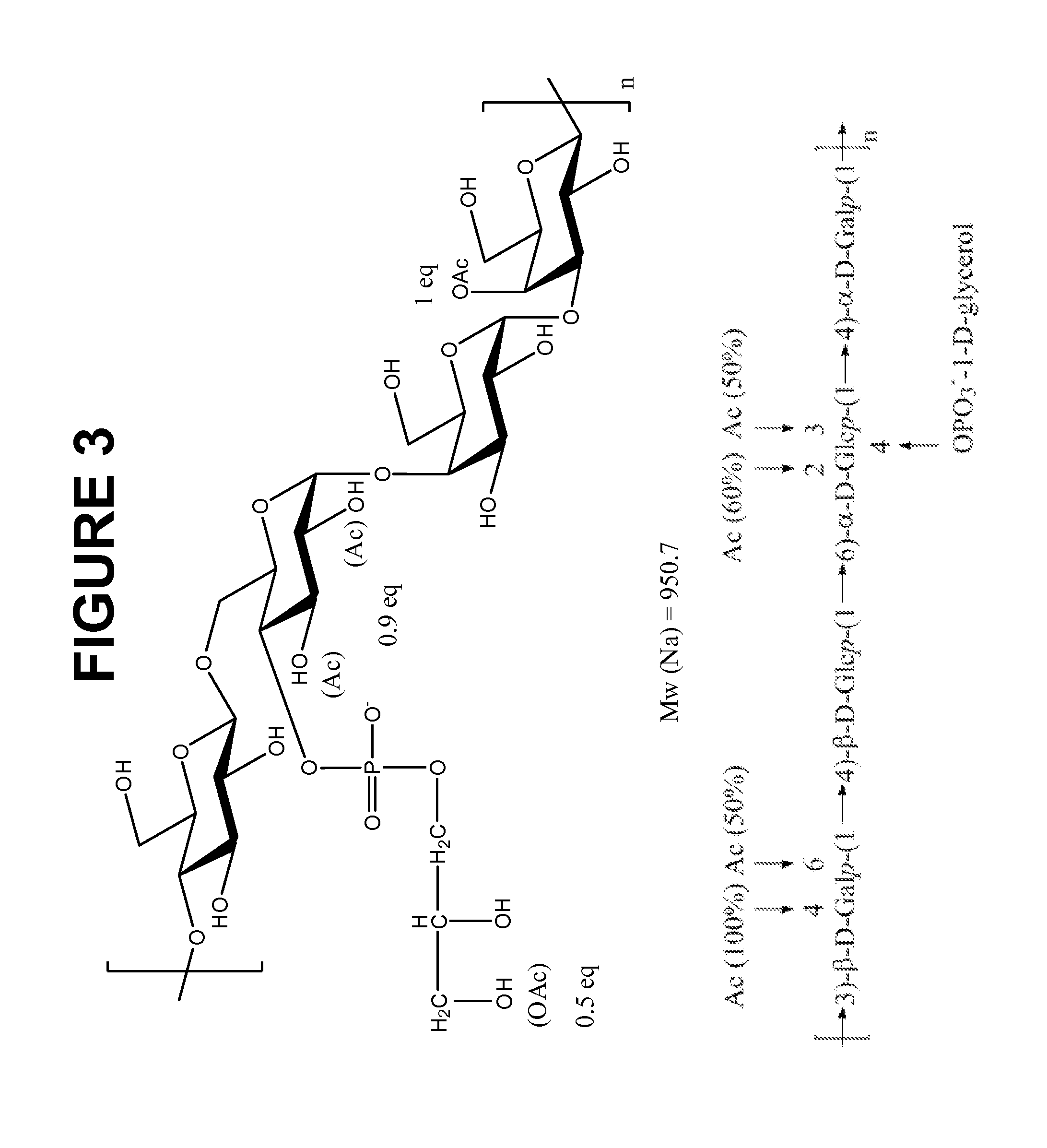
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 3 polysaccharide covalently linked to a carrier protein, the method including periodic acid oxidation of the polysaccharide in the presence of bivalent cations.
Owner:WYETH LLC
Immunogenic Compositions Comprising Conjugated Capsular Saccharide Antigens and Uses Thereof
ActiveUS20150202309A1Excellent characteristicsHigh yieldAntibacterial agentsMedical devicesStreptococcus pneumoniaeVaccination
The present invention relates to new immunogenic compositions comprising conjugated Streptococcus pneumoniae capsular saccharide antigens (glycoconjugates) and uses thereof. Immunogenic compositions of the present invention will typically comprise at least one glycoconjugate from a S. pneumoniae serotype not found in PREVNAR®, SYNFLORIX® and / or PREVNAR 13®. The invention also relates to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said novel immunogenic compositions.
Owner:PFIZER INC
Vaccine comprising streptococcus pneumoniae capsular polysaccharide conjugates
InactiveUS20100209450A1Antibacterial agentsSenses disorderConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various conjugated capsular saccharides from different S. pneumoniae serotypes conjugated to 2 or more different carrier proteins, where the composition comprises serotype 19F capsular saccharide conjugated to diphtheria toxoid. Methods of making and uses thereof are also described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
15-valent pneumococcal polysaccharide-protein conjugate vaccine composition
Owner:MERCK SHARP & DOHME LLC
Vaccine Comprising Streptococcus Pneumoniae Capsular Polysaccharide Conjugates
InactiveUS20090017059A1Antibacterial agentsSenses disorderStreptococcus pneumoniae capsular polysaccharideStreptococcus mitis
The present invention discloses an immunogenic composition comprising S. pneumoniae capsular saccharide conjugates from serotypes 19A and 19F wherein 19A is conjugated to a first bacterial toxoid and 19F is conjugated to a second bacterial toxoid. Vaccines, methods of making vaccines and uses of the vaccines are also described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
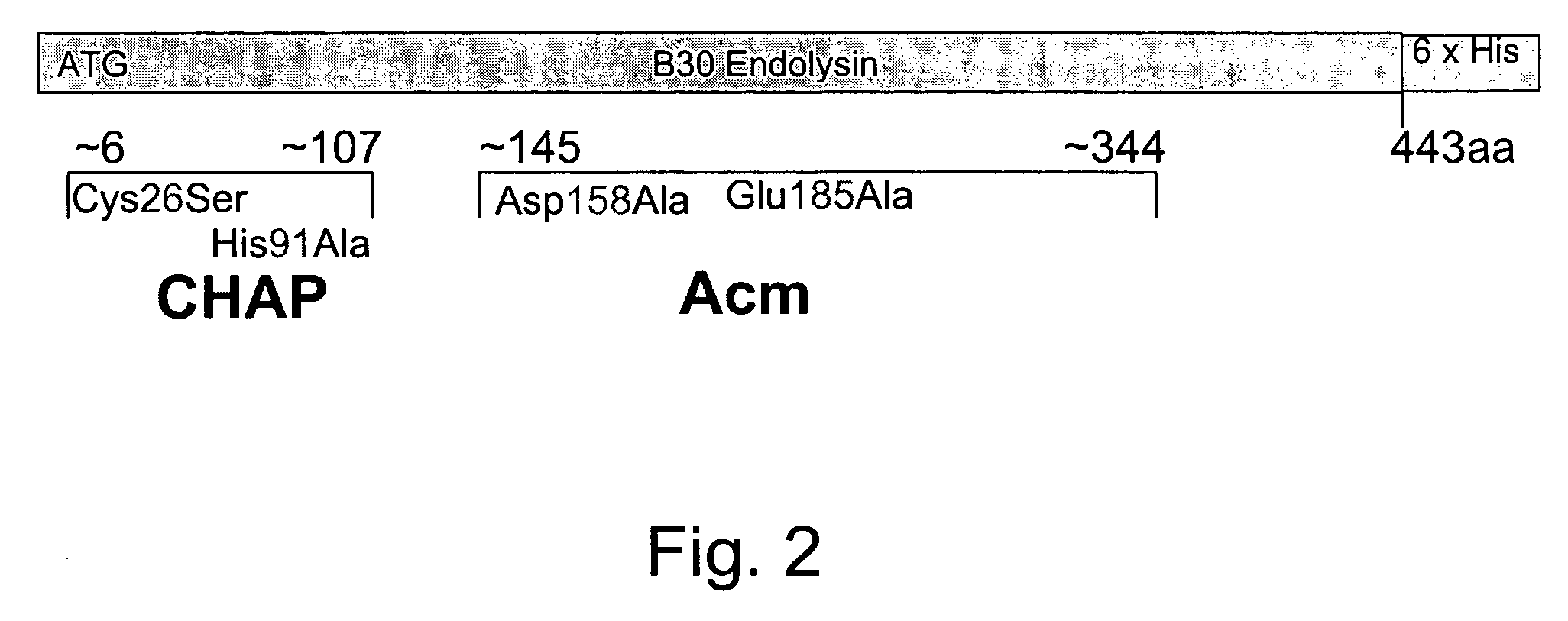
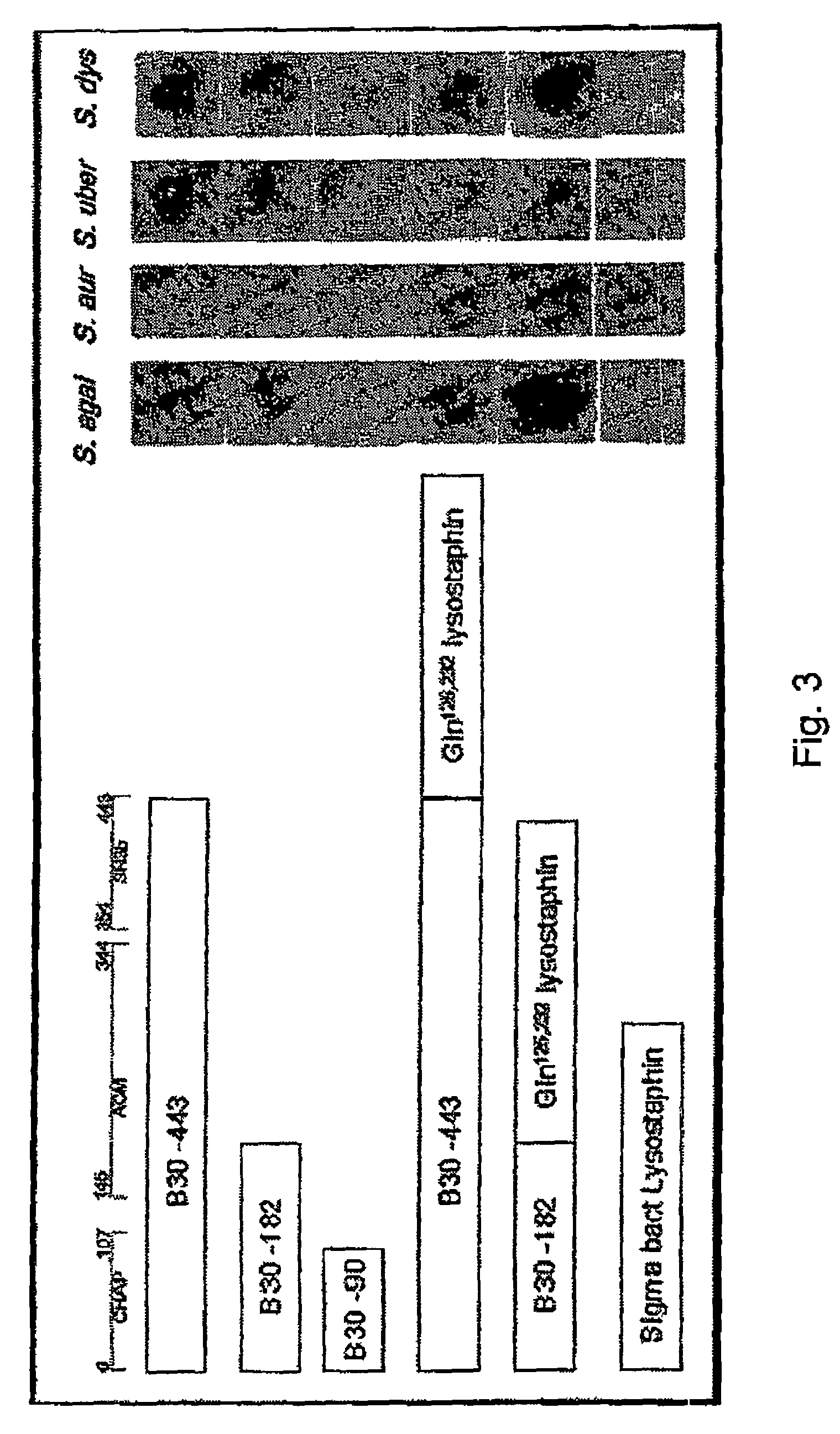
Nucleic acid encoding endolysin fusion protein
The invention concerns a recombinant nucleic acid molecule encoding an antimicrobial fusion peptidoglycan endopeptidase. The recombinant nucleic acid molecule according to the invention is formed from a nucleic acid encoding a bacterial endopeptidase (lysostaphin) from Staphylococcus simulans and a nucleic acid encoding a second endopeptidase (endolysin) module from Group B streptococcal bacteriophage B30. The encoded fusion endopeptidase has antimicrobial activity and kills both Staphylococcus bacteria and Streptococcus bacteria.
Owner:US SEC AGRI
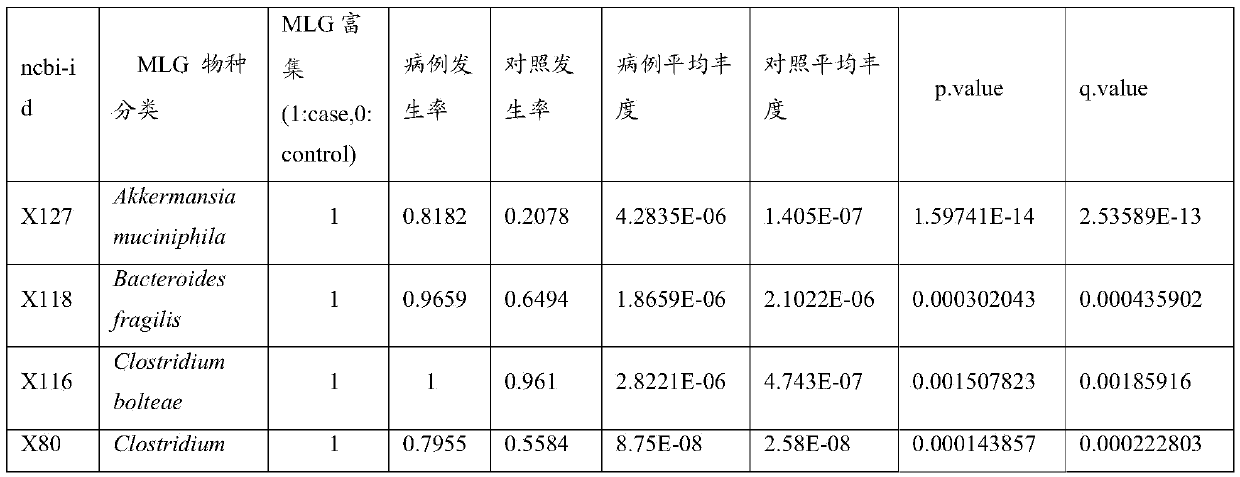
Biomarker capable of detecting diseases and application of biomarker
ActiveCN105368944AMonitor Treatment EffectsGood treatment effectBioreactor/fermenter combinationsBiological substance pretreatmentsStreptococcus infantisLactobacillus salivarius
The invention discloses a biomarker capable of detecting diseases. The biomarker at least comprises one of the following microbes or the combination of the following microbes: Akkermansia muciniphila, Bacteroides fragilis, Clostridium bolteae, Clostridium hathewayi, Clostridium sp.HGF2, Clostridium symbiosum, Eubacterium limosum, Lactobacillus fermentum, Lactobacillus salivarius, Ruminococcus torques, Streptococcus anginosus, Streptococcus infantis, Bacteroides stercoris, Bacteroides uniformis, Bilophila wadsworthia and Clostridiales sp.SS3 / 4. The invention further discloses an application of the biomarker for determining inflammatory bowel diseases. The invention also discloses a method, a system and a kit for detecting the biomarker in a tested object. According to the invention, through the research on intestinal flora, and through the high-throughput genome sequencing, the biomarker with high relativity with the inflammatory bowel diseases is screened out, and then the biomarker is utilized for accurately and efficiently detecting and diagnosing the inflammatory bowel diseases, and further used for monitoring the treatment effect for the disease.
Owner:AGRO BIOLOGICAL GENE RES CENT GUANGDONG ACADEMY OF AGRI SCI +2
Immunogenic Compositions Comprising Conjugated Capsular Saccharide Antigens and Uses Thereof
ActiveUS20180099039A1Excellent characteristicsHigh yieldPowder deliveryBacterial antigen ingredientsStreptococcus pneumoniaeVaccination
The present invention relates to new immunogenic compositions comprising conjugated Streptococcus pneumoniae capsular saccharide antigens (glycoconjugates) and uses thereof. Immunogenic compositions of the present invention will typically comprise at least one glycoconjugate from a S. pneumoniae serotype not found in PREVNAR®, SYNFLORIX® and / or PREVNAR 13®. The invention also relates to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said novel immunogenic compositions.
Owner:PFIZER INC
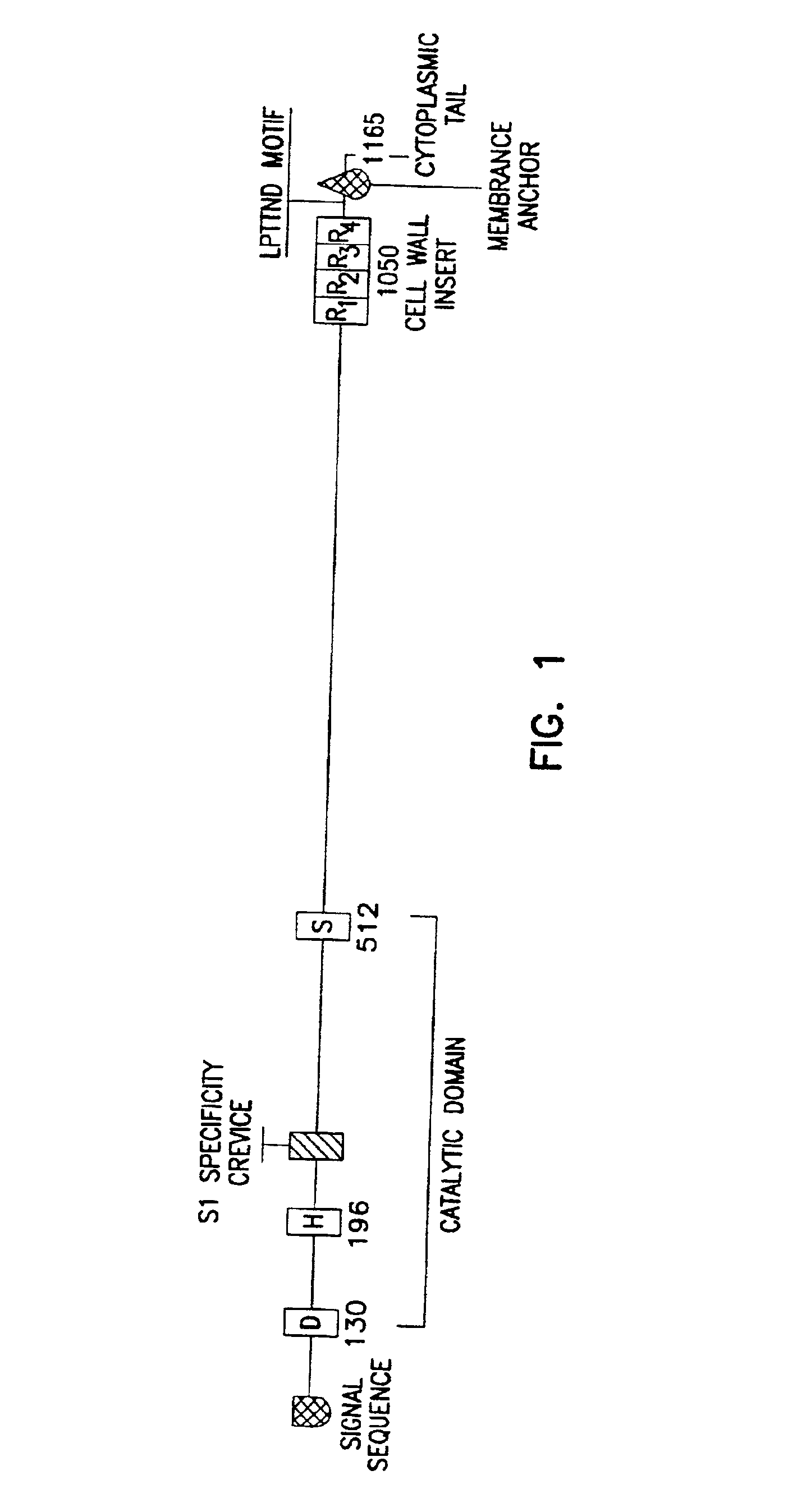
Method for isolating a C3 binding protein of streptococcus pneumoniae
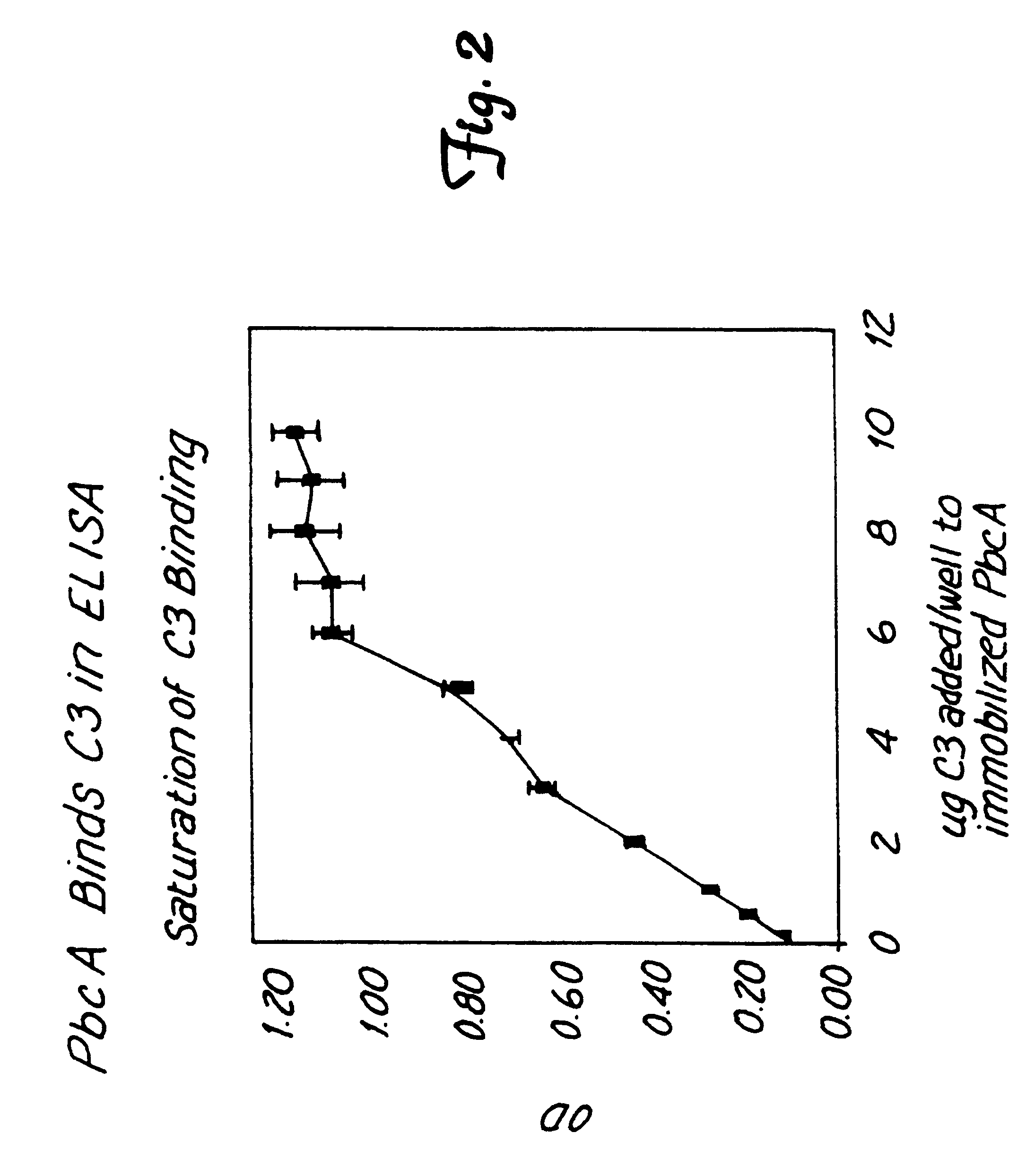
This invention relates to the identification of a human complement C3 binding protein from Streptococcus pneumoniae and to its sequence and to methods for its purification and use. The protein binds but does not degrade or cleave C3 and is implicated in S. pneumoniae virulence. The protein is recognized by antibodies produced by humans recovering from pneumococcal infection.
Owner:RGT UNIV OF MINNESOTA
Secreted Streptococcus Pneumoniae Proteins
Novel proteins from Streptococcus pneumoniae are described, together with nucleic acid sequences encoding them. Their use in vaccines and in screening methods is also described.
Owner:SANOFI PASTEUR LTD
Polypeptides And Immunogenic Conjugates Capable of Inducing Antibodies Against Pathogens, and Uses Thereof
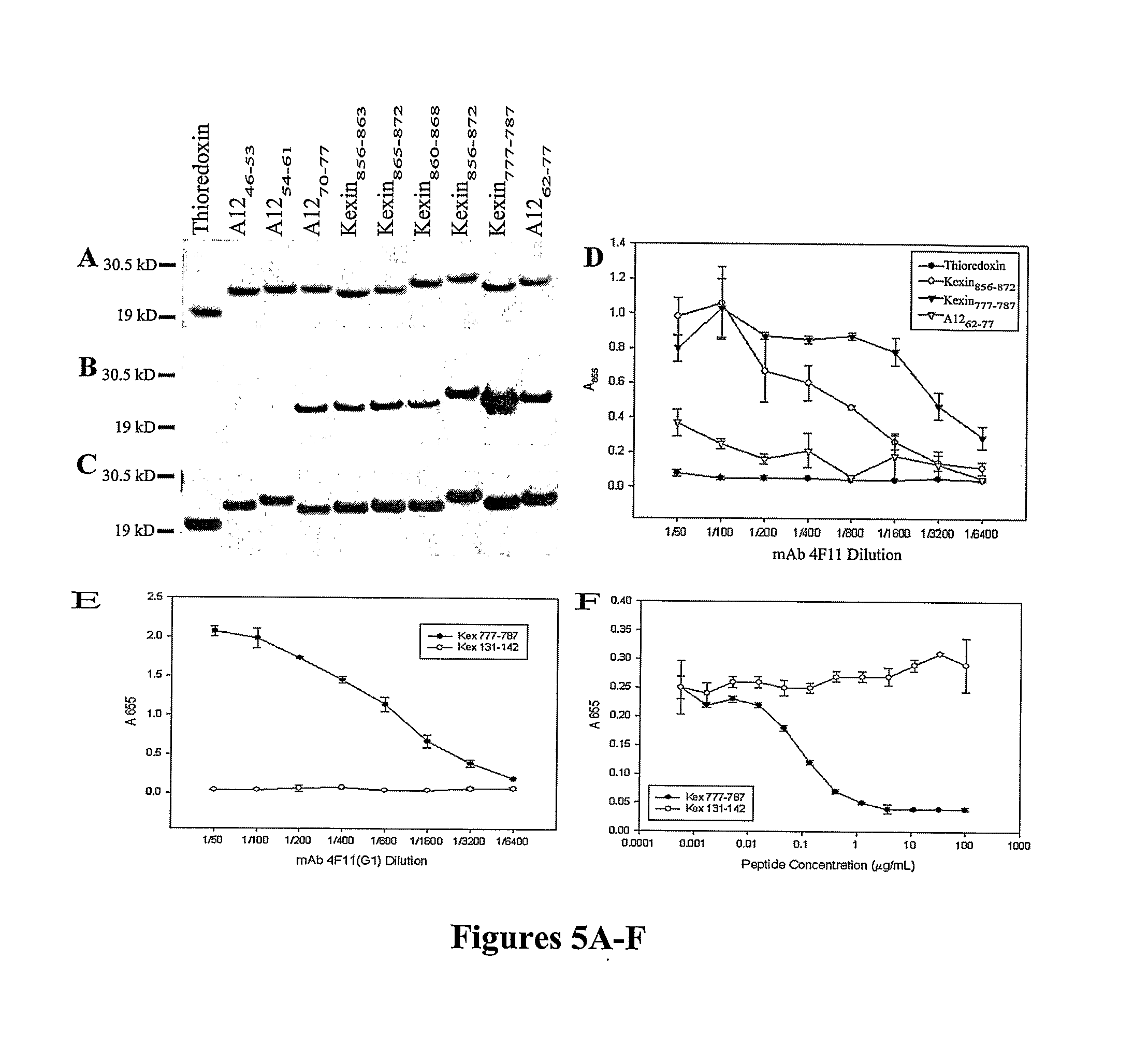
InactiveUS20080171053A1Efficient responseAntibacterial agentsBacterial antigen ingredientsKexinDNA construct
A number of immunologically active agents are described, including an isolated protein or polypeptide that includes the amino acid sequence of SEQ ID NO: 1, immunogenic conjugates containing either the protein or polypeptide, a full-length Pneumocystis kexin, or a full length Streptococcus pneumoniae pneumococcal surface protein A (PspA), antibodies recognizing the protein or polypeptide or the immunogenic conjugates (particularly the epitope of SEQ ID NO: 1), and nucleic acid molecules that encode the protein or polypeptide, as well as DNA constructs, expression vectors, and host cells that contain the nucleic acid molecules. Disclosed uses of the antibodies, immunogenic conjugates, and DNA constructs include inducing passive or active immunity to treat or prevent pathogen infections, particularly by a Pneumocystis organism, in a patient.
Owner:UNIVERSITY OF ROCHESTER
Purification of bacterial antigens
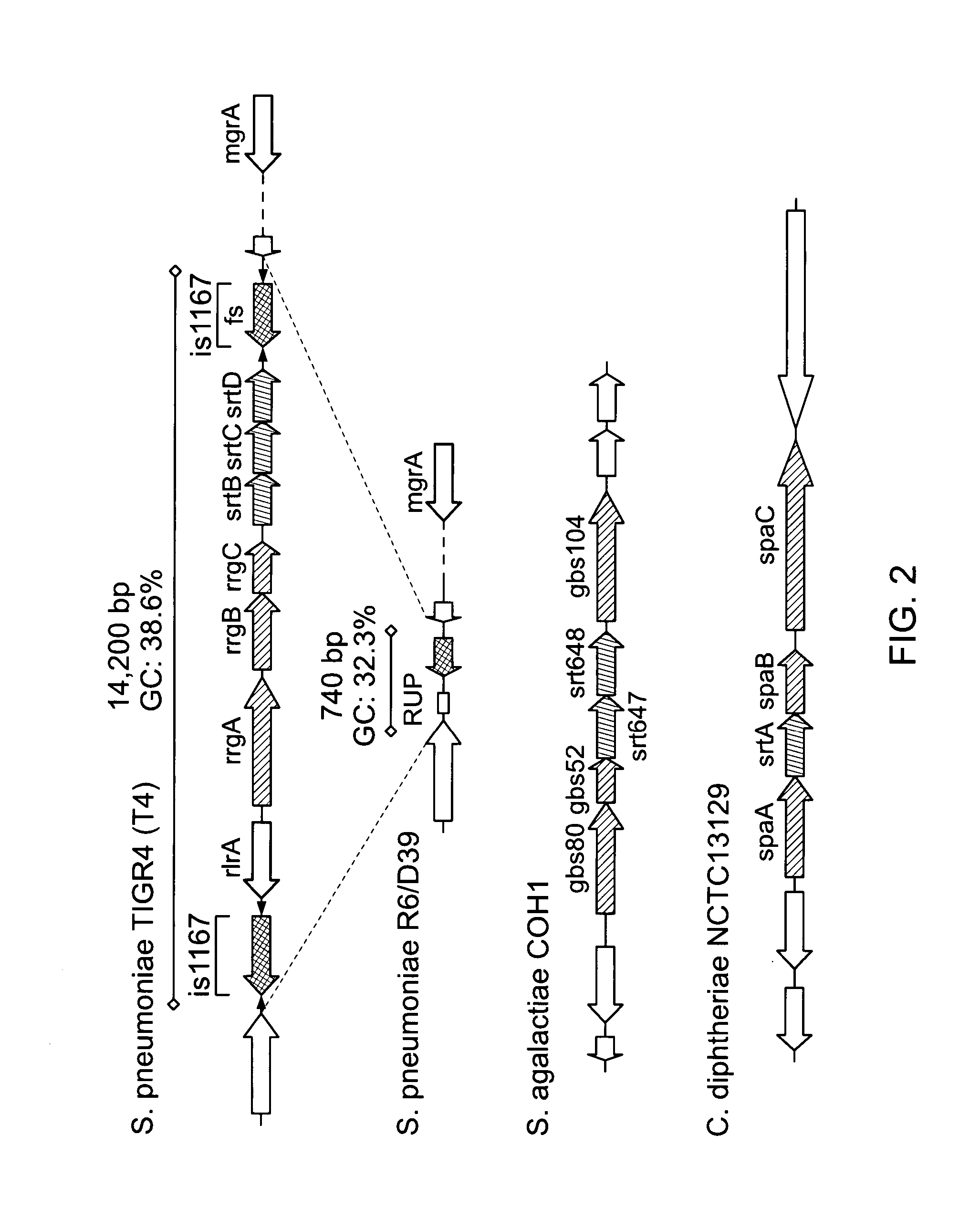
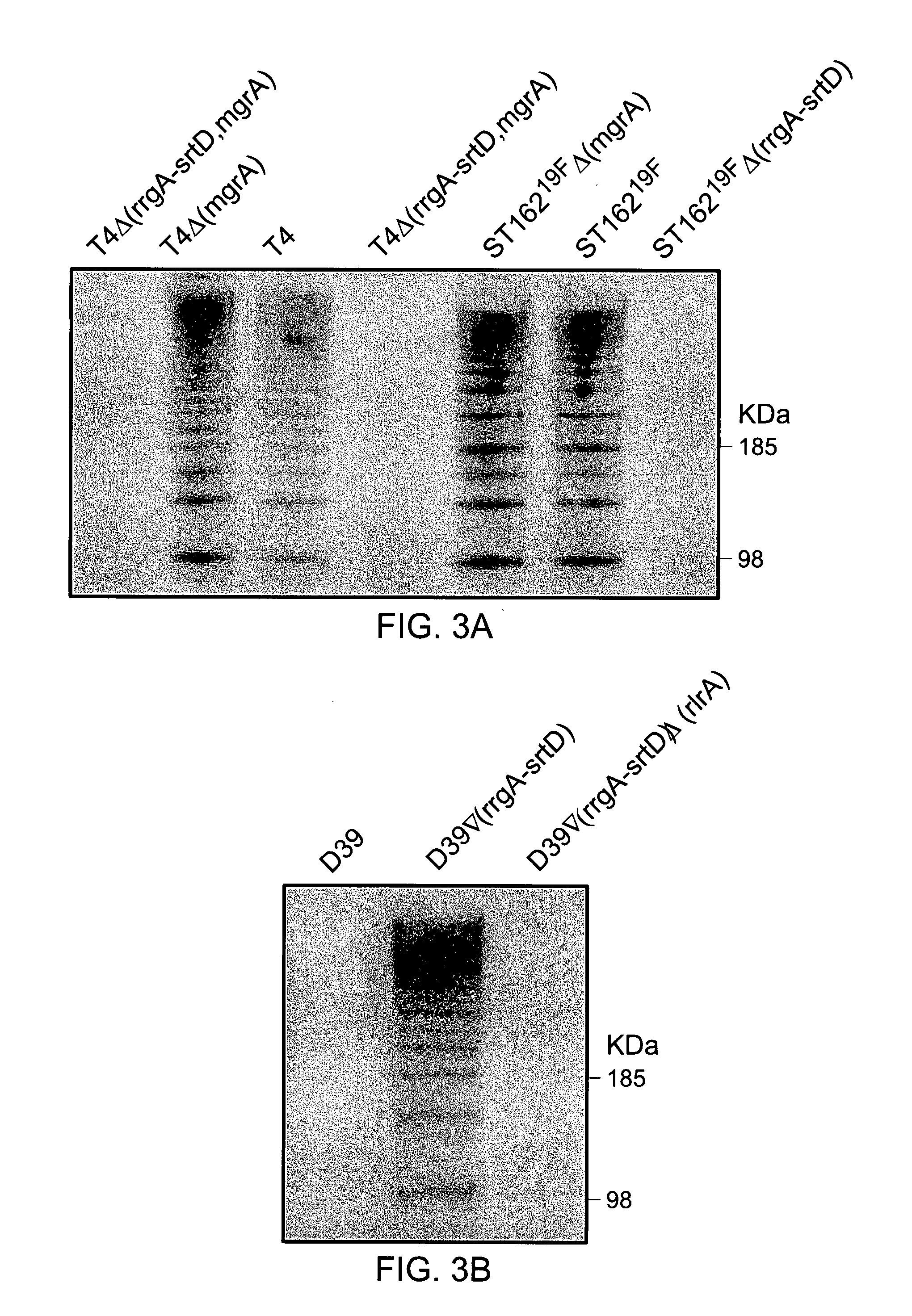
Presented are methods of isolation of pili and pilus-like structures from Gram-positive bacteria including Streptococcus pneumoniae and compositions that include such isolated pili. These compositions are useful as immunogenic compositions for the production of antibodies and immunostimulation. Also presented are methods of inhibiting Streptococcus pneumoniae, and methods of identifying inhibitors of Streptococcus pneumoniae.
Owner:NOVARTIS AG
Streptococcus pneumoniae vaccines
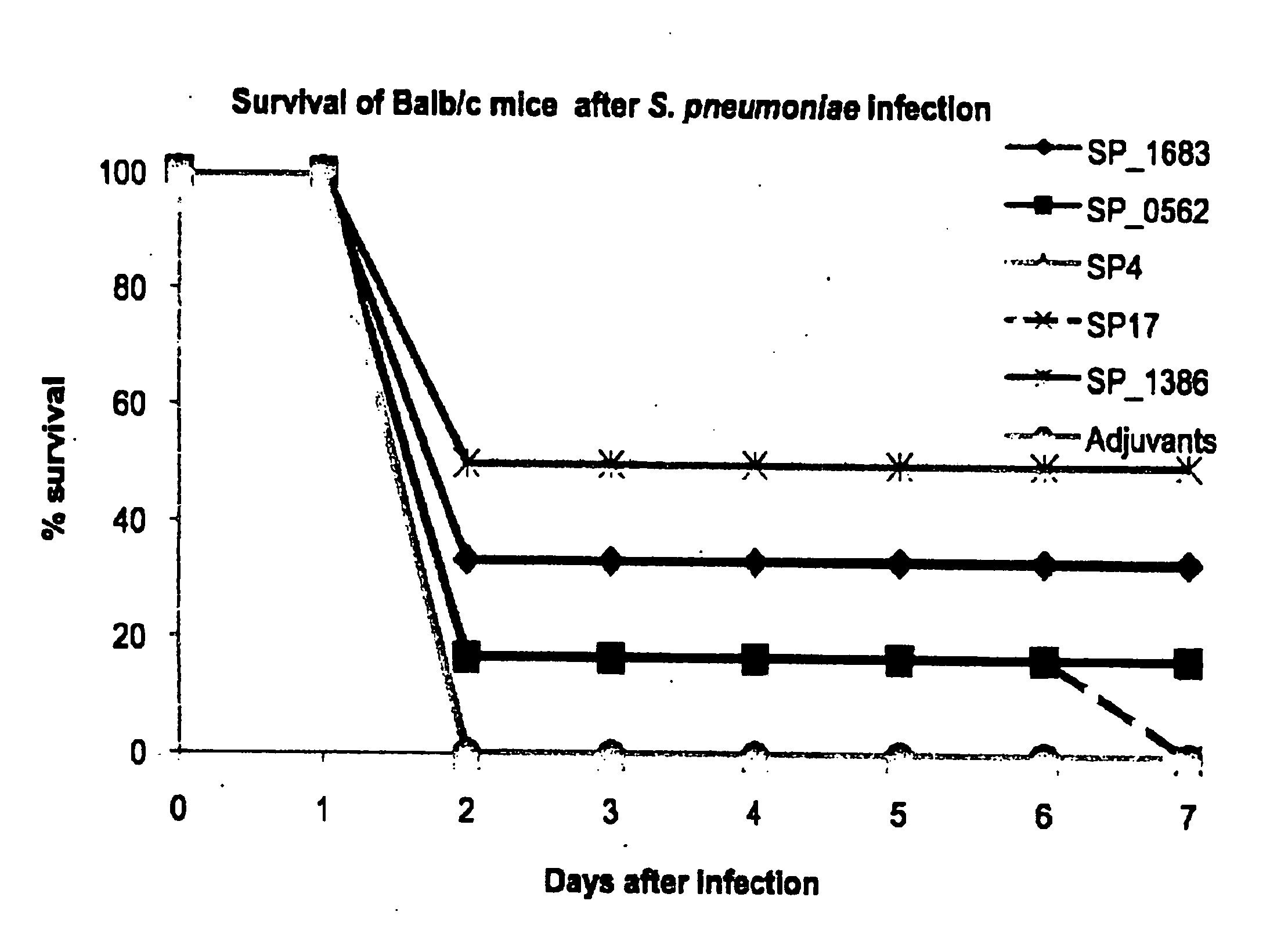
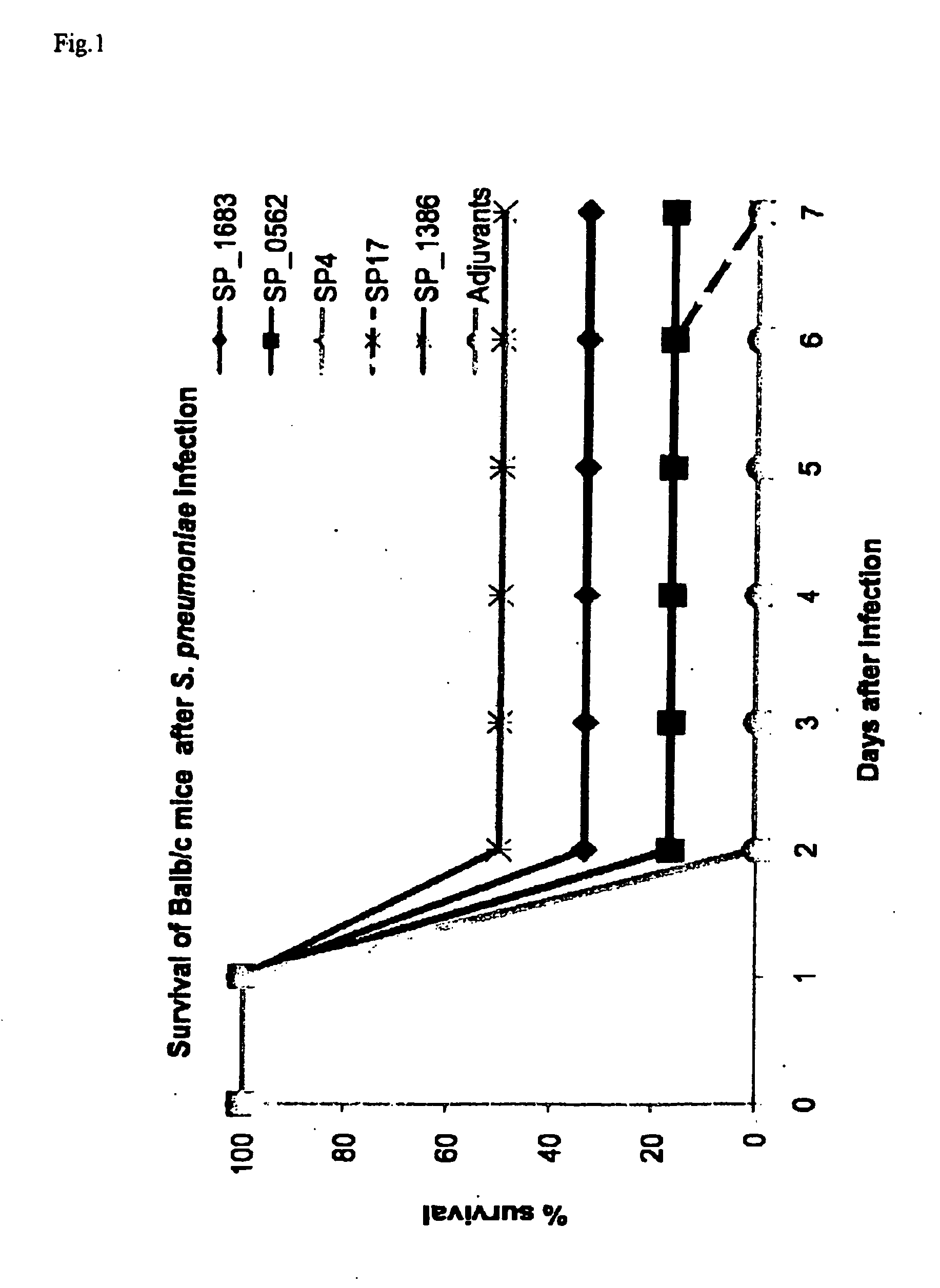
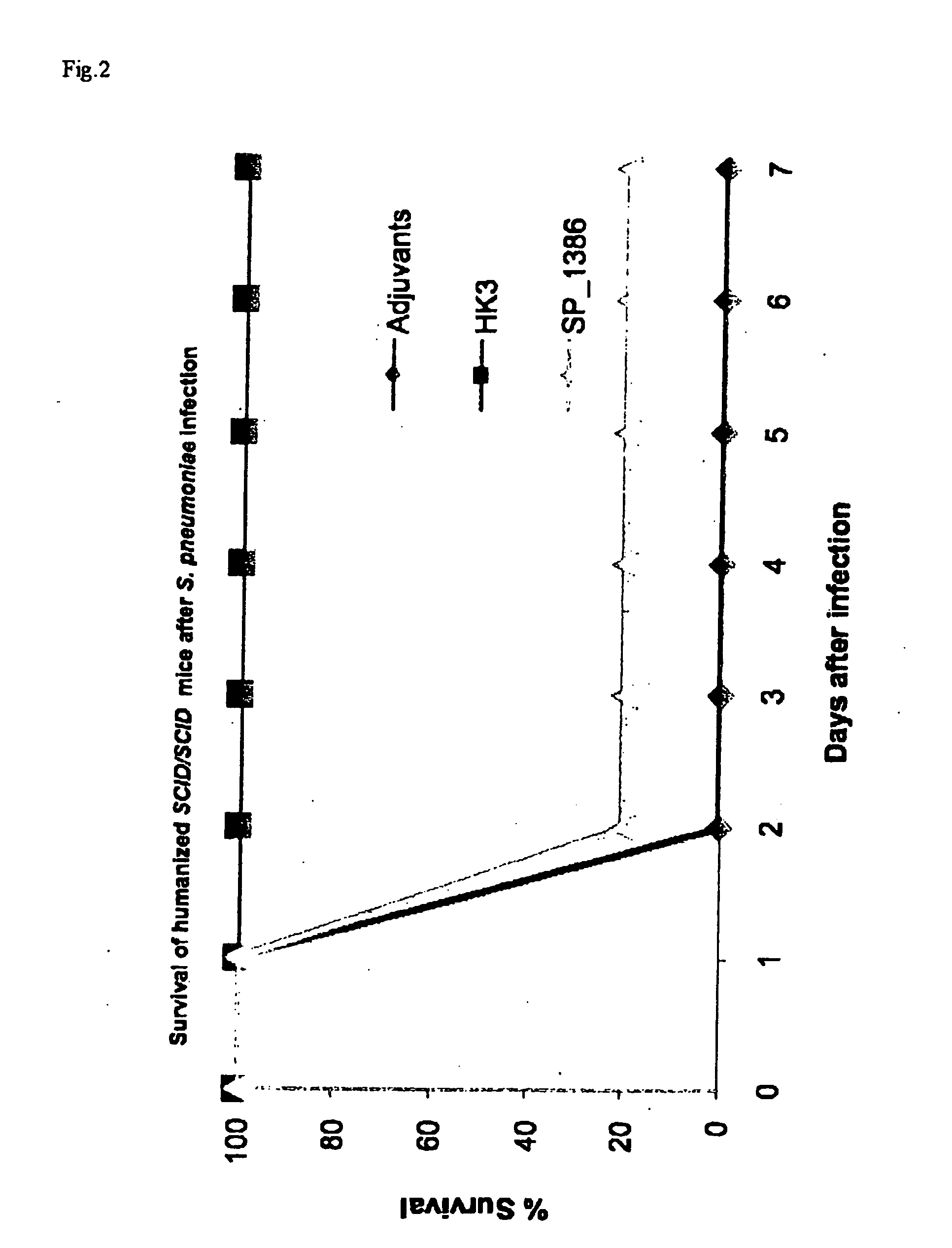
Streptococcus pneumoniae is a major cause of pneumoniae, meningitis, and major cause of morbidity and mortality throughout the world by bacterial otitis media, pneumoniae, meningitis, and bacteraemia. It is an important agent of disease in man especially among infants, the elderly and immunocompromised persons. The present invention provides a solution to this problem by providing a substantially pure or isolated disease related antigen selected from the group consisting of the isolated, recombinant or synthetic S. pneumoniae human immunogenic antigens of SP_0562, SP_0965, SP_0082 (in particular the fragments SP4 and SP 17 of said SP_0082), and a Periplasmic Binding Protein (PBP) (in particular SP_1683 or SP_1386), or a fragments thereof or substantially identical antigen for use in a treatment to induce a immunological memory in a human against S. pneumoniae cells for use in a vaccination treatment of S. pneumoniae disorder in human or against S. pneumoniae in a human. In a particular embodiment, the present invention provides an isolated, recombinant or synthetic S. pneumoniae Periplasmic Binding Protein (PBP) (in particular SP_1683 or SP_1386) as a disease related antigen for use in a treatment to induce a immunological memory in a human against S. pneumoniae cells for use in a vaccination treatment of S. pneumoniae disorder in human or for use in the treatment of an S. pnewnoniae infection in a human. It further provides antibodies that specifically bind to the S. pneumoniae disease related antigens identified herein for use in the treatment of a an S. pneumoniae infection in a human, such as for example in a treatment to induce immunological memory in a human against S. pneumoniae, i.e. in a vaccination treatment of S. pneumoniae. It is also an aspect of the present invention to provide the use of any one of the S. pneumoniae disease related antigens as identified herein, or of the antibodies specific for said antigens in methods to diagnose for a S. pneumoniae disorder in a human.
Owner:KATHOLIEKE UNIV LEUVEN
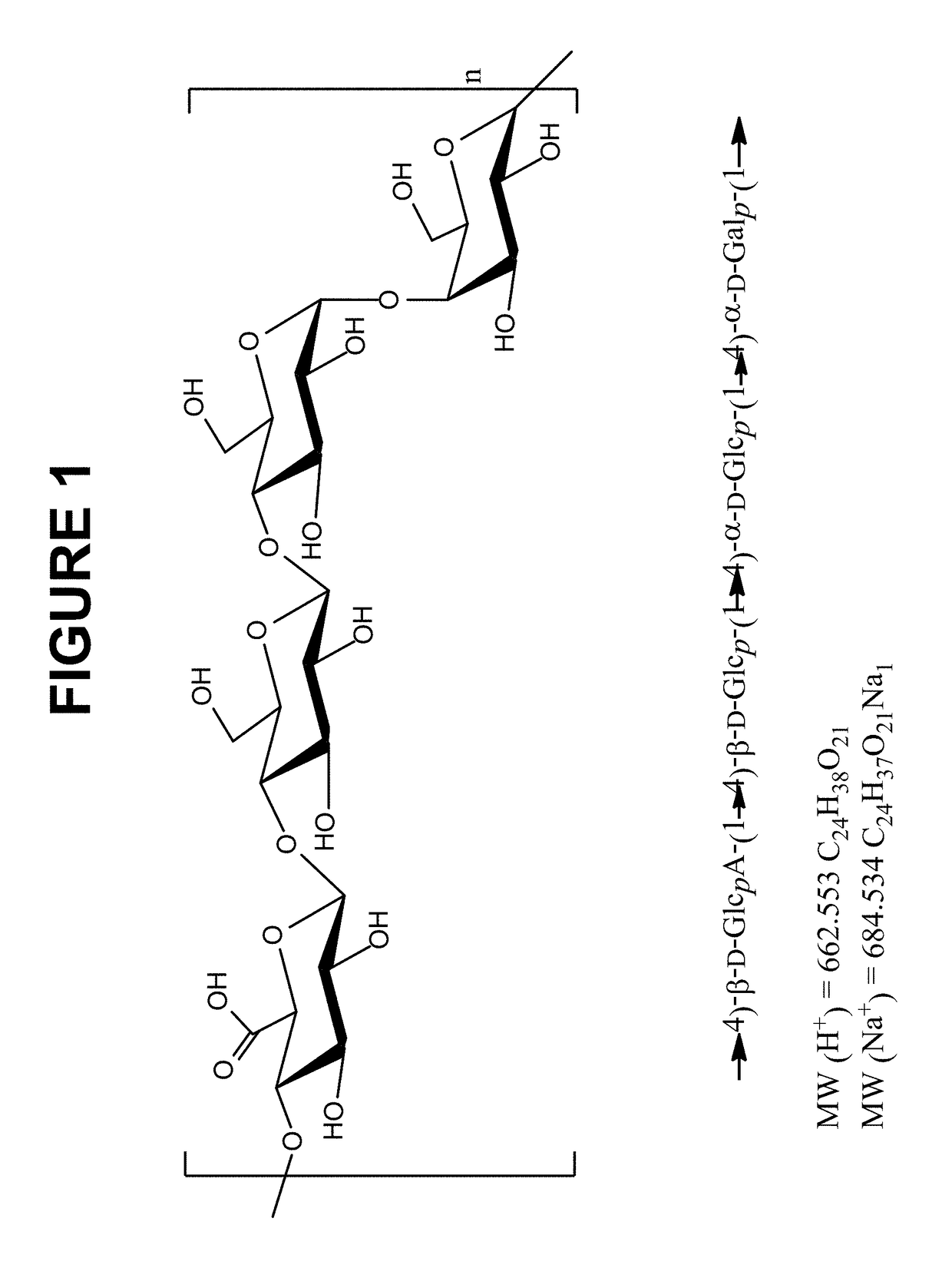
Streptococcus pneumoniae capsular polysaccharides and conjugates thereof
InactiveUS20160324949A1Antibacterial agentsOrganic active ingredientsStreptococcus pneumoniae capsular polysaccharideCarrier protein
The invention relates to isolated Streptococcus pneumoniae serotype 15B capsular polysaccharide and processes for their preparation. The invention also relates to immunogenic conjugates comprising Streptococcus pneumoniae serotype 15B capsular polysaccharide covalently linked to a carrier protein, processes for their preparation and immunogenic compositions comprising them.
Owner:PFIZER INC
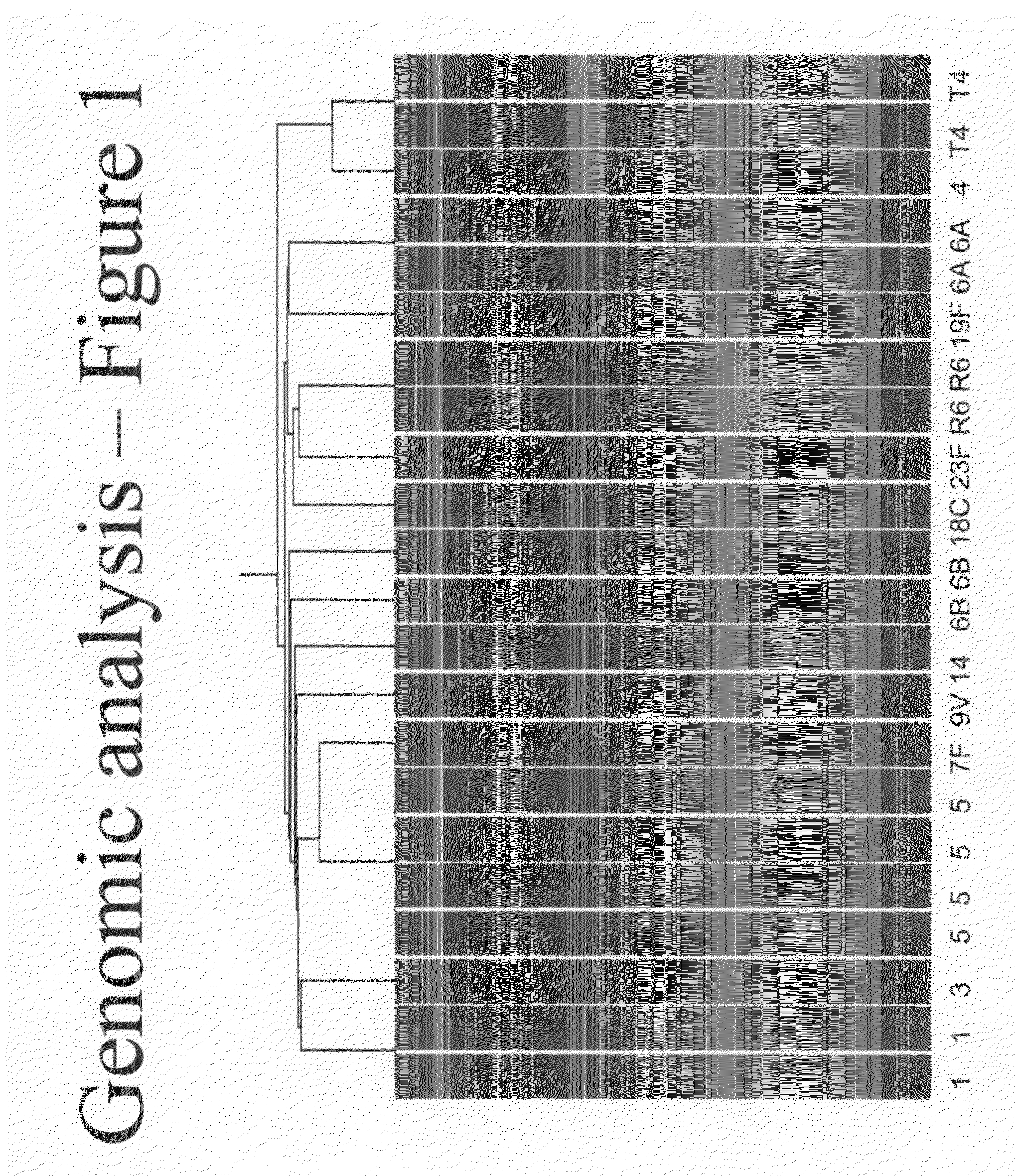
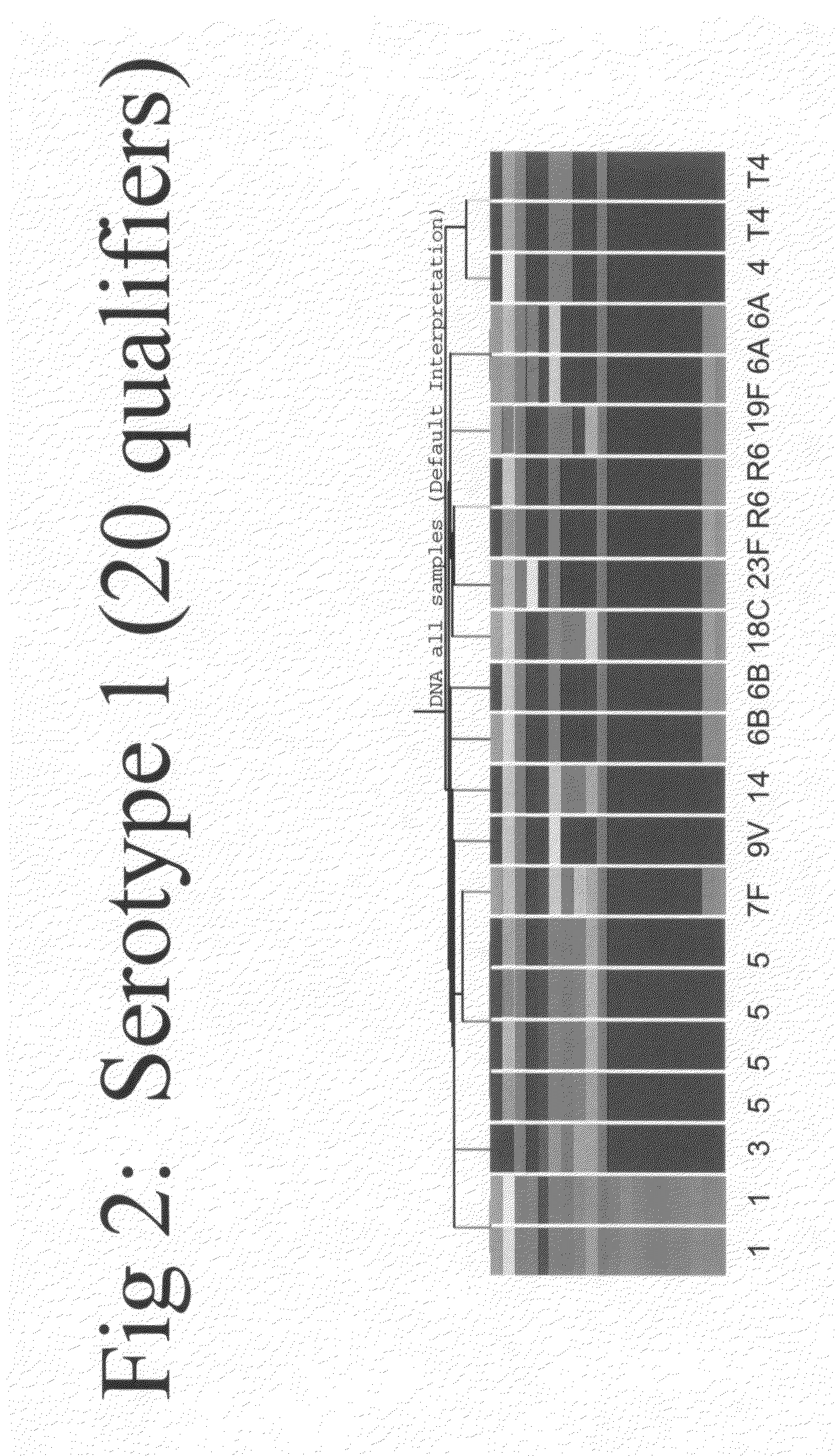
Microarray for monitoring gene expression in multiple strains of Streptococcus pneumoniae
InactiveUS20110177960A1Understanding of genetic expression patternRapid and accurate and discriminable detectionNucleotide librariesMicrobiological testing/measurementStreptococcus pneumoniaeStreptococcus mitis
The present invention features an array capable of monitoring gene expression patterns of multiple strains of Streptococcus pneumoniae including a substrate having a plurality of addresses, each of which has a probe disposed thereon.
Owner:WYETH LLC
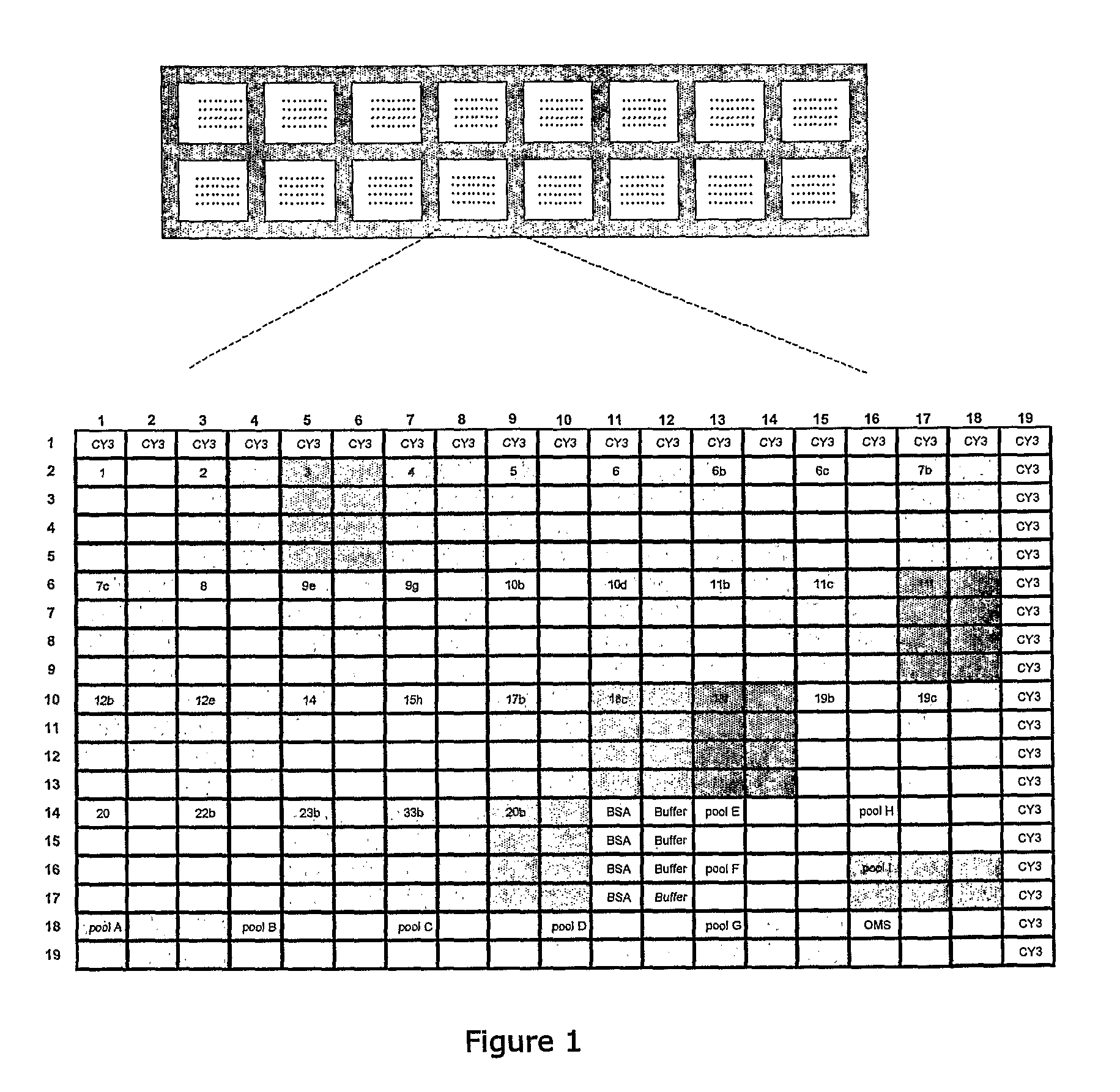
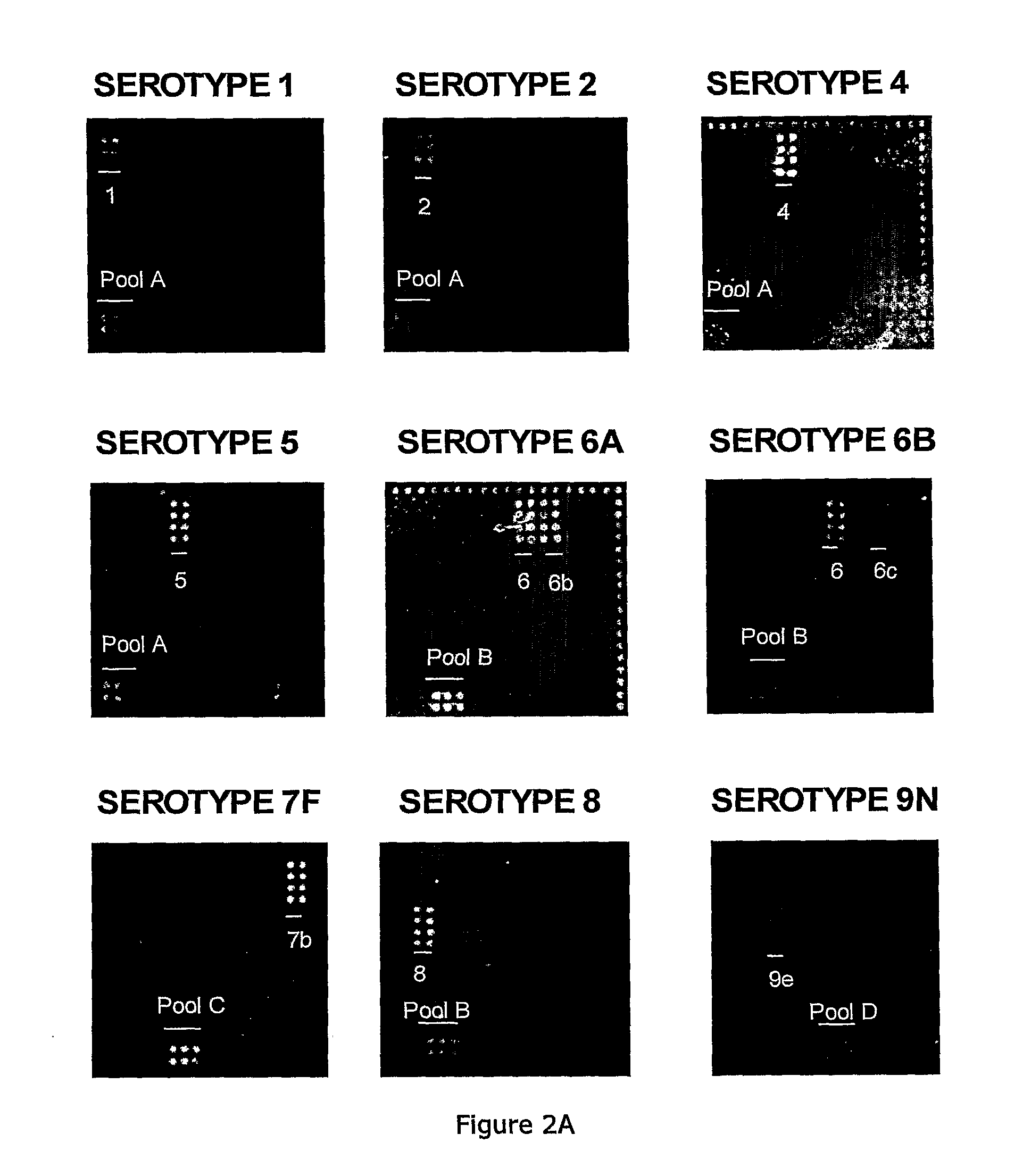
Methods and products for identifying strains of bacteria
InactiveUS20110053793A1Reduce and avoid needRemove and diminishes needPeptide librariesLibrary tagsSerum igeCapture antibody
Methods for identifying strains of bacteria, particularly methods for serotyping Streptococcus pneumoniae in a sample, methods for detecting and / or classifying S. pneumoniae infection by serotype, array devices and kits for use in such methods are disclosed. Array devices comprise a set of capture antibodies immobilised on a substrate at pre-determined array positions, wherein the set of capture antibodies comprises serotype-distinguishing antibodies which differ in their binding specificity for different S. pneumoniae serotypes. Serotyping methods may employ whole cell detection utilising one or more detectable labels, including in situ labelling of array-bound cells.
Owner:PROTEOMIKA +1
Streptococcus pneumoniae vaccine
InactiveCN1635904AReduced responseAntibacterial agentsBacterial antigen ingredientsStreptococcus mitisStreptococcus pneumoniae vaccine
The present invention provides an optimal formulation of multi-serotype Streptococcus pneumoniae conjugate vaccine.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Vaccine
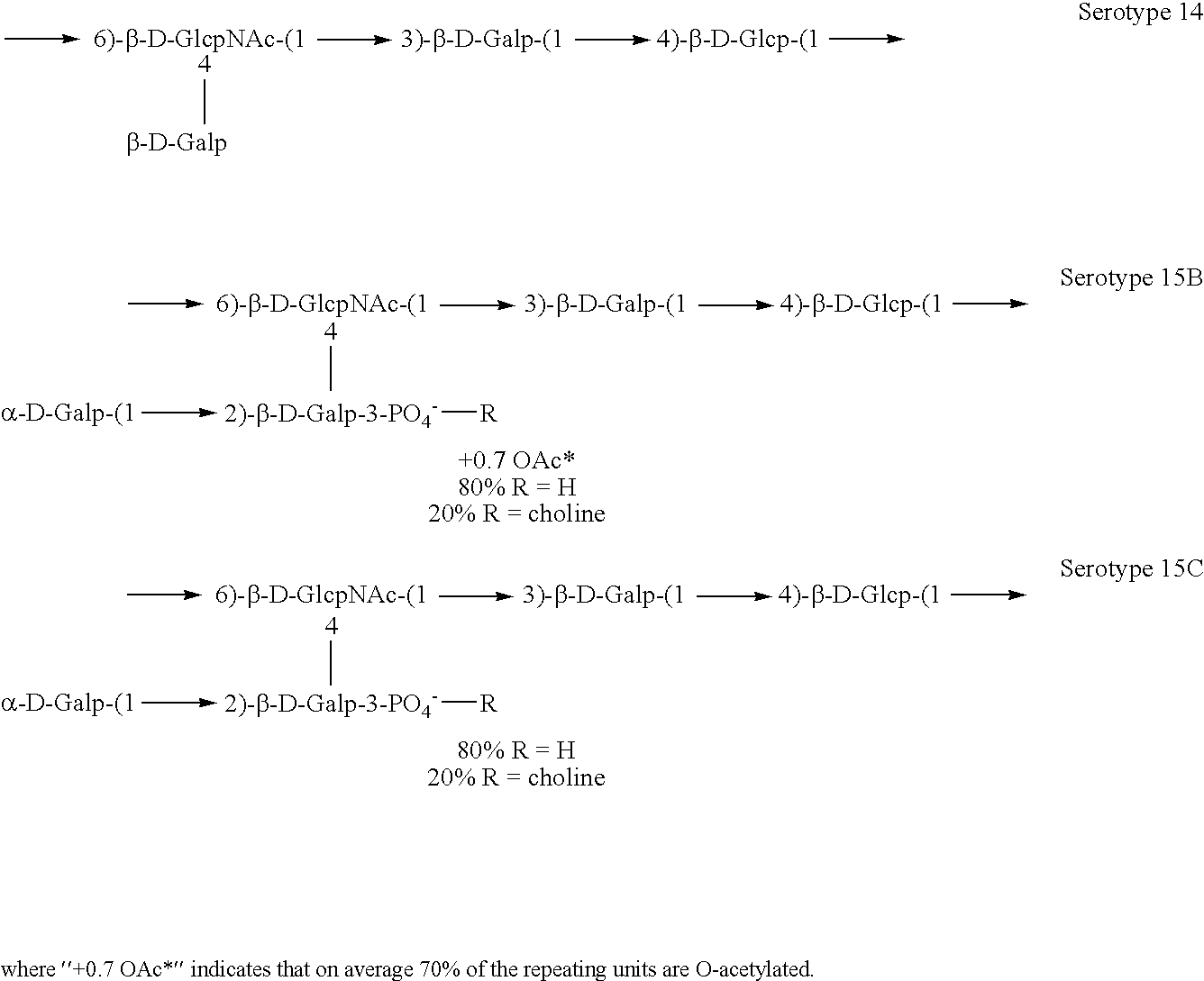
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various (for instance 9 or more) conjugated capsular saccharides from different S. pneumoniae serotypes, wherein the composition comprises conjugated capsular saccharide 18C which is less than 80, 70, 60, 50, 40, 30, 20, 15 or 10% O-Acetylated.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Method for controlling streptococcus pneumoniae serotype 19a polysaccharide molecular weight
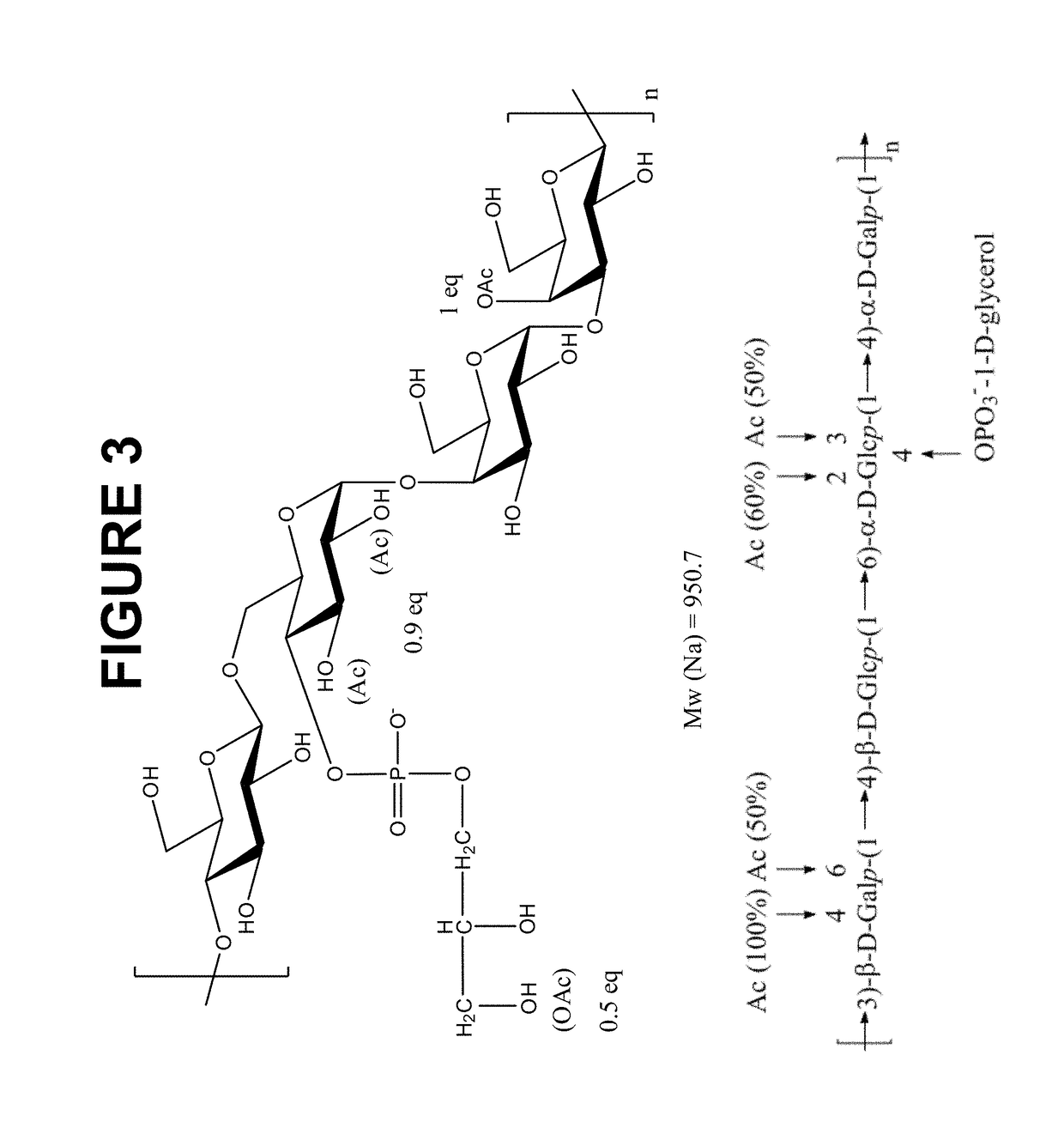
The present invention provides improved methods for producing a solution containing high molecular weight isolated Streptococcus pneumoniae 19A capsular polysaccharides. In certain methods, a fermentation culture of Streptococcus pneumoniae bacterial cells that produce serotype 19A capsular polysaccharides is fermented for less than 6 hours before the bacterial cells are lysed the capsular polysaccharides are harvested. In other methods, CO2 is supplied to the fermentation culture. Supplying CO2 to the fermentation culture includes adding bicarbonate ions to the fermentation culture, adding carbonate ions to the fermentation culture, adding mixtures of bicarbonate and carbonate ions to the fermentation culture, and overlaying the fermentation culture with CO2.
Owner:WYETH LLC
Recombinant Salmonella choleraesuis for expressing surface antigen gene sao of streptococcus suis type 2, vaccine and application
ActiveCN101979501AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacterial antigen ingredientsBacteroidesAntigen
The invention belongs to the field of animal bacterium gene engineering, and in particular relates to construction of resistance marker-free recombinant Salmonella choleraesuis for expressing surface antigen sao gene segment of streptococcus suis type 2, preparation of a vaccine and application. The resistance marker-free recombinant Salmonella choleraesuis for expressing the surface antigen sao gene segment of the streptococcus suis type 2, namely asd-C500 / Pya-saoA is obtained, and the collection number is CCTCC NO: M2010156. The asd gene of the Salmonella choleraesuis is deleted in the recombinant strain, and the recombinant strain contains plasmid pYA-saoA capable of expressing the asd and the sao gene segment of the streptococcus suis type 2. The invention also discloses a method for preparing the recombinant strain and the vaccine, and application in preparing Salmonella choleraesuis-streptococcus suis type 2 vaccines.
Owner:HUAZHONG AGRI UNIV +1
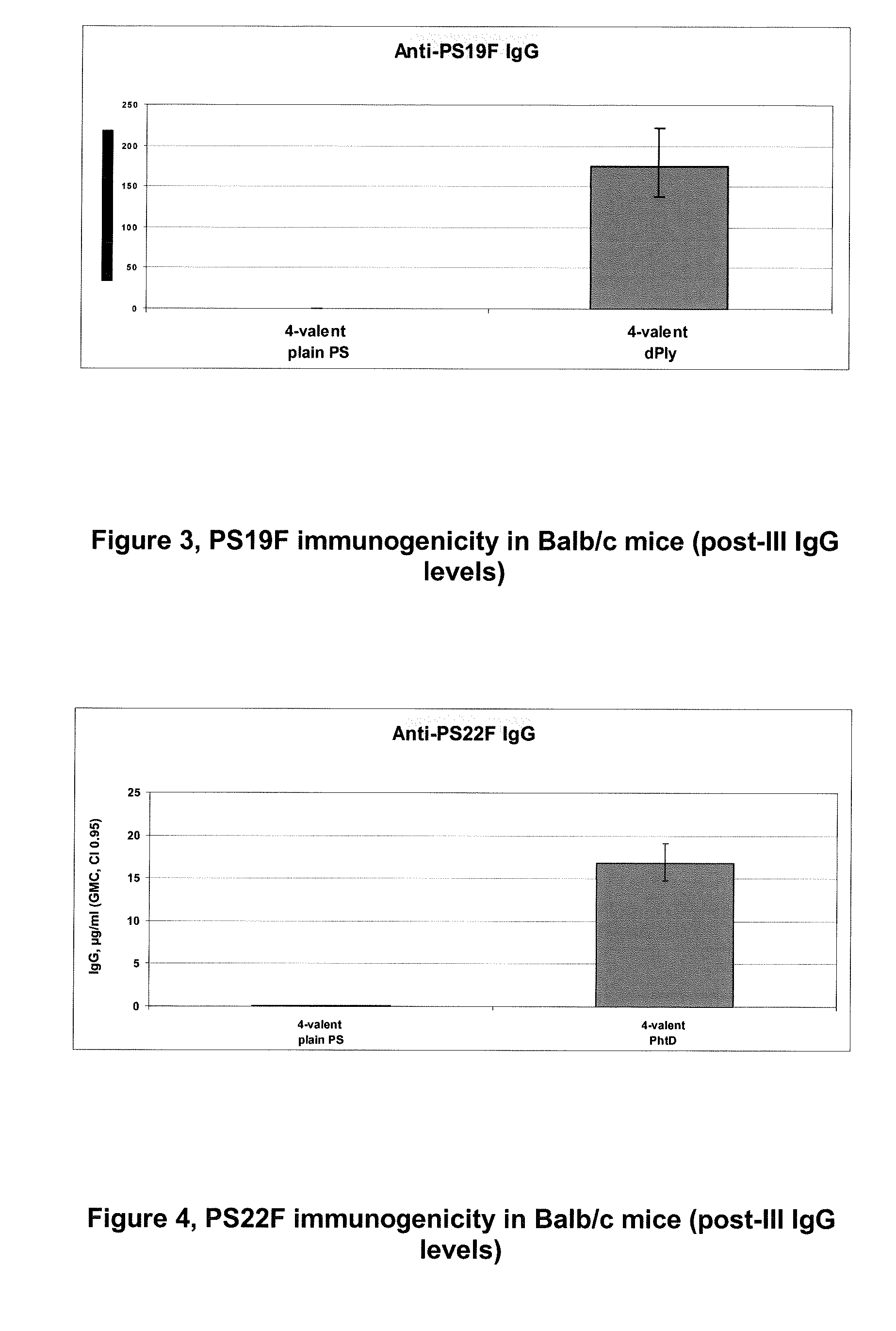
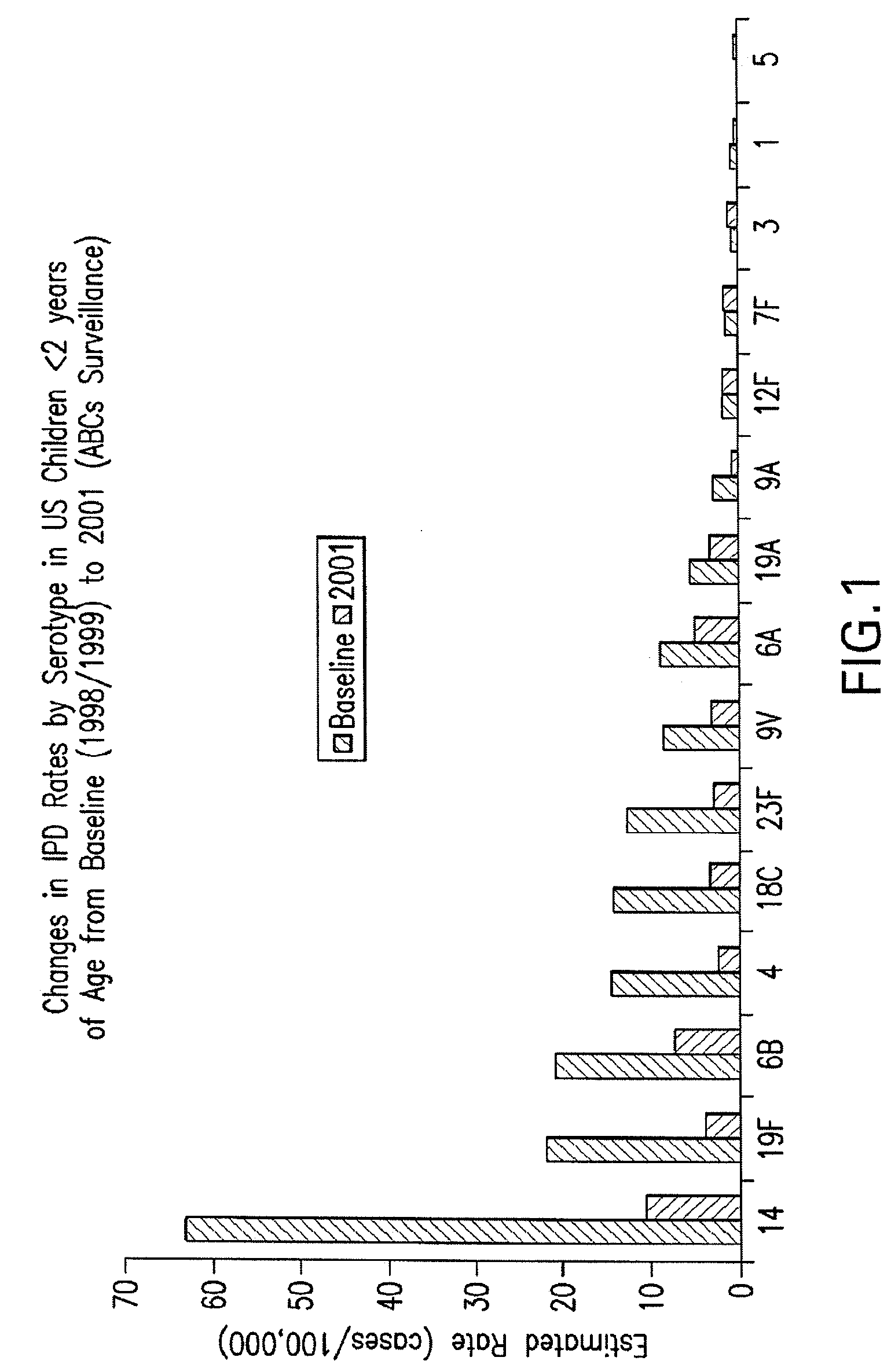
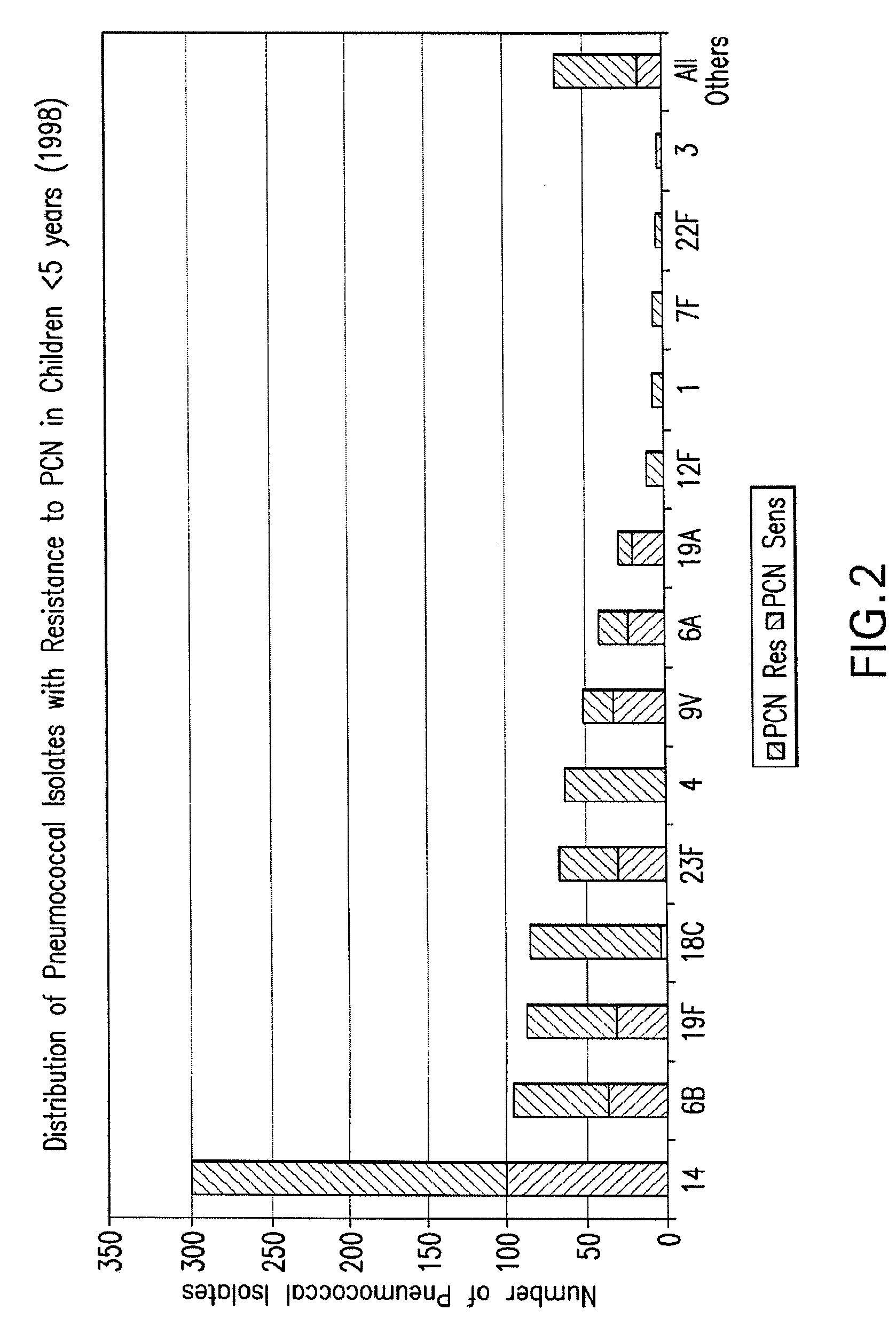
Pneumococcal serotypes
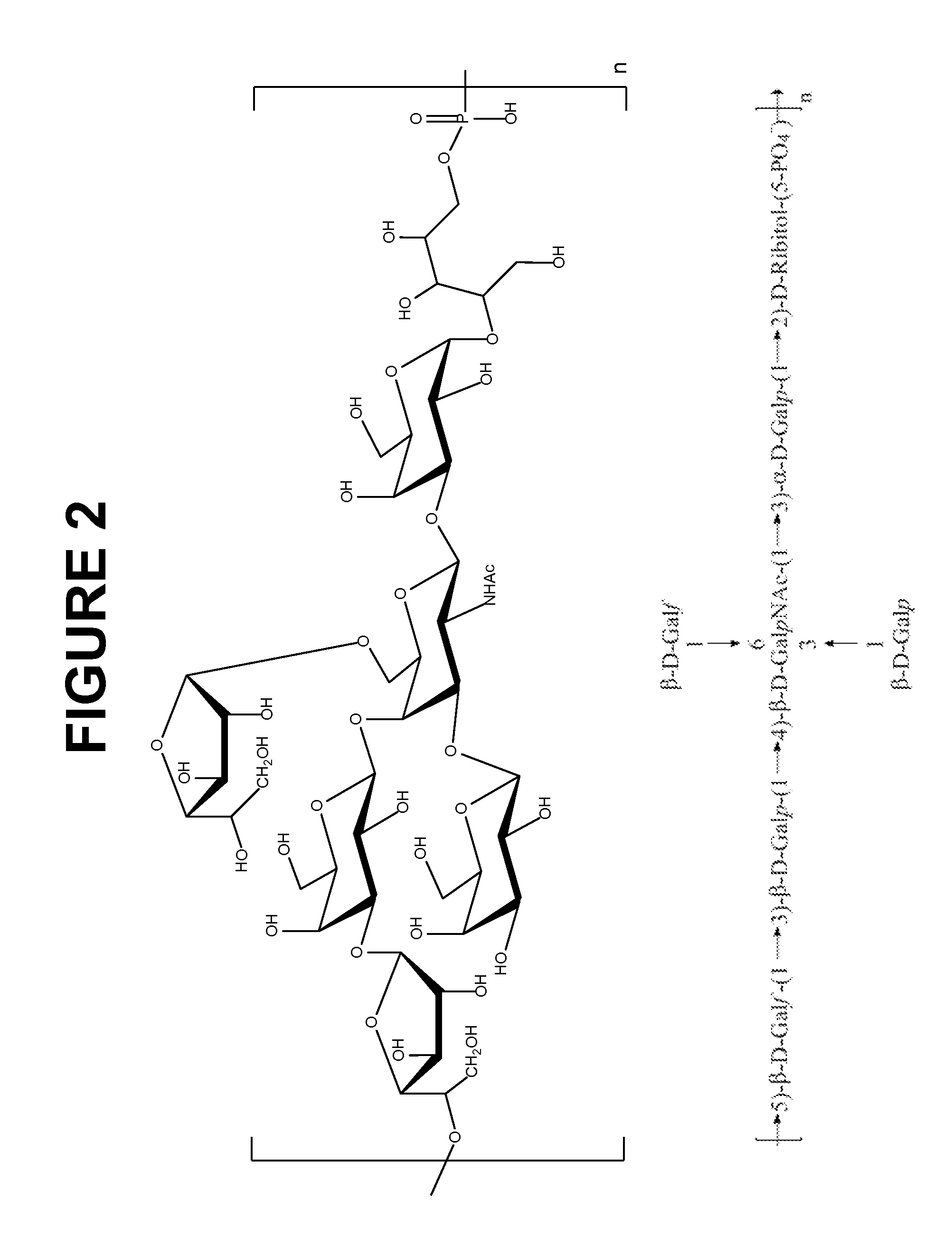
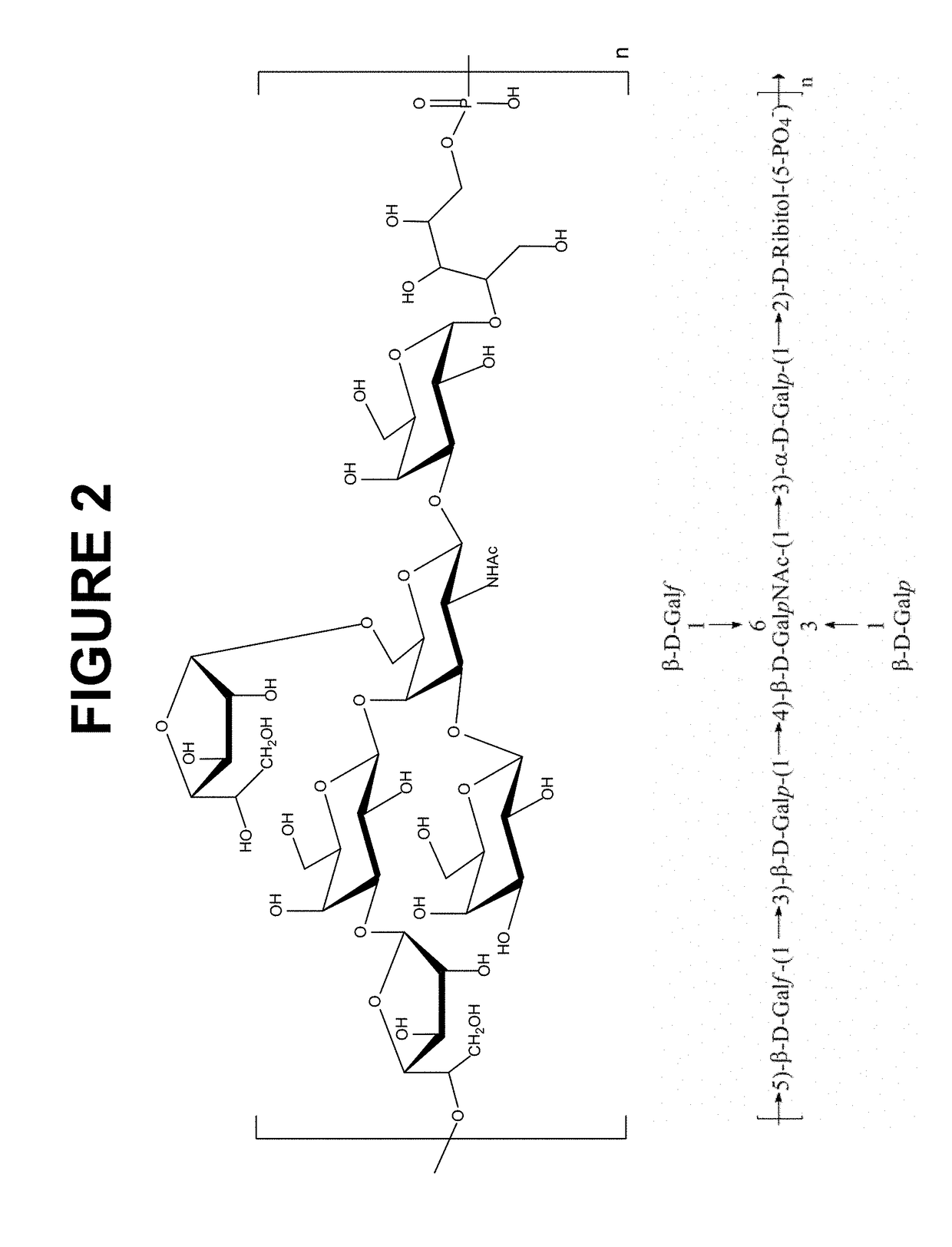
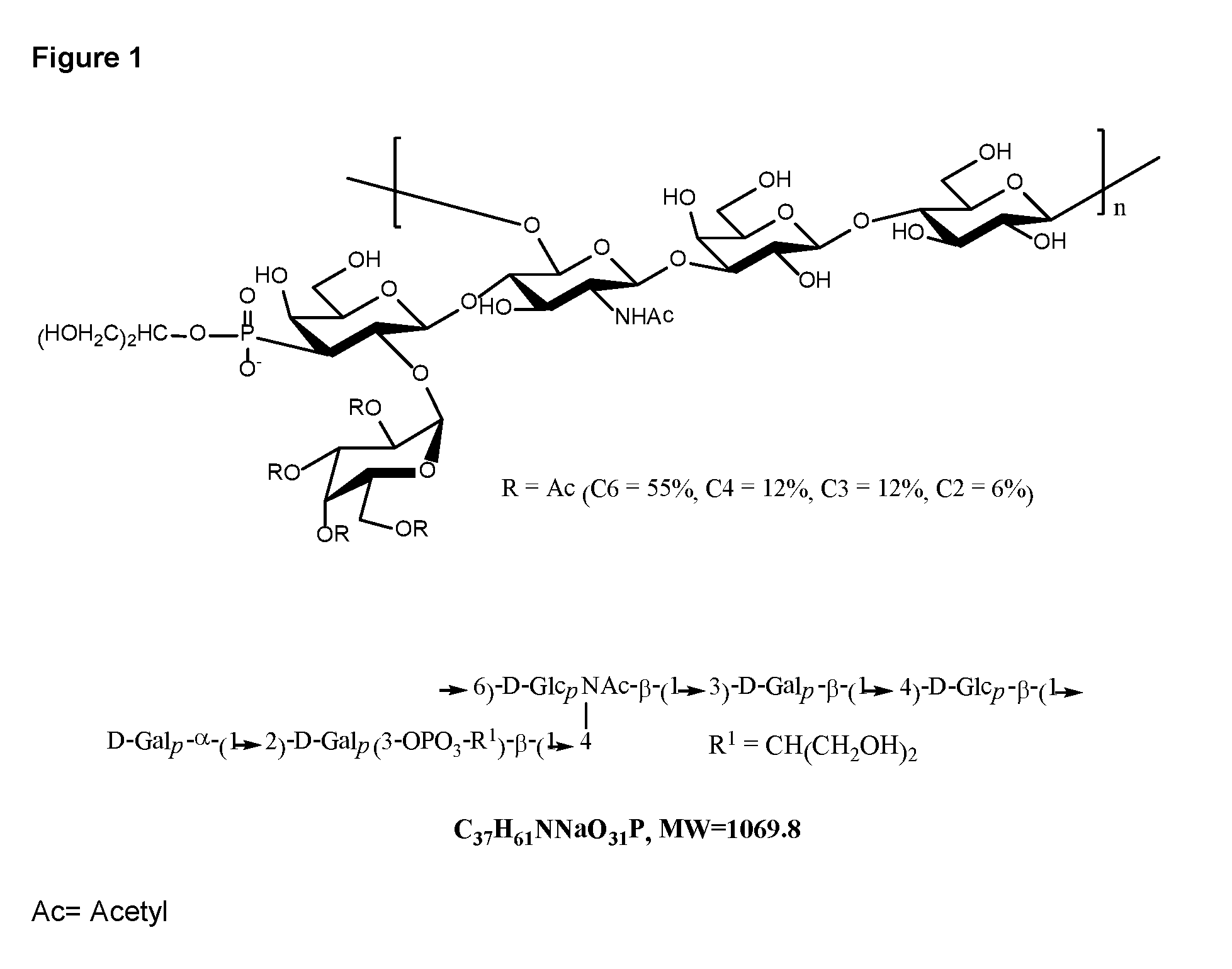
Disclosed is a new and emerging serotype of Streptococcus pneumoniae designated serotype 6C, and assays and monoclonal antibodies useful in identifying same. Also disclosed is a novel pneumococcal polysaccharide with the repeating unit {→2) glucose 1 (1→3) glucose 2 (1→3) rhamnose (1→3) ribitol (5→phosphate}. This new serotype may be included in pneumococcal vaccines.
Owner:INST ADOLFO LUTZ +1
Inactivated vaccine for streptococcus suis and pasteurella multocida diseases and preparation method thereof
ActiveCN101745105AAvoid infectionAchieve the effect of multiple defenses with one injectionAntibacterial agentsBacterial antigen ingredientsDiseaseSerum ige
The invention provides an inactivated vaccine for streptococcus suis and pasteurella multocida diseases and a preparation method thereof, which can prevent streptococcus suis infection caused by a plurality of different sero-group streptococcus and infection caused by a plurality of different capsular serotype pasteurella multocida, achieves multiple preventions with one injection clinically, and is lowered in cost, free from the hidden hazard of virus dispersion, safe and reliable. The invention further provides a method for preparing the inactivated vaccine for streptococcus suis and pasteurella multocida diseases.
Owner:广东永顺生物制药股份有限公司
Induction of an immune response against streptococcus pneumoniae polyaccharides
InactiveUS20090317412A1Antibacterial agentsOrganic active ingredientsDiseaseStreptococcus pneumoniae
The present invention provides conjugates of pan DR binding peptides with Streptococcus pneumoniae polysaccharides, and methods of preventing and treating diseases associated with Streptococcus pneumoniae infection with such conjugates.
Owner:ALEXANDER JEFFERY L +2
Protein-based streptococcus pneumoniae vaccines
InactiveUS20090252756A1Avoid infectionOvercome disadvantagesBacterial antigen ingredientsCell membraneCell wall
Vaccine compositions and methods for protecting a mammalian subject against infection with S. pneumoniae are disclosed. The vaccines and methods comprise an effective amount of one or more Streptococcus pneumoniae cell wall and / or cell membrane proteins and / or immunogenically-active fragments, derivatives or modifications thereof, wherein said proteins are selected from a defined group of proteins associated with age-dependent immunological responses.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
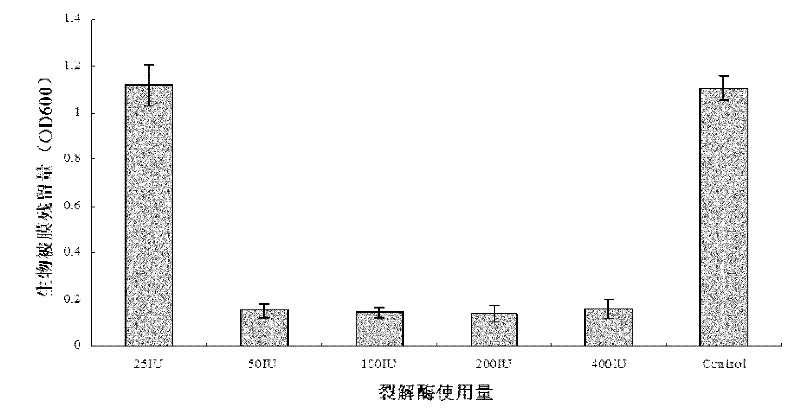
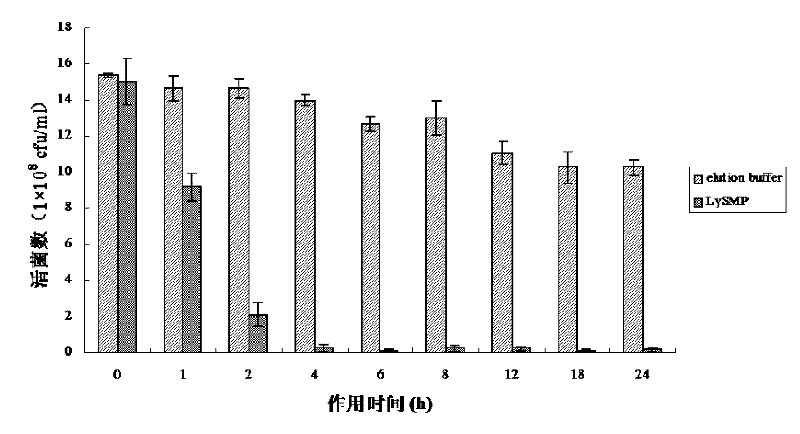
Method for degrading streptococcus suis biofilm by applying phage lyase
ActiveCN102198265AEfficient lysisClean up thoroughlyAntibacterial agentsPeptide/protein ingredientsBiofilmLyase
The invention provides a method for degrading a streptococcus suis biofilm by applying phage lyase, belonging to the field of biotechnology. The method comprises the following steps of: expressing lyase LySMP of which the molecular weight is 55kDa by adopting expression bacteria BL21-lys, purifying with a Ni column to obtain lyase, and degrading the streptococcus suis biofilm obtained by culture on a cell culture plate by using the lyase in vitro. The method provided by the invention can sterilize streptococcus suis SS2-4 and SS2-H and can simultaneously destroy the structure of the biofilm to achieve the effect on thoroughly clearing the biofilm.
Owner:SHANGHAI JIAO TONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com