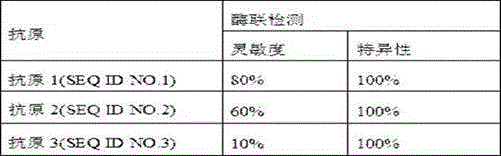
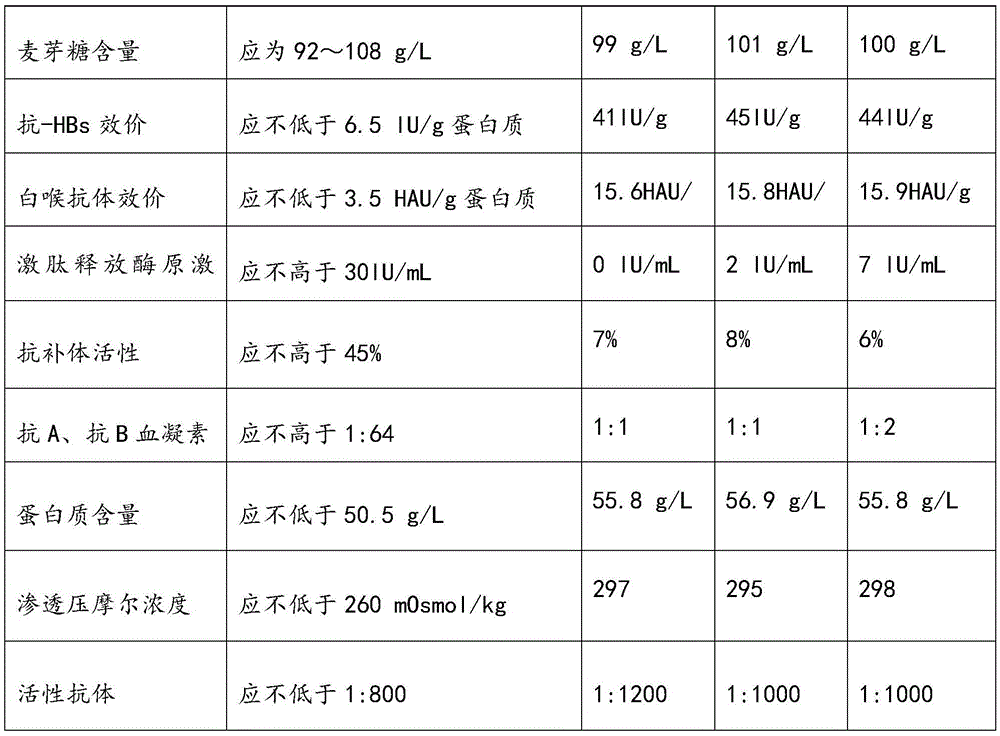
Patents
Literature
102 results about "Virus-neutralizing Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An antibody that binds to a virus and interferes with its ability to infect a cell.
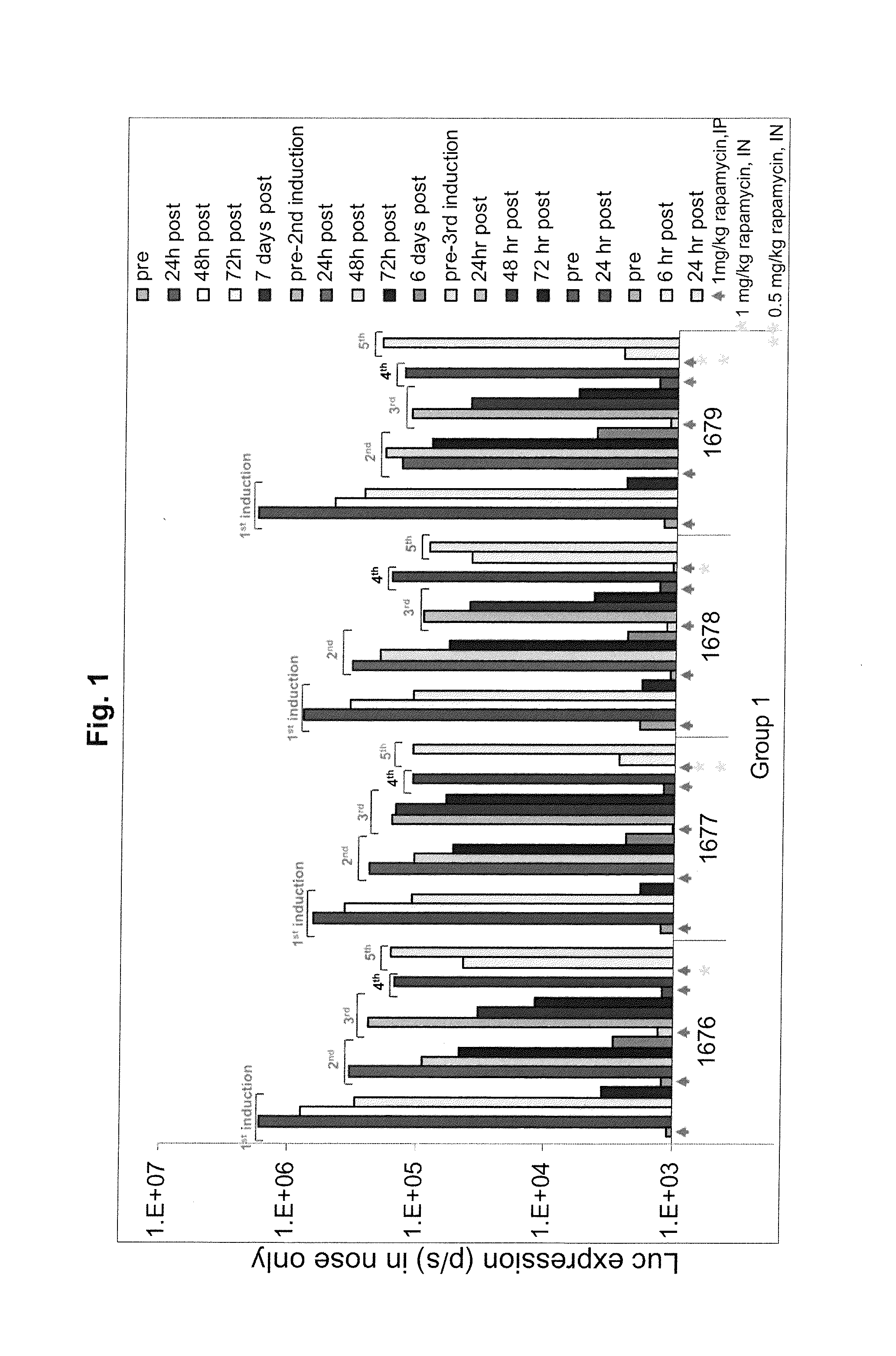
Regimens and Compositions for AAV-Mediated Passive Immunization of Airborne Pathogens
InactiveUS20140031418A1Organic active ingredientsBacterial antigen ingredientsRegimenHigh level expression
A prophylactic regimen for passively preventing infection with a pathogen which has a typical route of infection through the nasopharynx region of a subject, e.g., an airborne virus typically transmitted through coughing or sneezing. The method involves specifically targeting a subject's nasopharynx with a viral vector comprising an AAV capsid and carrying a nucleic acid sequence encoding an anti-viral neutralizing antibody construct operably linked to expression control sequences, in order to provide for high levels of expression of the anti-viral neutralizing antibody construct in the nasal airway cells. Optionally, the neutralizing antibody construct is expressed under a promoter which is regulated or induced by a small molecule which is delivered separately from the viral vector. In one embodiment, the method permits transfection of a subject's nasopharynx even where the subject has circulating neutralizing antibodies against the AAV capsid.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Recombinant cell line for stably expressing classical swine fever virus E2 protein, and applications of the same in preparation of subunit vaccines and diagnosis reagents of classical swine fever
ActiveCN103751774AStable in natureFight infectionMicroorganism based processesAntiviralsMaternal antibodyAntigen
The present invention discloses a strain of a recombinant cell line for stably expressing classical swine fever virus E2 protein, and applications of the recombinant cell line in preparation of subunit vaccines and diagnosis reagents of classical swine fever, wherein specifically the recombinant cell line is BCSFV-E2, is preserved in the China General Microbiological Culture Collection Center, and has the preservation number of CGMCC No.7719. The classical swine fever subunit vaccine prepared by using the recombinant cell line has characteristics of high safety, good immunization effect, easy mass production, less being susceptible to exogenous virus pollution or influence of antibodies, and no influence of the maternal antibody on immunization of swine, and can induce and produce high level classical swine fever virus neutralization antibodies after the swine is immunized. In addition, the present invention further discloses a method for constructing the recombinant mammalian cell line, a method for preparing the classical swine fever subunit vaccine, and applications of the antigen expressed by the recombinant cell line in preparation of classical swine fever prevention vaccines and diagnosis reagents.
Owner:HARBIN WEIKE BIOTECH DEV +1
Recombinant cell line for stable expression of porcine epidemic diarrhea virus S1 protein, vaccine and application
InactiveCN107619819AEase of mass productionGood antigenicityAntiviralsAntibody medical ingredientsEpidemic diarrheaAdjuvant
The invention discloses a recombinant cell line for stable expression of porcine epidemic diarrhea virus S1 protein, a vaccine and an application. The recombinant cell line is constructed by transfecting HEK-293T cells by virtue of recombinant plasmid which is constructed by carrying a target gene on a lentiviral vector, and then transfecting the HEK-293T cells by virtue of generated high-titer virus particles; and the recombinant cell line, which can achieve stable expression, can still keep an excellent protein expression level after several passages. The recombinant cell line for stable expression of the porcine epidemic diarrhea virus S1 protein provided by the invention has the characteristics of being easy for culture, rapid in proliferation, unlimited in expansion, stable in property and high in protein expression amount; and when the vaccine, which is prepared from the expression protein and adjuvants, is used for immunizing pigs, the generation of a high-titer porcine epidemicdiarrhea virus neutralizing antibody can be induced from animal bodies, and the piglets (the pigs) can resist strong attack of porcine epidemic diarrhea viruses.
Owner:GUANGZHOU BONIZZI BIOTECH CO LTD
Recombinant enterovirus 71 neutralizing antibodies and applications thereof
Provided is an antibody against enterovirus 71 comprising the amino acid sequence shown in SEQ ID NOS: 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, or 26 or functionally active homologues thereof. Also provided are methods for obtaining the antibody comprising (a) selecting a yeast expressing such an antibody from a yeast library, (b) culturing the yeast under conditions that the antibody is expressed, and (c) recovering the antibody from the culture. Also provided is a process for producing the antibody comprising (a) culturing a host cell under conditions that the antibody is expressed, (b) recovering the antibody from the culture, wherein the host cells are transformed or transfected for expressing the antibody against enterovirus 71. Also provided are pharmaceutical compositions comprising an antibody against enterovirus 71 and a pharmaceutically acceptable carrier or diluent, wherein the antibody has an anti-virus agent or detectable label attached thereto.
Owner:DEV CENT FOR BIOTECHNOLOGY
Type O foot-and-mouth disease virus mutant and preparation method and application thereof
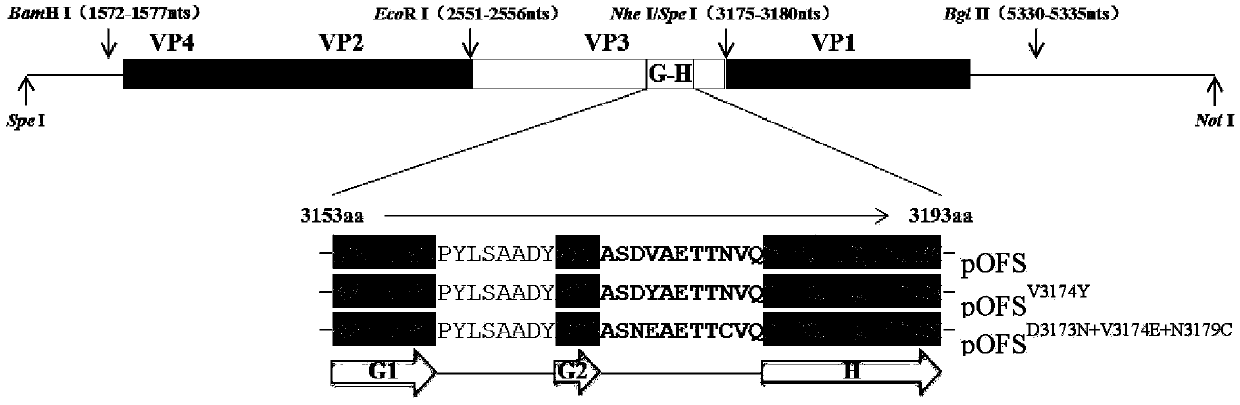
The invention provides a type O foot-and-mouth disease virus mutant and a preparation method and application thereof, and belongs to the technical field of vaccine candidate strains. According to thetype O foot-and-mouth disease virus mutant disclosed by the invention, an rHN virus strain is used as a female parent virus strain, and the following amino acids in a G-H ring of VP3 protein are mutated: the 173rd aspartic acid is mutated into asparagine, the 174th valine is mutated into glutamic acid and the 179th asparagine is mutated into cysteine. The virus mutant has heredity stability and obtains the capacity of caveolin for performing mediated infestation on CHO-K1cells. The result of detecting the cross neutralization capacity of immune positive serum indicates that compared with a female parent virus strain, the virus mutant has the advantages that the cross protection capacity of the virus mutant for inducing organisms to produce foot-and-mouth disease virus neutralizing antibodies is notably improved, and the virus mutant shows excellent antigen broad spectrum properties. Vaccines prepared through inactivation of the virus mutant can be used for preventing infection with type O foot-and-mouth disease viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Porcine circovirus II-type (PCV2) epitope peptide vaccine and preparation method thereof
ActiveCN103536912AStrong specificityEasy to saveViral antigen ingredientsAntiviralsDiseaseCircovirus
The invention relates to a porcine circovirus II-type (PCV2) epitope peptide vaccine and a preparation method of the vaccine. The vaccine contains three b cell epitopes, the lysine of the vaccine is four-branch peptide with the same core matrix structure epitope monomer and is connected with a general T ancillary cell (Th) epitope in series, and the vaccine has the molecular weight of 13kDa; the epitope peptide is named PCV CP98-156-228; after being vaccinated, a mice has stronger immune response, and a high potency virus neutralizing antibody can be generated. The epitope peptide vaccine has the advantages of being low in price, safe, high in specificity and easy to store and apply, and plays an important role in preventing and controlling the porcine circovirus disease. The PCV2 epitope peptide vaccine is prepared by four steps including predicting and screening epitope peptide, designing the epitope peptide vaccine, synthesizing, purifying and authenticating the epitope peptide as well as preparing the epitope peptide vaccine; the method is easy in raw material obtaining, lower in cost and easy to control, and has better operability, thus being suitable for being popularized and applied.
Owner:CHONGQING UNIV OF TECH +2
Recombinant receptor binding protein and recombinant receptor protein for detecting new coronavirus neutralizing antibody
ActiveCN112707968AEasy to mass produceMeet the needs of tracking and testingPolypeptide with localisation/targeting motifSsRNA viruses positive-senseReceptorImmunogenicity
The invention belongs to the field of biological medicines, and relates to a recombinant receptor binding protein and a recombinant receptor protein for detecting a new coronavirus neutralizing antibody, in particular to the recombinant receptor binding protein, the recombinant receptor protein, a nucleic acid molecule, a recombinant expression vector, a recombinant host cell, a reagent composition, a kit and application. The recombinant receptor binding protein is an RBD trimer protein, the RBD trimer protein has an optimized spatial structure and higher immunogenicity, the recombinant receptor protein is an ACE2 dimer protein, the ACE2 dimer protein has a natural protein conformation capable of simulating ACE2, after the recombinant receptor binding protein and the recombinant receptor protein are combined, the new coronavirus neutralizing antibody can be detected through the principle of enzyme linked immunosorbent assay, the sensitivity, specificity and stability are remarkably improved, and the requirement for tracking detection of the new coronavirus neutralizing antibody on the market can be greatly met.
Owner:SUZHOU CUREMED BIOMEDICAL TECH CO LTD
Anti-COVID-19 virus neutralizing antibody mhC3 as well as humanized antibody and application thereof
The invention discloses an anti-COVID-19 virus neutralizing antibody mhC3 as well as a humanized antibody and application thereof. The amino acid sequences of HCDR1, HCDR2 and HCDR3 in a heavy chain variable region of the antibody provided by the invention are shown as SEQ ID No.1, SEQ ID No.2 and SEQ ID No.3 in sequence; amino acid sequences of LCDR1, LCDR2 and LCDR3 in a light chain variable region of the antibody are shown as SEQ ID No. 4, SEQ ID No. 5 and SEQ ID No. 6 in sequence. The antibody disclosed by the invention can be specifically combined with novel coronavirus RBD protein and can neutralize novel coronavirus (SARS-CoV-2). The antibody provided by the invention can be used for preventing and treating coronavirus infection, and has important biological and medical significance.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Kit for testing neutralizing antibody racing ELISA in human and animal rabies
InactiveCN101251537AAccurate quantitative determinationEasy to operateBiological testingAntigenRabies
The invention discloses a reagent box for detecting hydrophobia neutralizing antibody competition ELISA of human beings and animals, wherein the reagent box can easily, quickly, accurately and quantitatively detect the hydrophobia neutralizing antibody in blood serums of human beings and animals by marking the hydrophobia neutralizing antibody, the standard serum and the envelope antigen. By using hydrophobia virosome or virus glycoprotein to coat enzyme synapticulae, the enzyme labeling hydrophobia neutralizing antibody is mixed with the blood serum to be tested and the standard serum respectively according to a certain ratio and reacts with the hydrophobia virus glycoprotein antigen coated on the enzyme synapticulae, a standard curve is drawn according to the OD value of the standard blood serum reaction and the known neutralizing titer after the color development, and the titer of the corresponding neutralizing antibody is obtained from the standard curve according to the OD value of the reaction of the blood serum to be tested. The reagent box has the advantages of accurately and quantitatively detecting the neutralizing antibody of the hydrophobia virus, along with simple operation and short time; moreover, the test result of the invention keeps a good consistence with test results of neutralizing test methods recommended by WHO and OIE.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Recombination VTT and method for detecting vaccinia virus neutralizing antibody by using same
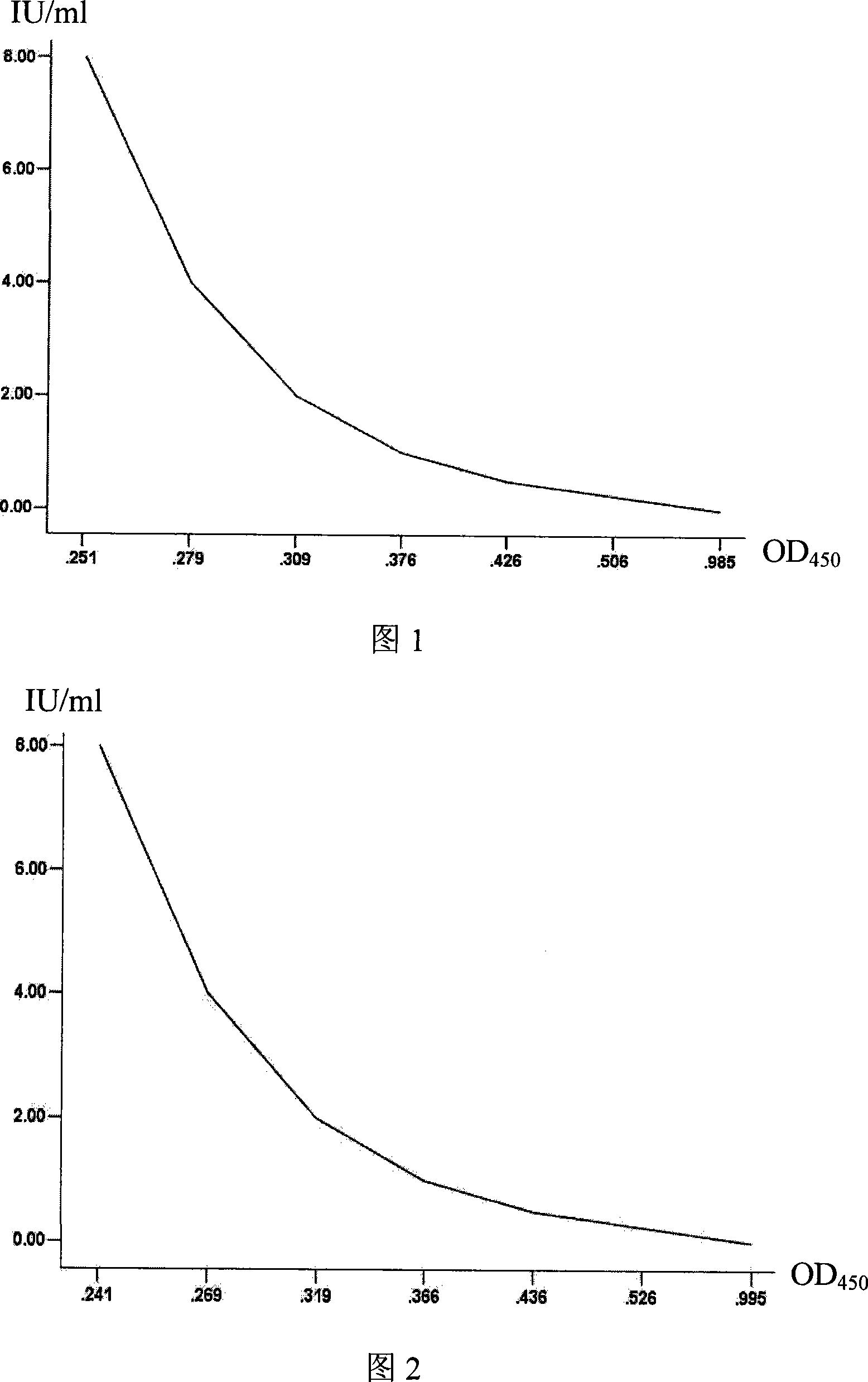
InactiveCN102391996AReduce demandShort detection cycleMicroorganism based processesViruses/bacteriophagesVacciniaLuciferases
The invention relates to recombination VTT (Vaccinia Tian Tan), and also relates to a vaccinia virus neutralizing antibody detection method and the purpose of the recombination VTT, wherein, the recombination VTT comprises luciferase genes and is used for detecting a vaccinia virus neutralizing antibody.
Owner:NAT INST FOR FOOD & DRUG CONTROL
H1n1 flu virus neutralizing antibodies
ActiveCN107922481ABiological material analysisImmunoglobulins against virusesH1n1 virusComplementarity determining region
An antibody, or a binding fragment of the antibody, against H1N1 virus, includes a heavy chain variable region and a light chain variable region, wherein the heavy chain variable region contains complementarity determining regions (CDR) that have the amino acid sequences of SEQ ID NO: 5, SEQ ID NO: 6, and SEQ ID NO: 7; and wherein the light chain variable region contains complementarity determining regions that have the amino acid sequences of SEQ ID NO: 8, SEQ ID NO: 9, and SEQ ID NO: 10. A method for treating or preventing H1N1 infection in a subject includes administering to the subject theantibody or the binding fragment of the antibody.
Owner:MEDIGEN BIOTECH
Novel coronavirus SARS-CoV-2 mRNA vaccines and preparation method and application thereof
ActiveCN113151312AProlong half-lifeEasy to getSsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationSpecific igg
The invention provides novel coronavirus SARS-CoV-2mRNA vaccines and a preparation method and application thereof. The invention provides three mRNA vaccines, namely RBD, S1 and S vaccines. The RBD vaccine disclosed by the invention can induce a high-titer antigen-specific IgG antibody and a virus neutralization antibody after immunization with one dose, the high-titer neutralization antibody can be maintained for at least 26 weeks, and remarkable immune protection can be provided for human ACE2 transgenic mice in serum adoptive transfer protection experiments. The RBD and S vaccine disclosed by the invention can induce immune protection capable of completely resisting SARS-CoV-2 virus infection in the human ACE2 transgenic mice after immunization with two doses. A large number of experimental results show that the mRNA vaccine provided by the invention has good immunogenicity, forms powerful immune protection after immunizing an organism, and has a huge development potential.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Regimens and Compositions for AAV-Mediated Passive Immunization of Airborne Pathogens
PendingUS20190216841A1Organic active ingredientsBacterial antigen ingredientsRegimenHigh level expression
A prophylactic regimen for passively preventing infection with a pathogen which has a typical route of infection through the nasopharynx region of a subject, e.g., an airborne virus typically transmitted through coughing or sneezing. The method involves specifically targeting a subject's nasopharynx with a viral vector comprising an AAV capsid and carrying a nucleic acid sequence encoding an anti-viral neutralizing antibody construct operably linked to expression control sequences, in order to provide for high levels of expression of the anti-viral neutralizing antibody construct in the nasal airway cells. Optionally, the neutralizing antibody construct is expressed under a promoter which is regulated or induced by a small molecule which is delivered separately from the viral vector. In one embodiment, the method permits transfection of a subject's nasopharynx even where the subject has circulating neutralizing antibodies against the AAV capsid.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
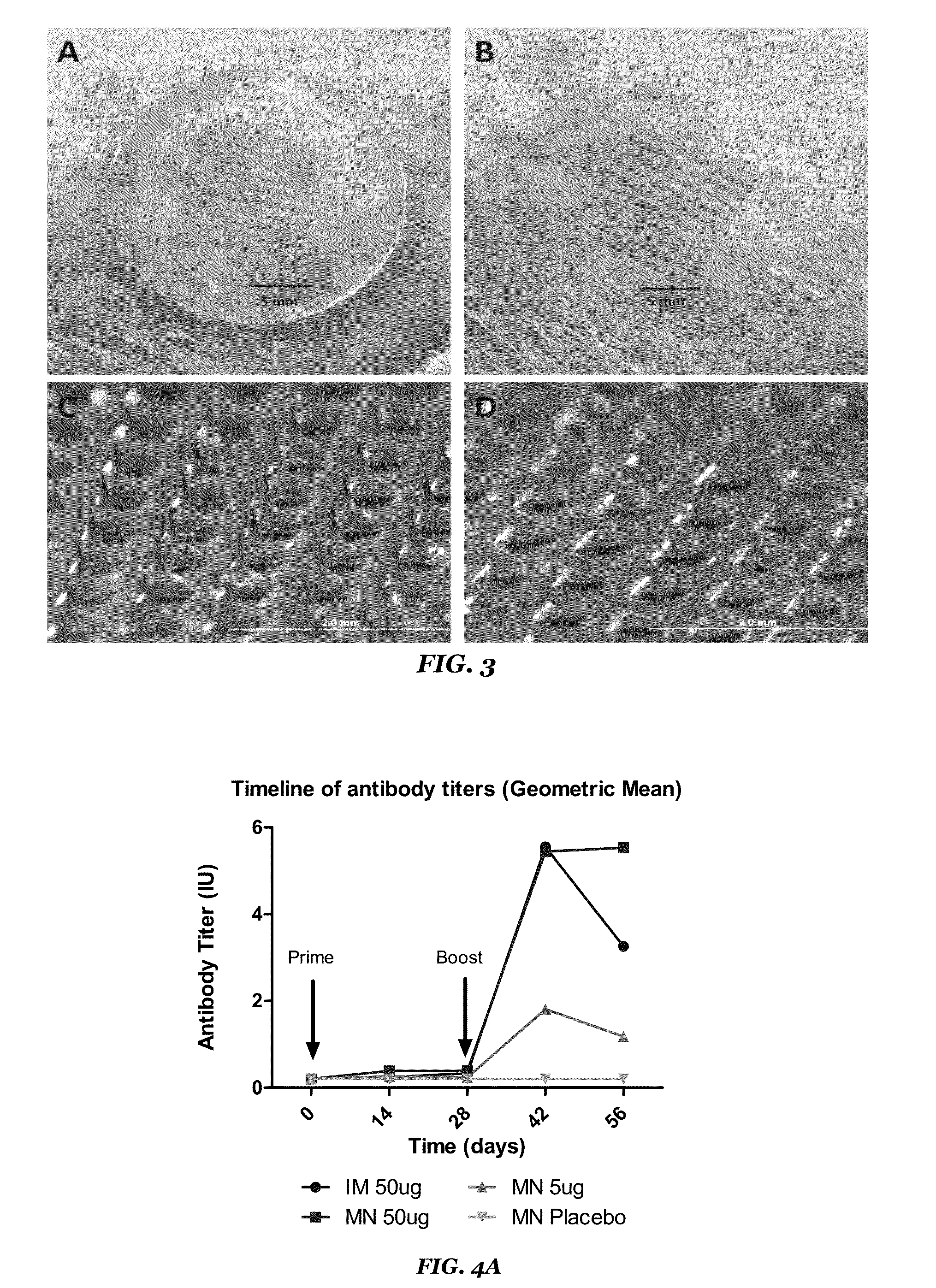
Methods of using Microneedle Vaccine Formulations to Elicit in Animals Protective Immunity against Rabies Virus
ActiveUS20160120799A1High level of stabilityEffective in eliciting protective immune responseSsRNA viruses negative-senseViral antigen ingredientsDiseaseAntigen
The present invention relates to microneedle vaccine formulations, as well as methods of use thereof to provide animals, including canines, protective immunity against infection and disease caused by rabies viruses. Once placed onto the skin of the animals, the microneedle formulations dissolve quickly into the surrounding skin, where the antigens then elicit in the animals high and protective levels of rabies virus neutralizing antibodies.
Owner:MERIAL INC
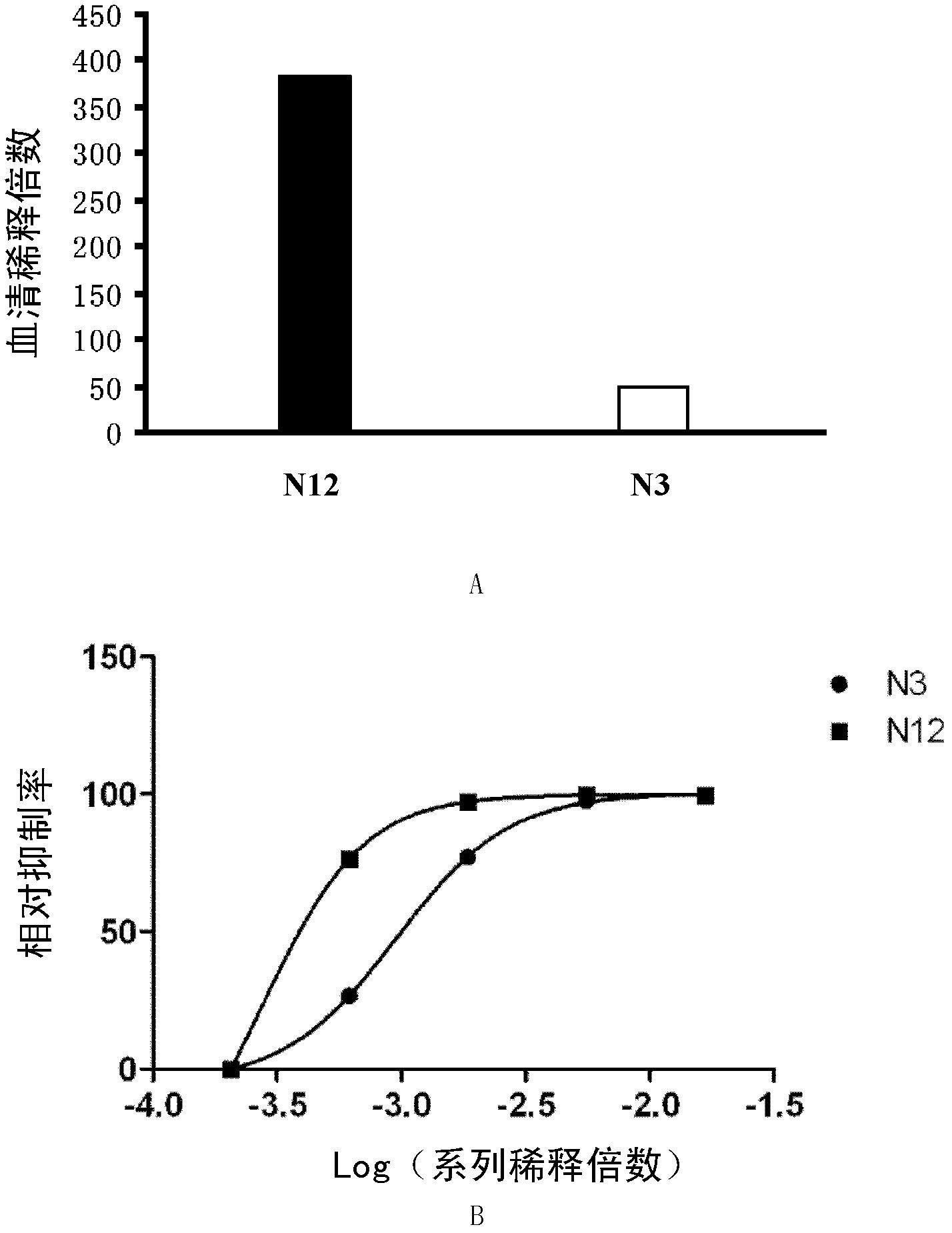
Method for detecting enterovirus neutralizing antibody and special recombinant virus for method
InactiveCN102911948ALower requirementSimple and fast operationFungiBacteriaHand-foot-and-mouth diseaseGenomic rna
The invention discloses a method for detecting an enterovirus neutralizing antibody and a special recombinant virus for the method and provides a DNA (deoxyribonucleic acid) molecule. The DNA molecule is a recombinant DNA obtained by substituting coding genes of all structural proteins in cDNA (complementary DNA) corresponding to genome RNA (ribonucleic acid) of an enterovirus for report genes. An EV71 FY pseudovirus system is used for the method for detecting the neutralizing antibody, and since pseudoviruses subjected to monocyclic infection are adopted, the safety problem during using of live viruses is avoided. After multiple tests, results show that the pseudovirus system is the method for detecting the neutralizing antibody and is safe, sensitive, rapid, specific, simple, convenient and low in cost. Based on the advantages, the pseudovirus system is extremely suitable for rapid and massive neutralizing antibody detection tests, and has important application values for virus vaccine development and detection of hand-foot-and-mouth disease specificity neutralizing antibody level of individual patients and patient populations.
Owner:NAT INST OF BIOLOGICAL SCI BEIJING
Respiratory syncytial virus resistance human immune globulin and preparation method thereof
ActiveCN105601736AStrong specificityExtended shelf lifeImmunoglobulins against virusesPeptide preparation methodsUltrafiltrationIon exchange
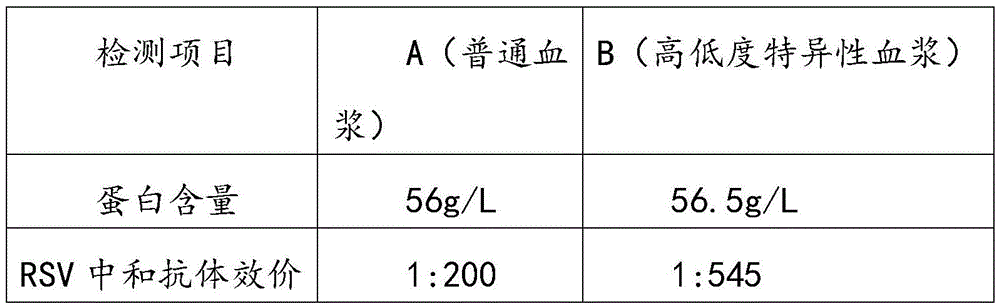
The invention relates to respiratory syncytial virus resistance human immune globulin and a preparation method thereof. The titer of the respiratory syncytial virus neutralizing antibody is larger than or equal to 1:900. The preparation method includes the steps of firstly, screening out privilege plasma with titer of the respiratory syncytial virus neutralizing antibody not lower than 1:200; secondly, mixing screened-out efficient privilege plasma; thirdly, separating the mixed plasma through a low-temperature ethyl alcohol filter press method, purifying and separating out a immune globulin component II through the combination with the ion-exchange column chromatography method, and obtaining immune globulin with the purity of 98-100% through filtration, chromatography, ultrafiltration, preparation, low-pH incubation virus inactivation, nano-membrane filtration virus removal and subpackaging. The titer, purity and recycling rate of the respiratory syncytial virus resistance human immune globulin obtained through the preparation and production method are high, treatment can be conducted for the respiratory syncytial virus, and the respiratory syncytial virus resistance human immune globulin and the preparation method have advantages of being safe, effective and the like.
Owner:哈尔滨派斯菲科生物制药有限公司
Varicella-zoster virus gE antigen and application thereof in detection of anti-varicella-zoster virus antibody
The invention discloses a varicella-zoster virus gE glycoprotein antigen capable of detecting an anti-varicella-zoster virus antibody, particularly an anti-varicella-zoster virus neutralizing antibody, and also discloses application of the antigen in the preparation of a detection reagent for detecting the anti-varicella-zoster virus neutralizing antibody and a corresponding detection reagent kit.
Owner:北京市华信行生物科技有限公司
Method for preparing animal cell galactosylated modification influenza HA (Hemagglutinin) glycoprotein from glycosyl engineering yeast
ActiveCN105671109AEfficient research and developmentIncrease production capacityFungiVirus peptidesHemagglutininYeast
The invention discloses a method for preparing animal cell galactosylated modification influenza HA (hemagglutinin) glycoprotein from glycosyl engineering yeast. The method provided by the invention comprises the following steps of expressing influenza virus HA genes in a yeast mutant to obtain recombinant yeast; and culturing the recombinant yeast for preparation to obtain an influenza virus HA glycoprotein with mammal glycoform structures but without fucose. Experiments prove that a vaccine prepared from influenza HA glycoprotein particles prepared by the method can be induced to generate high anti-influenza-virus neutralizing antibodies, and the problems of allergy and the like possibly caused by fungus galactosylated modification can be solved.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Novel coronavirus detection kit as well as detection method and application thereof
PendingCN112816712AHigh sensitivityHas practical application prospectsBiological testingImmunoassaysCapture antibodyViral antigens
The invention belongs to the field of biological medicines, and particularly relates to a novel coronavirus neutralizing antibody detection kit prepared by adopting a Pt-Pd alloy nano-particle labeled immunochromatography method, and a detection method and application of the novel coronavirus neutralizing antibody detection kit. The kit comprises a novel coronavirus SARS-CoV-2 neutralizing antibody Pt-Pd alloy nanoparticle test strip. The preparation method of the novel coronavirus SARS-CoV-2 neutralizing antibody Pt-Pd alloy nanoparticle test strip comprises the following steps: marking a novel coronavirus SARS-CoV-2 antigen as a capture antibody nano marker by using alloy nanoparticles, coating a purified A-type expression antigen and an antibody thereof on a cellulose membrane to respectively serve as a detection line T line and a quality control line C line, and performing condition optimization to establish the novel coronavirus neutralizing antibody Pt-Pd alloy nanoparticle test strip. The kit is convenient to operate and carry, has relatively high sensitivity, has operability and reproducibility, and can be used for diagnosing pneumonia caused by novel coronavirus, and the sensitivity of the kit can be improved by 24 times compared with that of colloidal gold.
Owner:王思亓
Human immunoglobulin resisting hand-foot-and-mouth disease and preparation method of human immunoglobulin
ActiveCN105669860AImprove survival rateStrong specificityImmunoglobulins against virusesPeptide preparation methodsEnterovirusUltrafiltration
The invention relates to human immunoglobulin resisting the hand-foot-and-mouth disease and a preparation method of the human immunoglobulin. The hand-foot-and-mouth virus-neutralizing antibody titer is larger than or equal to 1:800. The preparation method comprises steps as follows: (1) efficient positive plasma with the hand-foot-and-mouth virus-neutralizing antibody titer larger than or equal to 1:80 is screened; (2) the screened efficient positive plasma is mixed; (3) the mixed plasma is separated with a low-temperature ethanol filter pressing method, immunoglobulin components II are purified and separated with an ion-exchange column chromatography method, and the immunoglobulin with the purity ranging from 98.5% to 100% is obtained through filtration, chromatography, ultrafiltration, preparation, incubation of inactivated viruses at low pH, virus removal through nanofilm filtration and subpackaging. By means of the preparation and production method, the hand-foot-and-mouth virus-neutralizing antibody titer, the purity and the recovery rate are high, enteroviruses can be treated in a targeted manner, and the human immunoglobulin is an effective medicine for treating an enterovirus infectious disease, is safe and reliable and has higher social benefits and economic benefits.
Owner:哈尔滨派斯菲科生物制药有限公司
Grass carp hemorrhage vaccine prepared through yeast display and preparation method of grass carp hemorrhage vaccine
InactiveCN105879022AAvoid efficiencyAvoid attenuationFungiViral antigen ingredientsSurface displayYeast display
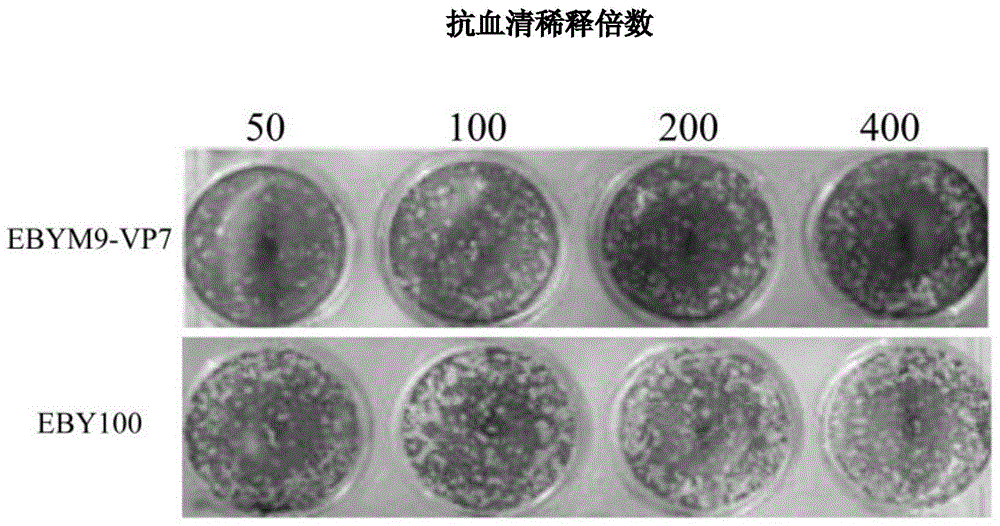
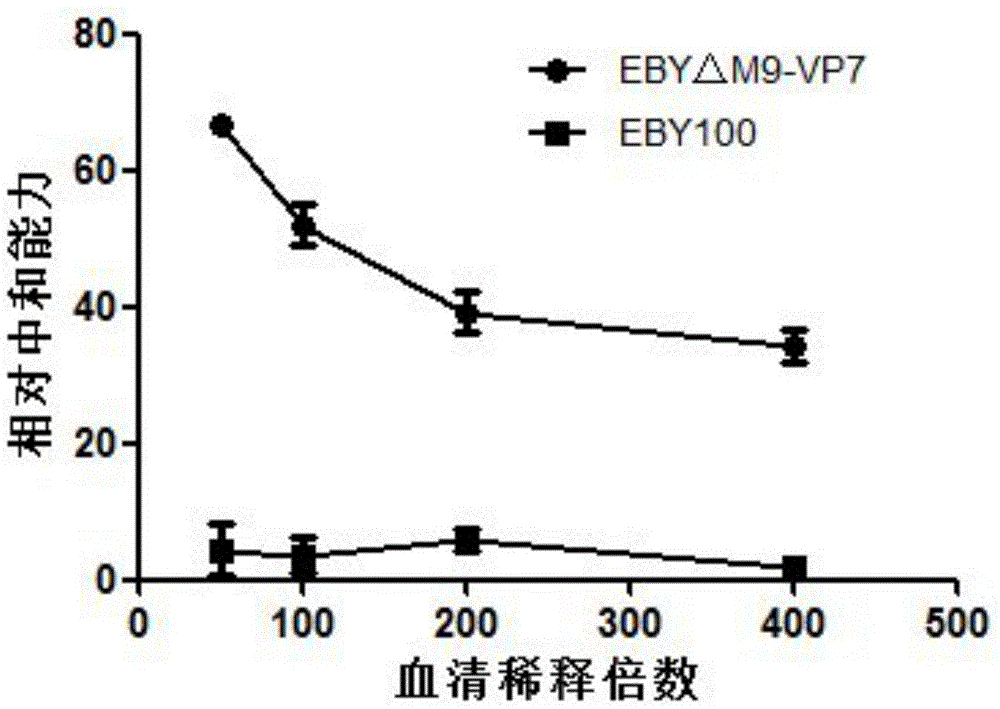
The invention discloses a grass carp hemorrhage vaccine prepared by adopting a yeast surface display technology, relating to the preparation of genetic engineering vaccines. The grass carp hemorrhage vaccine is prepared in a manner that GCRV-VP7 protein is displayed on the surface of brewer's yeast cells EBY100 delta Mnn9 with glycosylated genes knocked out. The brewer's yeast is an expression system of eukaryon, the galactosylated modification is carried out on the virus coat protein expressed by the brewer's yeast, and then an immune system of the grass carp is induced to generate virus-neutralizing antibodies. The grass carp hemorrhage vaccine has the advantages that a used yeast strain EBY100 delta Mnn9 with glycosylation deficiency can effectively remove the super glycosylation of the protein on the surface of the yeast, and thus the problem that the GCRV-VP7 antigen is covered, consequently, the vaccine efficiency loss and decrease are caused, is solved; the yeast cells are easy to culture and low in preparation cost, and thus the grass carp hemorrhage vaccines can be prepared in large scale at low cost.
Owner:INST OF AQUATIC LIFE ACAD SINICA +1
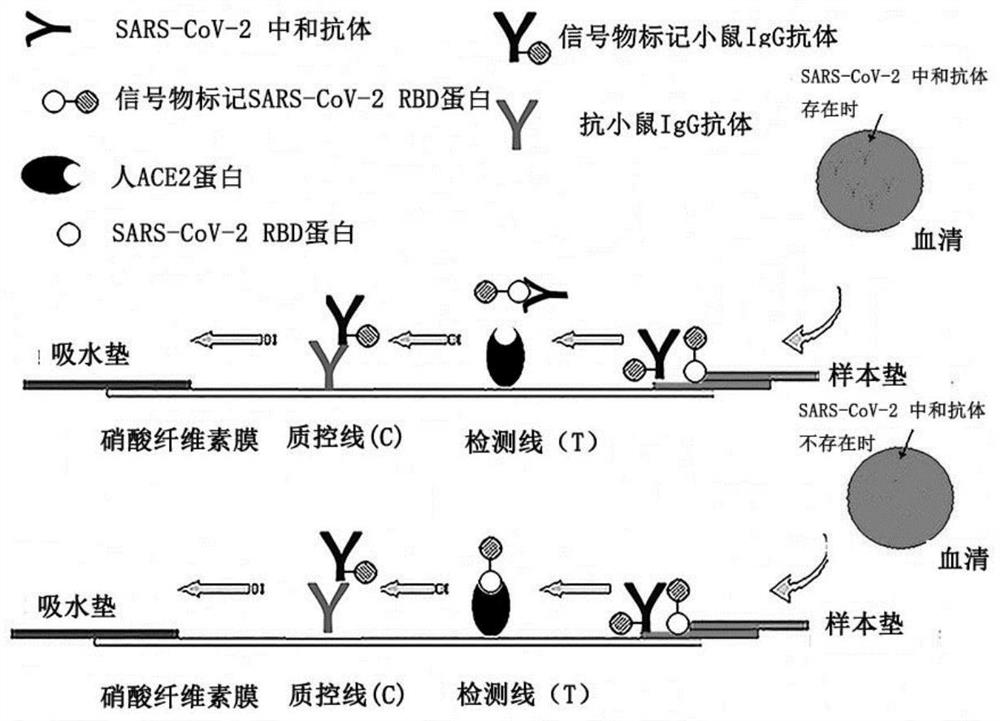
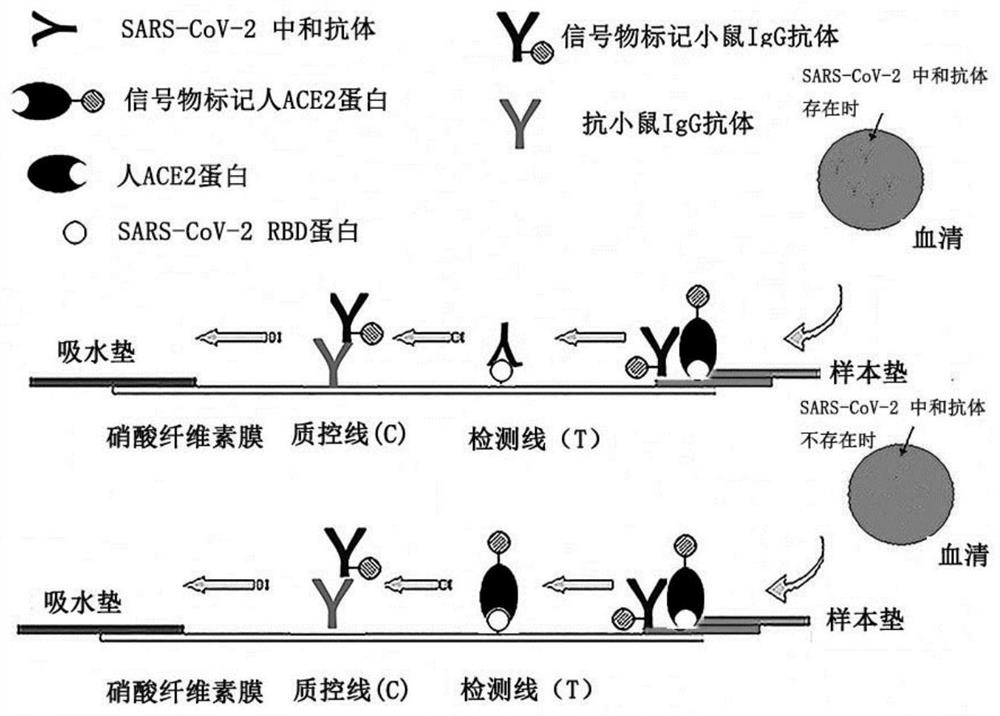
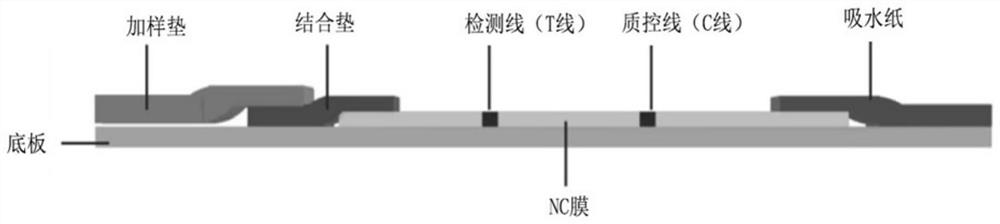
Immunochromatography device for detecting SARS-CoV-2 virus neutralizing antibody and application thereof
The invention relates to the field of antibody detection, in particular to an immunochromatography device for detecting an SARS-CoV-2 virus neutralizing antibody and application of the immunochromatography device, and further relates to a preparation method and a use method of the immunochromatography device for detecting the SARS-CoV-2 virus neutralizing antibody. The immunodetection device for detecting the coronavirus neutralizing antibody comprises a coronavirus spike protein part, ACE2 protein or a functional fragment thereof specifically combined with the coronavirus spike protein part and a solid-phase support, By detecting influence of a sample to be detected on the combination of the coronavirus spike protein part and the ACE2 protein or the functional fragment thereof on the solid-phase support, whether the coronavirus neutralizing antibody exists in the sample or not is judged. The immunochromatography device disclosed by the invention has specific, sensitive, rapid, simple and convenient detecting test paper, and is easy to popularize and apply in production practice.
Owner:NANJING GENSCRIPT BIOTECH CO LTD
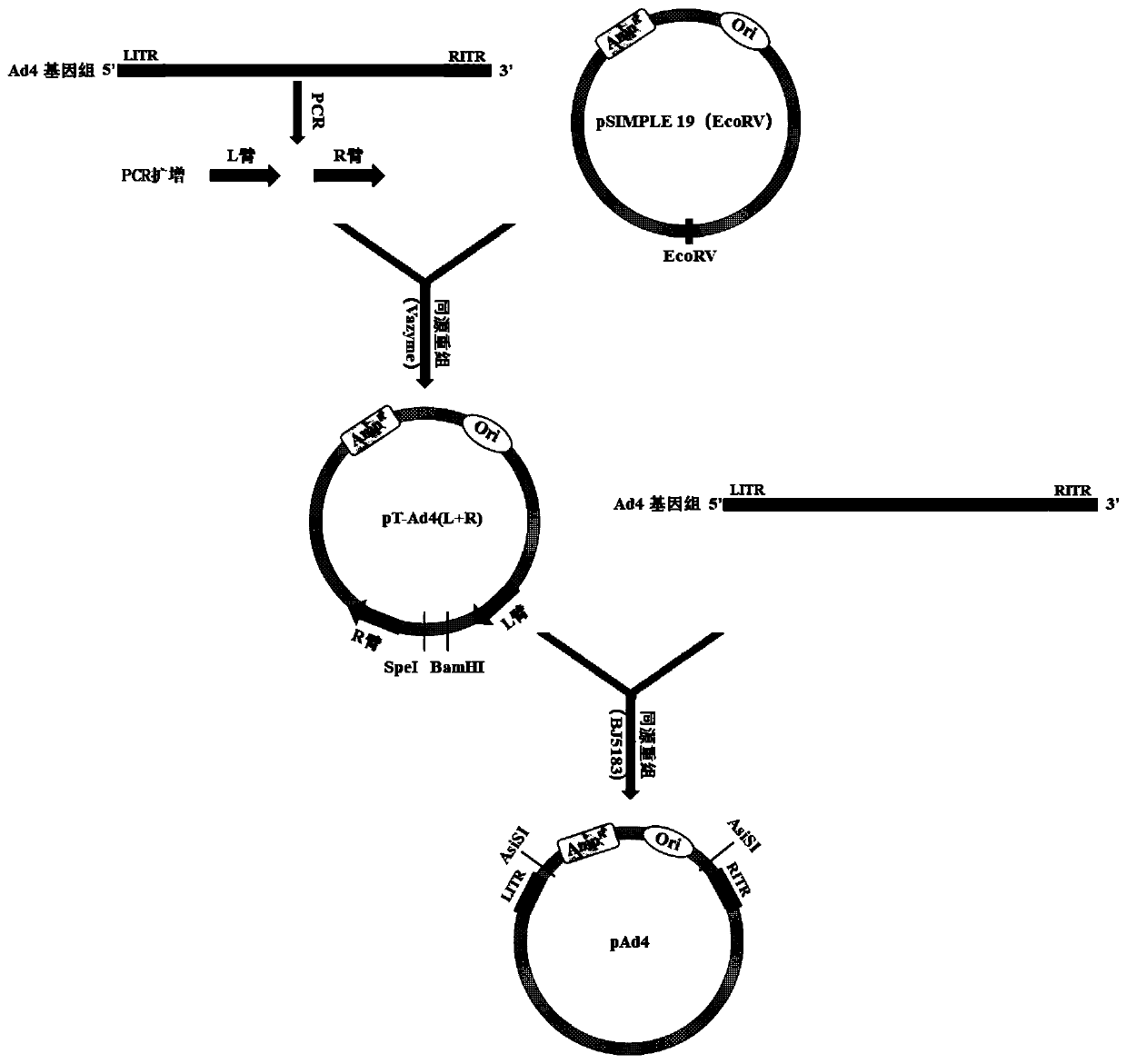
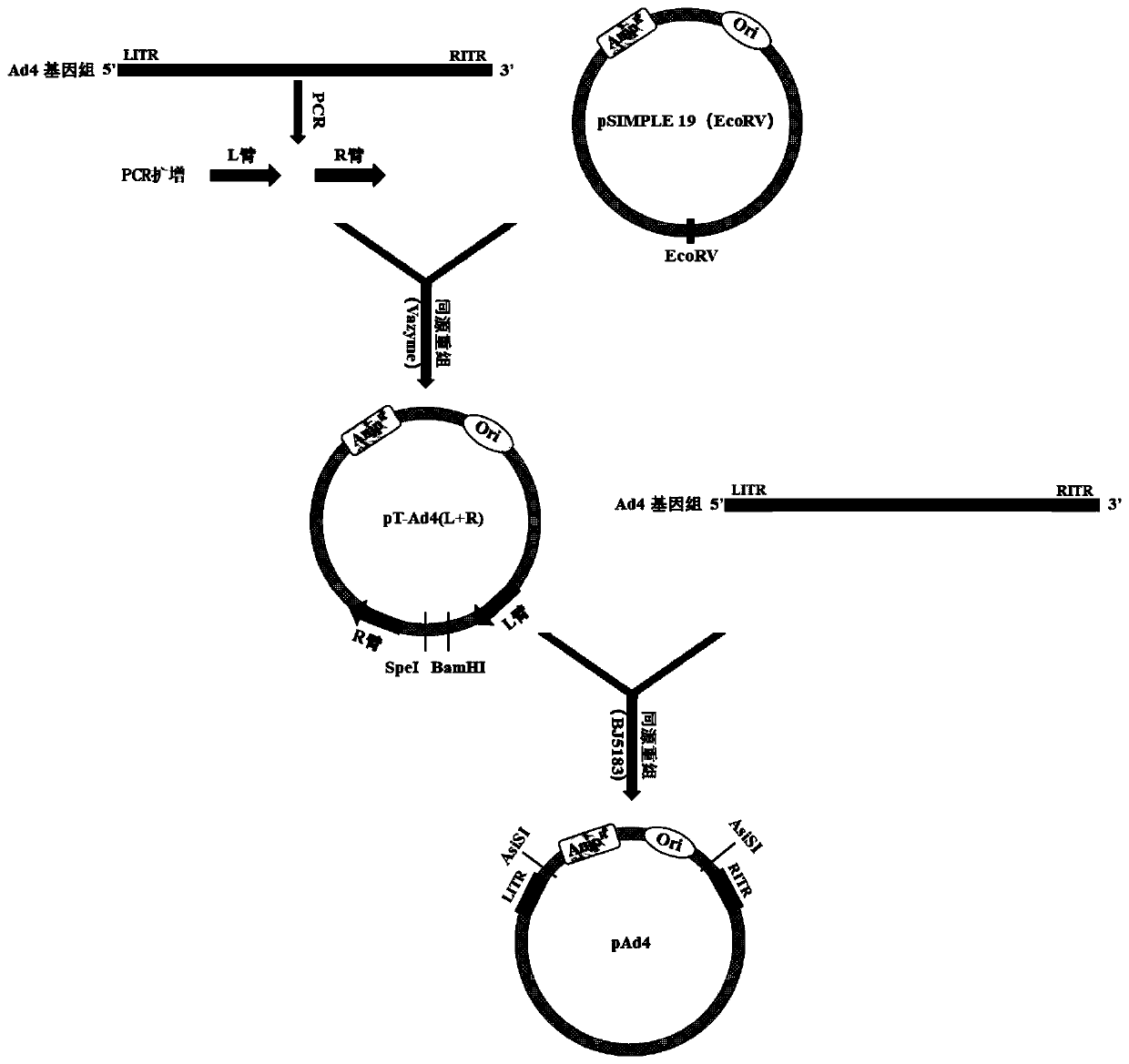
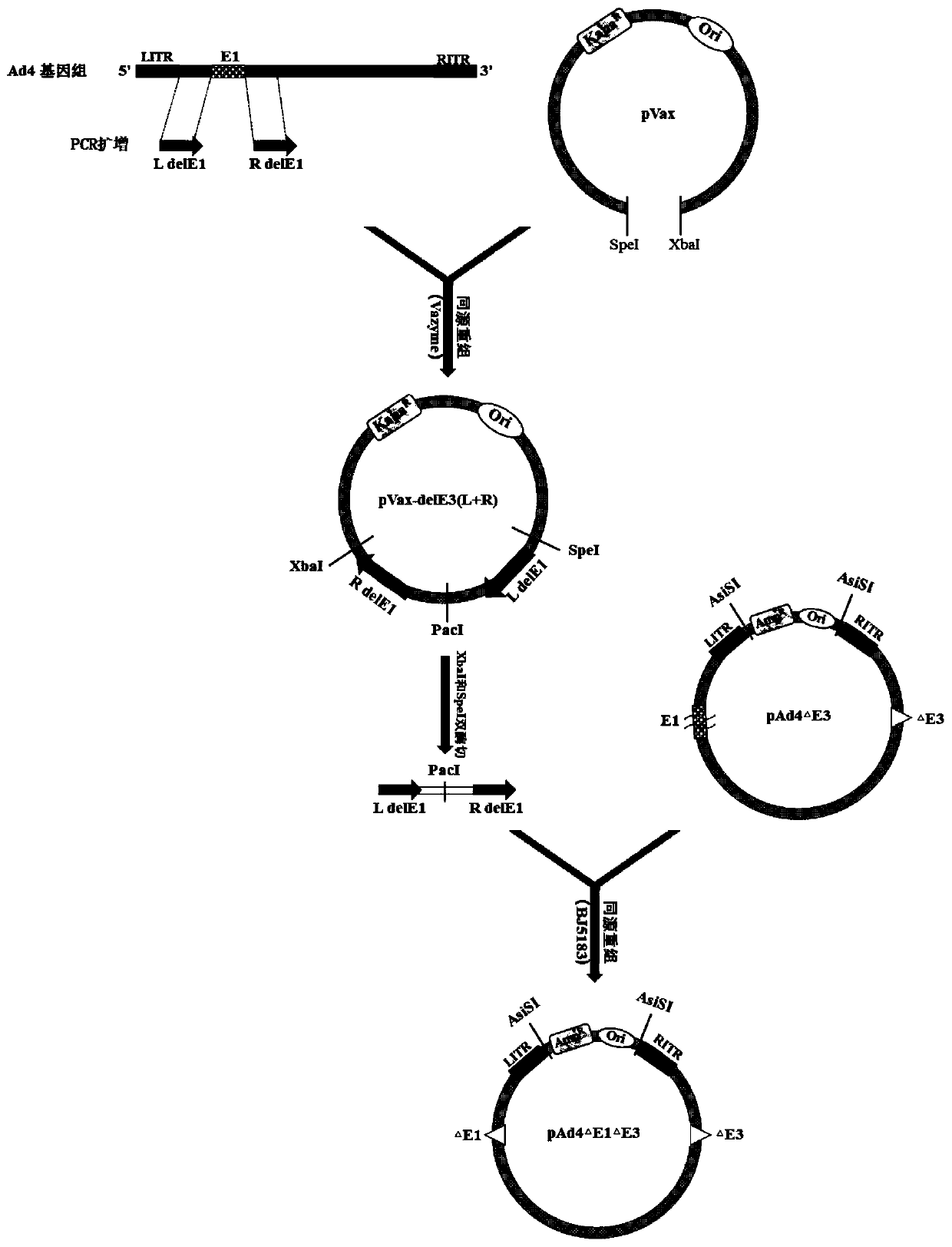
Replication-defective recombinant human-type-4 adenovirus, and preparation method and application thereof
PendingCN110551757AStrong immune responseViral antigen ingredientsImmunoglobulins against virusesOpen reading frameHuman type
The invention discloses a preparation method and application of replication-defective recombinant human-type-4 adenovirus. The replication-defective recombinant human-type-4 adenovirus is obtained bythe following method that a genome of the human-type-4 adenovirus is made to be like plasmids, genes E1 and E3 of the human-type-4 adenovirus are knocked out, and open reading frames 2, 3, 4, 5 and 6of an gene E4 of Ad4 are replaced with the corresponding open reading frames of an gene E4 of Ad5. The described replication-defective recombinant human-type-4 adenovirus vector can be potentially applied to the research and development of anti human-type-4 adenovirus vaccine, the screening of anti human type 4 adenovirus neutralizing antibody and drugs, the research and development of anti otherpathogen vaccine, report tracer system of biological research and the like.
Owner:GUANGZHOU N BIOMED LTD
Detection kit for varicella-herpes zoster virus neutralizing antibody and detection method for varicella-herpes zoster virus neutralizing antibody
The invention relates to a detection kit for a varicella-herpes zoster virus neutralizing antibody and a detection method for the varicella-herpes zoster virus neutralizing antibody. The detection kitcomprises a recombination expression varicella-herpes zoster virus glycoprotein E antigen, a sample dilution solution, 20-times concentrated washing solution, an enzyme-labelled second antibody, a substrate, a stop solution, standard serum and negative control. The recombination expression varicella-herpes zoster virus glycoprotein E antigen is a recombination expression plasmid transformation escherichia coli which is constructed by connecting a VZV-gE gene and a recombination expression vector pET-32a(+) vector, wherein the VZV-gE gene is optimized to be a escherichia coli preferred codon,and expressed recombinant protein is cloned, cultured and induced. The antigen used in the detection kit is the recombination expression varicella-herpes zoster virus glycoprotein E antigen, the safety is good, and pollution to the environment is not caused. The recombination expression varicella-herpes zoster virus glycoprotein E antigen is prepared by genetic engineering, the specificity is high, the quality is pure, and the cost is relatively low; and antigen site selection is accurate, coupling is good, nonspecific binding can be effectively excluded, the measurement error is reduced, andthe appearance of false negative and false positive is reduced.
Owner:WUHAN LIFE TECH
HIV-1 envelope protein associated with a broadly reactive neutralizing antibody response
The present invention relates to HIV-1 envelope proteins from a donor with non-progressive HIV-1 infection whose serum contains broadly cross-reactive, primary virus neutralizing antibody. The invention also relates to isolated or purified proteins and protein fragments that share certain amino acids at particular positions with the foregoing HIV-1 proteins.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Duck flavivirus subunit vaccine, as well as a preparation method and application thereof
ActiveCN102793918AAvoid infectionHigh amount of epitopeViral antigen ingredientsMicroorganism based processesMicroorganismCulture cell
The invention discloses a duck flavivirus subunit vaccine, as well as a preparation method and an application thereof. The duck flavivirus subunit vaccine contains stable transfected cell line expressed duck flavivirus prM-E protein and an adjuvant. The invention also discloses a method for establishing a stably expressed duck flavivirus prM-E protein cell line and culturing cell line expressed protein, and in particular relates to an expressed duck flavivirus prM-E protein cell line BHK-BYD-ME which is preserved in China General Microbiological Culture Collection Center with the number of CGMCC No.6342. The duck flavivirus subunit vaccine has high safety and a good immunization effect, and virus-neutralizing antibodies can be induced after a duck is immunized. Experiments prove that the vaccine can remarkably improve the resistance of a duck to the duck flavivirus, and can be used for preventing and controlling the duck flavivirus.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Neutralizing antibody high-sensitivity detection method and product
The invention relates to the field of antibody detection, in particular to an antibody detection method and product which have higher sensitivity when applied to neutralizing antibody detection, can further detect total antibodies and have wide market application value in current novel coronavirus neutralizing antibody and total antibody detection. The detection method provided by the invention comprises the step of contacting a sample with a ligand-containing fragment, a reagent 1 and a reagent 2. Of the reagent 1 and the reagent 2, one is connected with a marker, and the other is fixed on a solid-phase carrier.
Owner:GUANGDONG FAPON BIOTECH CO LTD
2019-nCoV S-antigens for generating 2019-nCoV neutralizing antibodies and method for preparing 2019-nCoV S-antigens
InactiveCN113248581AImprove immunityEffective immunitySsRNA viruses positive-senseSerum immunoglobulinsAntigenCell membrane
The invention discloses a 2019-nCoV S-antigens for generating 2019-nCoV neutralizing antibodies and a method for preparing the 2019-nCoV S-antigens. According to the 2019-nCoV S-antigens and the method, the 2019-nCoV S-antigens comprise 2019-nCoV S-antigen trimers combined with a cell membrane based on a Flag tag. The 2019-nCoV S-antigens can generate high-titer neutralizing antibodies so as to more effectively immunize animals.
Owner:江西浩然生物制药有限公司
Rabies virus-specific neutralizing human monoclonal antibodies and nucleic acids and related methods
Human monoclonal rabies virus neutralizing antibodies represent a safe and efficacious post-exposure prophylactic therapy for individuals exposed to a rabies virus. The nucleic acid and encoded amino acid sequences of the heavy and light chain immunoglobulins of human monoclonal rabies virus neutralizing antibodies, and their use, is described.
Owner:THOMAS JEFFERSON UNIV +1
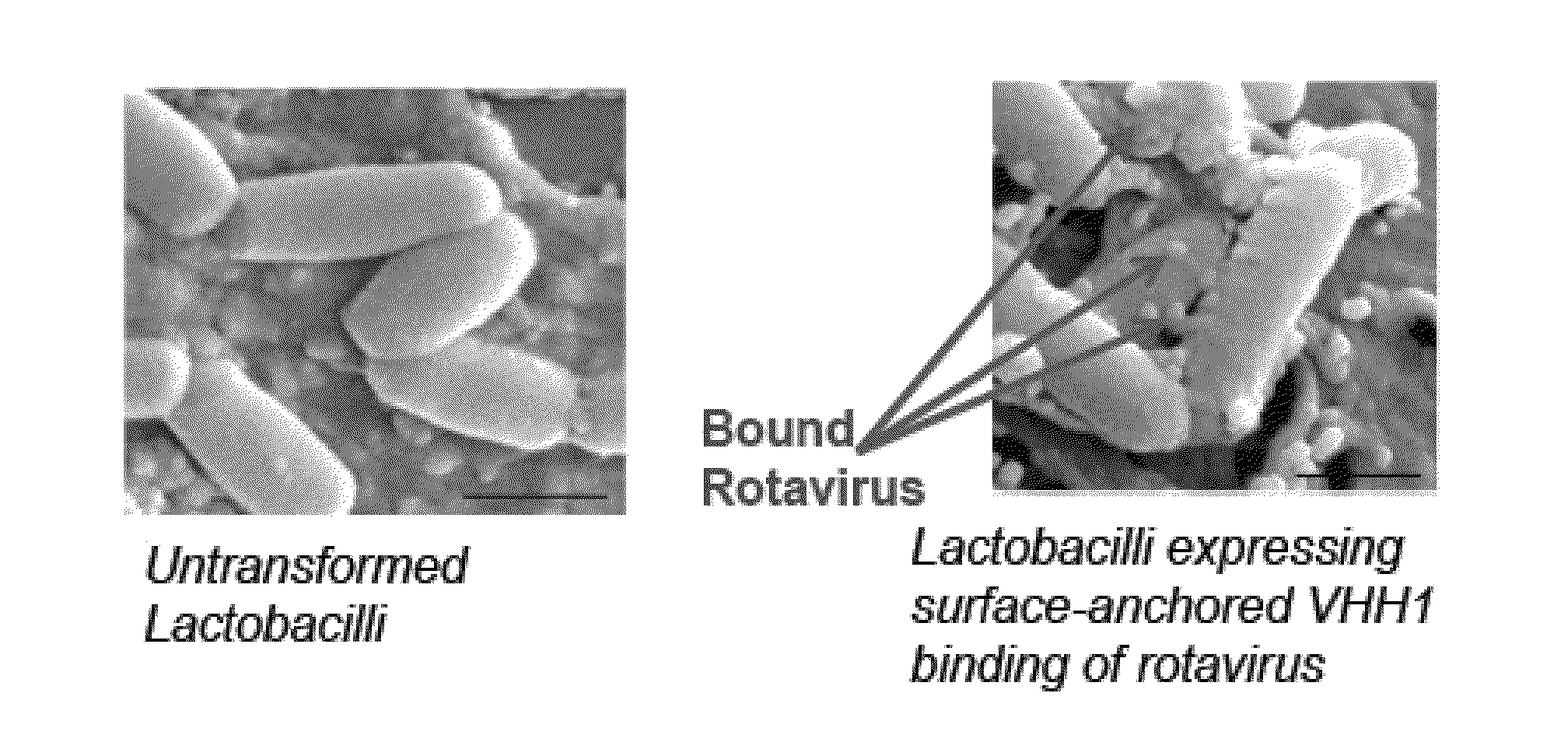
Compositions and Methods for Preventing or Relieving Symptoms of Infections
ActiveUS20150079115A1Rapid isolationCost-effectiveMicrobiological testing/measurementImmunoglobulins against virusesMicroorganismAntibody fragments
Food products and / or pharmaceutical preparations including (i) viral neutralizing antibodies or antibody fragments anchored to a probiotic microorganism and (ii) a carrier medium for delivering the viral neutralizing antibodies or antibody fragments anchored to probiotic microorganisms to the gut of a mammal. Also provided are methods of making food products and / or pharmaceutical preparations, which can be used to treat existing viral infections or prevent the spread or transmission of viral infection.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com