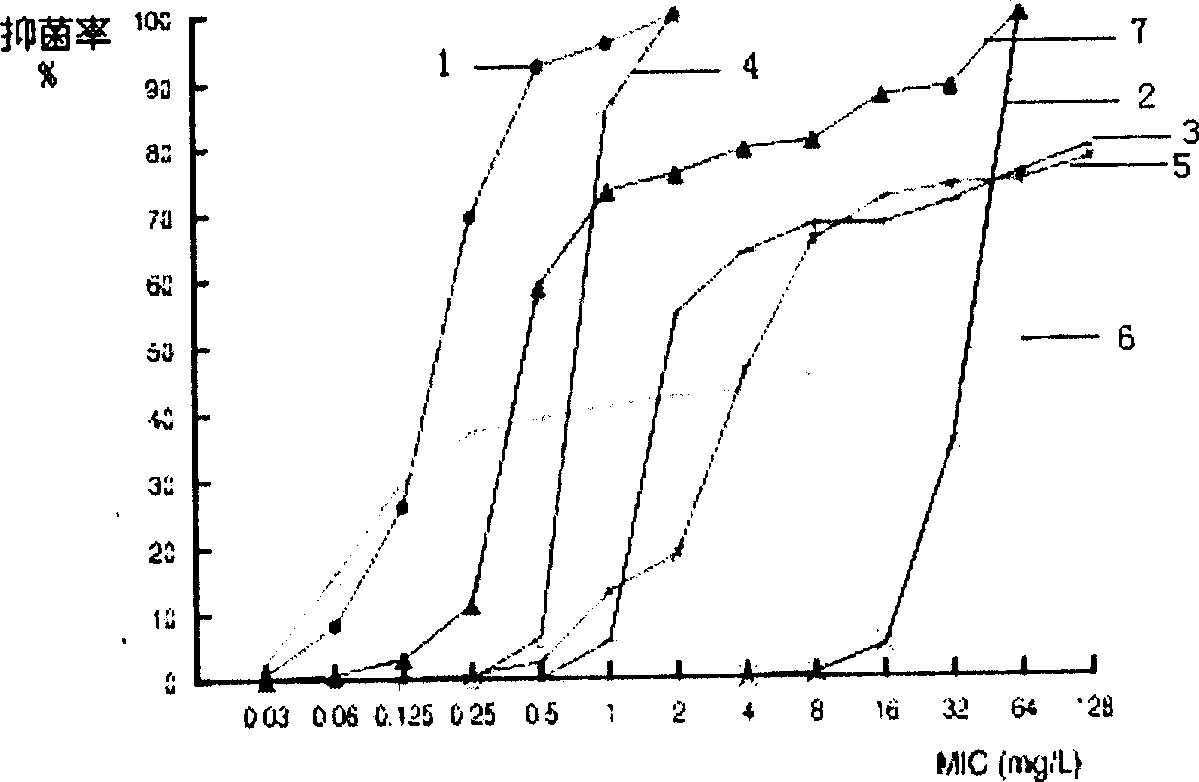
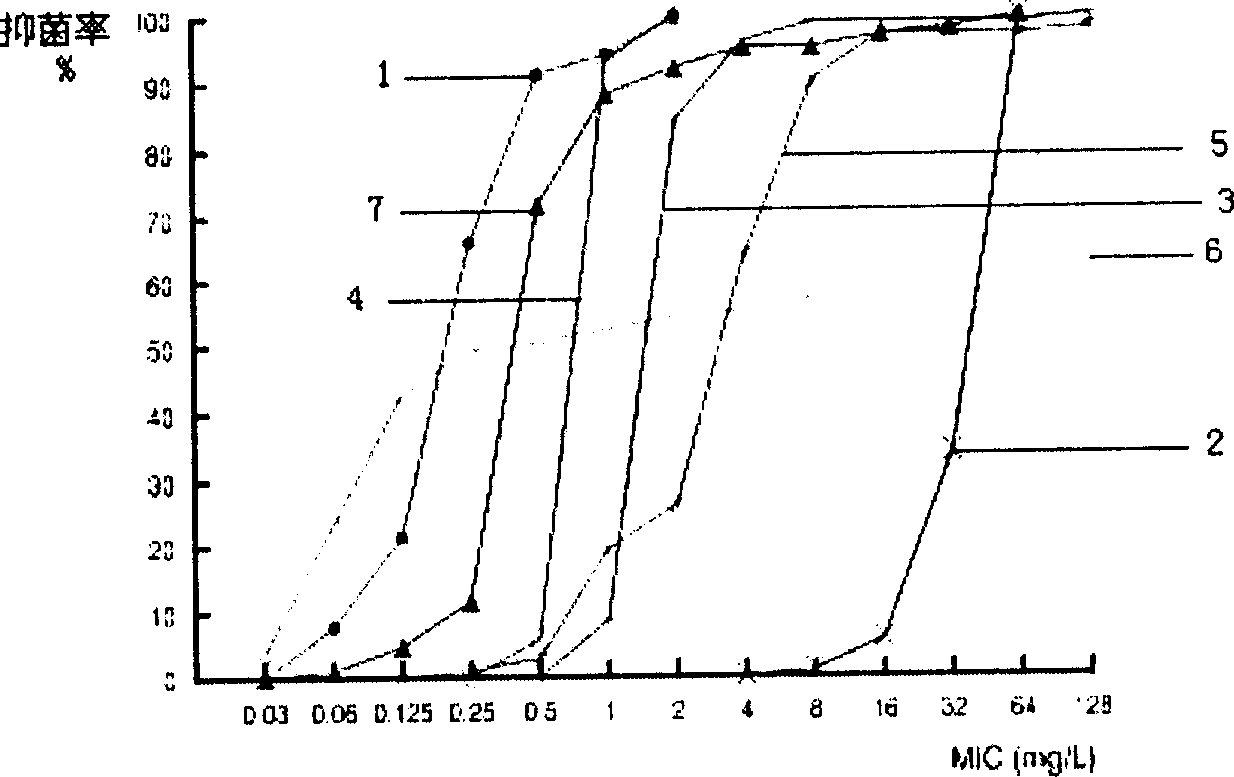
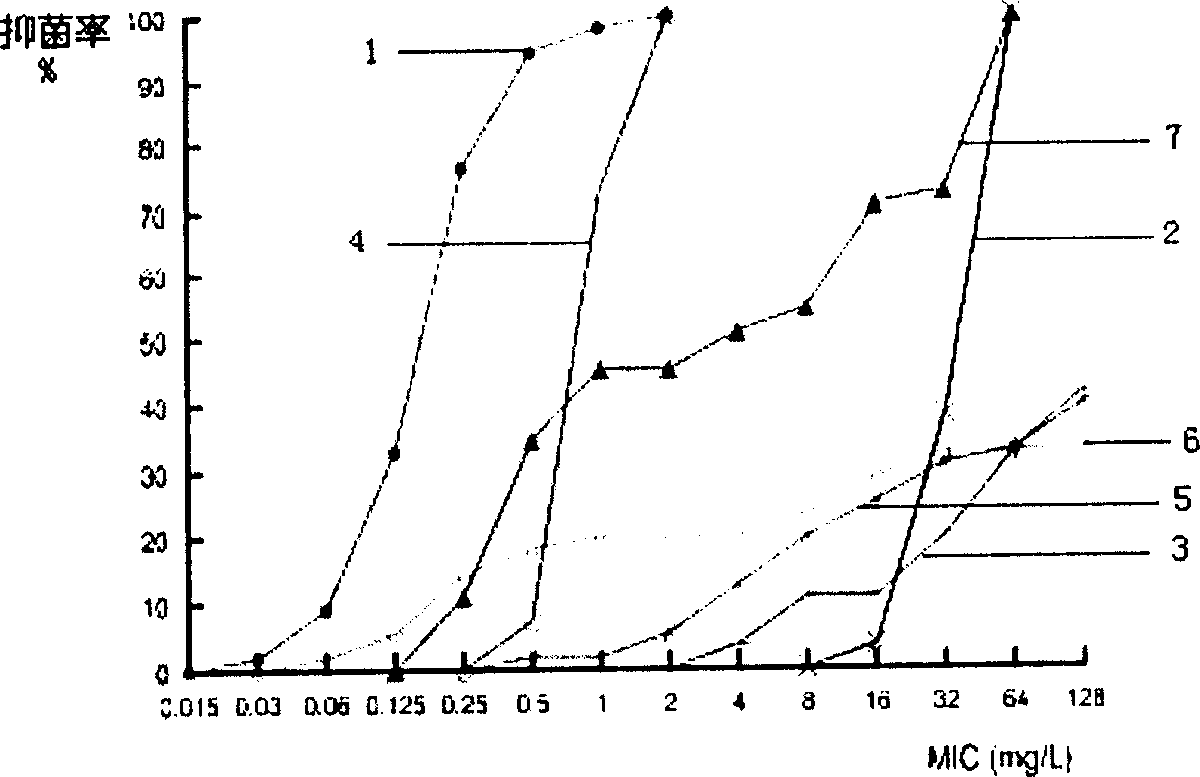
Lysostaphin freeze dried powder used for preventing and treating trauma surface infestation
A technology of lysostaphin and freeze-dried powder, which is applied in freeze-dried delivery, powder delivery, antibacterial drugs and other directions, can solve the problems of vancomycin not being used externally, high liver and kidney toxicity of patients, and toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 Preparation of lysostaphin freeze-dried powder
[0037] 1. Composition and ratio
[0038]Lysostaphin 0.4%; human serum albumin 10%; glycine 30%; mannitol 10%; potassium dihydrogen phosphate 15%; disodium hydrogen phosphate 30%.
[0039] 2. Preparation of semi-finished products
[0040] Weigh solid KH 2 PO 4 and Na 2 HPO 4 .12H 2 O, prepare 0.2mol / L phosphate buffer A (pH value 6.5 phosphate buffer, concentration is 0.2M). According to the formulation prescription, weigh the required glycine and mannitol, measure the required volume of human serum albumin, add it to the solution of part A, stir and dissolve, and obtain solution B. Measure the stock solution of recombinant lysostaphin, add it to solution B, make the biological activity of recombinant lysostaphin in the final solution be 400U / ml, add A solution to the final volume, and what is obtained at this time is the recombinant lysostaphin Semi-finished solution.
[0041] 3. Microf...
Embodiment 2
[0047] Example 2 Repeated Dosing Toxicity Test of Rabbit Subcutaneous Injection External Recombinant Lysostaphin
[0048] 1. Purpose of the experiment
[0049] The purpose of this experiment is to observe the nature, degree, development and recovery of toxic reactions in rabbits through subcutaneous injection of recombinant lysostaphin for external use, so as to provide reference materials for clinical safety drug dosage design and clinical toxicity monitoring.
[0050] 2. Experimental method
[0051] 24 New Zealand white rabbits, weighing 2.5-3.0kg. There are 3 dosage groups for recombinant lysostaphin for external use, and the dosages are 100, 20 and 4ml.kg -1 (equivalent to 241936, 48387 and 9677U·m-2), the solvent control group (0.9% sodium chloride injection), the subcutaneous injection volume is 1ml.kg -1 . Administered continuously 7 days a week for a total of 4 weeks.
[0052] 3. Experimental results
[0053] After administration, the general behavior and...
Embodiment 3
[0056] Example 3 Skin irritation test of transdermally administered topical recombinant lysostaphin in rabbits
[0057] 1. The purpose of the experiment:
[0058] To observe the local irritation reaction of intact skin and broken skin of rabbits after contacting externally applied recombinant lysostaphin, and to provide experimental data for the safety evaluation of its clinical use.
[0059] 2. Experimental method:
[0060] In the test, two test groups were established: intact skin (group I) and damaged skin peptide (group II), with 4 animals in each group, half male and half male, and each animal had a hairless area of 10 × 6 cm on the back of each animal with electric hair removal scissors. Prepare the skin once every other day during the medication period. Use a sterile needle to cut the epidermis of the skin in the shape of a "well" (1 cm apart) in the hair removal area for damaged skin to ooze blood. The drug concentration is based on the maximum dilution con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com