Suppository for treating mammal endometritis
A technology for endometritis and mammals, applied in the direction of suppository delivery, drug combination, medical preparations of non-active ingredients, etc., can solve the problems of small drug distribution area in the uterus, unfavorable penetration into the uterine mucosa, and the impact of uterine recovery. , to achieve non-toxic and side effects, overcome the effect of irritating and strong bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
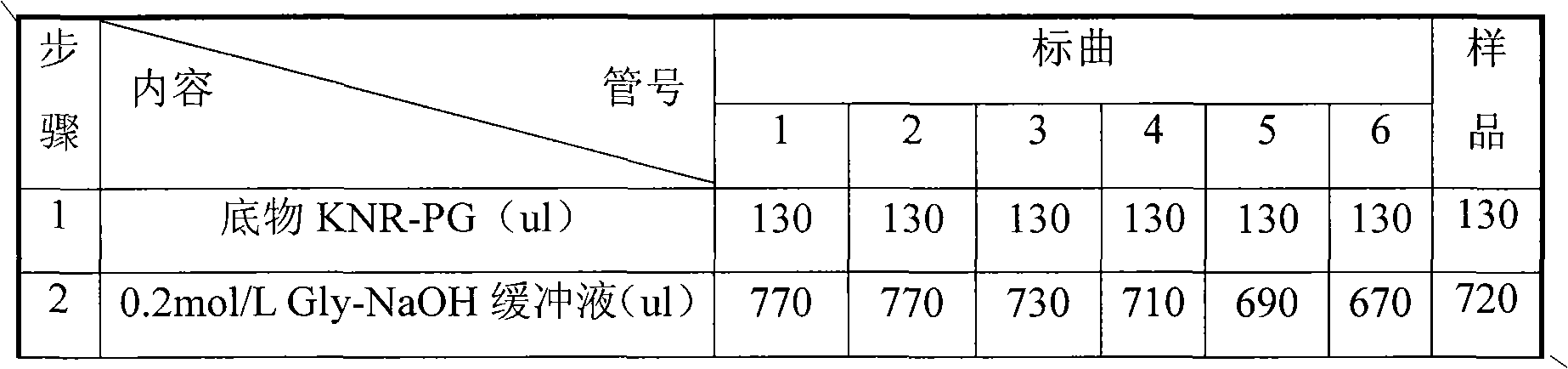
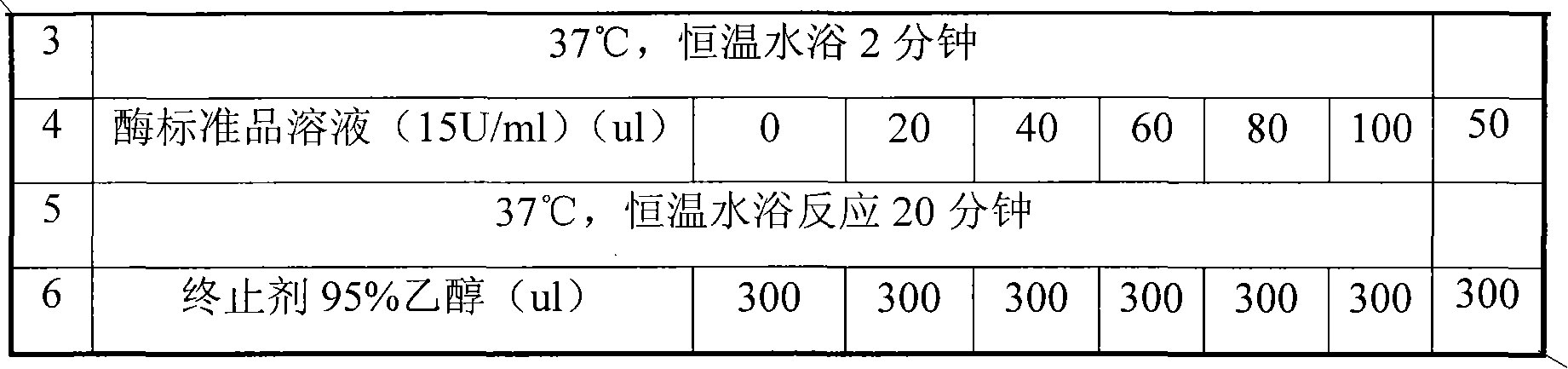
Examples
Embodiment 1
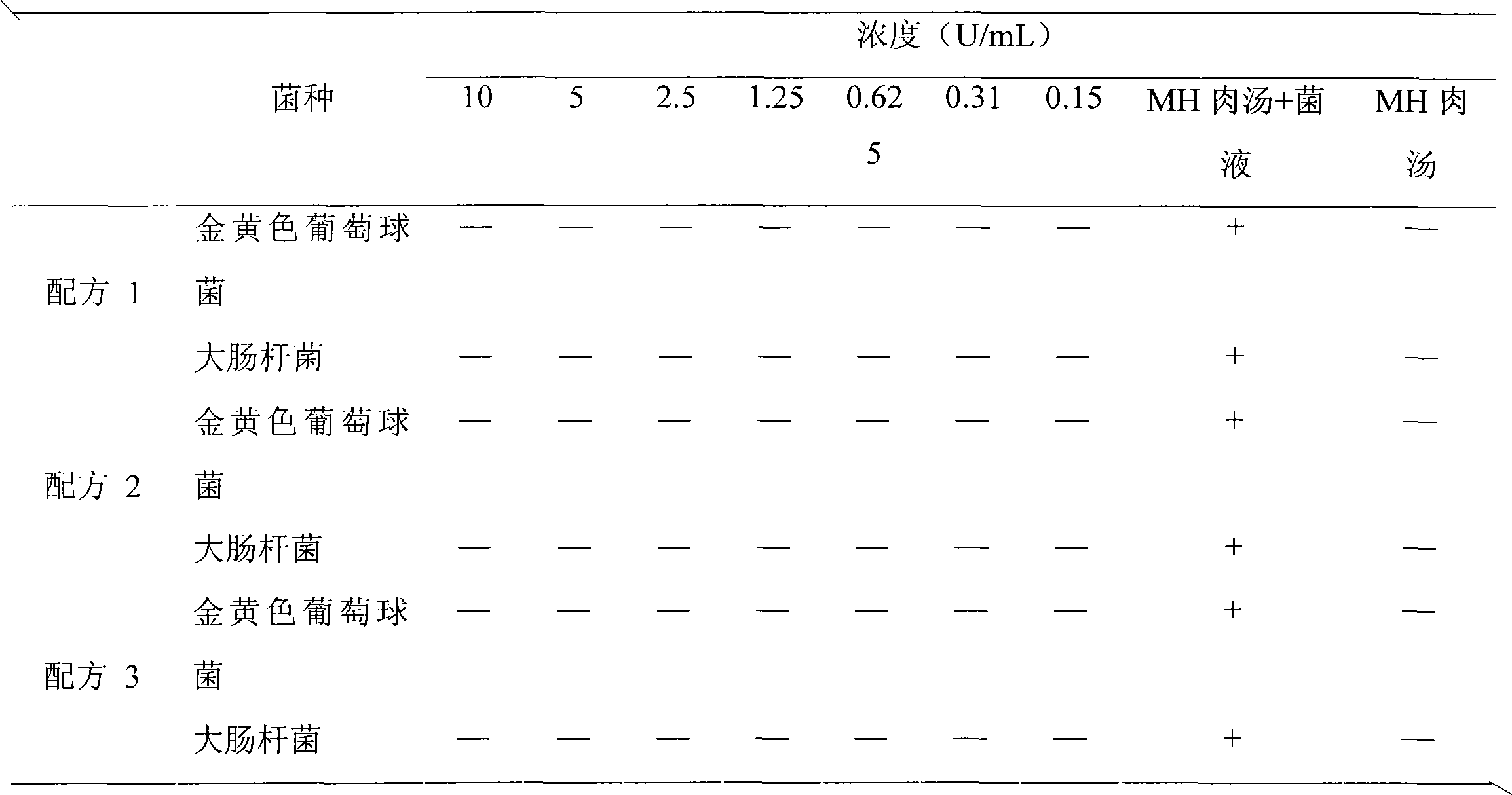
[0028] Embodiment 1 Composite enzyme suppository preparation method 1 (prescription 1)
[0029] 1. Composition and ratio
[0030] Lysostaphin 0.007%, lysozyme 0.1%, F6820%, HPMC 10%, sodium bicarbonate 10%, sodium dihydrogen phosphate 30%, semi-synthetic fatty acid glyceride 29.893%.
[0031] 2. Preparation
[0032]Weigh 0.007 g of lysostaphin, 0.1 g of lysozyme, 20 g of F68, 10 g of HPMC, 10 g% of sodium bicarbonate, and 30 g of sodium dihydrogen phosphate. The above raw materials are crushed through a 80-mesh sieve, mixed evenly, evenly mixed with 29.893 g of molten semi-synthetic fatty acid glycerides, and quickly poured into suppository molds to form.
Embodiment 2
[0033] Embodiment 2 Composite enzyme suppository preparation method 2 (prescription 2)
[0034] 1. Composition and ratio
[0035] Lysostaphin 0.004%, lysozyme 2%, F6810%, HPMC 20%, sodium bicarbonate 20%, sodium dihydrogen phosphate 10%, semi-synthetic fatty acid glyceride 37.996%.
[0036] 2. Preparation
[0037] Weigh 0.004 g of lysostaphin, 2 g of lysozyme, 10 g of F68, 20 g of HPMC, 20 g% of sodium bicarbonate, and 10 g of sodium dihydrogen phosphate. The above raw materials are crushed through an 80-mesh sieve, mixed evenly, evenly mixed with 37.996 g of molten semi-synthetic fatty acid glycerides, and quickly poured into a suppository mold to form.
Embodiment 3
[0038] Embodiment 3 Composite enzyme suppository preparation method 3 (prescription 3)
[0039] 1. Composition and ratio
[0040] Lysostaphin 0.01%, lysozyme 4%, F681%, HPMC 1%, sodium bicarbonate 30%, sodium dihydrogen phosphate 20%, semi-synthetic fatty acid glyceride 43.99%.
[0041] 2. Preparation
[0042] Weigh 0.01 g of lysostaphin, 4 g of lysozyme, F681 g, 1 g of HPMC, 30 g% of sodium bicarbonate, and 20 g of sodium dihydrogen phosphate. The above raw materials are crushed through an 80-mesh sieve, mixed evenly, evenly mixed with 43.99 g of molten semi-synthetic fatty acid glyceride, and quickly poured into a suppository mold to form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com