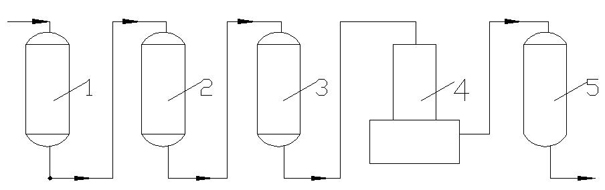
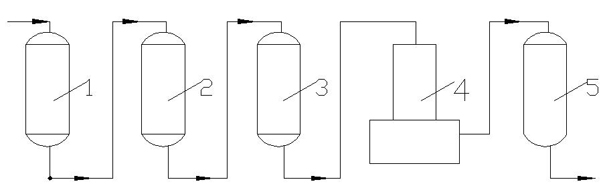
Ion exchange desalting method and device
A technology of ion exchange and ions, applied in the direction of ion exchange water/sewage treatment, etc., can solve the problems of inability to reduce the amount of alkali, silicic acid precipitation, etc., achieve the effect of reducing the amount of alkali, reducing the amount of alkali, and simplifying the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: The total number of influent cations is 5mmol / l, where Ca 2+ , Mg 2+ The content is 4mmol / l, the total number of anions is 5mmol / l, of which the strong acid anion (SO 4 2- , Cl - , NO 3 - etc.) with a content of 3mmol / l, bicarbonate HCO 3 - The content is 1.8mmol / l, and the silicate is 0.2mmol / l (the units are based on the monovalent ions commonly used in water treatment, the same below). The outlet water requires a conductivity of 1??s / cm.
[0040] After the feed water is exchanged by a weakly alkaline anion exchanger, the OH produced - The ion concentration is equal to the strong acidic anion concentration, that is, 3mmol / l, and bicarbonate HCO3 - combine to form carbonate CO 3 2- 3.6mmol / l, the formation of precipitate is also 3.6mmol / l, after filtering the precipitate through the filter, the content of cation entering the cation exchanger is 1.4mmol / l.
[0041] Ion exchange is an equivalent exchange. It can be known that the amount of acid r...
Embodiment 2
[0044] Example 2: The total number of influent cations is 6mmol / l, where Ca 2+ , Mg 2+ The content is 3mmol / l, the total number of anions is 6mmol / l, of which the strong acid anion (SO 4 2- , Cl - , NO 3 - etc.) with a content of 4mmol / l, bicarbonate HCO 3 - The content is 1.9mmol / l, and the silicate is 0.1mmol / l. The outlet water requires a conductivity of 10??s / cm.
[0045] After the feed water is exchanged by a weakly alkaline anion exchanger, the OH produced - The ion concentration is equal to the strong acidic anion concentration, that is, 4mmol / l, and bicarbonate HCO 3 - combine to form carbonate CO 3 2- 3.8mmol / l, the precipitate formed is 3mmol / l, after filtering the precipitate through the filter, the cation content entering the cation exchanger is 3mmol / l.
[0046] Ion exchange is an equivalent exchange. It can be seen that the amount of acid required in theory is 3 mmol for every 1 liter of water treated. While the traditional desalination system req...
Embodiment 3
[0049] Example 3: The total number of influent cations is 3.4mmol / l, where Ca 2+ , Mg 2+ The content is 2.8mmol / l, and the total number of anions is 3.4mmol / l, among which the strong acid anion (SO 4 2- , Cl - , NO 3 - etc.) with a content of 2mmol / l, bicarbonate HCO 3 - The content is 1.3mmol / l, and the silicate is 0.1mmol / l. The conductivity of the outlet water is required to be 0.1??s / cm.
[0050] After the feed water passes through the weakly basic anion exchanger, the OH produced - The ion concentration is equal to the strong acidic anion concentration, that is, 2mmol / l, and bicarbonate HCO 3 - combine to form carbonate CO 3 2- 2.6mmol / l, the precipitate was 2.6mmol / l.
[0051] The heavy carbonic acid is completely precipitated, no carbon remover is installed, and a mixed ion exchanger is required for high production water requirements. In order to reduce equipment investment, a strong alkaline anion exchanger may not be installed, and the water from the ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com