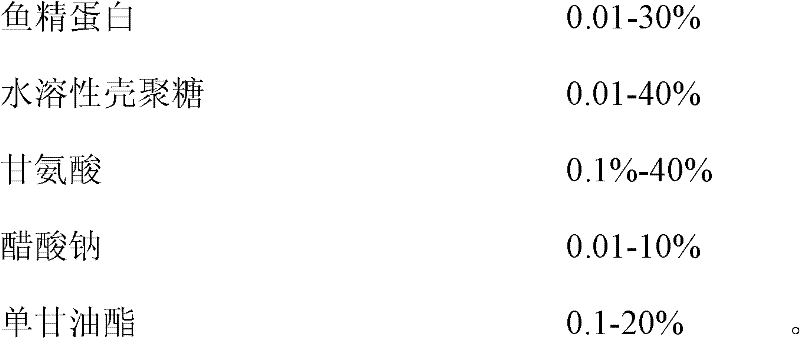Protamine compounded preparation, preparation method and application thereof
A protamine and preparation technology, applied to the application of the protamine compound preparation, the field of preparing the protamine compound preparation, can solve the problems of low efficiency, high cost, large toxic and side effects on human body and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] Protamine compound preparation of the present invention is made up of the content of following percentage by weight:
[0154] Proportion:
[0155]
[0156] Method: In a sterile room or ultra-clean workbench, accurately weigh the above reagents, protamine 0.3g, water-soluble chitosan 1.9g, glycine 1.1g, monoglyceride 0.5g, sodium acetate 0.1g, acetic acid 0.1g, 96ml (96g) of sterilized distilled water, put it into a clean glass or stainless steel container, stir gently, mix well, and then pack it into a sterile container.
[0157] Application: This formula can be used to make skin and mucous membrane bactericidal disinfectant, which can kill Staphylococcus aureus, Candida albicans, and Escherichia coli on the surface of skin and mucous membranes within 2-10 minutes, with a killing rate of 99.8%. It can treat abscesses, psoriasis, ulcers, bedsores, scalds, burns, boils, oral ulcers, cuts, vaginitis, oral diseases caused by various bacterial infections, and prevent sex...
Embodiment 2
[0159] Protamine compound preparation of the present invention is made up of the content of following percentage by weight:
[0160] Proportion:
[0161]
[0162] Method: Put 10g of acetic acid into a clean glass or stainless steel container in a sterile room or ultra-clean workbench, add 67ml of sterilized distilled water, stir gently, mix well, and then add 10g of water-soluble chitosan After homogenization, add 0.01g of protamine, 10g of glycine, 0.1g of monoglyceride, and 2.89g of sodium acetate, mix thoroughly and evenly, and pack it into a sterile container.
Embodiment 3
[0164] Protamine compound preparation of the present invention is made up of the content of following percentage by weight:
[0165] Proportion:
[0166]
[0167] Method: Mix it evenly in the same way as 1 and 2 above, put it into a sterile liquid-carrying container and seal it.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of carboxylation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com