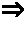Patents
Literature
274 results about "Bicarbonate Ion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A polyatomic ion whose formula is HCO3-.
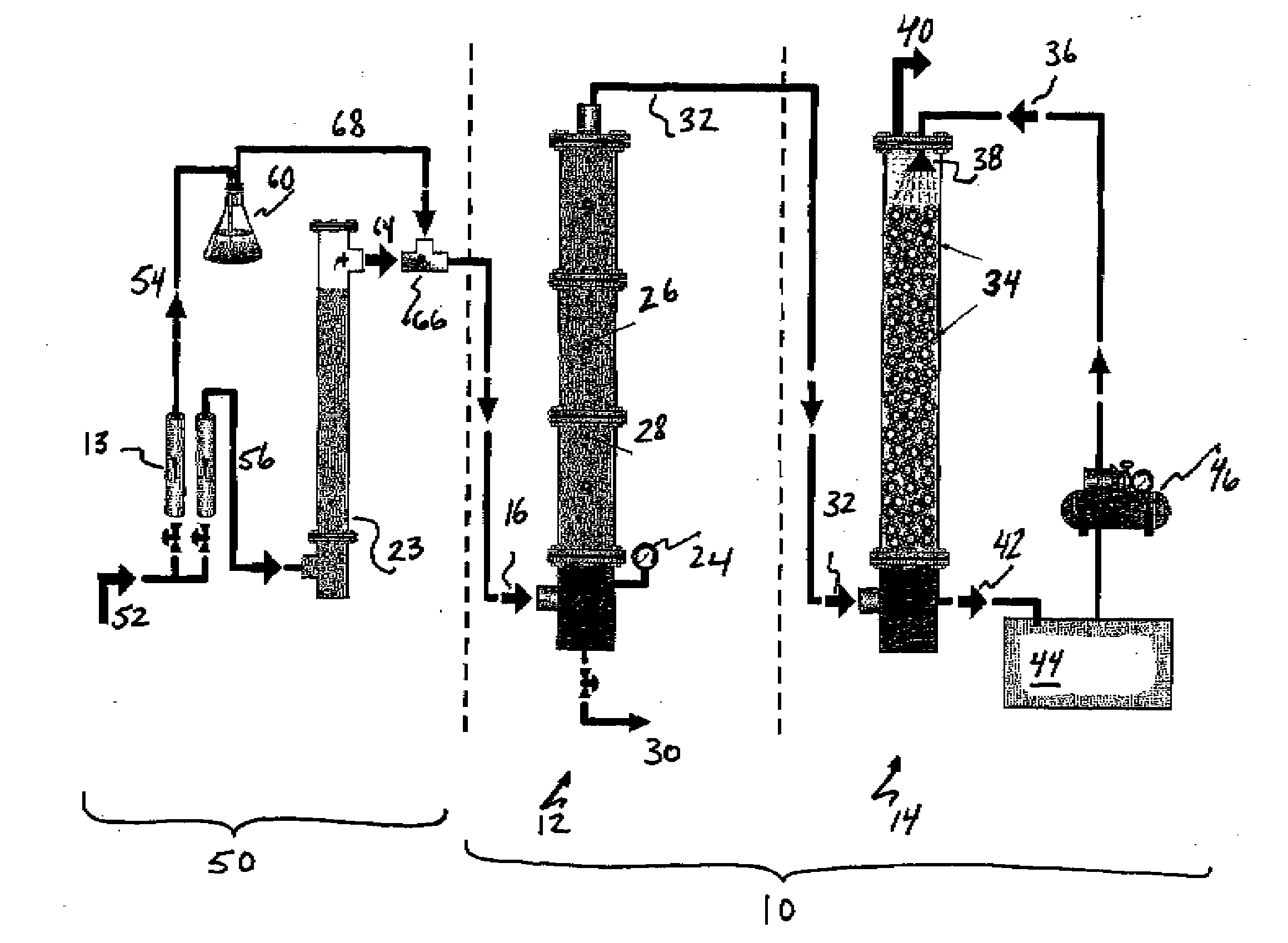
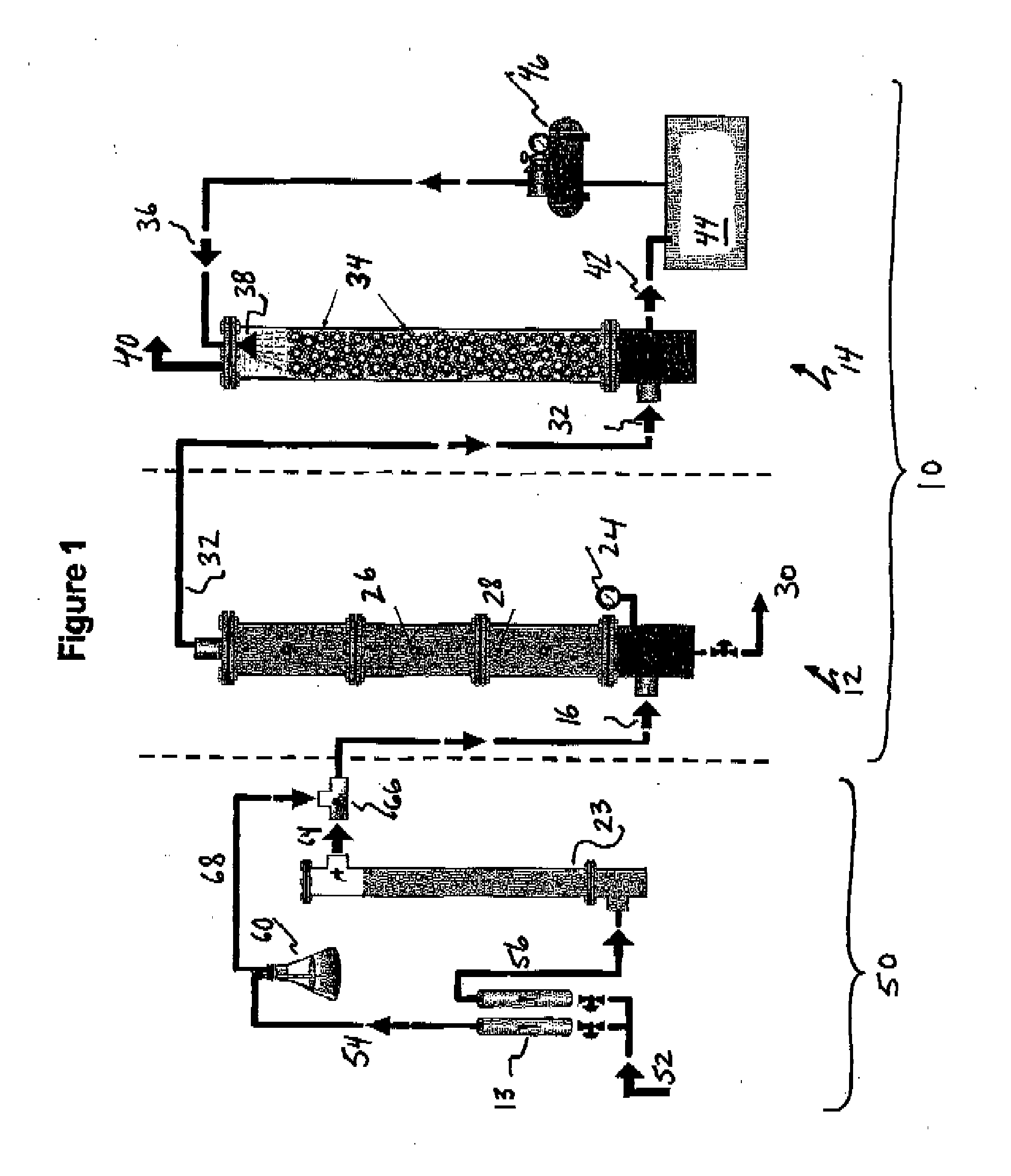
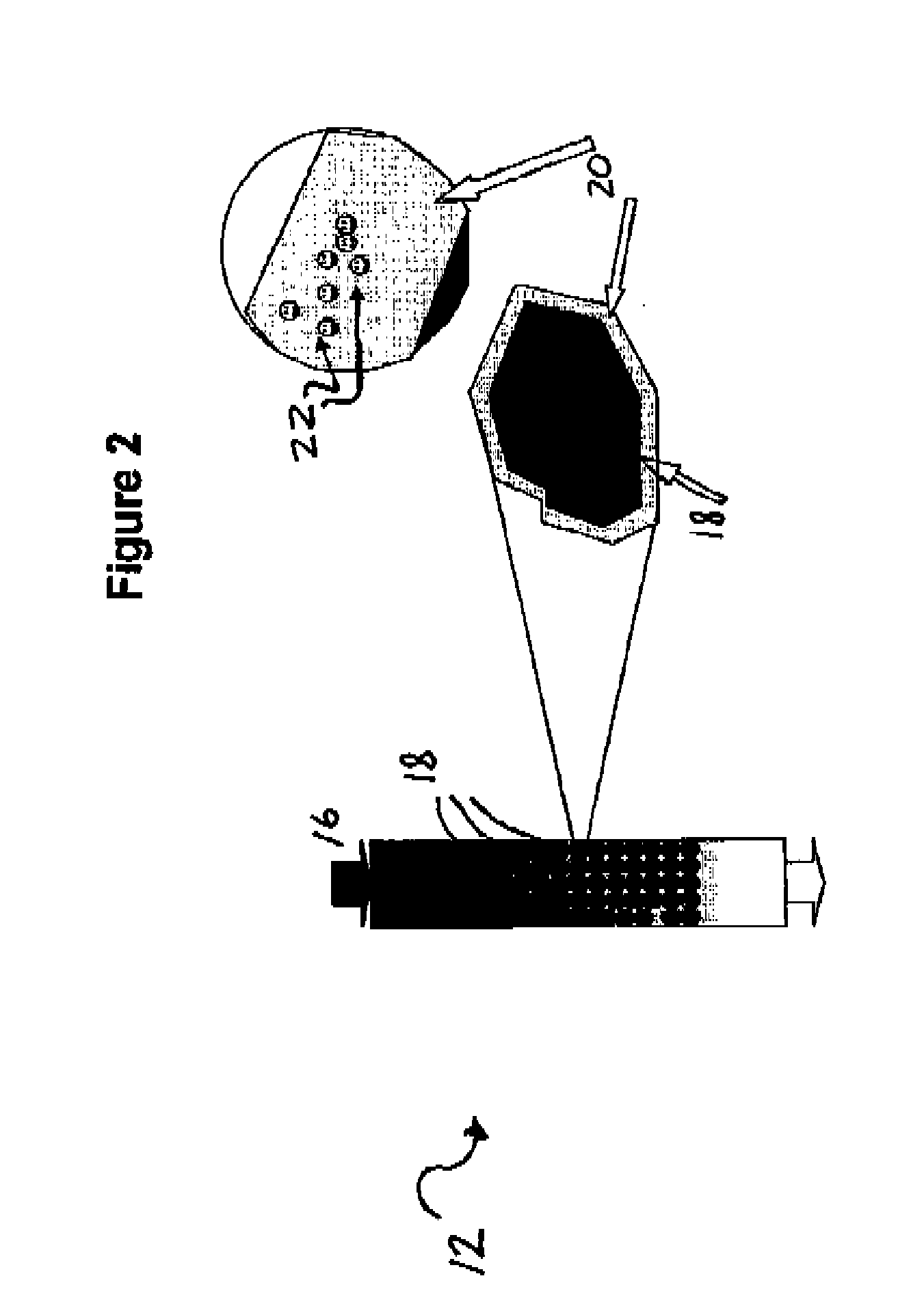
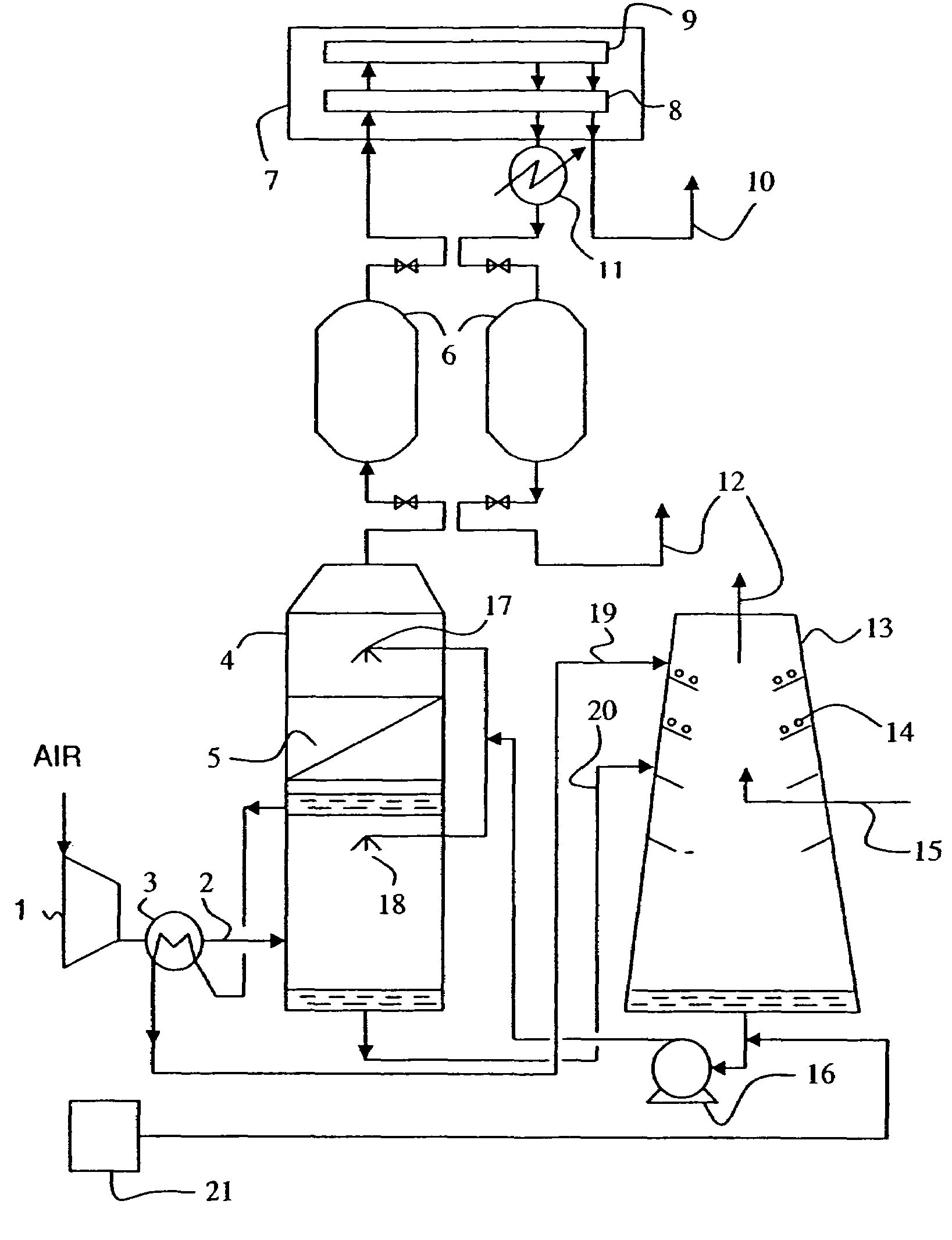
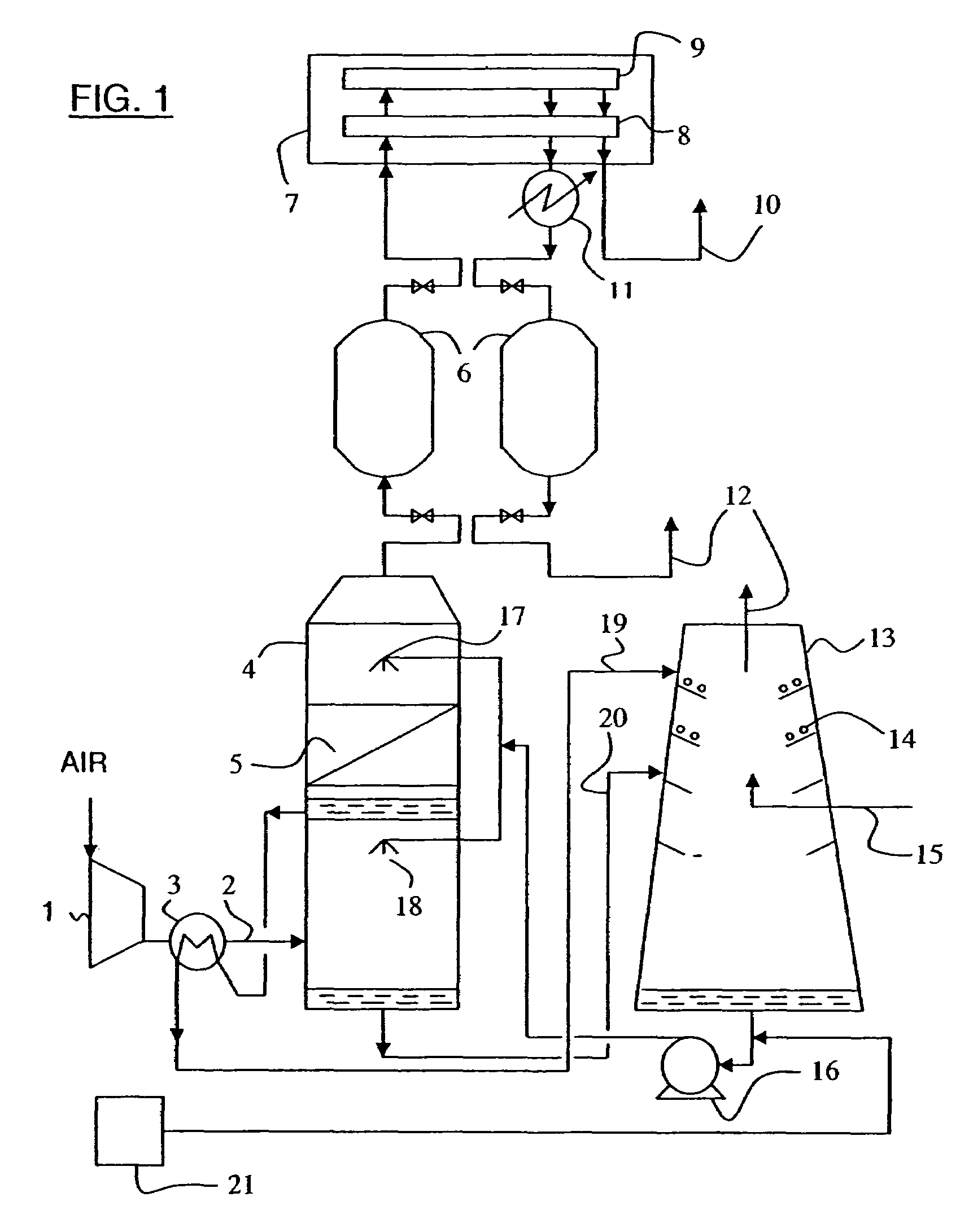
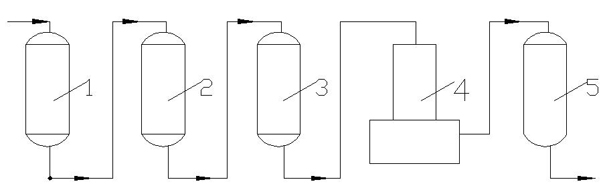
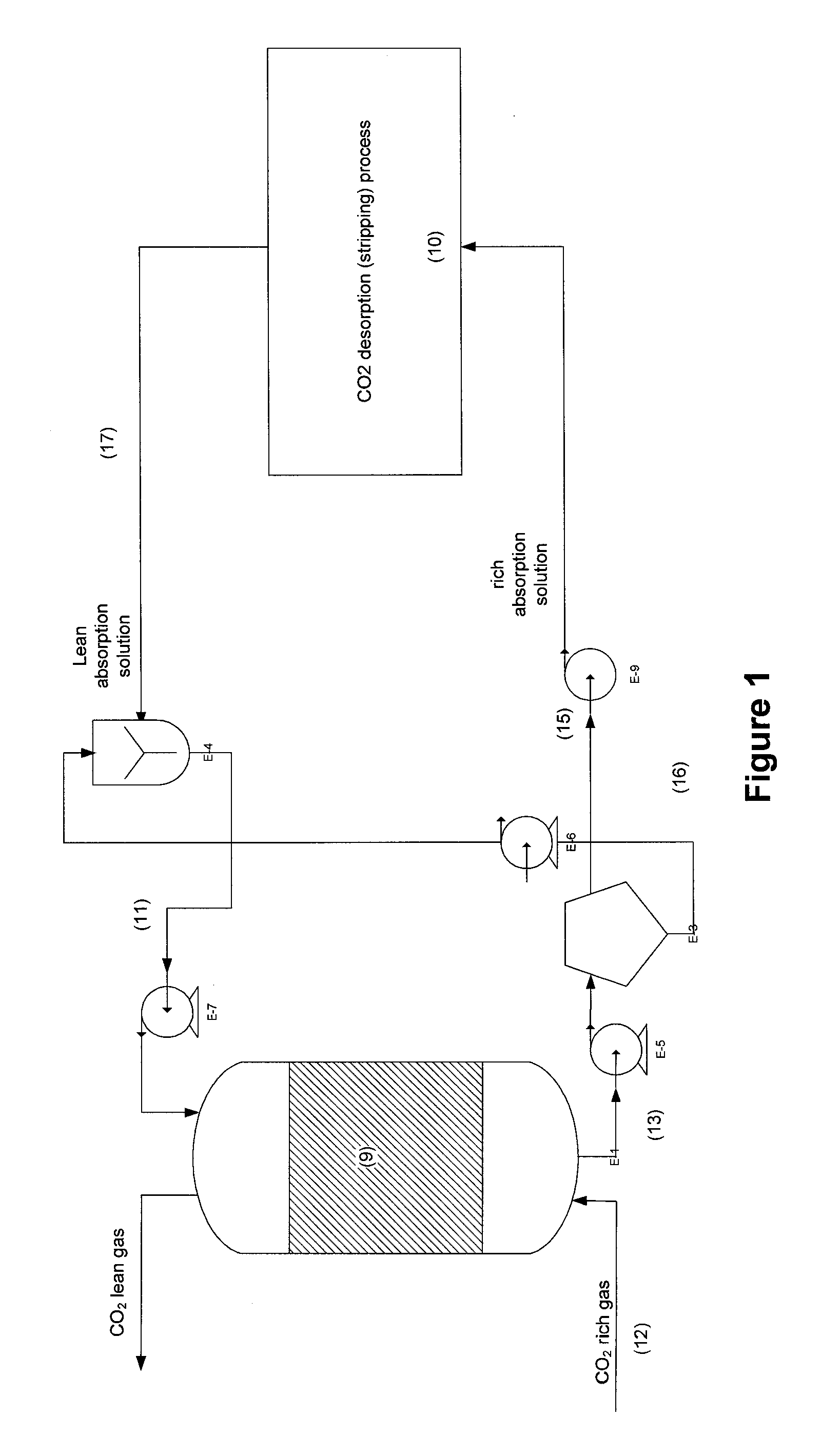
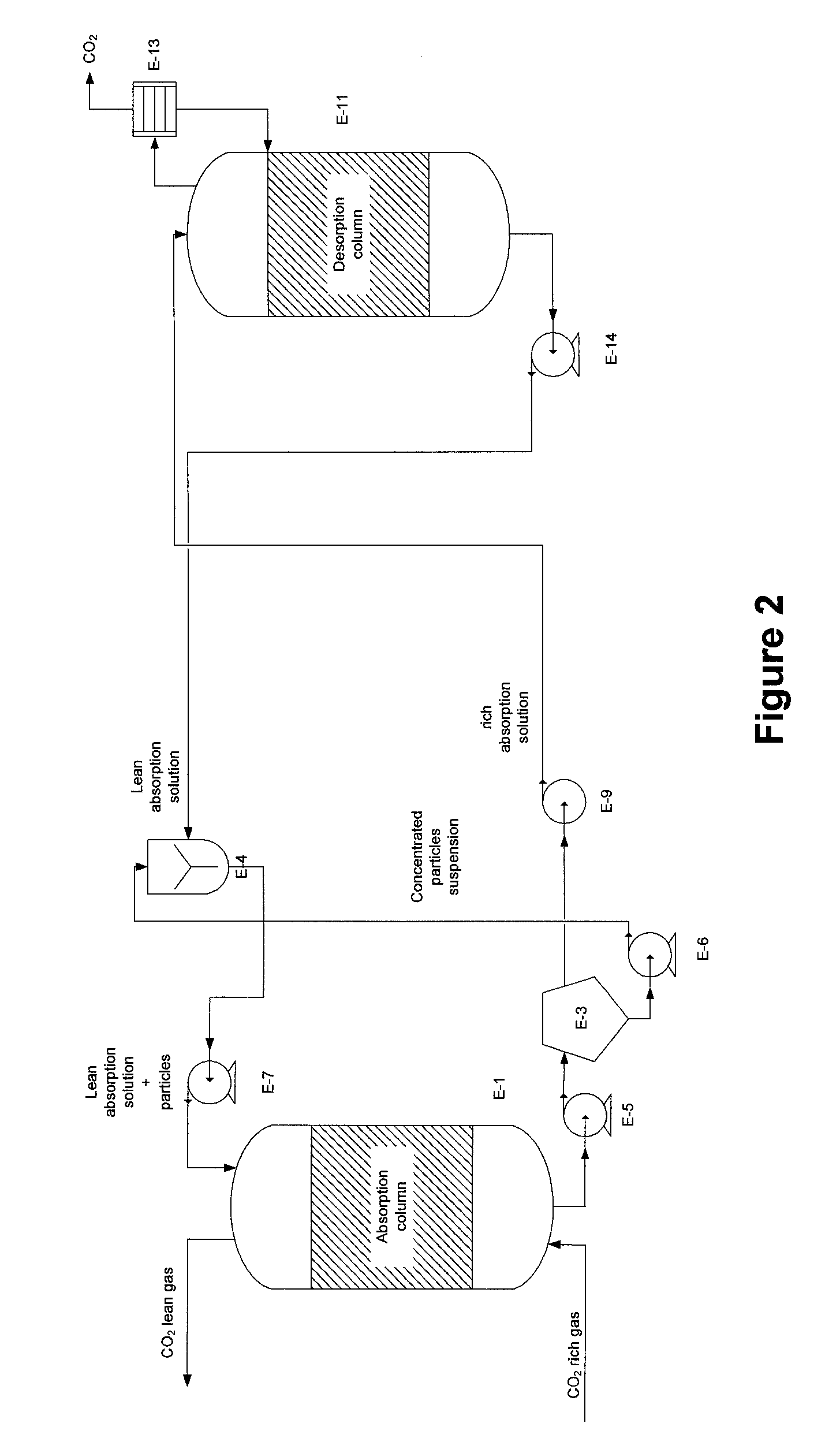
Process for desalination of saline water, especially water, having increased product yield and quality
InactiveUS6508936B1General water supply conservationSeawater treatmentTotal dissolved solidsDistillation
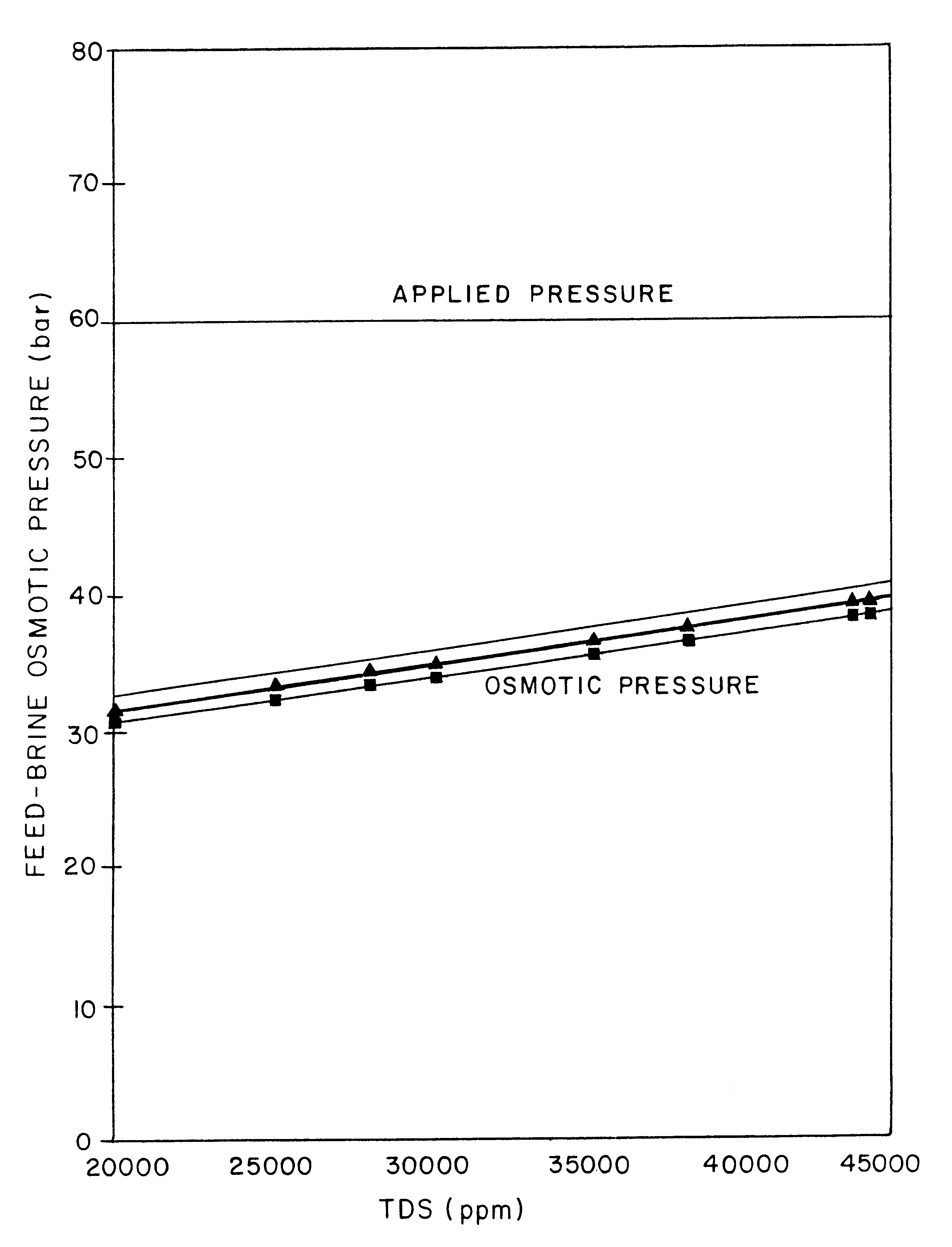
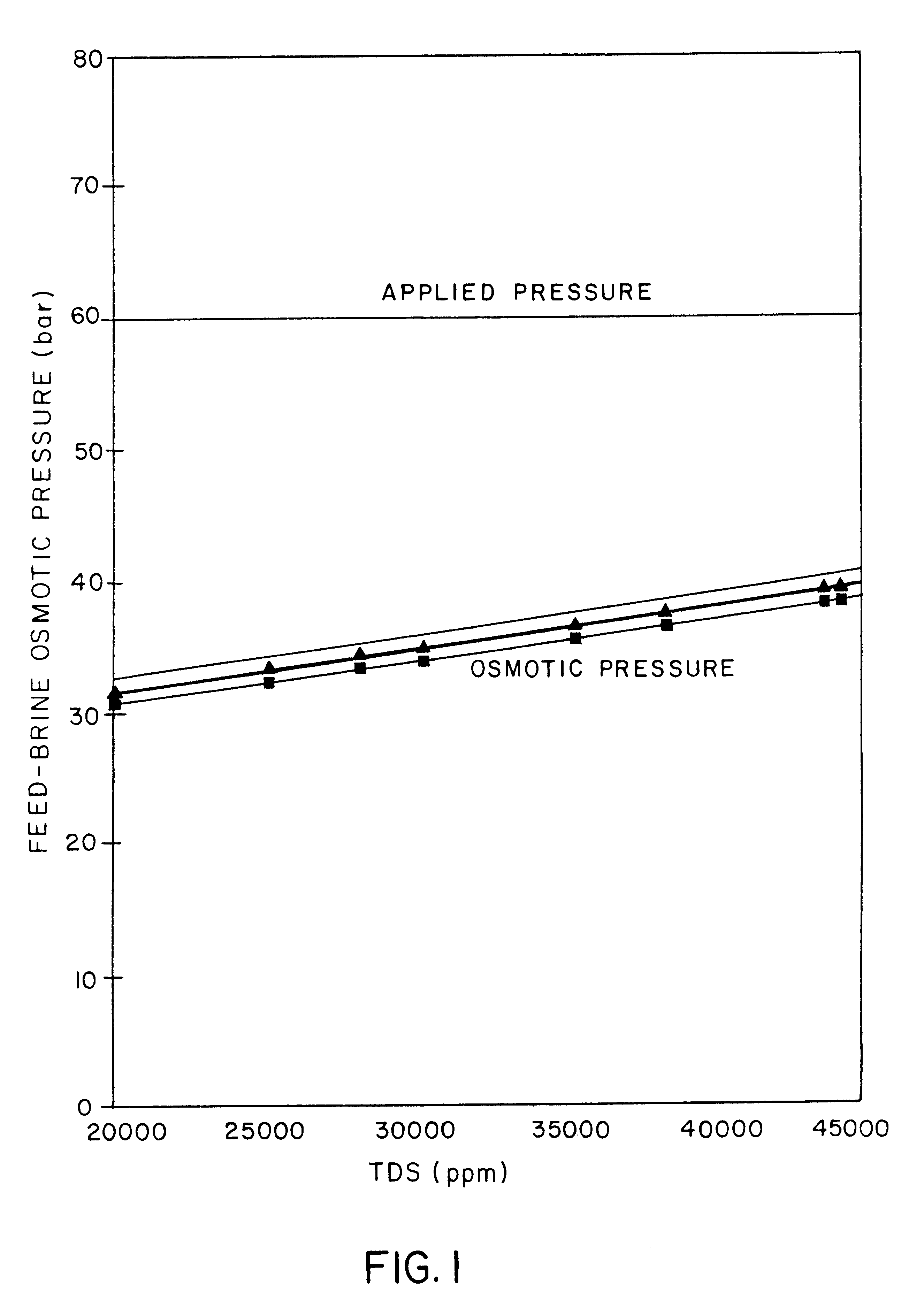
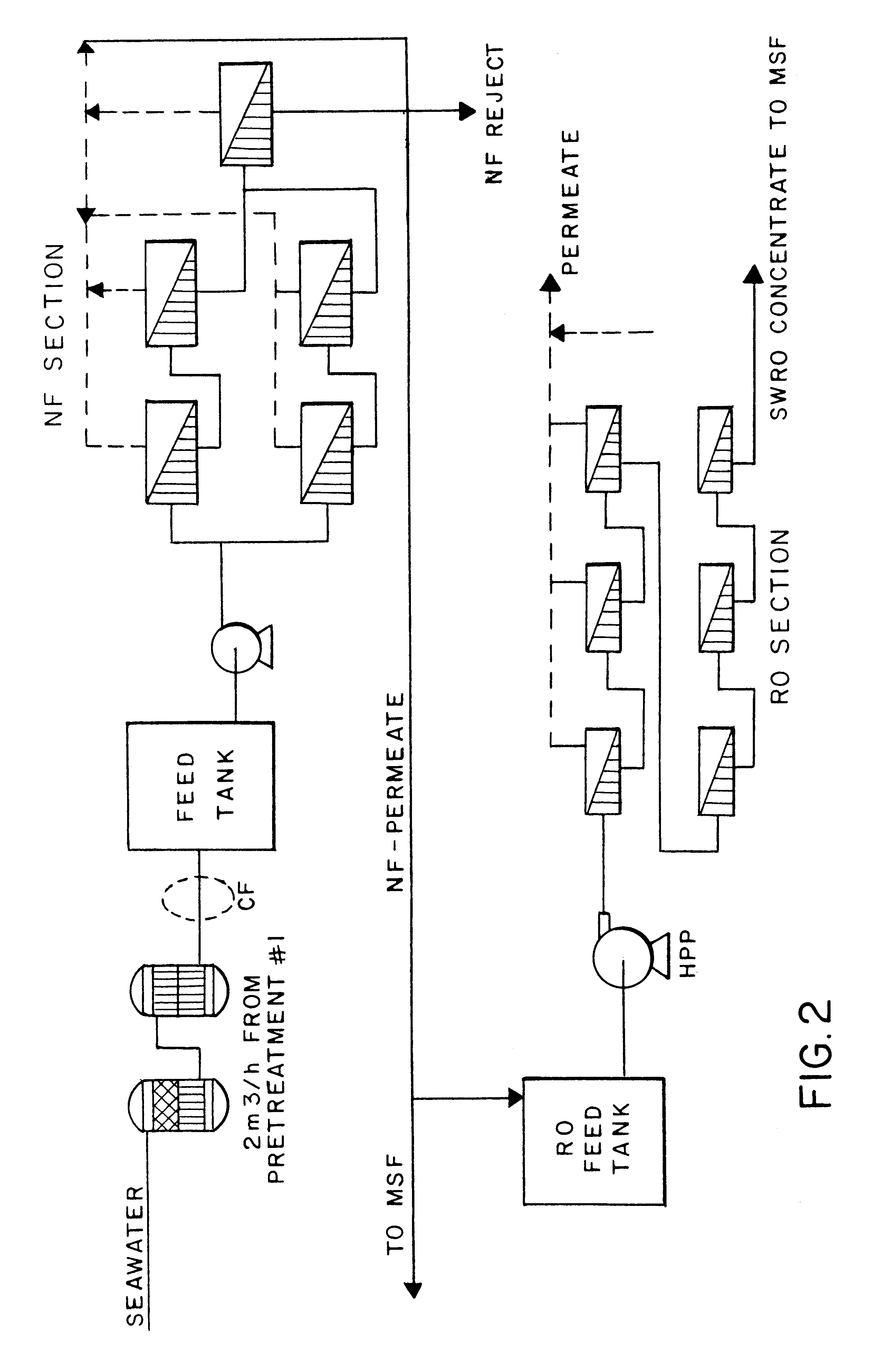
A desalination process is disclosed which combining two or more substantially different water treatment processes in a unique manner to desalinate saline water, especially sea water, to produce a high yield of high quality fresh water, including potable water, at an energy consumption equivalent to or less than much less efficient prior art desalination processes. In this process a nanofiltration step is synergistically combined with at least one of sea water reverse osmosis, multistage flash distillation. multieffect distillation of vapor compression distillation to provide an integrated desalination system by which sea water can be efficiently and economically converted to high quality potable water in yields which are at least 70%-80% greater than the yields available from the prior art processes. Typically a process of this invention using the nanofiltration initial step will produce, with respect to sea water feed properties, calcium, magnesium, sulfate and bicarbonate ion content reductions of 63%-94%, pH decreases of about 0.4-0.5 units and total dissolved solids content reductions of 35%-50%.
Owner:SALINE WATER CONVERSION CORP
Process and a plant for the production of Portland cement clinker
InactiveUS6908507B2Emission reductionReduce carbon dioxide emissionsGas treatmentDispersed particle separationCalcium in biologyHydrogen
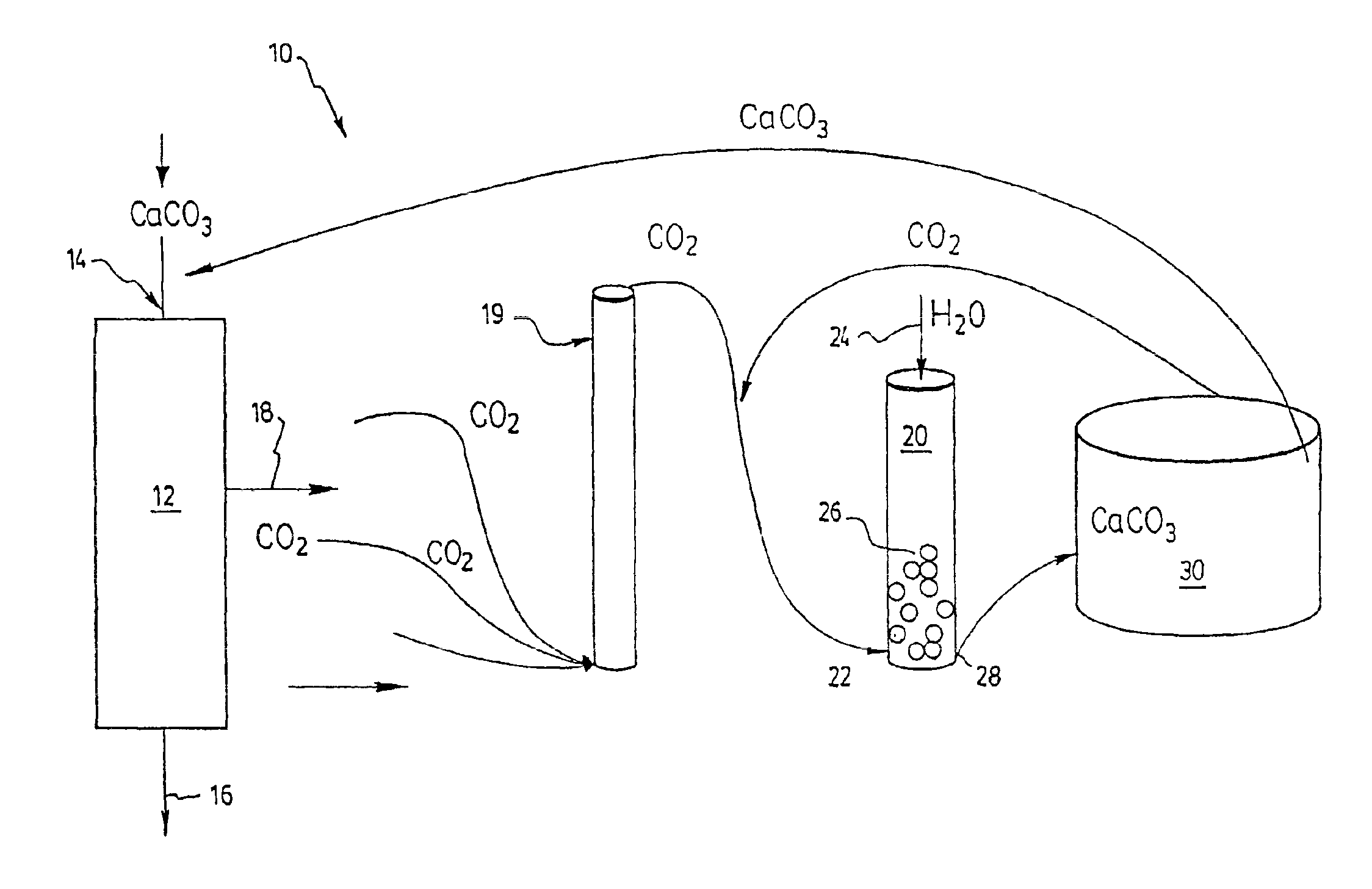
A process is disclosed for producing cement clinker, comprising the steps of: a) providing a mixture of ground calcareous materials and ground argillaceous materials; b) heating the mixture of step a) to a temperature sufficient to calcine and fuse the ground materials to form the cement clinker, and thereby producing an exhaust gas containing CO2; c) catalysing the hydration of at least a portion of the CO2 contained in the exhaust gas and producing a solution containing bicarbonate ions and hydrogen ions; and d) adding to the solution obtained in step c) metal ions, and adjusting the pH of the solution to precipitate a carbonate or said metal. Preferably, the metal ions are Ca++ obtained from the dissolution of a material selected from the group consisting of CaCl2, cement kiln dust and sea salts and the carbonate is CaCO3 which is advantageously recycled into the process by adding the CaCO3 to the mixture of step a). A cement plant for performing this process is also disclosed.
Owner:CO2 SOLUTION
Stable solution of zinc ions and bicarbonate and/or carbonate ions
InactiveUS6015547APrevention and counteractingInorganic/elemental detergent compounding agentsBiocideZinc ionBicarbonate Ion
A storage stable aqueous solution or aqueous gel of zinc ions in the presence of bicarbonate ions is disclosed. The solution comprises: (a) a source of zinc ion, (b) a source of a stabilizing anion which can stabilize soluble zinc and bicarbonate and / or carbonate in solution; (c) a source of bicarbonate ion; and (d) a solvent therefor. The solvent comprises a major proportion of water. The zinc salt is present in an amount suitable for the intended purpose; the stabilizing anion in an amount B of at least 1.2 equivalents per equivalent of zinc ion; and the bicarbonate ion cannot exceed certain levels which are related to the level of the stabilizing anion.
Owner:CHURCH & DWIGHT CO INC
Process and apparatus for the treatment of saline water
InactiveUS20060196836A1Reduce ZLD costReduce loadWaste water treatment from quariesGeneral water supply conservationTotal dissolved solidsEvaporation
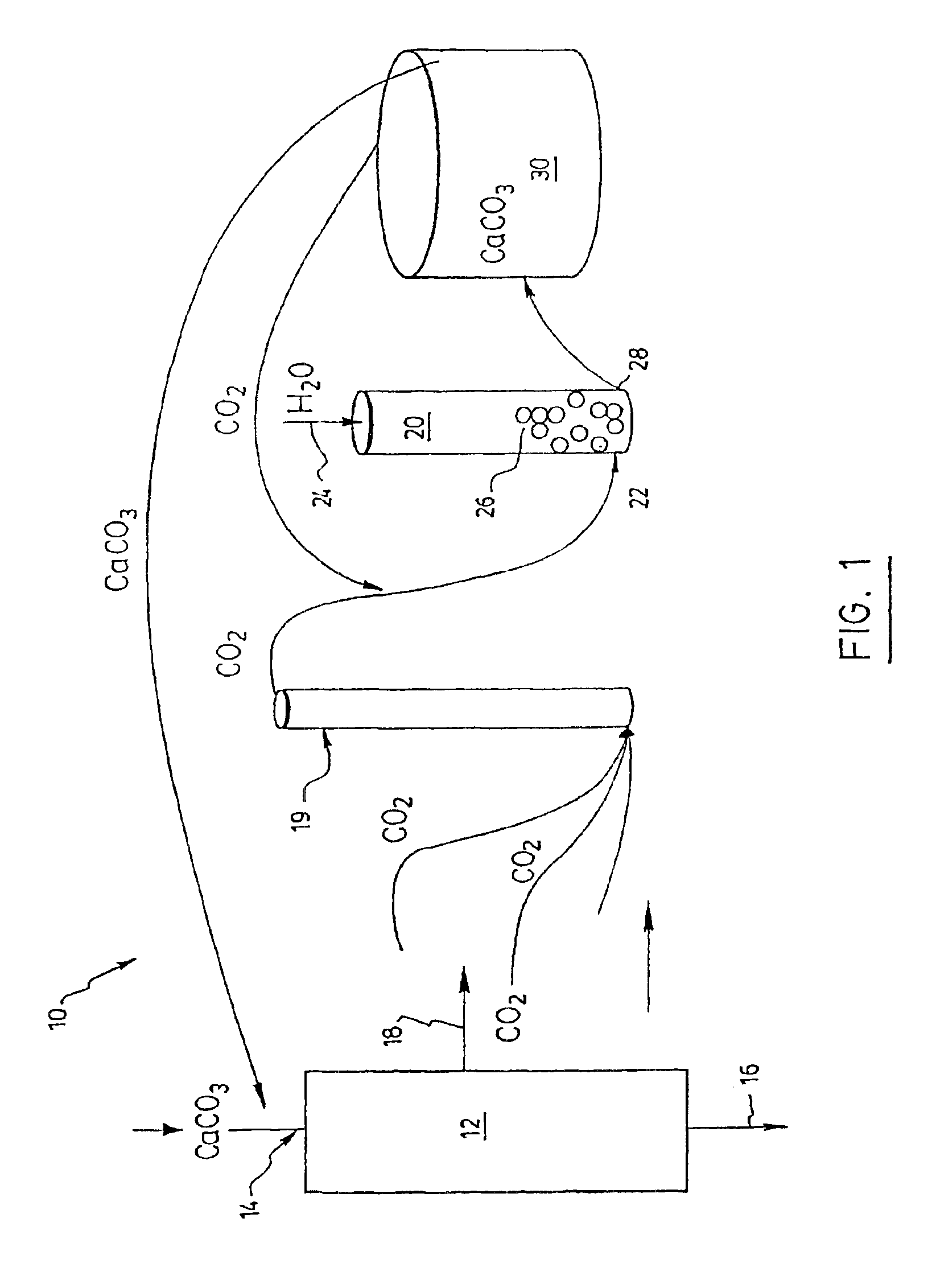
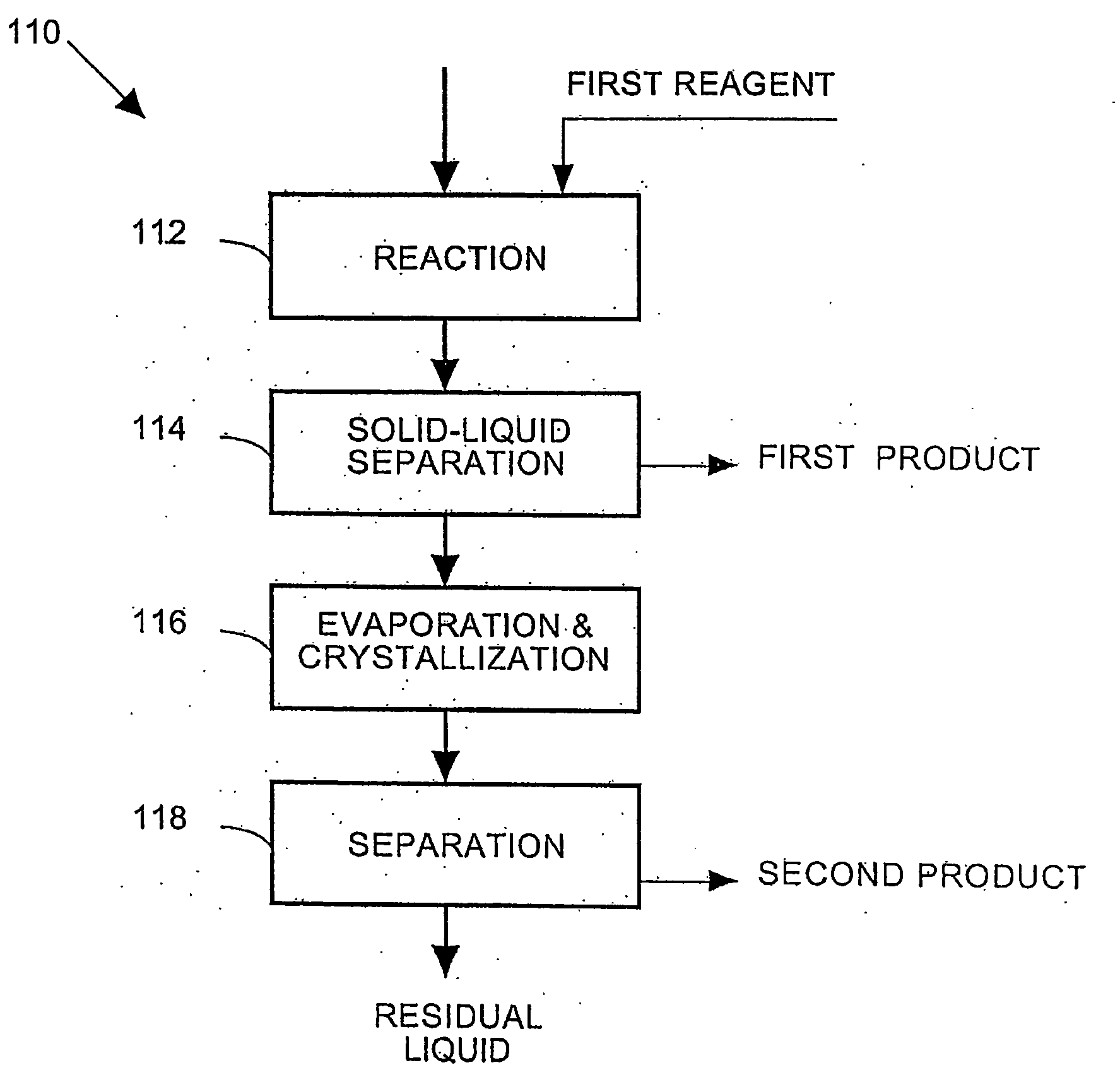
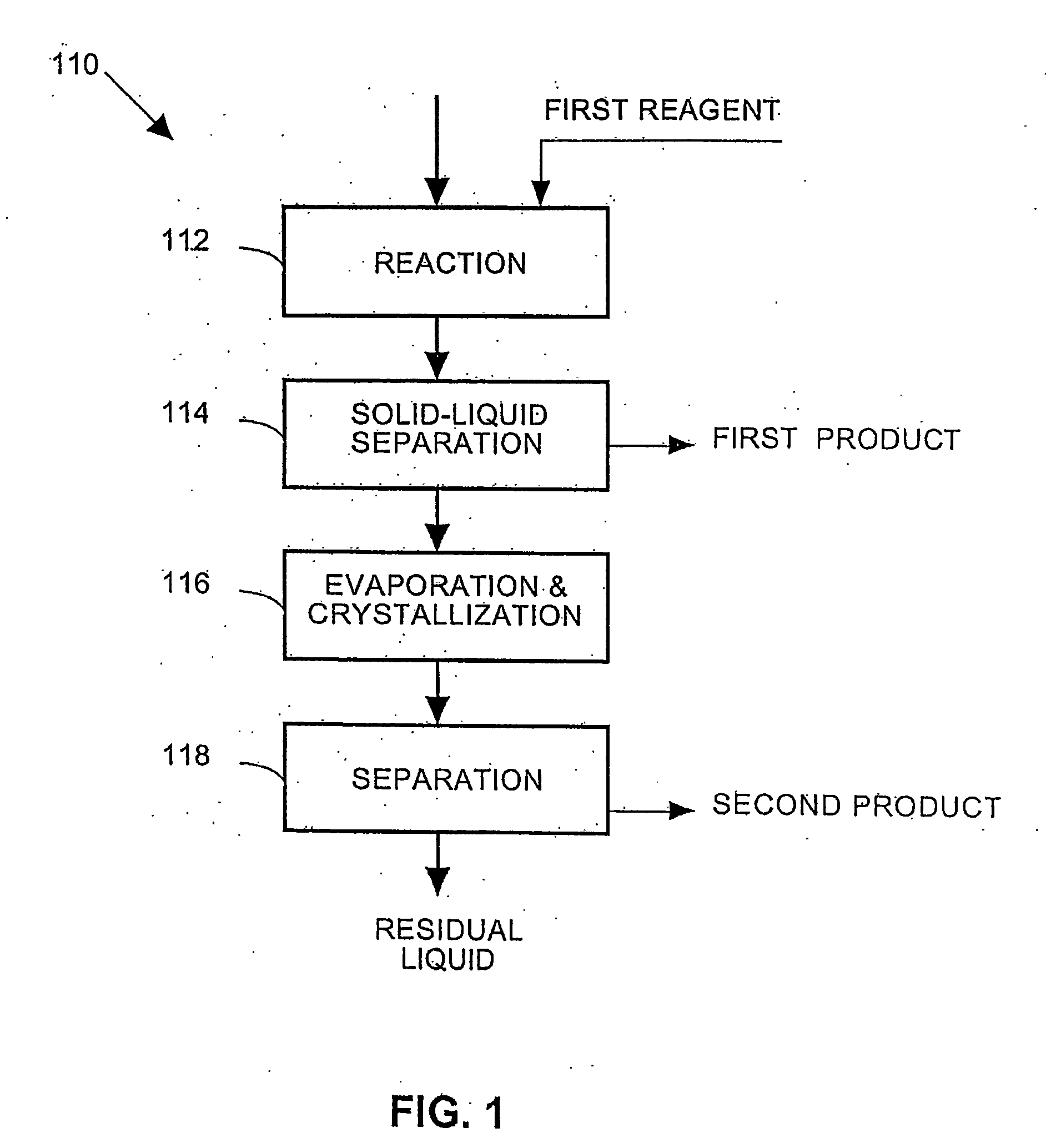
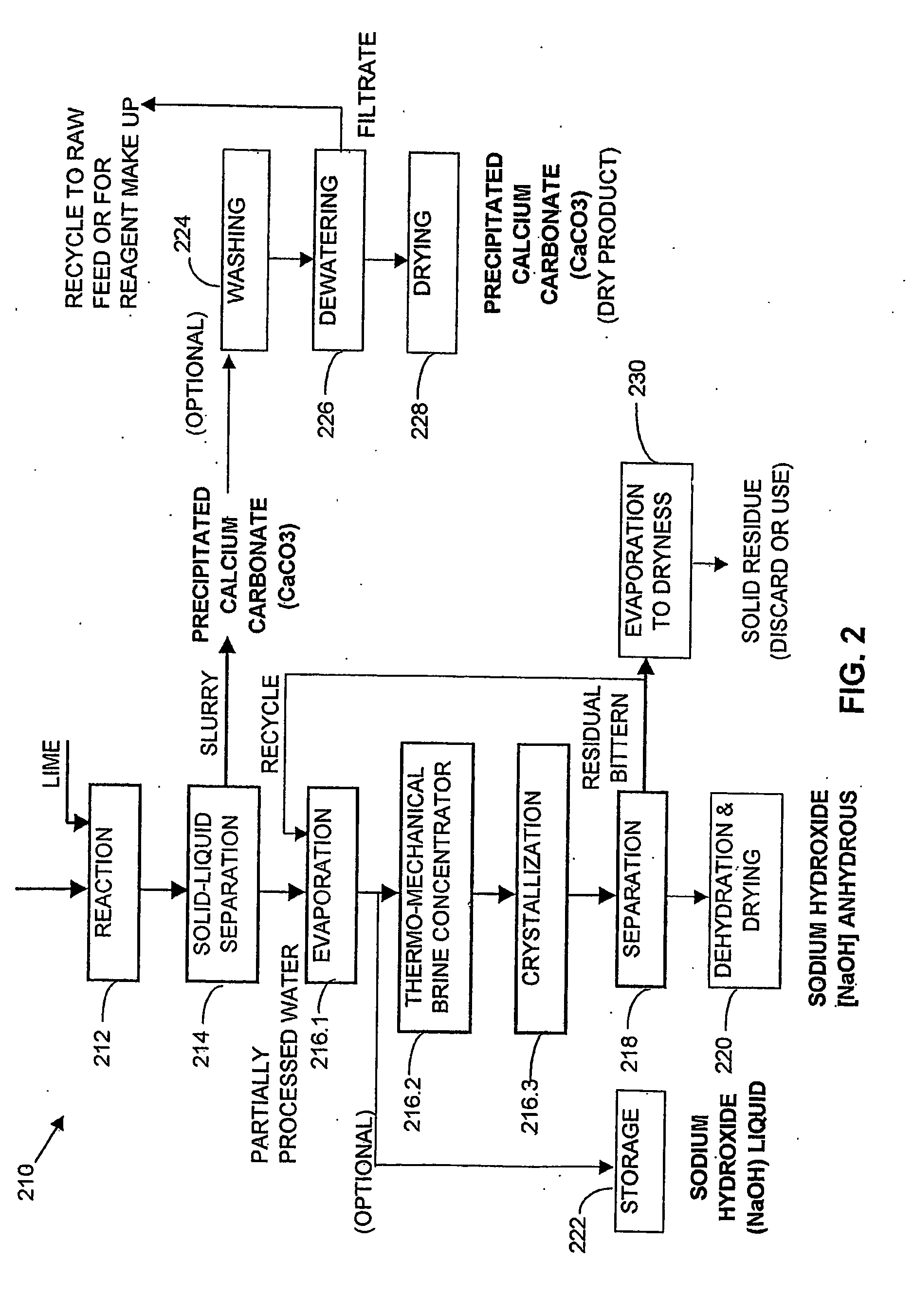
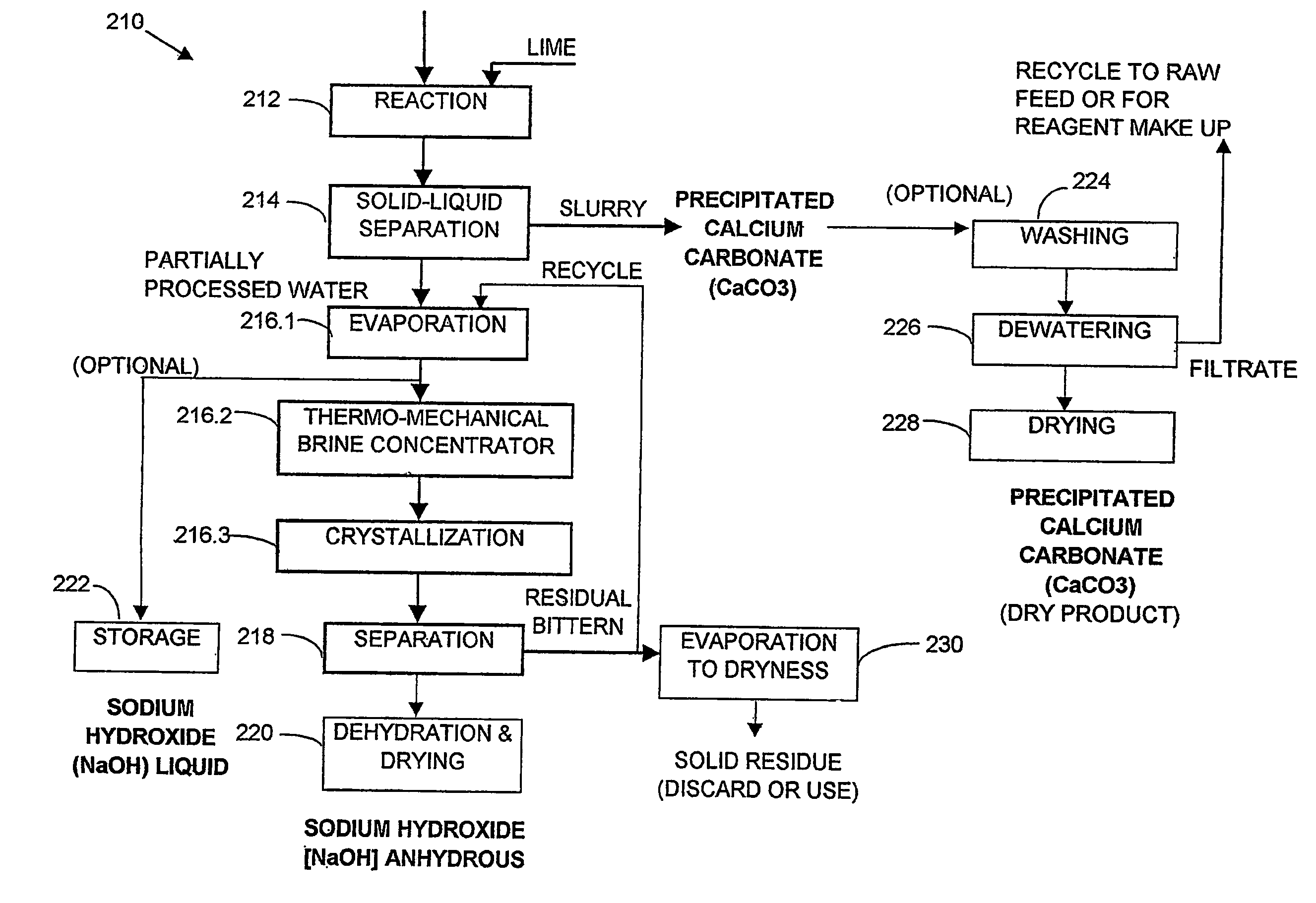
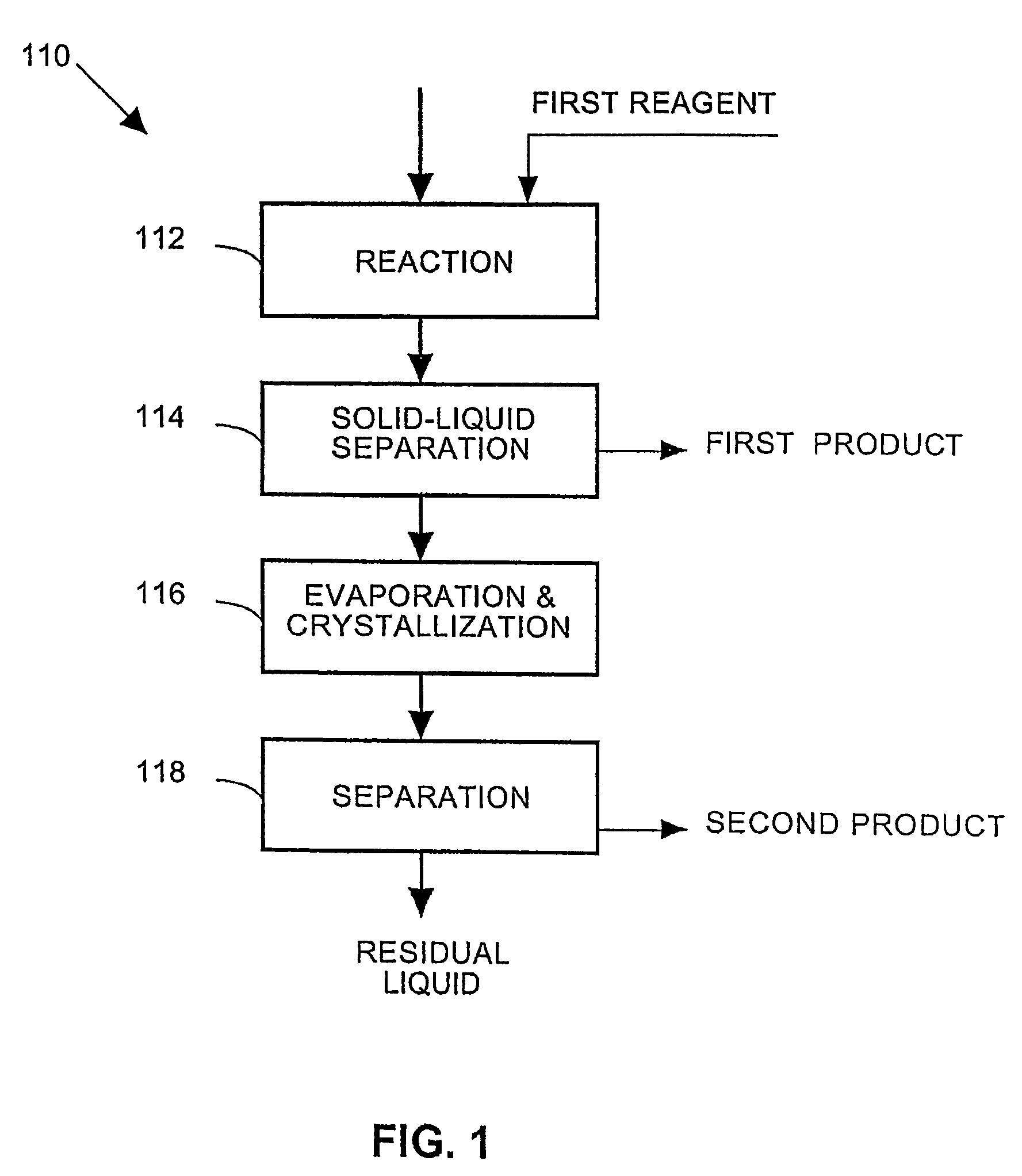
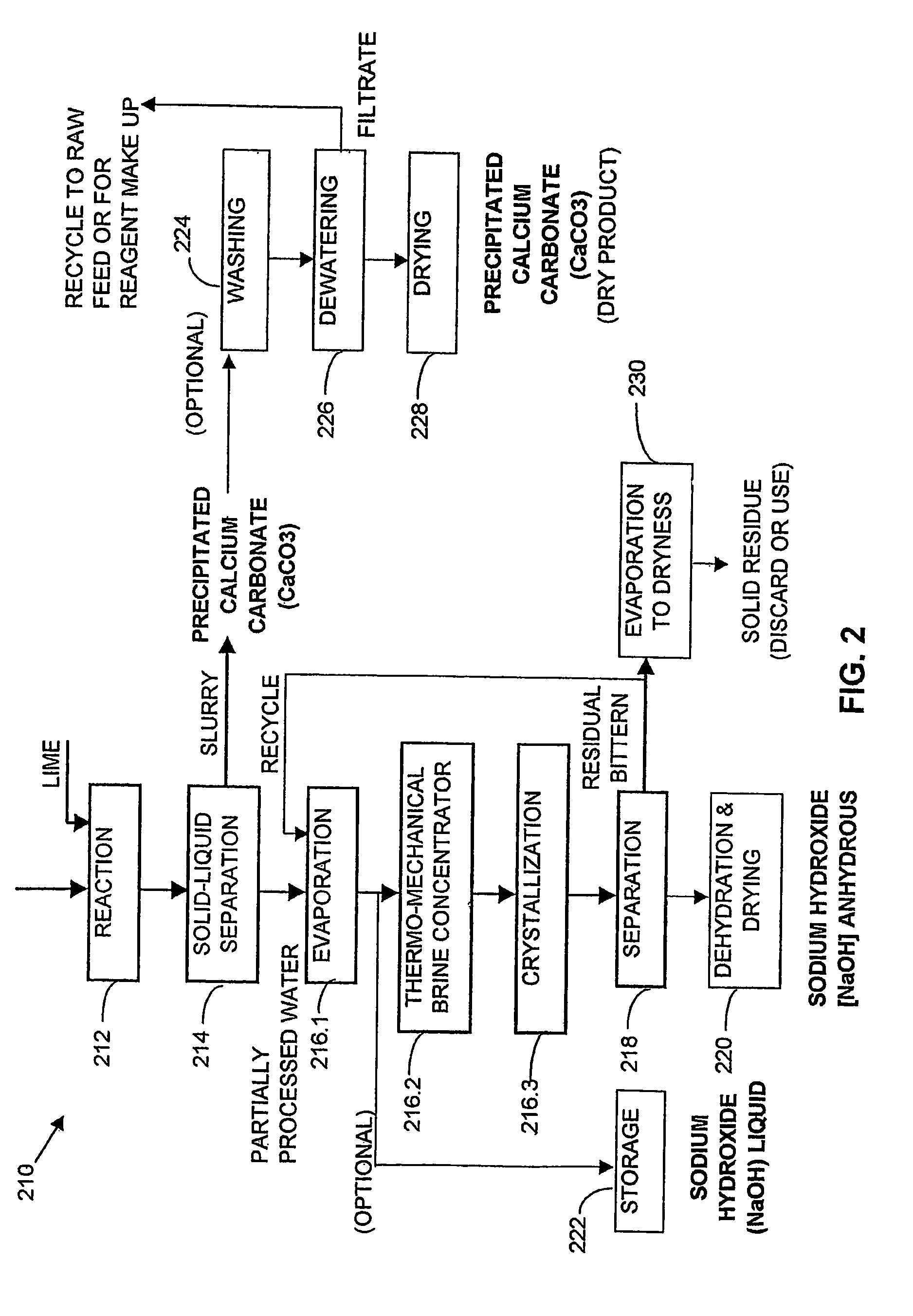
A process and an apparatus are described for treating seven types of saline waters each having a concentration of total dissolved solids exceeding 1 g / L, wherein the concentration of total dissolved solids, the ratio of the chloride ion concentration to the bicarbonate ion concentration and the ratio of the chloride ion concentration to the sulphate ion concentration of each of the water types are as indicated in Table 1. The process includes the steps of contacting the water with a first reagent comprising a source of calcium ions selected from calcium oxide and calcium hydroxide to form a first solid product which is recovered. The process includes a further step of subjecting at least a portion of the partially processed water to at least partial evaporation so as to promote the formation of a precipitate and a mother liquor. The precipitate is recovered as a second product.
Owner:GEO PROCESSORS
Process for the treatment of saline water
InactiveUS7595001B2Waste water treatment from quariesGeneral water supply conservationTotal dissolved solidsEvaporation
A process and an apparatus are described for treating seven types of saline waters each having a concentration of total dissolved solids exceeding 1 g / L, wherein the concentration of total dissolved solids, the ratio of the chloride ion concentration to the bicarbonate ion concentration and the ratio of the chloride ion concentration to the sulphate ion concentration of each of the water types are as indicated in Table 1. The process includes the steps of contacting the water with a first reagent comprising a source of calcium ions selected from calcium oxide and calcium hydroxide to form a first solid product which is recovered. The process includes a further step of subjecting at least a portion of the partially processed water to at least partial evaporation so as to promote the formation of a precipitate and a mother liquor. The precipitate is recovered as a second product.
Owner:GEO PROCESSORS
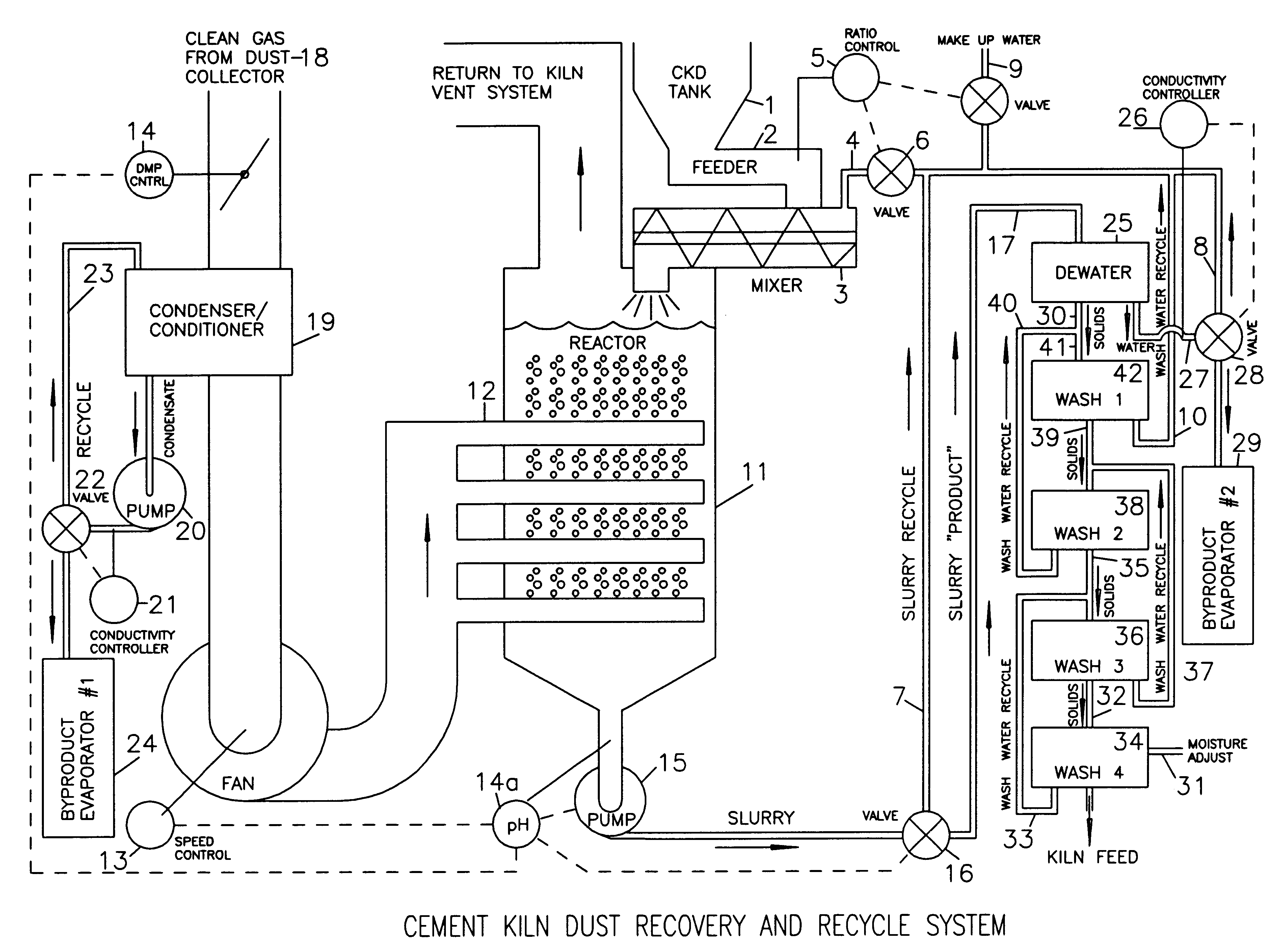
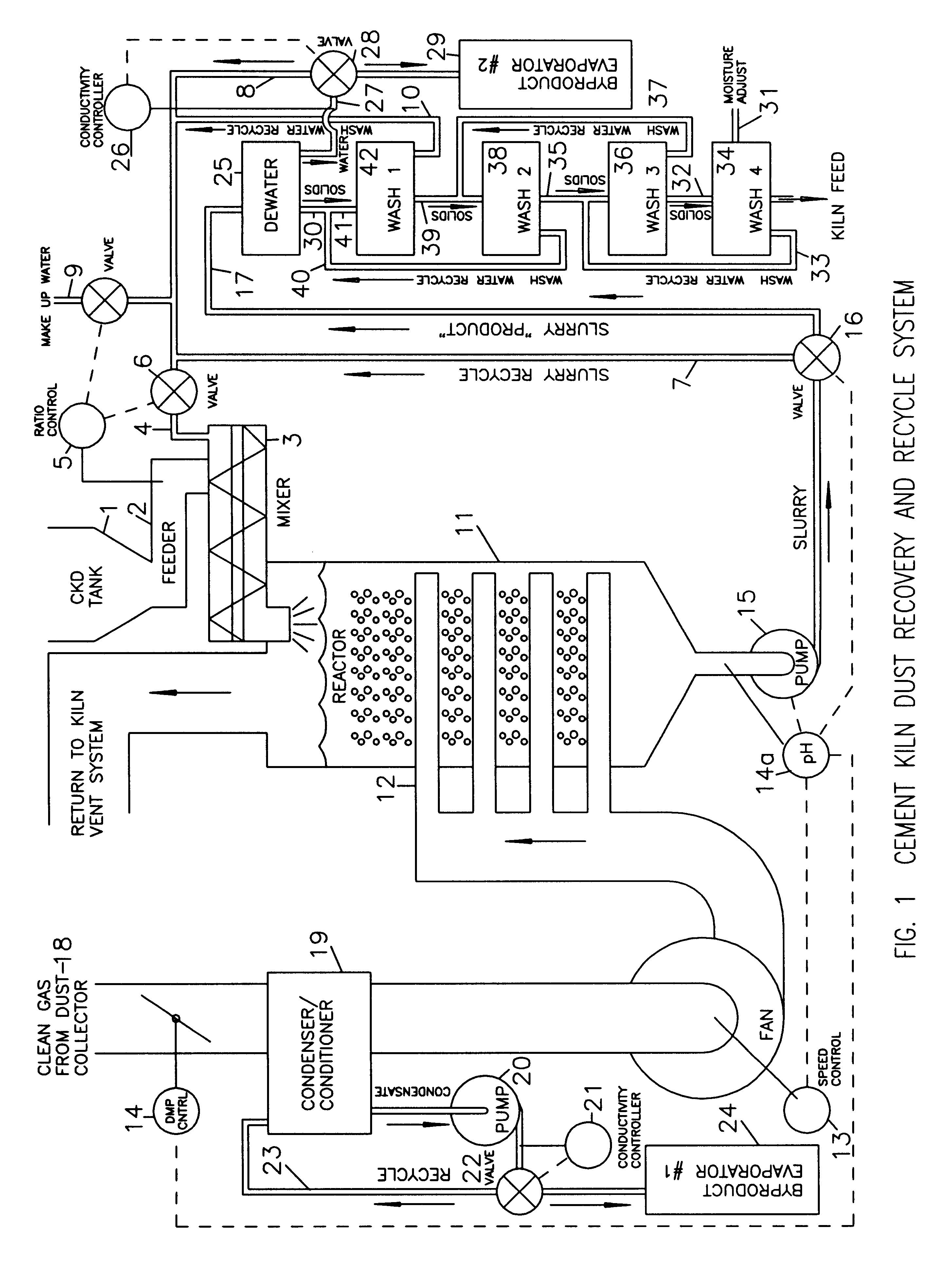
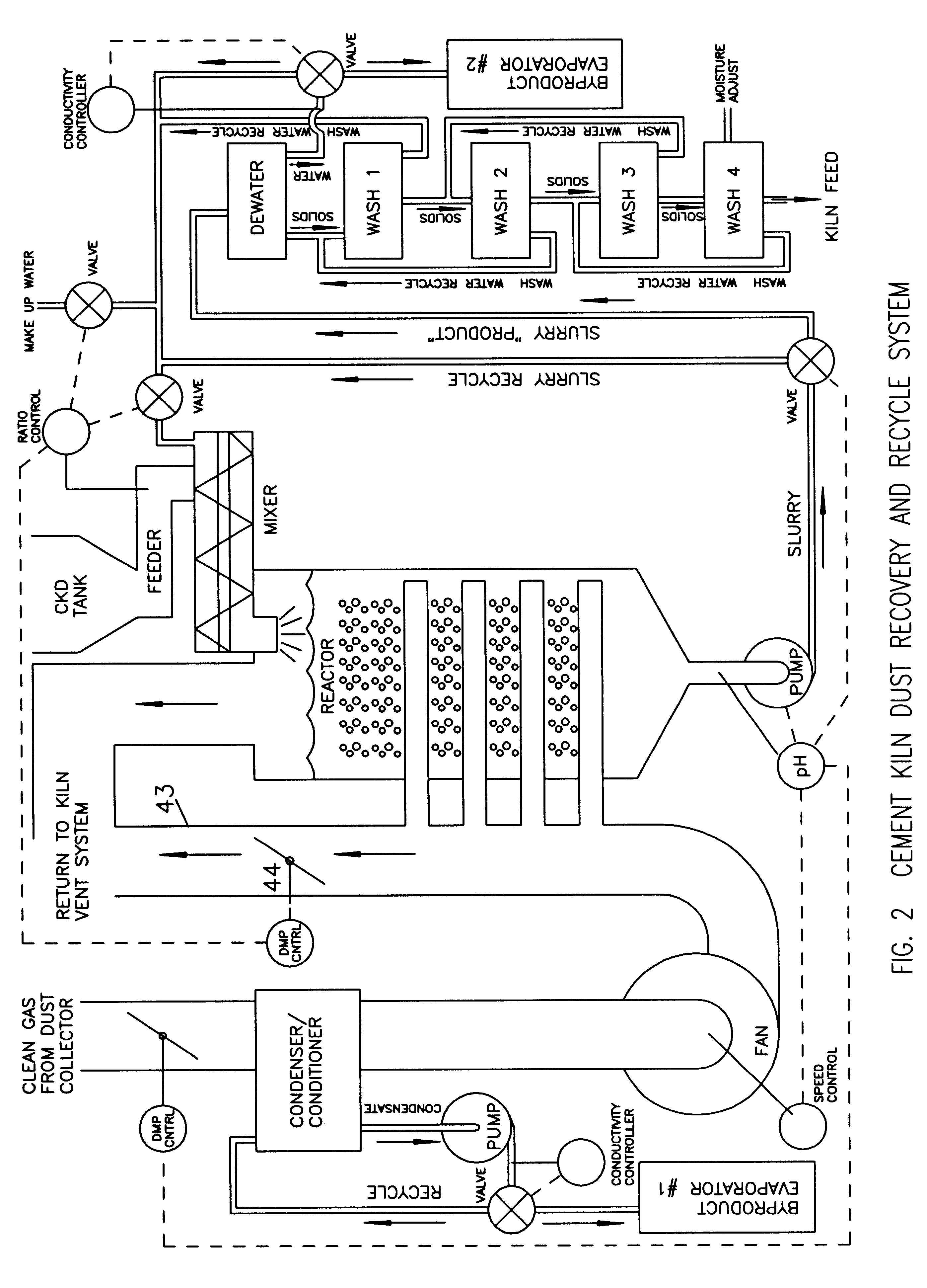
Method of treating cement kiln dust for recovery and recycle
Fresh or stockpiled cement kiln dust is moistened with sufficient water so that the amount of total free and combined water relative to dust is about 3 parts water to 1 part dust by mass, or less. The wet solids are treated with carbon dioxide to convert compounds, such as calcium hydroxide, to carbonates, such as calcium carbonate. The degree of carbonation is controlled so that the solubility of calcium becomes minimum for the dust being treated; this is also when hydroxyl and bicarbonate ions in solution are about at their minima. As the carbonation reactions occur, the water combined in hydroxides is released as free water so that the mixture becomes a slurry and the potentially soluble alkalies and sulfate (and any chlorides present) are released to the liquid phase. The solids are separated from the liquid, and the solids, which may be washed, provide a material suitable for return as feed to the kiln. The liquid, which contains the dissolved alkali compounds, is recycled to reclaim additional dust or treated to recover alkali salts when the alkali salts are sufficiently concentrated.While any source of carbon dioxide may be used, the preferred source is exit gases from the kiln. The gases are conditioned by condensation of water and removal of ammonium compounds, such as sulfate and chloride. The conditioning condensate may be treated to recover useful byproduct salts.
Owner:GEBHARDT RONALD FR
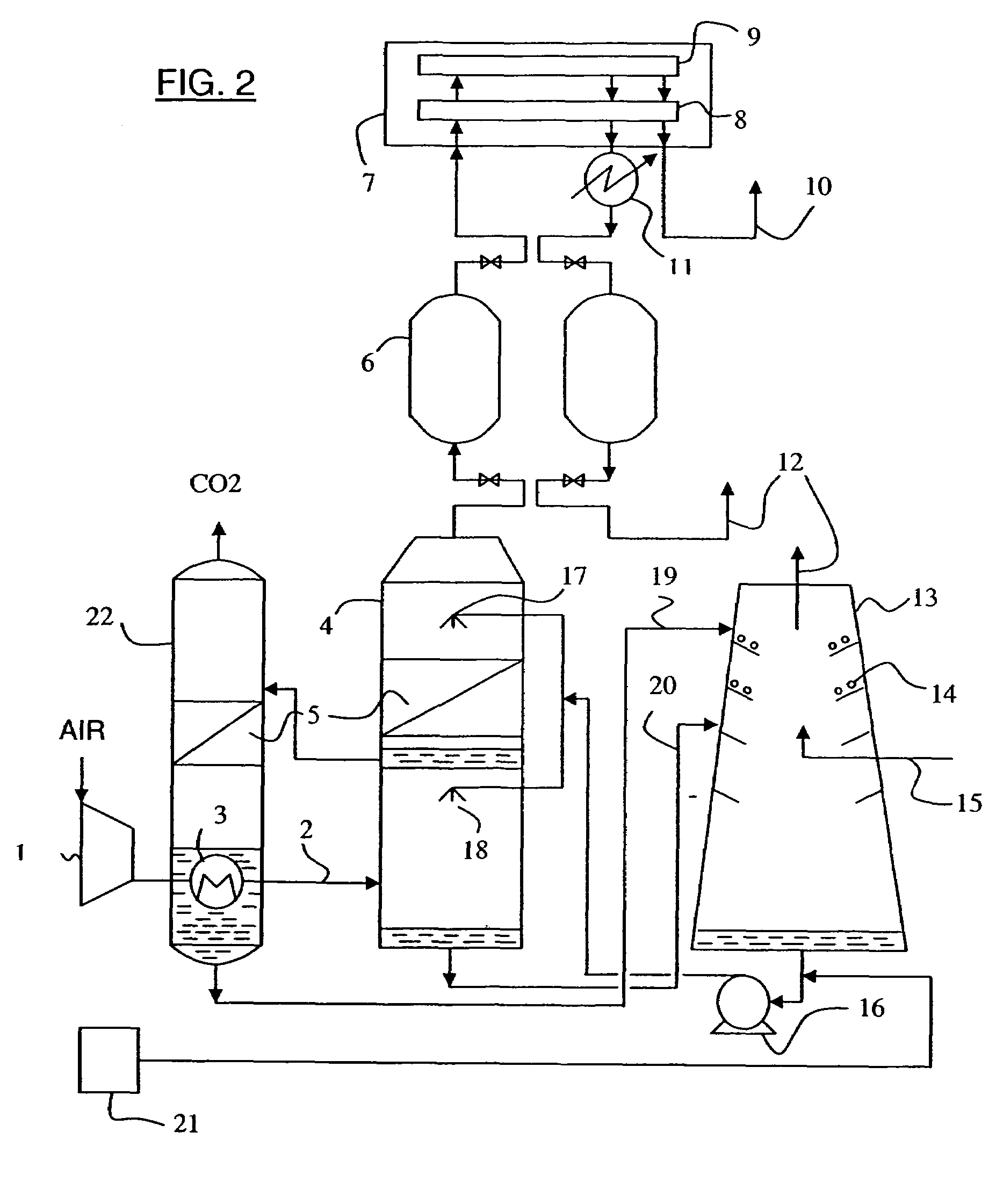
Process and installation for the fractionation of air into specific gases
InactiveUS20060213224A1Improve conversion performanceSolidificationLiquefactionHydration reactionCooling tower
Owner:CO2 SOLUTION
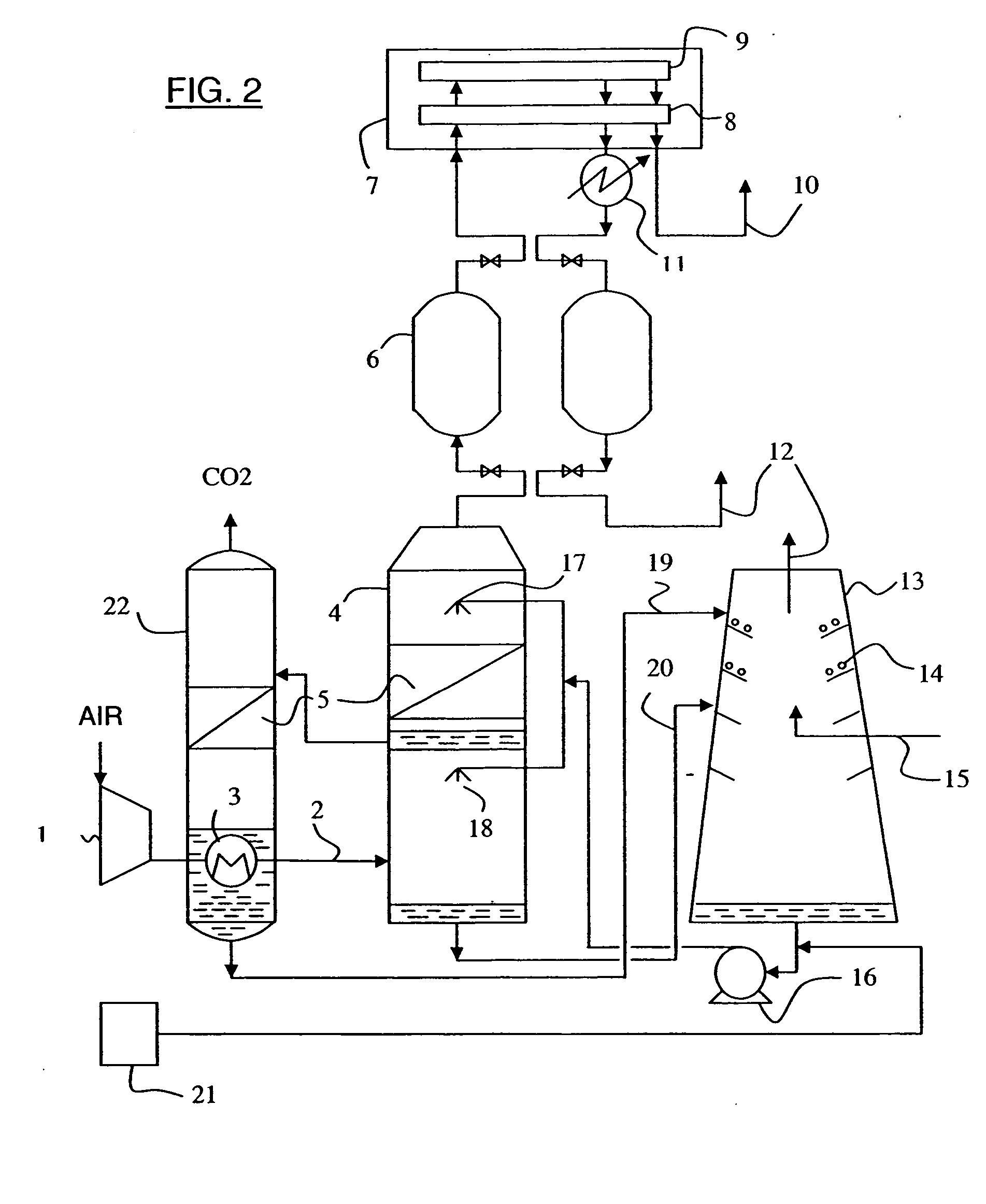
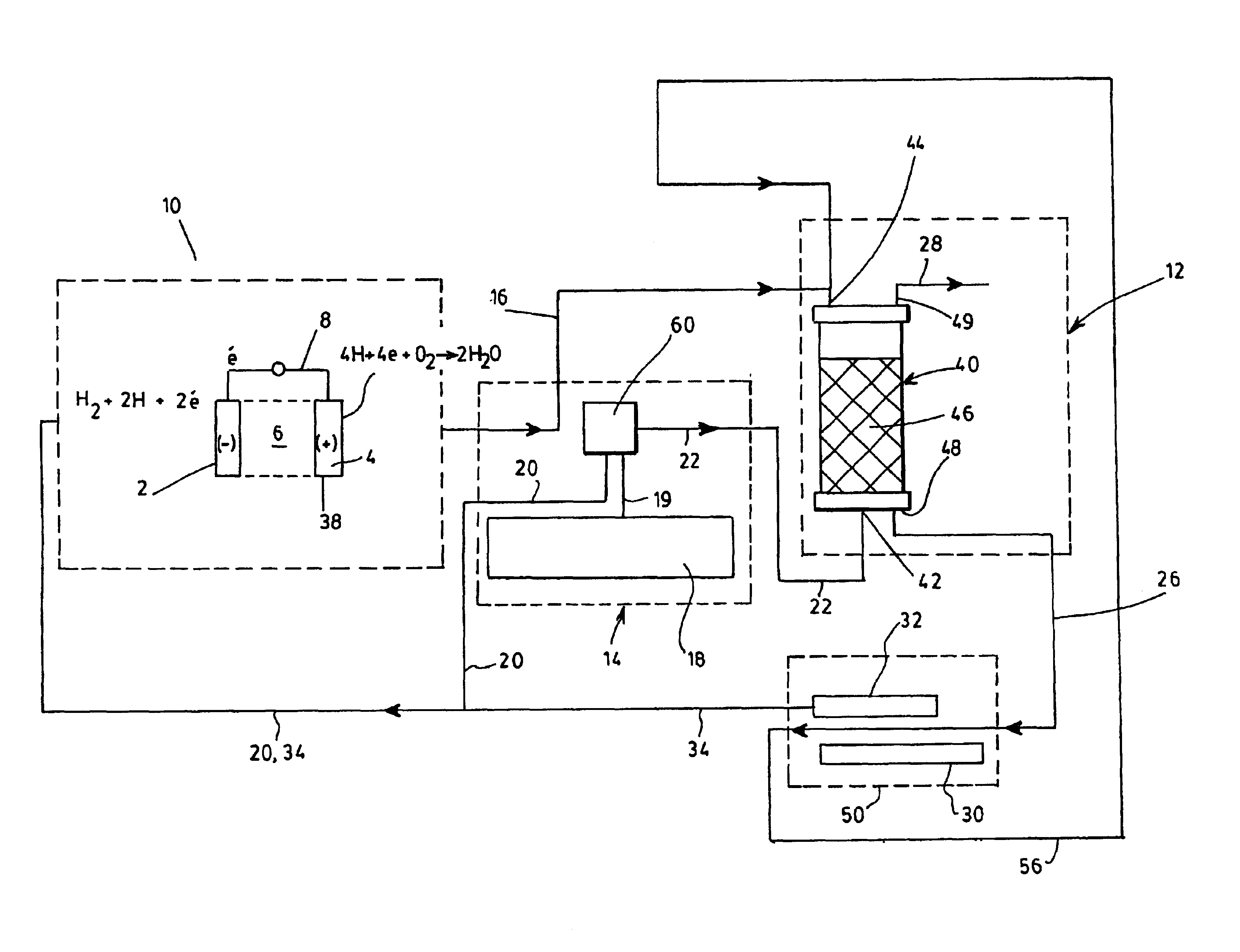
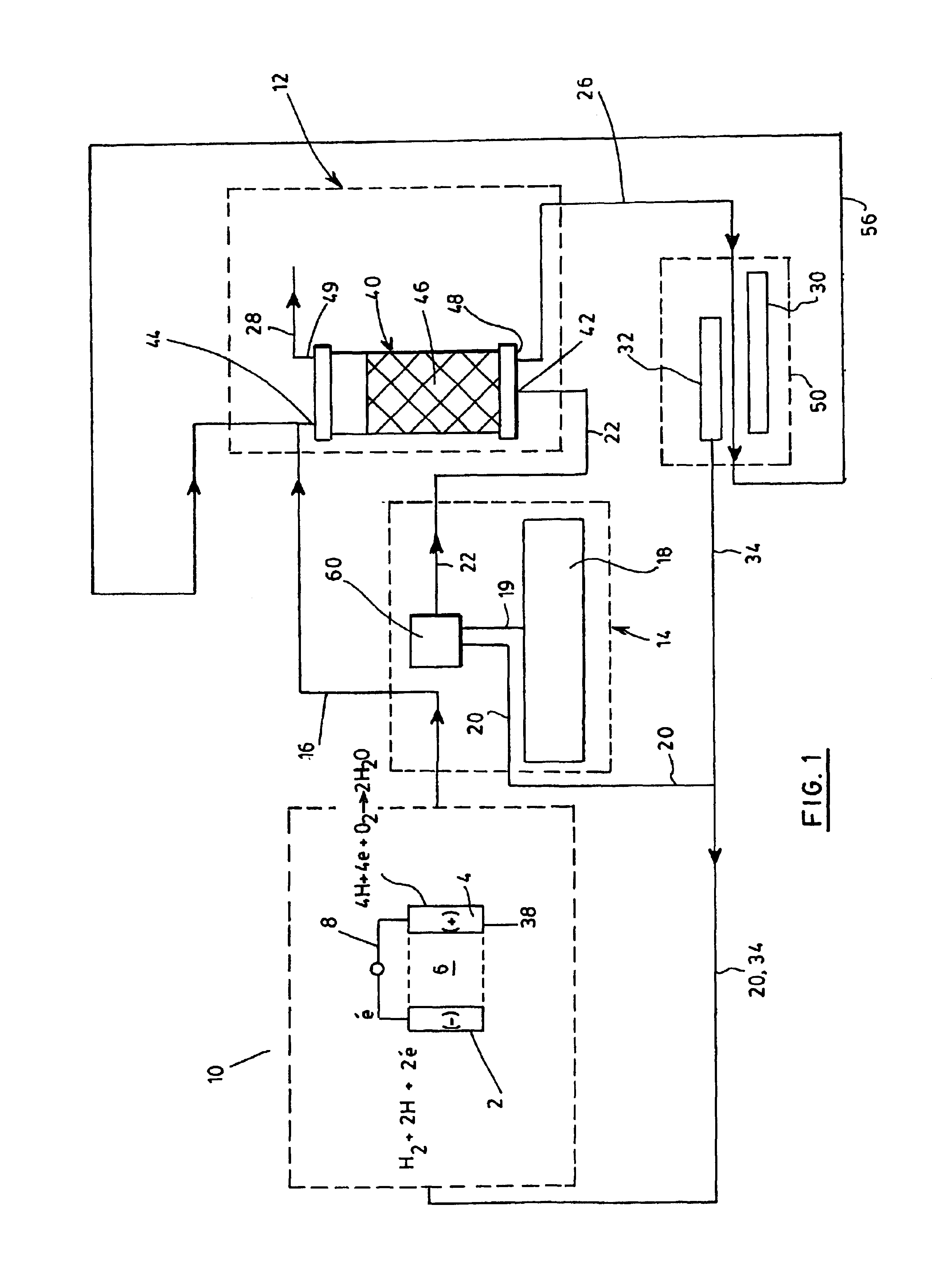
Process for generating electricity with a hydrogen fuel cell
InactiveUS6846584B2Improve hydrogen efficiencySimple and non-pollutingFinal product manufactureRegenerative fuel cellsElectricityCoupling
The object of the invention is the coupling of a hydrogen fuel cell to an enzymatic process for the production of electricity and the transformation and sequestration of CO2. Gaseous CO2 emissions from processes such as hydrocarbon reforming are transformed into carbonate or bicarbonate ions and hydrogen ions by the enzymatic system in order to prevent their contribution to the greenhouse effect. The hydrogen ions resulting from the enzymatic process are recovered and combined in order to supply the hydrogen fuel cell. Finally, water, a by-product of the oxidizing reaction of the hydrogen fuel cell, is recovered and recycled back into the aqueous enzymatic system.
Owner:CO2 SOLUTION
Improvement method for saline-alkali soil
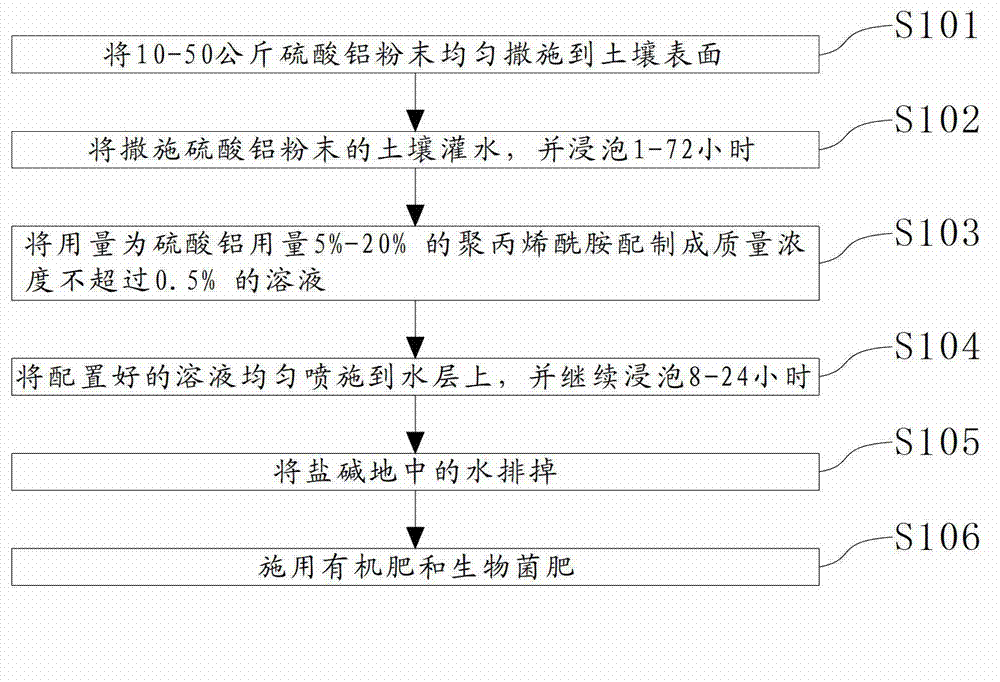
ActiveCN103109615AImprove propertiesImprove permeabilitySoil-working methodsAlkali soilPolyacrylamide
The invention relates to the agricultural field, in particular to an improvement method for saline-alkali soil. The improvement method for the saline-alkali soil comprises the following steps: sprinkling 10 to 50 kilograms of aluminum sulfate powders on the surface of the saline-alkali soil evenly; irrigating the soil with the aluminum sulfate powders and soaking the soil with the aluminum sulfate powders for 1 hour to 72 hours; compounding polyacrylamide with a dosage which is 5% to 20% of the dosage of the aluminum sulfate powders into a solution, wherein the mass concentration of the solution is no more than 0.5%; evenly spraying the compounded solution onto the water layer, and continuing to soak the soil with the aluminum sulfate powders for 8 hours to 24 hours; draining the water in the saline-alkali soil; and applying organic fertilizer and biological bacterial fertilizer to the saline-alkali soil. The improvement method for the saline-alkali soil can improve the sodium ions, the carbonate ions and the bicarbonate radical ions in the soil, so that the potential of hydrogen (pH) value of the solution of the soil can be reduced. The aluminum sulfate is increased, so that the soluble salts released by the soil particles are also increased, so that the salt materials in the soil can be reduced remarkably. Due to the fact that the macromolecule polyacrylamide is added, the soil can congeal into bigger particles like pea grains, so that permeability and water and fertilizer preserving capability of the soil can be improved.
Owner:黑龙江省汉通生物工程有限公司
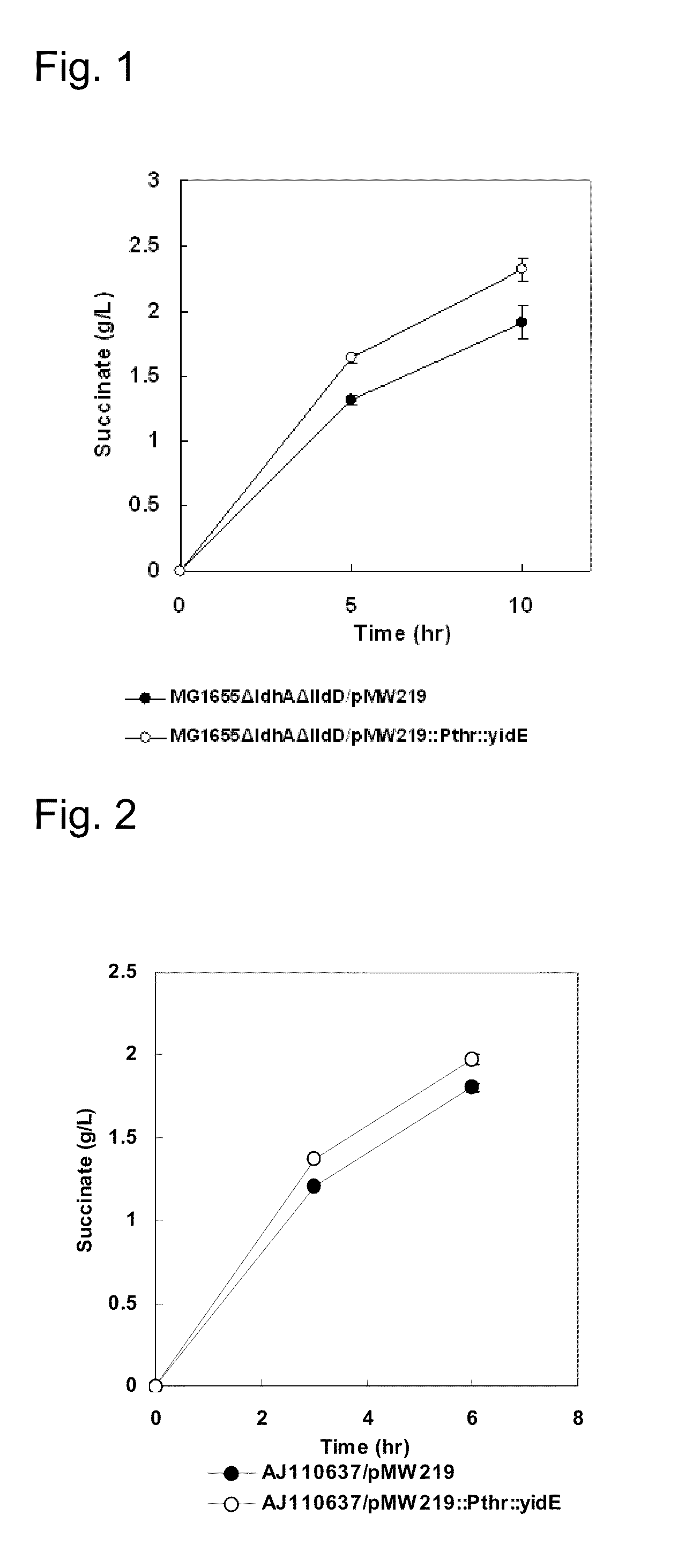
Process for producing succinic acid
ActiveUS20060205048A1Improve productivityProducing succinicBacteriaSugar derivativesPyruvate carboxylaseLactate dehydrogenase
Succinic acid is produced by allowing a bacterium modified to enhance fumarate reductase activity or cell preparation thereof to react with an organic raw material in a reaction solution containing one of a carbonate ion, a bicarbonate ion, and carbon dioxide gas to generate succinic acid. More preferably, succinic acid is produced by allowing a bacterium modified to enhance activities of fumarate reductase and pyruvate carboxylase and decrease lactate dehydrogenase activity or cell preparation thereof to react with an organic raw material in a reaction solution containing one of a carbonate ion, a bicarbonate ion, and carbon dioxide gas to generate succinic acid. Succinic acid is obtained by collecting the produced succinic acid.
Owner:AJINOMOTO CO INC
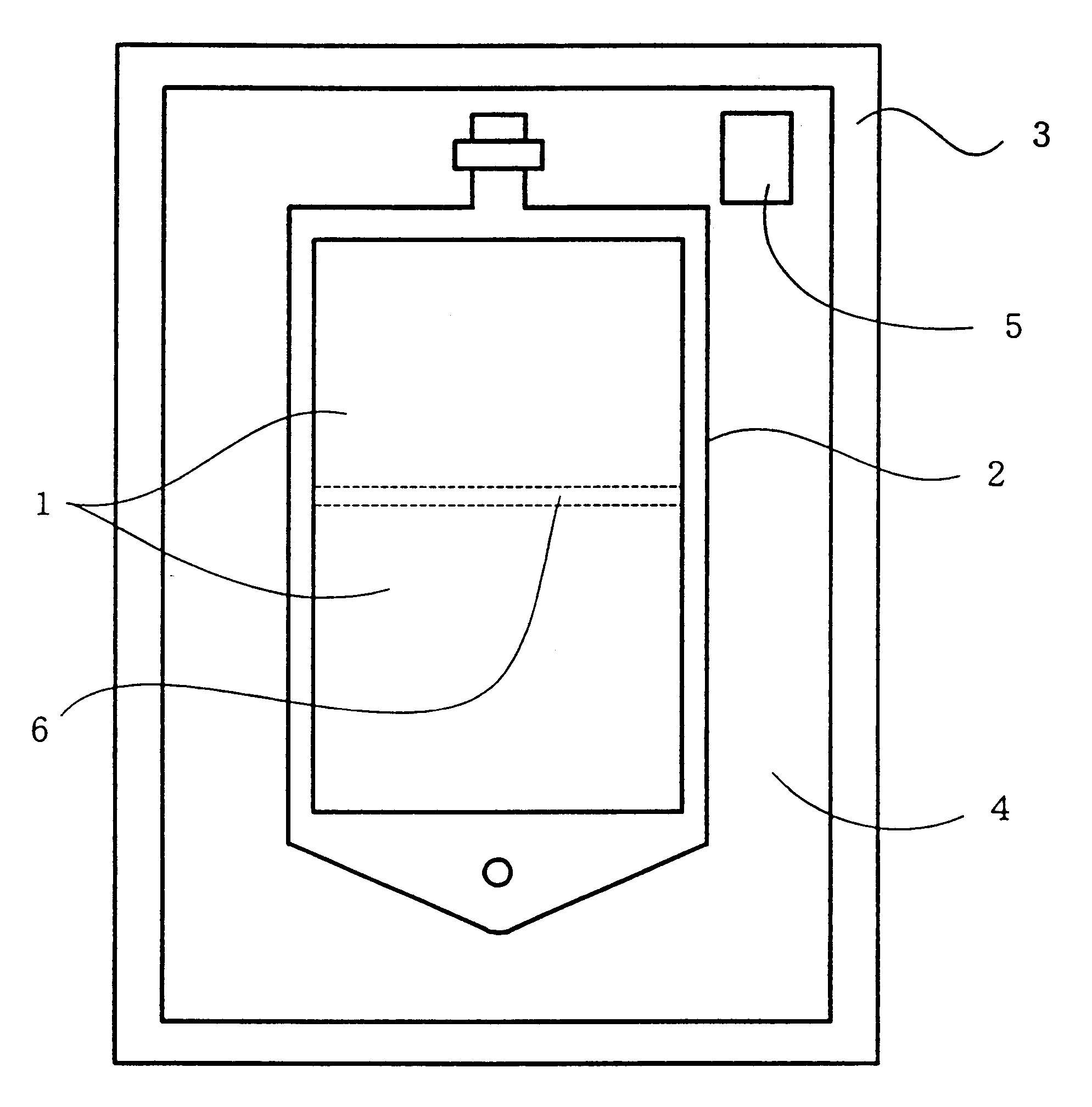
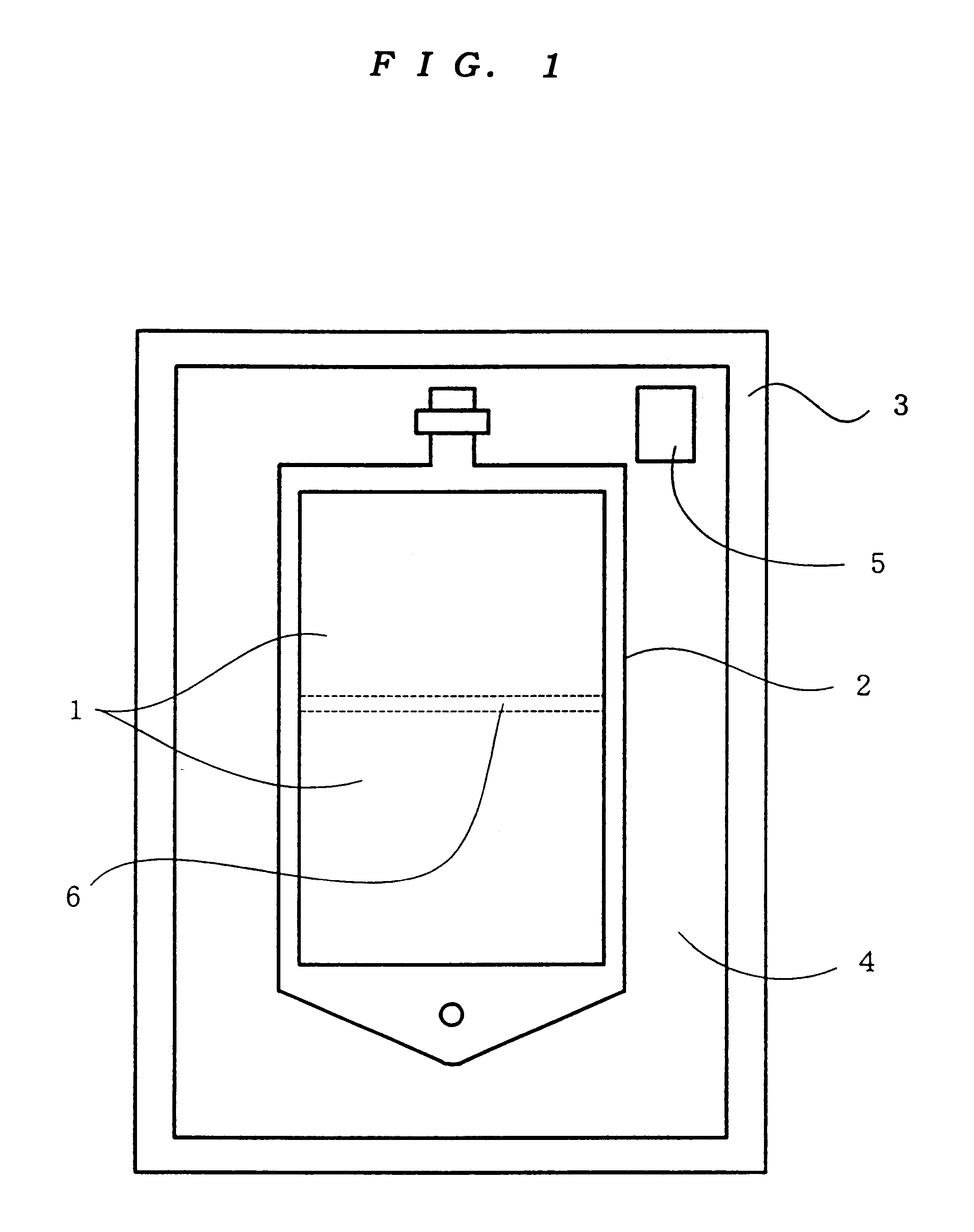
Reservoir assembly for container holding bicarbonate solution
InactiveUS20070265593A1Avoid changeEfficient detectionDiagnosticsSurgeryEngineeringCrosslinked polymers
A reservoir assembly can stably hold a bicarbonate solution. The reservoir assembly includes a gas-permeable plastic container holding a bicarbonate solution and a gas-impermeable casing for encasing the container. An effective way of detecting pinholes in the casing of the reservoir assembly is also provided. An oxygen absorber and an oxygen-detecting agent are placed in the space between the container and the casing. The oxygen absorber is of the type that does not affect the pH or the bicarbonate ion concentration of the solution and mainly contains a crosslinked polymer having carbon-carbon unsaturated bonds.
Owner:AJINOMOTO CO INC
Desulfurization waste water zero discharge processing system and processing method
InactiveCN106365371AQuality assuranceSave on medicine costsWater treatment parameter controlWater contaminantsSludgeClarifier
The invention discloses a desulfurization waste water zero discharge processing system and a processing method. The desulfurization waste water zero discharge processing system comprises a regulation sedimentation basin, triple connecting tanks, a defecator and a nanofilter which are connected in sequence, wherein the concentrated water outlet end of the nanofilter is connected with a mistorizer which sprays the concentrated water into a chimney flue; the triple connecting tanks include a neutralization tank, a reaction tank and a flocculation tank which are connected in sequence, wherein the neutralization tank is communicated with the water outlet of the regulation sedimentation basin, and is used for adjusting the pH value of desulfurization waste water, so that bicarbonate ions in the desulfurization waste water are removed; the reaction tank provides a reaction chamber for removing calcium ions in the desulfurization waste water; the flocculation tank is connected with the defecator. The desulfurization waste water zero discharge processing system and the processing method have the advantages of saving the cost of the agent for removing magnesium ions in the desulfurization waste water, reducing the quantity of sludge, lowering the operating cost, using no lime milk and improving the site working conditions. The sodium chloride salt in the waste water is purified and then crystallized, thus the recycle of the sodium chloride salt in the desulfurization waste water is achieved, the solid waste disposal cost is reduced, and certain economic benefits are produced.
Owner:SHANDONG SHANDA WIT ENVIRONMENTAL ENGINEERING CO LTD
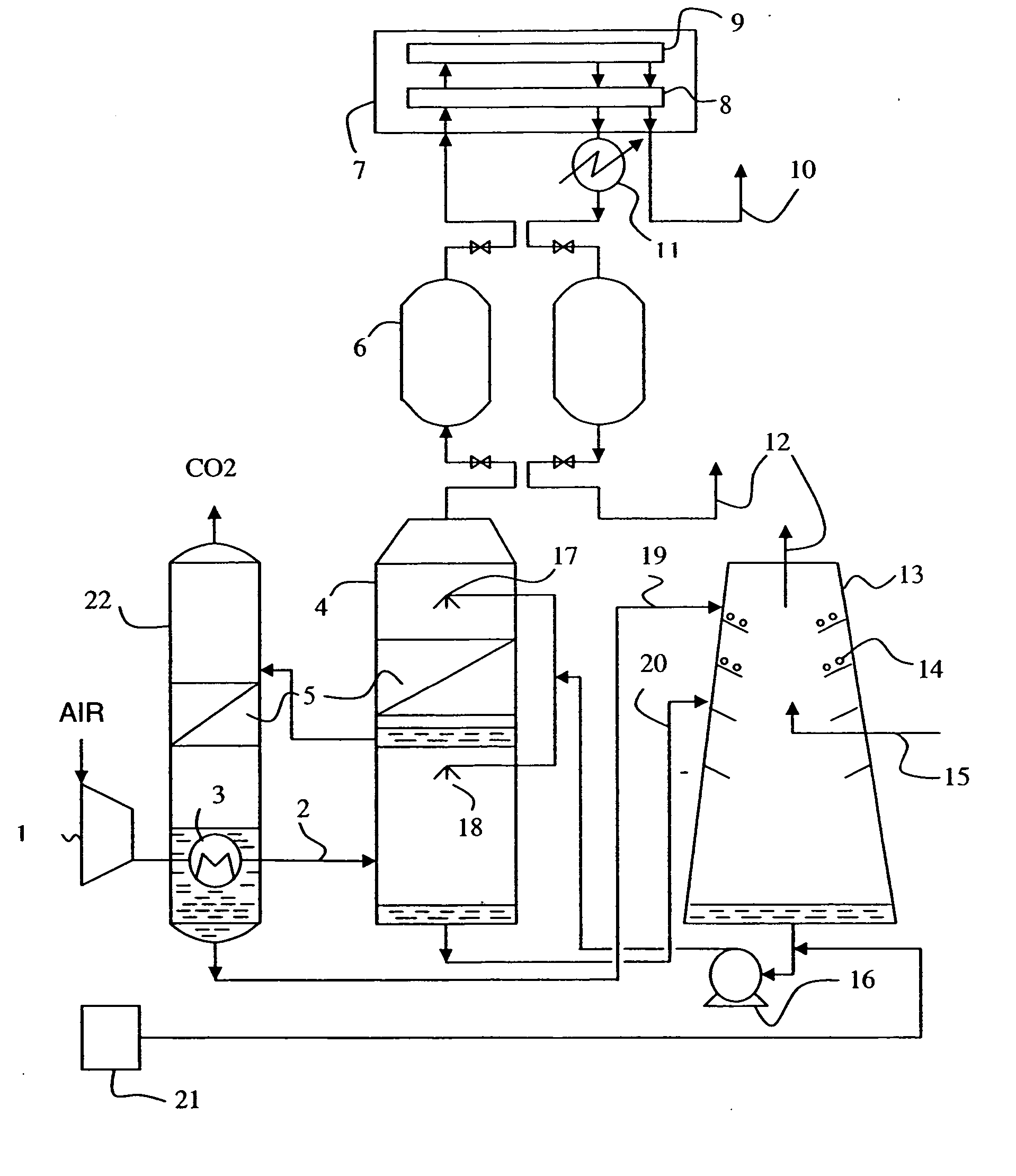
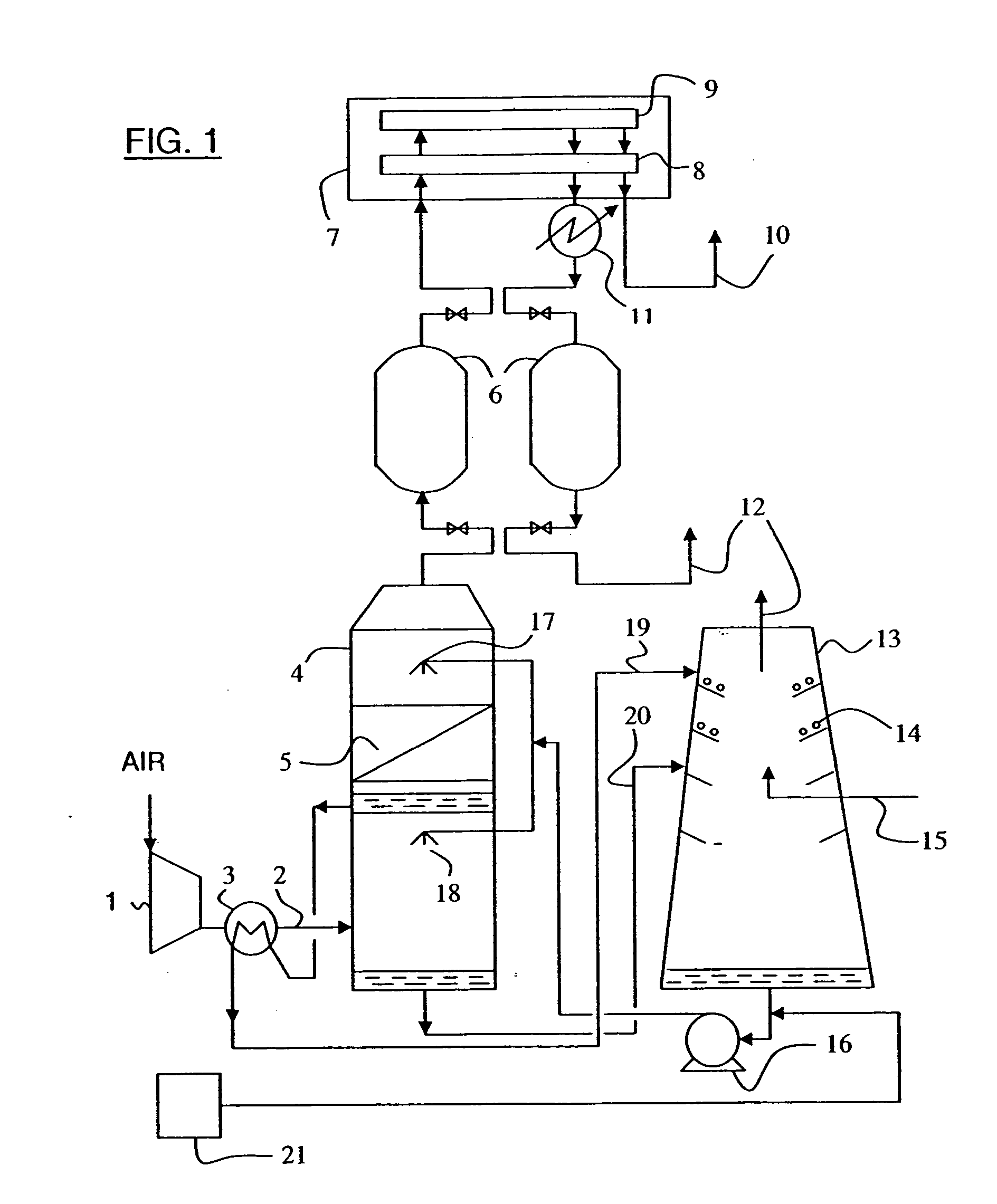
Gas purification apparatus and process using biofiltration and enzymatic reactions
InactiveUS20070048856A1Reduce the amount requiredGas treatmentDispersed particle separationHydrogenBicarbonate Ion
The invention provides a gas purification apparatus for purifying effluent gas by treating VOCs and the CO2 gas, comprising a biofiltration chamber containing micro-organisms for degrading the VOCs and producing a partially cleansed gas containing CO2 gas by-product; and an enzymatic chamber comprising an enzyme capable of catalyzing the hydration of the CO2 gas by-product into bicarbonate ions and hydrogen ions, to produce an ion rich solution and a purified gas. Additionally, a gas purification process is provided.
Owner:FPINNOVATIONS INC +1
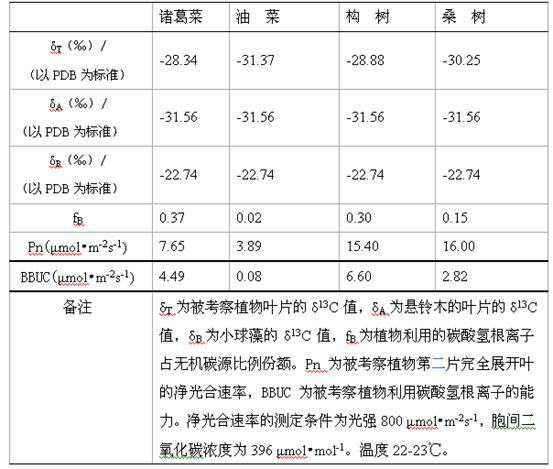
Method by utilizing double markers to acquire share of inorganic carbon source utilized by plants
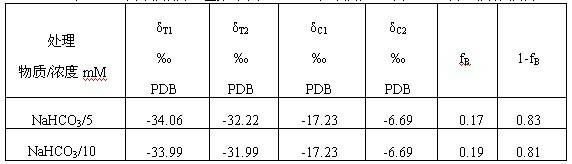
ActiveCN102511362AFew stepsSimple calculationCultivating equipmentsSoilless cultivationSodium bicarbonateCulture fluid
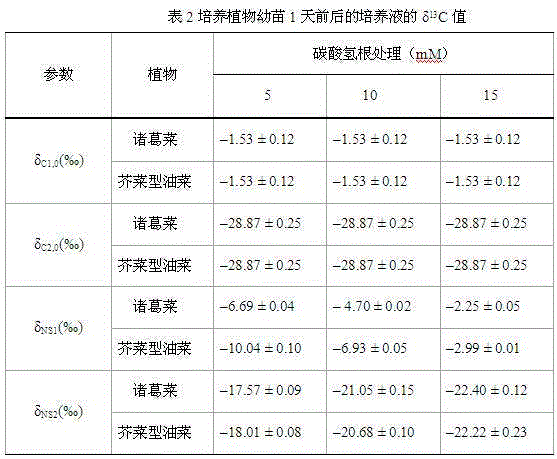
The invention discloses a method by utilizing double markers to acquire share of inorganic carbon source utilized by plants, which comprises the following steps that: A. sodium bicarbonate produced by different manufacturers is determined, two types of sodium bicarbonate with delta 13C difference being more than eight thousandths are used as an isotopic marker 1 and an isotopic marker 2 to be respectively added into nutrient fluid to culture plants; delta 13C value of bicarbonate ion in nutrient fluid of the isotopic marker 1 is delta C1, and delta 13C value of bicarbonate ion in nutrient fluid of the isotopic marker 2 is delta C2; and B. after more than four leaves appear, the values of delta 13C consisting of stable carbon isotopes of plant leaves to be observed correspondently culturedby the nutrient fluid of the two types of isotopic markers are respectively determined as delta T1 and delta T2; and C. delta C1, delta C2, delta T1 and delta T2 are brought into equation fB= to calculate the share fB of the carbonate ions utilized by the plants. Due to the adoption of the method, the share of the inorganic carbon source utilized by the plants can be rapidly acquired, the steps are fewer, simplicity in calculation can be realized, and data is reliable.
Owner:INST OF GEOCHEM CHINESE ACADEMY OF SCI
Process and installation for the fractionation of air into specific gases
InactiveUS7514056B2Improve conversion performanceBioreactor/fermenter combinationsSolidificationHydration reactionCooling tower
In order to reduce incoming atmospheric carbon dioxide levels in compressed air prior to cryogenic distillation, a water spray cooling tower equipped with biocatalytic packing, or fed with absorptive reagents, is used to convert gaseous carbon dioxide into bicarbonate ions which dissolve in the cooling water. The hydration reaction and refrigeration occur synergistically. The bicarbonate ions are subsequently removed from the solution using the heat from the compressed air in a regenerator re-boiler unit, and then fed to a percolation cooling tower for releasing CO2 and cooling.
Owner:CO2 SOLUTION
Carbon dioxide capture using resin-wafer electrodeionization
ActiveUS20100300894A1High methane contentReducing greenhouse gas emissionLiquid separation by electricityCarbon compoundsElectricityIon-exchange membranes
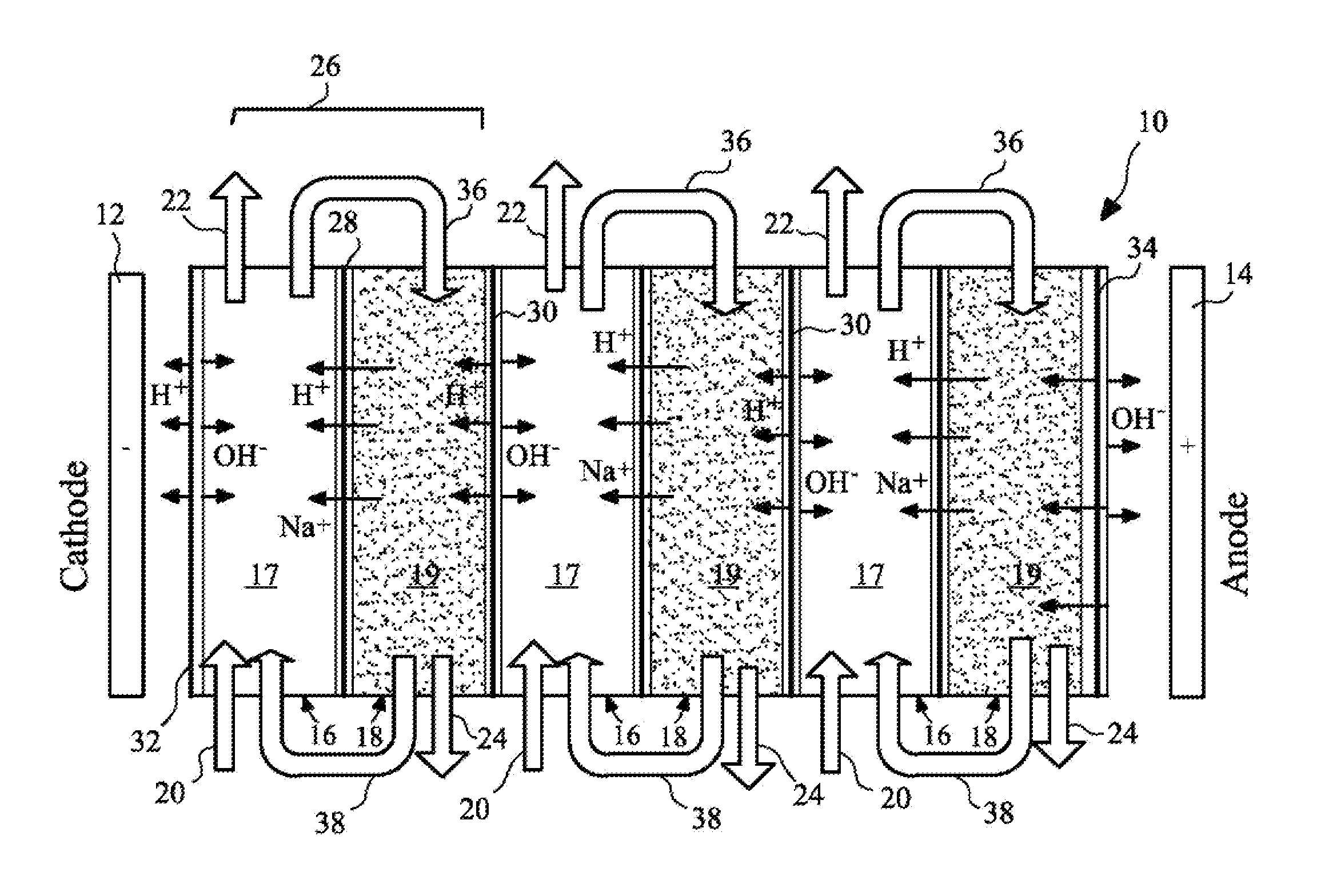
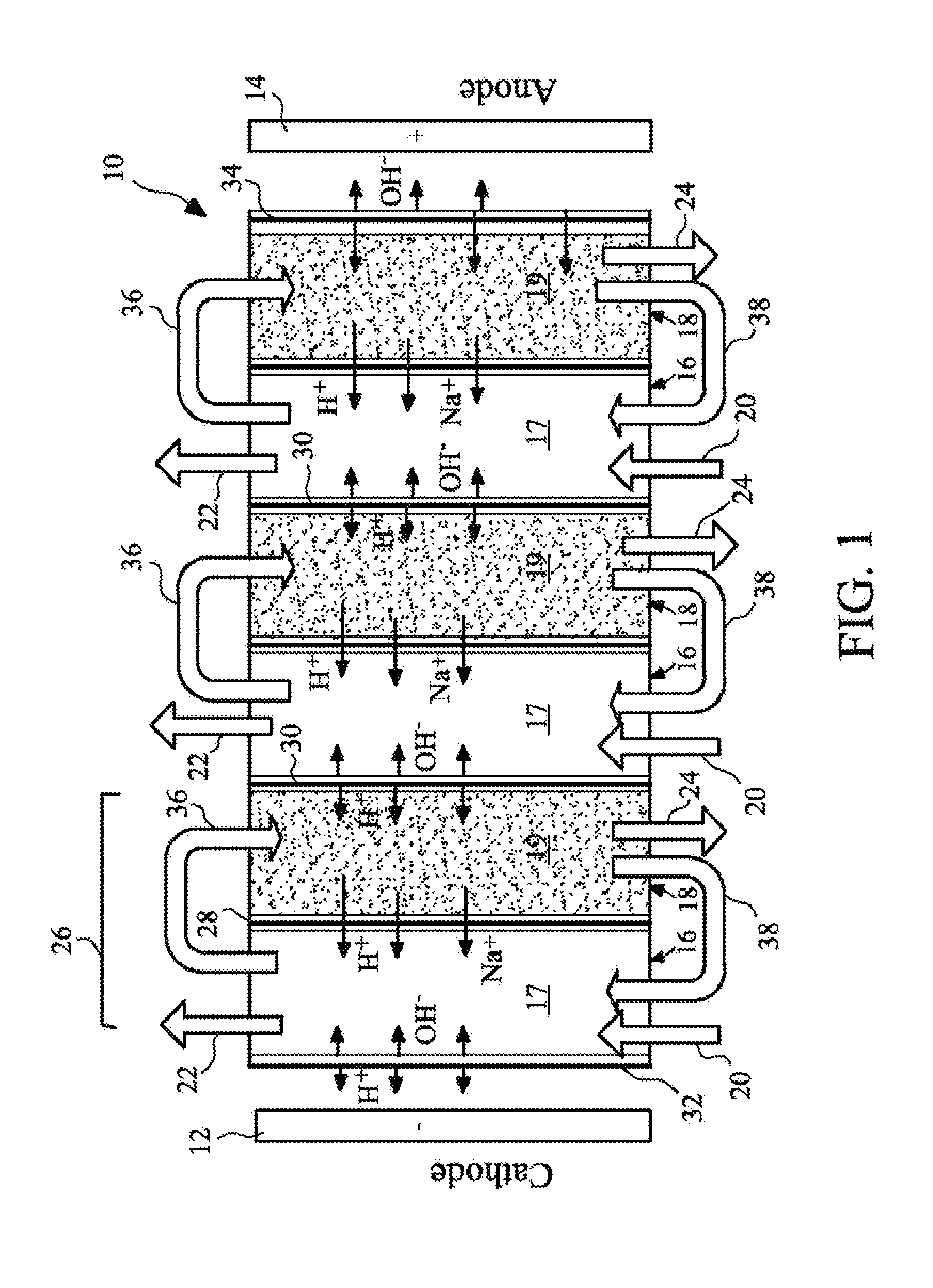
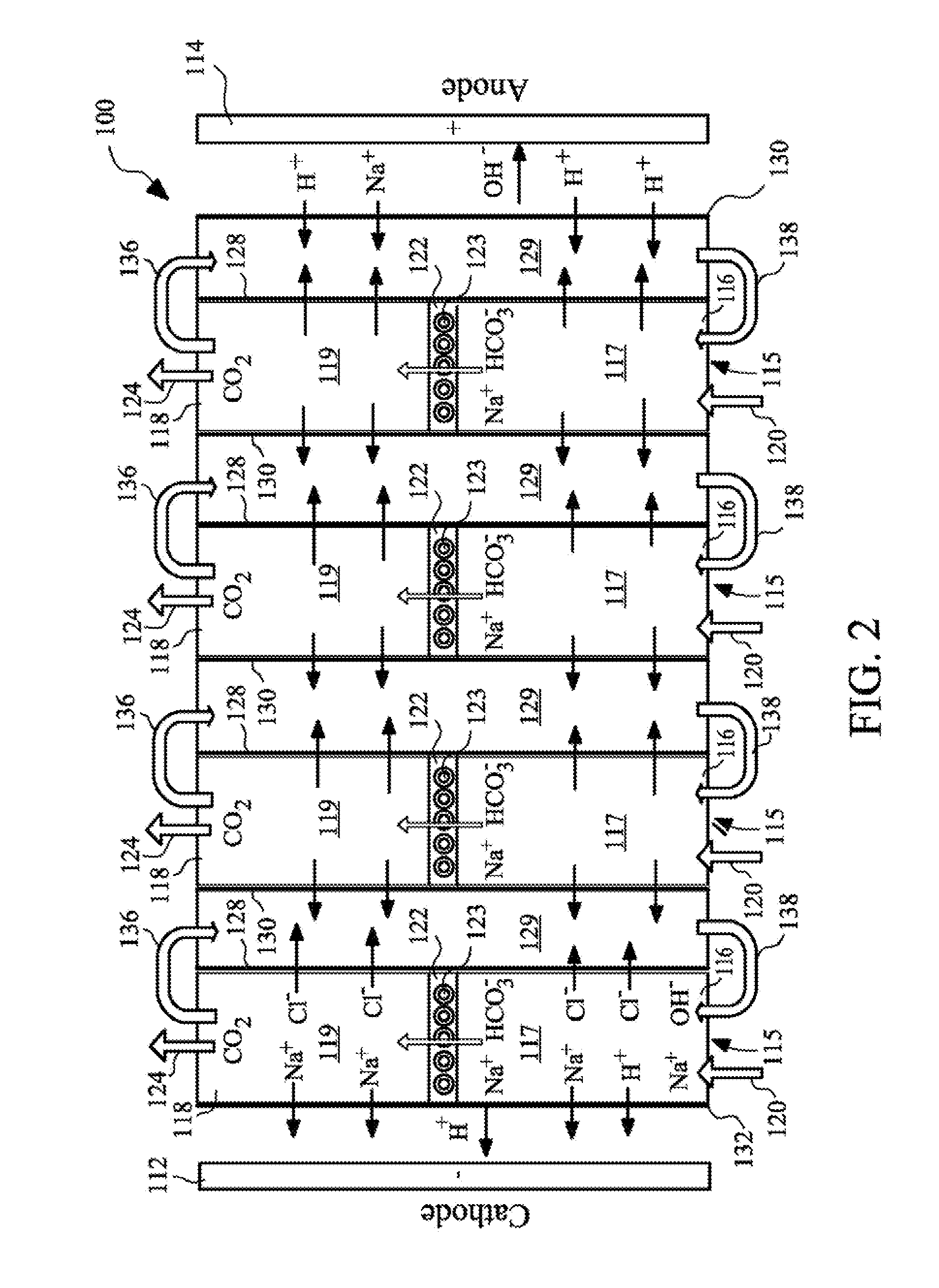
The present invention provides a resin-wafer electrodeionization (RW-EDI) apparatus including cathode and anode electrodes separated by a plurality of porous solid ion exchange resin wafers, which when in use are filled with an aqueous fluid. The apparatus includes one or more wafers comprising a basic ion exchange medium, and preferably includes one or more wafers comprising an acidic ion exchange medium. The wafers are separated from one another by ion exchange membranes. The fluid within the acidic and / or basic ion exchange wafers preferably includes, or is in contact with, a carbonic anhydrase (CA) enzyme to facilitate conversion of bicarbonate ion to carbon dioxide within the acidic medium. A pH suitable for exchange of CO2 is electrochemically maintained within the basic and acidic ion exchange wafers by applying an electric potential across the cathode and anode.
Owner:UCHICAGO ARGONNE LLC
Ion exchange desalting method and device
InactiveCN101979329AReduce dosageSimple processWater/sewage treatment by ion-exchangeFiltrationIon exchange
The invention discloses an ion exchange desalting method and an ion exchange desalting device. The method comprises the following steps of: 1) absorbing highly-acid anions in water by adopting weakly-alkaline anion exchange resin; 2) performing precipitation or filtration on the water; and 3) absorbing cations in the water by adopting cation exchange resin. The method and the device have the advantages that: OH<-> ions released by the weakly-alkaline anion exchange resin are bonded with bicarbonate ions HCO3<-> in the water to form carbonate CO3<2->, and the carbonate CO3<2-> forms insoluble electrolyte with calcium and magnesium ions in the water to partially remove dissolved electrolyte from the water and reduce the using amount of acids in a regeneration process; simultaneously, the weakly-alkaline anion exchange resin does not absorb silicate, so the use of excessive alkaline is avoided in the regeneration process and the using amount of the alkaline is reduced in the regeneration process; and a carbon-removal process can be selectively adopted, so a process flow is simplified.
Owner:CHONGQING MAYHEAT TECH
Packaged ophthalmic perfusate and cleaning fluid bag
Owner:SENJU PHARMA CO LTD +1
Method for measuring bicarbonate ion utilizing capability of plant
InactiveCN101926267ALittle plant materialSmall footprintHorticulture methodsMaterial analysisProportional shareIsotope
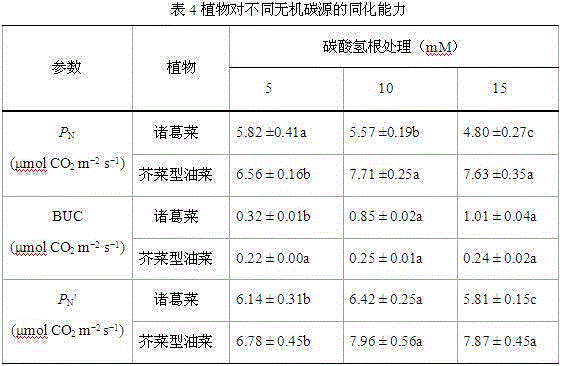
The invention discloses a method for measuring the bicarbonate ion utilizing capability of a plant. The method comprises the following steps of: (1) respectively measuring stable carbon isotope compositions, i.e. delta 13C values of leaves of a plant to be studied and a reference plant; (2) measuring the stable carbon isotope composition, i.e. delta 13C value of microalgae; (3) substituting the delta 13C value of the leaves of the plant to be studied, the delta 13C value of the leaves of the reference plant and the delta 13C value of the microalgae into a two-end-member model and calculating the proportional share of bicarbonate ions utilized by the plant to be studied in an inorganic carbon source; (4) measuring the net photosynthetic rate of the leaves of the plant to be studied; and (5) deriving the bicarbonate ion utilizing capability of the plant to be studied according to the net photosynthetic rate of the leaves of the plant to be studied and the proportional share of the bicarbonate ions utilized by the plant to be studied in the inorganic carbon source. The invention can be used for quantitatively measuring the bicarbonate ion utilizing capability of the plant with less plant materials and less steps and simple calculation.
Owner:INST OF GEOCHEM CHINESE ACADEMY OF SCI
Water filtration and treatment systems and methods
InactiveUS20140158638A1High affinityStable waterLiquid degasificationSolid sorbent liquid separationFiltrationTap water
Implementations of the present invention relate to systems, methods, and apparatus for filtering and treating water, such as tap water, well water, spring water, etc., and producing drinking, bathing, and swimming water. More specifically, such systems, methods, and apparatus can produce purified water by removing substantially all suspended as well as dissolved solids, undesirable acids, gasses and all and any contaminates from the water. Additionally, the systems, methods, and apparatus can produce reprogrammed high biophoton mineralized drinking water by chilling vortexing over proprietary lodestones, ingenious, sedimentary and metamorphic rocks and creating bicarbonate ions in the water introducing minerals and / or salts into the water.
Owner:PRISTINEHYDRO DEV
Formulation and process for co2 capture using carbonates and biocatalysts
ActiveUS20120129246A1Promote dissolutionEnhancing transformationGas treatmentOther chemical processesHydrogenDesorption
A formulation and process for capturing CO2 use an absorption mixture containing water, biocatalysts and a carbonate compound. The process includes contacting a CO2-containing gas with the absorption mixture to enable dissolution and transformation of CO2 into bicarbonate and hydrogen ions, thereby producing a CO2-depleted gas and an ion-rich solution, followed by subjecting the ion-rich solution to desorption. The biocatalyst improves absorption of the mixture comprising carbonate compounds and the carbonate compound promotes release of the bicarbonate ions from the ion-rich solution during desorption, producing a CO2 gas stream and an ion-depleted solution.
Owner:SAIPEM SPA
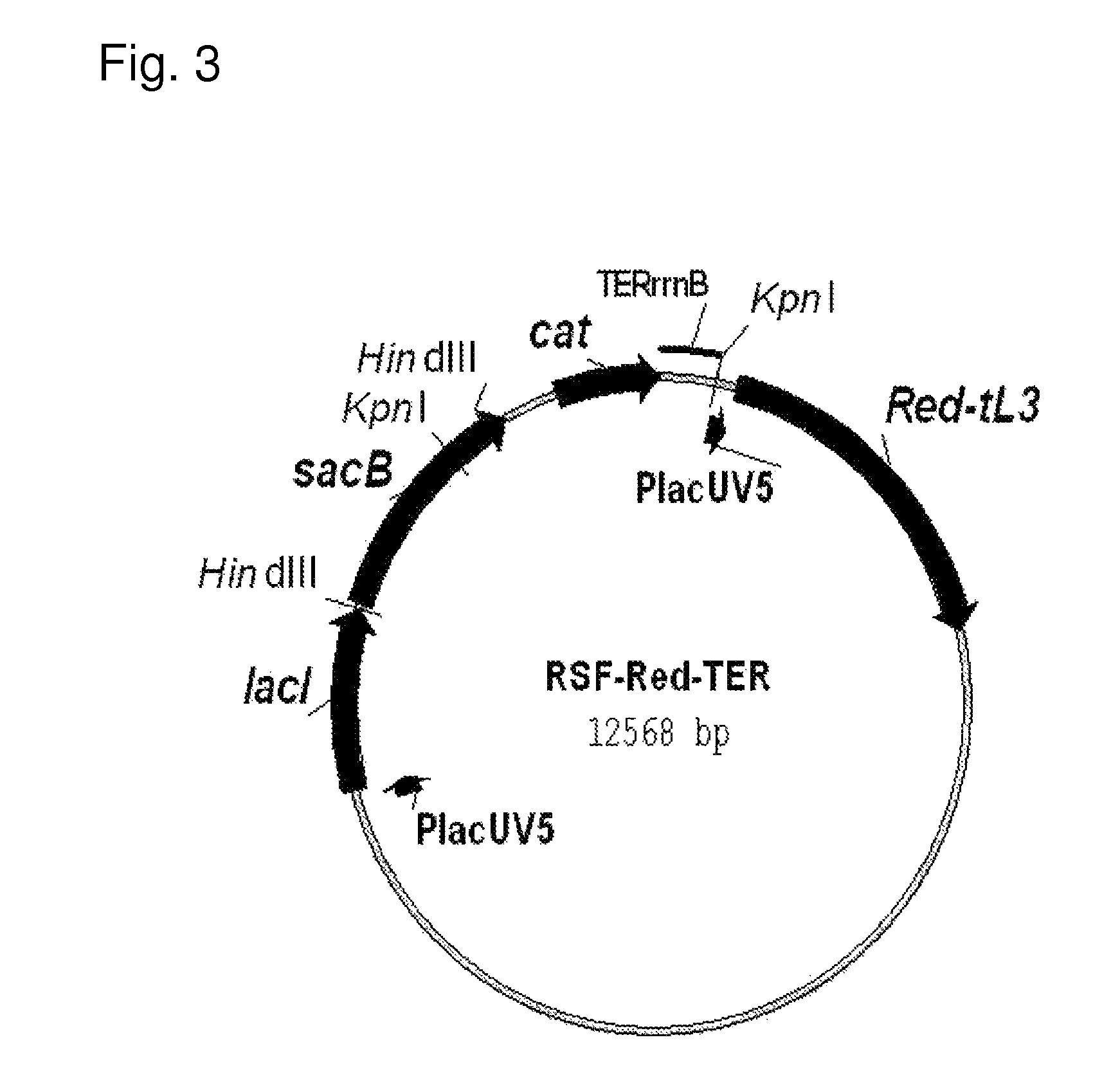
Method for producing an organic acid
An organic acid is produced by allowing a bacterium which has an ability to produce an organic acid and has been modified so that expression of yidE gene is enhanced, or a product obtained by processing the bacterium, to act on an organic raw material in a reaction mixture containing carbonate ions, bicarbonate ions, or carbon dioxide gas to produce the organic acid, and collecting the organic acid.
Owner:AJINOMOTO CO INC
Method and apparatus for producing a sterile fluid
InactiveUS6967002B1Avoid boilingInanimate material medical ingredientsSolid sorbent liquid separationBicarbonate IonElectrical and Electronics engineering
A method and apparatus for producing a sterile medical solution is disclosed. The method includes providing at least two components, a first component including bicarbonate ions and a second component including glucose or calcium ions, preheating the first component to a first temperature and the second component to a second temperature, heat sterilizing the first and second components separately to a third and fourth temperatures respectively, the third and fourth temperatures being sterilizing temperatures for the first and second components, maintaining the first and second components approximately at their respective third and fourth temperatures, so as to sterilize the first and second components, cooling the first and second components and delivering the first and second components to a mixing device.
Owner:GAMBRO LUNDIA AB
Low-sodium peritoneal dialysis liquid
InactiveCN101485683AImprove scalabilityPromote popularizationHydroxy compound active ingredientsPeptide/protein ingredientsMedicineChloride
The invention discloses a low-sodium peritoneal dialysis solution. The solution does not contain bicarbonate ions, and components of the solution calculated as mmol / L comprise the following: 124 to 128 mmol / L of sodium ions, 1 to 2 mmol / L of calcium ions, 0.2 to 0.8 mmol / L of magnesium ions, 90 to 110 mmol / L of chloride ions, 37 to 47 mmol / L of lactate ions, and a corresponding isotonic agent. The low-sodium peritoneal dialysis solution, during the preparation, overcomes the defect that a special membrane material must be used in order to solve the stability problem of producing pH by decomposing bicarbonate radicals, reduces the cost, and is favorable for further expansion and popularization of clinical application.
Owner:CHENGDU QINGSHAN LIKANG PHARMA CO LTD
Method for preparing calcium carbonate by using catalysis of microbial carbonic anhydrase
InactiveCN102121033AHigh purityAbundant resourcesMicroorganism based processesFermentationAtherion elymusFiltration
The invention provides a method for preparing calcium carbonate by using catalysis of microbial carbonic anhydrase, which comprises the following steps of: adding the carbonic anhydrase extracted from a microbial culture into a liquid system containing calcium ion and bicarbonate radical ion solution or a gas diffusion system containing calcium ion solution and releasing CO2 by using ammonium hydrogen carbonate solution, performing oscillating reaction at room temperature, controlling initial pH at 5 to 10, taking out the produced sediment, and performing filtration, washing and drying to obtain the calcium carbonate. The method makes full use of microbial resources and characteristics of specificity and high efficiency of enzyme catalytic reaction, prepares the calcium carbonate by accelerated deposition, and has the advantages of riche resources, simple process, clean environment, low cost, high efficiency, quickness and the like. The calcium carbonate prepared by deposition has high purity, is calcite crystal calcium carbonate, can be used as an industrial filler, and can also be used for repairing cracks of building engineering, buildings, stone landscapes and carved stone cultural relics.
Owner:HUAZHONG UNIV OF SCI & TECH
Method for producing an organic acid
An organic acid is produced by allowing a bacterium which has an ability to produce an organic acid and has been modified so that expression of the sucE1 and mdh genes are enhanced, or a product obtained by processing the bacterium, to act on an organic raw material in a reaction mixture containing carbonate ions, bicarbonate ions, or carbon dioxide gas, and collecting the organic acid.
Owner:AJINOMOTO CO INC
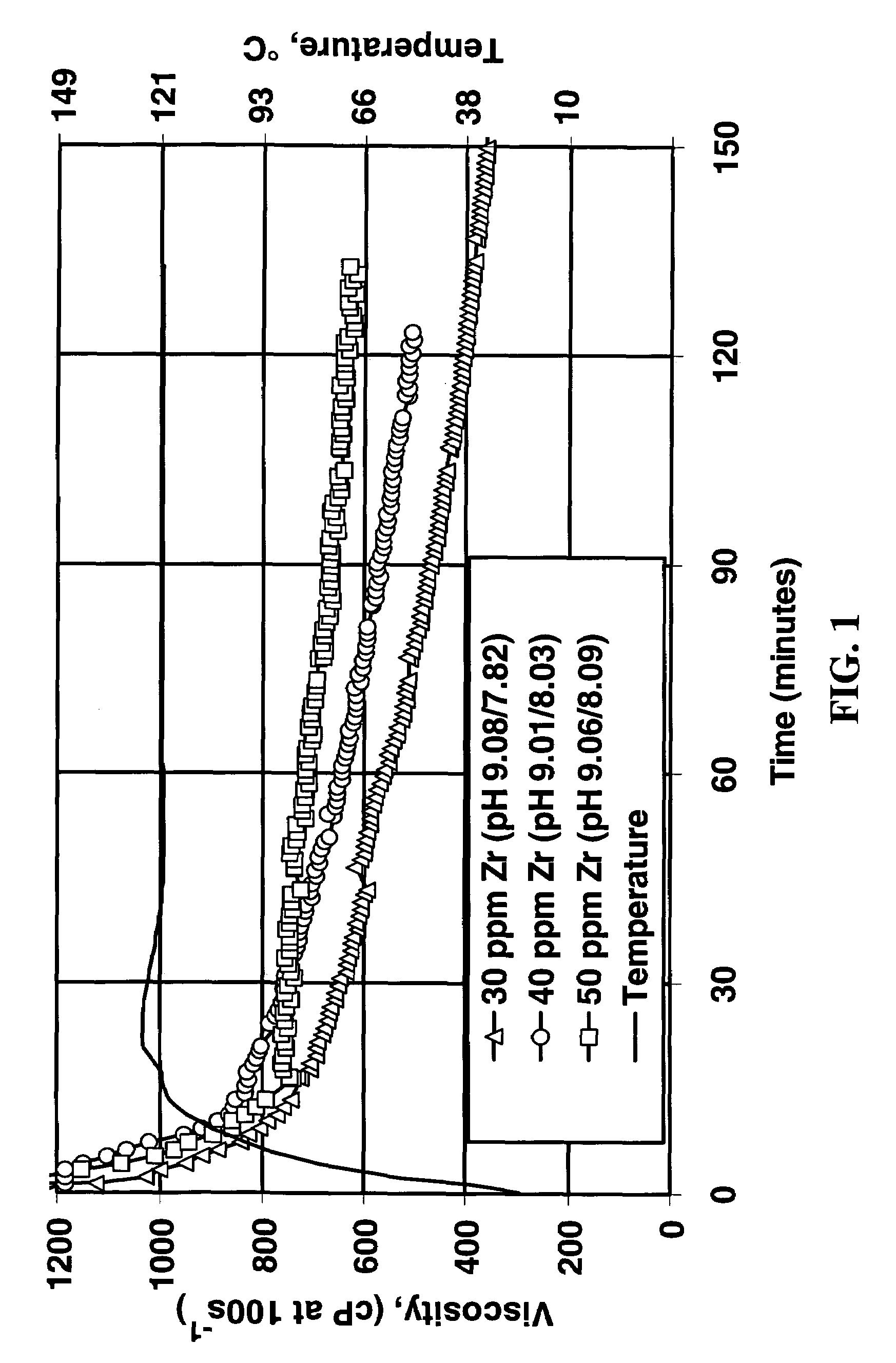
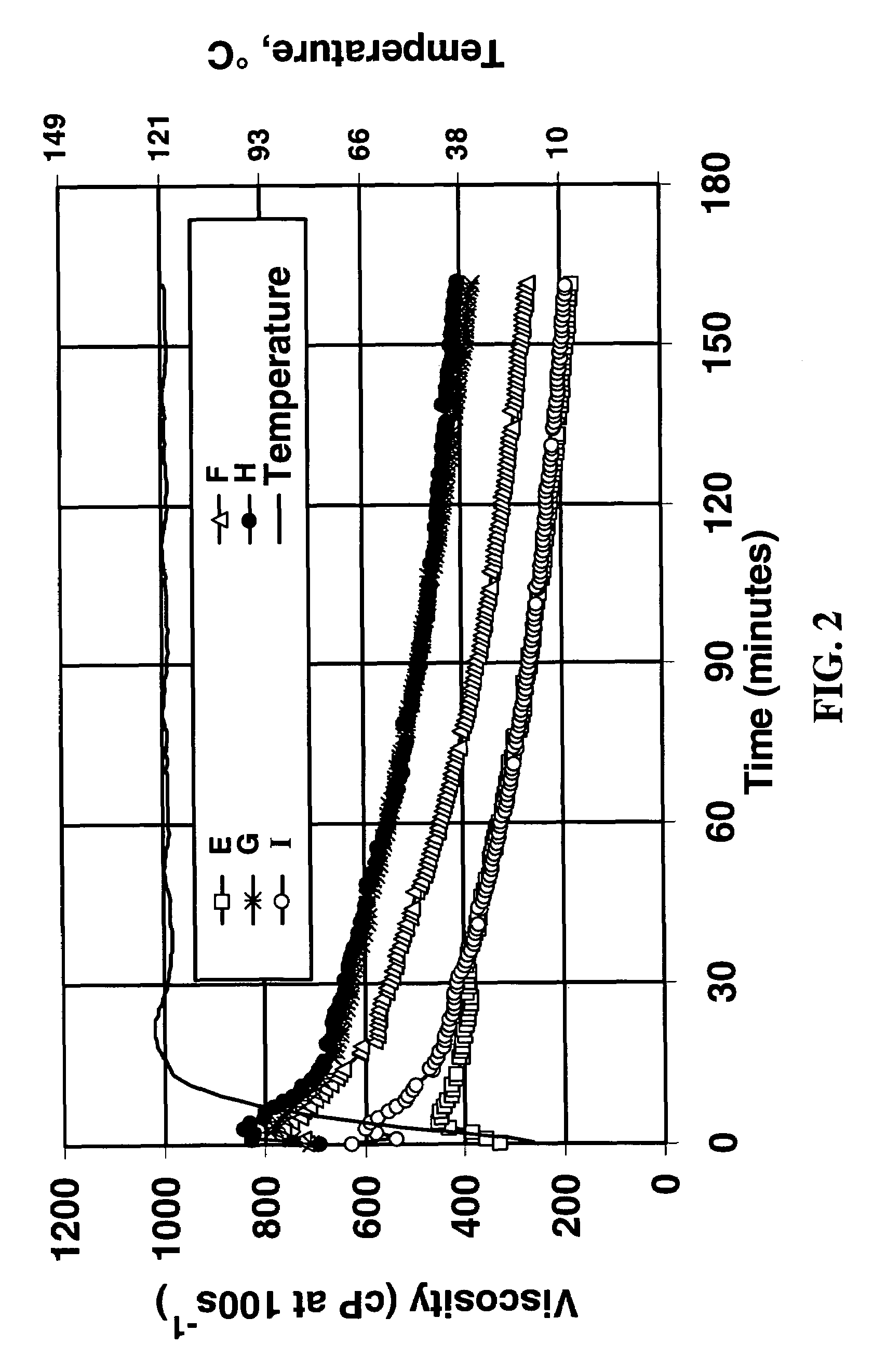
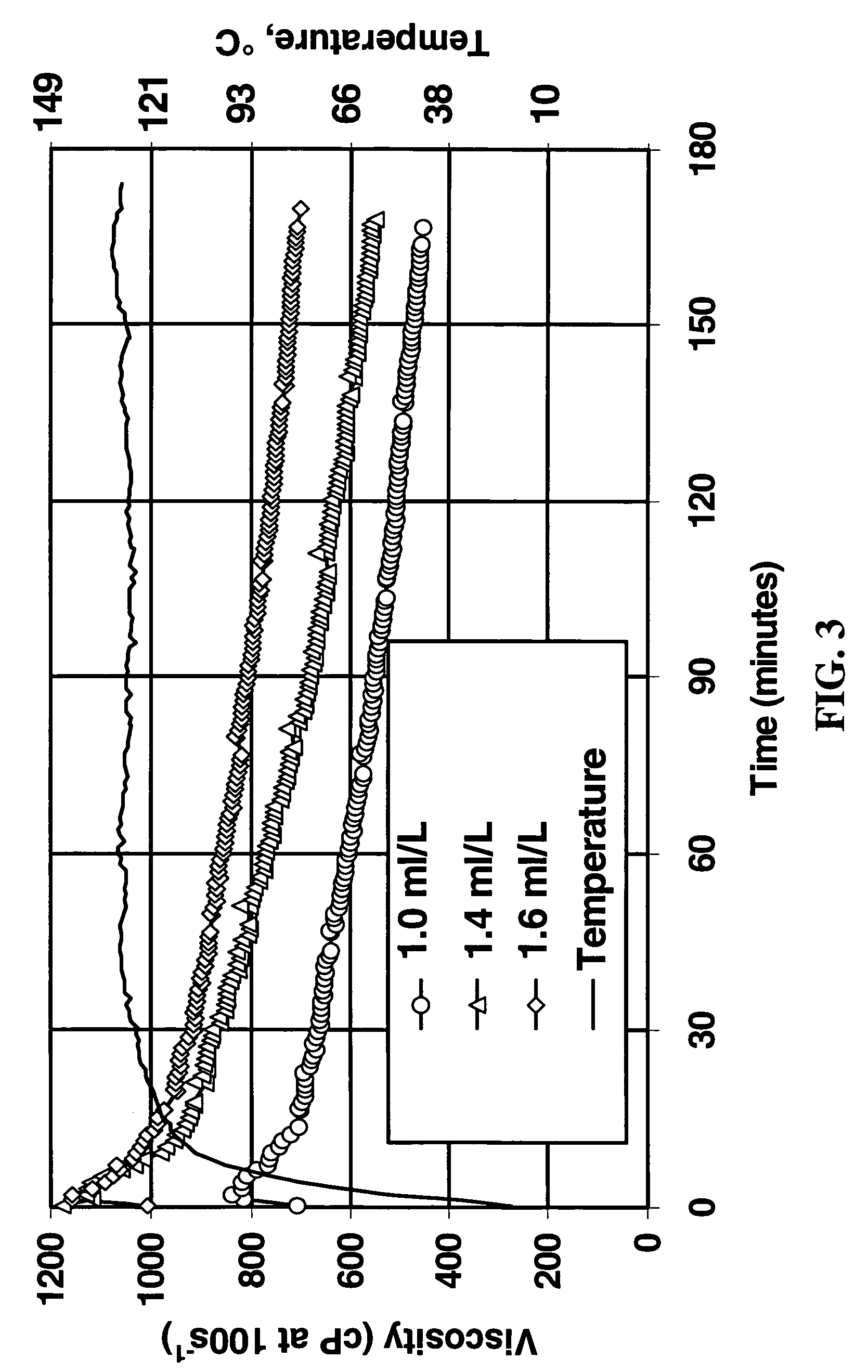
Polymer Crosslinking System Comprising Soluble Zr(IV), Carbonate and Bicarbonate Ions
A composition useful for making gelled fluids by crosslinking hydratable polymers is an aqueous solution of zirconium complexed with carbonate and bicarbonate as the only multidentate ligands complexed with zirconium. The composition also provides pH modifying capability and the crosslinking is delayed, so that the single composition replaces several liquid additives previously necessary for generation of fluids used, for example, in hydraulic fracturing.
Owner:SCHLUMBERGER TECH CORP
Bowel cleansing agent
It is provided a bowel cleansing preparation which is useful in the pretreatment prior to colonoscopy or colonic surgery containing a water-soluble polymer and electrolytes of 30-150 mEq / L of sodium ion, 3-20 mEq / L of potassium ion, and 10-30 mEq / L of bicarbonate ion, wherein 0.1-3 g / dL of magnesium ion as magnesium element is contained, and polyethylene glycol having an average molecular weight of about 7,300 to about 9,300 is used as the water-soluble polymer.
Owner:AJINOMOTO CO INC
Process for making lithographic printing plate
InactiveUS20110091814A1Improve uniformityLow running costSemiconductor/solid-state device manufacturingPhotomechanical exposure apparatusWater solubleBicarbonate Ion
To provide a process for making a lithographic printing plate that exhibits safety, and excellent developability and processing capacity in single solution processing using a processing solution having a pH in a weakly alkaline region, and preferably a pH of 8.5 to 10.5. A process for making a lithographic printing plate, the process including a preparation step of preparing a lithographic printing plate precursor having above a hydrophilic support an image-forming layer that contains an infrared-absorbing agent, a polymerization initiator, an ethylenically unsaturated compound, and a binder polymer, an exposure step of imagewise exposing the lithographic printing plate precursor, and a processing step of processing the imagewise exposed lithographic printing plate precursor using a processing solution containing carbonate ion, bicarbonate ion, and a water-soluble polymer compound.
Owner:FUJIFILM CORP
Method used for determining plant total photosynthesis carbon assimilation capacity
ActiveCN105067772AOvercome the lack of ability to exploitEasy accessMaterial analysisCarbon assimilationIsotopic labeling
The invention discloses a method used for determining plant total photosynthesis carbon assimilation capacity. According to the method, double-way isotope labeling and a double source isotope mixed module are adopted to determine using ratio of plant on added bicarbonate ions and using ratio of plant on bicarbonate ions come from atmospheric carbon dioxide dissolving; a photosynthesis device is adopted to determine photosynthesis assimilation capacity of plant on atmospheric carbon dioxide; and plant total photosynthesis carbon assimilation capacity is obtained finally. The method is used for measuring using capacity of plant on added bicarbonate ions, and measuring using capacity of plant on bicarbonate ions come from atmospheric carbon dioxide dissolving; and influences of old carbon on determination results are avoided; so that obtained plant total photosynthesis carbon assimilation capacity data is reliable, and result accuracy is high.
Owner:INST OF GEOCHEM CHINESE ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com