Protein drug solid preparation and preparation method thereof
A protein drug and solid preparation technology, which is applied in the direction of drug combination, pharmaceutical formula, peptide/protein composition, etc., to achieve good protein encapsulation efficiency and maintain the effect of protein drug activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 amphiphilic polymer
[0042] Prepare amphiphilic polymers as follows:
[0043] S01. Dissolving the hydrophobic polymer material in an organic solvent, adding a hydrophilic polymer material for a mixed reaction, removing the organic solvent and other impurities in the reaction system to obtain a mixture A;
[0044] S02. The mixture A was redissolved in water, and the product was collected after purification.
[0045] S03. Collect the dialysis product, freeze overnight at -80°C, and then freeze-dry on a freeze dryer to obtain a white flocculent substance.
[0046] Hydrophilic polymer materials include polyethylene glycol, polyoxyethylene, mPEG-NH2-400, mPEG-NH2-600, mPEG-NH2-800, mPEG-NH2-1000, mPEG-NH2-2000, mPEG-NH2- 4000, mPEG-NH2-5000 or mPEG-NH 2 -At least one in 10000; Select polyethylene glycol for use in the present embodiment.
[0047]The hydrophobic polymer material preferably comprises polymaleic anhydride, poly(maleic anhydr...
Embodiment 2
[0055] Example 2 Preparation of Amphiphilic Polymer and Detection of Self-Assembly
[0056] Follow the steps below to prepare amphiphilic polymers
[0057] S01. Dissolve poly(maleic anhydride-ALT-1-octadecene) in organic solvent, add mPEG-NH in proportion 2 Mixing reaction, removing the organic solvent and other impurities in the reaction system, to obtain mixture A;
[0058] S02. Reconstitute the mixture A with water, put it into a dialysis bag and perform dialysis in pure water;
[0059] S03. Collect the dialysis product, freeze overnight at -80°C, and then freeze-dry on a freeze dryer to obtain a white flocculent substance.
[0060] The organic solvent generally selects dichloromethane, dimethyl sulfoxide, acetone, ethanol, and selects dichloromethane in this embodiment.
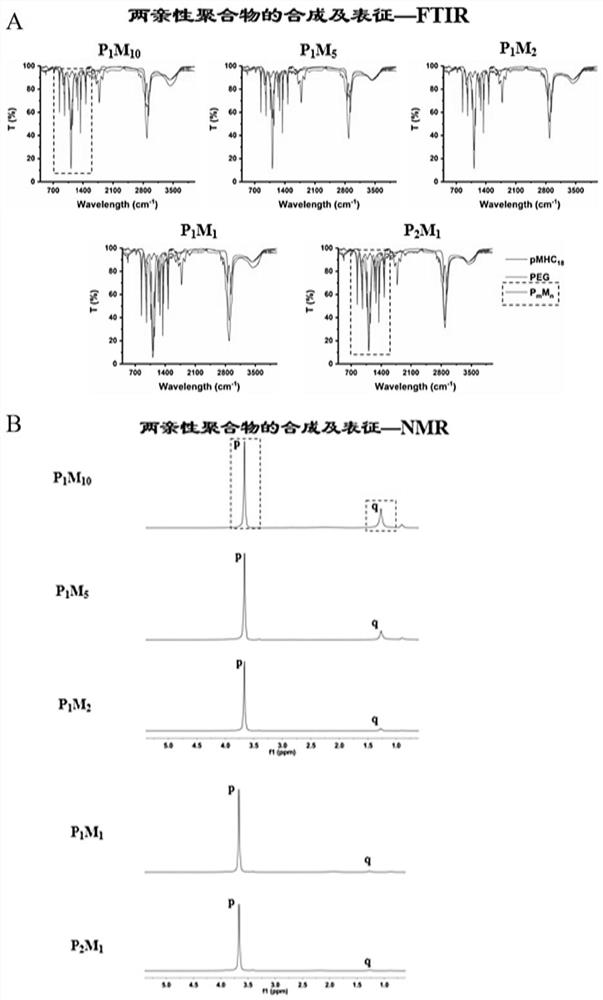
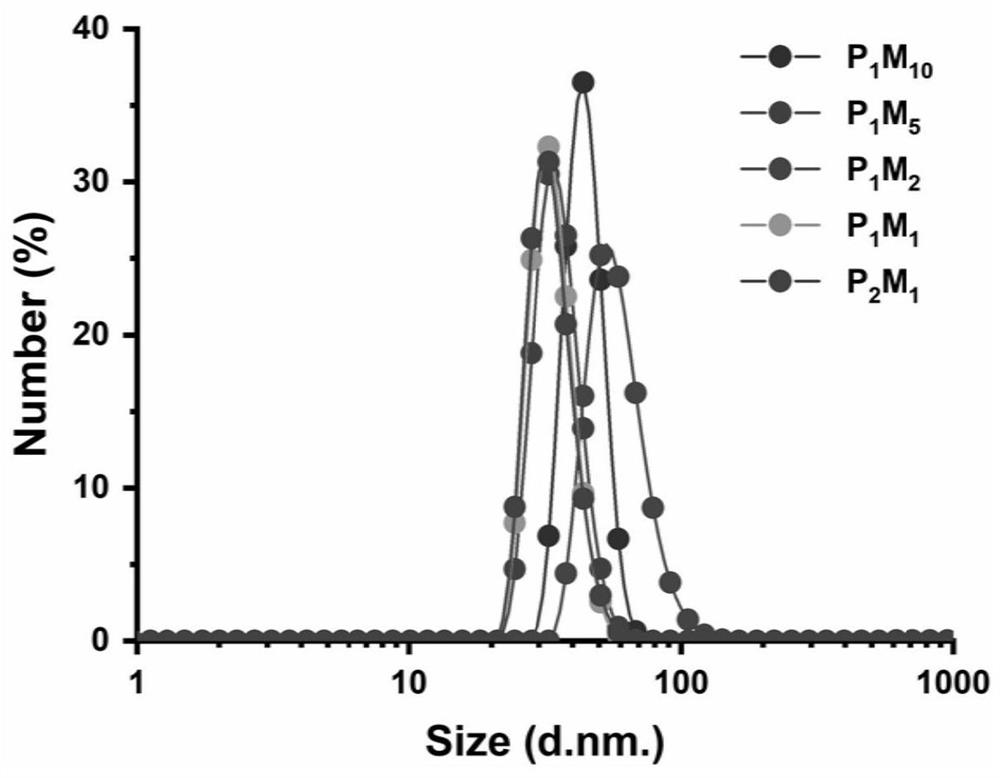
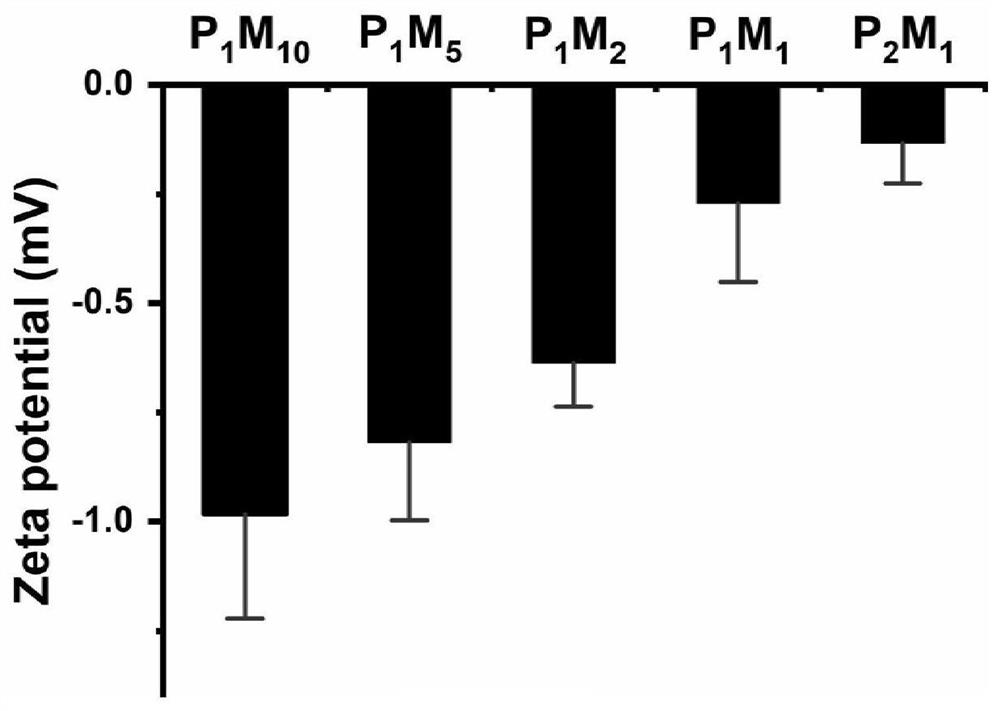
[0061] mPEG-NH 2 Grafting ratio P with poly(maleic anhydride-ALT-1-octadecene) m m n For (1:10)~(2:1), respectively prepare P 1 m 10 ,P 1 m 5 ,P 2 m 1 ,P 1 m 1 ,P 2 m 1 Five amphiphilic po...
Embodiment 3
[0067] The preparation of embodiment 3 insulin solid preparations
[0068] Insulin pharmaceutical preparations are prepared according to the following steps:
[0069] S11. Dissolving insulin in a buffer solution to obtain a protein drug solution;
[0070] S12. adding the amphiphilic polymer prepared in Example 1 to the protein drug solution described in step S11 to obtain a mixture;
[0071] S13. Put the mixture into a dialysis bag for dialysis in pure water, collect the dialysis product, and freeze-dry to obtain a solid insulin preparation.
[0072] The volume ratio of the amphiphilic polymer solution to the protein solution is (1:10)˜(10:1).
[0073] A magnetic stirrer can be used for mixing, the rotation speed of the magnetic stirrer is about 800rpm, and the reaction time is about 24h. The "approximately" mentioned in the summary of the invention all represent the original number and the upper and lower error is 20%, for example, "about 800rpm" is 800±160rpm.
[0074] F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com