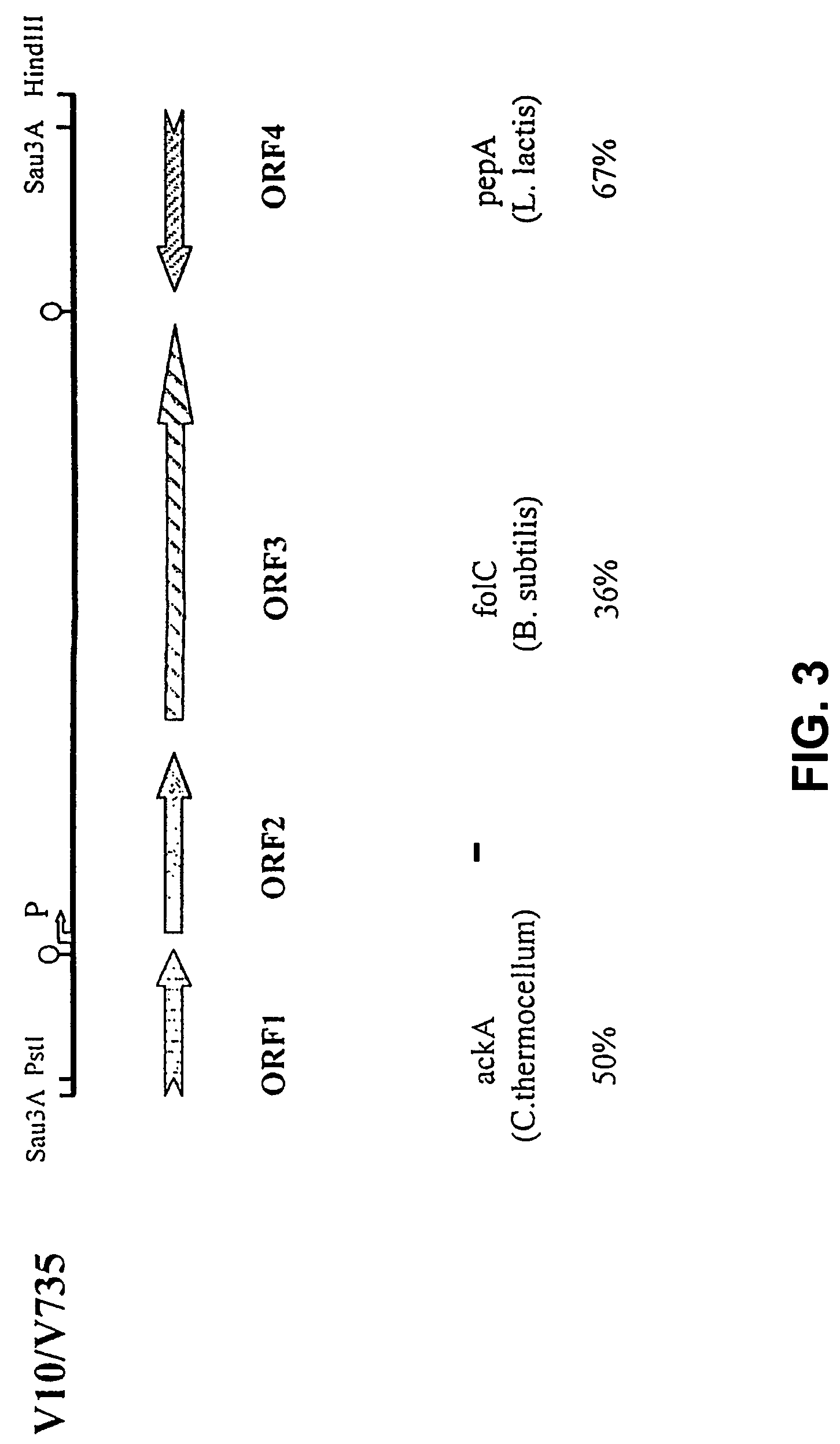
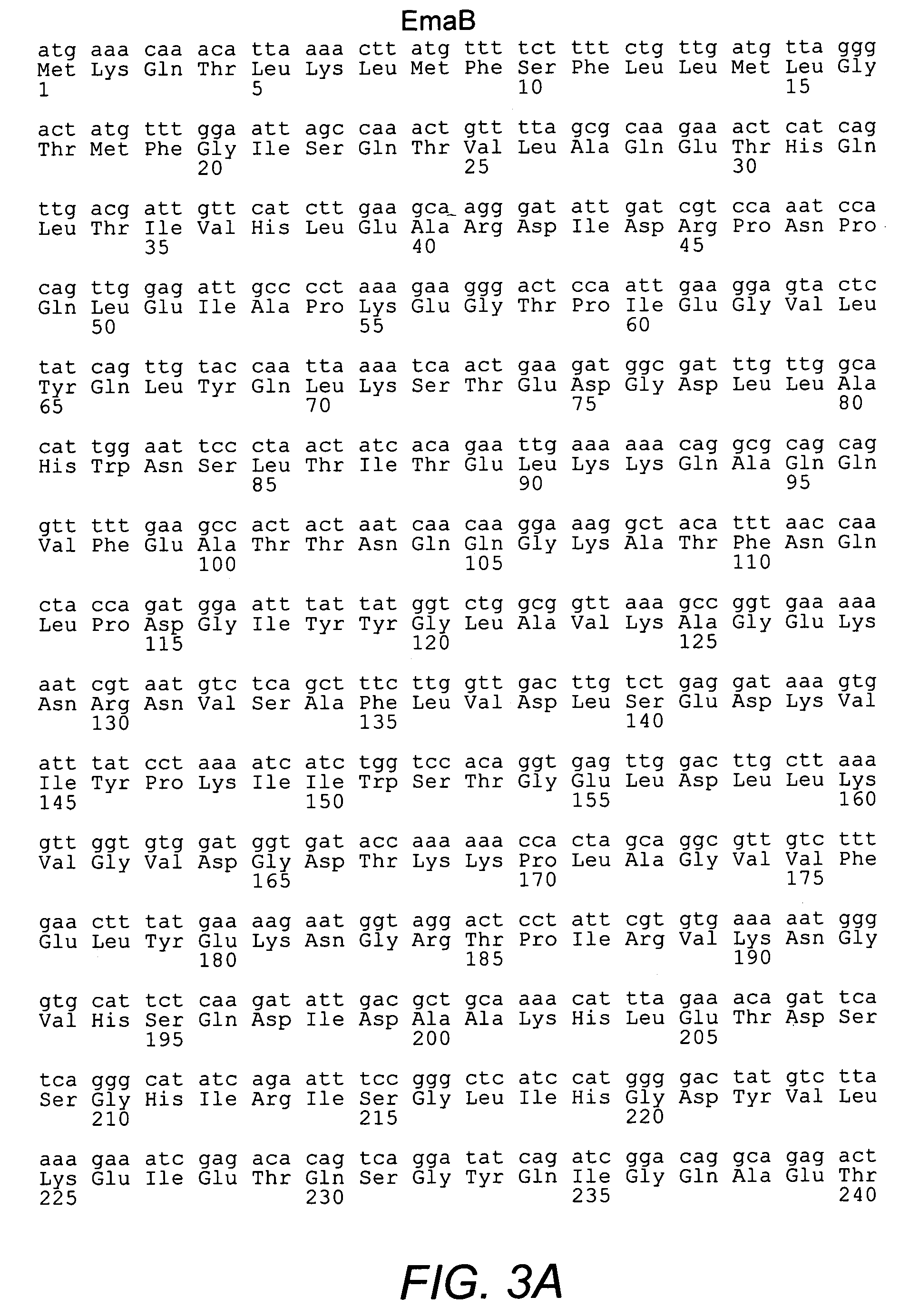Patents
Literature
300 results about "Streptococcus constellatus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Streptococcus constellatus is a species of Streptococcus part of the normal flora in the oral cavity, urogenital region, and intestinal tract. However, it can frequently cause purulent infections in other parts of the body. DNA homology studies and 16S rRNA sequence analysis demonstrate S. constellatus belongs to the Streptococcus anginosus group (milleri group) along with Streptococcus intermedius and Streptococcus anginosus.
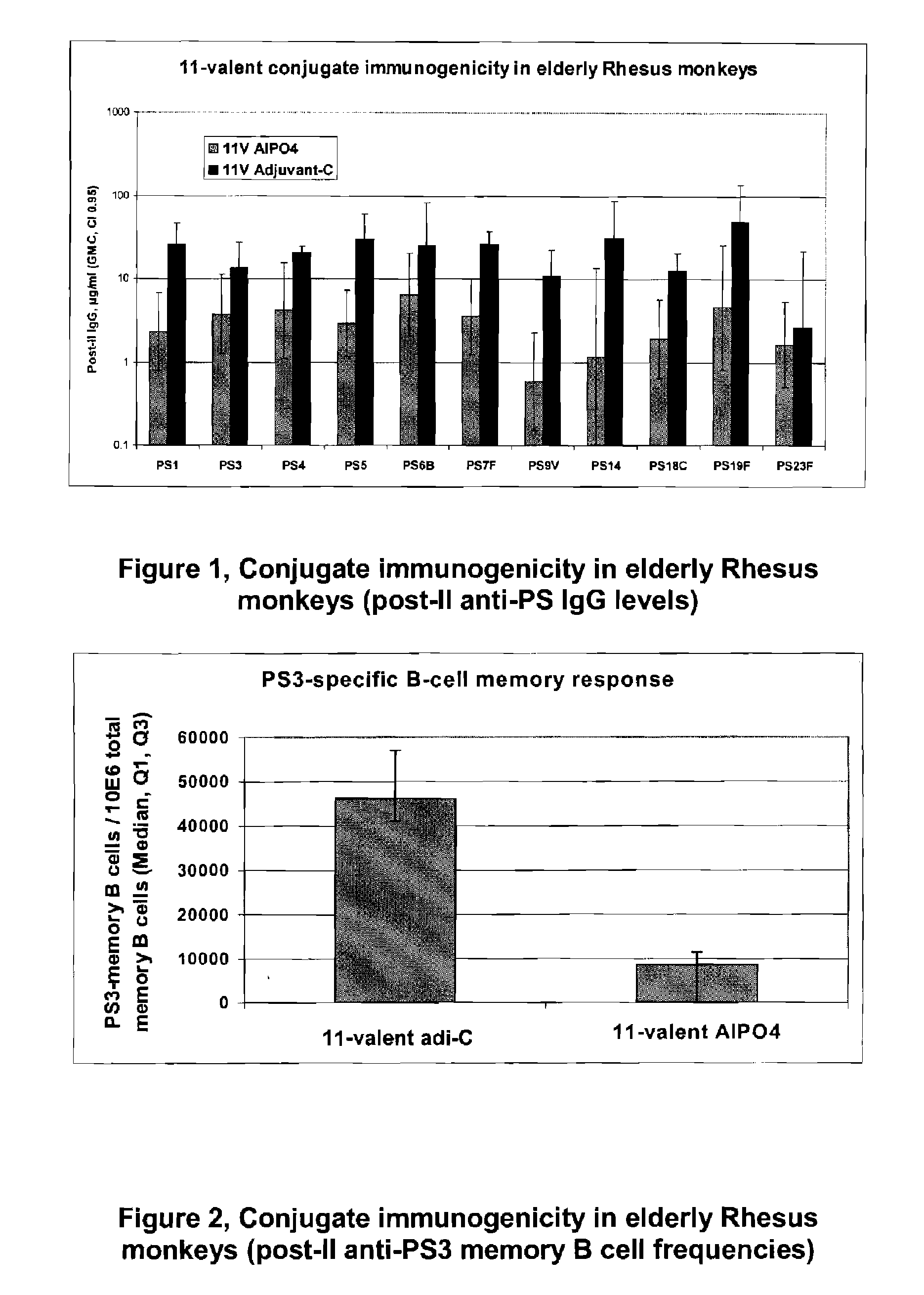
Multivalent pneumococcal polysaccharide-protein conjugate composition
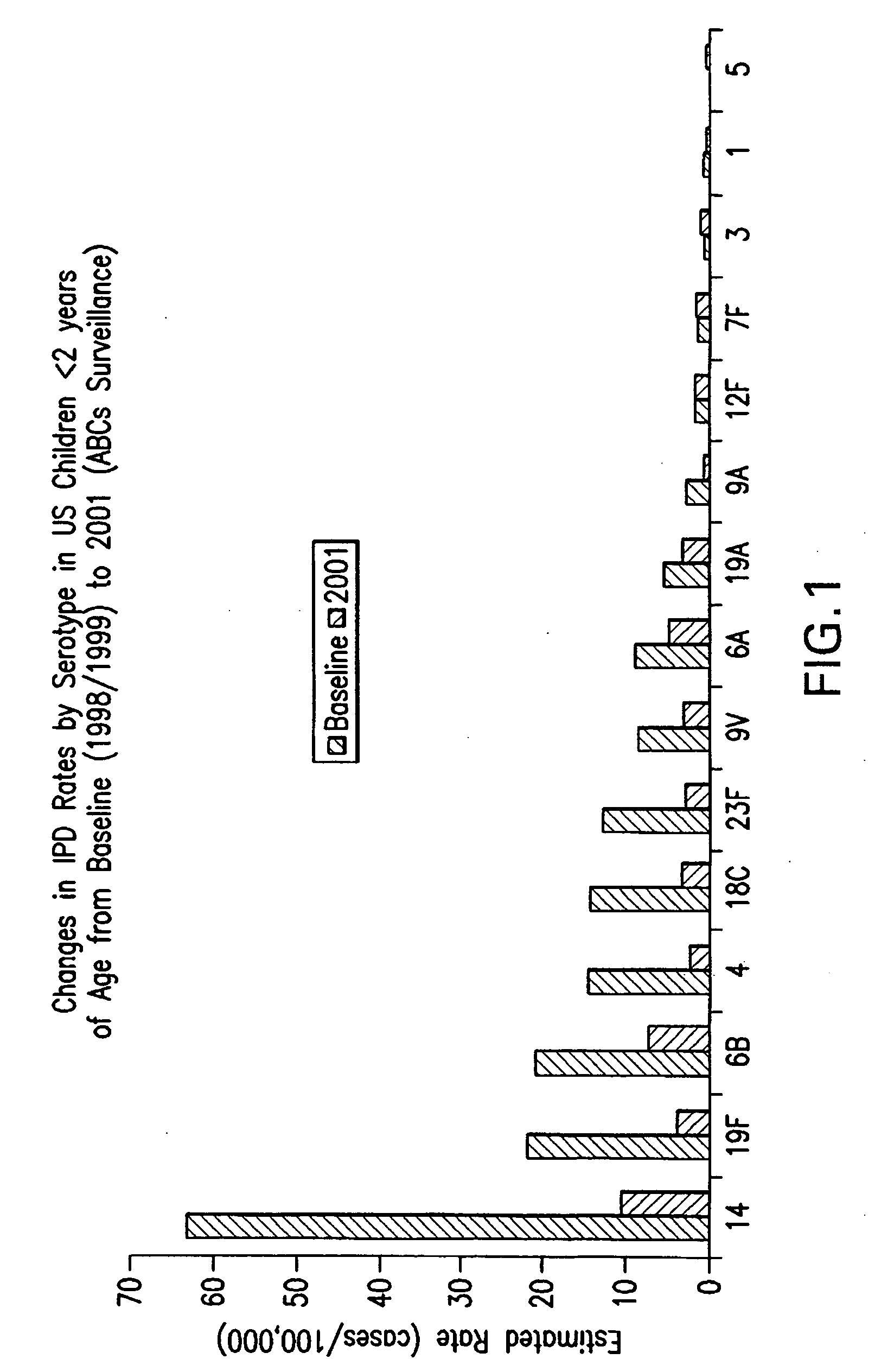
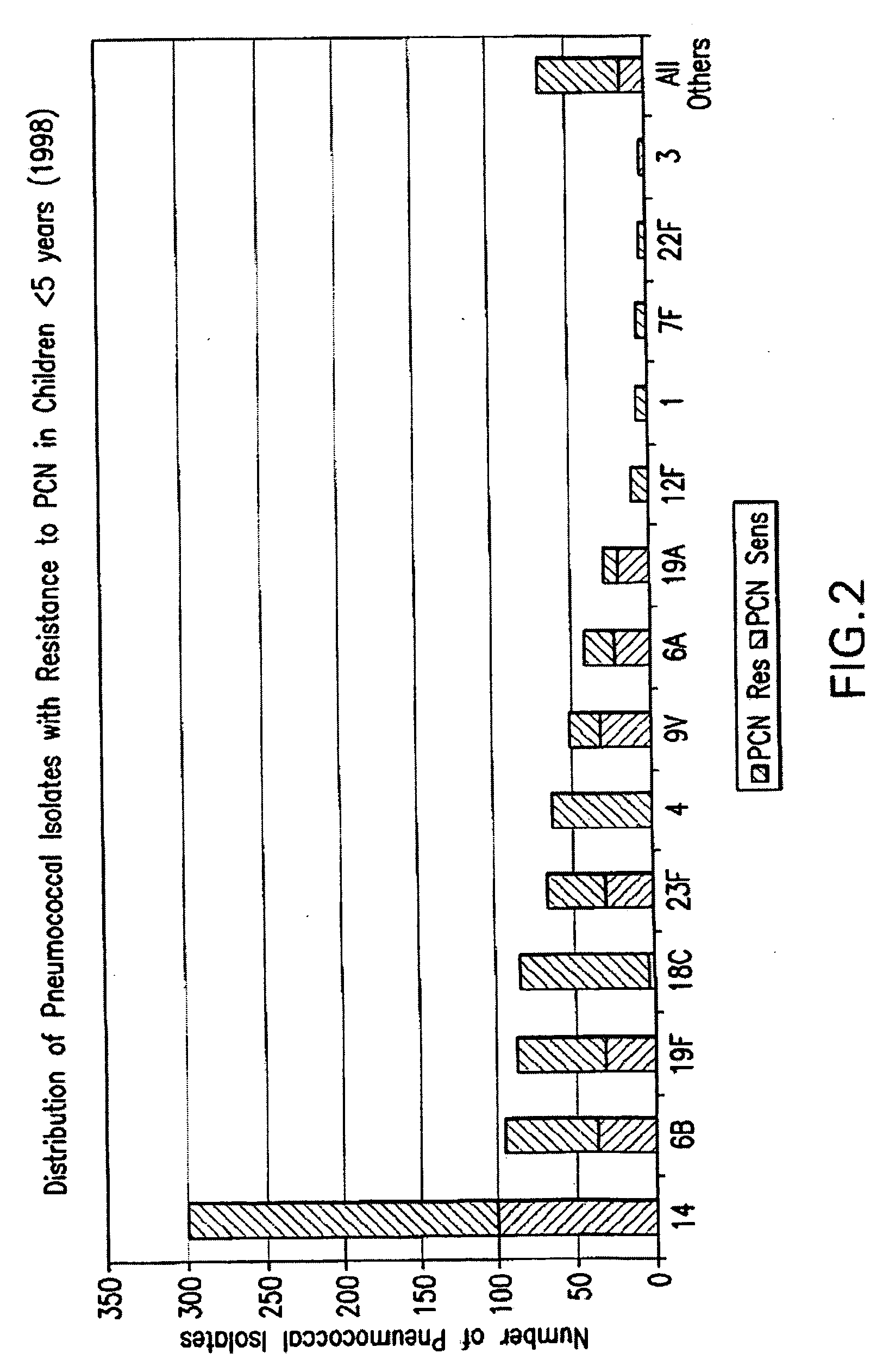
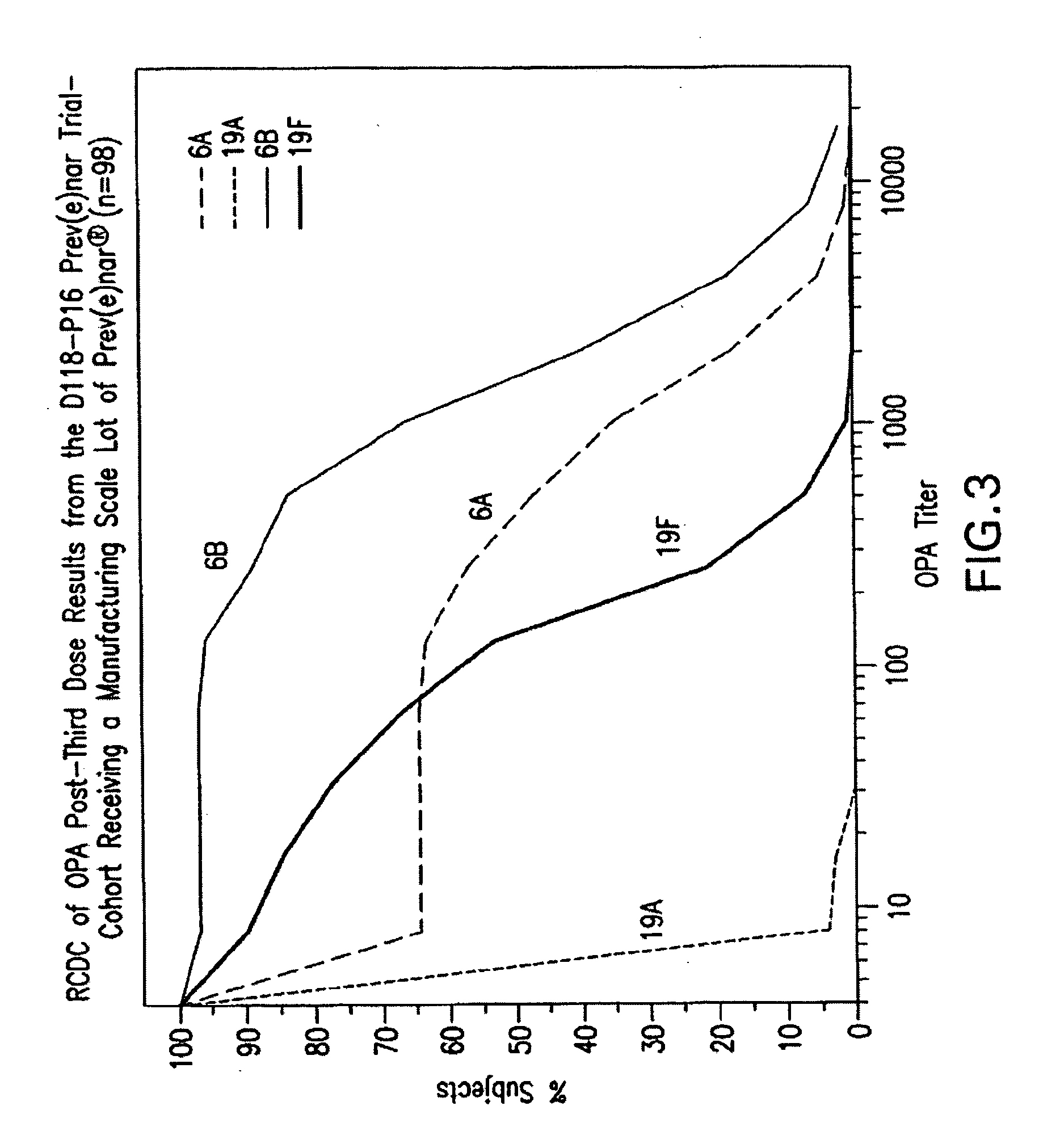
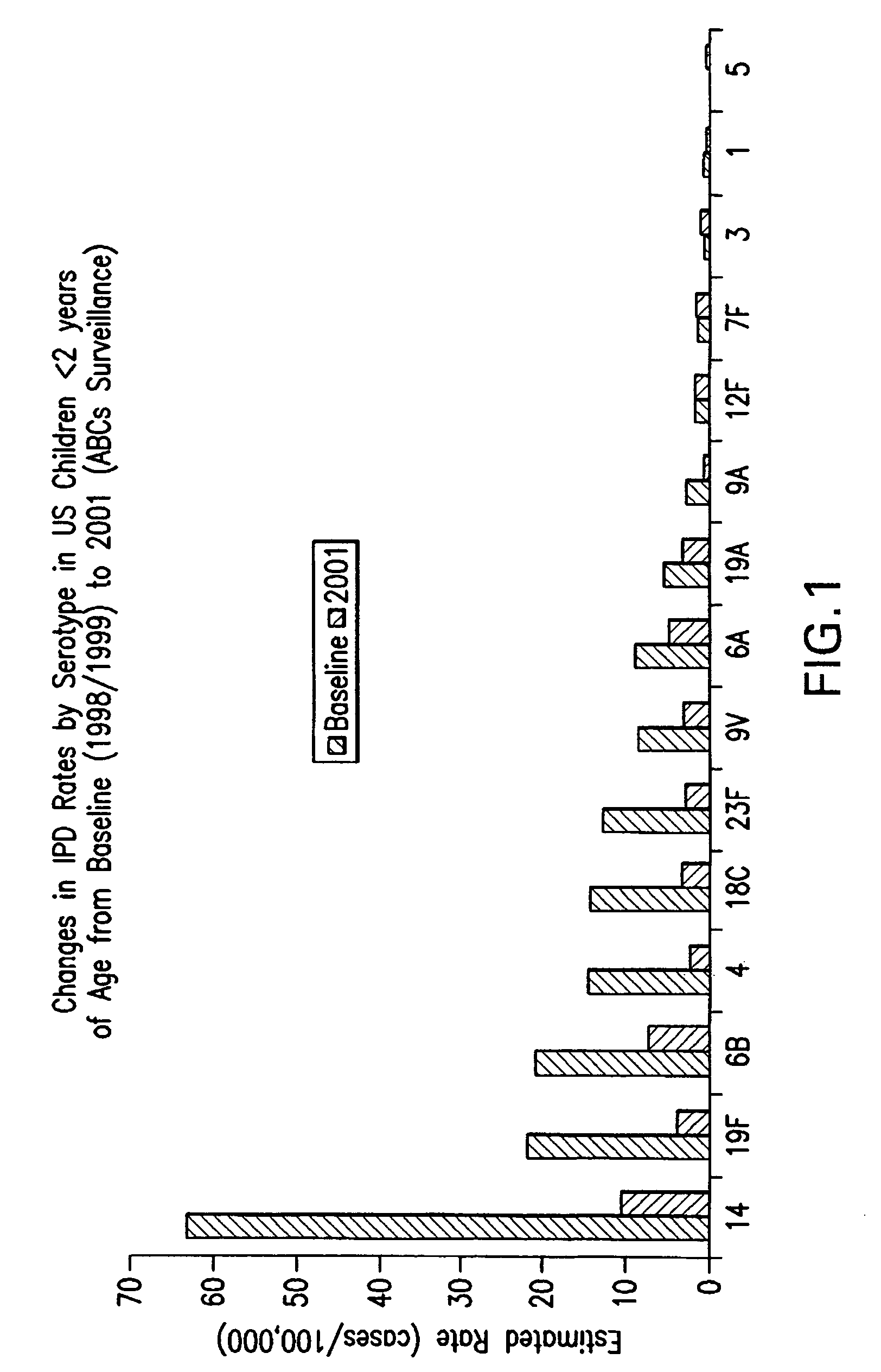
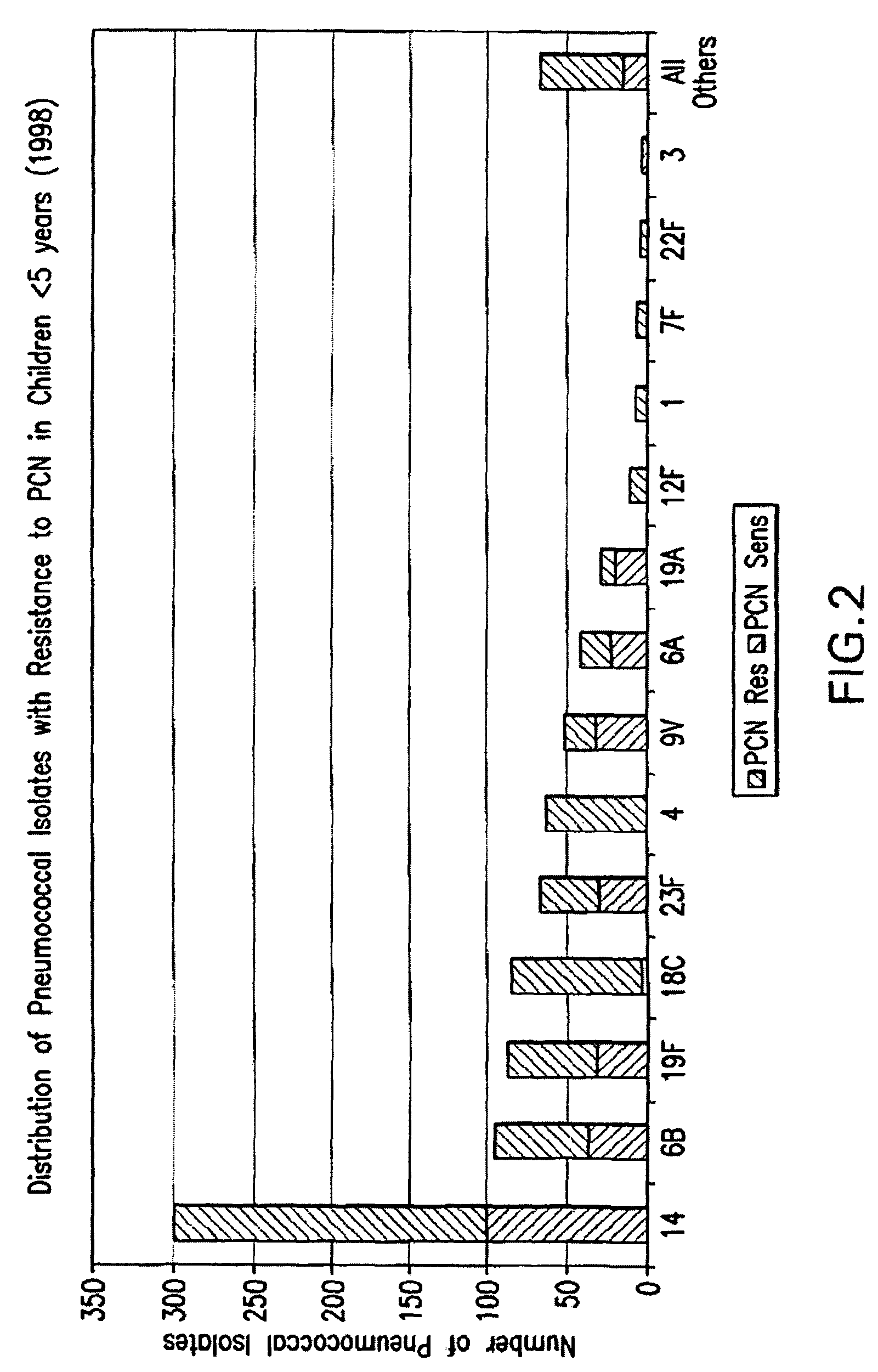
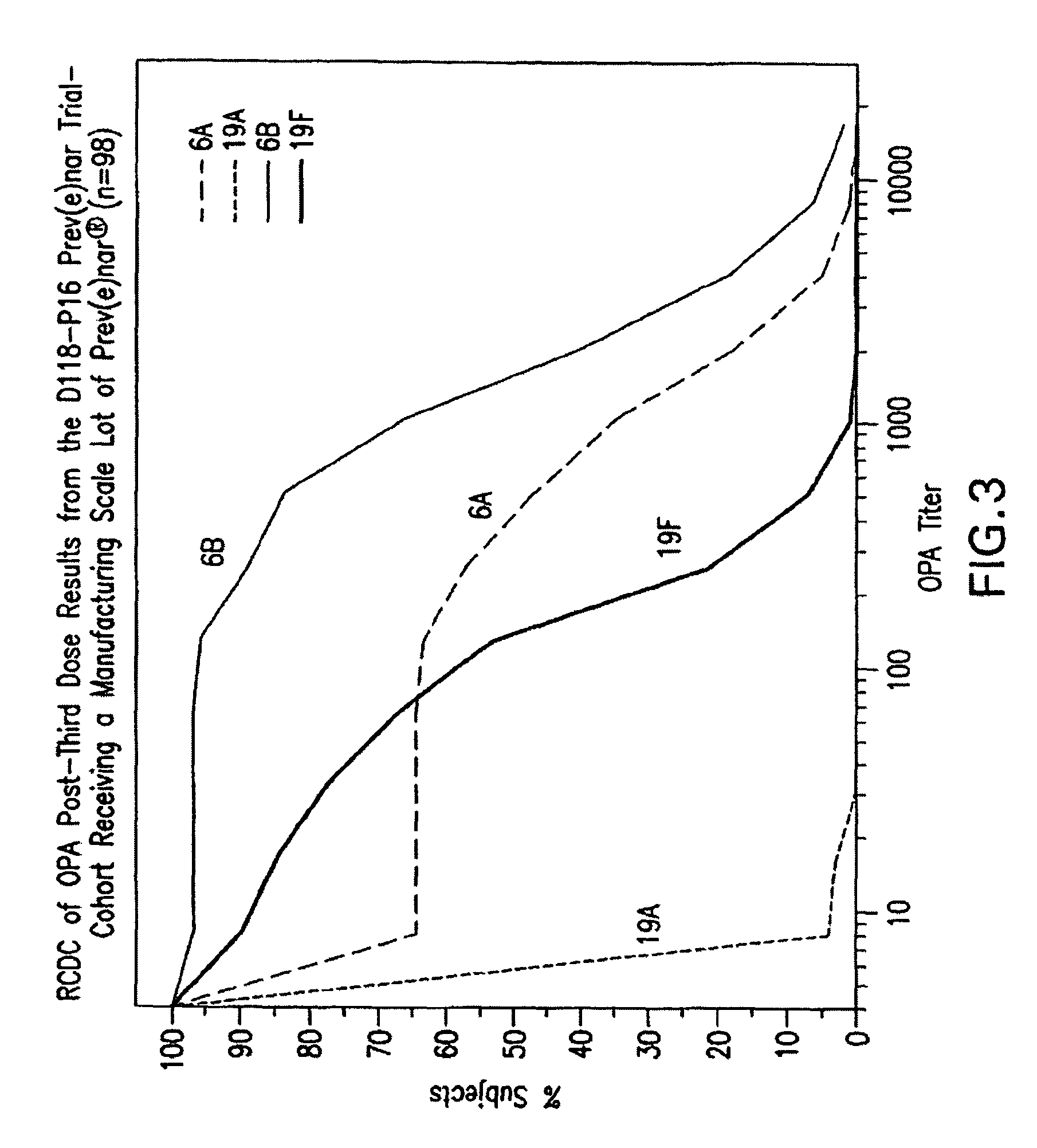
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Methods for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 19A polysaccharide are also provided in which the serotype 19A polysaccharide is co-lyophilized with a carrier protein and conjugation is carried out in dimethyl sulfoxide (DMSO) via a reductive amination mechanism.
Owner:WYETH LLC
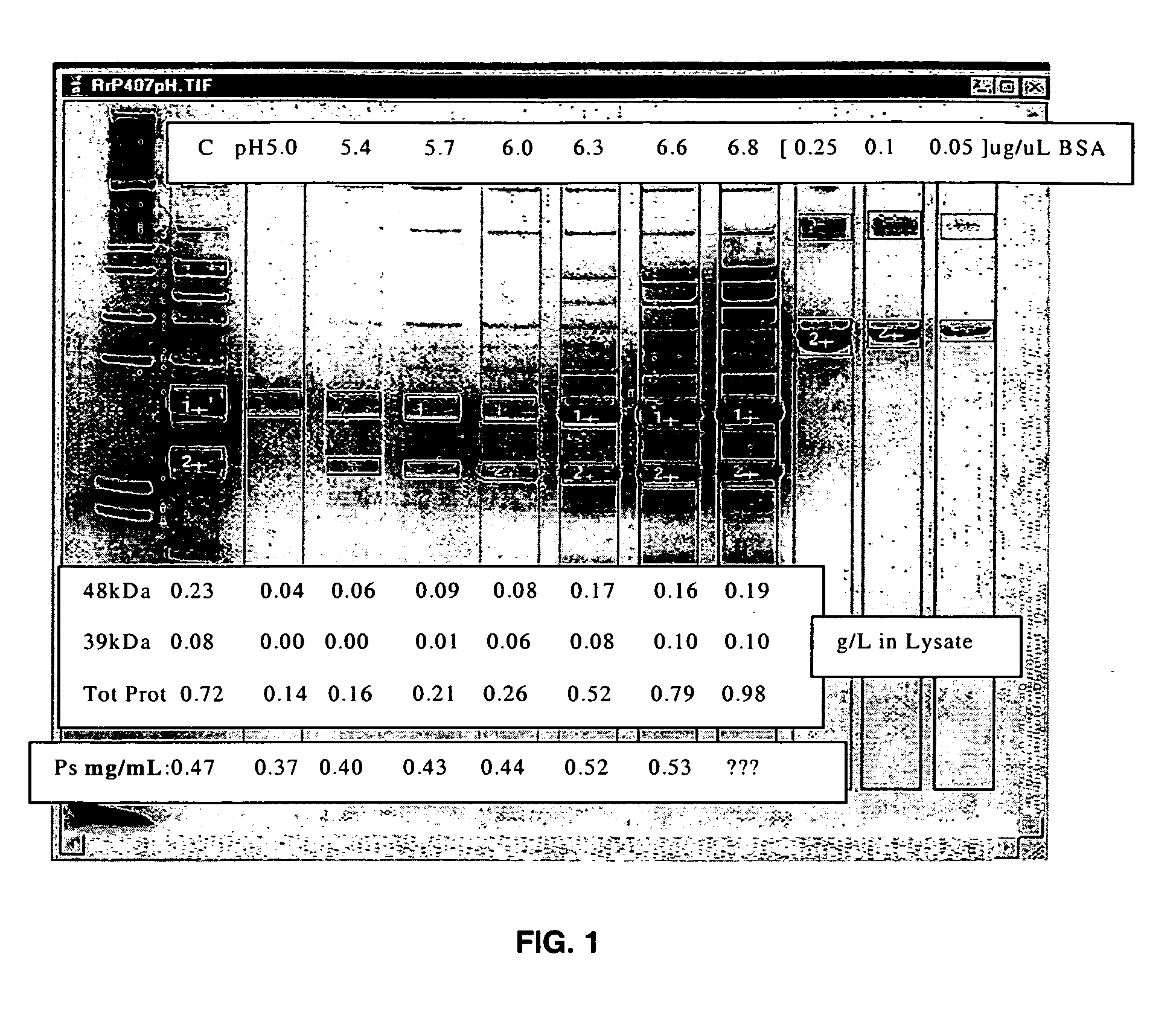
Separation of contaminants from Streptococcus pneumoniae polysaccharide by pH manipulation
ActiveUS20060228381A1Soluble protein is effectively reducedBacterial antigen ingredientsSugar derivativesStreptococcus pneumoniaeLysis
A process for reducing the protein content and preserving the capsular polysaccharide content in a complex cellular Streptococcus pneumoniae lysate broth prior to purification is described. Utilizing pH reduction after cellular lysis has resulted in a purified polysaccharide that consistently meets the protein specification, and higher recovery yields of polysaccharide during the purification process.
Owner:WYETH LLC
Streptococcus pneumoniae 37-kDa surface adhesin a protein
The invention provides a nucleic acid encoding the 37-kDa protein from Streptococcus pneumoniae. Also provided are isolated nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. The invention also provides purified polypeptides encoded by the nucleic acid encoding the 37-kDa protein from and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Also provided are antibodies which selectively binds the polypeptides encoded by the nucleic acid encoding the 37-kDa protein and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Also provided are vaccines comprising immunogenic polypeptides encoded by the nucleic acid encoding the 37-kDa protein and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Further provided is a method of detecting the presence of Streptococcus pneumoniae in a sample comprising the steps of contacting a sample suspected of containing Streptococcus pneumoniae with nucleic acid primers capable of hybridizing to a nucleic acid comprising a portion of the nucleic acid encoding the 37-kDa protein, amplifying the nucleic acid and detecting the presence of an amplification product, the presence of the amplification product indicating the presence of Streptococcus pneumoniae in the sample. Further provided are methods of detecting the presence of Streptococcus pneumoniae in a sample using antibodies or antigens, methods of preventing and treating Streptococcus pneumoniae infection in a subject.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Methods for the separation of streptococcus pneumoniae type 3 polysaccharides
ActiveUS20080102498A1Reducing and removing impurityImprove filtering effectAntibacterial agentsOrganic active ingredientsStreptococcus pneumoniaeLysis
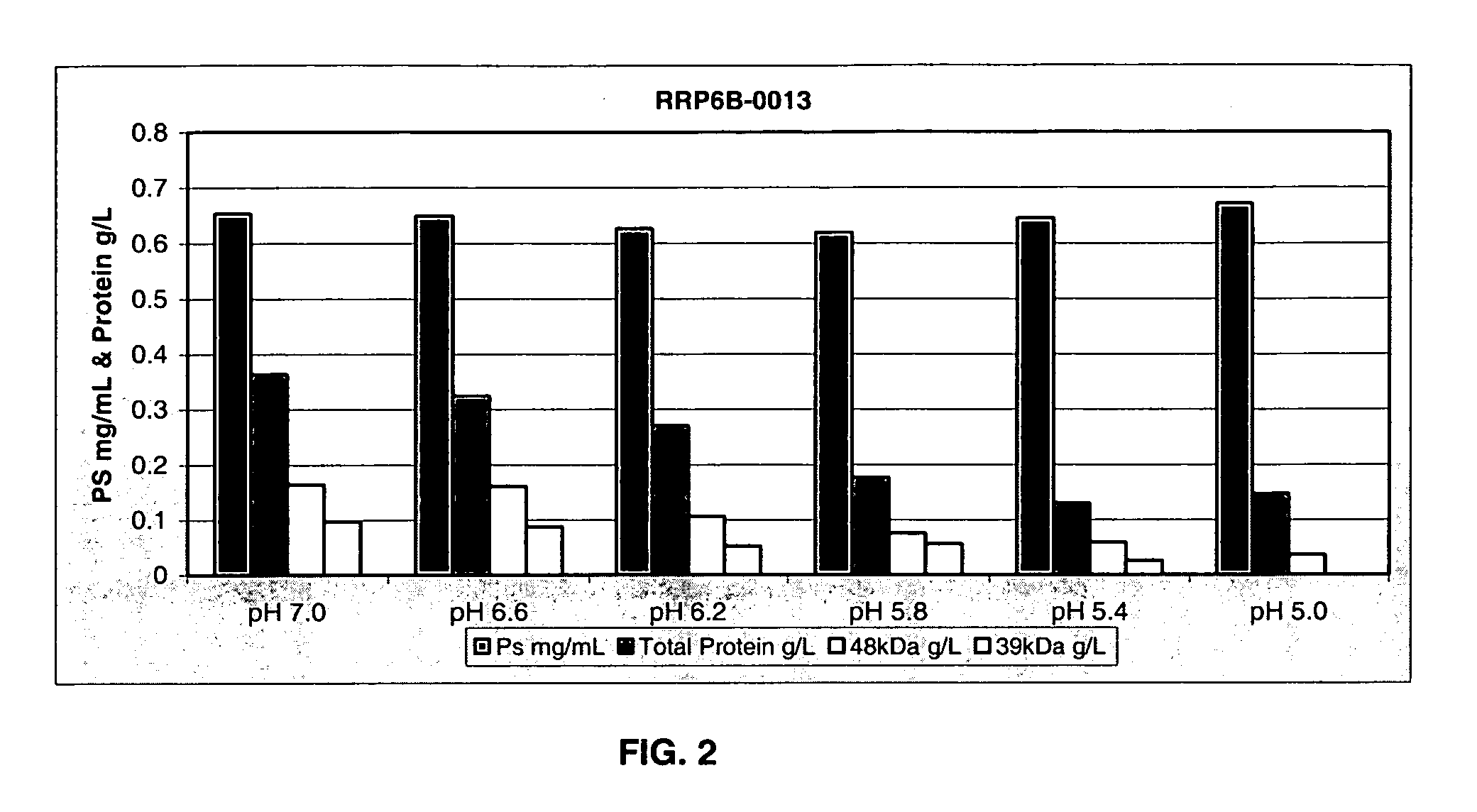
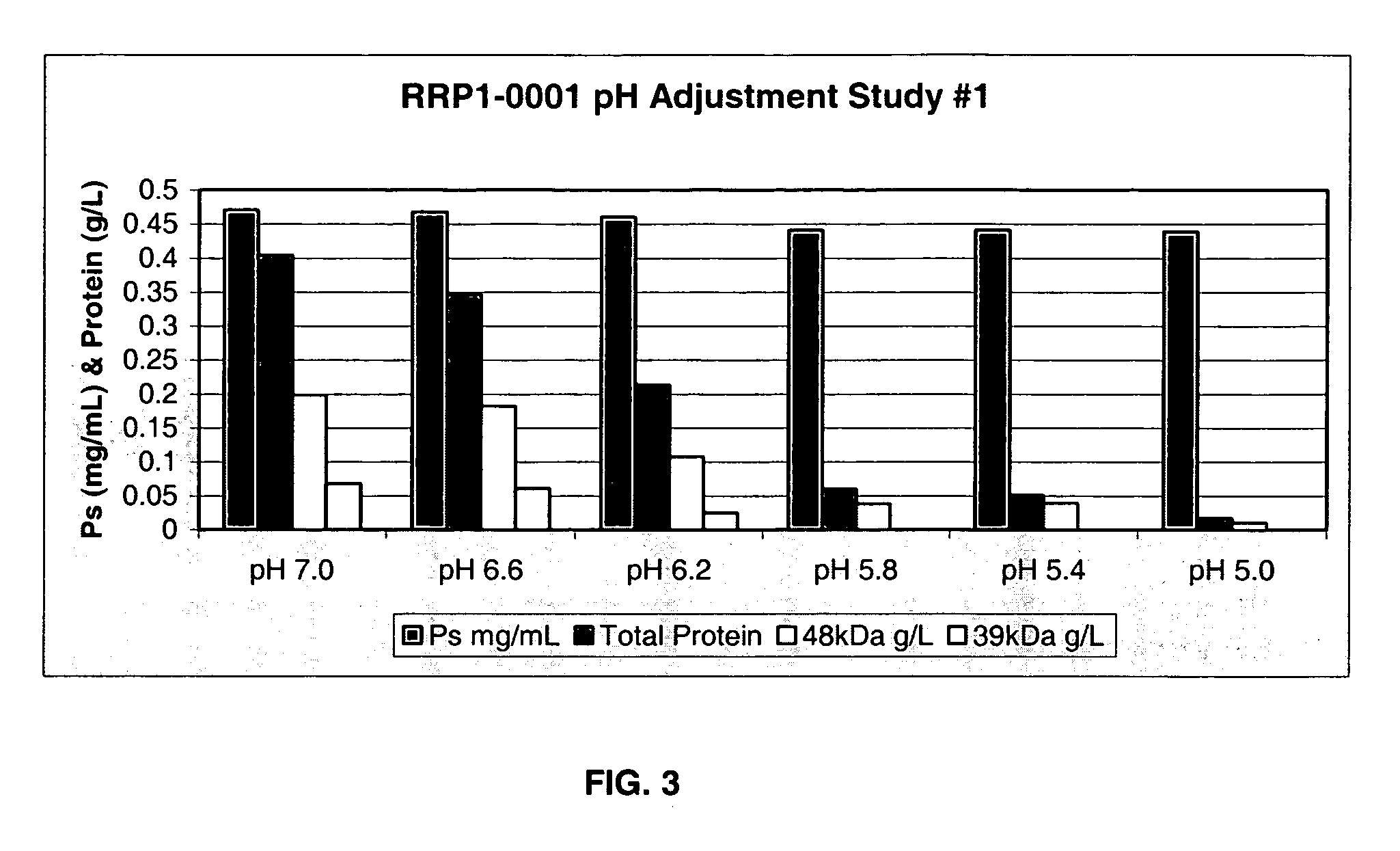
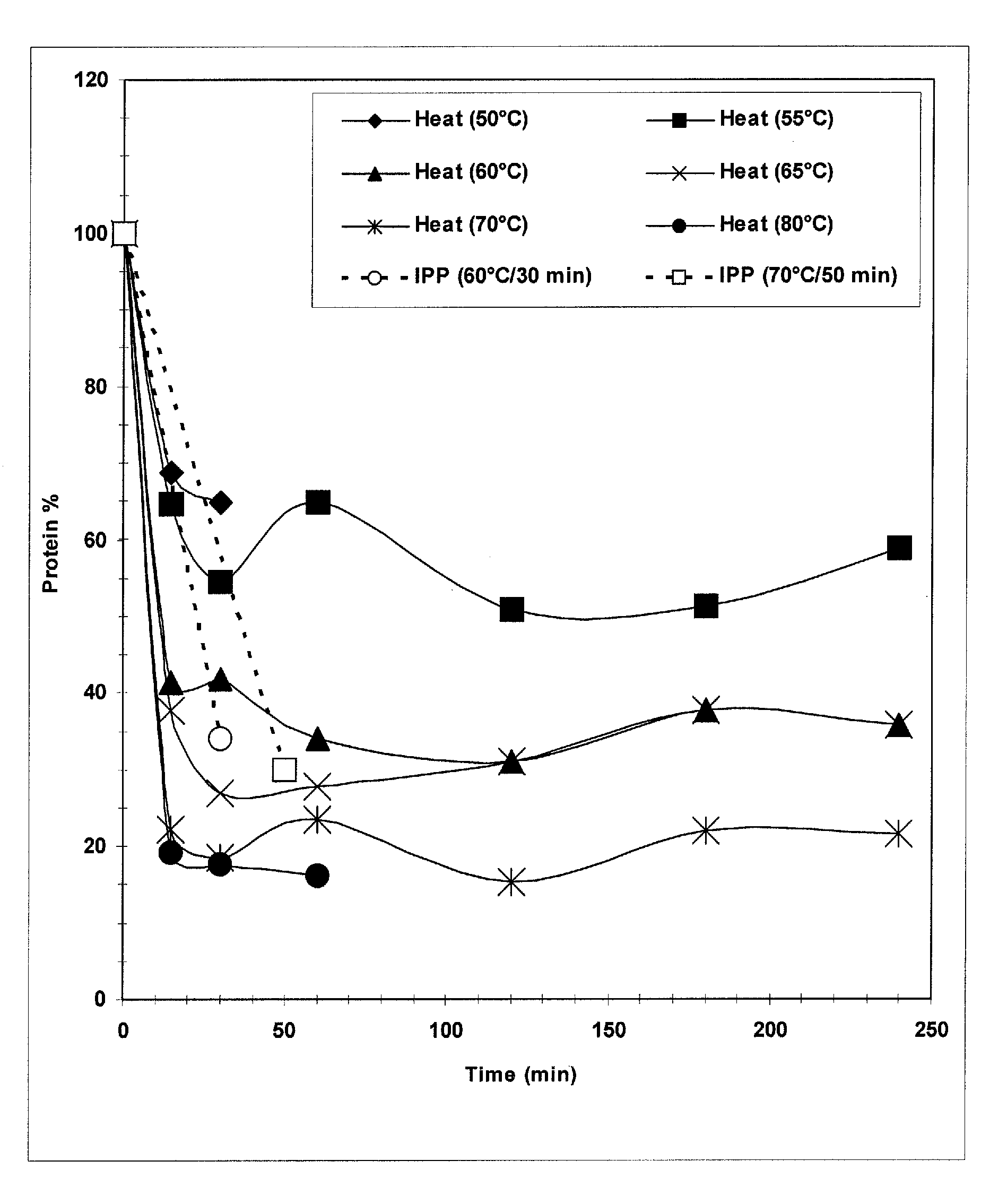
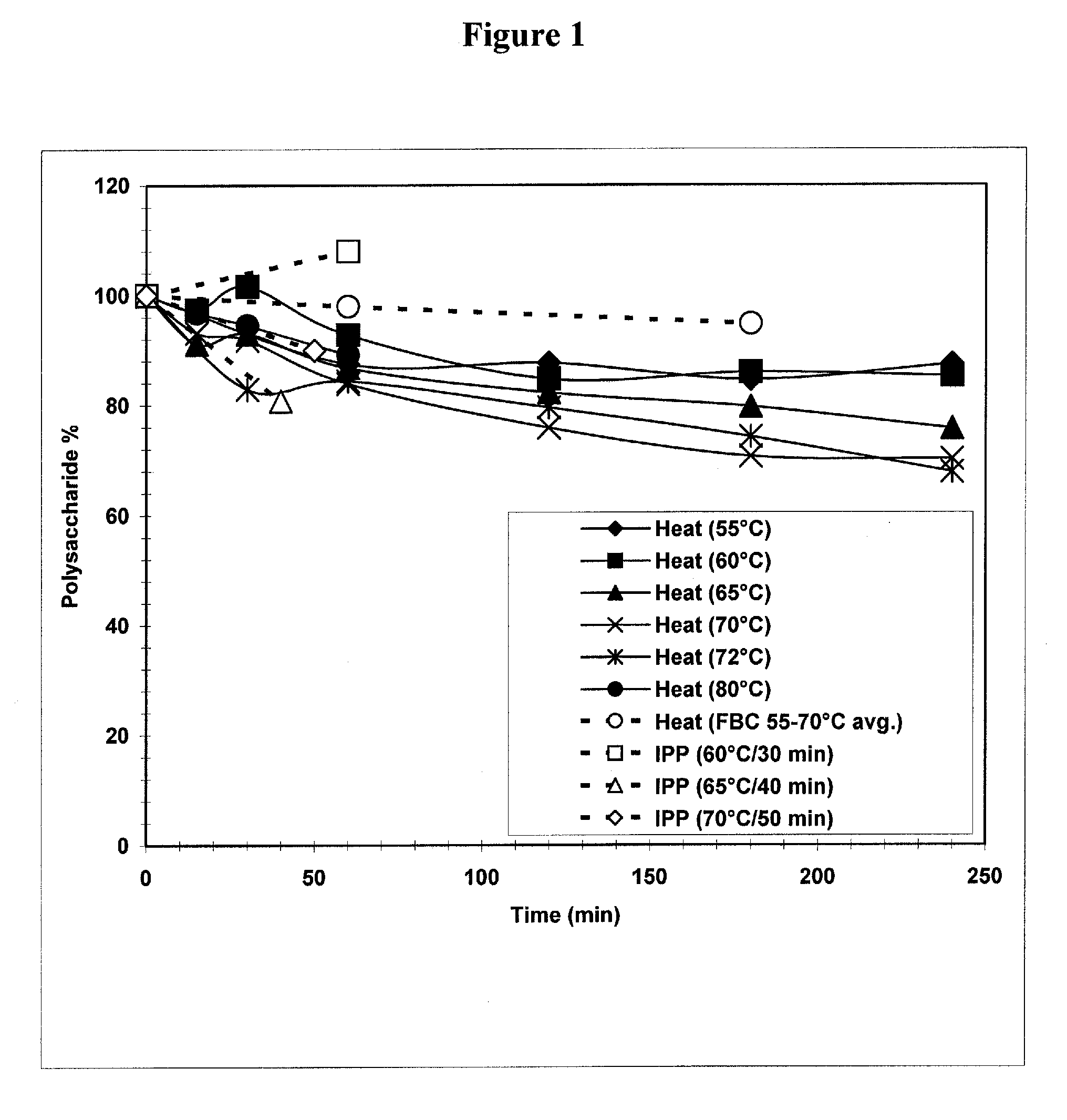
The present invention provides improved methods for the reduction or removal of protein impurities from a complex cellular Streptococcus pneumoniae lysate or centrate comprising serotype 3 polysaccharides involving steps relating to post-lysis heating or pH adjustment. In certain methods, the lysate is heated for a time and at a temperature sufficient to denature proteins present in the lysate and cause their aggregation and precipitation. In one embodiment, the lysate is heated to at least 60° C. for at least 30 minutes to cause protein aggregation and precipitation, more particularly about 60° C. to about 70° C. for about 30 to about 50 minutes, and even more particularly about 65° C. for about 40 minutes. In other methods, the pH of the lysate or centrate is increased to at least 8.0 to improve filterability, more particularly about 8.0 to 8.4, and even more particularly about 8.2. In further methods, heating and pH adjustment steps are combined to cause the aggregation and precipitation of proteins as well as to improve filterability of the lysates or centrates. In other methods, the pH of the lysate or centrate is lowered to about 3.0 to about 5.0 to cause protein aggregation and precipitation. Such methods allow for the production of substantially purified serotype 3 polysaccharide-containing lysates or centrates.
Owner:WYETH LLC
Streptococcus pneumoniae SP036 polynucleotides
The present invention relates to novel vaccines for the prevention or attenuation of infection by Streptococcus pneumoniae. The invention further relates to isolated nucleic acid molecules encoding antigenic polypeptides of Streptococcus pneumoniae. Antigenic polypeptides are also provided, as are vectors, host cells and recombinant methods for producing the same. The invention additionally relates to diagnostic methods for detecting Streptococcus nucleic acids, polypeptides and antibodies in a biological sample.
Owner:HUMAN GENOME SCI INC
Nucleic acid and amino acid sequences relating to Streptococcus pneumoniae for diagnostics and therapeutics
InactiveUS7098023B1Easy to adaptSugar derivativesBacteriaStreptococcus pneumoniaeNucleic acid sequencing
The invention provides isolated polypeptide and nucleic acid sequences derived from Streptococcus pneumoniae that are useful in diagnosis and therapy of pathological conditions; antibodies against the polypeptides; and methods for the production of the polypeptides. The invention also provides methods for the detection, prevention and treatment of pathological conditions resulting from bacterial infection.
Owner:SANOFI PASTEUR LTD
Nucleic acid and amino acid sequences relating to Streptococcus pneumoniae for diagnostics and therapeutics
InactiveUS7074914B1Easy to adaptAntibacterial agentsOrganic active ingredientsStreptococcus pneumoniaeNucleic acid sequencing
Owner:SANOFI PASTEUR LTD
Nucleic acid and amino acid sequences relating to Streptococcus pneumoniae for diagnostics and therapeutics
InactiveUS7081530B1Easy to adaptBacteriaSugar derivativesStreptococcus pneumoniaeNucleic acid sequencing
The invention provides isolated polypeptide and nucleic acid sequences derived from Streptococcus pneumoniae that are useful in diagnosis and therapy of pathological conditions; antibodies against the polypeptides; and methods for the production of the polypeptides. The invention also provides methods for the detection, prevention and treatment of pathological conditions resulting from bacterial infection.
Owner:SANOFI PASTEUR LTD
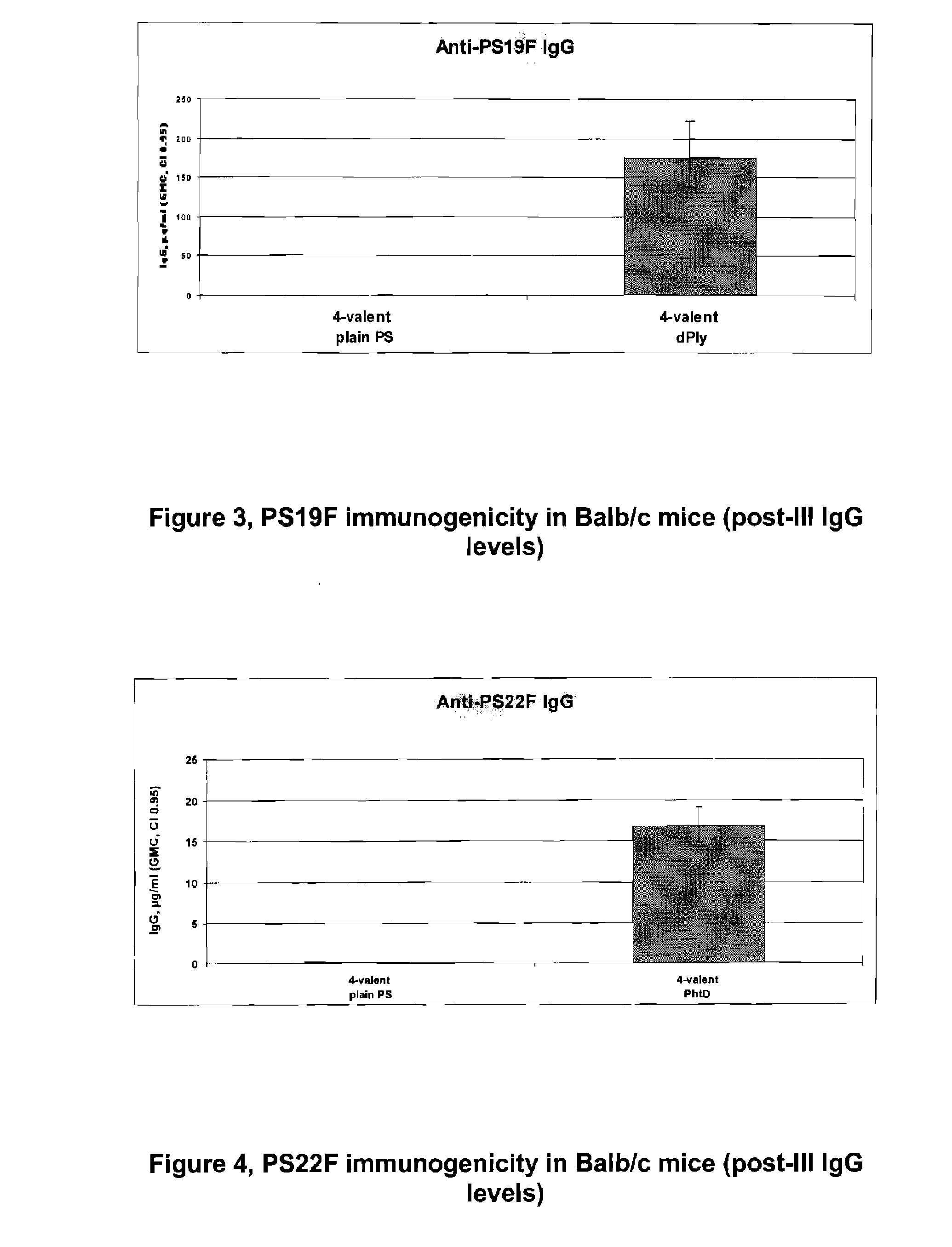
Vaccine comprising streptococcus pneumoniae capsular polysaccharide conjugates
InactiveUS20100209450A1Antibacterial agentsSenses disorderConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various conjugated capsular saccharides from different S. pneumoniae serotypes conjugated to 2 or more different carrier proteins, where the composition comprises serotype 19F capsular saccharide conjugated to diphtheria toxoid. Methods of making and uses thereof are also described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Vaccine Comprising Streptococcus Pneumoniae Capsular Polysaccharide Conjugates
InactiveUS20090017059A1Antibacterial agentsSenses disorderStreptococcus pneumoniae capsular polysaccharideStreptococcus mitis
The present invention discloses an immunogenic composition comprising S. pneumoniae capsular saccharide conjugates from serotypes 19A and 19F wherein 19A is conjugated to a first bacterial toxoid and 19F is conjugated to a second bacterial toxoid. Vaccines, methods of making vaccines and uses of the vaccines are also described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Species-specific, genus-specific and universal DNA probes and amplification primers to rapidly detect and identify common bacterial and fungal pathogens and associated antibiotic resistance genes from clinical specimens for diagnosis in microbiology laboratories
InactiveUS20040185478A1Reduce usageDetermine rapidly the bacterial resistance to antibioticsMicrobiological testing/measurementFermentationBacteroidesNeisseria meningitidis
DNA-based methods employing amplification primers or probes for detecting, identifying, and quantifying in a test sample DNA from (i) any bacterium, (ii) the species Streptococcus agalactiae, Staphylococcus saprophyticus, Enterococcus faecium, Neisseria meningitidis, Listeria monocytogenes and Candida albicans, and (iii) any species of the genera Streptococcus, Staphylococcus, Enterococcus, Neisseria and Candida are disclosed. DNA-based methods employing amplification primers or probes for detecting, identifying, and quantifying in a test sample antibiotic resistance genes selected from the group consisting of blatem, blarob, blashv, blaoxa, blaZ, aadB, aacC1, aacC2, aacC3, aacA4, aac6'-lla, ermA, ermB, ermC, mecA, vanA, vanB, vanC, satA, aac(6')-aph(2''), aad(6'), vat, vga, msrA, sul and int are also disclosed. The above microbial species, genera and resistance genes are all clinically relevant and commonly encountered in a variety of clinical specimens. These DNA-based assays are rapid, accurate and can be used in clinical microbiology laboratories for routine diagnosis. These novel diagnostic tools should be useful to improve the speed and accuracy of diagnosis of microbial infections, thereby allowing more effective treatments. Diagnostic kits for (i) the universal detection and quantification of bacteria, and / or (ii) the detection, identification and quantification of the above-mentioned bacterial and fungal species and / or genera, and / or (iii) the detection, identification and quantification of the above-mentioned antibiotic resistance genes are also claimed.
Owner:GENEOHM SCI CANADA
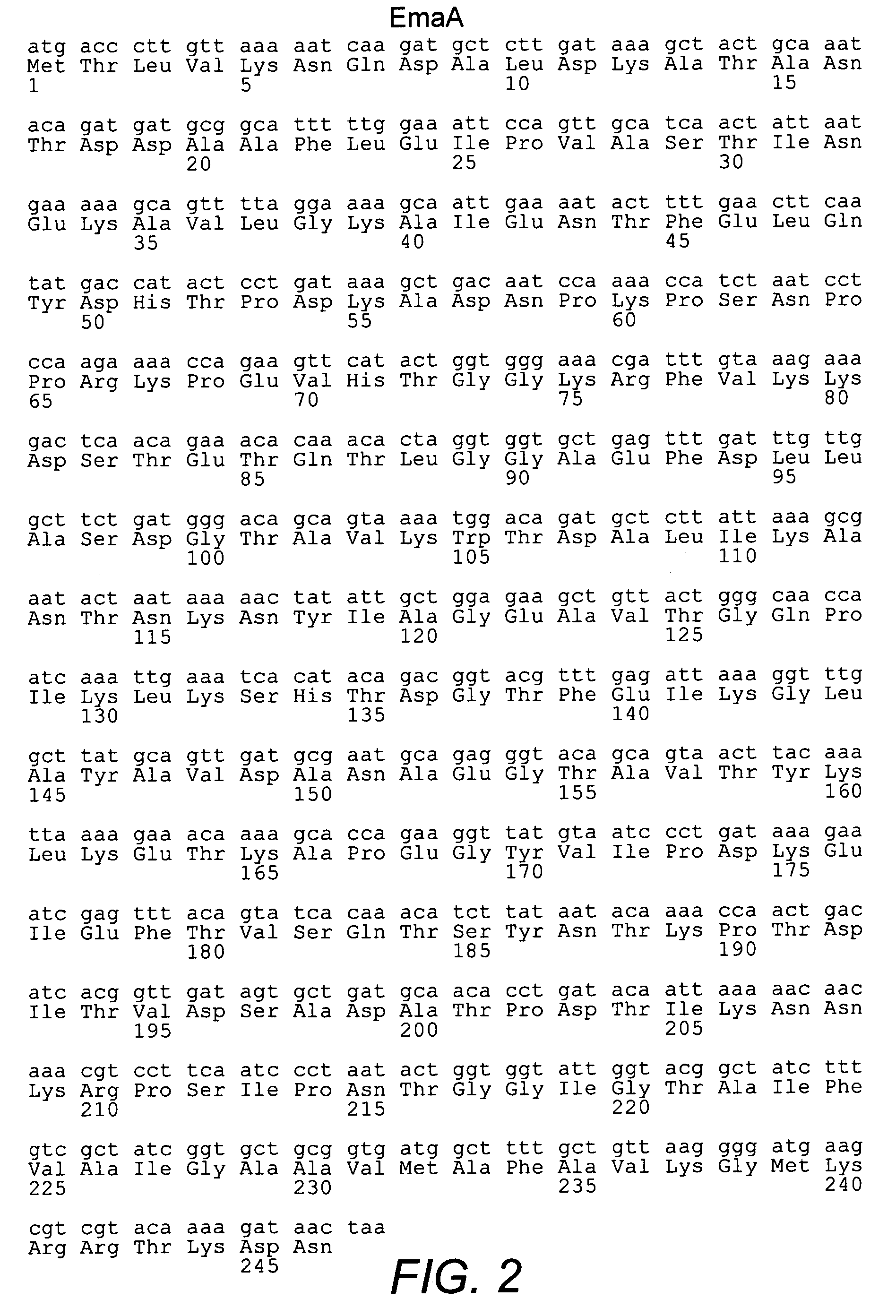
Group B streptococcus polypeptides nucleic acids and therapeutic compositions and vaccines thereof
InactiveUS7128919B2Modulate activityAvoid infectionBacteriaWhole-cell/virus/DNA/RNA ingredientsBacteroidesMutant
This invention provides isolated nucleic acids encoding polypeptides comprising amino acid sequences of streptococcal matrix adhesion (Ema) polypeptides. The invention provides nucleic acids encoding Group B streptococcal Ema polypeptides EmaA, EmaB, EmaC, EmaD and EmaE. The present invention provides isolated polypeptides comprising amino acid sequences of Group B streptococcal polypeptides EmaA, EmaB, EmaC, EmaD and EmaE, including analogs, variants, mutants, derivatives and fragments thereof. Ema homologous polypeptides from additional bacterial species, including S. pneumoniae, S. pyogenes, E. faecalis and C. diptheriae are also provided. Antibodies to the Ema polypeptides and immunogenic fragments thereof are also provided. The present invention relates to the identification and prevention of infections by virulent forms of streptococci. This invention provides pharmaceutical compositions, immunogenic compositions, vaccines, and diagnostic and therapeutic methods of use of the isolated polypeptides, antibodies thereto, and nucleic acids. Assays for compounds which modulate the polypeptides of the present invention for use in therapy are also provided.
Owner:UNIV OF UTAH RES FOUND +1
Product containing Lactobacillus reuteri strain ATTC PTA-4965 or PTA-4964 for inhibiting bacteria causing dental caries
InactiveUS6872565B2Reduce in quantityPrevent and reduce and treat dental cariesAntibacterial agentsCosmetic preparationsBacteroidesBacilli
Strains of Lactobacillus that have been selected for their capability of reducing the number of Streptococcus mutans in the mouth of mammals through inhibiting activity in combination with good binding to the oral mucins and dental plaque, Administering the strains in a product such as food, reduces and treats dental caries. Preferred strains are Lactobacillus reuteri strain ATTC PTA-4965 or Lactobacillus reuteri strain ATTC PTA-4964.
Owner:BIOGAIA AB
Choline binding proteins for anti-pneumococcal vaccines
InactiveUS6245335B1Improve efficiencySufficient quantityAntibacterial agentsBacteriaBacteroidesCholine binding protein
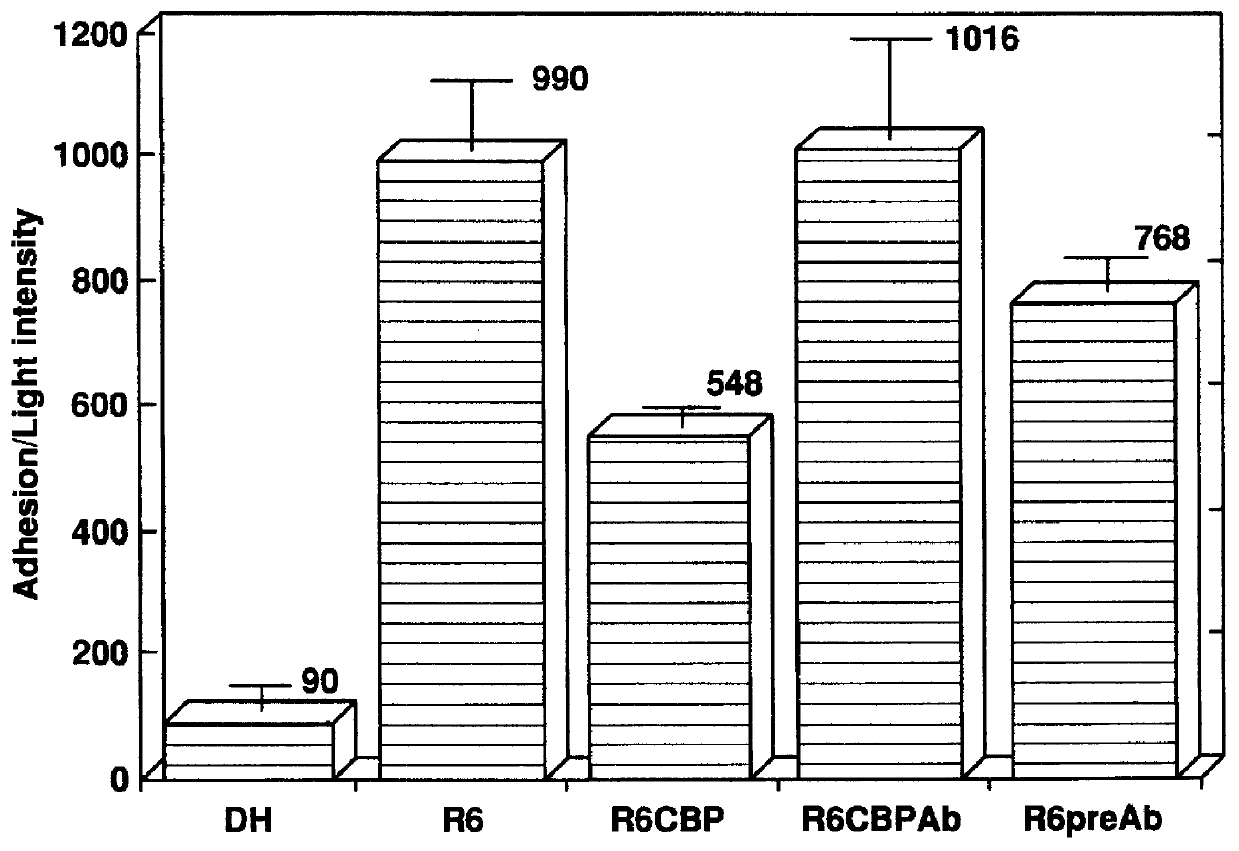
The invention relates to bacterial choline binding proteins (CBPs) which bind choline. Such proteins are particularly desirable for vaccines against appropriate strains of Gram positive bacteria, particularly streptococcus, and more particularly pneumococcus. Also provided are DNA sequences encoding the bacterial choline binding proteins or fragment thereof, antibodies to the bacterial choline binding proteins, pharmaceutical compositions comprising the bacterial choline binding proteins, antibodies to the bacterial choline binding proteins suitable for use in passive immunization, and small molecule inhibitors of choline binding protein mediated adhesion. Methods for diagnosing the presence of the bacterial choline binding protein, or of the bacteria, are also provided. In a specific embodiment, a streptococcal choline binding protein is an enolase, which demonstrates strong affinity for fibronectin.
Owner:THE ROCKEFELLER UNIV
Streptococcus suis vaccines and diagnostic tests
InactiveUS7125548B2ImmunogenicityImproving immunogenicityAntibacterial agentsBacteriaAntigenSpecific detection
The invention relates to Streptococcus suis infection in pigs, vaccines directed against those infections and tests for diagnosing Streptococcus suis infections. The invention provides an isolated or recombinant nucleic acid encoding a capsular gene cluster of Streptococcus suis or a gene or gene fragment derivated thereof. The invention further provides a nucleic acid probe or primer allowing species or serotype-specific detection of Streptococcus suis. The invention also provides a Streptococcus suis antigen and vaccine derived thereof.
Owner:STICHTING DIENST LANBOUWKUNDIG ONDERZOEK
Species-specific, genus-specific and universal DNA probes and amplification primers to rapidly detect and identify common bacterial and fungal pathogens and associated antibiotic resistance genes from clinical specimens for diagnosis in microbiology laboratories
InactiveUS20030049636A1Low costRapid positioningMicrobiological testing/measurementFermentationBacteroidesNeisseria meningitidis
DNA-based methods employing amplification primers or probes for detecting, identifying, and quantifying in a test sample DNA from (i) any bacterium, (ii) the species Streptococcus agalactiae, Staphylococcus saprophyticus, Enterococcus faecium, Neisseria meningitidis, Listeria monocytogenes and Candida albicans, and (iii) any species of the genera Streptococcus, Staphylococcus, Enterococcus, Neisseria and Candida are disclosed. DNA-based methods employing amplification primers or probes for detecting, identifying, and quantifying in a test sample antibiotic resistance genes selected from the group consisting of blatem, blarob, blashv, blaoxa, blaZ, aadB, aacC1, aacC2, aacC3, aacA4, aac6'-lla, ermA, ermB, ermC, mecA, vanA, vanB, vanC, satA, aac(6')-aph(2''), aad(6'), vat, vga, msrA, sul and int are also disclosed. The above microbial species, genera and resistance genes are all clinically relevant and commonly encountered in a variety of clinical specimens. These DNA-based assays are rapid, accurate and can be used in clinical microbiology laboratories for routine diagnosis. These novel diagnostic tools should be useful to improve the speed and accuracy of diagnosis of microbial infections, thereby allowing more effective treatments. Diagnostic kits for (i) the universal detection and quantification of bacteria, and / or (ii) the detection, identification and quantification of the above-mentioned bacterial and fungal species and / or genera, and / or (iii) the detection, identification and quantification of the above-mentioned antibiotic resistance genes are also claimed.
Owner:BERGERON MICHEL G +3
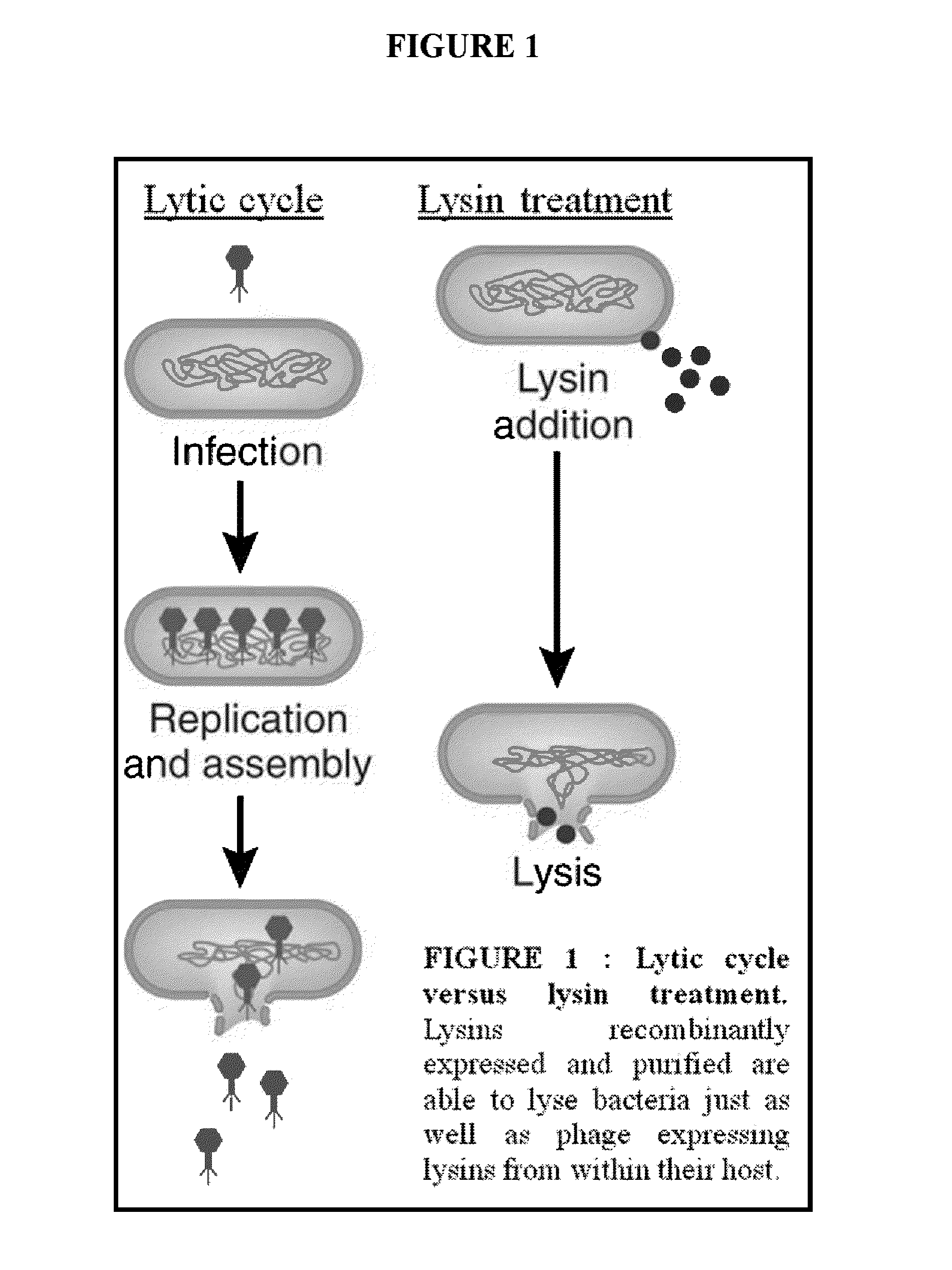
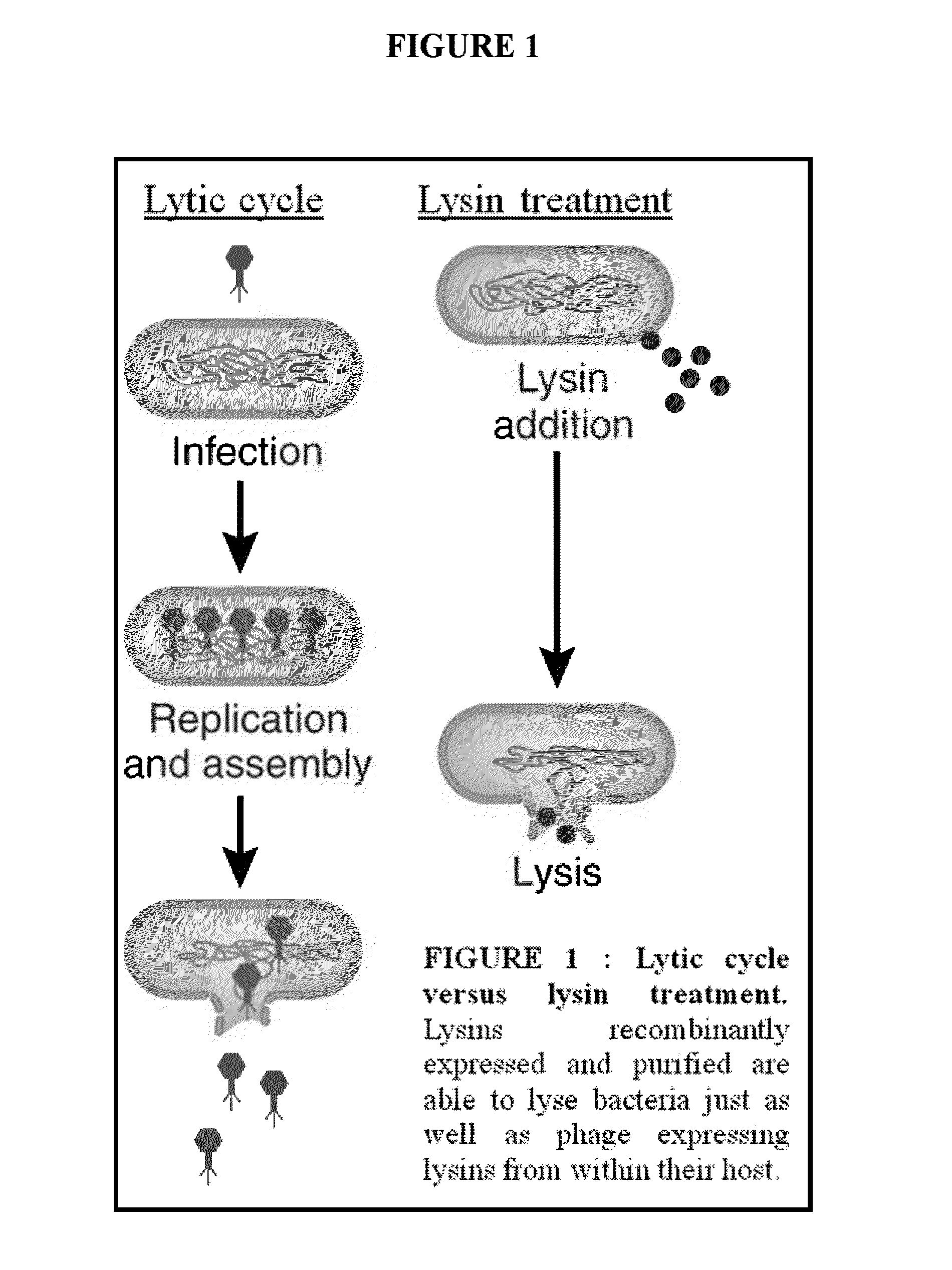
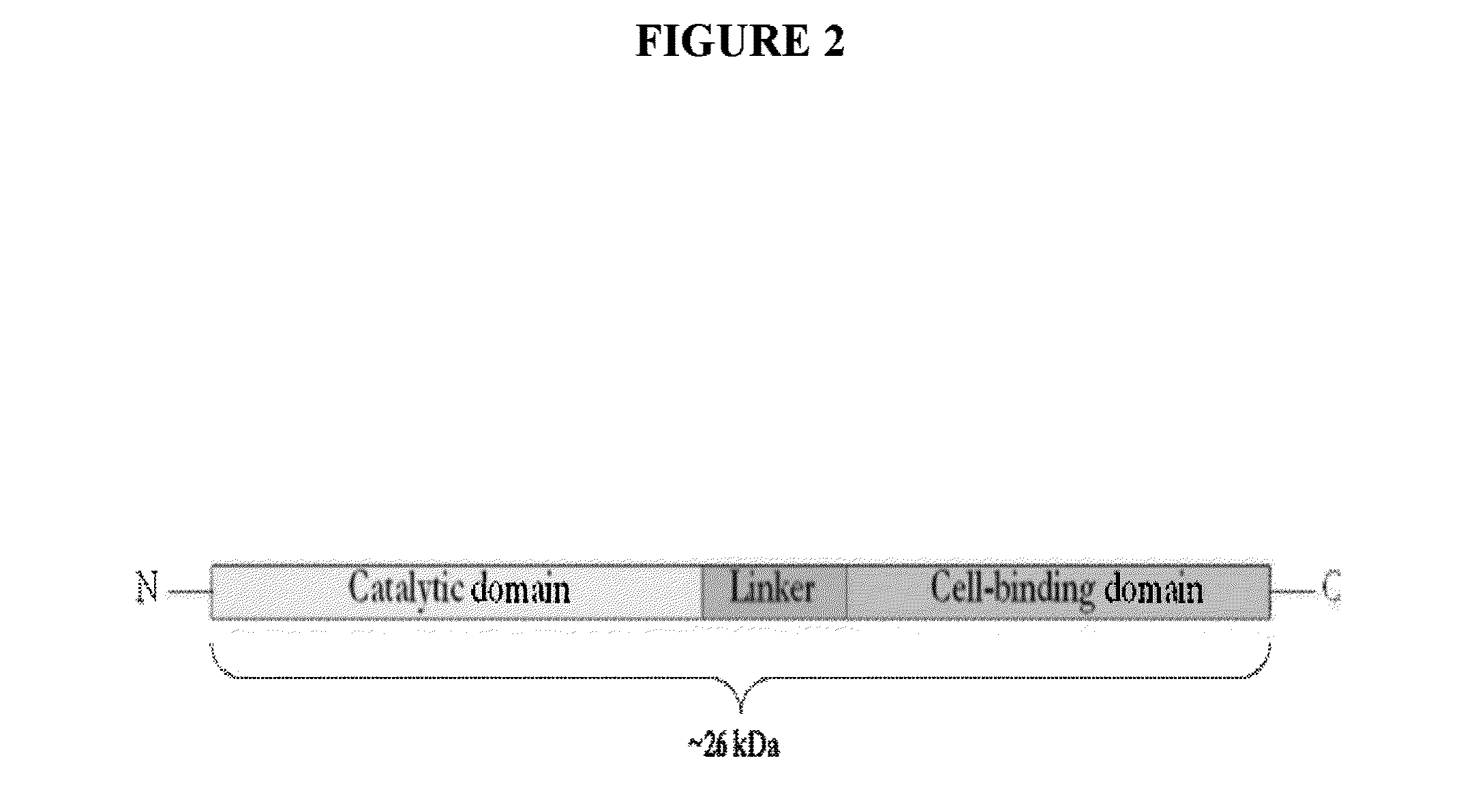
Streptococcus bacteriophage lysins for detection and treatment of gram positive bacteria
ActiveUS9034322B2Efficient killingReduce in quantityAntibacterial agentsPowder deliveryStreptococcus constellatusStaphylococcus xylosus
The present invention provides methods, compositions and articles of manufacture useful for the prophylactic and therapeutic amelioration and treatment of gram-positive bacteria, including Streptococcus and Staphylococcus, and related conditions. The invention provides compositions and methods incorporating and utilizing Streptococcus suis derived bacteriophage lysins, particularly PlySs2 and / or PlySs1 lytic enzymes and variants thereof, including truncations thereof. Methods for treatment of humans are provided.
Owner:THE ROCKEFELLER UNIV
Multiple vaccination including serogroup C meningococcus
Various improvements to vaccines that include a serogroup C meningococcal conjugate antigen, including: (a) co-administration with acellular B. pertussis antigen; (b) co-administration with an inactivated poliovirus antigen; (c) supply in a kit together with a separate pneumococcal conjugate component, which may be in a liquid form; and (d) use in combination with a pneumococcal conjugate antigen but without an aluminum phosphate adjuvant. A kit may have: (a) a first immunogenic component that comprises an aqueous formulation of a conjugated capsular saccharide from Streptococcus pneumoniae; (b) a second immunogenic component that comprises a conjugated capsular saccharide from Neisseria meningitidis serogroup C.
Owner:GSK VACCINES GMBH
Polypeptides And Immunogenic Conjugates Capable of Inducing Antibodies Against Pathogens, and Uses Thereof
InactiveUS20080171053A1Efficient responseAntibacterial agentsBacterial antigen ingredientsKexinDNA construct
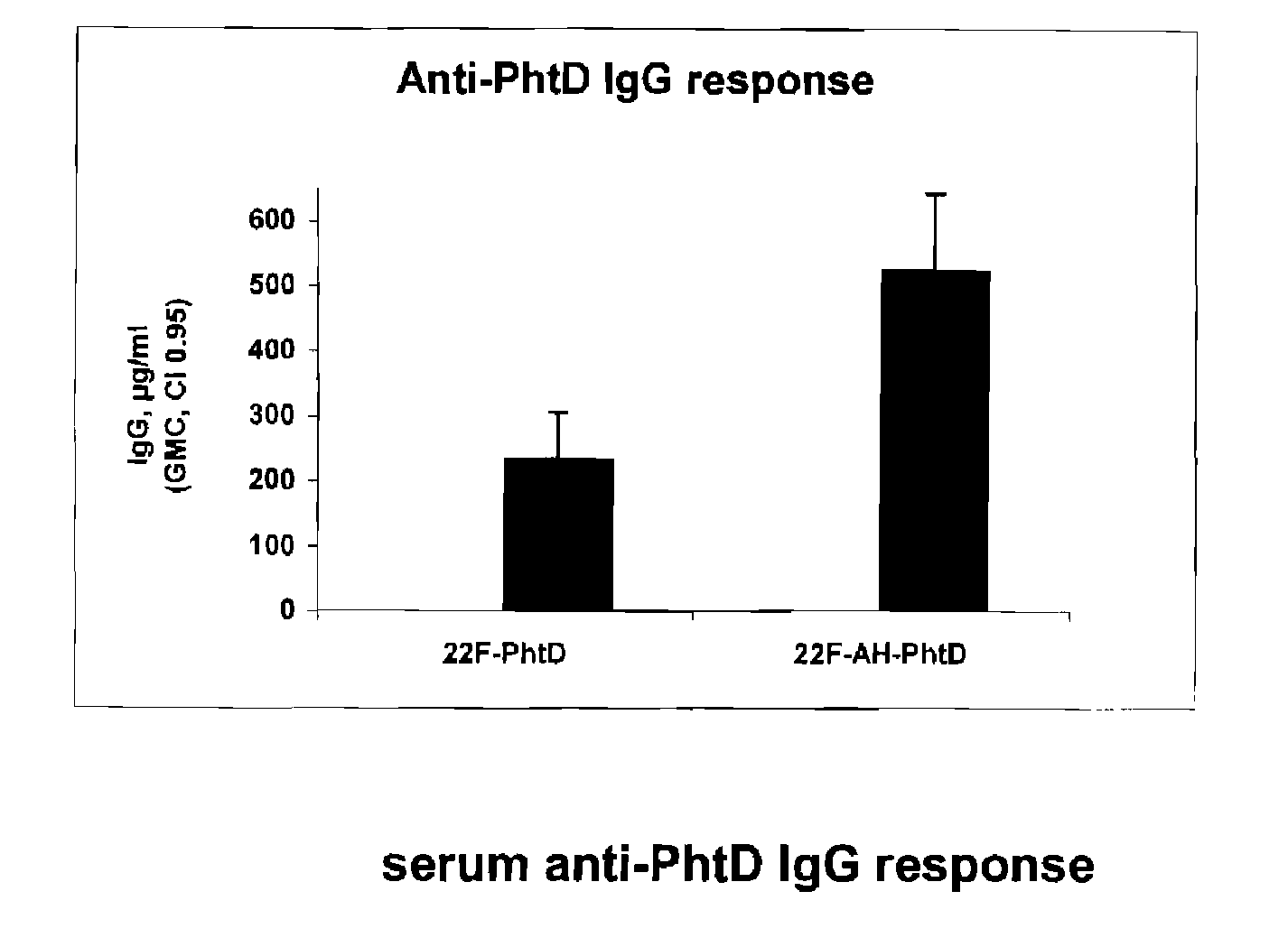
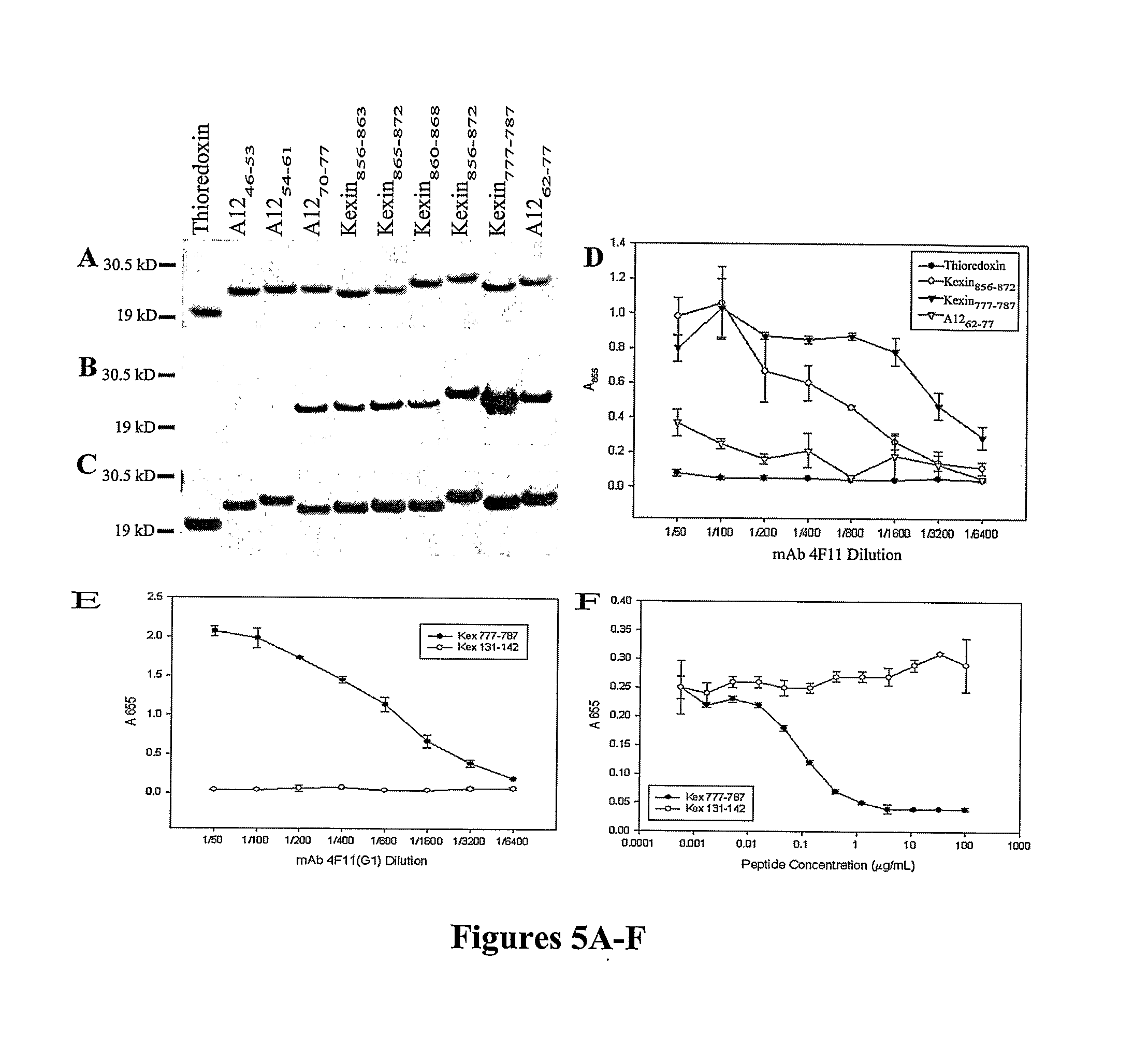
A number of immunologically active agents are described, including an isolated protein or polypeptide that includes the amino acid sequence of SEQ ID NO: 1, immunogenic conjugates containing either the protein or polypeptide, a full-length Pneumocystis kexin, or a full length Streptococcus pneumoniae pneumococcal surface protein A (PspA), antibodies recognizing the protein or polypeptide or the immunogenic conjugates (particularly the epitope of SEQ ID NO: 1), and nucleic acid molecules that encode the protein or polypeptide, as well as DNA constructs, expression vectors, and host cells that contain the nucleic acid molecules. Disclosed uses of the antibodies, immunogenic conjugates, and DNA constructs include inducing passive or active immunity to treat or prevent pathogen infections, particularly by a Pneumocystis organism, in a patient.
Owner:UNIVERSITY OF ROCHESTER
Pneumo-streptococcal-polysaccharide adventitia jointed vaccine and preparing method
InactiveCN101024079AEasy to manufactureImprove immunityAntibacterial agentsBacterial antigen ingredientsAntigenDisease
The present invention relates to a streptococcus pneumoriae polysaccharide-outer membrane protein combined vaccine and its preparation method, belonging to the field of streptococcus pneumoriae vaccine. The main antigen component of said vaccine is a streptococcus pneumoriae capsular polysaccharide-outer membrane protein combined product obtained by covalently connecting the capsular polysaccharide produced by streptococcus pneumoriae with its outer membrane protein. Said capsular polysaccharide is the capsular polysaccharide of one or several kinds of streptococcus pneumoriae, its molecular weight is about 200-500 KDa, every polysaccharide molecule has about 300-700 repeating units. The outer membrane protein is the outer membrane protein of one or several kinds of streptococcus pneumoriae, its molecular weight is about 30-100 Kda. Said vaccine can be used for preventing or curing the diseases induced by streptococcus pneumoriae.
Owner:CHANGHUI BIOLOGICAL ENG FUZHOU
Purification of bacterial antigens
Presented are methods of isolation of pili and pilus-like structures from Gram-positive bacteria including Streptococcus pneumoniae and compositions that include such isolated pili. These compositions are useful as immunogenic compositions for the production of antibodies and immunostimulation. Also presented are methods of inhibiting Streptococcus pneumoniae, and methods of identifying inhibitors of Streptococcus pneumoniae.
Owner:NOVARTIS AG
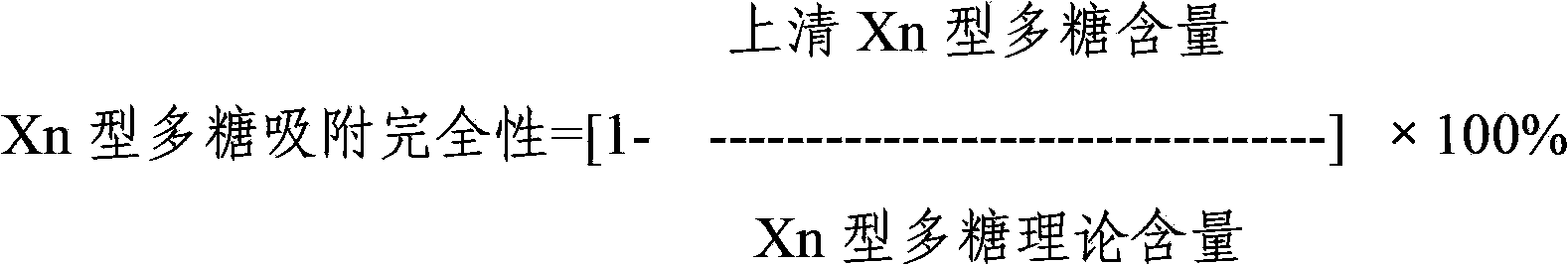
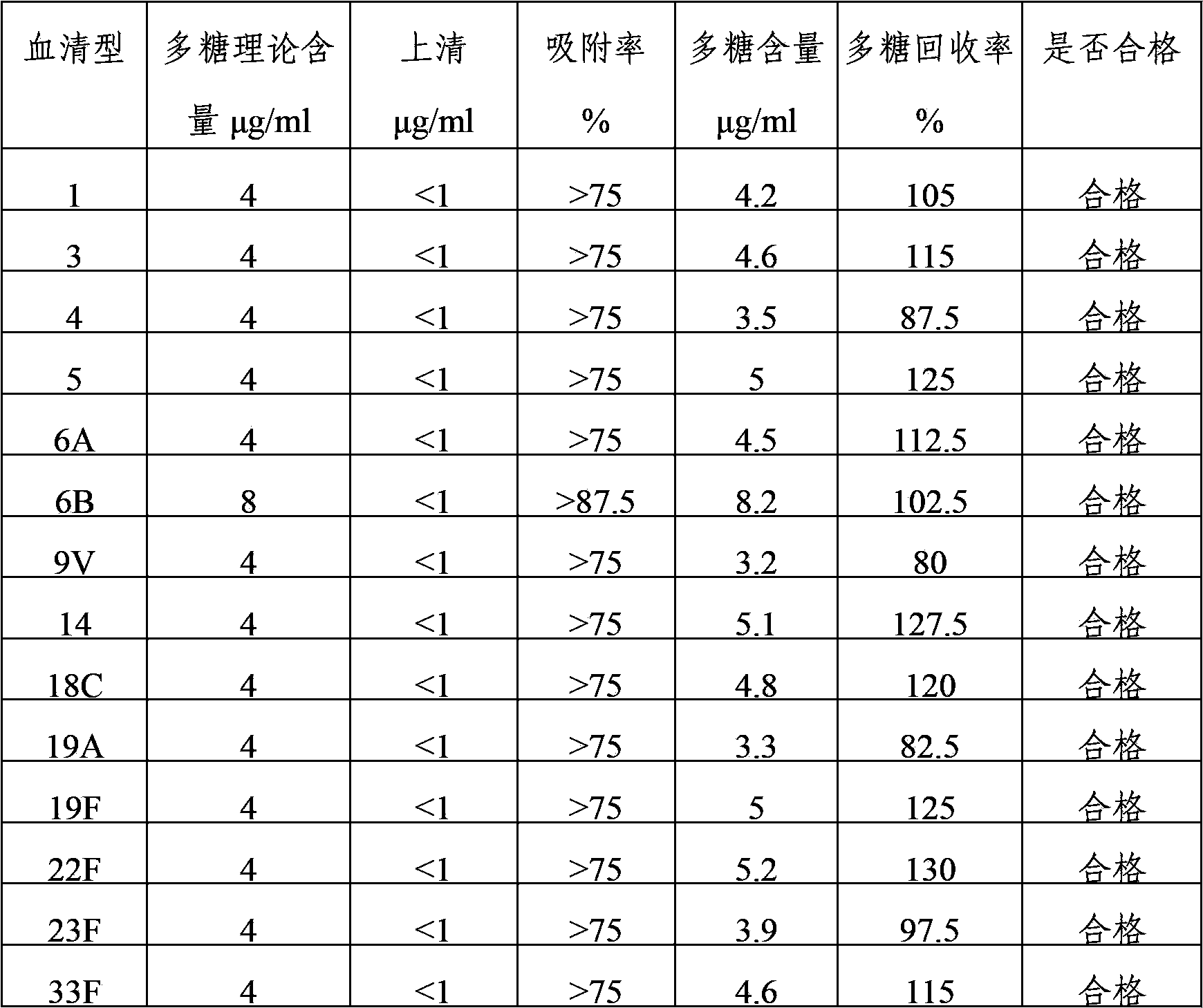
Multivalent pneumococcus capsular polysaccharide-protein conjugated composition and preparation method thereof
InactiveCN103623401AImprove adsorption capacityImprove stabilityAntibacterial agentsBacterial antigen ingredientsConjugate vaccineDisease
The invention provides a multivalent pneumococcus capsular polysaccharide-protein conjugated composition and a preparation method thereof. The conjugated composition is formed through covalent linkages between pneumococcus capsular polysaccharides of 14 different serotypes and a carrier protein, wherein the 14 different serotypes are: 1, 3, 4, 5, 6A, 6B, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F. The conjugated composition has a good absorption effect and stability, and has multiple immunogenicity and protective property aiming at the invasion of pneumococcus of the 14 serotypes; the effect of the conjugated composition is better than that of the low price and low quality pneumonia-treating compositions on the market, and moreover the immune response of the conjugated composition is higher than that of the composition that has not been combined. By using the multivalent pneumococcus capsular polysaccharide conjugate vaccine containing the conjugated composition, the inoculation times can be reduced, the immune procedure is simplified, diseases of humans and animals caused by the pneumococcus of the 14 serotypes can be effectively prevented, moreover the disease coverage is wider, and the immune effect is better.
Owner:SINOVAC RES & DEV
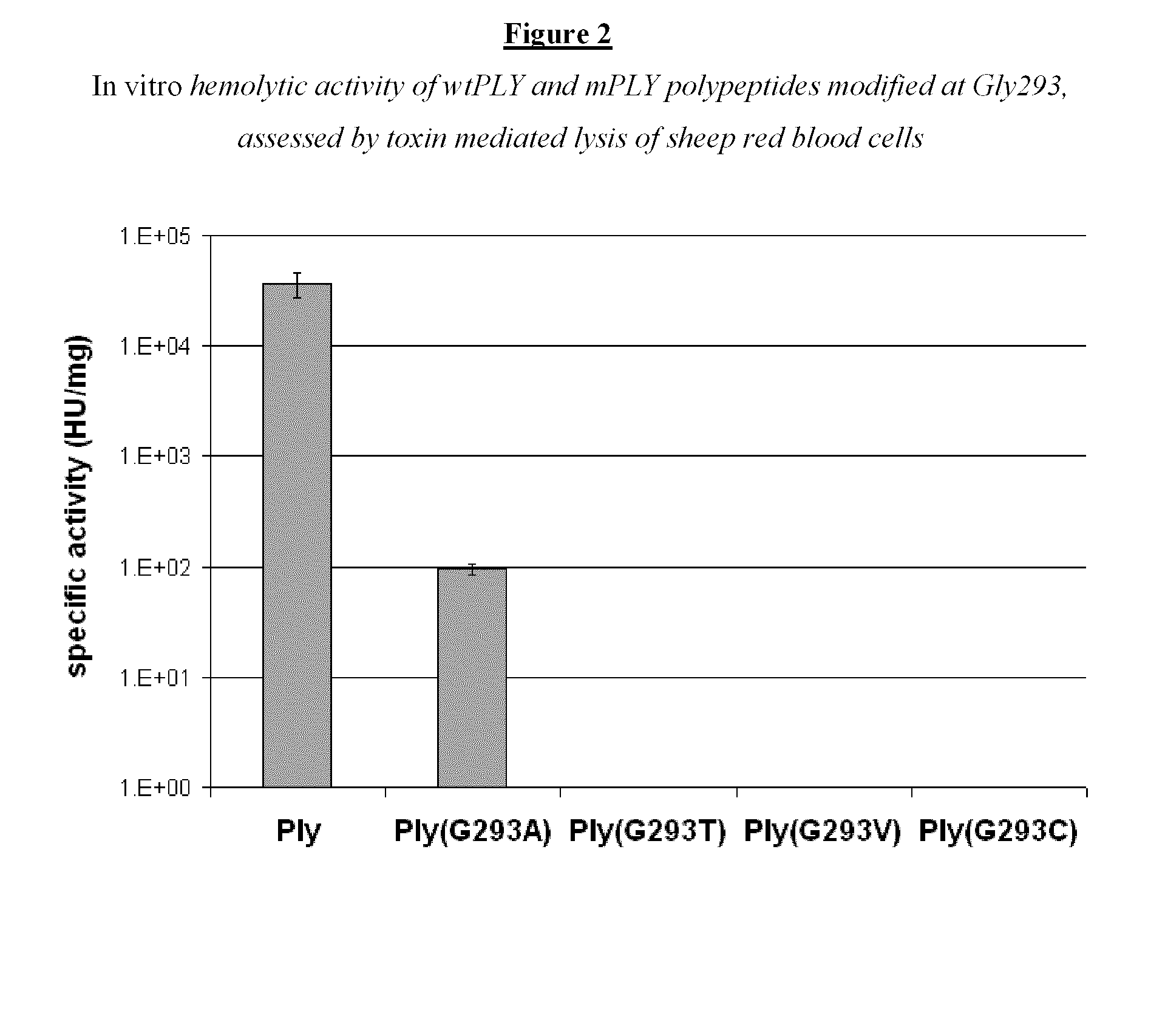
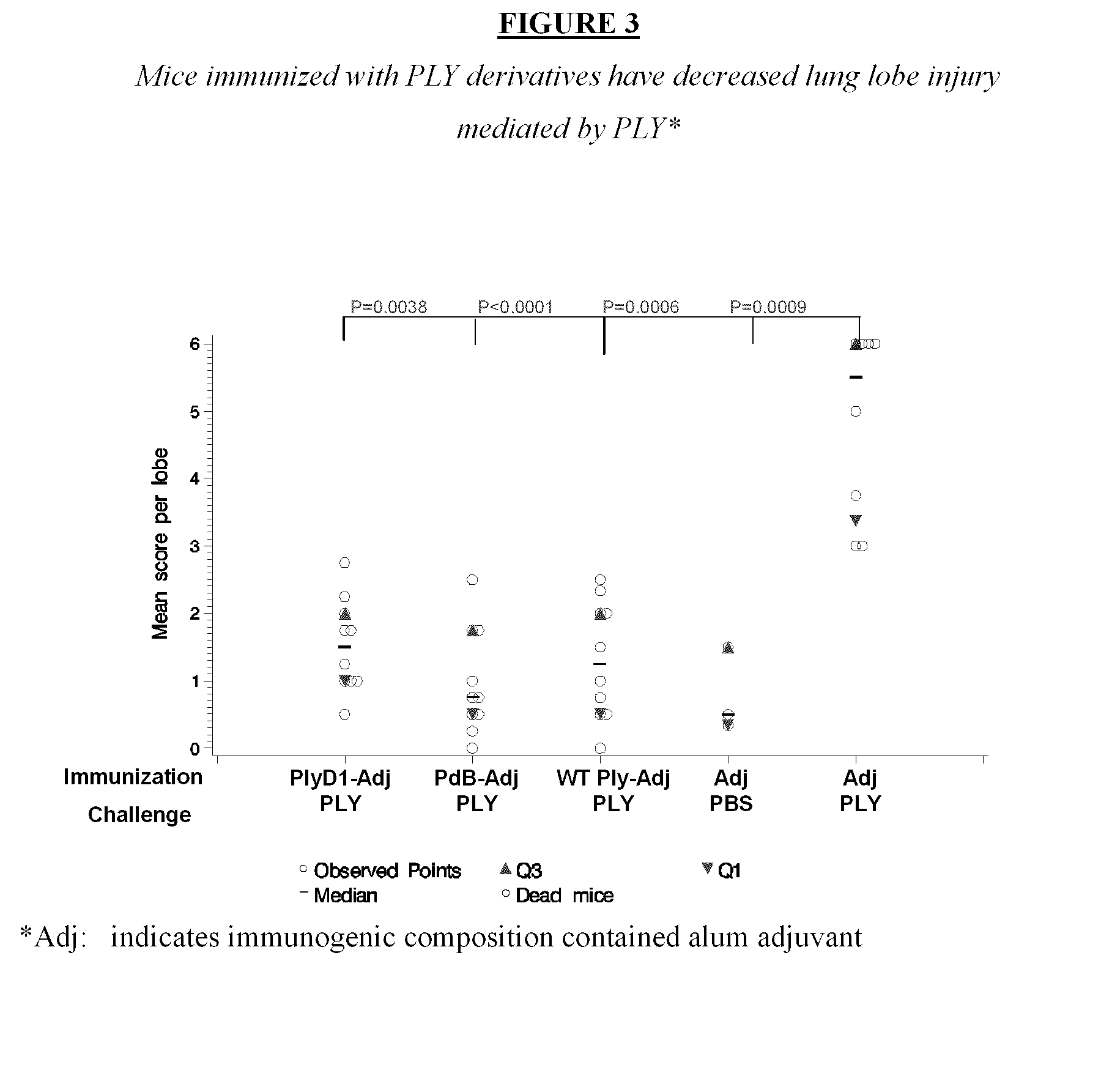
Modified streptococcus pneumonia pneumolysin (PLY) polypeptides
InactiveUS20110287046A1Low hemolytic activityInhibit hemolytic activityAntibacterial agentsBacteriaDiseaseSynechococcus
This disclosure relates to modified Streptococcus pneumonia pneumolysm (PLY) proteins which lack hemolytic activity and can be used as immunogens in an immunogenic composition or vaccine against invasive pneumococcol diseases caused by S. pneumonia. The modified pneumolysm proteins comprise ammo acid substitutions at threonine 65, glycine 293 and cysteine 428 Nucleic acids, polypeptides encoded thereby, compositions containing the same, methods for using such nucleic acids, polypeptides and compositions are also provided
Owner:THE KINGDOM OF THE NETHERLANDS REPRESENTED BY THE MIN OF HEALTH WELFARE & SPORT ON BEHALF OF THE MIN THE NAT INST OF PUBLIC HEALTH & THE ENVIRONMENT
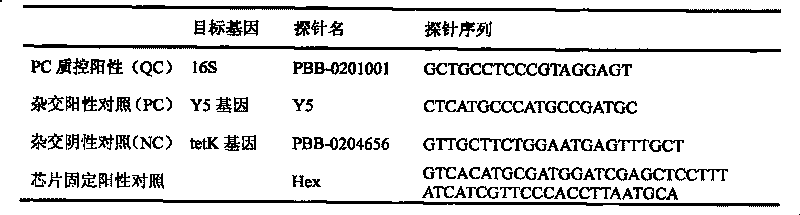
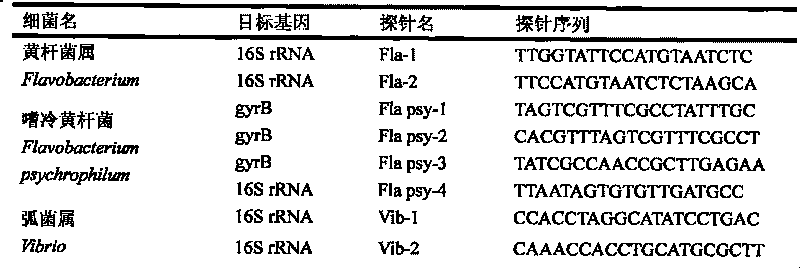
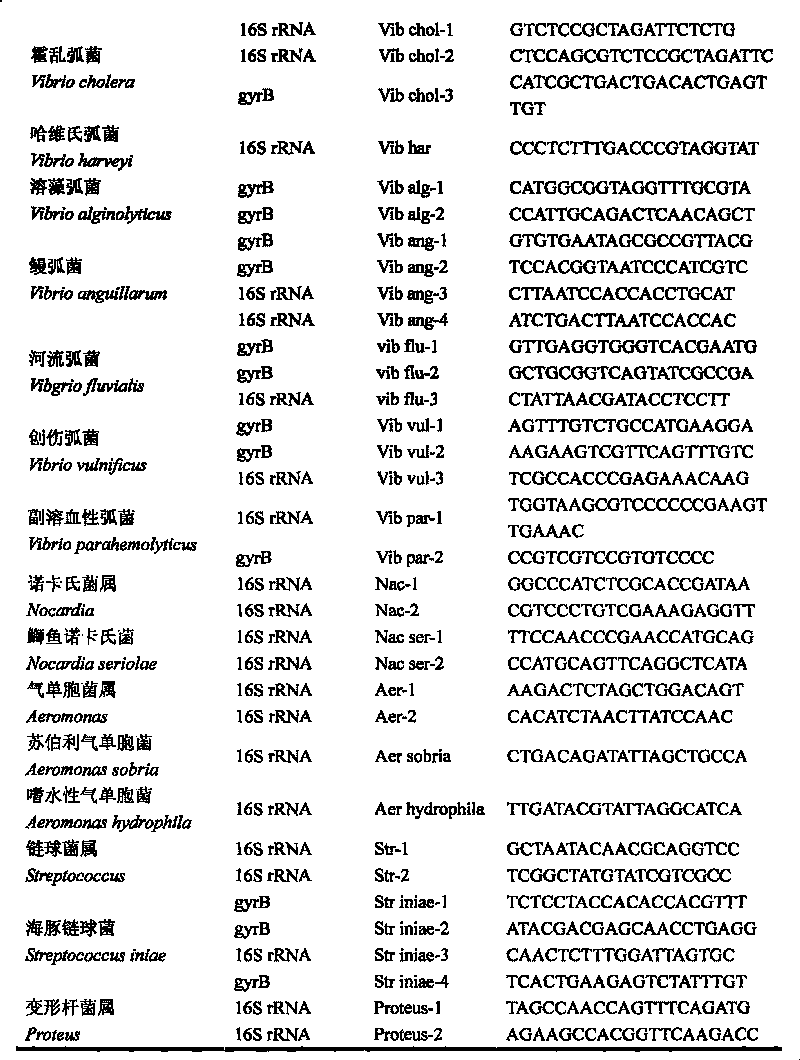
Gene chip of aquatic product cultivation pathogenic bacterium
InactiveCN101691608AReduce volumeRapid Test InterpretationNucleotide librariesMicrobiological testing/measurementBacteroidesPositive control
The invention discloses a gene chip of aquatic product cultivation pathogenic bacterium, comprising a solid phase carrier which is modified chemically, a detection probe and a quality control probe are distributed on the solid phase carrier in a dot matrix way; the detection probe comprises specificity 16S rDNA sequences and / or gyrB gene sequences of vibrio, comma bacillus, vibrio harveyi, vibrio alginolyicus, vibrio anguillarum, vibrio parahemolyticus, nocardia, nacardia seriolea, aeromonas, hydrophilic aeromonas, streptococcus and dolphin streptococcus, which are to be detected, the quality control probe includes PCR positive, chip fixed positive control, chip hybridizing negative control, chip hybridizing positive control and chip hybridizing blank control; the gene chip has the advantages of small volume and high flux, can detect known and unknown germs of the vibrio, the nocardia, the aeromonas and the streptococcus, and can detect specific germs with multiple kinds, and the simpleness and rapidness and specificity of the germs can be detected, and automatic detection can be carried out after detection software is additionally arranged.
Owner:NINGBO UNIV +2
Protein-based Streptococcus pneumoniae vaccines
InactiveUS7504110B2Avoid infectionAntibacterial agentsBacterial antigen ingredientsCell membraneCell wall
The present invention is primarily directed to a method for preventing infection of a mammalian subject with S. pneumoniae, wherein said method comprises administering to said subject an effective amount of one or more S. pneumoniae cell wall and / or cell membrane proteins and / or immunogenically-active fragments, derivatives or modifications thereof, wherein said proteins are selected from a defined group of immunogenic proteins. The present invention further provides vaccine compositions containing said cell wall and / or cell membrane proteins.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Virulence of streptococci
InactiveUS7670835B2Increase virulenceMinimal effectAntibacterial agentsBacterial antigen ingredientsGenomic SegmentVaccination
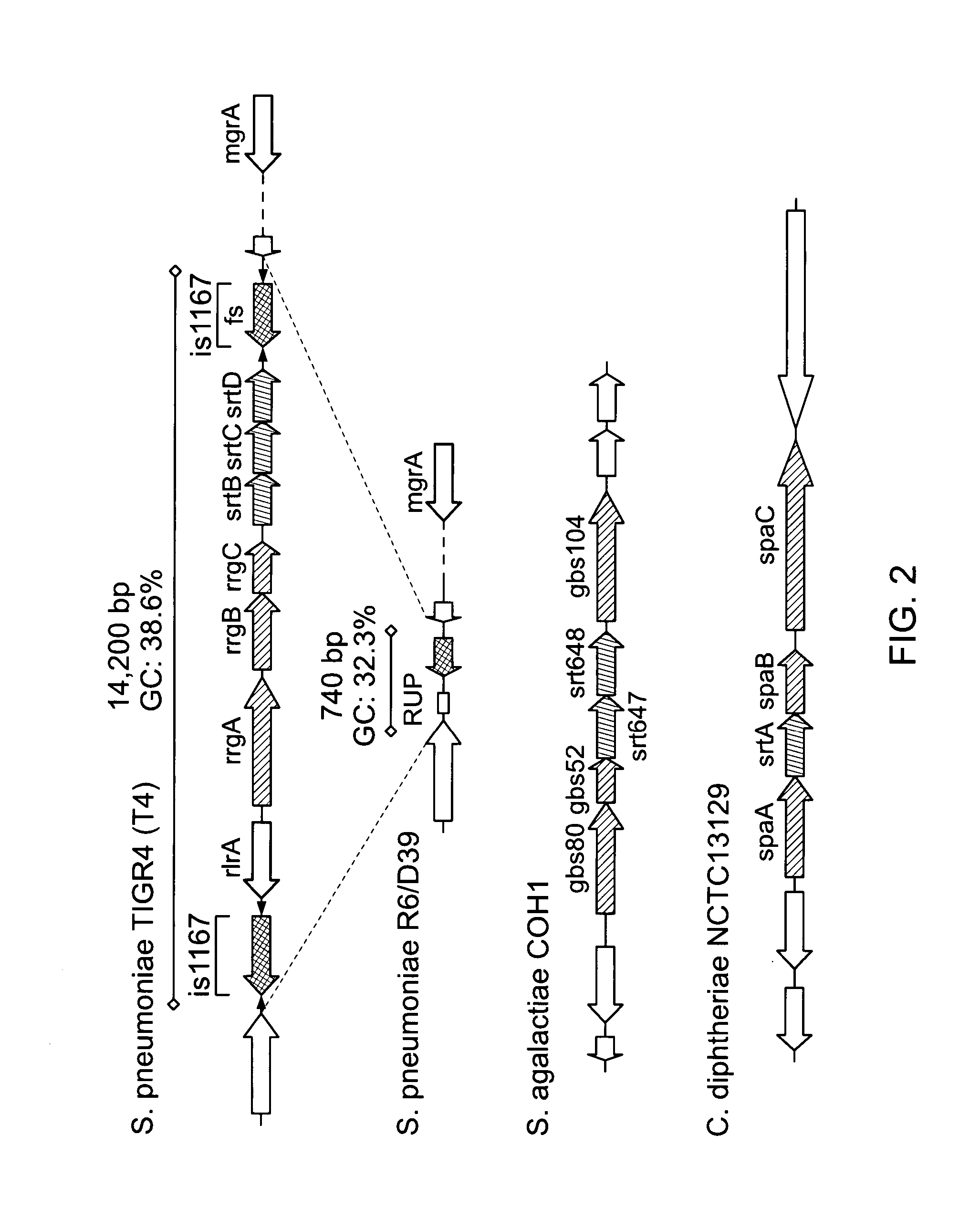
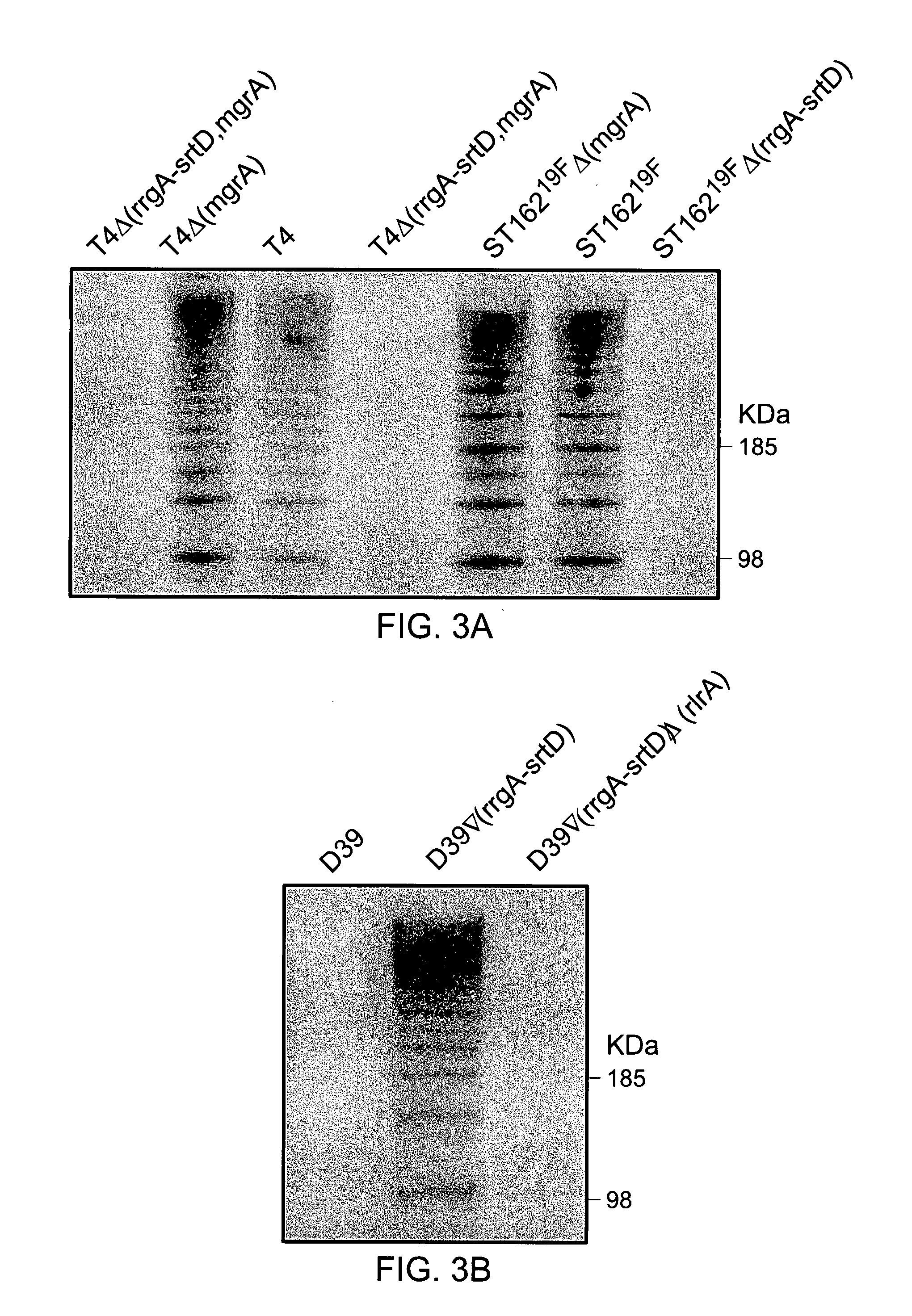
The invention relates to the field of diagnosis of and vaccination against Streptococcal infections and to the detection of virulence markers of Streptococci. The invention discloses a method for modulating virulence of a Streptococcus, the method comprising modifying a genomic fragment of Streptococcus wherein the genomic fragment comprises at least a functional part of a fragment identifiable by hybridization in Streptococcus suis to a nucleic acid or fragment thereof as shown in FIG. 5.
Owner:STICHTING WAGENINGEN RES
Streptococcus bacteriophage lysins for detection and treatment of gram positive bacteria
Owner:THE ROCKEFELLER UNIV
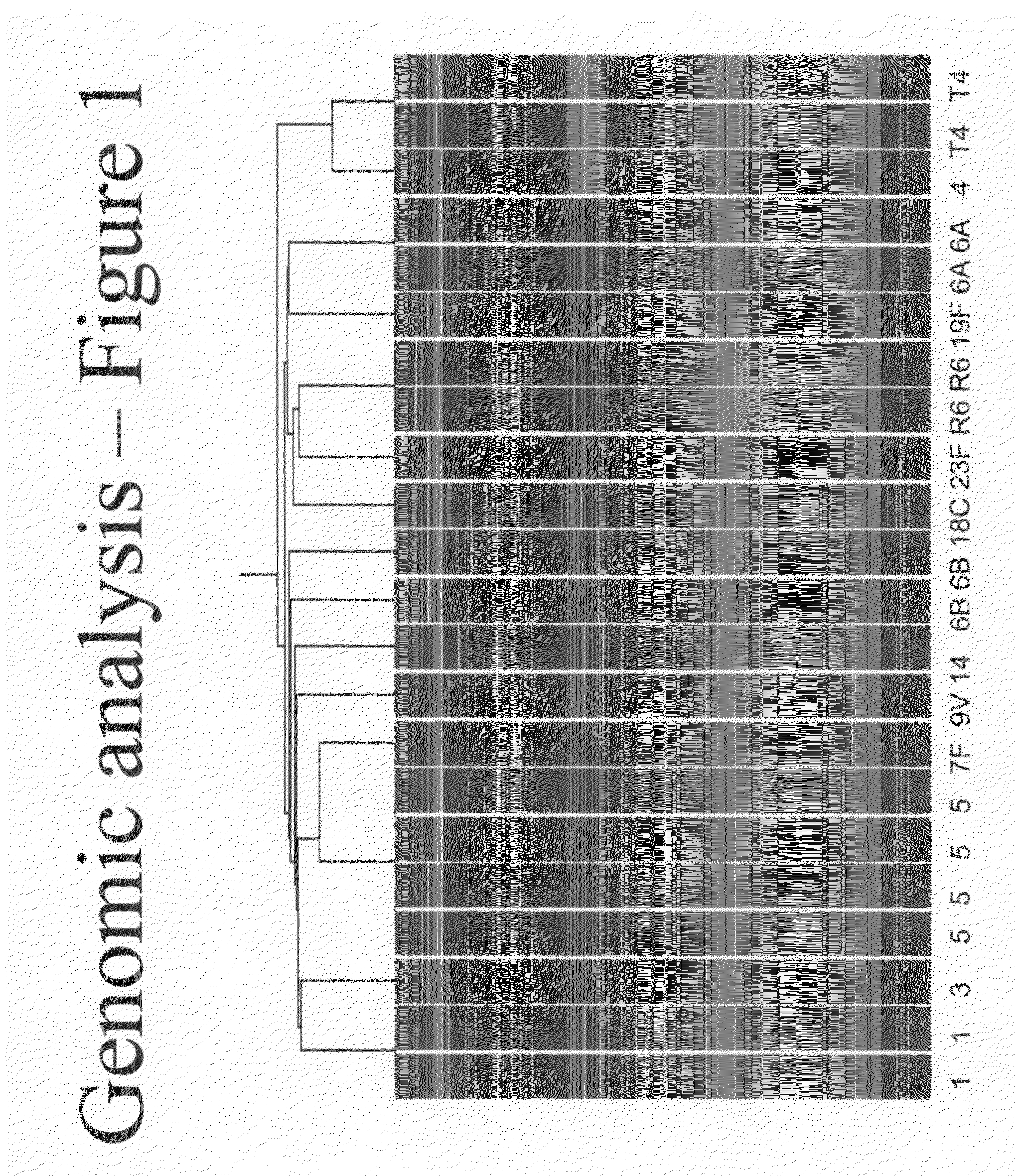
Microarray for monitoring gene expression in multiple strains of Streptococcus pneumoniae
InactiveUS20110177960A1Understanding of genetic expression patternRapid and accurate and discriminable detectionNucleotide librariesMicrobiological testing/measurementStreptococcus pneumoniaeStreptococcus mitis
The present invention features an array capable of monitoring gene expression patterns of multiple strains of Streptococcus pneumoniae including a substrate having a plurality of addresses, each of which has a probe disposed thereon.
Owner:WYETH LLC
Methods and products for identifying strains of bacteria
InactiveUS20110053793A1Reduce and avoid needRemove and diminishes needPeptide librariesLibrary tagsSerum igeCapture antibody
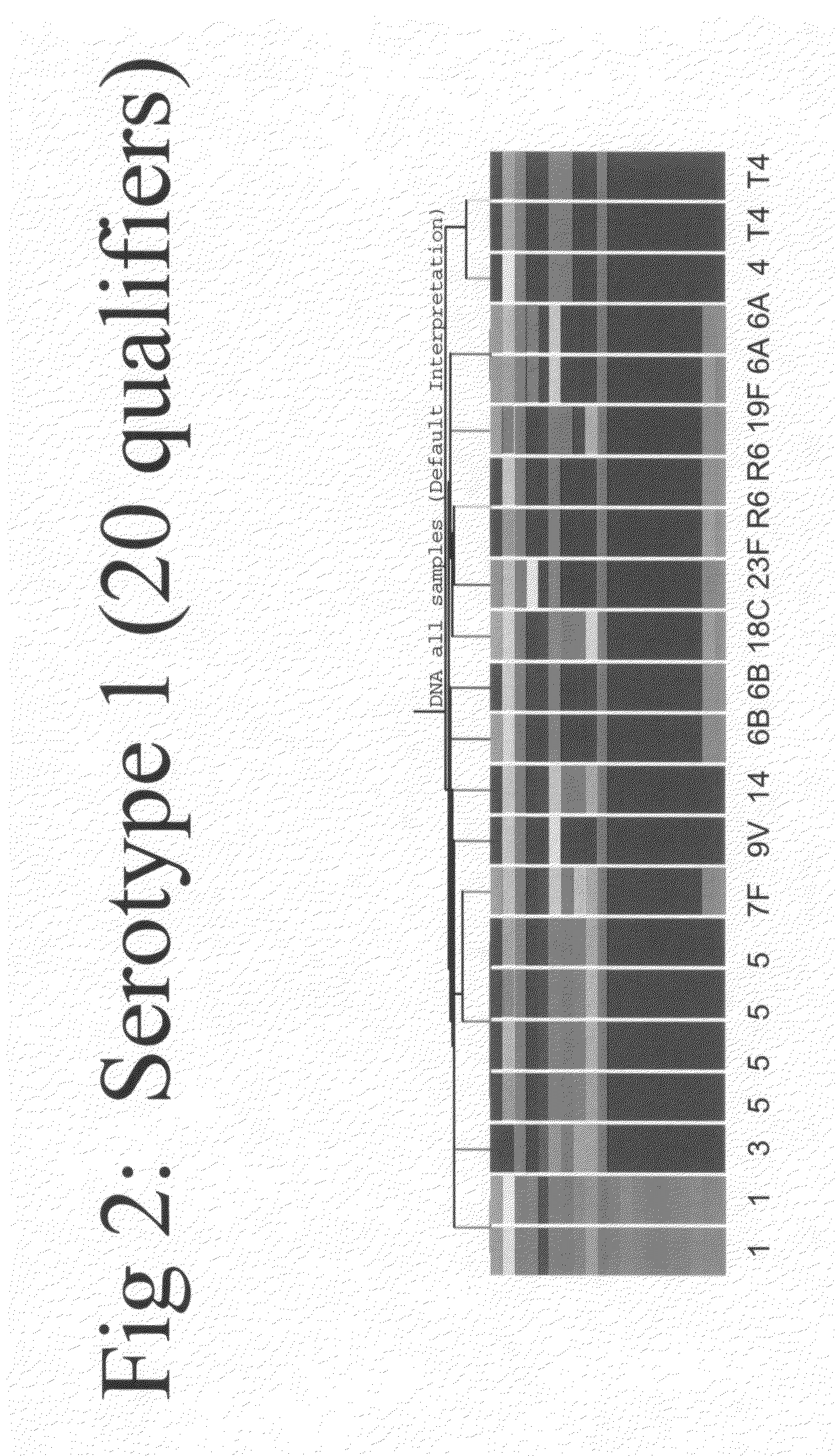
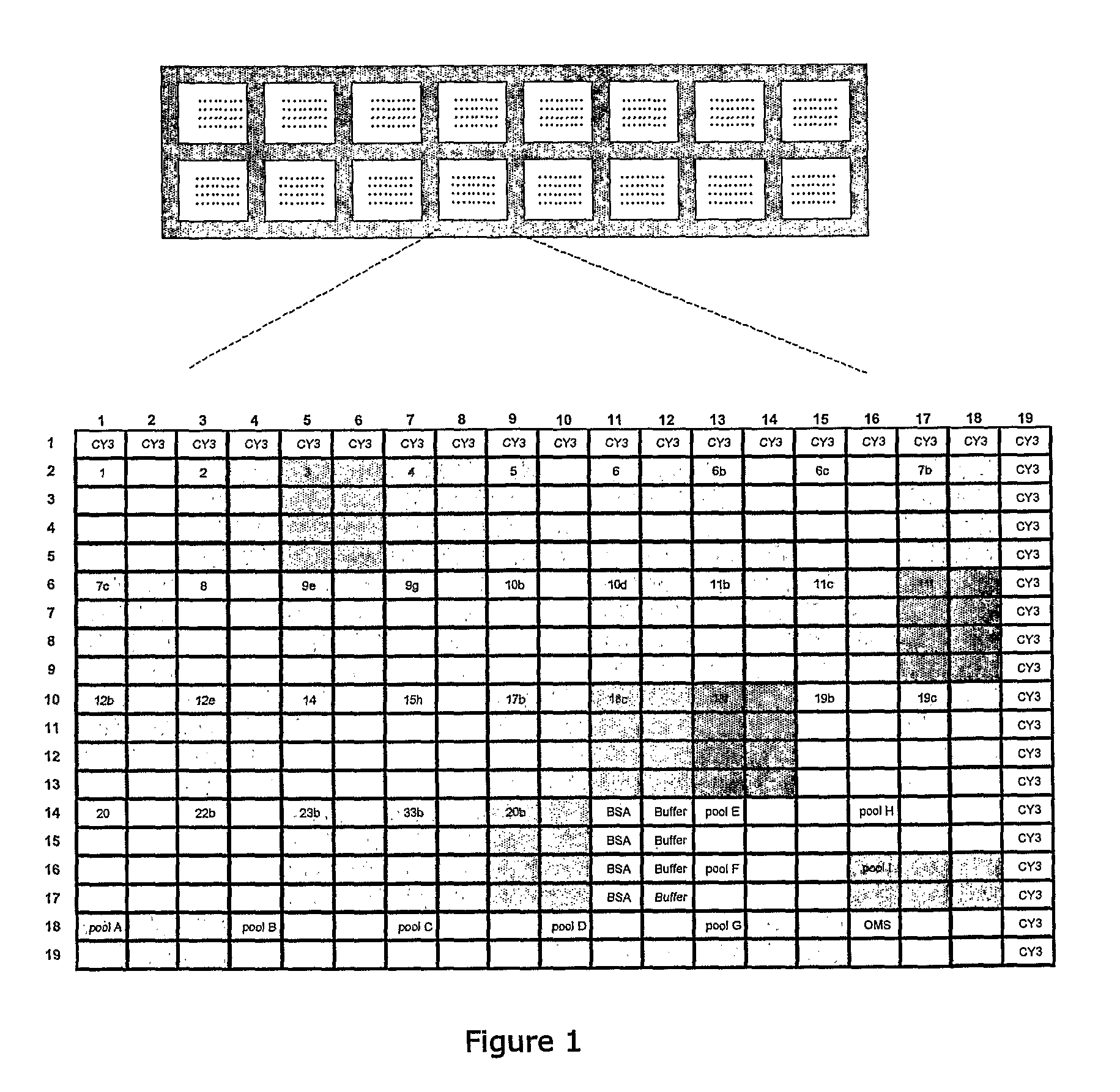
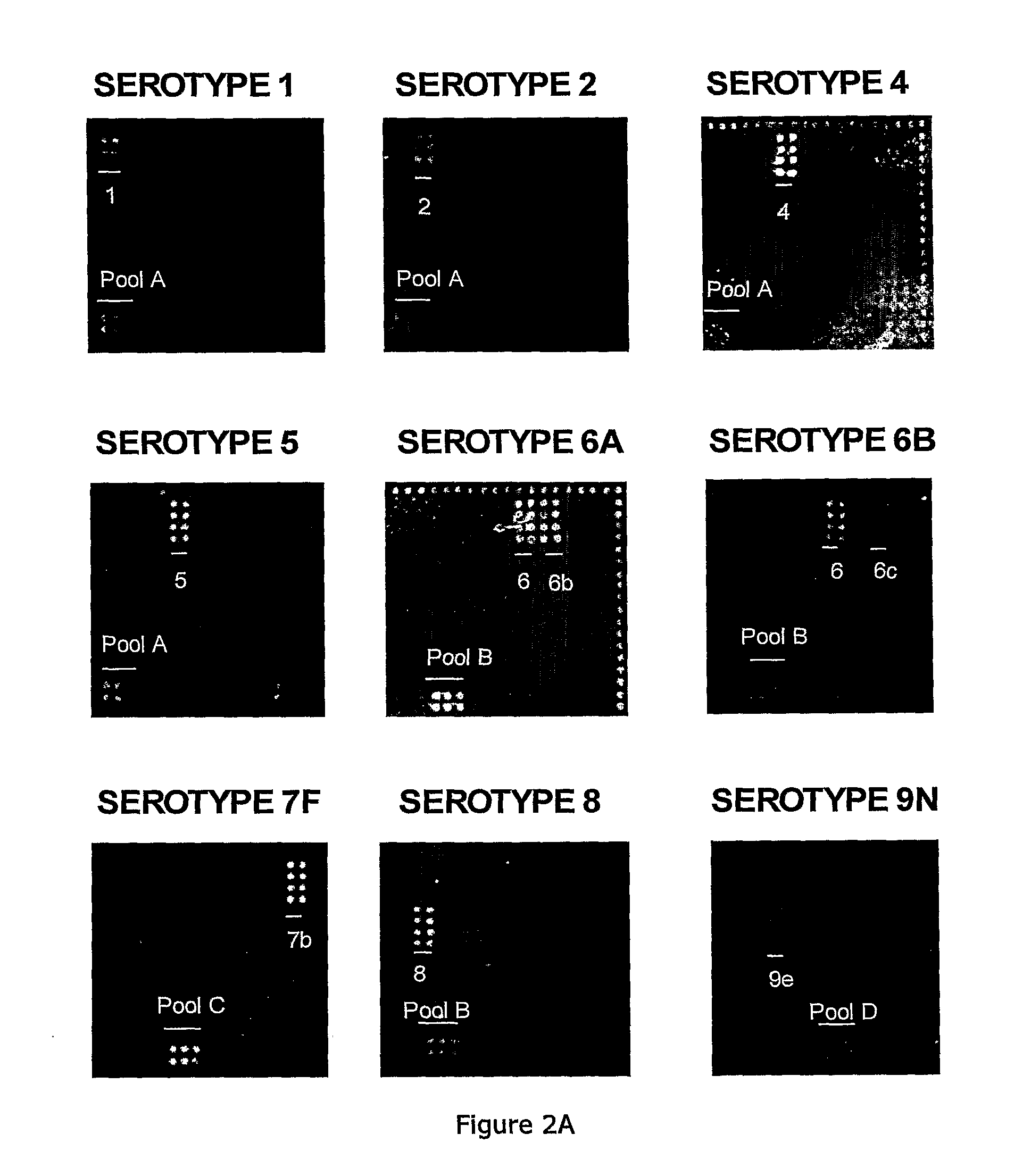
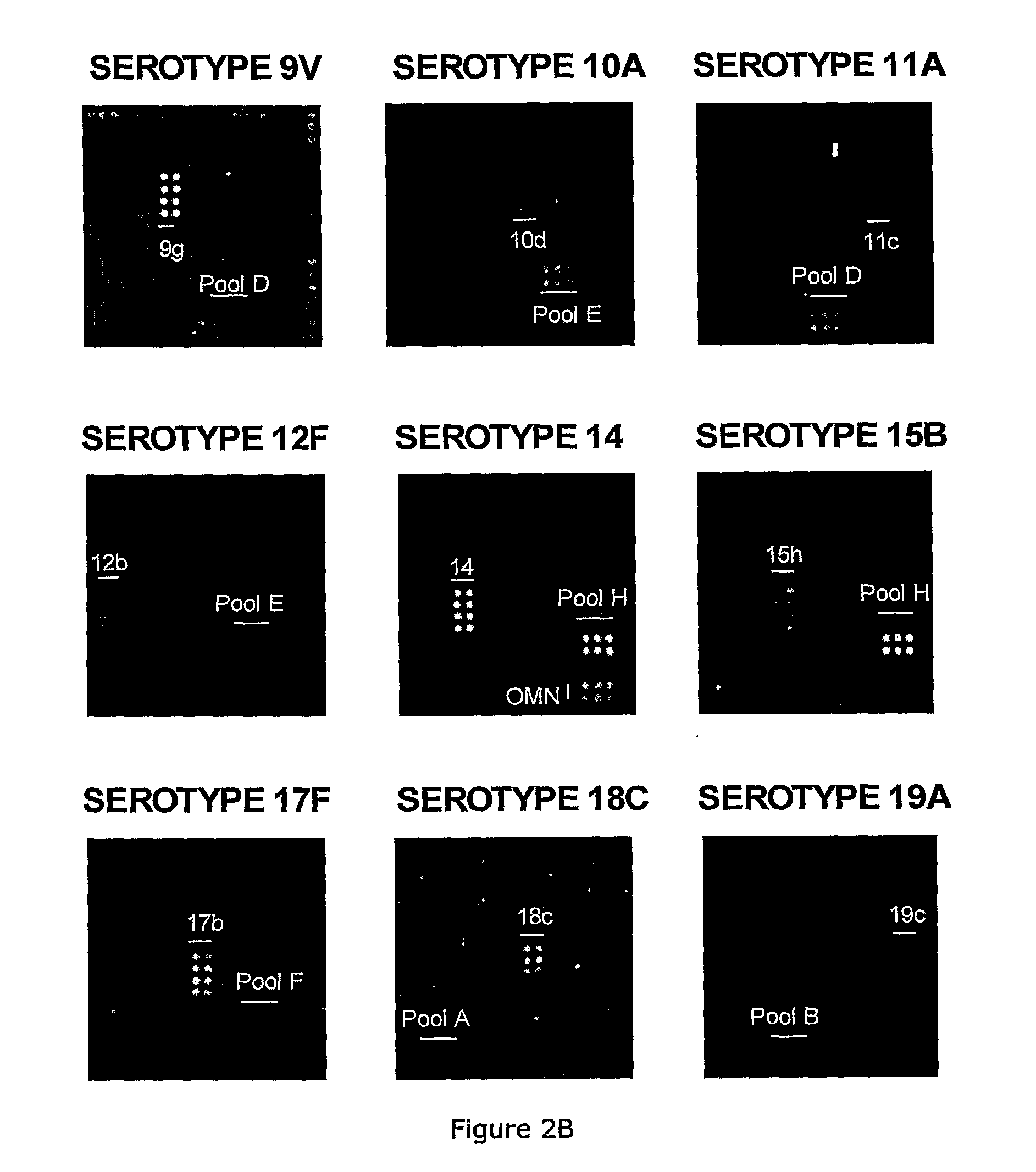
Methods for identifying strains of bacteria, particularly methods for serotyping Streptococcus pneumoniae in a sample, methods for detecting and / or classifying S. pneumoniae infection by serotype, array devices and kits for use in such methods are disclosed. Array devices comprise a set of capture antibodies immobilised on a substrate at pre-determined array positions, wherein the set of capture antibodies comprises serotype-distinguishing antibodies which differ in their binding specificity for different S. pneumoniae serotypes. Serotyping methods may employ whole cell detection utilising one or more detectable labels, including in situ labelling of array-bound cells.
Owner:PROTEOMIKA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com